- 1Department of Anesthesia, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Mesenchymal stem cells (MSCs) are multipotent stem cells, whose paracrine and immunomodulatory potential has made them a promising candidate for central nervous system (CNS) regeneration. Numerous studies have demonstrated that MSCs can promote immunomodulation, anti-apoptosis, and axon re-extension, which restore functional neural circuits. The therapeutic effects of MSCs have consequently been evaluated for application in various CNS diseases including spinal cord injury, cerebral ischemia, and neurodegenerative disease. In this review, we will focus on the research works published in the field of mechanisms and therapeutic effects of MSCs in CNS regeneration.
Introduction
Injuries and neurodegenerative diseases often bring about loss of neurons and axonal damage to central nervous system (CNS). Neurons fail to regenerate spontaneously in the mature mammalian CNS. Tremendous effort has been devoted to recognizing the mechanism of CNS regenerative failure, yet a complete understanding is still lacking. A broad spectrum of regeneration strategies, particularly by increasing neuronal survival and axon re-extension, have been met with mixed success (Varadarajan et al., 2022).
Mesenchymal stem cells (MSCs) are among the most widely studied multipotent stem cells, which reside in multiple organs and can be derived from various tissues. Their capability of differentiation into almost any end-stage lineage cells and strong paracrine effects make MSCs a promising candidate for endogenous regeneration. Moreover, the MSCs can be transplanted safely and effectively by systemic and local delivery route (Liu et al., 2020). However, the choice of MSC source, including the bone marrow (BM), adipose tissue (AT), and umbilical cord blood (UCB), is critical in determining the therapeutic potential of MSCs (Bortolotti et al., 2015). To date, BM-MSCs and AT-MSCs are the most extensively studied cell sources for CNS repair, because both of them showed similar neuronal differentiation potential (Chung et al., 2013). BM-MSCs can differentiate into astrocytes, neurons and Schwann cell like cells in the peripheral nervous system (PNS) to promote neural regeneration (Tohill and Terenghi, 2004). Meanwhile, some studies have shown that AT-MSCs can secrete various kinds of growth factor, such as brain-derived neurotrophic factor (BDNF), neural growth factor (NGF), and glia cell-line derived neurotropic factor (GDNF), which promotes neuron survival and axonal regeneration (Villoslada et al., 2000; Blesch and Tuszynski, 2003; Kerschensteiner et al., 2003). Compared to BM-MSCs, AT-MSCs produced a significantly larger amount of cytokines and growth factors, which mediate paracrine actions that promote cellular survival pathways and tissue-repair mechanisms (Zhou et al., 2013).
Numerous studies demonstrate that transplantation of MSCs can regulate neuron growth and axon re-extension, and ameliorate nervous system function after CNS injury or degeneration. In this review, we discuss the therapeutic effects of MSCs in CNS regeneration and the potential involved mechanisms.
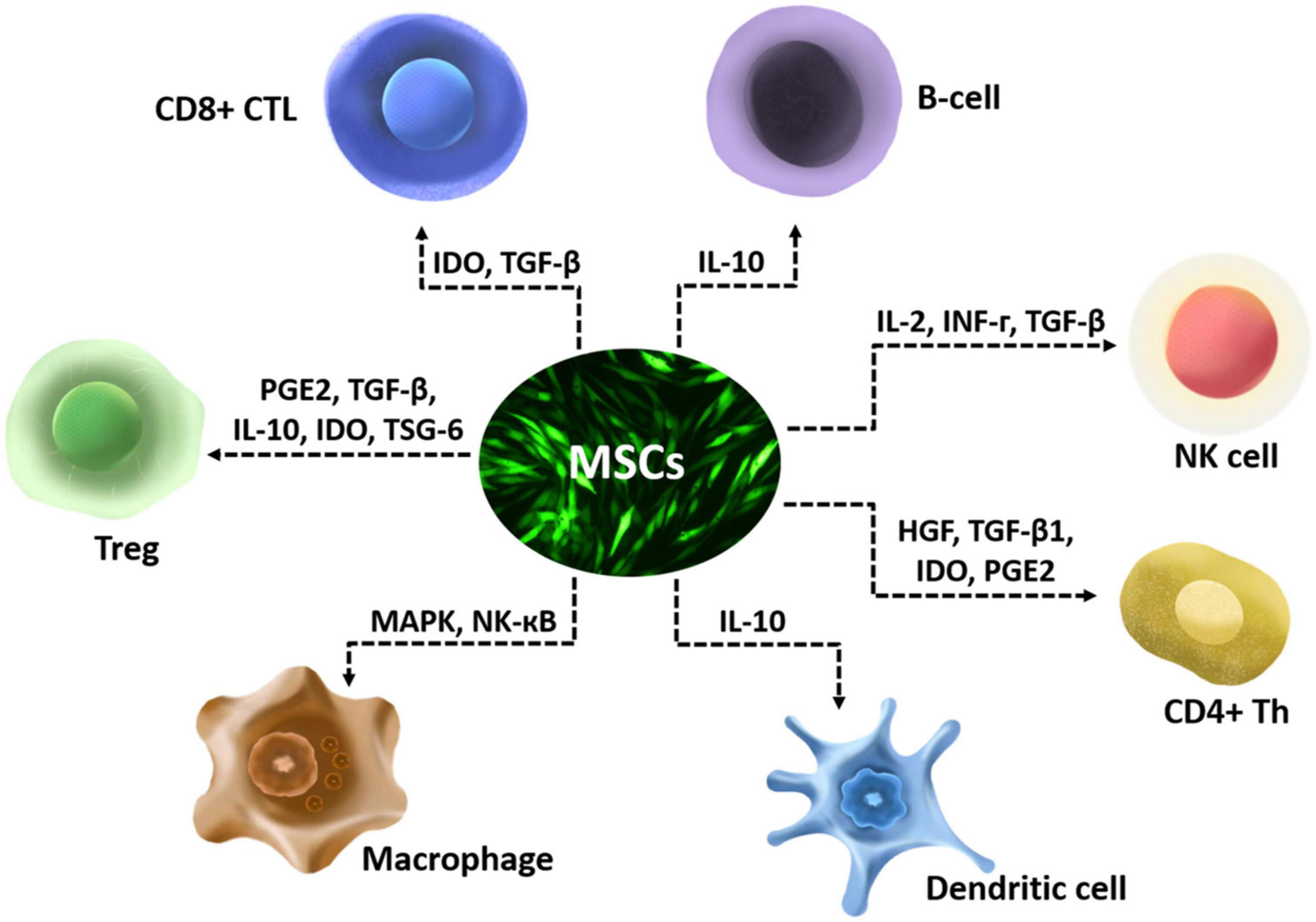
Immunomodulation effects of mesenchymal stem cells
The anti-inflammatory effect of MSCs is mostly executed via secretion of various enzymes and soluble factors and their paracrine actions on T lymphocytes, including naïve CD4+ T-cells, Th1 cells, Th2 Cells, Th17 Cells, CD4+ FoxP3+ Regulatory T-Cells (Tregs), and CD8+ T-cells (Mattar and Bieback, 2015). They also have multiple anti-inflammatory effects that include affecting the chemotactic properties of B cells (Corcione et al., 2006), suppressing interleukin-2 (IL-2) induced natural killer (NK) cell activation (Spaggiari et al., 2006), downregulating NK-activating receptors (Yen et al., 2009), and affect functions of myeloid cells such as monocytes (Jiang et al., 2005), dendritic cells (Ramasamy et al., 2007), and macrophages (Ylöstalo et al., 2012; Figure 1). MSCs modulate immune cells by disrupting their activation, proliferation, maturation, cytolytic activity, cytokine production, or antibody production (Gao et al., 2016). The CNS and its barriers are replete with innate and adaptive immune cells, which interact with glia in diseases. Interactions between immune cells and glia have been shown to perform critical roles in the regenerative capacity of CNS (Greenhalgh et al., 2020). The effects of MSCs on immune cells may participate in the interactions between immune cells and glia, then influence the regeneration of CNS.
