- 1Department of Molecular, Cellular and Biomedical Sciences, CUNY School of Medicine, New York, NY, United States
- 2IRCCS Centro Neurolesi Bonino Pulejo-Piemonte, Messina, Italy
Movement-related oscillations in the beta range (from 13 to 30 Hz) have been observed over sensorimotor areas with power decrease (i.e., event-related desynchronization, ERD) during motor planning and execution followed by an increase (i.e., event-related synchronization, ERS) after the movement’s end. These phenomena occur during active, passive, imaged, and observed movements. Several electrophysiology studies have used beta ERD and ERS as functional indices of sensorimotor integrity, primarily in diseases affecting the motor system. Recent literature also highlights other characteristics of beta ERD and ERS, implying their role in processes not strictly related to motor function. Here we review studies about movement-related ERD and ERS in diseases characterized by motor dysfunction, including Parkinson’s disease, dystonia, stroke, amyotrophic lateral sclerosis, cerebral palsy, and multiple sclerosis. We also review changes of beta ERD and ERS reported in physiological aging, Alzheimer’s disease, and schizophrenia, three conditions without overt motor symptoms. The review of these works shows that ERD and ERS abnormalities are present across the spectrum of the examined pathologies as well as development and aging. They further suggest that cognition and movement are tightly related processes that may share common mechanisms regulated by beta modulation. Future studies with a multimodal approach are warranted to understand not only the specific topographical dynamics of movement-related beta modulation but also the general meaning of beta frequency changes occurring in relation to movement and cognitive processes at large. Such an approach will provide the foundation to devise and implement novel therapeutic approaches to neuropsychiatric disorders.
Introduction
Almost a century after Berger’s first description of sensorimotor rhythms (Berger, 1929), a growing number of papers has established solid links between movement and specific dynamics of brain oscillations. Numerous is the evidence, obtained by non-invasive cortical recording techniques, such as electroencephalography (EEG) and magnetoencephalography (MEG), as well as by invasive cortical (electrocorticogram, ECoG) and subcortical recording techniques (from deep brain stimulation, DBS, devices). Changes in the electric or magnetic field over the sensorimotor cortex have been classified mostly based on their frequency. The first bandwidth described related to movement called rolandic mu rhythm or “central alpha” (8–12 Hz) (Magnus, 1954). Despite being characterized by similar frequency and amplitude as the occipital alpha oscillations, this rhythm was observed on centrally located scalp electrodes and its amplitude decreased (Gastaut, 1952), or desynchronized, over the sensorimotor area contralaterally to the moving effector for voluntary, imaged, passive, reflexive, and observed movements (McFarland et al., 2000). In the last 40 years, recordings with different electrophysiological techniques have shown that movements are also accompanied by changes over the sensorimotor areas in both beta (13–30 Hz) and gamma (30–200 Hz) bands (Pfurtscheller, 1981; Feige et al., 1996; Kilavik et al., 2013; Nowak et al., 2018). While gamma activity has a prokinetic nature since it increases during movement planning and execution (Muthukumaraswamy et al., 2010; Cheyne and Ferrari, 2013; Tatti et al., 2022), beta activity may have some “anti-kinetic” significance (Brown, 2006; Kilavik et al., 2013). Indeed, recordings over the sensorimotor areas show that beta power decreases or desynchronizes during the planning and the execution of movements, a phenomenon known as event-related desynchronization (ERD). Once movement is terminated, beta power starts increasing and reaches a peak with values greater than those at baseline, an occurrence known as event-related synchronization (ERS) (Pfurtscheller, 1981; Engel and Fries, 2010; Kilavik et al., 2013; Zaepffel et al., 2013; Spitzer and Haegens, 2017). These oscillations are present both over the sensorimotor cortex and in the basal ganglia, although controversies exist on whether they originate independently or are inter-dependent expression of the cortico-basal ganglia network activity (Spitzer and Haegens, 2017; Schmidt et al., 2019; Barone and Rossiter, 2021).
Despite extensive work in humans and animals, what is the functional role of the sensorimotor beta oscillations is a question still in need of an answer. The classic “idling” hypothesis views beta rhythm as an inhibitory frequency of the sensorimotor system (Pfurtscheller et al., 1996; Zaepffel et al., 2013). Accordingly, beta ERS and ERD would represent an “idle” state and an activation of motor cortex neurons, respectively. However, this hypothesis does not explain why similar modulation of beta power can also be observed during passive movements, motor imagery, and movement observation (Kilavik et al., 2013; Zaepffel et al., 2013; Tatti et al., 2019), as well as why its amplitude is not associated with specific movement parameters or effectors (Tatti et al., 2019), suggesting that beta modulation may occur in different domains, including the sensorimotor and cognitive domains (Engel and Fries, 2010).
Two new lines of evidence support this wider view of beta ERD/ERS, beyond their significance in motor planning and execution. First, during movements, the beta ERD/ERS dynamic has been observed not only over sensorimotor areas but also over frontal and pre-frontal areas (Moisello et al., 2015; Nelson et al., 2017; Ricci et al., 2019b; Tatti et al., 2021, 2020). In addition, that same pattern of beta ERD/ERS oscillation accompanies also working memory (Lundqvist et al., 2016; Miller et al., 2018) and focused attention tasks (Hanslmayr et al., 2014; Zavala et al., 2017), suggesting that beta ERD/ERS are not mere reflection of motor-related activity but their significance must extend to processes common to motor and cognitive functions. In this perspective, beta ERD would serve to release cortical inhibition, thus enabling movement execution or cognitive flow, while beta ERS would signal the need to maintain the current motor or cognitive set (Engel and Fries, 2010; Zaepffel et al., 2013; Spitzer and Haegens, 2017). Based on those observations, it has also been proposed that beta modulation might be a common denominator for attention-related processes, in both motor and cognitive domains, needed to filter out and cancel confounders or phenomena that are irrelevant to the event (Schmidt et al., 2019). Second, beta ERD-ERS could be interpreted from a metabolic perspective: a recent series of studies has shown that movement-related beta modulation, and in particular ERS amplitude, increases with practice with a cumulative effect that was more visible over the most involved brain areas and that returned to baseline after simple rest without sleep (Moisello et al., 2015; Nelson et al., 2021, 2017; Tatti et al., 2021, 2020). Therefore, ERS—and beta modulation in general—may represent an expression of energy consumption that is necessary for use-dependent and plasticity processes (Ghilardi et al., 2021). This view is supported by evidence in animals and humans that have been linking beta power changes to GABA and lactate changes (Jensen et al., 2005; Roopun et al., 2006; Yamawaki et al., 2008; Hall et al., 2011, 2010; Muthukumaraswamy et al., 2013; Rossiter et al., 2014b; Grønli et al., 2016) as well as to learning and plasticity (Hsu et al., 2011; Tan et al., 2016; Nelson et al., 2017).
No matter the precise significance of beta ERD, ERS, and movement-related beta modulation in general, all past studies have considered them as hallmark of the sensorimotor system functioning (Espenhahn et al., 2017) and thus, as possible biomarkers of motor symptoms in several neurological and psychiatric diseases. Indeed, movement-related beta activity has been extensively studied in patients with Parkinson’s disease (PD), the second most common neurodegenerative disorder that is still diagnosed with the appearance of motor symptoms, especially bradykinesia. Conversely, only a few studies focused on cognitive and behavioral disorders that are not specifically characterized by motor symptoms, such as Alzheimer’s disease (AD), schizophrenia, and physiological aging, conditions where subclinical motor or sensorimotor abnormalities can be experimentally detected. With this review, we wished to answer the following questions: Are abnormalities of movement-related beta ERD and ERS clearly and specifically linked to motor symptoms? Are these abnormalities present in pathological conditions other than classical motor disorders?
We thus focused on studies investigating movement-related ERD and ERS in the beta range during motor tasks performed with a limb and recorded with electrophysiological techniques with high time resolution (such as EEG, MEG, ECog, and DBS) that allows for differentiating phases of planning, execution and post-movement. The reviewed papers were on pathologies both specifically affecting the motor system [e.g., PD, dystonia, stroke, amyotrophic lateral sclerosis (ALS), cerebral palsy (CP), and multiple sclerosis, (MS)] and in conditions that are not strictly associated with motor disorders, including physiological aging, AD, schizophrenia, and obsessive-compulsive disorders (OCD). The research of the literature did not disclose studies about beta ERD or ERS in other psychiatric conditions, such as major depression.
The results of this review indicate that abnormalities of movement-related beta ERD and ERS have been described not only in classical motor disorders but also in pathologies that do not present with overt motor signs. Most importantly, such electrophysiological abnormalities do not appear to be specifically linked to motor symptoms but, in some instances, they were rather associated to non-motor problems. Altogether, the review of those studies suggests that beta ERD and ERS cannot be considered pure biomarkers of specific motor aspects, but they rather represent neural mechanisms common to features of motor and non-motor functions.
Movement-related beta event-related desynchronization and event-related synchronization in neuropsychiatric diseases
Throughout the present paper, we use the terminology of beta ERD to indicate decreases of beta power before and during movement and beta ERS for increases of beta power after movements. A description of all the retrieved studies is reported in Tables 1–4. A summary of the results are in Table 5.

Table 2. Review of studies on movement-related beta ERD and ERS over cortical and subcortical structures in Parkinson’s disease with EEG, MEG, cortical (ECoG) and subcortical (STN DBS) recordings.
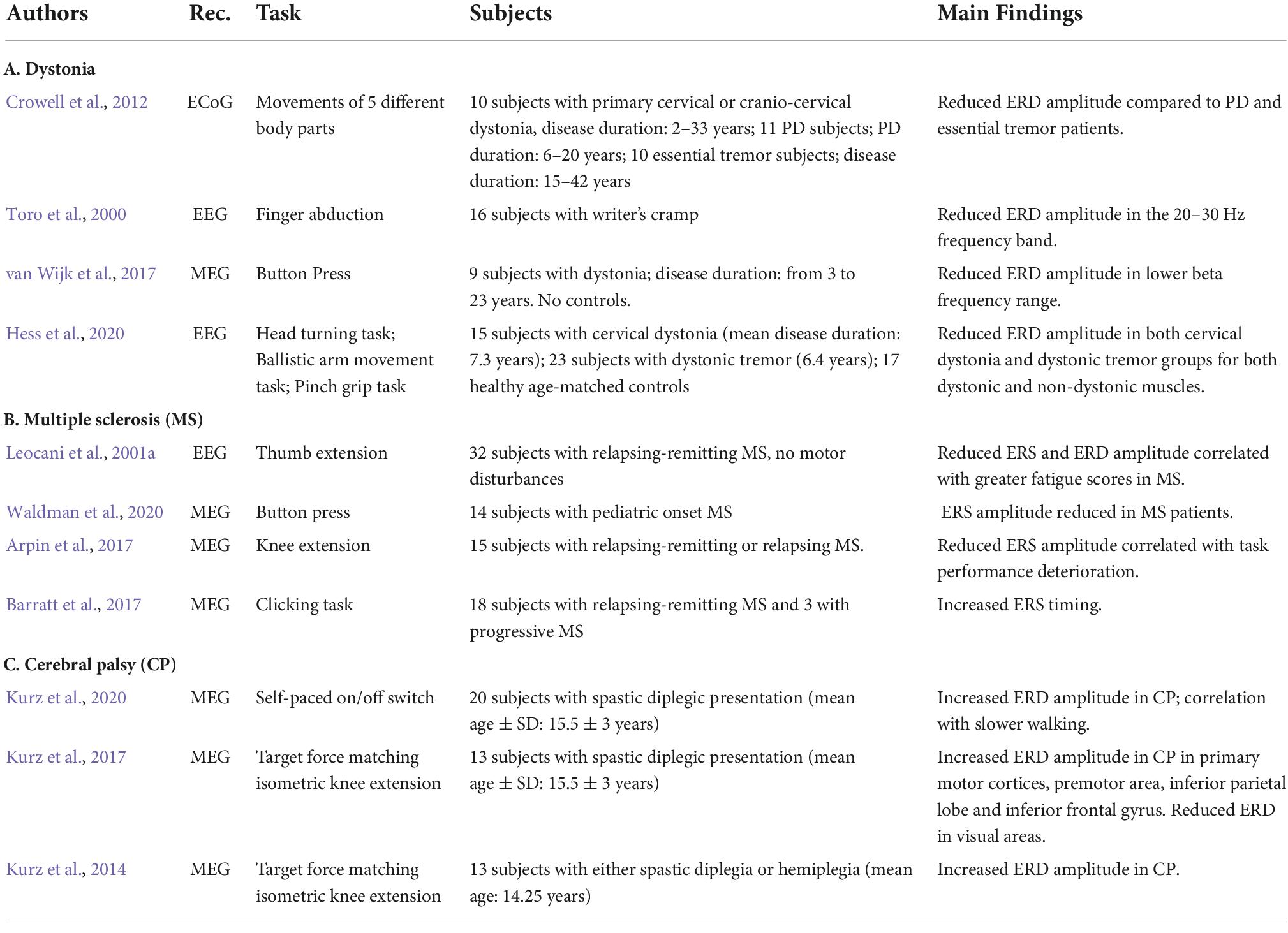
Table 3. Review of studies on movement-related beta ERD and ERS in dystonia, multiple sclerosis, and cerebral palsy.
