
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 13 June 2022
Sec. Brain Imaging Methods
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.918513
This article is part of the Research Topic Multi-Parameter and Quantitative MRI in Brain Diseases and Brain Development: Methodology and Application View all 4 articles
A correction has been applied to this article in:
Corrigendum: Alterations of cerebral perfusion and functional connectivity in children with idiopathic generalized epilepsy
 Guiqin Chen†
Guiqin Chen† Jie Hu†
Jie Hu† Haifeng Ran
Haifeng Ran Lei Nie
Lei Nie Wenying Tang
Wenying Tang Xuhong Li
Xuhong Li Qinhui Li
Qinhui Li Yulun He
Yulun He Junwei Liu
Junwei Liu Ganjun Song
Ganjun Song Gaoqiang Xu
Gaoqiang Xu Heng Liu*
Heng Liu* Tijiang Zhang*
Tijiang Zhang*Background: Studies have demonstrated that adults with idiopathic generalized epilepsy (IGE) have functional abnormalities; however, the neuropathological pathogenesis differs between adults and children. This study aimed to explore alterations in the cerebral blood flow (CBF) and functional connectivity (FC) to comprehensively elucidate the neuropathological mechanisms of IGE in children.
Methods: We obtained arterial spin labeling (ASL) and resting state functional magnetic resonance imaging data of 28 children with IGE and 35 matched controls. We used ASL to determine differential CBF regions in children with IGE. A seed-based whole-brain FC analysis was performed for regions with significant CBF changes. The mean CBF and FC of brain areas with significant group differences was extracted, then its correlation with clinical variables in IGE group was analyzed by using Pearson correlation analysis.
Results: Compared to controls, children with IGE had CBF abnormalities that were mainly observed in the right middle temporal gyrus, right middle occipital gyrus (MOG), right superior frontal gyrus (SFG), left inferior frontal gyrus (IFG), and triangular part of the left IFG (IFGtriang). We observed that the FC between the left IFGtriang and calcarine fissure (CAL) and that between the right MOG and bilateral CAL were decreased in children with IGE. The CBF in the right SFG was correlated with the age at IGE onset. FC in the left IFGtriang and left CAL was correlated with the IGE duration.
Conclusion: This study found that CBF and FC were altered simultaneously in the left IFGtriang and right MOG of children with IGE. The combination of CBF and FC may provide additional information and insight regarding the pathophysiology of IGE from neuronal and vascular integration perspectives.
Epilepsy is a chronic disorder of the nervous system disease caused by excessive brain neuronal firing or abnormal synchronous activity that causes brain dysfunction (Falco-Walter et al., 2018). It affects more than 70 million people globally (Thijs et al., 2019). According to the 2017 seizure classification of the International League Against Epilepsy, epilepsy is divided into focal epilepsy, generalized epilepsy, and epilepsy of unknown cause. Idiopathic generalized epilepsy (IGE) refers to an epilepsy syndrome involving genetic or speculated genetic defects (Scheffer et al., 2017). Children comprise approximately 0.5 to 1% of all IGE patients (Aaberg et al., 2017). Furthermore, it has been observed that the impact of IGE on the patient’s life is more pronounced at younger ages. This may lead to abnormal brain functions in children and could place their lives at risk. The etiology, pathogenesis, clinical treatment, and prognosis of epilepsy for children differ from those for adults because the brains of children are still developing. Compared to adults, children are more likely to have epilepsy more frequently, and it is difficult to locate the epileptic foci in children. Repeated seizures can cause changes in and reconstruction of the brain functions of children, leading to cognitive dysfunction and behavioral abnormalities (Holmes, 2016; Stirling et al., 2021). Recently, neuroimaging studies, especially those that used functional magnetic resonance imaging (fMRI), including arterial spin labeling (ASL) and resting state fMRI (rs-fMRI), have become increasingly popular.
Arterial spin labeling perfusion imaging is a non-invasive MRI technique for measuring CBF without using contrast agents (Ho, 2018). Because it does not require the injection of contrast agents, it has the advantages of easy access, no radiation, short scanning times, and low costs. Recently, ASL has been used widely for the study of neurological disorders, such as epilepsy (Schertz et al., 2020), Alzheimer’s disease (Iturria-Medina et al., 2016; Benedictus et al., 2017), Parkinson’s disease (Lin et al., 2017), and preoperative brain tumor grading (Shen et al., 2016; Ningning et al., 2017). Furthermore, ASL is capable of explaining the neural basis of IGE and enabling early diagnosis. Some previous studies based on ASL have shown that IGE patients have insufficient cerebral perfusion, mainly in the thalamus, superior middle brain, and left cerebellum (Sone et al., 2017). Other studies have shown increased cerebral perfusion mainly in the left parahippocampal gyrus, left middle temporal gyrus, and left fusiform gyrus (Chen et al., 2016). However, because of differences in image acquisition, samples, and analysis methods, detailed results across studies have been inconsistent.
With rs-fMRI, changes in blood oxygen level-dependent (BOLD) signals are detected. These can be used to study the spontaneous neural activity of the brain in the resting state and to evaluate the FC and networks of various brain regions. Furthermore, because rs-fMRI does not involve the need to perform specific tasks, participants can cooperate well. It has been used in the field of neuropsychiatry, where it is especially suitable for studying neuropsychiatric diseases of patients with cognitive and attentional disorders, or the inability to cooperate, including bipolar disorder (Bellani et al., 2020), psychosis (Collin et al., 2020), Alzheimer’s disease, and mild cognitive impairment (Ibrahim et al., 2021). Based on the region of interest (ROI) analysis method, Luo et al. (2011) studied the default mode network (DMN) of patients with absence epilepsy and observed that the functional connections of the frontal, parietal, and temporal lobes were significantly reduced. Kim et al. (2014) found a decrease in FC was found in both the precuneus cortex and medial prefrontal cortex of adult IGE patients. Based on the independent component analysis method, Li et al. (2015) showed FC changes in the dorsal attention network, salience network, and DMN in children with IGE-associated and dementia.
Based on these previous studies, CBF and FC are abnormal in IGE patients. However, the neurobiological mechanism of IGE in children is unclear, and there are no effective treatments available. Moreover, the correlation between CBF and internal brain function changes is unclear. Recently, Liang et al. (2013) combined ASL and rs-fMRI and found a close relationship between CBF and brain FC in the default network and executive control network of healthy adults. Tan et al. (2020) combined ASL and rs-fMRI and observed abnormal alterations in left amygdala perfusion and FC in patients with attention deficit/hyperactivity disorder; they also reported that neuronal and vascular perspectives of attention deficit/hyperactivity disorder will provide a deeper understanding of its pathophysiology. Joint ASL and rs-fMRI studies of patients with disorders of the nervous system, such as Alzheimer’s disease (Zheng et al., 2019), schizophrenia (Zhu et al., 2017), and depression (He et al., 2019), have been performed. Therefore, we speculated that areas showing CBF abnormalities and brain dysfunction in IGE patients may overlap. However, the simultaneous alteration of CBF and FC in IGE patients remains largely unknown. Therefore, this study aimed to explore cerebral CBF and FC in children with IGE by combining ASL with rs-fMRI and analyzing the correlations between CBF and FC changes and clinical variables to help elucidate the pathophysiological mechanism of IGE in children.
