- 1Department of Anesthesiology and Critical Care, Soroka University Medical Center, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 2Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Jacksonville, FL, United States
- 3Department of Radiology, Soroka University Medical Center, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 4Department of Physiology, Faculty of Biology, Ecology and Medicine, Dnepropetrovsk State University, Dnepropetrovsk, Ukraine
- 5Department of Psychology, Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Be’er Sheva, Israel
Depression is a common and serious complication following traumatic brain injury (TBI). Both depression and TBI have independently been associated with pathologically elevated extracellular brain glutamate levels. In the setting of TBI, blood glutamate scavenging with pyruvate has been widely shown as an effective method to provide neuroprotection by reducing blood glutamate and subsequent brain glutamate levels. Here we evaluate pyruvate as a novel approach in the treatment and prevention of post-TBI depression-like behavior in a rat model. Rats were divided into five groups: (1) sham-operated control with pyruvate, (2) sham-operated control with placebo, (3) post-TBI with placebo, (4) post-TBI given preventative pyruvate, and (5) post-TBI treated with pyruvate. These groups had an equal number of females and males. Rats were assessed for depressive-like behavior, neurological status, and glutamate levels in the blood and brain. Post-TBI neurological deficits with concurrent elevations in glutamate levels were demonstrated, with peak glutamate levels 24 h after TBI. Following TBI, the administration of either prophylactic or therapeutic pyruvate led to reduced glutamate levels, improved neurologic recovery, and improved depressive-like behavior. Glutamate scavenging with pyruvate may be an effective prophylactic and therapeutic option for post-TBI depression by reducing associated elevations in brain glutamate levels.
Introduction
The majority of survivors of moderate and severe traumatic brain injury (TBI) suffer from chronic neuropsychiatric consequences, including cognitive defects, depression, anxiety, social withdrawal and aggression (Tateno et al., 2003; McAllister, 2008; Jorge and Arciniegas, 2014; Hicks et al., 2019; Rauen et al., 2020). While these behavioral sequelae may at first be attributable to the emotional burdens of physical disability, these symptoms are not correlated with the severity of the initial injury or with pain (Bodnar et al., 2019) and can persist for decades (Hoofien et al., 2001; Koponen et al., 2002). Despite their significant impacts on functional recovery, quality of life, and resumption of employment (Rivara et al., 2011), these chronic neuropsychiatric conditions following TBI are often overlooked, undiagnosed and untreated.
Depressive disorders are generally treated by targeting the serotoninergic, adrenergic, and/or dopaminergic systems with medication that increases synaptic access of these neurotransmitters (Robinson et al., 1984; Currier et al., 1992; Andersen et al., 1994; Wiart et al., 2000). However, treatment for depressive disorders is effective in approximately two thirds of patients. For those suffering from depression following TBI, the selective serotonin reuptake inhibitor Sertraline (Zoloft) was found to be no more effective than placebo (Fann et al., 2017). As post-TBI depression remains difficult to manage, novel therapeutic approaches that specifically target this and related neuropsychiatric conditions have been of great clinical interest.
A growing body of evidence points to the involvement of the glutamatergic system in the etiology and treatment of TBI and depression, both independently and in parallel (O’Neil et al., 2018). Glutamate levels in the brain have been shown to contribute to the pathophysiology and neurological dysfunction seen after TBI (Zauner et al., 1996b; Koura et al., 1998; Zhang et al., 2001; Shutter et al., 2004; Mao et al., 2019). Post-TBI excess extracellular glutamate release leads to cell swelling, apoptosis, and neuronal death (Zauner et al., 1996a; Koura et al., 1998), and the maintenance of glutamate homeostasis is critical in improving neurological outcome (Zauner et al., 1996b; Hong et al., 2001; Zhang et al., 2001; Shutter et al., 2004; Mao et al., 2019). Depression and many mood disorders are similarly affected by the glutamatergic system (Levine et al., 2000; Krystal et al., 2002; Sanacora et al., 2003, 2012; Mitani et al., 2006; Maeng and Zarate, 2007; Pittenger et al., 2007; Mitchell and Baker, 2010; Zarate et al., 2010; Machado-Vieira et al., 2012; McCarthy et al., 2012; Tokita et al., 2012) of evidence indicates that future therapeutic options for depression will be comprised of modalities based on this system (Sanacora et al., 2008; Gruenbaum et al., 2020). Recent literature suggests that a susceptibility to depression may be caused by glutamatergic disturbances after TBI (O’Neil et al., 2018). Therefore, limiting excess glutamate concentrations following TBI may be a vital strategy to target both the neurologic and psychiatric progression of the condition.
Neurological motor symptoms of TBI have been shown to be attenuated by decreasing glutamate levels or function in the brain with dextorphan (Faden et al., 1989), N-methyl-D-aspartate (NMDA) antagonists (Mei et al., 2018), stimulation of excitatory amino acid transporters (EAATs) (Goodrich et al., 2013), or antibiotics and other drugs that block calcium channels or glutamate release (McConeghy et al., 2012; Hicks et al., 2019). However, these treatments can also limit the essential effects of glutamate, leading to adverse side effects (Ikonomidou and Turski, 2002; Hardingham and Bading, 2003; Muir, 2006). For example, human clinical trials of NMDA receptor antagonists have not only failed to demonstrate clinical neuroprotective efficacy but led to worsened neurological outcome and an increased mortality rate following TBI (Morris et al., 1998; Muir, 2006). Moreover, other preclinical studies have found that direct or indirect stimulation of NMDA receptors mitigated the severity of neurological deficits in hippocampal-based memory in adult rats (Temple and Hamm, 1996; Biegon et al., 2004) and in rat pups (Sta Maria et al., 2017; Biegon et al., 2018) after TBI.
An alternative approach is to eliminate excess toxic glutamate, rather than interfering with ongoing excitatory transmission via receptor antagonists. This can be accomplished by enhancing the brain-to-blood glutamate efflux, which occurs naturally via the endothelial transport systems, to eliminate excess glutamate from the brain’s interstitial fluid (Teichberg, 2007; Teichberg et al., 2009). Glutamate co-substrates pyruvate and oxaloacetate convert glutamate into its inactive form 2-ketoglutarate via blood resident enzymes glutamate-pyruvate transaminase and glutamate-oxaloacetate transaminase (Gonzalez et al., 2005; Leibowitz et al., 2012; Gray et al., 2014). Previous studies have established an association between brain glutamate and blood glutamate levels (Shaw et al., 1995; Ferrarese et al., 2001). A reduction in blood glutamate helps to form an ideal glutamate concentration gradient that causes excess glutamate to move from the brain’s extracellular fluid into the blood (Zlotnik et al., 2011a,2012a; Rogachev et al., 2012; Boyko et al., 2014). This process impedes secondary brain injury that can occur as a result of glutamate neurotoxicity (O’Kane et al., 1999; Teichberg et al., 2009; Boyko et al., 2014).
Glutamate reduction, unlike the use of NMDA receptor antagonists, does not impact glutamate receptors or glutamate-mediated synaptic activity. Instead, this process only removes pathologically-elevated glutamate levels in the brain without impeding the function of neural circuits that depend on glutamate transmission (Leibowitz et al., 2012; Boyko et al., 2014; Zhumadilov et al., 2015). Known as blood glutamate scavenging, this method for reduction of excess glutamate has been proposed as an effective method to ameliorate neurological conditions after TBI (Zlotnik et al., 2007, 2008, 2009, 2010, 2012b) and depressive symptoms after stroke (Frank et al., 2019a; Gruenbaum et al., 2020). The aim of this study was to employ a novel approach of blood glutamate scavenging with pyruvate for the prevention and treatment of post-TBI depressive-like behaviors in a rat model. We further analyzed the impact of gender differences on the development of post-TBI depressive-like behaviors and on subsequent treatment with blood glutamate scavenging.
Materials and Methods
Animals
The experiments were conducted in accordance with the recommendation of the Declarations of Helsinki and Tokyo and the Guidelines for the Use of Experimental Animals of the European Community. The experiments were approved by the Animal Care Committee of Ben-Gurion University of the Negev (Beer-Sheva, Israel). A total of 134 male and 133 female Sprague-Dawley rats were used in this experiment. All rats weighed between 300 and 350 g. Purina Chow and water were made available ad libitum. The temperature in the room was maintained at 22°C, with a 12 h light–dark cycle. All the tests were conducted in the dark phase between 8 am and 4 pm.
Experimental Design
The timeline of the experiment is illustrated in Figure 1. All rats were divided into two main groups, sham-operated and TBI. The rats were randomly assigned, but each group had an equal number of females and males (Table 1). 24 h after induction of TBI or sham surgery, all rats were divided into five groups: (1) sham-operated control group given pyruvate, (2) sham-operated control group given placebo, (3) post-TBI control group given placebo, (4) post-TBI group given preventative pyruvate, (5) post-TBI group treated with pyruvate (Table 1). Each of the five groups was randomly divided into two subgroups: (A) a group for behavioral tests and (B) a group for testing blood and cerebrospinal fluid (CSF), and outcomes from magnetic resonance imaging (MRI) with anesthesia (Table 1). At 24 h after TBI or sham protocol, we collected a sample of CSF and blood from the rats in subgroup B. On day 3 of the study, two groups (the post-TBI group given preventative pyruvate and the sham-operated control group given pyruvate) began to receive pyruvate for 30 days (Figure 1, Axis A). Within subgroup A, behavioral tests were performed after the completion of treatment at 1-month post-TBI, and 2 months after the completion of treatment. After the TBI induction or sham operation, the rats from the therapeutic protocol received no treatment for a month. After 1-month, behavioral tests (subgroup A) or blood CSF measurements (subgroup B) were taken, followed by treatment with pyruvate (Figure 1, Axis B) at a dose described below. Behavioral tests at 6 months were performed only for the sham-operated control group given placebo and post-TBI rats given placebo (Figure 1).
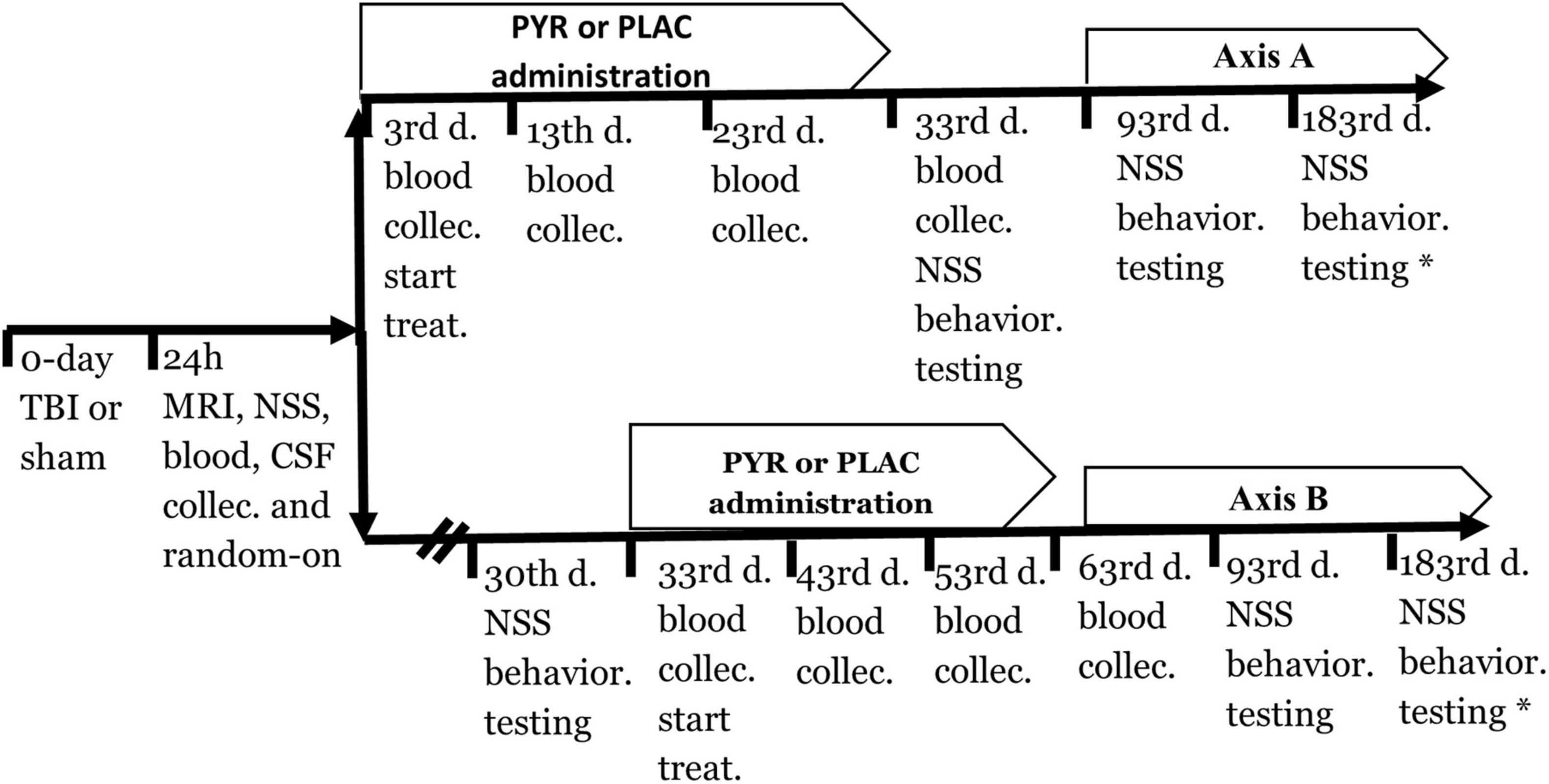
Figure 1. A timeline of the protocol for preventative (axis A) and treatment (axis B) approach. CSF, Cerebrospinal fluid; NSS, Neurological severity score; PLAC, Placebo; PYR, Pyruvate; TBI, Traumatic brain injury.
