
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurosci. , 14 April 2022
Sec. Neurogenesis
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.872794
This article is part of the Research Topic Insights in Neurogenesis: 2021 View all 10 articles
Human neurogenesis occurs mainly in embryonic, fetal, and neonatal stages and generates tremendously diverse neural cell types that constitute the human nervous system. Studies on human neurogenesis have been limited due to a lack of access to human embryonic and fetal tissues. Brain organoids derived from human pluripotent stem cells not only recapitulate major developmental processes during neurogenesis, but also exhibit human-specific features, thus providing an unprecedented opportunity to study human neurodevelopment. First, three-dimensional brain organoids resemble early human neurogenesis with diverse stem cell pools, including the presence of primate-enriched outer radial glia cells. Second, brain organoids recapitulate human neurogenesis at the cellular level, generating diverse neuronal cell types and forming stratified cortical layers. Third, brain organoids also capture gliogenesis with the presence of human-specific astrocytes. Fourth, combined with genome-editing technologies, brain organoids are promising models for investigating functions of human-specific genes at different stages of human neurogenesis. Finally, human organoids derived from patient iPSCs can recapitulate specific disease phenotypes, providing unique models for studying developmental brain disorders of genetic and environmental causes, and for mechanistic studies and drug screening. The aim of this review is to illustrate why brain organoids are good models to study various steps of human neurogenesis, with a focus on corticogenesis. We also discuss limitations of current brain organoid models and future improvements.
The human brain is one of the most complex and challenging organs to study. Higher intellectual and cognitive functions require all neural cell types with appropriate quantities and properties to form intricate networks for information processing. Neurogenesis, a process starting with the proliferation of neural stem cells and progenitor cells, followed by differentiation into neurons, is the foundation of brain development and function. Due to ethical reasons, accessibility to embryonic and fetal tissue is limited and it is difficult to apply many experimental approaches to primary human brain tissue. Thus, many key questions remain to be answered in human neurogenesis.
Recent advances in three dimensional (3D) organoid culture provide unique tools to overcome hurdles in studying human neurogenesis (Eiraku et al., 2008; Kadoshima et al., 2013; Lancaster et al., 2013; Qian et al., 2016). Brain organoids are self-organized cell aggregates derived from stem cells or induced pluripotent stem cells (iPSCs) that mimic fetal brain development. They have the potential to generate different brain cell types and structures relying on both intrinsic signals and external patterning cues (Lancaster and Knoblich, 2014; Qian et al., 2016; Monzel et al., 2017; Renner et al., 2017; Xiang et al., 2017). Using factors related to developmental patterning signals, organoids can be directed to model brain regions including the cortex, hippocampus, ganglionic eminences, thalamus, hypothalamus and cerebellum (Lancaster et al., 2013; Muguruma et al., 2015; Sakaguchi et al., 2015; Qian et al., 2016; Bagley et al., 2017; Birey et al., 2017; Monzel et al., 2017; Xiang et al., 2019; Huang et al., 2021), and even brain subregions, such as the arcuate nucleus of the hypothalamus (Huang et al., 2021). This model provides the opportunity to understand not only evolutionarily conserved, but also late evolved human-specific features of brain development as well as neurodevelopmental disorders. We focus on cortical organoids as an example as they are most studied.
Most of our understanding of neurogenesis comes from studies using in vivo models of flies, fish and rodents, which have contributed invaluable insights into neurogenic processes. Among these, the mouse is the most widely used animal model given its many conserved features with human neurogenesis (Table 1). The availability of diverse genetic tools in mice has been a great advantage, which allows scientists to manipulate evolutionally conserved genes or introduce human-specific genes essential for neurogenesis (Mira and Morante, 2020; Navabpour et al., 2020). Decades of studies in rodent models have led to the identification of mechanisms underlying many key features of neurogenesis, such as patterning cues, stem cell proliferation, neural cell production and migration, and circuitry formation. However, mouse models lack human-specific features critical for human neurogenesis. During evolution, the cerebral cortex of the human brain, which contributes to higher cognitive functions and distinguished linguistic abilities, has greatly expanded (Taverna et al., 2014; Hartenstein and Stollewerk, 2015). Human-specific genes and cell types are major contributors to this unique neurodevelopmental process (Bakken et al., 2016; O’Neill et al., 2018; Hodge et al., 2019; Prodromidou and Matsas, 2019; Benito-Kwiecinski et al., 2021). Moreover, mice are lissencephalic and lack the folded neocortical surface as in primates and humans (Rakic, 2009; Fietz et al., 2010; Hansen et al., 2010; Lui et al., 2011; Betizeau et al., 2013; Hartenstein and Stollewerk, 2015; Liu and Silver, 2021). Furthermore, due to differences in species-specific genes and gene regulation, mice are not always a reliable model for human disorders and translational studies (Denayer et al., 2014).
Another widely used model of neurogenesis is two-dimensional (2D) cell culture in vitro, including neural stem cell cultures or neuronal cell cultures (Table 1). While cell cultures have the advantage of being easy to maintain and valuable in studying a more homogenous cell population to unravel cellular and molecular mechanisms (Gaspard et al., 2008; Shi et al., 2012), these cellular models have limitations in representing many essential features of the brain. During neurodevelopment, the generation of different types of neurons are temporally controlled and spatially organized. In addition, cell-cell interactions and cytoarchitecture provide external signals that further direct the proliferation, differentiation, migration and circuitry formation during development (Scuderi et al., 2021). These features cannot be modeled in monolayer cultures.
