
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 20 September 2022
Sec. Perception Science
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.940343
This article is part of the Research TopicAcupuncture for Pain ManagementView all 20 articles
Neuropathic pain, caused by a lesion or disease of the somatosensory system, is common and distressing. In view of the high human and economic burden, more effective treatment strategies were urgently needed. Acupuncture has been increasingly used as an adjuvant or complementary therapy for neuropathic pain. Although the therapeutic effects of acupuncture have been demonstrated in various high-quality randomized controlled trials, there is significant heterogeneity in the underlying mechanisms. This review aimed to summarize the potential mechanisms of acupuncture on neuropathic pain based on the somatosensory system, and guided for future both foundational and clinical studies. Here, we argued that acupuncture may have the potential to inhibit neuronal activity caused by neuropathic pain, through reducing the activation of pain-related ion channels and suppressing glial cells (including microglia and astrocytes) to release inflammatory cytokines, chemokines, amongst others. Meanwhile, acupuncture as a non-pharmacologic treatment, may have potential to activate descending pain control system via increasing the level of spinal or brain 5-hydroxytryptamine (5-HT), norepinephrine (NE), and opioid peptides. And the types of endogenously opioid peptides was influenced by electroacupuncture-frequency. The cumulative evidence demonstrated that acupuncture provided an alternative or adjunctive therapy for neuropathic pain.
Neuropathic pain, caused by a lesion or disease of the somatosensory system, is one of the most intractable human complaints (Jensen et al., 2011). Patients commonly experienced spontaneous pain and/or evoked pain. The former is described as shooting, lancinating or burning pain and the latter is characterized by hyperalgesia to mechanical and cold stimulus (Bouhassira, 2019; Bannister et al., 2020). In addition to the obvious pain-related suffering, neuropathic pain may lead to negative effects such as depression, anxiety, and reduce the quality of life in patients (Laumet et al., 2015). Epidemiological studies have shown that more than 7% of the general population who undergo neuropathic pain, accounting for 20 to 25% of individuals with chronic pain (Torrance et al., 2006; Bouhassira et al., 2008). The high morbidity brought enormous psychological and economic burden to patients, families, and society. However, treatment of neuropathic pain has been extremely challenging, and treatment options are often unmanageable and limited due to the side effects and tolerability (Vranken, 2012; Finnerup et al., 2015). Conventional options to manage neuropathic pain leave much to be desired and more complementary therapies are sorely needed.
The somatosensory system consists of a number of neural pathways that carry various senses from the starting point in skin, muscles, tendons, and internal organs to the central nervous system and ultimately to consciousness (Gomez-Ramirez et al., 2016). Neuropathic pain is a direct consequence of alterations in the somatosensory system (Laedermann et al., 2013). Acupuncture as a non-invasive strategy for nerve stimulation, was a valuable therapy to improve neuropathic pain with a low incidence of adverse events (Barnes et al., 2008; Kelly and Willis, 2019). The somatosensory system mediates the sensation of de qi in acupuncture and plays an important role in the analgesic mechanism of acupuncture (Su et al., 2014). Aβ, Aδ, and C fibers are the most important types of primary afferent nerves in transmitting the acupuncture signal (Huo R. et al., 2020). The pain relief effects of acupuncture are also mediated by activity in the brain and spinal cord (Han, 2004). Along with the increasing application of acupuncture, the analgesic mechanisms of acupuncture through the somatosensory system has been increasingly discovered and progressively confirmed. This review synthesized relevant studies to gain a comprehensive understanding of the production, transmission and processing of acupuncture-like signals from the periphery to the central nervous system.
We searched PubMed, Web of Science, and Embase for available information describing issues related to acupuncture and neuropathic pain. The searches identified English language papers published from 2,000 up to the present time. Keywords included [“Acupuncture” or “Electroacupuncture” or “EA”] and [“Neuropathic pain” or “Peripheral neuropathic pain” or “Chronic neuropathic pain after peripheral nerve injury” or “Trigeminal neuralgia” or “Painful radiculopathy” or “Postherpetic neuralgia” or “painful diabetic neuropathy” or “Neuropathy after radiotherapy” or “Neuropathy after chemotherapy” or “Central post-stroke pain” or “Spinal cord injury pain” or “Multiple sclerosis pain” or “Parkinson’s disease pain”].
The inclusion criteria were designed as follows: (1) Research focused on acupuncture for neuropathic pain. (2) The intervention measures of the treatment group were acupuncture (manual acupuncture or electroacupuncture). (3) Literatures contained clinical research, systematic review/meta-analysis, and basic research. Meanwhile, studies that did not meet the above mentioned criteria were excluded.
Searches retrieved 918 articles. After careful evaluation of the data, we sought to summarize the information identified through the literature search. The search was performed by screening the reference lists of articles that met our inclusion criteria based on the titles and abstracts. Of these articles, we excluded 191 articles due to the absence of the full text, leaving 727 articles. Then, we excluded 287 articles including 77 case reports, 121 study protocols, and 89 review articles. Through the title and abstract, we excluded 52 articles that intervention measures were special acupuncture methods other than manual acupuncture or electroacupuncture. Finally, 388 articles were included after reading the full text, including 22 clinical studies, 59 meta-analyses/systematic reviews, and 307 basic studies. A flow chart of the search and filter process is shown in Figure 1.
Manual acupuncture (MA) and electroacupuncture (EA) were the two most common interventions for pain with acupuncture. In MA, the acupuncture needles are inserted into the acupoints and twisted up and down by hand. MA emphasizes the occurrence of de qi sensations, which can be induced by correct and effective manual manipulation (Spaeth et al., 2013). In EA, stimulating current is delivered to acupoints via the needle connected to an electrical stimulator. The therapeutic benefit of EA depends on the frequency, current amplitude, and pulse width of stimulation. Changing manipulations of MA or parameters of EA may produce different therapeutic effects (Xu et al., 2020). Comparing the changes in pain thresholds for different frequencies of rotational MA, strong stimulation of MA (4 r/s MA) was more effective than mild MA (2 r/s MA) on pain model rats, which was associated with C fiber activation (Song et al., 2021). The analgesia induced by 2 Hz EA was mediated by the endomorphin and that of 100 Hz EA by dynorphin (Han, 2004). In the neuropathic pain model rats, low-frequency (2 Hz) EA had a considerably greater effect on mechanical and thermal pain than high-frequency (100 Hz) EA (Sun et al., 2002). 2/100 Hz (at 2 Hz and 100 Hz frequencies alternately) stimulation increased the release of both endomorphin and dynorphin. It was thus obvious that a proper combination of different frequencies might produce a maximal release of a cocktail of neuropeptides for better therapeutic effects (Han, 2003). Despite of evidence supported the effectiveness of acupuncture for neuropathic pain and showed benefits of MA and EA. It was controversial whether EA does more effective than MA. EA results in more reproducible stimulation and may be substantially more advantageous than MA in continuous stimulation and reducing response times (Zhao et al., 2019). However, another study suggested that EA was not superior to MA treatment. Both therapies had similar efficacy in reducing chronic pain (Comachio et al., 2020).
There are several ways to select acupoints for neuropathic pain: local, regional, and distal points (Millstine et al., 2017). Common local points are where patients feel the most intense pain or pressure (or both) along the pain distribution, in light of the fact that the location of these acupoints are anatomically identical to those of humans. E.g., trigeminal neuralgia take Yuyao (Ex-HN 4), Cuanzhu (BL 2), and Yangbai (GB 14) (Figure 2A), which are located around the eyes (Gao et al., 2019); radiculalgia (lumbar) take Dachangshu (BL 25), and Guanyuanshu (BL 26) (Figure 2C) which parallel to the fourth and fifth lumbar spinous processes (Yu et al., 2021). Regional points are taken by following the meridians or according to the nerve distribution characteristics of the pain area. For trigeminal neuralgia, maxillary branch includes Quanliao (SI 18), Sibai (ST 2), and Juliao (ST 3) (Figure 2A), and mandibular branch includes Xiaguan (ST 7) and Jiache (ST 6) (Figure 2B), which all belonged to the stomach meridian of foot-yangming (Yu et al., 2021). Depending on the distribution of pain, such as persistent lower limb pain caused by nerve root compression, pain confined to the side of the affected leg will be treated through acupoints on the gallbladder meridian, including Huantiao (GB 30), Fengshi (GB 31), Xiyangguan (GB 33), Yanglingquan (GB 34), and Xuanzhong (GB 39) (Figures 2C,D). Pain confined to the posterior part of the affected leg will be treated through points on the bladder meridian, including Zhibian (BL 54), Chengfu (BL 36), Weizhong (BL 40), Chengshan (BL 57), and Kunlun (BL 60) (Figures 2C,D; Yu et al., 2021). Distal regions, such as Neiting (ST 44), Hegu 4 (LI 4), and Sanjian (LI 3) (Figure 2D), are taken for trigeminal neuralgia (Gao et al., 2019). It is an acupoint selection method based on traditional Chinese medicine theory. The location of acupoint is determined using basic theoretical frameworks (e.g., traditional Chinese medicine, TCM) or anatomical structures (e.g., innervation). Currently, acupuncturists tend to use a hybrid approach when providing TCM-based acupoint localization, and they may combine their practice with localized treatments based on current anatomical knowledge, such as the principles of acupoint selection mentioned earlier.
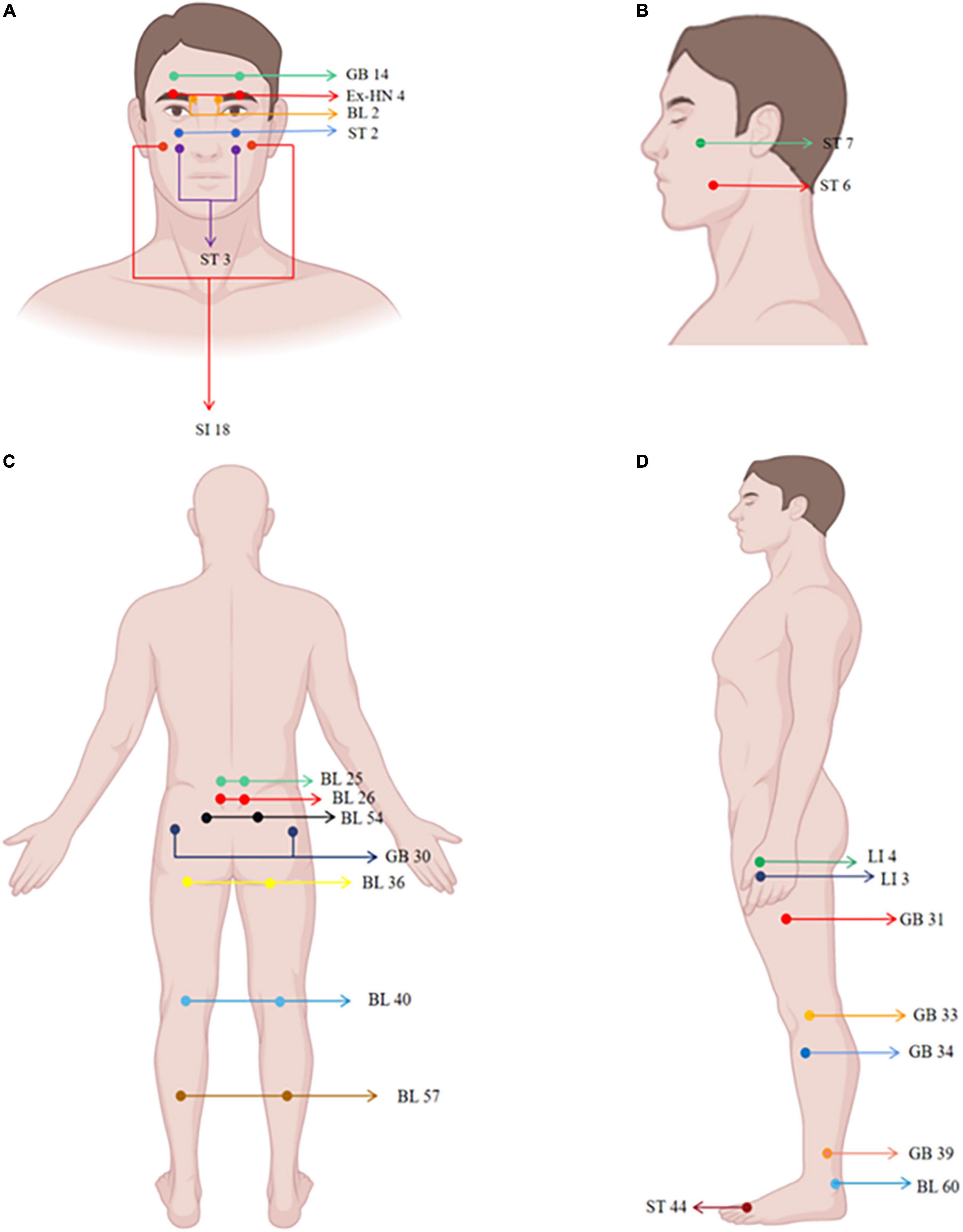
Figure 2. Human maps of acupoints used in neuropathic pain studies. The locations of acupoints are marked in the figure.
A variety of diseases including peripheral and/or central nerve injury, neuropathic pain induced by diabetes, anticancer chemotherapy, post-stroke pain and Parkinson’s have been used to study the effect of acupuncture on neuropathic pain (Figure 3; Becker et al., 2017; Fan et al., 2018; Zhi et al., 2018; Lovaglio et al., 2019; Yu et al., 2019; Wang et al., 2020; Edwards and Shaw, 2021; Zhang et al., 2021; Sollie et al., 2022). Table 1 listed acupuncture treatments for neuropathic pain caused by different diseases. In addition to the difference in the treatment of acupoints, the depth of acupuncture, the needle manipulation and the course of treatment may also be varied. These methods were not the only ones that physicians also made determinations based on their clinical experience. With the deepening of clinical research, the efficacy of acupuncture on neuropathic pain has been confirmed by several systemic reviews and meta-analyses, which were listed in Table 2 (Heo et al., 2013; Dimitrova et al., 2017; Hu et al., 2019; Liu et al., 2019; Pei et al., 2019; Yun et al., 2020; Cui et al., 2021; Yu et al., 2021). A study described the effectiveness and safety of acupuncture for the treatment of chemotherapy-induced peripheral neuropathy. After 8 weeks of treatment and follow-up, the acupuncture group showed a greater reduction in pain score than the vitamin B1 or gabapentin group. Moreover, the nerve conduction study was improved best in the acupuncture group and no adverse events were observed (Iravani et al., 2020). Based on currently available evidences, acupuncture appears more effective than pharmacotherapy or surgery with high degree of safety for improving neuropathic pain (Huang Z. et al., 2019; Yu et al., 2019; Iravani et al., 2020; Lee et al., 2020).
