- 1Department of Rehabilitation Medicine, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Department of Neurosurgery, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Clinical Medical of Acupuncture Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
Objective: The objectives of the study were to evaluate the efficacy of repetitive transcranial magnetic stimulation (rTMS) treatment for poststroke dysphagia (PSD) and explore the optimal stimulation parameters.
Method: The databases of Medline, Embase, Web of Science, and Cochrane Library were searched from the establishment to June 2021. All randomized controlled trials about rTMS treatment for PSD were enrolled. Dysphagia Grade (DG) and Penetration Aspiration Scale (PAS) were applied as the major dysphagia severity rating scales to evaluate the outcomes.
Results: A total of 12 clinical randomized controlled studies were included in our study. The summary effect size indicated that rTMS had a positive effect on PSD (SMD = −0.67, p < 0.001). The subgroup analysis for treatment duration and different stroke stages showed significant differences (treatment duration >5 days: SMD = −0.80, p < 0.001; subacute phase after stroke: SMD = −0.60, p < 0.001). Furthermore, no significant differences were observed among the other stimulation parameter subgroups (including stimulation frequency, location, and a single stimulation time) (p > 0.05).
Conclusion: rTMS is beneficial to the recovery of PSD patients, while an intervention of more than 5 days and in the subacute phase after stroke might bring new strategies and rational therapeutics to the treatment of PSD.
Systematic Review Registration: http://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022299469.
Introduction
Poststroke dysphagia (PSD) is a common after stroke, with an incidence ranging from 27 to 78% (Rofes et al., 2013; Benjamin et al., 2018; Zhong et al., 2021). The clinical complications of PSD include malnutrition, aspiration pneumonia, dehydration, social difficulties, etc. (Ekberg et al., 2002; Clavé et al., 2012; Kalra et al., 2015), which could lead to an increase in medical expenses and mortality, and impairment of the quality of life (Altman et al., 2010; Sasegbon and Hamdy, 2017). The regular treatment of PSD mainly includes oropharyngeal exercises, expiratory muscle training, compensation strategies (Langmore and Miller, 1994; Rogus-Pulia and Robins, 2013; Zhong et al., 2021), neuromuscular electrical stimulation (MMES) (Speyer et al., 2010; Mao et al., 2021; Zhong et al., 2021), invasive procedures, and noninvasive brain stimulation (NIBS) (Ludlow et al., 2007).
Two major NIBS techniques are repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), which offer the advantage of relatively wide availability, comfort, convenience, and lower side effects for the PSD patient as they are noninvasive interventions. Since rTMS and tDCS can also induce long-lasting effects, these techniques can be also used as adjuvant strategies for the treatment of psychiatric disorders (such as depression and bipolar disorder) and the rehabilitation of other neurological deficits (Fregni and Pascual-Leone, 2007; Sandrini et al., 2015; De Risio et al., 2020; Konstantinou et al., 2021; Pateraki et al., 2022).
The rTMS is an electromagnetic technique in which rapidly changing magnetic fields are used to induce the change in electrical currents and synaptic plasticity in the brain continuously (Pascual-Leone et al., 1998; Ridding and Rothwell, 2007). These changes can further alter cortical excitability and associated behaviors (Fiske et al., 2009; Schneider et al., 2010). Over the past decade, rTMS as a practical technique has proved effective and been widely used to promote the recovery of PSD (Verin and Leroi, 2009). The balance of neural activities between the bilateral cerebral hemispheres is dramatically disturbed and impaired after stroke—which is caused by the altered corpus callosum suppression, while rTMS could be used to restore this balance and improve the swallowing function (Gow et al., 2004; Du et al., 2016; Ünlüer et al., 2019; Zhong et al., 2021).
Various stimulation parameters in rTMS were demonstrated to be highly related to different effects, including intensity, frequency, threshold, etc. (Khedr et al., 2009; Park, 2013; Lee et al., 2015; Du et al., 2016). However, it seems that no consensus has been reached on the optimal parameters of rTMS on PSD at present (Khedr et al., 2009; Verin and Leroi, 2009). Therefore, we conducted this systematic analysis of RCT studies to assess the clinical efficacy of rTMS on PSD and explore the optimal parameters of rTMS to provide evidence for the clinical treatment of PSD.
Materials and Methods
Search Strategies
Medline, Embase, Web of Science, and the Cochrane Library were systematically searched from the establishment to June 2021 in English. We manually searched the related systematic reviews and further references, and searched databases with the MeSH term “dysphagia” or “swallowing disorders” or “deglutition” and “transcranial magnetic stimulation” or “rTMS” of the searching strategies for four electronic database searches. The search was limited to “randomized controlled trials,” and the full search strategy is detailed in the Supplementary Appendix. The retrieval was based on the subject terms, keywords, or titles. References from studies with potential relevance and review articles were manually checked. This study was registered with PROSPERO (CRD42022299469).
Inclusion and Exclusion Criteria
Studies that were included in this meta-analysis met the following criteria: (1) randomized controlled trials (RCT), including cross-over and cluster RCTs; (2) patients with PSD (confirmed by VFSS, FEES, swallowing questionnaire, or dysphagic outcome and severity scale); (3) patients enrolled in studies with age ≥18 years; (4) the treatment of the experimental group was rTMS, and the researchers provided original data or sufficient information about dysphagia that occurred pre- and posttreatment in experimental trials and control trials. Exclusion criteria were: (1) publications that did not offer original data, such as reviews, meta-analysis, systematic review; (2) publications that did not meet the inclusion criteria.
Outcome Measures
The primary efficacy outcome was the change in dysphagia severity rating scales of the PSD from baseline to postintervention, which was measured by Dysphagia Grade (DG), Penetration Aspiration Scale (PAS) at the present study.
Data Extraction and Quality Assessment
Data were independently extracted by two well-trained evaluators (JQ and ZLD) to review original texts, supplementary appendices, and protocols. The disagreements were solved by the third author's assistance. The study characteristics included publication year, first author, journal, and patient characteristics included diagnostic criteria, age, sample size, gender, etiology, lesion side, Barthel index, and different stroke stages. The treatment duration, stimulation frequency, location, threshold and a single stimulation time were also extracted. Furthermore, the adverse events of rTMS were included. The methodological quality of the researches was assessed in accordance with the risk of bias tool described in the Cochrane Handbook for systematic reviews of interventions (Cochrane Handbook 5.2) (Higgins and Green, 2019). Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other potential risks of bias were assessed. Trials that exceeded one risk assessment were considered to have a high risk of bias. Trials with all low-risk assessments were judged to have a low risk of bias; otherwise, they were considered to be at unclear risk of bias.
Statistical Analysis
The principal emphasis of this meta-analysis was to analyze the efficacy of rTMS for PSD and assess the optimal parameters for rTMS on PSD. The measurements were expressed as standardized mean difference (SMD) with 95% confidence intervals (CI) for continuous outcomes. The funnel plots were used to detect possible publication bias. A heterogeneity test was performed using the I2 statistic; when I2 ≥ 50%, the random-effect models were used for data analysis. Otherwise, when I2 < 50%, the fixed effect model was performed (Yang et al., 2015). I2 < 25% was recognized as low heterogeneity, 25% < I2 < 75% as moderate heterogeneity, and I2 > 75% as high heterogeneity (Ampt et al., 2018). Subgroup analyses were performed to explore the optimal parameters of rTMS on PSD, including the treatment duration, different stroke stages, stimulation frequency, a single stimulation time, stimulation location, and stimulation pattern. We also performed sensitivity analyses for efficacy outcomes, in which low-quality studies would be excluded when I2 > 50%. Analyses were conducted using Review Manager (RevMan) version 5.3 (Cochrane Collaboration, London, UK). A p-value <0.05 was considered statistically significant.
