- 1Faculty of Health Sciences, Torrens University, Sydney, NSW, Australia
- 2School of Medical Sciences, University of Sydney, Camperdown, NSW, Australia
- 3Department of Research and Governance, San Hospital, Wahroonga, NSW, Australia
- 4NICM Health Research Institute, University of Western Sydney, Westmead, NSW, Australia
- 5Department of Neurology, San Hospital, Wahroonga, NSW, Australia
- 6Australian National University, Canberra, ACT, Australia
- 7Centre for Healthy Futures, Torrens University, Sydney, NSW, Australia
- 8Faculty of Medicine, Human and Health Sciences, Macquarie University, Sydney, NSW, Australia
- 9College of Health and Medicine, Australian National University, Canberra, ACT, Australia
- 10Cardiac Health Institute, Sydney, NSW, Australia
Introduction: Parkinson’s disease (PD) is the second most common, progressive, and debilitating neurodegenerative disease associated with aging and the most common movement disorder. Photobiomodulation (PBM), the use of non-thermal light for therapeutic purposes using laser or light emitting diodes (LED) is an emerging non-invasive treatment for a diverse range of neurological conditions. The main objectives of this clinical trial are to investigate the feasibility, safety, tolerability, and efficacy of a novel transcranial LED helmet device (the “PDNeuro”) in the alleviation of symptoms of PD.
Methods and analysis: This is a 24-week, two-arm, triple-blinded randomized placebo-controlled clinical trial of a novel transcranial “PDNeuro” LED Helmet, comparing an active helmet to a sham helmet device. In a survey, 40 PD participants with Hoehn and Yahr Stage I–III during ON periods will be enrolled and randomly assigned into two groups. Both groups will be monitored weekly for the safety and tolerability of the “PDNeuro” LED Helmet. Clinical signs and symptoms assessed will include mobility, fine motor skills and cognition, with data collected at baseline, 12 weeks, and 24 weeks. Assessment tools include the TUG, UPDRS, and MoCA all validated for use in PD patients. Patient’s adherence to the device usage and participant drop out will be monitored weekly. At 12 weeks both placebo and treatment groups will crossover and placebo participants offered the treatment. The main indicator for clinical efficacy of the “PDneuro” Helmet is evidence of sustained improvements in motor and non-motor symptoms obtained from participant self-reported changes, carer reporting of changes and objective reassessment by the investigators. The outcomes will assist in a future larger randomized trial design.
Clinical Trial Registration: [https://www.anzctr.org.au], identifier [12621001722886].
Background
Parkinson’s disease (PD) is a complex, heterogenous neurodegenerative disorder that presents with both motor and non-motor symptoms (DeMaagd and Philip, 2015). Cognitive decline can be a late manifestation of the disease and as these symptoms usually progress slowly, patients can be living for many years with impaired cognition (Mack and Marsh, 2017). Treatment for PD has often involved multi-modalities including pharmacotherapy, surgical intervention, and physiotherapy (Iarkov et al., 2020). Although significant inroads in PD treatment have been made, to date, optimal treatment for a range of motor, non-motor, and non-dopaminergic symptoms remains a major therapeutic challenge (Hayes et al., 2019).
Consequently, identifying alternative non-invasive, therapeutic methods is needed. Photobiomodulation (PBM) is a non-invasive treatment modality that allows wavelengths of red or near-infrared light to reach tissues beyond the surface of the skin. This therapy has been demonstrated to produce a range of beneficial physiological changes (Heiskanen and Hamblin, 2018). Various light sources, including lasers and light emitting diodes (LEDs) can be applied. PBM therapy (PBMt) was traditionally delivered with hand-held lasers and more recently via LED devices, for a wide variety of conditions (Heiskanen and Hamblin, 2018).
To affect the brain using an LED transcranial device, the light must penetrate the skin and reach the tissues of the brain with a sufficient dose to interact with neurons in the brain. The effectiveness of PBMt is therefore dependent on the amount and rate of light energy that can penetrate the human scalp and skull. PBM penetration to the brain has been investigated with results suggesting that near infrared (NIR) light can penetrate to varying depths (Haeussinger et al., 2011; Tedford et al., 2015; Henderson and Morries, 2019; Salehpour et al., 2019). Salehpour et al. (2019), performed a review of the literature and found that on average, penetration of transcranial red/NIR (630–810 nm) is between 0.2 and 10% in humans. In addition, in a cadaver model, transcranial light at 808 nm wavelength has been demonstrated to penetrate the human skull to a depth of 40 mm (Tedford et al., 2015). Haeussinger et al. (2011) described a mean penetration depth of approximately 23 mm. As well, using human cadaver heads (formalin fixed), light has been shown to reach brain parenchyma with the percent penetrance ranging from 12% in the occipital region and 1% in the temporal region (Jagdeo et al., 2012). Used as a proxy for human head, studies on sheep skull showed that at 10–15 W power range, 810 and 980 nm NIR can provide biologically meaningful depth penetration of 30 mm (Henderson and Morries, 2015).
A sleuth of literature exists on the direct effects of PBMt on neuronal cellular metabolism (Hamblin, 2018; Dompe et al., 2020; Hamblin and Liebert, 2022; Wu et al., 2022) with light absorption within the mitochondrial electron transport chain increasing ATP production, leading to a reduction in neuronal death, reduced neuroinflammation, increased cell survival and down regulation of proinflammatory markers.
Specifically, the effect of transcranial LED’s on brain function has been well-studied with changes to various parameters noted in animal models and humans (Shaw et al., 2010; Naeser and Hamblin, 2011; Jahan et al., 2019; Zomorrodi et al., 2019; Longo et al., 2020; El Khoury et al., 2021; Yao et al., 2021). The effects reported in human studies include increased activity in areas associated with attention and novelty (El Khoury et al., 2021), beneficial effects in attentional performance (Jahan et al., 2019), positive effects on modulating brain activity (Yao et al., 2021), increased organization of neural function (Zomorrodi et al., 2019) and a beneficial effect on myelin repair pathways (Longo et al., 2020).
There are also studies that demonstrate improvement in neurological symptoms in patients with PD and other conditions when treated with transcranial PBM. Light therapy in PD has been found to be of clinical benefit in a PD case series (Hamilton et al., 2019). This and other case studies using investigational PBMt devices on neural and cognitive function have presented promising results such as increased energy (Hamblin and Huang, 2019), improved gait and cognition (Saltmarche et al., 2017; Vargas et al., 2017), improved speech, and reduction in freezing episodes (Maloney et al., 2010). A small pilot double blind, placebo-controlled trial (n = 11) that examined the effect of transcranial PBMt on mild cognitive dysfunction also reported favorable clinical outcomes (Berman and Nichols, 2019). Recently, Nizamutdinov et al. (2021) demonstrated improvement across several brain functions in dementia patients treated with transcranial LED when compared with a sham treated group. A recent small study on PBMt delivered via a transcranial device on PD patients documented its safety and tolerability and demonstrated measurable improvements in several PD related signs and symptoms (Liebert et al., 2021). Transcranial helmet devices appear an ideal solution and an important mode of delivery for PBMt due to ease of self-application and ability to irradiate large area of tissue (Heiskanen and Hamblin, 2018).
