
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 19 October 2022
Sec. Auditory Cognitive Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.976095
This article is part of the Research TopicTowards an Understanding of Tinnitus Heterogeneity, Volume IIView all 27 articles
Objective: This study aimed to investigate the differences in intra-regional brain activity and inter-regional functional connectivity between patients with recent-onset tinnitus (ROT) and persistent tinnitus (PT) using resting-state functional magnetic resonance imaging (rs-fMRI), including the amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), and voxel-wise functional connectivity (FC).
Method: We acquired rs-fMRI scans from 82 patients (25 without recent-onset tinnitus, 28 with persistent tinnitus, and 29 healthy controls). Age, sex, and years of education were matched across the three groups. We performed ALFF, ReHo, and voxel-wise FC analyses for all patients.
Results: Compared with the control group, participants with ROT and PT manifested significantly reduced ALFF and ReHo activity within the left and right dorsolateral superior frontal gyrus (SFG) and gyrus rectus (GR). Additional voxel-wise FC revealed decreased connectivity between the dorsolateral SFG (left and right) and the right superior parietal gyrus (SPG), right middle frontal gyrus (MFG), and left medial superior frontal gyrus (mSFG) within these two groups. Significant differences were observed between the ROT and PT groups, with the ROT group demonstrating reduced FC.
Conclusion: Our data suggest that patients with PT have more difficulty monitoring external stimuli and reorienting attention than patients with ROT. In addition, patients who perceive higher levels of disruption from tinnitus are more likely to develop persistent and debilitating tinnitus once the tinnitus lasts longer than six months. Therefore, we strongly recommend that clinicians implement effective tinnitus management strategies in patients with ROT as soon as possible.
Subjective tinnitus is a conscious auditory perception without a corresponding external source and is one of the most common yet distressing otologic pathologies, affecting approximately 8–20% of the adult population (Roberts et al., 2010). Studies have reported that subjective tinnitus is commonly associated with hearing loss, cerumen impaction, middle and inner ear-related pathologies, noise exposure, exposure to ototoxic medications and chemicals, aging, insomnia, anxiety, depression, head and neck injuries, and temporomandibular joint (TMJ) dysfunction (Baguley et al., 2013; Tunkel et al., 2014; Makar, 2021). In addition, tinnitus can be persistent, bothersome, and costly for patients and society in general. Cases of patients with extraordinarily persistent and debilitating tinnitus accompanied by severe anxiety or depression attempting suicide have been reported (Szibor et al., 2019).
It is generally believed that lesions in the peripheral hearing system and neuronal changes in the central nervous system contribute to tinnitus. Kapolowicz and Thompson (2020) reported that tinnitus might be closely related to an imbalance between auditory neuronal excitation and the inhibition network, leading to plasticity changes in the central auditory system. Knipper et al. (2021) proposed that hearing loss may contribute to a top-down mechanism that leads to tinnitus perception (Knipper et al., 2021). Khan et al. (2021) suggested that tinnitus might be a compensatory response to peripheral hearing system damage (Khan et al., 2021). Cai et al. (2020) reported abnormal functional connectivity (FC) in the auditory and non-auditory cortices in patients with hearing loss and tinnitus (Cai et al., 2020). Zhou et al. (2019) suggested that patients with hearing loss and tinnitus demonstrate abnormal intra-regional neural activity and disrupted connectivity in the hub regions of some non-auditory networks, including the default mode network (DMN), optical network, dorsal and ventral attention network (DAN and VAN), and central executive network (CEN) (Zhou et al., 2019). Minami et al. (2018) reported that tinnitus patients with hearing loss showed a statistically significant reduction in auditory-related FC compared with the control group. Finally, our previous project, using amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), and voxel-wise functional connectivity (FC) technologies, revealed that disruptions in the brain regions responsible for attention and stimuli monitoring and orientations could lead to tinnitus. Tinnitus has different forms, degrees of severity, and onset duration, which can only be described by patients’ testimony and corresponding symptoms. When categorizing tinnitus based on its onset duration (recent-onset or persistent), numerous studies have concentrated on developing pathophysiological models for chronic tinnitus (tinnitus that has an onset duration of at least six months). However, few studies have investigated the neuronal changes that occur from recent-onset to persistent tinnitus (PT) (Stolzberg et al., 2013; Cai et al., 2020; Lan et al., 2020). To the best of our knowledge, no studies have investigated the aforementioned issue using resting-state functional magnetic resonance imaging (rs-fMRI) technologies. Furthermore, investigating this issue is critical for identifying the contributing neural mechanisms and possible interventions to stop this transition. Therefore, our project aims were to uncover the differences in brain activity between recent-onset tinnitus (ROT) patients and PT patients using resting-state functional magnetic resonance imaging (rs-fMRI) technologies and to apply our findings to existing tinnitus management strategies.
This study was approved by the Research Ethics Committee of the Affiliated Zhongda Hospital, Southeast University. All participants provided written informed consent before participating in the study. We recruited 82 participants (all right-handed, with at least eight years of education), including 25 tinnitus participants with recent-onset tinnitus (ROT), 28 tinnitus participants with persistent tinnitus (PT), and 29 healthy participants as the control group, through our outpatient clinics between September 2011 and September 2013. The patients were group matched in terms of age, sex, and education level. Twenty-five participants perceived bilateral tinnitus and the remaining, 28 participants, perceived unilateral tinnitus. We defined the time course of tinnitus (recent-onset or persistent) according to the Tinnitus Clinical Practice Guidelines of the American Academy of Otolaryngology-Head and Neck Surgery. According to the guideline, if the overall duration of onset equals or is less than six months, tinnitus will be determined to be recent-onset. If the overall duration of onset is more than six months, tinnitus will be defined as persistent (Tunkel et al., 2014).
We performed pure-tone audiometric testing (PTA at 250; 500; 1,000; 2,000; 4,000; 6,000, and 8,000 Hz) for all recruited participants. Participants with a 7-frequency PTA < 25 dB HL were considered to have clinically normal hearing. In addition, we performed comprehensive tympanometry, diagnostic distortion-product otoacoustic emission (DPOAE), and diagnostic auditory brainstem response (ABR) to rule out middle ear pathologies and auditory neuropathy (ANSD). Furthermore, we collected crucial information about the duration of tinnitus and presence of insomnia in all participants.
To assess the distress associated with tinnitus, we distributed the Iowa version of the tinnitus handicap questionnaire (THQ) to both the ROT and PT groups. We also distributed the Self-Rating Depression Scale (SDS) and Self-Rating Anxiety Scale (SAS) questionnaires to all participants for anxiety and depression screening (Zung, 1971). No significant group differences were found in the patients’ gender, age, and educational background (p > 0.05). However, we found a statistically significant difference in the THQ total score, SAS, and SDS scores between the groups (p < 0.05). The demographic and clinical characteristics of the participants in each group are summarized in Table 1.
The exclusion criteria for this study included Meniere’s disease, objective tinnitus, pulsatile tinnitus, history of alcohol consumption, severe smoking, head and neck injuries, epilepsy, stroke, Alzheimer’s disease, Parkinson’s disease, cancer, MRI contraindications, primary psychiatric conditions including generalized anxiety disorder (GAD), depression, schizophrenia, and severe visual impairment. None of our participants failed depression or anxiety screening.
