- 1Laboratory of Laser Sports Medicine, School of Sports Science, South China Normal University, Guangzhou, China
- 2Department of Rehabilitation Medicine, Guangdong Women and Children Hospital, Guangzhou, China
- 3Department of Rehabilitation Medicine, Xiangtan Central Hospital, Xiangtan, China
Objective: Controversy exists regarding the impact of metformin and whether it prevents or promotes the incidence of cognitive dysfunction. This systematic review and meta-analysis were conducted to identify the effect of metformin therapy on cognitive function in patients with diabetes.
Methods: Electronic databases (PubMed, EMBASE, PsycINFO, the Cochrane Library, and Web of Science) were systematically searched by two investigators from the date of inception until March 1, 2022. The study followed PRISMA guidelines. Inclusion criteria were defined according to the PECOS model. Eligible studies investigated cognitive dysfunction in metformin users compared with non-users in adults with diabetes. Only observational study designs (such as cohort, cross-section, and case-control) were included.
Results: A systematic search identified 1,839 articles, of which 28 (17 cohort, 8 case-control, and 3 cross-sectional studies) were included in the meta-analysis. Metformin reduced the occurrence of cognitive impairment in patients with diabetes [unadjusted hazard ratio (HR) = 0.67, 95% CI: 0.62–0.73; adjusted hazard ratio (aHR) = 0.92, 95% CI: 0.85–0.99]. In addition, the use of metformin was associated with a decreased risk of dementia (HR = 0.64, 95% CI: 0.59–0.69; aHR = 0.90, 95% CI: 0.84–0.96), while a random-effects meta-analysis indicated no significant effect of metformin on the risk of Alzheimer's disease (AD) (HR = 0.85, 95% CI: 0.60–1.22; aHR = 1.10, 95% CI: 0.95–1.28).
Conclusion: Metformin therapy decreased the occurrence risk of cognitive decline in patients with diabetes mellitus. Moreover, the use of metformin by adults with diabetes for the prevention of dementia, but not AD, is supported by the available evidence.
Introduction
Cognitive dysfunction, which includes delirium, mild cognitive deficits, and dementia, is characterized by a significant decline from a previously attained cognitive functional level (Sachdev et al., 2014; Zhang et al., 2020). Numerous epidemiological studies have increasingly recognized cognitive impairment as important comorbidity and complication of diabetes and it has become a major public health concern (Gispen and Biessels, 2000; Biessels and Despa, 2018; Biessels and Whitmer, 2020). A systematic review reported that patients with diabetes have a 73% increase in the risk of dementia and a 56% increase in the risk of Alzheimer's disease (AD) (Diniz Pereira et al., 2021). Moreover, the etiology of cognitive impairment in patients with diabetes is potentially multifactorial (Campbell et al., 2018; Jash et al., 2020; Yuan et al., 2021). For example, poor glycemic control and the presence of microvascular complications, such as neuropathy and retinopathy, have also been associated with cognitive dysfunction (Moheet et al., 2015); insulin resistance also may increase the occurrence risk of AD (Baker et al., 2011; Lyu et al., 2020).
Metformin is a primary oral hypoglycemic agent widely used for treating diabetes since 1950s (Flory and Lipska, 2019). Metformin functions predominantly by improving the sensitivity of insulin receptors to insulin, which enhances glucose uptake and decreases hepatic glycogen synthesis at low glucose (Hundal et al., 2000; Satoh, 2014). However, the function of metformin is not confined to glucose reduction (Liu et al., 2014). Increasing evidence has emerged indicating that metformin can penetrate the blood-brain barrier to improve cerebral energy metabolism in some regions of the brain associated with semantic memory and some white matter in adults with diabetes (Huang et al., 2014; Sritawan et al., 2020). Moreover, an animal experiment supported the fact that metformin treatment prevents amyloid plaque deposition and reduces memory impairment (Ou et al., 2018).
Several studies have reported that metformin could negatively impact cognitive function (Hsiao et al., 2014; Ha et al., 2021). For example, Chen et al. (2009) found that the activation of AMP-activated protein kinase (AMPK) by metformin raised the production of β-secretase to promote the deposition of β-amyloid peptides (Aβ), which leads to cognitive dysfunction (Chen et al., 2009). In addition, a case-control study of patients aged 65 years or older indicated that the long-term metformin use increased the risk of AD [(OR): 1.71, 95% CI: 1.12–2.60] (Imfeld et al., 2012). Therefore, the effect of metformin and whether it is associated with the prevention or promotion of the incidence of cognitive impairment is controversial.
This meta-analysis aimed to analyze the available evidence on the use of metformin and cognitive function in adults with diabetes and ascertain the relationship between the two.
Methods
Search strategy
The databases (PubMed, EMBASE, PsycINFO, Cochrane Library, and Web of Science) were screened independently by two investigators (JHZ and YQS) from their inception date until March 1, 2022. The search strategy for the PubMed database is presented in Table 1. In addition, reference lists from identified and relevant reviews were manually searched.
Selection criteria
This systematic review and meta-analysis were conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2009). The protocol for this systematic review and meta-analysis was registered at INPLASY (registration number: INPLASY202250065). Studies were only selected for inclusion in accordance with the following PECOS criteria. Participants: all patients are individuals with diabetes aged 18 years or older and have no history of cognitive disorder. Exposure: taking metformin monotherapy at any dosage for any duration. Comparator: participants received other antidiabetic drugs rather than metformin or no therapy as the control group. Outcomes: studies that investigated the risk (or incidence) of cognitive dysfunction were eligible for inclusion. Study: only published observational study designs—such as cohort, case-control, or cross-sectional studies—were eligible for inclusion. In addition, detailed meeting summary information was included. Studies of randomized controlled trials (RCTs), case reports/series, basic science, and reviews were excluded.
Data extraction and quality assessment of each study
Data from all eligible studies were extracted onto a standardized Excel spreadsheet independently by two investigators (JHZ and YQS). The following data were abstracted from each included study: publication details (such as first author and year of publication), study design, number of participants, participant characteristics (mean age and age range), gender, comparator, exposure, number of events, years enrolled, and outcomes (diagnosis and diagnostic criteria). For any discrepancies, a consensus was reached via discussion between the two investigators; if any uncertainty remained regarding inclusion, a senior author (XYZ) was consulted. To acquire relevant missing data from included studies, the first and/or corresponding authors of the studies were contacted.
Subsequently, JHZ and YQS independently evaluated the quality of each included study. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used for cohort studies and case-control studies (Stang, 2010), while cross-sectional studies were appraised by the Agency for Healthcare Research and Quality (AHRQ) (Li et al., 2020).
Data synthesis
The Review Manager software (version 5.4) was used to conduct the meta-analysis and sensitivity analysis, and publication bias was performed by STATA software (version 15.0). In accordance with the study of Jatho et al. (2021), the odds ratio (OR), relative risk (RR), or hazard ratio (HR) with a 95% confidence interval (CI) were selected as the effect size for included studies. Adjusted OR/HR/RR (accounting for confounding variables) and unadjusted OR/HR/RR were conducted. Heterogeneity was assessed using Higgins I-squared (I2) (I2 > 50% was regarded as significant heterogeneity) (Higgins et al., 2003). Publication bias was examined by Begg's funnel plot and Egger's test (Macaskill et al., 2001). Publication bias is present if Begg's funnel plot shows asymmetry or the p-value of Egger's test is less than 0.05. A sensitivity analysis was conducted by moving each study individually. All statistical significance was set at p < 0.05 (two-tailed).