Drugs and Doses
Pyruvate (Sigma Israel Chemicals, Rehovot, Israel, catalog number P2256) was kept at a temperature of 2–4°C prior to use. Immediately before administration, it was dissolved in drinking water. Doses of 180 mg/kg/day were administered to rats in the experimental groups divided into two daily doses of 90 mg/kg for 30 days. A fresh solution of pyruvate was made every 12 h. The placebo groups received an equal dose of water without pyruvate. The dose of pyruvate was based on previous data that demonstrated by magnetic resonance spectroscopy that a dose of 180 mg/kg/day was optimal for reducing blood and brain glutamate by about 25–35% (Frank et al., 2019a).
Traumatic Brain Injury
Traumatic brain injury was performed, as previously described (Jones et al., 2008; Kabadi et al., 2010; Frank et al., 2021a,b). Rats received inhaled isoflurane as anesthetic with 5% for induction and 1.5–2.5% for maintenance, with equal parts medical air and oxygen. Prior to incision, the scalp was infiltrated with 0.5% bupivacaine. It was then perforated and reflected laterally with the left temporal muscle, while the underlying periosteum was dissected to reveal the skull. Craniotomy was performed at 5-mm using a trephine (Roboz Surgical Instrument Co., Gaithersburg, MD, United States) fastened to the drill bit of an electrical drill (Stoelting, Wood Dale, IL, United States). The center of the craniotomy was positioned 4 mm lateral and 4 mm posterior to bregma. A Luer 3-way stopcock was fixed and additionally held in place by cyanoacrylate adhesive and dental acrylic. The injury was then effected by a pressure pulse of 2.2 atmospheres (Jones et al., 2008; Kabadi et al., 2010). TBI was induced by a fluid-percussion device over 21–23 ms through the 3-way stopcock. The fluid pulse from the piston plunger, through involvement by the pendulum, was enacted via continuous saline fluid into the dura to allow for efficient transmission of the pressure pulse. Rats in the sham-operated control groups underwent the same procedure but without the administration of the fluid pulse.
Rats were monitored by a pulse-oximeter during the surgery to ensure uninterrupted measurements of heart rate and blood oxygen levels. After TBI induction, the incision was sutured, and the rats were allowed to recover from anesthesia.
Neurological Severity Score
Two blinded observers calculated Neurological Severity Score (NSS), as previously described (Boyko et al., 2011a,2013a; Ohayon et al., 2012; Zlotnik et al., 2012a; Frank et al., 2021b). Points were assigned for motor function and behavioral changes for an overall score between 0, indicating an intact neurological state, and 25, representing highest neurological impairment. The following criteria were evaluated: the ability to exit a circle (3-point scale), gait on a wide surface (3-point scale), gait on a narrow surface (4-point scale), effort to remain on a narrow surface (2-point scale), reflexes (5-point scale), seeking behavior (2-point scale), beam walking (3-point scale), and beam balance (3-point scale).
Sucrose Preference Test
The sucrose preference test was performed as described previously as a method to evaluate anhedonia, which reflects depressive-like symptoms, in a rodent model (Boyko et al., 2013a,2015). Two bottles of sucrose solution were placed in each rat’s cage, consisting of 1% (w/v) solution. The rat became acclimated to having two bottles in the cage, which allowed the rat to avoid neophobia during the sucrose preference test, for which two bottles were necessary. Similarly, one of the bottles was replaced by water for 24 h so that the rat could adjust to having one bottle of water and one bottle of sucrose. After this habituation, the rats were deprived of food and water for 12 h. At 9:00 am, the sucrose preference test was performed. The rats were housed in individual cages with free access to two bottles, one with 100 ml of sucrose solution (1% w/v) and the other with 100 ml of water, for 4 h. After this period, the volume (ml) of the consumed sucrose solution and water was recorded. Sucrose preference was calculated as sucrose preference (%) = sucrose consumption (ml)/[sucrose consumption (ml)+water consumption (ml)] × 100% (Boyko et al., 2013b,2019b).
Open Field Test
The standard open field test evaluates locomotor, exploratory, and anxiety-related depressive-like behaviors in animal models based on novel conditions (Boyko et al., 2013a). The open field test measures exploratory activity in a novel environment. The open field boxes were round black plastic arenas 2 m in diameter, 60 cm high walls situated in a darkened room. For analysis, the arena was cleaned with 10% ethanol after each behavioral recording.
A video camera was mounted 200 cm above the open field arena and recorded all experiments. Locomotor activity was recorded for 5 min by a Logitech HD Pro Webcam C920. Analysis after the recording was performed with Ethovision XT software (Noldus, Wageningen, Netherlands) (Frank et al., 2019b). The recordings were analyzed based on total distance traveled.
Magnetic Resonance Imaging
Diffusion-weighted imaging and T2 MRI were performed at 48 h following TBI, as described previously (Frank et al., 2019a). The rats underwent general anesthesia and were maintained with 1.5% isoflurane in oxygen. A 3T MRI was used (Ingenia, Philips Medical Systems, Best, Netherlands) using an eight-channel receive-only coil. Localizing T2w turbo spin echo (TSE) sequences were obtained in sagittal and coronal planes with TR/TE = 3,000/80 ms, turbo factor = 15, water-fat shift = 1.6 pixels, resolution (freq × phase × slice) = 0.47 mm × 0.41 mm × 2.0 mm and one average for a scan time of 1:00 min. In the axial direction the scan parameters included repetition time/echo time (TR/TE) = 3,000/80 ms, turbo factor = 14, water-fat shift = 1.6 pixels, resolution (freq × phase × slice) = 0.37 mm × 0.33 mm × 2.0 mm. Four averages were acquired for a scan time of 4:54 min. Diffusion tensor imaging in 6 directions was performed in the axial direction using a multi-shot STimulated Echo Acquisition Mode (STEAM) spin-echo, echo-planar sequence with repetition time/mixing time/echo time (TR/TM/TE) = 1,355/15.0/143 ms, SENSitivity Encoding (SENSE) reduction factor = 1.5, turbo factor = 19, b = 1,000 s/mm2, resolution (freq × phase × slice) = 0.55 mm × 0.55 mm × 2.0 mm with spectrally-selective fat suppression. Five signal averages were acquired for a scan time of 8:40 min. T2 perfusion studies were obtained using a dynamic, single-shot gradient-echo epi sequence with spectrally-selective fat suppression. The scan parameters were TR/TE = 1,300/40 ms, resolution (freq × phase × slice) = 0.64 mm × 0.69 mm × 2.0 mm, and one signal average giving a scan time of 1.3sec/dynamic. A total of 150 dynamics were acquired for a scan time of 3:19 min. We utilized the Intellispace Portal workstation (V5.0.0.20030, Philips Medical Systems, Best, Netherlands) for the post-processing of the perfusion studies.
Magnetic Resonance Imaging Analysis
An expert blinded to the groups performed image analysis. We generated quantitative apparent diffusion coefficient (ADC) maps, in units of square millimeters per second, in Philips software package (Ingenia, Philips Medical Systems, Best, Netherlands). Analysis was performed using ImageJ software (version 1.50i, National Institutes of Health, Bethesda, Maryland), as previously described (Boyko et al., 2019c). These thresholds indicated all pixels of ADC characteristics on each slice. The viability thresholds were 0.53X10-3mm2/s for ADC images (Bardutzky et al., 2005; Boyko et al., 2019c). Calculation of lesion volume was performed by the RICH method and included the correction for tissue swelling, according to the following formula (Boyko et al., 2013b):
Calculation of brain edema was also performed by the RICH method. The calculation of brain edema by the RICH technique was done by comparing the contralateral and ipsilateral hemispheres, and performed using the following formula (Boyko et al., 2011a):
The lesion volume and brain edema were measured as a percentage of the total brain (Boyko et al., 2019a).
Determination of Blood Glutamate
Whole blood (200 μl aliquot) had its protein removed by adding an equal volume of ice-cold 1 M perchloric acid, followed by utilization of a centrifuge at 10,000 × g for 10 min at 4°C. The supernatant was obtained for future analysis if necessary, and adjusted to pH 7.2, with 2 M K2CO3, and stored at −80°C.
To measure the glutamate concentration, the fluorometric method of Graham and Aprison (1966) was used (Graham and Aprison, 1966). A 60 μl aliquot from the perchloric acid supernatant was combined with 90 μl of a 0.3 M glycine; 0.25 M hydrazine hydrate buffer adjusted to pH 8.6 with 1 M H2SO4 and containing 11.25 U of glutamate dehydrogenase in 10 mM Nicotinamide adenine dinucleotide. After incubation for 30 to 45 min at room temperature, the fluorescence was measured at 460 nm with excitation at 350 nm. A glutamate standard curve was established with concentrations ranging from 0 to 6 μM. All determinations were done at least in duplicates (Boyko et al., 2011b).
Blood Sample Collection
Blood was collected from the tail vein for the determination of blood glutamate levels via a 24- guage Neoflon (Becton Dickinson, Helsingborg, Sweden) catheter. After the blood sample was collected, the catheter was removed from the vein (Boyko et al., 2012).
Cerebrospinal Fluid Sample Collection
Rats were anesthetized and the cisterna magna was cannulated, as previously described (Boyko et al., 2012), and 0.1 to 0.2 ml of CSF were gently aspirated.
Determination of Cerebrospinal Fluid Glutamate
Fresh CSF (110 μl) was mixed with perchloric acid (25 μl) of 0.3 M, and then centrifuged at 10,000 × g for 10 min at 4°C. The pellet was discarded and the supernatant was collected, adjusted to pH 7.2 with 12.5 μl of 2 M K2CO3 and stored at −80°C for later analysis (Boyko et al., 2012). Analysis was performed by fluorometric method as described above for blood samples.
Statistical Analysis
Statistical analysis was performed with the SPSS 20 package (SPSS Inc., Chicago, IL, United States). The Kolmogorov–Smirnov test was used, to consider the number of rats in each group for deciding the appropriate test for the comparisons between the different parameters. For non-parametric data, we used the transformation test or other suitable tests. The significance of comparisons between groups were determined using the Kruskal–Wallis and Mann–Whitney (for nonparametric data) and one-way ANOVA with Bonferroni post hoc test or the Student’s t-tests (for parametric data). Mortality rate was analyzed with chi-square and Fisher’s exact tests. Results were considered statistically significant when P < 0.05, and highly significant when P < 0.01.
