
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 13 June 2022
Sec. Sleep and Circadian Rhythms
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.902895
This article is part of the Research Topic Fundamentals in Neurological Sleep Disorders: What Are the Causes and How the Disease Progresses View all 4 articles
Background: The purpose of the study was to examine the association of long and short sleep duration with risk of Parkinson’s disease (PD) across RORA rs2028122 genotypes.
Methods: In the present prospective study with a large sized UK Biobank cohort, we performed multivariate logistic regression analyses, generalized additive model, interaction terms, stratification analysis, and mediation analysis to evaluate the association of long and short sleep duration with risk of PD across RORA rs2028122 genotypes.
Results: The GG genotype [1.16 (1.01, 1.33)], a short sleep duration [1.23 (1.10, 1.37)], and a long sleep duration [1.19 (1.03, 1.37)] were identified as the independent risk factors for PD. Sleep duration exhibited a curvilinear U-shaped correlation with the risk of PD; first, the risk of PD gradually decreased as the length of sleep increase, but then, the risk began to increase as the length of sleep increase. Among habitual long sleepers, AG carriers had a higher risk of PD compared with AA carriers [1.67 (1.09, 2.55)]. Among AG carriers, both habitual short [1.28 (1.09, 1.50)] and long [1.38 (1.13, 1.69)] sleepers increased the risk of PD compared with habitual normal sleepers. Among GG carriers, habitual short sleepers have a higher risk of PD [1.26 (1.06, 1.50)] compared with habitual normal sleepers. A mediation model suggested that the rs2028122 genotype partially mediated the causal pathway of sleep duration leading to the development of PD on a positive effect.
Conclusion: Our study demonstrated that the association between sleep duration and PD risk varied across different RORA rs2028122 genotypes. Our findings could help individuals to identify their potential risk profile and take timely actions to prevent the PD.
Previous studies demonstrated that about 64% of patients with Parkinson’s disease (PD) suffered from sleep problems (e.g., short and long sleep duration) (Barone et al., 2009; Mantovani et al., 2018; Doręgowska and Rudzińska-Bar, 2019). Although there has been a notable increase in the number of studies investigating the association between sleep duration and PD, it has not gleaned a consistent conclusion. Previous studies have shown that participants who were habitual short sleepers were associated with a higher risk of developing PD; however, this association was not observed for participants who were habitual long sleepers (Kay et al., 2018; Lysen et al., 2019). In contrast, Chen reported that habitual long sleep duration was associated with a higher risk of PD, but habitual short sleep duration did not increase this risk (Chen et al., 2006). However, these studies were based on a relatively small sample size, and analyses of these studies were not controlled for sufficient confounders (e.g., sleep-related covariates). Therefore, there is a clear need to set up a specific study, with a larger sample size and more confounders, to investigate whether and how long and/or short sleep duration affect the risk of PD.
The retinoic acid receptor-related orphan receptor A (RORA) gene is known to play a key role in circadian rhythm and sleep disorders (Möller-Levet et al., 2013) and was considered as a promising candidate gene for regulating sleep duration (Carvalho et al., 2020; Hou et al., 2020) and sleep latency (Kripke et al., 2015). De novo dominant toxic RORA gene variants have found to be associated with ataxia and cerebellar atrophy (Guissart et al., 2018). Knockout of the RORA gene in mice could lead to certain symptoms that are commonly seen in patients with PD, including the tremor and body imbalance (Dussault et al., 1998). These studies present direct evidence for a link of the RORA gene (e.g., rs2028122) with sleep duration and PD.
Therefore, we hypothesized that the RORA gene may play a role in the regulation of sleep duration and PD, and that the association between short and long sleep duration and risk of PD may vary across different RORA genotypes (e.g., rs2028122 genotypes). To test the hypothesis, in the present large observational study with one of the largest cohort worldwide: the UK Biobank, we specifically investigated the association between RORA rs2028122 genotypes, sleep duration, and PD. First, we used multivariate logistic regression analyses and a generalized additive model to evaluate the association of sleep duration and RORA rs2028122 genotypes with the risk of PD. Second, we used interaction terms and stratification analyses to investigate the potential interactions between rs2028122 genotypes and sleep duration on the risk of PD. Finally, mediation analysis was performed to evaluate whether the rs2028122 genotypes mediated in the causal path of sleep duration leading to the development of PD.
We analyzed the data of 502,505 participants from 22 centers between March 2006 and December 2010 in the UK Biobank (Table 1), whom were classified into a PD group (74.85 ± 5.51 years; 61.5% men) and a non-PD group (68.42 ± 8.11 years; 45.5% men). The definition of PD included Parkinson’s disease, secondary Parkinsonism, and Parkinsonism, according to the International Classification of Diseases, version 10 (ICD-10) terms from the UK Biobank data field 41,270 (ICD-10 codes G20-G22). A total of 3,163 (0.63%) PD events were observed during follow-up appointments up to 30 December 2020, which was higher than the prevalence rate of 0.1–0.3% that were reported among the general population (von Campenhausen et al., 2005; Pringsheim et al., 2014; Elbaz et al., 2016). Ethical approval was obtained from the Northwest Multi-Centre Research Ethics Committee (REC reference: 16/NW/0274).