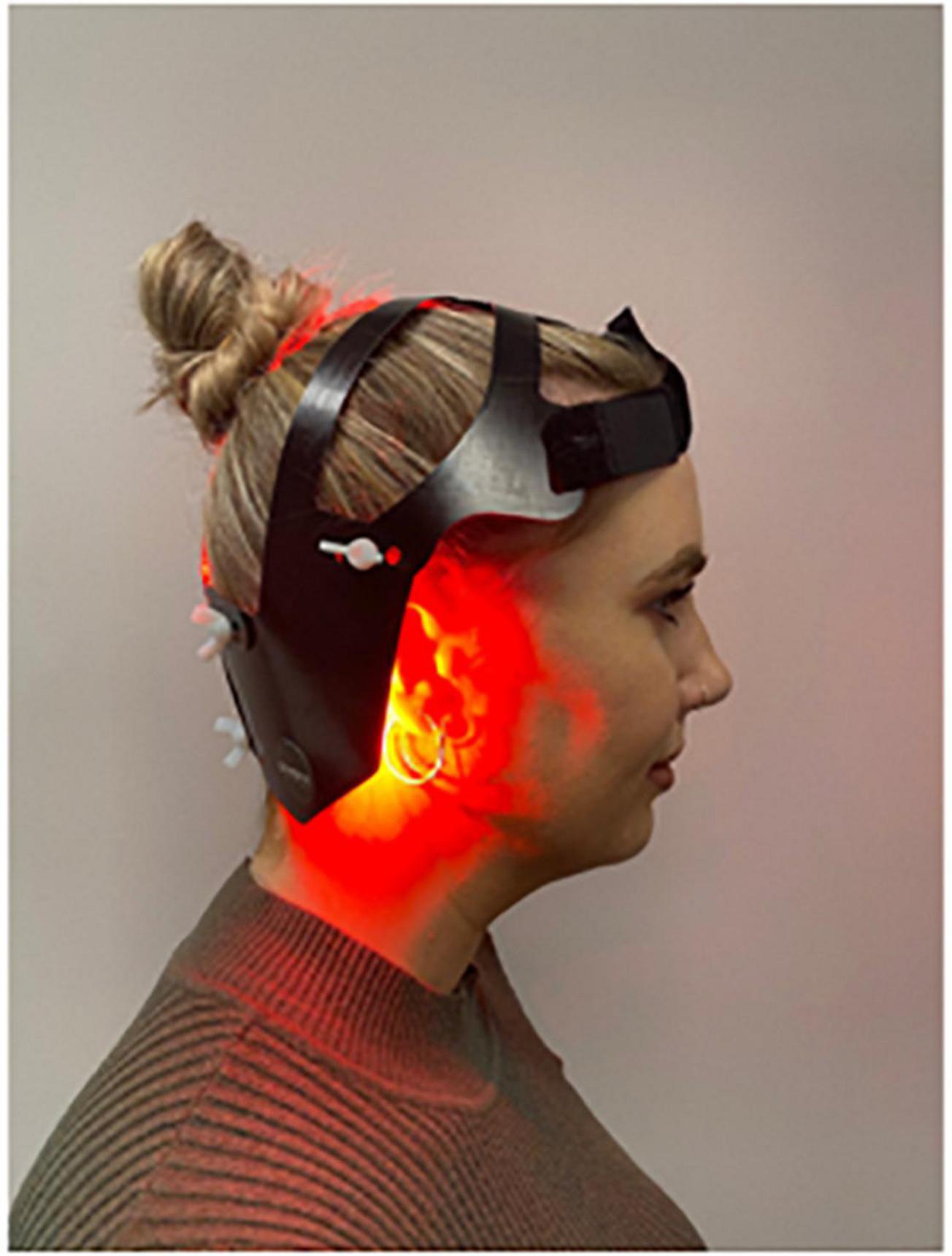
The “PDNeuro” Helmet (see Figures 1, 2) allows transcranial application of light at 20 locations, with each location including one infra-red (IR) 810 nm LED and one red 630–670 nm LED. The locations of the “PDNeuro” Helmet LEDs were selected based on anatomic points that have been used clinically and in published studies on neurodegenerative disease (Saltmarche et al., 2017; Hamilton et al., 2019; Liebert et al., 2021). The points include the mastoid process and the second cervical vertebrae points of the sub-occipital region. They are intended to target the corresponding dermatome to permit sensory input to converge onto the trigeminocervical nuclei and the neural connections of the brain stem and cerebellum pathways, including the putative endorestiform nucleus (Bradman and Barry, 2013; Paxinos et al., 2020).
This triple blinded, randomized controlled, at-home 24-week trial (RCT) with a crossover of the placebo group, has been designed to test the safety and effectiveness of the “PDNeuro” Helmet device in modifying clinical signs and symptoms experienced by PD patients. This compliments a previous experimental study that showed efficacy of a combined treatment protocol (transcranial with an abdominal, neck, and nasal application) in PD patients (Liebert et al., 2021). In previous published studies, the authors have demonstrated observable changes after 4-weeks of intervention, with statistical significance reported at 12-weeks utilizing both transcranial and remote PBMt, suggesting that 24-weeks will be sufficient to demonstrate significant effects with the present study being a randomized controlled trial with increased total number of participants (Liebert et al., 2021).
Hypothesis and aims
The main objectives of the study described here are to; investigate the feasibility, safety, tolerability, and efficacy of a novel transcranial LED helmet device (the “PDNeuro” Helmet) and determine the therapeutic effects of PBMt when applied 6 days per week over 12 weeks.
Hypotheses
1. Primary Hypothesis: Transcranial LED PBMt is a safe, non-invasive therapeutic intervention for patients with diagnosed PD.