Results
Mortality
The survival rate was calculated in the first 3 days following TBI or sham-operated procedure. During this period, the rats were not administered pyruvate. The mortality rate in sham-operated control rats was 0% in both gender groups, which was significantly lower than male (10.71%, p = 2.6E-02, chi-square and Fisher’s exact test, 2-sided) and female (9.64%, p = 2.5E-02, chi-square and Fisher’s exact test, 2-sided) rats following TBI.
Neurological Severity Score
There were no baseline neurological deficits observed in any of the rats before TBI or sham-operated procedure. The sham-operated control groups did not show any neurological deficit at any time point throughout the experiment. Compared to sham-operated controls, the NSS at 24 h was significantly greater in male [4(2–5) n = 75 vs. 0(0-0) n = 50, U = 45, p = 2.7E-21, r = 0.85] and female [4(3–6) n = 75 vs. 0(0-0) n = 50, U = 0, p = 4.5E-22, r = 0.86] rats after TBI, according to Mann–Whitney test (Figures 2A,B). No statistically significant differences were found between the 15 male and female groups, at time points of 30, 90, and 180 days, according to Kruskal–Wallis one-way analysis (see Figure 1 and Table 1). The data are measured as a count and expressed as median and 25–75 percentile range.
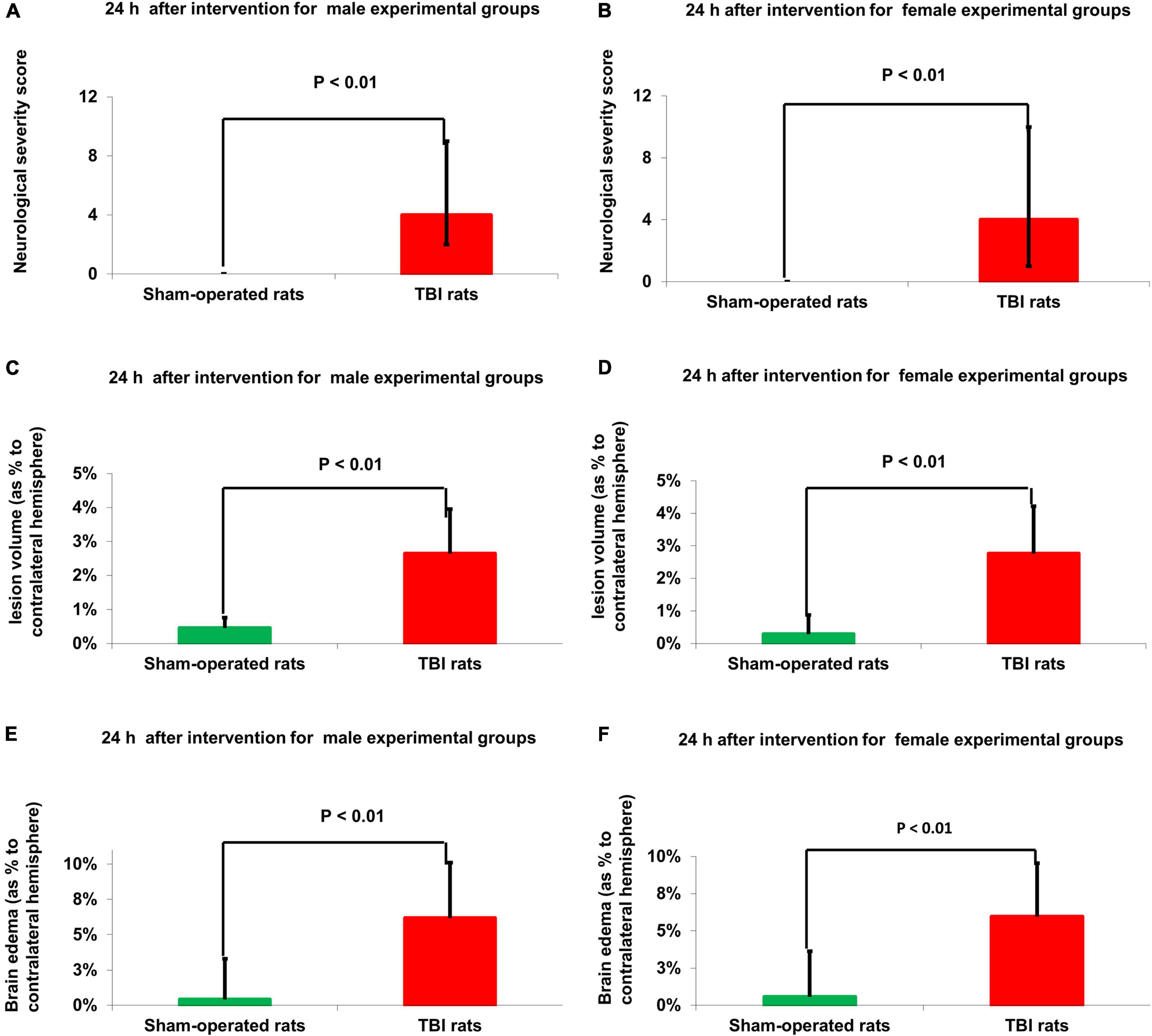
Figure 2. Neurological outcome (A,B) and MRI-determined lesion volume (C,D) and brain edema (E,F). Compared to sham-operated controls, the NSS at 24 h was significantly greater in male [p < 0.01 (A)] and female [p < 0.01 (B)] rats after TBI. The data are measured as a count and expressed as median and 25–75 percentile range. Compared to sham-operated rats, the lesion volume at 24 h was significantly greater in the male [p < 0.01 (C)] and female [p < 0.01 (D)] TBI groups. The data is expressed as a mean percentage of the contralateral hemisphere ± SD. Compared to sham-operated rats, the brain edema at 24 h was significantly greater in the male [p < 0.01, (E)] and female [p < 0.01 (F)] TBI groups. The data are expressed as a mean percentage of the contralateral hemisphere ± SD. TBI: Traumatic brain injury.
Magnetic Resonance Imaging-Determined Lesion Volume
Compared to sham-operated rats, the lesion volume at 24 h was significantly greater in the female [2.71% ± 1.29% vs.0.46% ± 0.24%, t(48) = −7.21, p = 3.4E-09] and male [2.68% ± 1.32% vs. 0.45% ± 0.28%, t(48) = −7.26, p = 3E-09] TBI groups, according to Student’s t-test (Figures 2C,D). The data are expressed as a mean percentage of the contralateral hemisphere ± SD.
Magnetic Resonance Imaging-Determined Brain Edema
Compared to sham-operated rats, the brain edema at 24 h was significantly greater in the female [5.98% ± 2.83% vs. 0.57% ± 0.29%, t(48) = −5.5, p = 1.4E-06] and male [6.15% ± 3.26% vs. 0.41% ± 0.24%, t(48) = −5.6, p = 1E-06] TBI groups, according to Student’s t-test (Figures 2E,F). The data are expressed as a mean percentage of the contralateral hemisphere ± SD.
Concentration of Cerebrospinal Fluid Glutamate
Compared to sham-operated rats, the concentration of CSF glutamate at 24 h was significantly greater in the female [25.27 μM/L ± 13.13 μM/L vs. 3.6 μM/L ± 6.28 μM/L, t(48) = −6.9, p = 1.2E-08] and male [26.27 μM/L ± 16.39 μM/L vs. 2.1 μM/L ± 5.76 μM/L, t(48) = −6.4, p = 6.1E-08] TBI groups, according to Student’s t-test (Figures 3A,B). The data are measured in μM/L and expressed as mean ± SD.
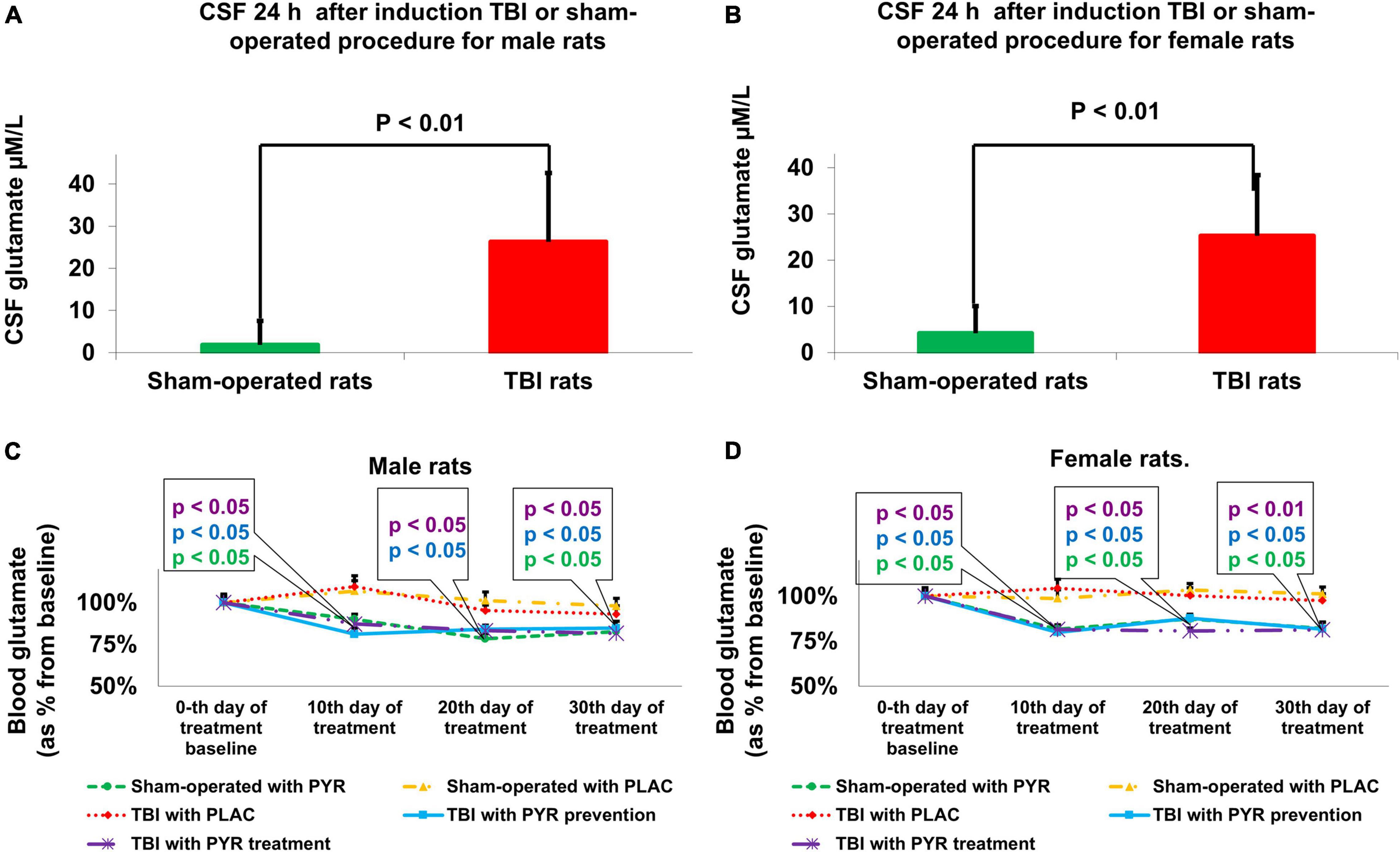
Figure 3. Brain (A,B) and blood (C,D) glutamate concentrations. Compared to sham-operated rats, the concentrations of CSF glutamate at 24 h was significantly greater in the male [p < 0.01, (A)] and female [p < 0.01 (B)] TBI groups. The data are measured in μM/L and expressed as mean ± SD. Blood glutamate levels were decreased in male [p < 0.05 (C)] and female [p < 0.05 (D)] groups that received preventative or therapeutic pyruvate. The data are measured in μM/L presented as a percentage from baseline and expressed as mean ± SEM. CSF, Cerebrospinal fluid; PLAC, Placebo; PYR, Pyruvate; TBI, Traumatic brain injury.
Concentration of Blood Glutamate
At baseline, there were no significant differences in blood glutamate concentration between treatment groups.
