- 1Department of Neurology, Chang Gung Memorial Hospital-Linkou Medical Center, Taoyuan City, Taiwan
- 2School of Medicine, College of Medicine, Chang Gung University, Taoyuan City, Taiwan
Epilepsy is a common disabling chronic neurological disorder characterized by an enduring propensity for the generation of seizures that result from abnormal hypersynchronous firing of neurons in the brain. Over 20–30% of epilepsy patients fail to achieve seizure control or soon become resistant to currently available therapies. Prolonged seizures or uncontrolled chronic seizures would give rise to neuronal damage or death, astrocyte activation, reactive oxygen species production, and mitochondrial dysfunction. Stem cell therapy is potentially a promising novel therapeutic strategy for epilepsy. The regenerative properties of stem cell-based treatment provide an attractive approach for long-term seizure control, particularly in drug-resistant epilepsy. Embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), neural stem cells (NSCs), induced pluripotent stem cells (iPSCs), and adipose-derived regenerative cells (ADRCs) are capable of differentiating into specialized cell types has been applied for epilepsy treatment in preclinical animal research and clinical trials. In this review, we focused on the advances in stem cell therapy for epilepsies. The goals of stem cell transplantation, its mechanisms underlying graft effects, the types of grafts, and their therapeutic effects were discussed. The cell and animal models used for investigating stem cell technology in epilepsy treatment were summarized.
Introduction
Epilepsy is a spectrum of heterogenous chronic brain disorders characterized by an enduring propensity for the generation of seizures which are the recurrent abnormal and hypersynchronous firing of neurons from dysfunctional brain circuits. Epilepsy remains one of the most prevalent neurological conditions affecting approximately 50–60 million people worldwide (WHO, 2022). Epilepsy can be classified into three major categories based on the possible underlying etiologies: idiopathic (genetic), symptomatic (acquired) including structural, infectious, metabolic, and immune etiologies, as well as unknown/cryptogenic (presume symptomatically) (Engel, 2011; Scheffer et al., 2017). Pharmacological therapy is the mainstay of treatment for epilepsy and many new anti-seizure medications (ASMs) have been developed in the past decades (Galanopoulou et al., 2012; Loscher, 2017). However, nearly one-third of epilepsy patients still fail to achieve seizure control or soon become resistant to currently available therapies (Kwan and Brodie, 2000, 2004; Shorvon, 2009a,b). Presently available medications are based on symptomatic strategies aiming to suppress or reduce the frequency and severity of seizures. These approaches do not affect and modify the underlying processes of epilepsy (Galanopoulou et al., 2012; Loscher et al., 2013). There is an unmet need to develop a therapeutic strategy for epilepsy that more specifically targets dysfunctional epileptic circuitry, particularly for medically refractory epilepsy and for suppressing the development and progression of epilepsy.
Stem cells are “undifferentiated” cells that have not yet engaged in a developmental path to form a specific cell type or tissue. These cells have the potential to be differentiated into a variety of specialized post-mitotic cell lineages (Kolios and Moodley, 2013). Stem cells can be classified into four major categories according to their origins: (1) Embryonic stem cells (ESCs), (2) Infant stem cells such as umbilical cord stem cells, (3) Adult stem cells (ASCs) including tissue-resident stem cells (e.g. ectoderm), neural stem cells, mesenchymal stem cells (MSCs) which can be obtained from bone marrow and adipose tissue, and (4) induced pluripotent stem cells (iPSCs) which are genetically reprogrammed adult stem cells to exhibit characteristics of ESCs (Fortier, 2005; Kolios and Moodley, 2013; EL Barky et al., 2017). These stem cells possess different differentiation potencies that are totipotent, pluripotent, multipotent, unipotent, and oligopotent cells (EL Barky et al., 2017). Totipotent cells have the capacity to differentiate into any cell type, form any tissue and produce an entire organism. Pluripotent cells have the potential to give rise to nearly all cells containing the three germ layers of the embryo, endoderm, mesoderm, and ectoderm. Multipotent cells can restrictedly differentiate to cell types related to their origin tissues. Unipotent cells and oligopotent cells can only be specialized in limited cell lineages of their own kind (EL Barky et al., 2017). As stem cells have the ability of self-replication and transform into specialized cell types, they play an important role in tissue repair and regeneration and provide the potential to restore and integrate the dysfunctional brain circuits to a normal state.
