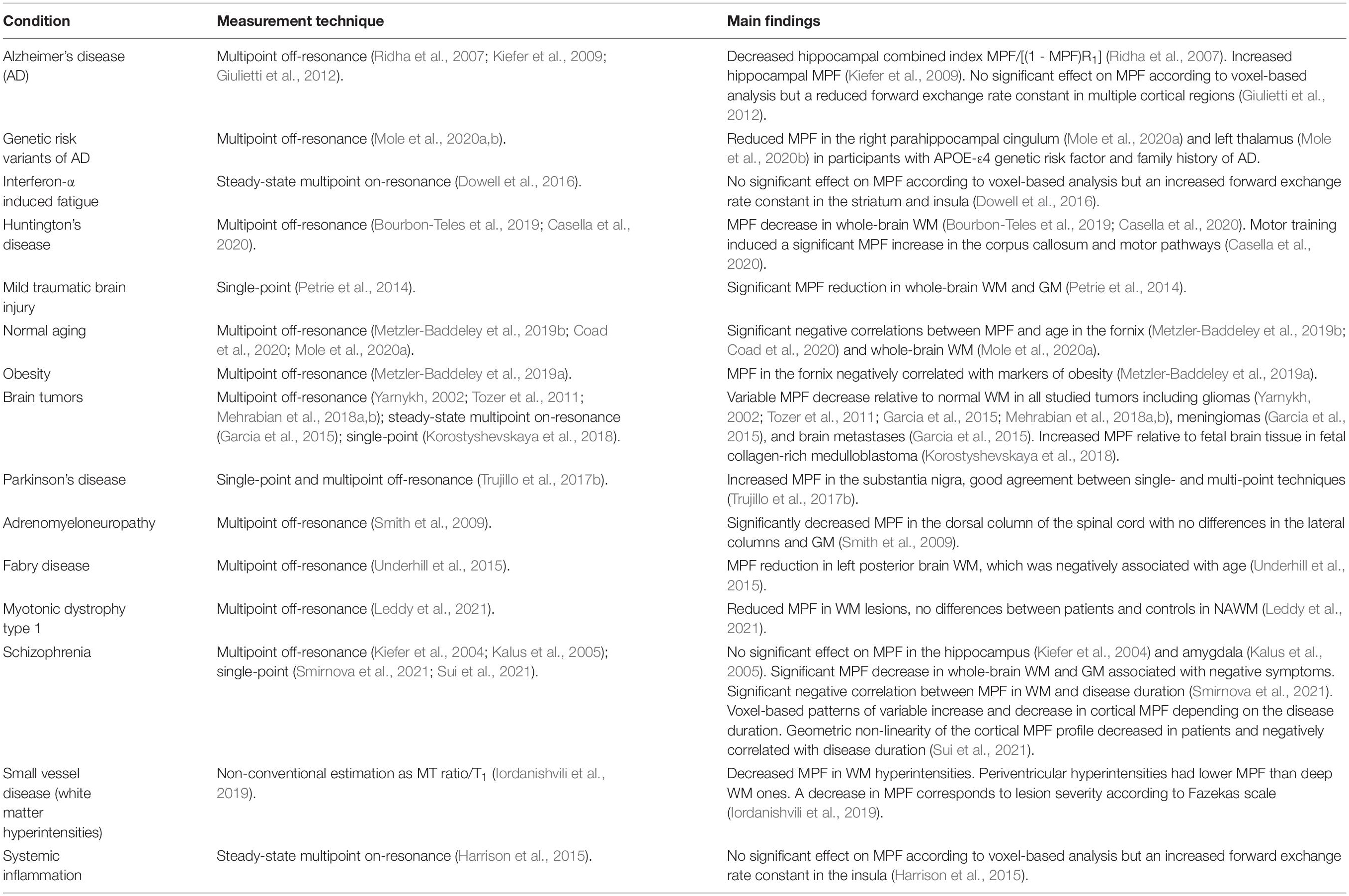
- 1Department of Radiology, University of Washington, Seattle, WA, United States
- 2Laboratory of Neurobiology, Tomsk State University, Tomsk, Russia
Macromolecular proton fraction (MPF) is a quantitative MRI parameter describing the magnetization transfer (MT) effect and defined as a relative amount of protons bound to biological macromolecules with restricted molecular motion, which participate in magnetic cross-relaxation with water protons. MPF attracted significant interest during past decade as a biomarker of myelin. The purpose of this mini review is to provide a brief but comprehensive summary of MPF mapping methods, histological validation studies, and MPF applications in neuroscience. Technically, MPF maps can be obtained using a variety of quantitative MT methods. Some of them enable clinically reasonable scan time and resolution. Recent studies demonstrated the feasibility of MPF mapping using standard clinical MRI pulse sequences, thus substantially enhancing the method availability. A number of studies in animal models demonstrated strong correlations between MPF and histological markers of myelin with a minor influence of potential confounders. Histological studies validated the capability of MPF to monitor both demyelination and re-myelination. Clinical applications of MPF have been mainly focused on multiple sclerosis where this method provided new insights into both white and gray matter pathology. Besides, several studies used MPF to investigate myelin role in other neurological and psychiatric conditions. Another promising area of MPF applications is the brain development studies. MPF demonstrated the capabilities to quantitatively characterize the earliest stage of myelination during prenatal brain maturation and protracted myelin development in adolescence. In summary, MPF mapping provides a technically mature and comprehensively validated myelin imaging technology for various preclinical and clinical neuroscience applications.
Introduction
Investigation of myelin damage, repair, and development in the central nervous system (CNS) for the understanding of pathological mechanisms and treatment monitoring in various neurological and psychiatric conditions using non-invasive imaging methods attracted substantial attention over past two decades (Heath et al., 2018; Piredda et al., 2021). Myelin has been recognized as a key source of brain tissue contrast in MRI due to its strong effect on the nuclear magnetic resonance relaxation times T1 and T2 (Piredda et al., 2021). However, conventional MRI does not allow quantitation of the myelin content changes and lacks specificity to myelin. Recent progress in quantitative MRI methods resulted in the development of several specialized techniques with improved specificity to myelin, which potentially can be used as sources of myelin biomarkers. The underlying biophysical tissue properties affected by myelin include single- or multi-component relaxation, magnetization transfer, anisotropic diffusion, and magnetic susceptibility (Heath et al., 2018; Piredda et al., 2021). Extensive overview of the current myelin imaging methods can be found in recent reviews (Heath et al., 2018; Piredda et al., 2021). Several recently published meta-analyses (Mancini et al., 2020; Lazari and Lipp, 2021; van der Weijden et al., 2021) compared multiple histological validation studies of prospective myelin imaging biomarkers. While there is no evidence of a superiority of any single myelin imaging method to date, the above studies consistently identified the macromolecular proton fraction (MPF) among the parameters enabling the strongest correlations with myelin histology.
MPF is a parameter describing the magnetization transfer (MT) effect and defined as a relative amount of protons bound to biological macromolecules with restricted molecular motion, which participate in magnetic cross-relaxation with free water protons (Yarnykh, 2012). MPF offers several important advantages as a myelin biomarker. Particularly, MPF has fewer physiological confounders as compared to myelin measures based on diffusion, relaxation, and susceptibility. Diffusion indexes associated with myelination, such as radial diffusivity and fractional anisotropy (Song et al., 2002), are affected by microstructural tissue organization and spatial orientation of myelinated fibers (Wheeler-Kingshott and Cercignani, 2009), whereas MPF is independent of these factors (Underhill et al., 2009; Stikov et al., 2011). Relaxation times T1, T2, and T2* and magnetic susceptibility in neural tissues are mainly determined by both myelination and concentration of iron (Stüber et al., 2014; Duyn and Schenck, 2017). As a consequence, a popular myelin biomarker, myelin water fraction measured from multi-component relaxation analysis (MacKay et al., 1994; Deoni et al., 2008; Hwang et al., 2010) is also influenced by the iron content (Birkl et al., 2019). In contrast, MPF is not affected by iron or other paramagnetic ions in tissues (Li et al., 2016; Trujillo et al., 2017a; Yarnykh et al., 2018a). MPF is also independent of magnetic field strength. In vivo MPF measurements in the brain white matter (WM) and gray matter (GM) appeared quantitatively consistent in a wide range of magnetic fields from 0.5 T (Anisimov et al., 2020) to 11.7 T (Naumova et al., 2017). Therefore, MPF provides an attractive approach as a uniform quantitative scale of myelin measurements across a variety of human and animal MRI platforms. Finally, MPF maps can be obtained using routine MRI equipment without modification of original manufacturers’ pulse sequences (Yarnykh et al., 2018b; Korostyshevskaya et al., 2019; Smirnova et al., 2021) thus facilitating clinical translation of this technology.