A majority of acupuncturists emphasized de qi which is a feeling of numbness, fullness, and sometimes soreness, when they performed acupuncture treatments. It seemed that acupuncture analgesia was manifest when the de qi feeling occured in patients following manipulation of acupuncture (Hui et al., 2005). The complete somatosensory system was the prerequisite for the appearance of de qi (Cao, 2002). Previous research confirmed that the de qi sensation and pressure pain threshold increased according to the depth and rotation of acupuncture (Choi et al., 2013). Treatment for chronic neuropathy pain usually lasts more than 4 weeks. Acupuncture may be a slow-acting agent and has a specific pattern of the dynamics for the entire coupled nervous system. The therapeutic effect of acupuncture is a gradual accumulation process, that is, as the number of acupuncture courses increases, the therapeutic effect gradually increases (Bai et al., 2009). Therefore, the above factors should be considered in acupuncture analgesia.
Animal models of neuropathic pain are critical for understanding the underlying mechanism and further development of acupuncture therapy. A variety of neuropathic pain animal models which induced by central or peripheral nerve injury have been used to study the acupuncture mechanism (Table 3; Zhang Y. et al., 2018; Li et al., 2019b; Huo B.B. et al., 2020; Du et al., 2021; Jang et al., 2021; Jiang et al., 2021; Wei et al., 2021; Xu et al., 2021; Zou et al., 2021). In addition to animal models of nerve ligation, there are injectable chemotherapy drug-induced neuropathy, post-herpetic neuralgia, and diabetes-induced peripheral nerve injury (Colleoni and Sacerdote, 2010). The former is caused by a primary injury or dysfunction of the nervous system, while the latter is caused by diseases such as diabetes, shingles, and cancer chemotherapy. Although every model possesses its own unique characteristics, different etiologies of neuropathic pain appear to lead to similar behavioral endpoints.
Neuropathic pain is divided into two major categories: peripheral and central, depending on the location of the lesion or disease (Finnerup et al., 2016; Scholz et al., 2019; Figure 4). At the peripheral level, alterations in receptors and ion channels impact neuronal function, resulting in spontaneous (ectopic) activity and pain (Khan et al., 2019). The pathological hallmarks of different types of peripheral nerve lesions have individual characteristics. Some may damage to the entire nerve, causing axonal neuropathy; others may damage to part of the axon or myelin sheath, causing demyelinating neuropathy (Hoffmann et al., 2008; Held et al., 2019). In the central nervous system, there are a variety of conditions that can cause central neuralgia, including damage to the spinal cord or brain, such as trauma, ischemic stroke, cerebral hemorrhage, or multiple sclerotic plaques (Apkarian et al., 2005; Siddall and Middleton, 2015). The mechanism of acupuncture for neuropathic pain is mediated by the somatosensory system (Figure 4). Acupuncture modulated the alterations of receptors and ion channels, inhibited activation of protein kinases and glia and activated the descending pain control system (Goldman et al., 2010; Li et al., 2013; Xu et al., 2020). This review synthesized these studies to provide a comprehensive understanding of how acupuncture alleviates pain through the somatosensory system.
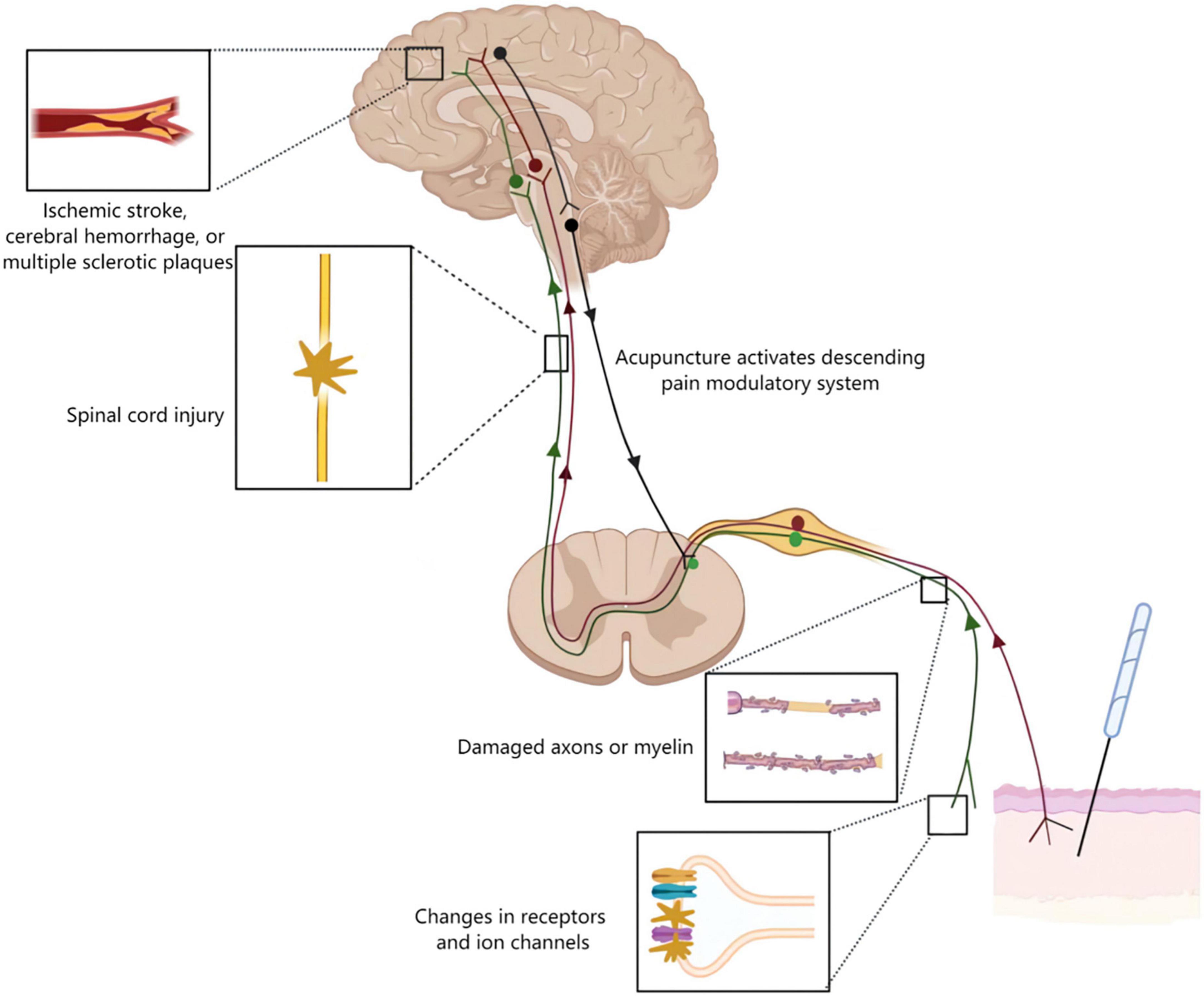
Figure 4. Acupuncture mechanisms on neuropathic pain. Neurological damage from peripheral to the cortical brain may lead to neuropathic pain. The figure shows four typical examples of lesions. Alterations in receptors and ion channels impact neuronal function, resulting in spontaneous (ectopic) activity and pain. Along the peripheral nerve different types of lesions may damage either the entire nerve or selectively the axons or myelin causing axonal or demyelinating neuropathies, respectively. Lesions of the spinal cord or brain as seen for example following traumatic injury, ischemic stroke, cerebral hemorrhage, or multiple sclerotic plaques may lead to central neuropathic pain. Acupuncture inhibited pain transmission via the somatosensory system, and activated the descending pain control system.
Peripheral nerve endings perceive nociceptive stimuli and activate pain pathways. In order for this interaction to happen, mechanical or other stimuli may affect the cytoplasmic membrane potential of axon which as soon exceeds a certain threshold level triggering action potentials. Nociceptors sense thermal, mechanical, and chemical stimuli through the expression of different ion channels such as the transient receptor potential (TRP) family of ion channels as well as ATP-gated purinergic channels (P2X). At this point, diverse types of voltage-gated sodium channels come into play to amplify transient receptor potentials and thus reach depolarization levels sufficient to initiate action potentials (Bannister et al., 2020; Sharif et al., 2020). Transient receptor potential vanilloid 1 (TRPV1) belongs to the family of TRP, that are intensively expressed in the peripheral nervous system and involved in a variety of physiological and pathophysiological processes in mammals (Nilius et al., 2007; Mickle et al., 2015). There is pharmacological evidence that blocking TRPV1 channel, alleviates neuropathic hypersensitivity in rodent models (Basso and Altier, 2017). P2X, specifically the C-fiber localized P2X3 receptor (P2X3R) subtypes, are expressed in the dorsal root ganglion (DRG) and involved in the initiation and maintenance of neuropathic pain (Tang Y. et al., 2016; Khan et al., 2019). Besides, P2X4 and P2X7 in DRG were also involved in thermal nociceptive hypersensitivity (Masoodifar et al., 2021). Voltage-gated sodium channels Nav1.1, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 are expressed in peripheral sensory neurons in different patterns and function as key regulators of sensory nerve excitability (Bennett et al., 2019). Mutations in voltage-gated sodium channels are associated with a variety of pain disorders. In neuropathic conditions, Nav1.8 is most highly expressed in small-diameter neuron subtypes (Dib-Hajj and Waxman, 2019). Another family of excitatory channels associated with neuropathic pain is hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels. HCN2 was specifically deleted in nociceptors expressing Nav1.8 in mice, but nerve lesion did not cause hyperalgesia to thermal or mechanical stimuli (Emery et al., 2011). HCN2 antagonist attenuated neuropathic hypersensitivity in neuropathic rats and inhibited spontaneous activity of C-nociceptors, but not Aβ fiber (Djouhri et al., 2018).
Electroacupuncture (EA) effectively reduced nociceptive sensitization in spared nerve injury (SNI) and spinal nerve ligation (SNL) by downregulating the expression ratio of TRPV1 in DRG (Fang et al., 2021). Administration of TRPV1 agonists reversed EA analgesia (Jiang et al., 2013; Du et al., 2021). Ca2+ imaging revealed that TRPV1 channel activity was increased in DRG neurons of paclitaxel-treated rats, whereas EA suppressed the increased TRPV1 channel activity. Pharmacological blockade of TRPV1 was similar to the analgesic effect of EA on pain allergy, while capsaicin reversed the effect of EA (Li et al., 2019b). EA might inhibit the activation of P2X3Rs in neuropathic pain and block primary afferent transmission mediated through P2X3Rs to alleviate mechanical and thermal nociceptive sensitization (Fei et al., 2020). Additionally, EA was more potent in reducing both mechanical allodynia and thermal hyperalgesia in combination with intrathecal A-317491 (a selective P2X3 and P2X2/3 receptor antagonist) (Wang et al., 2014). Therefore, EA and A-317491 might potentially have an additive effect in inhibiting the transmission of pain mediated by the P2X3 receptor. The protein levels of P2X4 and P2X7 in diabetes-induced neuropathy rats were significantly increased. 2 Hz EA improved the paw withdrawal latency and reduced the expression of P2X4 and P2X7 in DRG (Hu et al., 2022). EA attenuated Nav1.7 and Nav1.8 protein expression levels in the DRG during painful states (Yen et al., 2018). Nav1.3 was lacking in DRG neurons of normal adult rats, but was highly expressed in damaged sensory neurons (Waxman et al., 1994). EA diminished spinal cord injury (SCI)-induced upregulation of Nav1.3 (Liu and Wu, 2017). EA also reduced mechanical allodynia and face-grooming in trigeminal neuropathic pain rats through downregulation of HCN expression in the gasserian ganglion (Yang et al., 2019). These results suggested that acupuncture blocks pain-related ion channels and increases pain thresholds (Figure 5).
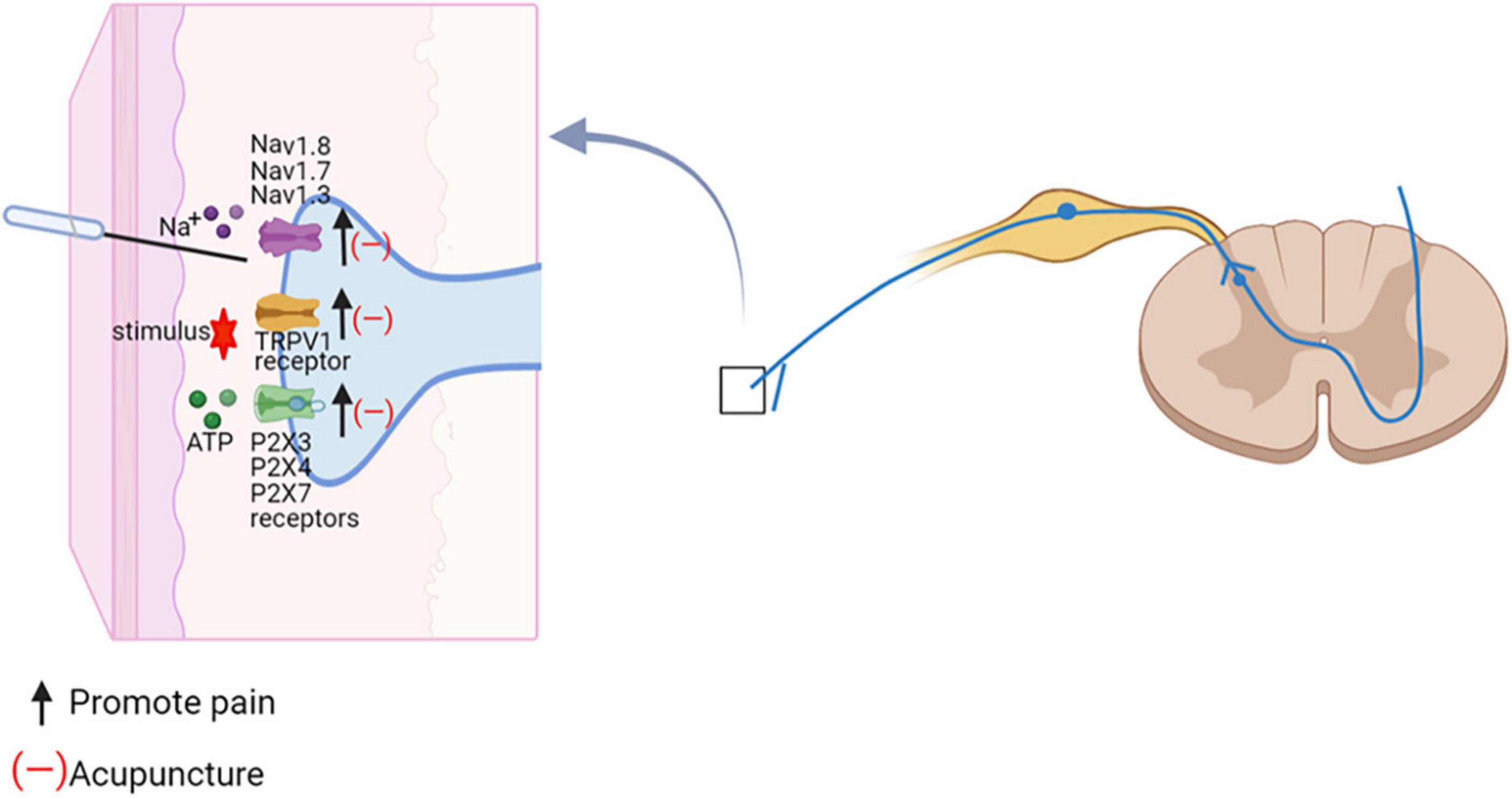
Figure 5. Acupuncture regulates ion-channel dysregulation in neuropathic pain. The effect of acupuncture on the spinal cord. Na+, sodium ion; Navs, Voltage-gated sodium channels; TRPV1, TRP vanolloid 1; ATP, adenosine triphosphate; P2X3, ATP-gated purinergic channels 3.