The earliest neural stem cells in cortical development are the neuroepithelial cells (NEs) in the ventricular zone (VZ), generated shortly after the formation and closure of neural tube. The initial number and symmetric proliferation of NEs determine the size of the cortex and set the size difference between the mouse and human brain at the very beginning of brain development (Meyer et al., 2000; Haubensak et al., 2004; Lui et al., 2011). At the onset of corticogenesis, NEs transform into apical radial glial cells (aRGs). aRGs reside in the VZ and proliferate to expand the progenitor pool. RGs give rise to intermediate progenitor cells (IPCs) that colonize in the subventricular zone (SVZ) and divide symmetrically to produce pairs of neurons. RGs extend processes to the apical and pial surfaces of the cortex and serve as scaffolds to guide the migration of newly born neurons to the cortical plate. The symmetric and asymmetric division of progenitor cells and migration of neurons give rise to the laminar structure of developing cortex consisting of the VZ, SVZ, intermediate zone, sub-plate, cortical plate, and marginal zone (Meyer et al., 2000; Kosodo et al., 2004; Götz and Huttner, 2005; Lui et al., 2011; Figure 1). Although these processes have been extensively studied in mice, they are not well investigated in the genetic background and cellular context of the human brain. The SVZ notably expanded in primates and humans with two morphologically distinguished regions–inner and outer SVZ (iSVZ and oSVZ). Outer radial glial (oRG) cells are prevalent progenitor cells in oSVZ and predominantly contribute to the expansion and folding or gyrification of the developing human cortex.
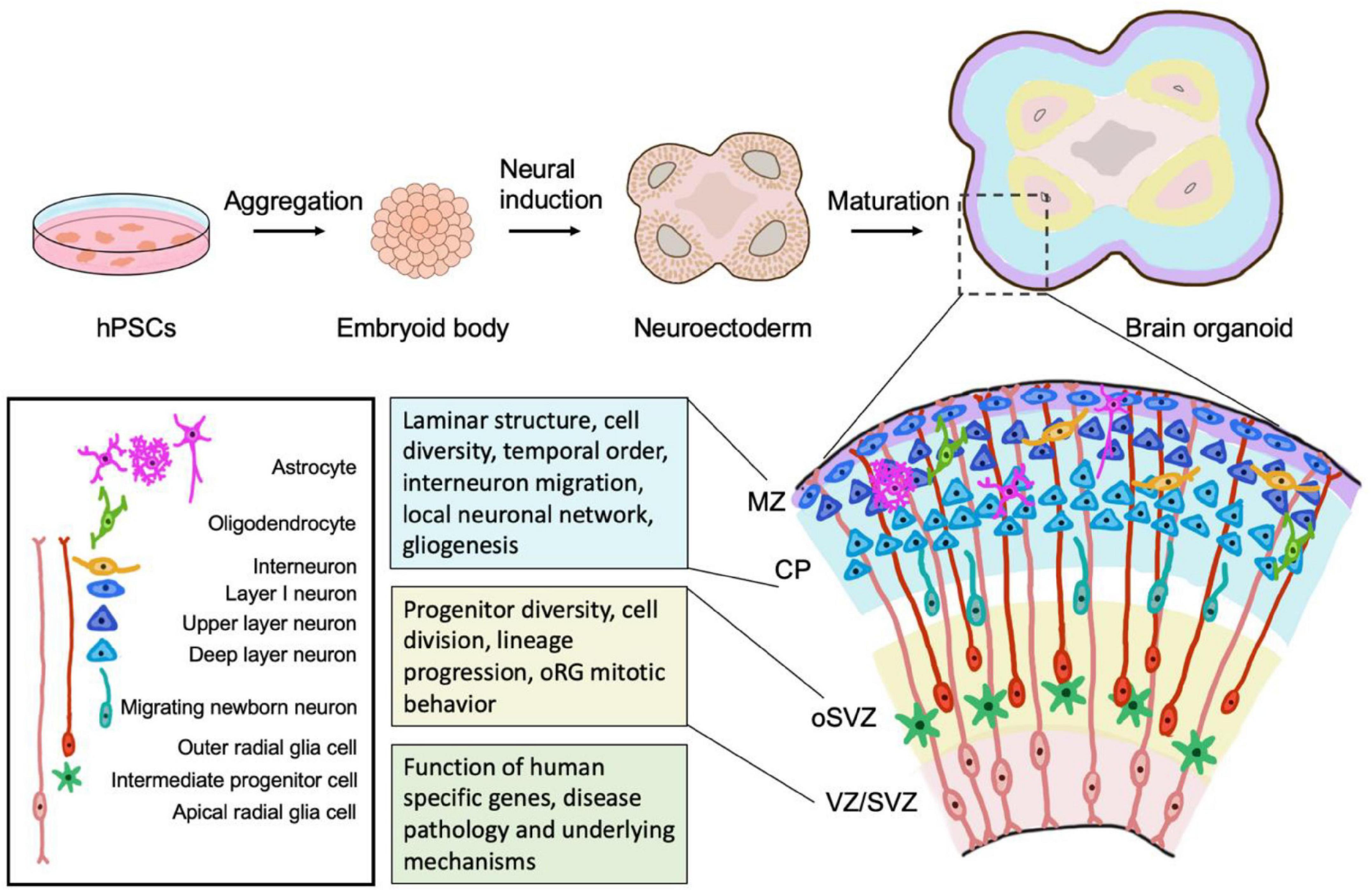
Figure 1. Brain organoids as models to study human neurogenesis. hPSCs are aggregated to form embryoid body and patterned to induce neuroectoderm fate. Neuroectodermal cells organize in the structure of rosette and subsequently develop into brain organoids. Organoids recapitulate the cell diversity and cytoarchitectural organization of the developing human brain. Organoids contain the major progenitor cell pools, including aRGs (apical radial glia cells) in the VZ/SVZ and human specific oRGs (outer radial glia cells) in the oSVZ, with distinct mitotic behavior and lineage progression. Organoids also maintain the structure of the cortical plate in human fetal brain development and thus can be used to investigate the laminar structure formation, generation of principal neuron types (upper layer- and deep layer- neurons), migration, neurogenesis-gliogenesis transition, and the temporal progression and maturation during the neurogenic process. Organoids also provide an opportunity to study the formation of local neuronal networks. Combined with modern genome editing tools and patient-derived iPSCs, organoids can be applied to study the functional contribution of human-specific genes and disease pathology and underlying mechanisms. MZ, marginal zone. CP, cortical plate. VZ, ventricular zone. SVZ, subventricular zone, oSVZ, outer subventricular zone.