Compared to sham-operated rats, the concentration of blood glutamate at 24 h was significantly greater in the female [121% ± 20% vs. 100% ± 18%, t(48) = 3.87, p < 0.01] and male [114% ± 18% vs. 100% ± 17%, t(48) = 2.75, p < 0.01] TBI groups, according to Student’s t-test. The data are measured in μM/L presented as a percentage from sham-operated rats and expressed as mean ± SD.
For male rats, at day 10 after pyruvate administration or placebo protocol, there were significant differences in blood glutamate levels between sham-operated rats given placebo (107% ± 8.6%), sham-operated rats given pyruvate (80.4% ± 4.4%), post-TBI given placebo (109.5% ± 9.4%), post-TBI rats given preventative pyruvate (77.6% ± 4.5%), and post-TBI rats treated with pyruvate (80.3% ± 6.1%) [Kruskal–Wallis, χ2 (4) = 12.9, p = 1.2E-02]. A subsequent Mann–Whitney test indicated that male blood glutamate levels were significantly decreased in the sham-operated rats given pyruvate (U = 21, p = 2.8E-02, r = –0.49), post-TBI rats given preventative pyruvate (U = 18, p = 1.6E-02, r = −0.02), and post-TBI rats treated with pyruvate (U = 22, p = 3.4E-02, r = −0.47), compared to sham-operated rats given placebo. At day 20, there were significant differences in male blood glutamate levels between sham-operated rats given placebo (101.2% ± 7.3%), sham-operated rats given pyruvate (82.7% ± 5.1%), post-TBI given placebo (105.2% ± 4.8%), post-TBI rats given preventative pyruvate (80.6% ± 3.5%) and post-TBI rats treated with pyruvate (77.4% ± 3.5%) [Kruskal–Wallis, χ2 (4) = 11, p = 2.6E-02]. A subsequent Mann–Whitney test indicated that male blood glutamate levels were significantly decreased in the post-TBI rats given preventative pyruvate (U = 24, p = 4.9E-02, r = −0.44) and post-TBI rats treated with pyruvate (U = 20, p = 2.3E-02, r = −0.51), compared to the sham-operated control group given placebo. Also on day 20, male blood glutamate levels in the sham-operated rats given pyruvate were lower than in the sham-operated control rats given placebo, although this difference did not reach statistical significance. At day 30, there were significant differences in male blood glutamate levels between sham-operated rats given placebo (99.4% ± 5.9%), sham-operated rats given pyruvate (75.9% ± 5.2%), post-TBI given placebo (93.1% ± 7.1%), post-TBI rats given preventative pyruvate (77.9% ± 5.4%) and post-TBI rats treated with pyruvate (79.9% ± 3.6%) [Kruskal–Wallis, χ2 (4) = 10.1, p = 3.9E-02]. A subsequent Mann–Whitney test indicated that male blood glutamate levels were significantly decreased in the sham-operated controls given pyruvate (U = 16, p = 1E-02, r = −0.58), post-TBI rats given preventative pyruvate (U = 75, p = 2.3E-02, r = −0.51) and post-TBI rats treated with pyruvate (U = 75, p = 2.3E-02, r = −0.51), compared to sham-operated rats given placebo (Figure 3C).
For female rats, at day 10, there were significant differences in blood glutamate levels between sham-operated rats given placebo (98.7% ± 6.7%), sham-operated rats given pyruvate (80% ± 4.3%), post-TBI given placebo (104.5% ± 7%), post-TBI rats given preventative pyruvate (80.1% ± 3%) and post-TBI rats treated with pyruvate (78.7% ± 4.4%) [Kruskal–Wallis, χ2 (4) = 12.8, p = 1.2E-02]. A subsequent Mann–Whitney test indicated that at day 10, female blood glutamate levels were significantly decreased in the sham-operated controls given pyruvate (U = 23, p = 4.1E-02, r = −0.46), post-TBI rats given preventative pyruvate (U = 23, p = 4.1E-02, r = −0.46), and post-TBI rats treated with pyruvate (U = 24, p = 4.9E-02, r = −0.45), compared to sham-operated rats given placebo. At day 20, there were significant differences in female blood glutamate levels between sham-operated rats given placebo (103.4% ± 5.1%), sham-operated rats given pyruvate (81.1% ± 5.7%), post-TBI given placebo (100.3% ± 6.1%), post-TBI rats given preventative pyruvate (86.4% ± 4.9%) and post-TBI rats treated with pyruvate (83.2% ± 5%) [Kruskal–Wallis, χ2 (4) = 11.8, p = 1.9E-02]. A subsequent Mann–Whitney test indicated that at day 20, female blood glutamate levels were significantly decreased in the sham-operated controls given pyruvate (U = 18, p = 1.6E-02, r = −0.54), post-TBI rats given preventative pyruvate (U = 24, p = 4.9E-02, r = −0.44), and post-TBI rats treated with pyruvate (U = 18, p = 1.6E-02, r = −0.54), compared to sham-operated rats given placebo. At day 30, there were significant differences in female blood glutamate levels between sham-operated rats given placebo 101.2% ± 5.4%), sham-operated rats given pyruvate (82.9% ± 4%), post-TBI given placebo (97.6% ± 5.3%), post-TBI rats given preventative pyruvate (80.4% ± 5.6%) and post-TBI rats treated with pyruvate (74.2% ± 3.7%) [Kruskal–Wallis, χ2 (4) = 14.6, p = 5.7E-03]. A subsequent Mann–Whitney test indicated that at day 30, female blood glutamate levels were significantly decreased in the sham-operated controls given pyruvate (U = 19, p = 1.9E-02, r = −0.52), post-TBI rats given preventative pyruvate (U = 21, p = 2.8E-02, r = −0.49) and post-TBI rats treated with pyruvate (U = 11 p = 3.2E-02, r = −0.66), compared to sham-operated controls given placebo (Figure 3D).
As expected (Puig et al., 2000), blood glutamate levels in post-TBI rats treated with placebo were not statistically significantly different than in the sham-operated control rats treated with placebo. The data are measured in μfvM/L presented as a percentage from baseline and expressed as mean ± SEM.
Sucrose Preference
For male rats at day 30, a one-way ANOVA showed a significant difference in the percentage of sucrose preference between the study groups F(4,65) = 13.5, p = 4.8E-08. Post hoc analysis with a Bonferroni test showed a significant decrease between post-TBI rats given placebo (76.2% ± 1.9%, p = 4E-05) and post-TBI rats treated with pyruvate (75.4% ± 2%, p = 8.7E-06) compared to sham-operated controls given placebo (91.1% ± 1.2%). At day 90, a one-way ANOVA showed a significant difference in the percentage of sucrose preference between the study groups F(4,65) = 11.11, p = 6.3E-07. Post hoc analysis with a Bonferroni test showed a significant decrease in post-TBI rats given placebo (73% ± 3.1%, p = 6.5E-05) compared to sham-operated controls given placebo (89.9% ± 1.7%) (Figures 4A,C,E).
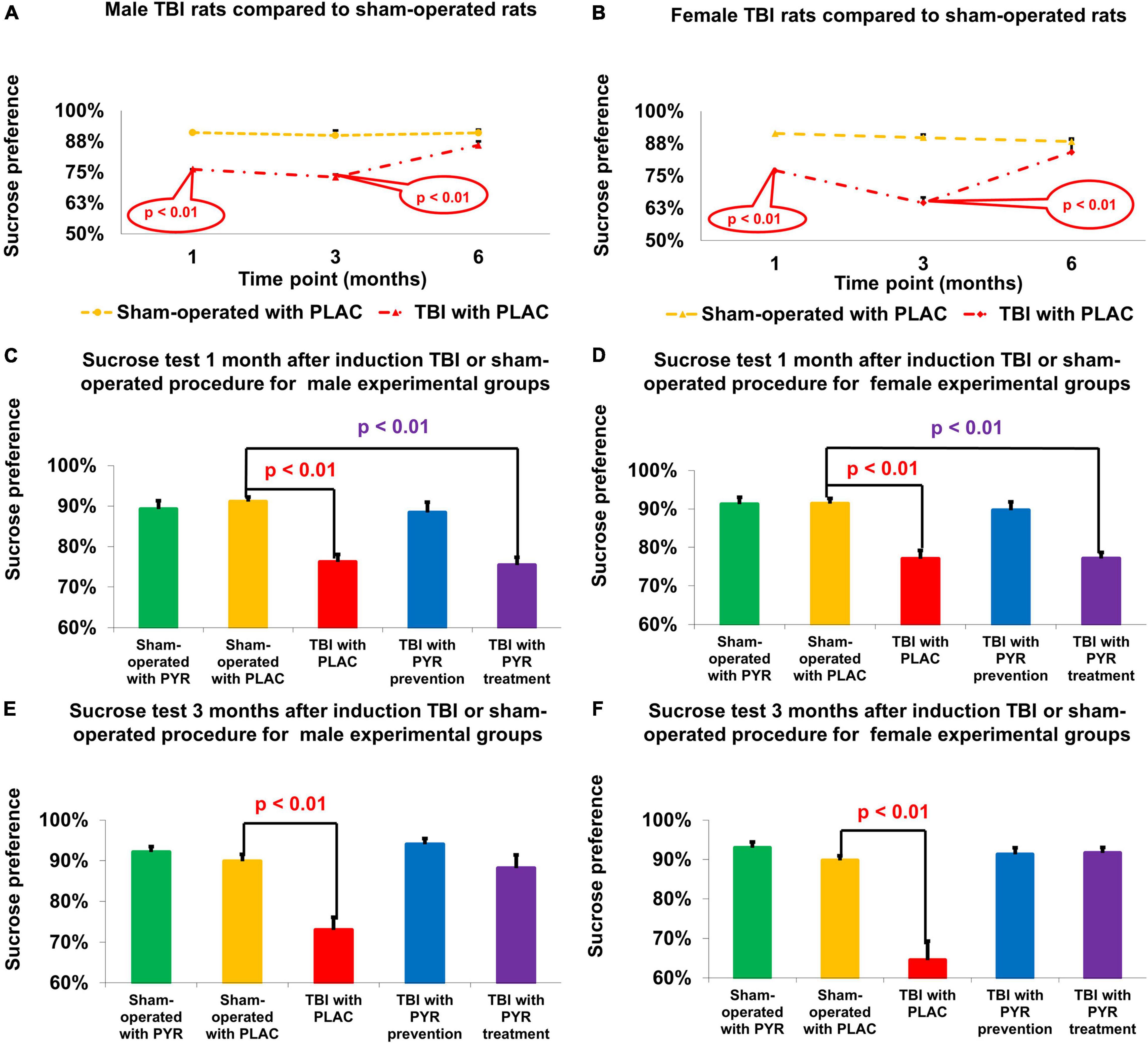
Figure 4. Sucrose preference test. There was a decrease in sucrose preference at 1 and 3 months following TBI for male (A) and female (B) rats given placebo compared to sham-operated controls (p < 0.01). For male (C) and female (D) rats at day 30, there was a significant decrease in sucrose preference in post-TBI rats given placebo (p < 0.01) and post-TBI rats treated with pyruvate (p < 0.01) compared to sham-operated controls given placebo. For male (E) and female (F) rats at day 90, there was a significant decrease in sucrose preference in post-TBI rats given placebo (p < 0.01) compared to sham-operated controls given placebo. The data are measured as a percentage and presented as mean ± SEM. PLAC, Placebo; PYR, Pyruvate; TBI, Traumatic brain injury.
For female rats at day 30, a one-way ANOVA showed a significant difference in the percentage of sucrose preference between the study groups F(4, 68) = 16.27, p = 2.2E-09. Post hoc analysis with a Bonferroni test showed a significant decrease in post-TBI rats given placebo (77% ± 2.2%, p = 7.5E-06) and post-TBI rats treated with pyruvate (77.1% ± 1.6%, p = 8.2E-06), compared to sham-operated controls given placebo (91.4% ± 1.3%). At day 90, a one-way ANOVA showed a significant difference in the percentage of sucrose preference between the study groups F(4,65) = 21.29, p = 3.0E-11. Post hoc analysis with a Bonferroni test showed a significant decrease in post-TBI rats given placebo (64.5% ± 4.8%, p = 1.4E-08) compared to sham-operated controls given placebo (89.8% ± 1.2%) (Figures 4B,D,F). The data are measured in ml presented as percentage and expressed as mean ± SEM.