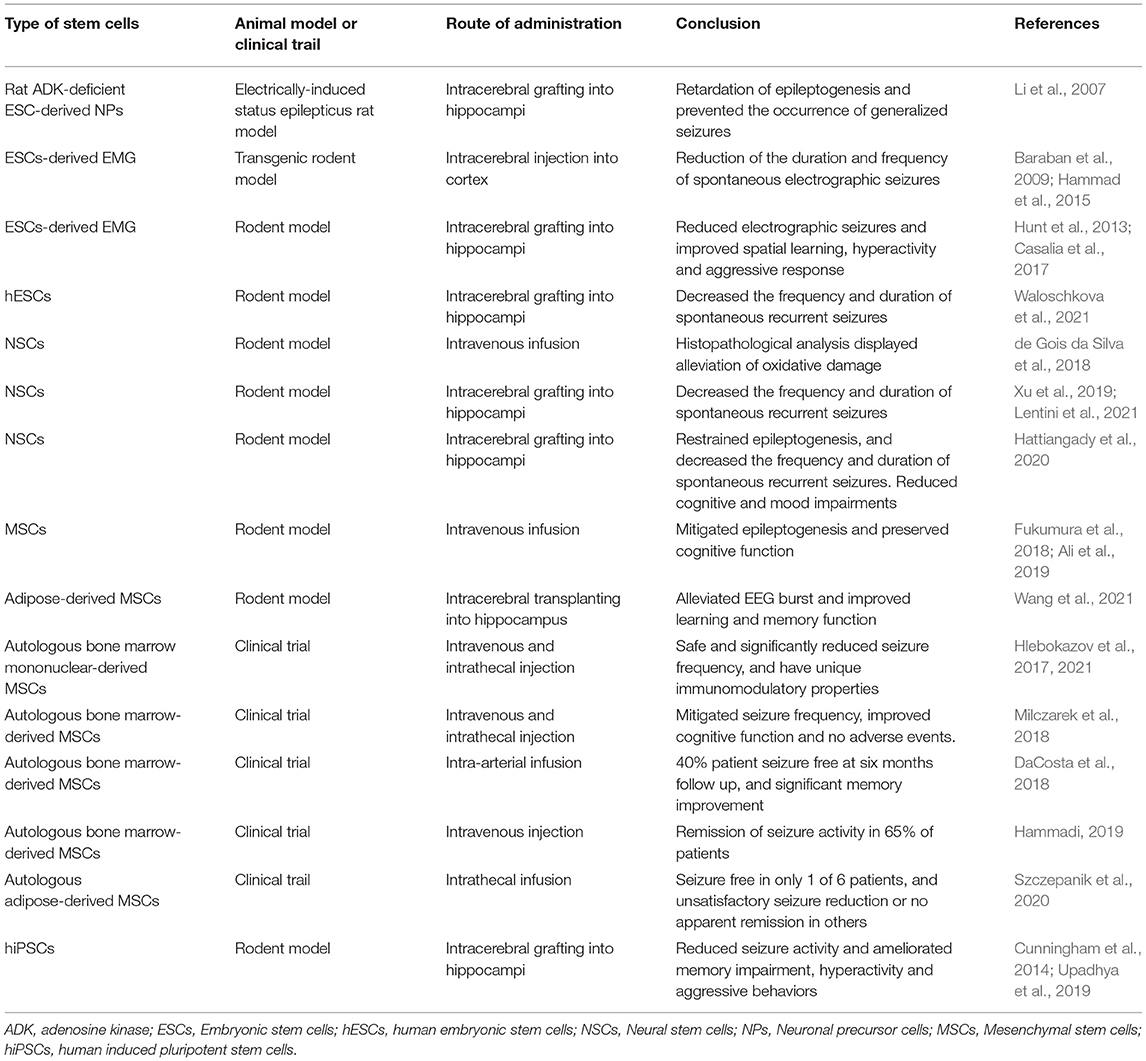
The therapeutic use of various stem cell types for the treatment of epilepsy has been increasingly explored in experimental and clinical research (Table 1). The pathophysiological mechanisms of epilepsy are attributed to a dysfunctional receptor or molecule expression of neural cells within the brain, neuronal damage or loss, astrocyte activation, reactive oxygen species production, and mitochondrial dysfunction. Stem cell-based treatment provides an attractive approach for replacement of the damaged or lost cells, correcting the imbalance of excitatory and inhibitory electrical activity in epileptic focus and brain circuits. Growing studies show that stem cell transplantation therapy is a novel promising therapeutic strategy for epilepsy. In this review, we focus on recent advancements in stem cell therapy for epilepsies and discuss the potential mechanisms, various graft sources, and their effects. We also review the disease models used for studying stem cell technology in epilepsy treatment.
Mechanisms of Action of Neurotransplantation for Epilepsy
The fundamental pathophysiologic basis of epilepsy is the transformation of the brain from a normal neuronal network to a long-lasting hyperexcitable state owing to an imbalance between the excitatory neurons and inhibitory neurons in the brain. Theoretically, stem cell transplantation could have disease-modifying effects to restore the malfunction of the epileptic brain via diverse mechanisms including specific cell substitution, rescue and repair of degeneration cells, reorganization of synapses, modulation of the secretion of neurotransmitters or beneficial neurotrophic factors (Li et al., 2007; Roper and Steindler, 2013; Hattiangady et al., 2020).
Cell Substitution
Epilepsy is often associated with either programmed or unprogrammed cell death. Replacement of damaged and lost cells or specific cell lines such as implantation of GABAergic interneurons integrating into pre-existing brain circuitry to achieve the suppression of hyperexcitation of neural networks (Waldau et al., 2010; Shetty and Upadhya, 2016).