Imaging data using a 3.0 T MRI scanner (Siemens MAGENETOM Trio, Erlangen, Germany) with a standard head coil. All participants were provided with foam paddings and earmuffs to minimize head motion and noise exposure during the scanning process. The participants were instructed to remain calm during the scan with their eyes closed, without falling asleep or thinking of anything particular. Functional images were obtained axially using a gradient echo-planar sequence sensitive to BOLD contrast, as follows: repetition time (TR), 2,000 ms; echo time (TE), 25 ms; slices, 36; thickness, 4 mm; gap, 0 mm; field of view (FOV), 240 mm × 240 mm; acquisition matrix, 64 × 64; and flip angle (FA), 90°.
Resting-state ALFF reflects spontaneous neural activity and yields physiologically meaningful results. Pre-processing of the ALFF images was performed using the Data Processing Assistant for Resting-State fMRI (DPARSF 5.2), Statistical Parametric Mapping (SPM12), and MATLAB 2021b. The first ten volumes were removed from each time series to account for the participants’ adaptation to the scanning environment. Slice timing and realignment for head motion correction were performed on the remaining 175 images. Subsequently, we performed the following procedures: spatially normalized into the stereotactic space of the Montreal Neurological Institute (MNI) (resampling voxel size = 3 Ö3 Ö3 mm3) and smoothed using a Gaussian kernel of 6 mm full width at half-maximum (FWHM), de-trending, and filtering (0.01–0.08 Hz). The participants with a head motion of more than 2.0 mm displacement or a 2.0-degree rotation in the x, y, or z directions were excluded from this study.
We then analyzed the ALFF data by transforming the time domain to the frequency domain using a fast Fourier transform. Next, we computed the square root of the power spectrum and averaged squared across 0.01–0.08 Hz at each voxel. The calculated average square root was used as the ALFF. Finally, the ALFF of each voxel was divided by the global mean ALFF value for standardization.
Regional homogeneity calculates the synchronization of low-frequency fluctuations between a given voxel and neighboring voxels, reflecting the neural function synchronization in the local brain region. Pre-processing of ReHo images was performed using DPARSF 5.2, SPM12, and MATLAB 2021b. The first ten volumes were removed from each time series to account for the participants’ adaptation to the scanning environment. Slice timing and realignment for head motion correction were performed on the remaining 175 images. The following procedures were performed: spatial normalization into the stereotactic space of the MNI (resampling voxel size = 3 Ö3 Ö3 mm3), de-trending, and filtering (0.01–0.08 Hz).
After the pre-processing stage, we performed the image calculation using the Kendall coefficient of concordance of the time series of a given voxel with its 27 nearest neighbors. Next, ReHo analyses were performed using DPARSF 5.2 software. The ReHo value of each voxel was standardized by partitioning the primal value using the global mean ReHo value. Finally, the data were smoothed with a Gaussian kernel of 6 mm FWHM for further statistical analysis.
We performed voxel-wise FC analysis using DPARSF 5.2, SPM12, and MATLAB 2021b. The first ten volumes were removed from each time series to account for the participants’ time to adapt to the scanning environment. Slice timing and realignment for head motion correction were then performed for the remaining 170 images. Subsequently, the procedures were carried out as follows: spatially normalized into the stereotactic space of the MNI (resampling voxel size = 3 Ö3 Ö3 mm3), and smoothed using a Gaussian kernel of 6 mm FWHM, de-trending, and filtering (0.01–0.08 Hz). Participants with a head motion with more than 2.0 mm displacement or a 2.0-degree rotation in the x, y, or z directions were excluded.
We extracted the ALFF and ReHo differences in brain regions between ROT participants and PT participants for voxel-wise FC analysis and defined them as seeds. We then used the average time series of seeds as a reference and calculated the Pearson correlation coefficient between the average signal change of each seed and the time sequences of the other voxels in the brain. Finally, we converted the correlation coefficient to a z-value by using Fisher’s z-transformation.
One-way analysis of variance (ANOVA) was first conducted to test the mean differences in ALFF, ReHo, and FC between the CG, the ROT group, and the group with PT (MATLAB 2021b). Statistically significant differences between groups were determined at p < 0.05. The participants’ age and sex were included as nuisance covariates. Next, we applied family wise error (FWE) correction for multiple comparisons using voxel-level inference at p < 0.001 and cluster-level inference at p < 0.05. Two-sample t-tests were then conducted to investigate the ALFF, ReHo, and FC differences between participants with ROT and the control group, participants with PT and the control group, and participants with ROT and participants with PT. Again, a statistically significant difference between groups was determined at p < 0.05. Finally, we used MRIcroGL software to draw 2-dimensional brain images to display the brain areas with statistically significant differences.
We found significant differences in ALFF values in the left and right dorsolateral superior frontal gyrus (SFG) and left gyrus rectus (GR) in the ROT and PT groups compared to those in the control group (Figure 1). Compared with the control group, the t-values of the ALFF for both the ROT and PT groups in the left GR were significantly lower than the global mean values from the control group (p < 0.05). The t-value of the ALFF in the ROT group was lower than that in the PT group (p < 0.05).
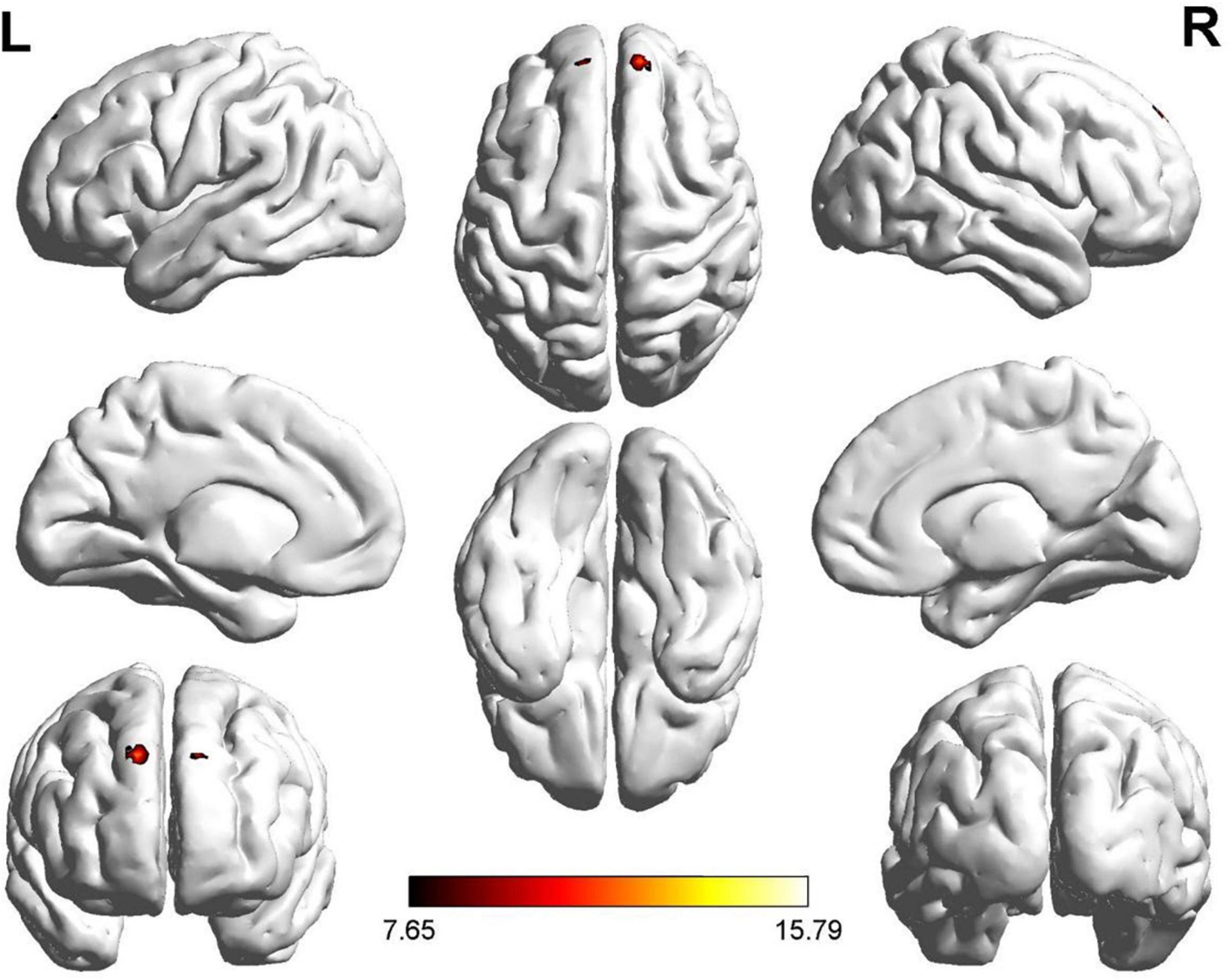
Figure 1. Significant differences in ALFF values were observed in the left and right dorsolateral SFG and rectus gyrus for both Recent-onset Tinnitus (ROT) and Persistent Tinnitus (PT) groups, compared to the healthy control group.