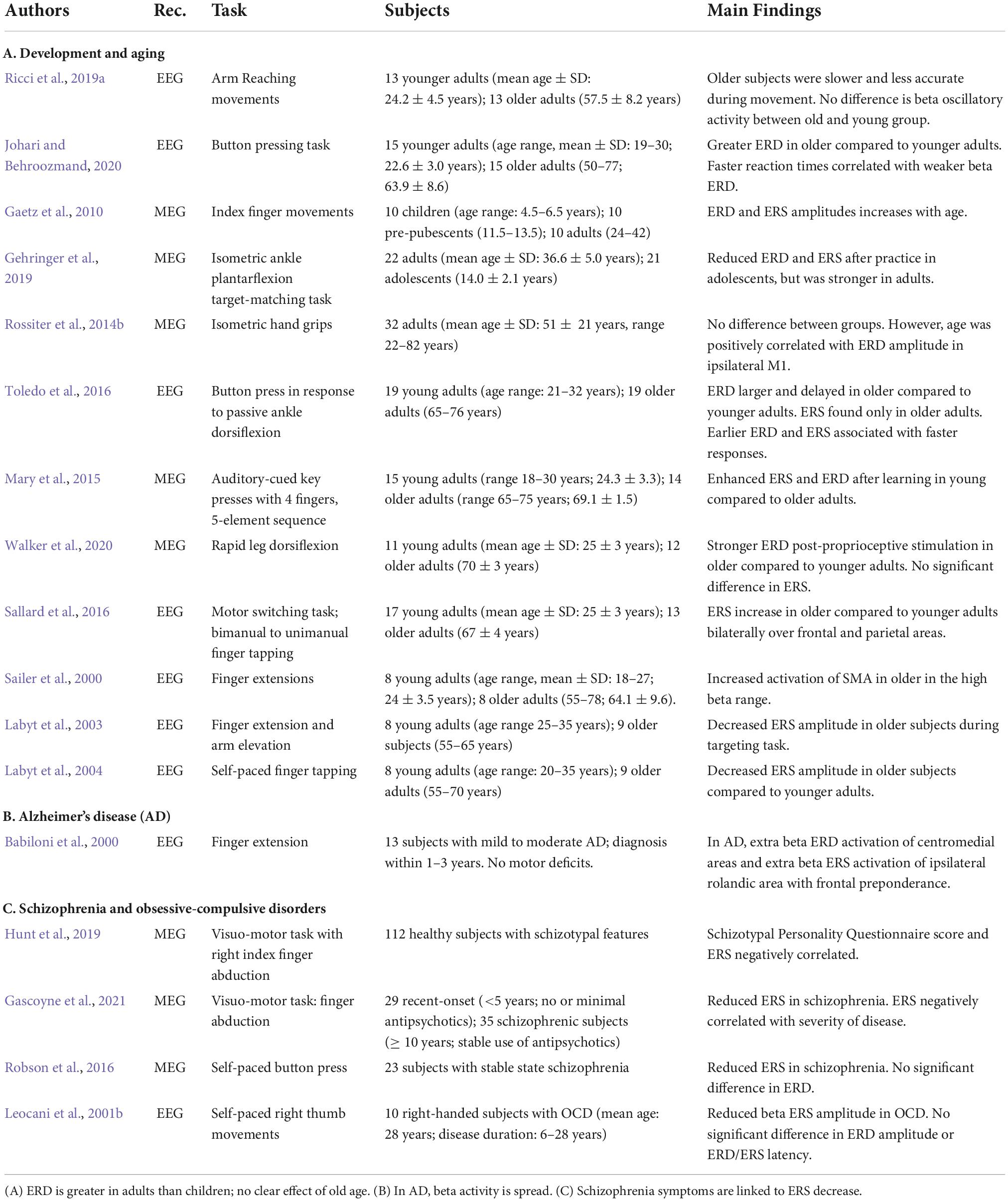
Table 4. Review of studies on movement-related beta ERD and ERS in development and aging, Alzheimer’s disease, and schizophrenia.
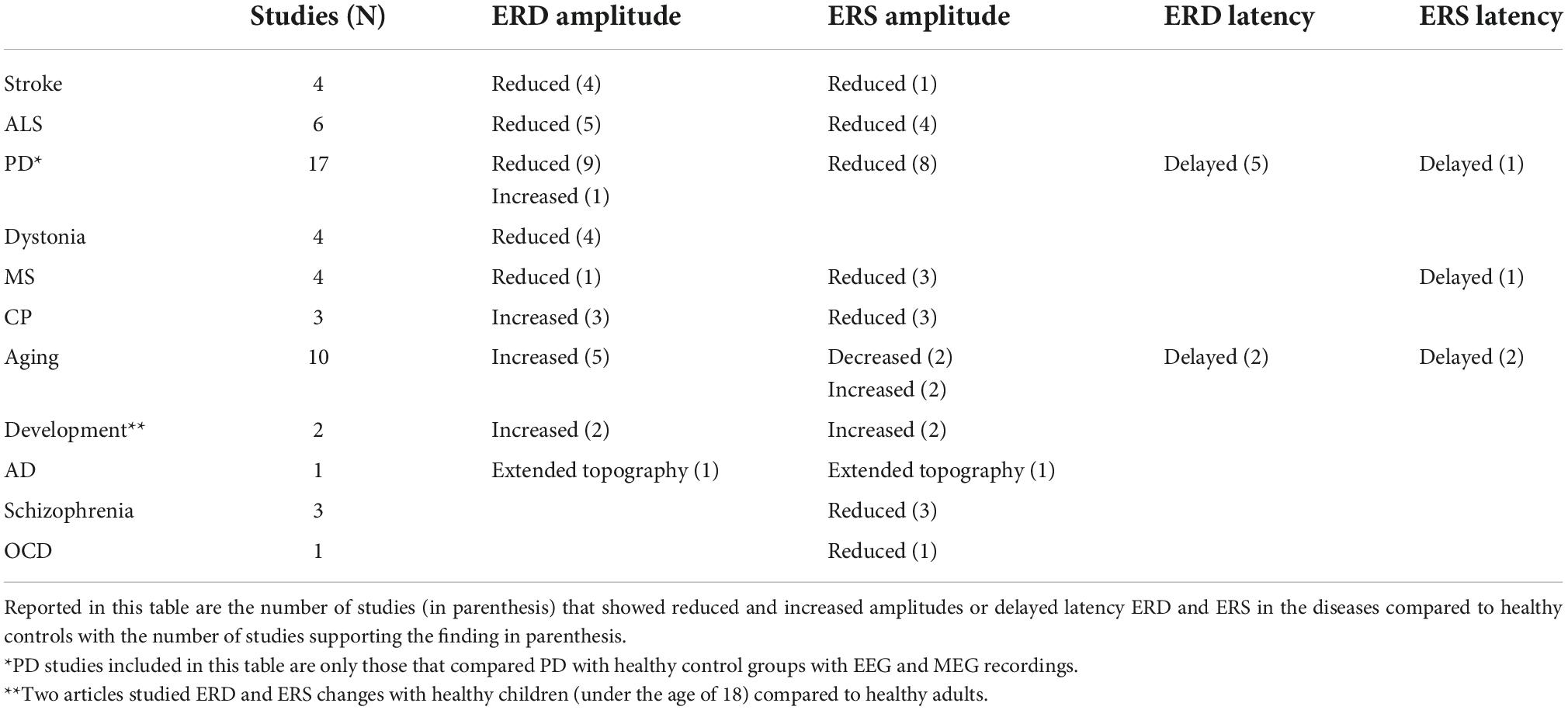
Stroke
Stroke is a leading cause of disability in the United States. Both ischemic and hemorrhagic stroke elicit widespread complications that depend on the degree of damage and the brain areas involved. Approximately 90% of post-stroke patients experience motor deficits that lead to disability and impediments in their daily living (Williams et al., 1999). Understanding the changes of beta oscillations and their functional relevance could thus help in tailoring effective rehabilitative strategies, including non-invasive brain stimulation techniques (Motolese et al., 2022). Here we discuss the findings of papers on movement-related beta ERD and ERS recorded with EEG or MEG in post-stroke patients during different types of motor tasks (Table 1A).
The majority of these studies focused on ERD, the biomarker that shows the most dramatic changes with stroke (Table 1A). Indeed, results show that stroke patients display reduced ERD in the ipsilesional hemisphere. In one MEG study, patients with various levels of impairment and lesion sites were tested from less than a month to 17 years after their first stroke (Rossiter et al., 2014a). In stroke patients, the amplitude of the ERD amplitude over the ipsilesional primary motor cortex was reduced and negatively correlated with the degree of motor impairment. In line with these results, an EEG study using a continuous tracking task and a simple wrist flexion-extension task found that the magnitude of ERD was on average 10% smaller in chronic stroke patients compared to controls (Espenhahn et al., 2020). Another MEG study in chronic stroke patients performing a finger tapping task confirmed the ERD amplitude reduction, while ERS was sometimes undetectable (Shiner et al., 2015). Greater ERD and ERS amplitudes were associated with better performance. In line with those findings, an EEG study focused on spastic co-contraction in post-stroke patients and reported a significant ERD decrease during elbow extensions. The ERD decrease correlated with an increased activation of elbow flexors that, in turn, reduced the active elbow motion. The authors concluded that these phenomena likely gave rise to the spastic co-contraction that is present in many stroke patients (Chalard et al., 2020).
In summary, the ERD reduction reported by these studies could be due to the inability to decrease beta power and thus to properly activate the motor cortex, thereby negatively impacting the descending motor signals and the resulting movement (Rossiter et al., 2014a). Adam et al. (2015) also suggested that stroke lesions may increase the variability of ERD onset, peak, and duration across trials, thus causing a reduced amplitude of the averaged ERD. Also, since increase of GABA-A in the primary motor cortex correlates with enhanced ERD, stroke lesions may affect GABA-A receptors or decrease the GABA concentration at the synaptic level (Adam et al., 2015).
Another important finding of these studies is that, following a stroke, the contralesional hemisphere seems to play a larger role during movement. In fact, a MEG study showed that the ERD amplitude ratio (ERD of lesioned M1/ERD of contralesional M1), an expression of interhemispheric asymmetry, was usually lower in patients compared to controls and negatively correlated with the degree of motor impairment (Rossiter et al., 2014a). A plausible explanation is that, in normal conditions, contralateral M1 activation also inhibits the opposite M1 in order to produce an appropriate movement. In case of stroke, the lesioned M1 cannot efficiently inhibit the contralesional hemisphere, thus explaining the increased ERD over the spared M1. On the other hand, the increased recruitment of the contralesional hemisphere shortly after stroke could function as a recovery or compensatory mechanism (Quandt et al., 2019).
In addition to amplitude, the temporal characteristics of movement-related beta ERD and ERS may also be of some significance to understand stroke-related changes and recovery mechanisms. Interestingly, Shiner et al. (2015) found that, in chronic stroke, the shorter the ERD duration the better was the degree of motor function. Conversely, ERS duration (which was not quantifiable in patients with greater motor impairment) was positively correlated with the degree of motor function (Shiner et al., 2015). While there is no clear explanation for these phenomena, one could speculate that the prolonged ERD is due to the loss of the appropriate neuronal population to be recruited, so that greater neuronal loss is associated with longer ERD duration and poorer motor performance.
In conclusion, the reviewed studies suggest that that abnormalities of ERD and ERS in stroke patients are somewhat related to abnormal motor function in general or specifically during the motor task.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is a progressive disorder characterized by the selective degeneration of both upper and lower motor neurons while the somatosensory system is spared by the degenerative process. The onset of clinical manifestation is insidious with focal weakness that progressively spreads to most muscles, eventually resulting in atrophy. Symptoms of ALS typically begin in the limbs, although about one third of cases involve bulbar muscles, heralded by difficulty chewing, swallowing, or speaking (Brown and Al-Chalabi, 2017). Because motor execution may not be plausible due to motor limitations, many studies resorted to motor imagery, a condition that still elicits ERD/ERS patterns albeit with reduced magnitudes in both normal controls (Pfurtscheller and Neuper, 1997) and in patients with ALS (Bai et al., 2010). The studies on ALS are reported in Table 1B.