2. Secondary Hypothesis: Treatment by Transcranial LED PBMt attenuates motor and non-motor symptoms of PD.
Methods and analysis
Trial design
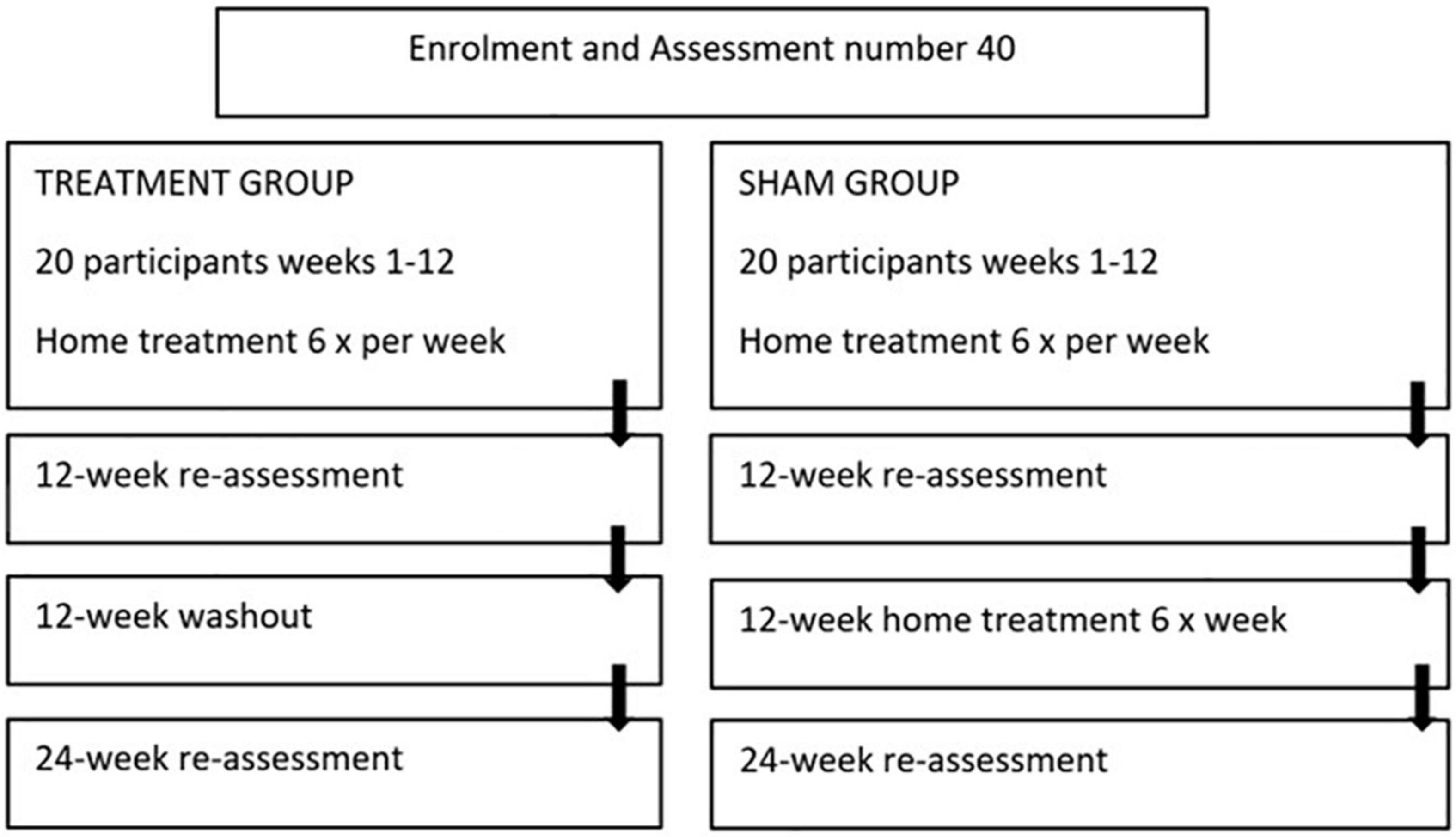
The study is a 24-week, two-arm, triple blinded randomized placebo-controlled trial evaluating the safety and effectiveness of the “PDNeuro” Helmet (see Figures 1, 2) compared with a sham helmet device. Treatment will consist of PBMt to the head six times per week for 12 weeks (see Figure 3), with Group 1 (the active group) receiving 12 min of infra-red and 12 min red LED, and Group 2 (the sham group) receiving sham treatment for 24 min. After the initial 12 weeks, the sham patients will be given the active treatment for 12 weeks while the active treatment group will have a 12 weeks washout period and then be re-assessed in 12 weeks (see Figure 3). Outcome measures will be collected by the study investigators that have received specialized training in assessment and data collection.
The study received ethics approval from Sydney Adventist Health (SAH) human ethics research committee (HREC) (approval number 2019-032). All experiments are carried out in accordance with the approved ethics guidelines.
Eligibility and recruitment
The study consists of 40 PD patients (20 male + 20 female) recruited via media advertisements on TV and local media.
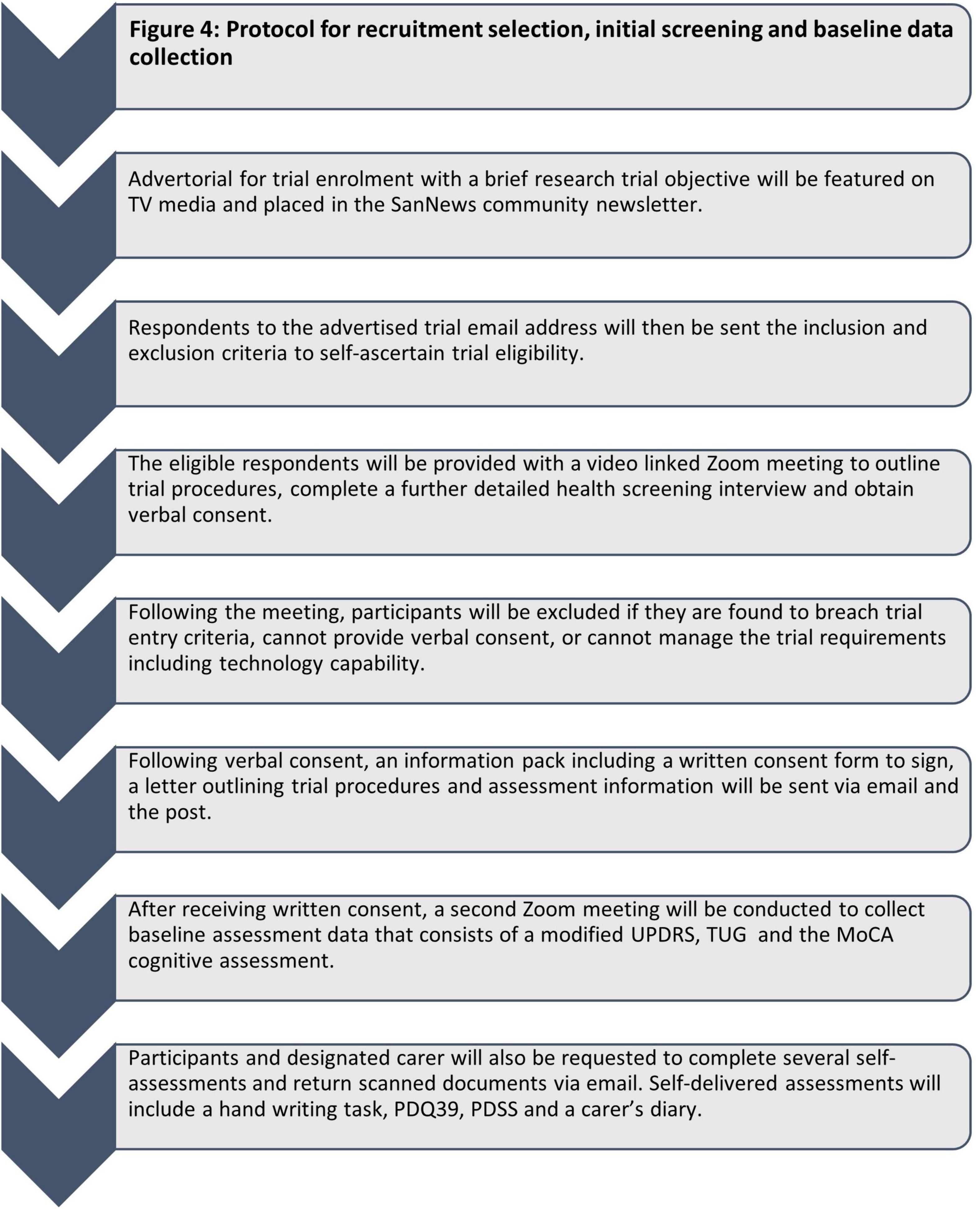
Due to the remote (virtual) nature of this trial, only PD participants categorized as Hoehn and Yahr Stage I-III during ON periods, and willing to have a carer present during all assessments and treatment sessions are included. Consent forms are signed by hand, and both emailed and posted back (see Figure 4 for overview of recruitment process).