No statistically significant difference was observed for the left dorsolateral SFG between the ROT and PT groups (p > 0.05). However, compared with the control group, the ALFF t-values for both the ROT and PT groups in the right dorsolateral SFG were significantly lower than the global mean values from the control group (p < 0.05).
No statistically significant difference was observed for the right GR between the ROT and PT groups (p > 0.05). However, compared with the control group, the t-values of the ALFF for both the ROT and PT groups in the left GR were significantly lower than the global mean values from the control group (p < 0.05). The results are presented in Table 2A.
We also discovered significant differences in ReHo values in the right dorsolateral SFG for both the ROT and PT groups compared with the control group (Figure 2). Regarding ReHo’s t-value, both the ROT and PT groups in the right dorsolateral SFG revealed significantly lower values than the global mean values in the control group (p < 0.05) (Table 2B). A two-sample t-test did not reveal any statistical differences between the ROT and PT groups in the right dorsolateral SFG (p > 0.05).

Figure 2. Significant differences in ReHo values were observed in the right dorsolateral SFG for recent-onset tinnitus (ROT) and persistent tinnitus (PT) groups, compared to the healthy control group.
Two regions identified in the ALFF analysis (dorsolateral SFG, left and right) were used as seeds for further FC analysis. Brain regions with significant FC pattern differences for ALFF analysis in clusters 2 and 3 are shown in Figures 3, 4, respectively. In contrast to the control group, both the ROT and PT groups exhibited a reduction in connectivity between the seed region in the left dorsolateral SFG (ALFF cluster 2) and the right superior parietal gyrus (SPG), right dorsolateral SFG, and left medial SFG (p < 0.05) (Table 3A). No significant difference was observed between the ROT and PT groups (P > 0.05). At the same time, both the ROT and PT groups exhibited decreased connectivity between the seed regions in the right dorsolateral SFG (ALFF cluster 3) and right middle frontal gyrus (MFG), left medial SFG, and right SPG (p < 0.05) (Table 3B). No difference was observed between the ROT and PT groups (p > 0.05), except for a reduced connectivity pattern between the right dorsolateral SFG (ALFF cluster 3) and the right MFG in the PT group compared to the ROT group.
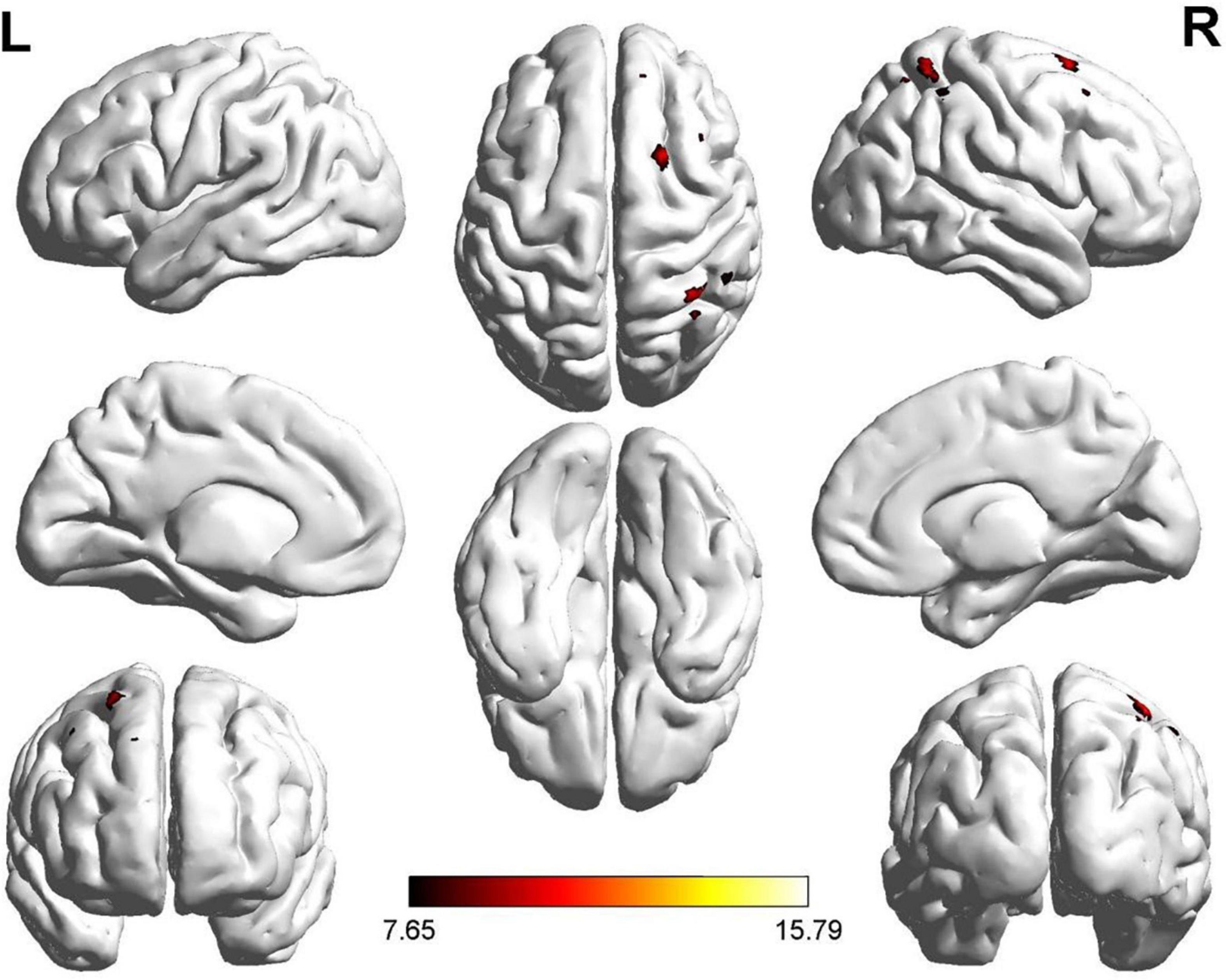
Figure 3. Significant FC patterns between the ROIs (dorsolateral SFG, left) and right SFG, left medial superior frontal gyrus (SFG), and right superior parietal gyrus (SPG) in the recent-onset tinnitus (ROT) and persistent tinnitus (PT) groups compared to the healthy control group.