Potential sources of heterogeneity were explored by conducting a subgroup analysis after sensitivity analysis. The following categorical variables were examined in the subgroup analysis: (1) dementia: oral metformin vs. oral other hypoglycemic drugs rather than metformin in patients with diabetes; (2) Alzheimer's disease: oral metformin vs. oral other hypoglycemic drugs rather than metformin in patients with diabetes.
Results
Study selection
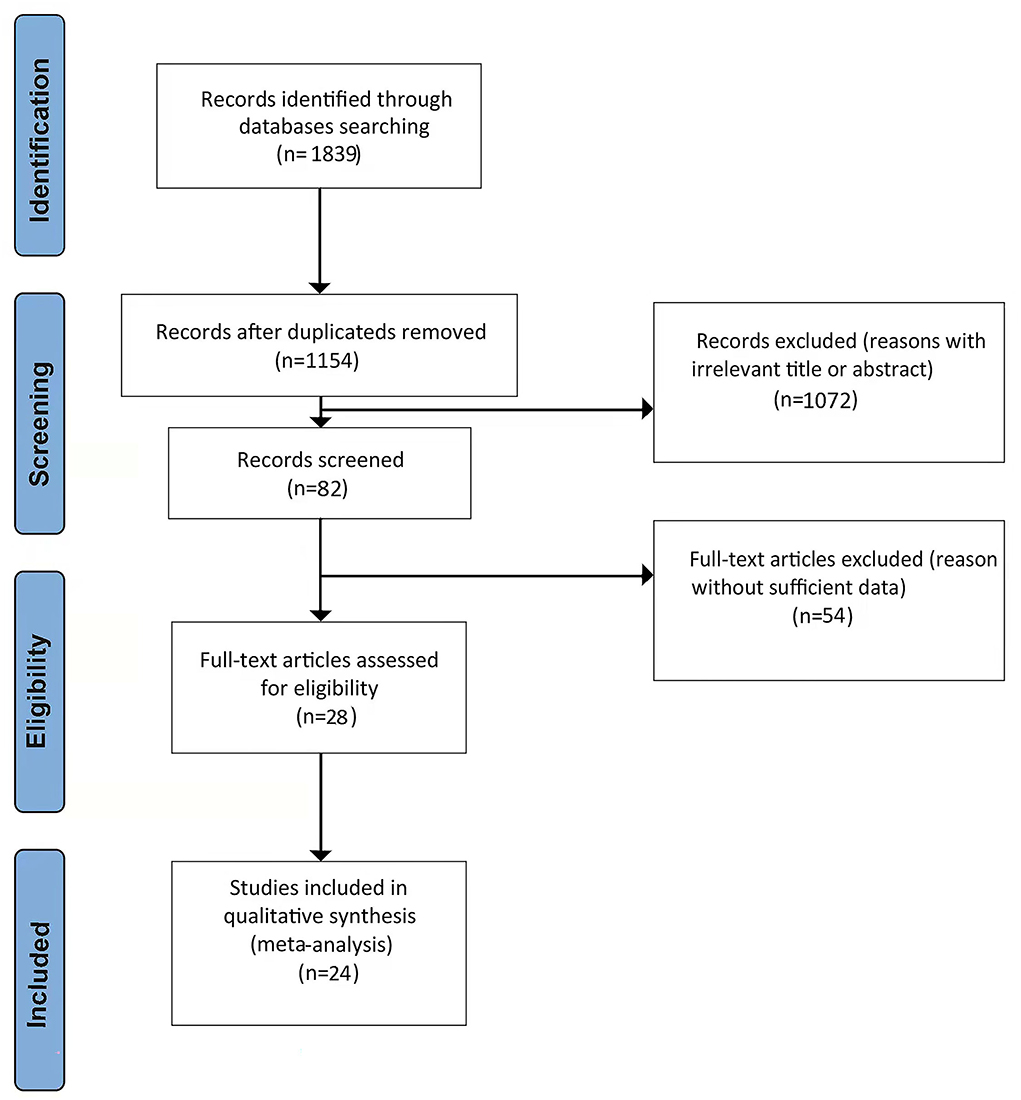
Figure 1 shows a PRISMA flow diagram of the literature search. A total of 1,839 potentially relevant studies were initially identified from the literature search. Of these, 685 duplicate articles that had been retrieved through electronic databases were removed. Another 1,072 irrelevant references were discarded after screening the titles and abstracts, and a further 54 studies were excluded for having insufficient data. Finally, 28 studies (Hsu et al., 2011; Imfeld et al., 2012; Moore et al., 2013; Whitmer et al., 2013; Cheng et al., 2014; Hsiao et al., 2014; Ng et al., 2014; Yokoyama et al., 2015; Liccini et al., 2016; Naharci et al., 2016; Orkaby et al., 2017; Bohlken et al., 2018; Kim et al., 2019; Koo et al., 2019; Porter et al., 2019; Scherrer et al., 2019a,b; Shi et al., 2019; Tseng, 2019; Weinstein et al., 2019; Wium-Andersen et al., 2019; Akimoto et al., 2020; Salas et al., 2020; Secnik et al., 2020; Sluggett et al., 2020; Ha et al., 2021; Sečník, 2021; Teng et al., 2021) remained and 24 studies were deemed appropriate in the pooled analysis after the screening of the initial 1,839 articles.