Inclusion criteria
• Females and males aged 59–85 years
• Diagnosed with Idiopathic PD (by United Kingdom Brain Bank Criteria) with Modified Hoehn and Yahr Stage I–III during ON periods
• ≥3 weeks of stable anti-PD medications
• Sufficient space (around 9 m2) to be able to perform motor assessments
• Suitable and sufficiently fast home-based internet connection for uninterrupted video calls and video conferencing
• Knowledge (self or carer) of using a phone and/or tablet applications on either IOS or Android platforms
• Attendance of a “carer” during each Zoom meeting and during all participant treatment sessions
Exclusion criteria – Participants will be excluded from the study if they:
• Are not capable of self-care
• History of significant psychotic episode(s) within the previous 12 months
• History of suicidal ideation or attempted suicide within previous 12 months
• Take potentially photosensitizing medications, in particular imipramine, hypericum, phenothiazine, lithium, chloroquine, hydrochlorothiazide, or tetracycline
• Have history of structural brain disease, active epilepsy, stroke, factors affecting gait performance and stance unrelated to PD, such as due to severe joint disease, orthopedic injuries, weakness, peripheral neuropathy with proprioceptive deficits, severe peripheral vascular occlusive disease, severe musculoskeletal disorders, uncorrected vision, vestibular problems or other acute illness or severe condition that would:
- Preclude the use of PBM therapy
- Place the patient at risk during evaluation of their PD, or
- Interfere with the evaluation of their PD
• Are currently participating in other clinical trials, including treatment of PD
• Are currently using any form of self-administered light therapy
• Have evidence of severe and unstable dysautonomia
• Have significant cardiac disease
⚬ Cardiac interventions (in the past 3-months)
⚬ Unstable arrhythmias (in the past 3-months)
⚬ Diagnosis of cardiac dysautonomia
Randomization
Following virtual screening and informed consent, 40 participants will be randomized into two groups and then allocated into a treatment or sham group via an independent research administrator and thereafter identified via an anonymous SN (serial number). To reduce the chance of participants inadvertently realizing that they are in the sham group, the HREC approved that the participants be informed there are four groups: active infrared, inactive infrared, active red + infrared, inactive red + infrared. Group 1 will receive active treatment and Group 2 will receive a sham helmet, which is identical in appearance. Participants will be instructed on helmet use by a trained technician via Zoom video communication.
By intention there will be equal numbers of males and females in each group. While it is well-known that gender differences effect human disease including PD, little research has been performed into the different gender responses to PBMt (Liebert et al., 2022). Therefore, the efficacy and tolerability outcomes of PBMt transcranial delivery will be subject to sub analysis based on gender.
Intervention
The participants will be mailed their “PDNeuro” or sham helmet with all treatment to be self-administered at home in the presence of their carer. Study participants will be provided with detailed written and visual instructions on correct helmet fitment and treatment protocol. This will be supplemented with a Zoom video consultation with a trained technician and scientist to ensure correct device fitment and operation. During this Zoom consultation the helmet will be viewed from the frontal, sagittal, and transverse plane to ensure correct fitment. Participants will be subsequently monitored on a weekly basis with a compulsory consultation with the same trained research personnel to ensure continued correct fitment of the study device and adherence to the study protocol. In addition, all participants will be encouraged to reach out to the research team outside of these monitorisation periods for any trial related queries via either phone or email which are monitored 24-h per day, 7-days per week.
The PBMt intervention will consist of six 24 min-treatment sessions per week for 12 weeks (see Figure 3). Participants in Group 1, the active treatment group, will receive transcranial light treatment with a “PDNeuro” LED Helmet device with 20 LED stimulus sites, each having one IR (810 nm) LED and one Red (630–670 nm) LED. Average optical power for the IR LED (810 nm) is 52 mW and for the RED LED (630–670 nm) 27 mW. Treatment dose will consist of 0.052 W × 720 s to total 37.44 joules for IR and 0.027 W × 720 s to total 19.44 joules for red. Therefore, with 37.44 and 19.44 joules delivered over 20 diodes a total of 1,137 joules will be administered per session.
Participants in the placebo/sham group (Group 2) will receive the same apparent treatment as Group 1, except that they will be “treated” with sham transcranial LED devices that deliver no light. After the 12 weeks the placebo group will be offered a further 12 weeks of treatment with the active “PDNeuro” Helmet.