Open-Field Test
For male rats at day 30, a one-way ANOVA showed a significant difference in the total distance traveled between the study groups F(4,72) = 6.49, p = 1.6E-08. Post hoc analysis with a Bonferroni test showed a significant increase in post-TBI rats given placebo (31.21 m ± 2.5 m, p = 2.9E-03) and post-TBI rats treated with pyruvate (28.93 m ± 4.25 m, p = 2.9E-02), compared to sham-operated controls given placebo (19.3 m ± 0.77 m). At day 90, a one-way ANOVA showed a significant difference in the total distance traveled between the study groups F(4,70) = 19.74, p = 6.5E-11. Post hoc analysis with a Bonferroni test showed a significant increase in post-TBI rats given placebo (30.52 m ± 1.94 m, p = 8.1E-10) compared to sham-operated controls given placebo (18.84 m ± 0.72 m) (Figures 5A,C,E).
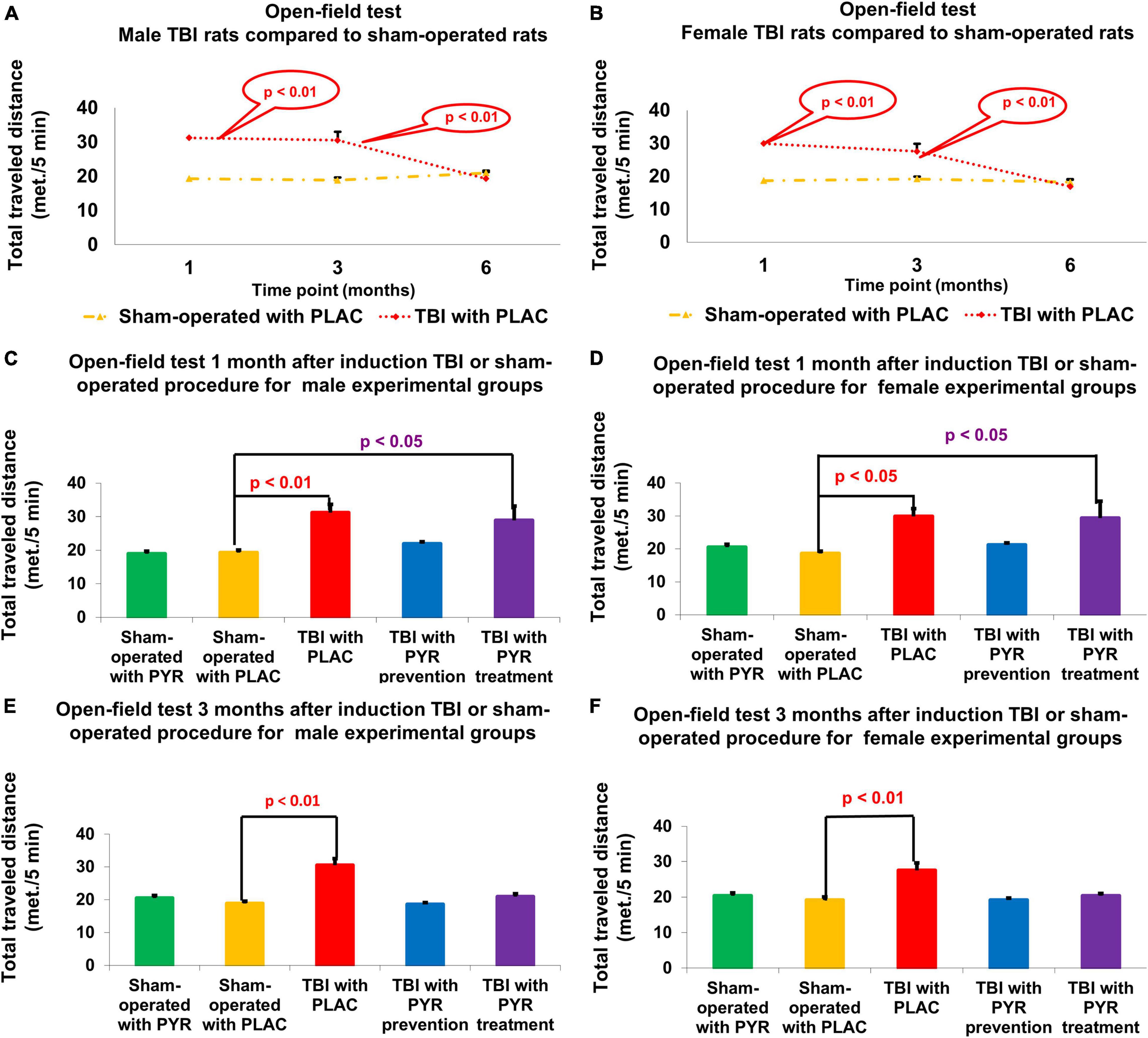
Figure 5. Open-field test. There was an increase in the total traveled distance at 1 and 3 months following TBI for male (A) and female (B) rats given placebo compared to sham-operated controls (p < 0.05). For male (C) and female (D) rats at day 30, there was a significant increase in total distance traveled in post-TBI rats given placebo (p < 0.01) and post-TBI rats treated with pyruvate (p < 0.01) compared to sham-operated controls given placebo. For male (E) and female (F) rats at day 90, there was a significant increase in total distance traveled in post-TBI rats given placebo (p < 0.01) compared to sham-operated controls given placebo. The data are measured as cm/5 min and presented as mean ± SEM. PLAC, Placebo; PYR, Pyruvate; TBI, Traumatic brain injury.
For female rats at day 30, a one-way ANOVA showed a significant difference in the total distance traveled between the study groups F(4,72) = 4.4, p = 3.1E-03. Post hoc analysis with a Bonferroni test showed a significant increase in post-TBI rats given placebo (29.89 m ± 2.33 m, p = 2.3E-02) and post-TBI rats treated with pyruvate (29.37 m ± 5.12 m, p = 3.5E-02), compared to sham-operated controls given placebo (18.65 m ± 0.69 m). At day 90, a one-way ANOVA showed significant difference in the total distance traveled between the study groups F(4,69) = 9.25, p = 4.8E-06. Post hoc analysis with a Bonferroni test showed a significant increase in distance traveled by post-TBI rats given placebo (27.55 m ± 2.06 m, p = 1.6E-05) compared to sham-operated controls given placebo (19.19 m ± 0.88 m) (Figures 5B,D,F). The data are measured as meter/5 min and presented as mean ± SEM.
Discussion
In this study, we investigated blood glutamate scavenging activity from pyruvate administration and its mechanisms as a viable option for antidepressant treatment in a rat model of post-traumatic depression. Specifically, we studied the effects of pyruvate on anhedonia and elevated locomotor activity (Bhatt et al., 2017) as a consequence of post-traumatic behavioral mood disorders. Additionally, we considered brain glutamate levels and blood glutamate scavenging, MRI findings, and neurological outcomes between male and female rodent groups, and we used both a prophylactic and a therapeutic pyruvate treatment protocol. Our results determined that pyruvate likely has an antidepressant effect on the brain via its participation in blood glutamate scavenging.
Our hypotheses on the following neurological conditions were confirmed by our study. We determined that the mortality rate in the TBI group was higher than in the sham group. Cerebral edema and lesion volume were significantly higher in the TBI group compared to the sham group (Figures 2C–F). We observed that neurological deficits were significantly greater in the TBI group compared to the sham group, which spontaneously recovered by 1 month (Figures 2A,B and section results > neurological performance). In addition, the concentration of glutamate was increased in the cerebrospinal fluid at 24 h as a consequence of TBI (Figures 3A,B). We have previously shown that the administration of pyruvate is effective in reducing cerebrospinal fluid glutamate levels in rodent models of subarachnoid hemorrhage (Boyko et al., 2012) and stroke (Frank et al., 2019a; Gruenbaum et al., 2020). A significant process of blood glutamate scavenging occurred in groups that received pyruvate treatment compared to placebo groups (Figures 3C–D). All the above results applied to both the male and female cohorts (Figures 2, 3). At days 10–30 following TBI, the glutamate levels in rats given placebo did not differ from the levels in naïve rats treated with placebo. In previous studies in the setting of stroke, an increase in blood glutamate levels was seen in the first 24 h, but the levels dropped to baseline at 48 h and beyond (Puig et al., 2000).
To study the efficacy of pyruvate as an antidepressant therapy, we used two behavioral tests. The first was the sucrose preference test that measures the level of anhedonia, one of the most common symptoms of depressive disorders (American Psychiatric Pub, 2013). The sucrose preference test is a standard test for assessing anhedonia in rat models and has allowed for the development of new therapeutic antidepressant treatments (Gururajan et al., 2019). In our study, the TBI rats developed anhedonia at higher rates compared to sham rats and then spontaneously recovered 6 months after TBI (Figures 4A,B). In contrast, the post-TBI rats who were administered pyruvate prophylactically showed no symptoms of anhedonia and did not differ from the results of the sham rat group at 1 and 3 months following TBI (Figures 4B–F).
Traumatic brain injury rats that received pyruvate only after they developed anhedonia symptoms, showed a therapeutic effect of the treatment at 3 months. Thus, pyruvate has proven to be effective in two approaches: in the prophylactic treatment protocol as well as the therapy protocol (Figure 4). Pyruvate showed equal rates of efficacy in both the male and female cohorts (Figure 4).
The second behavioral test which we used was an open field test, a common method used to detect high emotionality and locomotor hyperactivity (Ramamoorthy et al., 2008) in post-TBI rats (Lewen et al., 1999; Pandey et al., 2009) and mice (Li et al., 2006; Pullela et al., 2006; Tucker et al., 2016). These manifestations are associated with depressive status (Pandey et al., 2009) and show high response rates to antidepressant drugs (Lewen et al., 1999; Pandey et al., 2009; Bhatt et al., 2017; Jindal et al., 2017). The hyperlocomotion that we recorded in the TBI group during the open field test is attributable to damage caused by the brain insult to the cerebral cortex, striatum, and olfactory bulbs (Viggiano, 2008). An increase in total distance traveled in the olfactory bulbectomized model of depression is well documented in the literature (Kalueff and Tuohimaa, 2004). Assessment of hyperlocomotive behavior as a consequence of TBI is also used to verify new models of TBI (Kane et al., 2012).
Our study supports the hypothesis that hyperlocomotion after TBI is associated with dysregulation of the glutamatergic system, in particular by high levels of extracellular glutamate. The association between dysregulation of the glutamatergic system and hyperlocomotion has been widely reported (Takahata and Moghaddam, 2003; Abekawa et al., 2007; Hackler et al., 2010; Egerton et al., 2020). It was previously observed that activation of metabotropic glutamate receptors increases both horizontal and vertical locomotor activity and this activity is impeded by administration of a receptor antagonist, fluphenazine (Kim and Vezina, 1997). Gainetdinov et al. (2001) showed that drugs that enhance glutamatergic transmission, such as positive modulators of L-α-amino-3-hydroxy-5-methylisoxazole-4-propionate glutamate receptors, suppress the hyperactivity of mice lacking the dopamine transporter (Gainetdinov et al., 2001). The involvement of the glutamate system in the development of attention-deficit disorder, hyperactivity, and other behavioral motor disorders has also been previously described (Procaccini et al., 2013; Maksimovic et al., 2014a,b; Miller, 2019; Aitta-Aho et al., 2019). Halberstadt et al. (2011) demonstrated that loss of mGlu5 receptor activity either pharmacologically or through gene deletion leads to locomotor hyperactivity in mice. These studies strongly indicate that dysregulation of the glutamatergic system, alone or in combination with other major neurotransmitter systems such as dopamine, GABA, and the serotonin system, may induce hyper-locomotive effects that are controlled by drugs that regulate glutamate homeostasis (Tucker et al., 2016). Although the precise brain circuitry and pharmacological targets involved in the suppression of locomotor behavior require further elucidation, our data support the possibility that glutamatergic transmission in the hippocampus could be therapeutically applied to dampen the hyper-excitable hippocampus and other brain circuitries.
