- 1Center for Advanced Neuroimaging, University of California, Riverside, Riverside, CA, United States
- 2Department of Neurosciences, University of California, San Diego, San Diego, CA, United States
- 3Department of Bioengineering, University of California, Riverside, Riverside, CA, United States
- 4Department of Neurology, Emory University, Atlanta, GA, United States
Introduction: Striatal dopamine transporter (DAT) imaging using 123I-ioflupane single photon positron emitted computed tomography (SPECT) (DaTScan, GE) identifies 5−20% of newly diagnosed Parkinson’s disease (PD) subjects enrolling in clinical studies to have scans without evidence of dopaminergic deficit (SWEDD). These individuals meet diagnostic criteria for PD, but do not clinically progress as expected, and they are not believed to have neurodegenerative Parkinsonism. Inclusion of SWEDD participants in PD biomarker studies or therapeutic trials may therefore cause them to fail. DaTScan can identify SWEDD individuals, but it is expensive and not widely available; an alternative imaging approach is needed. Here, we evaluate the use of neuromelanin-sensitive, iron-sensitive, and diffusion contrasts in substantia nigra pars compacta (SNpc) to differentiate SWEDD from PD individuals.
Methods: Neuromelanin-sensitive, iron-sensitive, and diffusion imaging data for SWEDD, PD, and control subjects were downloaded from the Parkinson’s progression markers initiative (PPMI) database. SNpc volume, SNpc iron (R2), and SNpc free water (FW) were measured for each participant.
Results: Significantly smaller SNpc volume was seen in PD as compared to SWEDD (P < 10–3) and control (P < 10–3) subjects. SNpc FW was elevated in the PD group relative to controls (P = 0.017). No group difference was observed in SNpc R2.
Conclusion: In conclusion, nigral volume and FW in the SWEDD group were similar to that of controls, while a reduction in nigral volume and increased FW were observed in the PD group relative to SWEDD and control participants. These results suggest that these MRI measures should be explored as a cost-effective alternative to DaTScan for evaluation of the nigrostriatal system.
Introduction
Neuronal loss in structures within the nigrostriatal system is a core feature of Parkinson’s disease (PD) (Fearnley and Lees, 1991; Damier et al., 1999). Much of this loss occurs in substantia nigra pars compacta (SNpc) during the prodromal stages of PD, and an estimated 30–50% of melanized neurons are lost by the time of symptom onset (Fearnley and Lees, 1991; Cheng et al., 2010). Accordingly, efforts to develop neuroprotective therapies for PD increasingly focus on early-stage patients because the potential to prevent future neurodegeneration is greatest in this group. So far, no neuroprotective therapy has been established for PD at any stage (Athauda and Foltynie, 2015), but early-stage PD patients have the most subtle features and can be especially challenging to diagnose. Thus, there is a pressing need for the development of imaging markers to assist selection of patients for recruitment into PD neuroprotection trials, particularly when these studies focus on early-stage PD patients. Imaging tools are also needed to monitor progression of neurodegeneration, and for development as surrogate outcome measures for these trials.
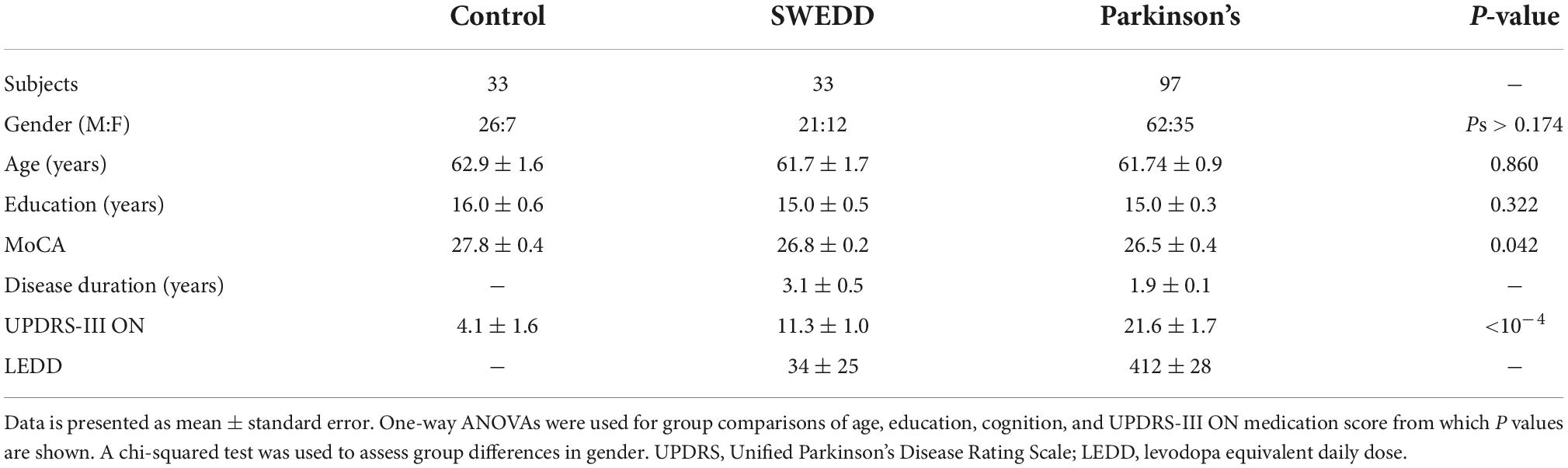
Striatal dopamine transporter (DAT) imaging using 123I-ioflupane single photon positron emitted computed tomography (SPECT), also called DaTScan (GE Healthcare, Chicago, IL, USA), and 18F-fluorodopa positron emission tomography (PET) have found that between 5 and 20% of newly diagnosed PD subjects enrolling in clinical PD studies have scans without evidence of dopaminergic deficit (SWEDD) (Batla et al., 2014; Erro et al., 2016). Individuals with SWEDD meet clinical diagnostic criteria for PD but lack imaging evidence of nigrostriatal degeneration, the hallmark pathology of PD. A comparison of DaTScan images in a control participant, SWEDD participant, and PD participant is shown in Figure 1. Longitudinal study of SWEDD patients has found that they do not clinically progress significantly (Marek et al., 2014), and they are insensitive to treatment with levodopa (Fahn and Parkinson Study, 2005). Further, the highest percentage of SWEDD participants is observed in studies enrolling just after diagnosis (Parkinson Study, 2002; Fahn et al., 2004; Marek et al., 2014). While SWEDDs may be a heterogeneous group with a range of etiologies underlying their presentation with apparent clinical PD (Erro et al., 2016), they are unlikely to have idiopathic PD (Marek et al., 2014; Lee et al., 2021). Therefore, inclusion of participants with SWEDD in biomarker studies or therapeutic trials for PD may cause them to fail due to inclusion of individuals without PD in the PD group.