Outcome measures
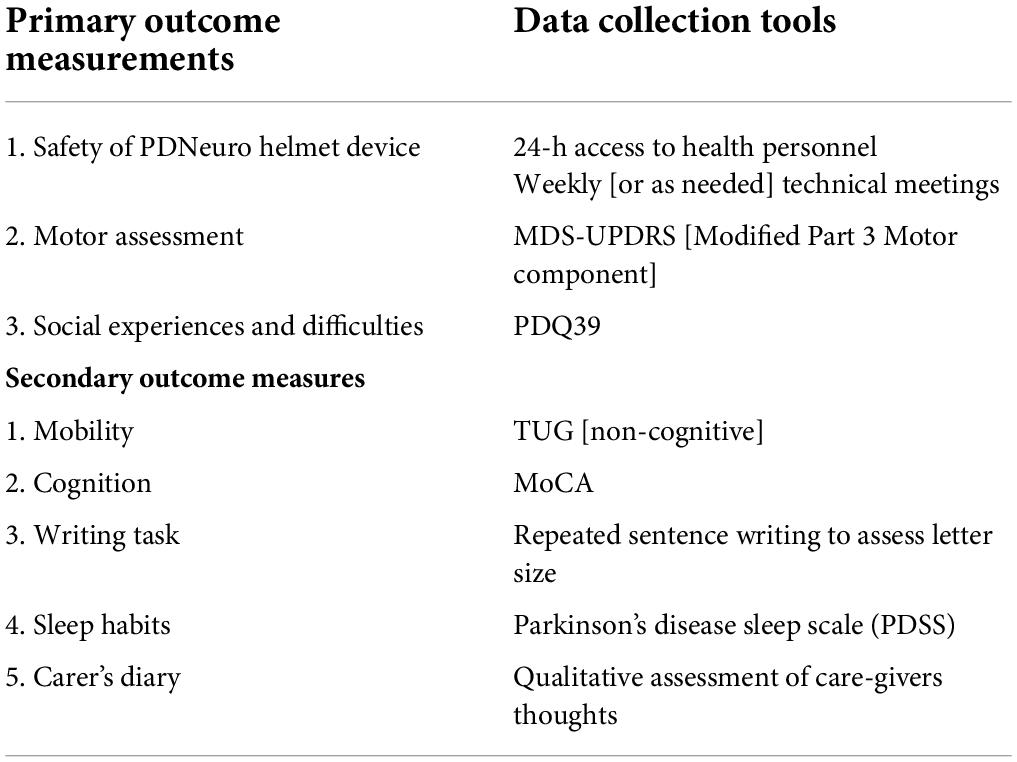
Due to the broad clinical signs and symptoms of PD, a range of outcome measures were selected to determine functional improvement (Table 1). Many PD clinical rating scales have been developed since the 1960s that assess both motor and non-motor functions (Ramaker et al., 2002) and all outcome measures to be used in this study have been validated for use in PD remotely (Abdolahi et al., 2013; Stillerova et al., 2016). The primary outcome measures will be conducted and recorded using a combination of self-reported assessment and visual assessment obtained via Zoom video link. Outcome measures will be supervised, and data will be collected via specialist examiners and physiotherapists all trained in conducting each assessment. Outcome measures will be obtained at baseline, 12-week and 24-week intervals (see Figure 3).
Primary outcome measurements
Safety of the “PDNeuro” Helmet device, MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) modified Part 3 and social experiences and difficulties as measured with the Parkinson’s disease-specific quality of life questionnaire, the PDQ-39.
Secondary outcome measurements
1. Movement mobility (TUG [non-cognitive])
2. Cognition (MoCA)
3. Writing task (assess writing size)
4. Sleep habits self-assessment (PDSS sleep scale)
5. Carer’s diary of daily living
UPDRS
The primary motor measure chosen is the motor assessment (Part 3) of the UPDRS as it is one of the most common movement assessment outcomes used in PD (Ivey et al., 2012), and demonstrates high internal consistency and inter-rater reliability while showing moderate construct validity (Rodriguez-Blazquez et al., 2017).
Furthermore, the UPDRS assessment has been shown to be clinically valid when conducted remotely (Schneider et al., 2020). Indeed, the feasibility, reliability and value of implementing remote telemedicine/video-based assessments for Parkinson’s disease patients for both research (Tarolli et al., 2020) and ongoing healthcare (Cubo et al., 2020) have been previously evaluated and deemed valid.
Sixteen of the eighteen items from the UPDRS motor were included. Rigidity was excluded as this is unable to be determined without manual assessment. As well, postural stability was not performed due to the potential risk of falling if performed without adequate supervision. In the UPDRS all items are scored on a scale from 0 (normal) to 4 (severe), and total scores obtained from the sum of the corresponding item scores, stage the severity of PD. These scores are to be collected using video link with attention being paid to positioning the camera and participant to ensure clear vision. The carer will be required to manipulate the camera to ensure that the participant is clearly visible. All assessors are trained in the administration of the UPDRS assessment tool to maximize the outcome measure.
PDQ39
It is well-documented that quality of life (QOL) plays an important role in patient self-efficacy, is instrumental for independent living and, in chronic conditions such as PD is frequently greatly impaired (Megari, 2013). The questionnaire tool PDQ39 is a self-administered questionnaire that assesses PD specific quality of health during a specific time-period. It assesses across eight life dimensions: mobility, ADL’s, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort (Hagell and Nygren, 2007). The PDQ39 will be administered by the patient during the same three time points: pre-treatment, following 12 weeks and 6 months post trial commencement.
Timed up and go test
To measure patients’ functional mobility, timed up and go (TUG) test will be used. The TUG test is a validated and effective tool to assess the participants functional mobility and the gait related motor symptoms common in PD (da Silva et al., 2017). The TUG involves sequential motor tasks that incorporate arising from a chair, walking, and turning around all of which are affected by PD. All assessors are trained in the administration of the TUG.
Montreal cognitive assessment
Cognitive impairment is a significant non-motor symptom of PD with as many as 80% of patients exhibiting decline in function (Flores-Torres et al., 2021). A frequently used assessment tool is the Montreal Cognitive Assessment (MoCA) a brief tool that assesses several cognitive domains. The MoCA is validated for use in 55–85-year-olds and recommended as a scale for cognitive screening in PD (Chou et al., 2010; Fang et al., 2020). The assessment consists of a 30-point test on a single side of A4 paper and can be administered in 10 min. All assessors are trained in the administration of the MoCA.
Writing task
Micrographia is a common clinical feature associated with PD and a measure of PD progression (Smits et al., 2014; Hamilton et al., 2019). Participants will be asked to write “the quick brown fox jumps over the lazy dog” and sign their name ten times and note of potential change in size will be recorded over the same three time-points.
Carer’s diary
A carer’s diary can provide qualitative data on patient experiences, as well as note changes in clinical manifestations that are not observed in the clinical environment. This information in the areas of physical, cognitive, and emotional domains can reveal both positive and negative changes and may provide a more detailed picture of the effect of treatment.
Safety considerations
Photobiomodulation is considered a safe treatment. In over 50 years of research on the effects of PBMt, there have been limited published results of harm when used within the correct dose window. Cassano et al. (2019) noted transitory and benign side effects of transcranial PBMt and Maiello et al. (2019) reported one case of severe headache, which led to discontinuation of treatment.
Dosing protocols for the PD trial have been based on both clinical experience by members of the research team and others (Dompe et al., 2020). To provide for patient safety, a suspected adverse event committee was formed consisting of the coordinating chief investigator, a neurologist from the research team, and an independent neurologist. A member of this team will be available via phone 24/7 to address any concerns that arise.