Results
Characteristics of Studies
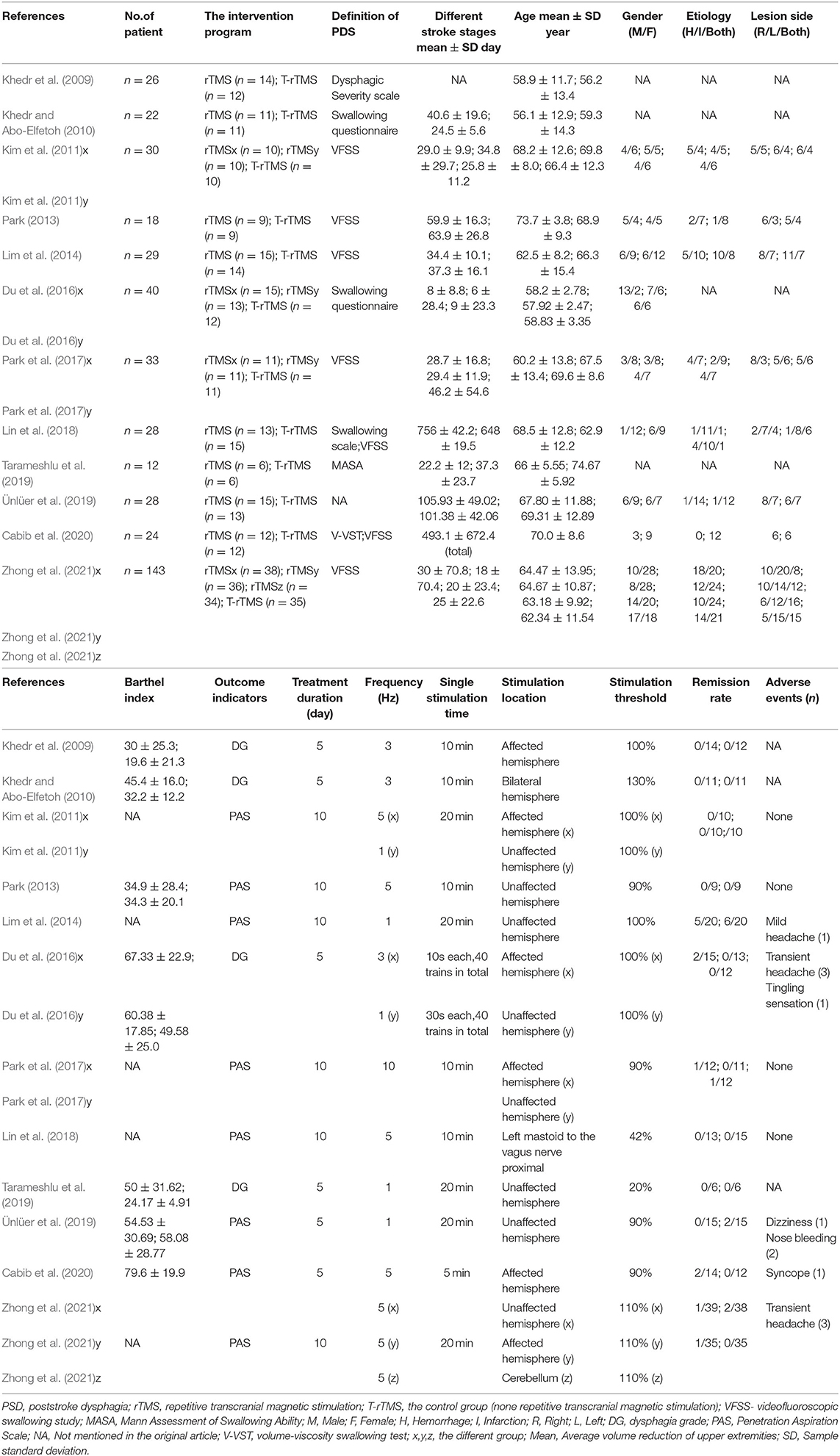
The initial search identified 1,448 studies from four databases, of which 41 full-text articles were carefully screened by the authors. The PRISMA statement flowchart shows the process of literature screening, study selection, and reasons with exclusion (Figure 1). Only 12 high-quality articles included in our meta-analysis met the stringent criteria. The final study group was comprised of 433 patients, including 273 in the treatment group and 160 in the control group. The duration of treatment ranged from 1 to 10 days, and the stimulation frequency ranged from 1 to 10 Hz. The time for stroke onset of the included subjects ranged from 8 to 756 days. The single stimulation time varied from 5 to 20 min (Table 1).
Adverse Events
A total of five studies reported the presence of discomfort after rTMS, including mild or transient headache, tingling sensation, dizziness, nose bleeding, and syncope (Lim et al., 2014; Du et al., 2016; Ünlüer et al., 2019; Cabib et al., 2020; Zhong et al., 2021). To sum up, 7 of 273 participants (2.5%) had mild or transient headache, 1 (0.4%) had tingling sensation, 1 (0.4%) had dizziness, 2 (0.7%) had nose bleeding, and 1 (0.4%) with syncope after rTMS intervention. Reassuringly, four studies reported no adverse events (Kim et al., 2011; Park, 2013; Park et al., 2017; Lin et al., 2018), whereas the remaining studies did not mention the adverse events after rTMS (Khedr et al., 2009; Khedr and Abo-Elfetoh, 2010; Tarameshlu et al., 2019).
Efficacy Outcomes
The overall pooled SMD analyzed by fixed model showed a significant advantage of rTMS interventions compared with control conditions (SMD = −0.67; 95% CI −0.88 to −0.45, p < 0.001), with moderate heterogeneity (I2 = 42%, p = 0.06), suggesting that the changes in PAS and DG in the rTMS group were much better than those in the control conditions (Figure 2A). Although outcome analysis indicated that 16 participants dropped out in the rTMS group compared with seven participants in the control conditions (Figure 2B), there was no statistical difference between the two groups (RR = 1.20, 95% CI 0.54–2.66, p = 0.65), with low heterogeneity (I2 = 0%, p = 0.69).
