
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 28 February 2022
Sec. Autonomic Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.844564
This article is part of the Research Topic Disruption of the Microbiota-Gut-Brain Axis in Functional Dyspepsia and Gastroparesis: Mechanisms and Clinical Implications View all 9 articles
Functional dyspepsia is one of the most commonly diagnosed disorders of the gut-brain interaction worldwide. The precise pathogenesis of functional dyspepsia is complex and remains incompletely understood. Therefore, advances in the understanding of functional dyspepsia could change clinical practice. The aim of this review is to highlight the relevance of psychotherapy and probiotics in the context of the microbiota-gut-brain axis in the pathophysiology and especially in the treatment of functional dyspepsia. Therefore, studies which have been conducted to investigate the role of psychotherapy and probiotics in FD and the microbiota-gut-brain axis in the pathophysiology of functional dyspepsia were examined, and the outcomes of this research summarized. There might be a link between changes in the microbiome and functional dyspepsia. Even though, specific alterations in the microbiome that may be pathognomonic in functional dyspepsia remain unclear, the use of probiotics became a viable treatment option for patients with functional dyspepsia. Since mental illness also plays an important role in the pathophysiology of functional dyspepsia, psychotherapy is a useful treatment method, with additional study results indicating that psychotherapy may also shift the microbiome in a favorable direction. Moreover, other findings suggest that probiotics can be used not only to alleviate gastrointestinal symptoms in functional dyspepsia, but also to treat or even prevent mental disorders in these patients. In summary, in this review we highlight the bi-directionality of the microbiota-gut-brain axis in the pathophysiology of functional dyspepsia. Although there are multiple treatment approaches, the burden of disease in patients with functional dyspepsia is still enormous and a definitive therapy to cure this disease does not (yet) exist. Lastly, there is a lack of studies on the impact of dysbiosis, mental health and probiotics on pathophysiology and symptomatology in functional dyspepsia which should be investigated in future studies.
Functional dyspepsia (FD) is one of the most commonly diagnosed disorders of gut-brain interaction (DGBI) worldwide (Stanghellini et al., 2016) and affects up to 7.2% of people in the community (Sperber et al., 2021). The Rome IV diagnostic provide the basis for defining and classifying patients with DGBI (Schmulson and Drossman, 2017). Here, dyspepsia encompasses various symptoms referable to the gastrointestinal (GI) tract such as postprandial fullness, epigastric pain, or early satiety. In approximately 80% of patients suffering from dyspepsia there is no structural explanation for their symptoms, which is then termed FD (Ford et al., 2015). FD can be further divided into the subgroups epigastric pain syndrome (EPS), characterized by epigastric pain or burning, and postprandial distress syndrome (PDS), characterized by postprandial fullness or early satiety, with overlaps also constituting a subgroup (Stanghellini et al., 2016).
The pathogenesis of FD remains still largely unclarified. However, it is assumed that a disturbed gut-brain axis leads to motility disorders, visceral hypersensitivity, and changes in mucosal and immune function (Vanheel and Farré, 2013; Stanghellini et al., 2016; Jung and Talley, 2018). Moreover, it has now been recognized that FD is closely associated with duodenal eosinophilia, epithelial barrier disruption (Nakagawa et al., 2020) as well as mucosal inflammation accompanied by higher levels of mast cells (Wang et al., 2015) with post-infectious FD possibly representing an own entity (Tack and Talley, 2013; Futagami et al., 2015). As epidemiological studies show, the prevalence of anxiety and depression is higher in patients suffering from FD than in healthy people, indicating that mental disorders may play a role in the development of FD (van Oudenhove and Aziz, 2013). Risk factors suspected so far include female gender, smoking, Helicobacter pylori (HP) infection, acute gastroenteritis, mental disorders, and use of non-steroidal anti-inflammatory drugs (Ford et al., 2015).
Moreover, some patients with DGBI who do not have elevated anxiety scores at baseline show increased anxiety scores later, indicating that the central nervous system and the gut interact in a bidirectional way in functional GI disorders. Moreover, the gut might directly cause brain dysfunction in a subset of these patients (Qin et al., 2014). Furthermore, dysbiosis is a potential factor in the pathophysiology of FD (Ding et al., 2020).
There is little evidence that lifestyle change or physical activity improves symptoms, and although some foods have been linked to the development of symptoms, there is little data on the impact of diet in patients with FD (Duncanson et al., 2018). Drug therapy is therefore the mainstay of treatment but, apart from patients who respond to HP eradication, offers only modest and often temporary relief. Advances in the understanding of FD could change clinical practice, and treatment of microbiome alterations could lead to a cure for a subset of these patients in the future. As the GI microbiome plays a key role in the gut-brain axis, this review discusses the role of the microbiota-gut-brain axis in FD, focusing on possible mechanisms and treatment approaches.
The aim of this narrative review is to highlight the relevance of the microbiome-gut-brain axis in the pathophysiology and especially in the treatment of FD. Many studies have shown that the microbiome is altered in patients with FD and that psychotherapy is also a useful treatment option – we aim to show a link between these two approaches via the microbiome-gut-brain axis and to highlight a potential triad between disordered microbiome, mental illness and FD that could stimulate further research in the future.
Scientific literature on this topic up to October 2021 was collected and screened in the databases Pubmed and PsychInfo using the search terms “functional dyspepsia” or “dyspepsia” combined with “microbiome,” “microbiota,” “dysbiosis,” “bacterial,” “bacterial overgrowth,” “probiotics,” “psychological,” “psychotherapy,” or “cognitive behavioral therapy.” All studies containing material relevant to the topic were considered. There was no date restriction (all studies until October 24th, 2021), but the search was limited to texts in English. After searching the database with the above keywords, 3.122 articles were found. Excluded were review articles, surveys, case reports, commentaries, letters or posters, and studies that deviated from the main topic. Ultimately, 46 articles were selected for this narrative review.
The last decade has witnessed a tremendous increase in research on the trillions of microorganisms living in the human body and their interaction with the host. Since the members of the microbiota are considered essential to the host’s physiological functions, dysregulation can lead to a disruption of microbial-host homeostasis and cause disease (Zmora et al., 2019). Therefore, it is crucial to further elucidate the relevance of the gut microbiota in the development of human disease. The gut microbiota could be a potential target for effective personalized medicine for several diseases in the future. Recent observations have shown that patients with FD show low levels of duodenal and systemic inflammation, especially duodenal eosinophilia (Talley et al., 2007; Liebregts et al., 2011) and increased duodenal permeability (Vanheel et al., 2014), so changes in the microbiota are a factor of interest.
A previous study compared the gastric fluid composition of patients with FD with healthy controls and reported a significant decrease in the frequency of the genus Prevotella in the FD compared to the control group (Nakae et al., 2016). The cause of this dysbiosis could be delayed gastric emptying, which potentially alters the acidity, mucus consistency and partial oxygenation of the stomach, altering the bacterial colonization of the stomach. Furthermore, microbiota of the gastric fluid in patients suffering from FD showed an increased ratio of Bacteroidetes to Proteobacteria, while no Acidobacteria could be detected. However, the gastric fluid of healthy individuals contained Acidobacteria and was characterized by a lower ratio of Bacteroidetes to Proteobacteria (Igarashi et al., 2017). In addition, a greater increase in the proportion of bile acid-positive gastric fluid samples was found in patients with FD than in the control group. Since the reflux of bile acids from the duodenum into the stomach physiologically occurs during gastric motility (Sjövall, 2011), patients with FD may suffer from a disturbed gastric motility. Furthermore, the increased species richness suggests that the mass and diversity of the gastric fluid microbiota is large enough for the metabolites and components of the bacteria to affect the stomach. Thus, it could be suggested that the toxic bacterial cellular components of the gut, such as lipopolysaccharides, stimulate leukocytes to produce pro-inflammatory cytokines, triggering gastric inflammation and consequently increase mucosal permeability, which may lead to gastric (enteric) nervous system dysfunction (Galanos et al., 1985). Since lipopolysaccharides and bile acids increase the permeability of the mucosa, inflammation in the duodenal region in patients with FD could be caused by the refluxed fluid containing such potentially toxic substances (Igarashi et al., 2017).
However, when comparing the microbiome of the upper GI tract in patients with FD and healthy control subjects the levels of Streptococcus were higher in the oral cavity, esophagus, stomach, and duodenum of the FD group. Moreover, Streptococcus abundance as well as OTU 90 frequencies correlated positively with upper GI complaints suggesting a link between Streptococcus and GI symptoms in patients with FD. In addition, the FD group showed higher levels of phylum Firmicutes (Fukui et al., 2020). In line with these findings, in a different study comparing the microbiota of the duodenal mucosa in patients with FD and in healthy subjects, the most prevalent genus in the duodenal mucosa was also Streptococcus in both groups. Here, an inverse relationship was noted between the relative frequency of Streptococcus and Prevotella, Veillonella and Actinomyces, which were significantly lower in patients with FD. In addition, quality of life and total bacterial load were also evaluated in patients with FD. Here, a negative correlation was shown between the bacterial load of the duodenal mucosa and quality of life. Furthermore, as the bacterial load on the mucosa increased, so did the severity of symptoms after a standardized meal and bacterial diversity declined with increasing total bacterial count (Zhong et al., 2017). Aforementioned studies illustrate that microbial alterations in FD are not confined to one site in the GI tract, highlighting the potential importance of homeostatic imbalance in the pathogenesis of these diseases.
Moreover, an investigation of the composition of the microbiome by analyzing fecal samples of rats with FD and liver depression-spleen deficiency syndrome, showed elevated levels of Firmicutes, Proteobacteria and Cyanobacteria in this model, while a lower frequency of Bacteroidetes was seen in the model compared to the control group (Qiu et al., 2017). When interpreting these results, it should be noted that the model may not exactly represent a model for FD and rather mimics chronic stress. Therefore, cautious interpretation of these data is warranted.
In another study, small intestinal bacterial overgrowth (SIBO) – an overload in the number and/or type of colonic bacteria in the upper GI tract (Bures et al., 2010; Sachdev and Pimentel, 2013) – was detected in 2 out of 38 (5.3%) patients with DGBI (one subject suffered from FD, the other from an overlap of FD and irritable bowel syndrome [IBS]) using the glucose breath test (Shimura et al., 2016). IBS is also a DGBI and is diagnosed according to the Rome IV criteria based on recurrent abdominal pain associated with defecation or in association with an altered stool frequency or form (Mearin et al., 2016). However, regarding the usefulness of the hydrogen breath test in diagnosis of FD, it should be noted that an H2 increase can also occur in patients without GI symptoms or complaints.