Safety will be assessed at weekly intervals in pre-organized Zoom meetings and at any other time as required. All suspected adverse events (SAE) will be followed up with the Committee coordinating chief investigator and all relevant medical history documented and maintained in a file of “identified” subjects.
All device issues will be directed to the device support officer. In the case of helmet failure, the faulty device will be repaired by a qualified medical devices electrical engineer, or a new device will be provided.
Patient timeline
The schedule of clinical research activities is illustrated in Table 2. Participant investigator interaction will consist of four Zoom meetings for screening and assessment and Zoom meetings weekly for initial and ongoing device management. As well, ongoing email, telephone calls, and Zoom meetings will be provided as necessary during the period of device usage.
Data collection
Once accepted on the trial all data will be stored in a secure location both in paper and electronic form. Data collected will include patient’s sociodemographic data and clinical history. Next, the primary outcomes and the secondary outcomes of the study will be collected. At the end of 12 weeks, the primary and secondary outcomes of the study participants will be reassessed by the same evaluator who performed the baseline assessment. Further data will be collected following 24 weeks when Group 2 (the sham group) have also had the chance to experience the treatment and Group 1 (the initial treatment group) have had a 12-week washout. The investigators responsible for collecting all outcome measurements will be blinded to the treatment being administered to the patients.
Statistical analysis
This is a feasibility study trialing the efficacy of the “PDNeuro” Helmet transcranial device. The sample size of 40 participants falls within the recommendation of between 12 and 50 for feasibility clinical studies (Julious, 2005; Sim and Lewis, 2012). As a series of N = 1 studies, it is anticipated that only basic statistical analysis will be performed. For each outcome measure, descriptive data (mean, standard deviation) will be calculated. From this data, “minimally important difference” (MID) scores will be computed based on 1/2 SD of each measure. This is a common MID measure (Norman et al., 2003) based on the distribution of the participant scores at baseline and provides a sensitive indicator of significant change over time for N = 1 case studies. This has also been used with good effect in previous PBMt PD trials (Liebert et al., 2021). As such, it does not suffer from a lack of statistical power that would be evident with more traditional ANOVA approaches with small sample sizes. The number of participants showing improvement (i.e., difference between two time points > MID) can be compared between time points with chi-square analyses.
In total, 162 participants (81 + 81) were proposed for recruitment over a 2-year period. However, due to limitations involving in no small part to the substantial COVID-19 restrictions during the conception of this trial, the decision was made to reduce the total number of participants to 40, with the intention of increasing this by 122 at a later date.
Discussion
Dissemination of results
The results addressing study objectives will be disseminated to relevant research, clinical, health services, and patient communities through requisite publications in peer-reviewed journals and presentations at scientific and clinical conferences, as well as media channels. These results will build upon safety and efficacy data to aid in the management of PD patients and in the development of a sufficiently powered larger randomized RCT.
Advantages and limitations
This is a triple blinded RCT designed to minimize bias and maximize the validity of any differences observed between the treatment and sham groups. While the triple blinding of this RCT is an advantage, restrictions resulting from SARS-CoV-2 affected the initial protocol, creating several limitations. Due to restrictions in Australia during the conception of this trial, it was not feasible to invite participants into a clinic setting for in-person intervention and assessments due to government regulations at the time. Furthermore, the increasing rate of infections and hospitalizations during this period meant that movement of research staff and participants to and from a clinic/hospital location would introduce an increased risk of potential COVID-19 infection. Therefore, the decision was made to pivot the study to a home-based trial to minimize as much person-to-person contact as possible, and to mitigate any risk of infection to our vulnerable population.
The scientific/clinical validity and feasibility of conducting our RCT remotely had been assessed prior to commencement of the study. For example, the UPDRS assessment used for the RCT has been shown to be clinically valid when conducted remotely (Schneider et al., 2020). Indeed, the feasibility, reliability, and value of implementing remote telemedicine/video-based assessments for Parkinson’s disease patients for both research (Tarolli et al., 2020) and ongoing healthcare (Cubo et al., 2020) have been previously evaluated and deemed valid. Furthermore, all outcome measures will be assessed by medical professionals (physicians, nurses, physiotherapists) who have been trained to conduct clinical assessments in Parkinson’s disease patients, with supervision of leading neurologist to ensure assessment accuracy and reliability.
The resulting limitation is that treatment is delivered unsupervised with only a carer present. This means that there is a reliance of self-reporting and participant honesty that the “PDNeuro” Helmet is being applied correctly for the duration of the treatment period. Assessments are also being recorded via video communication and although cameras are effective in providing visual feedback it is possible that fine tremors may be missed and reliance of carers to manipulate the camera and give good visual observation may present a challenge.
Both motor and non-motor symptoms will be assessed using validated assessment tools for PD patients. Assessors are all trained in the delivery of each assessment tool. Furthermore, both initial and follow-up assessment are to be performed by the same assessor to increase Intrarater reliability.
Another advantage is that this study will use both quantitative and qualitative data to explore the effect of multiple signs and symptoms to evaluate efficacy of PBMt.
Summary
This manuscript details the protocol for a prospective, single-centre study of a novel, portable LED neuro-helmet to evaluate its safety, efficacy, and tolerability. This study is important for the field of neurodegenerative disease, in particular PD, for several reasons.
The overall aging of the general population, together with the increasing prevalence of PD, means that diverse treatment options need to be explored. If the “PDNeuro” LED Helmet device is demonstrated to be safe and effective, then it potentially offers a portable, non-invasive, and inexpensive non-pharmaceutical treatment modality with minimal side effects that can be conveniently administrated at home or in an office/clinic.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Sydney Adventist Health (SAH) Human Ethics Research Committee (HREC), approval number (2019-032). The patients/participants will provide their written informed consent to participate in this study. Written informed consent will be obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AL and HK: conceptualization. CM: writing – original draft preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was received funding from SYMBYX Pty Ltd., which also supplied the “PDNeuro” and sham helmets for the trial. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this manuscript, or the decision to submit it for publication.
Acknowledgments
The sponsor and the supplier of devices for this study is SYMBYX Biome Pty Ltd. (2/50 Yeo Street, Neutral Bay, NSW 2089, Australia).