Participants were genotyped on the Affymetrix UK Biobank Lung Exome Evaluation (UK BiLEVE) Axiom array or the Applied Biosystems UK Biobank Axiom Array. Quality control and imputation, using the Haplotype Reference Consortium, UK10K and 1000 Genomes phase 3, and reference panels were conducted centrally at the UK Biobank, thus resulting in a total of 96 million SNPs (Bycroft et al., 2018). The RORA rs2028122 SNP (located on chromosome 15) was among the directly genotyped SNPs from the UK Biobank, including AA, AG, and GG genotypes. The minor allele frequency of the rs2028122 G risk allele was 59.1% in European.
To investigate the sleep duration (ID 1160), participants were asked to answer the question “About how many hours sleep do you get in every 24 h? (Please include naps)” with a response by an exact value. “Do not know” or “Prefer not to answer” responses were considered as missing values. The self-reported sleep duration was classified into three categories for analysis: a short sleep duration (≤6 h), a normal sleep duration (7–8 h), and a long sleep duration (≥9 h). Participants who usually reported short, normal, and long sleep duration were considered as habitual short, normal, and long sleepers, respectively.
Confounding factors included the following: demographic variables b[age (ID 21022), sex (ID 31), educational level (ID 6138), body mass index (BMI) (ID 21001), overall health rating (ID 21780), employment (ID 20277)], lifestyles [smoking (ID 20116), frequency of alcohol consumption (ID 20117), international physical activity questionnaire (IPAQ) (ID 22032), summated metabolic equivalent task (MET) minutes per week for all activity (ID 22040)], and sleep variables [getting up easily in the morning (ID 1170), chronotype (ID 1180), nap during the day (ID 1190), narcolepsy (ID 1220), sleeplessness or insomnia (ID 1200), snoring (ID 1210)]. Educational level (ID 6138) was classified into two categories depending on whether the patient held a college or university degree. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Alcohol consumption was divided into a low frequency (<3 times a week), a high frequency (≥3 times a week), and never (never and special occasions only). The overall health rating (ID 21780) was classified into excellent/good and fair/poor health rating.
Actigraphy devices (Axivity AX3) were worn 2.8–9.7 years after the study baseline from the UK Biobank for up to 7 days. Samples were excluded if they satisfied at least one of the following conditions: a non-zero or missing value in data field 90002 (data problem indicator), good wear time flag (field 90015) set to 0, good calibration flag (field 90016) set to 0, calibrated on own data flag (field 90017) set to 0, or overall wear duration (field 90051) less than 5 days. Finally, 98765 samples remained.
Sleep episodes within the sleep period time window were defined as periods of at least 5 min with no changes larger than 5° associated with the z-axis of the accelerometer. The summed duration of all sleep episodes was used as an indicator of sleep duration.
Categorical variables are presented as a number (percentage). We used unpaired t-tests, Mann–Whitney U-tests, and χ2-tests to compare the differences between groups where appropriate.
Logistic regression analysis was used to evaluate the associations of exposure variables (the rs2028122 genotype and sleep duration) with the risk of PD. Univariate and multivariate models were applied to evaluate the odds ratios (ORs) and 95% confidence intervals (95% CIs). A total of four schemes were analyzed by logistic regression analysis: (a) model 1, adjusted for age and sex; (b) model 2, further adjusted for education level, ethnicity, BMI, overall health rating, frequency of alcohol consumption, and employment status; (c) model 3, further adjusted for IPAQ and summated MET minutes per week for all activities; and (d) model 4, further adjusted for sleep variables. To compare the goodness of fit between statistical models, we calculated the Nagelkerke’s R-squared value for each logistic regression model.
Next, the sleep duration was treated as a continuous variable. We used penalized cubic splines in a generalized additive model (GAM) to evaluate the non-linear association between sleep duration and the risk of PD. Non-linearity was evaluated using the likelihood ratio test. If a p-value for non-linearity was < 0.05, it indicated the existence of evidence against the linearity assumption.
Furthermore, interaction terms were employed for the overall sample to explore potential interactions between the rs2028122 genotype and the sleep duration (“rs2028122 genotype * sleep duration”) on the risk of PD after controlling for demographic variables, lifestyles, and sleep variables. Mediation analysis was used to evaluate whether the rs2028122 genotype mediated the causal path of sleep duration leading to the development of PD.
All analyses were conducted with SPSS version 24.0 and R statistical software version 4.0. A two-tailed p-value < 0.05 was considered significant.
The demographic characteristics of the study population are presented in Table 1. Compared with non-PD group, PD group had a significant higher proportion of G risk allele (χ2 = 9.87, p = 0.007), and that of short/long sleep duration (χ2 = 180.60, p< 0.001). Compared with non-PD group, PD group was characterized by a lower educational level, a higher proportion of obesity, a poorer overall health rating, a poorer employment status, a lesser physical activity, and more sleep problems. The distribution of sleep duration across different rs2028122 genotypes is shown in Table 2 (χ2 = 108.129, p < 0.001).
The prevalence of PD exhibited a curvilinear U-shaped correlation with the length of sleep (Figure 1), which suggests that the prevalence of PD gradually decreased as the increase of sleep length, and then, it began to increase as the increase of sleep length at the inflexion about 7 h. Carriers of AG genotype had the highest incidence of PD among the rs2028122 genotypes at both ends of the sleep length spectrum.