Studies on microglia offer further insight into the role of glia and the immune cells in the CNS regeneration since microglia can be defined as both glia and immune cells (Greenhalgh et al., 2020). A recent study has shown that AT-MSCs are able to reprogram microglia/macrophage from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype (Shao et al., 2020). Zhang et al. (2013) observed that intravenous BM-MSCs transplantation in brain was associated with a lower density of microglia/macrophages and reduced levels of proinflammatory cytokines. Another study that investigated the therapeutic effects of BM-MSCs by systemic transplantation into traumatic brain injury (TBI) model of rats found that MSCs reduced microglia and increased neurogenesis (Kota et al., 2016). Besides, MSCs derived exosomes inhibit microglia inflammatory in the damaged regions in cerebral ischemia models (Zhao et al., 2020).
Extensive data found that MSCs could secrete a variety of soluble molecules include hepatocyte growth factor (HGF), transforming growth factor-β1 (TGF-β1), indoleamine-pyrrole 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), interleukin (IL)-13, IL-10, IL-12p70, IL-17E, and IL-27 to exert anti-inflammatory potential (Ryan et al., 2007; Ren et al., 2008; Sobacchi et al., 2017). Human MSCs isolated from BM, AT, dental pulp, Wharton’s jelly (WJ) and placenta paracrine anti-inflammatory factors, such as TGF-β, to promote neuroprotective effects (Ryan et al., 2007; Tomic et al., 2011; Zhou et al., 2011; Carrillo-Galvez et al., 2015; Heo et al., 2016). And the production of TGF-β by MSCs can be increased by proinflammatory cytokines, substrate rigidity, glucose levels and hypoxia (de Araujo Farias et al., 2018). On the other hand, TGF-β has also been shown to signal via SMAD2/3 phosphorylation in MSCs to regulate the biology of MSCs themselves (Choy and Derynck, 2003). By the ability to secrete bioactive and trophic factors, MSCs exert a significant influence on cellular regeneration and new tissue growth (Scuteri et al., 2011).
Although all the MSCs share basic properties, there are subtle differences among MSC types that may affect their immunomodulation. A recent study that compared the immunomodulatory effects of MSCs derived from BM, AT, and WJ of the umbilical cord on T-lymphocytes by co-culture, found that AT-MSCs showed the strongest effect on downregulating CD38 expression on activated T-lymphocytes, whereas BM-MSCs had the weakest effect (Najar et al., 2010). Meanwhile, Ribeiro et al. (2013) found that AT-MSCs emerged as the most immunosuppressive population, as hamper T-cell proliferation by arresting them in the non-activated compartment. However, another research demonstrated that AT-MSCs and BM-MSCs showed equal ability to induce Th0 differentiation into Th1 and Th2 (Xishan et al., 2013). Meanwhile, in a mouse model study, UCB derived MSCs and BM-MSCs showed a similar inhibition of Th17 cells (Li et al., 2013). Although the precise mechanism of these anti-inflammatory effects remains unclear, current clinical trials show that intravenous administration of MSC is a safe and effective treatment for immune disease (Li et al., 2021).
Anti-apoptotic effects of mesenchymal stem cells
An in vitro study showed that BM-MSC could modulate neuronal and glial response to apoptosis in amyotrophic lateral sclerosis (ALS) (Sun et al., 2013). Other studies also demonstrated that intracerebral (Kim K. et al., 2015; Zhou et al., 2016), intravenous (Wang et al., 2012; Chen et al., 2015), or intracerebroventricular (Park W. S. et al., 2016) transplantation of MSCs could ameliorate apoptosis of endogenous neural cells. The infiltrated inflammation-associated immune cells released numerous reactive oxygen species that led to programmed cell death in the injured area. MSCs may suppress oxidative stress and increase the anti-apoptotic Bcl-2 gene expression in brain (Gu et al., 2014). Previous studies showed that miRNA including miR-134 (Xiao et al., 2019), miR-138-5p (Deng et al., 2019), miR132-3p (Pan et al., 2020), miR-21-3p (Li et al., 2019), and miR-22-3p (Zhang et al., 2021) play important roles in these MSCs mediated anti-apoptosis effects in brain. miR-22-3p derived from AT-MSCs prevents neuron apoptosis by inhibiting KDM6B mediated BMP2/BMF axis (Zhang et al., 2021). These effects were abolished by inhibition of miR-22-3p. After intravenous transplantation, AT-MSCs inhibit neural apoptosis by reducing the abnormally high level of miR-21-3p in middle cerebral artery occlusion rat models (Li et al., 2019). It is demonstrated that miR-21-3p directly inhibits the MAT2B expression in neural cells, and miR-21-3p inhibition in neurons attenuated hypoxia/reoxygenation induced impairments. Meanwhile, BM-MSCs deliver anti-apoptotic miRNAs to protect oligodendrocytes, astrocytes, and endothelial cells from apoptosis, which facilitate axon re-extension (Deng et al., 2019; Xiao et al., 2019; Pan et al., 2020).
Axon re-extension effects of mesenchymal stem cells
Axon regeneration after injury is defined as axon regrowth and the subsequent innervation of injured region, resulting in recovery of function to the CNS. Axon re-extension is defined as axon lengthy regrowth that carry out de novo growth over long distances to reach their targets. It has been widely accepted that many extrinsic factors derived from the external environment around damaged areas limit axonal re-extension, such as chondroitin sulfate proteoglycan (CSPG) (Shen et al., 2009), myelin-associated glycoprotein (MAG) (Hasegawa et al., 2004), oligodendrocyte-myelin glycoprotein (von Büdingen et al., 2015), and Nogo-A (Schwab and Strittmatter, 2014). Preventing these inhibitory signals has been considered as a promising approach to promote axon re-extension.