In contrast, a recent meta-analysis including seven studies showed that the prevalence of SIBO is significantly higher in patients with FD compared to healthy individuals. The studies used breath tests (glucose breath test and lactulose breath test) to detect SIBO, and overall, the prevalence of SIBO in patients with FD was 32.7%. The prevalence of SIBO within the different subgroups of FD (EPS, PDS, overlap of EPS and PDS) was also investigated - no significant differences were identified here (Gurusamy et al., 2021). A case-control study showed that the prevalence of SIBO in patients with FD was 14%, compared to 10% in healthy controls. An analysis of the different FD subgroups did not take place here. Although the results of this study were not statistically significant, the researchers concluded that SIBO may cause more discomfort in patients suffering from FD than in healthy individuals due to increased visceral sensitivity (Chuah et al., 2021). Similarly, a recent study in patients with FD using quantitative cultures of the proximal small intestine to detect SIBO showed a higher overall prevalence of SIBO in patients with FD than in healthy subjects. Moreover, analysis of the different subtypes of FD showed a prevalence of SIBO of 12.5% in EPS, 20.8% in PDS and 31.6% in EPS-PDS overlap (Tziatzios et al., 2021). The results of these studies indicate that SIBO may be associated with the development or exacerbation of symptoms in a subgroup of patients with FD. Whether SIBO is the causal factor or a consequence of another process, e.g., impaired mucosal permeability, requires further research. However, it may be important to consider SIBO as a differential diagnosis in the examination of patients with refractory GI symptoms in routine clinical care. Even though these results do not provide a specific analysis of the composition of the microbiome, the findings do point toward a potential significance of GI dysbiosis in the pathophysiology of FD.
In addition, in a small subgroup (5%) of patients with FD, infection with HP appears to be the cause of dyspepsia (Moayyedi et al., 2000; Ding et al., 2020). Since HP infection often leads to chronic mucosal inflammation of the stomach and duodenum, disturbances in GI motility and sensitivity often follow (Suzuki and Moayyedi, 2013). Considering that dysregulation of the gut microbiota and gut-brain axis may play a crucial role in the pathogenesis of FD, the basis of microbiota-gut-brain axis dysfunction in the treatment of FD cannot be neglected.
In contrast, a recent study showed that the microbial composition of patients with FD was similar to those of healthy controls, covering a largely overlapping spectrum (Vasapolli et al., 2021). Thus, there appears to be limited consensus on the existence of FD-associated microbiome signatures. This may be due to the different methodologies used in assessing the microbiome, different sampling sites and 16S rRNA sequencing compared to shotgun metagenomics. While the use of 16S profiles of the microbiota is biased due to the selection of primers targeting specific variable regions of the 16S rRNA gene, shotgun metagenome sequencing assures that all DNA signatures are captured, in addition to allowing prediction of the functional capacity of the microbiota (Teh et al., 2021). All studies mentioned here utilized 16S rRNA sequencing with different primer targets, which limits the comparability of the results (Table 1).
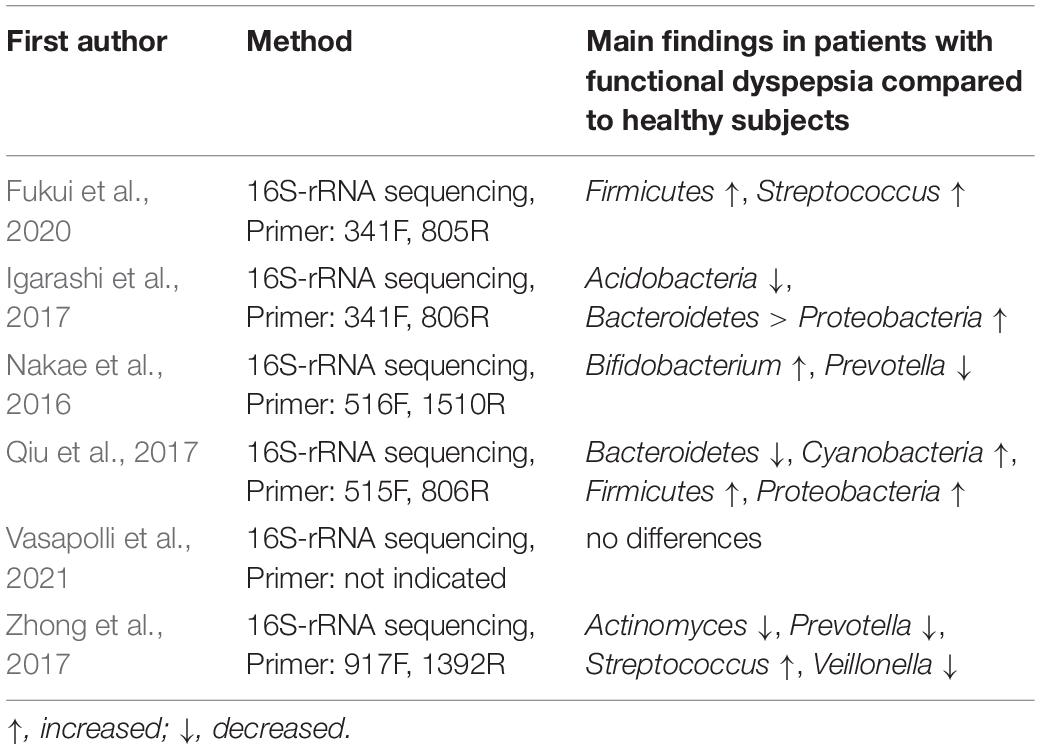
Table 1. Methods used for assessing the microbiome and outcomes of the studies discussed in this review (in alphabetical order).
When interpreting all these aforementioned alterations in the microbiome composition, it should always be kept in mind that there is no evidence of a causal relationship between dysbiosis, and FD and that further research is needed. Even though the studies discussed above show several changes in the microbiota composition and suggest a link, it should be taken into account that a microbiome shift can also be caused by pharmacotherapeutic treatment, e.g., proton pump inhibitors (PPIs) (Perry et al., 2020) or antidepressants (McGovern et al., 2019) for pain management.
If we assume dysbiosis as a cause of FD symptoms, probiotics might be considered as a possible treatment. Therefore, several studies have been conducted to shed further light on this possible option: One study included 131 participants suffering from HP-associated FD who were randomly assigned to receive Lactobacillus gasseri OLL2716 (LG21)-containing yogurt or placebo yogurt once daily for 12 weeks. Although the primary endpoint of this randomized double-blind trial, namely a decrease in HP load, was not significant, the results showed that postprandial fullness was significantly reduced in the LG21 group at the end of the study compared to baseline and compared to the placebo group (Takagi et al., 2016). Similar results were found in another randomized double-blind controlled study, where HP uninfected participants consumed yogurt containing LG21 for 12 weeks. Here, the LG21 group showed a statistically non-significant improvement in gastric symptoms compared to the control group, suggesting that this probiotic may be a useful therapeutic treatment for patients with FD (Ohtsu et al., 2017). However, no improvements were found in EPS-like symptoms by LG21. Instead, the results suggest that LG21 has a stronger positive effect on PDS symptoms than on EPS-like symptoms. Therefore, it can be assumed that prokinetic agents may have a positive effect on PDS (Matsueda et al., 2010), and the positive effects of LG21 may focus mainly on delayed gastric emptying and impaired adaptability to relaxation. Even though the effects of yogurt on gut microbiota are still controversial (Elli et al., 2006), the improvement in upper abdominal symptoms could be due to a reduction in secretory bile acids in the duodenum (Beeckmans et al., 2020). More precisely, hydrophobic bile salts affect the integrity of the small intestine and increase intestinal permeability (Erickson and Epsten, 1988; Stenman et al., 2013), which can consequently lead to bacterial translocation (Banerjee et al., 2016; Bednarska et al., 2017). A recent study found a positive correlation between the ratio of secondary to primary bile salts and duodenal permeability in patients with FD (Beeckmans et al., 2020). These results underline that the effects of certain bile salts on duodenal permeability and their potential role in the pathophysiology of FD should be further investigated in the future.
However, not only the symptom severity decreases, but also the microbiome shows a transformation with LG21 administration: In patients with FD, the dysbiosis in the gastric fluid was resolved by consuming yogurt containing LG21. Interestingly, an increase in the frequency of Prevotella was associated with a decrease in the severity of PDS symptoms in patients with FD receiving LG21 yogurt. This observation leads to the hypothesis that the frequency of the genus Prevotella could be used as a biomarker for the outcome of FD treatment (Nakae et al., 2016). In line with these findings, a different study reported that probiotic therapy with yogurt containing LG21 in patients with FD resulted in a change in the composition of the gastric fluid microbiota toward that observed in healthy subjects (Igarashi et al., 2017).
A different study that administered Lactobacillus paracasei L-37 in form of a drink for 14 and 28 days showed that this administration reduces the severity of symptoms in patients with FD while significantly alleviating clinical symptoms such as abdominal pain and belching. In addition, the number of probiotic bacteria such as Lactobacillus, Lactococcus and Weissella increased, while the number of pathogenic bacteria such as Lachnoclostridium significantly diminished (Sun et al., 2021). Interestingly, this study also measured gut metabolites. Here it was shown that consumption of Lactobacillus paracasei L-37 was associated with an increase in beneficial metabolites such as pelargonic acid, benzoic acid, and short-chain fatty acids (SCFAs), while harmful gut metabolites such as hippuric acid decreased (Sun et al., 2021).
Another randomized double-blind, placebo-controlled study investigated the effect of Bacillus coagulans MY01 and Bacillus subtilis MY02 administration in FD patients. The primary endpoint, a decrease in the score of the Leuven Postprandial Distress Scale, was achieved in the intervention group. Interestingly, while this study did not find a pronounced change in microbiome composition, it did show that an increase in Faecalibacterium could be associated with probiotic efficacy. In addition, a reduction in SIBO was shown in the intervention group, which can be inferred from the reduced proportion of positive glycolic acid breath tests in patients with FD taking PPIs (Wauters et al., 2021). Although studies are still scarce, the findings described above showing that probiotics have a positive effect on the severity of FD symptoms could imply a link between dysbiosis as a cause and FD symptoms as a consequence.
Moreover, there are several studies investigating the efficacy of treating FD with traditional Chinese medicine, which also point to a role of the microbiome in the pathophysiology of FD (He et al., 2019; Zhang et al., 2019, 2020; Gao et al., 2020). These studies should be interpreted with caution when it comes to the transferability of the results to humans with FD. The findings are not discussed in detail because the animal models used here likely do not reflect the complex pathophysiology of FD.
Now it remains unclear which specific mechanisms are behind the potentially positive effects of probiotics on FD symptomatology. Probiotics are defined by The Food Agricultural Organization/World Health Organization as “Live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” It is speculated that probiotics inhibit pathogenic bacteria in the gut, decrease epithelial permeability, decrease visceral hypersensitivity, improve gut motility, and alter the mucosal stress response, thereby reducing symptom severity in GI disorders (Hosseini et al., 2012). Another hypothesis is that probiotics act in the upper GI tract by reducing the levels of Escherichia/Shigella, a main source of toxic lipopolysaccharides, leading to restoration of alterations in the gastric microbiome (Igarashi et al., 2017). In addition, probiotics appear to reduce visceral hypersensitivity by regulating pain receptor expression in the GI tract (Rousseaux et al., 2007). Another trial suggests that a reduction in glycogen synthesis and associated blood lipid lowering is induced by Lactobacillus paracasei, and consequently leads to an improvement in gut motility (Gudiña et al., 2010). It is known that increased permeability of the duodenal mucosa plays a role in the pathophysiology of FD (Vanheel et al., 2014). Recently, probiotics have been reported to reduce this increased permeability by ameliorating abnormalities directly in gut microbiota or by producing SCFAs via fermentation (Dalile et al., 2019).
