- 1School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Tuina and Pain Management, Dongzhimen Hospital of Beijing University of Chinese Medicine, Beijing, China
- 3Department of Acupuncture, Oriental Hospital of Beijing University of Chinese Medicine, Beijing, China
Previous studies have proved and investigated the mechanism of the analgesic effect of tuina treatment on neuropathic pain. The purpose of this study was to analyze changes in gene expression in the dorsal root ganglia (DRG) and spinal dorsal horn (SDH) after 1-time tuina intervention to investigate the immediate analgesic mechanism by tuina. An improvement in nociceptive behavior in minor chronic constriction injury (CCI) rats after 1-time tuina was observed. 1-time tuina was more effective in the amelioration of thermal hyperalgesia, but no changes were found in the ultrastructure of DRG and SDH. Sixty-five differentially expressed genes (DEGs) modulated by tuina were detected in the DRG and 123 DEGs were detected in the SDH. Potential immediate analgesic mechanisms of tuina were analyzed by the Kyoto Encyclopedia of Genes and Genomes. DEGs were enriched in 75 pathways in DRG, and 107 pathways in SDH. The immediate analgesic mechanism of tuina is related to the calcium signaling pathway, thermogenesis, and regulation of lipolysis in adipocytes.
Introduction
Neuropathic pain (NP) is a syndrome caused by injury or disease of the somatosensory nervous system, either in the periphery or central, and is characterized by spontaneous pain, hyperalgesia, allodynia, and paresthesia (Baron et al., 2010). NP has a significant influence on the physical and psychological health of patients, and the prevalence in the whole population is approximately 10%, which varies by disease. Due to the complicated pathogenesis, current treatments such as medication did not show obvious effects (Schaefer et al., 2014; Fayaz et al., 2016; Scholz et al., 2019; Zheng et al., 2020).
Tuina is an alternative medical therapy which is safe and has virtually no side effects (Field, 2016). Clinical studies have proved that tuina has either immediate or cumulative analgesic effects, which can ameliorate the symptoms of NP (Gok Metin et al., 2017; Izgu et al., 2019). Its cumulative analgesic mechanism is based on the down-regulation of inflammatory cytokines (such as tumor necrosis factor-α, interleukin-1, and interleukin-1β) and the inhibition of microglial activation (Liu et al., 2021). Our previous studies confirmed the effectiveness of tuina analgesia, and found differentially expressed genes (DEGs) in DRG and SDH of rats with sciatic nerve injury after 20-time tuina treatment, which were mainly related to regulation of protein binding, response to pressure, and neuron projection (Lv, 2020; Lv et al., 2020a). However, the immediate analgesic mechanism of tuina is unknown. Clinically, 1 or 2 time tuina treatment can effectively relieve pain, achieve immediate analgesia and prevent disease progression in patients (Li et al., 2004; Qing et al., 2022). Therefore, searching for immediate effective targets and exploring mechanisms of tuina analgesia can provide new evidence and methods for the therapeutic targets of NP, as well as help tuina to be accepted and applied in more countries and regions.
As the sensory neurons of the first and second levels, the dorsal root ganglia (DRG) and the spinal dorsal horn (SDH) are the primary portals for nerve afferents and integration of pain information. They are important for both inducing central sensitization and processing NP development (Simeoli et al., 2017; Inoue and Tsuda, 2018; Shepherd et al., 2018). Here, we investigate the immediate analgesic mechanism of 1-time tuina and changes in RNA expression in DRG and SDH using the minor chronic constriction injury (CCI) model, which is a recognized model for simulating clinical peripheral neuropathic pain (Jaggi et al., 2011). To this end, we behaviorally assessed mechanical and thermal allodynia changes after the tuina treatment and non-treatment conditions in the CCI rat model. We also analyzed the gene expression differences and functions before and after intervention in the DRG and SDH of the surgical side by RNA-Seq.
Results
Changes in symptoms of hyperalgesia and allodynia in minor chronic constriction injury rats
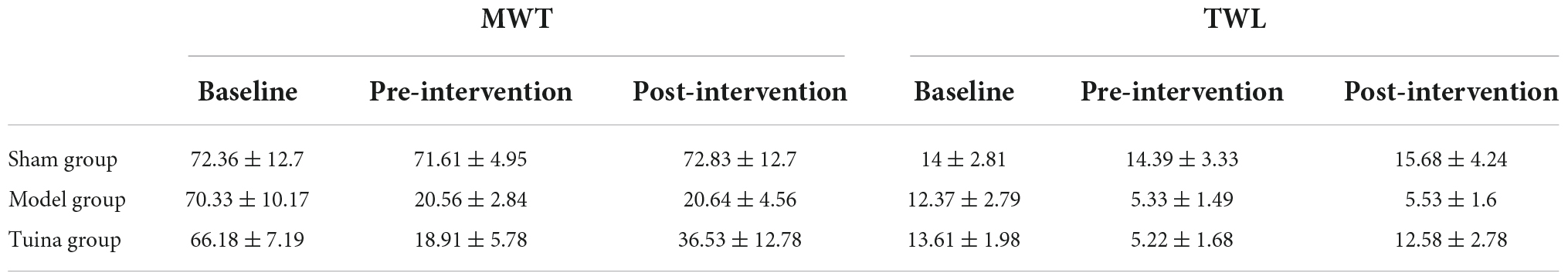
The rats were in good condition and recovered well from the surgical incision. The posture of rats in the sham group was normal when walking and resting. Rats in the model and tuina group had curled hind paws on the surgical side, limping when walking, and mostly lying on the left side when resting. There was no statistical difference in behavior tests between groups before modeling, and before intervention. Mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were decreased in both the model (P = 0.00, P = 0.00) and tuina groups (P = 0.00, P = 0.00) compared with the sham group. After a 1-time tuina intervention, MWT and TWL increased in the tuina group (P = 0.014, P = 0.00) compared with the model group (Figure 1 and Table 1).