Mesenchymal stem cells have been demonstrated to help neurites to overcome the inhibitory effects of Nogo-A, MAG, and CSPG. In MSC/neuronal cocultures, MSCs promote spinal neuronal adhesion and neurite extension over Nogo-A and MAG (Wright et al., 2014). miR17-92 derived from MSCs overcome the inhibitory effect of CSPGs, when cultured together (Zhang et al., 2017). In spinal cord injury (SCI) dogs, induced using compression method, local transplanted AT-MSCs prevent the accumulation of CSPG and enhance axonal extension (Park et al., 2012). In addition to inhibiting of the extrinsic factors, MSCs provides a favorable microenvironment for re-establishment of functional local circuits with HGF, epidermal growth factor (EGF), neurotrophin-3 (NT-3), and GDNF (Bai et al., 2012; Lv et al., 2021).
The potential role of mesenchymal stem cells in central nervous system regeneration
Mesenchymal stem cells originate from BM, AT, UCB, and synovium are capable of differentiation along mesodermal lineages other than that of their tissue of origin, so they were investigated mostly in clinical (Dawn and Bolli, 2005). MSC expression of neuronal or astrocytic marker has been observed in vitro (Fesharaki et al., 2018) and in vivo (Ma et al., 2018). Meanwhile it is generally accepted that MSCs can secrete several growth factors, such as BDNF, NGF, vascular endothelial growth factor (VEGF), GDNF and insulin-like growth factor 1 (IGF-1), which can facilitate neurogenesis, and create a favorable microenvironment for re-extension and remyelination during reconstruction to play a crucial role in nourishing and protecting neurons (Zhang et al., 2004; Vercelli et al., 2008; Uccelli et al., 2011; Muto et al., 2012). So MSCs have been widely studied and applied in regenerative medicine in nervous system. In this section, we summarize reports concerning the latest preclinical and clinical trials of various MSC types for tissue engineering in CNS. In the area of CNS regeneration, MSC based therapy mainly focuses on damage of CNS caused by severe trauma and continuous ischemia and CNS dysfunction caused by neurologic disease.
Spinal cord injury
Spinal cord injury results in immediate loss of nervous tissue followed by permanent deficits in sensory and motor functions below the injured spinal cord segment. The common promising experimental therapies for SCI include neurotrophic factors, enzymes and antibodies against inhibitory molecules, activated macrophages, bridging scaffolds and stem cell transplantation. The therapeutic approach differs depending on the stage after SCI. Traumatic SCI can be divided into acute phase, subacute phase, and chronic phase. The acute phase of SCI starts after injury and persists for hours to days. The acute phase involves the release of excitotoxicity, the breakdown of the blood-brain barrier, localized edema, and accelerated apoptosis (Emery et al., 1998). The chronic phase of SCI is associated with local inflammation, apoptosis, and ongoing demyelination (Schwab and Bartholdi, 1996; Fleming et al., 2006). Since most SCI patients remains in chronic phase, this phase attracts the greatest research interest among scientists and doctors. In animal models of SCI, stem cell-based regenerative approach has been demonstrated to elicit anatomical repair often accompanied by functional recovery (Ritfeld et al., 2012; Forraz et al., 2013). Stem cell-based regenerative medicine has become a new promising therapeutic approach for treating SCI (Lv et al., 2021; Nakazaki et al., 2021).
Mesenchymal stem cells have the potential to create a reparative environment, which is the main motivation for exploring MSCs for regenerative medicine in nervous system (Yang et al., 2008; Caplan, 2009). In vivo experiments employing different SCI models and various routes of MSCs administration revealed significant functional recovery. After transplantation of human WJ-MSCs into lesion site of complete spinal cord transection rats, the numbers of regenerated axons in the corticospinal trace and neurofilament positive fibers around the lesion site were increased (Yang et al., 2008). It was also reported that intraspinal grafting of rats BM-MSCs into the construction injured spinal cord promotes axonal regrowth and reduces the lesion volume (Gu et al., 2010). Meanwhile, MCSs that overexpress some molecules, such as NT-3 (Stewart et al., 2018), IL-10 (Gao et al., 2022), IL-13 (Dooley et al., 2016), and hemeoxygenase-1 (Khan et al., 2019), can elicit improved axon regeneration and promote motor functional recovery in SCI models.
Since AT-MSCs produced a significantly larger number of cytokines and growth factors than BM-MSCs, some publications suggest AT-MSCs to be an alternative to BM-MSCs for the cellular therapy of SCI (Forostyak et al., 2013). However, while AT-MSCs have been evaluated in animal SCI models, there remains a paucity of large and longitudinal clinical trials. The obstacles for clinical translation of MSCs are the low engraftment and poor survival (Qin and Zhao, 2020), and whether the MSCs can really provide benefit to patients (Staff et al., 2019). El-Kheir et al. (2014) conducted a phase I/II controlled single-blind clinical trial, in which SCI patients received an intrathecal injection of autologous BM-MSC combined with physical therapy showed functional improvements and no long-term cell therapy related side effects over patients received physical therapy alone. Vaquero et al. conducted a phase I, single center, non-randomized, uncontrolled clinical trial in span (NCT02165904). This study evaluated the effects and safety of the subarachnoid transplantation of autologous BM-MSC in patients with chronic SCI reported that most patients showed sensitivity improvement using American Spinal Injury Association score, and BM-MSC was associated with bronchitis in one patient.
Cerebral ischemia
Ischemic stroke induces an extensive neuro-inflammatory response, which seems to be responsible for the propagation of brain damage. However, experimental therapies aimed at reducing immunological reactions after ischemic stroke using cell inhibitors or mediators have not been successful. In this situation, new therapeutic strategies using stem cells have emerged as a promising tool. The most frequently used stem cells are the MSCs, because of their great trophic capabilities (Laso-Garcia et al., 2019). The possible mechanisms involved in potential therapeutic activity of MSCs including neuroprotection, immunomodulation, and activation of neurogenesis, synaptogenesis, astrogenesis, oligodendrogenesis, and angiogenesis in stroke (Dabrowska et al., 2019). Current research suggests that the beneficial effects exerted by MSCs are mainly related to differentiation and immune modulatory mechanism (Zachar et al., 2016).
Chen et al. (2001) reported that BM-MSC transplanted rats showed significant recovery in somatosensory behavior and neurological severity score after cerebral ischemia. Rat WJ-MSCs were shown to have a protective action when transplanted 3 days before a cardiac arrest induced global ischemia by an extracellular signaling mechanism (Jomura et al., 2007). This recovery was accompanied by a decrease in inflammatory reaction after global ischemia. When transplanted with human UCB-MSCs, cerebral ischemia animals presented reduced lesion size and higher extent of vascularization in ischemic areas. Meanwhile, the expression of SDF-1, BDNF, and GNF was higher in ischemic tissues following MSCs treatment (Ding et al., 2007). The authors of a 2018 meta-analysis concluded, “in preclinical studies, Median quality score 4.90/10; confidence interval 95% and large effect size were observed, that strongly supports the translation potential of MSCs therapy for ischemic stroke (Sarmah et al., 2018).”