Although the exact mechanisms mediating the effects of probiotics are still largely unknown, SCFAs are considered as possible mediators. SCFAs are metabolites of bacterial fermentation of dietary fibers in the gut and whose neuroactive properties may play an important role in communication along the microbiota-gut-brain axis (Stilling et al., 2016). As mentioned above, probiotics may lead to higher levels of SCFAs via the proliferation of beneficial SCFA-producing bacteria or the fermentation of complex carbohydrates. SCFAs can influence gut-brain communication and brain function directly or indirectly through immunological, endocrine, vagal and other pathways (Dalile et al., 2019). In addition, SCFAs affect systemic inflammation by regulating the secretion of interleukins and interact with vagal afferents (Lal et al., 2001; Corrêa-Oliveira et al., 2016). Data on studies investigating these direct effects in humans are still scarce. However, we know that SCFAs exert a range of effects to improve gut health, such as maintaining intestinal barrier integrity (Daly and Shirazi-Beechey, 2006; Lewis et al., 2010), protection against intestinal inflammation (Lewis et al., 2010) and increase of mucin secretion (Barcelo et al., 2000). Furthermore, SCFAs relax the proximal stomach in humans (Ropert et al., 1996) and additionally influence intestinal motility through the activation of SCFA receptors (Dass et al., 2007), the release of the intestinal hormone peptide YY (PYY) (Cherbut et al., 1998) or SCFA-induced serotonin release from enterochromaffin cells (Fukumoto et al., 2003). Nevertheless, the exact mechanism of action of individual probiotics in FD treatment can only be hypothesized so far.
Taken together, although data are still sparse, there might be a link between changes in the microbiome and FD. Even though specific alterations in the microbiome that may be pathognomonic in FD remain unclear and require further research, the efficacy of probiotics, which are naturally designed to alter the microbiome and reverse dysbiosis, became a viable treatment option for patients with FD. Thus, it appears that dysbiosis may play a considerable role in the pathophysiology of FD.
A study of the Swedish population showed that anxiety at baseline increases the risk of developing FD by almost 8 times after 10 years follow-up (Aro et al., 2015). Interestingly, multiple studies highlighted that the prevalence of anxiety and depression is significantly increased in patients with FD compared to healthy people (Talley et al., 1986). These observations indicate that mental illness plays a significant role in the pathogenesis of FD. Furthermore, pathophysiological research indicates that psychosocial factors and mental disorders may play a role in FD by modulating both visceral signal processing in the brain (van Oudenhove and Aziz, 2013; Browning and Travagli, 2014) and the effects of stress hormones on pain perception (Hannibal and Bishop, 2014). Furthermore, it is known that psychosocial factors and stress hormones also affect other aspects of the GI tract such as motility (Venkova et al., 2010), immune system activation (Konturek et al., 2011), permeability (Gareau et al., 2008) and microbiota (Tannock and Savage, 1974; Bailey and Coe, 1999). On the other hand, FD symptoms are thought to induce anxiety or depression due to a cytokine response in low-grade intestinal inflammation, which plays an important role in the development of psychological distress in patients with FD (Koloski et al., 2012). Therefore, psychological therapies have recently been adapted for the treatment of disturbed brain-gut interactions such as FD. Although we will primarily discuss the link between mental health and FD in the following, it is important to note that a prospective study from 2016 showed that two-thirds of patients with IBS or FD who did not suffer from anxiety or depression at baseline showed elevated anxiety and depression scores at 1-year follow-up (Koloski et al., 2016). These results underline that gut-to-brain alterations are relevant in a subgroup of these patients.
A meta-analysis of 2021 investigated the potential beneficial effects of various psychological therapies on the global symptom severity in patients with FD (Rodrigues et al., 2021). Even though the sample sizes in the studies included were mostly small, the effectiveness of psychological therapies on global FD symptom scores was clearly demonstrated (Rodrigues et al., 2021). Most studies included investigated the effect of cognitive behavioral therapy (CBT) on FD symptoms (Wilhelmsen et al., 1994; Cheng et al., 2007; Dehghanizade et al., 2015; Orive et al., 2015; Xiong et al., 2019) and concluded that CBT leads to a significant improvement in the severity of symptoms (Wilhelmsen et al., 1994; Cheng et al., 2007; Orive et al., 2015; Xiong et al., 2019), pain intensity (Orive et al., 2015) and further lowers the impact of the disease on patients’ lives (Dehghanizade et al., 2015). Moreover, a significant increase in gastric emptying rate and alterations in gastric motility parameters were monitored in the intervention group compared to the control group (Xiong et al., 2019). Based on these results, CBT can be considered as an effective therapeutic option in patients suffering from FD. Psychoanalysis also shows positive effects on patients with FD (Hamilton et al., 2000; Faramarzi et al., 2013, 2015) by reducing both FD-associated symptoms rated by patients (Hamilton et al., 2000; Faramarzi et al., 2013, 2015) and treating gastroenterologists (Hamilton et al., 2000). Other psychotherapeutic methods, such as hypnotherapy, are also effective in relieving symptoms (Calvert et al., 2002; Chiarioni et al., 2006) and improving quality of life (Calvert et al., 2002) in patients with FD. Moreover, gut-oriented hypnosis has been shown efficient in shortening gastric emptying time in both dyspeptic and healthy subjects, while patients with FD additionally reported a reduction in symptoms of epigastric fullness and abdominal discomfort (Chiarioni et al., 2006).
We have now highlighted two different factors that might play a role in the pathogenesis of FD – one is dysbiosis, and the other is disordered mental health. Now, one might wonder where the positive effects of psychological therapies in FD come from. On the one hand, as already mentioned above (Koloski et al., 2016), a subgroup of patients with FD first develops a mental disorder in the course of the disease, on which psychotherapeutic approaches can have a positive effect. Secondly, by combining both approaches, dysbiosis and disturbed mental health, one could hypothesize that psychotherapy alleviates mental disorders by shifting the microbiome in a favorable direction. This approach has already been explored in studies, although not specifically in FD models: Here, one study investigated the effect of mindful awareness practice in elderly patients with mild cognitive impairment and found that improvement in the patients’ cognition is associated with alterations in fecal microbiome profile. These results imply that signals from the brain may directly or indirectly modulate the composition of the gut microbiome (Khine et al., 2020). Similarly, interesting results were obtained from an investigation of outdoor nature-related activities and their effects on the intestinal microbiota of preschool children. The children’s gut microbiota changed, especially in terms of Roseburia frequency and fecal serotonin levels (Sobko et al., 2020). In contrast, a 12-week aerobic exercise intervention in adolescents with subthreshold mood syndromes and healthy adolescents showed no significant positive effects on gut microbiota in either group. Precisely, there were no differences in intestinal microbiota diversity, genus, or frequencies detected, which could also be due to a potentially inadequate exercise intensity (Wang et al., 2021). In line with these results, the combination of three months of dietary education combined with CBT also showed no changes in the diversity or composition of the gut microbiome compared to usual care in patients with IBS (Kamp et al., 2021).
An important aspect that should be mentioned in the context of mental condition on dysbiosis and GI discomfort is the role of the vagus nerve. It is known that stress increases gut permeability and alters the composition of the GI microbiome via various neuromodulators (Taché and Bonaz, 2007; Tache et al., 2018), a mechanism that also plays a role in the pathophysiology of IBS. A study in rats has shown that chronic stress alters intestinal permeability, which consequently leads to dysbiosis and later to visceral hypersensitivity (Moussaoui et al., 2017). Interestingly, stress can reduce vagus nerve activity (Taché and Bonaz, 2007; Wood and Woods, 2007), which may promote GI inflammation (Bonaz et al., 2016). Although there are no data yet on the effect of vagal stimulation on the GI microbiome, it can be speculated that the vagus nerve may have an impact on the gut microbiome through its effects on intestinal permeability (Meregnani et al., 2011; Karl et al., 2017). The efferents of the vagus nerve may have an anti-inflammatory effect in the gut and at the same time reduce intestinal permeability – both effects may be attributed to a strengthening of the tight junctions through vagal activity. Study results imply that vagus nerve activity exerts a protective function on the intestinal epithelial barrier (Cheadle et al., 2014; Yu and Li, 2014), but the exact mechanism is still unclear. Conversely, this means that reduced vagal activity increases epithelial permeability and consequently promotes systemic inflammation and chronic disease. Stress could therefore abolish the protective effect of the vagus nerve on the epithelial barrier as a whole and thus promote dysbiosis by disrupting epithelial homeostasis (McEwen, 2008). Furthermore, a reduction in vagal tone has been demonstrated in IBS and inflammatory bowel disease (Pellissier et al., 2014). The effects of psychotherapy on the microbiome and GI symptoms in FD could therefore also be explained by the influence of the vagus nerve. Consequently, monitoring and targeting vagal tone in patients with DGBI could be of value to potentially restore a homeostatic microbiota-gut-brain axis (Bonaz et al., 2018).
Now one can wonder whether the prevalence of psychological morbidity varies within the different subgroups of FD. Even before the Rome III subdivision of FD into subgroups, it was shown that mood and anxiety disorders were more common in patients with non-pain-dominant FD than in patients with pain-dominant FD (Handa et al., 1999). Another study, however, showed a correlation between epigastric pain and neuroticism, abuse, and somatization (Fischler et al., 2003). In contrast, other studies observed a relationship between PDS and somatization, anxiety, and depression, which was not observed in EPS (Aro et al., 2009; Hsu et al., 2009). Moreover, a study from 2012 showed that depression was only associated with PDS (Clauwaert et al., 2012). These findings highlight the urgent need for future studies focusing on a possible link between psychological comorbidity and specific symptoms in patients with FD, so that treatment approaches for FD in the future will hopefully be more personalized and based not only on symptoms but also on underlying pathophysiology and -psychology.
In conclusion, a precise answer to the hypothesis that psychotherapy alleviates mental disorders by shifting the microbiome in a favorable direction cannot be given based on the current data. The studies mentioned here included only small sample sizes, and the results are poorly comparable due to different patient characteristics and different interventions. To further confirm or reject this hypothesis, there is an urgent need for studies that further investigate these interesting effects of psychotherapy on the microbiome specifically in patients with FD, so that we are able to better understand this direction of the brain-gut-microbiome axis in the future.
We are aware that patients with FD often suffer from mental illness (Talley et al., 1986) and that anxiety can increase the risk for FD (Aro et al., 2015). The increased prevalence of anxiety and depression in patients with FD could be due to the disease burden or the FD symptoms themselves, which could trigger anxiety or depression through a cytokine reaction resulting in low-grade intestinal inflammation.
