- 1Department of Radiology, Nanjing Integrated Traditional Chinese and Western Medicine Hospital Affiliated With Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 3College of Information Science and Technology, Nanjing Forestry University, Nanjing, China
- 4Department of Radiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Purpose: Whether the intrinsic functional connectivity pattern of the default mode network (DMN) is involved in the progression of cognitive decline in Parkinson’s disease (PD) remains unclear. This study aimed to investigate the intrinsic functional connectivity (FC) pattern of the DMN anchored on the posterior cingulate cortex (PCC) in patients with PD by resting-state functional magnetic resonance imaging (fMRI).
Methods: Fifty patients with PD and 50 healthy controls (HCs) were included for resting-state fMRI scanning. A seed-based FC method was used to reveal FC patterns in the DMN with region of interest (ROI) in the PCC. Relationships between FC patterns and disease severity (UPDRS-III) were detected.
Results: Compared with the HCs, the patients with PD showed increased FC between the PCC and the right precuneus, left cuneus, and right angular gyrus. In the PD group, the increased FC values in the right precuneus were significantly and positively correlated with motor severity as assessed with UPDRS-III scores (rho = 0.337, p = 0.02).
Conclusion: Our result highlights that the patients with PD showed increased FC between the PCC and the right precuneus, left cuneus, and right angular gyrus in the DMN. The altered connectivity pattern in the DMN may play a crucial role in the neurophysiological mechanism of cognitive decline in patients with PD. These findings might provide new insights into neural mechanisms of cognitive decline in PD.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by movement disturbances such as bradykinesia, resting tremor, rigidity, and postural instability (Nemade et al., 2021; Tolosa et al., 2021). According to a survey in Europe, the prevalence rate of PD is 65.6 per 100,000–12,500 per 100,000 (Bloem et al., 2021; Le Heron and Macaskill, 2021). PD is not only accompanied by motor disorder, but it also leads to cognitive decline. Ding et al. demonstrated that three cognitive impairments, namely, executive function deficits, memory deficits, and visuospatial deficits, are most commonly observed in patients with PD (Ding et al., 2015). Stav et al. (2015) reported that lower amyloid-β42 in cerebrospinal fluid biomarkers was a potential biomarker for cognitive decline functions in early PD, mainly connected to medial temporal lobe-based cognitive functions. Besides, patients with PD are at high risk for dementia (Hanagasi et al., 2017), and the etiology of the neurophysiological mechanism of this increased risk remains unclear.
Recent advances in functional magnetic resonance imaging (fMRI) approaches have provided a non-invasive method for characterization of central nervous system changes in some specific brain regions in PD (Mitchell et al., 2021), including the basal ganglia (Szewczyk-Krolikowski et al., 2014; Rolinski et al., 2015; Filyushkina et al., 2019), hippocampus (Zeng et al., 2017; Guo et al., 2021), cingulate gyrus (Zhang et al., 2017; Wang et al., 2021), and so on. Zhang et al. (2017) found that patients with PD and fatigue had amplitude of low-frequency fluctuation (ALFF) changes in the right middle frontal gyrus within the attention network and in the left insula as well as right middle cingulate gyrus within the salience network. Meanwhile, Hou et al. (2014) demonstrated that patients with PD had decreased ALFF in the striatum and increased ALFF in the midbrain in slow-4 (0.027–0.073 Hz) relative to HCs. Moreover, Zeng et al. (2017) demonstrated that patients with PD showed decrease in regional homogeneity (ReHo) in the sensorimotor cortex, default mode network, and left cerebellum but increased ReHo in the supplementary motor area, bilateral temporal gyrus, and hippocampus with disease progression. Furthermore, PD also leads to brain network dysfunction. Various neuroimaging studies demonstrated that basal ganglia network dysfunction played an important role in impaired motor function in patients with PD (Filyushkina et al., 2019). Rolinski et al. demonstrated that decreased connectivity in the basal ganglia was detected in patients with PD (Rolinski et al., 2015). Szewczyk-Krolikowski et al. (2014) reported that patients with PD were associated with decreased functional connectivity in the basal ganglia network relative to HCs. However, these findings mainly focused on the abnormal local neural activity and basal ganglia network in patients with PD. Few studies on neurocognitive network alterations in patients with PD, especially the default mode network (DMN), have been reported.