The available evidence indicated that afferent nerve fibers and different receptors in the acupoints might played a key role in mediating the effects of acupuncture. Acupuncture inhibited neuronal activity caused by neuropathic pain, through reducing pain-related ion channels and receptors activation. At present, however, the specific pathway of changes in receptors and ion channels mediated by acupuncture stimulation points cannot be explained. More types of sham controls need to be employed to thoroughly evaluate the effects of acupoint specificity in future studies.
The spinal cord neuronal activity caused by neuropathic pain is partially attributed to increased synaptic efficacy. Ionic and metabotropic glutamate receptors exhibit phosphorylation or translocation changes, resulting in increased excitatory postsynaptic potential (EPSP) frequency and amplitude (Kiyoyuki et al., 2015; Hildebrand et al., 2016). Multiple protein kinases have been implicated in regulating neuronal plasticity and pain sensitization following intense noxious stimuli or injuries. There is increasing evidence suggesting that serine/threonine kinases family especially Protein kinase A (PKA), Protein kinase C (PKC), mitogen-activated protein kinases (MAPKs) (Ma et al., 2021), is critical for the induction and maintenance of pain hypersensitivity after injuries (Ji et al., 2007). Extracellular signal regulated kinase (ERK) (Kondo and Shibuta, 2020), p38 mitogen-activated protein kinases (p38 MAPK) (Lin et al., 2014), and calmodulin-dependent protein kinase II (CaMKII) (Qian et al., 2019) are downstream to many kinases. These kinases are activated in primary sensory and dorsal horn neurons by nociceptive activity, contributing to the induction and maintenance of pain sensitization (Choi et al., 2019). Compared with the normal rat, more PKA-positive cells were observed in the spinal dorsal horn of SNL rat (Wu et al., 2021). PKC activation depolarized unmyelinated afferent neurons, which enhance currents in afferent neurons activated by nociceptive stimuli, and PKC inhibitors blocked sensitization of afferent neurons (Velázquez et al., 2007). p38 MAPK and ERK are present in spinal dorsal horn, and their inhibitors inhibit neuropathic pain (Inoue and Tsuda, 2018). The activation pattern of ERK in the spinal cord correlated with neuropathic pain behavior at different time points after SNL. Intrathecal injection of the non-competitive ERK inhibitor PD98059 attenuated SNL-induced mechanical nociceptive hypersensitivity (Kondo and Shibuta, 2020). Inhibition of spinal CaMKII expression has been shown to prevent thermal hyperalgesia and mechanical allodynia (Fang et al., 2002).
Electroacupuncture (EA) reduced the expression of p38 MAPK and inhibited pain transmission in rat spinal cord dorsal horn (Wei and Hsieh, 2020; Jin et al., 2021). PKA expression levels are elevated and involved in neuropathic pain by activating the p38 MAPK pathway to mediate apoptosis in spinal cord cells (Deng et al., 2020). EA exerted analgesic effects by decreasing the expression of PKA in SNL model rats (Wu et al., 2021). 2 Hz EA reduced the expression of P2X3 receptors by inhibiting the PKC pathway thus relieved pain (Zhou et al., 2018), CaMKII is crucially involved in synaptic plasticity and long-term potentiation (LTP) (Luo et al., 2014). It was found that EA reduced p-CaMKII levels in the spinal cord and was blocked by pretreatment with 5-hydroxytryptamine (5-HT) 1A receptor antagonists, suggesting that 5-HT1A receptors were involved in the inhibitory effect of EA on spinal p-CaMKII (Zhang Y. et al., 2018). These studies clearly showed that acupuncture blocked multiple protein kinases activation to reduce spinal cord neuronal activity in painful conditions and achieved pain relief.
Proliferation, shape change and activation of microglial populations in the spinal dorsal horn has been reported in several models of neuropathic pain (Ji et al., 2016). Diverse ensuing changes in the transcriptional and secretory profile of microglia have been linked to neuropathic pain, including release of inflammatory factor, ATP, chemokines, amongst others (Inoue and Tsuda, 2018). Moreover, astrocyte activation further promotes neuronal activity (Ji et al., 2016). It is known that p38 MAPK and ERK play important roles in the maintenance of neuropathic pain (Deng et al., 2020; Kondo and Shibuta, 2020). P-p38 MAPK and p-ERK upregulation are localized to activate microglia within the dorsal horn of the lumbar region after spinal cord injury. CX3C chemokine fractalkine (CX3CL1) released from damaged neurons activates CX3C-chemokine receptor 1 (CX3CR1) on microglial cells and leads to tumor necrosis factor α (TNF-α), and Interleukin-1β (IL-1β) secretion via p38 MAPKs/ERK. Released IL-1β and TNF-α acts on spinal dorsal horn neurons to enhance glutamate excitatory synaptic transmission and decreaseγ-aminobutyric acid (GABA)-mediated and glycine-mediated synaptic inhibition (Gim et al., 2011; Inoue and Tsuda, 2018). Furthermore, ERK in activated microglia mediates the release of prostaglandin E2 (PGE2), which binds to prostaglandin E receptor 2 (EP2) expressed in spinal cord neurons, inducing a change in their excitatory state and thus causing neuropathic pain (Zhao et al., 2007). Spinal brain-derived neurotrophic factor (BDNF) is a key neuromodulator of pain transmission, and P2X4R activates spinal microglia to induce p38 MAPK phosphorylation to release BDNF, which transmits noxious signals to layer I neurons, thereby contributing to the pathogenesis of pain (Cappoli et al., 2020).
Microglia and astrocytes, are important targets for acupuncture analgesic (Figure 6). EA reduced mechanical and thermal pain in rat models of neuropathic pain by preventing microglia, astrocyte activation (Liang et al., 2016). EA and intrathecal injection of the glial metabolism suppressant fluorocitrate might synergize against pain (Sun et al., 2006). Therefore, the inactivation of glial cells may be partly responsible for the acupuncture analgesic. In a SCI model, acupuncture applied to GB34 inhibited p38 MAPK and ERK phosphorylation in microglia of L4-5 spinal cord (Choi D. C. et al., 2012). EA down-regulated the neuronal chemokine CX3CL1, which acted on CX3CR1 in microglia, and prevented the p38 MAPKs/ERK signaling pathway, leading to reduce the release of inflammatory cytokines, resulting in pain relief (Li et al., 2019a). Acupuncture attenuated the ERK-dependent PGE2 releasing from activated microglia (Choi D. C. et al., 2012). EA prevented BDNF binding to spinal cord neuronal tyrosine kinase receptor B (Trk B) by decreasing microglia activation and BDNF expression, thereby reducing nociceptive hyperalgesia and neuropathic pain (Tu et al., 2018).
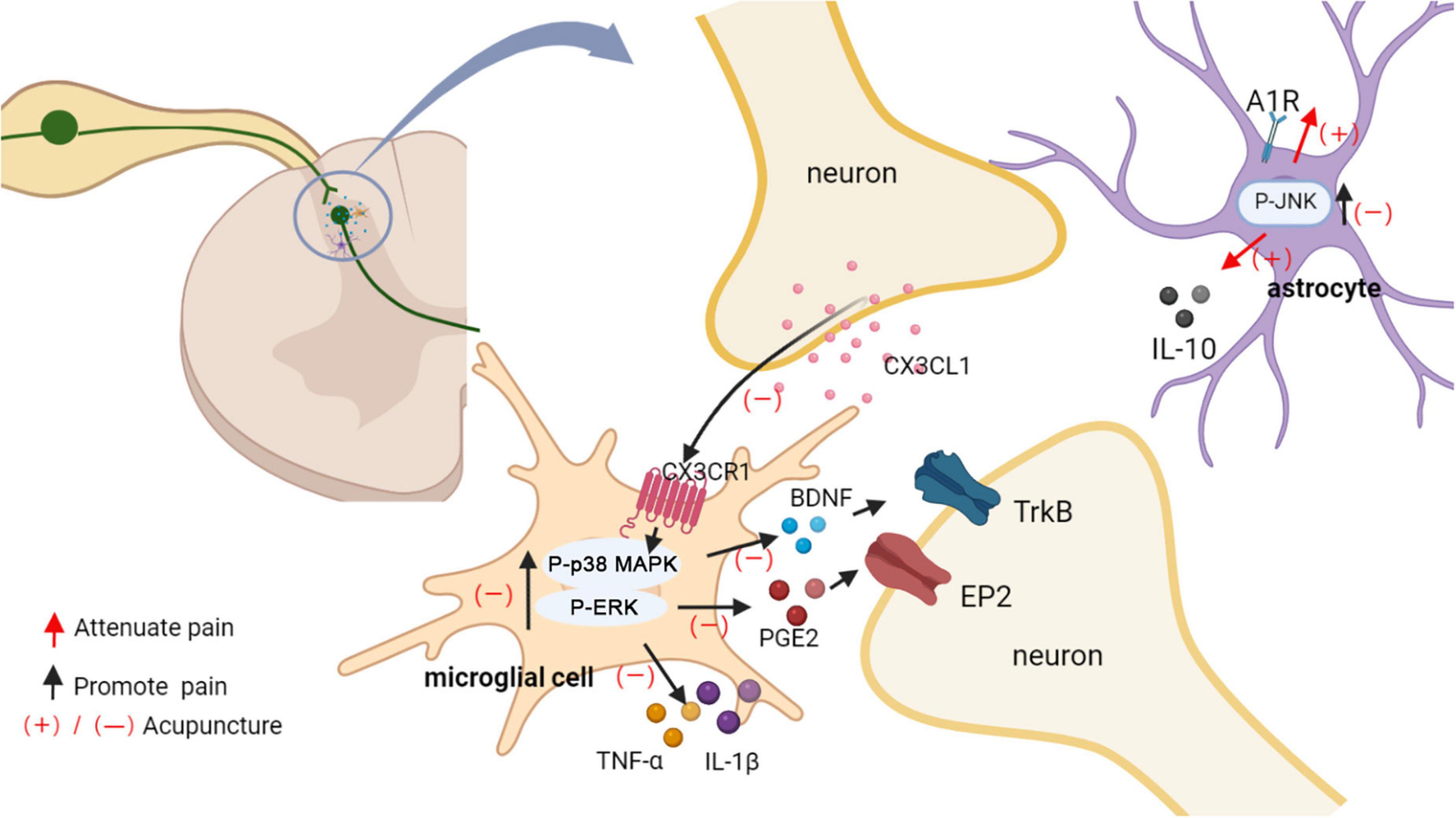
Figure 6. Acupuncture affects glial activation. EA down-regulated the neuronal chemokine CX3CL1 and prevented the p38 MAPK/ERK signaling pathway, leading to reduced release of TNF-α and IL-1β, BDNF, PGE2. Released IL-1β and TNF-α acts on spinal dorsal horn neurons to enhance the neuronal excitability and inflammatory response. PGE2 and BDNF bind to spinal cord neuronal EP2 and Trk B, respectively, inducing a change in their excitatory state and thus causing neuropathic pain. EA induced an increase in adenosine levels in the spinal cord, which in turn activated astrocyte A1Rs to produce an analgesic effect. Additionally, analgesic effect of acupuncture was mediated in part through inhibiting the activation of JNK and promoting the release of IL10 in astrocytes.
Adenosine is present at the extracellular space within the spinal cord dorsal horn and engaged in the processing of nociceptive sensory signals. Systemic or spinal administration of exogenous adenosine produces a potent analgesia against pathological pain. In rat spinal cord slices, adenosine increases postsynaptic inhibitory currents mediated by glycine receptors (GlyRs), and this synaptic potentiation is dependent on activation of adenosine A1 receptors (A1Rs) (Bai et al., 2017). Another study found that spinal A1R contributed to the inhibitory effects of EA on astrocyte activation as well as TNF-α upregulation (Zhang M. et al., 2018). The c-Jun N-terminal kinase (JNK), a major member of the MAPK family, has been shown to play a key role in intracellular signaling and contributes to central sensitization of chronic pain. Peripheral inflammation or nerve injury leads to JNK activation in spinal astrocytes. Activation of the JNK pathway lead to the production and release of several pro-inflammatory cytokines that play an important role as biological mediators in chronic pain (Wang et al., 2017).