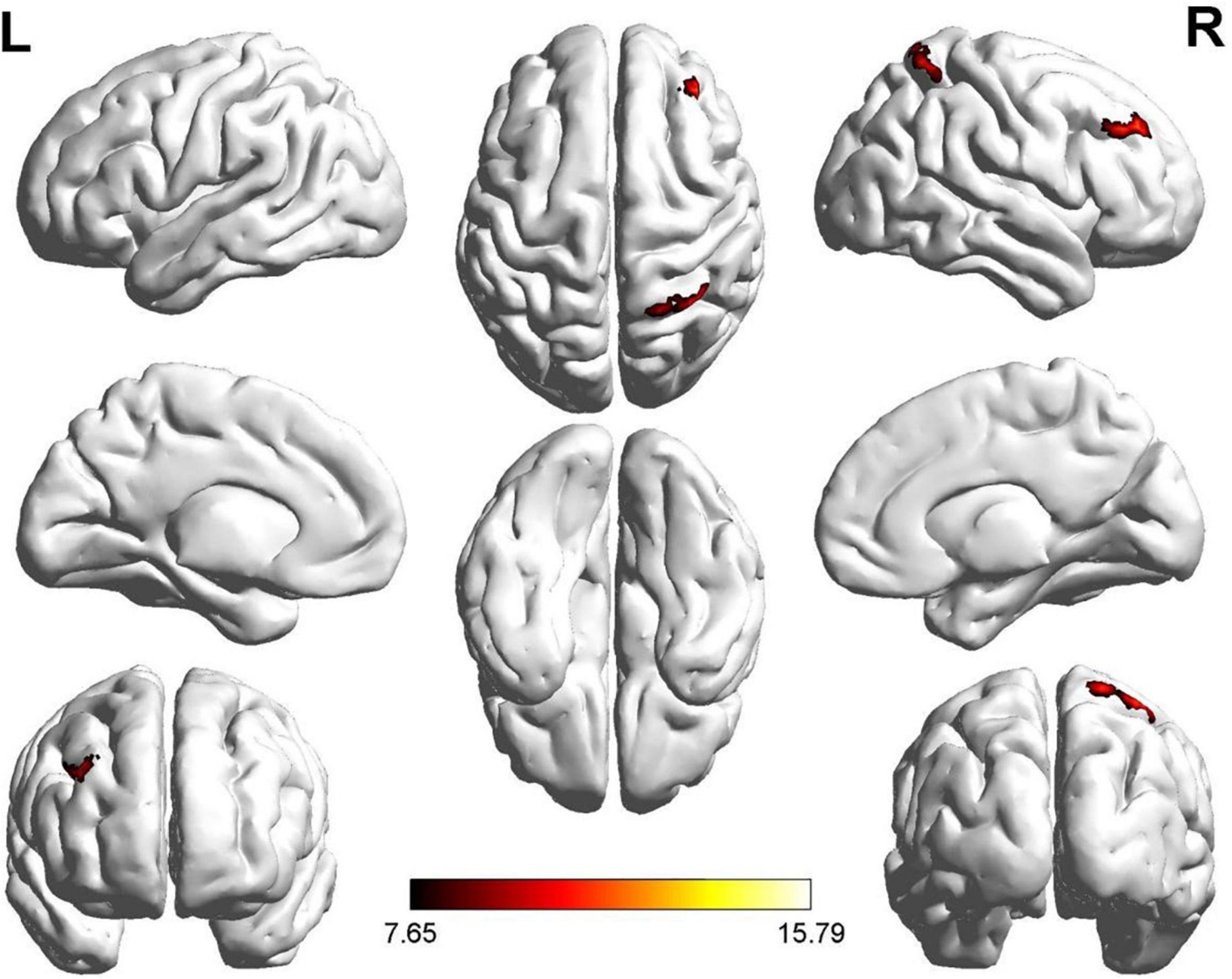
Figure 4. Significant FC patterns were observed between the right dorsolateral SFG and the right middle frontal gyrus (MFG), right medial superior frontal gyrus (SFG), and right superior parietal gyrus (SPG) in the recent-onset tinnitus (ROT) and persistent tinnitus (PT) groups compared to the CN healthy control group.
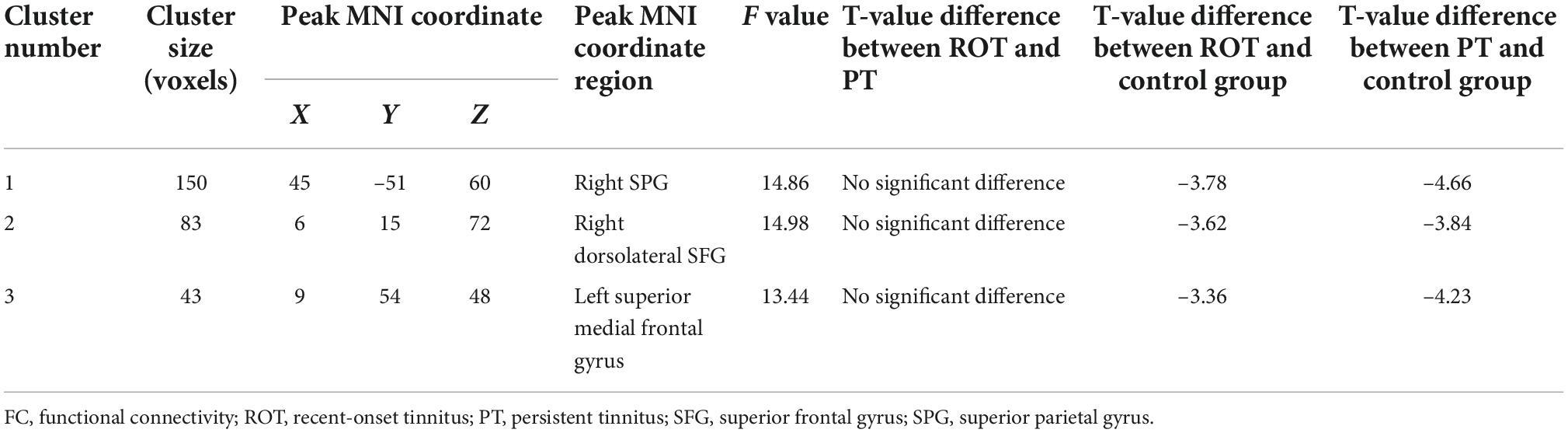
Table 3A. Decreased activities in voxel-wise FC ALFF cluster 2 for both ROT and PT groups than in the control group.