The human brain is a complex dynamic system capable of generating low-frequency oscillations (LFOs) at rest (Wise et al., 2004). Low-frequency fluctuations (< 0.01 Hz) in blood oxygenation level-dependent signals during rest reflect spontaneous neural activity, which can be conceptualized as a network of anatomically linked regions. The DMN is regarded as an endogenous neural network that shows consistently higher blood oxygenation level-dependent activity during rest. The DMN shows consistently higher BOLD activity during rest in several brain regions such as the middle temporal gyrus (MTG), medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), anterior cingulate cortex, and inferior parietal lobe (Greicius et al., 2003). The DMN plays an important role in various higher cognitive functions such as memory, prospection, and self-processing (Spreng and Grady, 2010). The PCC is a key region of the DMN with strong connectivity in primates with entorhinal cortex, parahippocampal gyrus, and, thus, hippocampal memory system, which is involved in autobiographical memory and imagining the future; and spatial navigation and scene processing (Leech and Sharp, 2014). Several prior studies have reported disrupted functional connectivity of the DMN in patients with PD by resting-state fMRI (Guo et al., 2021; Shang et al., 2021b; Wang et al., 2021) or fluorodeoxyglucose positron emission tomography (FDG-PET) (Ruppert et al., 2021; Schindlbeck et al., 2021; Shang et al., 2021a). However, how DMN dysfunction draws a relationship to cognitive symptoms in patients with PD still remains unknown.
To address this issue, we performed a voxel-level resting-state functional-connectivity neuroimaging analysis of the PCC with all other voxels in the brain between groups. This study aimed to determine whether patients with PD with impaired cognition exhibited an abnormal functional connectivity (FC) pattern of the DMN using a seed-based approach. Moreover, we investigated relationships between abnormal functional connectivity in the DMN and clinical variables in the PD group. Our findings might provide new insights into neural mechanisms of cognitive decline in PD.
Materials and Methods
Participants
Fifty patients with PD were enrolled from the Department of Neurology Affiliated to Nanjing First Hospital. The diagnostic criteria of PD are according to the clinical criteria of Movement Disorder Society (Postuma et al., 2015). For the PD group, disease severity and stage were evaluated using the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and Hoehn and Yahr (H-Y) scale, respectively. Interviews and clinical assessments were conducted structurally by two experienced neurologists.
The exclusion criteria for all the participants were as follows: (1) family history of PD, dementia, or hypertension; (2) complaint of cognitive impairment with MMSE score < 27 or MoCA score < 26; (3) additional neuropsychological disorders (e.g., Alzheimer’s disease, schizophrenia, depression, and epilepsy) or cerebrovascular accidents (e.g., stroke and intracranial hemorrhage); (4) any complications or lesions involving the central nervous system (e.g., diabetes mellitus, hyperthyroidism, tumor, and brain trauma); (5) history of alcoholism or substance abuse; any condition contraindicating MRI scanning (e.g., metal foreign body or implant, claustrophobia, and hyperpyrexia) or inducing severe head movement (e.g., hearing or visual loss); (6) years of education < 9. Fifty age- and gender-matched healthy controls (HCs) were also recruited for this study. Studies involving human participants are reviewed and approved according to the Declaration of Helsinki and by the institutional review board of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
All the patients with PD underwent a cognitive assessment, including global cognitive tests and an extensive neuropsychological test battery, to assess the neurocognitive state. The global cognitive assessments contained the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005).
Magnetic Resonance Imaging Acquisition
The subjects were scanned under resting conditions using a 3.0 Tesla MRI scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel receiver array head coil. During scanning, the subjects were supposed to lie quietly with their eyes closed and avoid head movement but not to fall asleep or think about anything special. To reduce head motion and scanner noise, foam pads and earplugs were used. Resting-state functional images were obtained axially using a gradient echo-planar imaging (EPI) sequence as follows: repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, slices = 33, thickness = 4 mm, gap = 0 mm, field of view (FOV) = 192 mm × 192 mm, acquisition matrix = 64 × 64, and flip angle (FA) = 90°. Recently, simultaneous multi-slice (SMS) imaging techniques have been used to highly accelerate the time of acquisition (Feinberg and Setsompop, 2013; Chen et al., 2015). Thus, SMS-accelerated EPI reconstruction was applied in this study. Structural images were obtained using a magnetization-prepared rapid gradient echo (MP2RAGE) sequence and following scan parameters: TR/TE = 5,000/2.98 ms, slices = 176, thickness = 1 mm, gap = 0 mm, FA = 90°, acquisition matrix = 256 × 256, and FOV = 256 mm × 256 mm.
Data Processing
The functional images were preprocessed using Statistical Parametric Mapping SPM121 and the toolbox for Data Processing Assistant and Analysis for Brain Imaging (DPABI)2 implemented in MATLAB 2013b (MathWorks, Natick, MA, United States) and in brief the following steps: (1) remove the first ten volumes function images, slice timing effects and head motion corrected; (2) Individual T1-weighted MP2RAGE structural images were registered to the mean fMRI data (Yan et al., 2016). Normalized data in Montreal Neurological Institute (MNI) 152 space were reliced at a resolution of 3 × 3 × 3mm3 and spatially smoothed with an 8-mm full width at half-maximum Gaussian kernel; (3) The linear regression to reduce the contribution of non-neuronal fluctuations; (4) Band-pass was filtered (0.01–0.08 Hz).