Cell Rescue
The regenerative properties of grafted stem cells are capable to ameliorate host cells from the degenerative processes, repair myelin sheaths of axons in the impaired neurons and enhance neurogenesis, and restrain anomalous mossy fiber sprouting in the epileptic brain (Uchida et al., 2012; Kodali et al., 2019; Hattiangady et al., 2020). Stem cells also exhibit neuroprotective capacity by attenuating oxidative stress and glutamate excitotoxicity associated with epilepsy (de Gois da Silva et al., 2018; Papazian et al., 2018; Luo et al., 2019).
Supplement of Beneficial Neurotrophic Factors
Grafted stem cells can differentiate into glial cells which have an important role in secreting many neurotrophic factors and molecules including brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), insulin-like growth factor-1 (IGF-1) and microRNA to support neuron survival, neurogenesis and maintain normal neuronal function (Waldau et al., 2010; Lai et al., 2014; Ali et al., 2019; Hattiangady et al., 2020; Wang et al., 2021).
Anti-Inflammatory Properties
MSCs and NSCs could suppress the neuroinflammation process via inhibiting the secretion of proinflammatory factors and promoting the release of anti-inflammatory factors, as well as modulate neuroimmune responses through direct cell contact (Ryu et al., 2009; Cao et al., 2011; Wang et al., 2014; Kim et al., 2020).
Therapeutic Applications and Effects of Stem Cells in Epilepsy
The utility of in vitro stem cell technology by patient-derived iPSCs to high-throughput screening of anti-seizure medications can be applied for personalized epilepsy therapy (Jiao et al., 2013; Kaufmann et al., 2015; Elitt et al., 2018; Lybrand et al., 2020). The potential major roles of stem cell therapy in epilepsy are seizure remission, inhibition of the progression of the epileptogenic process, prophylaxis against the development of chronic epilepsy, and improvement of cognitive function (Aligholi et al., 2021; Sadanandan et al., 2021; Ramos-Fresnedo et al., 2022). A phase I clinical trial study showed a favorable seizure reduction, a good safety profile, and improvement of pathological hallmarks in epilepsy patients treated with autologous MSCs (Hlebokazov et al., 2017).
Sources and Types of Stem Cells
The pathogenesis of epilepsy is not only limited to dysfunction of neurons (such as glutamatergic and GABAergic cells) but also involves glia such as astrocytes and microglia. Various sources and many types of cells have been tested as a novel treatment in epilepsy to explore the therapeutic potency and safety of stem cell therapy, particularly for drug-resistant epilepsy.
Embryonic Stem Cells (ESCs)
Embryonic stem cells are pluripotent that can be differentiated into almost any cell lineage. Cortical inhibitory interneurons mainly originate from the embryonic medial and caudal ganglionic eminences in the forebrain during development (Wichterle et al., 1999; Xu et al., 2004; Butt et al., 2005). Grafting medial ganglionic eminence (MEG) progenitors into the brain exhibits normal migratory activity and can functionally differentiate as GABAergic inhibitory neurons and integrate into the neural circuitry to suppress seizures (Baraban et al., 2009; Hunt et al., 2013; Casalia et al., 2017). However, transplantation of rodent embryonic caudal ganglionic eminence (CGE) was not shown a reduction in seizure activity (Casalia et al., 2017). Despite the embryonic stem cells owning a great differentiation capacity to various cell types, the ethical concerns limit the utility of embryonic stem cells harvested from humans for stem cells.
Neural Stem Cells (NSCs)
Neural stem cells are multipotent cells capable of self-renewing and generating three major cell types of the central nervous system: neurons, astrocytes, and oligodendrocytes (Al-Mayyahi et al., 2018). In the adult brain, only two regions have the ability of neurogenesis: the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus (Parent et al., 1997; Miltiadous et al., 2013). NSCs can be harvested from several sources such as fetal, postnatal, and adult brains, and also can be generated from ESCs and iPSCs (Hattiangady and Shetty, 2011; Noebels et al., 2012). In the animal model of temporal lobe epilepsy, transplanting NSCs into the hippocampus appears responsive effects on seizure remission and has the potential to mitigate and repair epilepsy-induced pathological changes (Hattiangady and Shetty, 2012; Noebels et al., 2012; Uchida et al., 2012; de Gois da Silva et al., 2018; Xu et al., 2019). While NSCs show promising potential for treating mesial temporal lobe epilepsy, clinical research is paucity and a lack of data to demonstrate the effectiveness of NSC therapy on other forms of epilepsy.