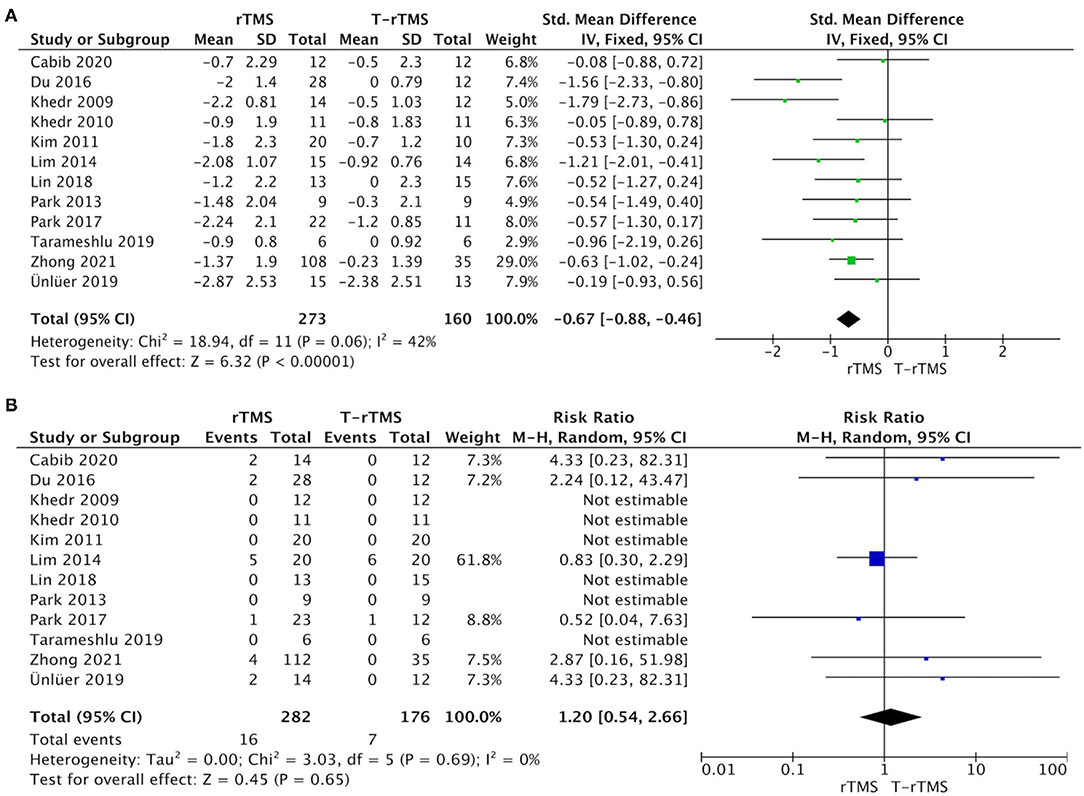
Figure 2. Primary efficacy outcomes and acceptability outcomes. (A) Forest plot of the standardized mean difference (SMD) for change scores in dysphagia severity rating scales. (B) Forest plot of risk ratios (with 95% confidence intervals) of dropout for any reason.
Subgroup Analyses
We conducted multiple subgroups to explore the optimal parameters for rTMS on PSD, including the treatment duration (>5 vs. <5 days), different stroke stages (subacute vs. recovery phase), stimulation frequency (1 vs. ≥3 Hz), single stimulation time (≤10 vs. >10 min), stimulation location (affected vs. unaffected hemisphere), stimulation pattern (high frequency in the affected hemisphere, high frequency in the unaffected hemisphere, low frequency in the unaffected hemisphere), and age (≤65 vs. >65 years).
Treatment Duration
Duration subgroup analysis demonstrated that treatment >5 days showed a higher effect size (SMD = −0.80; 95% CI, −1.08 to −0.52, p < 0.001), with low heterogeneity (I2 = 11%, p = 0.34) than the control conditions. However, the result in the <5-day group was opposite (SMD = −0.50; 95% CI, −1.26 to 0.26, p = 0.20), showing a moderate heterogeneity (I2 = 70%, p = 0.02) (Figure 3A).
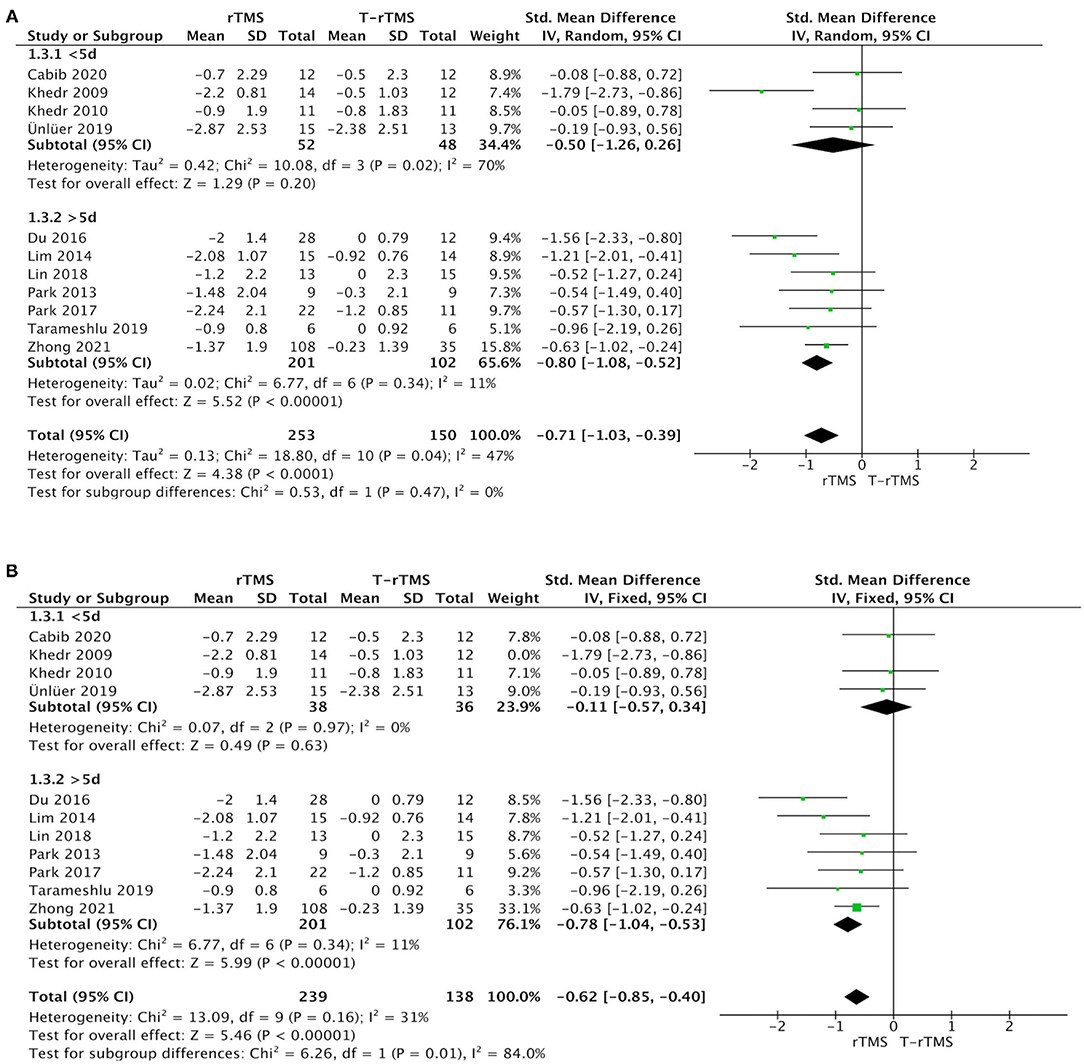
Figure 3. Subgroup analysis of primary outcomes. (A) Forest plot of the standardized mean difference (SMD) for change scores in dysphagia severity rating scales by the treatment duration. (B) Sensitive analysis (excluded the Khedr et al., 2009). PSD, poststroke dysphagia; rTMS, repetitive transcranial magnetic stimulation; T-rTMS, the control group; Mean, Average volume reduction of upper extremities; SD, Sample standard deviation.
Different Stroke Stages
Stroke stage subgroup analysis showed that in the subacute phase (<60 days) after stroke (Bath et al., 2018), rTMS group was significantly more beneficial than control conditions (SMD = −0.60; 95% CI, −0.85 to −0.35, p < 0.001), with moderate heterogeneity (I2 = 43%, p = 0.10). However, rTMS at the recovery phase (>60 days) failed to show a higher effect size than the control conditions (SMD = −0.32; 95% CI, −0.72 to 0.08, p = 0.12), with low heterogeneity (I2 = 0%, p = 0.82) (Figure 4).
