
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci. , 06 June 2022
Sec. Brain Imaging Methods
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.826759
Background: Gray matter volume (GMV) alteration in specific brain regions has been widely regarded as one of the most important neuroplasticity features in chronic pain patients with depressive symptoms (CP-D). However, the consistent and significant results were still lacking. Thus, further exploration was suggested to be performed.
Objectives: This study aimed to comprehensively collect the voxel-based morphometry (VBM) studies on GMV alteration between CP-D and healthy controls (HCs). And a systemic review and meta-analysis were made to explore the characteristic brain regions in chronic pain and depression comorbidity.
Methods: Search of PubMed, MEDLINE, Web of Science, and Cochrane Library databases updated to July 13, 2021. The altered GMV between CP-D and HCs in VBM studies was included in this meta-analysis. In total, 18 studies (20 datasets) and 1320 participants (520 patients and 800 HCs) were included. The significant coordinate information (x, y, z) reported in standard space and the effect size (t-value or z-score) were extracted and analyzed by anisotropic effect size-signed differential mapping (AES-SDM) 5.15 software.
Results: According to the main analysis results, CP-D showed significant and consistent increased GMV in the left hippocampus (HIP. L) and decreased GMV in the medial part of the left superior frontal gyrus (SFG. L, BA 10) compared to HCs. Subgroup analysis showed significant decreased GMV in the medial orbital part of SFG.R (BA 10) in neuropathic pain, as well as significant increased GMV in the right parahippocampal gyrus (PHG.R, BA 35), left hippocampus (HIP.L, BA 20), and right middle frontal gyrus (MFG.R) in musculoskeletal pain. Furthermore, meta-regression showed a positive relationship between the decreased GMV in the medial part of SFG.L and the percentage of female patients.
Conclusion: GMV abnormality in specific brain areas (e.g., HIP.L and SFG) was robust and reproducible, which could be significantly involved in this comorbidity disease. The findings in this study may be a valuable reference for future research.
Systematic Review Registration: [www.crd.york.ac.uk/prospero/].
Chronic pain has been a major problem and a heavy burden on human health for decades (Kuner and Kuner, 2021). It was estimated that about 20∼50% of adults in developed countries suffered from chronic pain (Fayaz et al., 2016; Grace et al., 2021). More than 1 billion people suffered from headaches, low back pain, and neck pain all of which were considered a significant risk of disability (Mills et al., 2019; Ashina, 2020). Many types of chronic pain (e.g., neuropathic pain and fibromyalgia) can induce emotional disorders, and the incidence of which is about 30∼80% (Yalcin and Barrot, 2014). Depression is one of the most common emotional disorders induced by a persistent stress state of chronic pain. The prevalence of this comorbidity in chronic pain patients is 40∼50%(Rizvi et al., 2021). Poor management and treatment outcomes of chronic pain exacerbate depression, which contributes to human suffering and disability (Yalcin and Barrot, 2014; Sheng et al., 2017; Kuner and Kuner, 2021). In turn, bad emotional states worsen pain (Bushnell et al., 2013; Porreca and Navratilova, 2017). Therefore, further studies on the underlying mechanisms and characteristics of neural remodeling are of great significance for the development of clinical diagnosis and treatment.
Voxel-based morphometry (VBM) has been one of the most important methods to explore the brain structure alteration in neuroimaging studies in recent decades (Ashburner and Friston, 2000), which can find the specific abnormality in pathological conditions, including chronic pain and depression. The gray matter volume (GMV) alteration is one of the most important objects of VBM studies (Borsook et al., 2014). Previous studies have shown that the altered GMV in cortical brain areas was associated with both chronic pain and depression (Gustin et al., 2013). However, the results of many neuroimaging studies are inconsistent. For instance, GMV reduction in the amygdala was reported in fibromyalgia patients with anxiety/depressive symptoms (Burgmer et al., 2009). But the GMV of the amygdala in low back pain patients with depressive symptoms was larger than healthy controls (HCs) (Mao et al., 2013). Likewise, increased GMV in the hippocampus has been reported in several studies to play a crucial role in chronic pain (e.g., burning mouth syndrome, fibromyalgia, and muskuloskeletal pain) with depressive symptoms (Khan et al., 2014; Fayed et al., 2017; James et al., 2018). However, some studies have also reported no changes in hippocampal GMV in chronic pain and depression comorbidity (Seminowicz et al., 2013; Mole et al., 2014). Since the findings of GMV alteration in chronic pain patients with depressive symptoms (CP-D) are heterogenous and controversial, a systemic review and meta-analysis are warranted.
In this systemic review and meta-analysis, we aimed to consider the chronic pain-associated depressive disorder and explore the characteristic brain regions involved in the process of chronic pain and depression comorbidity.
This study followed the PRISMA statement (Liberati et al., 2009). The detailed checklist is shown in Supplementary Table 1. The protocol of this systemic review and meta-analysis was registered in PROSPERO (CRD42021267592).
A comprehensive search of studies published from PubMed, MEDLINE, Web of Science, and Cochrane Library databases from its inception to July 13, 2021, was done. Text words: (“chronic pain”) AND (“depressive” OR “depression”) AND (“VBM” OR “voxel-based morphometry” OR “gray matter” OR “gray matter volume”) were used in this study (Mazine et al., 2018). All studies that had been searched were selected by title and abstract in the first step. Residual studies were checked by the detailed information of the article after removing the unrelated studies. In addition, the references of the included studies and relevant review articles were checked for additional relevant studies. The detailed search information is shown in Supplementary Data 1.
Studies that satisfied the following conditions were included in the meta-analysis: (1) Reported the changed GMV in chronic pain patients; all patients were evaluated by the depression-related scale and showed significantly higher depression scores than HCs; (2) compared the GMV between chronic pain patients with HCs; (3) adult participants (age > 18 years old); (4) showed the available coordinates information in standard space [such as Montreal Neurological Institute (MNI) or Talairach (TAL) space] and the effect size (such as t-value or z-score). Datasets were excluded if they satisfied the following conditions: (1) animal research; (2) children or adolescents; (3) not VBM method; (4) studies did not compare the changed GMV between chronic pain patients and HCs; (5) without depressive symptoms, not chronic pain, or other unrelated studies.
The 12-point checklist involved in the previous meta-analysis of VBM studies was used to assess the quality of each study selected for this meta-analysis (Tang et al., 2020), which is shown in Supplementary Table 2. Literature search, study evaluation, and selection were independently performed by two investigators (TM and Y-YJ), and all controversial discrepancies were resolved by a third investigator (L-FY) for the final decision.