Recently developed 3D brain organoid cultures derived from human pluripotent stem cells represent invaluable tools to address questions related to human-specific stem cell types and cytoarchitectures that are difficult to answer in mouse or 2D cell culture models (Table 1). For example, cortical organoids mimic the organization of neural stem cells and progenitor cells in the shape of rosettes (Kadoshima et al., 2013; Lancaster et al., 2013; Lancaster and Knoblich, 2014; Qian et al., 2016). Typical human cortical organoids contain the VZ, iSVZ and oSVZ radial scaffold and diverse RG cell types. At the apical surface of a lumen structure, SOX2+ aRGs can be found forming polarized radial structures, whereas TBR2+ IPCs are present in the SVZ region (Figure 1). oSVZ is also significantly expanded during organoid development and populated with oRGs characterized by HOPX, FAM107a and tenascin C (TNC) marker expression (Lancaster et al., 2013; Lancaster and Knoblich, 2014; Watanabe et al., 2017; Qian et al., 2020). Much effort has been invested to find out the extent to which organoids recapitulate human neurogenesis, and these studies showed consistently that organoids are capable of generating most brain cell types with similar transcriptomic profiles as their counterparts in the developing human brain (Camp et al., 2015; Quadrato et al., 2017; Amiri et al., 2018; Nascimento et al., 2019). Indeed, cortical organoids have been used to understand mechanisms underlying key aspects of human neurogenesis, such as evolutionary expansion of NE cells, progenitor diversity, cell division modes and lineage progression (Bershteyn et al., 2017; Subramanian et al., 2017; Andrews et al., 2020; Benito-Kwiecinski et al., 2021). Novel insight has been gained into the division pattern of oRGs and the role of the mTOR pathway in regulating oRG cellular morphology, migration, and mitotic behavior (Bershteyn et al., 2017; Andrews et al., 2020). These studies suggest that cortical organoids are a valuable system to study human-specific features and allow further investigation of questions about the molecular mechanisms underlying the transition of aRGs to oRGs and the potency of oRGs in generating diverse neuronal cell populations.
The driver of the evolutionary differences in the cerebral cortex among humans, non-human primates and other mammals has remained elusive. Cortical organoids offer an unprecedented opportunity to study human-specific genes during evolution (Otani et al., 2016; Fiddes et al., 2018; Kanton et al., 2019; Pollen et al., 2019; Benito-Kwiecinski et al., 2021; Liu and Silver, 2021). The human-specific NOTCH2NL gene was found to be highly expressed in RGs. An organoid model showed that NOTCH2NL activates the NOTCH signaling pathway, expands the progenitor population and results in the expansion of the overall organoid size (Fiddes et al., 2018). Organoids derived from human, gorilla, and chimpanzee cells showed that NE cells are the major contributor to human brain expansion. Differences in the cell shape, differentiation capacity, interkinetic nuclear migration and cell cycle length are key factors shaping the developing human brain (Benito-Kwiecinski et al., 2021).
In addition to the most widely used cortical organoids, specific areas of the central nervous system can be modeled by generating organoids using different patterning methods (Jacob et al., 2020). For example, hippocampus organoids show continuous structures consisting of choroid plexus, cortical hem and medial pallium tissues, and recapitulate the cell types and gene expression profiles similar to that of human hippocampus (Kadoshima et al., 2013; Pellegrini et al., 2020). Thalamic and hypothalamic organoids generate typical stem cell types along the developmental trajectory (Qian et al., 2016; Xiang et al., 2019). Midbrain organoids show structurally similar neuromelanin-like granules (Jo et al., 2016). Cerebellar organoids exhibit a layered structure containing cerebellar plate neuroepithelium, deep cerebellar nuclei, Purkinje and granule neurons (Muguruma et al., 2015). Spinal cord organoids generate intermediate and ventral spinal cord-like tissues with somatosensory neurons and spinal motor neurons (Ogura et al., 2018). However, these models sometimes lack the tissue architecture seen in vivo and are relatively simplified. Still, these region-specific organoids are emerging as powerful tools for studying neurogenesis in distinct regions of the human nervous system and modeling related disorders.
The cortical plate of the human brain is composed of neurons and glial cells, organized in a laminar structure of six layers. The asymmetric division of aRGs in the VZ/SVZ and symmetric division of oRGs in the oSVZ generate the majority of excitatory neurons that migrate radially to colonize different layers (Götz and Huttner, 2005; Cheung et al., 2010; Lui et al., 2011). The radial migration and graded maturation of neurons in an inside-out sequence in corticogenesis are conserved in mammals. Cajal–Retzius cells form the marginal zone and secrete Reelin for later layer establishment. The early born neurons form the deepest layer VI, characterized by the expression of TBR1. The later born neurons migrate and bypass early neurons to form more superficial layers with projections to different brain regions. Layer V is occupied by CTIP2+ neurons that project mostly to spinal cord, whereas layers II-VI are SATB2+ intratelencephalic neurons that project within forebrain. Layers II and III pyramidal neurons are also characterized by the expression of BRN1/2 and CUX1/2 (Meyer et al., 2000; Kast and Levitt, 2019; Bhaduri et al., 2021). The extraordinarily diverse inhibitory interneurons in cortex are generated mostly in the medial and caudal ganglionic eminences. They migrate tangentially to form connections with excitatory neurons and integrate to local neural networks (Gorski et al., 2002; Letinic et al., 2002). Although the basic architecture is conserved in human and mouse, the cell number, cell type and gene expression in each compartment are vastly different. The overall neuron population, especially the upper cortical layers, are profoundly expanded in primates and humans. The proportion of GABAergic neurons generated locally or from ganglionic eminences have prominent differences between rodents and primates (Letinic and Rakic, 2001; Anderson et al., 2002).
While human iPSCs can be directed to differentiate into most neuronal types under 2D culture conditions (Gaspard et al., 2008; Shi et al., 2012), the regionally organized columnar and laminar structures are completely lost, and the more complex neural networks are absent. Cortical organoids provide an attractive complement to animal and 2D cell culture models for studying structural organization, cell diversity, as well as the temporal order along the developmental trajectory. The basic laminar structure of cortical layers has been demonstrated in organoid models. At the onset of neuronal differentiation in cortical organoids, early born TBR1+ and CTIP2+ neurons, as well as Reelin+ Cajal–Retzius cells first appear to form a dense neuronal layer. SATB2+ and CUX1+ upper layer neurons are found at a later stage and localize close to the surface (Lancaster et al., 2013; Lancaster and Knoblich, 2014; Qian et al., 2016, 2020). Single cell sequencing data show that the diversity of neurons generated in organoids is similar to human fetal tissues (Quadrato et al., 2017; Tanaka et al., 2020). The dynamic production, migration and maturation of neuronal populations is also accompanied by neuronal network formation with a surge in electrical activity (Quadrato et al., 2017; Giandomenico et al., 2019; Trujillo et al., 2019). This holds great potential to deepen our understanding of neuronal circuits and network activity in the developing human cortex.