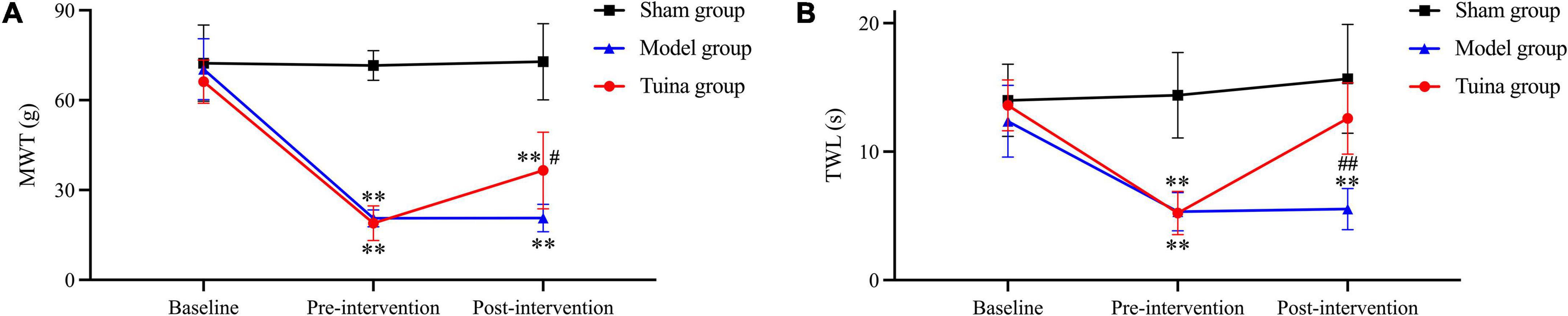
Figure 1. Results of behavioral tests of rats in each group. (A) MWT; (B) TWL. Sham group (n = 6); model group (n = 6); tuina group (n = 6). **P < 0.01, vs. the sham group; #P < 0.05, ##P < 0.01, vs. the model group.
Changes in the ultrastructure of dorsal root ganglia and spinal dorsal horn
The electron microscopy results of DRG in the sham group showed that the mitochondrial morphology was intact, and the mitochondrial matrix was homogeneous (Figure 2A). The results of the model and tuina groups showed mitochondrial swelling, disorganized mitochondrial cristae arrangement with matrix dissolution and dilated mitochondria with occasional vacuolization (Figures 2B,C). The electron microscopic results of SDH in the sham group showed the nuclear membrane was smooth and intact, the perinuclear gap was not significantly widened, and the chromatin was sparse (Figure 2D). The images of the model and tuina groups showed that the nucleus was irregular in shape, the double-layered nuclear membrane was intact, the perinuclear gap was not significantly widened, and the chromatin was aggregated (Figures 2E,F).
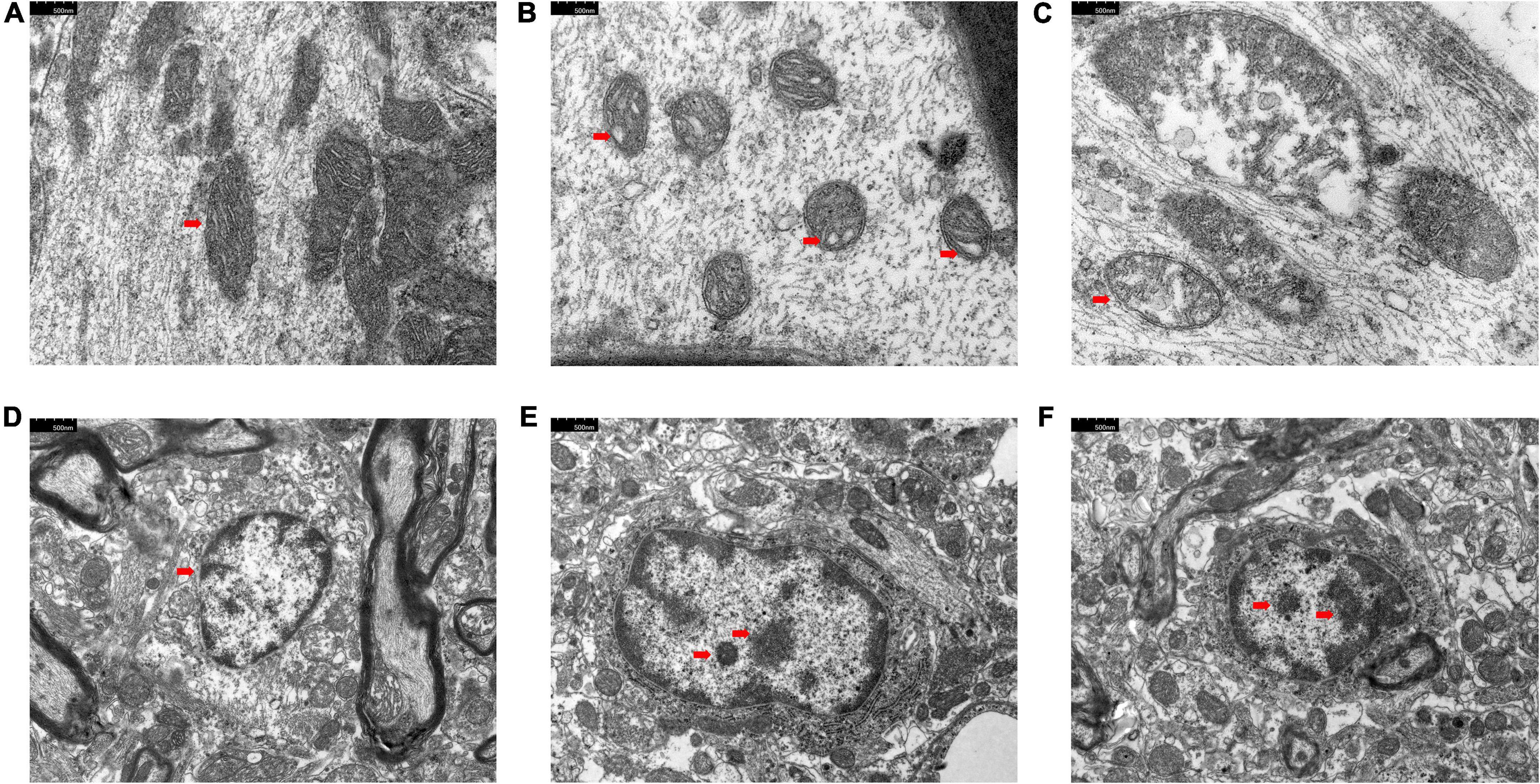
Figure 2. Electron microscopy results of DRG and SDH. Sham group (n = 3); model group (n = 3); tuina group (n = 3). Panels (A–C) were DRG; the arrow showed the mitochondrial vacuoles; panels (D–F) were SDH; the arrow shows the chromatin aggregation. (A,D) Sham group; (B,E) Model group; (C,F) Tuina group.