A growing body of evidence suggests that the gut microbiota communicates with the central nervous system, possibly through neural (vagus nerve, spinal cord), endocrine (HPA axis), metabolic (SCFAs, bile acids, tryptophan and many more), and immunological pathways (cytokines), thereby influencing brain function (Cryan and Dinan, 2012). Furthermore, microbiota release neuroactive compounds, such as GABA, serotonin, dopamine, and acetylcholine, thereby acting locally on the enteric nervous system (Lyte, 2011; Sarkar et al., 2016). Some of these neuroactive substances access the brain through the blood and the circumventricular organs or via the vagus nerve. Therefore, it could be hypothesized that a disturbed microbiome might affect mental health, followed by anxiety and depression. Thus, the mental disorders might be a consequence of the dysbiosis and therefore promote the development of FD, which may explain the findings that anxiety increases the risk of FD (Aro et al., 2015) and observations indicating that psychosocial factors and mental disorders may play a role in FD by modulating visceral signal processing in the brain (van Oudenhove and Aziz, 2013; Browning and Travagli, 2014).
Although the following section focuses on the impact of the microbiome on mental health and the associated potential of probiotics and fecal microbiota transplantation (FMT) in the treatment of FD, it should be noted that this axis also works in the other direction: Psychosocial stress is known to affect the GI tract at multiple sites. Psychosocial stress not only affects the microbiome (Tannock and Savage, 1974; Bailey and Coe, 1999), but also other aspects of the GI tract, such as motility (Venkova et al., 2010), immune system activation (Konturek et al., 2011), and permeability [74.].
In a study on patients with IBS 65% of the participants suffered from increased psychological distress, 31% from anxiety, and 21% suffered from depression. Interestingly, microbial composition was significantly associated with distress and depression suggesting a link between certain taxa and mental condition (Peter et al., 2018). Furthermore, while depression was negatively associated with Lachnospiraceae frequency, participants who scored higher on distress, anxiety, depression, and stress perception had significantly higher abundances of Proteobacteria. In addition, patients with anxiety showed elevated levels of Bacteroidaceae (Peter et al., 2018). The microbiome signature observed in this study in psychologically distressed patients with IBS could also exist for patients with FD. The in-depth characterization of a microbiome signature specific to psychologically distressed patients with FD could lead to the discovery of new biomarkers and therapeutics. Future studies are needed to further investigate this theory. However, in such future studies, it should be kept in mind that the precise microbial signature of FD has not yet been fully elucidated, so the results must be interpreted with caution: Here, it might be challenging to distinguish whether a potential microbial alteration is associated with mental disorders or with FD, which could consequently lead to the signature of mental disorders being falsely attributed to FD. It is therefore of great relevance not only to demonstrate an FD-specific microbial signature, but also to demonstrate a clear link between microbial alterations and both specific FD symptoms and established pathophysiological manifestations.
Several studies show that probiotics have positive effects on cognitive abilities (Allen et al., 2016; Roman et al., 2018; Lew et al., 2019; Berding et al., 2021; Bloemendaal et al., 2021) and mood (Allen et al., 2016; Roman et al., 2018; Lew et al., 2019; Berding et al., 2021), therefore they were termed ‘psychobiotics’. Moreover, probiotics have also been shown to alter brain activity in magnetoencephalography (Wang et al., 2019).
There are also studies, although not specifically in the context of FD, that underline how probiotics influence anxiety, depression, and other mental disorders: In depressed patients, administration of a probiotic containing Lactobacillus helveticus and Bifidobacterium longum leads to an improvement in depression scores compared to the placebo group (Kazemi et al., 2019). In addition, administration of the same probiotic formula to healthy volunteers for thirty days resulted in relief of the subjects’ psychological distress associated with decreased cortisol levels (Messaoudi et al., 2011). Furthermore, administration of a probiotic containing Lactobacillus plantarum PS128 (PS128) to a group of patients with self-reported insomnia resulted in significant improvement of depression scores, fatigue, brainwave activity and awakening during deep sleep (Ho et al., 2021). Consistent with these findings, a study on sixty Japanese medical students taking Lactobacillus gasseri CP2305-containing tablets or placebo tablets observed a significant decrease in anxiety and sleep disturbances compared to the placebo group (Nishida et al., 2019). Similar results were found in a group of stressed adults where the administration of Lactobacillus plantarum P8 led to a decrease in anxiety accompanied by a reduction of pro-inflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α (Lew et al., 2019). Another randomized double-blind study conducted on 423 women investigated the effect of Lactobacillus rhamnosus HN001 (HN001) on postnatal mood. Here, taking HN001 resulted in lower depression and anxiety scores, suggesting that this probiotic may be effective in treating postnatal depression and anxiety (Slykerman et al., 2017). In contrast, one study investigated the effect of a probiotic containing Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58 compared to placebo in depressed patients and could not find a difference regarding depressive symptoms between groups (Chahwan et al., 2019).
Another frequently discussed approach, instead of probiotic therapy, is fecal stool transfer to restore the balance of the gut microbiota: To address this hypothesis, a study was conducted to investigate the effect of FMT from healthy patients to patients with IBS. Interestingly, four weeks after FMT, patients with IBS showed a significant increase in the diversity of their microbiota, while their psychological status also improved. The researchers concluded that FMT is a potential method to restore the balance of the gut microbiota in patients with IBS (Mizuno et al., 2017). This finding should be confirmed in larger follow up randomized controlled studies. These results also raise the question of whether FMT could be a promising treatment for FD-associated dysbiosis and, at the same time, therapy or even prevention of common mental disorders in patients with FD. To our knowledge, there are no FD-related data to date that have investigated this interesting approach.
Where do the beneficial effects of probiotics (and possibly also FMT) on mental disorders come from? There are several mechanisms by which the gut microbiome influences brain function (Mayer et al., 2015). For example, it has been shown that gut bacteria can alter systemic levels of neurotransmitter precursors and thus influence central neurotransmitters (Desbonnet et al., 2010). It has also been shown that Candida, Streptococcus and Enterococcus can produce neurotransmitters such as serotonin (Lyte, 2013, 2014; Wall et al., 2014; Yano et al., 2015), Bacillus and Saccharomyces species can produce noradrenaline (Dinan and Cryan, 2017), while Lactobacillus and Bifidobacterium species can synthesize and release GABA (Dinan and Cryan, 2017). These microbially synthesized neurotransmitters can act locally and also cross the intestinal mucosa to act locally but potentially also the central nervous system via nerval signaling.
The positive effects of probiotics and FMT on anxiety and depression may be explained by the competitive exclusion of harmful gut pathogens, the decrease in proinflammatory cytokines and communication with the central nervous system via vagal sensory fibers, leading to changes in neurotransmitter levels or function (Yan and Polk, 2002; Lammers et al., 2003; Ramiah et al., 2008; Forsythe et al., 2010). Interestingly, Lactobacillus helveticus R0052 was shown to protect the microflora of the digestive tract from invasion by pathogenic bacteria (Wine et al., 2009). It is also known that Clostridium and Bacteroides spp. produce propionic acid, a SCFA that increases aggression and fear in animals (Hanstock et al., 2004). In addition, Lactobacillus plantarum has been shown to suppress intestinal pathogens, increase the population of beneficial gut microbiota, accompanied by increased levels of SCFAs in adults (Wang et al., 2014; Kwok et al., 2015).
As mentioned earlier, SCFAs are thought to play a major role in the interplay with the gut-brain axis. However, SCFAs do not only act on the gut, but also in psychopathological processes, which could explain the link between the microbiome and mental health. For example, fecal SCFA levels have been shown to be lower in depressed patients than in controls (Skonieczna-Żydecka et al., 2018; Szczesniak et al., 2016), while another study reported that fecal butyrate correlated with reported emotional problems in children (Michels et al., 2017). Furthermore, sodium butyrate has been shown to reverse depression-like and mania-like behaviors in rats (Resende et al., 2013). We do know that SCFAs can directly affect the brain, as they can cross the blood-brain barrier (Mitchell et al., 2011). Although their uptake into the brain appears to be minimal, they can centrally modulate neurotrophic factor levels (Schroeder et al., 2007), modulate neurotransmission (Frost et al., 2014), and promote serotonin biosynthesis (Fukumoto et al., 2003; Yano et al., 2015). Taken together, the interaction of SCFAs with these gut-brain pathways may directly or indirectly modulate processes associated with neuronal function, learning, memory, and mood (Intlekofer et al., 2013; Varela et al., 2015). To date, studies investigating the direct effect of SCFAs on brain function have been limited to in vitro and animal studies, making transferability to humans difficult. In the future, it should be further clarified whether the SCFAs derived from the microbiota can reach physiologically relevant concentrations in the human CNS at all and whether these SCFAs (derived from the microbiota) can also trigger these effects.
Moreover, the role of inflammatory processes in emotion is supported by evidence of a link between depression and elevated levels of Interleukin (IL)-6, TNF and C-reactive protein (Alesci et al., 2005). In addition, antidepressants are thought to work in part through the production of the immunoregulatory cytokine IL-10, thus suppressing inflammation and depressive mood (Maes, 2001). Interestingly, Lactobacillus and Bifidobacterium stems attenuated inflammatory responses or led to IL-10 production in rodents (Desbonnet et al., 2008; Duncker et al., 2008; Karimi et al., 2009) and showed anti-inflammatory activities in human cell lines (Wallace et al., 2003). In addition, Bifidobacterium administration has been shown to suppress an increase in plasma tryptophan, which has been associated with depression in animal models (Desbonnet et al., 2008). While the role of cytokines in FD or their association with symptom severity is still unclear, a significant increase in TNF-α and IL-1β was observed in one study (Liebregts et al., 2011). Moreover, while there was no difference in IL-6 levels between patients with FD and healthy subjects (Kindt et al., 2009; Liebregts et al., 2011), IL-6 levels were associated with increased abdominal pain (Kindt et al., 2009). Interestingly, it was found that patients with higher duodenal cytokine levels are more likely to benefit from therapeutic interventions targeting gut dysbiosis, particularly probiotics (Leventogiannis et al., 2019). However, these findings need to be further elucidated in future studies.
In addition, a previous study on Lactobacillus plantarum PS128 (PS128) showed that taking PS128 can increase dopamine and serotonin levels in the brains of mice and both neurotransmitters are affected by common antidepressants (Markou et al., 1998). Moreover, normal hypothalamus-pituitary-adrenal (HPA) axis activity is regulated by circadian excitatory inputs, stress-induced stimulation and several negative feedback loops mediated by corticotrophin-releasing factor (CRF), adrenocorticotropic hormone and cortisol (Munck et al., 1984). In one of the studies discussed here, daily administration of probiotics resulted in a significant reduction in urinary free cortisol levels (Messaoudi et al., 2011). Although the mechanism underlying the stress-reducing effect of Lactobacillus gasseri CP2305 is unclear (Nishida et al., 2019), the strain is known to colonize the gut, and it has been reported that administration of heat-inactivated cells to the stomach or intestine of rats can activate the afferent vagus nerve (Nishida et al., 2019). Thus, these properties may stimulate the gut-brain axis directly or indirectly and alter the activity of the HPA axis, leading to lower stress-related symptoms and an improvement of the gut environment.