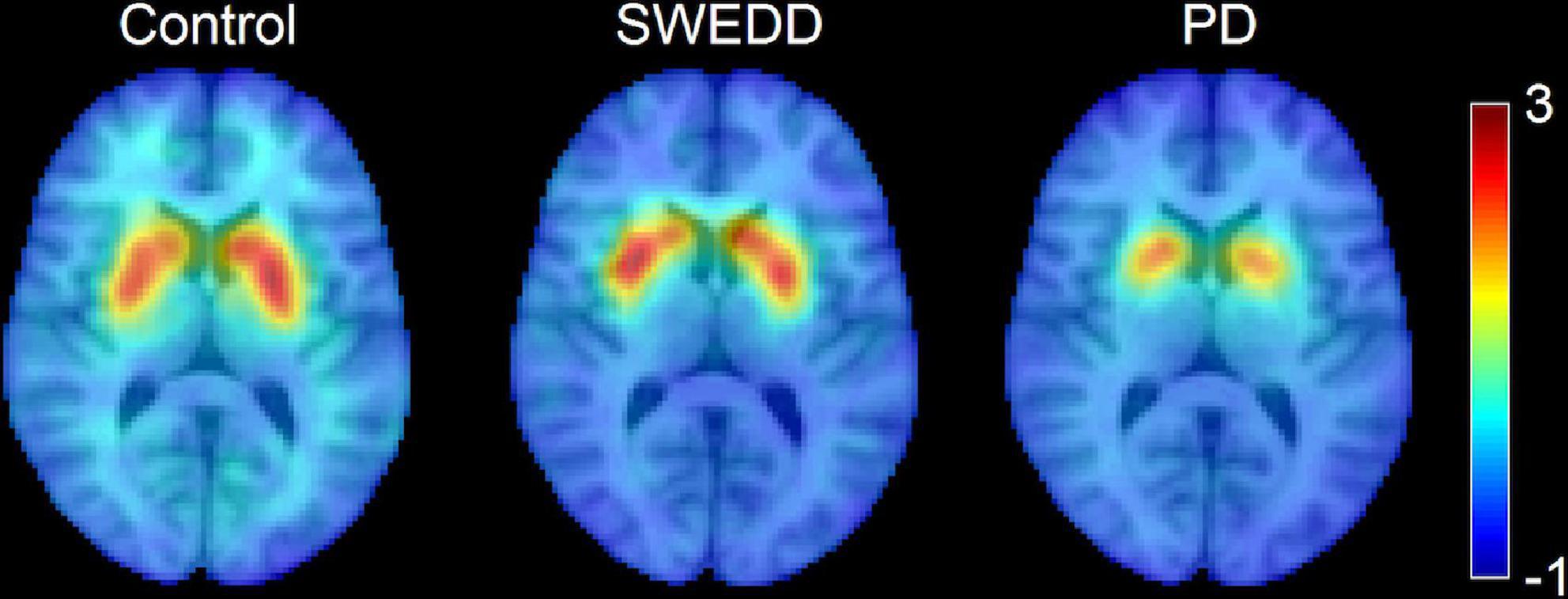
Figure 1. A comparison of striatal binding ratio (SBR) derived from DaTScan images at the level of the striatum in a control participant (left), scans without evidence of dopaminergic deficit (SWEDD) participant (middle), and PD participant (right).
DaTScan has been used in numerous clinical trials and biomarker studies to confirm the presence of neurodegenerative Parkinsonism. However, limited availability, high cost, substantial time requirement (a 3–6 h delay is needed between radionuclide injection and scan), and safety concerns relating to the use of radioisotopes impedes the widespread adoption of DaTScan (de la Fuente-Fernandez, 2012). The scan-rescan reproducibility of DaTScan is also sub-optimal for quantitative analysis of DaTScan as an outcome measure (Bhattacharjee et al., 2019). An MRI-based imaging marker to corroborate the presence of neurodegenerative Parkinsonism in individuals meeting diagnostic criteria for PD could improve biomarker study and trial designs in terms of cost-effectiveness, safety, and availability, and it may improve the odds of success of PD clinical trials.
Neuromelanin-sensitive MRI can evaluate dopaminergic neurodegeneration in SNpc in vivo without ionizing radiation. Neuromelanin-sensitive MRI images with explicit or incidental magnetization transfer (MT) effects allow quantification of neuromelanin-associated contrast in SNpc (Schwarz et al., 2011; Chen et al., 2014) and MT contrast has been shown to colocalize with melanized neurons (Kitao et al., 2013; Keren et al., 2015). Application of MT effects to examine nigral depigmentation has revealed PD-related reductions in nigral volume (Ogisu et al., 2013; Castellanos et al., 2015) or SNpc area in a single slice (Reimao et al., 2015). Neuromelanin-sensitive MRI methods have also been shown to have high scan-rescan reproducibility (Langley et al., 2017a; Wengler et al., 2020; van der Pluijm et al., 2021).