In a non-randomized small trial with BM-MSCs, the authors found improvements in clinical outcome (European Stroke Scale, National Institutes of Health Stroke Scale, and Fugl-Meyer total score) with stroke patients (Steinberg et al., 2016). Meanwhile Levy et al. (2019) reported that intravenous transfusion of allogenetic MSCs in patients with chronic stroke suggested behavioral gains in a randomized, placebo-controlled study. Another phase 1 clinical trial also demonstrated the safeness of intravenous BM-MSCs use for cerebral ischemia in human (Vahidy et al., 2019). However, all the clinical trials were small trials, so their results should be taken with caution.
Neurodegenerative diseases
The increasing prevalence of CNS disorders has been attributed to neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and multiple system atrophy (MSA) (Przedborski et al., 2003). A common characteristic among such disorders is progressive neuronal death that leads to debilitating neurologic impairments. Although our understanding of the neurodegenerative disease pathology has been improved these years, a precise and reliable treatment has not been accomplished. Current common treatments just relieve symptoms without affecting the major pathological characteristics of these diseases. MSCs hold great potential for cell therapy as they can differentiate toward neural fates and secrete a broad range of factors, which are able to promote neuroprotective or regenerative mechanisms. Moreover, upon transplantation, MSCs possess the capability to home toward neural lesions, implying their potential use as vehicles for therapeutic agents administration (Volkman and Offen, 2017).
Mesenchymal stem cells transplantation often improved survival rates, declined pathology, and rescued cognitive function decline in multiple rodent models of neurodegenerative diseases (Volkman and Offen, 2017). Preclinical studies found that MSCs from BM (Babaei et al., 2012), AT (Kim et al., 2012), UCB (Lee H. J. et al., 2012), and the placenta (Yun et al., 2013) have the ability to regulate amyloid pathology through neuroinflammation, which plays a crucial role in the progression of several neurodegenerative diseases. Bayat et al. (2021) demonstrated that intracerebral transplantation of human olfactory ecto derived MSCs could promote behavioral and anatomical recovery in a HD rat model. Study on conditioned medium of human amniotic membrane derived MSCs found that intraperitoneally injection of this conditioned medium could significantly decrease microglia activation in the R6/2 HD mouse model (Giampa et al., 2019). It is also demonstrated that human UCB-MSCs decreased secretion levels of the proinflammatory cytokines TNF-α and IL-1β, and increased level of the anti-inflammatory markers of IL-4, AMCase, YM-1, and Arg-1 in an AD mouse model (Lee H. J. et al., 2012). Following work showed that human BM-MSCs promote secretion of IL-4 from microglia cells and stimulated α-synuclein clearance in a PD mouse model (Park H. J. et al., 2016). Fontanilla et al. (2015) found that NGF might be responsible for the effects of AT-MSCs in SOD1 G93A mice, defined as the preservation of motor neurons and inflammatory pathway inhibition. To enhance their typical trophic support, MSCs have been genetically engineered to overexpress neurotropic factors, such as NGF, BDNF, and GDNF, whose neuroprotective actions are widely acknowledged (Lo Furno et al., 2018). Meanwhile, BDNF engineered MSCs have been considered for studies of regeneration in ALS, AD, PD, and HD, and even in SCI, TBI, and peripheral nerve injury (Deng et al., 2016).
Clinical studies indicated MSC-based therapy as a safe and feasible technique for patients with AD (Kim H. J. et al., 2015), PD (Venkatesh and Sen, 2017), ALS (Sykova et al., 2017), and MSA (Lee P. H. et al., 2012). Since preclinical and clinical studies have demonstrated the effectiveness of MSCs for the treatment of neurodegenerative disease, many researches begin to focus on the method to enhance the effects.
Other central nervous system disease
Although the clinical application of MSCs therapy in CNS disease currently remains infancy, MSCs research has rapidly expanded over the past decade. Besides neurodegenerative diseases, cerebral ischemia, and SCI, numerous animal model studies have also demonstrated the effects of MSCs in epilepsy (Agadi and Shetty, 2015). BM-MSCs can reduce epileptogenesis by inhibiting neuronal cell death and suppressing aberrant mossy fiber sprouting in a rat model of epilepsy (Fukumura et al., 2018). UCB-MSCs might enhance GABA neurotransmitter levels and ameliorate oxidative stress damage in pentylenetetrazole-induced chronic epilepsy in rats (Mohammed et al., 2014). Moreover, in a phase 1 open label study, MSCs can be a safe and promising candidate for cell therapy in anti-epileptic drugs resistant epilepsy patients (Hlebokazov et al., 2017). To improve the therapeutic effect of MSCs in a mouse model of epilepsy, genetically engineered MSCs, such as IL-13 engineered MSCs, which showed enhanced neuroprotective and disease-modifying effects, has been used (Ali et al., 2017).
Approaches to enhance therapeutic effects of mesenchymal stem cells
Although MSCs represent a promising candidate for CNS regeneration, low therapeutic efficacy limits their clinical use. Different culture conditions may result in altered survival, homing, and key functional features of MSCs. Madrigal et al. (2014) found that cell culture under hypoxic conditions has potential effects on MSCs therapeutic property by increasing the secretion of HGF, TGF-b, VEGF, TSG-6, which is important in CNS regeneration. Others demonstrated that pro-inflammatory stimuli and tri-dimensional growth stimulate trophic factors secretion of MSCs (Vizoso et al., 2017). It is evident that culture conditions will considerably affect the therapeutic efficacy of MSCs. Apart from culture medium, developed therapeutic strategies may also enhance therapeutic effects of MSCs such as delivery route and timing. Although there is no consensus on the optimum delivery route of MSCs, intracerebroventricular transplantation may be the most efficacious. By reviewing previous pre-clinical and clinical studies, Park et al. (2018) found that intracerebroventricular transplantation of MSCs may be associated with enhancement of endogenous, compared to intravenous and intraparenchymal routes for CNS regeneration. The intracerebroventricular transplanted MSCs attenuated brain injury in a time-dependent manner. Significant neuroprotection was demonstrated when administered from 2 to 7 days after induction in intraventricular hemorrhage rat models (Park H. J. et al., 2016).
Conclusion
Mounting evidence suggests that MSCs can be a potential therapy to promote CNS regeneration and functional restoration. The therapeutic role of MSCs is extremely complex. Demonstrating their exact interaction with other cells during neuronal survival, axon re-extension, synapse re-formation, and re-myelination may help researchers to optimize the effects of MSCs based therapies. Optimal conditioned culture, delivery route, and timing of MSCs may be a promising strategy to improve therapeutic effects. In conclusion, study MSCs in CNS provides insight into the exact mechanism of CNS regeneration and repair, helps optimize cell based therapy.