Bioinformatic analysis of dorsal root ganglia and spinal dorsal horn on the surgical side of rats
Results of the sample quality control
The results of performing quality control on the raw data indicated that the data were qualified and ready for subsequent analysis. We obtained a total of 920 million reads, including an average of 49.84 million reads per DRG sample and 53.32 million reads per SDH sample. The results of the error rate, Q20 and Q30 met the quality control standards, which showed that the sequencing results were reliable and credible (Table 2).
Changes of differentially expressed genes after tuina intervention
The results of principal component analysis (PCA) showed good intragroup aggregation in each group, indicating a high degree of similarity between the samples within the group. It also showed that there was good intergroup dispersion, which indicated significant differences between all groups. The results confirmed that the model was established successfully and tuina treatment was effective (Figure 3). Changes of DEGs in DRG and SDH on the surgical side were detected by RNA sequencing. A total of 964 DEGs were detected in the DRG of the three groups. There were 65 up-regulated DEGs and 277 down-regulated DEGs in the model vs. sham (Figure 4A), and 106 up-regulated DEGs and 129 down-regulated DEGs in the model vs. tuina (Figure 4B). Venn analysis showed a total of 122 DEGs between the model vs. sham and the model vs. tuina (Figure 5A). A total of 65 DEGs showed opposite expression trends between the model group and the tuina group. A total of 674 DEGs were detected in the SDH of the three groups. There were 80 up-regulated DEGs and 308 down-regulated DEGs in the model vs. sham (Figure 4C), and 52 up-regulated DEGs and 198 down-regulated DEGs in the model vs. tuina (Figure 4D). Venn analysis showed a total of 127 DEG between the model vs. sham and the model vs. tuina (Figure 5B). 123 DEGs showed opposite expression trends between the model and tuina group.
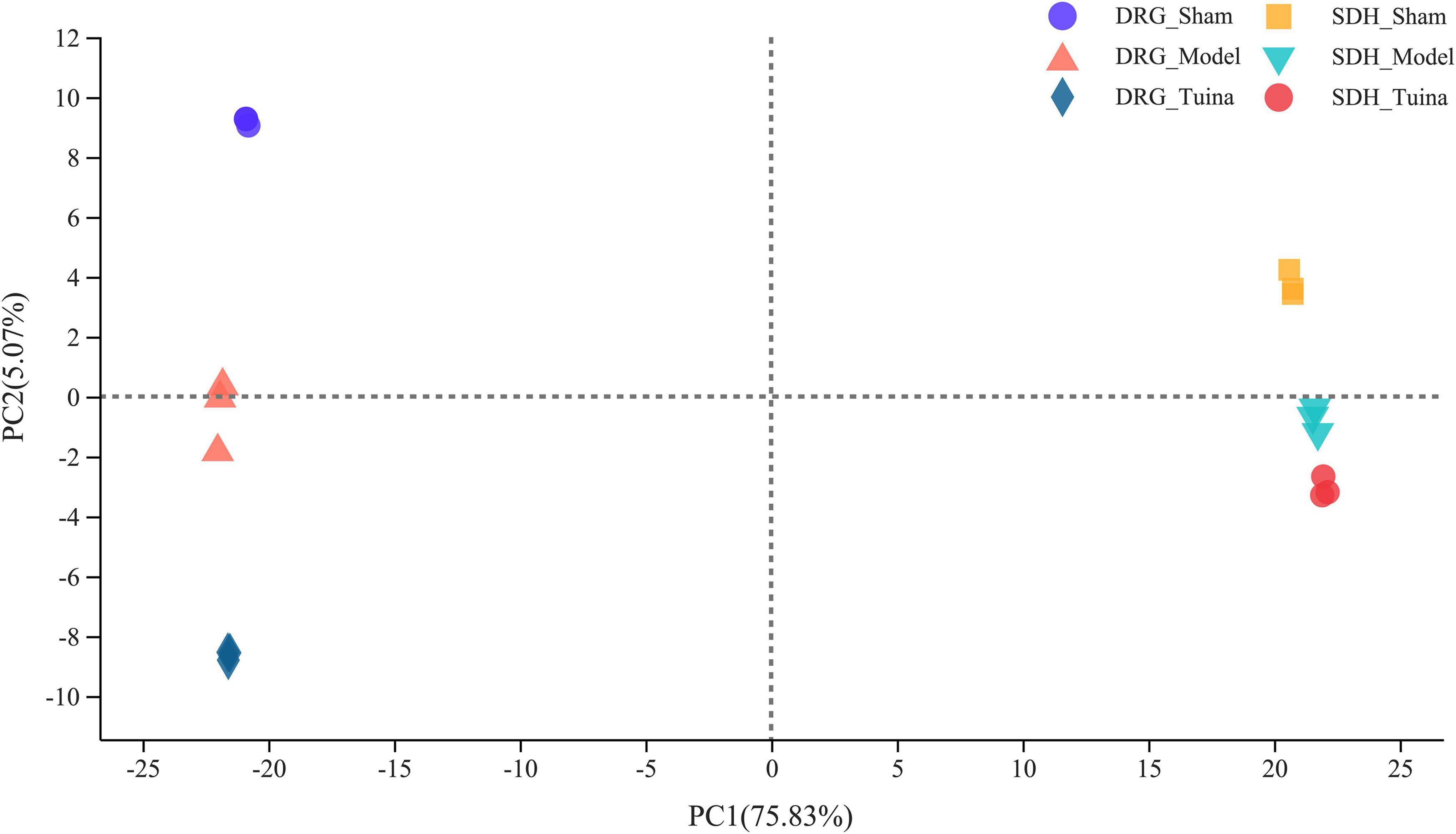
Figure 3. PCA analysis of DRG and SDH samples. Sham group (n = 3); model group (n = 3); tuina group (n = 3). PC1 = 75.83%, PC2 = 5.07%, a higher percentage means that this principal component is more capable of differentiating the samples. The distance between graphs represents how far the results of samples showed, and the farther distance indicates the lower similarity between samples.