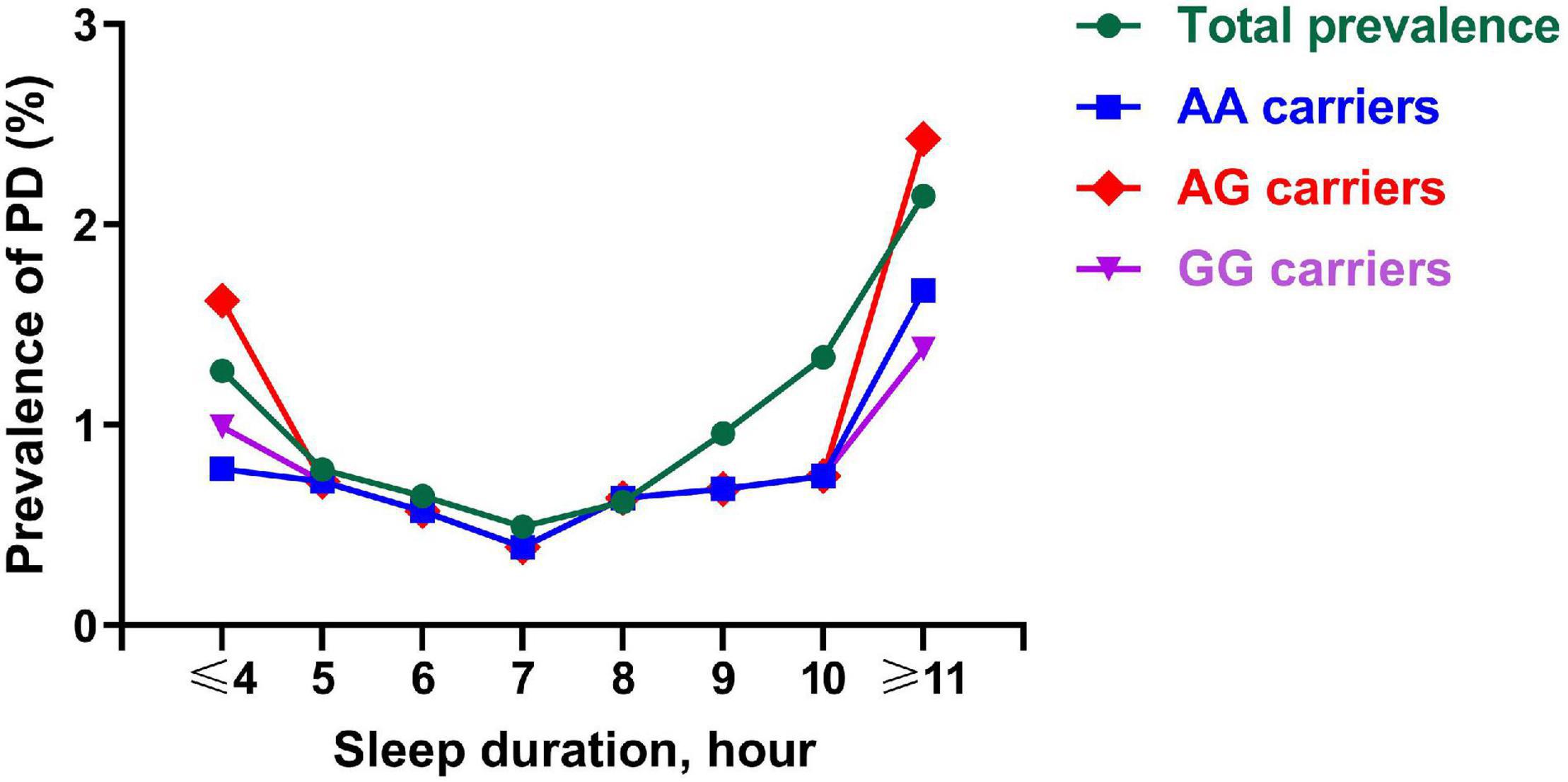
Figure 1. Prevalence of PD across a different sleep length spectrum. Each node represents the prevalence of PD of the corresponding sleep duration. This figure shows a curvilinear U-shaped correlation of sleep duration with prevalence of PD for AA carriers (blue line), AG carriers (red line), GG carriers (purple line), and total subjects (green line). PD, Parkinson’s disease.
A generalized additive model showed a non-linear association between the sleep duration and the risk of PD (Figure 2). Sleep duration also exhibited a curvilinear U-shaped correlation with the risk of PD, which was as the same as sleep duration with the prevalence of PD.