Histological studies have observed iron deposition alongside nigral depigmentation in PD (Dexter et al., 1987; Zucca et al., 2017). Magnetic resonance (MR) relaxometry measures the relaxation rate of the transverse magnetization (R2 or R2*) and is sensitive to iron (Langkammer et al., 2010). Increases in transverse relaxation rate in the substantia nigra regions of interest defined in T2- or T2*-weighted images (Kosta et al., 2006; Martin et al., 2008; Baudrexel et al., 2010; Peran et al., 2010; Du et al., 2011) or in regions of interest defined by neuromelanin-sensitive contrast (Langley et al., 2017b,2019; He et al., 2020, 2021; Jin et al., 2022) have been found. The regions of interest placed by neuromelanin-sensitive contrast are likely placed in the SNpc since neuromelanin-sensitive contrast co-localizes with melanized neurons (Kitao et al., 2013).
Diffusion MRI is sensitive to the diffusivity of water, and it allows researchers to probe tissue microstructure. Tissue microstructural changes in the setting of neurodegeneration likely include a local increase in extracellular fluid due to both cell loss and vasogenic edema caused by a neuroinflammatory response (Febo et al., 2020). Bi-compartment models applied to diffusion MRI data to separate restricted diffusion in intracellular compartments from extracellular fluid, i.e., the free water (FW) compartment, have also been applied to examine PD neurodegeneration-related changes in SNpc. Increases in nigral FW have been observed in PD patients as compared to controls (Ofori et al., 2015; Burciu et al., 2017; Guttuso et al., 2018; Langley et al., 2021). In this study, we use a multi-contrast approach to examine the nigrostriatal system in PD and SWEDD. Specifically, DaTScan is used to stratify the PD and SWEDD groups and to examine striatal DAT uptake, MT effects are used to examine nigral volume, relaxometry is used to examine iron deposition, and a bi-compartment model is used to examine nigral microstructure in PD patients, controls, and individuals with SWEDD.
Methods
Parkinson’s progression markers initiative overview
Data used in the preparation of this article were obtained from the Parkinson’s progression markers initiative (PPMI) database.1 For up-to-date information on the study, visit www.ppmi-info.org. Full inclusion and exclusion criteria for enrollment in PPMI can be found at www.ppmi-info.org. Each Institution’s Institutional Review Board approved the study and subjects gave written informed consent prior to enrolling in the study.
Participants
Explicit MT effects are generated by the application of fat saturation pulses prior to excitation (Schwarz et al., 2011) and incidental MT effects are generated by interleaved turbo spin echo (TSE) acquisitions (Dixon et al., 1990). These effects can be used to generate neuromelanin-sensitive images and evaluate the loss of neuromelanin-sensitive contrast in SNpc. The PPMI database was queried for individuals with T1-weighted MP-RAGE acquisitions, dual echo TSE acquisitions with a fat saturation pulse, and cardiac-gated diffusion tensor imaging (DTI) acquisitions.
Criteria for inclusion of subjects from the PPMI database used in this analysis were as follows: (1) participants must be scanned with cardiac-gated DTI and dual echo TSE with a fat saturation pulse and (2) participants must have DTI and TSE scans with scan parameters matching those in the PPMI imaging protocol. The 24-month time point was chosen for evaluation in the PD group since that time point contains the largest number of subjects with TSE acquisitions with a fat saturation pulse. A total of 163 subjects [33 controls (CO), 33 SWEDD, and 97 PD patients] met these criteria. All PD and SWEDD subjects underwent DaTScan to confirm diagnosis. Imaging data were downloaded in December 2019.
Magnetic resonance imaging acquisition
All MRI data used in this analysis were acquired on Siemens 3T MRI scanners (Erlangen, Germany). T1-weighted structural images in the PPMI cohort were used for registration to common space. Dual echo TSE images were acquired with the following parameters: TE1/TE2/TR = 11/101/3270 ms, FOV = 240 mm2 × 213 mm2, voxel size = 0.9 mm3 × 0.9 mm3 × 3 mm3, fat saturation pulse, 48 slices. The first echo of the TSE acquisition contains MT effects from the fat saturation pulse and interleaved TSE acquisition. Cardiac-gated diffusion-MRI data in the PPMI cohort were acquired using a monopolar diffusion encoding gradient with 64 unique gradient directions and the following parameters: TE/TR = 88/650−1100 ms, flip angle = 90, FOV = 229 mm2 × 229 mm2, voxel size = 1.98 mm3 × 1.98 mm3 × 2 mm3, b = 1,000 s/mm2, cardiac-triggered, with 72 slices.
Magnetic resonance imaging processing
Diffusion data was preprocessed with FSL (Smith et al., 2004). Diffusion MR data were corrected for motion and eddy-current distortions using EDDY in FSL. Next, susceptibility distortions were reduced by non-linearly fitting the b = 0 image to second echo from the TSE acquisition. Parameters derived from the diffusion data were estimated using DTIFIT in FSL. A bi-compartment model, as implemented in DIPY (Garyfallidis et al., 2014), was used to calculate FW images. Next, the b = 0 image was brain extracted and registered to the brain extracted T1-weighted image using a rigid body transform with a boundary-based registration cost function.
R2, an MRI measure sensitive to iron, was calculated from the dual echo TSE acquisition using a custom script in MATLAB by fitting a monoexponential model to the dual echo TSE images. For each voxel, R2 was calculated from the dual echo TSE acquisition using the following equation:
Where S(TEi) denotes the signal at echo time TEi. The resulting R2 maps were aligned to the T1-weighted image using a rigid body transform derived via the magnitude image from the first echo.
Regions of interest
An SNpc atlas, derived from MT images in a cohort of 76 healthy older participants (aged 66 ± 6 years), was used as a region of interest (ROI) in the diffusion analysis (Langley et al., 2020a). The SNpc atlas was transformed from Montreal Neurological Institute (MNI) space to subject space using linear and non-linear transforms in fMRIB Software Library (FSL) as previously described (Langley et al., 2020b). The SNpc atlas was thresholded at 60%, representing 60% of the population, and binarized. Mean FW and R2 was calculated in the SNpc for each subject.