Electroencephalography studies with either motor or imagery tasks have reported decreased ERD amplitude in patients with ALS (Bizovièar et al., 2014, McMackin et al., 2021). In particular, one study (Bizovièar et al., 2014) found that, despite normal task performance, patients with ALS displayed lower beta ERD and normal ERS amplitude compared to controls. However, unlike the controls, in ALS, ERS was absent over the hemisphere ipsilaterally to the moving finger. The authors explained this result as a consequence of degeneration of corpus callosum fibers (Van Zandijcke and Casselman, 1995; Filippini et al., 2010; Chapman et al., 2012). These findings differed from those of another study (Riva et al., 2012) reporting normal ERD in ALS and the presence of ERS asymmetry (Riva et al., 2012, see supplemental material) that was proportional to the degree of corticospinal damage. The contrasting results of the two studies could be due to differences in the selections of the range of beta band, the characteristics of ALS populations, and the tasks’ demands.
Decreased ERD amplitude in ALS have been also reported with motor imagery tasks (Kasahara et al., 2012; Hosni et al., 2019). One of those studies further showed a correlation of ERD amplitude with bulbar function—the worse the bulbar scale score, the smaller the ERD—but not with hand motor function (Kasahara et al., 2012). No clear ERS were detected in both imagery studies, probably due to the nature of the task (Kasahara et al., 2012; Hosni et al., 2019).
The reviewed studies indicate that, in general, the finding of abnormal ERD/ERS in ALS did not directly relate to motor dysfunction, the main problem of this disease. However, correlation between cognitive scores with ERS amplitude was reported at least in one study (McMackin et al., 2021). This suggests that movement-related beta ERD/ERS may reflect cognitive problems that often accompany ALS in the absence of motor task performance disruption.
Parkinson’s disease
Despite mounting evidence that PD is preceded and accompanied by non-motor symptoms, its diagnosis is still made upon the appearance of motor signs. It is thus not surprising that movement-related beta oscillatory activity in PD has been the object of many investigations. Moreover, because of approved surgical and DBS therapeutical approaches, these investigations have also been conducted with invasive electrophysiological techniques. Briefly, most studies have revealed that PD is generally associated with increased beta power at rest throughout the basal ganglia-thalamo-cortical motor network (Moran et al., 2011; Oswal et al., 2013; Stein and Bar-Gad, 2013; Heinrichs-Graham et al., 2014a). With a few exceptions, most PD studies have also shown that treatments with levodopa and DBS restore beta power during the resting state to lower levels (Brown, 2006; Degardin et al., 2009; Stein and Bar-Gad, 2013; Vinding et al., 2019; Wu et al., 2019; Wang et al., 2020). Despite high levels of resting state beta power, the review of the papers reported in Table 2 indicates that the typical pattern of movement-related ERD-ERS beta modulation is usually maintained in PD. Nevertheless, many studies found abnormal movement-related beta activity over the sensorimotor cortices and also in subcortical structures (Heinrichs-Graham et al., 2014a; Moisello et al., 2015; Rowland et al., 2015; Canessa et al., 2016; Kondylis et al., 2016; Stegemöller et al., 2016; Nelson et al., 2017; Vinding et al., 2019; Wu et al., 2019). In Table 2 we summarize the results of studies about cortical and subcortical movement-related beta ERD and ERS in PD with invasive and non-invasive recordings.
With the introduction of recording electrodes in the subthalamic nucleus (STN) or globus pallidum (Gpi) in the context of DBS treatment, many studies have focused on defining the pattern of electrical activity that are characteristic of PD in these nuclei (Kühn et al., 2004; Brown, 2006; Weinberger et al., 2009; Giannicola et al., 2010; Jenkinson and Brown, 2011; Moran et al., 2011; Stein and Bar-Gad, 2013; Heinrichs-Graham et al., 2014a; Wang et al., 2014; Canessa et al., 2016). Studies have revealed prominent oscillations in the beta frequency range (11–30 Hz) at rest (Brown, 2006; Weinberger et al., 2009). The pattern of movement-related beta oscillations in the STN has been clearly recorded with Go/NoGo tasks and motor imagery and it closely resembles that recorded from cortical sites (Kühn et al., 2004, 2006). Dopaminergic medication and STN DBS modulate beta oscillatory activity in PD patients, ameliorating motor symptoms (Devos et al., 2003a,b; Doyle et al., 2005; Oswal et al., 2013). Indeed, following levodopa administration, ERD amplitude increases in the STN with faster response times, regardless of task complexity (Oswal et al., 2013), while ERD latency decreases (Doyle et al., 2005). Clearly, definite conclusions about the effect of the disease cannot be directly drawn from ECoG studies because group comparisons can be performed in subjects with pathological basal ganglia activity and not with normal subjects. The small number of patients (from seven to eleven in most of the studies) and the great variability of the clinical phenotype are additional limitations of these studies. Nonetheless, studies with ECoG and STN DBS are useful to determine accurate topography, to find proper and personalized tuning stimulation parameters and, at the same time, to measure the behavioral output. Therefore, in this context, they could provide valuable information to guide and implement novel therapeutic interventions, such as adaptive stimulation (see: Pozzi and Isaias, 2022).
Besides the results from DBS studies, most of the EEG, MEG, and ECoG studies have found ERD abnormalities in PD. One of the reported alterations is a delay in ERD occurrence (Magnani et al., 1998; Labyt et al., 2005; Wu et al., 2019) that can be corrected with levodopa administration (Magnani et al., 2002). Many studies reported reduced beta ERD amplitude over the sensorimotor cortices of PD patients compared to different control groups with EEG, MEG, and ECoG recordings (Praamstra and Pope, 2007; Heinrichs-Graham et al., 2014b; Moisello et al., 2015; Canessa et al., 2016; Nelson et al., 2017; Vinding et al., 2019; Wu et al., 2019; Weersink et al., 2020; McLinden et al., 2021; Weersink, 2021). However, a few studies have either found no difference between patients with PD and patients with essential tremor (Tamás et al., 2006) or larger ERD amplitude in PD compared to essential tremor, epilepsy (Kondylis et al., 2016), and normal controls (Smith et al., 2012; Kondylis et al., 2016; Stegemöller et al., 2016; Johari and Behroozmand, 2021). Briefly, many factors could have played a role in the diverse results, such as differences in the study design, the analytical approach, the choice of the control groups, the small sample of subjects, the great number of tested variables as well as large within- and between-group variabilities in terms of age, performance, and number of recordings.
Only a few PD studies focused on movement-related beta ERS with EEG or MEG recordings. The general finding is that ERS amplitude is decreased in PD patients. This has been observed with active limb movements during an advanced cued task (Heinrichs-Graham et al., 2014b), a reaction time task (Moisello et al., 2015; Nelson et al., 2017), a Go/No-Go task (Wu et al., 2019), as well as with passive movements (Vinding et al., 2019). Reductions of beta ERS amplitude are already visible in the early stages of PD (Pfurtscheller et al., 1998). Studies on the performance of the two upper limbs showed that ERS amplitude is lower over the hemisphere contralaterally to the more akinetic limb (Labyt et al., 2005, 2003). Like for ERD, both dopaminergic therapy and DBS can acutely increase beta ERS amplitude; such increases usually correlate with the degree of motor improvement, especially for bradykinesia (Canessa et al., 2016). However, a study with passive movements reported administration of levodopa improved motor symptoms but it did not change ERS amplitude (Vinding et al., 2019).
A few studies have recently investigated the effect of motor practice on movement-related beta ERS in patients with PD and in normal controls. The authors found that beta ERS amplitude and movement-related beta modulation (defined as the difference from peak ERD to peak ERS) increase with practice over frontal and sensorimotor regions (Moisello et al., 2015; Nelson et al., 2017; Marchesi et al., 2019; Ricci et al., 2019a,b; Tatti et al., 2021, 2020, 2019). Moreover, the increase of beta ERS and modulation depth achieved at the end of practice predicted motor improvement measured 24 h later (Nelson et al., 2017), thus suggesting that practice-related ERS increases reflect some aspects of plasticity. In line with this conclusion, in PD, which is usually associated with reduced plasticity and retention (Marinelli et al., 2017), the practice-related ERS increase is significantly less evident, despite normal values of ERS and beta modulation at baseline (Moisello et al., 2015; Nelson et al., 2017). In summary, these works support the hypothesis that practice-related increases of beta ERS may represent local processes needed to engage plasticity-related activity, as recently hypothesized (Ghilardi et al., 2021).
Dystonia
Dystonia is characterized by involuntary muscle contractions that may cause abnormal posture or repetitive movements with highly variable clinical features. The distribution and number of affected body parts range from a single location (focal dystonia), to two or more adjacent locations (segmental dystonia) or to all the areas of the body (general dystonia). While some motor manifestations of dystonia appear in isolation, others arise in association with other diseases or non-motor symptoms (Quartarone and Ghilardi, 2022). In Table 3A we report the summary of studies on movement-related beta oscillatory activity.
In general, patients with dystonia present with a broad reduction of beta ERD documented with different recording modalities. ERD amplitude reduction between 20 and 30 Hz was first reported in patients with writer’s cramp performing a finger abduction task during EEG recordings (Toro et al., 2000). Other studies have confirmed this result with many techniques. For instance, an EEG study investigated two cohorts of dystonic patients—one with cervical dystonia and the other one with dystonic tremor—while they performed a motor task; results showed that the amplitude of beta ERD was significantly reduced compared to healthy controls. An earlier ECoG study showed a similar reduction of ERD amplitude in patients with dystonia compared to patients with either PD or essential tremor (Crowell et al., 2012). In both studies, ERD decrease was present also for movements with non-affected muscles, suggesting that dystonia is associated with a generalized sensorimotor impairment in agreement with the results of kinematic and TMS studies (Pelosin et al., 2009; Quartarone and Hallett, 2013). Lower beta ERD amplitude in idiopathic dystonia was also confirmed by a study of local field potentials from GPi DBS with simultaneous MEG recording during a finger task (van Wijk et al., 2017). Greater coherence between DBS and MEG recordings in beta frequency range was associated with increased reaction time, confirming the role of beta oscillations in motor planning.
Multiple sclerosis
Multiple sclerosis is an inflammatory neurodegenerative disease characterized by demyelination and widespread damage to the central nervous system. Therefore, the symptoms associated with this disease are diverse and often include motor and sensory impairments, visual disturbances, and fatigue with a highly variable time course. Magnetic resonance imaging (MRI) is the gold standard for diagnosing MS and for monitoring structural changes overtime, although the degree of demyelination cannot always predict disease progression (Daumer et al., 2009; Hemond and Bakshi, 2018). Electrophysiological measures have been used to supply further information about functional changes over time and to assess the effects of treatments. In Table 3B we report MS studies on movement-related beta modulation.
In general, MS is not associated with ERD alterations, while abnormalities in ERS have been usually reported by both EEG and MEG studies. Indeed, two MEG studies with different motor tasks found no difference in ERD amplitude between healthy controls and patients with different forms of MS (Arpin et al., 2017; Waldman et al., 2020). These results are in agreement with those of an earlier EEG study investigating a group of normal subjects and two groups of patients with MS, one with fatigue and the other without fatigue assessed with the Fatigue Severity Scale (Leocani et al., 2001a). While ERD amplitude was similar in non-fatigued MS patients and healthy controls, the fatigued MS patients displayed a smaller ERD than the other two groups. The greater fatigue scores corresponded to a larger ERD reduction, suggesting that non-motor features may contribute to ERD amplitude.
All the three studies also reported a decrease of ERS amplitude in MS. In one of them (Arpin et al., 2017), weaker ERS in MS patients correlated with abnormal task performance parameters. The study about the effect of fatigue (Leocani et al., 2001a), besides decreased ERS in MS compared to normal controls, found the smallest ERS amplitude in the fatigued MS group and a negative correlation between fatigue scores and ERS amplitude. Finally, a MEG report (Barratt et al., 2017) studying the temporal profile of beta power dynamics during a visuomotor task showed increased ERS timing in MS patients, confirming a disruption of beta ERS mechanisms. The authors further suggested that neuronal damage in MS, either from decreased neuronal density or demyelination, may lead to a decrease in neuronal firing and thus to a reduced ability to synchronize.
Cerebral palsy
Cerebral palsy is the most prevalent cause of neurological impairment in the United States pediatric population, resulting from injury or improper development of part of the brain and presenting with both motor and sensory abnormalities. Periventricular white matter damage in CP during or shortly after birth may reduce the efficacy of information transmission along the thalamocortical and corticospinal tracts resulting in a wide variety of sensorimotor impairments and abnormalities in movement-related beta oscillations (Kurz et al., 2017).