It has been shown that EA first downregulated microglia activation (after 2 days of EA) and then astrocyte activation (after 1–2 weeks of EA treatment) (Wang et al., 2018). A1Rs expression in the L4–6 spinal segments were increased by EA, indicating that EA induced an increase in adenosine levels in the spinal cord, which in turn activated astrocyte A1Rs to produce an analgesic effect (Dai et al., 2020). In SCI rats, acupuncture inhibited JNK activation in astrocytes at the spinal cord L4-5 level. The level of p-c-Jun, a downstream molecule of JNK, was also decreased by acupuncture. In addition, the number of hypertrophic, activated astrocytes in the L4-5 dorsal horn I-II layers was significantly reduced in the acupuncture-treated group. It was suggested that the analgesic effect of acupuncture was mediated in part through inhibition of JNK activation in astrocytes after SCI (Lee J. Y. et al., 2013). Interleukin-10 (IL-10) is a powerful anti-inflammatory cytokine that improves inflammation and protects damaged nerve. It mainly distributes in the superficial spinal astrocytes. The anti-nociceptive effects of EA were blocked by the spinal IL-10 inhibitor, suggesting EA had a regulatory effect on IL-10 in spinal astrocytes (Dai et al., 2019).
The spinal cord is an important center for mediating the analgesic effects of acupuncture. EA down-regulated the neuronal chemokine CX3CL1 and prevented microglial p38 MAPK/ERK signaling pathway, leading to reduced release of TNF-α and IL-1β, BDNF, PGE2, and reducing the neuronal excitability and inflammatory response. The activation of microglial cells in the ipsilateral spinal cord dorsal horn increased within 1 day of nerve injury; however, astrocytes were activated later than microglial cells and were implicated in the maintenance of mechanical allodynia after spinal nerve injury. In the early and late stages of neuropathic pain, repeated EA therapy inhibited microglia and astrocyte activation, respectively (Liang et al., 2016). These results suggested that inhibition of spinal microglia activation was involved in the early stage of EA analgesia, while inhibition of astrocytes activation was involved in the maintenance of EA analgesia.
It is well-established that the descending pain control system of the midbrain and brainstem regulates the processing of nociceptive information in the spinal cord. The anterior cingulate cortex (ACC) promotes spinal excitatory synaptic transmission leading to nociceptive hyperalgesia. Descending corticospinal tract fibers originating from somatosensory cortex project not only to the spinal ventral horn, but also to the spinal dorsal horn and to the sensory synapse (Liu et al., 2018). Imaging studies in human volunteers showed that the rostral ACC likely mediated analgesia by activation of the periaqueductal gray (PAG) (Eippert et al., 2009). The PAG is known to be an important relay station receiving inputs from higher brain centers for descending modulation of pain through projections to the spinal cord via the rostroventral medulla (RVM) and the locus coeruleus (LC) (Alhadeff et al., 2018). In neuropathic pain, acupuncture plays a complex and critical role in the network of descending pathways projecting from brain structures to the spinal dorsal horn (Figure 7). The analgesic effect of descending pain control system relies on endogenous gamma-aminobutyric acid GABA, 5-HT, norepinephrine (NE), and endogenous opioids (Silva et al., 2013).
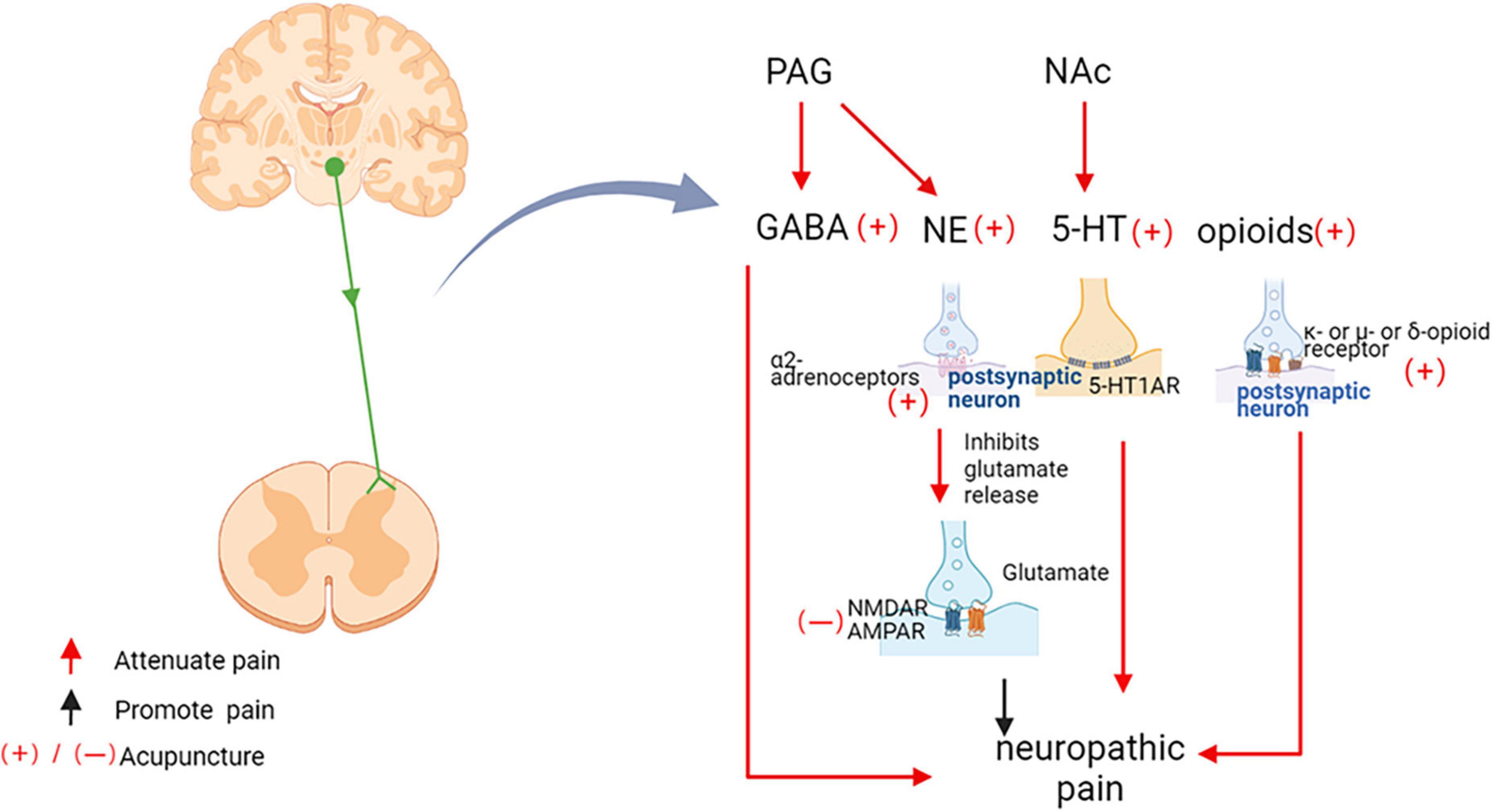
Figure 7. Acupuncture inhibits neuropathic pain through the downstream pathway. PAG, periaqueductal gray matter; nucleus accumbens, NAc; GABA, Gama aminobutyric acid; NE, Norepinephrine; 5-HT, 5-hydroxytryptamine; 5-HT1AR, 5-hydroxytryptamine 1A receptors; 5-HT3R, 5-hydroxytryptamine 3 receptors; NMDAR, N-methyl-D-aspartate receptors; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.
Gama aminobutyric acid (GABA) is an important inhibitory neurotransmitter. GABAergic projections of interneurons from the brainstem to the spinal cord control spinal cord nociceptive transmission (François et al., 2017). The PAG manages pain through downstream modulation of the spinal dorsal horn, and EA activation of PAG neurons involved the descending pain control system of GABAergic (Fusumada et al., 2007). In the neuropathic pain model induced by chronic constriction injury (CCI), EA up-regulated the level of GABA in the PAG (Huang C. P. et al., 2019). EA at Jiaji (EX-B2) acupoints improved neuropathic pain by increasing protein expression levels of GABA receptors in the spinal cord (Jiang et al., 2018). Using in vivo two-photon imaging in mice with chronic systolic injury, it was found that EA therapy systematically modulates Ca2+ activity in primary somatosensory cortical neural circuits, including inhibition of excitatory pyramidal neurons, enhancement of GABA-ergic somatostatin positive interneurons, thereby mediating improved mechanical or thermal hypersensitivity (Wei et al., 2021).
5-HT and its receptors are key substances that regulate pain and play a crucial role in EA analgesia. 5-HT released from the nucleus accumbens (NAc) were increased in rats receiving acupuncture, which was observed 20 min after acupuncture treatment and persisted until 40 min after the end of acupuncture (Yoshimoto et al., 2006). Exogenous lateral ventricular 5-HT have analgesic effects that partially mimic the analgesic effects of EA (Chang et al., 2004). Current research indicated that the alleviation of cold nociceptive hypersensitivity by 2 Hz EA was mediated by spinal 5-HT1A and 5-HT3 receptors (Kim et al., 2005). In addition, it has been shown that lateral ventricular injections of 5-HT1A receptors antagonists blocked the analgesic effects induced by low-and high-frequency EA (Chang et al., 2004). Blockade of 5-HT1A receptors in the ventral tegmental area reversed morphine-/dextromethorphan-induced analgesia in pain model rats (Seddighfar et al., 2019). Spinal 5-HT2A receptors (5-HT2AR) mediate the downstream vulnerability of 5-HTergic axons through multiple mechanisms. Inhibition of spinal dorsal horn 5-HT2AR expression prevents mechanical nociceptive hypersensitivity of the face and associated changes in PKCγ+ interneuron morphology (Alba-Delgado et al., 2018). However, there are no reports of neuropathic pain relief by acupuncture mediated by 5-HT2A, providing a direction for the next study.
Norepinephrine (NE) and α2-adrenergic receptors are widely distributed in the brain and spinal cord, and activation of the NEergic descending pain control system is involved in the anti-nociceptive effects of EA (Silva et al., 2011). Acupuncture at Zusanli (ST 36) and Shangjuxu (ST 37) activated neurons in the PAG to exert anti-nociceptive effects, increased the release of NE from the PAG. The α1-, α2-, β-adrenoceptors were found to be located in the PAG, and noradrenalin and α1-and α2-agonists were found to activate the lateral and ventrolateral PAG neurons. These findings suggested that the modulation of pain by EA in PAG involved NE (Murotani et al., 2010). Intrathecal injection of drugs that increase spinal NE utilization promotes the long-term anti-nociceptive effects of EA (Silva et al., 2013). Intrathecal injection of the α2–adrenergic antagonist yohimbine reduced EA-induced analgesia in a dose-dependent manner, suggesting that the analgesic effect of EA was dependent on the binding of norepinephrine to α2 receptors (Koo et al., 2008).
Endogenous opioids were closely related to the analgesic effect of acupuncture. Acupuncture reduced the SNL-induced hypersensitivity response, which blocked by naloxone, a non-selective opioid receptor antagonist (Cidral-Filho et al., 2011), suggesting that the analgesic effect of acupuncture was dependent on the opioid system. Low-frequency and high-frequency EA activated different types of opioid receptors. Mu-or delta-opioid receptor antagonists blocked the 2 Hz EA anti-mechanical nociceptive hypersensitivity (Kim et al., 2004). 100 Hz EA leaded to the release of dynorphin, which binded to kappa-opioid receptors in the spinal cord and provides pain relief (Huang et al., 2008). However, 2/100 Hz EA activated both mu/delta and kappa opioid receptors, inducing a synergistic analgesic effect that was more effective than constant frequency stimulation (Han, 2003).
Ionic glutamate receptors include n-methyl-d-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methyl-4-isoxazolidinepropanoic acid (AMPA) receptors. Glutamate is the main excitatory neurotransmitter acting on ionotropic glutamate receptors to direct central sensitization (Groc and Choquet, 2006). EA inhibited phosphorylation of AMPA receptor (AMPAR) GluR2 subunit (Tang Y. et al., 2016). Low-frequency EA exerted analgesic effects by modulating the phosphorylation status of spinal NMDA receptor subunits NR1 and NR2B (Jung et al., 2010). EA improved SNI-induced pain behavior and decreased glutamate release in the spinal dorsal horn (Vidal-Torres et al., 2012). 5-HT1BR (Choi I. S. et al., 2012) and α2-adrenoceptors (Li and Eisenach, 2001) activation reduced glutamate release from medullary dorsal horn neurons. However, in SNL-induced neuropathic pain rat model, 2 Hz EA activated the endogenous opioid system and induces NMDA receptor-dependent long-term depression (LTD) in the spinal dorsal horn (Xing et al., 2007). The different results may be explained by different acupoints and different parameters of EA.
After the initial injury healing, chronic pain will continue, which is related to brain remodeling (Kuner and Flor, 2016) and the treatment rely on post-therapeutic effect of EA. Brain processing of acupuncture stimuli in neuropathic pain patients or animals may underlie its beneficial effects. Previous study showed that neuropathic pain following brachial plexus avulsion injury (BPAI) induced metabolic connectivity changes significantly among sensorimotor-related areas and pain-related area in bilateral hemispheres (Huo R. et al., 2020). The decreased metabolic connectivity between ipsilateral dorsolateral thalamus and somatosensory cortex was related with BPAI-induced neuropathic pain. EA increased the thermal withdrawal latency of BPAI rat and improved the strength of connectivity among the above regions (Hou et al., 2020). Evidence suggested that neuropathic pain patients responded to acupuncture with more pronounced fMRI signal decrease in the amygdala and signal increase in the lateral hypothalamic area differently than healthy people. Acupuncture coordinated limbic response that included the hypothalamus and amygdala (Napadow et al., 2007). The hypothalamus is an important component of the central descending pain modulatory circuit from the cerebral cortex to the spinal cord (Ossipov et al., 2010), activation of hypothalamic neurons can inhibit the input of nociceptive signals to the pain centers in the cortex (Lin and Chen, 2008). CCI rat following EA intervention, there were 17 hypothalamic proteins identified with significant changes in the expression. EA attenuated pain may via regulation of expression of these proteins in the hypothalamus (Vogt, 2016). Although studies have indicated involvement of many brain structures in the modulation of acupuncture analgesia, hypothalamus might play a crucial role in this process. A study revealed that increased local synaptic activity in the ipsilateral somatosensory cortex and decreased in the contralateral somatosensory cortex after sciatic nerve injury. EA served as repeated sensory stimulation, might potentially induce increased synaptic activity in the corresponding cortices involving contralateral somatosensory cortex of sciatic nerve injury (Wu et al., 2018).