This study involved sixty-three subjects. There were 28 children with IGE and 35 healthy controls (HCs). The study protocol was approved and was performed in accordance with the recommendations of the Medical Research Ethics Committee of Zunyi Medical University’s Affiliated Hospital. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Children with IGE underwent a complete neuropsychological assessment using the Chinese version of the Wechsler Intelligence Scale for Children, including verbal, performance, and full-scale intelligence quotient (IQ) assessments, on the same day as the MRI scan.
Idiopathic generalized epilepsy patients were required to fulfill the following inclusion criteria: (I) diagnosis of IGE according to the 2010 International League Against Epilepsy diagnostic criteria (II) underwent a normal routine MRI examination of the brain (III) and age 6–16 years. The exclusion criteria were as follows: (I) other neuropsychiatric diseases, (II) history of drug abuse, (III) craniocerebral trauma or surgical history, (IV) intracranial organic lesions or serious artifacts; and (V) head motion exceeding ± 2 mm or ± 2°.
The following were inclusion criteria for the HCs: (I) underwent a normal routine MRI examination of the brain and (II) age 6–16 years. The exclusion criteria were as follows: (I) other neurological/psychiatric conditions, (II) history of drug abuse, (III) cognitive and memory disorders, and (IV) head motion exceeding ± 2 mm or ± 2.
A 3.0-T HDxt system (GE Healthcare, Milwaukee, WI, United States) was used to acquire all MRI results. ASL perfusion imaging was performed using pseudocontinuous labeling with three-dimensional fast spin-echo acquisition and background suppression. The following parameters were used: repetition time, 4,599 ms; echo time, 9.8 ms; post-label delay, 1,525 ms; spiral-in readout of eight arms with 512 sample points; field of view, 240 mm × 240 mm; slice number, 36; and slice thickness, 4 mm. The rs-fMRI data were acquired using an echo planar imaging sequence with the following parameters: repetition time, 2,000 ms; echo time, 30 ms; field of view, 240 mm × 240 mm; flip angle, 90°; slice number, 33; slice thickness, 4 mm; and volume, 210. The participants were instructed to keep their eyes closed, relax, remain still, think of nothing, and not fall asleep during the scanning process.
Preprocessing of the MRI data was performed with Data Processing Assistant for Resting-State Brain Imaging (DPARSF) (Chao-Gan and Yu-Feng, 2010). First, the first 10 parts of each participant’s recording were discarded, and the remaining parts were corrected for temporal differences and head motion. Next, functional data were realigned with echo planar imaging templates of the Montreal Neurological Institute (MNI). Standard spaces were spatially normalized using non-linear registration and then resampled to 3-mm isotropic voxels. Spatial smoothing was performed with a 6-mm full-width at half-maximum Gaussian kernel, followed by linear detrending of the time series. Thereafter, a regressing procedure was performed for the nuisance covariates derived from Friston’s 24 head movement estimates, white matter signals, and cerebrospinal fluid signals. Finally, functional images were temporally bandpass-filtered (0.01–0.1 Hz).
Cerebral blood flow images were obtained from the original ASL data using an MRI scanner post-processing workstation. We acquired CBF images and processed the data using SPM121. The CBF images of each patient’s structural space were transformed to the Montreal Neurological Institute standard space. Subsequently, we calculated a z-score from the spatially normalized CBF images of each participant by subtracting the mean and dividing by the standard deviation of all voxels across all subjects. Finally, all CBF images were smoothed using a 6-mm × 6-mm × 6-mm Gaussian kernel filter with full-width at half-maximum. Further statistical analyses using an independent two-sample t test were performed to compare the groups using age and sex as covariates. The significance threshold was set using the Gaussian random field (GFR) correction (P < 0.05). Based on Pearson’s correlation analysis, abnormal CBF was correlated with IQ, disease course, and age at onset. The significance level was set at P < 0.05.
A seed-based FC analysis was conducted. First, based on the CBF results, four regions with significant differences between groups were selected as masks and defined as ROIs during the FC analysis. Thereafter, using an independent two-sample t test with age and sex as covariates, we compared the two groups (IGE patients vs. HCs). The significance threshold was set using the GFR correction (P < 0.05, two-tailed). Finally, we extracted the abnormal FC of brain regions. Pearson’s correlation analysis was performed for brain regions with abnormal FC in children with IGE and IQ, disease course, and age at onset. The significance level was set at P < 0.05.
Demographic and clinical characteristics of the 28 children with IGE (14 females and 14 males; age, 11.71 ± 2.89 years) and 35 HCs (19 females and 16 males; age, 11.88 ± 2.48 years) are described in Table 1. There were no significant differences in sex (P = 0.958, P > 0.05, chi-square test), age (P = 0.801, P > 0.05, two-sample t-test), and education (P = 0.385, P > 0.05, two-sample t-test) between the IGE patients and HCs.
The spatial distribution map of CBF in the HCs and IGE patients are shown in Figure 1. Subtle differences were observed between these groups (IGE patients: 53.28 ± 8.09 ml/100 g/min; HCs: 53.85 ± 7.60 ml/100 g/min; two-sample t test; t = 0.29; P = 0.773) in the whole brain matter. Increased blood flow was detected mainly in the left inferior frontal gyrus (IFG), middle frontal gyrus, cingulate gyrus, bilateral middle temporal gyrus (MTG), thalamus, right middle frontal gyrus (MOG), and right medial prefrontal cortex.
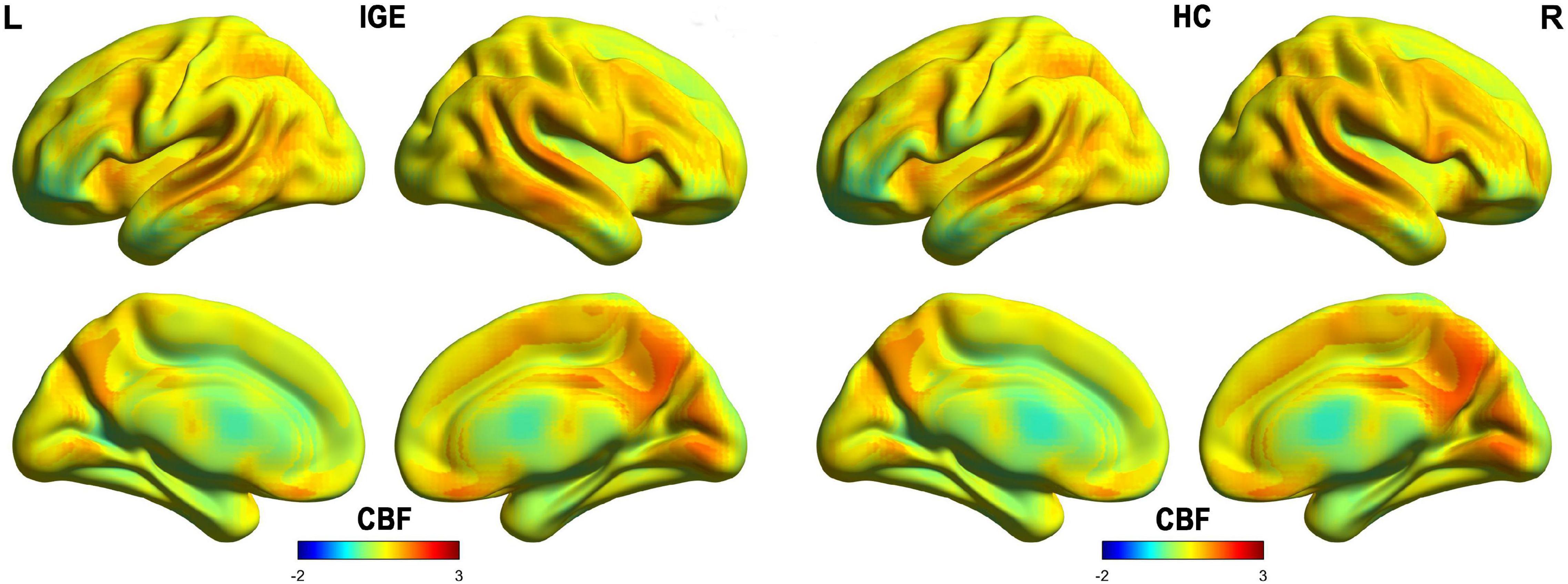
Figure 1. Spatial distribution maps of CBF at the group level. For each group (i.e., IGE or HC), the individual CBF maps were normalized to z-scores and then averaged across subjects to generate a group-level map. IGE, idiopathic generalized epilepsy; CBF, cerebral blood flow; HC, healthy controls.