Definition of the Seed Region of Interest and Functional Connectivity Analysis
According to previous studies (Goto et al., 2013), the coordinate is the posterior cingulate cortex (PCC) (x = 0, y = −53, z = 26) and is 6 mm in diameter. For seed-based FC analysis, a correlation analysis of time course was performed between the spherical seed region (PCC) and each voxel of the whole brain for each subject using the DPABI software.
Statistical Analysis
The normality distribution of demographic characteristics and clinical assessments was checked using the Kolmogorov–Smirnov method. The intergroup difference of age was analyzed by using independent-sample t-test. The comparison of sex between PD and HCs was conducted by χ2-test. Mann–Whitney U test was applied for comparisons between groups in years of education and scores of clinical scales. SPSS software (version 20.0, SPSS Inc., Chicago, IL, United States) was utilized for the above statistical analyses. A p-value < 0.05 was defined as statistically significant.
One-sample t-test was conducted to assess patterns of DMN maps using the DPABI software. Two-sample t-test was conducted to assess different zFC maps between groups using the Gaussian random field (GFR) method, which was used to correct for multiple comparisons using the DPABI software (Two-tailed, voxel-level: p < 0.01, GRF correction, cluster-level: p < 0.05). Finally, Pearson correlation coefficients between altered zFC values and clinical variables were analyzed using SPSS.
Results
Demographics Measurements
The detailed demographic characteristics and clinical assessments are shown in Table 1. The patients with PD were included at the early stage (H-Y stage, mean 1.41 ± 0.45) with a relatively short disease duration (mean 6.32 ± 4.63 years). No significant differences in age, gender, and years of education were observed between the patients with PD and the HCs (p > 0.05). The PD group had lower MoCA scores than the HCs (p < 0.001).
Functional Connectivity Differences of the Default Mode Network
The Spatial Pattern of Default Mode Network Between Parkinson’s Disease Group and Healthy Controls Group
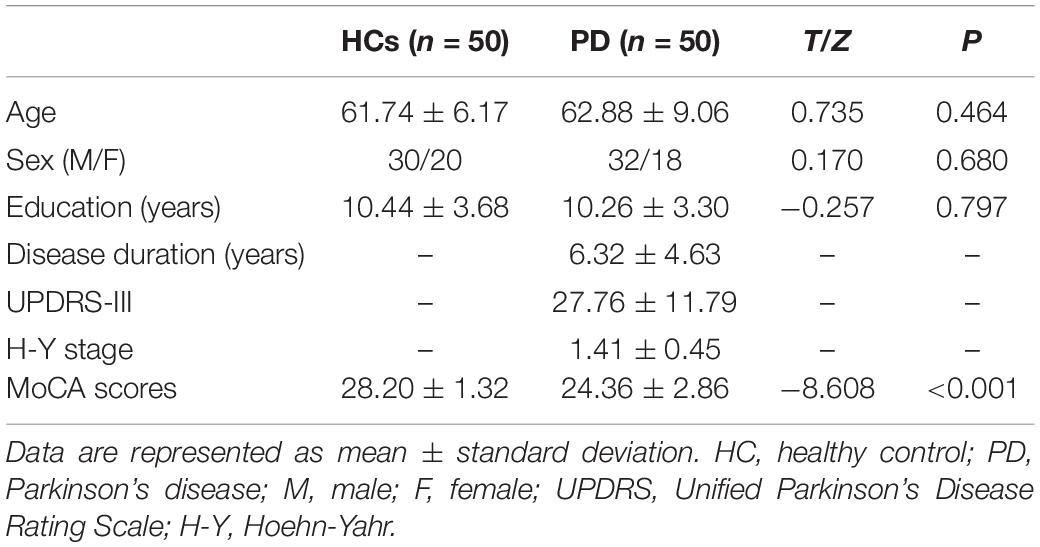
The PCC mainly showed positive FC in DMN regions, including the mPFC, inferior parietal lobule (IPL), and precuneus (Figure 1). Compared with the HCs, the patients with PD showed increased FC between the PCC and the right precuneus (PreCUN), left cuneus (CUN), and right angular gyrus (ANG) (Figure 2 and Table 2).