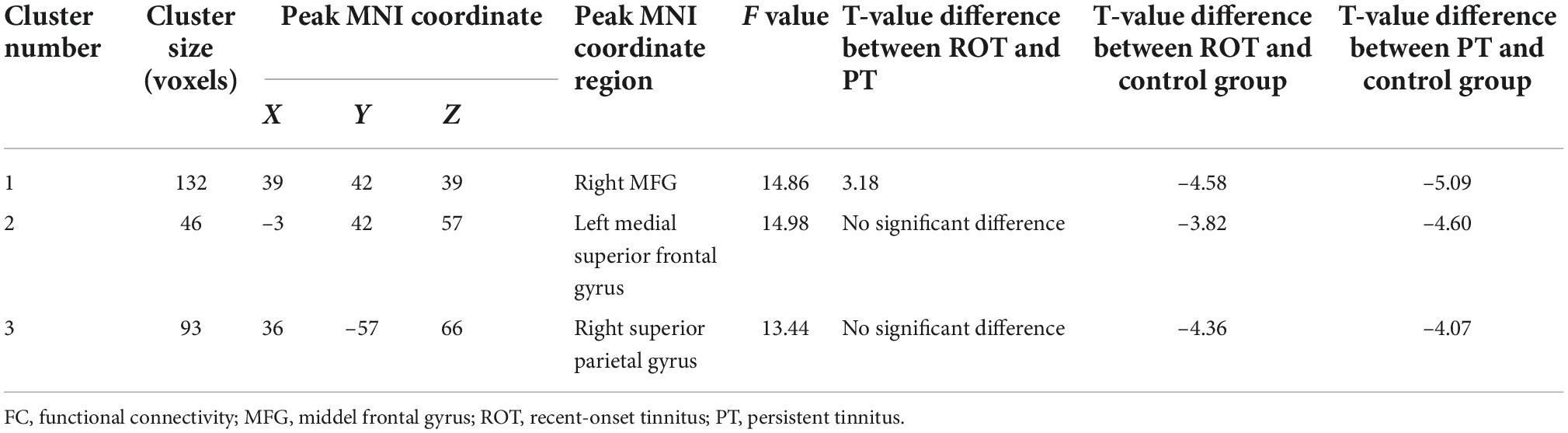
Table 3B. Decreased activities in voxel-wise FC ALFF cluster 3 for ROT and PT groups than in the control group.
One region identified in the ReHo analysis (right dorsolateral SFG) was used as the seed for further FC analysis. The brain regions with significant FC pattern differences are shown in Figure 5. In contrast to the control group, both the ROT and PT groups demonstrated lower connectivity levels between the seed region in the right dorsolateral SFG and right MFG, left medial superior frontal gyrus, and right superior parietal gyrus (SPG) (P < 0.05) (Table 3C). No difference was observed between the ROT and PT groups (p > 0.05), except for an elevated connectivity pattern between the right dorsolateral SFG (ReHo Cluster 1) and right MFG in the ROT group compared to the PT group.
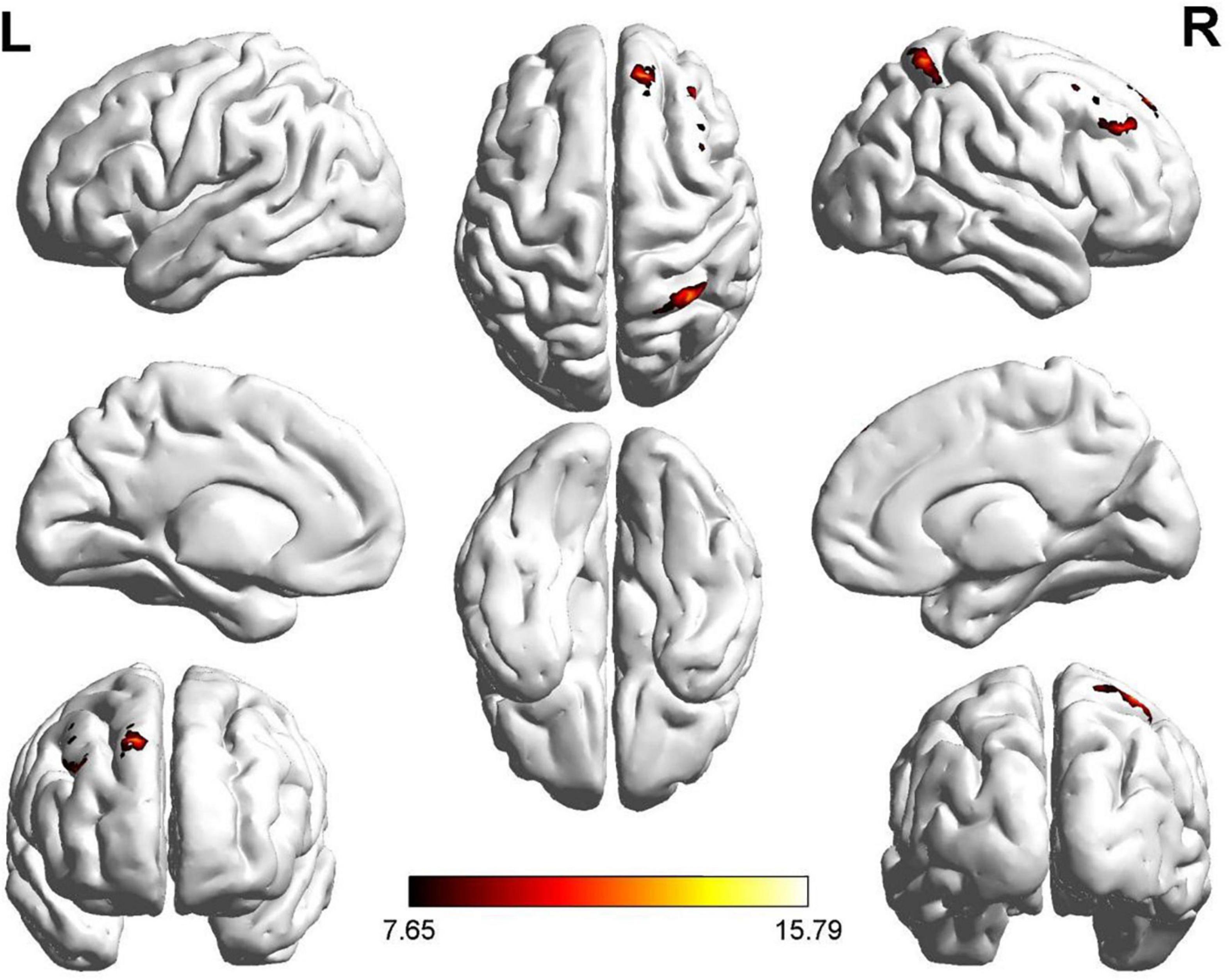
Figure 5. Significant FC patterns were observed between the right dorsolateral SFG and the right middle frontal gyrus (MFG), right superior parietal gyrus (SPG), and right dorsolateral SFG in the recent-onset tinnitus (ROT) and persistent tinnitus (PT) groups, compared to the healthy control group.
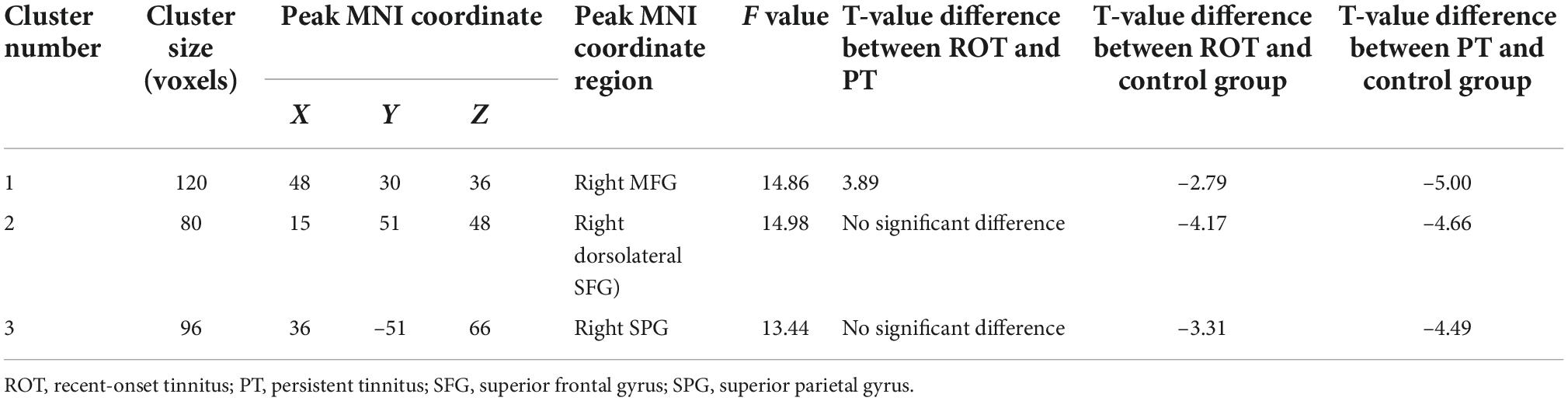
Table 3C. Decreased activities in voxel-wise functional connectivity (ReHo cluster 1) for both ROT and PT groups than in the control group.