The recent development of assembloid models furthered integration of interneurons to cortical neuronal networks. Several groups have fused human ganglionic eminence organoids and cortical organoids to investigate the migration and integration of interneurons (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017). By applying assembloids to model Timothy syndrome, it was found that interneurons carrying the genetic mutation display abnormal migratory saltations, providing insights into the cellular mechanism of this neurodevelopmental disease.
During the later stages of neurogenesis, RGs switch from neurogenic to gliogenic differentiation to generate glial cells, including astrocytes and oligodendrocytes that later populate throughout the cortical layers (Rash et al., 2019). Organoids show promising production of diverse astrocytes (Dezonne et al., 2017; Sloan et al., 2017; Qian et al., 2020). At later stages of organoid cultures, multiple astrocyte subtypes can be found in neural layers, showing morphology and distribution patterns similar to that of the human cerebral cortex (Qian et al., 2020). Transcriptome analysis show that these astrocytes closely resemble their counterparts in fetal tissue and display gradual maturation over time (Sloan et al., 2017). Another important glial cell type, oligodendrocytes, is also present in organoids (Madhavan et al., 2018; Marton et al., 2019). Oligodendrocytes are the myelin-forming cells in the central nervous system that generate myelin to wrap axons to ensure fast signal transmission and provide metabolic support. Multiple studies showed that oligodendrocytes in organoids display similar cellular and molecular features as human oligodendrocytes in vivo (Madhavan et al., 2018; Marton et al., 2019; Gordon et al., 2021). These studies demonstrate the applicability of organoids in understanding neural development and neurological disorders at a more complex level.
Cortical organoids recapitulate key features of neurogenesis, and thus represent an ideal model for investigating the etiology of neurodevelopmental disorders. They provide spatial and temporal information about neurogenesis in the context of 3D tissue along the developmental trajectory, bridging the gap between conventional 2D culture and disease pathogenesis. Moreover, organoids generated from patient-derived iPSCs enable modeling of both genetic and idiopathic disorders that are hard to study in animal models. Cumulative studies have used organoids to model genetic brain disorders including autism spectrum disorder (ASD) (Mariani et al., 2015; Wang et al., 2017; Ye et al., 2017; Mellios et al., 2018; Srikanth et al., 2018; Johnstone et al., 2019; Sun et al., 2019; Kang et al., 2021), schizophrenia (Stachowiak et al., 2017; Qian et al., 2020), microcephaly (Lancaster et al., 2013), macrocephaly (Li et al., 2017) and lissencephaly (Bershteyn et al., 2017; Karzbrun et al., 2018). These studies utilize iPSCs generated from patients or human stem cells containing mutations introduced by CRISPR-Cas9 based genome editing. With the advancement of genetic, sequencing and imaging tools, brain organoids facilitated the discovery of etiology and brought novel insight into molecular and cellular mechanisms of these brain disorders. For example, some ASD organoids exhibit accelerated cell cycle and overproduction of GABAergic inhibitory neurons, indicating disrupted excitatory-inhibitory neuronal networks in these patients. Further investigation revealed the transcription factor FOXG1 is responsible for the overproduction of GABAergic neurons and its expression is correlated with the severity of the disease (Mariani et al., 2015). In an Angelman syndrome organoid model, the mechanism of synaptic dysfunction caused by ubiquitin protein ligase E3A (UBE3A) was illuminated. UBE3A leads to degradation of calcium- and voltage-dependent big potassium channels and suppresses neuronal hyperexcitability. These disease models also allow researchers to test drug treatments based on phenotypes and to facilitate translational studies (Kang et al., 2021).
Organoids are also a valuable model for studying brain infectious diseases (Cugola et al., 2016; Nowakowski et al., 2016; Qian et al., 2016; Watanabe et al., 2017; Jacob et al., 2020). For example, utilizing organoid models, researchers found that ZIKV causes progenitor cell death and reduced proliferation, resulting in a microcephaly-like phenotype (Dang et al., 2016; Qian et al., 2016). In addition to uncovering the infection mechanism, organoids also facilitated drug design and screening to mitigate the damage of ZIKV on the developing brain (Dang et al., 2016; Watanabe et al., 2017). Together, this recent progress in applying organoids to multi-disciplinary research suggest that brain organoids provide a powerful platform for uncovering the etiology of neural developmental disorders, investigating disease mechanisms and testing drug efficacy for better treatments.
While valuable insights into neurogenesis have been gained from brain organoid studies, there are limitations of this in vitro model in fully recapitulating human brain development. Although organoids have shown advantages in regard to diverse progenitor and neuron types and the fidelity of organoid models has been heavily investigated by comparing the cell types and gene expression at the single cell level in the fetal brain, not all cellular subtypes are present and certain cells show altered gene expression at the molecular level (Amiri et al., 2018; Pollen et al., 2019; Jacob et al., 2021). Thus, further improvement of organoid protocols and culture conditions is needed to better recapitulate neurogenesis in the human brain.
Microglia, a resident immune cell type in the brain, is absent in cortical organoids due to their non-neural lineage. In rodent models, microglia have been shown to play essential roles in neurogenesis of the developing cortex. They adapt their functions in diverse states to regulate programmed cell death and synapse elimination, and have a profound impact on maintaining homeostasis in the brain (Cunningham et al., 2013; Butovsky and Weiner, 2018; Li and Barres, 2018). Thus, it would be beneficial to integrate microglia into brain organoids to better mimic the physiological environment and to study immune response in infectious neurological diseases. Indeed, several groups have attempted to differentiate microglia from stem cells or iPSCs and incorporate them into organoids (Ormel et al., 2018; Song et al., 2019; Xu et al., 2021). Another approach is transplantation of organoids into mouse brains. Progressive microglia integration from the host can be found in human organoid grafts in mouse cortex (Mansour et al., 2018). These methods provide great potential to broaden our understanding of the complexity of neurogenesis under normal and disease conditions.