Sample characteristics
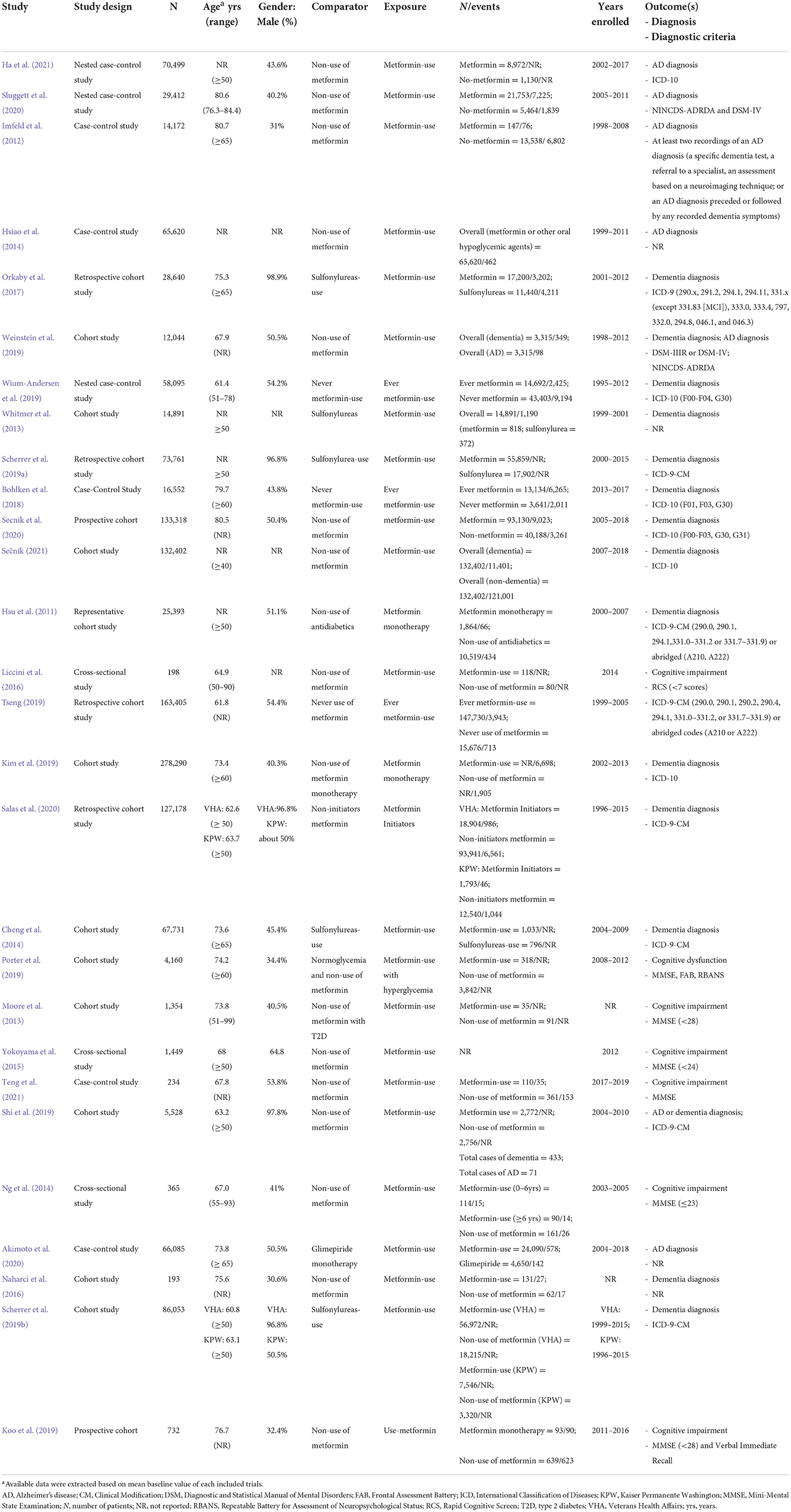
The characteristics of the 28 included studies on the occurrence risk of cognitive dysfunction in diabetes with oral metformin are shown in Table 2. The 28 studies comprised seventeen cohort studies (Hsu et al., 2011; Moore et al., 2013; Whitmer et al., 2013; Cheng et al., 2014; Naharci et al., 2016; Orkaby et al., 2017; Kim et al., 2019; Koo et al., 2019; Porter et al., 2019; Scherrer et al., 2019a,b; Shi et al., 2019; Tseng, 2019; Weinstein et al., 2019; Salas et al., 2020; Secnik et al., 2020; Sečník, 2021), eight case-control studies (Imfeld et al., 2012; Hsiao et al., 2014; Bohlken et al., 2018; Wium-Andersen et al., 2019; Akimoto et al., 2020; Sluggett et al., 2020; Ha et al., 2021; Teng et al., 2021), and three cross-sectional studies (Ng et al., 2014; Yokoyama et al., 2015; Liccini et al., 2016). The articles were all published between 2012 and 2021, and their enrolment periods ranged from 1995 to 2019 except for two articles (Moore et al., 2013; Naharci et al., 2016) for which the enrollment periods were not reported. Sample sizes varied from 278,290 (Kim et al., 2019) to 193 (Naharci et al., 2016). Additional details on the covariates that were adjusted for in the statistical analyses are included in Supplementary Table 1.
Quality assessment
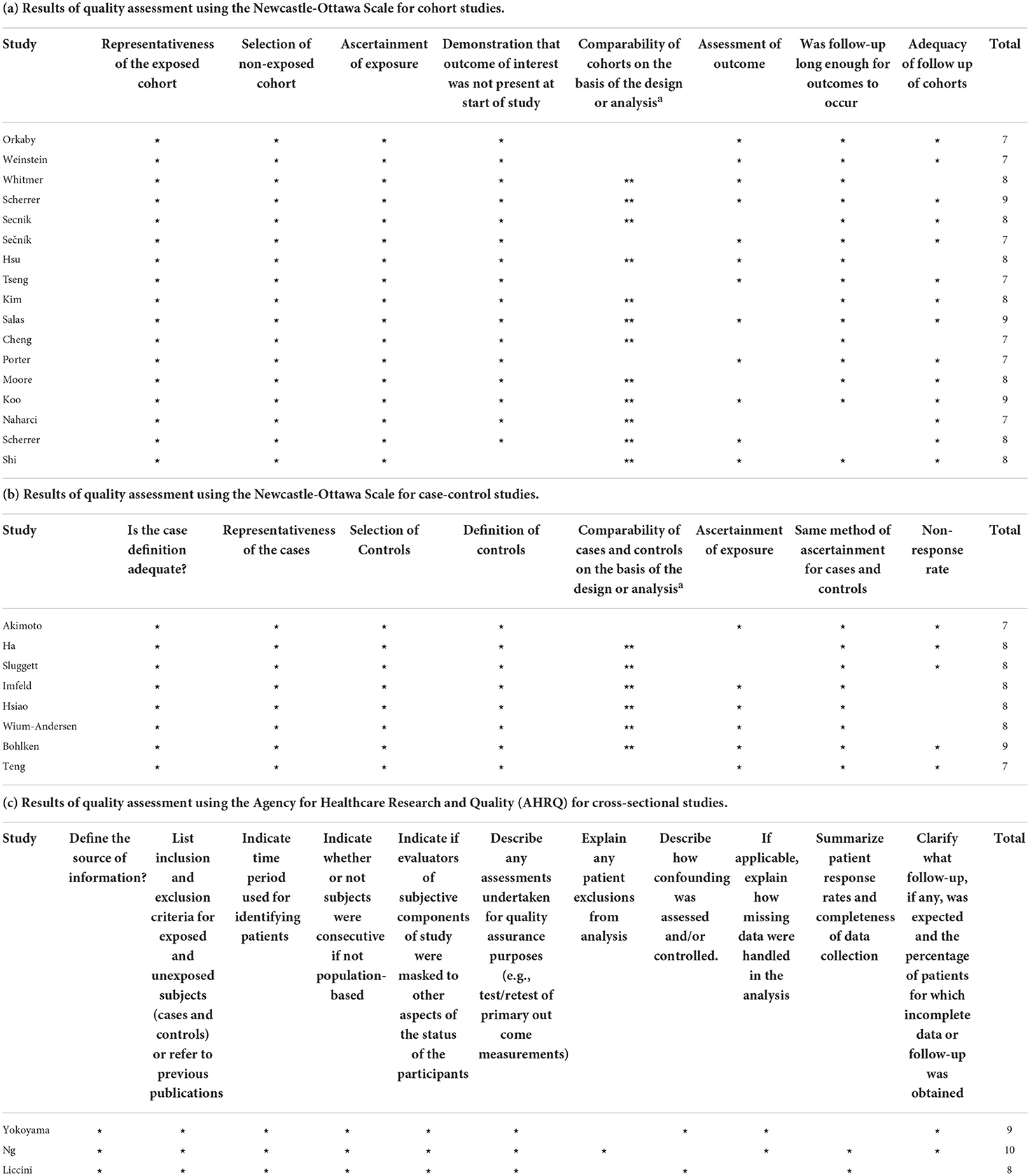
As shown in Table 3, the NOS score for all the cohort studies and case-control studies ranged from 7 to 9 points. The AHRQ score for each included cross-sectional study ranged from 8 to 10 points.