A meta-analysis of GMV differences between CP-D and HCs was conducted with the seed-based d mapping (SDM) software package (version 5.15) in a standard process (Radua et al., 2012, 2014). The SDM approach used effect sizes to combine reported peak coordinates extracted from databases with statistical parametric maps, and it recreated original maps of the effect size of GMV difference between patients and HCs (Chen et al., 2020; Sheng et al., 2020, 2021). The SDM process was briefly described as follows: First, extracted peak coordinates and effect size (e.g., t-values and z-score) of differences in GMV between CP-D and HCs from each dataset and the z-score could be changed to t-values with the online tool on SDM website1 (Aoki and Inokuchi, 2016; Aoki et al., 2017); a standard MNI map of the GMV differences was then separately recreated for each dataset using an anisotropic Gaussian kernel. The mean map was generated by voxel-wise calculation of the random-effects mean of the dataset maps, weighted by the sample size, intra-dataset variability, and between-dataset heterogeneity. To optimally balance false positives and negatives, we used the default SDM kernel size and thresholds to optimize sensitivity while controlling false positives [full width at half maximum (FWHM) = 20 mm, P = 0.005, peak height Z = 1, cluster extent = 10 voxels] (Radua et al., 2012). Then, a quantitative meta-analytic comparison of altered GMV was conducted by calculating differences between patients and controls in each voxel, and the statistical significance was determined using 50 randomization tests (Kolesar et al., 2019; Liu et al., 2021).
Significant peaks located in gray matter (GM) areas were visualized and discussed in detail in this study. Peaks located in white matter (WM) areas were objectively described and discussed in the end. According to the patient’s chronic pain type, we divided them into musculoskeletal pain and neuropathic pain (Fayed et al., 2017; Ashina, 2020; Finnerup et al., 2021). All the above analysis was repeated in subgroups. The visualization of changed GMV was performed with the MRIcroGL software https://www.nitrc.org/plugins/mwiki/index.php/mricrogl:MainPage/home (Chung and Park, 2019, 2020; Kumral et al., 2021; Park and Jung, 2021).
Following the mean analysis of the data, a whole-brain voxel-based jackknife sensitivity analysis was performed to test the robustness of the findings by iteratively repeating the same analysis, excluding one dataset each time. This analysis was to establish the extent to which the results could be replicated. If a brain region remained significant in all or most of (>50%) the combinations of studies, the finding would be considered highly replicable. Heterogeneity analysis was conducted using a random-effects model with Q statistics to explore unexplained between-study variability in the results. Heterogeneous brain regions were obtained using the default SDM kernel size and thresholds. In addition, publication bias was assessed with Egger’s test of the AES-SDM default by extracting the values from statistically significant relevant peaks between patients and HCs. The visualization of heterogeneity information was performed with GraphPad Prism software (version 9). The publication bias was visualized with a funnel plot performed by SDM default in Figure 4.
Meta-regression was made by the linear model (Select linear model-Meta-regression) and choose the threshold (Probability = 0.0005; Peak height threshold = 1.000; Extent threshold = 10), the correlation between SDM-estimate and factors (age, disease duration, female percentage of patients, and depression score) (Picó-Pérez et al., 2020; Tang et al., 2020).
A total of 18 studies, including three fibromyalgia (Burgmer et al., 2009; Robinson et al., 2011; Fayed et al., 2017), two mixed pain (Seminowicz et al., 2013; Ikeda et al., 2018), three back pain (Schmidt-Wilcke et al., 2006; Mao et al., 2013; Fritz et al., 2016), two trigeminal neuralgia (Wang et al., 2017; Zhang et al., 2018), one knee osteoarthritis (Liao et al., 2018), one musculoskeletal pain (James et al., 2018), one pelvic pain (As-Sanie et al., 2012), one burning mouth syndrome (Khan et al., 2014), one chronic facial pain (Schmidt-Wilcke et al., 2010), one neuropathic pain (Mole et al., 2014), and two migraines (Hubbard et al., 2014; Neeb et al., 2017), and a total of 1,320 participants (520 patients and 800 HCs) were included after systemic searching and selecting from 1,726 studies. The detailed flow diagram is shown in Figure 1. In subgroups, 4 of 18 studies were classified as musculoskeletal pain (Burgmer et al., 2009; Robinson et al., 2011; Fayed et al., 2017; James et al., 2018) and 10 of 18 studies were classified as neuropathic pain (Schmidt-Wilcke et al., 2006, 2010; Mao et al., 2013; Hubbard et al., 2014; Khan et al., 2014; Mole et al., 2014; Fritz et al., 2016; Neeb et al., 2017; Wang et al., 2017; Zhang et al., 2018). General clinical information (authors, publication date, participants, chronic pain types, depression rating, magnetic field, standard space, P-value, disease duration, and age) of the included studies was fully collected in Table 1. The detailed depression information of studies included is shown in Supplementary Table 3.
The main analysis of 18 studies included showed the altered GMV of two major brain areas could be significant and consistent (Table 2). The increased GMV in the left hippocampus (HIP.L) (MNI: x = –24, y = –18, z = –12, SDM-Z = 1.283, P < 0.005) and decreased GMV in the medial part of the left superior frontal gyrus (SFG.L) (BA 10, MNI: x = –4, y = 62, z = 10, SDM-Z = –2.436, P < 0.005). Information on brain areas is shown in Figures 2A,B. The heterogeneity analysis of the 18 studies showed heterogeneity between studies (Positive peaks: Q = 24.798, P < 0.005; Negative peaks: Q = 30.891, P < 0.005) (Supplementary Table 4 and Figures 3A,B). The sensitivity analysis of the main results showed 16 of 20 times of analyses were significant in the HIP.L and 19 of 20 times of analyses were significant in the medial part of SFG.L (Supplementary Table 5). Egger’s test for main results showed no publication bias in HIP.L and the medial part of SFG.L (BA 10) (P > 0.05) (Figures 4A,B).