Following the expansion of organoids during culture, a hypoxic necrotic core inevitably develops due to a lack of vascularization and inefficient oxygen and nutrient exchange. Single cell sequencing data also revealed upregulated glycolytic and ER stress genes in organoids (Amiri et al., 2018; Bhaduri et al., 2020). These cellular stressors are postulated to impair cellular diversity and neuronal differentiation. In addition, endothelial cells from vasculature are crucial for establishing a niche that stimulates the self-renewal of neural stem cells and IPCs (Qin et al., 2004; Wang et al., 2019). Vascularization can be established after engrafting organoids into the mouse brain, as mouse endothelial cells could invade human organoids and establish vasculature for nutrient supply (Daviaud et al., 2018; Mansour et al., 2018). Direct generation of vascularized organoids in vitro has also been attempted, while some features of the human fetal telencephalon can be found, delivery of oxygen and nutrients through the vasculature has not been achieved in vitro (Shi et al., 2020). Successful integration of vascular structure with active flow for nutrient exchange in organoids will not only support better survival, but also facilitate the homeostasis of many cell types, and bring new perspectives on human neurogenesis.
Another major caveat of the organoid model is the variation of morphology and inconsistency. This is caused by differences in the genetic background of stem cells and iPSCs, as well as methods of patterning and culturing. Although brain organoids display some hallmarks of structure and cell type diversity, their size and morphology vary dramatically, generating different qualities and quantities within and between batches. In addition, protocols for organoids differentiation are different across research groups, raising the question of whether the developmental trajectories and cell identities are equivalent using different methodologies. Many groups have addressed the heterogeneity of organoids by transcriptome comparison (Pollen et al., 2019; Velasco et al., 2019; Yoon et al., 2019). However, it is still a challenge to establish a universal organoid differentiation protocol with reproducibility and robustness.
In summary, brain organoids are valuable tools to study brain development and have greatly expanded our toolbox and knowledge of human neurogenesis. With further improvement in organoid technology and incorporation of bioengineering and molecular tools, such as engineered scaffolds, optogenetics and chemical genetics, and synaptic tracing, more advanced experiments can be performed using organoid models to generate novel insights into neurogenesis in the human brain.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
QY contributed the figure and the table. All authors contributed to the article and approved the submitted version.
The research in the authors’ laboratories were supported by grants from the National Institutes of Health (R35NS116843 to HS, and RF1MH123979, R01MH125528, R35NS097370, and U19AI131130 to G-LM) and Sheldon G. Adelson Medical Research Foundation (to G-LM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amiri, A., Coppola, G., Scuderi, S., Wu, F., Roychowdhury, T., Liu, F., et al. (2018). Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 362:eaat6720. doi: 10.1126/science.aat6720
Anderson, S. A., Kaznowski, C. E., Horn, C., Rubenstein, J. L. R., and McConnell, S. K. (2002). Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb. Cortex 12, 702–709. doi: 10.1093/cercor/12.7.702
Andrews, M. G., Subramanian, L., and Kriegstein, A. R. (2020). Mtor signaling regulates the morphology and migration of outer radial glia in developing human cortex. eLife 9, 1–21. doi: 10.7554/ELIFE.58737
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J., and Knoblich, J. A. (2017). Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751. doi: 10.1038/nmeth.4304
Bakken, T. E., Miller, J. A., Ding, S. L., Sunkin, S. M., Smith, K. A., Ng, L., et al. (2016). A comprehensive transcriptional map of primate brain development. Nature 535, 367–375. doi: 10.1038/nature18637
Benito-Kwiecinski, S., Giandomenico, S. L., Sutcliffe, M., Riis, E. S., Freire-Pritchett, P., Kelava, I., et al. (2021). An early cell shape transition drives evolutionary expansion of the human forebrain. Cell 184, 2084.e19–2102.e19. doi: 10.1016/j.cell.2021.02.050
Bershteyn, M., Nowakowski, T. J., Pollen, A. A., di Lullo, E., Nene, A., Wynshaw-Boris, A., et al. (2017). Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435.e4–449.e4. doi: 10.1016/j.stem.2016.12.007
Bhaduri, A., Andrews, M. G., Mancia Leon, W., Jung, D., Shin, D., Allen, D., et al. (2020). Cell stress in cortical organoids impairs molecular subtype specification. Nature 578, 142–148. doi: 10.1038/s41586-020-1962-0
Bhaduri, A., Sandoval-Espinosa, C., Otero-Garcia, M., Oh, I., Yin, R., Eze, U. C., et al. (2021). An atlas of cortical arealization identifies dynamic molecular signatures. Nature 598, 200–204. doi: 10.1038/s41586-021-03910-8
Birey, F., Andersen, J., Makinson, C. D., Islam, S., Wei, W., Huber, N., et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. doi: 10.1038/nature22330
Betizeau, M., Cortay, V., Patti, D., Pfister, S., Gautier, E., Bellemin-Ménard, A., et al. (2013). Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80, 442–457. doi: 10.1016/j.neuron.2013.09.032
Butovsky, O., and Weiner, H. L. (2018). Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635. doi: 10.1038/s41583-018-0057-5
Camp, J. G., Badsha, F., Florio, M., Kanton, S., Gerber, T., Wilsch-Bräuninger, M., et al. (2015). Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. U.S.A. 112, 15672–15677. doi: 10.1073/pnas.1520760112