According to the voxel-wise ASL analysis, decreased CBF in IGE patients was mainly present in the triangular part of the left IFG (IFGtriang) and increased CBF in IGE patients was mainly present in the right MTG, MOG, and superior FG (SFG) (Figure 2 and Table 2). By identifying the four brain regions, we were able to explore the whole brain FC of each region. We conducted two-sample t-tests to examine the differences in FC of the regions of interest in the IGE patients and HCs. Compared with the HCs, we found there was a significant decrease in connectivity in the left IFGtriang with the CAL (Figure 3), and that the FC between the right MOG and bilateral CAL was decreased in children with IGE (Figure 4).
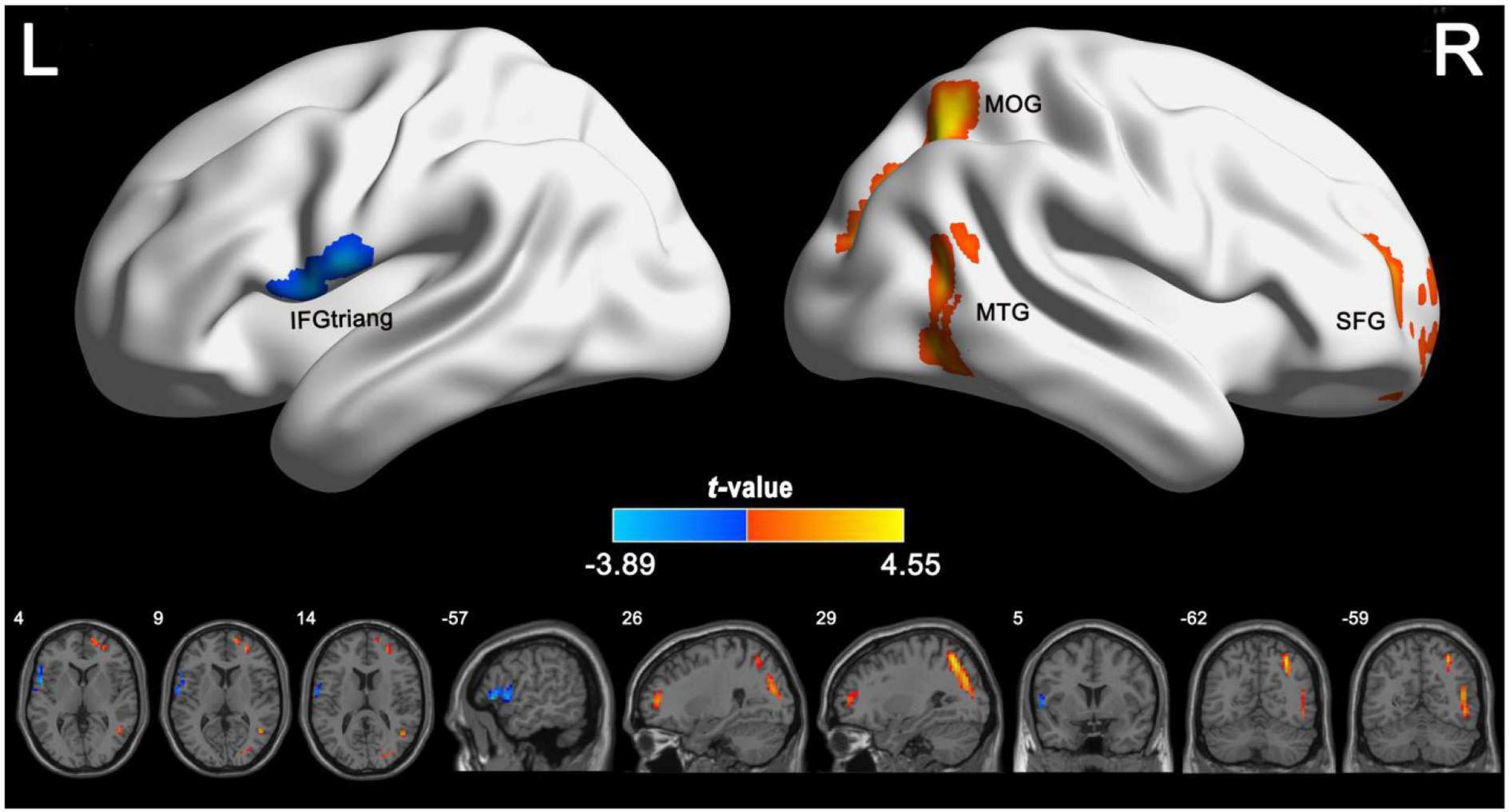
Figure 2. Group differences in CBF between IGE patients and healthy controls. The independent two-sample t test was conducted between the IGE group and the healthy control group. All results were corrected for multiple comparisons (GRF-corrected, P < 0.05). The cold colors denote significantly decreased CBF in the IGE patients. The warm colors denote significantly increased CBF in the IGE patients. CBF, cerebral blood flow; GRF, Gaussian random field; IGE, idiopathic generalized epilepsy.

Figure 3. Between-group comparisons for the seed-based functional connectivity with the seeds of left IFGtriang. The independent two-sample t test was conducted between the IGE group and HC. All results were corrected for multiple comparisons (GRF, P < 0.05). CBF, cerebral blood flow; HC, healthy controls; GRF, Gaussian random field; IGE, idiopathic generalized epilepsy. IFGtriang, inferior frontal gyrus, triangular part.