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells are multipotent cells that can be isolated from bone marrow, adipose tissue, umbilical cord blood, placenta, and amniotic fluid (Kolios and Moodley, 2013; Berebichez-Fridman and Montero-Olvera, 2018). MSCs possess self-renewal capacities and are able to differentiate into many specialized cell types including neurons (Tropel et al., 2006). Recently, several preclinical animal and clinical studies have investigated the applications of MSCs for the treatment of epilepsy and appear potentials to ameliorate the severity of epilepsy and attenuate epilepsy-induced excitotoxicity, oxidative stress, and neuroinflammation (Fukumura et al., 2018; Milczarek et al., 2018; Papazian et al., 2018; Kodali et al., 2019; Xian et al., 2019; Linares et al., 2020). Furthermore, stem cell therapy using MSCs allows either allogeneic or autologous transplantation that extends the clinical feasibility. Clinical trials with autologous MSCs obtained from human bone marrow or adipose-derived regenerative cells for the treatment of medically refractory epilepsy display variable efficacy of seizure reduction with the improvement of cognitive and psychomotor function and good safety of the therapy (Hlebokazov et al., 2017; Milczarek et al., 2018; Szczepanik et al., 2020).
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells can be non-invasively derived from adult somatic cells such as fibroblast or peripheral mononuclear blood cells undergoing genetic reprogramming to have pluripotent properties (Yu et al., 2007; Braganca et al., 2019). iPSCs are able to differentiate into many neural cell types including distinct subtypes of GABAergic interneurons, astrocytes, motor neurons, and Purkinje cells (Du and Parent, 2015; Wang et al., 2015; Watson et al., 2018). Specific cell types derived from iPSCs implanting into the epileptic brain can migrate extensively into different subfields of the target brain region with synaptic integration of grafted cells into the dysfunctional circuitry (Cunningham et al., 2014). Grafting maturing GABAergic interneurons derived from human-induced pluripotent stem cells (hiPSCs) in an epilepsy mouse model has been shown to mitigate seizures and improve cognitive function, aggressive behavior, and mood (Cunningham et al., 2014). Implanting hiPSCs-derived MGE-like interneuron precursors into epileptic rodent hippocampi showed anti-epileptic and anti-epileptogenic effects with significantly restraining seizure phenotypes and alleviating cell loss, abnormal neurogenesis, and aberrant mossy fiber sprouting (Noakes et al., 2019; Upadhya et al., 2019). Nonetheless, there is still a lack of clinical data showing the application of iPSCs for the treatment of human epilepsy.
Disease Models and Animal Model Studies
Use of iPSCs to Model Diseases
Patient-derived iPSCs are models of a patient's genetic background and can be used as a platform for modeling patient-specific diseases, human genetic epilepsies, high throughput drug screening, and studying the mechanisms of epilepsy (Sun et al., 2016; Elitt et al., 2018; Kim et al., 2018).
Stem Cell Transplants in Animal Models of Epilepsy
Mesial temporal lobe epilepsy (mTLE) which mainly involves the hippocampus is often medically refractory. Animal models mimic mesial temporal lobe epilepsy including kainic acid and pilocarpine rodent models are commonly used in chronic epilepsy models for stem cell research (Cunningham et al., 2014; Casalia et al., 2017; Upadhya et al., 2019; Lentini et al., 2021; Waloschkova et al., 2021). Electrically-induced status epilepticus model is another applicable rodent model of status epilepticus and chronic mTLE (Li et al., 2007). Genetic or transgenic mice such as Kv1.1 mutation which associates with human episodic ataxia type 1 and focal cortical epilepsy, and Stargazer (stg) transgenic mouse model of absence epilepsy have been applied to explore the therapeutic effects on epilepsy by transplantation of GABAergic interneurons into the brain (Baraban et al., 2009; Hammad et al., 2015). Pentylenetetrazole and picrotoxin rodent models are acute seizure models that can be used to evaluate the anticonvulsant effects of cell-based therapy (Handreck et al., 2014; de Gois da Silva et al., 2018).
Perspectives of Clinical Application of Neurotransplantation of iPSCs for Epilepsy
iPSCs obtained from patients themselves can be manipulated or corrected by novel genetic editing technology such as CRISPR/Cas9, then autologously implanted into patients to repair their underlying pathological circuits in the epileptic brain (Hockemeyer and Jaenisch, 2016; Dever et al., 2019). Combining the iPSCs and genetic engineering technology might serve as a potential disease-modifying treatment for epilepsy and personalized therapy.
Conclusions
Emerging evidence suggests that stem cell approaches could be a promising tool in epilepsy treatment. However, further studies are required to investigate and address the optimal regimen, microenvironment, and timing of stem cell differentiation and implantation. In addition, compared to ESCs and NSCs, MSCs and iPSCs do not raise ethical issues. Autologous cell transplants avoid the immune response and have better integration between the graft and host cells which would be a good option for the development of cell-based therapy. Advanced preclinical investigations in clinically relevant models of epilepsy and larger clinical trials need to be conducted to further demonstrate the efficacy and safety of stem cell therapy for epilepsy.