Cheung, A. F. P., Kondo, S., Abdel-Mannan, O., Chodroff, R. A., Sirey, T. M., Bluy, L. E., et al. (2010). The subventricular zone is the developmental milestone of a 6-layered neocortex: comparisons in metatherian and eutherian mammals. Cereb. Cortex 20, 1071–1081. doi: 10.1093/cercor/bhp168
Cugola, F. R., Fernandes, I. R., Russo, F. B., Freitas, B. C., Dias, J. L. M., Guimarães, K. P., et al. (2016). The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271. doi: 10.1038/nature18296
Cunningham, C. L., Martínez-Cerdeño, V., and Noctor, S. C. (2013). Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013
Dang, J., Tiwari, S. K., Lichinchi, G., Qin, Y., Patil, V. S., Eroshkin, A. M., et al. (2016). Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258–265. doi: 10.1016/j.stem.2016.04.014
Daviaud, N., Friedel, R. H., and Zou, H. (2018). Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. eNeuro 5:ENEURO.0219-18.2018. doi: 10.1523/ENEURO.0219-18.2018
Denayer, T., Stöhrn, T., and van Roy, M. (2014). Animal models in translational medicine: validation and prediction. New Horizons Transl. Med. 2, 5–11. doi: 10.1016/j.nhtm.2014.08.001
Dezonne, R. S., Sartore, R. C., Nascimento, J. M., Saia-Cereda, V. M., Romaõ, L. F., Alves-Leon, S. V., et al. (2017). Derivation of functional human astrocytes from cerebral organoids. Sci. Rep. 7:45091. doi: 10.1038/srep45091
Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura, S., Matsumura, M., et al. (2008). Self-Organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532. doi: 10.1016/j.stem.2008.09.002
Fiddes, I. T., Lodewijk, G. A., Mooring, M., Bosworth, C. M., Ewing, A. D., Mantalas, G. L., et al. (2018). Human-Specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 173, 1356.e22–1369.e22. doi: 10.1016/j.cell.2018.03.051
Fietz, S. A., Kelava, I., Vogt, J., Wilsch-Bräuninger, M., Stenzel, D., Fish, J. L., et al. (2010). OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699. doi: 10.1038/nn.2553
Gaspard, N., Bouschet, T., Hourez, R., Dimidschstein, J., Naeije, G., van den Ameele, J., et al. (2008). An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357. doi: 10.1038/nature07287
Giandomenico, S. L., Mierau, S. B., Gibbons, G. M., Wenger, L. M. D., Masullo, L., Sit, T., et al. (2019). Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679. doi: 10.1038/s41593-019-0350-2
Gordon, A., Yoon, S. J., Tran, S. S., Makinson, C. D., Park, J. Y., Andersen, J., et al. (2021). Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 24, 331–342. doi: 10.1038/s41593-021-00802-y
Gorski, J. A., Talley, T., Qiu, M., Puelles, L., Rubenstein, J. L., and Jones, K. R. (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002
Götz, M., and Huttner, W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788. doi: 10.1038/nrm1739
Hansen, D. V., Lui, J. H., Parker, P. R. L., and Kriegstein, A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. doi: 10.1038/nature08845
Hartenstein, V., and Stollewerk, A. (2015). The evolution of early neurogenesis. Dev. Cell 32, 390–407. doi: 10.1016/j.devcel.2015.02.004
Haubensak, W., Attardo, A., Denk, W., Huttner, W. B., and Simons, K. (2004). Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 101, 3196–3201. doi: 10.1073/pnas.0308600100
Hodge, R. D., Bakken, T. E., Miller, J. A., Smith, K. A., Barkan, E. R., Graybuck, L. T., et al. (2019). Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68. doi: 10.1038/s41586-019-1506-7
Huang, W. K., Wong, S. Z. H., Pather, S. R., Nguyen, P. T. T., Zhang, F., Zhang, D. Y., et al. (2021). Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28, 1657.e10–1670.e10. doi: 10.1016/j.stem.2021.04.006
Jacob, F., Pather, S. R., Huang, W. K., Zhang, F., Wong, S. Z. H., Zhou, H., et al. (2020). Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 27, 937.e9–950.e9. doi: 10.1016/j.stem.2020.09.016
Jacob, F., Schnoll, J. G., Song, H., and Ming, G. (2021). “Building the brain from scratch: engineering region-specific brain organoids from human stem cells to study neural development and disease. Curr. Topics Dev. Biol. 142, 477–530. doi: 10.1016/bs.ctdb.2020.12.011
Jo, J., Xiao, Y., Sun, A. X., Cukuroglu, E., Tran, H. D., Göke, J., et al. (2016). Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257. doi: 10.1016/j.stem.2016.07.005
Johnstone, M., Vasistha, N. A., Barbu, M. C., Dando, O., Burr, K., Christopher, E., et al. (2019). Reversal of proliferation deficits caused by chromosome 16p13.11 microduplication through targeting NFκB signaling: an integrated study of patient-derived neuronal precursor cells, cerebral organoids and in vivo brain imaging. Mol. Psychiatry 24, 294–311. doi: 10.1038/s41380-018-0292-1
Kadoshima, T., Sakaguchi, H., Nakano, T., Soen, M., Ando, S., Eiraku, M., et al. (2013). Correction Correction for “Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. U.S.A. 110, 20284–20289. doi: 10.1073/pnas
Kang, Y., Zhou, Y., Li, Y., Han, Y., Xu, J., Niu, W., et al. (2021). A human forebrain organoid model of fragile X syndrome exhibits altered neurogenesis and highlights new treatment strategies. Nat. Neurosci. 24, 1377–1391. doi: 10.1038/s41593-021-00913-6
Kanton, S., Boyle, M. J., He, Z., Santel, M., Weigert, A., Sanchís-Calleja, F., et al. (2019). Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422. doi: 10.1038/s41586-019-1654-9
Karzbrun, E., Kshirsagar, A., Cohen, S. R., Hanna, J. H., and Reiner, O. (2018). Human brain organoids on a chip reveal the physics of folding. Nat. Phys. 14, 515–522. doi: 10.1038/s41567-018-0046-7
Kast, R. J., and Levitt, P. (2019). Precision in the development of neocortical architecture: from progenitors to cortical networks. Progr. Neurobiol. 175, 77–95. doi: 10.1016/j.pneurobio.2019.01.003