Conflict of Interest
BB was employed by Sumolite Pty Ltd. VP was an employee and AL, BB, GH, CSM, and HK were shareholders of SYMBYX Pty Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdolahi, A., Scoglio, N., Killoran, A., Dorsey, E. R., and Biglan, K. M. (2013). Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Parkinson. Relat. Disord. 19, 218–221. doi: 10.1016/j.parkreldis.2012.10.008
Berman, M. H., and Nichols, T. W. (2019). Treatment of neurodegeneration: integrating Photobiomodulation and neurofeedback in Alzheimer’s dementia and Parkinson’s: a review. Photobiomodulation Photomed. Laser Surg. 37, 623–634. doi: 10.1089/photob.2019.4685
Bradman, L., and Barry, C. (2013). The role of the trigeminal sensory nuclear complex in the pathophysiology of craniocervical dystonia. J. Neurosci. 33, 18358–18367. doi: 10.1523/JNEUROSCI.3544-13.2013
Cassano, P., Caldieraro, M. A., Norton, R., Mischoulon, D., Trinh, N. H., Nyer, M., et al. (2019). Reported side effects, weight and blood pressure, after repeated sessions of transcranial photobiomodulation. Photobiomodulation Photomed. Laser Surg. 37, 651–656. doi: 10.1089/photob.2019.4678
Chou, K. L., Amick, M. M., Brandt, J., Camicioli, R., Frei, K., Gitelman, D., et al. (2010). A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov. Disord. 25, 2501–2507. doi: 10.1002/mds.23362
Cubo, E., Hassan, A., Bloem, B. R., and Mari, Z. (2020). Implementation of telemedicine for urgent and ongoing healthcare for patients with Parkinson’s disease during the COVID-19 pandemic: new expectations for the future. J. Parkinsons Dis. 10, 911–913. doi: 10.3233/JPD-202108
da Silva, B. A., Faria, C. D., Santos, M. P., and Swarowsky, A. (2017). Assessing Timed Up and Go in Parkinson’s disease: reliability and validity of Timed Up and Go Assessment of biomechanical strategies. J. Rehabil. Med. 49, 723–731. doi: 10.2340/16501977-225
DeMaagd, G., and Philip, A. (2015). Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 40:504.
Dompe, C., Moncrieff, L., Matys, J., Grzech-Leśniak, K., Kocherova, I., Bryja, A., et al. (2020). Photobiomodulation—underlying mechanism and clinical applications. J. Clin. Med. 9:1724. doi: 10.3390/jcm9061724
El Khoury, H. E., Mitrofanis, J., and Henderson, L. A. (2021). Does photobiomodulation influence the resting-state brain networks in young human subjects? Exp. Brain Res. 239, 435–449. doi: 10.1007/s00221-020-05981-x
Fang, C., Lv, L., Mao, S., Dong, H., and Liu, B. (2020). Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinsons Dis. 2020:2076942. doi: 10.1155/2020/2076942
Flores-Torres, M. H., Hughes, K. C., Molsberry, S., Gao, X., Kang, J. H., Schwarzschild, M. A., et al. (2021). Cognitive function in men with non-motor features of Parkinson’s disease. BMJ Neurol. Open 3:e000112. doi: 10.1136/bmjno-2020-000112
Haeussinger, F. B., Heinzel, S., Hahn, T., Schecklmann, M., Ehlis, A. C., and Fallgatter, A. J. (2011). Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One 6:e26377. doi: 10.1371/journal.pone.0026377
Hagell, P., and Nygren, C. (2007). The 39 item Parkinson’s disease questionnaire (PDQ-39) revisited: implications for evidence- based medicine. J. Neurol. Neurosurg. Psychiatry 78, 1191–1198. doi: 10.1136/jnnp.2006.111161
Hamblin, M. R. (2018). Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 94, 199–212. doi: 10.1111/php.12864
Hamblin, M. R., and Huang, Y. Y. (eds) (2019). Photobiomodulation in the Brain: Low-Level Laser (Light) Therapy in Neurology and Neuroscience. Cambridge, MA: Academic Press.
Hamblin, M. R., and Liebert, A. (2022). Photobiomodulation therapy mechanisms beyond cytochrome c oxidase. Photobiomodulation Photomed. Laser Surg. 40, 75–77. doi: 10.1089/photob.2021.0119
Hamilton, C., Hamilton, D., Nicklason, F., and Mitrofanis, J. (2019). “Transcranial photobiomodulation therapy: observations from four movement disorder patients,” in Photobiomodulation in the Brain, eds M. R. Hamblin and Y.-Y. Huang (Cambridge, MA: Academic Press), 463–472.
Hayes, M. W., Fung, V. S., Kimber, T. E., and O’Sullivan, J. D. (2019). Updates and advances in the treatment of Parkinson disease. Med. J. Austral. 211, 277–283. doi: 10.5694/mja2.50224
Heiskanen, V., and Hamblin, M. R. (2018). Photobiomodulation: lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 17, 1003–1017. doi: 10.1039/c8pp90049c
Henderson, T. A., and Morries, L. D. (2015). Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 11:2191. doi: 10.2147/NDT.S78182
Henderson, T. A., and Morries, L. D. (2019). “Near-infrared photonic energy penetration—principles and practice,” in Photobiomodulation in the Brain, eds M. R. Hamblin and Y.-Y. Huang (Cambridge, MA: Academic Press), 67–88.
Iarkov, A., Barreto, G. E., Grizzell, J. A., and Echeverria, V. (2020). Strategies for the treatment of Parkinson’s disease: beyond dopamine. Front. Aging Neurosci. 12:4. doi: 10.3389/fnagi.2020.00004
Ivey, F. M., Katzel, L. I., Sorkin, J. D., Macko, R. F., and Shulman, L. M. (2012). The Unified Parkinson’s disease rating scale as a predictor of peak aerobic capacity and ambulatory function. J. Rehabil. Res. Dev. 49:1269. doi: 10.1682/jrrd.2011.06.0103
Jahan, A., Nazari, M. A., Mahmoudi, J., Salehpour, F., and Salimi, M. M. (2019). Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers Med. Sci. 34, 1193–1200. doi: 10.1007/s10103-018-02710-3
Jagdeo, J. R., Adams, L. E., Brody, N. I., and Siegel, D. M. (2012). Transcranial red and near infrared light transmission in a cadaveric model. PLoS One 7:e47460. doi: 10.1371/journal.pone.0047460
Julious, S. A. (2005). Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut. Stat. J. Appl. Stat. Pharm. Ind. 4, 287–291. doi: 10.1002/pst.185
Liebert, A., Bicknell, B., Laakso, E. L., Heller, G., Jalilitabaei, P., Tilley, S., et al. (2021). Improvements in clinical signs of Parkinson’s disease using photobiomodulation: a prospective proof-of-concept study. BMC Neurol. 21:256. doi: 10.1186/s12883-021-02248-y
Liebert, A., Seyedsadjadi, N., Pang, V., Litscher, G., and Kiat, H. (2022). Evaluation of gender differences in response to photobiomodulation therapy, including laser acupuncture: a narrative review and implication to precision medicine. Photobiomodulation Photomed. Laser Surg. 40, 78–87. doi: 10.1089/photob.2021.0066
Longo, M. G. F., Tan, C. O., Chan, S. T., Welt, J., Avesta, A., Ratai, E., et al. (2020). Effect of transcranial low-level light therapy vs sham therapy among patients with moderate traumatic brain injury: a randomized clinical trial. JAMA Netw. Open 3:e2017337. doi: 10.1001/jamanetworkopen.2020.17337
Mack, J., and Marsh, L. (2017). Parkinson’s disease: cognitive impairment. Focus 15, 42–54. doi: 10.1176/appi.focus.20160043
Maiello, M., Losiewicz, O. M., Bui, E., Spera, V., Hamblin, M. R., Marques, L., et al. (2019). Transcranial photobiomodulation with near-infrared light for generalized anxiety disorder: a pilot study. Photobiomodulation Photomed. Laser Surg. 37, 644–650. doi: 10.1089/photob.2019.4677
Maloney, R., Shanks, S., and Maloney, J. (2010). Application of low-level laser therapy for the symptomatic care of late-stage Parkinson’s disease: a non-controlled, non-randomized study. Lasers Surg. Med. 185:61.