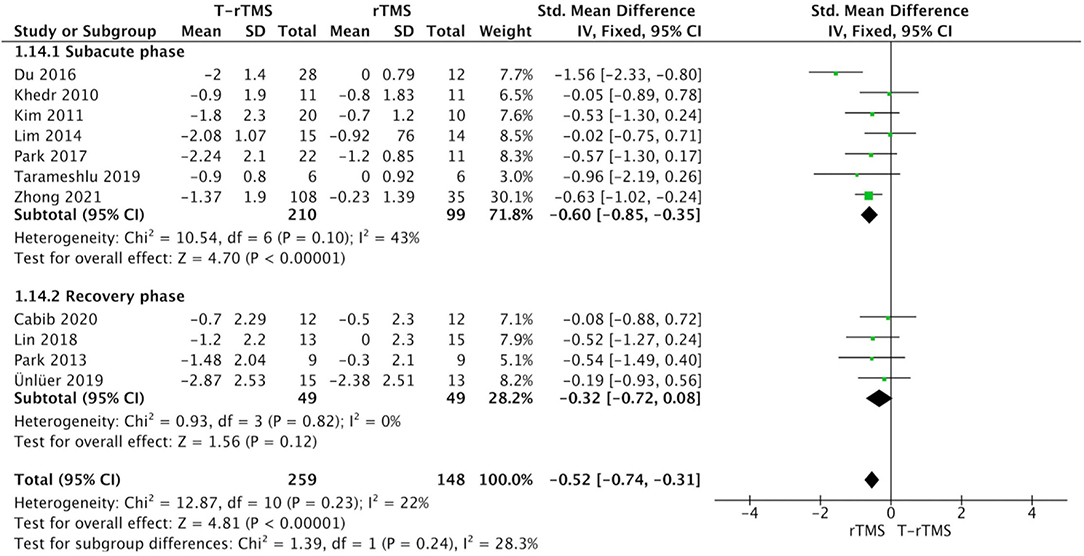
Figure 4. Subgroup analysis of primary outcomes: forest plot of the standardized mean difference (SMD) for change scores in dysphagia severity rating scales by the different stroke stages. PSD, poststroke dysphagia; rTMS, repetitive transcranial magnetic stimulation; T-rTMS, the control group; Mean, Average volume reduction of upper extremities; SD, Sample standard deviation.
Single Stimulation Time
No significant difference was found between ≤10- and >10-min groups (p = 0.77). According to the single stimulation time, studies in which there were ≤10 min (SMD = −0.71; 95% CI, −1.02 to −0.40, p < 0.001) and studies with >10 min (SMD = −0.64; 95% CI, −0.93 to −0.36, p < 0.001) both showed a higher effect size than the control conditions (Table 2).
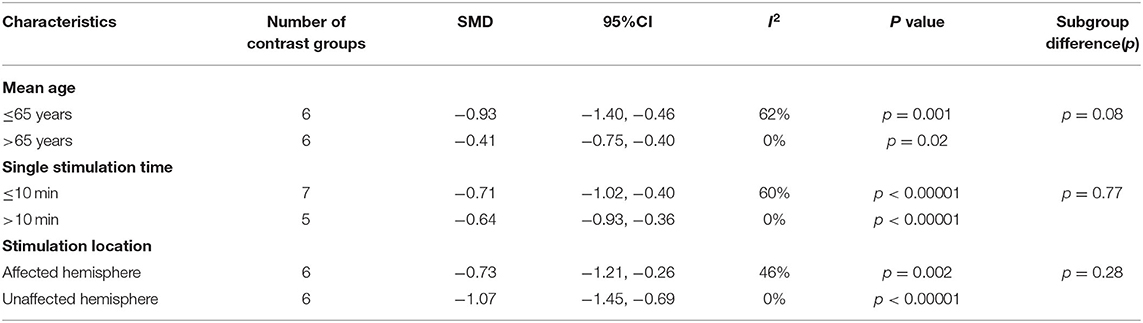
Table 2. Meta-analyses of studies examining the effects of rTMS interventions on mean age, single stimulation period, location and pattern compared with control conditions: overall results and subgroup analyses.
Stimulation Frequency
Stimulation frequency subgroup analysis showed that no significant difference was found between the low- (1 Hz) and high-frequency groups (≥3 Hz) (p = 0.23). Studies with low frequency (SMD = −1.01; 95% CI, −1.64 to −0.38, p = 0.002) and high frequency (SMD = −0.58; 95% CI, −0.91 to −0.25, p < 0.001) both indicated a higher effect size than the control conditions (Figure 5A).
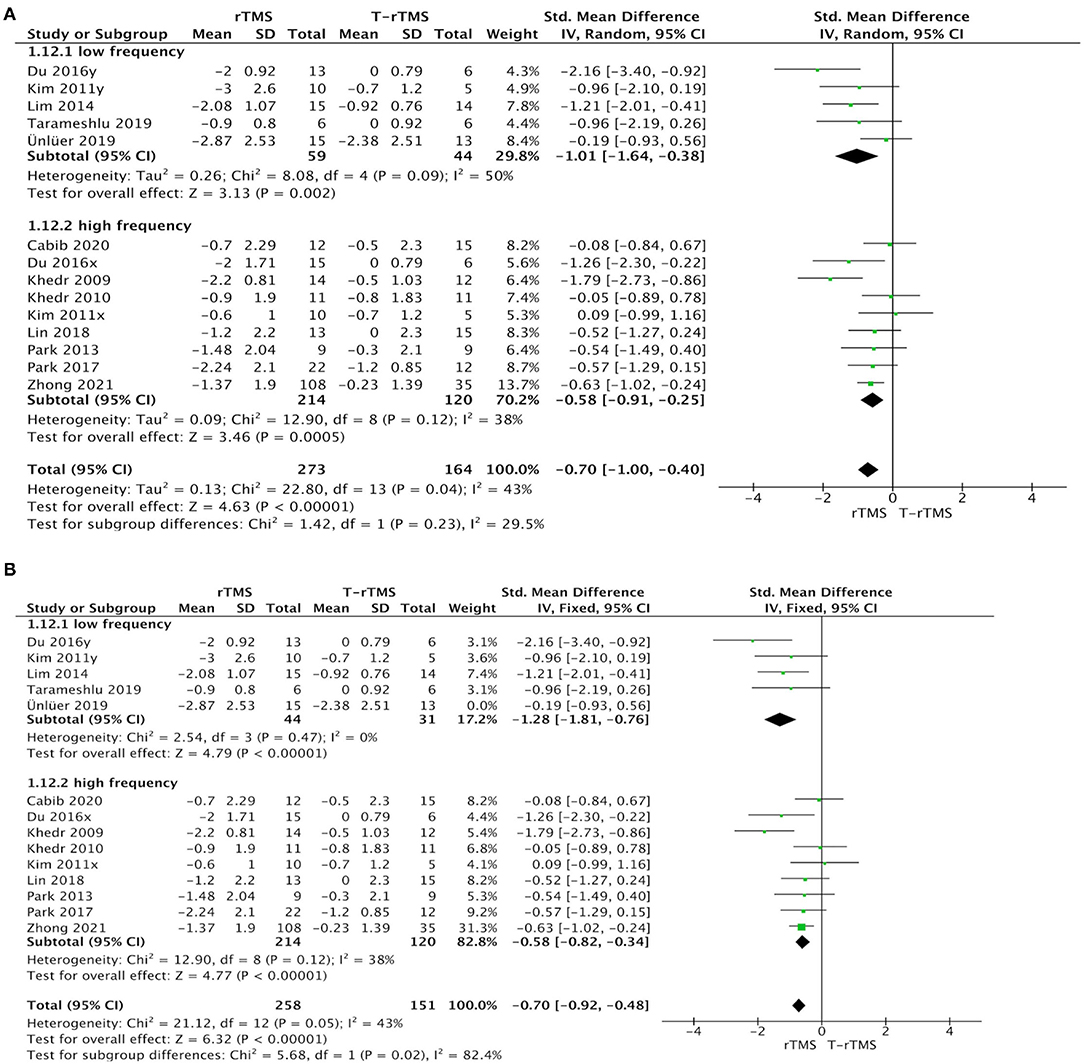
Figure 5. Subgroup analysis of primary outcomes. (A) Forest plot of the standardized mean difference (SMD) for change scores in dysphagia severity rating scales by the stimulation frequency. (B) Sensitive analysis (excluded the Ünlüer et al., 2019).