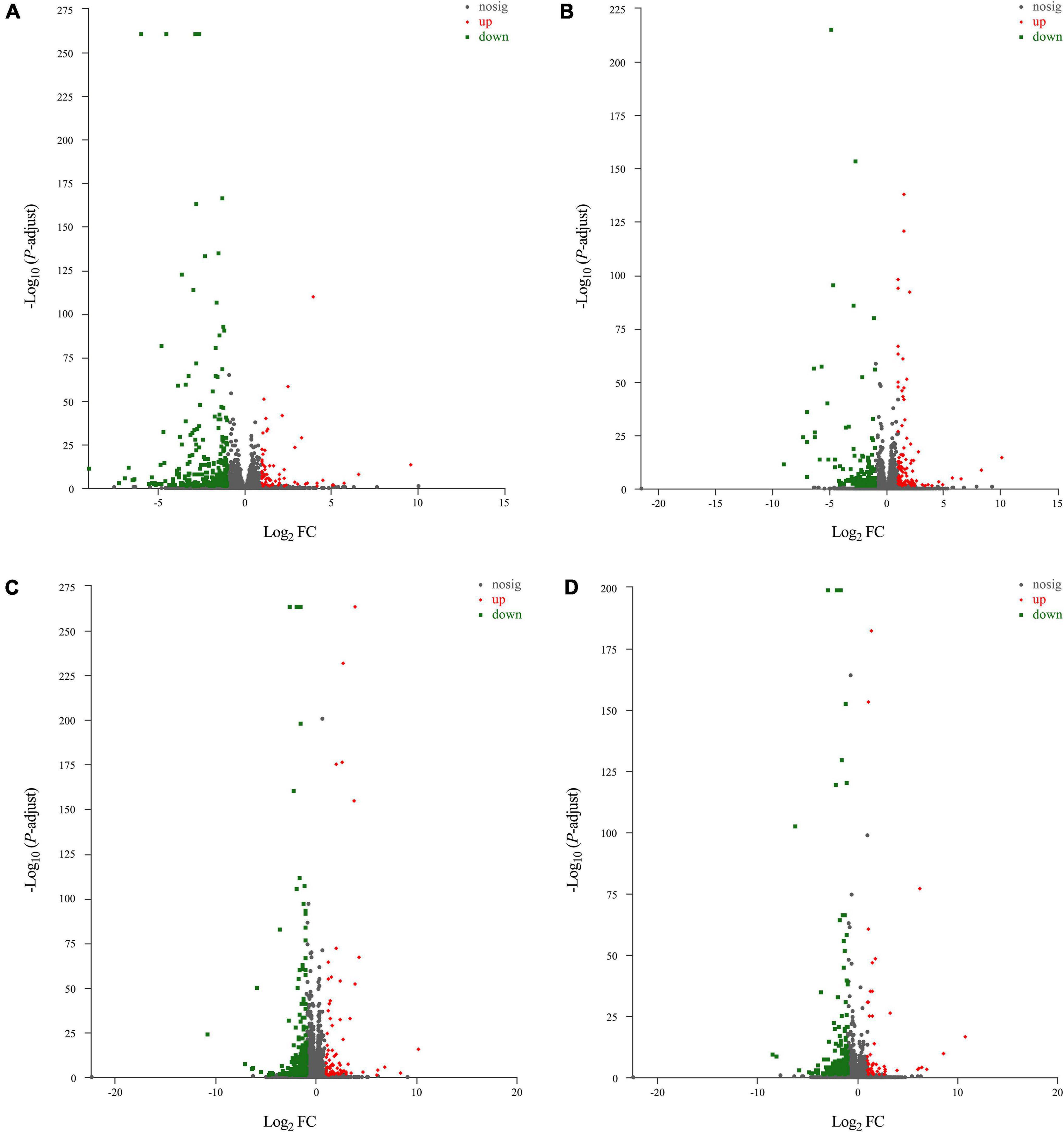
Figure 4. Volcano map results, the abscissa is the Log2 FC and the ordinate is the -LOG10, P-adjust <0.05. Results of DRG sequencing, (A) model vs. sham; (B) model vs. tuina; Results of SDH sequencing, (C) model vs. sham; (D) model vs. tuina. Gray are genes with no differential expression, red are genes with up-regulated expression, and green are genes with down-regulated expression.