Author contributions
ML wrote the manuscript and polished it up for publication. HC created the figures. MZ gave advice and edits. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Natural Science Foundation of China (No. 82001193).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agadi, S., and Shetty, A. K. (2015). Concise review: prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells 33, 2093–2103. doi: 10.1002/stem.2029
Ali, I., Aertgeerts, S., Le Blon, D., Bertoglio, D., Hoornaert, C., Ponsaerts, P., et al. (2017). Intracerebral delivery of the M2 polarizing cytokine interleukin 13 using mesenchymal stem cell implants in a model of temporal lobe epilepsy in mice. Epilepsia 58, 1063–1072. doi: 10.1111/epi.13743
Babaei, P., Soltani Tehrani, B., and Alizadeh, A. (2012). Transplanted bone marrow mesenchymal stem cells improve memory in rat models of Alzheimer’s disease. Stem Cells Int. 2012:369417. doi: 10.1155/2012/369417
Bai, L., Lennon, D. P., Caplan, A. I., DeChant, A., Hecker, J., Kranso, J., et al. (2012). Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 15, 862–870. doi: 10.1038/nn.3109
Bayat, A. H., Saeidikhoo, S., Ebrahimi, V., Mesgar, S., Joneidi, M., Soltani, R., et al. (2021). Bilateral striatal transplantation of human olfactory stem cells ameliorates motor function, prevents necroptosis-induced cell death and improves striatal volume in the rat model of Huntington’s disease. J. Chem. Neuroanat. 112:101903. doi: 10.1016/j.jchemneu.2020.101903
Blesch, A., and Tuszynski, M. H. (2003). Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J. Comp. Neurol. 467, 403–417. doi: 10.1002/cne.10934
Bortolotti, F., Ukovich, L., Razban, V., Martinelli, V., Ruozi, G., Pelos, B., et al. (2015). In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Rep. 4, 332–339. doi: 10.1016/j.stemcr.2015.01.001
Caplan, A. I. (2009). Why are MSCs therapeutic? New data: new insight. J. Pathol. 217, 318–324. doi: 10.1002/path.2469
Carrillo-Galvez, A. B., Cobo, M., Cuevas-Ocana, S., Gutierrez-Guerrero, A., Sanchez-Gilabert, A., Bongarzone, P., et al. (2015). Mesenchymal stromal cells express GARP/LRRC32 on their surface: effects on their biology and immunomodulatory capacity. Stem Cells 33, 183–195. doi: 10.1002/stem.1821
Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., et al. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32, 1005–1011. doi: 10.1161/01.str.32.4.1005
Chen, M., Li, X., Zhang, X., He, X., Lai, L., Liu, Y., et al. (2015). The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: contribution of TSG-6. J. Neuroinflammation 12:61. doi: 10.1186/s12974-015-0284-x
Choy, L., and Derynck, R. (2003). Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278, 9609–9619. doi: 10.1074/jbc.M212259200
Chung, C. S., Fujita, N., Kawahara, N., Yui, S., Nam, E., and Nishimura, R. (2013). A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J. Vet. Med. Sci. 75, 879–886. doi: 10.1292/jvms.12-0470
Corcione, A., Benvenuto, F., Ferretti, E., Giunti, D., Cappiello, V., Cazzanti, F., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372. doi: 10.1182/blood-2005-07-2657
Dabrowska, S., Andrzejewska, A., Lukomska, B., and Janowski, M. (2019). Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflammation 16:178. doi: 10.1186/s12974-019-1571-8
Dawn, B., and Bolli, R. (2005). Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res. Cardiol. 100, 494–503. doi: 10.1007/s00395-005-0552-5
de Araujo Farias, V., Carrillo-Galvez, A. B., Martin, F., and Anderson, P. (2018). TGF-beta and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 43, 25–37. doi: 10.1016/j.cytogfr.2018.06.002
Deng, P., Torrest, A., Pollock, K., Dahlenburg, H., Annett, G., Nolta, J. A., et al. (2016). Clinical trial perspective for adult and juvenile Huntington’s disease using genetically-engineered mesenchymal stem cells. Neural Regen. Res. 11, 702–705. doi: 10.4103/1673-5374.182682
Deng, Y., Chen, D., Gao, F., Lv, H., Zhang, G., Sun, X., et al. (2019). Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J. Biol. Eng. 13:71. doi: 10.1186/s13036-019-0193-0
Ding, D. C., Shyu, W. C., Chiang, M. F., Lin, S. Z., Chang, Y. C., Wang, H. J., et al. (2007). Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol. Dis. 27, 339–353. doi: 10.1016/j.nbd.2007.06.010
Dooley, D., Lemmens, E., Vangansewinkel, T., Le Blon, D., Hoornaert, C., Ponsaerts, P., et al. (2016). Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Rep. 7, 1099–1115. doi: 10.1016/j.stemcr.2016.11.005
El-Kheir, W. A., Gabr, H., Awad, M. R., Ghannam, O., Barakat, Y., Farghali, H. A., et al. (2014). Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 23, 729–745. doi: 10.3727/096368913X664540
Emery, E., Aldana, P., Bunge, M. B., Puckett, W., Srinivasan, A., Keane, R. W., et al. (1998). Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89, 911–920. doi: 10.3171/jns.1998.89.6.0911
Fesharaki, M., Razavi, S., Ghasemi-Mobarakeh, L., Behjati, M., Yarahmadian, R., Kazemi, M., et al. (2018). Differentiation of human scalp adipose-derived mesenchymal stem cells into mature neural cells on electrospun nanofibrous scaffolds for nerve tissue engineering applications. Cell J. 20, 168–176. doi: 10.22074/cellj.2018.4898
Fleming, J. C., Norenberg, M. D., Ramsay, D. A., Dekaban, G. A., Marcillo, A. E., Saenz, A. D., et al. (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129(Pt 12), 3249–3269. doi: 10.1093/brain/awl296
Fontanilla, C. V., Gu, H., Liu, Q., Zhu, T. Z., Zhou, C., Johnstone, B. H., et al. (2015). Adipose-derived stem cell conditioned media extends survival time of a mouse model of amyotrophic lateral sclerosis. Sci. Rep. 5:16953. doi: 10.1038/srep16953
Forostyak, S., Jendelova, P., and Sykova, E. (2013). The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 95, 2257–2270. doi: 10.1016/j.biochi.2013.08.004
Forraz, N., Wright, K. E., Jurga, M., and McGuckin, C. P. (2013). Experimental therapies for repair of the central nervous system: stem cells and tissue engineering. J. Tissue Eng. Regen. Med. 7, 523–536. doi: 10.1002/term.552