In the current study, we utilized various resting-state fMRI technologies, including ALFF, ReHo, and voxel-wise FC, to investigate the differences in intra-regional brain activity and inter-regional FC in patients with ROT and PT. To the best of our knowledge, this is the first study to reveal neuronal changes during the transition from ROT to PT using resting-state fMRI.
Our findings revealed that participants with ROT and PT demonstrated abnormal intra-regional neural activity and disrupted FC. In addition, regions of some non-auditory networks including the DMN, optical network, DAN, and CEN were affected (Chen et al., 2017). Furthermore, we discovered significant differences within the ALFF, ReHo, and FC activity levels between the ROT and PT groups, with the PT group demonstrating the lowest activity and connectivity levels among the three groups. In order to identify the differences in brain activity between recent-onset and PT participants, we explored the roles of each brain region revealed by the rs-FMRI analysis and identified possible strategies to prevent the transition from ROT to PT.
The GR is located on the medial margin of the inferior surface of the frontal lobe. Although its specific function remains unclear, clinical reports have indicated that patients who undergo surgical removal of the GR demonstrate temporary cognitive deficits, including a reduction in memory and personality changes (Joo et al., 2016). In addition, studies using resting-state FC technologies have revealed that patients with distressful tinnitus demonstrate abnormal brain activity within the bilateral GR (Ueyama et al., 2013, 2015). Furthermore, studies have also revealed that the GR demonstrates anatomical connections with the limbic system (Lan et al., 2022). Du et al. (2020) reported that the GR demonstrated strong FC with the anterior, medial, and posterior orbital gyrus, SFG, ventromedial prefrontal cortex, and anterior cingulate cortex (Du et al., 2020).
Our findings revealed that participants with ROT demonstrated reduced activity levels in the GR compared with participants with PT. In addition, compared with the healthy control group, participants from both tinnitus groups demonstrated reduced GR activity levels. Therefore, these results indicate that patients with recent-onset or PT might perceive temporary cognitive decline due to disruptions in the GR. Furthermore, for patients with ROT, the level of disruption to cognitive processing due to tinnitus may be higher than that in patients with PT due to the novelty of tinnitus.
Both ALFF and ReHo analyses revealed a reduction in activity level in the dorsolateral SFG on both sides in participants with PT or ROT. The results did not reveal significant differences in dorsolateral SFG activities between the recent-onset tinnitus (ROT) and PT groups. The main functions of the dorsolateral SFG are top-down processing and cognitive functions, including working memory, episodic memory, goal-driven attention, planning, problem solving, and task switching. These findings imply the role of the dorsolateral SFG in CEN manipulation (Kinoshita et al., 2012; Hu et al., 2016).
In addition, the dorsolateral SFG demonstrates FC with the DMN, especially with the precuneus. The existing literature indicates that the DMN specializes in internally oriented cognitive processes, such as conceptual processing, daydreaming, and future planning (Cloutman and Lambon Ralph, 2012; Lin et al., 2017). Therefore, we suggest that the dorsolateral SFG regulates the interaction between the CEN and DMN. Reduced dorsolateral SFG activity might disrupt the CEN, eventually reducing patients’ top-down attention-filtering capability. Furthermore, our results suggest that the overall duration of tinnitus does not contribute to reduced activity levels in the left and right dorsolateral SFG. Tinnitus patients can perceive difficulties switching their attention away from the tinnitus, regardless of experiencing recent-onset or PT.
The existing literature has revealed that the medial SFG has anatomical connections with the cingulate cortex (mainly the anterior and medial section of the cingulate cortex, ACC, and MCC) through the cingulum and that functional correlation with the MCC and the DMN (Nagahama et al., 1999). In addition, dense connections between the dorsolateral prefrontal cortex (DLPFC) (including the SFG) and, ACC, and MCC, have also been discovered in humans (Zhang et al., 2011; Cloutman and Lambon Ralph, 2012; Ueyama et al., 2013).
Moreover, rs-FCs between SFG, ACC, and MCC have been reported (Cloutman and Lambon Ralph, 2012; Yang et al., 2014; Khan et al., 2021). The anatomical and functional connections between the medial SFG and anterior MCC suggest that the medial SFG is involved in cognitive control because the anterior part of the MCC is related to cognitive control, including conflict monitoring, response selection, error detection, and attention manipulation. Additionally, the medial SFG demonstrates anatomic connections with the ACC, a core node of the DMN, and a functional correlation with the DMN, suggesting that the medial SFG is critical for DMN manipulation (Hu et al., 2021).
Our findings suggest that the overall duration of tinnitus onset does not play a role in generating FC differences in the left medial SFG. Nevertheless, participants from the ROT and PT groups demonstrated reduced FC between the bilateral dorsolateral SFG and left medial superior frontal gyrus compared with the healthy control group. Therefore, we propose that reduced FC between the dorsolateral SFG and medial SFG disrupts DMN regulation, further reducing the patients’ ability to manipulate attention. Furthermore, this significant change within the top-down attention-regulating mechanism increases tinnitus perception regardless of the overall duration of tinnitus onset.
As a critical component of the VAN, the right MFG serves as a convergence center for the DAN and VAN by working as a circuit breaker to interrupt ongoing endogenous attentional processes in the DAN and reorient attention to exogenous stimuli (Japee et al., 2015; Briggs et al., 2021). Furthermore, the right MFG actively engages in reorienting distinctive signals from unexpected locations (Carter et al., 2006).
Our findings revealed reduced FC between the dorsolateral and right MFG. This change could lead to a disruption between the VAN and DAN, which influences attention orientation to novel stimuli. This conclusion agrees with the typical description of patients with tinnitus that they unconsciously perceive their tinnitus to be more prominent in quieter situations, regardless of the tinnitus duration (Xu et al., 2019).
In addition, we also discovered that participants with tinnitus developed within six months (ROT group) demonstrated statistically higher FC than participants with PT. Participants with PT also demonstrated higher THQ, SAS, and SDS scores than participants with ROT. This result indicated that patients with tinnitus would experience more difficulties reorienting their attention away from tinnitus once it lasted longer than six months (from recent-onset to persistent).
The main functions of the SFG are spontaneous attention regulation and top-down processing. The existing literature suggests that the SPG becomes more active during a task-free resting-state. Since the SFG acts as a critical component of the superior parietal lobule (SPL), it demonstrates a strong connection with the occipital lobe and involves somatosensory and visuospatial stimulus integration, written language, and working memory (Berlucchi and Vallar, 2018). The existing literature also reported SPG’s implications of SPG in shifting attention between visual targets and spatial-related attention shift states (Lin et al., 2021). Our findings revealed no significant differences between the FC levels in the ROT and PT groups. However, both groups demonstrated reduced FC compared with the healthy control group. Thus, this finding indicates that reduced FC between the dorsolateral SFG and SPG could disrupt working memory in patients with tinnitus, regardless of tinnitus duration.