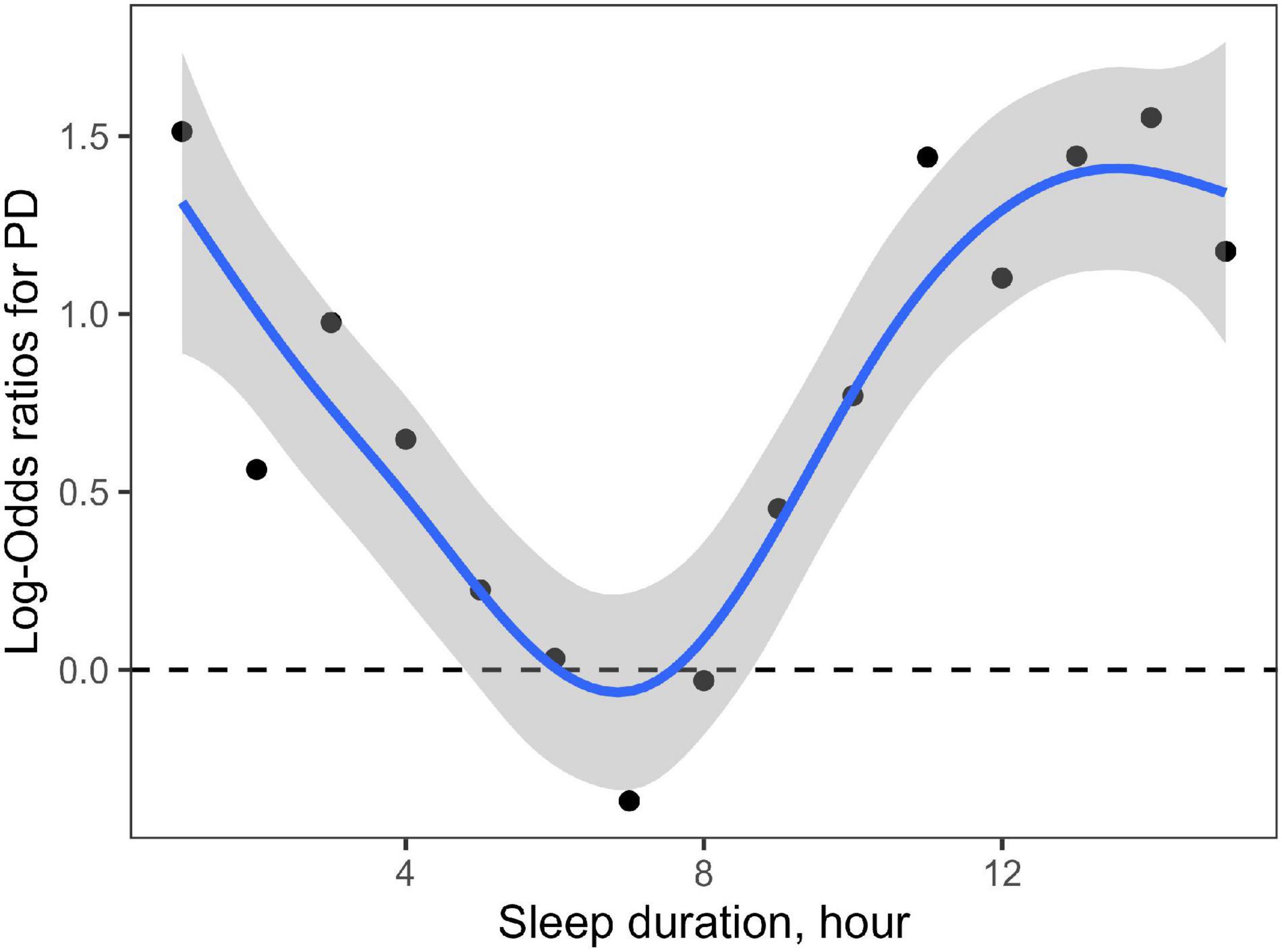
Figure 2. Non-linear association of sleep duration with risk of PD. Each node represents the logarithm of OR value of the corresponding sleep duration increasing the risk of PD. The shaded area represents the 95% confidence interval of the logarithm of OR value. This figure shows a curvilinear U-shaped correlation of sleep duration with the risk of PD.
Table 3 shows that there was a significant interaction effect between the RORA rs2028122 AG genotype and long sleep duration (AG genotype * long sleep duration) regarding the risk of PD without adjusting for any confounders [1.44 (1.01–2.05), p = 0.042]. This interaction effect remained significant after adjusting for age, sex, education level, employment status, ethnicity, BMI, overall health rating, lifestyles, and sleep-related factors [1.73 (1.10–2.72), p = 0.019]. However, there is no interaction effect between AG genotype and short sleep duration [1.23 (0.90–1.68), p = 0.19], between GG genotype and long sleep duration [1.48 (0.93–2.36), p = 0.10], and between GG genotype and short sleep duration [1.24 (0.91–1.71), p = 0.18].
The associations between the RORA genotype, sleep duration, and the risk of PD are presented in Table 4. Multivariate logistic regression model showed that compared with participants who carried the rs2028122 AA genotype, those GG carries had a higher risk of PD [OR (95% CI), 1.16 (1.01, 1.33), p = 0.038, Model 4]. The AG genotype was not associated with a higher risk of PD [1.07 (0.93, 1.22), p = 0.35]. Compared with participants who usually reported a normal sleep duration, those habitual long sleepers [1.19 (1.03, 1.37), p = 0.019] and habitual short sleepers [1.23 (1.10, 1.37), p< 0.001] were associated with a higher risk of PD.
We investigated the association between the RORA rs2028122 genotypes and the risk of PD, separated by sleep duration (Table 5a) and RORA rs2028122 genotype (Table 5b), respectively. When stratifying our analysis by sleep duration, we found that among habitual long sleepers, AG genotype increased the risk of PD compared with AA genotype [1.67 (1.09, 2.55), p = 0.019]. In contrast, among normal [0.96 (0.81, 1.13), p = 0.60] and short [1.13 (0.87, 1.47), p = 0.35] sleepers, AG genotype was not associated with the PD risk. Among habitual normal sleepers, the GG genotype was not associated with the risk of PD [1.06 (0.89, 1.26), p = 0.51].