Fukumura, S., Sasaki, M., Kataoka-Sasaki, Y., Oka, S., Nakazaki, M., Nagahama, H., et al. (2018). Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Res. 141, 56–63. doi: 10.1016/j.eplepsyres.2018.02.008
Gao, F., Chiu, S. M., Motan, D. A., Zhang, Z., Chen, L., Ji, H. L., et al. (2016). Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 7:e2062. doi: 10.1038/cddis.2015.327
Gao, T., Huang, F., Wang, W., Xie, Y., and Wang, B. (2022). Interleukin-10 genetically modified clinical-grade mesenchymal stromal cells markedly reinforced functional recovery after spinal cord injury via directing alternative activation of macrophages. Cell. Mol. Biol. Lett. 27:27. doi: 10.1186/s11658-022-00325-9
Giampa, C., Alvino, A., Magatti, M., Silini, A. R., Cardinale, A., Paldino, E., et al. (2019). Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor deficits in the R6/2 mouse model of Huntington’s disease. J. Cell. Mol. Med. 23, 1581–1592. doi: 10.1111/jcmm.14113
Greenhalgh, A. D., David, S., and Bennett, F. C. (2020). Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 21, 139–152. doi: 10.1038/s41583-020-0263-9
Gu, N., Rao, C., Tian, Y., Di, Z., Liu, Z., Chang, M., et al. (2014). Anti-inflammatory and antiapoptotic effects of mesenchymal stem cells transplantation in rat brain with cerebral ischemia. J. Stroke Cerebrovasc. Dis. 23, 2598–2606. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.032
Gu, W., Zhang, F., Xue, Q., Ma, Z., Lu, P., and Yu, B. (2010). Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology 30, 205–217. doi: 10.1111/j.1440-1789.2009.01063.x
Hasegawa, Y., Fujitani, M., Hata, K., Tohyama, M., Yamagishi, S., and Yamashita, T. (2004). Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J. Neurosci. 24, 6826–6832. doi: 10.1523/jneurosci.1856-04.2004
Heo, J. S., Choi, Y., Kim, H. S., and Kim, H. O. (2016). Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 37, 115–125. doi: 10.3892/ijmm.2015.2413
Hlebokazov, F., Dakukina, T., Ihnatsenko, S., Kosmacheva, S., Potapnev, M., Shakhbazau, A., et al. (2017). Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: an open label study. Adv. Med. Sci. 62, 273–279. doi: 10.1016/j.advms.2016.12.004
Jiang, X. X., Zhang, Y., Liu, B., Zhang, S. X., Wu, Y., Yu, X. D., et al. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126. doi: 10.1182/blood-2004-02-0586
Jomura, S., Uy, M., Mitchell, K., Dallasen, R., Bode, C. J., and Xu, Y. (2007). Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells 25, 98–106. doi: 10.1634/stemcells.2006-0055
Kerschensteiner, M., Stadelmann, C., Dechant, G., Wekerle, H., and Hohlfeld, R. (2003). Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann. Neurol. 53, 292–304. doi: 10.1002/ana.10446
Khan, I. U., Yoon, Y., Choi, K. U., Jo, K. R., Kim, N., Lee, E., et al. (2019). therapeutic effects of intravenous injection of fresh and frozen thawed HO-1-overexpressed Ad-MSCs in dogs with acute spinal cord injury. Stem Cells Int. 2019:8537541. doi: 10.1155/2019/8537541
Kim, H. J., Seo, S. W., Chang, J. W., Lee, J. I., Kim, C. H., Chin, J., et al. (2015). Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimers Dement 1, 95–102. doi: 10.1016/j.trci.2015.06.007
Kim, K., Park, H. W., Moon, H. E., Kim, J. W., Bae, S., Chang, J. W., et al. (2015). The effect of human umbilical cord blood-derived mesenchymal stem cells in a collagenase-induced intracerebral hemorrhage rat model. Exp. Neurobiol. 24, 146–155. doi: 10.5607/en.2015.24.2.146
Kim, S., Chang, K. A., Kim, J., Park, H. G., Ra, J. C., Kim, H. S., et al. (2012). The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One 7:e45757. doi: 10.1371/journal.pone.0045757
Kota, D. J., Prabhakara, K. S., van Brummen, A. J., Bedi, S., Xue, H., DiCarlo, B., et al. (2016). Propranolol and mesenchymal stromal cells combine to treat traumatic brain injury. Stem Cells Transl. Med. 5, 33–44. doi: 10.5966/sctm.2015-0065
Laso-Garcia, F., Diekhorst, L., Gomez-de Frutos, M. C., Otero-Ortega, L., Fuentes, B., Ruiz-Ares, G., et al. (2019). Cell-based therapies for stroke: promising solution or dead end? Mesenchymal stem cells and comorbidities in preclinical stroke research. Front. Neurol. 10:332. doi: 10.3389/fneur.2019.00332
Lee, H. J., Lee, J. K., Lee, H., Carter, J. E., Chang, J. W., Oh, W., et al. (2012). Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 33, 588–602. doi: 10.1016/j.neurobiolaging.2010.03.024
Lee, P. H., Lee, J. E., Kim, H. S., Song, S. K., Lee, H. S., Nam, H. S., et al. (2012). A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann. Neurol. 72, 32–40. doi: 10.1002/ana.23612
Levy, M. L., Crawford, J. R., Dib, N., Verkh, L., Tankovich, N., and Cramer, S. C. (2019). Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke 50, 2835–2841. doi: 10.1161/STROKEAHA.119.026318
Li, A., Guo, F., Pan, Q., Chen, S., Chen, J., Liu, H. F., et al. (2021). Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front. Immunol. 12:728190. doi: 10.3389/fimmu.2021.728190
Li, C., Fei, K., Tian, F., Gao, C., and Yang, S. (2019). Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat Med. J. 60, 439–448. doi: 10.3325/cmj.2019.60.439
Li, L., Liu, S., Xu, Y., Zhang, A., Jiang, J., Tan, W., et al. (2013). Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology 92, 257–264. doi: 10.1159/000354883
Liu, Z., Mikrani, R., Zubair, H. M., Taleb, A., Naveed, M., Baig, M., et al. (2020). Systemic and local delivery of mesenchymal stem cells for heart renovation: challenges and innovations. Eur. J. Pharmacol. 876:173049. doi: 10.1016/j.ejphar.2020.173049
Lo Furno, D., Mannino, G., and Giuffrida, R. (2018). Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell Physiol. 233, 3982–3999. doi: 10.1002/jcp.26192
Lv, B., Zhang, X., Yuan, J., Chen, Y., Ding, H., Cao, X., et al. (2021). Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res. Ther. 12:36. doi: 10.1186/s13287-020-02090-y
Ma, Y. H., Zeng, X., Qiu, X. C., Wei, Q. S., Che, M. T., Ding, Y., et al. (2018). Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials 160, 37–55. doi: 10.1016/j.biomaterials.2018.01.015