While MPF mapping has been in use for almost 20 years, the current literature lacks a review specifically focused on MPF as a myelin biomarker. In this review, we sought to provide a brief but comprehensive summary of existing MPF mapping techniques, histological validation studies, and MPF applications in neuroscience. The review is based on PubMed literature search including synonyms of MPF (such as “bound pool fraction,” “bound proton fraction,” “semisolid pool fraction,” “semisolid proton fraction,” and “bound water fraction”) and a similar quantity, pool size ratio (PSR) (Gochberg and Gore, 2003) related to MPF as PSR = MPF/(1−MPF). The term MPF is uniformly used below, although different notations can be found in original publications. The search methodology is detailed in Supplementary Material.
Literature Review
Macromolecular Proton Fraction Mapping Methods
The group of methods allowing reconstruction of MPF maps alone or in combination with other MT parametric maps is commonly referred to as quantitative MT (qMT). The two-pool model of MT (Morrison and Henkelman, 1995) provides a general theoretical framework for all qMT techniques. Within this model, tissue is represented by two proton magnetization reservoirs (free water and macromolecular pool), where the process of magnetization exchange is described by the cross-relaxation rate constant and MPF. Each pool has own transverse and longitudinal relaxation times. We recommend reviews (Henkelman et al., 2001; Sled, 2018) for more details of biophysics of the MT effect. The model parameters can be estimated using the two basic strategies: analysis of a signal behavior in response to off-resonance radiofrequency (RF) saturation with variable offset frequency and power (Z-spectroscopy) and analysis of temporal signal evolution after initial semi-selective perturbation of either water or macromolecular magnetization, which is described by the bi-exponential function (cross-relaxometry). In the contemporary formulation, the two-pool model for tissues includes the super Lorentzian spectral line-shape of the macromolecular pool and Lorentzian line-shape of the free water pool (Morrison and Henkelman, 1995). This model was adapted to the pulsed steady-state saturation regimen, which are achieved using MRI pulse sequences, and enabled the first experimental demonstrations of MPF maps of the human brain along with the maps of other model parameters using multi-parameter voxel-wise fit of Z-spectral images (Sled and Pike, 2001; Yarnykh, 2002). Also, several methods based on the analysis of bi-exponential longitudinal relaxation were reported. The RF pulse excitation schemes in these techniques may vary and may be preferentially targeted at either the free water or macromolecular protons. Within this paradigm, the techniques based on selective inversion of free water magnetization (Gochberg and Gore, 2003, 2007; Dortch et al., 2011; Cronin et al., 2020), stimulated echo preparation (Ropele et al., 2003; Soellinger et al., 2011), and broadband saturation of the macromolecular pool (van Gelderen et al., 2017) were developed. On-resonance saturation of water protons caused by readout RF pulses in fast gradient-echo sequences also can be used as a tool for MPF mapping (Gloor et al., 2008; Garcia et al., 2010; Bayer et al., 2021) based on steady-state models, where the signal is sampled as a function of the excitation pulse duration and flip angle.
The two-pool model contains six independent parameters. None of existing qMT methods can simultaneously measure all of them, and certain assumptions are required. In Z-spectroscopic methods, T1 of the pools are unavailable from the model fit and are mathematically coupled with cross-relaxation parameters. Accordingly, separate T1 mapping is usually performed, and T1 of the free water pool (T1F) is calculated under some assumption about T1 of the macromolecular pool (T1M). The common assumptions include equating them (Yarnykh, 2002) or setting T1M = 1 s (Morrison and Henkelman, 1995). T1 measurements also may need to be corrected for the cross-relaxation effect (Mossahebi et al., 2014). In cross-relaxometric experiments, T2 of the pools cannot be extracted from data and are usually estimated by simulations in order to approximate the initial magnetization state (Gochberg and Gore, 2003; Ropele et al., 2003; Gloor et al., 2008; van Gelderen et al., 2017).
Simultaneous estimation of many parameters (usually 4 or 3) in qMT techniques requires a large number of source images, which result in impractically long data acquisition (>30 min for the human whole-brain examination), particularly in earlier methods (Sled and Pike, 2001; Ramani et al., 2002; Yarnykh, 2002; Gloor et al., 2008; Dortch et al., 2011). Additional B0 and B1 maps are also frequently used for correction of errors caused by field non-uniformities (Sled and Pike, 2001; Gloor et al., 2008; Yarnykh, 2012; Boudreau et al., 2018), thus further increasing examination time. Reduction of the acquisition time can be achieved using several strategies including optimized schedules of variable experimental parameters (Cercignani and Alexander, 2006; Li et al., 2010; Levesque et al., 2011; Battiston et al., 2018; Boudreau and Pike, 2018; Cronin et al., 2020), specialized sequences enabling acquisition of several data points within a single scan (Soellinger et al., 2011; van Gelderen et al., 2017; Battiston et al., 2019), and a reduction of the model dimension by constraining certain parameters or their combinations (Ropele et al., 2003; Yarnykh and Yuan, 2004; Cercignani et al., 2005; Yarnykh, 2012). The last approach resulted in the most radical solution providing MPF estimation from a single spoiled gradient-echo image with off-resonance saturation (Yarnykh, 2012) and a T1 map calculated using the two-point variable flip angle method (Deoni et al., 2005). The single-point method exploits negligible variability of the cross-relaxation rate constant (in the macromolecules-to-water direction), T2 of macromolecular protons, and the product of observed R1 = 1/T1 and T2 of the free water pool in brain tissues (Yarnykh, 2012). These quantities are fixed in the reconstruction algorithm, thus making MPF the only adjustable parameter. Further acceleration of the single-point technique included elimination of a reference image (Yarnykh, 2016), which is usually needed in most qMT techniques, exclusion of B0 mapping due to a negligible effect of B0-related errors (Yarnykh et al., 2020), and a new data-driven algorithm for B1 non-uniformity correction (Yarnykh, 2021), which obviates commonly used in qMT protocols B1 mapping sequences. With these improvements, the entire single-point MPF mapping protocol consists of only three spoiled gradient-echo sequences providing MT-, T1-, and proton-density-weighted images. Acceleration achieved with the single-point MPF mapping method can be converted into either high-resolution acquisition with a generally acceptable for human neuroscience applications scan time (about 15 min for a whole-brain dataset with isotropic 1.25 mm3 resolution; Yarnykh, 2021) or fast clinically targeted protocols (3.5 min for a whole-brain dataset with 1.5 × 1.5 × 5.0 mm3 resolution; Yarnykh et al., 2018b). Due to lesser sensitivity to noise, single-point MPF mapping showed improved reproducibility compared to multi-parameter techniques. Particularly, reported coefficients of variation of repeated measurements in the human brain were 1–2% for the single-point method (Yarnykh et al., 2020; Smirnova et al., 2021) and about 5% for multi-parameter qMT (Davies et al., 2004; Levesque et al., 2010b).
Software availability is an important aspect of future MPF mapping applications. While most studies to date utilized custom-written software tools, we identified two open-source freely available software packages enabling MPF map reconstruction. Quantitative MRI analysis MATLAB (MathWorks, Inc.; Natick, MA, United States) library “qMRLab” (Karakuzu et al., 2020; qMRLab, 2021) allows MPF map reconstruction based on several widely used multi-parameter qMT fit models (Sled and Pike, 2001; Gloor et al., 2008; Li et al., 2010). Specifically targeted at the single-point method (Yarnykh, 2012, 2016) C++ language software “MPF_map” is available from the website (Macromolecular Proton Fraction [MPF], 2022).