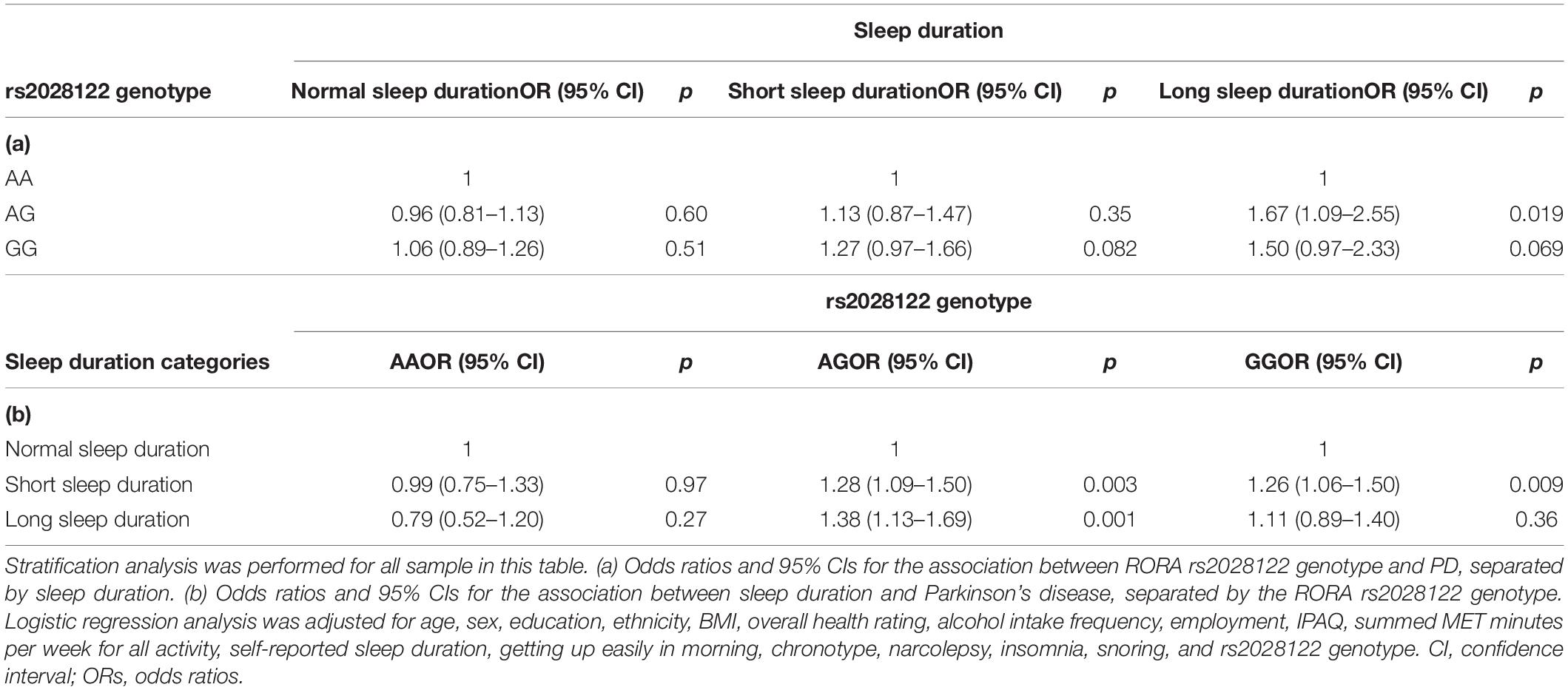
Table 5. Odds ratios and 95% CIs for the association between RORA rs2028122 genotype and PD, separated by sleep duration and RORA rs2028122 genotype, respectively.
When stratifying our analysis by RORA rs2028122 genotype, we found that among AG carriers, compared with habitual normal sleepers, habitual short sleepers [1.28 (1.09, 1.50), p = 0.003] and habitual long sleepers [1.38 (1.13, 1.69), p = 0.001] were associated with a higher risk of PD, respectively. Among GG carriers, only habitual short sleepers were associated with a higher risk of PD [1.26 (1.06, 1.50), p = 0.009].
We also analyzed the association of sleep duration with the risk of PD among those participants who were worn the actigraphy devices by multivariate logistic regression, interaction terms, and stratification analysis after adjusting for all confounders. We found a consistent finding of the association of sleep duration with the risk of PD. Compared with habitual normal sleepers, long [1.46 (1.09, 1.97), p = 0.012] and short [1.54 (1.19, 2.00), p = 0.001] sleepers were also associated with a higher risk of PD.
When stratifying our analysis by RORA rs2028122 genotype, we found that among the AG carriers, compared with habitual normal sleepers, those habitual short [1.87 (1.27, 2.74), p = 0.002] and long [1.79 (1.16, 2.77), p = 0.009] sleepers were associated with a higher risk of PD, respectively.
The mediation model suggests that the rs2028122 genotype partially mediates the causal pathway of sleep duration leading to the development of PD on a positive effect (Figure 3). In the mediation model, the average causal mediation effect (p = 0.002), average direct effect (p = 0.002), and total effect (p < 0.001) were all statistically significant.