Meta-analysis on metformin and cognitive dysfunction
Overall, 24 studies examined the effects of metformin use on cognitive performance in patients with diabetes. Forest plots are shown in Figures 2A,B. A total of 11 unadjusted studies that could be pooled in a meta-analysis showed that diabetes with oral metformin was associated with a reduced risk of cognitive impairment (HR: 0.67, 95% CI: 0.62–0.73, I2 = 86%, p < 0.00001) (Figure 2A) (Hsu et al., 2011; Imfeld et al., 2012; Orkaby et al., 2017; Kim et al., 2019; Koo et al., 2019; Scherrer et al., 2019a,b; Tseng, 2019; Salas et al., 2020; Sečník, 2021; Teng et al., 2021). Similarly, the meta-analysis of 23 studies with available data revealed that metformin was associated with a reduced risk of cognitive dysfunction in adults with diabetes after adjusting for potential confounding factors (aHR: 0.92, 95% CI: 0.85–0.99, I2 = 89%, p < 0.00001) (Figure 2B) (Hsu et al., 2011; Imfeld et al., 2012; Moore et al., 2013; Whitmer et al., 2013; Cheng et al., 2014; Hsiao et al., 2014; Naharci et al., 2016; Orkaby et al., 2017; Bohlken et al., 2018; Kim et al., 2019; Porter et al., 2019; Scherrer et al., 2019a,b; Shi et al., 2019; Tseng, 2019; Weinstein et al., 2019; Wium-Andersen et al., 2019; Akimoto et al., 2020; Salas et al., 2020; Secnik et al., 2020; Sluggett et al., 2020; Sečník, 2021; Teng et al., 2021).

Figure 2. (A) Meta-analysis of unadjusted covariates between metformin and the occurrence of cognitive dysfunction. (B) Meta-analysis of adjusted covariates between metformin and the occurrence of cognitive dysfunction.
Subgroup analysis
Meta-analysis on metformin and dementia
There were 15 studies with data available to examine the effect of metformin use on the incidence of dementia in adults diagnosed with diabetes. As depicted in Figure 3A, a total of 7 unadjusted studies showed that diabetes with oral metformin was associated with a decreased risk of dementia in patients with diabetes (HR: 0.64, 95% CI: 0.59–0.69, I2 = 81%, p < 0.0001) (Hsu et al., 2011; Kim et al., 2019; Scherrer et al., 2019a,b; Tseng, 2019; Salas et al., 2020; Sečník, 2021). Similarly, as shown in Figure 3B, a total of 14 studies with available data indicated that metformin was associated with a reduced risk of dementia after adjusting for potential confounding factors (aHR: 0.90, 95% CI: 0.84–0.96, I2 = 82%, p < 0.00001) (Hsu et al., 2011; Whitmer et al., 2013; Cheng et al., 2014; Orkaby et al., 2017; Bohlken et al., 2018; Scherrer et al., 2019a,b; Shi et al., 2019; Tseng, 2019; Weinstein et al., 2019; Wium-Andersen et al., 2019; Salas et al., 2020; Secnik et al., 2020; Sečník, 2021).
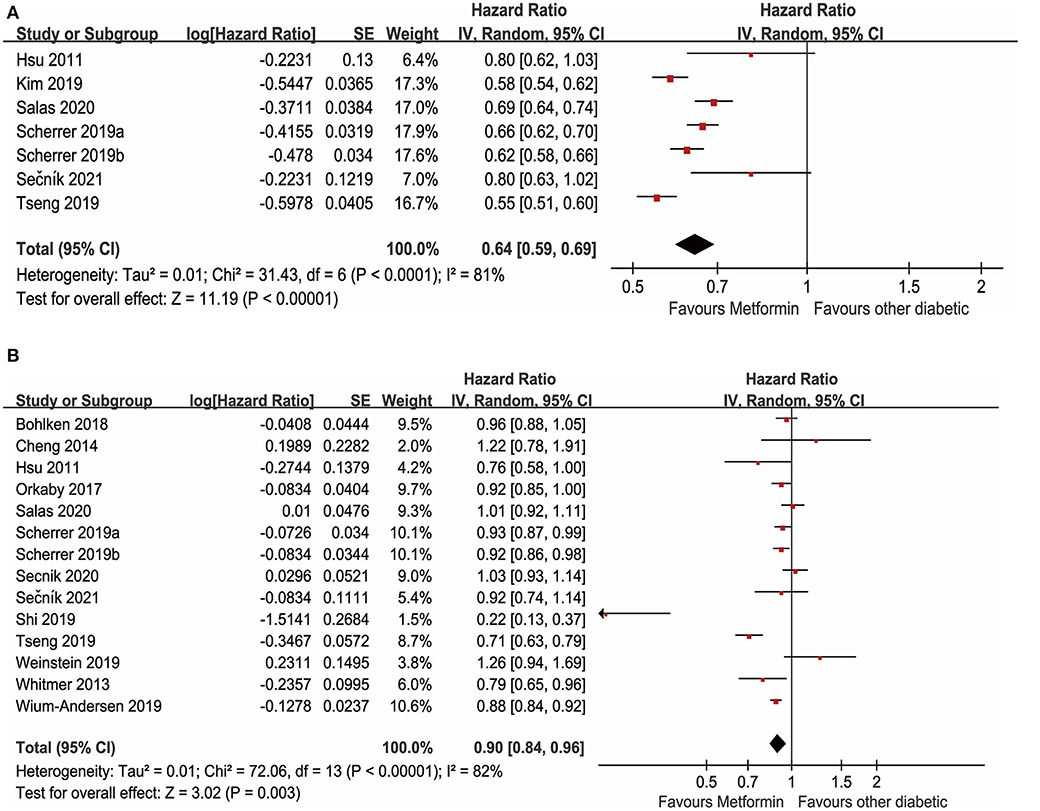
Figure 3. (A) Meta-analysis of unadjusted covariates between metformin and the occurrence of dementia. (B) Meta-analysis of adjusted covariates between metformin and the occurrence of dementia.