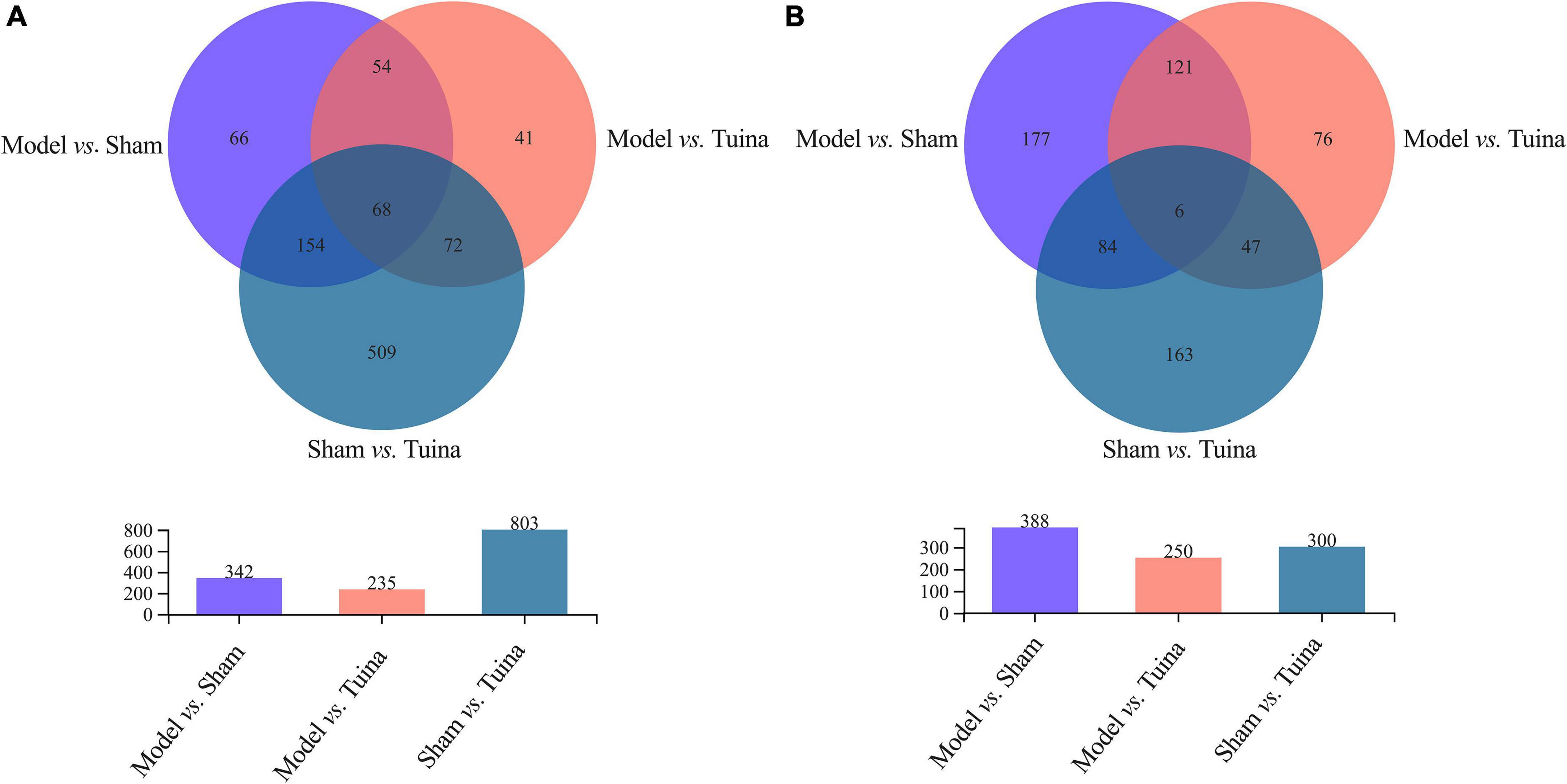
Figure 5. Results of the Venn analysis, the abscissa is the group, and the ordinate is the number of DEGs. (A) DRG sequencing; (B) SDH sequencing. The overlapping regions are identically expressed genes, and genes in the overlapping regions of model vs. sham and model vs. tuina are associated with the therapeutic effects of tuina.
Pathways analysis of differentially expressed genes regulated by tuina in dorsal root ganglia and spinal dorsal horn
To understand the function and role of DEGs in detail, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses (Figure 6). DEGs in DRG were enriched in 75 pathways, including the calcium signaling pathway, vascular smooth muscle contraction, leukocyte transendothelial migration, regulation of lipolysis in adipocytes, gonadotropin-releasing hormone (GnRH) secretion, cGMP-protein kinase G (cGMP-PKG) signaling pathway, apelin signaling pathway, and thermogenesis pathways. DEGs in SDH were enriched in 107 pathways, including the hypoxia-inducible factor (HIF) -1 signaling pathway, interleukin-17 signaling pathway, Toll-like receptor signaling pathway, GnRH signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, and T helper (Th) 17 cell differentiation pathways.