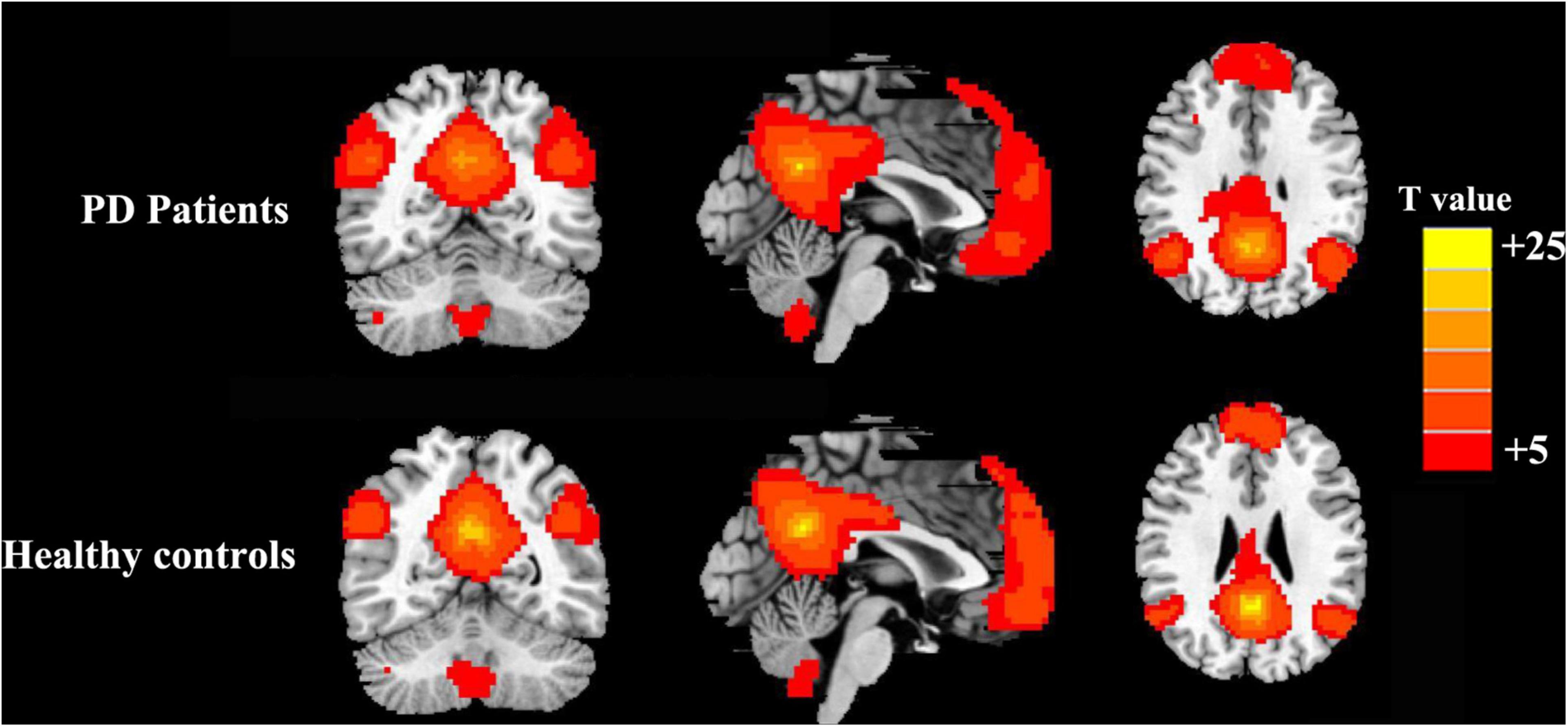
Figure 1. Results of the two components representing the default mode network (DMN) by one-sample t-test in patients with Parkinson’s disease (PD) and healthy controls.
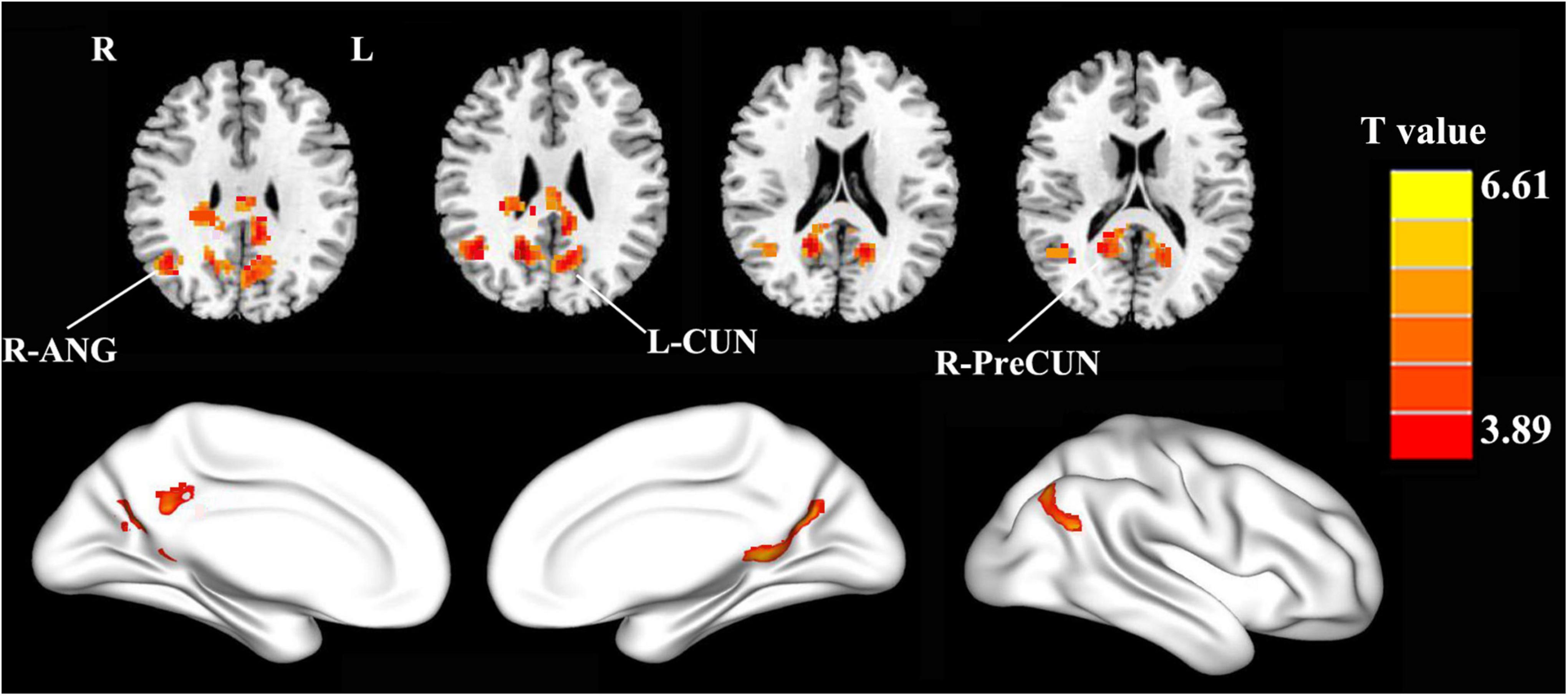
Figure 2. Compared with the healthy controls, the patients with PD exhibited enhanced FC between the PCC and the right precuneus (R-PreCUN), left cuneus (L-CUN), and right angular gyrus (R-ANG) (two-tailed, voxel-level: p < 0.01, GRF correction, cluster-level: p < 0.05).
Correlation Analysis
After the correlation analysis, in the PD group, the increased FC values in the right PreCUN were significantly