Meta-analysis on metformin and Alzheimer's disease
In the meta-analysis, seven studies with available data for evaluating the relationship between metformin use and AD in adults with diabetes were included. Forest plots are shown in Figures 4A,B. A total of two unadjusted studies showed that diabetes with oral metformin did not decrease the risk of AD (HR: 0.85, 95% CI: 0.60–1.22, I2 = 79%, p = 0.03) (Figure 4A) (Imfeld et al., 2012; Orkaby et al., 2017). Similarly, a total of seven studies indicated that oral metformin was not associated with a decreased occurrence of AD in individuals with diabetes after adjusting for potential confounding factors (aHR: 1.10, 95% CI: 0.95–1.28, I2 = 69%, p = 0.004) (Figure 4B) (Imfeld et al., 2012; Hsiao et al., 2014; Orkaby et al., 2017; Shi et al., 2019; Weinstein et al., 2019; Akimoto et al., 2020; Sluggett et al., 2020).
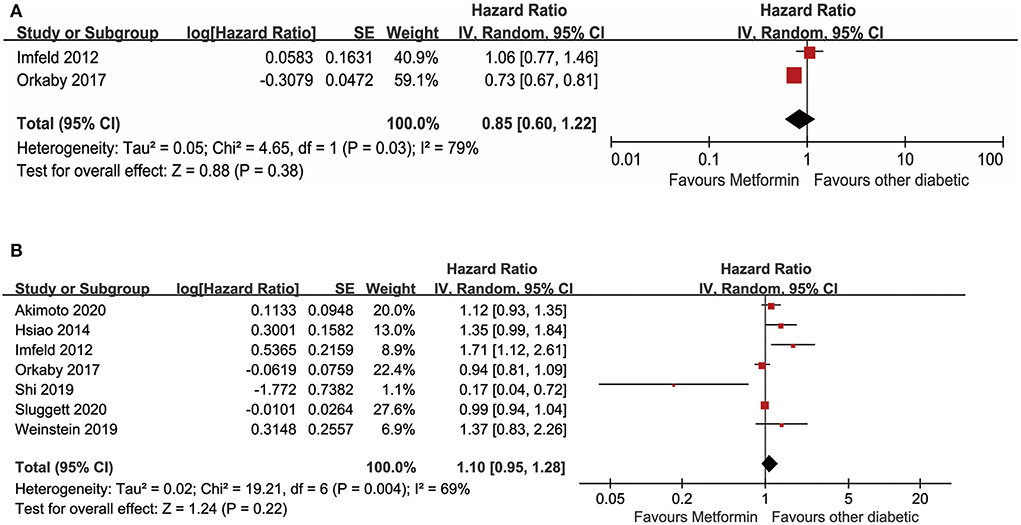
Figure 4. (A) Meta-analysis of unadjusted covariates between metformin and the occurrence of Alzheimer' s disease. (B) Meta-analysis of adjusted covariates between metformin and the occurrence of Alzheimer' s disease.
Sensitivity analysis and publication bias
Sensitivity analyses were performed to investigate the influence of each individual study on the overall meta-analysis summary estimate and the validity of the effect size. Excluding the included studies one by one demonstrated that no single study had a significant impact on the outcome of the combined analysis, suggesting that the results of this meta-analysis were stable (Figure 5). Egger's test and Begg's funnel plot did not find evidence of publication bias (p < 0.05) (Figure 6).
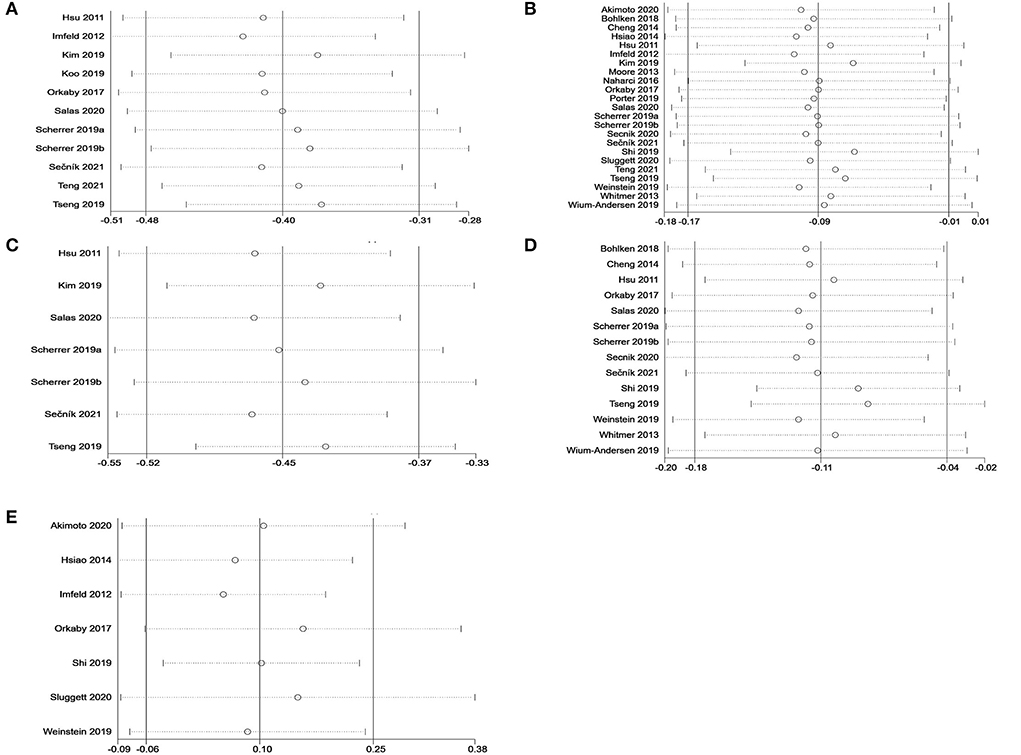
Figure 5. Sensitivity analysis of metformin on cognitive dysfunction in patients with diabetes in a random-effects meta-analysis of included observational studies. (A) All studies (unadjusted covariates); (B) All studies (adjusted covariates); (C) Subgroup meta-analysis on dementia (unadjusted covariates); (D) Subgroup meta-analysis on dementia (adjusted covariates); (E) Subgroup meta-analysis on AD (adjusted covariates).