Substantia nigra pars compacta was segmented using a thresholding method. A reference region was drawn in the cerebral peduncle in MNI common space and then transformed to individual NM-MRI images and used to threshold. Thresholding was restricted to the anatomic location of SNpc using the probabilistic standard space mask (Langley et al., 2020a). Voxels with intensity >μref + 3σref were considered part of SNpc.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics software version 24 (IBM Corporation, Somers, NY, USA) and results are reported as mean ± standard error (SE). A P value of 0.05 was considered significant for all statistical tests performed in this work. A chi-squared test was used to evaluate gender differences in group. Normality of SNpc volumes and diffusion metrics was assessed using the Shapiro-Wilk test for each group and all data was found to be normal. The effect of group (SWEDD, PD, control) was tested with separate analysis of variance (ANOVA) for SNpc volume, mean SNpc FW, and mean SNpc R2. Since iron correlates with single-compartment diffusion indices, mean diffusivity and fractional anisotropy, were not used in this analysis (Langley et al., 2020b). ANOVA was used to test the effect of group (SWEDD, PD, control) on the ratio of caudate nucleus striatal binding ratio (SBR) to the putamen SBR. Caudate and putamen striatal binding ratios (SBRs) were obtained from summary variables in the PPMI database. For all ANOVAs, if the interaction was significant, post hoc comparisons between each pair of groups were performed using respective two-tailed t-tests. Correlations between SNpc volume, mean SNpc FW, and mean SNpc R2 and clinical measures [Unified Parkinson’s Disease Rating Scale (UPDRS) and disease duration] were assessed in the PD and SWEDD groups. A correlation was considered significant if P < 0.05 after multiple comparison correction. To test the feasibility of using MRI metrics to differentiate SWEDD from PD, receiver operator characteristic (ROC) curves were obtained for SNpc volume, SNpc FW, and SNpc R2 values.
Results
Participants
Demographic data for each group is shown in Table 1. The ANOVA found no significant group effect in participant age (P = 0.860; F = 0.151), and education (P = 0.322; F = 1.41). No difference in gender was observed between PD and control groups (P = 0.184), PD and SWEDD groups (P = 0.747), and SWEDD and control groups (P = 0.174). A significant group effect was seen in MoCA (P = 0.042; F = 3.218). The CO group had a higher MoCA score as compared to the SWEDD (P = 0.019) and PD (P = 0.034) groups. No difference was seen in MoCA between SWEDD and PD groups (P = 0.439). A significant group effect was seen in UPDRS-III ON score (P < 10–4; F = 45.506) with the PD group having a higher UPDRS-III ON score than the CO (P < 10–4) and SWEDD (P < 10–4) groups. The SWEDD group had a higher UPDRS-III ON score as compared to the CO group (P = 0.003).
Effects of group
A comparison of SNpc contrast in the first echo from the TSE acquisition for control, SWEDD, and PD subjects is shown in Figure 2. The effect of group (PD, SWEDD, control) was tested with ANOVAs in each MRI measure (SNpc volume, SNpc R2, SNpc FW). A significant main effect in group (P < 10–4; F = 33.475) was seen for SNpc volume. Pairwise-comparisons of means showed decreases in SNpc volume in the PD group (PD: 308 ± 14 mm3) relative to the control group (CO: 483 ± 24 mm3; P < 10–3) and SWEDD (SWEDD: 493 ± 24 mm3; P < 10–3) groups. No difference was seen in SNpc volume between SWEDD and control groups (p = 0.235). No significant main effect was found in SNpc R2 (P = 0.564; F = 0.570). A significant main effect in group was seen in SNpc FW (p = 0.027; F = 3.710) with group means showing increases in SNpc FW in the PD group (PD: 0.198 ± 0.006) relative to control group (CO: 0.169 ± 0.010; P = 0.017), and a non-significant trend toward increased SNpc FW in PD vs. SWEDD (SWEDD: 0.177 ± 0.010; P = 0.074) was observed. No difference was seen in SNpc FW between the SWEDD and control (P = 0.579) groups. Group comparisons are shown in Figure 3.
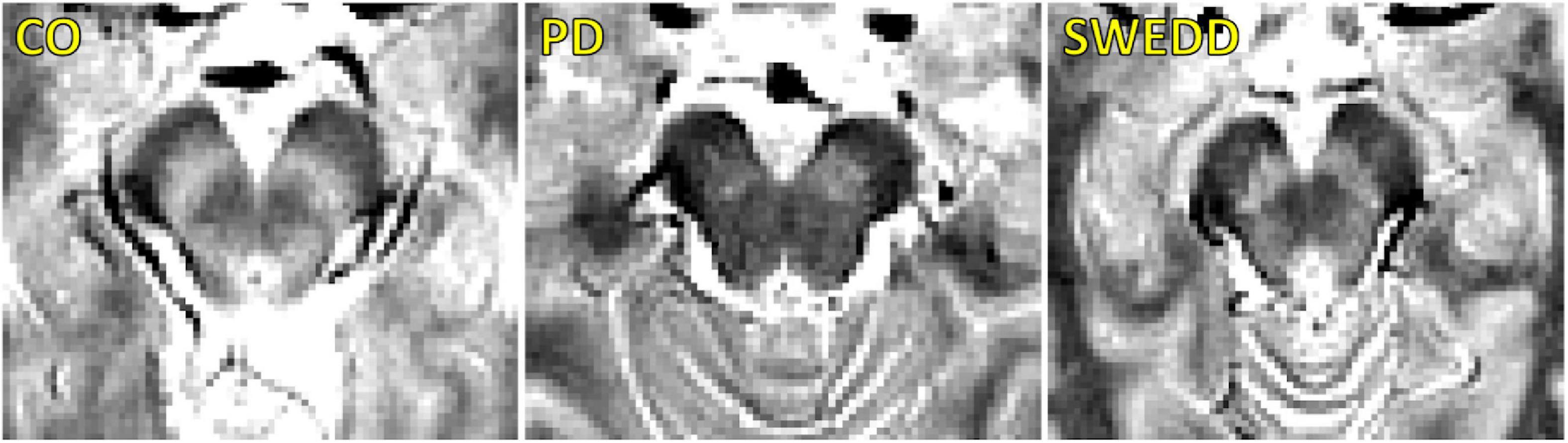
Figure 2. A comparison of neuromelanin-sensitive contrast from the first echo of the TSE acquisition in controls (left), PD (middle), and SWEDD (right) subjects. The PD subject exhibits a loss of contrast as compared to the control and SWEDD subjects.