A review of papers on movement-related beta modulation in CP (see Table 3C) shows that CP is in general associated with increased beta ERD. The MEG studies by Kurz and colleagues with spastic diplegic and hemiplegic CP patients during a goal-directed knee extension task (Kurz et al., 2014, 2017, 2020) all reported greater ERD amplitude in subjects with CP compared to age-matched controls. When auditory tones paced the movements, the ERD increase was widely spread, from the medial post-central gyrus to the superior parietal lobe (Kurz et al., 2014). ERD increases were also present in the primary motor cortices, premotor area, inferior parietal lobe, and inferior frontal gyrus when a visual feedback of their performance was given (Kurz et al., 2017). The authors concluded that the higher ERD amplitude within the M1 may indicate difficulty in monitoring the consequences of the ongoing motor actions and in matching their actions to the targets. The same study also showed decreased ERD amplitude over the occipital and other visual areas that was associated with slower reaction times and target matching error, thus suggesting that such reduction may reflect greater difficulty in performing visuomotor tasks. A similar MEG study with a task that did not provide online visual feedback but only knowledge of the results, confirmed the presence of ERD with greater amplitudes in the sensorimotor areas in CP compared to controls but no differences in ERD amplitudes over the occipital areas (Kurz et al., 2020). The authors stated that the lack of difference in terms of occipital beta ERD is possibly related to intact occipital function in this group of participants compared to the previous one. An alternative explanation should take into account the lack of online visual feedback during the task compared to the other studies. Finally, this study found that, in CP, greater ERD amplitude was associated with slower walking cadence, a relationship not found in controls, suggesting that increased ERD amplitude in CP may result from abnormalities in energy regulation.
Normal aging
While several works have investigated the effect of aging with different types of motor tasks, only a few studies have addressed the evolution of movement-related beta oscillations during development (Table 4A). In particular, a MEG study by Gaetz et al. (2010) demonstrated that the amplitudes of beta ERD and ERS change as a function of age: both beta ERD and ERS are weaker in children (aged from 4 to 6 years) compared to adolescents (from 11 to 13 years), which, in turn, display weaker values than adults (from 24 to 42 years). The authors concluded that weaker beta ERD and ERS in children may reflect reduced motor cortical inhibition and thus high plasticity, which are typical of childhood and may serve to promote normal development. These findings are in agreement with the results of a more recent MEG study investigating differences between adolescents (mean age: 14.0 years) and adults (36.6 years) after practice sessions in a leg force task (Gehringer et al., 2019). At baseline, adolescents exhibited weaker beta ERD and ERS than the adult group over the contralateral sensorimotor cortex; after practice, beta ERD and ERS increased mostly in the adults (Gehringer et al., 2019).
With aging, and mostly in the later part of adult life, beside cognitive decline, motor abilities usually deteriorate, as shown in a variety of experimental tasks (Salthouse, 1984; Ranganathan et al., 2001). There are also reports indicating that, with aging, loss of motor cortical and spinal neurons can occur (Doherty et al., 1993; Eisen et al., 1996) together with sarcopenia (Priyadarsini et al., 2022). Aging-related performance deterioration can be accompanied by changes in movement-related beta oscillations (Mary et al., 2015; Sallard et al., 2016; Toledo et al., 2016; Walker et al., 2020), although a few studies failed to find any age effects (Labyt et al., 2003; Rossiter et al., 2014b; Ricci et al., 2019a; Table 4A). Briefly, reports of increased beta ERD amplitude in older compared to younger subjects have been described by both EEG and MEG studies in a variety of motor tasks over areas contralateral to the involved effector: during finger tapping (Sailer et al., 2000; Mary et al., 2015), button press (Johari and Behroozmand, 2020), speech (Johari and Behroozmand, 2020), ankle movements (Toledo et al., 2016), and involuntary stretch of leg muscles (Walker et al., 2020). Many of these papers also reported delayed timing of ERD.
Controversial results have been found for beta ERS. Early studies by Labyt et al. (2004, 2003) reported decreased ERS after self-paced finger movements in older compared to younger subjects. On the other hand, during assessment of ankle proprioception, the presence of ERS was noted only in the older group, but not in the younger group (Toledo et al., 2016). The authors interpreted this finding as a mechanism to overcome age-related deterioration of peripheral and central structures to generate an adequate sensorimotor output. In line with these findings, Sallard and coworkers (Sallard et al., 2016) found that older populations exhibited stronger beta ERS than younger populations over frontal and sensorimotor regions during finger tapping tasks. In contrast with these results, a study investigating age-related differences after proprioceptive stimulation showed increased ERD in the older group, but no group differences in terms of beta ERS (Walker et al., 2020). Despite differences in ERS timings, movement accuracy, and speed, reaching movements studies did not find age-related differences for both ERD and ERS amplitude (Ricci et al., 2019a). In addition, the study showed that the amplitude of ERS increased with practice in both groups at the same rate (Ricci et al., 2019a).
The contrasting results of these papers could be due to differences in tasks, sample size, and age ranges. Most importantly, age may not be the best predictor of cortical changes since other individual factors play important roles in aging processes, such as motor practice, lifestyle, gender, genetics, and differences in plasticity and energy mechanisms (Dachtler and Fox, 2017; Ricci et al., 2019a).
Alzheimer’s disease
Alzheimer’s disease is one of the most common neurodegenerative disorders, characterized by a progressive decline in memory and cognitive function. Usually, motor dysfunctions are not clinically evident in either the early stages of AD or Mild Cognitive Impairment. Clinically evident motor impairment usually occurs in later stages of AD and manifests as apraxia, the inability to conceptualize and perform meaningful actions. Nevertheless, research studies have shown that subtle signs of motor impairment, such as increased reaction time (Koller et al., 1984; Müller et al., 1991), slow movements, impaired walking and balance (Buchner and Larson, 1987; Alexander et al., 1995; Ott et al., 1995) are already present early on in the disease. In particular, there is evidence that, in the early phases of AD, reaching movements are slower with a great reliance on continuous on-line visual cues and a scarce dependence on motor planning (Bellgrove et al., 1997; Ghilardi et al., 2000, 1999). Indeed, while accuracy of visually guided movements is similar in patients with AD and controls, movement speed and transport phase are significantly reduced in AD. When visual feedback is withheld, patients with AD also show a decay of movement accuracy. The degrees of motor performance disruption and cognitive abnormalities are highly correlated, thus pointing to impairments in frontal and parietal regions, a pathological hallmark of AD (Ghilardi et al., 2000, 1999). Therefore, early motor abnormalities may represent initial, subclinical manifestations of apraxia in AD. Despite the evidence of early impairment of motor function, there is only one EEG study in AD (Table 4B) directly investigating its neural bases and its possible association with movement-related beta oscillations (Babiloni et al., 2000).
In that study, EEG was recorded in patients with mild to moderate AD while performing movement extensions with the right middle finger (Babiloni et al., 2000). The results were compared to a group of age-matched healthy controls and a younger group. In agreement with previous reports, the movements of patients were slower than those of both control groups. Nevertheless, beta ERD and ERS amplitudes in the centroparietal electrodes contralaterally to the movement were similar in all three groups. Further analyses with surface Laplacian estimates of ERD and ERS showed a more extended activation in the AD group; differently from the control groups, ERD and ERS were spread to frontal areas and contralateral sites. No correlation was found between motor performance indices and ERD/ERS abnormalities. The authors concluded that to perform simple finger movements, AD patients may engage additional areas outside the classical motor cortical network, a pattern that is normally used for more complex movements. That supplementary recruitment is probably due to depleted resources in the sensorimotor network, suggesting that AD is “a global brain network disease” that includes abnormal “processing of sensorimotor information despite no overt movement disorder” (Babiloni et al., 2000).
Schizophrenia and obsessive-compulsive disorders
Schizophrenia is defined by a range of symptoms that include distortion of reality, disorganized thought and behavior, and imbalance of dopaminergic pathways. Beside disorganization and psychomotor motor poverty, subtle signs of motor and sensory abnormalities can be present early on in the disease in a variety of motor activities, including gaze (Calkins et al., 2008), force adjustment and fine motor function (Rosen et al., 1991; Exner et al., 2006; Teremetz et al., 2014; Walther and Mittal, 2016; Térémetz et al., 2017), as well as gait and posture (Kent et al., 2012; Bernard et al., 2014). While antipsychotic medication could play a role (Putzhammer et al., 2005; Nowak et al., 2013), sensorimotor impairments can be seen in unmedicated patients and just after the first episode of psychosis (Caligiuri and Lohr, 1994; Wolff and O’Driscoll, 1999; Ayehu et al., 2014; Teremetz et al., 2014). On these bases, to explore the neural bases of schizophrenia-related sensorimotor abnormalities, a few studies investigated movement-related beta ERD and ERS in patients with schizophrenia and in normal subjects with different scores of schizotypal personality (Table 4C).
There are only two works investigating movement-related beta oscillations in patients with clinically diagnosed schizophrenia. Both studies were conducted with MEG and showed similar beta ERD amplitudes in patients and controls. Importantly, they both also reported a reduction of beta ERS amplitude that correlated with disease severity. In the first study, patients with stable schizophrenia and without motor problems performed self-paced button clicking movements after target presentation (Robson et al., 2016). The other study included a group with recently developed schizophrenia and absent or minimal antipsychotic medications and another with established psychosis (Gascoyne et al., 2021). They performed a finger abduction task in response to a stimulus. Both studies found decreased ERS which was associated with disease progression, with no significant role of medication. The evidence that the prime driver of beta ERS reduction is not medication but rather disease progression is also suggested by the results of another more recent study on schizotypal personality (Hunt et al., 2019). Schizotypy has been recognized as a set of traits quantitatively within normal range, but qualitatively similar to those of neurodevelopmental and schizophrenia spectrum disorders. In a sample of more than 100 normal subjects, the authors found that the degree of schizotypal personality was related to ERS amplitude decreases, in that lower ERS amplitude corresponded to higher Schizotypal Personality Questionnaire scores, with higher variance accounted for by the scores of disorganization and interpersonal factors (Hunt et al., 2019).
Obsessive-compulsive disorders is an anxiety disorder characterized by inability to suppress intrusive thoughts and repetitive actions with dysfunction of orbitofrontal cortex, anterior cingulate, basal ganglia, and other limbic structures. One study has shown decreased amplitude of beta ERS in a small cohort of subjects with OCD, a finding that was interpreted as impairment of post-movement deactivation mechanisms likely due to orbito-frontal cortex dysfunction (Leocani et al., 2001b; Table 4C).
Discussion
This review suggests that neurological and psychiatric disorders with or without overt motor problems present with abnormalities of movement-related beta ERD and ERS and that such abnormalities are, in many cases, not directly related to motor symptoms. As summarized in Table 5, most studies found decreased ERD amplitude in stroke, ALS, dystonia, and PD; decreased ERD and ERS amplitudes were described in MS, especially in patients with fatigue; conversely, ERD with abnormally increased amplitude was reported in CP. As far as physiological maturation, a process accompanied by changes in motor and cognitive function, ERD and ERS amplitudes increase during development, reaching a plateau in adulthood, a finding that has been linked to the reduced cortical inhibition and high plasticity typical of childhood. However, contrasting results about the effects of old age suggest that age per se is not associated with changes in movement-related beta oscillations and other factors related to lifestyle, history, cognition, and perhaps genetics may play significant roles. Only a few studies have investigated possible changes in neuropsychiatric conditions without clinically evident sensorimotor impairment. In AD, ERD, and ERS associated with simple finger movements were spread beyond the normal motor cortical network over areas that, in normal subjects, are engaged only for complex movements. Such an enlarged recruitment suggests that first, neurodegeneration in AD must also affect the sensorimotor function, at least in terms of cortical re-wiring, and, second, that overt apraxia, which is often present later on in the disease, may stem from these early changes. Decreased ERS amplitude correlated with the progression of schizophrenia and also with the degree of schizotypal personality; in OCD, similar ERS findings were not related to motor performance. In summary, abnormalities of movement-related beta ERD and ERS were found in all the neuropsychiatric diseases we have reviewed and such abnormalities were not necessarily dependent on the presence of motor symptoms.
Limitations and caveats
A few factors limit the interpretation of the findings of the reviewed studies. Some effects could have been underestimated because in many studies a small number of subjects were tested, while in others there was high variability of either the lesions’ location (as in the case of stroke) or the clinical characteristics of the disease (as in PD). Limitations include: different definitions of the beta band (for instance, in some studies from 13 to 25 Hz, in others from 15 to 35 Hz); ad hoc analyses focused on selected sub-bands (e.g., Johari and Behroozmand, 2021); single reports that require further confirmation, as in the case of AD, CP, and OCD. Another caveat is the interpretation of LFP recordings from cortical and subcortical sites in terms of ERD and ERS as reflecting the modulation of the strength of a sustained oscillation. Indeed, evidence suggests that beta ERD and ERS may likely represent the probability of occurrence of a brief bursting transient event (Canolty et al., 2012; Rule et al., 2017). Therefore, the common and current approach of averaging trials may not provide a thorough picture of beta activity and thus limits the understanding of beta ERS and ERD in the sensorimotor system. Also, ECoG and subcortical recording studies can provide information on a restricted topography and with comparisons of patients affected by other brain pathologies, thus limiting conclusions about the effects of the pathology itself. An example is the comparison of dystonia and PD recordings in the STN. The two diseases share many pathophysiologic features, such as alterations in movement-related beta oscillations, although STN DBS recordings showed lower beta ERD in dystonia compared to PD (Crowell et al., 2012). Nevertheless, these studies are useful to assess the effects of pharmacological or other therapy when associated to the clinical evaluation of the patient. Finally, we could not retrieve any study regarding beta modulation in other psychiatric conditions. These pathologies warrant further research to provide more information about the link between beta modulation and behavioral symptoms at large. This review did not take into account clinical studies related to traumatic brain injuries (TBI). To our knowledge, there are no systematic studies on TBI. Nevertheless, ERD/ERS could provide an effective mean for follow up studies in larger cohorts of patients with TBI or other diseases, as suggested by a single case study with a patient with open TBI (D’Arcy et al., 2020).