Author Contributions
B-LC: conceptualization and funding acquisition. B-LC and K-HC: writing—original draft and writing—review and editing. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Chang Gung Memorial Hospital, Taipei, Taiwan (CMRPG3I0521, CMRPG3K1021, CMRPG3L0661, and BMRPJ26) and the Ministry of Science and Technology, Taiwan (MOST 108-2314-B-182A-153 -, MOST 109-2314-B-182-079-, and MOST 110-2314-B-182-055-).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, S. O., Shahin, N. N., Safar, M. M., and Rizk, S. M. (2019). Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: role of autophagy. J. Adv. Res. 18, 101–112. doi: 10.1016/j.jare.2019.01.013
Aligholi, H., Safahani, M., and Asadi-Pooya, A. A. (2021). Stem cell therapy in patients with epilepsy: a systematic review. Clin. Neurol. Neurosurg. 200, 106416. doi: 10.1016/j.clineuro.2020.106416
Al-Mayyahi, R. S., Sterio, L. D., Connolly, J. B., Adams, C. F., Al-Tumah, W. A., Sen, J., et al. (2018). A proteomic investigation into mechanisms underpinning corticosteroid effects on neural stem cells. Mol. Cell Neurosci. 86, 30–40. doi: 10.1016/j.mcn.2017.11.006
Baraban, S. C., Southwell, D. G., Estrada, R. C., Jones, D. L., Sebe, J. Y., Alfaro-Cervello, C., et al. (2009). Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 106, 15472–15477. doi: 10.1073/pnas.0900141106
Berebichez-Fridman, R., and Montero-Olvera, P. R. (2018). Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ. Med. J. 18, e264–e277. doi: 10.18295/squmj.2018.18.03.002
Braganca, J., Lopes, J. A., Mendes-Silva, L., and Almeida Santos, J. M. (2019). Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J. Stem Cells 11, 421–430. doi: 10.4252/wjsc.v11.i7.421
Butt, S. J., Fuccillo, M., Nery, S., Noctor, S., Kriegstein, A., Corbin, J. G., et al. (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48, 591–604. doi: 10.1016/j.neuron.2005.09.034
Cao, W., Yang, Y., Wang, Z., Liu, A., Fang, L., Wu, F., et al. (2011). Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity 35, 273–284. doi: 10.1016/j.immuni.2011.06.011
Casalia, M. L., Howard, M. A., and Baraban, S. C. (2017). Persistent seizure control in epileptic mice transplanted with gamma-aminobutyric acid progenitors. Ann. Neurol. 82, 530–542. doi: 10.1002/ana.25021
Cunningham, M., Cho, J. H., Leung, A., Savvidis, G., Ahn, S., Moon, M., et al. (2014). hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15, 559–573. doi: 10.1016/j.stem.2014.10.006
DaCosta, J. C., Portuguez, M. W., Marinowic, D. R., Schilling, L. P., Torres, C. M., DaCosta, D. I., et al. (2018). Safety and seizure control in patients with mesial temporal lobe epilepsy treated with regional superselective intra-arterial injection of autologous bone marrow mononuclear cells. J. Tissue Eng. Regen. Med. 12, e648–e656. doi: 10.1002/term.2334
de Gois da Silva, M. L., da Silva Oliveira, G. L., de Oliveira Bezerra, D., da Rocha Neto, H. J., Feitosa, M. L. T., Argolo Neto, N. M., et al. (2018). Neurochemical properties of neurospheres infusion in experimental-induced seizures. Tissue Cell 54, 47–54. doi: 10.1016/j.tice.2018.08.002
Dever, D. P., Scharenberg, S. G., Camarena, J., Kildebeck, E. J., Clark, J. T., Martin, R. M., et al. (2019). CRISPR/Cas9 genome engineering in engraftable human brain-derived neural stem cells. iScience 15, 524–535. doi: 10.1016/j.isci.2019.04.036
Du, X., and Parent, J. M. (2015). Using patient-derived induced pluripotent stem cells to model and treat epilepsies. Curr. Neurol. Neurosci. Rep. 15, 71. doi: 10.1007/s11910-015-0588-3
EL Barky, A. R., Mohamed Ali, E. M., and Mohamed, T. M. (2017). Stem cells, classifications and their clinical applications. Am. J. Pharmacol. Therapeut. 1, 1–7. Available online at: https://www.scireslit.com/Pharmacology/AJPT-ID11.php
Elitt, M. S., Barbar, L., and Tesar, P. J. (2018). Drug screening for human genetic diseases using iPSC models. Hum. Mol. Genet. 27, R89–R98. doi: 10.1093/hmg/ddy186
Engel, J. Jr. (2011). The etiologic classification of epilepsy. Epilepsia 52, 1195–1197; discussion: 1205. doi: 10.1111/j.1528-1167.2011.03065.x