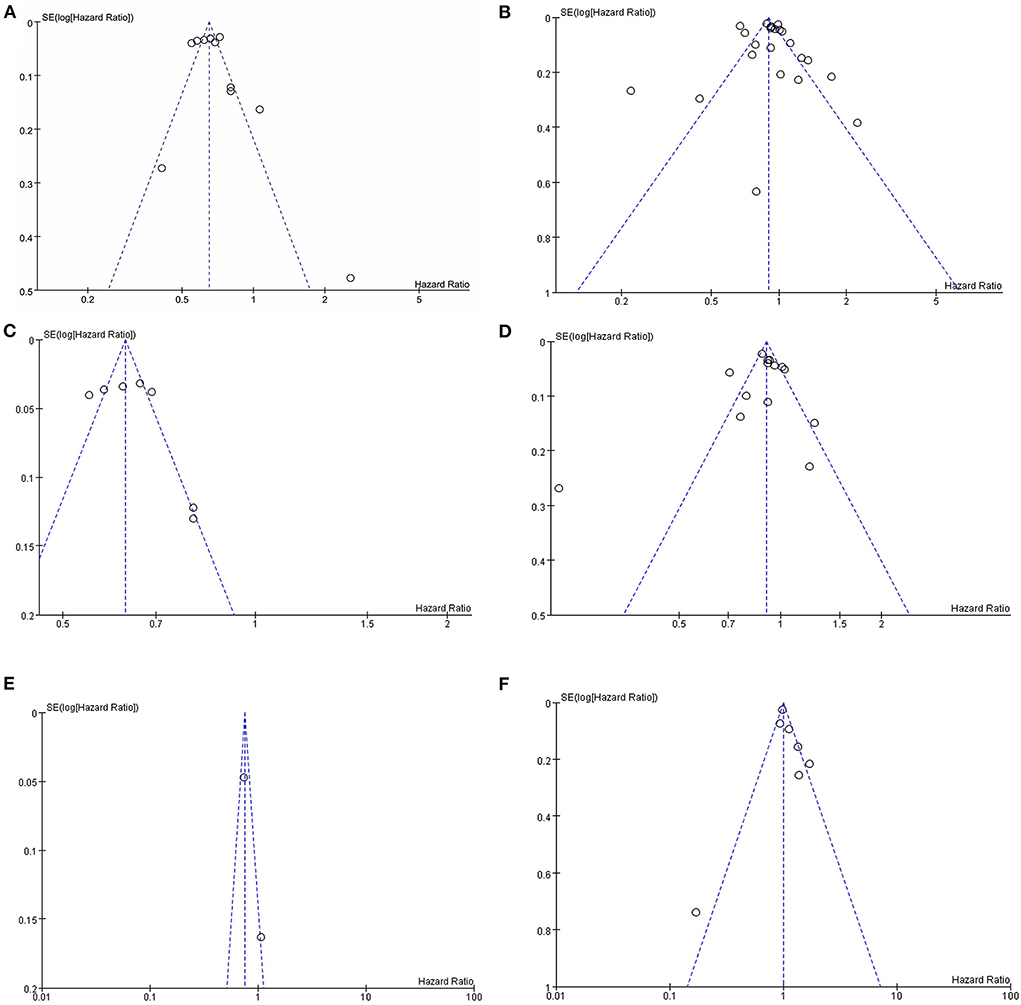
Figure 6. Assessment of publication bias by funnel plots of metformin on cognitive dysfunction in patients with diabetes included observational studies. (A) All studies (unadjusted covariates); (B) All studies (adjusted covariates); (C) Subgroup meta-analysis on dementia (unadjusted covariates); (D) Subgroup meta-analysis on dementia (adjusted covariates); (E) Subgroup meta-analysis on AD (unadjusted covariates); (F) Subgroup meta-analysis on AD (adjusted covariates).
Discussion
The aim of this systematic review and meta-analysis was to evaluate the impact of metformin on cognitive impairment in adults diagnosed with diabetes. A total of 28 observational studies met the inclusion criteria, and 24 studies were deemed appropriate in the pooled analysis for this systematic review and meta-analysis, the main findings of which included: (1) a meta-analysis reported that the protective effect of metformin therapy decreases the risk of cognitive dysfunction in patients with diabetes; (2) subgroup analyses found that oral metformin was associated with a decreased risk of dementia in patients with diabetes; (3) a subgroup analysis of meta-analysis on metformin and AD found that metformin could be associated with no significant effect on the decreased risk of AD.
Congruent with the findings that oral metformin was associated with a lower prevalence of dementia in the current study, there is some research suggesting that metformin initiation is associated with a substantially lower risk of dementia among younger African American patients (Scherrer et al., 2019a). However, the results from Salas et al. (2020) did not support initiating metformin earlier to prevent cognitive decline. The discrepancies in the results of these studies might be attributed to patient populations that differed in clinical and demographic characteristics and treatment timing. Therefore, RCTs with an optimal sample size need to be performed on the use of metformin for diabetes to confirm and extend these findings.
A subgroup analysis in the current study, based on observational studies, indicated that metformin was not significantly associated with a decreased risk of AD. In contrast, Ha et al. (2021) reported that metformin use was related to an increased risk of AD after adjusting for comorbidities and cardiometabolic risk profile by multivariable regression analyses. In addition, the treatment of diabetes with metformin cumulatively for more than 4 years significantly increased the risk of developing AD (Hsiao et al., 2014). However, Sluggett et al. (2020) showed that long-term (≥10 years) and high-dose metformin therapy had a lower risk of incidence of AD in older people with diabetes. Taken together, these results show that future RCTs with a larger sample size focusing on AD and metformin use in adults diagnosed with diabetes are warranted to explain these mixed findings.
The potential mechanisms of the relationship between metformin and cognitive performance have yet to be elucidated. Previous studies in animals have indicated that metformin could reduce cognitive impairment by reversing the harmful effects of impaired insulin signaling that causes a cascade of deleterious events, such as oxidative stress, inflammation, and tau hyper-phosphorylation (Farr et al., 2019; Gorgich et al., 2021). Moreover, the current study provides primary evidence suggesting that adults with diabetes facing a high risk of cognitive dysfunction should consider metformin as a first-line therapy.
Heterogeneity was detected in the meta-analysis of the present study. This variation could potentially be related to differences in the cumulative dose and duration of metformin, the race of the participants, and the duration and severity of diabetes. In addition, the uncertain accuracy of AD diagnoses in administrative data should be considered. Studies were included that used reliable neuropsychological cognitive assessment tools (i.e., Repeatable Battery for the Assessment of Neuropsychological Status and Frontal Assessment Battery) to report cognitive impairment instead of Minimum Mental State Examination (MMSE) in metformin users. However, the current study showed that the effect of metformin on the incidence of cognitive impairment remained effective when data were adjusted for potential confounding factors.
Limitations
There were three main limitations in this meta-analysis. First, the findings of this review provided only very weak support for the hypothesis that metformin could prevent cognitive impairment in people without diabetes. However, the subgroup analysis of these factors could not be conducted due to the limited amount of data. Second, a subgroup analysis of studies adequately controlled for diabetes severity at baseline could not be conducted due to the limited amount of data. Finally, studies with vs. without an active comparator were not performed in the subgroup analysis in this meta-analysis.