As can be seen, the descending pain modulation system, including the ACC, the PAG, the NAc, and the hypothalamus, plays an important role in EA analgesia. EA might reverse the maladaptive brain plasticity, promote the release of endogenous substances such as GABA, NE, 5-HT, and opioids. This could be an important mechanism underlying the post-therapeutic effect of EA, and it deserves further study.
Acupuncture, which has a history of 2,000-year, is a useful adjunct therapy or an acceptable alternative treatment of pain (Wells et al., 2017). The research base for acupuncture is rapidly expanding. Somatosensory stimulation, including acupuncture, could thus act as an additional input to re-arrange the neural loop, nociceptive, and acupuncture signals was integrated at spinal and supraspinal levels (Figure 4). Acupuncture suppressed pain, probably due to effects on the afferent nerve. The gate control theory might play a role in acupuncture analgesia. Non-painful input by acupuncture closed the “gates” to painful input, which prevented pain sensations from traveling to the central nervous system (Lemmon, 2018). Modulation of sensory input occurs at the primary afferent neuron and spinal dorsal horn during an acupuncture treatment, which may depend on acupoints at the same spinal section as the pain site. We speculated, in this case, acupuncture inhibited neuronal activity caused by neuropathic pain, through reducing pain-related ion channels and protein kinases activation (Figure 5). In the spinal dorsal horn, EA down-regulated the neuronal chemokine CX3CL1 and prevented microglial p38 MAPK/ERK signaling pathway, leading to reduced release of TNF-α and IL-1β, BDNF, PGE2, and reducing the neuronal excitability and inflammatory response (Figure 6). Furthermore, functional magnetic resonance imaging studies have shown that acupuncture at specific acupoints modulated areas of the brain (Huang et al., 2021), and acupuncture activated descending pain control system. The distal acupoints such as Neiting (ST 44), Hegu 4 (LI 4), and Sanjian (LI 3) probably work in this way. Research has found that, in nerve injury model, contralateral but not ipsilateral acupuncture produced clear analgesia. This difference might be due to the damaged nerve blocks the conduction of acupuncture signals, while contralateral acupuncture inhibited pain through the central descending inhibitory, independent of the influence of local damaged nerves (Zhang H. et al., 2018). GABA, NE, 5-HT, and endogenous opioids are involved centrally (Figure 7). Taken together, acupuncture, as a distinctive therapeutic modality to pain, produced physiologic changes in the brain, spinal cord, and at the periphery. Medication treatment was often associated with side effects, and many patients did not achieve adequate pain relief at tolerated doses (Finnerup et al., 2015). On the contrary, acupuncture is a relatively safe and well-tolerated treatment, with most patients experiencing no adverse effects at all (Pfister et al., 2010).
As a matter of fact, the optimal prescription of acupuncture treatment (acupuncture point, degree of stimulation, frequency of treatment, and a number of treatment sessions) for neuropathic pain is a controversial issue amongst acupuncture experts. EA and MA are two acupuncture manipulations commonly used in clinical practice. Our previous study found that EA and MA effectively improved pain symptoms in patients with osteoarthritis of the knee, but EA had a faster onset of action than MA (Xu et al., 2020). Current evidence does not yet support an efficacy difference between MA and EA in the treatment of neuropathic pain. MA involves inserting an acupuncture needle into an acupuncture point and then twisting it up and down with the hand. EA delivers an electric current to the acupoint through an inserted needle. In terms of “needling feeling,” EA is often described as painful and numb, while MA is dominated by heaviness and distension in the deep tissue beneath the acupuncture point. MA activated all types of afferent fibers (Aβ, Aδ, and C) (Kagitani et al., 2010). The current intensity of EA was sufficient to excite Aβ-and some Aδ-fibers inducing analgesic effects (Zhao, 2008). Reason for this difference might be that MA and EA activated distinct ion-channels, but this inference had not been confirmed. In clinical practice, the intense intensity of EA was not suitable for patient analgesia, because the excitation of C fibers by synchronous strong electrical pulses would inevitably cause unbearable pain. The parameters of the EA could be precisely characterized. A study characterized the generation and transmission of electrical signals in Aβ-and some Aδ-fibers induced by acupuncture-like stimuli. EA in frequency-specific modes (2/15 Hz or 2/100 Hz) best mimicked MA (Huo R. et al., 2020). The efficacy of MA and EA also may be influenced by disease state, acupuncture duration, acupuncture parameters and acupoints. Therefore, clarifying the analgesic mechanisms of MA and EA and selecting the appropriate acupuncture modality are essential to improve clinical efficacy.
The frequency-dependent study for analgesia at high-and low-frequency highlighted the best operating parameters. Low-frequency (2 Hz) EA caused the release of neuropeptides such as encephalin and endorphin, which acedt on mu and/or delta opioid receptors to mediate analgesia; high-frequency (100 Hz) EA caused the release of dynorphin, which was mediated by kappa opioid receptors to mediate analgesia. Certain brain regions have been found to be associated with the release of various types of central opioid peptides (Zhang et al., 2003), but it was unclear how these brain regions were modulated by the 2 Hz and 100 Hz EA, respectively. The studies screened frequencies of EA, and the results indicated that in rats with neuropathic pain, 2 Hz EA induced a robust and longer lasting analgesic than 100 Hz EA (He et al., 2017; Xia et al., 2019). In type 2 diabetic neuropathic pain rat, EA at both 2, and 100 Hz down-regulated CGRP (Calcitonin gene related peptide) and P2X3 receptors overexpression in DRGs, but the analgesic effect of EA was stronger at 2 Hz (He et al., 2017). Another study showed that compared with 100 Hz EA, 2 Hz EA effectively regulated the expression level of genes in the arcuate nucleus region of the hypothalamus, especially those related to neurogenesis (Wang et al., 2012).
Acupoints have a characteristic that they become sensitive and even painful when exposed to pathological processes (Zhou and Benharash, 2014). The analysis of anatomical have revealed that acupoints have a number of elements such as a high density of nerve endings, A-and C-afferent fibers and vascular, which could perceive stimulation (Li et al., 2004). When stimulating acupoints, the local of acupoints may release biomolecules to exert the role of analgesia or neuromodulation. Acupuncture stimulates the somatic afferent nerves of the skin and muscles under the acupoints. Then, the somatic sensory information is carried to the spinal cord and cortex area of the brain that modulate spinal signal transmission and pain perception in the brain (Wang et al., 2008). Therefore, acupuncture analgesia was essentially a manifestation of integrative processes at different levels of the nervous system between afferent impulses from the pain regions and impulses from acupoints. The infiltration of procaine, a local anesthetic, into the deep tissues around the point of acupuncture entirely abolished the analgesic effect, suggesting that nerves was mediators of this response (Zhou and Benharash, 2014). The selection of acupoints may make the effect of acupuncture more targeted in different diseases and different pain sites. But there are no studies to explain in the treatment of pain why acupoints are effective and non-acupoints are not, or why this acupoint is effective and other acupoints are not. Therefore, the specificity of the acupoints should be studied further.
Acupuncture has been used to treat neuropathic pain caused by different diseases. In addition to nerve injury-induced peripheral neuropathic pain, a meta-analysis showed that benefit for acupuncture over control in the treatment of neuropathic pain caused by diabetes, human immunodeficiency virus (HIV), Bell’s palsy, and carpal tunnel syndrome (Dimitrova et al., 2017). Here, it should be pointed out that diabetic neuropathy, a major complication of diabetes mellitus, refers to a collection of clinically diverse disorders affecting the nervous system. Despite most of diabetes peripheral neuropathy is characterized by hypoesthesia, it also may present with pain. Of all diabetic peripheral neuropathy patients, 20% develop neuropathic pain (Sloan et al., 2018). Acupuncture was considered as a treatment option for diabetic neuropathic pain. Zusanli (ST 36), Feishu (BL 13), Pishu (BL 20), Sanyinjiao (SP 6), and Yinlingquan (SP 9) were the most widely used acupoints (Cho and Kim, 2021). And acupuncture could have a beneficial effect on neurological and motor function recovery (Fan et al., 2018). Previous research indicated that acupuncture also appeared to improve motor and sensory nerve conduction parameters, curing the disease from both sensory and functional aspects (Dimitrova et al., 2017). In spinal cord injury patients, acupuncture could be useful to improve pain and other complications if patients experience side effects or have no (or a weak) response to a conventional treatment (Heo et al., 2013). However, the analgesic effect of acupuncture on neuropathic pain induced by spinal cord injury has an obvious selectivity, which depends on the location and type of pain, as well as the type of injury (Siddall and Middleton, 2015). Acupuncture recipients with incomplete damage to central nervous system pathways that remained intact appeared to recover better than those with complete damage, and patients with musculoskeletal pain responded better to treatment compared with those with central pain. In addition, participants with moderate pain were more likely to achieve long-term pain relief than those suffering from severe pain (Mehta et al., 2013).
In clinical and preclinical models, some forms of neuropathic pain persist as a result of sympathetic nerve activity, and local sympathetic blockade or lesion is used to treat it (Xie et al., 2016, 2020). After peripheral nerve injury, sympathetic neurons sprout within DRG, sensitizing nociceptive neurons to adrenergic stimulation, although this remains controversial (Hoffman et al., 2018). A new study provided evidence that sympathetic sprouting in the DRG played a role in spontaneous pain in the SNI and related neuropathic pain models (Zheng et al., 2022). They concluded that norepinephrine released from sympathetic induced DRG neuronal clustering discharge, which correlated directly with spontaneous pain behavior caused by nerve injury. Research has shown that acupuncture reduced sympathetic nerve hyperactivity (Li et al., 2009). However, EA for neuropathic pain by modulating sympathetic nerves has not been studied.
In neuropathic pain conditions, acupuncture may improve pain through somatosensory system including both central and peripheral mechanism. In the periphery, acupuncture inhibited neuronal activity caused by neuropathic pain, through reducing pain-related ion channels and receptors activation (Figure 5). In the spinal dorsal horn, EA down-regulated the neuronal chemokine CX3CL1 and prevented microglial p38 MAPK/ERK signaling pathway, leading to reduced release of TNF-α and IL-1β, BDNF, PGE2, and reducing the neuronal excitability and inflammatory response (Figure 6). Furthermore, acupuncture activated descending pain control system (Figure 7). The cumulative evidence demonstrated that acupuncture provided an alternative or adjunctive therapy for neuropathic pain.
In traditional Chinese medicine, the choice of appropriate acupoints is the key to acupuncture treatment. Intensities, frequencies, and the course of treatment of acupuncture all affect analgesic effects. However, the differences between MA and EA, the effects of EA frequency on relevant brain regions and the possible systematic differences between acupoint and non-acupoint are currently unknown. Conducting these studies in the future will provide better evidence to guide clinical on acupuncture modalities, acupuncture parameters and acupoints for the treatment of neuropathic pain. In the light of addressing the above issues, future studies shall be conducted in a broader context. High-quality, multifaceted basic research that explores the mechanisms of acupuncture analgesia will provide more possibilities for pain management.
XM, H-PL, and C-ZL put forward the idea of performing the review. XM wrote the initial manuscript. H-PL and C-ZL revised and edited the manuscript. WC and X-WH draw the manuscript. N-NY, LW, and C-XT summarized the tables. All authors have approved the submitted version.
This work was supported by funds from the National Key R&D Program of China (2019YFC1712104).
Figures were created with BioRender software.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alba-Delgado, C., Mountadem, S., Mermet-Joret, N., Monconduit, L., Dallel, R., Artola, A., et al. (2018). 5-HT2A receptor-induced morphological reorganization of PKCγ-expressing interneurons gates inflammatory mechanical allodynia in rat. J. Neurosci. 38, 10489–10504. doi: 10.1523/JNEUROSCI.1294-18.2018
Alhadeff, A. L., Su, Z., Hernandez, E., Klima, M. L., Phillips, S. Z., Holland, R. A., et al. (2018). A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152.e15. doi: 10.1016/j.cell.2018.02.057
Apkarian, A. V., Bushnell, M. C., Treede, R.-D., and Zubieta, J.-K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 9, 463–484. doi: 10.1016/j.ejpain.2004.11.001
Bai, H.-H., Liu, J.-P., Yang, L., Zhao, J.-Y., Suo, Z.-W., Yang, X., et al. (2017). Adenosine A1 receptor potentiated glycinergic transmission in spinal cord dorsal horn of rats after peripheral inflammation. Neuropharmacology 126, 158–167. doi: 10.1016/j.neuropharm.2017.09.001
Bai, L., Qin, W., Tian, J., Liu, P., Li, L., Chen, P., et al. (2009). Time-varied characteristics of acupuncture effects in fMRI studies. Hum. Brain Mapp. 30, 3445–3460. doi: 10.1002/hbm.20769
Bannister, K., Sachau, J., Baron, R., and Dickenson, A. H. (2020). Neuropathic pain: Mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 60, 257–274. doi: 10.1146/annurev-pharmtox-010818-021524
Barnes, P. M., Bloom, B., and Nahin, R. L. (2008). Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 12, 1–23.