Figure 3. Group comparisons for substantia nigra pars compacta (SNpc) volume (A), SNpc free water (FW) (B), SNpc R2 (C), left DaT striatal uptake ratio (D), and right DaT striatal uptake ratio (E) in control, SWEDD, and PD groups. AUC curves for SNpc volume, mean SNpc FW, and mean SNpc R2 are shown in panel (F). SNpc volume outperformed mean SNpc FW and mean SNpc R2 as a diagnostic marker to differentiate SWEDD from PD. All box plots, the top and bottom of the box denote the 25th and 75th percentiles, respectively, with the line denoting the median value. In panels (A–F) *P < 0.05 and **P < 0.01.
Using the DaTScan data, the effect of group (PD, SWEDD, control) was tested with ANOVAs on SBRs, i.e., the ratio of striatal uptake to occipital uptake. Specifically, the ratio of caudate nucleus SBR to putamen SBR was determined for each participant, and a significant main effect between groups was observed for the left (F = 68.585, P < 10–3) and right (F = 41.538, P < 10–3) ratios. Pairwise-comparisons of means showed increases in the ratio of caudate SBR to putamen SBR in the PD group (left: 2.7 ± 0.8; right: 2.7 ± 1.0) relative to control group (left: 1.5 ± 0.2; right: 1.3 ± 0.2) as well as relative to the SWEDD group (left: 1.4 ± 0.4; right: 1.5 ± 0.4) on both left and right sides (Ps < 10–3). No difference was seen in the ratio of caudate SBR to putamen SBR between control and SWEDD groups (left: P = 0.819; right: P = 0.496).
Clinical correlations
No significant correlations were observed between any MRI measure (SNpc volume, SNpc FW, or SNpc R2) and any clinical measure (UPDRS-III and disease duration) in the PD group (Ps > 0.267) or in the SWEDD group (Ps > 0.179).
Receiver operator characteristic analysis
Substantia nigra pars compacta volume differentiated SWEDD from PD better than SNpc FW or SNpc R2. The area under the ROC curve (AUC) for SNpc volume was 0.786 [SE = 0.048; 95% confidence interval (CI): 0.692–0.880; P < 10–4]. The AUC for mean SNpc FW was 0.489 (SE = 0.060; 95% CI: 0.371–0.607; P = 0.861) while the AUC for mean SNpc R2 was 0.405 (SE = 0.060; 95% CI: 0.272–0.539; P = 0.163). AUC curves are shown in Figure 3.
Discussion
Participants with SWEDD are individuals who have clinical features of Parkinsonism and meet diagnostic criteria for PD, but they do not exhibit presynaptic dopaminergic deficits on DaTScan (see Figure 1). To date, the underlying pathophysiology in patients with clinical features of Parkinsonism and SWEDD remains controversial. However, these individuals typically do not progress clinically or radiographically as expected in neurodegenerative Parkinsonism (Marek et al., 2014), and they appear to represent a heterogeneous group that does not have idiopathic PD (Lee et al., 2021). Currently, DaTScan is used as a screening tool to exclude SWEDDs in clinical PD studies. In research studies enrolling PD patients within 9 months of diagnosis approximately 5–20% of participants will be SWEDD, with the highest percentage occurring in studies recruiting newly diagnosed PD (Parkinson Study, 2002; Fahn et al., 2004; Marek et al., 2014). However, lack of availability of DaTScan, high cost, and exposure to ionizing radiation from the radioligand limit its use. Thus, there is a need for a widely available screening tool to exclude SWEDDs from enrollment in PD clinical studies, because these individuals are unlikely to have neurodegenerative Parkinsonism (Marek et al., 2014). This study examines the performance of nigral MRI metrics to differentiate SWEDD from PD. We found that nigral MRI metrics (SNpc volume, SNpc FW) in the SWEDD group resemble those derived in the control group and differ from PD.
The radiotracer used in DaTScan binds to DAT in the presynaptic membrane of dopamine neuron axon terminals and is sensitive to the loss of membrane DAT protein, axonal neuronal loss, or nigrostriatal neuronal loss (Makinen et al., 2016). SWEDD patients do not show deficits in striatal DAT uptake, and our analysis of caudate nucleus to putamen SBR found results that were consistent with this. This is an expected result, since the PD and SWEDD groups are stratified based on their DaTScan results, but it is a useful confirmation of the basis of this study design. Neuromelanin-sensitive contrast is correlated with DAT binding (Isaias et al., 2016; Biondetti et al., 2021) and co-localizes with pigmented catecholamine neurons (Kitao et al., 2013). These results imply that SNpc should not undergo neurodegeneration in SWEDD. In agreement with this, no difference was observed in nigral FW and nigral volume between SWEDD and control groups.
In contrast to the SWEDD group, a reduction in SNpc volume was observed in the PD group relative to the control group. This result is in agreement with earlier studies that found nigral volume is reduced in PD patients (Ogisu et al., 2013; Castellanos et al., 2015) or contrast is reduced in the lateral and ventral portion of SNpc (Huddleston et al., 2017). The reduction in volume may be caused by depletion of melanized neurons in nigrosome-1, the subregion of SNpc that undergoes the greatest loss neuronal loss (Damier et al., 1999), as earlier studies have noted reductions in neuromelanin-sensitive contrast in regions consistent with nigrosome-1 (Schwarz et al., 2011; Langley et al., 2020a). Increases in nigral FW were also seen in the PD group relative to the control group, which is consistent with other studies reporting nigral FW increases in PD (Ofori et al., 2015; Langley et al., 2021).