Conclusion
Metformin reduces the incidence of cognitive impairment but not AD in adults with diabetes. Future trials should examine the role of metformin in patients with diabetes in an RCT with a larger sample size, well-controlled confounding factors, sufficient follow-up time, and more accurate assessment of metformin exposure levels.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
J-HZ, X-YZ, and Y-QS contributed equally to the conception of this systematic review and meta-analysis. J-HZ, X-YZ, Y-QS, R-HL, MC, and ML conceived the study design. X-YZ made search strategy and discussed with Y-QS and J-HZ. J-HZ and Y-QS were conducted the search and data collection from the included studies and performed quality assessment and data analysis. X-YZ drafted the final version of this study. All authors have approved the publication of study and take the responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.984559/full#supplementary-material
References
Akimoto, H., Negishi, A., Oshima, S., Wakiyama, H., Okita, M., Horii, N., et al. (2020). Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am. J. Alzheimers Dis. Other Dementias 35, 1533317519899546. doi: 10.1177/1533317519899546
Baker, L. D., Cross, D. J., Minoshima, S., Belongia, D., Watson, G. S., and Craft, S. (2011). Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68, 51–57. doi: 10.1001/archneurol.2010.225
Biessels, G. J., and Despa, F. (2018). Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604. doi: 10.1038/s41574-018-0048-7
Biessels, G. J., and Whitmer, R. A. (2020). Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia 63, 3–9. doi: 10.1007/s00125-019-04977-9
Bohlken, J., Jacob, L., and Kostev, K. (2018). Association between the use of antihyperglycemic drugs and dementia risk: a case-control study. J. Alzheimers Dis. 66, 725–732. doi: 10.3233/JAD-180808
Campbell, J. M., Stephenson, M. D., de Courten, B., Chapman, I., Bellman, S. M., and Aromataris, E. (2018). Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J. Alzheimers Dis. 65, 1225–1236. doi: 10.3233/JAD-180263
Chen, Y., Zhou, K., Wang, R., Liu, Y., Kwak, Y. D., Ma, T., et al. (2009). Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc. Natl. Acad. Sci. U. S. A. 106, 3907–3912. doi: 10.1073/pnas.0807991106
Cheng, C., Lin, C. H., Tsai, Y. W., Tsai, C. J., Chou, P. H., and Lan, T. H. (2014). Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J. Gerontol. Series A Biol. Sci. Med. Sci. 69, 1299–1305. doi: 10.1093/gerona/glu073
Diniz Pereira, J., Gomes Fraga, V., Morais Santos, A. L., Carvalho, M. D. G., Caramelli, P., and Braga Gomes, K. (2021). Alzheimer's disease and type 2 diabetes mellitus: a systematic review of proteomic studies. J. Neurochem. 156, 753–776. doi: 10.1111/jnc.15166
Farr, S. A., Roesler, E., Niehoff, M. L., Roby, D. A., McKee, A., and Morley, J. E. (2019). Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. J. Alzheimers. Dis. 68, 1699–1710. doi: 10.3233/JAD-181240
Flory, J., and Lipska, K. (2019). Metformin in 2019. JAMA 321, 1926–1927. doi: 10.1001/jama.2019.3805
Gispen, W. H., and Biessels, G. J. (2000). Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 23, 542–549. doi: 10.1016/S0166-2236(00)01656-8
Gorgich, E. A. C., Parsaie, H., Yarmand, S., Baharvand, F., and Sarbishegi, M. (2021). Long-term administration of metformin ameliorates age-dependent oxidative stress and cognitive function in rats. Behav. Brain Res. 410, 113343. doi: 10.1016/j.bbr.2021.113343
Ha, J., Choi, D. W., Kim, K. J., Cho, S. Y., Kim, H., Kim, K. Y., et al. (2021). Association of metformin use with Alzheimer's disease in patients with newly diagnosed type 2 diabetes: a population-based nested case-control study. Sci. Rep. 11, 24069. doi: 10.1038/s41598-021-03406-5
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hsiao, H. W., Yang, Y. S., Lu, Y. L., Kornelius, E., Chiou, J. Y., and Huang, C. N. (2014). Risk for Alzheimer's disease in type 2 diabetic patients treated with metformin. Diabetes 63, A372–A372. doi: 10.2337/db14-1317-1629
Hsu, C. C., Wahlqvist, M. L., Lee, M. S., and Tsai, H. N. (2011). Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis. 24, 485–493. doi: 10.3233/JAD-2011-101524
Huang, Y. C., Hsu, C. C., Lin, W. C., Yin, T. K., Huang, C. W., Wang, P. W., et al. (2014). Effects of metformin on the cerebral metabolic changes in type 2 diabetic patients. ScientificWorldJournal. 2014, 694326. doi: 10.1155/2014/694326
Hundal, R. S., Krssak, M., Dufour, S., Laurent, D., Lebon, V., Chandramouli, V., et al. (2000). Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069. doi: 10.2337/diabetes.49.12.2063
Imfeld, P., Bodmer, M., Jick, S. S., and Meier, C. R. (2012). Metformin, other antidiabetic drugs, and risk of Alzheimer's disease: a population-based case-control study. J. Am. Geriatr. Soc. 60, 916–921. doi: 10.1111/j.1532-5415.2012.03916.x
Jash, K., Gondaliya, P., Kirave, P., Kulkarni, B., Sunkaria, A., and Kalia, K. (2020). Cognitive dysfunction: a growing link between diabetes and Alzheimer's disease. Drug Dev. Res. 81, 144–164. doi: 10.1002/ddr.21579
Jatho, A., Cambia, J. M., and Myung, S. K. (2021). Consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer: a meta-analysis of observational studies. Public Health Nutr. 24, 6122–6136. doi: 10.1017/S136898002100104X
Kim, J. Y., Ku, Y. S., Kim, H. J., Trinh, N. T., Kim, W., Jeong, B., et al. (2019). Oral diabetes medication and risk of dementia in elderly patients with type 2 diabetes. Diabetes Res. Clin. Pract. 154, 116–123. doi: 10.1016/j.diabres.2019.07.004
Koo, B. K., Kim, L. K., Lee, J. Y., and Moon, M. K. (2019). Taking metformin and cognitive function change in older patients with diabetes. Geriatr. Gerontol. Int. 19, 755–761. doi: 10.1111/ggi.13692
Li, C., Cheng, G., Sha, T., Cheng, W., and Yan, Y. (2020). The relationships between screen use and health indicators among infants, toddlers, and preschoolers: a meta-analysis and systematic review. Int. J. Environ. Res. Public Health 17, 7324. doi: 10.3390/ijerph17197324
Liccini, A., Malmstrom, T. K., and Morley, J. E. (2016). Metformin use and cognitive dysfunction among patients with diabetes mellitus. J. Am. Med. Dir. Assoc. 17, 1063–1065. doi: 10.1016/j.jamda.2016.08.026
Liu, Y., Tang, G., Li, Y., Wang, Y., Chen, X., Gu, X., et al. (2014). Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflammation 11, 177. doi: 10.1186/s12974-014-0177-4
Lyu, F., Wu, D., Wei, C., and Wu, A. (2020). Vascular cognitive impairment and dementia in type 2 diabetes mellitus: an overview. Life Sci. 254, 117771. doi: 10.1016/j.lfs.2020.117771
Macaskill, P., Walter, S. D., and Irwig, L. (2001). A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 20, 641–654. doi: 10.1002/sim.698
Moheet, A., Mangia, S., and Seaquist, E. R. (2015). Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 1353, 60–71. doi: 10.1111/nyas.12807
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Moore, E. M., Mander, A. G., Ames, D., Kotowicz, M. A., Carne, R. P., Brodaty, H., et al. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987. doi: 10.2337/dc13-0229
Naharci, M. I., Cintosun, U., Ozturk, A., Oztin, H., Bozoglu, E., and Doruk, H. (2016). Association of metformin therapy with the risk of dementia in older adults with type 2 diabetes mellitus. Eur. Geriatr. Med. 7, S62–S63.