Basso, L., and Altier, C. (2017). Transient receptor potential channels in neuropathic pain. Curr. Opin. Pharmacol. 32, 9–15. doi: 10.1016/j.coph.2016.10.002
Becker, H., Stuifbergen, A. K., Schnyer, R. N., Morrison, J. D., and Henneghan, A. (2017). Integrating acupuncture within a wellness intervention for women with multiple sclerosis. J. Holist. Nurs. 35, 86–96. doi: 10.1177/0898010116644833
Bennett, D. L., Clark, A. J., Huang, J., Waxman, S. G., and Dib-Hajj, S. D. (2019). The role of voltage-gated sodium channels in pain signaling. Physiol. Rev. 99, 1079–1151. doi: 10.1152/physrev.00052.2017
Bouhassira, D. (2019). Neuropathic pain: Definition, assessment and epidemiology. Revue Neurologique 175, 16–25. doi: 10.1016/j.neurol.2018.09.016
Bouhassira, D., Lantéri-Minet, M., Attal, N., Laurent, B., and Touboul, C. (2008). Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387. doi: 10.1016/j.pain.2007.08.013
Cao, X. (2002). Scientific bases of acupuncture analgesia. Acupunct. Electro Therap. Res. 27, 1–14. doi: 10.3727/036012902816026103
Cappoli, N., Tabolacci, E., Aceto, P., and Dello Russo, C. (2020). The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 349, 577406. doi: 10.1016/j.jneuroim.2020.577406
Chang, F.-C., Tsai, H.-Y., Yu, M.-C., Yi, P.-L., and Lin, J.-G. (2004). The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J. Biomed. Sci. 11, 179–185. doi: 10.1007/BF02256561
Cho, E., and Kim, W. (2021). Effect of acupuncture on diabetic neuropathy: A narrative review. Int. J. Mol. Sci. 22:8575. doi: 10.3390/ijms22168575
Choi, D. C., Lee, J. Y., Lim, E. J., Baik, H. H., Oh, T. H., and Yune, T. Y. (2012). Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp. Neurol. 236, 268–282. doi: 10.1016/j.expneurol.2012.05.014
Choi, I. S., Cho, J.-H., An, C.-H., Jung, J.-K., Hur, Y.-K., Choi, J.-K., et al. (2012). 5-HT(1B) receptors inhibit glutamate release from primary afferent terminals in rat medullary dorsal horn neurons. Br. J. Pharmacol. 167, 356–367. doi: 10.1111/j.1476-5381.2012.01964.x
Choi, S.-R., Beitz, A. J., and Lee, J.-H. (2019). Inhibition of cytochrome P450c17 reduces spinal astrocyte activation in a mouse model of neuropathic pain via regulation of p38 MAPK phosphorylation. Biomed. Pharmacother. 118:109299. doi: 10.1016/j.biopha.2019.109299
Choi, Y.-J., Lee, J.-E., Moon, W.-K., and Cho, S.-H. (2013). Does the effect of acupuncture depend on needling sensation and manipulation? Complement. Ther. Med. 21, 207–214. doi: 10.1016/j.ctim.2012.12.009
Cidral-Filho, F. J., da Silva, M. D., Moré, A. O. O., Córdova, M. M., Werner, M. F., and Santos, A. R. S. (2011). Manual acupuncture inhibits mechanical hypersensitivity induced by spinal nerve ligation in rats. Neuroscience 193, 370–376. doi: 10.1016/j.neuroscience.2011.07.076
Colleoni, M., and Sacerdote, P. (2010). Murine models of human neuropathic pain. Biochim. Biophys. Acta 1802, 924–933. doi: 10.1016/j.bbadis.2009.10.012
Comachio, J., Oliveira, C. C., Silva, I. F. R., Magalhães, M. O., and Marques, A. P. (2020). Effectiveness of manual and electrical acupuncture for chronic non-specific low back pain: A randomized controlled trial. J. Acupunct. Meridian Stud. 13, 87–93. doi: 10.1016/j.jams.2020.03.064
Cui, Y., Wang, F., Li, H., Zhang, X., Zhao, X., and Wang, D. (2021). Efficacy of acupuncture for herpes zoster: A systematic review and meta-analysis. Complement. Med. Res. 28, 463–472. doi: 10.1159/000515138
da Silva, J. R. T., da Silva, M. L., and Prado, W. A. (2013). Electroacupuncture at 2/100?hz activates antinociceptive spinal mechanisms different from those activated by electroacupuncture at 2 and 100?hz in responder rats. Evid. Based Complement. Alternat. Med. 2013:205316. doi: 10.1155/2013/205316
Dai, Q.-X., Huang, L.-P., Mo, Y.-C., Yu, L.-N., Du, W.-W., Zhang, A.-Q., et al. (2020). Role of spinal adenosine A1 receptors in the analgesic effect of electroacupuncture in a rat model of neuropathic pain. J. Int. Med. Res. 48:300060519883748. doi: 10.1177/0300060519883748
Dai, W.-J., Sun, J.-L., Li, C., Mao, W., Huang, Y.-K., Zhao, Z.-Q., et al. (2019). Involvement of interleukin-10 in analgesia of electroacupuncture on incision pain. Evid. Based Complement. Alternat. Med. 2019:8413576. doi: 10.1155/2019/8413576
Deng, Y., Yang, L., Xie, Q., Yang, F., Li, G., Zhang, G., et al. (2020). Protein kinase a is involved in neuropathic pain by activating the p38MAPK pathway to mediate spinal cord cell apoptosis. Mediat. Inflamm. 2020:6420425. doi: 10.1155/2020/6420425
Dib-Hajj, S. D., and Waxman, S. G. (2019). Sodium channels in human pain disorders: Genetics and pharmacogenomics. Annu. Rev. Neurosci. 42, 87–106. doi: 10.1146/annurev-neuro-070918-050144
Dimitrova, A., Murchison, C., and Oken, B. (2017). Acupuncture for the treatment of peripheral neuropathy: A systematic review and meta-analysis. J. Alternat. Complement. Med. 23, 164–179. doi: 10.1089/acm.2016.0155
Djouhri, L., Smith, T., Ahmeda, A., Alotaibi, M., and Weng, X. (2018). Hyperpolarization-activated cyclic nucleotide-gated channels contribute to spontaneous activity in L4 C-fiber nociceptors, but not Aβ-non-nociceptors, after axotomy of L5-spinal nerve in the rat in vivo. Pain 159, 1392–1402. doi: 10.1097/j.pain.0000000000001224
Du, J., Fang, J., Xiang, X., Yu, J., Le, X., Liang, Y., et al. (2021). Effects of low- and high-frequency electroacupuncture on protein expression and distribution of TRPV1 and P2X3 in rats with peripheral nerve injury. Acupunct. Med. 39, 478–490. doi: 10.1177/0964528420968845
Edwards, J. W., and Shaw, V. (2021). Acupuncture in the management of trigeminal neuralgia. Acupunct. Med. 39, 192–199. doi: 10.1177/0964528420924042
Eippert, F., Bingel, U., Schoell, E. D., Yacubian, J., Klinger, R., Lorenz, J., et al. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543. doi: 10.1016/j.neuron.2009.07.014
Emery, E. C., Young, G. T., Berrocoso, E. M., Chen, L., and McNaughton, P. A. (2011). HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333, 1462–1466. doi: 10.1126/science.1206243
Fan, Q., Cavus, O., Xiong, L., and Xia, Y. (2018). Spinal cord injury: How could acupuncture help? J. Acupunct. Merid. Stud. 11, 124–132. doi: 10.1016/j.jams.2018.05.002
Fang, J., Du, J., Xiang, X., Shao, X., He, X., Jiang, Y., et al. (2021). SNI and CFA induce similar changes in TRPV1 and P2X3 expressions in the acute phase but not in the chronic phase of pain. Exp. Brain Res. 239, 983–995. doi: 10.1007/s00221-020-05988-4
Fang, L., Wu, J., Lin, Q., and Willis, W. D. (2002). Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J. Neurosci. 22, 4196–4204.
Fei, X., He, X., Tai, Z., Wang, H., Qu, S., Chen, L., et al. (2020). Electroacupuncture alleviates diabetic neuropathic pain in rats by suppressing P2X3 receptor expression in dorsal root ganglia. Purinergic Signal. 16, 491–502. doi: 10.1007/s11302-020-09728-9
Finnerup, N. B., Attal, N., Haroutounian, S., McNicol, E., Baron, R., Dworkin, R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 14, 162–173. doi: 10.1016/S1474-4422(14)70251-0
Finnerup, N. B., Haroutounian, S., Kamerman, P., Baron, R., Bennett, D. L. H., Bouhassira, D., et al. (2016). Neuropathic pain: An updated grading system for research and clinical practice. Pain 157, 1599–1606. doi: 10.1097/j.pain.0000000000000492
François, A., Low, S. A., Sypek, E. I., Christensen, A. J., Sotoudeh, C., Beier, K. T., et al. (2017). A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839.e6. doi: 10.1016/j.neuron.2017.01.008
Fusumada, K., Yokoyama, T., Miki, T., Wang, Z.-Y., Yang, W., Lee, N.-S., et al. (2007). C-Fos expression in the periaqueductal gray is induced by electroacupuncture in the rat, with possible reference to GABAergic neurons. Okajimas Folia Anatomica Japonica 84, 1–9. doi: 10.2535/ofaj.84.1
Gao, J., Zhao, C., Jiang, W., Zheng, B., and He, Y. (2019). Effect of acupuncture on cognitive function and quality of life in patients with idiopathic trigeminal neuralgia. J. Nerv. Ment. Dis. 207, 171–174. doi: 10.1097/NMD.0000000000000937
Gim, G.-T., Lee, J.-H., Park, E., Sung, Y.-H., Kim, C.-J., Hwang, W.-W., et al. (2011). Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res. Bull. 86, 403–411. doi: 10.1016/j.brainresbull.2011.09.010
Goldman, N., Chen, M., Fujita, T., Xu, Q., Peng, W., Liu, W., et al. (2010). Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 13, 883–888. doi: 10.1038/nn.2562
Gomez-Ramirez, M., Hysaj, K., and Niebur, E. (2016). Neural mechanisms of selective attention in the somatosensory system. J. Neurophysiol. 116, 1218–1231. doi: 10.1152/jn.00637.2015
Groc, L., and Choquet, D. (2006). AMPA and NMDA glutamate receptor trafficking: Multiple roads for reaching and leaving the synapse. Cell Tissue Res. 326, 423–438. doi: 10.1007/s00441-006-0254-9
Han, J.-S. (2003). Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 26, 17–22. doi: 10.1016/s0166-2236(02)00006-1
Han, J.-S. (2004). Acupuncture and endorphins. Neurosci. Lett. 361, 258–261. doi: 10.1016/j.neulet.2003.12.019
He, X.-F., Wei, J.-J., Shou, S.-Y., Fang, J.-Q., and Jiang, Y.-L. (2017). Effects of electroacupuncture at 2 and 100 Hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J. Zhejiang Univ. Sci. B 18, 239–248. doi: 10.1631/jzus.B1600247
Held, M., Karl, F., Vlckova, E., Rajdova, A., Escolano-Lozano, F., Stetter, C., et al. (2019). Sensory profiles and immune-related expression patterns of patients with and without neuropathic pain after peripheral nerve lesion. Pain 160, 2316–2327. doi: 10.1097/j.pain.0000000000001623
Heo, I., Shin, B.-C., Kim, Y.-D., Hwang, E.-H., Han, C. W., and Heo, K.-H. (2013). Acupuncture for spinal cord injury and its complications: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2013:364216. doi: 10.1155/2013/364216
Hildebrand, M. E., Xu, J., Dedek, A., Li, Y., Sengar, A. S., Beggs, S., et al. (2016). Potentiation of synaptic GluN2B NMDAR currents by fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 17, 2753–2765. doi: 10.1016/j.celrep.2016.11.024
Hoffman, B. U., Baba, Y., Griffith, T. N., Mosharov, E. V., Woo, S.-H., Roybal, D. D., et al. (2018). Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100, 1401–1413.e6. doi: 10.1016/j.neuron.2018.10.034
Hoffmann, T., Sauer, S. K., Horch, R. E., and Reeh, P. W. (2008). Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J. Neurosci. 28, 6281–6284. doi: 10.1523/JNEUROSCI.1627-08.2008
Hou, A.-L., Zheng, M.-X., Hua, X.-Y., Huo, B.-B., Shen, J., and Xu, J.-G. (2020). Electroacupuncture-related metabolic brain connectivity in neuropathic pain due to brachial plexus avulsion injury in rats. Front. Neural Circuits 14:35. doi: 10.3389/fncir.2020.00035
Hu, H., Chen, L., Ma, R., Gao, H., and Fang, J. (2019). Acupuncture for primary trigeminal neuralgia: A systematic review and PRISMA-compliant meta-analysis. Complement. Ther. Clin. Pract. 34, 254–267. doi: 10.1016/j.ctcp.2018.12.013
Hu, Q.-Q., He, X.-F., Ma, Y.-Q., Ma, L.-Q., Qu, S.-Y., Wang, H.-Z., et al. (2022). Dorsal root ganglia P2X4 and P2X7 receptors contribute to diabetes-induced hyperalgesia and the downregulation of electroacupuncture on P2X4 and P2X7. Purinergic Signal. [Epub ahead of print]. doi: 10.1007/s11302-022-09844-8
Huang, C., Huang, Z.-Q., Hu, Z.-P., Jiang, S.-Z., Li, H.-T., Han, J.-S., et al. (2008). Electroacupuncture effects in a rat model of complete Freund’s adjuvant-induced inflammatory pain: Antinociceptive effects enhanced and tolerance development accelerated. Neurochem. Res. 33, 2107–2111. doi: 10.1007/s11064-008-9721-x
Huang, C. P., Lin, Y.-W., Lee, D.-Y., and Hsieh, C.-L. (2019). Electroacupuncture relieves CCI-induced neuropathic pain involving excitatory and inhibitory neurotransmitters. Evid. Based Complement. Alternat. Med. 2019:6784735. doi: 10.1155/2019/6784735
Huang, L., Xu, G., He, J., Tian, H., Zhou, Z., Huang, F., et al. (2021). Bibliometric analysis of functional magnetic resonance imaging studies on acupuncture analgesia over the past 20 years. J. Pain Res. 14, 3773–3789. doi: 10.2147/JPR.S340961
Huang, Z., Liu, S., Zhou, J., Yao, Q., and Liu, Z. (2019). Efficacy and safety of acupuncture for chronic discogenic sciatica, a randomized controlled sham acupuncture trial. Pain Med. 20, 2303–2310. doi: 10.1093/pm/pnz167
Hui, K. K. S., Liu, J., Marina, O., Napadow, V., Haselgrove, C., Kwong, K. K., et al. (2005). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27, 479–496. doi: 10.1016/j.neuroimage.2005.04.037
Huo, B.B., Zheng, M.-X., Hua, X.-Y., Shen, J., Wu, J.-J., and Xu, J.-G. (2020). Brain metabolism in rats with neuropathic pain induced by brachial plexus avulsion injury and treated via electroacupuncture. J. Pain Res. 13, 585–595. doi: 10.2147/JPR.S232030
Huo, R., Han, S.-P., Liu, F.-Y., Shou, X.-J., Liu, L.-Y., Song, T.-J., et al. (2020). Responses of primary afferent fibers to acupuncture-like peripheral stimulation at different frequencies: Characterization by single-unit recording in rats. Neurosci. Bull. 36, 907–918. doi: 10.1007/s12264-020-00509-3
Inoue, K., and Tsuda, M. (2018). Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 19, 138–152. doi: 10.1038/nrn.2018.2
Iravani, S., Kazemi Motlagh, A. H., Emami Razavi, S. Z., Shahi, F., Wang, J., Hou, L., et al. (2020). Effectiveness of acupuncture treatment on chemotherapy-induced peripheral neuropathy: A pilot, randomized, assessor-blinded, controlled trial. Pain Res. Manag. 2020:2504674. doi: 10.1155/2020/2504674