Madrigal, M., Rao, K. S., and Riordan, N. H. (2014). A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 12:260. doi: 10.1186/s12967-014-0260-8
Mattar, P., and Bieback, K. (2015). Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front. Immunol. 6:560. doi: 10.3389/fimmu.2015.00560
Mohammed, A. S., Ewais, M. M., Tawfik, M. K., and Essawy, S. S. (2014). Effects of intravenous human umbilical cord blood mesenchymal stem cell therapy versus gabapentin in pentylenetetrazole-induced chronic epilepsy in rats. Pharmacology 94, 41–50. doi: 10.1159/000365219
Muto, T., Miyoshi, K., Horiguchi, T., Hagita, H., and Noma, T. (2012). Novel genetic linkage of rat Sp6 mutation to amelogenesis imperfecta. Orphanet. J. Rare Dis. 7:34. doi: 10.1186/1750-1172-7-34
Najar, M., Raicevic, G., Boufker, H. I., Fayyad Kazan, H., De Bruyn, C., Meuleman, N., et al. (2010). Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 264, 171–179. doi: 10.1016/j.cellimm.2010.06.006
Nakazaki, M., Morita, T., Lankford, K. L., Askenase, P. W., and Kocsis, J. D. (2021). Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-beta upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell. Vesicles 10:e12137. doi: 10.1002/jev2.12137
Pan, Q., Kuang, X., Cai, S., Wang, X., Du, D., Wang, J., et al. (2020). miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res. Ther. 11:260. doi: 10.1186/s13287-020-01761-0
Park, H. J., Oh, S. H., Kim, H. N., Jung, Y. J., and Lee, P. H. (2016). Mesenchymal stem cells enhance alpha-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 132, 685–701. doi: 10.1007/s00401-016-1605-6
Park, S. E., Lee, N. K., Na, D. L., and Chang, J. W. (2018). Optimal mesenchymal stem cell delivery routes to enhance neurogenesis for the treatment of Alzheimer’s disease: optimal MSCs delivery routes for the treatment of AD. Histol. Histopathol. 33, 533–541. doi: 10.14670/HH-11-950
Park, S. S., Lee, Y. J., Lee, S. H., Lee, D., Choi, K., Kim, W. H., et al. (2012). Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 14, 584–597. doi: 10.3109/14653249.2012.658913
Park, W. S., Sung, S. I., Ahn, S. Y., Sung, D. K., Im, G. H., Yoo, H. S., et al. (2016). Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transplant. 25, 1131–1144. doi: 10.3727/096368915x689640
Przedborski, S., Vila, M., and Jackson-Lewis, V. (2003). Neurodegeneration: what is it and where are we? J. Clin. Invest. 111, 3–10. doi: 10.1172/JCI17522
Qin, H., and Zhao, A. (2020). Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell 11, 707–722. doi: 10.1007/s13238-020-00738-2
Ramasamy, R., Fazekasova, H., Lam, E. W., Soeiro, I., Lombardi, G., and Dazzi, F. (2007). Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 83, 71–76.
Ren, G., Zhang, L., Zhao, X., Xu, G., Zhang, Y., Roberts, A. I., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150. doi: 10.1016/j.stem.2007.11.014
Ribeiro, A., Laranjeira, P., Mendes, S., Velada, I., Leite, C., Andrade, P., et al. (2013). Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 4:125. doi: 10.1186/scrt336
Ritfeld, G. J., Nandoe Tewarie, R. D., Vajn, K., Rahiem, S. T., Hurtado, A., Wendell, D. F., et al. (2012). Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 21, 1561–1575. doi: 10.3727/096368912X640484
Ryan, J. M., Barry, F., Murphy, J. M., and Mahon, B. P. (2007). Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 149, 353–363. doi: 10.1111/j.1365-2249.2007.03422.x
Sarmah, D., Agrawal, V., Rane, P., Bhute, S., Watanabe, M., Kalia, K., et al. (2018). Mesenchymal stem cell therapy in ischemic stroke: a meta-analysis of preclinical studies. Clin. Pharmacol. Ther. 103, 990–998. doi: 10.1002/cpt.927
Schwab, M. E., and Bartholdi, D. (1996). Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76, 319–370.
Schwab, M. E., and Strittmatter, S. M. (2014). Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60. doi: 10.1016/j.conb.2014.02.011
Scuteri, A., Miloso, M., Foudah, D., Orciani, M., Cavaletti, G., and Tredici, G. (2011). Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr. Stem Cell Res. Ther. 6, 82–92. doi: 10.2174/157488811795495486
Shao, M., Jin, M., Xu, S., Zheng, C., Zhu, W., Ma, X., et al. (2020). Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation 43, 1536–1547. doi: 10.1007/s10753-020-01230-z
Shen, Y., Tenney, A. P., Busch, S. A., Horn, K. P., Cuascut, F. X., Liu, K., et al. (2009). PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596. doi: 10.1126/science.1178310
Sobacchi, C., Palagano, E., Villa, A., and Menale, C. (2017). Soluble factors on stage to direct mesenchymal stem cells fate. Front. Bioeng. Biotechnol. 5:32. doi: 10.3389/fbioe.2017.00032
Spaggiari, G. M., Capobianco, A., Becchetti, S., Mingari, M. C., and Moretta, L. (2006). Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107, 1484–1490. doi: 10.1182/blood-2005-07-2775
Staff, N. P., Jones, D. T., and Singer, W. (2019). Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin. Proc. 94, 892–905. doi: 10.1016/j.mayocp.2019.01.001
Steinberg, G. K., Kondziolka, D., Wechsler, L. R., Lunsford, L. D., Coburn, M. L., Billigen, J. B., et al. (2016). Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 47, 1817–1824. doi: 10.1161/STROKEAHA.116.012995
Stewart, A. N., Kendziorski, G., Deak, Z. M., Bartosek, N. C., Rezmer, B. E., Jenrow, K., et al. (2018). Transplantation of mesenchymal stem cells that overexpress NT-3 produce motor improvements without axonal regeneration following complete spinal cord transections in rats. Brain Res. 1699, 19–33. doi: 10.1016/j.brainres.2018.06.002
Sun, H., Bénardais, K., Stanslowsky, N., Thau-Habermann, N., Hensel, N., Huang, D., et al. (2013). Therapeutic potential of mesenchymal stromal cells and MSC conditioned medium in Amyotrophic Lateral Sclerosis (ALS)–in vitro evidence from primary motor neuron cultures, NSC-34 cells, astrocytes and microglia. PLoS One 8:e72926. doi: 10.1371/journal.pone.0072926
Sykova, E., Rychmach, P., Drahoradova, I., Konradova, S., Ruzickova, K., Vorisek, I., et al. (2017). Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 26, 647–658. doi: 10.3727/096368916X693716