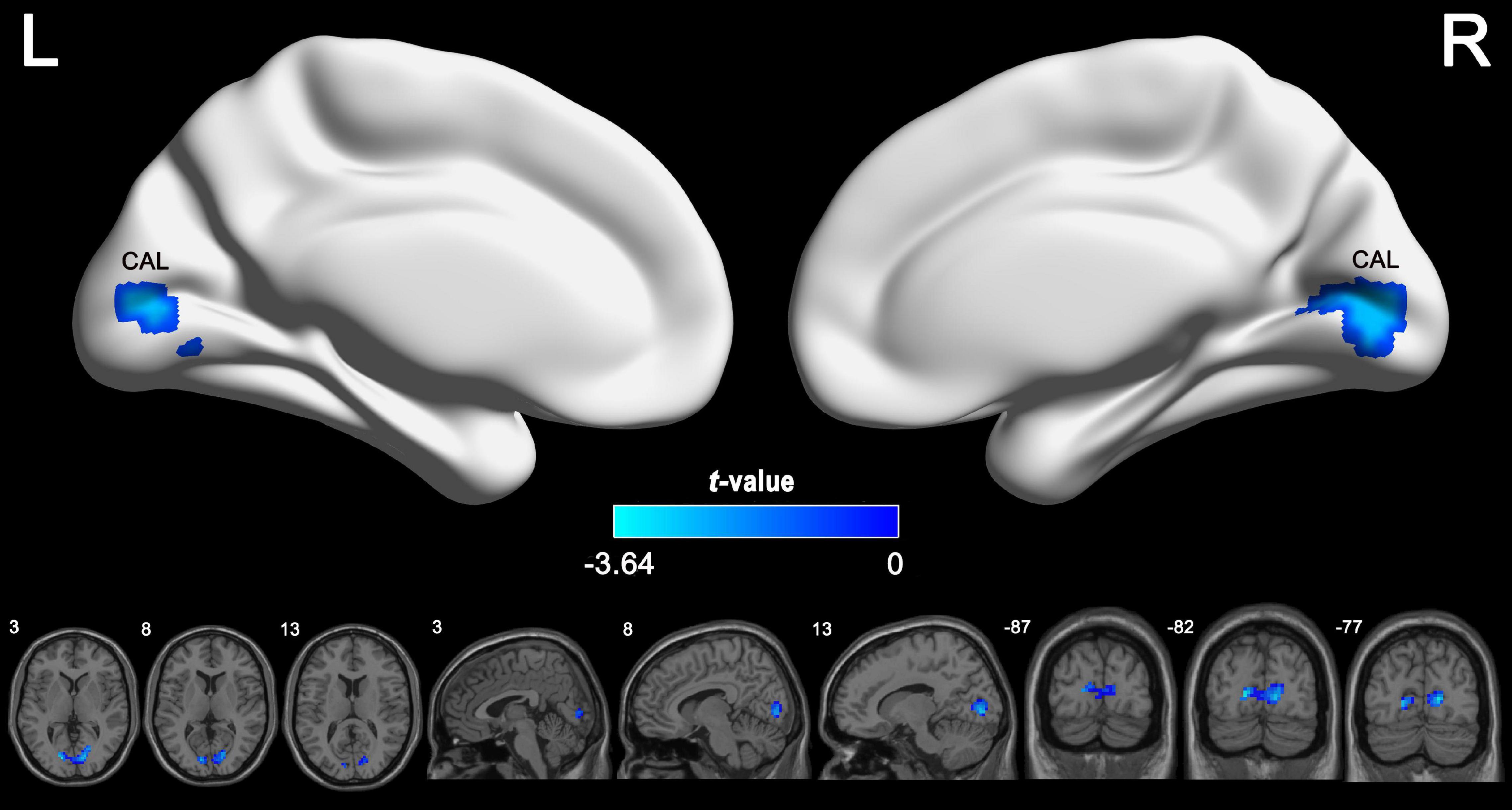
Figure 4. Between-group comparisons for the seed-based functional connectivity with the seeds of right MOG. The independent two-sample t test was conducted between the IGE group and HC. All results were corrected for multiple comparisons (GRF, P < 0.05). CBF, cerebral blood flow; HC, healthy controls; GRF, Gaussian random field; IGE, idiopathic generalized epilepsy; MOG, middle occipital gyrus.
Cerebral blood flow of the right SFG was negatively correlated with the age at onset in the IGE group (Figure 5). Additionally, FC of the left IFGtriang and ipsilateral CAL were negatively correlated with IGE duration (Figure 6). However, no significant correlations were observed between the intelligence scale and CBF or FC.
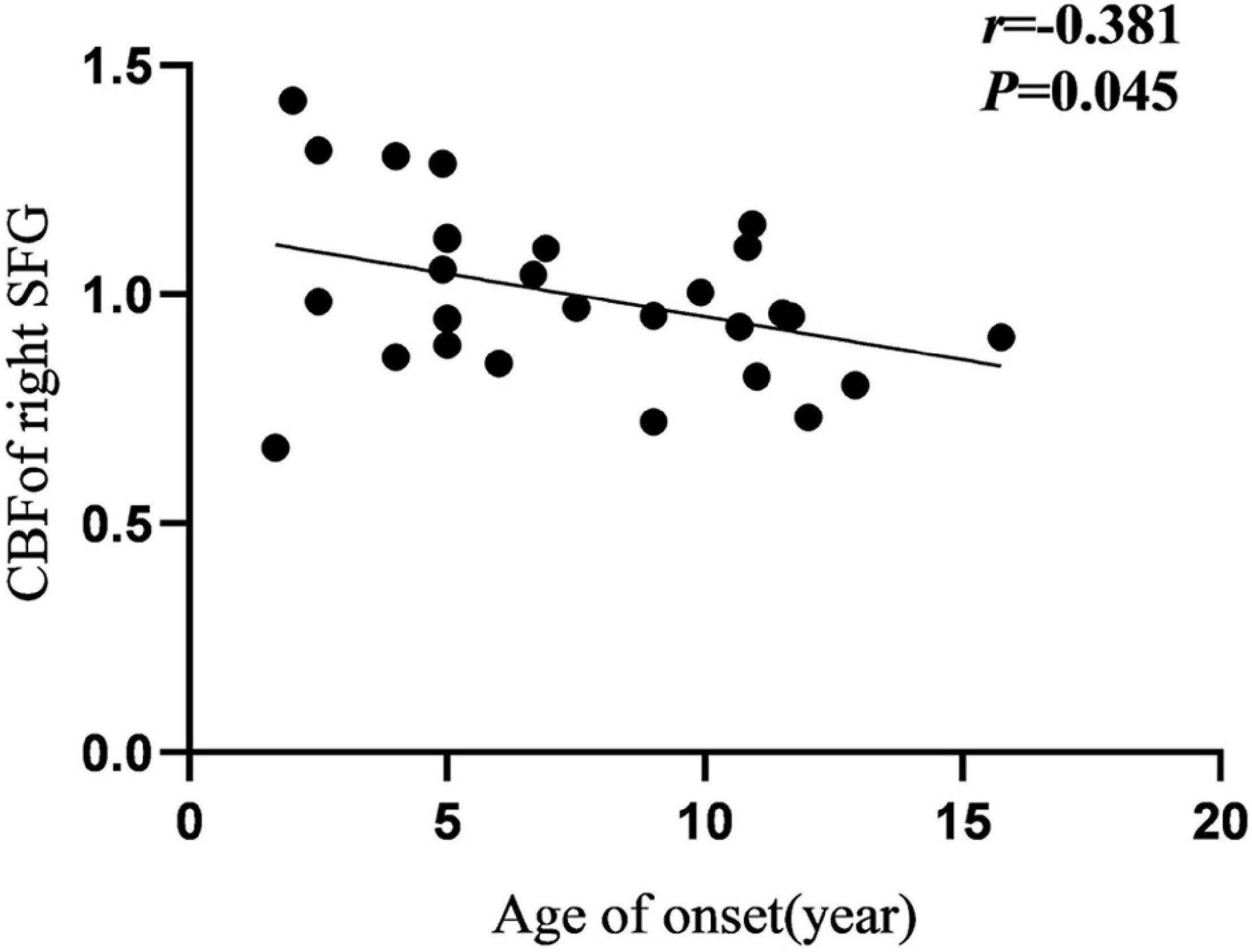
Figure 5. Pearson correlation between CBF of right SFG and age of onset in IGE patients. CBF, cerebral blood flow; SFG, superior frontal gyrus.