The existing literature indicates that the level of tinnitus distress within six months of the initial onset predicts the long-term level of tinnitus distress in patients after six months of onset. Patients who perceive higher levels of tinnitus disruption are more likely to develop persistent and debilitating tinnitus. Multiple findings from our study indicate that patients with ROT demonstrate reduced capability of top-down attention and stimuli monitoring and orientation. Therefore, clinicians should provide effective tinnitus management strategies for patients with ROT (Kleinstäuber and Weise, 2020).
Considering that the cause of tinnitus can be multifactorial, there is no standard treatment plan for tinnitus. Nevertheless, clinicians can effectively manage tinnitus using multidisciplinary options. According to clinical practice guidelines for tinnitus from the American Academy of Otolaryngology-Head and Neck Surgery, patient education and counseling, hearing amplification, sound therapy, and cognitive-behavioral therapy should be implemented individually or in combination for tinnitus management (Tunkel et al., 2014; Zenner et al., 2016; Liu et al., 2021; Osuji, 2021).
The small sample size of our study may have reduced our ability to detect causal relationships between abnormal connectivity patterns and tinnitus characteristics. Therefore, studies with a larger sampling size are strongly recommended. Furthermore, subjects were exposed to equipment noises during the scanning process. Even though noise cancelation was instrumented in all subjects, scanner noise may reduce subjects’ tinnitus levels, thereby changing rs-FC status. Therefore, reducing the noise of brain imaging equipment will be helpful in future tinnitus-related investigations.
Our project indicated a reduced activity level within the dorsolateral SFG (left and right) and GR using ALFF and ReHo analyses. Patients with PT demonstrated higher activity levels in the GR than those with ROT. Furthermore, our follow-up voxel-wise FC revealed decreased connection activity between the dorsolateral SFG (left and right) and right SPG, right MFG, and left mSFG for participants with ROT and PT, compared to the healthy control group. Patients with ROT demonstrated a higher level of FC than those with PT did. Our data suggest that patients with PT are more likely to experience difficulties in monitoring external stimuli and attention reorientation than patients with ROT. In addition, patients who perceive higher levels of tinnitus disruption are more likely to develop persistent and debilitating tinnitus. Therefore, we strongly recommend that clinicians implement effective tinnitus management strategies in patients with ROT as soon as possible.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Affiliated Zhongda Hospital of Southeast University. The patients/participants provided their written informed consent to participate in this study.
HD: conceptualization, methodology, investigation, formal analysis, and writing—original draft preparation. XF: resources, investigation, data curation, and writing—review and editing. XQ: supervision, project administration, writing—review and editing, and funding acquisition. JZ: resources, software, validation, visualization, and formal analysis. BL: resources, software, validation, and formal analysis. AL: funding support, resources, and validation. XG and ZH: supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81970884), National Natural Science Foundation of China Youth Science Foundation (82101223), Project of Invigorating Health Care through Science, Technology, and Education (ZDXKB2016015), and Fellowship of China Postdoctoral Science Foundation (2020M681561).
The authors express their gratitude to the Department of Otolaryngology-Head and Neck Surgery and the Department of Radiology at Nanjing Zhongda Hospital, Affiliated with Southeast University, for graciously sharing precious clinical data. We were supported by the Project of Invigorating Health Care through Science, Technology, and Education.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baguley, D., McFerran, D., and Hall, D. (2013). Tinnitus. Lancet 382, 1600–1607. doi: 10.1016/s0140-6736(13)60142-7
Berlucchi, G., and Vallar, G. (2018). The history of the neurophysiology and neurology of the parietal lobe. Handb. Clin. Neurol. 151, 3–30. doi: 10.1016/b978-0-444-63622-5.00001-2
Briggs, R. G., Lin, Y. H., Dadario, N. B., Kim, S. J., Young, I. M., Bai, M. Y., et al. (2021). Anatomy and White Matter Connections of the Middle Frontal Gyrus. World Neurosurg. 150, e520–e529. doi: 10.1016/j.wneu.2021.03.045
Cai, Y., Xie, M., Su, Y., Tong, Z., Wu, X., Xu, W., et al. (2020). Aberrant Functional and Causal Connectivity in Acute Tinnitus With Sensorineural Hearing Loss. Front. Neurosci. 14:592. doi: 10.3389/fnins.2020.00592
Carter, R. M., O’Doherty, J. P., Seymour, B., Koch, C., and Dolan, R. J. (2006). Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage 29, 1007–1012. doi: 10.1016/j.neuroimage.2005.09.011
Chen, Y. C., Xia, W., Chen, H., Feng, Y., Xu, J. J., Gu, J. P., et al. (2017). Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum. Brain Mapp. 38, 2384–2397. doi: 10.1002/hbm.23525
Cloutman, L. L., and Lambon Ralph, M. A. (2012). Connectivity-based structural and functional parcellation of the human cortex using diffusion imaging and tractography. Front. Neuroanat. 6:34. doi: 10.3389/fnana.2012.00034
Du, J., Rolls, E. T., Cheng, W., Li, Y., Gong, W., Qiu, J., et al. (2020). Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex 123, 185–199. doi: 10.1016/j.cortex.2019.10.012
Hu, J., Cui, J., Xu, J. J., Yin, X., Wu, Y., and Qi, J. (2021). The Neural Mechanisms of Tinnitus: A Perspective From Functional Magnetic Resonance Imaging. Front. Neurosci. 15:621145. doi: 10.3389/fnins.2021.621145
Hu, S., Ide, J. S., Zhang, S., and Li, C. S. R. (2016). The Right Superior Frontal Gyrus and Individual Variation in Proactive Control of Impulsive Response. J. Neurosci. 36, 12688–12696. doi: 10.1523/jneurosci.1175-16.2016
Japee, S., Holiday, K., Satyshur, M. D., Mukai, I., and Ungerleider, L. G. (2015). A role of right middle frontal gyrus in reorienting of attention: A case study. Front. Syst. Neurosci. 9:23. doi: 10.3389/fnsys.2015.00023
Joo, M. S., Park, D. S., Moon, C. T., Chun, Y. I., Song, S. W., and Roh, H. G. (2016). Relationship between Gyrus Rectus Resection and Cognitive Impairment after Surgery for Ruptured Anterior Communicating Artery Aneurysms. J. Cerebrovasc. Endovasc. Neurosurg. 18:223. doi: 10.7461/jcen.2016.18.3.223