Beta modulation in the sensorimotor cortex and beyond
While investigations in neuropsychiatric diseases have demonstrated impairments in movement-related beta oscillation, beta ERD and ERS cannot be considered markers of a specific status, disease, sensorimotor symptom, or brain lesion site. Indeed, the amplitude of ERD and ERS in normal subjects is not linked to specific kinematic characteristics (Tatti et al., 2019), a link that is instead evident for gamma power (Tatti et al., 2022), but it is probably related to other factors including practice and learning (Schmidt et al., 2019; Ghilardi et al., 2021). Therefore, it is likely that beta ERD and ERS have a wider meaning and reflect general abilities or mechanisms within the sensorimotor system –and associated areas at large- to ideate, assemble, program, promote and finalize a behavior as well as to update internal models related to these processes. Some findings reported in this review support this wider view. First, beta ERD and ERS can be elicited over the sensorimotor areas not only during the execution of movements but also during motor imagery without producing a measurable motor output, as confirmed by some ALS studies. Second, the ERD and ERS abnormalities found in AD (which did not correlate with any motor performance indices) may represent early phases of apraxia, a symptom likely resulting from progressive impairment of the objective Euclidean and body-centered spaces followed by the disruption of the actual space of object manipulation. Third, decreased beta ERD and ERS amplitudes were highly correlated with fatigue in MS, but not with motor disability. Indeed, fatigue is a symptom that cannot be confined to the motor domain but extends to mood and sleep regulation, as well as to other functions. Also, studies link fatigue to frontal areas dysfunction (Morgante et al., 2011). Fourth, movement-related beta ERS amplitude recorded over the sensorimotor cortex seems to be related to the degree of schizotypy and, in particular, to organizational and interpersonal factors (defined in the Schizotypal Personality Questionnaire) that heavily rely on frontal lobe function (Hunt et al., 2019). Finally, ERS abnormalities in ALS correlated with cognitive and behavioral scores and not with motor performance (McMackin et al., 2021). Other lines of evidence also support the notion that the beta ERD-ERS dynamics may have meanings that transcend motor function per se, linking it to attention-related and other processes. Indeed, movement-related beta modulation has been observed also over frontal and pre-frontal areas (Moisello et al., 2015; Ricci et al., 2019b; Tatti et al., 2019, 2020, 2021), suggesting a more diffuse involvement of cortical areas in movement programming, execution, stopping, and possibly in internal model updating. Also, ERD-ERS patterns in the beta range has been recorded in prefrontal areas even in non-motor tasks, specifically during both working memory (Lundqvist et al., 2016; Miller et al., 2018) and focused attention tasks (Hanslmayr et al., 2014; Zavala et al., 2017). For these reasons, as suggested by Schmidt et al. (2019), it is likely that cognition and movement share common processes –both regulated by beta modulation – that allow for focusing and suppressing irrelevant phenomena, with a sort of center-surround suppression mechanism of action. This view of common mechanisms underlying motor and cognitive processes, such as those expressed by beta modulation, transcends the conceptual separation of the different brain functions into motor and cognitive categories and should ultimately guide new research approaches for finding more effective therapeutical solutions.
Movement-related beta modulation and practice
Finally, an aspect that only a few studies highlighted is that in normal subjects, beta ERS and ERD amplitudes increase with practice across trials (Moisello et al., 2015; Nelson et al., 2017), possibly reflecting plasticity-related phenomena. This interpretation is in agreement with the results of MEG studies (Mary et al., 2015; Gehringer et al., 2019) showing enhanced beta ERS after intensive motor training and with the finding that iTBS, a protocol inducing LTP-like phenomena in the sensorimotor system, enhances movement-related beta ERS in a subsequent motor task (Hsu et al., 2011). Furthermore, the increase is local and task-related, as beta ERS is enhanced over both frontal and somatosensory areas following visuo-motor learning, but not after practice in a purely visual learning task (Tatti et al., 2020) or in a simpler motor task without visuo-motor learning (Tatti et al., 2021). Such practice-related beta increase vanishes after a similar period (about 90 min) of either quiet wake or napping (Tatti et al., 2020), suggesting that movement-related beta modulation may reflect phenomena needed to start, maintain, and finalize LTP processes (Ghilardi et al., 2021). These conclusions about a link between beta power with learning and plasticity are also in agreement with the work by Tan et al. (2016). Intriguingly, as animal studies have shown a relation between beta power during movement and lactate consumption (Grønli et al., 2016), it is possible that practice-related beta changes may represent an index of local energy consumption and availability (Ghilardi et al., 2021).
Future perspectives
Based upon this review and considerations derived from it, it is evident the need of further integrated research on beta ERD and ERS before these measures can be effectively used as biomarkers of specific brain functions in neuropsychiatric diseases. These future studies will be fundamental to determine: ERD/ERS general mechanisms and significance, the behavioral counterparts, the pharmacology, the topography and its dynamics, the biochemical, metabolic and electrophysiological processes involved at the subcellular, cellular, and system levels. For instance, from a pharmacological point of view, the amplitude of beta power and ERD have been linked to levels of GABA (Gaetz et al., 2010; Hall et al., 2011; Muthukumaraswamy et al., 2013) suggesting a relationship between beta oscillations and the balance between inhibitory and excitatory processes, and the potential for experience-dependent plasticity. From a metabolic point of view, it would be important to confirm in humans the finding of animal studies that changes of beta power reflect lactate consumption (Grønli et al., 2016). If true, this finding could be on one hand, the key to understand symptoms of neurological diseases in terms of energy regulation, from parkinsonian bradykinesia to fatigue (which is present not only in MS but also in several brain diseases) and to attention-related problems characteristic of neuropsychiatric disorders. On the other hand, it will also be particularly relevant for interventions geared to enhance the processes involved in LTP induction and maintenance that may deficient or abnormal in disorders characterized by neurodegeneration, inflammatory processes, maladaptive plasticity, and abnormal beta modulation. Topographical and connectivity studies about the temporal profile of beta ERD and ERS across sensorimotor (Meziane et al., 2015) and frontal areas during performance and practice would provide the bases for designing specific spatial and temporal neuromodulation interventions to improve performance. In terms of mechanisms, a firm definition of the link between beta power and center-surround suppression during motor and cognitive performance would be of paramount importance to understand attention-related problems in health and disease (Schmidt et al., 2019), and thus to address symptoms with novel approaches.
These are only a small sample of the many facets that need to be uncovered by research on movement-related beta ERD and ERS. Reaching these goals, an effort that needs a comprehensive multimodal translational approach, is essential to define targeted therapeutical interventions for the care of many motor and non-motor features of neuropsychiatric diseases.
Author contributions
ET, MFG, and AQ contributed to the conception of the study. MFG, FF, JP, SG, CC, TA, SS, and SAS wrote the manuscript. MFG, JP, FF, DM, ET, and AQ revised the manuscript. All authors read and approved the submitted version.
Funding
This work was supported by DOD W81XWH-19-1-0810 (AQ and MFG) and by Current Research Funds 2022, Ministry of Health, Italy.
Acknowledgments
We thank the reviewers that provided precious advises and helped improving and focusing the scope of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SR declared a past co-authorship with the author, ET to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adam, R., Isabella, S., and Chan, J. L. (2015). Insight into motor control and motor impairment from stroke and beta oscillations. J. Neurophysiol. 114, 3033–3035. doi: 10.1152/jn.00098.2015
Alexander, N. B., Mollo, J. M., Giordani, B., Ashton-Miller, J. A., Schultz, A. B., Grunawalt, J. A., et al. (1995). Maintenance of balance, gait patterns, and obstacle clearance in Alzheimer’s disease. Neurology 45, 908–914. doi: 10.1212/wnl.45.5.908
Arpin, D. J., Heinrichs-Graham, E., Gehringer, J. E., Zabad, R., Wilson, T. W., and Kurz, M. J. (2017). Altered sensorimotor cortical oscillations in individuals with multiple sclerosis suggests a faulty internal model. Hum. Brain Mapp. 38, 4009–4018. doi: 10.1002/hbm.23644
Ayehu, M., Shibre, T., Milkias, B., and Fekadu, A. (2014). Movement disorders in neuroleptic-naïve patients with schizophrenia spectrum disorders. BMC Psychiatry 14:280. doi: 10.1186/s12888-014-0280-1
Babiloni, C., Babiloni, F., Carducci, F., Cincotti, F., Del Percio, C., De Pino, G., et al. (2000). Movement-related electroencephalographic reactivity in Alzheimer disease. Neuroimage 12, 139–146. doi: 10.1006/nimg.2000.0602
Bai, O., Lin, P., Huang, D., Fei, D.-Y., and Floeter, M. K. (2010). Towards a user-friendly brain-computer interface: Initial tests in ALS and PLS patients. Clin. Neurophysiol. 121, 1293–1303. doi: 10.1016/j.clinph.2010.02.157
Barone, J., and Rossiter, H. E. (2021). Understanding the role of sensorimotor beta oscillations. Front. Syst. Neurosci. 15:655886. doi: 10.3389/fnsys.2021.655886
Barratt, E. L., Tewarie, P. K., Clarke, M. A., Hall, E. L., Gowland, P. A., Morris, P. G., et al. (2017). Abnormal task driven neural oscillations in multiple sclerosis: A visuomotor MEG study. Hum. Brain Mapp. 38, 2441–2453. doi: 10.1002/hbm.23531
Bellgrove, M. A., Phillips, J. G., Bradshaw, J. L., Hall, K. A., Presnell, I., and Hecht, H. (1997). Response programming in dementia of the Alzheimer type: A kinematic analysis. Neuropsychologia 35, 229–240. doi: 10.1016/s0028-3932(96)00081-4
Berger, H. (1929). Über das elektrenkephalogramm des menschen. Archiv. F. Psychiatrie 87, 527–570. doi: 10.1007/BF01797193
Bernard, J. A., Dean, D. J., Kent, J. S., Orr, J. M., Pelletier-Baldelli, A., Lunsford-Avery, J. R., et al. (2014). Cerebellar networks in individuals at ultra high-risk of psychosis: Impact on postural sway and symptom severity. Hum. Brain Mapp. 35, 4064–4078. doi: 10.1002/hbm.22458
Bizovièar, N., Dreo, J., Koritnik, B., and Zidar, J. (2014). Decreased movement-related beta desynchronization and impaired post-movement beta rebound in amyotrophic lateral sclerosis. Clin. Neurophysiol. 125, 1689–1699. doi: 10.1016/j.clinph.2013.12.108
Brown, P. (2006). Bad oscillations in Parkinson’s disease. J. Neural Transm. Suppl. 70, 27–30. doi: 10.1007/978-3-211-45295-0_6
Brown, P., and Marsden, C. D. (1999). Bradykinesia and impairment of EEG desynchronization in Parkinson’s disease. Mov. Disord. 14, 423–429. doi: 10.1002/1531-8257(199905)14:3<423::aid-mds1006>3.0.co;2-v
Brown, R. H., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172. doi: 10.1056/NEJMra1603471
Buchner, D. M., and Larson, E. B. (1987). Falls and fractures in patients with Alzheimer-type dementia. JAMA 257, 1492–1495.