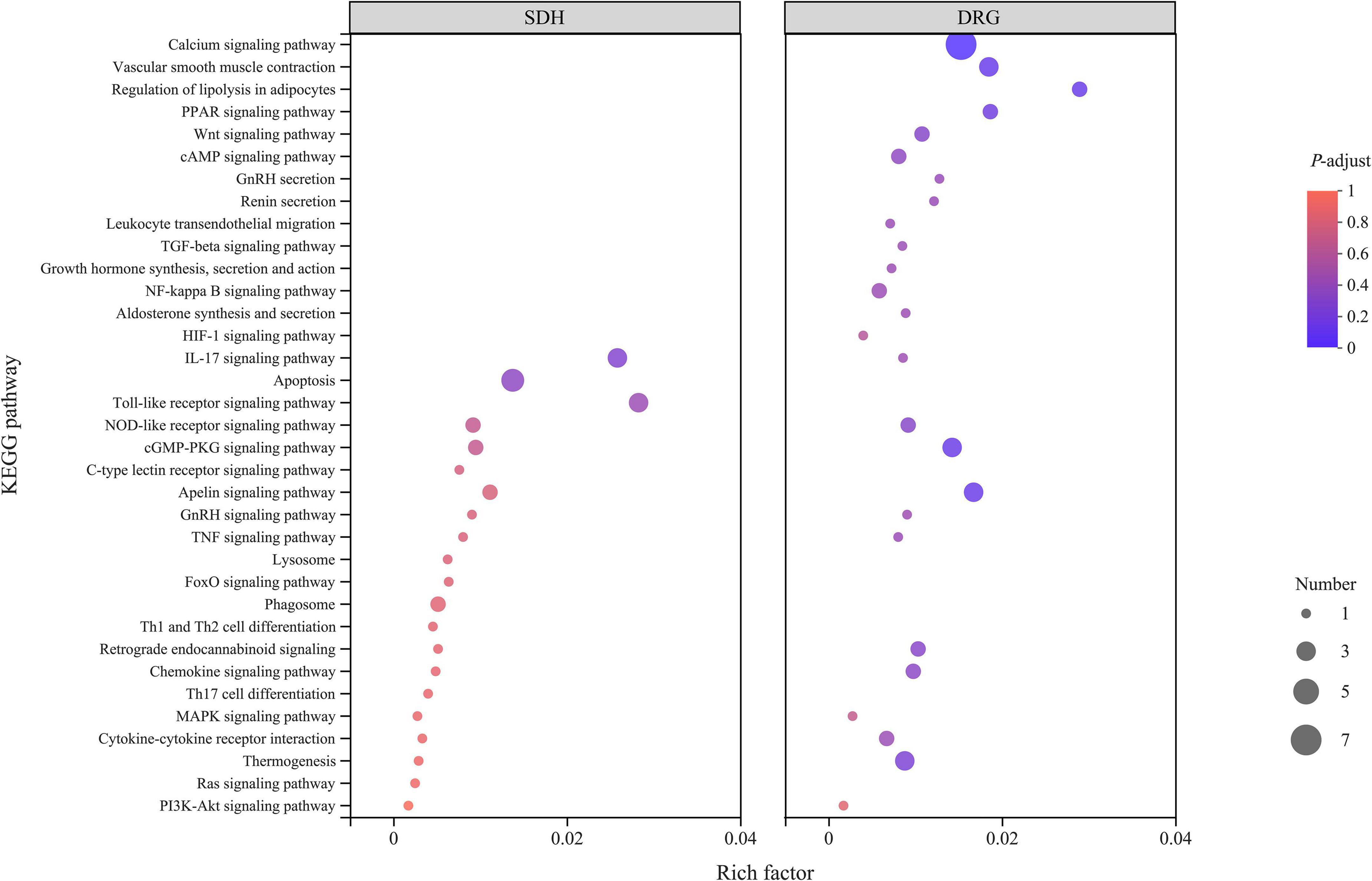
Figure 6. KEGG analysis results of DRG samples. SDH on the left and DRG on the right, the abscissa is the KEGG pathway, and the ordinate is the rich factor. The higher the rich factor, the greater the degree of enrichment, and the size of the dots indicates the number of genes in the pathway, while the color of the dots corresponds to the different P-adjust ranges.
Discussion
Tuina analgesia is effective and rapid, with practically no side effects (Wang et al., 2020; Rivaz et al., 2021; Liu et al., 2022). The goal of this study was to investigate the potential mechanisms in the induction of immediate analgesia by tuina. We assessed the immediate analgesic effect of tuina treatment by evaluating the changes in MWT and TWL before and after the treatment and screened DEGs using RNA-Seq. KEGG analysis was used to further explore the mechanisms.
Minor CCI model was used for simulating clinical NP. The minor CCI model causes nerve edema through ligation, producing chronic constriction and compression resulting in degeneration and necrosis of some nerve fibers which manifests as neuropathic pain with inflammatory pain after modeling, forming stable chronic pain in 3–5 days (Grace et al., 2011; MacDonald et al., 2021). In our preliminary study, consistent with the literature, we found that the model was stable at day 7 after modeling, so we chose that time point to give tuina intervention. The “Three-Manipulation and Three-Acupoint” is a combination of manipulations and acupoints that we have studied and proven to be effective (Li et al., 2019; Lv et al., 2020a). We found that after 20-time intervention, the MWT and TWL of rats with sciatic nerve injury were significantly improved, and demonstrated significant cumulative analgesic effects of the “Three-Manipulation and Three-Acupoint”.
In this experiment, we found that rats in the model and tuina groups showed significant allodynia, thermal hyperalgesia, and limp positions after modeling. The electron microscopy results of DRG and SDH in the model group also confirmed the difference in ultrastructure from the sham group. This suggests that the model replication was successful and consistent with the literature (Grace et al., 2010). While in previous experiments, we tested the therapeutic effect after a 20-time tuina intervention, the present study focuses on the immediate analgesic effect of tuina (Lv et al., 2020b). Behavioral tests were performed immediately after 1-time tuina treatment. Compared to the model group, the results of MWT and TWL in the tuina group were statistically significant. This result showed that 1-time tuina treatment can effectively alleviate hyperalgesia. Compared with mechanical hyperalgesia, the improvement in thermal hyperalgesia was more noticeable. The electron microscopic results of DRG and SDH revealed no difference in ultrastructure between the model and tuina groups, implying that short-term tuina intervention did not restore the morphology.