Kosodo, Y., Röper, K., Haubensak, W., Marzesco, A. M., Corbeil, D., and Huttner, W. B. (2004). Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mamalian neuroepithelial cells. EMBO J. 23, 2314–2324. doi: 10.1038/sj.emboj.7600223
Lancaster, M. A., and Knoblich, J. A. (2014). Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340. doi: 10.1038/nprot.2014.158
Lancaster, M. A., Renner, M., Martin, C. A., Wenzel, D., Bicknell, L. S., Hurles, M. E., et al. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. doi: 10.1038/nature12517
Letinic, K., and Rakic, P. (2001). Telencephalic origin of human thalamic GABAergic neurons. Nat. Neurosci. 4, 931–936. doi: 10.1038/nn0901-931
Letinic, K., Zoncu, R, and Rakic, P. (2002). Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. doi: 10.1038/nature00779
Li, Q., and Barres, B. A. (2018). Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242. doi: 10.1038/nri.2017.125
Li, Y., Muffat, J., Omer, A., Bosch, I., Lancaster, M. A., Sur, M., et al. (2017). Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 20, 385.e3–396.e3. doi: 10.1016/j.stem.2016.11.017
Liu, J., and Silver, D. L. (2021). Founder cells shape brain evolution. Cell 184, 1965–1967. doi: 10.1016/j.cell.2021.03.045
Lui, J. H., Hansen, D. V., and Kriegstein, A. R. (2011). Development and evolution of the human neocortex. Cell 146, 18–36. doi: 10.1016/j.cell.2011.06.030
Madhavan, M., Nevin, Z. S., Shick, H. E., Garrison, E., Clarkson-Paredes, C., Karl, M., et al. (2018). Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 15, 700–706. doi: 10.1038/s41592-018-0081-4
Mansour, A. A., Gonçalves, J. T., Bloyd, C. W., Li, H., Fernandes, S., Quang, D., et al. (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36, 432–441. doi: 10.1038/nbt.4127
Mariani, J., Coppola, G., Zhang, P., Abyzov, A., Provini, L., Tomasini, L., et al. (2015). FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390. doi: 10.1016/j.cell.2015.06.034
Marton, R. M., Miura, Y., Sloan, S. A., Li, Q., Revah, O., Levy, R. J., et al. (2019). Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 22, 484–491. doi: 10.1038/s41593-018-0316-9
Mellios, N., Feldman, D. A., Sheridan, S. D., Ip, J. P. K., Kwok, S., Amoah, S. K., et al. (2018). MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry 23, 1051–1065. doi: 10.1038/mp.2017.86
Meyer, G., Schaaps, J. P., Moreau, L., and Goffinet, A. M. (2000). Embryonic and early fetal development of the human neocortex. J. Neurosci. 20, 1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000
Mira, H., and Morante, J. (2020). Neurogenesis from embryo to adult – lessons from flies and mice. Front. Cell Dev. Biol. 8:533. doi: 10.3389/fcell.2020.00533
Monzel, A. S., Smits, L. M., Hemmer, K., Hachi, S., Moreno, E. L., van Wuellen, T., et al. (2017). Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 8, 1144–1154. doi: 10.1016/j.stemcr.2017.03.010
Muguruma, K., Nishiyama, A., Kawakami, H., Hashimoto, K., and Sasai, Y. (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550. doi: 10.1016/j.celrep.2014.12.051
Nascimento, J. M., Saia-Cereda, V. M., Sartore, R. C., da Costa, R. M., Schitine, C. S., Freitas, H. R., et al. (2019). Human cerebral organoids and fetal brain tissue share proteomic similarities. Front. Cell Dev. Biol. 7:303. doi: 10.3389/fcell.2019.00303
Navabpour, S., Kwapis, J. L., and Jarome, T. J. (2020). A neuroscientist’s guide to transgenic mice and other genetic tools. Neurosci. Biobehav. Rev. 108, 732–748. doi: 10.1016/j.neubiorev.2019.12.013
Nowakowski, T. J., Pollen, A. A., di Lullo, E., Sandoval-Espinosa, C., Bershteyn, M., and Kriegstein, A. R. (2016). Expression analysis highlights AXL as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell 18, 591–596. doi: 10.1016/j.stem.2016.03.012
Ogura, T., Sakaguchi, H., Miyamoto, S., and Takahashi, J. (2018). Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development (Cambridge) 145:dev162214. doi: 10.1242/dev.162214
O’Neill, A. C., Kyrousi, C., Klaus, J., Leventer, R. J., Kirk, E. P., Fry, A., et al. (2018). A primate-specific isoform of PLEKHG6 regulates neurogenesis and neuronal migration. Cell Rep. 25, 2729.e6–2741.e6. doi: 10.1016/j.celrep.2018.11.029
Ormel, P. R., Vieira de Sá, R., van Bodegraven, E. J., Karst, H., Harschnitz, O., Sneeboer, M. A. M., et al. (2018). Microglia innately develop within cerebral organoids. Nat. Commun. 9:4167. doi: 10.1038/s41467-018-06684-2
Otani, T., Marchetto, M. C., Gage, F. H., Simons, B. D., and Livesey, F. J. (2016). 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467–480. doi: 10.1016/j.stem.2016.03.003
Pellegrini, L., Bonfio, C., Chadwick, J., Begum, F., Skehel, M., and Lancaster, M. A. (2020). Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369:eaaz5626. doi: 10.1126/science.aaz5626
Pollen, A. A., Bhaduri, A., Andrews, M. G., Nowakowski, T. J., Meyerson, O. S., Mostajo-Radji, M. A., et al. (2019). Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743.e17–756.e17. doi: 10.1016/j.cell.2019.01.017
Prodromidou, K., and Matsas, R. (2019). Species-Specific miRNAs in human brain development and disease. Front. Cell. Neurosci. 13:559. doi: 10.3389/fncel.2019.00559
Qian, X., Nguyen, H. N., Song, M. M., Hadiono, C., Ogden, S. C., Hammack, C., et al. (2016). Brain-Region-Specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254. doi: 10.1016/j.cell.2016.04.032
Qian, X., Su, Y., Adam, C. D., Deutschmann, A. U., Pather, S. R., Goldberg, E. M., et al. (2020). Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 26, 766.e9–781.e9. doi: 10.1016/j.stem.2020.02.002