Validation in Animal Models
Macromolecular proton fraction measurements were compared with histological myelin assessment in a variety of animal models including normal animal brain (Underhill et al., 2011; Khodanovich et al., 2017) and spinal cord (Dula et al., 2010), cuprizone-induced demyelination in mice (Thiessen et al., 2013; Turati et al., 2015; Khodanovich et al., 2017, 2019; Soustelle et al., 2019, 2021), experimental autoimmune encephalomyelitis in rats (Rausch et al., 2009), lipopolysaccharide-induced focal demyelination in rats (Janve et al., 2013), hexachlorophene-induced intramyelinic edema in rats (Harkins et al., 2013), genetic hypomyelination (Ou et al., 2009a,b; West et al., 2018) and hypermyelination (West et al., 2018) in mice, ischemic stroke in rats (Khodanovich et al., 2018, 2021), spinal cord injury in primates (Wang et al., 2016, 2019; Wu et al., 2020), and demyelinated peripheral nerve ex vivo (Odrobina et al., 2005; Ou et al., 2009a). All studies reported qualitative correspondence of demyelinated anatomical zones with a reduced MPF and/or MPF reduction associated with demyelination relative to a control sample. When histology was quantitatively assessed, MPF demonstrated strong correlations with histological myelin markers characterized by correlation coefficients in a range 0.7–0.99 (Underhill et al., 2011; Janve et al., 2013; Thiessen et al., 2013; Turati et al., 2015; Khodanovich et al., 2017, 2018, 2019, 2021; West et al., 2018; Soustelle et al., 2019, 2021).
Several works focused on validation of MPF as a tool for monitoring re-myelination that is of critical importance for therapeutic intervention studies. An increase in MPF was correlated with re-myelination after withdrawal of cuprizone (Turati et al., 2015; Khodanovich et al., 2019) and replicated restoration of oligodendrogenesis (Khodanovich et al., 2019) in WM and GM of cuprizone-pretreated mice. In the stroke model, MPF showed a unique capability to identify local post-ischemic remyelination, which was unobservable with conventional imaging techniques (Khodanovich et al., 2021).
Animal studies provided important insights into specificity of MPF to myelin and a role of potential confounders. MPF in normal brain tissues is largely independent of the total cell count and axonal density (Underhill et al., 2011). The loss of axons and neurons in ischemic stroke did not affect MPF (Khodanovich et al., 2018). MPF in the ischemic infarct was also found to be insensitive to microglial (Khodanovich et al., 2018) and astroglial (Khodanovich et al., 2021) proliferation, which represent pathological hallmarks of sub-acute and chronic stroke lesions. At the same time, due to dilution of the macromolecular content, MPF is affected by edema (Stanisz et al., 2004; Harkins et al., 2013; Khodanovich et al., 2018), which may cause up to 10–15% overestimation of myelin loss by MPF in acute stroke (Khodanovich et al., 2018). Multi-modal approaches were proposed to correct the effect of water content changes on MPF, particularly using proton density (Giacomini et al., 2009; Mossahebi et al., 2015) or T2 measurements (Khodanovich et al., 2018), but they need more rigorous validation.
Animal models of brain development were not studied as extensively as demyelination models. Nevertheless, several publications indicate utility of MPF for monitoring normal or abnormal myelin development (Samsonov et al., 2012; Lu et al., 2018; Goussakov et al., 2019). Using MPF, these studies demonstrated dramatic distinctions in temporal myelination trajectories between the genetic canine demyelination model and normal animals (Samsonov et al., 2012), widespread effect of ischemia-hypoxia on postnatal myelination in murine WM and GM (Goussakov et al., 2019), and alterations in age-dependent myelin development caused by microbiota in mice (Lu et al., 2018).
Neuroscience Applications
The most common primary demyelinating disease, multiple sclerosis (MS) attracted significant interest as an area of clinical MPF applications. The earliest technical development studies (Sled and Pike, 2001; Yarnykh, 2002) demonstrated that MPF maps clearly depict MS lesions in WM as areas of low MPF. Subsequent reports identified the capability of MPF to detect microscopic demyelination in normal-appearing WM (NAWM) (Davies et al., 2003, 2004; Tozer et al., 2003, 2005; Narayanan et al., 2006; Cercignani et al., 2009; Spano et al., 2010; Yarnykh et al., 2015, 2018a; Bagnato et al., 2020). However, some studies did not find significant NAWM MPF differences between patients and controls (Bagnato et al., 2018; McKeithan et al., 2019), probably due to methodological distinctions in acquisition protocols. MPF provided new insights into lesion pathology in MS enabling studies of demyelination heterogeneity (Levesque et al., 2005; Clarke et al., 2021) and temporal evolution (Giacomini et al., 2009; Levesque et al., 2010a). Post-mortem MPF and histology studies of MS (Schmierer et al., 2007; Bagnato et al., 2018) confirmed good agreement between demyelination and a reduced MPF. The majority of MS studies utilized multipoint techniques with either off-resonance saturation (Sled and Pike, 2001; Yarnykh, 2002; Tozer et al., 2003, 2005; Davies et al., 2003, 2004; Levesque et al., 2005; Narayanan et al., 2006; Schmierer et al., 2007; Cercignani et al., 2009; Giacomini et al., 2009; Levesque et al., 2010a; Spano et al., 2010) or selective inversion-recovery preparation (Bagnato et al., 2018, 2020; McKeithan et al., 2019; Clarke et al., 2021) to obtain MPF maps. The single-point method (Yarnykh, 2012) extended the area of MPF applications to GM (Yarnykh et al., 2015, 2018a) and demonstrated strong associations of GM MPF with MS disability scales and disease phenotype (Yarnykh et al., 2015). The single-point method was also adapted to the spinal cord imaging (Smith et al., 2014) and showed a significant reduction of both NAWM and GM MPF in MS (Smith et al., 2017).
Applications of MPF in other conditions are scarce. The summary of non-MS clinical applications of MPF and key findings is provided in Table 1. Collectively, these studies indicate growing usage of MPF as an exploratory myelin imaging tool in diseases not primarily related to myelin pathology and suggest that MPF mapping adds a new dimension in quantitative clinical neuroimaging.
Brain development is another promising area of MPF applications since myelination is a fundamental component of CNS maturation. Fast single-point MPF mapping was used to investigate the earliest stage of myelin development in the fetal brain and showed close correlations with gestational age in the anatomic regions with known prenatal myelination onset (Yarnykh et al., 2018b; Korostyshevskaya et al., 2019). It was also demonstrated that the single-point method enables reliable measurements of very low MPF values in the fetal brain, which are about fivefold lower than MPF in adult WM (Yarnykh et al., 2018b; Korostyshevskaya et al., 2018, 2019). A recent large-scale study investigated spatiotemporal trajectories of protracted myelin development during adolescence using high-resolution MPF maps and identified that GM myelination is characterized by a significantly faster rate as compared to WM and correlates with puberty (Corrigan et al., 2021). In the technical aspect, MPF maps used in this study demonstrated unprecedented anatomical contrast, as illustrated by the study template in Figure 1.
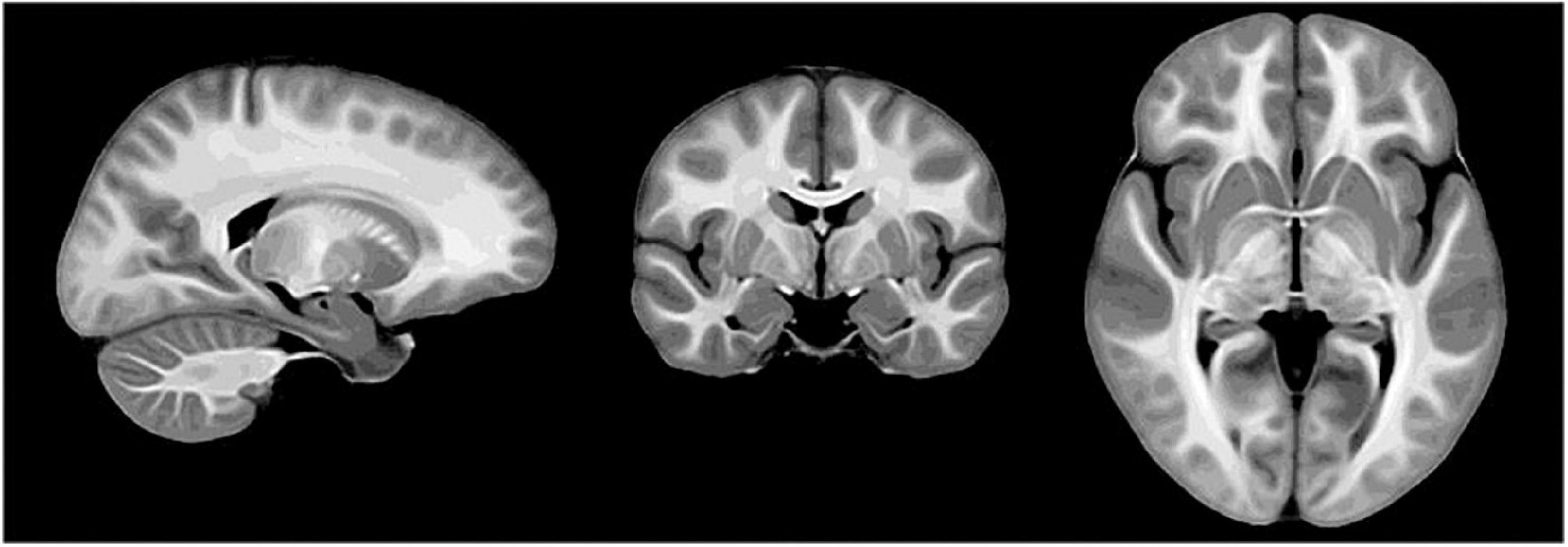
Figure 1. Study-specific template derived from macromolecular proton fraction (MPF) maps of 146 adolescent study subjects (reprinted from Corrigan et al. (2021); free PMC article).