Fortier, L. A. (2005). Stem cells: classifications, controversies, and clinical applications. Vet. Surg. 34, 415–423. doi: 10.1111/j.1532-950X.2005.00063.x
Fukumura, S., Sasaki, M., Kataoka-Sasaki, Y., Oka, S., Nakazaki, M., Nagahama, H., et al. (2018). Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Res. 141, 56–63. doi: 10.1016/j.eplepsyres.2018.02.008
Galanopoulou, A. S., Buckmaster, P. S., Staley, K. J., Moshe, S. L., Perucca, E., Engel, J. Jr., et al. (2012). Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia 53, 571–582. doi: 10.1111/j.1528-1167.2011.03391.x
Hammad, M., Schmidt, S. L., Zhang, X., Bray, R., Frohlich, F., and Ghashghaei, H. T. (2015). Transplantation of GABAergic interneurons into the neonatal primary visual cortex reduces absence seizures in stargazer mice. Cereb. Cortex 25, 2970–2979. doi: 10.1093/cercor/bhu094
Hammadi, A. A. (2019). Autologous bone marrow derived mononuclear cells for the treatment of drug resistant epilepsy. J. Stem Cell Res. Ther. 5, 23–25. doi: 10.15406/jsrt.2019.05.00129
Handreck, A., Backofen-Wehrhahn, B., Broer, S., Loscher, W., and Gernert, M. (2014). Anticonvulsant effects by bilateral and unilateral transplantation of GABA-producing cells into the subthalamic nucleus in an acute seizure model. Cell Transplant. 23, 111–132. doi: 10.3727/096368912X658944
Hattiangady, B., Kuruba, R., Shuai, B., Grier, R., and Shetty, A. K. (2020). Hippocampal neural stem cell grafting after status epilepticus alleviates chronic epilepsy and abnormal plasticity, and maintains better memory and mood function. Aging Dis. 11, 1374–1394. doi: 10.14336/AD.2020.1020
Hattiangady, B., and Shetty, A. K. (2011). Neural stem cell grafting in an animal model of chronic temporal lobe epilepsy. Curr. Protoc. Stem Cell Biol. Chapter 2, Unit: 2D7. doi: 10.1002/9780470151808.sc02d07s18
Hattiangady, B., and Shetty, A. K. (2012). Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl. Med. 1, 696–708. doi: 10.5966/sctm.2012-0050
Hlebokazov, F., Dakukina, T., Ihnatsenko, S., Kosmacheva, S., Potapnev, M., Shakhbazau, A., et al. (2017). Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: an open label study. Adv. Med. Sci. 62, 273–279. doi: 10.1016/j.advms.2016.12.004
Hlebokazov, F., Dakukina, T., Potapnev, M., Kosmacheva, S., Moroz, L., Misiuk, N., et al. (2021). Clinical benefits of single vs repeated courses of mesenchymal stem cell therapy in epilepsy patients. Clin. Neurol. Neurosurg. 207, 106736. doi: 10.1016/j.clineuro.2021.106736
Hockemeyer, D., and Jaenisch, R. (2016). Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18, 573–586. doi: 10.1016/j.stem.2016.04.013
Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A., and Baraban, S. C. (2013). GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 16, 692–697. doi: 10.1038/nn.3392
Jiao, J., Yang, Y., Shi, Y., Chen, J., Gao, R., Fan, Y., et al. (2013). Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 22, 4241–4252. doi: 10.1093/hmg/ddt275
Kaufmann, M., Schuffenhauer, A., Fruh, I., Klein, J., Thiemeyer, A., Rigo, P., et al. (2015). High-throughput screening using iPSC-derived neuronal progenitors to identify compounds counteracting epigenetic gene silencing in fragile X syndrome. J. Biomol. Screen 20, 1101–1111. doi: 10.1177/1087057115588287
Kim, H. S., Jeon, I., Noh, J. E., Lee, H., Hong, K. S., Lee, N., et al. (2020). Intracerebral transplantation of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) promotes migration, differentiation and functional recovery in a rodent model of Huntington's disease. Exp. Neurobiol. 29, 130–137. doi: 10.5607/en20011
Kim, H. W., Quan, Z., Kim, Y. B., Cheong, E., Kim, H. D., Cho, M., et al. (2018). Differential effects on sodium current impairments by distinct SCN1A mutations in GABAergic neurons derived from Dravet syndrome patients. Brain Dev. 40, 287–298. doi: 10.1016/j.braindev.2017.12.002
Kodali, M., Castro, O. W., Kim, D. K., Thomas, A., Shuai, B., Attaluri, S., et al. (2019). Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 21:181. doi: 10.3390/ijms21010181