The calcium signaling pathway of DRG is a key factor in the immediate analgesic effect of tuina. Many of the pathways enriched in this study were associated with cascade activation of the calcium signaling pathway, which is critical to initiate and maintain peripheral sensitization of NP (Cui et al., 2021). Peripheral injurious stimuli increase intracellular Ca2+ through calcium signaling pathways, N-methyl-D-aspartic acid receptors, and other metabolic-like receptors in response to each other, and it can further activate calcium/calmodulin-dependent protein kinase II, and protein kinase A, which enhances postsynaptic excitability and triggers NP, resulting in peripheral and central sensitization (Yang et al., 2004; Latremoliere and Woolf, 2009; Incontro et al., 2018; Liu et al., 2018). The study proved that the mechanical stimulation administered to the body by tuina could initiate the skeletal muscle calcium signaling pathway, regulate the uptake and release of Ca2+, and adjust the intracellular Ca2+ concentration, thus repairing the damaged cells (Lin et al., 2013). The 1-time tuina intervention was able to modulate the calcium signaling pathway, which was beneficial in reducing nerve damage, local inflammatory microenvironment-induced nociceptive hypersensitivity, and reducing the hyperexcitability of injury-sensing neurons (Luo et al., 2008; Chen et al., 2009).
Thermogenesis/regulation of lipolysis in adipocytes in DRG and SDH is associated with the immediate analgesic mechanism of tuina. The results of behavioral tests indicated that tuina was more effective for thermal nociceptive sensitization. Tuina is considered to have a warming effect in Chinese medical theory. In the KEGG enriched pathways, we have found that the expression of thermogenesis/regulation of lipolysis in adipocytes, and uncoupling of protein-1 mediates the dissipation of energetic chemicals then induces adaptive thermogenesis through brown fat tissue. The binding to beta-adrenergic receptors increases lipolysis and regulates local energy metabolism of nerve injury at the genetic level, thereby increasing TWL (Kissig et al., 2016; Mills et al., 2018). The immediate mechanism of tuina analgesia will continue to be validated in future research. Furthermore, the KEGG enrichment results revealed that the 1-time tuina intervention was able to activate the apelin signaling pathway. Apelin is expressed in fat tissue and skeletal muscle. It can induce skeletal muscle cell proliferation and inhibit skeletal muscle atrophy, suggesting that tuina exerts immediate analgesic effects while preventing possible motor dysfunction of NP in the long term (Vinel et al., 2018).
The main limitation of this study is the lack of verification for the target genes and pathways to further confirm that the results were reliable. We will further verify the calcium signaling pathway and differential genes, focus on the gene expression changes of DRG after different times of tuina (1, 2, 3 times) in the future, and explore gene expression patterns through different therapeutic styles combined with multiple omics approaches to clarify the unique mechanism of tuina analgesia.
Materials and methods
Animals and ethical approval
Eighteen male Sprague-Dawley (SD) rats of 8-week-old, weighing 200 ± 10 g, were obtained from SPF Biotechnology Co., LTD. (Beijing, China), and the certificate number is SCXK (JING) 2019-0010. Rats can drink and feed freely and are cultured in an environment with the appropriate temperature (25 ± 0.5°C), humidity (45 ± 5%), and light cycle (12/12 h). All animal experimental procedures followed the local principles of the Animal Ethics Committee of the Beijing University of Chinese Medicine (BUCM-4-2020111103-4087). We strive to reduce the number of animals used and ensure their welfare in the design of our experiments.
Modeling methods and study design
Pathological modeling started after 7 days of acclimatization. The method of modeling the minor CCI was as described in previous studies (Bennett and Xie, 1988; Grace et al., 2010). Briefly, rats were anesthetized with pentobarbital (1%, Sigma-Aldrich LLC., Germany). The sciatic nerve of the right side was exposed in front of the nerve branch by blunt dissection. A chromic intestinal suture (4-0, Shandong Boda Medical Products Co., Ltd., Shandong, China) was loosely tied around the nerve and did not interrupt the blood circulation of the epineural vasculature. The sciatic nerve was exposed for 3 min without any ligation in the sham group. The skin was then sewn together with two sutures. Seven days of acclimatization after operation, the 18 rats, which had similar levels of pain sensation, were randomly divided into the sham group (n = 6), model group (n = 6), and tuina group (n = 6).
Intervention methods
The intervention was performed after the model was stably established (7th day after modeling) according to the literature and previous studies (Ma et al., 2017; Lv et al., 2020b; Talaei et al., 2022). The tuina group received a 1-time tuina intervention. The procedure for the “Three-Manipulation and Three-Acupoint” treatment was performed as follows: Firstly, a rat was fixed on the message platform of the Tuina Manipulation Simulator (Self-developed machine, China invention patent number ZL200710187403.1). Secondly, the machine parameters were set to stimulate with a force of 4 N, 60 times per minute. Third, the stimulus rod was placed on BL 37, GB 34, and BL 57 of the surgical side, then finger pressing, plucking, and kneading manipulation were stimulated, respectively. Each acupoint and manipulation were operated for 1 min consecutively for 9 min in total (Lv et al., 2020a). The grip restraint intervention was performed in the sham and model groups.
Behavior tests
Behavioral tests were performed three times—baseline (before modeling), pre-intervention, and post-intervention. MWT and TWL were operated on the right hind paw as in the previous description (Bennett and Xie, 1988). The measurement was repeated three times with a 10-min interval between each measurement.