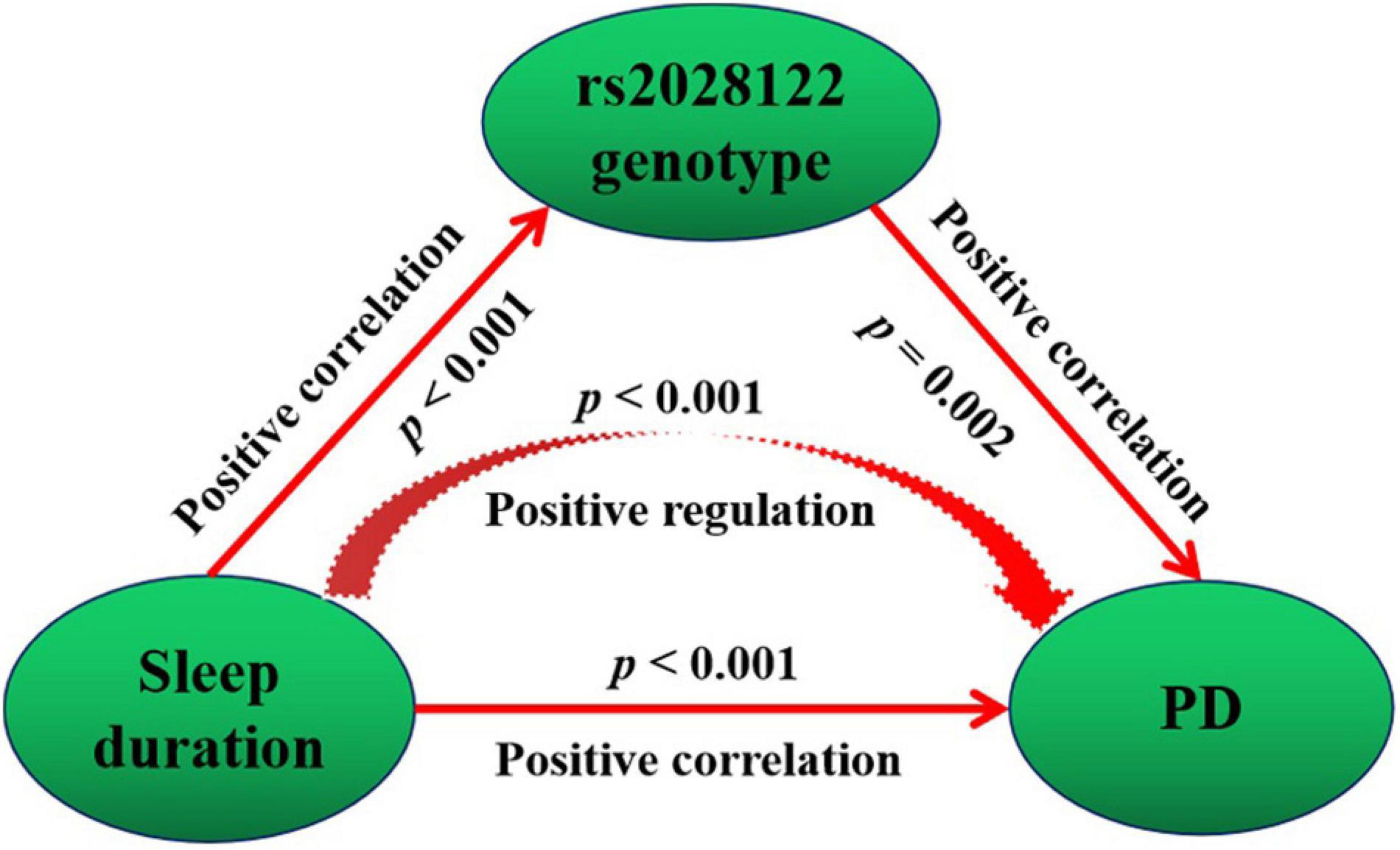
Figure 3. Mediating causality of the RORA rs2028122 in the causal path of sleep duration leading to PD. Mediation model suggests that the rs2028122 genotype partially mediates the causal path of sleep duration leading to development of PD on positive effect.
This prospective study of a large-sized UK Biobank cohort was the first epidemiological study to investigate whether (and how) sleep duration and RORA rs2028122 genotype could affect the risk of PD. Our study identified five main novel findings. First, the GG genotype, a short sleep duration, and a long sleep duration were identified as the independent risk factors for PD. Second, the RORA rs2028122 genotype mutation did not increase the risk of PD among normal sleepers; furthermore, neither a long nor a short sleep duration increased the risk of PD among AA carriers. Third, the RORA G risk allele increased the risk of PD. Short sleepers had a 26% higher risk of PD among GG carriers. Short sleepers and long sleepers had 28 and 38% higher risk of PD among AG carriers, respectively. Fourth, we identified a significant interaction effect between the AG genotype and a long sleep duration (there was a 73% higher risk of PD). Furthermore, the AG genotype increased 67% risk of PD among those habitual long sleepers. Finally, a mediation model suggested that the rs2028122 genotype partially mediated the causal pathway of sleep duration leading to the development of PD on a positive effect.