Stimulation Location
Stimulation location subgroup analysis showed that both the affected hemisphere stimulation (SMD = −0.73; 95% CI, −1.21 to −0.26, p = 0.002) and the unaffected hemisphere stimulation (SMD = −1.07; 95% CI, −1.45 to −0.69, p < 0.001) had a higher effect size than the control conditions. However, no significant difference was found between the two groups (p = 0.28) (Table 2).
Stimulation Pattern
The rTMS group exhibited a significant effect size than the control condition when patients adopted a high frequency in the affected hemisphere (SMD = −0.88; 95% CI, −1.51 to −0.24, p = 0.007), a high frequency in the unaffected hemisphere (SMD = −0.59; 95% CI, −1.07 to −0.10, p = 0.02), and a low frequency in the unaffected hemisphere (SMD = −1.01; 95% CI, −1.64 to −0.38, p = 0.002) (Figure 6).
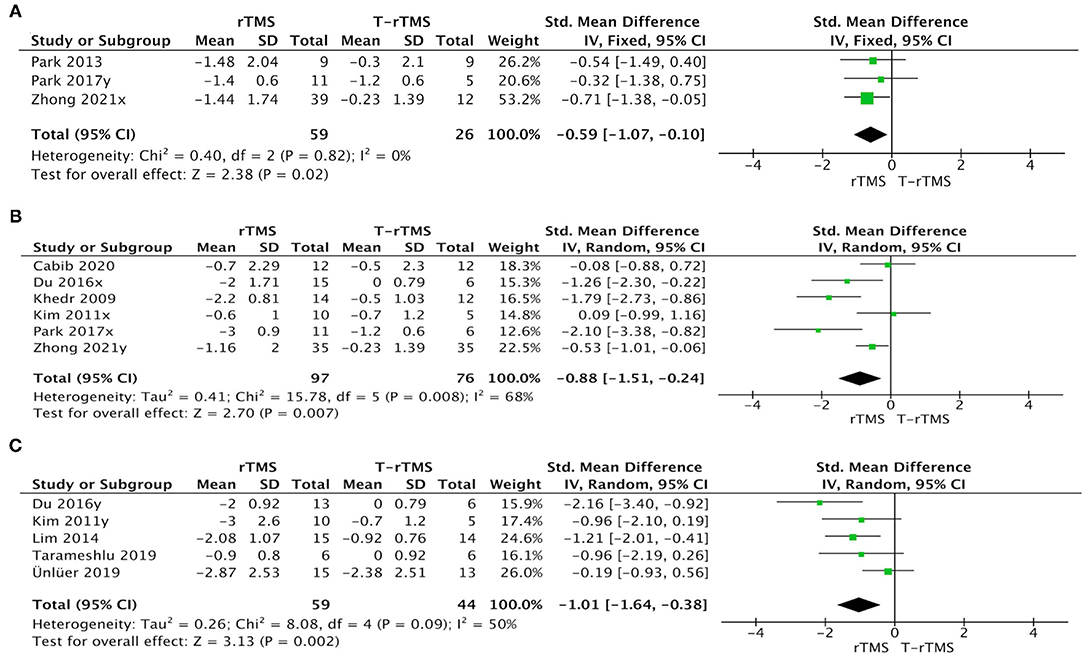
Figure 6. Subgroup analysis of primary outcomes: forest plot of the standardized mean difference (SMD) for change scores in dysphagia severity rating scales by the stimulation pattern. (A) High-frequency in unaffected hemisphere. (B) High-frequency in affected hemisphere. (C) Low-frequency in unaffected hemisphere.
Age
Subgroup analysis based on mean age also showed no significant difference (p = 0.08). Studies with a mean age of patients ≥65 years old (SMD = −0.41; 95% CI, −0.75 to −0.40, p = 0.02) and <65 years old (SMD = −0.93; 95% CI, −1.40 to −0.46, p = 0.001) both showed significant effect size compared with the control conditions (Table 2).
Sensitivity Analyses
Sensitivity analyses for dysphagia rating scales change in PSD by excluding those studies with low quality (Khedr et al., 2009; Ünlüer et al., 2019). The effect size was much lower (SMD = −0.11; 95% CI, −0.57 to 0.34, p = 0.63), with low heterogeneity (I2 = 0%, p = 0.97) in the <5-day group (Figure 3B). Similarly, the effect size was much higher (SMD = −1.28; 95% CI, −1.81 to −0.76, p < 0.001) in the low-frequency group, with low heterogeneity (I2 = 0%, p = 0.47) (Figure 5B).
Risk of Bias for Independent Studies
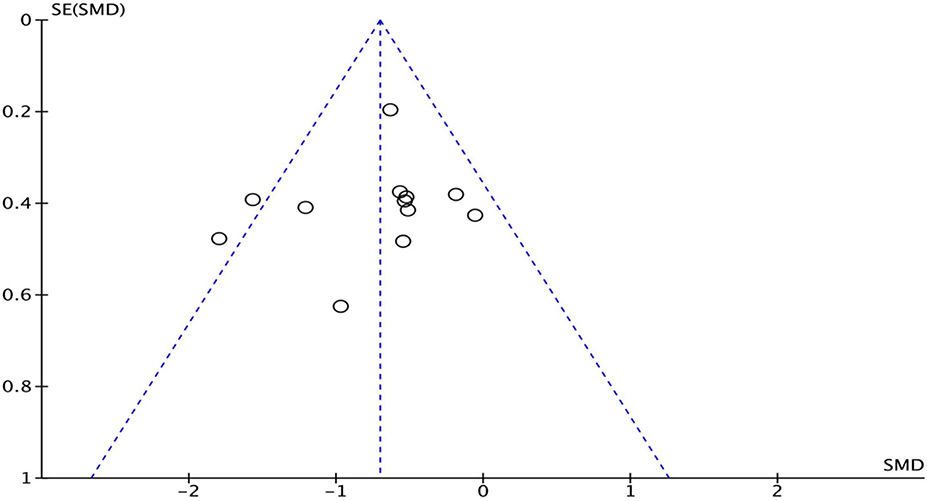
In the “risk of bias” graph, the overall quality of the literature was below the average (Figure 7). Eleven of the studies described the methods of random sequence generation (selection bias) in detail and were classified as low risk. The funnel plot indicated there may be publication bias (Figure 8), but the results must be viewed with caution due to the small number of publications.