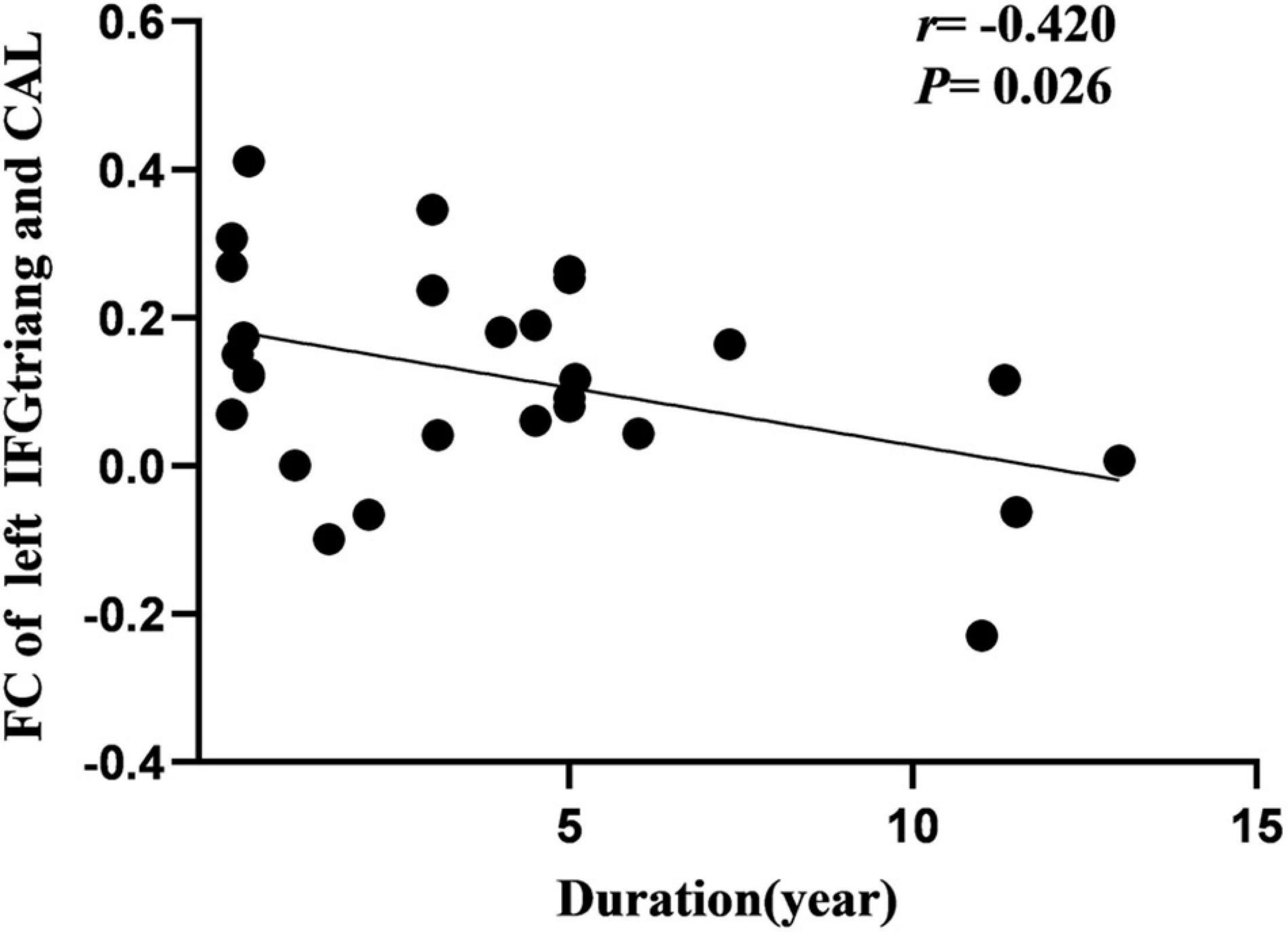
Figure 6. Pearson correlation between FC of left CAL and course of disease in IGE patients. FC, functional connectivity; CAL, calcarine fissure.
This study combined three-dimensional ASL perfusion with rs-fMRI to examine changes in CBF and potential FC disruptions in CBF-altered regions in children with IGE. Compared with HCs, children with IGE had significant abnormal perfusion in the right MOG and left IFGtriang. A seed-based FC analysis was used to evaluate the potential changes in these abnormal regions and found simultaneous decreases in the FC between the left IFGtriang and CAL and between the right MOG and bilateral CAL, which could help comprehensively elucidate the neuropathological mechanism of IGE in children.
Previous studies have consistently reported significant abnormal perfusion in the frontal, temporal, and occipital lobes of IGE patients. The regions showing perfusion abnormalities in the frontotemporal lobe also showed structural and functional abnormalities similar to those reported by other MRI studies (Ke et al., 2017; Deng et al., 2019; Li et al., 2020). In this study, hypoperfusion was found in the left IFGtriang. This is partially consistent with the findings reported by Joo et al. (2006), who used single-photon emission computed tomography to study CBF in the IGE group and observed that multiple brain regions, including the IFG, showed low perfusion. Moreover, we observed reduced CBF in the frontal lobe, indicating dysfunction of the nerves and blood vessels in that area. Pathological processes with IGE can be linked to neurovascular dysfunction, especially CBF abnormalities. More specifically, the stability of neuronal cells is reduced in patients with epilepsy. When epilepsy occurs, brain ischemia and hypoxia are aggravated, resulting in failure of the sodium pump. An increase in Na+ influx leads to depolarization, causing vascular neuronal damage to a certain extent. In particular, when the endothelial function of the local capillaries is damaged, local CBF is reduced (Barth and Mody, 2011; Gooshe et al., 2015; Tewolde et al., 2015). Therefore, we can speculate that the IFGtriang is vascularly dysfunctional, and that decreases in CBF lead to the destruction of the vascular barrier, glial hyperplasia, and inflammation and induce excessive excitement of neuronal networks, thereby promoting epilepsy and leading to reduced perfusion. Combined with a neurobiological analysis, this speculation may help explain CBF abnormalities in children with IGE more comprehensively.