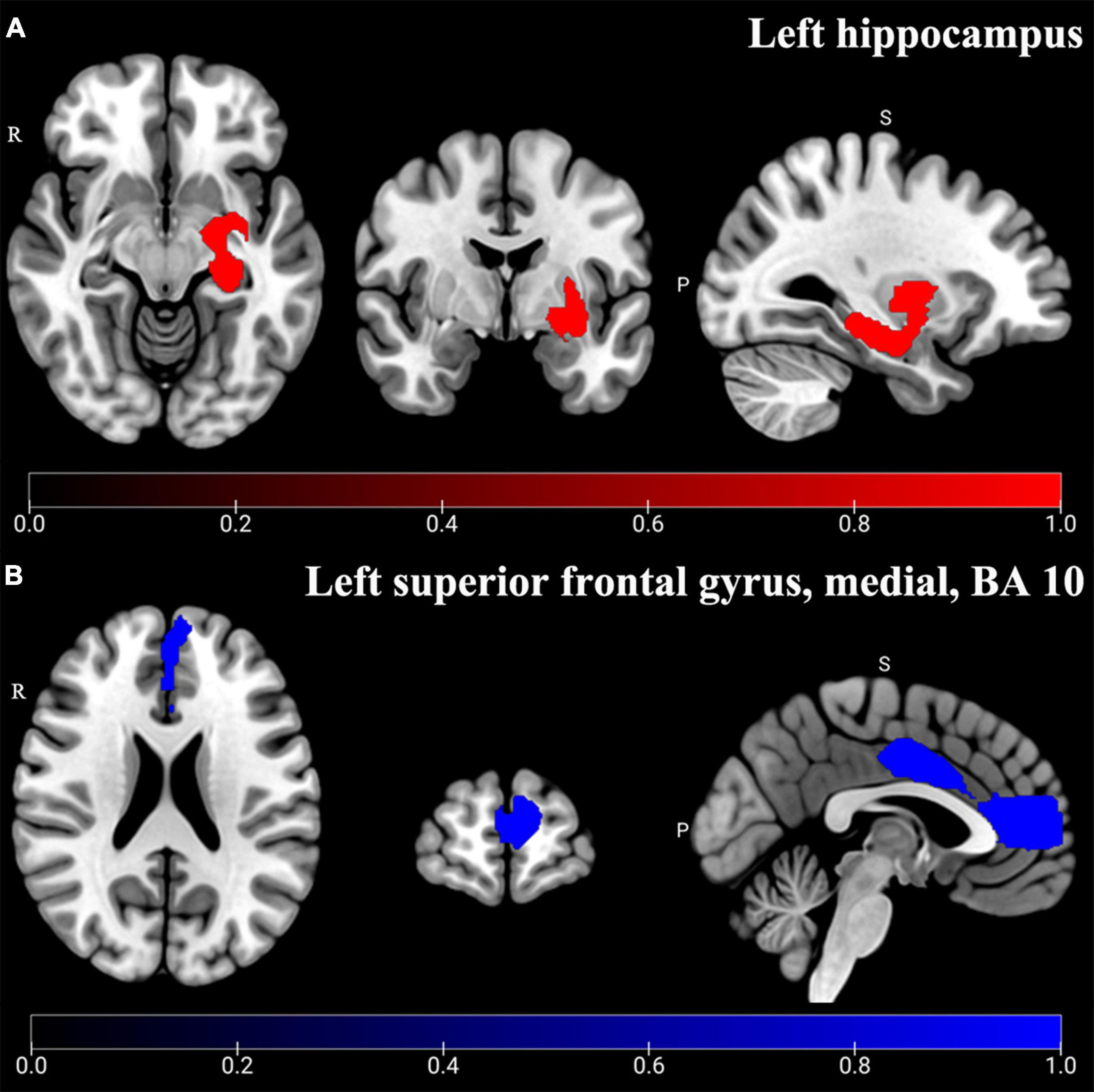
Figure 2. Altered GMV of main analysis results. Significant and consistent increased GMV (Red) in the left hippocampus (A) and decreased GMV (Blue) in the left superior frontal gyrus, medial, and BA 10 (B) were shown in CP-D compared with HCs (P < 0.005).
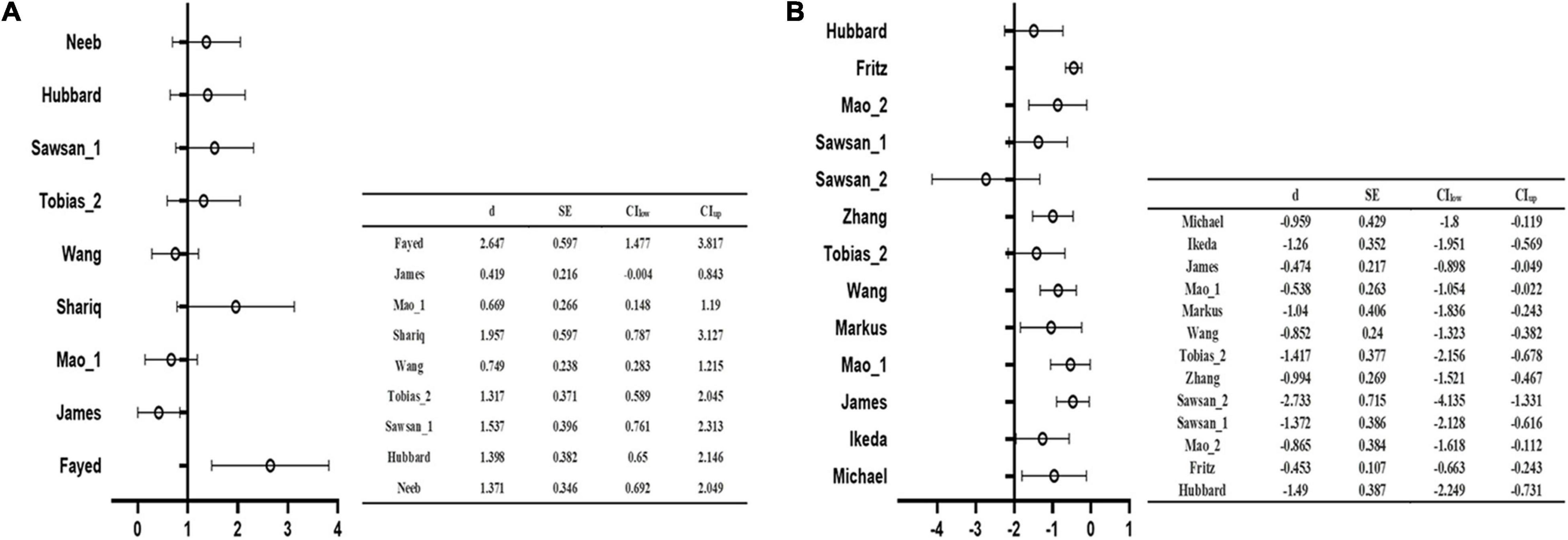
Figure 3. The forest plot of the main analysis results in heterogeneity assessment. Q test of AES-SDM default showed the main results existed heterogeneity in both positive (A) and negative (B) coordinate results (P < 0.05).