Jang, J.-H., Song, E.-M., Do, Y.-H., Ahn, S., Oh, J.-Y., Hwang, T.-Y., et al. (2021). Acupuncture alleviates chronic pain and comorbid conditions in a mouse model of neuropathic pain: The involvement of DNA methylation in the prefrontal cortex. Pain 162, 514–530. doi: 10.1097/j.pain.0000000000002031
Jensen, T. S., Baron, R., Haanpää, M., Kalso, E., Loeser, J. D., Rice, A. S. C., et al. (2011). A new definition of neuropathic pain. Pain 152, 2204–2205. doi: 10.1016/j.pain.2011.06.017
Ji, R. R., Kawasaki, Y., Zhuang, Z. Y., Wen, Y. R., and Zhang, Y. Q. (2007). Protein kinases as potential targets for the treatment of pathological pain. Handbook Exp. Pharmacol. 177, 359–389. doi: 10.1007/978-3-540-33823-9_13
Ji, R.-R., Chamessian, A., and Zhang, Y.-Q. (2016). Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577. doi: 10.1126/science.aaf8924
Jiang, M., Chen, X., Zhang, L., Liu, W., Yu, X., Wang, Z., et al. (2021). Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol. Res. 54:24. doi: 10.1186/s40659-021-00348-0
Jiang, S.-W., Lin, Y.-W., and Hsieh, C.-L. (2018). Electroacupuncture at Hua Tuo Jia Ji acupoints reduced neuropathic pain and increased GABAA receptors in rat spinal cord. Evid. Based Complement. Alternat. Med. 2018:8041820. doi: 10.1155/2018/8041820
Jiang, Y.-L., Yin, X.-H., Shen, Y.-F., He, X.-F., and Fang, J.-Q. (2013). Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid. Based Complement. Alternat. Med. 2013:170910. doi: 10.1155/2013/170910
Jin, Y., Zhou, J., Xu, F., Ren, Z., Hu, J., Zhang, C., et al. (2021). Electroacupuncture alleviates the transition from acute to chronic pain through the p38 MAPK/TNF-α signalling pathway in the spinal dorsal horn. Acupunct. Med. 39, 708–715. doi: 10.1177/09645284211020766
Jung, T. G., Lee, J. H., Lee, I. S., and Choi, B. T. (2010). Involvement of intracellular calcium on the phosphorylation of spinal N-methyl-D-aspartate receptor following electroacupuncture stimulation in rats. Acta Histochem. 112, 127–132. doi: 10.1016/j.acthis.2008.09.009
Kagitani, F., Uchida, S., and Hotta, H. (2010). Afferent nerve fibers and acupuncture. Autonom. Neurosci. Basic Clin. 157, 2–8. doi: 10.1016/j.autneu.2010.03.004
Khan, A., Khan, S., and Kim, Y. S. (2019). Insight into pain modulation: Nociceptors sensitization and therapeutic targets. Curr. Drug Targ. 20, 775–788. doi: 10.2174/1389450120666190131114244
Kim, J. H., Min, B.-I., Na, H. S., and Park, D. S. (2004). Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: Mediation by spinal opioid receptors. Brain Res. 998, 230–236. doi: 10.1016/j.brainres.2003.11.045
Kim, S. K., Park, J. H., Bae, S. J., Kim, J. H., Hwang, B. G., Min, B.-I., et al. (2005). Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: Mediation by spinal adrenergic and serotonergic receptors. Exp. Neurol. 195, 430–436. doi: 10.1016/j.expneurol.2005.06.018
Kiyoyuki, Y., Taniguchi, W., Okubo, M., Yamanaka, H., Kobayashi, K., Nishio, N., et al. (2015). Leukotriene enhances NMDA-induced inward currents in dorsal horn neurons of the rat spinal cord after peripheral nerve injury. Mol. Pain 11:53. doi: 10.1186/s12990-015-0059-5
Kondo, M., and Shibuta, I. (2020). Extracellular signal-regulated kinases (ERK) 1 and 2 as a key molecule in pain research. J. Oral Sci. 62, 147–149. doi: 10.2334/josnusd.19-0470
Koo, S. T., Lim, K. S., Chung, K., Ju, H., and Chung, J. M. (2008). Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain 135, 11–19. doi: 10.1016/j.pain.2007.04.034
Kuner, R., and Flor, H. (2016). Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 18, 20–30. doi: 10.1038/nrn.2016.162
Laedermann, C. J., Cachemaille, M., Kirschmann, G., Pertin, M., Gosselin, R.-D., Chang, I., et al. (2013). Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain. J. Clin. Invest. 123, 3002–3013. doi: 10.1172/JCI68996
Laumet, G., Garriga, J., Chen, S.-R., Zhang, Y., Li, D.-P., Smith, T. M., et al. (2015). G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat. Neurosci. 18, 1746–1755. doi: 10.1038/nn.4165
Lee, J. Y., Choi, D. C., Oh, T. H., and Yune, T. Y. (2013). Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS One 8:e73948. doi: 10.1371/journal.pone.0073948
Lee, S., Kim, J.-H., Shin, K.-M., Kim, J.-E., Kim, T.-H., Kang, K.-W., et al. (2013). Electroacupuncture to treat painful diabetic neuropathy: Study protocol for a three-armed, randomized, controlled pilot trial. Trials 14:225. doi: 10.1186/1745-6215-14-225
Lee, S., Lee, C.-S., Moon, J. Y., Song, H.-G., Yoo, Y., Kim, J., et al. (2020). Electroacupuncture may improve burning and electric shock-like neuropathic pain: A prospective exploratory pilot study. J. Alternat. Complement. Med. 26, 1136–1143. doi: 10.1089/acm.2020.0307
Li, A.-H., Zhang, J.-M., and Xie, Y.-K. (2004). Human acupuncture points mapped in rats are associated with excitable muscle/skin-nerve complexes with enriched nerve endings. Brain Res. 1012, 154–159. doi: 10.1016/j.brainres.2004.04.009
Li, C., Yang, J., Sun, J., Xu, C., Zhu, Y., Lu, Q., et al. (2013). Brain responses to acupuncture are probably dependent on the brain functional status. Evid. Based Complement. Alternat. Med. 2013:175278. doi: 10.1155/2013/175278
Li, P., Tjen-A-Looi, S. C., Guo, Z.-L., Fu, L.-W., and Longhurst, J. C. (2009). Long-loop pathways in cardiovascular electroacupuncture responses. J. Appl. Physiol. 106, 620–630. doi: 10.1152/japplphysiol.91277.2008
Li, X., and Eisenach, J. C. (2001). Alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J. Pharmacol. Exp. Therap. 299, 939–944.
Li, Y., Yin, C., Li, X., Liu, B., Wang, J., Zheng, X., et al. (2019b). Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory neurons. Int. J. Mol. Sci. 20:E5917. doi: 10.3390/ijms20235917
Li, Y., Fang, Z., Gu, N., Bai, F., Ma, Y., Dong, H., et al. (2019a). Inhibition of chemokine CX3CL1 in spinal cord mediates the electroacupuncture-induced suppression of inflammatory pain. J. Pain Res. 12, 2663–2672. doi: 10.2147/JPR.S205987
Liang, Y., Qiu, Y., Du, J., Liu, J., Fang, J., Zhu, J., et al. (2016). Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation. Acupunct. Med. 34, 40–47. doi: 10.1136/acupmed-2015-010773
Lin, J.-G., and Chen, W.-L. (2008). Acupuncture analgesia: A review of its mechanisms of actions. Am. J. Chin. Med. 36, 635–645. doi: 10.1142/S0192415X08006107
Lin, X., Wang, M., Zhang, J., and Xu, R. (2014). p38 MAPK: A potential target of chronic pain. Curr. Med. Chem. 21, 4405–4418. doi: 10.2174/0929867321666140915143040
Liu, J., and Wu, Y. (2017). Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed. Pharmacother. 89, 1125–1135. doi: 10.1016/j.biopha.2017.02.077
Liu, S., Zhang, C. S., Cai, Y., Guo, X., Zhang, A. L., Xue, C. C., et al. (2019). Acupuncture for post-stroke shoulder-hand syndrome: A systematic review and meta-analysis. Front. Neurol. 10:433. doi: 10.3389/fneur.2019.00433
Liu, Y., Latremoliere, A., Li, X., Zhang, Z., Chen, M., Wang, X., et al. (2018). Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550. doi: 10.1038/s41586-018-0515-2
Lovaglio, A. C., Socolovsky, M., Di Masi, G., and Bonilla, G. (2019). Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India 67, S32–S37. doi: 10.4103/0028-3886.250699
Luo, C., Kuner, T., and Kuner, R. (2014). Synaptic plasticity in pathological pain. Trends Neurosci. 37, 343–355. doi: 10.1016/j.tins.2014.04.002
Ma, R., Liu, X., Clark, J., Williams, G. M., and Doi, S. A. (2015). The impact of acupuncture on neurological recovery in spinal cord injury: A systematic review and meta-analysis. J. Neurotrauma 32, 1943–1957. doi: 10.1089/neu.2014.3866
Ma, Y., Chen, J., Yu, D., Wei, B., Jin, H., Zeng, J., et al. (2021). CAMP-PKA signaling is involved in regulation of spinal HCN channels function in diabetic neuropathic pain. Neurosci. Lett. 750:135763. doi: 10.1016/j.neulet.2021.135763
Masoodifar, M., Hajihashemi, S., Pazhoohan, S., Nazemi, S., and Mojadadi, M.-S. (2021). Effect of the conditioned medium of mesenchymal stem cells on the expression levels of P2X4 and P2X7 purinergic receptors in the spinal cord of rats with neuropathic pain. Purinergic Signal. 17, 143–150. doi: 10.1007/s11302-020-09756-5
Mehta, S., Orenczuk, K., McIntyre, A., Willems, G., Wolfe, D. L., Hsieh, J. T. C., et al. (2013). Neuropathic pain post spinal cord injury part 1: Systematic review of physical and behavioral treatment. Topics Spinal Cord Inj. Rehabil. 19, 61–77. doi: 10.1310/sci1901-61
Mickle, A. D., Shepherd, A. J., and Mohapatra, D. P. (2015). Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 131, 73–118. doi: 10.1016/bs.pmbts.2015.01.002
Millstine, D., Chen, C. Y., and Bauer, B. (2017). Complementary and integrative medicine in the management of headache. BMJ (Clinical Research Ed.) 357:j1805. doi: 10.1136/bmj.j1805
Murotani, T., Ishizuka, T., Nakazawa, H., Wang, X., Mori, K., Sasaki, K., et al. (2010). Possible involvement of histamine, dopamine, and noradrenalin in the periaqueductal gray in electroacupuncture pain relief. Brain Res. 1306, 62–68. doi: 10.1016/j.brainres.2009.09.117
Napadow, V., Kettner, N., Liu, J., Li, M., Kwong, K. K., Vangel, M., et al. (2007). Hypothalamus and amygdala response to acupuncture stimuli in Carpal Tunnel Syndrome. Pain 130, 254–266. doi: 10.1016/j.pain.2006.12.003
Nayak, S., Shiflett, S. C., Schoenberger, N. E., Agostinelli, S., Kirshblum, S., Averill, A., et al. (2001). Is acupuncture effective in treating chronic pain after spinal cord injury? Arch. Phys. Med. Rehabil. 82, 1578–1586. doi: 10.1053/apmr.2001.26624
Nilius, B., Owsianik, G., Voets, T., and Peters, J. A. (2007). Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217. doi: 10.1152/physrev.00021.2006
Ossipov, M. H., Dussor, G. O., and Porreca, F. (2010). Central modulation of pain. J. Clin. Invest. 120, 3779–3787. doi: 10.1172/JCI43766
Pei, W., Zeng, J., Lu, L., Lin, G., and Ruan, J. (2019). Is acupuncture an effective postherpetic neuralgia treatment? A systematic review and meta-analysis. J. Pain Res. 12, 2155–2165. doi: 10.2147/JPR.S199950
Pfister, D. G., Cassileth, B. R., Deng, G. E., Yeung, K. S., Lee, J. S., Garrity, D., et al. (2010). Acupuncture for pain and dysfunction after neck dissection: Results of a randomized controlled trial. J. Clin. Oncol. 28, 2565–2570. doi: 10.1200/JCO.2009.26.9860
Qian, Y., Xia, T., Cui, Y., Chu, S., Ma, Z., and Gu, X. (2019). The role of CaMKII in neuropathic pain and fear memory in chronic constriction injury in rats. Int. J. Neurosci. 129, 146–154. doi: 10.1080/00207454.2018.1512986
Ruengwongroj, P., Muengtaweepongsa, S., Patumanond, J., and Phinyo, P. (2020). Effectiveness of press needle treatment and electroacupuncture in patients with postherpetic neuralgia: A matched propensity score analysis. Complement. Ther. Clin. Pract. 40:101202. doi: 10.1016/j.ctcp.2020.101202
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 160, 53–59. doi: 10.1097/j.pain.0000000000001365
Seddighfar, M., Ghasemzadeh, Z., and Rezayof, A. (2019). The blockade of 5-HT1A receptors in the ventral tegmental area inhibited morphine/dextromethorphan-induced analgesia in pain rat models. Brain Res. 1715, 27–34. doi: 10.1016/j.brainres.2019.03.018
Sharif, B., Ase, A. R., Ribeiro-da-Silva, A., and Séguéla, P. (2020). Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 106, 940–951.e4. doi: 10.1016/j.neuron.2020.03.021
Siddall, P. J., and Middleton, J. W. (2015). Spinal cord injury-induced pain: Mechanisms and treatments. Pain Manag. 5, 493–507. doi: 10.2217/pmt.15.47
Silva, J. R. T., Silva, M. L., and Prado, W. A. (2011). Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J. Pain 12, 51–60. doi: 10.1016/j.jpain.2010.04.008
Silva, M. L., Silva, J. R. T., and Prado, W. A. (2013). Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the anterior pretectal nucleus. Life Sci. 93, 742–754. doi: 10.1016/j.lfs.2013.09.012
Sloan, G., Shillo, P., Selvarajah, D., Wu, J., Wilkinson, I. D., Tracey, I., et al. (2018). A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract. 144, 177–191. doi: 10.1016/j.diabres.2018.08.020
Sollie, M., Pind, R., Madsen, C. B., and Sørensen, J. A. (2022). Acupuncture (superficial dry-needling) as a treatment for chronic postherpetic neuralgia—a randomized clinical trial. Br. J. Pain 16, 96–108. doi: 10.1177/20494637211023075
Song, S., Xu, Y., Liu, J., Jia, Y., Lin, X., Liu, Y., et al. (2021). Strong twirling-rotating manual acupuncture with 4r/s Is superior to 2r/s in relieving pain by activating C-fibers in rat models of CFA-induced pain. Evid. Based Complement. Alternat. Med. 2021:5528780. doi: 10.1155/2021/5528780
Spaeth, R. B., Camhi, S., Hashmi, J. A., Vangel, M., Wasan, A. D., Edwards, R. R., et al. (2013). A longitudinal study of the reliability of acupuncture deqi sensations in knee osteoarthritis. Evid. Based Complement. Alternat. Med. 2013:204259. doi: 10.1155/2013/204259
Su, Y.-S., Yang, Z.-K., Xin, J.-J., He, W., Shi, H., Wang, X.-Y., et al. (2014). Somatosensory nerve fibers mediated generation of de-qi in manual acupuncture and local moxibustion-like stimuli-modulated gastric motility in rats. Evid. Based Complement. Alternat. Med. 2014:673239. doi: 10.1155/2014/673239
Sun, R.-Q., Wang, H.-C., and Wang, Y. (2002). [Effect of electroacupuncture with different frequencies on neuropathic pain in a rat model]. Zhongguo Ying Yong Sheng Li Xue Za 18, 128–131.