Despite a recent increase in studies concerning on whether long and/or short sleep duration are able to increase the risk of PD, there are still no consistent conclusions, and the results are required further replication in a larger sample size. Lysen et al. (2019) found that habitual short sleepers were significantly more likely to develop PD when compared to those habitual suitable sleepers; this relationship was not observed in habitual long sleepers. These findings were further confirmed in an independent external cohort (Kay et al., 2018). However, Chen et al. (2006) found that a habitual long sleep duration may be an earlier biomarker of PD among female nurses. In another study, Postuma et al. (2017) reported that a self-reported long sleep duration increased the risk for non-PD conversion to PD. In this study, we discovered that both long and short sleep duration increased the risk of PD, even after adjusting for age, sex, socioeconomic status, sleep status, lifestyle, and physical activity. Our sensitivity analyses also exhibited a consistent finding among those participants who were worn the actigraphy devices. The sample size of our prospective study was the largest among these studies, which may be one of the main reasons why both long and short sleep duration were identified as the risk factors. Furthermore, our analyses showed that the association between sleep duration and PD risk appears to vary across RORA rs2028122 genotypes, and that the rs2028122 genotype partially mediates the causal pathway of sleep duration leading to the development of PD on a positive effect. These findings suggest that the previously inconsistent findings about the association of long and short sleep duration with the risk of PD may be influenced by the specific type of gene mutation level.
The most striking and interesting finding was that the association between sleep duration and PD risk varied across different RORA rs2028122 genotypes. Circadian rhythm disorder has been associated with changes in habitual sleep duration (Rutenfranz et al., 1988) and may increase the risk of PD (Leng et al., 2019). The RORA gene is involved in the regulation of circadian rhythms (Jetten, 2009) and has been considered as a novel locus for sleep duration (Hou et al., 2020). In our study, the rs2028122 genotype partially mediated the causal pathway of sleep duration leading to the development of PD on a positive effect. These findings highlight the role of the RORA gene in the regulation of sleep duration and PD; however, such finding has not been studied. Previous studies mainly focused on the association between the RORA gene and mental disorders, and the physiological function and physiopathological role of the rs2028122 genotypes remained unknown. Our study is the first to comprehensively investigate whether and how the RORA rs2028122 genotype and long/short sleep duration affect the risk of PD. The RORA SNP rs2028122 allele A has been shown to have a dominant protective effect against sleep disorders in patients with depression (Lavebratt et al., 2010). In this study, we found that the RORA rs2028122 mutation did not increase the risk of PD among normal sleepers, and neither long nor short sleep duration increased the risk of PD among carriers of AA genotype. However, the interaction between the rs2028122 mutations and long/short sleep duration appears to lead to a variable risk of PD, especially for habitual long sleepers in carriers of AG genotype and habitual short sleepers in carriers of GG genotype. The exact reasons for such interactions need to be investigated further.
There are several limitations to our study that should be addressed. First, participants in the UK Biobank have a restricted age range; therefore, our data cannot represent the entire population (Wang et al., 2021). Second, our study was limited by its cross-sectional design. Thus, causality cannot be inferred from the associations observed. Third, the physiological function and physiopathological role of the rs2028122 genotype need to be investigated further.
In conclusion, our study demonstrated that long and short sleep duration, respectively, increased the risk of PD. The association between circadian profile and PD risk varied across different RORA rs2028122 genotypes. The RORA G risk allele, long sleep duration, and short sleep duration were identified as the risk factors of PD. Carriers of RORA rs2028122 AG and GG genotypes should alter their circadian profiles to reduce their risk of developing PD. A habitual short sleep duration is not recommended for carriers of rs2028122 GG genotype. Neither short nor long sleep duration are recommended for carriers of rs2028122 AG genotype. Our findings could help individuals to identify their potential risk profiles of certain individuals and provide guidance for individuals to take precise and timely actions to prevent the future incidence of PD. Public health guidance should focus on reducing the risk of PD by advocating healthy sleep habits.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found on UK Biobank: https://biobank.ndph.ox.ac.uk/ukb/.
The studies involving human participants were reviewed and approved by the Northwest Multi-Centre Research Ethics Committee (REC reference: 16/NW/0274). The patients/participants provided their written informed consent to participate in this study.
X-JD and YW had the idea for and designed this study, had full access to all the data in this study, take responsibility for the integrity of the data and the accuracy of the data analysis, and critically revised the manuscript for important intellectual content and gave final approval for the version to be published. YS and X-JD drafted the manuscript and did the analysis. JW took responsibility for double check of the data analysis. All authors agreed to be accountable for all the aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately resolved.
This research has been conducted using the UK Biobank Resource under application number 75732. This work was supported by the Guangdong Basic and Applied Basic Research Foundation (grant no. 2020A1515011469), Medical Scientific Research Foundation of Guangdong Province of China (grant no. B2021086), and Sanming Project of Medicine in Shenzhen (grant no. SZSM201812052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Barone, P., Antonini, A., Colosimo, C., Marconi, R., Morgante, L., Avarello, T. P., et al. (2009). The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 24, 1641–1649. doi: 10.1002/mds.22643
Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L. T., Sharp, K., et al. (2018). The UK biobank resource with deep phenotyping and genomic data. Nature 562, 203–209. doi: 10.1038/s41586-018-0579-z
Carvalho, F. G., Cunha, A. M. D., Tonon, A. C., Pereira, F. D. S., Matte, U., Callegari-Jacques, S. M., et al. (2020). Poor sleep quality associates with self-reported psychiatric and cardiometabolic symptoms independently of sleep timing patterns in a large sample of rural and urban workers. J. Sleep Res. 29:e12969. doi: 10.1111/jsr.12969
Chen, H., Schernhammer, E., Schwarzschild, M. A., and Ascherio, A. (2006). A prospective study of night shift work, sleep duration, and risk of Parkinson’s disease. Am. J. Epidemiol. 163, 726–730. doi: 10.1093/aje/kwj096
Doręgowska, M., and Rudzińska-Bar, M. (2019). Sleep disorders in Parkinson’s disease. Wiad. Lek. 72, 425–431.