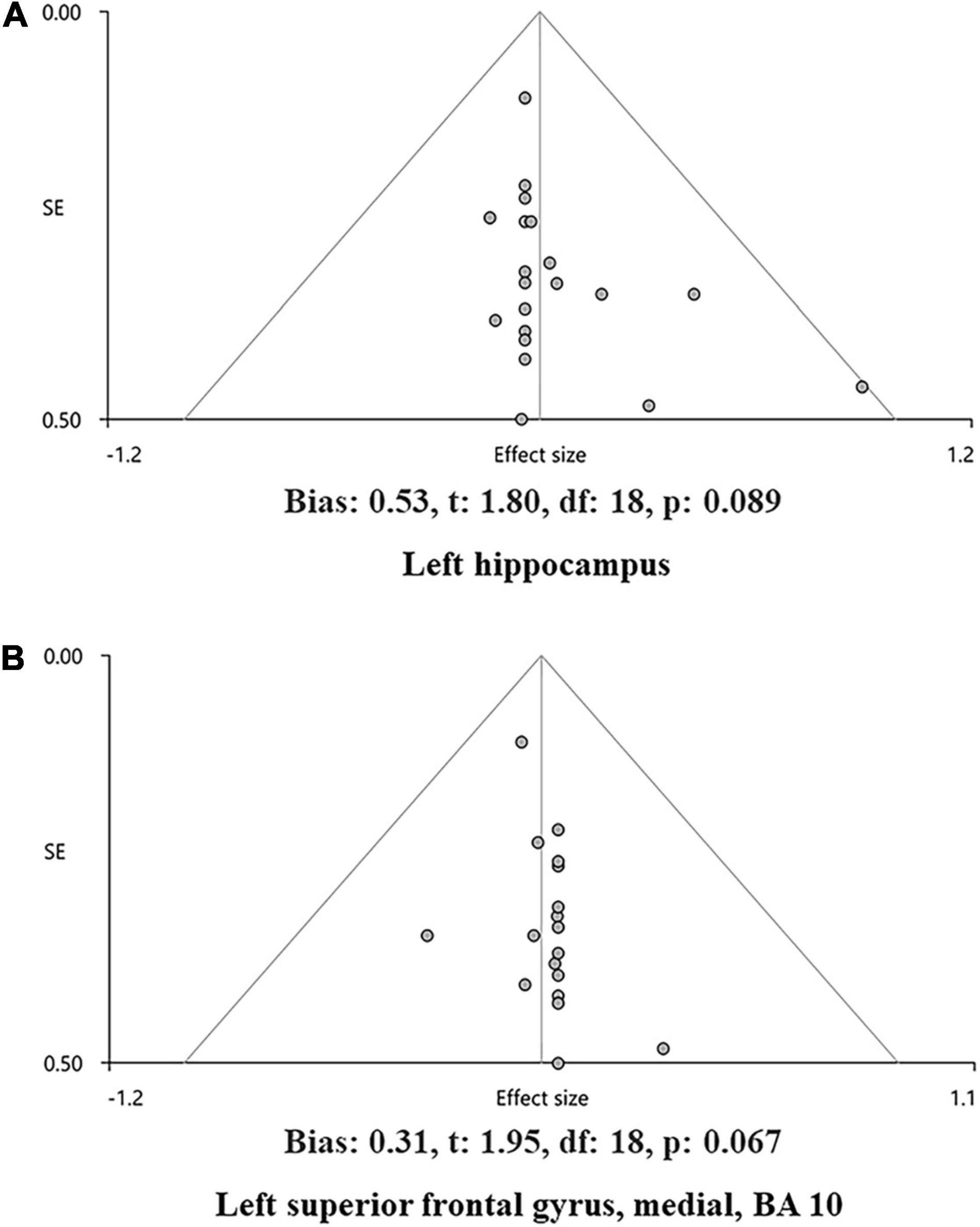
Figure 4. The funnel plot of the main analysis results in bias assessment. Main analysis results in the left hippocampus (A) and the left superior frontal gyrus, medial, and BA 10 (B) and showed no bias by Egger’s test of AES-SDM default (P > 0.05).
Chronic neuropathic pain patients with depressive symptoms (CNP-D) showed significant decreased GMV in the medial orbital part of SFG.R compared to HCs (BA 10, MNI: x = 12, y = 48, z = –8, SDM-Z = –2.685, P < 0.005) (Table 3 and Figure 5A). Jackknife analysis showed that 8 of 11 times were significant in the medial orbital part of SFG.R, which is shown in Supplementary Table 6.
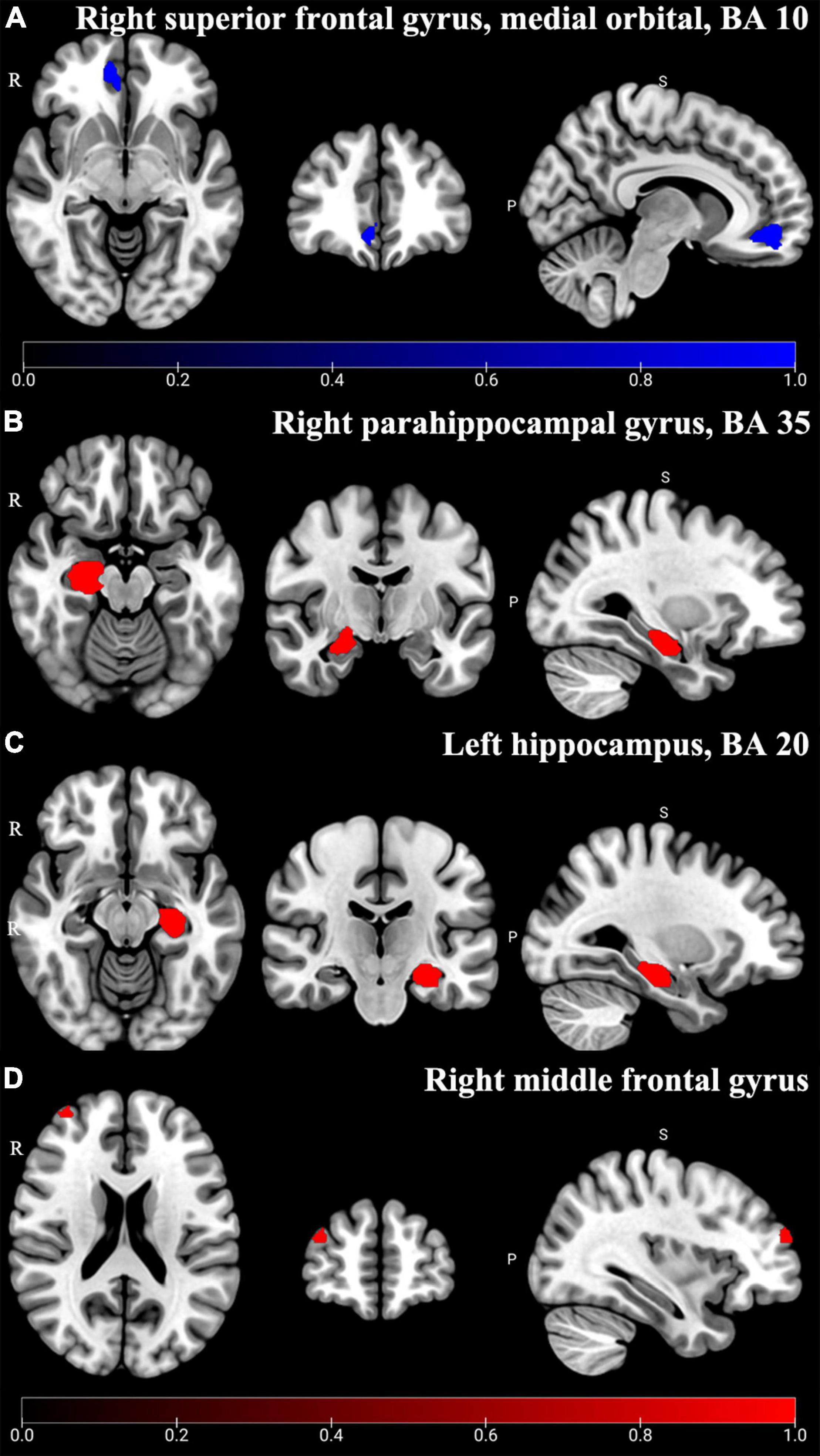
Figure 5. Altered GMV of subgroup analysis results. In CNP-D, the significant decreased (Blue) GMV was shown in the right superior frontal gyrus, medial orbital, and BA 10 (A) (P < 0.005); in CMP-D, the significant increased (Red) GMV was shown in the right parahippocampal gyrus, BA 35 (B), left hippocampus, BA 20 (C), and right middle frontal gyrus (D) (P < 0.005).