Discussion
Substantial body of evidence confirms high sensitivity and specificity of MPF to myelin. At the same time, early brain development (pre- or postnatal) remains an area where animal model studies could provide an important background for future clinical applications. Water content alterations remain a sole major confounder of MPF according to prior studies. Development of multimodal imaging approaches to mitigate the effect of water content changes would be of crucial value for MPF application in pathological conditions involving significant edema component, such as acute stroke or brain injury. In the perspective of clinical translation, MPF mapping should enable sufficiently fast acquisition and independence of a particular imaging platform. The last requirement can be met, if a technique employs standard pulse sequences provided by most MRI equipment manufacturers. In the current state of development, only two approaches [single-point synthetic-reference method (Yarnykh, 2016) and selective inversion-recovery with optimized sampling and accelerated acquisition (Cronin et al., 2020)] allow designs of MPF mapping protocols based on unmodified sequences and provide whole-brain acquisition in less than 10 min. Since fast MPF mapping employs constrained reconstruction algorithms, consensus is needed regarding the details of the fit procedure and values of constrained parameters to facilitate comparisons between multiple studies. This aspect may involve further model refinements, such as more accurate parameters modeling macromolecular protons (Helms and Hagberg, 2009; van Gelderen et al., 2016, 2017). Finally, multi-platform protocol harmonization and repeatability studies are needed to enable MPF applications in multicenter clinical trials.
Author Contributions
AK and AN contributed to the literature research, and manuscript drafting and formatting according to the journal guidelines. VY overviewed the general concept and design of the study and made final corrections to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors received support from the National Institutes of Health grants R21NS109727 and R24NS104098. AK was also supported by the Russian Science Foundation project No. 21-75-00038.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.819912/full#supplementary-material
References
Anisimov, N. V., Pavlova, O. S., Pirogov, Y. A., and Yarnykh, V. L. (2020). Three-dimensional fast single-point macromolecular proton fraction mapping of the human Brain at 0.5 Tesla. Quant. Imag. Med. Surg. 10, 1441–1449. doi: 10.21037/qims-19-1057
Bagnato, F., Hametner, S., Franco, G., Pawate, S., Sriram, S., Lassmann, H., et al. (2018). Selective inversion recovery quantitative magnetization transfer brain MRI at 7 T: clinical and postmortem validation in multiple sclerosis. J. Neuroimag. 28, 380–388. doi: 10.1111/jon.12511
Bagnato, F., Franco, G., Ye, F., Fan, R., Commiskey, P., Smith, S. A., et al. (2020). Selective inversion recovery quantitative magnetization transfer imaging: toward a 3 T Clinical application in multiple sclerosis. Mult. Scler. 26, 457–467. doi: 10.1177/1352458519833018
Battiston, M., Grussu, F., Ianus, A., Schneider, T., Prados, F., Fairney, J., et al. (2018). An optimized framework for quantitative magnetization transfer imaging of the cervical spinal cord in Vivo. Magn. Reson. Med. 79, 2576–2588. doi: 10.1002/mrm.26909
Battiston, M., Schneider, T., Grussu, F., Yiannakas, M. C., Prados, F., De Angelis, F., et al. (2019). Fast bound pool fraction mapping via steady-state magnetization transfer saturation using single-shot EPI. Magn. Reson. Med. 82, 1025–1040. doi: 10.1002/mrm.27792
Bayer, F. M., Bock, M., Jezzard, P., and Smith, A. K. (2021). Unbiased signal equation for quantitative magnetization transfer mapping in balanced steady-state free precession MRI. Magn. Reson. Med 2021:28940. doi: 10.1002/mrm.28940
Birkl, C., Birkl-Toeglhofer, A. M., Endmayr, V., Hoftberger, R., Kasprian, G., Krebs, C., et al. (2019). The Influence of brain iron on myelin water imaging. Neuroimage 199, 545–552. doi: 10.1016/j.neuroimage.2019.05.042
Bourbon-Teles, J., Bells, S., Jones, D. K., Coulthard, E., Rosser, A., and Metzler-Baddeley, C. (2019). Myelin breakdown in human huntington’s disease: multi-modal evidence from diffusion MRI and Quantitative Magnetization Transfer. Neuroscience 403, 79–92. doi: 10.1016/j.neuroscience.2017.05.042
Boudreau, M., and Pike, G. B. (2018). Sensitivity regularization of the cramer-rao lower bound to minimize b1 nonuniformity effects in quantitative magnetization transfer Imaging. Magn. Reson. Med. 80, 2560–2572. doi: 10.1002/mrm.27337
Boudreau, M., Stikov, N., and Pike, G. B. (2018). B1-Sensitivity analysis of quantitative magnetization transfer imaging. Magn. Reson. Med. 79, 276–285. doi: 10.1002/mrm.26673
Casella, C., Bourbon-Teles, J., Bells, S., Coulthard, E., Parker, G. D., Rosser, A., et al. (2020). Drumming motor sequence training induces apparent myelin remodelling in huntington’s disease: a longitudinal diffusion mri and quantitative magnetization transfer Study. J. Huntingt. Dis. 9, 303–320. doi: 10.3233/JHD-200424
Cercignani, M., and Alexander, D. C. (2006). Optimal acquisition schemes for in vivo quantitative magnetization Transfer MRI. Magn. Reson. Med. 56, 803–810. doi: 10.1002/mrm.21003
Cercignani, M., Symms, M. R., Schmierer, K., Boulby, P. A., Tozer, D. J., Ron, M., et al. (2005). Three-Dimensional quantitative magnetisation transfer imaging of the human brain. Neuroimage 27, 436–441. doi: 10.1016/j.neuroimage.2005.04.031
Cercignani, M., Basile, B., Spano, B., Comanducci, G., Fasano, F., Caltagirone, C., et al. (2009). Investigation of quantitative magnetization transfer parameters of lesions and normal appearing white matter in multiple sclerosis. NMR Biomed. 22, 646–653. doi: 10.1002/nbm.1379
Clarke, M. A., Lakhani, D. A., Wen, S., Gao, S., Smith, S. A., Dortch, R., et al. (2021). Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult. Scler. Relat. Disord. 49:102738. doi: 10.1016/j.msard.2021.102738
Coad, B. M., Craig, E., Louch, R., Aggleton, J. P., Vann, S. D., and Metzler-Baddeley, C. (2020). Precommissural and postcommissural fornix microstructure in healthy aging and Cognition. Brain Neurosci. Adv. 4:99316. doi: 10.1177/2398212819899316
Corrigan, N. M., Yarnykh, V. L., Hippe, D. S., Owen, J. P., Huber, E., Zhao, T. C., et al. (2021). Myelin development in cerebral gray and white matter during adolescence and late childhood. Neuroimage 227:117678. doi: 10.1016/j.neuroimage.2020.117678
Cronin, M. J., Xu, J., Bagnato, F., Gochberg, D. F., Gore, J. C., and Dortch, R. D. (2020). Rapid whole-brain quantitative magnetization transfer imaging using 3D Selective Inversion Recovery Sequences. Magn. Reson. Imag. 68, 66–74. doi: 10.1016/j.mri.2020.01.014