Dussault, I., Fawcett, D., Matthyssen, A., Bader, J. A., and Giguère, V. (1998). Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech. Dev. 70, 147–153.
Elbaz, A., Carcaillon, L., Kab, S., and Moisan, F. (2016). Epidemiology of Parkinson’s disease. Rev. Neurol. 172, 14–26. doi: 10.1016/j.neurol.2015.09.012
Guissart, C., Latypova, X., Rollier, P., Khan, T. N., Stamberger, H., McWalter, K., et al. (2018). Dual molecular effects of dominant RORA mutations cause two variants of syndromic intellectual disability with either autism or cerebellar ataxia. Am. J. Hum. Genet. 102, 744–759. doi: 10.1016/j.ajhg.2018.02.021
Hou, S. J., Tsai, S. J., Kuo, P. H., Liu, Y. L., Yang, A. C., Lin, E., et al. (2020). An association study in the Taiwan biobank reveals RORA as a novel locus for sleep duration in the Taiwanese population. Sleep Med. 73, 70–75. doi: 10.1016/j.sleep.2020.04.008
Jetten, A. M. (2009). Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7:e003. doi: 10.1621/nrs.07003
Kay, D. B., Tanner, J. J., and Bowers, D. (2018). Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 8:e00967. doi: 10.1002/brb3.967
Kripke, D. F., Kline, L. E., Nievergelt, C. M., Murray, S. S., Shadan, F. F., Dawson, A., et al. (2015). Genetic variants associated with sleep disorders. Sleep Med. 16, 217–224. doi: 10.1016/j.sleep.2014.11.003
Lavebratt, C., Sjöholm, L. K., Partonen, T., Schalling, M., and Forsell, Y. (2010). PER2 variantion is associated with depression vulnerability. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153b, 570–581. doi: 10.1002/ajmg.b.31021
Leng, Y., Musiek, E. S., Hu, K., Cappuccio, F. P., and Yaffe, K. (2019). Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 18, 307–318. doi: 10.1016/S1474-4422(18)30461-7
Lysen, T. S., Darweesh, S. K. L., Ikram, M. K., Luik, A. I., and Ikram, M. A. (2019). Sleep and risk of parkinsonism and Parkinson’s disease: a population-based study. Brain 142, 2013–2022. doi: 10.1093/brain/awz113
Mantovani, S., Smith, S. S., Gordon, R., and O’Sullivan, J. D. (2018). An overview of sleep and circadian dysfunction in Parkinson’s disease. J. Sleep Res. 27:e12673. doi: 10.1111/jsr.12673
Möller-Levet, C. S., Archer, S. N., Bucca, G., Laing, E. E., Slak, A., Kabiljo, R., et al. (2013). Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc. Natl. Acad. Sci. U.S.A. 110, E1132–E1141. doi: 10.1073/pnas.1217154110
Postuma, R. B., Gagnon, J. F., Pelletier, A., and Montplaisir, J. Y. (2017). Insomnia and somnolence in idiopathic RBD: a prospective cohort study. NPJ Parkinsons Dis. 3:9. doi: 10.1038/s41531-017-0011-7
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi: 10.1002/mds.25945
Rutenfranz, J., Plett, R., Knauth, P., Condon, R., De Vol, D., Fletcher, N., et al. (1988). Work at sea: a study of sleep, and of circadian rhythms in physiological and psychological functions, in watchkeepers on merchant vessels. II. Sleep duration, and subjective ratings of sleep quality. Int. Arch. Occup. Environ. Health 60, 331–339. doi: 10.1007/BF00405666
von Campenhausen, S., Bornschein, B., Wick, R., Bötzel, K., Sampaio, C., Poewe, W., et al. (2005). Prevalence and incidence of Parkinson’s disease in Europe. Eur. Neuropsychopharmacol. 15, 473–490. doi: 10.1016/j.euroneuro.2005.04.007
Keywords: Parkinson’s disease, sleep duration, RORA, rs2028122, UK Biobank
Citation: Shao Y, Dai X-j, Wang J and Wang Y (2022) Association Between Sleep Duration and Parkinson’s Disease Varied Across Related Orphan Receptor A rs2028122 Genotypes. Front. Neurosci. 16:902895. doi: 10.3389/fnins.2022.902895
Received: 24 March 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Aideen M. Sullivan, University College Cork, IrelandReviewed by:
Francisco Jose Fernandez Gomez, University of Murcia, SpainCopyright © 2022 Shao, Dai, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi-jian Dai, ZGFpeGpkb2N0b3JAMTI2LmNvbQ==; Yongjun Wang, d2FuZ3lqMTkzMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.