When comparing SWEDD and PD, nigral volume outperformed nigral FW and nigral R2 as a diagnostic marker. These findings suggest that nigral volume, or other nigrostriatal imaging measures, could be useful for removal of SWEDDs in clinical trials. While the SNpc volume discrimination of PD vs. SWEDD approaches a useful level for clinical trial participant selection with an AUC of 0.786 (AUC > 0.8 is a typically discussed threshold), further improvements in accuracy are needed to make clinical decisions at the individual level, which would require AUC > 0.9. Diagnostic accuracy may be improved by using neuromelanin-sensitive acquisitions based on explicit MT effects (Wengler et al., 2020; van der Pluijm et al., 2021) or incorporating other metrics, such as UPDRS score or MRI metrics from other structures, in machine learning classification algorithms. Metrics derived from neuromelanin-sensitive data show promise for differential diagnosis of Parkinsonian syndromes (Ohtsuka et al., 2014; Wang et al., 2019; Simoes et al., 2020) and further development of these metrics may allow for accurate discrimination of SWEDD from PD.
This study has several caveats. First, R2, a measure derived from a multiecho TSE sequence, was used to measure nigral iron deposition in PD and SWEDD. R2 is not as sensitive to iron deposition as R2* or susceptibility, which are derived from gradient-echo acquisitions (Langkammer et al., 2010). Further, R2* has been shown to be a robust marker for examining nigral iron deposition in PD (Langley et al., 2019) and may aid in differentiating SWEDD from PD. Second, incidental MT effects from an interleaved multislice TSE acquisition were used to generate contrast sensitive to neuromelanin. Neuromelanin-sensitive MRI approaches employing explicit MT effects exhibit high scan-rescan reproducibility in controls, outperforming methods based on incidental MT effects (Wengler et al., 2020; van der Pluijm et al., 2021). The use of explicit MT effects may improve diagnostic accuracy for differentiating SWEDD from PD. Finally, no correlations were observed between MRI measures (nigral R2, nigral FW, and nigral volume) and clinical features (disease duration, UPDRS-III ON score). The lack of correlation may also be explained in part by insensitivity of the imaging measures (incidental MT effects and R2). However, since the PD subjects in PPMI are recruited at the time of initial diagnosis and therefore have very similar disease duration and clinical states (e.g., similar UPDRS-III scores), the lack of correlation may be due to insufficient variation in the clinical variables.
In conclusion, nigral volume and nigral FW in the SWEDD group were similar to that of the control group while a reduction in nigral volume and an increase in FW were observed in the PD group relative to the SWEDD and control groups. The changes in nigral volume and FW in the PD group are consistent with SNpc neurodegeneration effects in PD. With further development, nigral volume and FW may become markers used for differential diagnosis of SWEDD from PD. These imaging markers may be used to exclude individuals with SWEDD from PD neuroprotection trials.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here, https://www.ppmi-info.org/access-data-specimens/data.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional IRB approved the study for each site and subjects gave written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JL: conceptualization, data curation, formal analysis, methodology, writing – original draft, and writing – review and editing. KH: formal analysis and writing – review and editing. XH and DH: conceptualization, methodology, and writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the NIH-NINDS 1K23NS105944-01A1 (DH), NIH-NIA 1U19 AG071754-01 (DH, XH, and JL), the Department of Veteran Affairs 1I01RX002967-01A2 (DH), the Emory American Parkinson’s Disease Association Center for Advanced Research (DH), the Emory Lewy Body Dementia Association Research Center of Excellence (DH), and the Michael J. Fox Foundation for Parkinson’s Research (MJF-10854 and MJFF-010556; DH, XH, and JL).
Acknowledgments
Parkinson’s progression markers initiative (PPMI) – a public-private partnership – was funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including (list the full names of all of the PPMI funding partners found at www.ppmi-info.org/about-ppmi/who-we-are/study-sponsors).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Athauda, D., and Foltynie, T. (2015). The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 11, 25–40. doi: 10.1038/nrneurol.2014.226
Batla, A., Erro, R., Stamelou, M., Schneider, S. A., Schwingenschuh, P., Ganos, C., et al. (2014). Patients with scans without evidence of dopaminergic deficit: a long-term follow-up study. Mov. Disord. 29, 1820–1825. doi: 10.1002/mds.26018
Baudrexel, S., Nurnberger, L., Rub, U., Seifried, C., Klein, J. C., Deller, T., et al. (2010). Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. Neuroimage 51, 512–520. doi: 10.1016/j.neuroimage.2010.03.005
Bhattacharjee, S., Paramanandam, V., and Bhattacharya, A. (2019). Analysis of the effect of dopamine transporter scan on the diagnosis and management in a tertiary neurology center. Neurohospitalist 9, 144–150. doi: 10.1177/1941874419829293
Biondetti, E., Santin, M. D., Valabregue, R., Mangone, G., Gaurav, R., Pyatigorskaya, N., et al. (2021). The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 144, 3114–3125. doi: 10.1093/brain/awab191
Burciu, R. G., Ofori, E., Archer, D. B., Wu, S. S., Pasternak, O., McFarland, N. R., et al. (2017). Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain 140, 2183–2192. doi: 10.1093/brain/awx146
Castellanos, G., Fernandez-Seara, M. A., Lorenzo-Betancor, O., Ortega-Cubero, S., Puigvert, M., Uranga, J., et al. (2015). Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov. Disord. 30, 945–952. doi: 10.1002/mds.26201
Chen, X., Huddleston, D. E., Langley, J., Ahn, S., Barnum, C. J., Factor, S. A., et al. (2014). Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn. Reson. Imaging 32, 1301–1306. doi: 10.1016/j.mri.2014.07.003
Cheng, H.-C., Ulane, C. M., and Burke, R. E. (2010). Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 67, 715–725. doi: 10.1002/ana.21995
Damier, P., Hirsch, E. C., Agid, Y., and Graybiel, A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8), 1437–1448.
de la Fuente-Fernandez, R. (2012). Is DaTSCAN really needed for accurate Parkinson’s disease diagnosis? Expert. Rev. Neurother. 12, 1375–1377. doi: 10.1586/ern.12.135
Dexter, D. T., Wells, F. R., Agid, F., Agid, Y., Lees, A. J., Jenner, P., et al. (1987). Increased nigral iron content in postmortem parkinsonian brain. Lancet 2, 1219–1220.