In addition, chronic musculoskeletal pain patients with depressive symptoms (CMP-D) showed significant increased GMV in the right parahippocampal gyrus (PHG.R) (BA 35, MNI: x = 22, y = –18, z = –18, SDM-Z = 1.184, P < 0.005), HIP.L (BA 20, MNI: x = –28, y = –20, z = –16, SDM-Z = 1.155, P < 0.005), and right middle frontal gyrus (MFG.R) (MNI: x = 36, y = 58, z = 22; SDM-Z = 1.347, P < 0.005) compared to HCs (Table 3 and Figures 5B–D). Jackknife analysis showed that 3 of 4 times were significant in the PHG.R, 3 of 4 times were significant in the HIP.L, and 3 of 4 times were significant in the MFG.R, which are shown in Supplementary Table 6.
The risk factors, including age, sex ratio (female), BDI score, HAMD score, and disease duration, were checked with meta-regression. And the result showed a positive relationship between the decreased GMV in the medial part of SFG.L and the percentage of female patients (r = 0.3450, P < 0.0005) (Figure 6). No significant results were shown in the other risk factors.
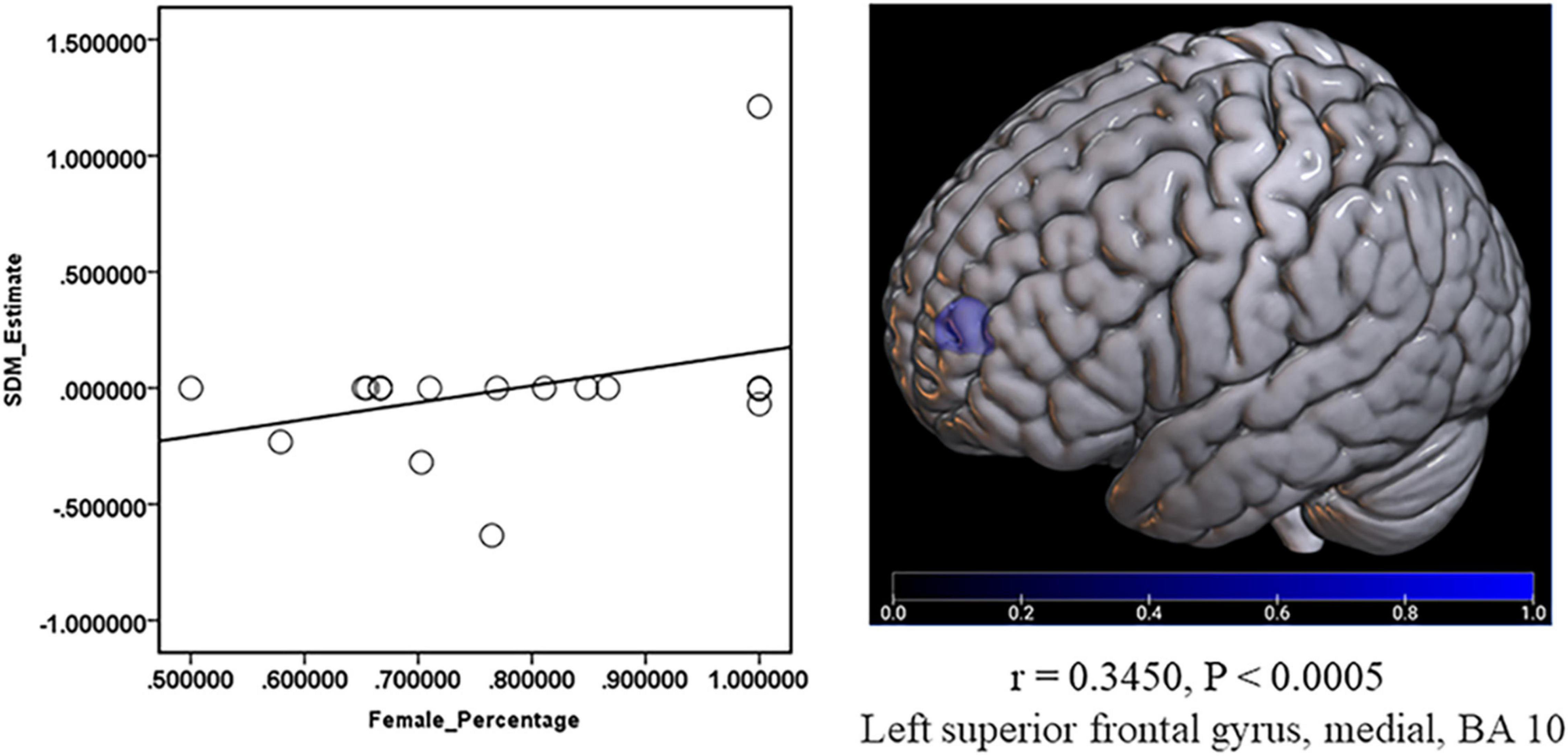
Figure 6. Meta-regression about the relationship between GMV and the percentage of female patients. The percentage of female patients showed a positive relationship with the decreased (Blue) GMV in the left superior frontal gyrus, medial, and BA 10 (r = 0.3450, P < 0.0005).