In our study, we found that TBI rats were more likely to travel farther distances in the open field compared to the sham group until 6 months following TBI. In addition, more TBI rats developed hyperlocomotion activity compared to sham rats and then spontaneously recovered 6 months after TBI. In contrast, the rats after TBI from the protocol of preventive treatment with pyruvate showed no symptoms of hyperlocomotive activity and did not differ from sham rats at 1 and 3 months following TBI. TBI rats that were in the treatment group and did not receive pyruvate after TBI developed symptoms of hyperlocomotion and only then began to receive pyruvate as a therapeutic approach. Thus, pyruvate showed its efficacy both as a prophylactic protocol and as a therapeutic protocol. Pyruvate showed equal effective results in both male and female cohorts.
The similarities between the male and female cohorts in the outcomes of the sucrose preference test and the open field test elucidate our understanding of gender differences concerning depression and anxiety. Women tend to suffer more often from major depressive disorder (Kovacs et al., 1989; Weissman et al., 1993; Bebbington, 1998; Merikangas et al., 2010) and anxiety (Angst and Dobler-Mikola, 1985; Kessler et al., 1994; Bruce et al., 2005). In rodent models, different rat strains can display significant gender disparities in models of depression (Kokras and Dalla, 2014), though it is generally observed that female rodents appear more active in the open field test, with less anxiety (Ter Horst et al., 2009; Kokras and Dalla, 2014). In our study, the use of the sucrose preference test in addition to the open field test assisted in developing more comprehensive neurological findings.
While it was outside of the scope of this study, we have previously observed that women display lower levels of blood glutamate concentration at baseline, and in conditions such as amyotrophic lateral sclerosis, rheumatoid arthritis, and growth hormone deficiency (Stover and Kempski, 2005). We have also determined that progesterone and estrogen have neuroprotective properties that act to reduce blood glutamate levels (Zlotnik et al., 2011b; Tsesis et al., 2013). We followed recommendations in the literature to include both sexes in this model (Rubin and Lipton, 2019), and, therefore our results accurately show the possible regulatory effects of pyruvate in similar ways across both groups. We hypothesize that more research on the topic of gender differences will support the use of pyruvate as a pharmacological approach that addresses depression for both men and women.
In our study, we began treatment on the third day after TBI. Usually, however, new therapeutic modalities are administered in the first hours after a brain injury (Tucker et al., 2016). We based our methodology on previous evidence that pyruvate has a neuroprotective effect in models of stroke and subarachnoid hemorrhage and, when administered in the first hours, reduces cerebral edema, infarction zone and blood brain barrier breakdown (Frank et al., 2019a). A reduction in damage to the brain tissue after pyruvate administration can potentially affect the development of behavioral outcomes after TBI. To neutralize the effect of histological outcomes on behavioral ones, we started pyruvate administration on the third day after TBI.
In summary, we have provided significant evidence that the process of blood glutamate scavenging by pyruvate induces antidepressant properties. These properties result in the prevention or treatment of anhedonia and hyperlocomotion that are caused by glutamate deregulation after TBI in rats. These conditions are symptoms of depressive-like conditions in rodent models. When analyzed in conjunction with previously observed neuroprotective properties of blood glutamate scavenging, it has become more apparent that blood glutamate scavengers should be considered as a viable treatment option for post-TBI depression.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the corresponding author (MB), upon reasonable request.
Ethics Statement
The animal study was reviewed and approved by the experiments were approved by the Animal Care Committee of Ben-Gurion University of the Negev (Be’er Sheva, Israel).
Author Contributions
DF, BG, OK, and MB: study conception, data collection, data analysis, manuscript writing and editing, and final approval of manuscript. IS, VZ, OS, RG, MD, and AZ: data collection, data analysis, manuscript editing, and final approval of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Adam Abu Gama, Department of Orthopedic Surgery, Soroka Medical Center, Ben-Gurion University, Be’er Sheva, Israel, Mamdoch Abu Rabia of the Department of Anesthesiology and Critical Care, Soroka University Medical Center, and Rom Kahana of the Department of Anesthesiology Assuta Medical Centers, Israel, for their outstanding help in behavioral examination and analysis of the results by computer software. We would also like to thank Stella Cherninson and Alena Muraveva of the Department of Radiology, Soroka University Medical Center, Ben-Gurion University of the Negev, Be’er Sheva, Israel, for their outstanding help with the analysis of MR images by computer software and for carrying out measurements. We would also like to thank Valeria Frishman, laboratory assistant and Amos Douvdevani Head, Research Lab., from the Department of Clinical Biochemistry, Soroka Medical Center, Ben-Gurion University, for his help with the biochemical analysis.
Abbreviations
ADC, apparent diffusion coefficient; CSF, cerebrospinal fluid; EAATs, excitatory amino acid transporters; MRI, magnetic resonance imaging; NSS, Neurological severity score; NMDA, N-methyl-D-aspartate; TR/TE, repetition time/echo time; TR/TM/TE, repetition time/mixing time/echo time; SENSE, SENSitivity Encoding; STEAM, STimulated Echo Acquisition Mode; TBI, traumatic brain injury; TSE, turbo spin echo.
References
Abekawa, T., Ito, K., and Koyama, T. (2007). Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at short-and long-term withdrawal from this antipsychotic. Naunyn Schmiedebergs Arch. Pharmacol. 375, 261–271. doi: 10.1007/s00210-007-0154-x
Aitta-Aho, T., Maksimovic, M., Dahl, K., Sprengel, R., and Korpi, E. R. (2019). Attenuation of novelty-induced hyperactivity of Gria1-/-mice by cannabidiol and hippocampal inhibitory chemogenetics. Front. Pharmacol. 10:309. doi: 10.3389/fphar.2019.00309
American Psychiatric Pub (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5§). Washington, DC: American Psychiatric Pub.
Andersen, G., Vestergaard, K., and Lauritzen, L. (1994). Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 25, 1099–1104. doi: 10.1161/01.str.25.6.1099
Angst, J., and Dobler-Mikola, A. (1985). The zurich study. V. Anxiety and phobia in young adults. Eur. Arch. Psychiatry Neurol. Sci. 235, 171–178. doi: 10.1007/BF00380989
Bardutzky, J., Shen, Q., Henninger, N., Bouley, J., Duong, T. Q., and Fisher, M. (2005). Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke 36, 2000–2005. doi: 10.1161/01.STR.0000177486.85508.4d
Bhatt, S., Mahesh, R., Jindal, A., and Devadoss, T. (2017). Neuropharmacological and neurochemical evaluation of Nn-propyl-3-ethoxyquinoxaline-2-carboxamide (6n): a novel serotonergic 5-HT3 receptor antagonist for co-morbid antidepressant-and anxiolytic-like potential using traumatic brain injury model in rats. J. Basic Clin. Physiol. Pharmacol. 28, 93–100. doi: 10.1515/jbcpp-2016-0057
Biegon, A., Fry, P. A., Paden, C. M., Alexandrovich, A., Tsenter, J., and Shohami, E. (2004). Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. U.S.A. 101, 5117–5122. doi: 10.1073/pnas.0305741101
Biegon, A., Liraz-Zaltsman, S., and Shohami, E. (2018). Stimulation of N-methyl-D-aspartate receptors by exogenous and endogenous ligands improves outcome of brain injury. Curr. Opin. Neurol. 31, 687–692. doi: 10.1097/WCO.0000000000000612
Bodnar, C. N., Roberts, K. N., Higgins, E. K., and Bachstetter, A. D. (2019). A systematic review of closed head injury models of mild traumatic brain injury in mice and rats. J. Neurotrauma 36, 1683–1706. doi: 10.1089/neu.2018.6127
Boyko, M., Azab, A. N., Kuts, R., Gruenbaum, B. F., Gruenbaum, S. E., Melamed, I., et al. (2013a). The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Res. 1491, 109–116. doi: 10.1016/j.brainres.2012.10.061
Boyko, M., Kutz, R., Gruenbaum, B. F., Cohen, H., Kozlovsky, N., Gruenbaum, S. E., et al. (2013b). The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn. Affect Behav. Neurosci. 13, 847–859. doi: 10.3758/s13415-013-0177-3
Boyko, M., Gruenbaum, S. E., Gruenbaum, B. F., Shapira, Y., and Zlotnik, A. (2014). Brain to blood glutamate scavenging as a novel therapeutic modality: a review. J. Neural. Transm. (Vienna) 121, 971–979. doi: 10.1007/s00702-014-1181-7
Boyko, M., Kuts, R., Gruenbaum, B. F., Tsenter, P., Grinshpun, J., Frank, D., et al. (2019a). An alternative model of laser-induced stroke in the motor cortex of rats. Biol. Proc. Online 21:9. doi: 10.1186/s12575-019-0097-x
Boyko, M., Kutz, R., Grinshpun, J., Zvenigorodsky, V., Gruenbaum, B. F., Gruenbaum, S. E., et al. (2019b). The effect of depressive-like behavior and antidepressant therapy on social behavior and hierarchy in rats. Behav. Brain Res. 370:111953. doi: 10.1016/j.bbr.2019.111953
Boyko, M., Zvenigorodsky, V., Grinshpun, J., Shiyntum, H. N., Melamed, I., Kutz, R., et al. (2019c). Establishment of novel technical methods for evaluating brain edema and lesion volume in stroked rats: a standardization of measurement procedures. Brain Res. 1718, 12–21. doi: 10.1016/j.brainres.2019.04.022
Boyko, M., Kutz, R., Grinshpun, J., Zvenigorodsky, V., Gruenbaum, S. E., Gruenbaum, B. F., et al. (2015). Establishment of an animal model of depression contagion. Behav. Brain Res. 281, 358–363. doi: 10.1016/j.bbr.2014.12.017
Boyko, M., Melamed, I., Gruenbaum, B. F., Gruenbaum, S. E., Ohayon, S., Leibowitz, A., et al. (2012). The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics 9, 649–657. doi: 10.1007/s13311-012-0129-6
Boyko, M., Ohayon, S., Goldsmith, T., Novack, L., Novack, V., Perry, Z. H., et al. (2011a). Morphological and neuro-behavioral parallels in the rat model of stroke. Behav. Brain Res. 223, 17–23. doi: 10.1016/j.bbr.2011.03.019
Boyko, M., Zlotnik, A., Gruenbaum, B. F., Gruenbaum, S. E., Ohayon, S., Kuts, R., et al. (2011b). Pyruvate’s blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur. J. Neurosci. 34, 1432–1441. doi: 10.1111/j.1460-9568.2011.07864.x
Bruce, S. E., Yonkers, K. A., Otto, M. W., Eisen, J. L., Weisberg, R. B., Pagano, M., et al. (2005). Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am. J. Psychiatry 162, 1179–1187. doi: 10.1176/appi.ajp.162.6.1179
Currier, M., Murray, G., and Welch, C. (1992). Electroconvulsive therapy for poststroke depressed geriatric patients. J. Neuropsychiatry Clin. Neurosci. 4, 140–144. doi: 10.1176/jnp.4.2.140