To further explore the underlying mechanism of how probiotics affect brain physiology and thus anxiety and depression, animal models were employed: Here, changes in the expression of GABA receptors in the brains of mice were demonstrated together with changes in anxiety-related behavior upon treatment with Lactobacillus rhamnosus (Bravo et al., 2011), and it was suggested that the vagus nerve might be the link between the gut and altered GABA receptor expression in the brain. Other studies examining similar effects in humans showed no differences in modifying stress-related measures underlining the difficulty of extrapolating from animal to human studies (Kelly et al., 2017).
In summary, these results are difficult to compare due to the use of differing questionnaires, heterogeneous patient groups and the different composition of probiotics. Nevertheless, the few IBS-related data give hope that probiotics are also a treatment option in patients with FD. The results mentioned above raise the idea that probiotics can be used not only to alleviate GI symptoms in FD, but also to treat or even prevent mental disorders in these patients. So far, there is a lack of FD-specific studies on this topic.
In summary, in this review we have highlighted the bi-directionality of the microbiota-gut-brain axis in the pathophysiology of FD: First, we demonstrated that there may be a link between changes in the microbiome and FD. Although the specific changes in the microbiome that may be pathognomonic in FD remain unclear and require further research, the efficacy of probiotics, which are inherently designed to alter the microbiome and reverse dysbiosis, is a viable treatment option for patients with FD. Thus, it appears that dysbiosis plays a potentially key role in the pathophysiology of FD (Figure 1).
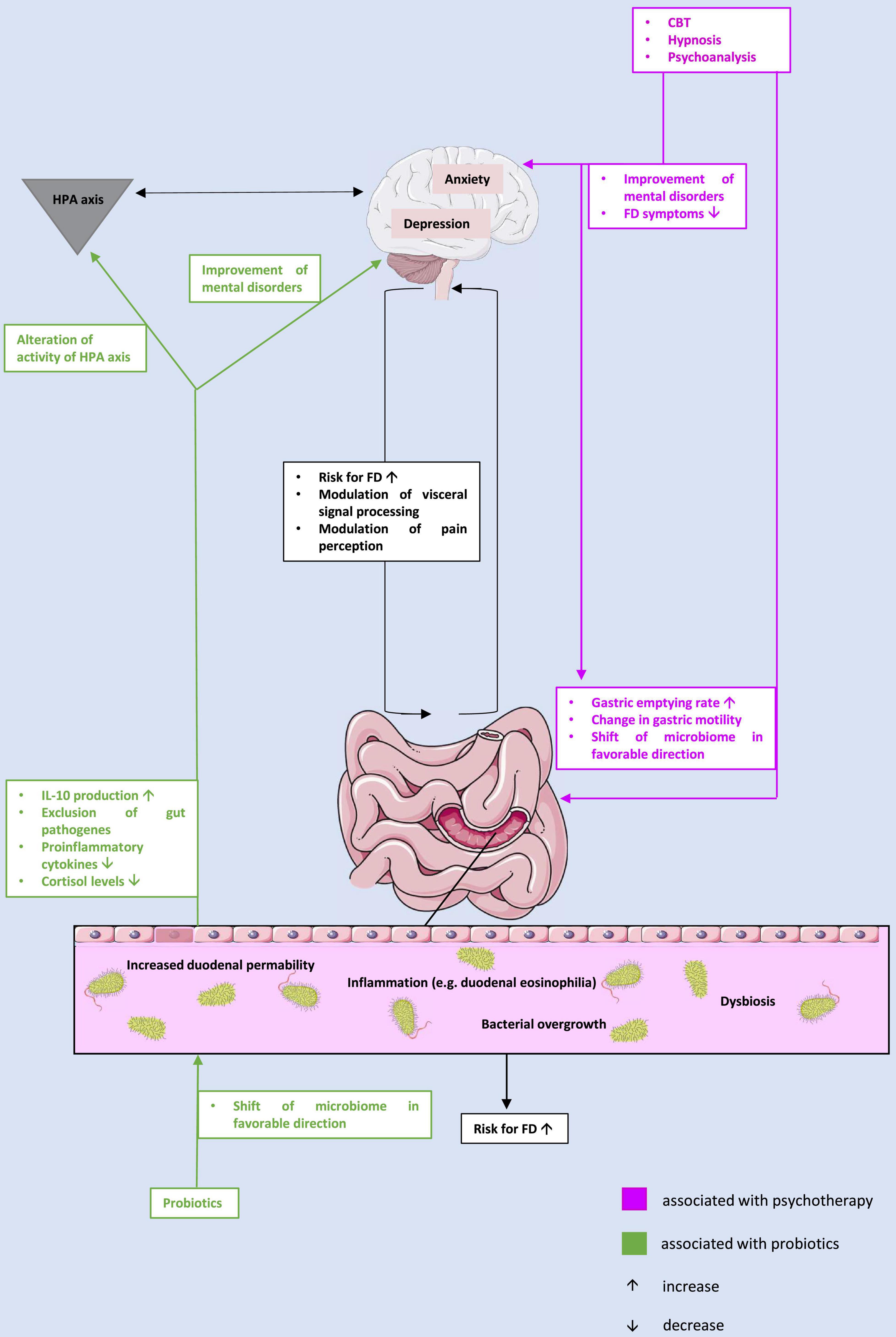
Figure 1. The potential interplay between microbiome, gut, and brain in the context of the pathophysiology of functional dyspepsia and treatment approaches. CBT, cognitive behavioral therapy; FD, functional dyspepsia; HPA, hypothalamus-pituitary-adrenal; IL, interleukin.
On the other hand, we have illuminated that mental illness both plays a role in the pathogenesis of FD and can occur for the first time in the majority of patients during the course of FD, which is why psychological therapies are also a promising approach in the treatment of FD symptoms. One could link both approaches, dysbiosis and mental disorders, and hypothesize that psychotherapy shifts the microbiome in a favorable direction. This latter approach has already been explored in studies, although not specifically in FD models. However, a precise answer to this hypothesis cannot be given based on the current data. To further confirm or reject this hypothesis, studies are urgently needed to further investigate these interesting effects of psychotherapy on the microbiome specifically in patients with FD, so that we can better understand this direction of the brain-gut-microbiome axis in the future.
There is growing evidence that the gut microbiota communicates with the central nervous system, possibly through neural, endocrine, and immunological pathways, and influences brain function. Therefore, it could be hypothesized that a disrupted microbiome could impact mental health, followed by anxiety and depression. Thus, the mental disorders could be a consequence of the dysbiosis and thus favor the development of FD, which could explain the findings that anxiety increases the risk of FD. The findings mentioned above raise the idea that probiotics can be used not only to alleviate GI symptoms in FD, but also to treat or even prevent mental disorders in these patients.
In summary, the complex pathophysiology of FD remains still largely unexplained. Although there are multiple treatment approaches, the burden of disease in patients with FD is still enormous and a definitive therapy to cure this disease does not yet exist. There is a lack of studies on the impact of dysbiosis, mental health and probiotics on pathophysiology and symptomatology in FD. Therefore, well-designed studies are needed in the future, to elucidate more precisely the underlying causes of dysbiosis, mental health disorders and FD or, where appropriate, to explore a potential direct link between them. Resolving one of these factors could also give rise to a possible link between one or more pathophysiological factors and FD symptomatology.
SR performed the database search, screened the papers, and wrote the first draft of the manuscript. AS planned the article and gave critical input throughout the study. Both authors finalized the manuscript.
We acknowledge support by Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AM declared a past collaboration with one of the author AS to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alesci, S., Martinez, P. E., Kelkar, S., Ilias, I., Ronsaville, D. S., Listwak, S. J., et al. (2005). Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J. Clin. Endocrinol. Metab. 90, 2522–2530. doi: 10.1210/jc.2004-1667
Allen, A. P., Hutch, W., Borre, Y. E., Kennedy, P. J., Temko, A., Boylan, G., et al. (2016). Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 6:e939. doi: 10.1038/tp.2016.191
Aro, P., Talley, N. J., Johansson, S. E., Agréus, L., and Ronkainen, J. (2015). Anxiety is linked to new-onset dyspepsia in the swedish population: a 10-year follow-up study. Gastroenterology 148, 928–937. doi: 10.1053/j.gastro.2015.01.039
Aro, P., Talley, N. J., Ronkainen, J., Storskrubb, T., Vieth, M., Johansson, S. E., et al. (2009). Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology 137, 94–100. doi: 10.1053/j.gastro.2009.03.039
Bailey, M. T., and Coe, C. L. (1999). Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35, 146–155.
Banerjee, S., Sindberg, G., Wang, F., Meng, J., Sharma, U., Zhang, L., et al. (2016). Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal. Immunol. 9, 1418–1428. doi: 10.1038/mi.2016.9
Barcelo, A., Claustre, J., Moro, F., Chayvialle, J. A., Cuber, J. C., and Plaisancié, P. (2000). Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46, 218–224. doi: 10.1136/gut.46.2.218
Bednarska, O., Walter, S. A., Casado-Bedmar, M., Ström, M., Salvo-Romero, E., Vicario, M., et al. (2017). Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 153, 948–960. doi: 10.1053/j.gastro.2017.06.051
Beeckmans, D., Farré, R., Riethorst, D., Keita, A. V., Augustijns, P., Soderholm, J. D., et al. (2020). Relationship between bile salts, bacterial translocation, and duodenal mucosal integrity in functional dyspepsia. Neurogastroenterol. Motil. 32:e13788. doi: 10.1111/nmo.13788
Berding, K., Long-Smith, C. M., Carbia, C., Bastiaanssen, T. F. S., van de Wouw, M., Wiley, N., et al. (2021). A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology 238, 149–163. doi: 10.1007/s00213-020-05665-y
Bloemendaal, M., Szopinska-Tokov, J., Belzer, C., Boverhoff, D., Papalini, S., Michels, F., et al. (2021). Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: exploratory analyses. Transl. Psychiatry 11:300. doi: 10.1038/s41398-021-01404-9
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12:49. doi: 10.3389/fnins.2018.00049
Bonaz, B., Sinniger, V., and Pellissier, S. (2016). Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 594, 5781–5790. doi: 10.1113/JP271539
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci USA 108, 16050–16055. doi: 10.1073/pnas.1102999108
Browning, K. N., and Travagli, R. A. (2014). Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 4, 1339–1368. doi: 10.1002/cphy.c130055
Bures, J., Cyrany, J., Kohoutova, D., Förstl, M., Rejchrt, S., Kvetina, J., et al. (2010). Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 16, 2978–2990. doi: 10.3748/wjg.v16.i24.2978
Calvert, E. L., Houghton, L. A., Cooper, P., Morris, J., and Whorwell, P. J. (2002). Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology 123, 1778–1785. doi: 10.1053/gast.2002.37071
Chahwan, B., Kwan, S., Isik, A., van Hemert, S., Burke, C., and Roberts, L. (2019). Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 253, 317–326. doi: 10.1016/j.jad.2019.04.097
Cheadle, G. A., Costantini, T. W., Bansal, V., Eliceiri, B. P., and Coimbra, R. (2014). Cholinergic signaling in the gut: a novel mechanism of barrier protection through activation of enteric glia cells. Surg. Infect. 15, 387–393. doi: 10.1089/sur.2013.103
Cheng, C., Yang, F. C., Jun, S., and Hutton, J. M. (2007). Flexible coping psychotherapy for functional dyspeptic patients: a randomized, controlled trial. Psychosom. Med. 69, 81–88. doi: 10.1097/01.psy.0000249734.99065.6f
Cherbut, C., Ferrier, L., Rozé, C., Anini, Y., Blottière, H., Lecannu, G., et al. (1998). Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiol. 275, G1415–G1422. doi: 10.1152/ajpgi.1998.275.6.G1415
Chiarioni, G., Vantini, I., De Iorio, F., and Benini, L. (2006). Prokinetic effect of gut-oriented hypnosis on gastric emptying. Aliment Pharmacol. Ther. 23, 1241–1249. doi: 10.1111/j.1365-2036.2006.02881.x
Chuah, K. H., Wong, M. S., Tan, P. O., Lim, S. Z., Beh, K. H., Chong, S. C. S., et al. (2021). Small intestinal bacterial overgrowth in various functional gastrointestinal disorders: a case-control study. Dig. Dis. Sci. 21:4. doi: 10.1007/s10620-021-07227-4
Clauwaert, N., Jones, M. P., Holvoet, L., Vandenberghe, J., Vos, R., Tack, J., et al. (2012). Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: are all symptoms equal? Neurogastroenterol. Motil. 24, 1088–e565. doi: 10.1111/j.1365-2982.2012.01985.x
Corrêa-Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T., and Vinolo, M. A. (2016). Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5:e73. doi: 10.1038/cti.2016.17
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
Daly, K., and Shirazi-Beechey, S. P. (2006). Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol. 25, 49–62. doi: 10.1089/dna.2006.25.49
Dass, N. B., John, A. K., Bassil, A. K., Crumbley, C. W., Shehee, W. R., Maurio, F. P., et al. (2007). The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol. Motil. 19, 66–74. doi: 10.1111/j.1365-2982.2006.00853.x
Dehghanizade, Z., Zargar, Y., Mehrabizadeh Honarmand, M., Kadkhodaie, A., and Eydi Baygi, M. (2015). The effectiveness of cognitive behavior stress management on functional dyspepsia symptoms. J. Adv. Med. Educ. Prof. 3, 45–49.