There have been reports of increased CBF during the postictal phase of typical absence seizures and the interictal phase of myoclonic epilepsy in adolescents (Nehlig et al., 2004; Tae et al., 2007). In another study, CBF increased in the left MTG, left parahippocampal gyrus, and left fusiform gyrus of IGE patients, and a negative correlation between age at onset and prolongation of arterial transit time of the left superior temporal gyrus was observed. That study showed that IGE patients may have cortical dysfunction in the temporal lobe and fusiform gyrus, which may be associated with epileptic activity (Chen et al., 2016). During our study, children with IGE had higher regional CBF in the right MTG and SFG regions, which is highly consistent with previously reported findings (Tae et al., 2007; Chen et al., 2016). Most epilepsy occurs in the frontotemporal lobe, and microstructural changes in these regions have been observed in IGE patients (Betting et al., 2010; Zhang et al., 2016). Another study revealed that epileptiform discharges in patients with juvenile myoclonic epilepsy are not generalized with bilateral synchronous diffusion onset. Additionally, during propagation, discharges have localized onset and a restricted cortical network including regions of the frontal cortex and temporal cortex (Holmes et al., 2010). Previous studies demonstrated that the involvement of leukocyte-endothelial interactions in epilepsy is inextricably linked to hyperperfusion-induced seizures (Fabene et al., 2008; Storti et al., 2014). Our results and the aforementioned evidence show that IGE patients have a pattern of high perfusion, and that abnormal CBF perfusion in the MTG and SFG may be associated with epileptiform activity in IGE patients.
The MOG is the visual sensory cortex, which is mainly responsible for processing visual information (Müller and Kleinschmidt, 2003). We observed an increase in CBF in the right MOG and speculated that this may be related to visual abnormalities before the attack, such as blurred vision and deformed visual changes, or to damage to the visual cortex, indicating abnormalities in the occipital network involved in the neurobiological mechanism of IGE, in some patients. However, this increase in CBF in the right MOG was inconsistent with previous results and may be related to the different sample sizes, IGE subtypes, and medications used during this study.
During the seed-based FC analysis, we found a significant decrease in the FC in the left IFGtriang with the CAL, and the FC between the right MOG and bilateral CAL was decreased in children with IGE.
Previous studies have reported abnormal FC of the frontal lobe with other regions, such as the thalamus, posterior cingulate gyrus, and orbitofrontal cortex in IGE patients (Hamandi et al., 2006; Bai et al., 2011; Kim et al., 2014). During our study, we found that the FC between the left IFGtriang and ipsilateral CAL was reduced. The IFGtriang area is a part of the prefrontal cortex that has a key role in executive functions such as attention and executive ability. The CAL is involved in visual information processing, and we speculated that cerebral perfusion of the frontal lobe was closely related to the FC of the ipsilateral CAL, suggesting that children with IGE might have visual impairment (i.e., abnormal frontal perfusion in children with IGE might be accompanied by abnormal visual function).
The MOG and CAL are important components of the visual sensory cortex and visual network, and they have a critical role in the processing of visual information. Salinas and Szabó (2015) observed that FC between the occipital cortex and multiple brain areas of the epilepsy network in the IGE group was significantly abnormal. Similarly, Szabó et al. (2007) observed that photosensitive baboons with IGE had frequent discharges in the parietal cortex and occipital cortex during seizures and interictal periods. Combined with the results of this study, the decrease in FC between the occipital lobe and CAL in children with IGE may be related to the photosensitivity (Ákos Szabó et al., 2016) of some patients with epilepsy, suggesting the presence of FC abnormality in the visual cortex network, which may be a potential cause of impaired visual function.
During our study, we found that areas with perfusion abnormalities in children with IGE had alterations in FC. The BOLD signal depends on the concentration of deoxyhemoglobin in the blood and is regulated by changes in the CBF and cerebral blood volume (Driver et al., 2017) and abnormal perfusion changes in the concentration of local deoxyhemoglobin in the brain, leading to changes in BOLD signal intensity and whole brain FC. Therefore, abnormal perfusion with IGE may lead to changes in brain FC.
During this study, we observed that CBF in the right SFG was negatively correlated with the age at IGE onset, signifying that a younger age at onset is associated with a greater CBF increase. The frontal lobe is the advanced center of brain development and embodiment of human wisdom. It is closely related to attention, cognition, and executive control function. Therefore, it can be proposed that CBF changes in the SFG may hurt the ability of the region to recruit when responding to task demands, and thus may cause impaired cognitive functioning and attention disturbances. Furthermore, between abnormal FC of the left IFGtriang and CAL was negatively correlated with IGE duration. The frontal lobe and CAL have important roles in cognitive and visual processing. Previous studies have found that changes in the prefrontal function correlate well with disease duration. Kay et al. (2013) performed fMRI studies of IGE and observed that there was a decrease in FC between the frontal lobe and posterior cingulate gyrus, and that FC was negatively correlated with disease duration. Using rs-fMRI, McGill (McGill et al., 2012) observed that the FC of the prefrontal cortex of the DMN was significantly negatively correlated with the duration of epilepsy, suggesting that DMN connectivity decreased with the prolonged duration of epilepsy. During this study, the FC between the IFGtriang and CAL decreased and was negatively correlated with the duration of the disease, suggesting that FC changes between the IFGtriang and CAL may indicate IGE disease progression, thus reflecting the chronic damage to the brain function network caused by epilepsy and the development of the brain functional networks in children with IGE.
There were some limitations to this study. First, intelligence of the HCs was not evaluated; however, they attended normal primary and secondary schools. The performance of the HCs during the MRI scanning sessions and at school was to be normal. Second, there were no data regarding the IGE subtypes. Third, previous studies showed that antiepileptic drugs may affect the results of functional MRI. Jansen et al. (2006) reported that the activation of language-mediated regions in the prefrontal cortex of epilepsy patients treated with topiramate was significantly lower than that of the untreated group. Moeller et al. (2008) found no signal changes in the striatum-thalamus-cortex in unmedicated children with absence epilepsy; however, signal changes in that region were found in the medication group. The children with IGE in this study were treated with antiepileptic drugs. Unfortunately, we did not collect enough data to investigate the effects of these drugs on IGE neural activity. Therefore, the findings of this study need to be interpreted carefully. In the future, we will attempt to design a study with stricter criteria and data about IGE subtypes and drugs to perform further analyses.