Sun, S., Chen, W.-L., Wang, P.-F., Zhao, Z.-Q., and Zhang, Y.-Q. (2006). Disruption of glial function enhances electroacupuncture analgesia in arthritic rats. Exp. Neurol. 198, 294–302. doi: 10.1016/j.expneurol.2005.11.011
Tang, Q., Jiang, Q., Sooranna, S. R., Lin, S., Feng, Y., Zhang, Q., et al. (2016). Effects of electroacupuncture on pain threshold of laboring rats and the expression of norepinephrine transporter and α2 adrenergic receptor in the central nervous system. Evid. Based Complement. Alternat. Med. 2016:9068257. doi: 10.1155/2016/9068257
Tang, Y., Yin, H.-Y., Rubini, P., and Illes, P. (2016). Acupuncture-induced analgesia: A neurobiological basis in purinergic signaling. Neuroscientist 22, 563–578. doi: 10.1177/1073858416654453
Torrance, N., Smith, B. H., Bennett, M. I., and Lee, A. J. (2006). The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain 7, 281–289. doi: 10.1016/j.jpain.2005.11.008
Tu, W.-Z., Li, S.-S., Jiang, X., Qian, X.-R., Yang, G.-H., Gu, P.-P., et al. (2018). Effect of electro-acupuncture on the BDNF-TrkB pathway in the spinal cord of CCI rats. Int. J. Mol. Med. 41, 3307–3315. doi: 10.3892/ijmm.2018.3563
Velázquez, K. T., Mohammad, H., and Sweitzer, S. M. (2007). Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 55, 578–589. doi: 10.1016/j.phrs.2007.04.006
Vidal-Torres, A., Carceller, A., Zamanillo, D., Merlos, M., Vela, J. M., and Fernández-Pastor, B. (2012). Evaluation of formalin-induced pain behavior and glutamate release in the spinal dorsal horn using in vivo microdialysis in conscious rats. J. Pharmacol. Sci. 120, 129–132. doi: 10.1254/jphs.12105sc
Vogt, B. A. (2016). Midcingulate cortex: Structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 74, 28–46. doi: 10.1016/j.jchemneu.2016.01.010
Vranken, J. H. (2012). Elucidation of pathophysiology and treatment of neuropathic pain. Cent. Nerv. Syst. Agents Med. Chem. 12, 304–314. doi: 10.2174/187152412803760645
Wang, J.-Y., Gao, Y.-H., Qiao, L.-N., Zhang, J.-L., Duan-Mu, C.-L., Yan, Y.-X., et al. (2018). Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats. BMC Complement. Alternat. Med. 18:74. doi: 10.1186/s12906-018-2134-8
Wang, K., Zhang, R., He, F., Lin, L.-B., Xiang, X.-H., Ping, X.-J., et al. (2012). Electroacupuncture frequency-related transcriptional response in rat arcuate nucleus revealed region-distinctive changes in response to low- and high-frequency electroacupuncture. J. Neurosci. Res. 90, 1464–1473. doi: 10.1002/jnr.23028
Wang, L., Gao, Z., Niu, X., Yuan, M., Li, Y., Wang, F., et al. (2020). Acupuncture for diabetic neuropathic pain: A protocol for systematic review and meta analysis. Medicine 99:e23244. doi: 10.1097/MD.0000000000023244
Wang, S.-M., Kain, Z. N., and White, P. (2008). Acupuncture analgesia: I. The scientific basis. Anesth. Analg. 106, 602–610. doi: 10.1213/01.ane.0000277493.42335.7b
Wang, W.-S., Tu, W.-Z., Cheng, R.-D., He, R., Ruan, L.-H., Zhang, L., et al. (2014). Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J. Neurosci. Res. 92, 1703–1713. doi: 10.1002/jnr.23451
Wang, Y.-R., Xu, H., Tao, M., Xu, L.-H., and Fu, X.-C. (2017). Ligustilide relieves complete Freund’s adjuvant-induced mechanical hyperalgesia through inhibiting the activation of spinal c-Jun N-terminal Kinase/c-Jun pathway in rats. Pharmacogn. Mag. 13, 634–638. doi: 10.4103/pm.pm_546_16
Waxman, S. G., Kocsis, J. D., and Black, J. A. (1994). Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 72, 466–470. doi: 10.1152/jn.1994.72.1.466
Wei, J.-A., Hu, X., Zhang, B., Liu, L., Chen, K., So, K.-F., et al. (2021). Electroacupuncture activates inhibitory neural circuits in the somatosensory cortex to relieve neuropathic pain. iScience 24:102066. doi: 10.1016/j.isci.2021.102066
Wei, T.-H., and Hsieh, C.-L. (2020). Effect of acupuncture on the p38 signaling pathway in several nervous system diseases: A systematic review. Int. J. Mol. Sci. 21:E4693. doi: 10.3390/ijms21134693
Wells, R. E., Baute, V., and Wahbeh, H. (2017). Complementary and integrative medicine for neurologic conditions. Med. Clin. North Am. 101, 881–893. doi: 10.1016/j.mcna.2017.04.006
Wu, J.-J., Lu, Y.-C., Hua, X.-Y., Ma, S.-J., Shan, C.-L., and Xu, J.-G. (2018). Cortical remodeling after electroacupuncture therapy in peripheral nerve repairing model. Brain Res. 1690, 61–73. doi: 10.1016/j.brainres.2018.04.009
Wu, Q., Chen, J., Yue, J., Ying, X., Zhou, Y., Chen, X., et al. (2021). Electroacupuncture improves neuronal plasticity through the A2AR/cAMP/PKA signaling pathway in SNL rats. Neurochem. Int. 145:104983. doi: 10.1016/j.neuint.2021.104983
Xia, Y.-Y., Xue, M., Wang, Y., Huang, Z.-H., and Huang, C. (2019). Electroacupuncture alleviates spared nerve injury-induced neuropathic pain and modulates HMGB1/NF-κB signaling pathway in the spinal cord. J. Pain Res. 12, 2851–2863. doi: 10.2147/JPR.S220201
Xie, W., Chen, S., Strong, J. A., Li, A.-L., Lewkowich, I. P., and Zhang, J.-M. (2016). Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J. Neurosci. 36, 8712–8725. doi: 10.1523/JNEUROSCI.4118-15.2016
Xie, W., Strong, J. A., and Zhang, J.-M. (2020). Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain 161, 1925–1936. doi: 10.1097/j.pain.0000000000001887
Xing, G.-G., Liu, F.-Y., Qu, X.-X., Han, J.-S., and Wan, Y. (2007). Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp. Neurol. 208, 323–332. doi: 10.1016/j.expneurol.2007.09.004
Xu, H., Yang, Y., Deng, Q.-W., Zhang, B.-B., Ruan, J.-W., Jin, H., et al. (2021). Governor vessel electro-acupuncture promotes the intrinsic growth ability of spinal neurons through activating calcitonin gene-related peptide/α-calcium/calmodulin-dependent protein kinase/neurotrophin-3 pathway after spinal cord injury. J. Neurotrauma 38, 734–745. doi: 10.1089/neu.2020.7155
Xu, S., Yu, L., Luo, X., Wang, M., Chen, G., Zhang, Q., et al. (2020). Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: Multicentre, randomised clinical trial. BMJ 368:m697. doi: 10.1136/bmj.m697
Yang, L., Ding, W., You, Z., Yang, J., Shen, S., Doheny, J. T., et al. (2019). Alleviation of trigeminal neuropathic pain by electroacupuncture: The role of hyperpolarization-activated cyclic nucleotide-gated channel protein expression in the Gasserian ganglion. Acupunct. Med. 37, 192–198. doi: 10.1177/0964528419841614
Yen, L.-T., Hsu, Y.-C., Lin, J.-G., Hsieh, C.-L., and Lin, Y.-W. (2018). Role of ASIC3, Nav1.7 and Nav1.8 in electroacupuncture-induced analgesia in a mouse model of fibromyalgia pain. Acupunct. Med. 36, 110–116. doi: 10.1136/acupmed-2016-011244
Yoshimoto, K., Fukuda, F., Hori, M., Kato, B., Kato, H., Hattori, H., et al. (2006). Acupuncture stimulates the release of serotonin, but not dopamine, in the rat nucleus accumbens. Tohoku J. Exp. Med. 208, 321–326. doi: 10.1620/tjem.208.321
Yu, B., Li, M., Huang, H., Ma, S., Huang, K., Zhong, Z., et al. (2021). Acupuncture treatment of diabetic peripheral neuropathy: An overview of systematic reviews. J. Clin. Pharm. Therap. 46, 585–598. doi: 10.1111/jcpt.13351
Yu, S.-W., Lin, S.-H., Tsai, C.-C., Chaudhuri, K. R., Huang, Y.-C., Chen, Y.-S., et al. (2019). Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Front. Neurol. 10:1114. doi: 10.3389/fneur.2019.01114
Yun, J.-M., Lee, S.-H., Cho, J.-H., Kim, K.-W., and Ha, I.-H. (2020). The effects of acupuncture on occipital neuralgia: A systematic review and meta-analysis. BMC Complement. Med. Ther. 20:171. doi: 10.1186/s12906-020-02955-y
Zhang, H., Sun, J., Xin, X., Huo, Z., and Li, D. (2018). Contralateral electroacupuncture relieves chronic neuropathic pain in rats with spared nerve injury. Med. Sci. Monit. 24, 2970–2974. doi: 10.12659/MSM.909741
Zhang, M., Dai, Q., Liang, D., Li, D., Chen, S., Chen, S., et al. (2018). Involvement of adenosine A1 receptor in electroacupuncture-mediated inhibition of astrocyte activation during neuropathic pain. Arquivos Neuro Psiquiatria 76, 736–742. doi: 10.1590/0004-282X20180128
Zhang, N., Wang, L.-Q., Li, J.-L., Su, X.-T., Yu, F.-T., Shi, G.-X., et al. (2021). The management of sciatica by acupuncture: An expert consensus using the improved delphi survey. J. Pain Res. 14, 13–22. doi: 10.2147/JPR.S280404
Zhang, W.-T., Jin, Z., Cui, G.-H., Zhang, K.-L., Zhang, L., Zeng, Y.-W., et al. (2003). Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: A functional magnetic resonance imaging study. Brain Res. 982, 168–178. doi: 10.1016/s0006-8993(03)02983-4
Zhang, Y., Li, A., Xin, J., Ren, K., Berman, B. M., Lao, L., et al. (2018). Electroacupuncture alleviates chemotherapy-induced pain through inhibiting phosphorylation of spinal CaMKII in rats. Eur. J. Pain 22, 679–690. doi: 10.1002/ejp.1132
Zhao, L., Li, D., Zheng, H., Chang, X., Cui, J., Wang, R., et al. (2019). Acupuncture as adjunctive therapy for chronic stable angina: A randomized clinical trial. JAMA Intern. Med. 179, 1388–1397. doi: 10.1001/jamainternmed.2019.2407
Zhao, P., Waxman, S. G., and Hains, B. C. (2007). Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 27, 2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007
Zhao, Z.-Q. (2008). Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85, 355–375. doi: 10.1016/j.pneurobio.2008.05.004
Zheng, Q., Xie, W., Lückemeyer, D. D., Lay, M., Wang, X.-W., Dong, X., et al. (2022). Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron 110, 209–220.e6. doi: 10.1016/j.neuron.2021.10.019
Zhi, W. I., Ingram, E., Li, S. Q., Chen, P., Piulson, L., and Bao, T. (2018). Acupuncture for bortezomib-induced peripheral neuropathy: Not just for pain. Integr. Cancer Ther. 17, 1079–1086. doi: 10.1177/1534735418788667
Zhou, W., and Benharash, P. (2014). Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Merid. Stud. 7, 190–193. doi: 10.1016/j.jams.2014.02.007
Zhou, Y.-F., Ying, X.-M., He, X.-F., Shou, S.-Y., Wei, J.-J., Tai, Z.-X., et al. (2018). Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 14, 359–369. doi: 10.1007/s11302-018-9617-4
Keywords: neuropathic pain, acupuncture, ion channels, glial cells, descending pain control system, somatosensory system
Citation: Ma X, Chen W, Yang N-N, Wang L, Hao X-W, Tan C-X, Li H-P and Liu C-Z (2022) Potential mechanisms of acupuncture for neuropathic pain based on somatosensory system. Front. Neurosci. 16:940343. doi: 10.3389/fnins.2022.940343
Received: 10 May 2022; Accepted: 29 August 2022;
Published: 20 September 2022.
Edited by:
Jian Kong, Massachusetts General Hospital, and Harvard Medical School, United StatesReviewed by:
Junying Wang, Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, ChinaCopyright © 2022 Ma, Chen, Yang, Wang, Hao, Tan, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cun-Zhi Liu, bGN6NjIzNzgwQDEyNi5jb20=; Hong-Ping Li, aG9uZ3BpbmdfbGlAYnVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.