Qin, S., Goderie, S. K., Li, J., Nithin, K., Yu, S., Natalia, A., et al. (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340. doi: 10.1126/science.1095505
Quadrato, G., Nguyen, T., Macosko, E. Z., Sherwood, J. L., Yang, S. M., Berger, D. R., et al. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53. doi: 10.1038/nature22047
Rakic, P. (2009). Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735. doi: 10.1038/nrn2719
Rash, B. G., Duque, A., Morozov, Y. M., Arellano, J. I., Micali, N., and Rakic, P. (2019). Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc. Natl. Acad. Sci. U.S.A. 116, 7089–7094. doi: 10.1073/pnas.1822169116
Renner, M., Lancaster, M. A., Bian, S., Choi, H., Ku, T., Peer, A., et al. (2017). Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 36, 1316–1329. doi: 10.15252/embj.201694700
Sakaguchi, H., Kadoshima, T., Soen, M., Narii, N., Ishida, Y., Ohgushi, M., et al. (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 6:8896. doi: 10.1038/ncomms9896
Scuderi, S., Altobelli, G. G., Cimini, V., Coppola, G., and Vaccarino, F. M. (2021). Cell-to-Cell adhesion and neurogenesis in human cortical development: a study comparing 2D monolayers with 3D organoid cultures. Stem Cell Rep. 16, 264–280. doi: 10.1016/j.stemcr.2020.12.019
Shi, Y., Kirwan, P., Smith, J., Robinson, H. P. C., and Livesey, F. J. (2012). Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 15, 477–486. doi: 10.1038/nn.3041
Shi, Y., Sun, L., Wang, M., Liu, J., Zhong, S., Li, R., et al. (2020). Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18:e3000705. doi: 10.1371/journal.pbio.3000705
Sloan, S. A., Darmanis, S., Huber, N., Khan, T. A., Birey, F., Caneda, C., et al. (2017). Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779.e6–790.e6. doi: 10.1016/j.neuron.2017.07.035
Song, L., Yuan, X., Jones, Z., Vied, C., Miao, Y., Marzano, M., et al. (2019). Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci. Rep. 9:11055. doi: 10.1038/s41598-019-47444-6
Srikanth, P., Lagomarsino, V. N., Muratore, C. R., Ryu, S. C., He, A., Taylor, W. M., et al. (2018). Shared effects of DISC1 disruption and elevated WNT signaling in human cerebral organoids. Transl. Psychiatry 8:77. doi: 10.1038/s41398-018-0122-x
Stachowiak, E. K., Benson, C. A., Narla, S. T., Dimitri, A., Chuye, L. E. B., Dhiman, S., et al. (2017). Cerebral organoids reveal early cortical maldevelopment in schizophrenia—computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 7:6. doi: 10.1038/s41398-017-0054-x
Subramanian, L., Bershteyn, M., Paredes, M. F., and Kriegstein, A. R. (2017). Dynamic behaviour of human neuroepithelial cells in the developing forebrain. Nat. Commun. 8:14167. doi: 10.1038/ncomms14167
Sun, A. X., Yuan, Q., Fukuda, M., Yu, W., Yan, H., Lim, G. G. Y., et al. (2019). Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 366, 1486–1492. doi: 10.1126/science.aav5386
Tanaka, Y., Cakir, B., Xiang, Y., Sullivan, G. J., and Park, I. H. (2020). Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30, 1682.e3–1689.e3. doi: 10.1016/j.celrep.2020.01.038
Taverna, E., Götz, M., and Huttner, W. B. (2014). The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502. doi: 10.1146/annurev-cellbio-101011-155801
Trujillo, C. A., Gao, R., Negraes, P. D., Gu, J., Buchanan, J., Preissl, S., et al. (2019). Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558.e7–569.e7. doi: 10.1016/j.stem.2019.08.002
Velasco, S., Kedaigle, A. J., Simmons, S. K., Nash, A., Rocha, M., Quadrato, G., et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527. doi: 10.1038/s41586-019-1289-x
Wang, J., Cui, Y., Yu, Z., Wang, W., Cheng, X., Ji, W., et al. (2019). Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25, 754.e9–767.e9. doi: 10.1016/j.stem.2019.09.009
Wang, P., Mokhtari, R., Pedrosa, E., Kirschenbaum, M., Bayrak, C., Zheng, D., et al. (2017). CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol. Autism 8:11. doi: 10.1186/s13229-017-0124-1
Watanabe, M., Buth, J. E., Vishlaghi, N., de la Torre-Ubieta, L., Taxidis, J., Khakh, B. S., et al. (2017). Self-organized cerebral organoids with human-specific features predict effective drugs to combat zika virus infection. Cell Rep. 21, 517–532. doi: 10.1016/j.celrep.2017.09.047
Xiang, Y., Tanaka, Y., Cakir, B., Patterson, B., Kim, K. Y., Sun, P., et al. (2019). hESC-Derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24, 487.e7–497.e7. doi: 10.1016/j.stem.2018.12.015
Xiang, Y., Tanaka, Y., Patterson, B., Kang, Y. J., Govindaiah, G., Roselaar, N., et al. (2017). Fusion of regionally specified hPSC-Derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383.e7–398.e7. doi: 10.1016/j.stem.2017.07.007
Xu, R., Boreland, A. J., Li, X., Erickson, C., Jin, M., Atkins, C., et al. (2021). Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 16, 1923–1937. doi: 10.1016/j.stemcr.2021.06.011
Ye, F., Kang, E., Yu, C., Qian, X., Jacob, F., Yu, C., et al. (2017). DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron 96, 1041.e5–1054.e5. doi: 10.1016/j.neuron.2017.10.010
Keywords: brain organoids, neurogenesis, neural development, stem cell, induced pluripotent stem cells
Citation: Yang Q, Hong Y, Zhao T, Song H and Ming G-l (2022) What Makes Organoids Good Models of Human Neurogenesis? Front. Neurosci. 16:872794. doi: 10.3389/fnins.2022.872794
Received: 10 February 2022; Accepted: 02 March 2022;
Published: 14 April 2022.
Edited by:
Gerd Kempermann, German Center for Neurodegenerative Diseases (DZNE), GermanyReviewed by:
Orly Reiner, Weizmann Institute of Science, IsraelCopyright © 2022 Yang, Hong, Zhao, Song and Ming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-li Ming, Z21pbmdAcGVubm1lZGljaW5lLnVwZW5uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.