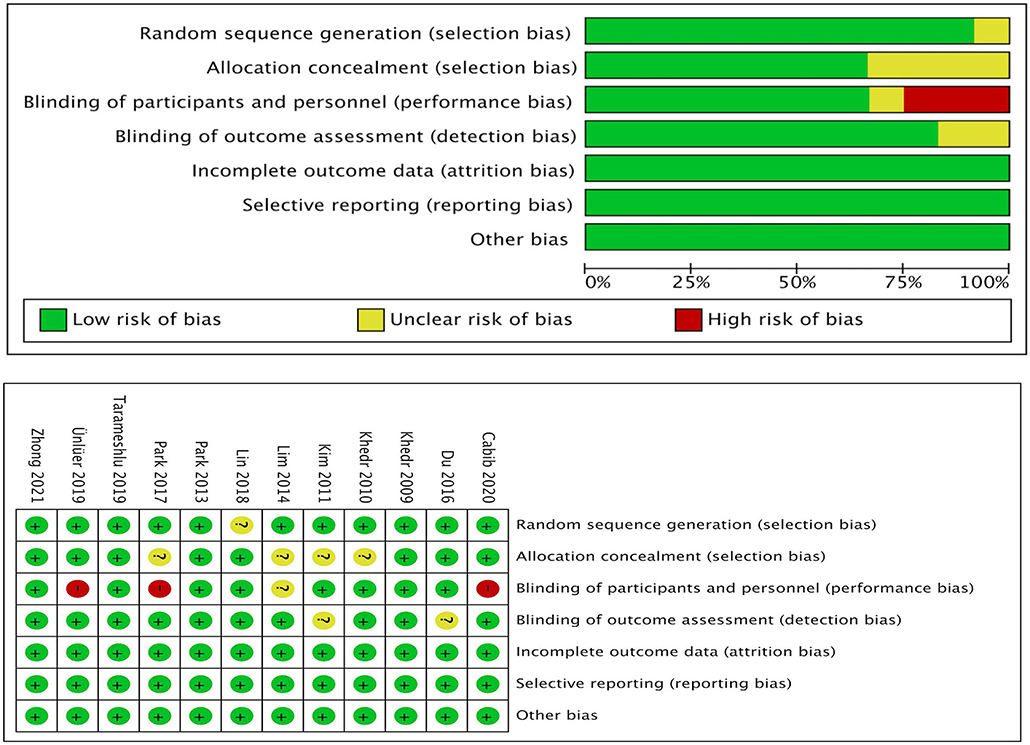
Figure 7. Risk of bias analysis and quality assessment of included trials. *Percentages of the assessments of each risk of bias item across all included studies.
Discussion
The important role of rTMS for PSD treatment has been frequently confirmed by previous studies (Liao et al., 2016; Chiang et al., 2018). Even though some mild adverse events have been reported, such as mild or transient headache, tingling sensation, dizziness (Lim et al., 2014; Du et al., 2016; Ünlüer et al., 2019; Cabib et al., 2020; Zhong et al., 2021), etc., the effect of rTMS on PSD remains worthy of recognition. We conducted a meeting with different subgroups and discussed the optimal parameters of rTMS for PSD, which might have the potential to guide the clinical trial design and treatment strategies. The two significant findings of this meta-analysis were the positive therapeutic effect in an intervention lasting for >5 days and in the subacute phase after stroke.
Subgroup analysis for treatment duration suggested that the effect of >5 days was significantly superior to the control conditions, and there remained no effect when stimulation duration was <5 days. Therefore, the former treatment duration seems to be the more suitable rTMS treatment strategy for PSD. Gow et al. (2004) found that rTMS could regulate the excitability of the swallowing-related cerebral cortex with different frequency stimulations. The cortical excitability would disappear when the N-methyl-D-aspartate receptor was blocked, indicating that the influence of rTMS may be mediated by cortical synaptic function (Stefan et al., 2002). Hence, rTMS intervention for >5 days may have a cumulative effect on the cortical synaptic function and lead to the rehabilitation of PSD after appropriate intervention duration. However, it was difficult to identify whether the longer-term intervention was the moderator of better treatment effects or not because most intervention durations were too short to carry out reliable analysis among the included studies (the longest was only10 days) (Kim et al., 2011; Park, 2013; Park et al., 2017; Zhong et al., 2021). More studies are urgently needed to explore the most suitable treatment duration for rTMS on the treatment of PSD.
Subgroup analysis for stroke stages revealed a statistically significant difference at the subacute phase after stroke in the rTMS group, while no difference at the recovery phase, suggesting that the earlier rTMS intervention may be meaningful for the treatment of PSD. Hamdy et al. (1998) found that an increase in cortical excitability in the unaffected hemisphere was linked to the recovery of swallowing function. The early rTMS intervention for PSD might promote this compensatory mechanism, making PSD patients recover better in the subacute phase after stroke. At present, research on rTMS applied to the acute phase of stroke remains insufficient (Carey et al., 2017). Due to data limitations, we were unable to complete the detailed analysis of rTMS in the acute phase, subacute phase, and recovery phase after PSD.
Subgroup analysis for stimulation frequency indicated that both low- and high-frequency stimulations exhibited a significant effect size. Different frequencies of rTMS have been used to achieve the purpose of treatment, in which low-frequency rTMS mainly inhibit neural activity (Hummel et al., 2005; Mansur et al., 2005; Fregni et al., 2006), while high-frequency could improve motor excitability (Peinemann et al., 2004). Two key parameters of rTMS are the stimulation frequency and location; thus, the selection of stimulation site is usually highly related to the applied rTMS frequency. Some studies adopted low-frequency rTMS for the unaffected hemisphere to reduce the excitability of cortical neurons and tend to recover interhemispheric imbalance after a stroke, while the others chose to utilize high-frequency rTMS to stimulate the affected hemisphere (Kim et al., 2011; Du et al., 2016; Liao et al., 2016; Tarameshlu et al., 2019; Ünlüer et al., 2019). Interestingly, in this meta-analysis, we found that both low- and high-frequency stimulation could achieve a therapeutic effect, although the suitable frequency of rTMS stimulation remained in question.
Subgroup analysis for stimulation pattern showed that low- and high-frequency interventions for the unaffected hemisphere both achieved desired clinical efficacy. rTMS could effectively improve the functional rehabilitation of PSD patients through the stimulation to the cerebral pharyngeal motor cortex (Verin and Leroi, 2009; Momosaki et al., 2014; Du et al., 2016). The output signals of affected hemispheres reduced dramatically, and the balance between the bilateral hemispheres would be broken after stroke. The relatively excessive inhibition from the affected hemispheres might further disturb the balance of the brain (Alia et al., 2017). Promoting and optimizing rehabilitation strategies for PSD is closely associated with the revision of the balance of bilateral hemispheres, and the excitatory stimulation to unaffected hemispheres could accelerate the recovery process of PSD (Hamdy et al., 1998). We found that delivering the intervention to the unaffected hemisphere with high and low, or high frequency in the affected hemisphere all achieved expected efficacy. As the mechanism of rTMS has not yet been fully understood, further high-quality studies are needed to verify it.