Kolios, G., and Moodley, Y. (2013). Introduction to stem cells and regenerative medicine. Respiration 85, 3–10. doi: 10.1159/000345615
Kwan, P., and Brodie, M. J. (2000). Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319. doi: 10.1056/NEJM200002033420503
Kwan, P., and Brodie, M. J. (2004). Drug treatment of epilepsy: when does it fail and how to optimize its use? CNS Spectr. 9, 110–119. doi: 10.1017/s1092852900008476
Lai, C. P., Tannous, B. A., and Breakefield, X. O. (2014). Noninvasive in vivo monitoring of extracellular vesicles. Methods Mol. Biol. 1098, 249–258. doi: 10.1007/978-1-62703-718-1_19
Lentini, C., d'Orange, M., Marichal, N., Trottmann, M. M., Vignoles, R., Foucault, L., et al. (2021). Reprogramming reactive glia into interneurons reduces chronic seizure activity in a mouse model of mesial temporal lobe epilepsy. Cell Stem Cell 28, 2104.e10–2121.e10. doi: 10.1016/j.stem.2021.09.002
Li, T., Steinbeck, J. A., Lusardi, T., Koch, P., Lan, J. Q., Wilz, A., et al. (2007). Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain 130(Pt 5), 1276–1288. doi: 10.1093/brain/awm057
Linares, G. R., Leng, Y., Maric, D., and Chuang, D. M. (2020). Overexpression of fibroblast growth factor-21 (FGF-21) protects mesenchymal stem cells against caspase-dependent apoptosis induced by oxidative stress and inflammation. Cell Biol. Int. 44, 2163–2169. doi: 10.1002/cbin.11409
Loscher, W. (2017). Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem. Res. 42, 1873–1888. doi: 10.1007/s11064-017-2222-z
Loscher, W., Klitgaard, H., Twyman, R. E., and Schmidt, D. (2013). New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 12, 757–776. doi: 10.1038/nrd4126
Luo, M. L., Pan, L., Wang, L., Wang, H. Y., Li, S., Long, Z. Y., et al. (2019). Transplantation of NSCs promotes the recovery of cognitive functions by regulating neurotransmitters in rats with traumatic brain injury. Neurochem. Res. 44, 2765–2775. doi: 10.1007/s11064-019-02897-z
Lybrand, Z. R., Goswami, S., and Hsieh, J. (2020). Stem cells: a path towards improved epilepsy therapies. Neuropharmacology 168, 107781. doi: 10.1016/j.neuropharm.2019.107781
Milczarek, O., Jarocha, D., Starowicz-Filip, A., Kwiatkowski, S., Badyra, B., and Majka, M. (2018). Multiple autologous bone marrow-derived CD271(+) mesenchymal stem cell transplantation overcomes drug-resistant epilepsy in children. Stem Cells Transl. Med. 7, 20–33. doi: 10.1002/sctm.17-0041
Miltiadous, P., Kouroupi, G., Stamatakis, A., Koutsoudaki, P. N., Matsas, R., and Stylianopoulou, F. (2013). Subventricular zone-derived neural stem cell grafts protect against hippocampal degeneration and restore cognitive function in the mouse following intrahippocampal kainic acid administration. Stem Cells Transl. Med. 2, 185–198. doi: 10.5966/sctm.2012-0074
Noakes, Z., Keefe, F., Tamburini, C., Kelly, C. M., Cruz Santos, M., Dunnett, S. B., et al. (2019). Human pluripotent stem cell-derived striatal interneurons: differentiation and maturation in vitro and in the rat brain. Stem Cell Rep. 12, 191–200. doi: 10.1016/j.stemcr.2018.12.014
Noebels, J. L., Avoli, M., and Rogawski, M. A. (2012). Jasper's Basic Mechanisms of the Epilepsies, 4th Edn. Bethesda, MD: National Center for Biotechnology Information.
Papazian, I., Kyrargyri, V., Evangelidou, M., Voulgari-Kokota, A., and Probert, L. (2018). Mesenchymal stem cell protection of neurons against glutamate excitotoxicity involves reduction of NMDA-triggered calcium responses and surface GluR1, and is partly mediated by TNF. Int. J. Mol. Sci. 19:651. doi: 10.3390/ijms19030651
Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S., and Lowenstein, D. H. (1997). Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 17, 3727–3738.
Ramos-Fresnedo, A., Perez-Vega, C., Domingo, R. A., Lee, S. J., Perkerson, R. B., Zubair, A. C., et al. (2022). Mesenchymal stem cell therapy for focal epilepsy: a systematic review of preclinical models and clinical studies. Epilepsia. doi: 10.1111/epi.17266. [Epub ahead of print].