Caligiuri, M. P., and Lohr, J. B. (1994). A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol. Psychiatry 35, 104–111. doi: 10.1016/0006-3223(94)91199-1
Calkins, M. E., Iacono, W. G., and Ones, D. S. (2008). Eye movement dysfunction in first-degree relatives of patients with schizophrenia: A meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 68, 436–461. doi: 10.1016/j.bandc.2008.09.001
Canessa, A., Pozzi, N. G., Arnulfo, G., Brumberg, J., Reich, M. M., Pezzoli, G., et al. (2016). Striatal dopaminergic innervation regulates subthalamic beta-oscillations and cortical-subcortical coupling during movements: Preliminary evidence in subjects with Parkinson’s disease. Front. Hum. Neurosci. 10:611. doi: 10.3389/fnhum.2016.00611
Canolty, R. T., Ganguly, K., and Carmena, J. M. (2012). Task-dependent changes in cross-level coupling between single neurons and oscillatory activity in multiscale networks. PLoS Comput. Biol. 8:e1002809. doi: 10.1371/journal.pcbi.1002809
Chalard, A., Amarantini, D., Tisseyre, J., Marque, P., and Gasq, D. (2020). Spastic co-contraction is directly associated with altered cortical beta oscillations after stroke. Clin. Neurophysiol. 131, 1345–1353. doi: 10.1016/j.clinph.2020.02.023
Chapman, M. C., Jelsone-Swain, L., Fling, B. W., Johnson, T. D., Gruis, K., and Welsh, R. C. (2012). Corpus callosum area in amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. 13, 589–591. doi: 10.3109/17482968.2012.708935
Cheyne, D., and Ferrari, P. (2013). MEG studies of motor cortex gamma oscillations: Evidence for a gamma “fingerprint” in the brain? Front. Hum. Neurosci. 7:575. doi: 10.3389/fnhum.2013.00575
Crowell, A. L., Ryapolova-Webb, E. S., Ostrem, J. L., Galifianakis, N. B., Shimamoto, S., Lim, D. A., et al. (2012). Oscillations in sensorimotor cortex in movement disorders: An electrocorticography study. Brain 135, 615–630. doi: 10.1093/brain/awr332
D’Arcy, R. C. N., Greene, T., Greene, D., Frehlick, Z., Fickling, S. D., Campbell, N., et al. (2020). Portable neuromodulation induces neuroplasticity to re-activate motor function recovery from brain injury: A high-density MEG case study. J. Neuroeng. Rehabil. 17, 158–169. doi: 10.1186/s12984-020-00772-5
Dachtler, J., and Fox, K. (2017). Do cortical plasticity mechanisms differ between males and females? J. Neurosci. Res. 95, 518–526. doi: 10.1002/jnr.23850
Daumer, M., Neuhaus, A., Herbert, J., and Ebers, G. (2009). Prognosis of the individual course of disease: The elements of time, heterogeneity and precision. J. Neurol. Sci. 287, (Suppl. 1), S50–S55. doi: 10.1016/S0022-510X(09)71301-2
Degardin, A., Houdayer, E., Bourriez, J.-L., Destée, A., Defebvre, L., Derambure, P., et al. (2009). Deficient “sensory” beta synchronization in Parkinson’s disease. Clin. Neurophysiol. 120, 636–642. doi: 10.1016/j.clinph.2009.01.001
Devos, D., Labyt, E., Derambure, P., Bourriez, J. L., Cassim, F., Guieu, J. D., et al. (2003a). Effect of L-Dopa on the pattern of movement-related (de)synchronisation in advanced Parkinson’s disease. Neurophysiol. Clin. 33, 203–212. doi: 10.1016/j.neucli.2003.10.001
Devos, D., Labyt, E., Cassim, F., Bourriez, J. L., Reyns, N., Touzet, G., et al. (2003b). Subthalamic stimulation influences postmovement cortical somatosensory processing in Parkinson’s disease. Eur. J. Neurosci. 18, 1884–1888. doi: 10.1046/j.1460-9568.2003.02925.x
Doherty, T. J., Vandervoort, A. A., and Brown, W. F. (1993). Effects of ageing on the motor unit: A brief review. Can. J. Appl. Physiol. 18, 331–358. doi: 10.1139/h93-029
Doyle, L. M. F., Kühn, A. A., Hariz, M., Kupsch, A., Schneider, G.-H., and Brown, P. (2005). Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s disease. Eur. J. Neurosci. 21, 1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x
Eisen, A., Entezari-Taher, M., and Stewart, H. (1996). Cortical projections to spinal motoneurons: Changes with aging and amyotrophic lateral sclerosis. Neurology 46, 1396–1404. doi: 10.1212/wnl.46.5.1396
Engel, A. K., and Fries, P. (2010). Beta-band oscillations–signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Espenhahn, S., de Berker, A. O., van Wijk, B. C. M., Rossiter, H. E., and Ward, N. S. (2017). Movement-related beta oscillations show high intra-individual reliability. Neuroimage 147, 175–185. doi: 10.1016/j.neuroimage.2016.12.025
Espenhahn, S., Rossiter, H. E., van Wijk, B. C. M., Redman, N., Rondina, J. M., Diedrichsen, J., et al. (2020). Sensorimotor cortex beta oscillations reflect motor skill learning ability after stroke. Brain Commun. 2:fcaa161. doi: 10.1093/braincomms/fcaa161
Exner, C., Weniger, G., Schmidt-Samoa, C., and Irle, E. (2006). Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr. Res. 84, 386–396. doi: 10.1016/j.schres.2006.03.013
Feige, B., Kristeva-Feige, R., Rossi, S., Pizzella, V., and Rossini, P. M. (1996). Neuromagnetic study of movement-related changes in rhythmic brain activity. Brain Res. 734, 252–260.
Filippini, N., Douaud, G., Mackay, C. E., Knight, S., Talbot, K., and Turner, M. R. (2010). Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75, 1645–1652. doi: 10.1212/WNL.0b013e3181fb84d1
Gaetz, W., MacDonald, M., Cheyne, D., and Snead, O. C. (2010). Neuromagnetic imaging of movement-related cortical oscillations in children and adults: Age predicts post-movement beta rebound. NeuroImage 51, 792–807. doi: 10.1016/j.neuroimage.2010.01.077
Gascoyne, L. E., Brookes, M. J., Rathnaiah, M., Katshu, M. Z. U. H., Koelewijn, L., Williams, G., et al. (2021). Motor-related oscillatory activity in schizophrenia according to phase of illness and clinical symptom severity. NeuroImage 29:102524. doi: 10.1016/j.nicl.2020.102524
Gastaut, H. (1952). [Electrocorticographic study of the reactivity of rolandic rhythm]. Rev. Neurol (Paris) 87, 176–182.
Gehringer, J. E., Arpin, D. J., Heinrichs-Graham, E., Wilson, T. W., and Kurz, M. J. (2019). Practice modulates motor-related beta oscillations differently in adolescents and adults. J. Physiol. 597, 3203–3216. doi: 10.1113/JP277326
Ghilardi, M. F., Alberoni, M., Marelli, S., Rossi, M., Franceschi, M., Ghez, C., et al. (1999). Impaired movement control in Alzheimer’s disease. Neurosci. Lett. 260, 45–48. doi: 10.1016/s0304-3940(98)00957-4
Ghilardi, M. F., Alberoni, M., Rossi, M., Franceschi, M., Mariani, C., and Fazio, F. (2000). Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res. 876, 112–123. doi: 10.1016/s0006-8993(00)02635-4
Ghilardi, M. F., Tatti, E., and Quartarone, A. (2021). Beta power and movement-related beta modulation as hallmarks of energy for plasticity induction: Implications for Parkinson’s disease. Parkinsonism Relat. Disord. 88, 136–139. doi: 10.1016/j.parkreldis.2021.05.018
Giannicola, G., Marceglia, S., Rossi, L., Mrakic-Sposta, S., Rampini, P., Tamma, F., et al. (2010). The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Exp. Neurol. 226, 120–127. doi: 10.1016/j.expneurol.2010.08.011
Grønli, J., Rempe, M. J., Clegern, W. C., Schmidt, M., and Wisor, J. P. (2016). Beta EEG reflects sensory processing in active wakefulness and homeostatic sleep drive in quiet wakefulness. J. Sleep Res. 25, 257–268. doi: 10.1111/jsr.12380
Hall, S. D., Barnes, G. R., Furlong, P. L., Seri, S., and Hillebrand, A. (2010). Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum. Brain Mapp. 31, 581–594. doi: 10.1002/hbm.20889
Hall, S. D., Stanford, I. M., Yamawaki, N., McAllister, C. J., Rönnqvist, K. C., Woodhall, G. L., et al. (2011). The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 56, 1506–1510. doi: 10.1016/j.neuroimage.2011.02.025
Hanslmayr, S., Matuschek, J., and Fellner, M.-C. (2014). Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr. Biol. 24, 904–909. doi: 10.1016/j.cub.2014.03.007
Heinrichs-Graham, E., Kurz, M. J., Becker, K. M., Santamaria, P. M., Gendelman, H. E., and Wilson, T. W. (2014a). Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson’s disease: A pharmaco-magnetoencephalography study. J. Neurophysiol. 112, 1739–1747. doi: 10.1152/jn.00383.2014
Heinrichs-Graham, E., Wilson, T. W., Santamaria, P. M., Heithoff, S. K., Torres-Russotto, D., Hutter-Saunders, J. A. L., et al. (2014b). Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb. Cortex 24, 2669–2678. doi: 10.1093/cercor/bht121
Hemond, C. C., and Bakshi, R. (2018). Cold Spring Harb. Perspect. Med. 8:a028969. doi: 10.1101/cshperspect.a028969
Hess, C. W., Gatto, B., Chung, J. W., Ho, R. L. M., Wang, W., Wagle Shukla, A., et al. (2020). Cortical oscillations in cervical dystonia and dystonic tremor. Cereb. Cortex Commun. 1:tgaa048. doi: 10.1093/texcom/tgaa048
Hosni, S. M., Deligani, R. J., Zisk, A., McLinden, J., Borgheai, S. B., and Shahriari, Y. (2019). An exploration of neural dynamics of motor imagery for people with amyotrophic lateral sclerosis. J. Neural Eng. 17:016005. doi: 10.1088/1741-2552/ab4c75
Hsu, Y.-F., Liao, K.-K., Lee, P.-L., Tsai, Y.-A., Yeh, C.-L., Lai, K.-L., et al. (2011). Intermittent theta burst stimulation over primary motor cortex enhances movement-related beta synchronisation. Clin. Neurophysiol. 122, 2260–2267. doi: 10.1016/j.clinph.2011.03.027
Hunt, B. A. E., Liddle, E. B., Gascoyne, L. E., Magazzini, L., Routley, B. C., Singh, K. D., et al. (2019). Attenuated post-movement beta rebound associated with schizotypal features in healthy people. Schizophr. Bull. 45, 883–891. doi: 10.1093/schbul/sby117
Jenkinson, N., and Brown, P. (2011). New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 34, 611–618. doi: 10.1016/j.tins.2011.09.003
Jensen, O., Goel, P., Kopell, N., Pohja, M., Hari, R., and Ermentrout, B. (2005). On the human sensorimotor-cortex beta rhythm: Sources and modeling. Neuroimage 26, 347–355. doi: 10.1016/j.neuroimage.2005.02.008
Johari, K., and Behroozmand, R. (2020). Event-related desynchronization of alpha and beta band neural oscillations predicts speech and limb motor timing deficits in normal aging. Behav. Brain Res. 393:112763. doi: 10.1016/j.bbr.2020.112763
Johari, K., and Behroozmand, R. (2021). Neural correlates of speech and limb motor timing deficits revealed by aberrant beta band desynchronization in Parkinson’s disease. Clin. Neurophysiol. 132, 2711–2721. doi: 10.1016/j.clinph.2021.06.022
Joundi, R. A., Brittain, J., Green, A. L., Aziz, T. Z., Brown, P., and Jenkinson, N. (2013). Persistent suppression of subthalamic beta-band activity during rhythmic finger tapping in Parkinson’s disease. Clin. Neurophysiol. 124, 565–573. doi: 10.1016/j.clinph.2012.07.029
Kasahara, T., Terasaki, K., Ogawa, Y., Ushiba, J., Aramaki, H., and Masakado, Y. (2012). The correlation between motor impairments and event-related desynchronization during motor imagery in ALS patients. BMC Neurosci. 13:66. doi: 10.1186/1471-2202-13-66
Kent, J. S., Hong, S. L., Bolbecker, A. R., Klaunig, M. J., Forsyth, J. K., O’Donnell, B. F., et al. (2012). Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One 7:e41808. doi: 10.1371/journal.pone.0041808
Kilavik, B. E., Zaepffel, M., Brovelli, A., MacKay, W. A., and Riehle, A. (2013). The ups and downs of β oscillations in sensorimotor cortex. Exp. Neurol. 245, 15–26. doi: 10.1016/j.expneurol.2012.09.014
Koller, W. C., Wilson, R. S., Glatt, S. L., and Fox, J. H. (1984). Motor signs are infrequent in dementia of the Alzheimer type. Ann. Neurol. 16, 514–516. doi: 10.1002/ana.410160418
Kondylis, E. D., Randazzo, M. J., Alhourani, A., Lipski, W. J., Wozny, T. A., Pandya, Y., et al. (2016). Movement-related dynamics of cortical oscillations in Parkinson’s disease and essential tremor. Brain 139, 2211–2223. doi: 10.1093/brain/aww144
Kühn, A. A., Doyle, L., Pogosyan, A., Yarrow, K., Kupsch, A., Schneider, G.-H., et al. (2006). Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson’s disease. Brain 129, 695–706. doi: 10.1093/brain/awh715
Kühn, A. A., Williams, D., Kupsch, A., Limousin, P., Hariz, M., Schneider, G.-H., et al. (2004). Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 127, 735–746. doi: 10.1093/brain/awh106
Kurz, M. J., Becker, K. M., Heinrichs-Graham, E., and Wilson, T. W. (2014). Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol. 56, 1072–1077. doi: 10.1111/dmcn.12513
Kurz, M. J., Bergwell, H., Spooner, R., Baker, S., Heinrichs-Graham, E., and Wilson, T. W. (2020). Motor beta cortical oscillations are related with the gait kinematics of youth with cerebral palsy. Ann.Clin. Transl. Neurol. 7, 2421–2432. doi: 10.1002/acn3.51246
Kurz, M. J., Proskovec, A. L., Gehringer, J. E., Heinrichs-Graham, E., and Wilson, T. W. (2017). Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage 15, 298–305. doi: 10.1016/j.nicl.2017.05.008
Labyt, E., Cassim, F., Devos, D., Bourriez, J.-L., Destée, A., Guieu, J.-D., et al. (2005). Abnormal cortical mechanisms in voluntary muscle relaxation in de novo parkinsonian patients. J. Clin. Neurophysiol. 22, 192–203.