Kapolowicz, M. R., and Thompson, L. T. (2020). Plasticity in Limbic Regions at Early Time Points in Experimental Models of Tinnitus. Front. Syst. Neurosci. 13:88. doi: 10.3389/fnsys.2019.00088
Khan, R. A., Sutton, B. P., Tai, Y., Schmidt, S. A., Shahsavarani, S., and Husain, F. T. (2021). A large-scale diffusion imaging study of tinnitus and hearing loss. Sci. Rep. 11:23395. doi: 10.1038/s41598-021-02908-6
Kinoshita, M., Shinohara, H., Hori, O., Ozaki, N., Ueda, F., Nakada, M., et al. (2012). Association fibers connecting the Broca center and the lateral superior frontal gyrus: A microsurgical and tractographic anatomy. J. Neurosurg. 116, 323–330. doi: 10.3171/2011.10.jns11434
Kleinstäuber, M., and Weise, C. (2020). Psychosocial Variables That Predict Chronic and Disabling Tinnitus: A Systematic Review. Curr. Top. Behav. Neurosci. 51, 361–380. doi: 10.1007/7854_2020_213
Knipper, M., Mazurek, B., van Dijk, P., and Schulze, H. (2021). Too Blind to See the Elephant? Why Neuroscientists Ought to Be Interested in Tinnitus. J. Assoc. Res. Otolaryngol. 22, 609–621. doi: 10.1007/s10162-021-00815-1
Lan, L., Chen, Y. C., Shang, S., Lu, L., Xu, J. J., Yin, X., et al. (2022). Topological features of limbic dysfunction in chronicity of tinnitus with intact hearing: New hypothesis for ‘noise-cancellation’ mechanism. Progress Neuro Psychopharmacol. Biol. Psychiatry 113:110459. doi: 10.1016/j.pnpbp.2021.110459
Lan, L., Li, J., Chen, Y., Chen, W., Li, W., Zhao, F., et al. (2020). Alterations of brain activity and functional connectivity in transition from acute to chronic tinnitus. Hum. Brain Mapp. 42, 485–494. doi: 10.1002/hbm.25238
Lin, P., Yang, Y., Gao, J., De Pisapia, N., Ge, S., Wang, X., et al. (2017). Dynamic Default Mode Network across Different Brain States. Sci. Rep. 7:46088. doi: 10.1038/srep46088
Lin, Y. H., Dadario, N. B., Hormovas, J., Young, I. M., Briggs, R. G., MacKenzie, A. E., et al. (2021). Anatomy and White Matter Connections of the Superior Parietal Lobule. Operat. Neurosurg. 21, E199–E214. doi: 10.1093/ons/opab174
Liu, H., Zhang, J., Yang, S., Wang, X., Zhang, W., Li, J., et al. (2021). Efficacy of sound therapy interventions for tinnitus management: A protocol for systematic review and network meta-analysis. Medicine 100:e27509. doi: 10.1097/MD.0000000000027509
Makar, S. K. (2021). Etiology and Pathophysiology of Tinnitus - A Systematic Review. Int. Tinnitus J. 25, 76–86. doi: 10.5935/0946-5448.20210015
Minami, S. B., Oishi, N., Watabe, T., Uno, K., and Ogawa, K. (2018). Auditory Related Resting State fMRI Functional Connectivity in Tinnitus Patients: Tinnitus Diagnosis Performance. Otol. Neurotol. 39, 1–5. doi: 10.1097/mao.0000000000001626
Nagahama, Y., Okada, T., Katsumi, Y., Hayashi, T., Yamauchi, H., Sawamoto, N., et al. (1999). Transient Neural Activity in the Medial Superior Frontal Gyrus and Precuneus Time Locked with Attention Shift between Object Features. NeuroImage 10, 193–199. doi: 10.1006/nimg.1999.0451
Osuji, A. E. (2021). Tinnitus, Use and Evaluation of Sound Therapy, Current Evidence and Area of Future Tinnitus Research. Int. Tinnitus J. 25, 71–75. doi: 10.5935/0946-5448.20210014
Roberts, L. E., Eggermont, J. J., Caspary, D. M., Shore, S. E., Melcher, J. R., and Kaltenbach, J. A. (2010). Ringing Ears: The Neuroscience of Tinnitus. J. Neurosci. 30, 14972–14979. doi: 10.1523/jneurosci.4028-10.2010
Stolzberg, D., Hayes, S. H., Kashanian, N., Radziwon, K., Salvi, R. J., and Allman, B. L. (2013). A novel behavioral assay for the assessment of acute tinnitus in rats optimized for simultaneous recording of oscillatory neural activity. J. Neurosci. Methods 219, 224–232. doi: 10.1016/j.jneumeth.2013.07.021
Szibor, A., Mäkitie, A., and Aarnisalo, A. A. (2019). Tinnitus and suicide: An unresolved relation. Audiol. Res. 9:222. doi: 10.4081/audiores.2019.222
Tunkel, D. E., Bauer, C. A., Sun, G. H., Rosenfeld, R. M., Chandrasekhar, S. S., and Cunningham, E. R. Jr., et al. (2014). Clinical practice guideline: Tinnitus. Otolaryngol. Head Neck Surg. 151, S1–S40. doi: 10.1177/0194599814545325
Ueyama, T., Donishi, T., Ukai, S., Ikeda, Y., Hotomi, M., Yamanaka, N., et al. (2013). Brain Regions Responsible for Tinnitus Distress and Loudness: A Resting-State fMRI Study. PLoS One 8:e67778. doi: 10.1371/journal.pone.0067778
Ueyama, T., Donishi, T., Ukai, S., Yamamoto, Y., Ishida, T., Tamagawa, S., et al. (2015). Alterations of Regional Cerebral Blood Flow in Tinnitus Patients as Assessed Using Single-Photon Emission Computed Tomography. PLoS One 10:e0137291. doi: 10.1371/journal.pone.0137291
Xu, J. J., Cui, J., Feng, Y., Yong, W., Chen, H., Chen, Y. C., et al. (2019). Chronic Tinnitus Exhibits Bidirectional Functional Dysconnectivity in Frontostriatal Circuit. Front. Neurosci. 13:1299. doi: 10.3389/fnins.2019.01299
Yang, H., Zheng, Y., Ou, Y., and Huang, X. (2014). Regional homogeneity on resting state fMRI in patients with tinnitus. J. Otol. 9, 173–178. doi: 10.1016/j.joto.2014.10.001
Zenner, H. P., Delb, W., Kröner-Herwig, B., Jäger, B., Peroz, I., Hesse, G., et al. (2016). A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur. Arch. Oto Rhino Laryngol. 274, 2079–2091. doi: 10.1007/s00405-016-4401-y
Zhang, S., Ide, J. S., and Li, C. S. R. (2011). Resting-State Functional Connectivity of the Medial Superior Frontal Cortex. Cereb. Cortex 22, 99–111. doi: 10.1093/cercor/bhr088
Zhou, G. P., Shi, X. Y., Wei, H. L., Qu, L. J., Yu, Y. S., Zhou, Q. Q., et al. (2019). Disrupted Intraregional Brain Activity and Functional Connectivity in Unilateral Acute Tinnitus Patients With Hearing Loss. Front. Neurosci. 13:1010. doi: 10.3389/fnins.2019.01010
Keywords: recent-onset tinnitus, persistent tinnitus, resting-state functional magnetic resonance imaging, amplitude of low-frequency fluctuation, regional homogeneity, voxel-wise functional connectivity
Citation: Du H, Feng X, Qian X, Zhang J, Liu B, Li A, Huang Z and Gao X (2022) Recent-onset and persistent tinnitus: Uncovering the differences in brain activities using resting-state functional magnetic resonance imaging technologies. Front. Neurosci. 16:976095. doi: 10.3389/fnins.2022.976095
Received: 23 June 2022; Accepted: 26 September 2022;
Published: 19 October 2022.
Edited by:
Patrick K. A. Neff, Swiss Federal Institute of Technology Lausanne, SwitzerlandReviewed by:
Zhenchang Wang, Affiliated Beijing Friendship Hospital, Capital Medical University, ChinaCopyright © 2022 Du, Feng, Qian, Zhang, Liu, Li, Huang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhichun Huang, aHVhbmcxOTYzNjE4QHNvaHUuY29t; Xia Gao, Z2FveGlhZW50QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.