positively correlated with motor severity as assessed with UPDRS-III scores (rho = 0.337, p = 0.02) (Figure 3A). Moreover, the enhanced FC values in the right ANG were also positively associated with the UPDRS-III scores (rho = 0.527, p < 0.001) (Figure 3B).
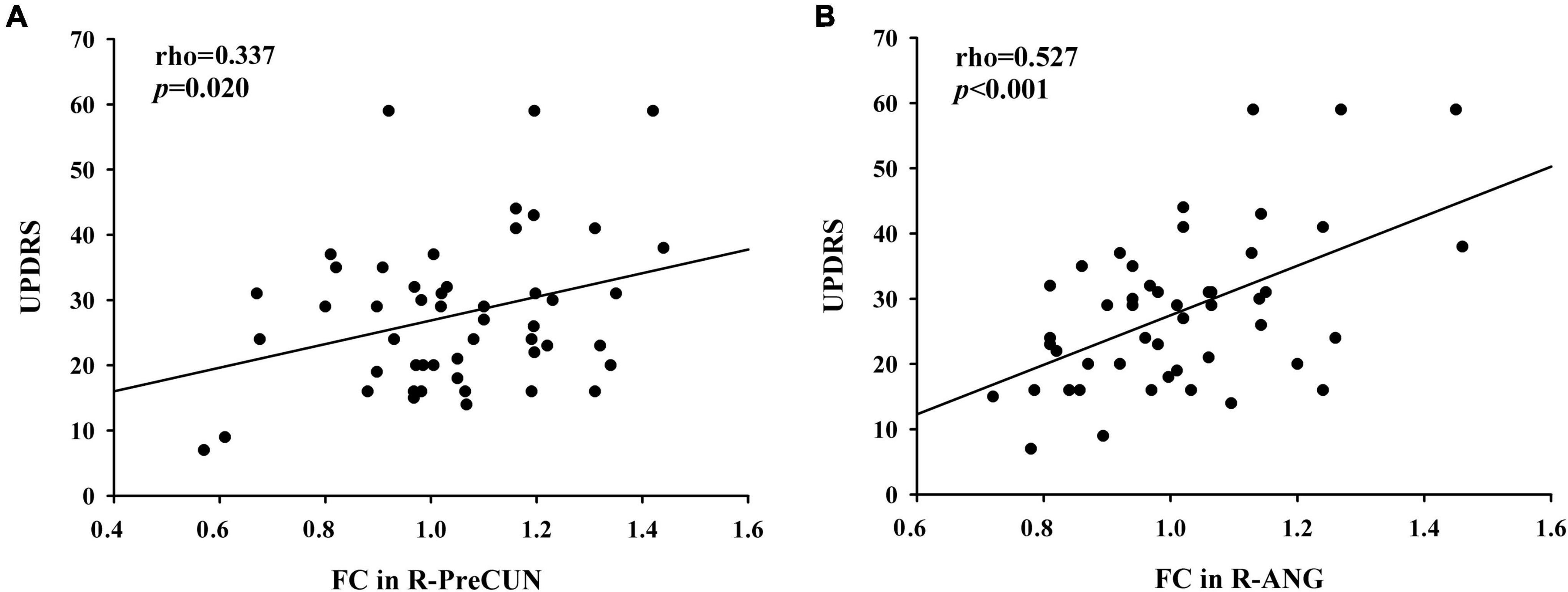
Figure 3. (A) Positive correlations between enhanced functional connectivity (FC) between the PCC and the right PreCUN and UPDRS-III scores in patients with PD (rho = 0.337, p = 0.02). (B) Moreover, the enhanced FC values in the right ANG were positively associated with the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) scores (rho = 0.527, p < 0.001).