In this study, depressive symptoms accompanying chronic pain were taken into consideration to explore the characteristic brain regions in this comorbid state. By extracting the significant peak coordinates of GMV alteration in different types of chronic pain and checking the depression scales, we found that the increased GMV in the HIP.L and decreased GMV in the medial part of SFG.L were significant and consistent in CP-D. In addition, the sex ratio (female) of patients was positively related to the decreased GMV in the medial part of SFG.L in meta-regression, which indicated that this factor might affect the brain structure in the process of CP-D.
Chronic pain and depression are generally divided into separate diseases in clinical experience (Rotenstein et al., 2016; Park and Zarate, 2019; Grace et al., 2021). However, accumulating studies found that similar brain areas were involved in the common mechanism of both diseases (Han and Pae, 2015; Sheng et al., 2017; Georgopoulos et al., 2019). In the main analysis of our study, CP-D showed a significant decreased GMV in the medial part of SFG.L compared to HCs. And the subgroup analysis also found that the decreased GMV in the medial orbital part of SFG.R was involved in the CNP-D, which was consistent with the results in previous systemic reviews and meta-analyses of neuropathic pain (Pan et al., 2015). Similarly, structure magnetic resonance imaging (sMRI) studies reported that the patients with depression had a significant GMV reduction in the frontal and orbitofrontal cortex (Kraus et al., 2017), as well as a decreased GMV in the medial orbital gyrus (Kandilarova et al., 2019). Thus, we could speculate that the specific brain region of SFG (medial) may play a crucial role in the process of chronic pain and depression comorbidity.
In addition to the congruent abnormality of specific brain areas in both chronic pain and depression, inconsistent brain regions remain (e.g., hippocampus, PHG.R, and MFG.R). In 2017, increased GMV in the bilateral hippocampus was found in fibromyalgia patients compared to HCs (Fayed et al., 2017), which was also shown in patients with burning mouth syndrome (Khan et al., 2014). Besides, previous systemic reviews and meta-analyses demonstrated the increased GMV in the HIP. R was involved in the process of chronic pain (Smallwood et al., 2013). Interestingly, neuroimaging studies reported that depressed patients have smaller hippocampal volumes than HCs (Kraus et al., 2017; Serrano-Sosa et al., 2020). However, the main and subgroup analysis in our study found increased GMV in the HIP. L was significantly involved in CP-D. Similarly, subgroup analysis showed that the significant increased GMV in PHG.R and MFG.R was involved in CMP-D. But previous systemic review and meta-analysis studies reported that patients with fibromyalgia had smaller GMV in PHG.R than HCs (Shi et al., 2016). The previous study has also reported decreased GMV in the MFG. R of patients with MDD, whereas increased GMV in the MFG and R of patients with bipolar disorder (BD) (Wise et al., 2017). Taken together, the GMV alteration in the hippocampus, PHG.R, and MFG.R may be heterogenous and controversial in CP-D. Therefore, it is essential to comprehensively explore the neuromechanisms of these brain regions in CP-D in future studies.
Furthermore, the main and subgroup analysis also revealed that some peak coordinates outside the gray matter mask appeared in WM areas, including the left inferior network, uncinate fasciculus (UF. L), right inferior network, inferior fronto-occipital (IFOF. R), and right superior longitudinal fasciculus II (SLF II. R). On one hand, calculations of voxels were not restricted within the specific masks by the algorithm of SDM, which might cause the peaks to be outside the mask in the recreated map (Radua et al., 2014); on the other hand, the potential function of the significant peaks in WM areas should not be ignored just because it might be due to an error. For instance, emerging evidence reported that the neurobiological significance of low-frequency BOLD fluctuations (LFBFs) was not just shown in GM but also in WM (Ji et al., 2017), and which has been explored in the Parkinson’s disease (PD) (Ji et al., 2018, 2021). Therefore, these findings extending to the WM areas can be accepted cautiously, and can also be learned in conjunction with the adjacent GM areas involved in CP-D. In addition, meta-regression indicated that the sexual difference might affect the GMV in the medial part of SFG.L in CP-D. However, other risk factors should not be ignored because of the limited data, which was suggested to be explored in future.
There were some limitations in this study. First, only four databases were searched. And the studies included were published, while unpublished studies and potential bias could not be ignored; Second, only the peak coordinate information reported in the studies was extracted to be analyzed, which was the common shortage in neuroimaging meta-analysis (Liu et al., 2021); Third, lack of data on the risk factors in meta-regression; Fourth, we only focused on the GMV abnormality to reduce bias. However, further studies should take a broader approach to studying this comorbid disease.
In conclusion, this systemic review and meta-analysis provide some specific brain areas involved in chronic pain and depressive symptoms comorbidity. The GMV alteration in SFG is consistent with previous studies, which show a stable reduction in CP-D. Although some results of this study are inconsistent with previous studies (especially the hippocampus), the important value of which CP-D cannot be ignored. Furthermore, it is recommended to further explore the neural mechanisms of the hippocampus in CP-D in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
G-BC, J-LL, and WW made the study and article framework design. G-BC, J-LL, and L-FY provided the funding support. TM, Y-YJ, and L-FY performed the systemic search, study selection, data analysis, and wrote the original draft. Z-YL checked the methods and results and provided the technical support of software. G-BC, J-LL, J-JL, and WW revised the article. All authors contributed to the article and approved the submitted version.
This study is supported by the National Natural Science Foundation of China (No. 81870866, J-LL) and the Military Medical Enhancement Program of Air Force Medical University (No. 2018HKPY03, G-BC and No. 2018JSTS13, L-FY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.826759/full#supplementary-material
Aoki, Y., Cortese, S., and Castellanos, F. X. (2017). Research Review: diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J. Child Psychol. Psychiatry 59, 193–202. doi: 10.1111/jcpp.12778
Aoki, Y., and Inokuchi, R. (2016). A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neurosci. Biobehav. Rev. 66, 119–126. doi: 10.1016/j.neubiorev.2016.04.021
Ashburner, J., and Friston, K. J. (2000). Voxel-Based morphometry—the methods. NeuroImage 11, 805–821. doi: 10.1006/nimg.2000.0582
As-Sanie, S., Harris, R. E., Napadow, V., Kim, J., Neshewat, G., Kairys, A., et al. (2012). Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 153, 1006–1014. doi: 10.1016/j.pain.2012.01.032
Borsook, D., Erpelding, N., and Becerra, L. (2014). Losses and gains: chronic pain and altered brain morphology. Expert Rev. Neurother. 13, 1221–1234. doi: 10.1586/14737175.2013.846218
Burgmer, M., Gaubitz, M., Konrad, C., Wrenger, M., Hilgart, S., Heuft, G., et al. (2009). Decreased gray matter volumes in the Cingulo-Frontal cortex and the amygdala in patients with fibromyalgia. Psychosom. Med. 71, 566–573. doi: 10.1097/PSY.0b013e3181a32da0
Bushnell, M. C., Ceko, M., and Low, L. A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 14, 502–511. doi: 10.1038/nrn3516
Chen, E. Y., Eickhoff, S. B., Giovannetti, T., and Smith, D. V. (2020). Obesity is associated with reduced orbitofrontal cortex volume: a coordinate-based meta-analysis. Neuroimage Clin. 28:102420. doi: 10.1016/j.nicl.2020.102420
Chung, B. S., and Park, J. S. (2019). Real-Color volume models made from Real-Color sectioned images of visible korean. J. Korean Med. Sci. 34:e86. doi: 10.3346/jkms.2019.34.e86
Chung, B. S., and Park, J. S. (2020). Automatic segmentation of true color sectioned images using FMRIB Software Library: first trial in brain, gray matter, and white matter. Clin. Anat. 33, 1197–1203. doi: 10.1002/ca.23564
Fayaz, A., Croft, P., Langford, R. M., Donaldson, L. J., and Jones, G. T. (2016). Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 6:e10364. doi: 10.1136/bmjopen-2015-010364
Fayed, N., Garcia-Marti, G., Sanz-Requena, R., Marti-Bonmati, L., and Garcia-Campayo, J. (2017). Difference in regional brain volume between fibromyalgia patients and Long-Term meditators. Actas Esp. Psiquiatr. 45, 268–276.