Roper, S. N., and Steindler, D. A. (2013). Stem cells as a potential therapy for epilepsy. Exp. Neurol. 244, 59–66. doi: 10.1016/j.expneurol.2012.01.004
Ryu, J. K., Cho, T., Wang, Y. T., and McLarnon, J. G. (2009). Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J. Neuroinflamm. 6, 39. doi: 10.1186/1742-2094-6-39
Sadanandan, N., Saft, M., Gonzales-Portillo, B., and Borlongan, C. V. (2021). Multipronged attack of stem cell therapy in treating the neurological and neuropsychiatric symptoms of epilepsy. Front. Pharmacol. 12, 596287. doi: 10.3389/fphar.2021.596287
Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L., et al. (2017). ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 58, 512–521. doi: 10.1111/epi.13709
Shetty, A. K., and Upadhya, D. (2016). GABA-ergic cell therapy for epilepsy: advances, limitations and challenges. Neurosci. Biobehav. Rev. 62, 35–47. doi: 10.1016/j.neubiorev.2015.12.014
Shorvon, S. D. (2009a). Drug treatment of epilepsy in the century of the ILAE: the first 50 years, 1909-1958. Epilepsia 50(Suppl 3), 69–92. doi: 10.1111/j.1528-1167.2009.02041.x
Shorvon, S. D. (2009b). Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959-2009. Epilepsia 50(Suppl 3), 93–130. doi: 10.1111/j.1528-1167.2009.02042.x
Sun, Y., Pasca, S. P., Portmann, T., Goold, C., Worringer, K. A., Guan, W., et al. (2016). A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. eLife 5, 13073. doi: 10.7554/eLife.13073
Szczepanik, E., Mierzewska, H., Antczak-Marach, D., Figiel-Dabrowska, A., Terczynska, I., Tryfon, J., et al. (2020). Intrathecal infusion of autologous adipose-derived regenerative cells in autoimmune refractory epilepsy: evaluation of safety and efficacy. Stem Cells Int. 2020, 7104243. doi: 10.1155/2020/7104243
Tropel, P., Platet, N., Platel, J. C., Noel, D., Albrieux, M., Benabid, A. L., et al. (2006). Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24, 2868–2876. doi: 10.1634/stemcells.2005-0636
Uchida, N., Chen, K., Dohse, M., Hansen, K. D., Dean, J., Buser, J. R., et al. (2012). Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci. Transl. Med. 4, 155ra136. doi: 10.1126/scitranslmed.3004371
Upadhya, D., Hattiangady, B., Castro, O. W., Shuai, B., Kodali, M., Attaluri, S., et al. (2019). Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc. Natl. Acad. Sci. U.S.A. 116, 287–296. doi: 10.1073/pnas.1814185115
Waldau, B., Hattiangady, B., Kuruba, R., and Shetty, A. K. (2010). Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 28, 1153–1164. doi: 10.1002/stem.446
Waloschkova, E., Gonzalez-Ramos, A., Mikroulis, A., Kudlacek, J., Andersson, M., Ledri, M., et al. (2021). Human stem cell-derived GABAergic interneurons establish efferent synapses onto host neurons in rat epileptic hippocampus and inhibit spontaneous recurrent seizures. Int. J. Mol. Sci. 22:13243. doi: 10.3390/ijms222413243
Wang, L., Zhao, Y., Pan, X., Zhang, Y., Lin, L., Wu, Y., et al. (2021). Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. 1750, 147121. doi: 10.1016/j.brainres.2020.147121
Wang, S., Wang, B., Pan, N., Fu, L., Wang, C., Song, G., et al. (2015). Differentiation of human induced pluripotent stem cells to mature functional Purkinje neurons. Sci. Rep. 5, 9232. doi: 10.1038/srep09232
Wang, Y., Chen, X., Cao, W., and Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016. doi: 10.1038/ni.3002
Watson, L. M., Wong, M. M. K., Vowles, J., Cowley, S. A., and Becker, E. B. E. (2018). A simplified method for generating Purkinje cells from human-induced pluripotent stem cells. Cerebellum 17, 419–427. doi: 10.1007/s12311-017-0913-2
WHO (2022). Epilepsy. WHO Fact Sheets. Available online at: https://www.who.int/en/news-room/fact-sheets/detail/epilepsy (accessed April 01, 2022).
Wichterle, H., Garcia-Verdugo, J. M., Herrera, D. G., and Alvarez-Buylla, A. (1999). Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 2, 461–466. doi: 10.1038/8131
Xian, P., Hei, Y., Wang, R., Wang, T., Yang, J., Li, J., et al. (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 9, 5956–5975. doi: 10.7150/thno.33872
Xu, K., Liu, F., Xu, W., Liu, J., Chen, S., and Wu, G. (2019). Transplanting GABAergic neurons differentiated from neural stem cells into hippocampus inhibits seizures and epileptiform discharges in pilocarpine-induced temporal lobe epilepsy model. World Neurosurg. 128, e1–e11. doi: 10.1016/j.wneu.2019.01.245
Xu, Q., Cobos, I., De La Cruz, E., Rubenstein, J. L., and Anderson, S. A. (2004). Origins of cortical interneuron subtypes. J. Neurosci. 24, 2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004
Keywords: stem cells, seizure, epilepsy, disease-modifying, genetic engineering, graft, disease models
Citation: Chang B-L and Chang K-H (2022) Stem Cell Therapy in Treating Epilepsy. Front. Neurosci. 16:934507. doi: 10.3389/fnins.2022.934507
Received: 02 May 2022; Accepted: 30 May 2022;
Published: 27 June 2022.
Edited by:
Yu-Chen Hu, National Tsing Hua University, TaiwanReviewed by:
Stephanie Schorge, University College London, United KingdomCopyright © 2022 Chang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao-Luen Chang, YmFvbHVlbkBnbWFpbC5jb20=
 Bao-Luen Chang
Bao-Luen Chang Kuo-Hsuan Chang
Kuo-Hsuan Chang