However, we must acknowledge some certain limitations in this study. First, publication bias was quite obvious (Figures 7, 8), and the number of included articles was extremely limited. Second, the PSD outcome indicators were mainly DG and PAS scores, which might not be the most reasonable tools for the evaluation of swallowing function. Finally, the severity of primary diseases was not distinguished, which may affect the effect size, reliability, validity, and quality of this meta-analysis. Therefore, the interpretation of the results should be made more cautious, and more high-quality RCT literature analyses are urgently needed to confirm the conclusions.
Conclusion
rTMS could bring new strategies and rational therapeutics to the management of PSD patients. Early intervention of rTMS after stroke and the treatment duration of more than 5 days may provide a meaningful effect for PSD patients returning to normal life and work activities. However, there are still controversies about the best stimulation hemisphere and frequency of rTMS at present.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JQ responsible for article retrieval and writing. Z-mW, Q-pY, and YD responsible for the selection of articles and analysis the data. Z-lD responsible for the review of articles and ensuring that all listed authors have approved the manuscript before submission. All authors contributed to the article and approved the submitted version.
Funding
This work has been sponsored by the Natural Science Foundation of China (NSFC, No. 81972159) and Natural Science Foundation of Guangdong Province (NSFP, No. 2020A1515010881).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.845737/full#supplementary-material
Abbreviations
PSD, poststroke dysphagia; rTMS, repetitive transcranial magnetic stimulation; DG, dysphagia grade; PAS, Penetration Aspiration Scale; T-rTMS, none repetitive transcranial magnetic stimulation; VFSS, Video Fluoroscopic Swallowing Study; RCT, randomized controlled trials; FEES, fiberoptic endoscopic evaluation of swallowing; NIBS, noninvasive brain stimulation; tDCS, transcranial direct current stimulation.
References
Alia, C., Spalletti, C., Lai, S., Panarese, A., Lamola, G., Bertolucci, F., et al. (2017). Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front. Cell. Neurosci. 16, 11. doi: 10.3389/fncel.2017.00076
Altman, K. W., Yu, G. P., and Schaefer, S. D. (2010). Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 136, 784–789. doi: 10.1001/archoto.2010.129
Ampt, F. H., Willenberg, L., Agius, P. A., Chersich, M., Luchters, S., and Lim, M. S. C. (2018). Incidence of unintended pregnancy among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 8, e021779. doi: 10.1136/bmjopen-2018-021779
Bath, P. M., Lee, H. S., and Everton, L. F. (2018). Swallowing therapy for dysphagia in acute and subacute stroke. Cochr. Database Syst. Rev. 10, CD000323. doi: 10.1002/14651858.CD000323.pub3
Benjamin, E. J., Virani, S. S., and Callaway, C. W. (2018). Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Ahacoeapscassc 137, 67–92. doi: 10.1161/CIR.0000000000000558
Cabib, C., Nascimento, W., Rofes, L., Arreola, V., Tomsen, N., Mundet, L., et al. (2020). Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motility 32, e13887. doi: 10.1111/nmo.13887
Carey, J. R., Chappuis, D. M., Finkelstein, M. J., Frost, K. L., Leuty, L. K., McNulty, A. L., et al. (2017). Importance and difficulties of pursuing rTMS research in acute stroke. Phys. Ther. 97, 310–319. doi: 10.1093/ptj/pzx005
Chiang, C. F., Lin, M. T., Hsiao, M. Y., Yeh, Y. C., Liang, Y. C., and Wang, T. G. (2018). Comparative efficacy of non-invasive neuros-timulation therapies for acute and subacute post-stroke dysphagia: a systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 100, 739–750. doi: 10.1016/j.apmr.2018.09.117
Clavé, P., Rofes, L., Carrión, S., Ortega, O., Cabré, M., Serra-Prat, M., et al. (2012). Pathophysiology relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr. Inst. Workshop Ser. 72, 57–66. doi: 10.1159/000339986
De Risio, L., Borgi, M., Pettorruso, M., Miuli, A., Ottomana, A. M., Sociali, A., et al. (2020). Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): a systematic review and meta-analysis of preclinical studies. Transl. Psychiatry 10, 393. doi: 10.1038/s41398-020-01055-2
Du, J., Yang, F., Liu, L., Hu, J., Cai, B., Liu, W., et al. (2016). Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double-blind clinical trial. Clin. Neurophysiol. 127, 1907–1913. doi: 10.1016/j.clinph.2015.11.045
Ekberg, O., Hamdy, S. H., and Woisard, V. (2002). Social and psychological burden of dysphagia: it's impact on diagnosis and treatment. Dysphagia 17, 139–146. doi: 10.1007/s00455-001-0113-5
Fiske, A., Wetherell, J. L., and Gatz, M. (2009). Depression in older adults. Annu. Rev. Clin. Psychol. 5, 363–389. doi: 10.1146/annurev.clinpsy.032408.153621
Fregni, F., Boggio, P. S., Valle, A. C., Rocha, R. R., Duarte, J., Ferreira, M. J., et al. (2006). A sham- controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 37, 15–22. doi: 10.1161/01.STR.0000231390.58967.6b
Fregni, F., and Pascual-Leone, A. (2007). Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 3, 383–393. doi: 10.1038/ncpneuro0530
Gow, D., Rothwell, J., Hobson, A., Thompson, D., and Hamdy, S. (2004). Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin. Neurophysiol. 115, 1044–1051. doi: 10.1016/j.clinph.2003.12.001
Hamdy, S., Aziz, Q., Rothwell, J. C., Power, M., Singh, K. D., Nicholson, D. A., et al. (1998). Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115, 1104–1112. doi: 10.1016/S0016-5085(98)70081-2
Higgins, J. P. T., and Green, S., (eds.). (2019). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration Oxford. Available online at: https://www.cochrane-handbook.org (accessed July 16, 2020).