Discussion
This study examining the DMN in patients with PD has generated several important results. The main findings were that the patients with PD showed increased FC between the PCC and the right PreCUN, left CUN, and right ANG relative to the HC group. Moreover, these abnormalities were significantly correlated with the disease severity of PD. In our study, we found that PD patients had higher FC between the PCC and the right PreCUN relative HC. The PreCUN is the core component of the DMN (Yu et al., 2017). The PreCUN plays an important role in self-centered mental imagery and episodic memory retrieval (Utevsky et al., 2014). Previous neuroimaging studies demonstrated that PreCUN dysfunction was observed in patients with PD, which is involved in memory deficits, attention and working memory deficits, and time perception deficits in patients with PD. The increased FC between the PCC and the PreCUN was also observed in other neurodegenerative diseases including Alzheimer’s disease (Churchill et al., 2021). In line with these findings, we speculated that enhanced FC between the PCC and the right PreCUN might indicate cognition dysfunction in patients with PD.
We also found that the patients with PD showed increased FC between the PCC and the right ANG relative to the HCs. Previous neuroimaging studies demonstrated that ANG plays an important role in semantic information processing (Boylan et al., 2015). Meanwhile, the ANG is also involved in mnemonic functions (Matchin et al., 2019). Moreover, the ANG is the core hub of the DMN (Ramanan et al., 2018). It was shown that lack of desynchronization of brain oscillatory activity in the PCC and right ANG disrupts the efficient processing in the fronto-parietal working memory network, leading to decline in visual working memory performance (Vatansever et al., 2017). Thus, we speculated that the increased FC between the PCC and the right ANG might indicate the working memory performance in patients with PD. Furthermore, in the PD group, the increased FC values in the right Pre CUN and right ANG were significantly positively correlated with the disease severity of PD. Li et al. observed that altered global synchronizations in DMN regions such as the IPL were correlated with UPDRS-III scores, which was similar with our current results (Li M. et al., 2019). The DMN is also thought to be associated with self-referential processing (Gusnard et al., 2001; Li W. et al., 2019). We hypothesized that higher global synchronizations of the DMN in PD may result in decreased ability to be self-referential, more likely to maintain the default mode state, and less control of interactions between brain regions. Therefore, increased FC in the DMN might be a potential biomarker for identifying neural mechanism dysfunction in patients with PD.
Several limitations must be acknowledged in our study. First, previous longitudinal data have indicated a dissociation of the DMN in PD, where reduced connectivity between the PCC and the mPFC is associated with future cognitive impairments (Zarifkar et al., 2021). However, our study is cross-sectional with a relatively small sample size. Thus, it is difficult to make direct causal inferences regarding the relationships between the aberrant FC and the disease severity of PD. Further longitudinal fMRI studies are required to establish the causal relationships and confirm the current findings. Second, our study lacked an assessment of various neurophysiological tests on patients with PD. Moreover, the choice of seed may bias connectivity findings toward specific, smaller, or overlapping sub-systems rather than larger distinct networks (Buckner et al., 2008). Some data-driven fMRI techniques, such as independent component analysis (ICA) or graph theory analysis, will be utilized in our future study. Finally, physiologic noise including respiratory, head motion, and cardiac fluctuations, might have compromised our results. These confounding factors should be taken into consideration in future studies.
Conclusion
This study highlights that patients with PD showed aberrant FC in the DMN. The enhanced connectivity pattern in the DMN may play a pivotal role in the neurophysiological mechanism of cognitive decline in patients with PD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LC and TH drafted the manuscript for the work. DM helped to acquire the clinical and fMRI data. Y-CC took care of the financial support, review, and final approval of the manuscript to be published. All authors have read and approved the final manuscript.