We observed simultaneous changes in the CBF and FC in the left IFGtriang and right MOG in children with IGE. The combination of ASL and rs-fMRI and the integration of neuronal and vascular information can add to our understanding of the pathophysiology of children with IGE.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Affiliated Hospital of Zunyi Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
GC and JH contributed to the conception, design, and radiological expertise, helped to select and assess cases, and drafted manuscript. JL, GS, and GX contributed radiological expertise and carried out the statistical analysis and designed the study. HR and LN contributed to the drafting and revision of the manuscript. WT, QL, XL, and YH offered data collection. TZ and HL reviewed and critiqued the manuscript and approved the final manuscript. All authors have read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82171919 and 81960312).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aaberg, K. M., Gunnes, N., Bakken, I. J., Lund Søraas, C., Berntsen, A., Magnus, P., et al. (2017). Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics 139:e20163908. doi: 10.1542/peds.2016-3908
Ákos Szabó, C., Salinas, F. S., Li, K., Franklin, C., Leland, M. M., Fox, P. T., et al. (2016). Modeling the effective connectivity of the visual network in healthy and photosensitive, epileptic baboons. Brain Struct. Funct. 221, 2023–2033. doi: 10.1007/s00429-015-1022-y
Bai, X., Guo, J., Killory, B., Vestal, M., Berman, R., Negishi, M., et al. (2011). Resting functional connectivity between the hemispheres in childhood absence epilepsy. Neurology 76, 1960–1967. doi: 10.1212/WNL.0b013e31821e54de
Barth, A. M., and Mody, I. (2011). Changes in hippocampal neuronal activity during and after unilateral selective hippocampal ischemia in vivo. J. Neurosci. 31, 851–860. doi: 10.1523/JNEUROSCI.5080-10.2011
Bellani, M., Bontempi, P., Zovetti, N., Gloria Rossetti, M., Perlini, C., Dusi, N., et al. (2020). Resting state networks activity in euthymic bipolar disorder. Bipolar Disord. 22, 593–601. doi: 10.1111/bdi.12900
Benedictus, M. R., Leeuwis, A. E., Binnewijzend, M. A., Kuijer, J. P., Scheltens, P., Barkhof, F., et al. (2017). Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur. Radiol. 27, 1169–1175.
Betting, L. E., Li, L. M., Lopes-Cendes, I., Guerreiro, M. M., Guerreiro, C. A., and Cendes, F. (2010). Correlation between quantitative EEG and MRI in idiopathic generalized epilepsy. Hum. Brain Mapp. 31, 1327–1338. doi: 10.1002/hbm.20944
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: A MATLAB toolbox for “Pipeline”. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Chen, G., Lei, D., Ren, J., Zuo, P., Suo, X., Wang, D. J., et al. (2016). Patterns of postictal cerebral perfusion in idiopathic generalized epilepsy: a multi-delay multi-parametric arterial spin labelling perfusion MRI study. Sci. Rep. 6:28867. doi: 10.1038/srep28867
Collin, G., Nieto-Castanon, A., Shenton, M. E., Pasternak, O., Kelly, S., Keshavan, M. S., et al. (2020). Brain functional connectivity data enhance prediction of clinical outcome in youth at risk for psychosis. Neuroimage Clin. 26:102108. doi: 10.1016/j.nicl.2019.102108
Deng, D. M., Chen, L. Z., Li, Y. W., Long, R., Tang, G. C., Han, F. G., et al. (2019). Cortical morphologic changes in recent-onset, drug-naïve idiopathic generalized epilepsy. Magn. Reson. Imaging 61, 137–142. doi: 10.1016/j.mri.2019.05.035
Driver, I. D., Wise, R. G., and Murphy, K. (2017). Graded Hypercapnia-Calibrated BOLD: Beyond the Iso-metabolic Hypercapnic Assumption. Front. Neurosci. 11:276. doi: 10.3389/fnins.2017.00276
Fabene, P. F., Navarro Mora, G., Martinello, M., Rossi, B., Merigo, F., Ottoboni, L., et al. (2008). A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 14, 1377–1383. doi: 10.1038/nm.1878
Falco-Walter, J. J., Scheffer, I. E., and Fisher, R. S. (2018). The new definition and classification of seizures and epilepsy. Epilepsy Res. 139, 73–79. doi: 10.1016/j.eplepsyres.2017.11.015
Gooshe, M., Abdolghaffari, A. H., Aleyasin, A. R., Chabouk, L., Tofigh, S., Hassanzadeh, G. R., et al. (2015). Hypoxia/ischemia a key player in early post stroke seizures: modulation by opioidergic and nitrergic systems. Eur. J. Pharmacol. 746, 6–13. doi: 10.1016/j.ejphar.2014.11.005
Hamandi, K., Salek-Haddadi, A., Laufs, H., Liston, A., Friston, K., Fish, D. R., et al. (2006). EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 31, 1700–1710. doi: 10.1016/j.neuroimage.2006.02.016
He, Z., Sheng, W., Lu, F., Long, Z., Han, S., Pang, Y., et al. (2019). Altered resting-state cerebral blood flow and functional connectivity of striatum in bipolar disorder and major depressive disorder. Prog Neuropsychopharmacol. Biol. Psychiatry 90, 177–185. doi: 10.1016/j.pnpbp.2018.11.009
Ho, M. L. (2018). Arterial spin labeling: clinical applications. J. Neuroradiol. 45, 276–289. doi: 10.1016/j.neurad.2018.06.003
Holmes, G. L. (2016). Effect of seizures on the developing brain and cognition. Semin. Pediatr. Neurol. 23, 120–126. doi: 10.1016/j.spen.2016.05.001
Holmes, M. D., Quiring, J., and Tucker, D. M. (2010). Evidence that juvenile myoclonic epilepsy is a disorder of frontotemporal corticothalamic networks. Neuroimage. 49, 80–93. doi: 10.1016/j.neuroimage.2009.08.004
Ibrahim, B., Suppiah, S., Ibrahim, N., Mohamad, M., Hassan, H. A., Nasser, N. S., et al. (2021). Diagnostic power of resting-state fMRI for detection of network connectivity in Alzheimer’s disease and mild cognitive impairment: a systematic review. Hum. Brain Mapp. 42, 2941–2968. doi: 10.1002/hbm.25369
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M., and Evans, A. C. (2016). Alzheimer’s disease neuroimaging initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7:11934. doi: 10.1038/ncomms11934
Jansen, J. F., Aldenkamp, A. P., Marian Majoie, H. J., Reijs, R. P., de Krom, M. C., Hofman, P. A., et al. (2006). Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav. 9, 181–185. doi: 10.1016/j.yebeh.2006.05.004
Joo, E. Y., Hong, S. B., Tae, W. S., Han, S. J., Seo, D. W., Lee, K. H., et al. (2006). Effect of lamotrigine on cerebral blood flow in patients with idiopathic generalised epilepsy. Eur. J. Nucl. Med. Mol. Imaging 33, 724–729. doi: 10.1007/s00259-005-0029-7
Kay, B. P., DiFrancesco, M. W., Privitera, M. D., Gotman, J., Holland, S. K., and Szaflarski, J. P. (2013). Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia 54, 461–470. doi: 10.1111/epi.12057
Ke, M., Jin, B., Liu, G., and Yang, X. (2017). Impairments of cingulated cortex in the generalized tonic-clonic seizure epilepsy by combining morphological and functional connectivity magnetic resonance imaging. J. Integr. Neurosci. 16, 429–439. doi: 10.3233/JIN-170026
Kim, J. B., Suh, S. I., Seo, W. K., Oh, K., Koh, S. B., and Kim, J. H. (2014). Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia 55, 592–600. doi: 10.1111/epi.12580
Li, Q., Cao, W., Liao, X., Chen, Z., Yang, T., Gong, Q., et al. (2015). Altered resting state functional network connectivity in children absence epilepsy. J. Neurol. Sci. 354, 79–85. doi: 10.1016/j.jns.2015.04.054
Li, Y., Chen, Q., and Huang, W. (2020). Disrupted topological properties of functional networks in epileptic children with generalized tonic-clonic seizures. Brain Behav. 10:e01890. doi: 10.1002/brb3.1890
Liang, X., Zou, Q., He, Y., and Yang, Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. U.S.A. 110, 1929–1934. doi: 10.1073/pnas.1214900110
Lin, W. C., Chen, P. C., Huang, C. C., Tsai, N. W., Chen, H. L., Wang, H. C., et al. (2017). Autonomic function impairment and brain perfusion deficit in Parkinson’s disease. Front. Neurol. 8:246. doi: 10.3389/fneur
Luo, C., Li, Q., Lai, Y., Xia, Y., Qin, Y., Liao, W., et al. (2011). Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum. Brain Mapp. 32, 438–449. doi: 10.1002/hbm.21034
McGill, M. L., Devinsky, O., Kelly, C., Milham, M., Castellanos, F. X., Quinn, B. T., et al. (2012). Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav. 23, 353–359. doi: 10.1016/j.yebeh.2012.01.013
Moeller, F., Siebner, H. R., Wolff, S., Muhle, H., Granert, O., Jansen, O., et al. (2008). Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia 49, 1510–1519. doi: 10.1111/j.1528-1167.2008.01626.x
Müller, N. G., and Kleinschmidt, A. (2003). Dynamic interaction of object- and space-based attention in retinotopic visual areas. J. Neurosci. 23, 9812–9816. doi: 10.1523/JNEUROSCI
Nehlig, A., Valenti, M. P., Thiriaux, A., Hirsch, E., Marescaux, C., and Namer, I. J. (2004). Ictal and interictal perfusion variations measured by SISCOM analysis in typical childhood absence seizures. Epileptic Disord. 6, 247–253.