Megari, K. (2013). Quality of life in chronic disease patients. Health Psychol. Res. 1:e27. doi: 10.4081/hpr.2013.e27
Naeser, M. A., and Hamblin, M. R. (2011). Potential for transcranial laser or LED therapy to treat stroke, traumatic brain injury, and neurodegenerative disease. Photomed. Laser Surg. 29, 443–446. doi: 10.1089/pho.2011.9908
Nizamutdinov, D., Qi, X., Berman, M. H., Dougal, G., Dayawansa, S., Wu, E., et al. (2021). Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. 12:954.
Norman, G. R., Sloan, J. A., and Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 41, 582–592. doi: 10.1097/01.MLR.0000062554.74615.4C
Paxinos, G., Furlong, T., and Watson, C. (2020). Human Brainstem: Cytoarchitecture, Chemoarchitecture, Myeloarchitecture. Cambridge, MA: Academic Press.
Ramaker, C., Marinus, J., Stiggelbout, A. M., and Van Hilten, B. J. (2002). Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov. Disord. 17, 867–876. doi: 10.1002/mds.10248
Rodriguez-Blazquez, C., Forjaz, M. J., and Martinez-Martin, P. (2017). “Rating scales in movement disorders,” in Movement Disorders Curricula, eds C. Falup-Pecurariu, J. Ferreira, P. Martinez-Martin, and K. R. Chaudhuri (Vienna: Springer), 65–75.
Salehpour, F., Cassano, P., Rouhi, N., Hamblin, M. R., De Taboada, L., Farajdokht, F., et al. (2019). Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: a review of literature. Photobiomodulation Photomed. Laser Surg. 37, 581–595. doi: 10.1089/photob.2019.4676
Saltmarche, A. E., Naeser, M. A., Ho, K. F., Hamblin, M. R., and Lim, L. (2017). Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed. Laser Surg. 35, 432–441. doi: 10.1089/pho.2016.4227
Schneider, R. B., Myers, T. L., Tarolli, C. G., Amodeo, K., Adams, J. L., Jensen-Roberts, S., et al. (2020). Remote administration of the MDS-UPDRS in the time of COVID-19 and beyond. J. Parkinsons Dis. 10, 1379–1382. doi: 10.3233/JPD-202121
Shaw, V. E., Spana, S., Ashkan, K., Benabid, A. L., Stone, J., Baker, G. E., et al. (2010). Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 518, 25–40. doi: 10.1002/cne.22207
Sim, J., and Lewis, M. (2012). The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 65, 301–308. doi: 10.1016/j.jclinepi.2011.07.011
Smits, E. J., Tolonen, A. J., Cluitmans, L., Van Gils, M., Conway, B. A., Zietsma, R. C., et al. (2014). Standardized handwriting to assess bradykinesia, micrographia and tremor in Parkinson’s disease. PLoS One 9:e97614. doi: 10.1371/journal.pone.0097614
Stillerova, T., Liddle, J., Gustafsson, L., Lamont, R., and Silburn, P. (2016). Remotely assessing symptoms of Parkinson’s disease using videoconferencing: a feasibility study. Neurol. Res. Int. 2016:4802570. doi: 10.1155/2016/4802570
Tarolli, C. G., Andrzejewski, K., Zimmerman, G. A., Bull, M., Goldenthal, S., Auinger, P., et al. (2020). Feasibility, reliability, and value of remote video-based trial visits in Parkinson’s disease. J. Parkinsons Dis. 10, 1779–1786. doi: 10.3233/JPD-202163
Tedford, C. E., DeLapp, S., Jacques, S., and Anders, J. (2015). Re: “Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue”. Lasers Surg. Med. 47, 312–322. doi: 10.1002/lsm.22377
Vargas, E., Barrett, D. W., Saucedo, C. L., Huang, L. D., Abraham, J. A., Tanaka, H., et al. (2017). Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 32, 1153–1162. doi: 10.1007/s10103-017-2221-y
Wu, H., Liu, Q., Meng, C., Xia, Q., Pan, Y., Zhang, H., et al. (2022). Web crawling and mRNA sequencing analyze mechanisms of photobiomodulation. Photobiomodulation Photomed. Laser Surg. 40, 252–260. doi: 10.1089/photob.2021.0142
Yao, L., Qian, Z., Liu, Y., Fang, Z., Li, W., and Xing, L. (2021). Effects of stimulating frequency of NIR LEDs light irradiation on forehead as quantified by EEG measurements. J. Innov. Optical Health Sci. 14:2050025. doi: 10.1142/S179354582050025X
Keywords: photobiomodulation, transcranial, Parkinson’s disease, cognitive dysfunction, mobility
Citation: McGee C, Liebert A, Herkes G, Bicknell B, Pang V, McLachlan CS and Kiat H (2022) Protocol for randomized controlled trial to evaluate the safety and feasibility of a novel helmet to deliver transcranial light emitting diodes photobiomodulation therapy to patients with Parkinson’s disease. Front. Neurosci. 16:945796. doi: 10.3389/fnins.2022.945796
Received: 16 May 2022; Accepted: 06 July 2022;
Published: 17 August 2022.
Edited by:
Santiago Perez-Lloret, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Soraya L. Valles, University of Valencia, SpainDaniel Martinez-Ramirez, Escuela de Medicina y Ciencias de la Salud TecSalud Tecnológico de Monterrey, Mexico
Copyright © 2022 McGee, Liebert, Herkes, Bicknell, Pang, McLachlan and Kiat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann Liebert, YW5uLmxpZWJlcnRAc3lkbmV5LmVkdS5hdQ==
 Claire McGee1
Claire McGee1 Ann Liebert
Ann Liebert Vincent Pang
Vincent Pang Hosen Kiat
Hosen Kiat