Funding
This study was supported by the 333 High-level Talents Training Project of Jiangsu Province (No. BRA2019122).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZX declared a past co-authorship with the author Y-CC to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Boylan, C., Trueswell, J. C., and Thompson-Schill, S. L. (2015). Compositionality and the angular gyrus: a multi-voxel similarity analysis of the semantic composition of nouns and verbs. Neuropsychologia 78, 130–141. doi: 10.1016/j.neuropsychologia.2015.10.007
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Chen, L. A. T. V., Xu, J., Moeller, S., Ugurbil, K., Yacoub, E., and Feinberg, D. A. (2015). Evaluation of highly accelerated simultaneous multi-slice EPI for fMRI. Neuroimage 104, 452–459. doi: 10.1016/j.neuroimage.2014.10.027
Churchill, N. W., Hutchison, M. G., Graham, S. J., and Schweizer, T. A. (2021). Long-term changes in the small-world organization of brain networks after concussion. Sci. Rep. 11:6862. doi: 10.1038/s41598-021-85811-4
Ding, W., Ding, L. J., Li, F. F., Han, Y., and Mu, L. (2015). Neurodegeneration and cognition in Parkinson’s disease: a review. Eur. Rev. Med. Pharmacol. Sci. 19, 2275–2281.
Feinberg, D. A., and Setsompop, K. (2013). Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 229, 90–100. doi: 10.1016/j.jmr.2013.02.002
Filyushkina, V., Popov, V., Medvednik, R., Ushakov, V., Batalov, A., Tomskiy, A., et al. (2019). Hyperactivity of basal ganglia in patients with parkinson’s disease during internally guided voluntary movements. Front. Neurol. 10:847. doi: 10.3389/fneur.2019.00847
Goto, M., Abe, O., Aoki, S., Hayashi, N., Miyati, T., Takao, H., et al. (2013). Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology 55, 869–875. doi: 10.1007/s00234-013-1193-2
Greicius, M. D., Krasnow, B., Reiss, A. L., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258.
Guo, W., Jin, W., Li, N., Gao, J., Wang, J., Chang, Y., et al. (2021). Brain activity alterations in patients with Parkinson’s disease with cognitive impairment based on resting-state functional MRI. Neurosci. Lett. 747:135672. doi: 10.1016/j.neulet.2021.135672
Gusnard, D. A., Raichle, M. E., and Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2, 685–694. doi: 10.1038/35094500
Hanagasi, H. A., Tufekcioglu, Z., and Emre, M. (2017). Dementia in Parkinson’s disease. J. Neurol. Sci. 374, 26–31.
Hou, Y., Wu, X., Hallett, M., Chan, P., and Wu, T. (2014). Frequency-dependent neural activity in Parkinson’s disease. Hum. Brain Mapp. 35, 5815–5833. doi: 10.1002/hbm.22587
Le Heron, C., and Macaskill, M. (2021). A multi-step model of parkinson’s disease pathogenesis. Mov. Disord. 36, 2530–2538. doi: 10.1002/mds.28719
Leech, R., and Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. doi: 10.1093/brain/awt162
Li, M., Liu, Y., Chen, H., Hu, G., Yu, S., Ruan, X., et al. (2019). Altered global synchronizations in patients with parkinson’s disease: a resting-state fMRI study. Front. Aging Neurosci. 11:139. doi: 10.3389/fnagi.2019.00139
Li, W., Qiao, L., Zhang, L., Wang, Z., and Shen, D. (2019). Functional brain network estimation with time series self-scrubbing. IEEE J. Biomed. Health Inform. 23, 2494–2504. doi: 10.1109/JBHI.2019.2893880
Matchin, W., Liao, C. H., Gaston, P., and Lau, E. (2019). Same words, different structures: an fMRI investigation of argument relations and the angular gyrus. Neuropsychologia 125, 116–128. doi: 10.1016/j.neuropsychologia.2019.01.019
Mitchell, T., Lehéricy, S., Chiu, S. Y., Strafella, A. P., Stoessl, A. J., and Vaillancourt, D. E. (2021). Emerging neuroimaging biomarkers across disease stage in parkinson disease: a review. JAMA Neurol. 78, 1262–1272. doi: 10.1001/jamaneurol.2021.1312
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment. MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Nemade, D., Subramanian, T., and Shivkumar, V. (2021). An update on medical and surgical treatments of parkinson’s disease. Aging Dis. 12, 1021–1035.
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601.