Egerton, A., Grace, A. A., Stone, J., Bossong, M. G., Sand, M., and Mcguire, P. (2020). Glutamate in schizophrenia: neurodevelopmental perspectives and drug development. Schizophr. Res. 223, 59–70. doi: 10.1016/j.schres.2020.09.013
Faden, A. I., Demediuk, P., Panter, S. S., and Vink, R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800. doi: 10.1126/science.2567056
Fann, J. R., Bombardier, C. H., Temkin, N., Esselman, P., Warms, C., Barber, J., et al. (2017). Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J. Head Trauma Rehabil. 32, 332–342. doi: 10.1097/HTR.0000000000000322
Ferrarese, C., Aliprandi, A., Tremolizzo, L., Stanzani, L., De Micheli, A., Dolara, A., et al. (2001). Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 57, 671–675. doi: 10.1212/wnl.57.4.671
Frank, D., Gruenbaum, B. F., Melamed, I., Grinshpun, J., Benjamin, I., Vzhetson, N., et al. (2021a). A metric test for assessing spatial working memory in adult rats following traumatic brain injury. J. Vis. Exp. 171, doi: 10.3791/62291
Frank, D., Gruenbaum, B. F., Shelef, I., Zvenigorodsky, V., Benjamin, Y., Shapoval, O., et al. (2021b). A novel histological technique to assess severity of traumatic brain injury in rodents: comparisons to neuroimaging and neurological outcomes. Front. Neurosci. 15:733115. doi: 10.3389/fnins.2021.733115
Frank, D., Kuts, R., Tsenter, P., Gruenbaum, B. F., Grinshpun, Y., Zvenigorodsky, V., et al. (2019a). The effect of pyruvate on the development and progression of post-stroke depression: a new therapeutic approach. Neuropharmacology 155, 173–184. doi: 10.1016/j.neuropharm.2019.05.035
Frank, D., Zlotnik, A., Kofman, O., Grinshpun, J., Severynovska, O., Brotfain, E., et al. (2019b). Early life stress induces submissive behavior in adult rats. Behav. Brain Res. 372:112025. doi: 10.1016/j.bbr.2019.112025
Gainetdinov, R. R., Mohn, A. R., Bohn, L. M., and Caron, M. G. (2001). Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 98, 11047–11054. doi: 10.1073/pnas.191353298
Gonzalez, S. V., Nguyen, N. H., Rise, F., and Hassel, B. (2005). Brain metabolism of exogenous pyruvate. J. Neurochem. 95, 284–293. doi: 10.1111/j.1471-4159.2005.03365.x
Goodrich, G. S., Kabakov, A. Y., Hameed, M. Q., Dhamne, S. C., Rosenberg, P. A., and Rotenberg, A. (2013). Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J. Neurotrauma 30, 1434–1441. doi: 10.1089/neu.2012.2712
Graham, L., and Aprison, M. (1966). Fluorometric determination of aspartate, glutamate, and γ-aminobutyrate in nerve tissue using enzymic method. Anal. Biochem. 15, 487–497. doi: 10.1016/0003-2697(66)90110-2
Gray, L. R., Tompkins, S. C., and Taylor, E. B. (2014). Regulation of pyruvate metabolism and human disease. Cell Mol. Life Sci. 71, 2577–2604. doi: 10.1007/s00018-013-1539-2
Gruenbaum, B. F., Kutz, R., Zlotnik, A., and Boyko, M. (2020). Blood glutamate scavenging as a novel glutamate-based therapeutic approach for post-stroke depression. Ther. Adv. Psychopharmacol. 10:2045125320903951. doi: 10.1177/2045125320903951
Gururajan, A., Reif, A., Cryan, J. F., and Slattery, D. A. (2019). The future of rodent models in depression research. Nat. Rev. Neurosci. 20, 686–701. doi: 10.1038/s41583-019-0221-6
Hackler, E., Byun, N., Jones, C., Williams, J., Baheza, R., Sengupta, S., et al. (2010). Selective potentiation of the metabotropic glutamate receptor subtype 2 blocks phencyclidine-induced hyperlocomotion and brain activation. Neuroscience 168, 209–218. doi: 10.1016/j.neuroscience.2010.02.057
Halberstadt, A. L., Lehmann-Masten, V. D., Geyer, M. A., and Powell, S. B. (2011). Interactive effects of mGlu5 and 5-HT 2A receptors on locomotor activity in mice. Psychopharmacology 215, 81–92. doi: 10.1007/s00213-010-2115-1
Hardingham, G. E., and Bading, H. (2003). The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 26, 81–89. doi: 10.1016/S0166-2236(02)00040-1
Hicks, A. J., Clay, F. J., Hopwood, M., James, A. C., Jayaram, M., Perry, L. A., et al. (2019). The efficacy and harms of pharmacological interventions for aggression after traumatic brain injury—systematic review. Front. Neurol. 10:1169. doi: 10.3389/fneur.2019.01169
Hong, Z., Xinding, Z., Tianlin, Z., and Liren, C. (2001). Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin. Chem. 47, 1458–1462.
Hoofien, D., Gilboa, A., Vakil, E., and Donovick, P. J. (2001). Traumatic brain injury (TBI) 10? 20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 15, 189–209. doi: 10.1080/026990501300005659
Ikonomidou, C., and Turski, L. (2002). Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1, 383–386. doi: 10.1016/s1474-4422(02)00164-3
Jindal, A., Mahesh, R., Bhatt, S., and Pandey, D. (2017). Molecular modifications by regulating cAMP signaling and oxidant-antioxidant defence mechanisms, produce antidepressant-like effect: a possible mechanism of etazolate aftermaths of impact accelerated traumatic brain injury in rat model. Neurochem. Int. 111, 3–11. doi: 10.1016/j.neuint.2016.12.004
Jones, N. C., Cardamone, L., Williams, J. P., Salzberg, M. R., Myers, D., and O’brien, T. J. (2008). Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J. Neurotrauma 25, 1367–1374. doi: 10.1089/neu.2008.0641
Jorge, R. E., and Arciniegas, D. B. (2014). Mood disorders after TBI. Psychiatr. Clin. 37, 13–29. doi: 10.1016/j.psc.2013.11.005
Kabadi, S. V., Hilton, G. D., Stoica, B. A., Zapple, D. N., and Faden, A. I. (2010). Fluid-percussion–induced traumatic brain injury model in rats. Nat. Protoc. 5:1552. doi: 10.1038/nprot.2010.112
Kalueff, A. V., and Tuohimaa, P. (2004). Experimental modeling of anxiety and depression. Acta Neurobiol. Exp. 64, 439–448.
Kane, M. J., Angoa-Pérez, M., Briggs, D. I., Viano, D. C., Kreipke, C. W., and Kuhn, D. M. (2012). A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 203, 41–49. doi: 10.1016/j.jneumeth.2011.09.003
Kessler, R. C., Mcgonagle, K. A., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., et al. (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19. doi: 10.1001/archpsyc.1994.03950010008002
Kim, J.-H., and Vezina, P. (1997). Activation of metabotropic glutamate receptors in the rat nucleus accumbens increases locomotor activity in a dopamine-dependent manner. J. Pharmacol. Exp. Ther. 283, 962–968.
Kokras, N., and Dalla, C. (2014). Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 171, 4595–4619. doi: 10.1111/bph.12710
Koponen, S., Taiminen, T., Portin, R., Himanen, L., Isoniemi, H., Heinonen, H., et al. (2002). Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am. J. Psychiatry 159, 1315–1321. doi: 10.1176/appi.ajp.159.8.1315
Koura, S., Doppenberg, E., Marmarou, A., Choi, S., Young, H., and Bullock, R. (1998). “Relationship between excitatory amino acid release and outcome after severe human head injury,” in Proceedings of the Intracranial Pressure and Neuromonitoring in Brain Injury (Berlin: Springer), 244–246. doi: 10.1007/978-3-7091-6475-4_70
Kovacs, M., Gatsonis, C., Paulauskas, S. L., and Richards, C. (1989). Depressive disorders in childhood: IV. A longitudinal study of comorbidity with and risk for anxiety disorders. Arch. Gen. Psychiatry. 46, 776–782. doi: 10.1001/archpsyc.1989.01810090018003
Krystal, J. H., Sanacora, G., Blumberg, H., Anand, A., Charney, D. S., Marek, G., et al. (2002). Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol. Psychiatry 7 (Suppl. 1), S71–S80. doi: 10.1038/sj.mp.4001021
Leibowitz, A., Boyko, M., Shapira, Y., and Zlotnik, A. (2012). Blood glutamate scavenging: insight into neuroprotection. Int. J. Mol. Sci. 13, 10041–10066. doi: 10.3390/ijms130810041
Levine, J., Panchalingam, K., Rapoport, A., Gershon, S., Mcclure, R. J., and Pettegrew, J. W. (2000). Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 47, 586–593. doi: 10.1016/s0006-3223(99)00284-x
Lewen, A., Fredriksson, A., Li, G. L., Olsson, Y., and Hillered, L. (1999). Behavioural and morphological outcome of mild cortical contusion trauma of the rat brain: influence of NMDA-receptor blockade. Acta. Neurochirurgica 141, 193–202. doi: 10.1007/s007010050286
Li, S., Kuroiwa, T., Katsumata, N., Ishibashi, S., Sun, L. Y., Endo, S., et al. (2006). Transient versus prolonged hyperlocomotion following lateral fluid percussion injury in mongolian gerbils. J. Neurosci. Res. 83, 292–300. doi: 10.1002/jnr.20720
Machado-Vieira, R., Ibrahim, L., Henter, I. D., and Zarate, C. A. Jr. (2012). Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol. Biochem. Behav. 100, 678–687. doi: 10.1016/j.pbb.2011.09.010
Maeng, S., and Zarate, C. A. Jr. (2007). The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr. Psychiatry Rep. 9, 467–474. doi: 10.1007/s11920-007-0063-1
Maksimovic, M., Aitta-Aho, T., and Korpi, E. R. (2014a). Reversal of novelty-induced hippocampal c-Fos expression in GluA1 subunit-deficient mice by chronic treatment targeting glutamatergic transmission. Eur. J. Pharmacol. 745, 36–45. doi: 10.1016/j.ejphar.2014.10.005
Maksimovic, M., Vekovischeva, O. Y., Aitta-Aho, T., and Korpi, E. R. (2014b). Chronic treatment with mood-stabilizers attenuates abnormal hyperlocomotion of GluA1-subunit deficient mice. PLoS One 9:e100188. doi: 10.1371/journal.pone.0100188
Mao, Y., Zhuang, Z., Chen, Y., Zhang, X., Shen, Y., Lin, G., et al. (2019). Imaging of glutamate in acute traumatic brain injury using chemical exchange saturation transfer. Quant. Imaging Med. Surg. 9:1652. doi: 10.21037/qims.2019.09.08
McAllister, T. W. (2008). Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry 7, 3–10. doi: 10.1002/j.2051-5545.2008.tb00139.x
McCarthy, D. J., Alexander, R., Smith, M. A., Pathak, S., Kanes, S., Lee, C. M., et al. (2012). Glutamate-based depression GBD. Med. Hypotheses 78, 675–681. doi: 10.1016/j.mehy.2012.02.009
McConeghy, K. W., Hatton, J., Hughes, L., and Cook, A. M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636. doi: 10.2165/11634020-000000000-00000
Mei, Z., Qiu, J., Alcon, S., Hashim, J., Rotenberg, A., Sun, Y., et al. (2018). Memantine improves outcomes after repetitive traumatic brain injury. Behav. Brain Res. 340, 195–204. doi: 10.1016/j.bbr.2017.04.017
Merikangas, K. R., He, J.-P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., et al. (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–989.
Miller, E. M., Quintero, J. E., Pomerleau, F., Huettl, P., Gerhardt, G. A., and Glaser, P. E. (2019). Chronic methylphenidate alters tonic and phasic glutamate signaling in the frontal cortex of a freely-moving rat model of ADHD. Neurochem. Res. 44, 89–101.
Mitani, H., Shirayama, Y., Yamada, T., Maeda, K., Ashby, C. R. Jr., and Kawahara, R. (2006). Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1155–1158. doi: 10.1016/j.pnpbp.2006.03.036
Mitchell, N. D., and Baker, G. B. (2010). An update on the role of glutamate in the pathophysiology of depression. Acta. Psychiatr. Scand. 122, 192–210. doi: 10.1111/j.1600-0447.2009.01529.x
Morris, G. F., Juul, N., Marshall, S. B., Benedict, B., and Marshall, L. F. (1998). Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Executive Committee of the International Selfotel Trial. Neurosurgery 43, 1369–1372; discussion 1372–1364.