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J., and Dinan, T. G. (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174. doi: 10.1016/j.jpsychires.2008.03.009
Desbonnet, L., Garrett, L., Clarke, G., Kiely, B., Cryan, J. F., and Dinan, T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 170, 1179–1188. doi: 10.1016/j.neuroscience.2010.08.005
Dinan, T. G., and Cryan, J. F. (2017). Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 595, 489–503. doi: 10.1113/JP273106
Ding, J. H., Jin, Z., Yang, X. X., Lou, J., Shan, W. X., Hu, Y. X., et al. (2020). Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 26, 6141–6162. doi: 10.3748/wjg.v26.i40.614
Duncanson, K. R., Talley, N. J., Walker, M. M., and Burrows, T. L. (2018). Food and functional dyspepsia: a systematic review. J. Hum. Nutr. Diet. 31, 390–407. doi: 10.1111/jhn.12506
Duncker, S. C., Wang, L., Hols, P., and Bienenstock, J. (2008). The D-alanine content of lipoteichoic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model. Neurogastroenterol. Motil. 20, 843–850. doi: 10.1111/j.1365-2982.2008.01085.x
Elli, M., Callegari, M. L., Ferrari, S., Bessi, E., Cattivelli, D., Soldi, S., et al. (2006). Survival of yogurt bacteria in the human gut. Appl. Environ. Microbiol. 72, 5113–5117. doi: 10.1128/AEM.02950-05
Erickson, R. A., and Epsten, R. M. Jr. (1988). Oral chenodeoxycholic acid increases small intestinal permeability to lactulose in humans. Am. J. Gastroenterol. 83:541.
Faramarzi, M., Azadfallah, P., Book, H. E., Rasolzadeh Tabatabai, K., Taherim, H., and Kashifard, M. (2015). The effect of psychotherapy in improving physical and psychiatric symptoms in patients with functional dyspepsia. Iran J. Psychiatry 10, 43–49.
Faramarzi, M., Azadfallah, P., Book, H. E., Tabatabaei, K. R., Taheri, H., and Shokri-shirvani, J. (2013). A randomized controlled trial of brief psychoanalytic psychotherapy in patients with functional dyspepsia. Asian J. Psychiatr. 6, 228–234. doi: 10.1016/j.ajp.2012.12.012
Fischler, B., Tack, J., De Gucht, V., Shkedy, Z. I., Persoons, P., Broekaert, D., et al. (2003). Heterogeneity of symptom pattern, psychosocial factors, and pathophysiological mechanisms in severe functional dyspepsia. Gastroenterology 124, 903–910. doi: 10.1053/gast.2003.50155
Ford, A. C., Marwaha, A., Sood, R., and Moayyedi, P. (2015). Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 64, 1049–1057. doi: 10.1136/gutjnl-2014-307843
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H., and Bienenstock, J. (2010). Mood and gut feelings. Brain Behav. Immun. 24, 9–16. doi: 10.1016/j.bbi.2009.05.058
Frost, G., Sleeth, M. L., Sahuri-Arisoylu, M., Lizarbe, B., Cerdan, S., Brody, L., et al. (2014). The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5:3611. doi: 10.1038/ncomms4611
Fukui, A., Takagi, T., Naito, Y., Inoue, R., Kashiwagi, S., Mizushima, K., et al. (2020). Higher Levels of streptococcus in upper gastrointestinal mucosa associated with symptoms in patients with functional Dyspepsia. Digestion 101, 38–45. doi: 10.1159/000504090
Fukumoto, S., Tatewaki, M., Yamada, T., Fujimiya, M., Mantyh, C., Voss, M., et al. (2003). Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1269–R1276. doi: 10.1152/ajpregu.00442.2002
Futagami, S., Itoh, T., and Sakamoto, C. (2015). Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol. Ther. 41, 177–188. doi: 10.1111/apt.13006
Galanos, C., Lüderitz, O., Rietschel, E. T., Westphal, O., Brade, H., Brade, L., et al. (1985). Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 148, 1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x
Gao, L., Niu, X., Niu, T., Wang, X., Lu, X., Feng, Q., et al. (2020). Curative efficacy of extract from Ganjiangdazao recipe on functional dyspepsia in rats. J. Tradit. Chin. Med. 40, 654–663. doi: 10.19852/j.cnki.jtcm.2020.04.012
Gareau, M. G., Silva, M. A., and Perdue, M. H. (2008). Pathophysiological mechanisms of stress-induced intestinal damage. Curr. Mol. Med. 8, 274–281. doi: 10.2174/156652408784533760
Gudiña, E. J., Teixeira, J. A., and Rodrigues, L. R. (2010). Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid. Surf B Biointer. 76, 298–304. doi: 10.1016/j.colsurfb.2009.11.008
Gurusamy, S. R., Shah, A., Talley, N. J., Koloski, N., Jones, M. P., Walker, M. M., et al. (2021). Small intestinal bacterial overgrowth in functional dyspepsia: a systematic review and meta-analysis. Am. J. Gastroenterol. 116, 935–942. doi: 10.14309/ajg.0000000000001197
Hamilton, J., Guthrie, E., Creed, F., Thompson, D., Tomenson, B., Bennett, R., et al. (2000). A randomized controlled trial of psychotherapy in patients with chronic functional dyspepsia. Gastroenterology 119, 661–669. doi: 10.1053/gast.2000.16493
Handa, M., Mine, K., Yamamoto, H., Tsutsui, S., Hayashi, H., Kinukawa, N., et al. (1999). Esophageal motility and psychiatric factors in functional dyspepsia patients with or without pain. Dig. Dis. Sci. 44, 2094–2098. doi: 10.1023/a:1026690806330
Hannibal, K. E., and Bishop, M. D. (2014). Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 94, 1816–1825. doi: 10.2522/ptj.20130597
Hanstock, T. L., Clayton, E. H., Li, K. M., and Mallet, P. E. (2004). Anxiety and aggression associated with the fermentation of carbohydrates in the hindgut of rats. Physiol. Behav. 82, 357–368. doi: 10.1016/j.physbeh.2004.04.002
He, Y., Yang, C., Wang, P., Yang, L., Wu, H., Liu, H., et al. (2019). Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. J. Ethnopharmacol. 240:111953. doi: 10.1016/j.jep.2019.111953
Ho, Y. T., Tsai, Y. C., Kuo, T. B. J., and Yang, C. C. H. (2021). Effects of lactobacillus plantarum ps128 on depressive symptoms and sleep quality in self-reported insomniacs: a randomized. double-blind, placebo-controlled pilot trial. Nutrients 13:2820. doi: 10.3390/nu13082820
Hosseini, A., Nikfar, S., and Abdollahi, M. (2012). Probiotics use to treat irritable bowel syndrome. Expert Opin. Biol. Ther. 12, 1323–1334. doi: 10.1517/14712598.2012.707179
Hsu, Y. C., Liou, J. M., Liao, S. C., Yang, T. H., Wu, H. T., Hsu, W. L., et al. (2009). Psychopathology and personality trait in subgroups of functional dyspepsia based on Rome III criteria. Am. J. Gastroenterol. 104, 2534–2542. doi: 10.1038/ajg.2009.328
Igarashi, M., Nakae, H., Matsuoka, T., Takahashi, S., Hisada, T., Tomita, J., et al. (2017). Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 4:e000144. doi: 10.1136/bmjgast-2017-000144
Intlekofer, K. A., Berchtold, N. C., Malvaez, M., Carlos, A. J., McQuown, S. C., Cunningham, M. J., et al. (2013). Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 38, 2027–2034. doi: 10.1038/npp.2013.104
Jung, H. K., and Talley, N. J. (2018). Role of the duodenum in the pathogenesis of functional dyspepsia: a paradigm shift. J. Neurogastroenterol. Motil. 24, 345–354. doi: 10.5056/jnm18060
Kamp, K. J., Plantinga, A. M., Cain, K. C., Burr, R. L., Barney, P., Jarrett, M., et al. (2021). Comprehensive self-management program with diet education does not alter microbiome characteristics in women with irritable bowel syndrome. Biol. Res. Nurs. 23, 471–480. doi: 10.1177/1099800420984543
Karimi, K., Inman, M. D., Bienenstock, J., and Forsythe, P. (2009). Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 179, 186–193. doi: 10.1164/rccm.200806-951OC
Karl, J. P., Margolis, L. M., Madslien, E. H., Murphy, N. E., Castellani, J. W., Gundersen, Y., et al. (2017). Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol Gastrointest Liver Physiol. 312, G559–G571. doi: 10.1152/ajpgi.00066.2017
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 38, 522–528. doi: 10.1016/j.clnu.2018.04.010
Kelly, J. R., Allen, A. P., Temko, A., Hutch, W., Kennedy, P. J., Farid, N., et al. (2017). Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 61, 50–59. doi: 10.1016/j.bbi.2016.11.018
Khine, W. W. T., Voong, M. L., Ng, T. K. S., Feng, L., Rane, G. A., Kumar, A. P., et al. (2020). Mental awareness improved mild cognitive impairment and modulated gut microbiome. Aging 12, 24371–24393. doi: 10.18632/aging.202277
Kindt, S., Van Oudenhove, L., Broekaert, D., Kasran, A., Ceuppens, J. L., Bossuyt, X., et al. (2009). Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol. Motil. 21, 389–398. doi: 10.1111/j.1365-2982.2008.01220.x
Koloski, N. A., Jones, M., Kalantar, J., Weltman, M., Zaguirre, J., and Talley, N. J. (2012). The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 61, 1284–1290. doi: 10.1136/gutjnl-2011-300474
Koloski, N. A., Jones, M., and Talley, N. J. (2016). Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol. Ther. 44, 592–600. doi: 10.1111/apt.13738
Konturek, P. C., Brzozowski, T., and Konturek, S. J. (2011). Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 62, 591–599.