Hummel, F., Celnik, P., Giraux, P., Floel, A., Wu, W. H., Gerloff, C., et al. (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 128, 490–499. doi: 10.1093/brain/awh369
Kalra, L., Irshad, S., Hodsoll, J., Simpson, M., Gulliford, M., Smithard, D., et al. (2015). Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): A prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. 386, 1835–1844. doi: 10.1016/S0140-6736(15)00126-9
Khedr, E. M., and Abo-Elfetoh, N. (2010). Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J. Neurol. Neurosurg. Psychiatr. 81, 495–499. doi: 10.1136/jnnp.2009.188482
Khedr, E. M., Abo-Elfetoh, N., and Rothwell, J. C. (2009). Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol. Scand. 119, 155–161. doi: 10.1111/j.1600-0404.2008.01093.x
Kim, L., Chun, M. H., and Kim, B. R. (2011). Effect of repetitive transcranial magnetic stimulation on patients with brain injury and dysphagia. Ann. Rehabil. Med. 35, 765–771. doi: 10.5535/arm.2011.35.6.765
Konstantinou, G., Hui, J., Ortiz, A., Kaster, T. S., Downar, J., Blumberger, D. M., et al. (2021). Repetitive transcranial magnetic stimulation (rTMS) in bipolar disorder: a systematic review. Bipolar Disord. 24, 10–26. doi: 10.1111/bdi.13099
Langmore, S. E., and Miller, R. M. (1994). Behavioral treatment for adults with oropharyngeal dysphagia. Arch. Phys. Med. Rehabil. 75, 1154–1160. doi: 10.1016/0003-9993(94)90094-9
Lee, J. H., Kim, S. B., Lee, K. W., Lee, S. J., and Lee, J. U. (2015). Effect of repetitive transcranial magnetic stimulation according to the stimulation site in stroke patients with dysphagia. Ann. Rehabil. Med. 39, 432–439. doi: 10.5535/arm.2015.39.3.432
Liao, X., Xing, G., Guo, Z., Jin, Y., Tang, Q., He, B., et al. (2016). Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: a systematic review and meta-analysis. Clin. Rehabil. 31, 289–298. doi: 10.1177/0269215516644771
Lim, K. B., Lee, H. J., Yoo, J., and Kwon, Y. G. (2014). Effect of low-frequency rTMS and NMES on subacute unilateral hemispheric stroke with dysphagia. Ann. Rehabil. Med. 38, 592–602. doi: 10.5535/arm.2014.38.5.592
Lin, W. S., Chou, C. L., Chang, M. H., Chung, Y. M., Lin, F. G., and Tsai, P. Y. (2018). Vagus nerve magnetic modulation facilitates dysphagia recovery in patients with stroke involving the brainstem-A proof of concept study. Brain Stimul. 11, 264–270. doi: 10.1016/j.brs.2017.10.021
Ludlow, C. L., Humbert, I., Saxon, K., Poletto, C., Sonies, B., and Crujido, L. (2007). Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia 22, 1–10. doi: 10.1007/s00455-006-9029-4
Mansur, C. G., Fregni, F., Boggio, P. S., Riberto, M., Gallucci-Neto, J., Santos, C. M., et al. (2005). A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64, 1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92
Mao, H., Lyu, Y., Li, Y., Gan, L., Ni, J., Liu, L., et al. (2021). Clinical study on swallowing function of brainstem stroke by tDCS. Neurol. Sci. 43, 477–484. doi: 10.1007/s10072-021-05247-6
Momosaki, R., Abo, M., Watanabe, S., Kakuda, W., Yamada, N., and Mochio, K. (2014). Functional magnetic stimulation using a parabolic coil for dysphagia after stroke. Neuromodulation 17, 637–641. doi: 10.1111/ner.12137
Park, E., Kim, M. S., Chang, W. H., Oh, S. M., Kim, Y. K., Lee, A., et al. (2017). Effects of bilateral repetitive transcranial magnetic stimulation on post-stroke dysphagia. Brain Stimul. 10, 75–82. doi: 10.1016/j.brs.2016.08.005
Park, J. W. (2013). The effect of 5 HZ high-frequency repetitive transcranial magnetic stimulation on contra-lesional swallowing motor cortex in post-stroke dysphagic patients: a randomized controlled study. Dysphagia 28, 299–300.
Pascual-Leone, A., Tormos, J. M., Keenan, J., Tarazona, F., Cañete, C., and Catalá, M. D. (1998). Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 15, 333–343. doi: 10.1097/00004691-199807000-00005
Pateraki, G., Anargyros, K., Aloizou, A. M., Siokas, V., Bakirtzis, C., Liampas, I., et al. (2022). Therapeutic application of rTMS in neurodegenerative and movement disorders: a review. J. Electromyogr. Kinesiol. 62, 102–622. doi: 10.1016/j.jelekin.2021.102622
Peinemann, A., Reimer, B., Löer, C., Quartarone, A., Münchau, A., Conrad, B., et al. (2004). Long-lasting increase in corticospinal excita- bility after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 115, 1519–1526. doi: 10.1016/j.clinph.2004.02.005
Ridding, M. C., and Rothwell, J. C. (2007). Is there a future for therapeutic use of transcranial magnetic stimulation? Nat. Rev. Neurosci. 8, 559. doi: 10.1038/nrn2169
Rofes, L., Vilardell, N., and Clave, P. (2013). Post-stroke dysphagia: progress at last. Neurogastroenterol. Motil. 25, 278–282. doi: 10.1111/nmo.12112
Rogus-Pulia, N., and Robins, J. A. (2013). Approaches to the rehabilitation of dysphagia in acute poststroke patients. Semin. Speech Lang. 34, 154–169. doi: 10.1055/s-0033-1358368
Sandrini, M., Cohen, L. G., and Censor, N. (2015). Modulating reconsolidation: a link to causal systems-level dynamics of human memories. Trends Cogn. Sci. 19, 475–482. doi: 10.1016/j.tics.2015.06.002
Sasegbon, A., and Hamdy, S. (2017). The anatomy and physiology of normal and abnormal swallowing in oropharyngeal dysphagia. Neurogastroenterol. Motil. 29, 2533–2546. doi: 10.1111/nmo.13100
Schneider, S. A., Pleger, B., Draganski, B., Cordivari, C., Rothwell, J. C., Bhatia, K. P., et al. (2010). Modulatory effects of 5Hz rTMS over the primary somatosensory cortex in focal dystonia-An fMRI-TMS study. Mov. Disord. 25, 76–83. doi: 10.1002/mds.22825
Speyer, R., Baijens, L., Heijnen, M., and Zwijnenberg, I. (2010). Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia 25, 40–65. doi: 10.1007/s00455-009-9239-7
Stefan, K., Kunesch, E., Benecke, R., Cohen, L. G., and Classen, J. (2002). Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. 543, 699–708. doi: 10.1113/jphysiol.2002.023317
Tarameshlu, M., Ansari, N. N., Ghelichi, L., and Jalaei, S. (2019). The effect of repetitive transcranial magnetic stimulation combined with traditional dysphagia therapy on poststroke dysphagia: a pilot double-blinded randomized-controlled trial. Int. J. Rehabil. Res. 42, 133–138. doi: 10.1097/MRR.0000000000000336
Ünlüer, N. Ö., Temuçin, Ç. M., Demir, N., Serel Arslan, S., and Karaduman, A. A. (2019). Effects of low-frequency repetitive transcranial magnetic stimulation on swallowing function and quality of life of post-stroke patients. Dysphagia 34, 360–371. doi: 10.1007/s00455-018-09965-6
Verin, E., and Leroi, A. (2009). Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia 24, 204. doi: 10.1007/s00455-008-9195-7
Yang, S. N., Pyun, S. B., Kim, H. J., Ahn, H. S., and Rhyu, B. J. (2015). Effectiveness of noninvasive brain stimulation in dysphagia subsequent to stroke: a systemic review and meta-analysis. Dysphagia 30, 383–391. doi: 10.1007/s00455-015-9619-0
Keywords: poststroke dysphagia (PSD), repetitive transcranial magnetic stimulation (rTMS), randomized controlled trial, meta-analysis, parameters
Citation: Qiao J, Ye Q-p, Wu Z-m, Dai Y and Dou Z-l (2022) The Effect and Optimal Parameters of Repetitive Transcranial Magnetic Stimulation on Poststroke Dysphagia: A Meta-Analysis of Randomized Controlled Trials. Front. Neurosci. 16:845737. doi: 10.3389/fnins.2022.845737
Received: 30 December 2021; Accepted: 24 February 2022;
Published: 28 April 2022.
Edited by:
Maurizio Acampa, Siena University Hospital, ItalyReviewed by:
Seung Nam Yang, Korea University, South KoreaPingping Meng, The Affiliated Hospital of Qingdao University, China
Leila Ghelichi, Iran University of Medical Sciences, Iran
Copyright © 2022 Qiao, Ye, Wu, Dai and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zu-lin Dou, ZG91enVsaW4mI3gwMDA0MDttYWlsLnN5c3UuZWR1LmNu
 Jia Qiao
Jia Qiao Qiu-ping Ye1
Qiu-ping Ye1 Zhi-min Wu
Zhi-min Wu