Davies, G. R., Ramani, A., Dalton, C. M., Tozer, D. J., Wheeler-Kingshott, C. A., Barker, G. J., et al. (2003). Preliminary magnetic resonance study of the macromolecular proton fraction in white matter: a potential marker of Myelin? Mult. Scler. 9, 246–249. doi: 10.1191/1352458503ms911oa
Davies, G. R., Tozer, D. J., Cercignani, M., Ramani, A., Dalton, C. M., Thompson, A. J., et al. (2004). Estimation of the macromolecular proton fraction and bound pool T2 in Multiple Sclerosis. Mult. Scler. 10, 607–613. doi: 10.1191/1352458504ms1105oa
Deoni, S. C. L., Peters, T. M., and Rutt, B. K. (2005). High-Resolution T1 and T2 Mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn. Reson. Med. 53, 237–241. doi: 10.1002/mrm.20314
Deoni, S. C. L., Rutt, B. K., Arun, T., Pierpaoli, C., and Jones, D. K. (2008). Gleaning Multicomponent T1 and T2 information from steady-state imaging data. Magn. Reson. Med. 60, 1372–1387. doi: 10.1002/mrm.21704
Dortch, R. D., Li, K., Gochberg, D. F., Welch, E. B., Dula, A. N., Tamhane, A. A., et al. (2011). Quantitative magnetization transfer imaging in human brain at 3 T via Selective Inversion Recovery. Magn. Reson. Med. 66, 1346–1352. doi: 10.1002/mrm.22928
Dowell, N. G., Cooper, E. A., Tibble, J., Voon, V., Critchley, H. D., Cercignani, M., et al. (2016). acute changes in striatal microstructure predict the development of interferon-Alpha Induced Fatigue. Biol. Psychiatry 79, 320–328. doi: 10.1016/j.biopsych.2015.05.015
Dula, A. N., Gochberg, D. F., Valentine, H. L., Valentine, W. M., and Does, M. D. (2010). Multiexponential T2, magnetization transfer, and quantitative histology in white Matter Tracts of rat Spinal Cord. Magn. Reson. Med. 63, 902–909. doi: 10.1002/mrm.22267
Duyn, J. H., and Schenck, J. (2017). Contributions to Magnetic Susceptibility of Brain Tissue. NMR Biomed. 30:3546. doi: 10.1002/nbm.3546
Garcia, M., Gloor, M., Wetzel, S. G., Radue, E. W., Scheffler, K., and Bieri, O. (2010). Characterization of normal appearing brain structures using high-resolution quantitative magnetization transfer steady-state free precession imaging. Neuroimage 52, 532–537. doi: 10.1016/j.neuroimage.2010.04.242
Garcia, M., Gloor, M., Bieri, O., Radue, E.-W., Lieb, J. M., Cordier, D., et al. (2015). Imaging of primary brain tumors and metastases with fast quantitative 3-dimensional Magnetization Transfer. J. Neuroimag. 25, 1007–1014. doi: 10.1111/jon.12222
Giacomini, P. S., Levesque, I. R., Ribeiro, L., Narayanan, S., Francis, S. J., Pike, G. B., et al. (2009). Measuring demyelination and remyelination in acute multiple sclerosis lesion Voxels. Arch. Neurol. 66, 375–381. doi: 10.1001/archneurol.2008.578
Giulietti, G., Bozzali, M., Figura, V., Spanò, B., Perri, R., Marra, C., et al. (2012). Quantitative magnetization transfer provides information complementary to grey matter atrophy in alzheimer’s Disease Brains. Neuroimage 59, 1114–1122. doi: 10.1016/j.neuroimage.2011.09.043
Gloor, M., Scheffler, K., and Bieri, O. (2008). Quantitative magnetization transfer imaging using balanced SSFP. Magn. Reson. Med. 60, 691–700. doi: 10.1002/mrm.21705
Gochberg, D., and Gore, J. (2003). Quantitative imaging of magnetization transfer using an inversion recovery sequence. Magn. Reson. Med. 49, 501–505. doi: 10.1002/mrm.10386
Gochberg, D., and Gore, J. (2007). Quantitative magnetization transfer imaging via selective inversion recovery with short repetition Times. Magn. Reson. Med. 57, 437–441. doi: 10.1002/mrm.21143
Goussakov, I., Synowiec, S., Yarnykh, V., and Drobyshevsky, A. (2019). Immediate and delayed decrease of long term potentiation and memory deficits after neonatal intermittent Hypoxia. Int. J. Dev. Neurosci. 74, 27–37. doi: 10.1016/j.ijdevneu.2019.03.001
Harkins, K. D., Valentine, W. M., Gochberg, D. F., and Does, M. D. (2013). In-Vivo Multi-Exponential T2, magnetization transfer and quantitative histology in a rat model of intramyelinic Edema. Neuroimage Clin. 2, 810–817. doi: 10.1016/j.nicl.2013.06.007
Harrison, N. A., Cooper, E., Dowell, N. G., Keramida, G., Voon, V., Critchley, H. D., et al. (2015). Quantitative magnetization transfer imaging as a biomarker for effects of systemic Inflammation on the Brain. Biol. Psychiatry 78, 49–57. doi: 10.1016/j.biopsych.2014.09.023
Heath, F., Hurley, S. A., Johansen-Berg, H., and Sampaio-Baptista, C. (2018). Advances in Noninvasive Myelin Imaging. Dev. Neurobiol. 78, 136–151. doi: 10.1002/dneu.22552
Helms, G., and Hagberg, G. E. (2009). In Vivo quantification of the bound pool T1 in human white matter using the binary spin-bath model of progressive magnetization transfer Saturation. Phys. Med. Biol. 54, N529–N540. doi: 10.1088/0031-9155/54/23/N01
Henkelman, R. M., Stanisz, G. J., and Graham, S. J. (2001). Magnetization Transfer in MRI: a Review. NMR Biomed. 14, 57–64. doi: 10.1002/nbm.683
Hwang, D., Kim, D.-H., and Du, Y. P. (2010). In Vivo multi-slice mapping of myelin water Content Using T2* Decay. Neuroimage 52, 198–204. doi: 10.1016/j.neuroimage.2010.04.023
Iordanishvili, E., Schall, M., Loução, R., Zimmermann, M., Kotetishvili, K., Shah, N. J., et al. (2019). Quantitative MRI of cerebral white matter hyperintensities: a new approach towards understanding the underlying pathology. Neuroimage 202:116077. doi: 10.1016/j.neuroimage.2019.116077
Janve, V. A., Zu, Z., Yao, S. Y., Li, K., Zhang, F. L., Wilson, K. J., et al. (2013). The Radial Diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of Type III Multiple Sclerosis (MS) Lesions. Neuroimage 74, 298–305. doi: 10.1016/j.neuroimage.2013.02.034
Karakuzu, A., Boudreau, M., Duval, T., Boshkovski, T., Leppert, I. R., and Cabana, J. F. (2020). qMRLab: Quantitative MRI Analysis, under one Umbrella. J. Open Sour. Softw. 5:2343. doi: 10.21105/joss.02343
Kalus, P., Slotboom, J., Gallinat, J., Wiest, R., Ozdoba, C., Federspiel, A., et al. (2005). The Amygdala in schizophrenia: a trimodal magnetic resonance imaging Study. Neurosci. Lett. 375, 151–156. doi: 10.1016/j.neulet.2004.11.004
Khodanovich, M. Y., Sorokina, I. V., Glazacheva, V. Y., Akulov, A. E., Nemirovich-Danchenko, N. M., Romashchenko, A. V., et al. (2017). Histological validation of fast macromolecular proton fraction mapping as a quantitative myelin imaging method in the cuprizone demyelination Model. Sci. Rep. 7:46686. doi: 10.1038/srep46686
Khodanovich, M. Y., Kisel, A. A., Akulov, A. E., Atochin, D. N., Kudabaeva, M. S., Glazacheva, V. Y., et al. (2018). Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction Mapping. J. Cereb. Blood Flow Metab. 38, 919–931. doi: 10.1177/0271678X18755203
Khodanovich, M., Pishchelko, A., Glazacheva, V., Pan, E., Akulov, A., Svetlik, M., et al. (2019). Quantitative imaging of white and gray matter remyelination in the cuprizone demyelination model using the macromolecular proton fraction. Cells 8:1204. doi: 10.3390/cells8101204