Kwok, L. Y., Guo, Z., Zhang, J., Wang, L., Qiao, J., Hou, Q., et al. (2015). The impact of oral consumption of Lactobacillus plantarum P-8 on faecal bacteria revealed by pyrosequencing. Benef. Microbes. 6, 405–413. doi: 10.3920/BM2014.0063
Lal, S., Kirkup, A. J., Brunsden, A. M., Thompson, D. G., and Grundy, D. (2001). Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907
Lammers, K. M., Brigidi, P., Vitali, B., Gionchetti, P., Rizzello, F., Caramelli, E., et al. (2003). Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 38, 165–172. doi: 10.1016/S0928-8244(03)00144-5
Leventogiannis, K., Gkolfakis, P., Spithakis, G., Tsatali, A., Pistiki, A., Sioulas, A., et al. (2019). Effect of a preparation of four probiotics on symptoms of patients with irritable bowel syndrome: association with intestinal bacterial overgrowth. Prob. Antimicrob. Prot. 11, 627–634. doi: 10.1007/s12602-018-9401-3
Lew, L. C., Hor, Y. Y., Yusoff, N. A. A., Choi, S. B., Yusoff, M. S. B., Roslan, N. S., et al. (2019). Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 38, 2053–2064. doi: 10.1016/j.clnu.2018.09.010
Lewis, K., Lutgendorff, F., Phan, V., Söderholm, J. D., Sherman, P. M., and McKay, D. M. (2010). Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel Dis. 16, 1138–1148. doi: 10.1002/ibd.21177
Liebregts, T., Adam, B., Bredack, C., Gururatsakul, M., Pilkington, K. R., Brierley, S. M., et al. (2011). Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am. J. Gastroenterol. 106, 1089–1098. doi: 10.1038/ajg.2010.512
Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581. doi: 10.1002/bies.201100024
Lyte, M. (2013). Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9:e1003726. doi: 10.1371/journal.ppat.1003726
Lyte, M. (2014). Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut. Microb. 5, 381–389. doi: 10.4161/gmic.28682
Maes, M. (2001). The immunoregulatory effects of antidepressants. Hum. Psychopharmacol. 16, 95–103. doi: 10.1002/hup.191
Markou, A., Kosten, T. R., and Koob, G. F. (1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18, 135–174. doi: 10.1016/S0893-133X(97)00113-9
Matsueda, K., Hongo, M., Tack, J., Aoki, H., Saito, Y., and Kato, H. (2010). Clinical trial: dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia - 100 mg t.i.d. is an optimal dosage. Neurogastroenterol. Motil. 22, 618–e173. doi: 10.1111/j.1365-2982.2009.01449.x
Mayer, E. A., Labus, J. S., Tillisch, K., Cole, S. W., and Baldi, P. (2015). Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 12, 592–605. doi: 10.1038/nrgastro.2015.121
McEwen, B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185. doi: 10.1016/j.ejphar.2007.11.071
McGovern, A. S., Hamlin, A. S., and Winter, G. (2019). A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust. NZ J. Psychiatry 53, 1151–1166. doi: 10.1177/0004867419877954
Mearin, F., Lacy, B. E., Chang, L., Chey, W. D., Lembo, A. J., Simren, M., et al. (2016). Bowel Disorders. Gastroenterology 18:31. doi: 10.1053/j.gastro.2016.02.031
Meregnani, J., Clarençon, D., Vivier, M., Peinnequin, A., Mouret, C., Sinniger, V., et al. (2011). Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 160, 82–89. doi: 10.1016/j.autneu.2010.10.007
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., et al. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764. doi: 10.1017/S0007114510004319
Michels, N., Van de Wiele, T., and De Henauw, S. (2017). Chronic psychosocial stress and gut health in children: associations with calprotectin and fecal short-chain fatty acids. Psychosom. Med. 79, 927–935. doi: 10.1097/PSY.0000000000000413
Mitchell, R. W., On, N. H., Del Bigio, M. R., Miller, D. W., and Hatch, G. M. (2011). Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 117, 735–746. doi: 10.1111/j.1471-4159.2011.07245.x
Mizuno, S., Masaoka, T., Naganuma, M., Kishimoto, T., Kitazawa, M., Kurokawa, S., et al. (2017). Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 96, 29–38. doi: 10.1159/000471919
Moayyedi, P., Forman, D., Braunholtz, D., Feltbower, R., Crocombe, W., Liptrott, M., et al. (2000). The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Leeds HELP Study Group. Am. J. Gastroenterol. 95, 1448–1455. doi: 10.1111/j.1572-0241.2000.2126_1.x
Moussaoui, N., Jacobs, J. P., Larauche, M., Biraud, M., Million, M., Mayer, E., et al. (2017). Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J. Neurogastroenterol. Motil. 23, 135–143. doi: 10.5056/jnm16105
Munck, A., Guyre, P. M., and Holbrook, N. J. (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 5, 25–44. doi: 10.1210/edrv-5-1-25
Nakae, H., Tsuda, A., Matsuoka, T., Mine, T., and Koga, Y. (2016). Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol. 3:e000109. doi: 10.1136/bmjgast-2016-000109
Nakagawa, K., Hara, K., Fikree, A., Siddiqi, S., Woodland, P., Masamune, A., et al. (2020). Patients with dyspepsia have impaired mucosal integrity both in the duodenum and jejunum: in vivo assessment of small bowel mucosal integrity using baseline impedance. J. Gastroenterol. 55, 273–280. doi: 10.1007/s00535-019-01614-5
Nishida, K., Sawada, D., Kuwano, Y., Tanaka, H., and Rokutan, K. (2019). Health benefits of lactobacillus gasseri cp2305 tablets in young adults exposed to chronic stress: a randomized. double-blind, placebo-controlled study. Nutrients 11:1859. doi: 10.3390/nu11081859
Ohtsu, T., Takagi, A., Uemura, N., Inoue, K., Sekino, H., Kawashima, A., et al. (2017). The Ameliorating effect of lactobacillus gasseri OLL2716 on functional dyspepsia in helicobacter pylori-uninfected individuals: a randomized controlled study. Digestion 96, 92–102. doi: 10.1159/000479000
Orive, M., Barrio, I., Orive, V. M., Matellanes, B., Padierna, J. A., Cabriada, J., et al. (2015). A randomized controlled trial of a 10 week group psychotherapeutic treatment added to standard medical treatment in patients with functional dyspepsia. J. Psychosom. Res. 78, 563–568. doi: 10.1016/j.jpsychores.2015.03.003
Pellissier, S., Dantzer, C., Mondillon, L., Trocme, C., Gauchez, A. S., Ducros, V., et al. (2014). Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One 9:e105328. doi: 10.1371/journal.pone.0105328
Perry, I. E., Sonu, I., Scarpignato, C., Akiyama, J., Hongo, M., and Vega, K. J. (2020). Potential proton pump inhibitor-related adverse effects. Ann. NY Acad. Sci. 1481, 43–58. doi: 10.1111/nyas.14428
Peter, J., Fournier, C., Durdevic, M., Knoblich, L., Keip, B., Dejaco, C., et al. (2018). Microbial signature of psychological distress in irritable bowel syndrome. Psychosom. Med. 80, 698–709. doi: 10.1097/PSY.0000000000000630
Qin, H. Y., Cheng, C. W., Tang, X. D., and Bian, Z. X. (2014). Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 20, 14126–14131. doi: 10.3748/wjg.v20.i39.14126
Qiu, J. J., Liu, Z., Zhao, P., Wang, X. J., Li, Y. C., Sui, H., et al. (2017). Gut microbial diversity analysis using Illumina sequencing for functional dyspepsia with liver depression-spleen deficiency syndrome and the interventional Xiaoyaosan in a rat model. World J. Gastroenterol. 23, 810–816. doi: 10.3748/wjg.v23.i5.810
Ramiah, K., van Reenen, C. A., and Dicks, L. M. (2008). Surface-bound proteins of Lactobacillus plantarum 423 that contribute to adhesion of Caco-2 cells and their role in competitive exclusion and displacement of Clostridium sporogenes and Enterococcus faecalis. Res. Microbiol. 159, 470–475. doi: 10.1016/j.resmic.2008.06.002
Resende, W. R., Valvassori, S. S., Réus, G. Z., Varela, R. B., Arent, C. O., Ribeiro, K. F., et al. (2013). Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav. Pharmacol. 24, 569–579. doi: 10.1097/FBP.0b013e32836546fc
Rodrigues, D. M., Motomura, D. I., Tripp, D. A., and Beyak, M. J. (2021). Are psychological interventions effective in treating functional dyspepsia? A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 2047–2057. doi: 10.1111/jgh.15566
Roman, P., Estévez, A. F., Miras, A., Sánchez-Labraca, N., Cañadas, F., Vivas, A. B., et al. (2018). Pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci. Rep. 8:10965. doi: 10.1038/s41598-018-29388-5
Ropert, A., Cherbut, C., Rozé, C., Le Quellec, A., Holst, J. J., and Fu-Cheng, X. (1996). Bruley des Varannes S, Galmiche JP. Colonic fermentation and proximal gastric tone in humans. Gastroenterology 111, 289–296. doi: 10.1053/gast.1996.v111.pm8690193
Rousseaux, C., Thuru, X., Gelot, A., Barnich, N., Neut, C., Dubuquoy, L., et al. (2007). Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 13, 35–37. doi: 10.1038/nm1521
Sachdev, A. H., and Pimentel, M. (2013). Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther. Adv. Chronic. Dis. 4, 223–231. doi: 10.1177/2040622313496126
Sarkar, A., Lehto, S. M., Harty, S., Dinan, T. G., Cryan, J. F., and Burnet, P. W. J. (2016). Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 39, 763–781. doi: 10.1016/j.tins.2016.09.002
Schmulson, M. J., and Drossman, D. A. (2017). What is new in rome IV. J. Neurogastroenterol. Motil. 23, 151–163. doi: 10.5056/jnm16214
Schroeder, F. A., Lin, C. L., Crusio, W. E., and Akbarian, S. (2007). Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 62, 55–64. doi: 10.1016/j.biopsych.2006.06.036
Shimura, S., Ishimura, N., Mikami, H., Okimoto, E., Uno, G., Tamagawa, Y., et al. (2016). Small Intestinal bacterial overgrowth in patients with refractory functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 22, 60–68. doi: 10.5056/jnm15116