Ramanan, S., Piguet, O., and Irish, M. (2018). Rethinking the role of the angular gyrus in remembering the past and imagining the future: the contextual integration model. Neuroscientist 24, 342–352. doi: 10.1177/1073858417735514
Rolinski, M., Griffanti, L., Szewczyk-Krolikowski, K., Menke, R. A., Wilcock, G. K., Filippini, N., et al. (2015). Aberrant functional connectivity within the basal ganglia of patients with Parkinson’s disease. Neuroimage Clin. 8, 126–132. doi: 10.1016/j.nicl.2015.04.003
Ruppert, M. C., Greuel, A., Freigang, J., Tahmasian, M., Maier, F., Hammes, J., et al. (2021). The default mode network and cognition in Parkinson’s disease: a multimodal resting-state network approach. Hum. Brain Mapp. 42, 2623–2641. doi: 10.1002/hbm.25393
Schindlbeck, K. A., Vo, A., Mattis, P. J., Villringer, K., Marzinzik, F., Fiebach, J. B., et al. (2021). Cognition-Related functional topographies in parkinson’s disease: localized loss of the ventral default mode network. Cereb. Cortex 31, 5139–5150. doi: 10.1093/cercor/bhab148
Shang, S., Zhang, H., Feng, Y., Wu, J., Dou, W., Chen, Y. C., et al. (2021b). Region-Specific neurovascular decoupling associated with cognitive decline in parkinson’s disease. Front. Aging Neurosci. 13:770528. doi: 10.3389/fnagi.2021.770528
Shang, S., Li, D., Tian, Y., Li, R., Zhao, H., Zheng, L., et al. (2021a). Hybrid PET-MRI for early detection of dopaminergic dysfunction and microstructural degradation involved in Parkinson’s disease. Commun. Biol. 4:1162. doi: 10.1038/s42003-021-02705-x
Spreng, R. N., and Grady, C. L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22, 1112–1123. doi: 10.1162/jocn.2009.21282
Stav, A. L., Aarsland, D., Johansen, K. K., Hessen, E., Auning, E., and Fladby, T. (2015). Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease. Parkinsonism Relat. Disord. 21, 758–764.
Szewczyk-Krolikowski, K., Menke, R. A., Rolinski, M., Duff, E., Salimi-Khorshidi, G., Filippini, N., et al. (2014). Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology 83, 208–214.
Tolosa, E., Garrido, A., Scholz, S. W., and Poewe, W. (2021). Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397.
Utevsky, A. V., Smith, D. V., and Huettel, S. A. (2014). Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–940.
Vatansever, D., Manktelow, A. E., Sahakian, B. J., Menon, D. K., and Stamatakis, E. A. (2017). Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 38, 41–52. doi: 10.1002/hbm.23341
Wang, Q., He, W., Liu, D., Han, B., Jiang, Q., and Niu, J. (2021). Functional connectivity in parkinson’s disease patients with mild cognitive impairment. Int. J. Gen. Med. 14, 2623–2630.
Wise, R. G., Ide, K., Poulin, M. J., and Tracey, I. (2004). Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21, 1652–1664. doi: 10.1016/j.neuroimage.2003.11.025
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351.
Yu, E., Liao, Z., Mao, D., Zhang, Q., Ji, G., Li, Y., et al. (2017). Directed functional connectivity of posterior cingulate cortex and whole brain in alzheimer’s disease and mild cognitive impairment. Curr. Alzheimer Res. 14, 628–635. doi: 10.2174/1567205013666161201201000
Zarifkar, P., Kim, J., La, C., Zhang, K., Yorkwilliams, S., Levine, T. F., et al. (2021). Cognitive impairment in Parkinson’s disease is associated with default mode network subsystem connectivity and cerebrospinal fluid Aβ. Parkinsonism Relat. Disord. 83, 71–78. doi: 10.1016/j.parkreldis.2021.01.002
Zeng, Q., Guan, X., Law Yan Lun, J. C. F., Shen, Z., Guo, T., Xuan, M., et al. (2017). Longitudinal alterations of local spontaneous brain activity in parkinson’s disease. Neurosci. Bull. 33, 501–509. doi: 10.1007/s12264-017-0171-9
Keywords: Parkinson’s disease, cognitive decline, functional connectivity, default mode network, functional magnetic resonance imaging
Citation: Chen L, Huang T, Ma D and Chen Y-C (2022) Altered Default Mode Network Functional Connectivity in Parkinson’s Disease: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurosci. 16:905121. doi: 10.3389/fnins.2022.905121
Received: 26 March 2022; Accepted: 10 May 2022;
Published: 03 June 2022.
Edited by:
Shuo Hu, Central South University, ChinaReviewed by:
Zhenyu Xiong, Rutgers, The State University of New Jersey, United StatesJyothilakshmi Vasavan, Algonquin College, Canada
Copyright © 2022 Chen, Huang, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Chen Chen, Y2hlbnl1Y2hlbjE5ODlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Lu Chen1†
Lu Chen1† Di Ma
Di Ma Yu-Chen Chen
Yu-Chen Chen