Khodanovich, M. Y., Gubskiy, I. L., Kudabaeva, M. S., Namestnikova, D. D., Kisel, A. A., Anan’ina, T. V., et al. (2021). Long-term monitoring of chronic demyelination and remyelination in a rat ischemic stroke model using macromolecular proton fraction Mapping. J. Cereb. Blood Flow Metab. 41, 2856–2869. doi: 10.1177/0271678X211020860
Kiefer, C., Slotboom, J., Buri, C., Gralla, J., Remonda, L., Dierks, T., et al. (2004). Differentiating hippocampal subregions by means of quantitative magnetization transfer and relaxometry: preliminary Results. Neuroimage 23, 1093–1099. doi: 10.1016/j.neuroimage.2004.07.066
Kiefer, C., Brockhaus, L., Cattapan-Ludewig, K., Ballinari, P., Burren, Y., Schroth, G., et al. (2009). Multi-Parametric classification of alzheimer’s disease and mild cognitive impairment: the impact of quantitative magnetization transfer MR Imaging. Neuroimage 48, 657–667. doi: 10.1016/j.neuroimage.2009.07.005
Korostyshevskaya, A. M., Savelov, A. A., Papusha, L. I., Druy, A. E., and Yarnykh, V. L. (2018). Congenital medulloblastoma: fetal and postnatal longitudinal observation with Quantitative MRI. Clin. Imag. 52, 172–176. doi: 10.1016/j.clinimag.2018.06.001
Korostyshevskaya, A. M., Prihodko, I., Savelov, A. A., and Yarnykh, V. L. (2019). Direct comparison between apparent diffusion coefficient and macromolecular proton fraction as quantitative biomarkers of the human Fetal Brain Maturation. J. Magn. Reson. Imag. 50, 52–61. doi: 10.1002/jmri.26635
Lazari, A., and Lipp, I. (2021). Can MRI measure myelin? systematic review, qualitative assessment, and meta-analysis of studies validating microstructural imaging with myelin Histology. Neuroimage 230:117744. doi: 10.1016/j.neuroimage.2021.117744
Leddy, S., Serra, L., Esposito, D., Vizzotto, C., Giulietti, G., Silvestri, G., et al. (2021). Lesion distribution and substrate of white matter damage in myotonic dystrophy type 1: comparison with Multiple Sclerosis. Neuroimag. Clin. 29:102562. doi: 10.1016/j.nicl.2021.102562
Levesque, I., Sled, J. G., Narayanan, S., Santos, A. C., Brass, S. D., Francis, S. J., et al. (2005). The role of edema and demyelination in chronic T1 Black Holes: a Quantitative Magnetization Transfer Study. J. Magn. Reson. Imag. 21, 103–110. doi: 10.1002/jmri.20231
Levesque, I. R., Giacomini, P. S., Narayanan, S., Ribeiro, L. T., Sled, J. G., Arnold, D. L., et al. (2010a). quantitative magnetization transfer and myelin water imaging of the evolution of Acute Multiple Sclerosis Lesions. Magn. Reson. Med. 63, 633–640. doi: 10.1002/mrm.22244
Levesque, I. R., Sled, J. G., Narayanan, S., Giacomini, P. S., Ribeiro, L. T., Arnold, D. L., et al. (2010b). Reproducibility of quantitative magnetization-transfer imaging parameters from repeated measurements. Magn. Reson. Med. 64, 391–400. doi: 10.1002/mrm.22350
Levesque, I. R., Sled, J. G., and Pike, G. B. (2011). Iterative optimization method for design of quantitative magnetization transfer imaging experiments. Magn. Reson. Med. 66, 635–643. doi: 10.1002/mrm.23071
Li, K., Zu, Z., Xu, J., Janve, V. A., Gore, J. C., Does, M. D., et al. (2010). Optimized inversion recovery sequences for quantitative t1 and magnetization transfer imaging. Magn. Reson. Med. 64, 491–500. doi: 10.1002/mrm.22440
Li, K., Li, H., Zhang, X. Y., Stokes, A. M., Jiang, X., Kang, H., et al. (2016). Influence of water compartmentation and heterogeneous relaxation on quantitative magnetization transfer imaging in rodent Brain Tumors. Magn. Reson. Med. 76, 635–644. doi: 10.1002/mrm.25893
Lu, J., Synowiec, S., Lu, L., Yu, Y., Bretherick, T., Takada, S., et al. (2018). Microbiota influence the development of the brain and behaviors in C57BL/6J Mice. PLoS One 13:e0201829. doi: 10.1371/journal.pone.0201829
MacKay, A., Whittall, K., Adler, J., Li, D., Paty, D., and Graeb, D. (1994). In Vivo visualization of myelin water in brain by magnetic resonance. Magn. Reson. Med. 31, 673–677. doi: 10.1002/mrm.1910310614
Macromolecular Proton Fraction [MPF] (2022). Quantitative Myelin Mapping Technique for Neuroscience. Available online at: https://www.macromolecularmri.org (Accessed January 13, 2022)
Mancini, M., Karakuzu, A., Cohen-Adad, J., Cercignani, M., Nichols, T. E., and Stikov, N. (2020). An Interactive Meta-Analysis of MRI Biomarkers of Myelin. Elife 9:e61523. doi: 10.7554/eLife.61523
McKeithan, L. J., Lyttle, B. D., Box, B. A., O’Grady, K. P., Dortch, R. D., Conrad, B. N., et al. (2019). 7 T Quantitative Magnetization Transfer (qMT) of cortical gray matter in multiple sclerosis correlates with cognitive impairment. Neuroimage 203:116190. doi: 10.1016/j.neuroimage.2019.116190
Mehrabian, H., Lam, W. W., Myrehaug, S., Sahgal, A., and Stanisz, G. J. (2018a). Glioblastoma (GBM) Effects on Quantitative MRI of Contralateral Normal Appearing White Matter. J. Neurooncol. 139, 97–106. doi: 10.1007/s11060-018-2846-0
Mehrabian, H., Myrehaug, S., Soliman, H., Sahgal, A., and Stanisz, G. J. (2018b). Quantitative magnetization transfer in monitoring glioblastoma (GBM) Response to Therapy. Sci. Rep. 8:2475. doi: 10.1038/s41598-018-20624-6
Metzler-Baddeley, C., Mole, J. P., Leonaviciute, E., Sims, R., Kidd, E. J., and Ertefai, B. (2019a). Sex-specific effects of central adiposity and inflammatory markers on limbic Microstructure. Neuroimage 189, 793–803. doi: 10.1016/j.neuroimage.2019.02.007
Metzler-Baddeley, C., Mole, J. P., Sims, R., Fasano, F., Evans, J., Jones, D. K., et al. (2019b). Fornix white matter glia damage causes hippocampal gray matter damage during age-dependent Limbic Decline. Sci. Rep. 9:1060. doi: 10.1038/s41598-018-37658-5
Mole, J. P., Fasano, F., Evans, J., Sims, R., Hamilton, D. A., Kidd, E., et al. (2020a). Genetic risk of dementia modifies obesity effects on white matter myelin in cognitively healthy adults. Neurobiol. Aging 94, 298–310. doi: 10.1016/j.neurobiolaging.2020.06.014
Mole, J. P., Fasano, F., Evans, J., Sims, R., Kidd, E., Aggleton, J. P., et al. (2020b). APOE-ε4-Related differences in left thalamic microstructure in cognitively healthy Adults. Sci. Rep. 10:19787. doi: 10.1038/s41598-020-75992-9
Morrison, C., and Henkelman, R. M. (1995). A model for magnetization transfer in tissues. Magn. Reson. Med. 33, 475–482. doi: 10.1002/mrm.1910330404
Mossahebi, P., Yarnykh, V. L., and Samsonov, A. (2014). Analysis and Correction of Biases in Cross-Relaxation MRI due to Biexponential Longitudinal Relaxation. Magn. Reson. Med. 71, 830–838. doi: 10.1002/mrm.24677
Mossahebi, P., Alexander, A. L., Field, A. S., and Samsonov, A. A. (2015). Removal of cerebrospinal fluid partial volume effects in quantitative magnetization transfer imaging using a three-pool model with nonexchanging water component. Magn. Reson. Med. 74, 1317–1326. doi: 10.1002/mrm.25516