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101, 259–301. doi: 10.1152/physrev.00045.2019
Fritz, H., McAuley, J. H., Wittfeld, K., Hegenscheid, K., Schmidt, C. O., Langner, S., et al. (2016). Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a Population-Based cohort study. J. Pain 17, 111–118. doi: 10.1016/j.jpain.2015.10.003
Grace, P. M., Tawfik, V. L., Svensson, C. I., Burton, M. D., Loggia, M. L., and Hutchinson, M. R. (2021). The neuroimmunology of chronic pain: from rodents to humans. J. Neurosci. 41, 855–865. doi: 10.1523/JNEUROSCI.1650-20.2020
Gustin, S. M., Peck, C. C., Macey, P. M., Murray, G. M., and Henderson, L. A. (2013). Unraveling the Effects of Plasticity and Pain on Personality. J. Pain 14, 1642–1652. doi: 10.1016/j.jpain.2013.08.005
Han, C., and Pae, C. (2015). Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 12:1. doi: 10.4306/pi.2015.12.1.1
Hubbard, C. S., Khan, S. A., Keaser, M. L., Mathur, V. A., Goyal, M., and Seminowicz, D. A. (2014). Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. ENeuro 1, e14–e20. doi: 10.1523/ENEURO.0006-14.2014
Ikeda, E., Li, T., Kobinata, H., Zhang, S., and Kurata, J. (2018). Anterior insular volume decrease is associated with dysfunction of the reward system in patients with chronic pain. Eur. J. Pain 22, 1170–1179. doi: 10.1002/ejp.1205
James, H. B., Marina, S. A., Antoni, K. A., Sarah, C. A., and Richard, W. C. D. (2018). Structural network differences in chronic muskuloskeletal pain: beyond fractional anisotropy. NeuroImage 182, 441–455. doi: 10.1016/j.neuroimage.2017.12.021
Ji, G., Liao, W., Chen, F., Zhang, L., and Wang, K. (2017). Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: more than just noise. Sci. Bull. 62, 656–657. doi: 10.1016/j.scib.2017.03.021
Ji, G. J., Liu, T., Li, Y., Liu, P., Sun, J., Chen, X., et al. (2021). Structural correlates underlying accelerated magnetic stimulation in Parkinson’s disease. Hum. Brain Mapp. 42, 1670–1681. doi: 10.1002/hbm.25319
Ji, G. J., Ren, C., Li, Y., Sun, J., Liu, T., Gao, Y., et al. (2018). Regional and network properties of white matter function in Parkinson’s diseaseHum. Brain Mapp. 40, 1253–1263. doi: 10.1002/hbm.24444
Kandilarova, S., Stoyanov, D., Sirakov, N., Maes, M., and Specht, K. (2019). Reduced grey matter volume in frontal and temporal areas in depression: contributions from voxel-based morphometry study. Acta Neuropsychiatr. 31, 252–257. doi: 10.1017/neu.2019.20
Khan, S. A., Keaser, M. L., Meiller, T. F., and Seminowicz, D. A. (2014). Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155, 1472–1480. doi: 10.1016/j.pain.2014.04.022
Kolesar, T. A., Bilevicius, E., Wilson, A. D., and Kornelsen, J. (2019). Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 24:102016. doi: 10.1016/j.nicl.2019.102016
Kraus, C., Castren, E., Kasper, S., and Lanzenberger, R. (2017). Serotonin and neuroplasticity - Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 77, 317–326. doi: 10.1016/j.neubiorev.2017.03.007
Kumral, E., Bayam, F. E., and Özdemir, H. N. (2021). Cognitive and behavioral disorders in patients with precuneal infarcts. Eur. Neurol. 84, 157–167. doi: 10.1159/000513098
Kuner, R., and Kuner, T. (2021). Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev. 101, 213–258. doi: 10.1152/physrev.00040.2019
Liao, X., Mao, C., Wang, Y., Zhang, Q., Cao, D., Seminowicz, D., et al. (2018). Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine 97:e145. doi: 10.1097/MD.0000000000010145
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. b2700 doi: 10.1136/bmj
Liu, X., Lai, H., Li, J., Becker, B., Zhao, Y., Wang, S., et al. (2021). Gray matter structures associated with neuroticism: a meta-analysis of whole-brain voxel-based morphometry studies. Hum. Brain Mapp. 42, 2706–2721. doi: 10.1002/hbm.25395
Mao, C., Wei, L., Zhang, Q., Liao, X., Yang, X., and Zhang, M. (2013). Differences in brain structure in patients with distinct sites of chronic pain: a voxel-based morphometric analysis. Neural Regen. Res. 8, 2981–2990. doi: 10.3969/j.issn.1673-5374.2013.32.001
Mazine, A., Rocha, R. V., El-Hamamsy, I., Ouzounian, M., Yanagawa, B., Bhatt, D. L., et al. (2018). Ross Procedure vs Mechanical Aortic Valve Replacement in Adults: a Systematic Review and Meta-analysis. JAMA Cardiol. 3:978. doi: 10.1001/jamacardio.2018.2946
Mills, S. E. E., Nicolson, K. P., and Smith, B. H. (2019). Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 123, e273–e283. doi: 10.1016/j.bja.2019.03.023
Mole, T. B., MacIver, K., Sluming, V., Ridgway, G. R., and Nurmikko, T. J. (2014). Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. Neuroimage Clin. 5, 28–35. doi: 10.1016/j.nicl.2014.05.014
Neeb, L., Bastian, K., Villringer, K., Israel, H., Reuter, U., and Fiebach, J. B. (2017). Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache 57, 400–416. doi: 10.1111/head.13012
Pan, P. L., Zhong, J. G., Shang, H. F., Zhu, Y. L., Xiao, P. R., Dai, Z. Y., et al. (2015). Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur. J. Pain 19, 1224–1231. doi: 10.1002/ejp.670
Park, J. S., and Jung, Y. W. (2021). Peeled images and sectioned images from real-color volume models of foot. Surg. Radiol. Anat. 43, 37–43. doi: 10.1007/s00276-020-02534-3