Sjövall, H. (2011). Meaningful or redundant complexity - mechanisms behind cyclic changes in gastroduodenal pH in the fasting state. Acta Physiol. 201, 127–131. doi: 10.1111/j.1748-1716.2010.02155.x
Skonieczna-Żydecka, K., Grochans, E., Maciejewska, D., Szkup, M., Schneider-Matyka, D., Jurczak, A., et al. (2018). Faecal short chain fatty acids profile is changed in polish depressive Women. Nutrients 10:1939. doi: 10.3390/nu10121939
Slykerman, R. F., Hood, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). Probiotic in pregnancy study group. effect of lactobacillus rhamnosus hn001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Sobko, T., Liang, S., Cheng, W. H. G., and Tun, H. M. (2020). Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: the Play&Grow randomized controlled trial. Sci. Rep. 10:21993. doi: 10.1038/s41598-020-78642-2
Sperber, A. D., Bangdiwala, S. I., Drossman, D. A., Ghoshal, U. C., Simren, M., Tack, J., et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology 160, 99–114. doi: 10.1053/j.gastro.2020.04.014
Stanghellini, V., Chan, F. K., Hasler, W. L., Malagelada, J. R., Suzuki, H., Tack, J., et al. (2016). Gastroduodenal Disorders. Gastroenterology 150, 1380–1392. doi: 10.1053/j.gastro.2016.02.011
Stenman, L. K., Holma, R., Eggert, A., and Korpela, R. (2013). A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G227–G234. doi: 10.1152/ajpgi.00267.2012
Stilling, R. M., van de Wouw, M., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2016). The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 99, 110–132. doi: 10.1016/j.neuint.2016.06.011
Sun, E., Zhang, X., Zhao, Y., Li, J., Sun, J., Mu, Z., et al. (2021). Beverages containing Lactobacillus paracasei LC-37 improved functional dyspepsia through regulation of the intestinal microbiota and their metabolites. J. Dairy Sci. 104, 6389–6398. doi: 10.3168/jds.2020-19882
Suzuki, H., and Moayyedi, P. (2013). Helicobacter pylori infection in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 168–174. doi: 10.1038/nrgastro.2013.9
Szczesniak, O., Hestad, K. A., Hanssen, J. F., and Rudi, K. (2016). Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 19, 279–283. doi: 10.1179/1476830515Y.0000000007
Taché, Y., and Bonaz, B. (2007). Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J. Clin. Invest. 117, 33–40. doi: 10.1172/JCI30085
Tache, Y., Larauche, M., Yuan, P. Q., and Million, M. (2018). Brain and Gut CRF Signaling: biological actions and role in the gastrointestinal tract. Curr. Mol. Pharmacol. 11, 51–71. doi: 10.2174/1874467210666170224095741
Tack, J., and Talley, N. J. (2013). Functional dyspepsia–symptoms, definitions and validity of the Rome III criteria. Nat. Rev. Gastroenterol. Hepatol. 10, 134–141. doi: 10.1038/nrgastro.2013.14
Takagi, A., Yanagi, H., Ozawa, H., Uemura, N., Nakajima, S., Inoue, K., et al. (2016). Effects of Lactobacillus gasseri OLL2716 on helicobacter pylori-associated dyspepsia: a multicenter randomized double-blind controlled trial. Gastroenterol. Res. Pract. 2016:7490452. doi: 10.1155/2016/7490452
Talley, N. J., Fung, L. H., Gilligan, I. J., McNeil, D., and Piper, D. W. (1986). Association of anxiety, neuroticism, and depression with dyspepsia of unknown cause. A case-control study. Gastroenterology 90, 886–892. doi: 10.1016/0016-5085(86)90864-4
Talley, N. J., Walker, M. M., Aro, P., Ronkainen, J., Storskrubb, T., Hindley, L. A., et al. (2007). Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin. Gastroenterol. Hepatol. 5, 1175–1183. doi: 10.1016/j.cgh.2007.05.015
Tannock, G. W., and Savage, D. C. (1974). Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 9, 591–598. doi: 10.1128/iai.9.3.591-598.1974
Teh, J. J., Berendsen, E. M., Hoedt, E. C., Kang, S., Zhang, J., Zhang, F., et al. (2021). Novel strain-level resolution of Crohn’s disease mucosa-associated microbiota via an ex vivo combination of microbe culture and metagenomic sequencing. ISME J. 15, 3326–3338. doi: 10.1038/s41396-021-00991-1
Tziatzios, G., Gkolfakis, P., Papanikolaou, I. S., Mathur, R., Pimentel, M., Damoraki, G., et al. (2021). High prevalence of small intestinal bacterial overgrowth among functional dyspepsia patients. Dig. Dis. 39, 382–390. doi: 10.1159/000511944
van Oudenhove, L., and Aziz, Q. (2013). The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 158–167. doi: 10.1038/nrgastro.2013.10
Vanheel, H., and Farré, R. (2013). Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 142–149. doi: 10.1038/nrgastro.2012.255
Vanheel, H., Vicario, M., Vanuytsel, T., Van Oudenhove, L., Martinez, C., Keita, A. V., et al. (2014). Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 63, 262–271. doi: 10.1136/gutjnl-2012-303857
Varela, R. B., Valvassori, S. S., Lopes-Borges, J., Mariot, E., Dal-Pont, G. C., Amboni, R. T., et al. (2015). Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr Res. 61, 114–121. doi: 10.1016/j.jpsychires.2014.11.003
Vasapolli, R., Schulz, C., Schweden, M., Casèn, C., Kirubakaran, G. T., Kirste, K. H., et al. (2021). Gut microbiota profiles and the role of anti-CdtB and anti-vinculin antibodies in patients with functional gastrointestinal disorders (FGID). Eur. J. Clin. Invest. 51:e13666. doi: 10.1111/eci.13666
Venkova, K., Johnson, A. C., Myers, B., and Greenwood-Van Meerveld, B. (2010). Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology 58, 1161–1167. doi: 10.1016/j.neuropharm.2010.02.012
Wall, R., Cryan, J. F., Ross, R. P., Fitzgerald, G. F., Dinan, T. G., and Stanton, C. (2014). Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 817, 221–239. doi: 10.1007/978-1-4939-0897-4_10
Wallace, T. D., Bradley, S., Buckley, N. D., and Green-Johnson, J. M. (2003). Interactions of lactic acid bacteria with human intestinal epithelial cells: effects on cytokine production. J. Food Prot. 66, 466–472. doi: 10.4315/0362-028x-66.3.466
Wang, H., Braun, C., Murphy, E. F., and Enck, P. (2019). Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am. J. Gastroenterol. 114, 1152–1162. doi: 10.14309/ajg.0000000000000203
Wang, L., Zhang, J., Guo, Z., Kwok, L., Ma, C., Zhang, W., et al. (2014). Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 30, 776–783. doi: 10.1016/j.nut.2013.11.018
Wang, R., Cai, Y., Li, J., Yau, S. Y., Lu, W., Stubbs, B., et al. (2021). Effects of aerobic exercise on gut microbiota in adolescents with subthreshold mood syndromes and healthy adolescents: A 12-week, randomized controlled trial. J. Affect. Disord. 293, 363–372. doi: 10.1016/j.jad.2021.06.025
Wang, X., Li, X., Ge, W., Huang, J., Li, G., Cong, Y., et al. (2015). Quantitative evaluation of duodenal eosinophils and mast cells in adult patients with functional dyspepsia. Ann. Diagn. Pathol. 19, 50–56. doi: 10.1016/j.anndiagpath.2015.02.001
Wauters, L., Slaets, H., De Paepe, K., Ceulemans, M., Wetzels, S., Geboers, K., et al. (2021). Efficacy and safety of spore-forming probiotics in the treatment of functional dyspepsia: a pilot randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 6, 784–792. doi: 10.1016/S2468-1253(21)00226-0
Wilhelmsen, I., Haug, T. T., Ursin, H., and Berstad, A. (1994). Effect of short-term cognitive psychotherapy on recurrence of duodenal ulcer: a prospective randomized trial. Psychosom. Med. 56, 440–448. doi: 10.1097/00006842-199409000-00009
Wine, E., Gareau, M. G., Johnson-Henry, K., and Sherman, P. M. (2009). Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 300, 146–152. doi: 10.1111/j.1574-6968.2009.01781.x
Wood, S. K., and Woods, J. H. (2007). Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert. Opin. Ther. Targets 11, 1401–1413. doi: 10.1517/14728222.11.11.1401
Xiong, Y., Xing, H., Hu, L., Xie, J., Liu, Y., and Hu, D. (2019). Effects of comfort care on symptoms, gastric motility, and mental state of patients with functional dyspepsia. Medicine 98:e16110. doi: 10.1097/MD.0000000000016110
Yan, F., and Polk, D. B. (2002). Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277, 50959–50965. doi: 10.1074/jbc.M207050200
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Yu, Y. B., and Li, Y. Q. (2014). Enteric glial cells and their role in the intestinal epithelial barrier. World J. Gastroenterol. 20, 11273–11280. doi: 10.3748/wjg.v20.i32.11273
Zhang, S., Lin, L., Liu, W., Zou, B., Cai, Y., Liu, D., et al. (2019). Shen-Ling-Bai-Zhu-San alleviates functional dyspepsia in rats and modulates the composition of the gut microbiota. Nutr. Res. 71, 89–99. doi: 10.1016/j.nutres.2019.10.001
Zhang, S., Xu, Z., Cao, X., Xie, Y., Lin, L., Zhang, X., et al. (2020). Shenling Baizhu San improves functional dyspepsia in rats as revealed by 1H-NMR based metabolomics. Anal. Methods 12, 2363–2375. doi: 10.1039/d0ay00580k
Zhong, L., Shanahan, E. R., Raj, A., Koloski, N. A., Fletcher, L., Morrison, M., et al. (2017). Dyspepsia and the microbiome: time to focus on the small intestine. Gut 66, 1168–1169. doi: 10.1136/gutjnl-2016-312574
Keywords: functional dyspepsia, gut-brain axis, microbiome, psychotherapy, probiotics
Citation: Rupp SK and Stengel A (2022) Bi-Directionality of the Microbiota-Gut-Brain Axis in Patients With Functional Dyspepsia: Relevance of Psychotherapy and Probiotics. Front. Neurosci. 16:844564. doi: 10.3389/fnins.2022.844564
Received: 28 December 2021; Accepted: 07 February 2022;
Published: 28 February 2022.
Edited by:
Lucas Wauters, KU Leuven, BelgiumCopyright © 2022 Rupp and Stengel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Stengel, YW5kcmVhcy5zdGVuZ2VsQG1lZC51bmktdHVlYmluZ2VuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.