Ng, T. P., Feng, L., Yap, K. B., Lee, T. S., Tan, C. H., and Winblad, B. (2014). Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimers Dis. 41, 61–68. doi: 10.3233/JAD-131901
Orkaby, A. R., Cho, K., Cormack, J., Gagnon, D. R., and Driver, J. A. (2017). Metformin vs sulfonylurea use and risk of dementia in US veterans aged $65 years with diabetes. Neurology 89, 1877–1885. doi: 10.1212/WNL.0000000000004586
Ou, Z., Kong, X., Sun, X., He, X., Zhang, L., Gong, Z., et al. (2018). Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 69, 351–363. doi: 10.1016/j.bbi.2017.12.009
Porter, K. M., Ward, M., Hughes, C. F., O'Kane, M., Hoey, L., McCann, A., et al. (2019). Hyperglycemia and metformin use are associated with B vitamin deficiency and cognitive dysfunction in older adults. J. Clin. Endocrinol. Metab. 104, 4837–4847. doi: 10.1210/jc.2018-01791
Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., et al. (2014). Classifying neurocognitive disorders: the DSM-5 approach. Nat. Rev. Neurol. 10, 634–642. doi: 10.1038/nrneurol.2014.181
Salas, J., Morley, J. E., Scherrer, J. F., Floyd, J. S., Farr, S. A., Zubatsky, M., et al. (2020). Risk of incident dementia following metformin initiation compared with noninitiation or delay of antidiabetic medication therapy. Pharmacoepidemiol. Drug Saf. 29, 623-634. doi: 10.1002/pds.5014
Satoh, T. (2014). Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int. J. Mol. Sci. 15, 18677–18692. doi: 10.3390/ijms151018677
Scherrer, J. F., Morley, J. E., Salas, J., Floyd, J. S., Farr, S. A., and Dublin, S. (2019a). Association between metformin initiation and incident dementia among African American and White Veterans health administration patients. Ann. Fam. Med. 17, 352–362. doi: 10.1370/afm.2415
Scherrer, J. F., Salas, J., Floyd, J. S., Farr, S. A., Morley, J. E., and Dublin, S. (2019b). Metformin and sulfonylurea use and risk of incident dementia. Mayo Clin. Proc. 94, 1444–1456. doi: 10.1016/j.mayocp.2019.01.004
Sečník, J. (2021). Diabetes mellitus in patients with dementia: clinical care and pharmacological treatment (Ph. D. thesis). Karolinska Institutet, Stockholm, Sweden. p. 1–82. Available online at: https://openarchive.ki.se/xmlui/bitstream/handle/10616/47435/Thesis_Juraj_Secnik.pdf?sequence=1&isAllowed=y
Secnik, J., Xu, H., Schwertner, E., Hammar, N., Alvarsson, M., Winblad, B., et al. (2020). Dementia diagnosis is associated with changes in antidiabetic drug prescription: an open-cohort study of ~130,000 Swedish subjects over 14 Years. J. Alzheimers Dis. 76, 1581–1594. doi: 10.3233/JAD-200618
Shi, Q., Liu, S., Fonseca, V. A., Thethi, T. K., and Shi, L. (2019). Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open 9, e024954. doi: 10.1136/bmjopen-2018-024954
Sluggett, J. K., Koponen, M., Bell, J. S., Taipale, H., Tanskanen, A., Tiihonen, J., et al. (2020). Metformin and risk of Alzheimer's disease among community-dwelling people with diabetes: a national case-control study. J. Clin. Endocrinol. Metab. 105, E963–E972. doi: 10.1210/clinem/dgz234
Sritawan, N., Prajit, R., Chaisawang, P., Sirichoat, A., Pannangrong, W., Wigmore, P., et al. (2020). Metformin alleviates memory and hippocampal neurogenesis decline induced by methotrexate chemotherapy in a rat model. Biomed. Pharmacother. 131, 110651. doi: 10.1016/j.biopha.2020.110651
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Teng, Z., Feng, J., Qi, Q., Dong, Y., Xiao, Y., Xie, X., et al. (2021). Long-term use of metformin is associated with reduced risk of cognitive impairment with alleviation of cerebral small vessel disease burden in patients with type 2 diabetes. Front. Aging Neurosci. 13, 773797. doi: 10.3389/fnagi.2021.773797
Tseng, C.-H. (2019). Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis. 10, 37–48. doi: 10.14336/AD.2017.1202
Weinstein, G., Davis-Plourde, K. L., Conner, S., Himali, J. J., Beiser, A. S., Lee, A., et al. (2019). Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer's disease: pooled analysis from 5 cohorts. PLoS ONE 14, e0212293. doi: 10.1371/journal.pone.0212293
Whitmer, R., Quesenberry Jr, C., Allison, J., and Karter, A. (2013). Anti-hyperglycemic therapy and risk of dementia: a new user cohort study. Alzheimers Dementia 9, P136. doi: 10.1016/j.jalz.2013.04.077
Wium-Andersen, I. K., Osler, M., Jørgensen, M. B., Rungby, J., and Wium-Andersen, M. K. (2019). Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur. J. Endocrinol. 181, 499–507. doi: 10.1530/EJE-19-0259
Yokoyama, H., Ogawa, M., Honjo, J., Okizaki, S., Yamada, D., Shudo, R., et al. (2015). Risk factors associated with abnormal cognition in Japanese outpatients with diabetes, hypertension or dyslipidemia. Diabetol. Int. 6, 268–274. doi: 10.1007/s13340-014-0194-7
Yuan, C. L., Yi, R., Dong, Q., Yao, L. F., and Liu, B. (2021). The relationship between diabetes-related cognitive dysfunction and leukoaraiosis. Acta Neurol. Belg. 121, 1101–1110. doi: 10.1007/s13760-021-01676-4
Keywords: metformin, dementia, Alzheimer's disease, diabetes mellitus, cognitive dysfunction
Citation: Zhang J-H, Zhang X-Y, Sun Y-Q, Lv R-H, Chen M and Li M (2022) Metformin use is associated with a reduced risk of cognitive impairment in adults with diabetes mellitus: A systematic review and meta-analysis. Front. Neurosci. 16:984559. doi: 10.3389/fnins.2022.984559
Received: 02 July 2022; Accepted: 29 July 2022;
Published: 25 August 2022.
Edited by:
Raymond Scott Turner, Georgetown University, United StatesReviewed by:
Kaiyuan Wang, Tianjin Medical University Cancer Institute and Hospital, ChinaJeffrey Scherrer, Saint Louis University, United States
Copyright © 2022 Zhang, Zhang, Sun, Lv, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Yang Zhang, OTQ5OTk1MTYxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Jia-Hao Zhang
Jia-Hao Zhang Xin-Yang Zhang
Xin-Yang Zhang Yan-Qiu Sun
Yan-Qiu Sun Ren-Hua Lv3
Ren-Hua Lv3