Labyt, E., Szurhaj, W., Bourriez, J.-L., Cassim, F., Defebvre, L., Destée, A., et al. (2004). Influence of aging on cortical activity associated with a visuo-motor task. Neurobiol. Aging 25, 817–827. doi: 10.1016/j.neurobiolaging.2003.08.010
Labyt, E., Szurhaj, W., Bourriez, J.-L., Cassim, F., Defebvre, L., Destée, A., et al. (2003). Changes in oscillatory cortical activity related to a visuomotor task in young and elderly healthy subjects. Clin Neurophysiol 114, 1153–1166. doi: 10.1016/s1388-2457(03)00058-0
Leocani, L., Colombo, B., Magnani, G., Martinelli-Boneschi, F., Cursi, M., Rossi, P., et al. (2001a). Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement—EEG evidence. Neuroimage 13, 1186–1192. doi: 10.1006/nimg.2001.0759
Leocani, L., Locatelli, M., Bellodi, L., Fornara, C., Henin, M., Magnani, G., et al. (2001b). Abnormal pattern of cortical activation associated with voluntary movement in obsessive-compulsive disorder: An EEG study. Am. J. Psychiatry 158, 140–142. doi: 10.1176/appi.ajp.158.1.140
Lundqvist, M., Rose, J., Herman, P., Brincat, S. L., Buschman, T. J., and Miller, E. K. (2016). Gamma and beta bursts underlie working memory. Neuron 90, 152–164. doi: 10.1016/j.neuron.2016.02.028
Magnani, G., Cursi, M., Leocani, L., Volonté, M. A., and Comi, G. (2002). Acute effects of L-dopa on event-related desynchronization in Parkinson’s disease. Neurol. Sci. 23, 91–97. doi: 10.1007/s100720200033
Magnani, G., Cursi, M., Leocani, L., Volonté, M. A., Locatelli, T., Elia, A., et al. (1998). Event-related desynchronization to contingent negative variation and self-paced movement paradigms in Parkinson’s disease. Mov. Disord. 13, 653–660. doi: 10.1002/mds.870130408
Magnus, O. (1954). The central alpha-rhythm (“rhythme en arceau”). Electroencephalogr. Clin. Neurophysiol. 6, 349–350.
Marchesi, G., Albanese, G. A., Ferrazzoli, D., George, S., Ricci, S., Tatti, E., et al. (2019). Effects of rTMS and intensive rehabilitation in Parkinson’s disease on learning and retention. IEEE Int. Conf. Rehabil. Robot. 2019, 1260–1265. doi: 10.1109/ICORR.2019.8779471
Marinelli, L., Quartarone, A., Hallett, M., Frazzitta, G., and Ghilardi, M. F. (2017). The many facets of motor learning and their relevance for Parkinson’s disease. Clin. Neurophysiol. 128, 1127–1141. doi: 10.1016/j.clinph.2017.03.042
Mary, A., Bourguignon, M., Wens, V., Op de Beeck, M., Leproult, R., De Tiège, X., et al. (2015). Aging reduces experience-induced sensorimotor plasticity. A magnetoencephalographic study. Neuroimage 104, 59–68. doi: 10.1016/j.neuroimage.2014.10.010
McFarland, D. J., Miner, L. A., Vaughan, T. M., and Wolpaw, J. R. (2000). Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr. 12, 177–186. doi: 10.1023/a:1023437823106
McLinden, J., Deligani, R., Abtahi, M., Akbar, U., Mankodiya, K., and Shahriari, Y. (2021). Disruptions of cortico-kinematic interactions in Parkinson’s disease. Behav. Brain Res. 404:113153. doi: 10.1016/j.bbr.2021.113153
McMackin, R., Dukic, S., Costello, E., Pinto-Grau, M., Keenan, O., Fasano, A., et al. (2021). Sustained attention to response task-related beta oscillations relate to performance and provide a functional biomarker in ALS. J. Neural. Eng. 18:026006. doi: 10.1088/1741-2552/abd829
Meziane, H. B., Moisello, C., Perfetti, B., Kvint, S., Isaias, I. U., Quartarone, A., et al. (2015). Movement preparation and bilateral modulation of beta activity in aging and Parkinson’s disease. PLoS One 10:e0114817. doi: 10.1371/journal.pone.0114817
Miller, E. K., Lundqvist, M., and Bastos, A. M. (2018). Working Memory 2.0. Neuron 100, 463–475. doi: 10.1016/j.neuron.2018.09.023
Moisello, C., Blanco, D., Lin, J., Panday, P., Kelly, S. P., Quartarone, A., et al. (2015). Practice changes beta power at rest and its modulation during movement in healthy subjects but not in patients with Parkinson’s disease. Brain Behav. 5:e00374. doi: 10.1002/brb3.374
Moran, R. J., Mallet, N., Litvak, V., Dolan, R. J., Magill, P. J., Friston, K. J., et al. (2011). Alterations in brain connectivity underlying beta oscillations in parkinsonism. PLoS Comput. Biol. 7:e1002124. doi: 10.1371/journal.pcbi.1002124
Morgante, F., Dattola, V., Crupi, D., Russo, M., Rizzo, V., Ghilardi, M. F., et al. (2011). Is central fatigue in multiple sclerosis a disorder of movement preparation? J. Neurol. 258, 263–272. doi: 10.1007/s00415-010-5742-x
Motolese, F., Capone, F., and Di Lazzaro, V. (2022). New tools for shaping plasticity to enhance recovery after stroke. Handb. Clin. Neurol. 184, 299–315. doi: 10.1016/B978-0-12-819410-2.00016-3
Müller, G., Richter, R. A., Weisbrod, S., and Klingberg, F. (1991). Reaction time prolongation in the early stage of presenile onset Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 241, 46–48. doi: 10.1007/BF02193754
Muthukumaraswamy, S. D., Myers, J. F. M., Wilson, S. J., Nutt, D. J., Lingford-Hughes, A., Singh, K. D., et al. (2013). The effects of elevated endogenous GABA levels on movement-related network oscillations. NeuroImage 66, 36–41. doi: 10.1016/j.neuroimage.2012.10.054
Muthukumaraswamy, S. D., Singh, K. D., Swettenham, J. B., and Jones, D. K. (2010). Visual gamma oscillations and evoked responses: Variability, repeatability and structural MRI correlates. Neuroimage 49, 3349–3357. doi: 10.1016/j.neuroimage.2009.11.045
Nelson, A. B., Moisello, C., Lin, J., Panday, P., Ricci, S., Canessa, A., et al. (2017). Beta oscillatory changes and retention of motor skills during practice in healthy subjects and in patients with Parkinson’s disease. Front. Hum. Neurosci. 11:104. doi: 10.3389/fnhum.2017.00104
Nelson, A. B., Ricci, S., Tatti, E., Panday, P., Girau, E., Lin, J., et al. (2021). Neural fatigue due to intensive learning is reversed by a nap but not by quiet waking. Sleep 44:zsaa143. doi: 10.1093/sleep/zsaa143
Nowak, D. A., Dafotakis, M., and Fink, G. R. (2013). Kinematic analysis of grasping in focal dystonia of the face and neck. Neuroscience 237, 216–222. doi: 10.1016/j.neuroscience.2013.01.065
Nowak, M., Zich, C., and Stagg, C. J. (2018). Motor cortical gamma oscillations: What have we learnt and where are we headed? Curr. Behav. Neurosci. Rep. 5, 136–142. doi: 10.1007/s40473-018-0151-z
Oswal, A., Litvak, V., Brücke, C., Huebl, J., Schneider, G.-H., Kühn, A. A., et al. (2013). Cognitive factors modulate activity within the human subthalamic nucleus during voluntary movement in Parkinson’s disease. J. Neurosci. 33, 15815–15826. doi: 10.1523/JNEUROSCI.1790-13.2013
Ott, B. R., Ellias, S. A., and Lannon, M. C. (1995). Quantitative assessment of movement in Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 8, 71–75.
Pelosin, E., Bove, M., Marinelli, L., Abbruzzese, G., and Ghilardi, M. F. (2009). Cervical dystonia affects aimed movements of nondystonic segments. Mov. Disord. 24, 1955–1961. doi: 10.1002/mds.22693
Pfurtscheller, G. (1981). Central beta rhythm during sensorimotor activities in man. Electroencephalogr. Clin. Neurophysiol. 51, 253–264. doi: 10.1016/0013-4694(81)90139-5
Pfurtscheller, G., and Neuper, C. (1997). Motor imagery activates primary sensorimotor area in humans. Neurosci. Lett. 239, 65–68. doi: 10.1016/s0304-3940(97)00889-6
Pfurtscheller, G., Pichler-Zalaudek, K., Ortmayr, B., Diez, J., and Reisecker, F. (1998). Postmovement beta synchronization in patients with Parkinson’s disease. J. Clin. Neurophysiol. 15, 243–250. doi: 10.1097/00004691-199805000-00008
Pfurtscheller, G., Stancák, A., and Neuper, C. (1996). Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr. Clin. Neurophysiol. 98, 281–293. doi: 10.1016/0013-4694(95)00258-8
Pozzi, N. G., and Isaias, I. U. (2022). “Adaptive deep brain stimulation: Retuning Parkinson’s disease,” in Handbook of clinical neurology, neuroplasticity, eds A. Quartarone, M. F. Ghilardi, and F. Boller (Amsterdam: Elsevier), 273–284.