Park, L. T., and Zarate, C. J. (2019). Depression in the primary care setting. N. Engl. J. Med. 380, 559–568. doi: 10.1056/NEJMcp1712493
Picó-Pérez, M., Moreira, P. S., de Melo Ferreira, V., Radua, J., Mataix-Cols, D., Sousa, N., et al. (2020). Modality-specific overlaps in brain structure and function in obsessive-compulsive disorder: multimodal meta-analysis of case-control MRI studies. Neurosci. Biobehav. Rev. 112, 83–94. doi: 10.1016/j.neubiorev.2020.01.033
Porreca, F., and Navratilova, E. (2017). Reward, motivation, and emotion of pain and its relief. Pain 158, S43–S49. doi: 10.1097/j.pain.0000000000000798
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Rubia, K., Canales-Rodríguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for Coordinate-Based Meta-Analyses of neuroimaging studies. Front. Psychiatry 5:13. doi: 10.3389/fpsyt.2014.00013
Rizvi, S. J., Gandhi, W., and Salomons, T. (2021). Reward processing as a common diathesis for chronic pain and depression. Neurosci. Biobehav. Rev. 127, 749–760. doi: 10.1016/j.neubiorev.2021.04.033
Robinson, M. E., Craggs, J. G., Price, D. D., Perlstein, W. M., and Staud, R. (2011). Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J. Pain 12, 436–443. doi: 10.1016/j.jpain.2010.10.003
Rotenstein, L. S., Ramos, M. A., Torre, M., Segal, J. B., Peluso, M. J., Guille, C., et al. (2016). Prevalence of depression, depressive symptoms, and suicidal ideation among medical students. JAMA 316:2214. doi: 10.1001/jama.2016.17324
Schmidt-Wilcke, T., Hierlmeier, S., and Leinisch, E. (2010). Altered regional brain morphology in patients with chronic facial pain. Headache 50, 1278–1285. doi: 10.1111/j.1526-4610.2010.01637.x
Schmidt-Wilcke, T., Leinisch, E., Gänbauer, S., Draganski, B., Bogdahn, U., Altmeppen, J., et al. (2006). Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125, 89–97. doi: 10.1016/j.pain.2006.05.004
Seminowicz, D. A., Shpaner, M., Keaser, M. L., Krauthamer, G. M., Mantegna, J., Dumas, J. A., et al. (2013). Cognitive-Behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 14, 1573–1584. doi: 10.1016/j.jpain.2013.07.020
Serrano-Sosa, M., Sampathgiri, K., Spuhler, K. D., DeLorenzo, C., Parsey, R., and Huang, C. (2020). The importance of identifying functional Val158Met polymorphism in catechol-O-Methyltransferase when assessing MRI-based volumetric measurements in major depressive disorder. Brain Imaging Behav. 14, 2762–2770. doi: 10.1007/s11682-019-00225-1
Sheng, J., Liu, S., Wang, Y., Cui, R., and Zhang, X. (2017). The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017, 1–10. doi: 10.1155/2017/9724371
Sheng, L., Ma, H., Shi, Y., Dai, Z., Zhong, J., Chen, F., et al. (2020). Cortical thickness in migraine: a Coordinate-Based Meta-Analysis. Front. Neurosci. 14:600423. doi: 10.3389/fnins.2020.600423
Sheng, L., Zhao, P., Ma, H., Radua, J., Yi, Z., Shi, Y., et al. (2021). Cortical thickness in Parkinson’s disease: a coordinate-based meta-analysis. Medicine 13, 4007–4023. doi: 10.18632/aging.202368
Shi, H., Yuan, C., Dai, Z., Ma, H., and Sheng, L. (2016). Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin. Arthritis Rheum. 46, 330–337. doi: 10.1016/j.semarthrit.2016.06.002
Smallwood, R. F., Laird, A. R., Ramage, A. E., Parkinson, A. L., Lewis, J., Clauw, D. J., et al. (2013). Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J. Pain 14, 663–675. doi: 10.1016/j.jpain.2013.03.001
Tang, Y., Wang, M., Zheng, T., Yuan, F., Yang, H., Han, F., et al. (2020). Grey matter volume alterations in trigeminal neuralgia: a systematic review and meta-analysis of voxel-based morphometry studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 98:109821. doi: 10.1016/j.pnpbp.2019.109821
Wang, Y., Cao, D. Y., Remeniuk, B., Krimmel, S., Seminowicz, D. A., and Zhang, M. (2017). Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 158, 1561–1570. doi: 10.1097/j.pain.0000000000000951
Wise, T., Radua, J., Via, E., Cardoner, N., Abe, O., Adams, T., et al. (2017). Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry 22, 1455–1463. doi: 10.1038/mp.2016.72
Yalcin, I., and Barrot, M. (2014). The anxiodepressive comorbidity in chronic pain. Curr. Opin. Anaesthesiol. 27, 520–527. doi: 10.1097/ACO.0000000000000116
Keywords: chronic pain, depressive symptom, voxel-based morphometry, gray matter volume, meta-analysis
Citation: Ma T, Ji Y-Y, Yan L-F, Lin J-J, Li Z-Y, Wang W, Li J-L and Cui G-B (2022) Gray Matter Volume Abnormality in Chronic Pain Patients With Depressive Symptoms: A Systemic Review and Meta-Analysis of Voxel-Based Morphometry Studies. Front. Neurosci. 16:826759. doi: 10.3389/fnins.2022.826759
Received: 22 February 2022; Accepted: 19 April 2022;
Published: 06 June 2022.
Edited by:
Gong-Jun Ji, Anhui Medical University, ChinaReviewed by:
Drozdstoy Stoyanov Stoyanov, Plovdiv Medical University, BulgariaCopyright © 2022 Ma, Ji, Yan, Lin, Li, Wang, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wang, d2FuZ3dlbkBmbW11LmVkdS5jbg==; Jin-Lian Li, amlubGlhbkBmbW11LmVkdS5jbg==; Guang-Bin Cui, Y3VpZ2J0ZEBmbW11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.