Tohill, M., and Terenghi, G. (2004). Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol. Appl. Biochem. 40, 17–24. doi: 10.1042/BA20030173
Tomic, S., Djokic, J., Vasilijic, S., Vucevic, D., Todorovic, V., Supic, G., et al. (2011). Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 20, 695–708. doi: 10.1089/scd.2010.0145
Uccelli, A., Benvenuto, F., Laroni, A., and Giunti, D. (2011). Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol. 24, 59–64. doi: 10.1016/j.beha.2011.01.004
Vahidy, F. S., Haque, M. E., Rahbar, M. H., Zhu, H., Rowan, P., Aisiku, I. P., et al. (2019). Intravenous bone marrow mononuclear cells for acute ischemic stroke: safety, feasibility, and effect size from a phase I clinical trial. Stem Cells 37, 1481–1491. doi: 10.1002/stem.3080
Varadarajan, S. G., Hunyara, J. L., Hamilton, N. R., Kolodkin, A. L., and Huberman, A. D. (2022). Central nervous system regeneration. Cell 185, 77–94. doi: 10.1016/j.cell.2021.10.029
Venkatesh, K., and Sen, D. (2017). Mesenchymal stem cells as a source of dopaminergic neurons: a potential cell based therapy for Parkinson’s Disease. Curr. Stem Cell Res. Ther. 12, 326–347. doi: 10.2174/1574888X12666161114122059
Vercelli, A., Mereuta, O. M., Garbossa, D., Muraca, G., Mareschi, K., Rustichelli, D., et al. (2008). Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 31, 395–405. doi: 10.1016/j.nbd.2008.05.016
Villoslada, P., Hauser, S. L., Bartke, I., Unger, J., Heald, N., Rosenberg, D., et al. (2000). Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of t helper cell type 1 and 2 cytokines within the central nervous system. J. Exp. Med. 191, 1799–1806. doi: 10.1084/jem.191.10.1799
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., and Perez-Fernandez, R. (2017). Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18:1852. doi: 10.3390/ijms18091852
Volkman, R., and Offen, D. (2017). Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells 35, 1867–1880. doi: 10.1002/stem.2651
von Büdingen, H. C., Mei, F., Greenfield, A., Jahn, S., Shen, Y. A., Reid, H. H., et al. (2015). The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J. Cell Biol. 210, 891–898. doi: 10.1083/jcb.201504106
Wang, S. P., Wang, Z. H., Peng, D. Y., Li, S. M., Wang, H., and Wang, X. H. (2012). Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: reduced apoptosis and enhanced neuroprotection. Mol. Med. Rep. 6, 848–854. doi: 10.3892/mmr.2012.997
Wright, K. T., Uchida, K., Bara, J. J., Roberts, S., El Masri, W., and Johnson, W. E. (2014). Spinal motor neurite outgrowth over glial scar inhibitors is enhanced by coculture with bone marrow stromal cells. Spine J. 14, 1722–1733. doi: 10.1016/j.spinee.2014.01.021
Xiao, Y., Geng, F., Wang, G., Li, X., Zhu, J., and Zhu, W. (2019). Bone marrow–derived mesenchymal stem cells–derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J. Cell. Biochem. 120, 2109–2118. doi: 10.1002/jcb.27519
Xishan, Z., Baoxin, H., Xinna, Z., and Jun, R. (2013). Comparison of the effects of human adipose and bone marrow mesenchymal stem cells on T lymphocytes. Cell Biol. Int. 37, 11–18. doi: 10.1002/cbin.10002
Yang, C. C., Shih, Y. H., Ko, M. H., Hsu, S. Y., Cheng, H., and Fu, Y. S. (2008). Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One 3:e3336. doi: 10.1371/journal.pone.0003336
Yen, B. L., Chang, C. J., Liu, K. J., Chen, Y. C., Hu, H. I., Bai, C. H., et al. (2009). Brief report–human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 27, 451–456. doi: 10.1634/stemcells.2008-0390
Ylöstalo, J. H., Bartosh, T. J., Coble, K., and Prockop, D. J. (2012). Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30, 2283–2296. doi: 10.1002/stem.1191
Yun, H. M., Kim, H. S., Park, K. R., Shin, J. M., Kang, A. R., il Lee, K., et al. (2013). Placenta-derived mesenchymal stem cells improve memory dysfunction in an Abeta1-42-infused mouse model of Alzheimer’s disease. Cell Death Dis. 4:e958. doi: 10.1038/cddis.2013.490
Zachar, L., Bacenkova, D., and Rosocha, J. (2016). Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 9, 231–240. doi: 10.2147/JIR.S121994
Zhang, J., Li, Y., Chen, J., Yang, M., Katakowski, M., Lu, M., et al. (2004). Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 1030, 19–27. doi: 10.1016/j.brainres.2004.09.061
Zhang, R., Liu, Y., Yan, K., Chen, L., Chen, X. R., Li, P., et al. (2013). Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation 10:106. doi: 10.1186/1742-2094-10-106
Zhang, Y., Chopp, M., Liu, X. S., Katakowski, M., Wang, X., Tian, X., et al. (2017). Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol. 54, 2659–2673. doi: 10.1007/s12035-016-9851-0
Zhang, Y., Liu, J., Su, M., Wang, X., and Xie, C. (2021). Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res. Ther. 12:111. doi: 10.1186/s13287-020-02091-x
Zhao, Y., Gan, Y., Xu, G., Yin, G., and Liu, D. (2020). MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neurochem. Res. 45, 1180–1190. doi: 10.1007/s11064-020-02998-0
Zhou, C., Yang, B., Tian, Y., Jiao, H., Zheng, W., Wang, J., et al. (2011). Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 272, 33–38. doi: 10.1016/j.cellimm.2011.09.010
Zhou, H., Zhang, H., Yan, Z., and Xu, R. (2016). Transplantation of human amniotic mesenchymal stem cells promotes neurological recovery in an intracerebral hemorrhage rat model. Biochem. Biophys. Res. Commun. 475, 202–208. doi: 10.1016/j.bbrc.2016.05.075
Keywords: mesenchymal stem cells (MeSH ID D059630), regenerative medicine, central nervous system, immunomodulation, anti-apoptosis
Citation: Li M, Chen H and Zhu M (2022) Mesenchymal stem cells for regenerative medicine in central nervous system. Front. Neurosci. 16:1068114. doi: 10.3389/fnins.2022.1068114
Received: 14 October 2022; Accepted: 28 November 2022;
Published: 13 December 2022.
Edited by:
Mudasir Bashir Gugjoo, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, IndiaReviewed by:
Rawaa Al-Mayyahi, University of Basrah, IraqRegan Hamel, University of Cambridge, United Kingdom
Copyright © 2022 Li, Chen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxin Zhu, bXh6aHVAdGpoLnRqbXUuZWR1LmNu
 Man Li1
Man Li1 Mingxin Zhu
Mingxin Zhu