Praamstra, P., and Pope, P. (2007). Slow brain potential and oscillatory EEG manifestations of impaired temporal preparation in Parkinson’s disease. J. Neurophysiol. 98, 2848–2857. doi: 10.1152/jn.00224.2007
Priyadarsini, N., Nanda, P., Devi, S., and Mohapatra, S. (2022). Sarcopenia: An age-related multifactorial disorder. Curr. Aging Sci. 15, 209–217. doi: 10.2174/1874609815666220304194539
Putzhammer, A., Perfahl, M., Pfeiff, L., and Hajak, G. (2005). Correlation of subjective well-being in schizophrenic patients with gait parameters, expert-rated motor disturbances, and psychopathological status. Pharmacopsychiatry 38, 132–138. doi: 10.1055/s-2005-864125
Quandt, F., Bönstrup, M., Schulz, R., Timmermann, J. E., Mund, M., Wessel, M. J., et al. (2019). The functional role of beta-oscillations in the supplementary motor area during reaching and grasping after stroke: A question of structural damage to the corticospinal tract. Hum. Brain Mapp. 40, 3091–3101. doi: 10.1002/hbm.24582
Quartarone, A., and Ghilardi, M. F. (2022). “Neuroplasticity in dystonia: Motor symptoms and beyond,” in Handbook of clinical neurology, neuroplasticity, eds A. Quartarone, M. F. Ghilardi, and F. Boller (Amsterdam: Elsevier), 207–218. doi: 10.1016/B978-0-12-819410-2.00031-X
Quartarone, A., and Hallett, M. (2013). Emerging concepts in the physiological basis of dystonia. Mov. Disord. 28, 958–967. doi: 10.1002/mds.25532
Ranganathan, V. K., Siemionow, V., Sahgal, V., and Yue, G. H. (2001). Effects of aging on hand function. J. Am. Geriatr. Soc. 49, 1478–1484. doi: 10.1046/j.1532-5415.2001.4911240.x
Ricci, S., Tatti, E., Mehraram, R., Panday, P., and Ghilardi, M. F. (2019b). Beta band frequency differences between motor and frontal cortices in reaching movements. IEEE Int. Conf. Rehabil. Robot. 2019, 1254–1259. doi: 10.1109/ICORR.2019.8779373
Ricci, S., Mehraram, R., Tatti, E., Nelson, A. B., Bossini-Baroggi, M., Panday, P., et al. (2019a). Aging does not affect beta modulation during reaching movements. Neural. Plasticity 2019:e1619290. doi: 10.1155/2019/1619290
Riva, N., Falini, A., Inuggi, A., Gonzalez-Rosa, J. J., Amadio, S., Cerri, F., et al. (2012). Cortical activation to voluntary movement in amyotrophic lateral sclerosis is related to corticospinal damage: Electrophysiological evidence. Clin. Neurophysiol. 123, 1586–1592. doi: 10.1016/j.clinph.2011.12.013
Robson, S. E., Brookes, M. J., Hall, E. L., Palaniyappan, L., Kumar, J., Skelton, M., et al. (2016). Abnormal visuomotor processing in schizophrenia. NeuroImage 12, 869–878. doi: 10.1016/j.nicl.2015.08.005
Roopun, A. K., Middleton, S. J., Cunningham, M. O., LeBeau, F. E. N., Bibbig, A., Whittington, M. A., et al. (2006). A beta2-frequency (20-30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc. Natl. Acad. Sci. U.S.A. 103, 15646–15650. doi: 10.1073/pnas.0607443103
Rosen, A. J., Lockhart, J. J., Gants, E. S., and Westergaard, C. K. (1991). Maintenance of grip-induced muscle tension: A behavioral marker of schizophrenia. J. Abnorm. Psychol. 100, 583–593. doi: 10.1037//0021-843x.100.4.583
Rossiter, H. E., Davis, E. M., Clark, E. V., Boudrias, M.-H., and Ward, N. S. (2014b). Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 91, 360–365. doi: 10.1016/j.neuroimage.2014.01.012
Rossiter, H. E., Boudrias, M.-H., and Ward, N. S. (2014a). Do movement-related beta oscillations change after stroke? J. Neurophysiol. 112, 2053–2058. doi: 10.1152/jn.00345.2014
Rowland, N., de Hemptinne, C., Swann, N., Qasim, S., Miocinovic, S., Ostrem, J., et al. (2015). Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: Changes in beta and gamma bands. Front. Hum. Neurosci. 9:512. doi: 10.3389/fnhum.2015.00512
Rule, M. E., Vargas-Irwin, C. E., Donoghue, J. P., and Truccolo, W. (2017). Dissociation between sustained single-neuron spiking and transient β-LFP oscillations in primate motor cortex. J. Neurophysiol. 117, 1524–1543. doi: 10.1152/jn.00651.2016
Sailer, A., Dichgans, J., and Gerloff, C. (2000). The influence of normal aging on the cortical processing of a simple motor task. Neurology 55, 979–985. doi: 10.1212/wnl.55.7.979
Sallard, E., Tallet, J., Thut, G., Deiber, M.-P., and Barral, J. (2016). Age-related changes in post-movement beta synchronization during a selective inhibition task. Exp. Brain Res. 234, 3543–3553. doi: 10.1007/s00221-016-4753-y
Salthouse, T. A. (1984). Effects of age and skill in typing. J. Exp. Psychol. 113, 345–371. doi: 10.1037/0096-3445.113.3.345
Schmidt, R., Ruiz, M. H., Kilavik, B. E., Lundqvist, M., Starr, P. A., and Aron, A. R. (2019). Beta oscillations in working memory. Executive control of movement and thought, and sensorimotor function. J. Neurosci. 39, 8231–8238. doi: 10.1523/JNEUROSCI.1163-19.2019
Shiner, C. T., Tang, H., Johnson, B. W., and McNulty, P. A. (2015). Cortical beta oscillations and motor thresholds differ across the spectrum of post-stroke motor impairment, a preliminary MEG and TMS study. Brain Res. 1629, 26–37. doi: 10.1016/j.brainres.2015.09.037
Smith, B. A., Jacobs, J. V., and Horak, F. B. (2012). Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Exp. Brain Res. 222, 455–470. doi: 10.1007/s00221-012-3232-3
Spitzer, B., and Haegens, S. (2017). Beyond the status quo: A role for beta oscillations in endogenous content (re)activation. eNeuro 4:ENEURO.0170-17.2017. doi: 10.1523/ENEURO.0170-17.2017
Stegemöller, E. L., Allen, D. P., Simuni, T., and MacKinnon, C. D. (2016). Motor cortical oscillations are abnormally suppressed during repetitive movement in patients with Parkinson’s disease. Clin. Neurophysiol 127, 664–674. doi: 10.1016/j.clinph.2015.05.014
Stein, E., and Bar-Gad, I. (2013). Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol. Special Issue 245, 52–59. doi: 10.1016/j.expneurol.2012.07.023
Tamás, G., Pálvölgyi, L., Takáts, A., Szirmai, I., and Kamondi, A. (2006). Movement-related beta responses in essential tremor and Parkinson’s disease. Ideggyogy. Sz. 59, 417–424.
Tan, H., Wade, C., and Brown, P. (2016). Post-movement beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J. Neurosci. 36, 1516–1528. doi: 10.1523/JNEUROSCI.3204-15.2016
Tatti, E., Ferraioli, F., Cacciola, A., Chan, C., Quartarone, A., and Ghilardi, M. F. (2022). Modulation of gamma spectral amplitude and connectivity during reaching predicts peak velocity and movement duration. Front. Neurosci. 16:836703. doi: 10.3389/fnins.2022.836703
Tatti, E., Ferraioli, F., Peter, J., Alalade, T., Nelson, A. B., Ricci, S., et al. (2021). Frontal increase of beta modulation during the practice of a motor task is enhanced by visuomotor learning. Sci. Rep. 11:17441. doi: 10.1038/s41598-021-97004-0
Tatti, E., Ricci, S., Mehraram, R., Lin, N., George, S., Nelson, A. B., et al. (2019). Beta modulation depth is not linked to movement features. Front. Behav. Neurosci. 13:49. doi: 10.3389/fnbeh.2019.00049
Tatti, E., Ricci, S., Nelson, A. B., Mathew, D., Chen, H., Quartarone, A., et al. (2020). Prior practice affects movement-related beta modulation and quiet wake restores it to baseline. Front. Syst. Neurosci. 14:61. doi: 10.3389/fnsys.2020.00061
Teremetz, M., Amado, I., Bendjemaa, N., Krebs, M.-O., Lindberg, P. G., and Maier, M. A. (2014). Deficient grip force control in schizophrenia: Behavioral and modeling evidence for altered motor inhibition and motor noise. PLoS One 9:e111853. doi: 10.1371/journal.pone.0111853
Térémetz, M., Carment, L., Brénugat-Herne, L., Croca, M., Bleton, J.-P., Krebs, M.-O., et al. (2017). Manual dexterity in schizophrenia-A neglected clinical marker? Front. Psychiatry 8:120. doi: 10.3389/fpsyt.2017.00120
Toledo, D. R., Manzano, G. M., Barela, J. A., and Kohn, A. F. (2016). Cortical correlates of response time slowing in older adults: ERP and ERD/ERS analyses during passive ankle movement. Clin. Neurophysiol. 127, 655–663. doi: 10.1016/j.clinph.2015.05.003
Toro, C., Deuschl, G., and Hallett, M. (2000). Movement-related electroencephalographic desynchronization in patients with hand cramps: Evidence for motor cortical involvement in focal dystonia. Ann. Neurol. 47, 456–461.
van Wijk, B. C. M., Neumann, W.-J., Schneider, G.-H., Sander, T. H., Litvak, V., and Kühn, A. A. (2017). Low-beta cortico-pallidal coherence decreases during movement and correlates with overall reaction time. NeuroImage 159, 1–8. doi: 10.1016/j.neuroimage.2017.07.024
Van Zandijcke, M., and Casselman, J. (1995). Involvement of corpus callosum in amyotrophic lateral sclerosis shown by MRI. Neuroradiology 37, 287–288. doi: 10.1007/BF00588334
Vinding, M. C., Tsitsi, P., Piitulainen, H., Waldthaler, J., Jousmäki, V., Ingvar, M., et al. (2019). Attenuated beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci. Rep. 9:2604. doi: 10.1038/s41598-019-39204-3
Waldman, A. T., Sollee, J. R., Datta, R., Lavery, A. M., Liu, G., Aleman, T. S., et al. (2020). Structural correlates of atypical visual and motor cortical oscillations in pediatric-onset multiple sclerosis. Hum. Brain Mapp. 41, 4299–4313. doi: 10.1002/hbm.25126
Walker, S., Monto, S., Piirainen, J. M., Avela, J., Tarkka, I. M., Parviainen, T. M., et al. (2020). Older age increases the amplitude of muscle stretch-induced cortical beta-band suppression but does not affect rebound strength. Front. Aging Neurosci. 12:117. doi: 10.3389/fnagi.2020.00117
Walther, S., and Mittal, V. A. (2016). Why we should take a closer look at gestures. Schizophr. Bull. 42, 259–261. doi: 10.1093/schbul/sbv229
Wang, J., Hirschmann, J., Elben, S., Hartmann, C. J., Vesper, J., Wojtecki, L., et al. (2014). High-frequency oscillations in Parkinson’s disease: Spatial distribution and clinical relevance. Mov. Disord. 29, 1265–1272. doi: 10.1002/mds.25962
Wang, Q., Meng, L., Pang, J., Zhu, X., and Ming, D. (2020). Characterization of EEG data revealing relationships with cognitive and motor symptoms in Parkinson’s disease: A systematic review. Front. Aging Neurosci. 12:587396. doi: 10.3389/fnagi.2020.587396
Weersink, J. (2021). Arm swing in healthy and Parkinsonian gait: Explorations on brain, muscle and movement level (Thesis fully internal (DIV)). [Groningen]: University of Groningen, doi: 10.33612/diss.190721932
Weersink, J. B., Gefferie, S. R., van Laar, T., Maurits, N. M., and de Jong, B. M. (2020). Pre-movement cortico-muscular dynamics underlying improved parkinson gait initiation after instructed arm swing. J. Parkinsons Dis. 10, 1675–1693. doi: 10.3233/JPD-202112
Weinberger, M., Hutchison, W. D., and Dostrovsky, J. O. (2009). Pathological subthalamic nucleus oscillations in PD: Can they be the cause of bradykinesia and akinesia? Exp. Neurol. 219, 58–61. doi: 10.1016/j.expneurol.2009.05.014
Williams, G. R., Jiang, J. G., Matchar, D. B., and Samsa, G. P. (1999). Incidence and occurrence of total (First-Ever and Recurrent) stroke. Stroke 30, 2523–2528. doi: 10.1161/01.STR.30.12.2523
Wolff, A. L., and O’Driscoll, G. A. (1999). Motor deficits and schizophrenia: The evidence from neuroleptic-naïve patients and populations at risk. J. Psychiatry Neurosci. 24, 304–314.
Wu, H.-M., Hsiao, F.-J., Chen, R.-S., Shan, D.-E., Hsu, W.-Y., Chiang, M.-C., et al. (2019). Attenuated NoGo-related beta desynchronisation and synchronisation in Parkinson’s disease revealed by magnetoencephalographic recording. Sci. Rep. 9:7235. doi: 10.1038/s41598-019-43762-x
Yamawaki, N., Stanford, I. M., Hall, S. D., and Woodhall, G. L. (2008). Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience 151, 386–395. doi: 10.1016/j.neuroscience.2007.10.021
Zaepffel, M., Trachel, R., Kilavik, B. E., and Brochier, T. (2013). Modulations of EEG beta power during planning and execution of grasping movements. PLoS One 8:e60060. doi: 10.1371/journal.pone.0060060
Keywords: beta oscillations, EEG, MEG, movement, neurological diseases, psychiatric disorders, ERD, ERS
Citation: Peter J, Ferraioli F, Mathew D, George S, Chan C, Alalade T, Salcedo SA, Saed S, Tatti E, Quartarone A and Ghilardi MF (2022) Movement-related beta ERD and ERS abnormalities in neuropsychiatric disorders. Front. Neurosci. 16:1045715. doi: 10.3389/fnins.2022.1045715
Received: 15 September 2022; Accepted: 31 October 2022;
Published: 23 November 2022.
Edited by:
Julia Stephen, The Mind Research Network (MRN), United StatesReviewed by:
Helen M. Bronte-Stewart, Stanford University, United StatesMario Versaci, Mediterranea University of Reggio Calabria, Italy
Simone Rossi, University of Siena, Italy
Ahmed Naglah, University of Louisville, United States
Copyright © 2022 Peter, Ferraioli, Mathew, George, Chan, Alalade, Salcedo, Saed, Tatti, Quartarone and Ghilardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa Tatti, ZWxpc2F0YXR0aUBtc24uY29t; Angelo Quartarone, YXF1YXJ0YXI2NUBnbWFpbC5jb20=; M. Felice Ghilardi, bGljZS5tZzc5QGdtYWlsLmNvbQ==
†Present Address: Francesca Ferraioli, Department of Cognitive Science, Psychological, Pedagogical and Cultural Studies, University of Messina, Messina, Italy
 Jaime Peter
Jaime Peter Francesca Ferraioli
Francesca Ferraioli Dave Mathew
Dave Mathew Shaina George1
Shaina George1 Cameron Chan
Cameron Chan Tomisin Alalade
Tomisin Alalade Sheilla A. Salcedo
Sheilla A. Salcedo Shannon Saed
Shannon Saed Elisa Tatti
Elisa Tatti Angelo Quartarone
Angelo Quartarone M. Felice Ghilardi
M. Felice Ghilardi