Narayanan, S., Francis, S. J., Sled, J. G., Santos, A. C., Antel, S., Levesque, I., et al. (2006). Axonal injury in the cerebral normal-appearing white matter of patients with multiple sclerosis is related to concurrent demyelination in lesions but not to concurrent demyelination in normal-appearing white matter. Neuroimage 29, 637–642. doi: 10.1016/j.neuroimage.2005.07.017
Naumova, A. V., Akulov, A. E., Khodanovich, M. Y., and Yarnykh, V. L. (2017). High-resolution three-dimensional macromolecular proton fraction mapping for quantitative neuroanatomical imaging of the rodent brain in ultra-high magnetic Fields. Neuroimage 147, 985–993. doi: 10.1016/j.neuroimage.2016.09.036
Odrobina, E., Lam, T., Pun, T., Midha, R., and Stanisz, G. (2005). MR Properties of Excised Neural Tissue Following Experimentally Induced Demyelination. NMR Biomed. 18, 277–284. doi: 10.1002/nbm.951
Ou, X., Sun, S. W., Liang, H. F., Song, S. K., and Gochberg, D. F. (2009a). Quantitative magnetization transfer measured pool-size ratio reflects optic nerve myelin content in Ex Vivo Mice. Magn. Reson. Med. 61, 364–371. doi: 10.1002/mrm.21850
Ou, X., Sun, S. W., Liang, H. F., Song, S. K., and Gochberg, D. F. (2009b). The MT Pool Size Ratio and the DTI Radial diffusivity may reflect the myelination in shiverer and Control Mice. NMR Biomed. 22, 480–487. doi: 10.1002/nbm.1358
Petrie, E. C., Cross, D. J., Yarnykh, V. L., Richards, T., Martin, N. M., Pagulayan, K., et al. (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in iraq and afghanistan war Veterans. J. Neurotrauma 31, 425–436. doi: 10.1089/neu.2013.2952
Piredda, G. F., Hilbert, T., Thiran, J. P., and Kober, T. (2021). Probing Myelin Content of the Human Brain with MRI: a Review. Magn. Reson. Med. 85, 627–652. doi: 10.1002/mrm.28509
qMRLab (2021). Quantitative MRI. Under one Umbrella. Available online at: http://qmrlab.org (Accessed January 13, 2022)
Ramani, A., Dalton, C., Miller, D. H., Tofts, P. S., and Barker, G. J. (2002). Precise Estimate of Fundamental In-Vivo MT parameters in human brain in clinically feasible times. Magn. Reson. Imag. 20, 721–731. doi: 10.1016/s0730-725x(02)00598-2
Rausch, M., Tofts, P. S., Lervik, P., Walmsley, A. R., Mir, A., Schubart, A., et al. (2009). Characterization of white matter damage in animal models of multiple sclerosis by magnetization transfer ratio and quantitative mapping of the apparent bound proton Fraction F. Mult. Scler. 15, 16–27. doi: 10.1177/1352458508096006
Ridha, B. H., Tozer, D. J., Symms, M. R., Stockton, K. C., Lewis, E. B., Siddique, M. M., et al. (2007). Quantitative magnetization transfer imaging in Alzheimer Disease. Radiology 244, 832–837. doi: 10.1148/radiol.2443061128
Ropele, S., Seifert, T., Enzinger, C., and Fazekas, F. (2003). Method for Quantitative Imaging of the Macromolecular 1H Fraction in Tissues. Magn. Reson. Med. 49, 864–871. doi: 10.1002/mrm.10427
Samsonov, A., Alexander, A. L., Mossahebi, P., Wu, Y. C., Duncan, I. D., and Field, A. S. (2012). Quantitative MR imaging of two-pool magnetization transfer model parameters in myelin mutant shaking Pup. Neuroimage 62, 1390–1398. doi: 10.1016/j.neuroimage.2012.05.077
Schmierer, K., Tozer, D. J., Scaravilli, F., Altmann, D. R., Barker, G. J., Tofts, P. S., et al. (2007). Quantitative magnetization transfer imaging in postmortem multiple sclerosis Brain. J. Magn. Reson. Imaging 26, 41–51. doi: 10.1002/jmri.20984
Sled, J. G. (2018). Modelling and interpretation of magnetization transfer imaging in the Brain. Neuroimage 182, 128–135. doi: 10.1016/j.neuroimage.2017.11.065
Sled, J. G., and Pike, G. B. (2001). Quantitative imaging of magnetization transfer exchange and relaxation properties In Vivo Using MRI. Magn. Reson. Med. 46, 923–931. doi: 10.1002/mrm.1278
Smirnova, L. P., Yarnykh, V. L., Parshukova, D. A., Kornetova, E. G., Semke, A. V., Usova, A. V., et al. (2021). Global hypomyelination of the brain white and gray matter in schizophrenia: quantitative imaging using macromolecular proton Fraction. Transl. Psychiatry 11:365. doi: 10.1038/s41398-021-01475-8
Smith, S. A., Golay, X., Fatemi, A., Mahmood, A., Raymond, G. V., Moser, H. W., et al. (2009). Quantitative magnetization transfer characteristics of the human cervical spinal cord in vivo: application to adrenomyeloneuropathy. Magn. Reson. Med. 61, 22–27. doi: 10.1002/mrm.21827
Smith, A. K., Dortch, R. D., Dethrage, L. M., and Smith, S. A. (2014). Rapid, High-resolution quantitative magnetization transfer MRI of the Human Spinal Cord. Neuroimage 95, 106–116. doi: 10.1016/j.neuroimage.2014.03.005
Smith, A. K., By, S., Lyttle, B. D., Dortch, R. D., Box, B. A., Mckeithan, L. J., et al. (2017). Evaluating single-point quantitative magnetization transfer in the cervical spinal cord: application to multiple sclerosis. Neuroimage Clin. 16, 58–65. doi: 10.1016/j.nicl.2017.07.010
Soellinger, M., Langkammer, C., Seifert-Held, T., Fazekas, F., and Ropele, S. (2011). Fast bound pool fraction mapping using stimulated Echoes. Magn. Reson. Med. 66, 717–724. doi: 10.1002/mrm.22846
Song, S.-K., Sun, S.-W., Ramsbottom, M. J., Chang, C., Russell, J., and Cross, A. H. (2002). Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage 17, 1429–1436. doi: 10.1006/nimg.2002.1267
Soustelle, L., Antal, M. C., Lamy, J., Rousseau, F., Armspach, J. P., and Loureiro de Sousa, P. (2019). Correlations of Quantitative MRI Metrics with Myelin Basic Protein (MBP) Staining in a Murine Model of Demyelination. NMR Biomed. 32:e4116. doi: 10.1002/nbm.4116
Soustelle, L., Antal, M. C., Lamy, J., Harsan, L. A., and Loureiro de Sousa, P. (2021). Determination of optimal parameters for 3D Single-point macromolecular proton fraction mapping at 7 t in healthy and demyelinated mouse Brain. Magn. Reson. Med. 85, 369–379. doi: 10.1002/mrm.28397
Spano, B., Cercignani, M., Basile, B., Romano, S., Mannu, R., Centonze, D., et al. (2010). Multiparametric MR Investigation of the Motor pyramidal system in patients with ‘Truly Benign’. Multiple Sclerosis. Mult. Scler. 16, 178–188. doi: 10.1177/1352458509356010
Stanisz, G. J., Webb, S., Munro, C. A., Pun, T., and Midha, R. (2004). MR Properties of Excised neural tissue following experimentally induced inflammation. Magn. Reson. Med. 51, 473–479. doi: 10.1002/mrm.20008
Stikov, N., Perry, L. M., Mezer, A., Rykhlevskaia, E., Wandell, B. A., Pauly, J. M., et al. (2011). Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. Neuroimage 54, 1112–1121. doi: 10.1016/j.neuroimage.2010.08.068
Stüber, C., Morawski, M., Schäfer, A., Labadie, C., Wähnert, M., Leuze, C., et al. (2014). Myelin and iron concentration in the human brain: a quantitative study of mri contrast. Neuroimage 93, 95–106. doi: 10.1016/j.neuroimage.2014.02.026
Sui, Y. V., Bertisch, H., Lee, H.-H., Storey, P., Babb, J. S., Goff, D. C., et al. (2021). Quantitative macromolecular proton fraction mapping reveals altered cortical myelin profile in schizophrenia spectrum Disorders. Cereb. Cortex Commun. 2:tgab015. doi: 10.1093/texcom/tgab015