Dixon, W. T., Engels, H., Castillo, M., and Sardashti, M. (1990). Incidental magnetization transfer contrast in standard multislice imaging. Magn. Reson. Imaging 8, 417–422.
Du, G., Lewis, M. M., Styner, M., Shaffer, M. L., Sen, S., Yang, Q. X., et al. (2011). Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov. Disord. 26, 1627–1632. doi: 10.1002/mds.23643
Erro, R., Schneider, S. A., Stamelou, M., Quinn, N. P., and Bhatia, K. P. (2016). What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J. Neurol. Neurosurg. Psychiatry 87, 319–323. doi: 10.1136/jnnp-2014-310256
Fahn, S., Oakes, D., Shoulson, I., Kieburtz, K., Rudolph, A., Lang, A., et al. (2004). Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 351, 2498–2508. doi: 10.1056/NEJMoa033447
Fahn, S., and Parkinson Study, G. (2005). Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J. Neurol. 252(Suppl. 4), IV37–IV42. doi: 10.1007/s00415-005-4008-5
Fearnley, J. M., and Lees, A. J. (1991). Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5), 2283–2301.
Febo, M., Perez, P. D., Ceballos-Diaz, C., Colon-Perez, L. M., Zeng, H., Ofori, E., et al. (2020). Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-gamma. Brain Struct. Funct. 225, 427–439. doi: 10.1007/s00429-019-02017-1
Garyfallidis, E., Brett, M., Amirbekian, B., Rokem, A., van der Walt, S., Descoteaux, M., et al. (2014). Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 8:8. doi: 10.3389/fninf.2014.00008
Guttuso, T. Jr., Bergsland, N., Hagemeier, J., Lichter, D. G., Pasternak, O., and Zivadinov, R. (2018). Substantia Nigra free water increases longitudinally in Parkinson disease. AJNR Am. J. Neuroradiol. 39, 479–484. doi: 10.3174/ajnr.A5545
He, N., Ghassaban, K., Huang, P., Jokar, M., Wang, Y., Cheng, Z., et al. (2021). Imaging iron and neuromelanin simultaneously using a single 3D gradient echo magnetization transfer sequence: Combining neuromelanin, iron and the nigrosome-1 sign as complementary imaging biomarkers in early stage Parkinson’s disease. Neuroimage 230:117810. doi: 10.1016/j.neuroimage.2021.117810
He, N., Langley, J., Huddleston, D. E., Chen, S., Huang, P., Ling, H., et al. (2020). Increased iron-deposition in lateral-ventral substantia nigra pars compacta: A promising neuroimaging marker for Parkinson’s disease. Neuroimage Clin. 28:102391. doi: 10.1016/j.nicl.2020.102391
Huddleston, D. E., Langley, J., Sedlacik, J., Boelmans, K., Factor, S. A., and Hu, X. P. (2017). In vivo detection of lateral-ventral tier nigral degeneration in Parkinson’s disease. Hum. Brain Mapp. 38, 2627–2634. doi: 10.1002/hbm.23547
Isaias, I. U., Trujillo, P., Summers, P., Marotta, G., Mainardi, L., Pezzoli, G., et al. (2016). Neuromelanin imaging and dopaminergic loss in Parkinson’s disease. Front. Aging Neurosci. 8:196. doi: 10.3389/fnagi.2016.00196
Jin, Z., Wang, Y., Jokar, M., Li, Y., Cheng, Z., Liu, Y., et al. (2022). Automatic detection of neuromelanin and iron in the midbrain nuclei using a magnetic resonance imaging-based brain template. Hum. Brain Mapp. 43, 2011–2025. doi: 10.1002/hbm.25770
Keren, N. I., Taheri, S., Vazey, E. M., Morgan, P. S., Granholm, A. C., Aston-Jones, G. S., et al. (2015). Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. Neuroimage 113, 235–245. doi: 10.1016/j.neuroimage.2015.03.020
Kitao, S., Matsusue, E., Fujii, S., Miyoshi, F., Kaminou, T., Kato, S., et al. (2013). Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology 55, 947–953. doi: 10.1007/s00234-013-1199-9
Kosta, P., Argyropoulou, M. I., Markoula, S., and Konitsiotis, S. (2006). MRI evaluation of the basal ganglia size and iron content in patients with Parkinson’s disease. J. Neurol. 253, 26–32. doi: 10.1007/s00415-005-0914-9
Langkammer, C., Krebs, N., Goessler, W., Scheurer, E., Ebner, F., Yen, K., et al. (2010). Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257, 455–462. doi: 10.1148/radiol.10100495
Langley, J., He, N., Huddleston, D. E., Chen, S., Yan, F., Crosson, B., et al. (2019). Reproducible detection of nigral iron deposition in 2 Parkinson’s disease cohorts. Mov. Disord. 34, 416–419. doi: 10.1002/mds.27608
Langley, J., Huddleston, D. E., Crosson, B., Song, D. D., Factor, S. A., and Hu, X. (2020a). Multimodal assessment of nigrosomal degeneration in Parkinson’s disease. Parkinson. Relat. Disord. 80, 102–107. doi: 10.1016/j.parkreldis.2020.09.021
Langley, J., Huddleston, D. E., and Hu, X. (2021). Nigral diffusivity, but not free water, correlates with iron content in Parkinson’s disease. Brain Commun. 3:fcab251. doi: 10.1093/braincomms/fcab251
Langley, J., Huddleston, D. E., Liu, C. J., and Hu, X. (2017a). Reproducibility of locus coeruleus and substantia nigra imaging with neuromelanin sensitive MRI. MAGMA 30, 121–125. doi: 10.1007/s10334-016-0590-z