Ningning, D., Haopeng, P., Xuefei, D., Wenna, C., Yan, R., Jingsong, W., et al. (2017). Perfusion imaging of brain gliomas using arterial spin labeling: correlation with histopathological vascular density in MRI-guided biopsies. Neuroradiology 59, 51–59. doi: 10.1007/s00234-016-1756-0
Salinas, F. S., and Szabó, C. Á (2015). Resting-state functional connectivity in the baboon model of genetic generalized epilepsy. Epilepsia 56, 1580–1589. doi: 10.1111/epi.13115
Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L., et al. (2017). ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 58, 512–521. doi: 10.1111/epi.13709
Schertz, M., Benzakoun, J., Pyatigorskaya, N., Belkacem, S., Sahli-Amor, M., Navarro, V., et al. (2020). Specificities of arterial spin labeling (ASL) abnormalities in acute seizure. J. Neuroradiol. 47, 20–26. doi: 10.1016/j.neurad.2018.11.003
Shen, N., Zhao, L., Jiang, J., Jiang, R., Su, C., Zhang, S., et al. (2016). Intravoxel incoherent motion diffusion-weighted imaging analysis of diffusion and microperfusion in grading gliomas and comparison with arterial spin labeling for evaluation of tumor perfusion. J. Magn. Reson. Imaging. 44, 620–632. doi: 10.1002/jmri.25191
Sone, D., Watanabe, M., Ota, M., Kimura, Y., Sugiyama, A., Maekawa, T., et al. (2017). Thalamic hypoperfusion and disrupted cerebral blood flow networks in idiopathic generalized epilepsy: arterial spin labeling and graph theoretical analysis. Epilepsy Res. 129, 95–100. doi: 10.1016/j.eplepsyres.2016.12.009
Stirling, R. E., Cook, M. J., Grayden, D. B., and Karoly, P. J. (2021). Seizure forecasting and cyclic control of seizures. Epilepsia 62(Suppl. 1), S2–S14. doi: 10.1111/epi.16541
Storti, S. F., Boscolo Galazzo, I., Del Felice, A., Pizzini, F. B., Arcaro, C., Formaggio, E., et al. (2014). Combining ESI, ASL and PET for quantitative assessment of drug-resistant focal epilepsy. Neuroimage 102 (Pt 1), 49–59. doi: 10.1016/j.neuroimage.2013.06.028
Szabó, C. A., Narayana, S., Kochunov, P. V., Franklin, C., Knape, K., Davis, M. D., et al. (2007). PET imaging in the photosensitive baboon: case-controlled study. Epilepsia 48, 245–253. doi: 10.1111/j.1528-1167.2006.00949.x
Tae, W. S., Joo, E. Y., Han, S. J., Lee, K. H., and Hong, S. B. (2007). CBF changes in drug naive juvenile myoclonic epilepsy patients. J. Neurol. 254, 1073–1080. doi: 10.1007/s00415-006-0491-6
Tan, Y. W., Liu, L., Wang, Y. F., Li, H. M., Pan, M. R., Zhao, M. J., et al. (2020). Alterations of cerebral perfusion and functional brain connectivity in medication-naïve male adults with attention-deficit/hyperactivity disorder. CNS Neurosci. Ther. 26, 197–206. doi: 10.1111/cns.13185
Tewolde, S., Oommen, K., Lie, D. Y., Zhang, Y., and Chyu, M. C. (2015). Epileptic seizure detection and prediction based on continuous cerebral blood flow monitoring–a review. J. Healthc. Eng. 6, 159–178. doi: 10.1260/2040-2295.6.2.159
Thijs, R. D., Surges, R., O’Brien, T. J., and Sander, J. W. (2019). Epilepsy in adults. Lancet 393, 689–701. doi: 10.1016/S0140-6736(18)32596-0
Zhang, Y., Gao, Y., Zhou, M., Wu, J., Zee, C., and Wang, D. A. (2016). Diffusional kurtosis imaging study of idiopathic generalized epilepsy with unilateral interictal epileptiform discharges in children. J. Neuroradiol. 43, 339–345. doi: 10.1016/j.neurad.2016.05.001
Zheng, W., Cui, B., Han, Y., Song, H., Li, K., He, Y., et al. (2019). Disrupted regional cerebral blood flow, functional activity and connectivity in Alzheimer’s disease: a combined ASL perfusion and resting state fMRI study. Front. Neurosci. 13:738. doi: 10.3389/fnins.2019.00738
Keywords: idiopathic generalized epilepsy, cerebral blood flow, functional connectivity, arterial spin labeling, resting state fMRI
Citation: Chen G, Hu J, Ran H, Nie L, Tang W, Li X, Li Q, He Y, Liu J, Song G, Xu G, Liu H and Zhang T (2022) Alterations of Cerebral Perfusion and Functional Connectivity in Children With Idiopathic Generalized Epilepsy. Front. Neurosci. 16:918513. doi: 10.3389/fnins.2022.918513
Received: 12 April 2022; Accepted: 25 May 2022;
Published: 13 June 2022.
Edited by:
Xu Yan, Siemens Healthineers, ChinaReviewed by:
Jiadi Xu, Kennedy Krieger Institute, United StatesCopyright © 2022 Chen, Hu, Ran, Nie, Tang, Li, Li, He, Liu, Song, Xu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tijiang Zhang, dGlqemhhbmdAMTYzLmNvbQ==; Heng Liu, em1jbGl1aEAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.