Muir, K. W. (2006). Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 6, 53–60. doi: 10.1016/j.coph.2005.12.002
Ohayon, S., Boyko, M., Saad, A., Douvdevani, A., Gruenbaum, B. F., Melamed, I., et al. (2012). Cell-free DNA as a marker for prediction of brain damage in traumatic brain injury in rats. J. Neurotrauma 29, 261–267. doi: 10.1089/neu.2011.1938
O’Kane, R. L., Martinez-Lopez, I., Dejoseph, M. R., Vina, J. R., and Hawkins, R. A. (1999). Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J. Biol. Chem. 274, 31891–31895. doi: 10.1074/jbc.274.45.31891
O’Neil, D. A., Nicholas, M. A., Lajud, N., Kline, A. E., and Bondi, C. O. (2018). Preclinical models of traumatic brain injury: emerging role of glutamate in the pathophysiology of depression. Front. Pharmacol. 9:579. doi: 10.3389/fphar.2018.00579
Pandey, D. K., Yadav, S. K., Mahesh, R., and Rajkumar, R. (2009). Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 205, 436–442. doi: 10.1016/j.bbr.2009.07.027
Pittenger, C., Sanacora, G., and Krystal, J. H. (2007). The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol. Disord. Drug Targets 6, 101–115. doi: 10.2174/187152707780363267
Procaccini, C., Maksimovic, M., Aitta-Aho, T., Korpi, E. R., and Linden, A. M. (2013). Reversal of novelty-induced hyperlocomotion and hippocampal c-Fos expression in GluA1 knockout male mice by the mGluR2/3 agonist LY354740. Neuroscience 250, 189–200. doi: 10.1016/j.neuroscience.2013.07.010
Puig, N., Dávalos, A., Adan, J., Piulats, J., Martínez, J. M., and Castillo, J. (2000). Serum amino acid levels after permanent middle cerebral artery occlusion in the rat. Cerebrovasc. Dis. 10, 449–454. doi: 10.1159/000016106
Pullela, R., Raber, J., Pfankuch, T., Ferriero, D. M., Claus, C. P., Koh, S.-E., et al. (2006). Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Devel. Neurosci. 28, 396–409. doi: 10.1159/000094166
Ramamoorthy, R., Radhakrishnan, M., and Borah, M. (2008). Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. Behav. Pharmacol. 19, 29–40. doi: 10.1097/FBP.0b013e3282f3cfd4
Rauen, K., Reichelt, L., Probst, P., Schäpers, B., Müller, F., Jahn, K., et al. (2020). Quality of life up to 10 years after traumatic brain injury: a cross-sectional analysis. Health Qual. Life Outcomes 18, 1–12. doi: 10.1186/s12955-020-01391-3
Rivara, F. P., Koepsell, T. D., Wang, J., Temkin, N., Dorsch, A., Vavilala, M. S., et al. (2011). Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics 128, e1129–e1138. doi: 10.1542/peds.2011-0840
Robinson, R. G., Lipsey, J. R., and Pearlson, G. D. (1984). The occurrence and treatment of poststroke mood disorders. Compr. Ther. 10, 19–24.
Rogachev, B., Ohayon, S., Saad, A., Vorobiovsky, V., Gruenbaum, B. F., Leibowitz, A., et al. (2012). The effects of hemodialysis on blood glutamate levels in chronic renal failure: implementation for neuroprotection. J. Crit. Care 27, 743 e741–e747. doi: 10.1016/j.jcrc.2012.07.002
Rubin, T. G., and Lipton, M. L. (2019). Sex differences in animal models of traumatic brain injury. J. Exp. Neurosci. 13:1179069519844020. doi: 10.1177/1179069519844020
Sanacora, G., Rothman, D. L., Mason, G., and Krystal, J. H. (2003). Clinical studies implementing glutamate neurotransmission in mood disorders. Ann. N. Y. Acad. Sci. 1003, 292–308. doi: 10.1196/annals.1300.018
Sanacora, G., Treccani, G., and Popoli, M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. doi: 10.1016/j.neuropharm.2011.07.036
Sanacora, G., Zarate, C. A., Krystal, J. H., and Manji, H. K. (2008). Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 7, 426–437. doi: 10.1038/nrd2462
Shaw, P. J., Forrest, V., Ince, P. G., Richardson, J. P., and Wastell, H. J. (1995). CSF and plasma amino acid levels in motor neuron disease: elevation of CSF glutamate in a subset of patients. Neurodegeneration 4, 209–216. doi: 10.1006/neur.1995.0026
Shutter, L., Tong, K. A., and Holshouser, B. A. (2004). Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. J. Neurotrauma 21, 1693–1705. doi: 10.1089/neu.2004.21.1693
Sta Maria, N. S., Reger, M. L., Cai, Y., Baquing, M. A. T., Buen, F., Ponnaluri, A., et al. (2017). D-cycloserine restores experience-dependent neuroplasticity after traumatic brain injury in the developing rat brain. J. Neurotrauma 34, 1692–1702. doi: 10.1089/neu.2016.4747
Stover, J. F., and Kempski, O. S. (2005). Anesthesia increases circulating glutamate in neurosurgical patients. Acta Neurochir. (Wien) 147, 847–853. doi: 10.1007/s00701-005-0562-y
Takahata, R., and Moghaddam, B. (2003). Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology 28, 1117–1124. doi: 10.1038/sj.npp.1300127
Tateno, A., Jorge, R. E., and Robinson, R. G. (2003). Clinical correlates of aggressive behavior after traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 15, 155–160. doi: 10.1176/jnp.15.2.155
Teichberg, V., Cohen-Kashi-Malina, K., Cooper, I., and Zlotnik, A. (2009). Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 158, 301–308. doi: 10.1016/j.neuroscience.2008.02.075
Teichberg, V. I. (2007). From the liver to the brain across the blood–brain barrier. Proc. Natl. Acad. Sci.U.S.A. 104, 7315–7316. doi: 10.1073/pnas.0702450104
Temple, M. D., and Hamm, R. J. (1996). Chronic, post-injury administration of D-cycloserine, an NMDA partial agonist, enhances cognitive performance following experimental brain injury. Brain Res. 741, 246–251. doi: 10.1016/s0006-8993(96)00940-7
Ter Horst, G. J., Wichmann, R., Gerrits, M., Westenbroek, C., and Lin, Y. (2009). Sex differences in stress responses: focus on ovarian hormones. Physiol. Behav. 97, 239–249. doi: 10.1016/j.physbeh.2009.02.036
Tokita, K., Yamaji, T., and Hashimoto, K. (2012). Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol. Biochem. Behav. 100, 688–704. doi: 10.1016/j.pbb.2011.04.016
Tsesis, S., Gruenbaum, B. F., Ohayon, S., Boyko, M., Gruenbaum, S. E., Shapira, Y., et al. (2013). The effects of estrogen and progesterone on blood glutamate levels during normal pregnancy in women. Gynecol. Endocrinol. 29, 912–916. doi: 10.3109/09513590.2013.813467
Tucker, L. B., Fu, A. H., and Mccabe, J. T. (2016). Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma 33, 880–894. doi: 10.1089/neu.2015.3977
Viggiano, D. (2008). The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 194, 1–14. doi: 10.1016/j.bbr.2008.06.033
Weissman, M. M., Bland, R., Joyce, P. R., Newman, S., Wells, J. E., and Wittchen, H.-U. (1993). Sex differences in rates of depression: cross-national perspectives. J. Affect. Disord. 29, 77–84. doi: 10.1016/0165-0327(93)90025-f
Wiart, L., Petit, H., Joseph, P. A., Mazaux, J. M., and Barat, M. (2000). Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke 31, 1829–1832. doi: 10.1161/01.str.31.8.1829
Zarate, C. Jr., Machado-Vieira, R., Henter, I., Ibrahim, L., Diazgranados, N., and Salvadore, G. (2010). Glutamatergic modulators: the future of treating mood disorders? Harv. Rev. Psychiatry 18, 293–303. doi: 10.3109/10673229.2010.511059
Zauner, A., Bullock, R., Kuta, A., Woodward, J., and Young, H. (1996a). “Glutamate release and cerebral blood flow after severe human head injury,” in Proceedings of the Clinical Aspects of Microdialysis (Berlin: Springer), 40–44. doi: 10.1007/978-3-7091-6894-3_9
Zauner, A., Bullock, R., Kuta, A. J., Woodward, J., and Young, H. F. (1996b). Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir. Suppl. 67, 40–44. doi: 10.1007/978-3-7091-6894-3_9
Zhang, H., Zhang, X., Zhang, T., and Chen, L. (2001). Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin. Chem. 47, 1458–1462. doi: 10.1093/clinchem/47.8.1458
Zhumadilov, A., Boyko, M., Gruenbaum, S. E., Brotfain, E., Bilotta, F., and Zlotnik, A. (2015). Extracorporeal methods of blood glutamate scavenging: a novel therapeutic modality. Expert. Rev. Neurother. 15, 501–508. doi: 10.1586/14737175.2015.1032259
Zlotnik, A., Gruenbaum, B. F., Klin, Y., Gruenbaum, S. E., Ohayon, S., Sheiner, E., et al. (2011a). The effects of insulin, glucagon, glutamate, and glucose infusion on blood glutamate and plasma glucose levels in naive rats. J. Neurosurg. Anesthesiol. 23, 323–328. doi: 10.1097/ANA.0b013e3182299b15
Zlotnik, A., Gruenbaum, B. F., Mohar, B., Kuts, R., Gruenbaum, S. E., Ohayon, S., et al. (2011b). The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol. Reprod. 84, 581–586. doi: 10.1095/biolreprod.110.088120
Zlotnik, A., Gruenbaum, S. E., Artru, A. A., Rozet, I., Dubilet, M., Tkachov, S., et al. (2009). The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: evidence from the use of maleate. J. Neurosurg. Anesthesiol. 21, 235–241. doi: 10.1097/ANA.0b013e3181a2bf0b
Zlotnik, A., Gurevich, B., Cherniavsky, E., Tkachov, S., Matuzani-Ruban, A., Leon, A., et al. (2008). The contribution of the blood glutamate scavenging activity of pyruvate to its neuroprotective properties in a rat model of closed head injury. Neurochem. Res. 33, 1044–1050. doi: 10.1007/s11064-007-9548-x
Zlotnik, A., Gurevich, B., Tkachov, S., Maoz, I., Shapira, Y., and Teichberg, V. I. (2007). Brain neuroprotection by scavenging blood glutamate. Exp. Neurol. 203, 213–220. doi: 10.1016/j.expneurol.2006.08.021
Zlotnik, A., Klin, Y., Gruenbaum, B. F., Gruenbaum, S. E., Ohayon, S., Leibowitz, A., et al. (2012a). beta2 adrenergic-mediated reduction of blood glutamate levels and improved neurological outcome after traumatic brain injury in rats. J. Neurosurg. Anesthesiol. 24, 30–38. doi: 10.1097/ANA.0b013e318232deaa
Zlotnik, A., Sinelnikov, I., Gruenbaum, B. F., Gruenbaum, S. E., Dubilet, M., Dubilet, E., et al. (2012b). Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology 116, 73–83. doi: 10.1097/ALN.0b013e31823d7731
Keywords: depression, glutamate scavenging, neuroprotection, pyruvate, traumatic brain injury
Citation: Frank D, Gruenbaum BF, Shelef I, Zvenigorodsky V, Severynovska O, Gal R, Dubilet M, Zlotnik A, Kofman O and Boyko M (2022) Blood Glutamate Scavenging With Pyruvate as a Novel Preventative and Therapeutic Approach for Depressive-Like Behavior Following Traumatic Brain Injury in a Rat Model. Front. Neurosci. 16:832478. doi: 10.3389/fnins.2022.832478
Received: 09 December 2021; Accepted: 07 January 2022;
Published: 14 February 2022.
Edited by:
Barbara Budzynska, Medical University of Lublin, PolandReviewed by:
Monika Gawrońska-Grzywacz, Medical University of Lublin, PolandPushpa Sharma, Uniformed Services University of the Health Sciences, United States
Copyright © 2022 Frank, Gruenbaum, Shelef, Zvenigorodsky, Severynovska, Gal, Dubilet, Zlotnik, Kofman and Boyko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew Boyko, bWF0dGhld2JveWtvcmVzZWFyY2hAZ21haWwuY29t
†These authors have contributed equally to this work
 Dmitry Frank
Dmitry Frank Benjamin F. Gruenbaum2†
Benjamin F. Gruenbaum2† Ilan Shelef
Ilan Shelef Vladislav Zvenigorodsky
Vladislav Zvenigorodsky Ora Kofman
Ora Kofman Matthew Boyko
Matthew Boyko