Langley, J., Huddleston, D. E., Sedlacik, J., Boelmans, K., and Hu, X. P. (2017b). Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov. Disord. 32, 441–449. doi: 10.1002/mds.26883
Langley, J., Hussain, S., Flores, J. J., Bennett, I. J., and Hu, X. (2020b). Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta. Neurobiol. Aging 87, 89–97. doi: 10.1016/j.neurobiolaging.2019.11.016
Lee, J. W., Song, Y. S., Kim, H., Ku, B. D., and Lee, W. W. (2021). Patients with scans without evidence of dopaminergic deficit (SWEDD) do not have early Parkinson’s disease: Analysis of the PPMI data. PLoS One 16:e0246881. doi: 10.1371/journal.pone.0246881
Makinen, E., Joutsa, J., Johansson, J., Maki, M., Seppanen, M., and Kaasinen, V. (2016). Visual versus automated analysis of [I-123]FP-CIT SPECT scans in parkinsonism. J. Neural. Transm. 123, 1309–1318. doi: 10.1007/s00702-016-1586-6
Marek, K., Seibyl, J., Eberly, S., Oakes, D., Shoulson, I., Lang, A. E., et al. (2014). Longitudinal follow-up of SWEDD subjects in the PRECEPT study. Neurology 82, 1791–1797. doi: 10.1212/WNL.0000000000000424
Martin, W. R., Wieler, M., and Gee, M. (2008). Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70(16 Pt 2), 1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5
Ofori, E., Pasternak, O., Planetta, P. J., Burciu, R., Snyder, A., Febo, M., et al. (2015). Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol. Aging 36, 1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029
Ogisu, K., Kudo, K., Sasaki, M., Sakushima, K., Yabe, I., Sasaki, H., et al. (2013). 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease. Neuroradiology 55, 719–724. doi: 10.1007/s00234-013-1171-8
Ohtsuka, C., Sasaki, M., Konno, K., Kato, K., Takahashi, J., Yamashita, F., et al. (2014). Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinson. Relat. Disord. 20, 755–760. doi: 10.1016/j.parkreldis.2014.04.005
Parkinson Study, G. (2002). Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287, 1653–1661. doi: 10.1001/jama.287.13.1653
Peran, P., Cherubini, A., Assogna, F., Piras, F., Quattrocchi, C., Peppe, A., et al. (2010). Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133, 3423–3433. doi: 10.1093/brain/awq212
Reimao, S., Pita Lobo, P., Neutel, D., Correia Guedes, L., Coelho, M., Rosa, M. M., et al. (2015). Substantia nigra neuromelanin magnetic resonance imaging in de novo Parkinson’s disease patients. Eur. J. Neurol. 22, 540–546. doi: 10.1111/ene.12613
Schwarz, S. T., Rittman, T., Gontu, V., Morgan, P. S., Bajaj, N., and Auer, D. P. (2011). T1-Weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson’s disease. Mov. Disord. 26, 1633–1638.
Simoes, R. M., Castro Caldas, A., Grilo, J., Correia, D., Guerreiro, C., Pita Lobo, P., et al. (2020). A distinct neuromelanin magnetic resonance imaging pattern in parkinsonian multiple system atrophy. BMC Neurol. 20:432. doi: 10.1186/s12883-020-02007-5
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl. 1), S208–S219. doi: 10.1016/j.neuroimage.2004.07.051
van der Pluijm, M., Cassidy, C., Zandstra, M., Wallert, E., de Bruin, K., Booij, J., et al. (2021). Reliability and reproducibility of Neuromelanin-sensitive imaging of the Substantia Nigra: a comparison of three different sequences. J. Magn. Reson. Imaging 53, 712–721. doi: 10.1002/jmri.27384
Wang, J., Huang, Z., Li, Y., Ye, F., Wang, C., Zhang, Y., et al. (2019). Neuromelanin-sensitive MRI of the substantia nigra: An imaging biomarker to differentiate essential tremor from tremor-dominant Parkinson’s disease. Parkinson. Relat. Disord. 58, 3–8. doi: 10.1016/j.parkreldis.2018.07.007
Wengler, K., He, X., Abi-Dargham, A., and Horga, G. (2020). Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. Neuroimage 208:116457. doi: 10.1016/j.neuroimage.2019.116457
Keywords: scans without evidence of dopaminergic deficit, Parkinson’s disease, neuromelanin, substantia nigra pars compacta, free water
Citation: Langley J, Hwang KS, Hu XP and Huddleston DE (2022) Nigral volumetric and microstructural measures in individuals with scans without evidence of dopaminergic deficit. Front. Neurosci. 16:1048945. doi: 10.3389/fnins.2022.1048945
Received: 20 September 2022; Accepted: 28 October 2022;
Published: 24 November 2022.
Edited by:
Maria Teresa Pellecchia, University of Salerno, ItalyReviewed by:
Sulev Kõks, Murdoch University, AustraliaAntonio Martin-Bastida, University of Navarra, Spain
Copyright © 2022 Langley, Hwang, Hu and Huddleston. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping P. Hu, eGh1QGVuZ3IudWNyLmVkdQ==; Daniel E. Huddleston, ZGFuaWVsLmh1ZGRsZXN0b25AZW1vcnkuZWR1
 Jason Langley
Jason Langley Kristy S. Hwang
Kristy S. Hwang Xiaoping P. Hu1,3*
Xiaoping P. Hu1,3*