Thiessen, J. D., Zhang, Y., Zhang, H., Wang, L., Buist, R., Del Bigio, M. R., et al. (2013). Quantitative MRI and ultrastructural examination of the cuprizone mouse model of Demyelination. NMR Biomed. 26, 1562–1581. doi: 10.1002/nbm.2992
Tozer, D., Ramani, A., Barker, G. J., Davies, G. R., Miller, D. H., and Tofts, P. S. (2003). Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn. Reson. Med. 50, 83–91. doi: 10.1002/mrm.10514
Tozer, D. J., Davies, G. R., Altmann, D. R., Miller, D. H., and Tofts, P. S. (2005). Correlation of apparent myelin measures obtained in multiple sclerosis patients and controls from magnetization transfer and multicompartmental T2 analysis. Magn. Reson. Med. 53, 1415–1422. doi: 10.1002/mrm.20479
Tozer, D. J., Rees, J. H., Benton, C. E., Waldman, A. D., Jager, H. R., and Tofts, P. S. (2011). Quantitative magnetisation transfer imaging in glioma: preliminary Results. NMR Biomed. 24, 492–498. doi: 10.1002/nbm.1614
Trujillo, P., Summers, P. E., Ferrari, E., Zucca, F. A., Sturini, M., Mainardi, L. T., et al. (2017a). Contrast mechanisms associated with neuromelanin-MRI. Magn. Reson. Med. 78, 1790–1800. doi: 10.1002/mrm.26584
Trujillo, P., Summers, P. E., Smith, A. K., Smith, S. A., Mainardi, L. T., Cerutti, S., et al. (2017b). Pool Size ratio of the substantia nigra in parkinson’s disease derived from two different quantitative magnetization transfer approaches. Neuroradiology 59, 1251–1263. doi: 10.1007/s00234-017-1911-2
Turati, L., Moscatelli, M., Mastropietro, A., Dowell, N. G., Zucca, I., Erbetta, A., et al. (2015). In Vivo quantitative magnetization transfer imaging correlates with histology during de- and remyelination in cuprizone-treated Mice. NMR Biomed. 28, 327–337. doi: 10.1002/nbm.3253
Underhill, H. R., Yuan, C., and Yarnykh, V. L. (2009). Direct quantitative comparison between cross-relaxation imaging and diffusion tensor imaging of the human Brain at 3.0 T. Neuroimage 47, 1568–1578. doi: 10.1016/j.neuroimage.2009.05.075
Underhill, H. R., Rostomily, R. C., Mikheev, A. M., Yuan, C., and Yarnykh, V. L. (2011). Fast bound pool fraction imaging of the in vivo rat brain: association with myelin content and validation in the C6 Glioma Model. Neuroimage 54, 2052–2065. doi: 10.1016/j.neuroimage.2010.10.065
Underhill, H. R., Golden-Grant, K., Garrett, L. T., Uhrich, S., Zielinski, B. A., and Scott, C. R. (2015). Detecting the effects of fabry disease in the adult human brain with diffusion tensor imaging and fast bound-pool fraction imaging. J. Magn. Reson. Imaging 42, 1611–1622. doi: 10.1002/jmri.24952
van der Weijden, C. W. J., García, D. V., Borra, R. J. H., Thurner, P., Meilof, J. F., van Laar, P. J., et al. (2021). Myelin Quantification with MRI: a Systematic Review of Accuracy and Reproducibility. Neuroimage 226:117561. doi: 10.1016/j.neuroimage.2020.117561
van Gelderen, P., Jiang, X., and Duyn, J. H. (2016). Effects of Magnetization Transfer on T1 contrast in human brain white matter. Neuroimage 128, 85–95. doi: 10.1016/j.neuroimage.2015.12.032
van Gelderen, P., Jiang, X., and Duyn, J. H. (2017). Rapid measurement of brain macromolecular proton fraction with transient saturation Transfer MRI. Magn. Reson. Med. 77, 2174–2185. doi: 10.1002/mrm.26304
Wang, F., Li, K., Mishra, A., Gochberg, D., Min Chen, L., and Gore, J. C. (2016). Longitudinal assessment of spinal cord injuries in nonhuman primates with quantitative magnetization Transfer. Magn. Reson. Med. 75, 1685–1696. doi: 10.1002/mrm.25725
Wang, F., Wu, T. L., Li, K., Chen, L. M., and Gore, J. C. (2019). Spatiotemporal trajectories of quantitative magnetization transfer measurements in injured spinal cord using Simplified Acquisitions. Neuroimag. Clin. 23:101921. doi: 10.1016/j.nicl.2019.101921
West, K. L., Kelm, N. D., Carson, R. P., Gochberg, D. F., Ess, K. C., and Does, M. D. (2018). Myelin Volume Fraction Imaging with MRI. Neuroimage 182, 511–521. doi: 10.1016/j.neuroimage.2016.12.067
Wheeler-Kingshott, C. A., and Cercignani, M. (2009). About “Axial” and “Radial” Diffusivities. Magn. Reson. Med. 61, 1255–1260. doi: 10.1002/mrm.21965
Wu, T. L., Byun, N. E., Wang, F., Mishra, A., Janve, V. A., Chen, L. M., et al. (2020). Longitudinal assessment of recovery after spinal cord injury with behavioral measures and diffusion, quantitative magnetization transfer and functional magnetic resonance imaging. NMR Biomed. 33:e4216. doi: 10.1002/nbm.4216
Yarnykh, V. L. (2002). Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human mri: theory and clinical applications. Magn. Reson. Med. 47, 929–939. doi: 10.1002/mrm.10120
Yarnykh, V. L. (2012). Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn. Reson. Med. 68, 166–178. doi: 10.1002/mrm.23224
Yarnykh, V. L. (2016). Time-Efficient, high-resolution, whole brain three-dimensional macromolecular proton fraction mapping. Magn. Reson. Med. 75, 2100–2106. doi: 10.1002/mrm.25811
Yarnykh, V. L. (2021). Data-Driven Retrospective Correction of B1 Field Inhomogeneity in Fast Macromolecular Proton Fraction and R1 Mapping. IEEE Trans. Med. Imag. 2021:3088258. doi: 10.1109/TMI.2021.3088258
Yarnykh, V. L., and Yuan, C. (2004). Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human Brain. Neuroimage 23, 409–424. doi: 10.1016/j.neuroimage.2004.04.029
Yarnykh, V. L., Bowen, J. D., Samsonov, A., Repovic, P., Mayadev, A., Qian, P., et al. (2015). Fast whole-brain three-dimensional macromolecular proton fraction mapping in multiple Sclerosis. Radiology 274, 210–220. doi: 10.1148/radiol.14140528
Yarnykh, V. L., Kisel, A. A., and Khodanovich, M. Y. (2020). Scan-Rescan Repeatability and Impact of B0 and B1 Field non-uniformity corrections in single-point whole-brain macromolecular proton fraction mapping. J. Magn. Reson. Imaging 51, 1789–1798. doi: 10.1002/jmri.26998
Yarnykh, V. L., Krutenkova, E. P., Aitmagambetova, G., Repovic, P., Mayadev, A., Qian, P., et al. (2018a). Iron-Insensitive quantitative assessment of subcortical gray matter demyelination in multiple sclerosis using the macromolecular proton fraction. AJNR Am. J. Neuroradiol. 39, 618–625. doi: 10.3174/ajnr.A5542
Keywords: macromolecular proton fraction (MPF), myelin, magnetization transfer (MT), central nervous system, brain, spinal cord, MRI, quantitative imaging
Citation: Kisel AA, Naumova AV and Yarnykh VL (2022) Macromolecular Proton Fraction as a Myelin Biomarker: Principles, Validation, and Applications. Front. Neurosci. 16:819912. doi: 10.3389/fnins.2022.819912
Received: 22 November 2021; Accepted: 17 January 2022;
Published: 09 February 2022.
Edited by:
Jiazheng Wang, Philips Research, NetherlandsReviewed by:
Silvia De Santis, Cardiff University, United KingdomCopyright © 2022 Kisel, Naumova and Yarnykh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vasily L. Yarnykh, eWFybnlraEB1dy5lZHU=
 Alena A. Kisel
Alena A. Kisel Anna V. Naumova
Anna V. Naumova Vasily L. Yarnykh
Vasily L. Yarnykh