MWT: Measurement started when exploratory and grooming behaviors stopped, and the rats were motionless and relaxed. An electronic Von Frey instrument (BIO-EVF5; Bioseb, USA) was used, avoiding the metal grid, and the same position on the plantar surface of the hind paws was stimulated with the disposable measurement tip. The intensity of the applied force was adapted, recording the animal’s response (e.g., curling hind paws, licking hind paws) and the maximum value automatically was recorded by the system.
TWL: Using a thermal analgesia device (PL-200; Chengdu Techman Software Co., Ltd., China), the heat intensity was set to 50%, and the latency was cut off at 30 seconds to prevent skin damage. The infrared source beneath the plantar surface of the hind paws was put in the right position. The recording of the animal’s response and the time was taken automatically by the system.
Electron microscopy
After the last behavioral test, three rats in each group were randomly selected and deeply anesthetized with pentobarbital (1%), then fixed with 4% paraformaldehyde. The DRGs and SDHs of the fourth to sixth lumbar (L4–6) on the right side were removed and fixed for 2 h in 0.1 M PB with 1% osmic acid. The SDH was then subjected to gradient dehydration in ethyl alcohol and acetone, with concentrations of 30, 50, 70, 80, 95, 100, 100, 100 (acetone), 100 (acetone), for 15∼20 min each. After being embedded overnight in the resin, the spinal dorsal horn was sectioned at a thickness of approximately 70 nm. Ultrathin sections were stained for 8 min with 2% uranyl acetate followed by 2.6% lead citrate for 8 min, and dehydrated overnight at room temperature. Transmission electron microscopy (Hitachi TEM system, Japan) was used for observation.
RNA-seq
Three rats were rapidly sacrificed using the same anesthetic method. The RNA-Seq experimental procedure consists of 5 parts: (1) RNA extraction, (2) transcriptome library preparation and sequencing, (3) read mapping of raw data, (4) differential expression analysis and (5) analysis of functional enrichment and alternative splice events identification. The DRG and SDH of L4-6 segments on the right side were removed and separated from the cauda equina (stored at –80°C). Total RNA was extracted from DRG and SDH with TRIzol reagent according to the manufacturer’s protocol (Invitrogen, CA, USA), then RNA was determined in quality and quantity. Only high-quality RNA samples were selected for the next experiment. The cDNA transcriptome library was constructed following a TruSeq RNA sample preparation kit (Illumina, CA, USA) according to the protocol of the manufacturer (Chomczynski and Sacchi, 2006). The final double-stranded cDNA samples were synthesized with a SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA, USA). After end-repair, phosphorylation, ‘A’ base addition, and amplification, sequencing was performed on an HiSeq X Ten platform (Illumina, CA, USA). The raw paired-end reads were trimmed, and quality controlled using SeqPrep and Sickle. The differential expression genes (DEGs) can be identified and considered statistically significant with the P-adjust ≤0.05 and fold change >2.0. Finally, all the alternative splice events that occurred in the samples were identified using rMATS (Shen et al., 2014).
Statistical analysis
Data analysis was performed with SPSS Statistics software (version 26.0). MWT and TWL results were presented as mean ± SD. One-way ANOVA was used for comparisons between groups, and the LSD multiple comparison test was used for multiple comparisons. P < 0.05 was treated as statistically significant. The KEGG enrichment analysis of DEGs was performed with KOBAS.1
Conclusion
In this study, we found that 1-time tuina intervention could alleviate the hyperalgesia of minor CCI rats, and especially ease thermal hyperalgesia more effectively. However, 1-time tuina treatment did not change the ultrastructure in DRG and SDH when observed using electron microscopy technology. The immediate analgesic mechanism of tuina is mainly related to the calcium signaling pathway, thermogenesis, and regulation of lipolysis in adipocytes.
Data availability statement
The original contributions presented in this study are publicly available. The raw data of RNA-Seq can be found on NCBI at the following link: https://www.ncbi.nlm.nih.gov/bioproject/884185 with accession number: PRJNA884185. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of the Beijing University of Chinese Medicine.
Author contributions
TY and ZL: study conception and design of the work. HW, YZ, YX, YJ, QG, and DL: animal experiments and data acquisition. HW and ZL: analysis, data interpretation, and drafting of the manuscript. HW, ZL, and TY: approval of the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82074573 and 81674094).
Acknowledgments
We thank Xiaoying Tian for her help in language polishing and Kaimin Li for RNA-Seq data processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Baron, R., Binder, A., and Wasner, G. (2010). Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9, 807–819. doi: 10.1016/S1474-4422(10)70143-5
Bennett, G. J., and Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107. doi: 10.1016/0304-3959(88)90209-6
Chen, Y., Luo, F., Yang, C., Kirkmire, C. M., and Wang, Z. J. (2009). Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J. Pharmacol. Exp. Ther. 330, 650–659. doi: 10.1124/jpet.109.152165
Chomczynski, P., and Sacchi, N. (2006). The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 1, 581–585. doi: 10.1038/nprot.2006.83
Cui, W., Wu, H., Yu, X., Song, T., Xu, X., and Xu, F. (2021). The calcium channel α2δ1 subunit: Interactional targets in primary sensory neurons and role in neuropathic pain. Front. Cell Neurosci. 15:699731. doi: 10.3389/fncel.2021.699731
Fayaz, A., Croft, P., Langford, R. M., Donaldson, L. J., and Jones, G. T. (2016). Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open. 6:010364. doi: 10.1136/bmjopen-2015-010364
Field, T. (2016). Massage therapy research review. Complement. Ther. Clin. Pract. 24, 19–31. doi: 10.1016/j.ctcp.2016.04.005
Gok Metin, Z., Arikan Donmez, A., Izgu, N., Ozdemir, L., and Arslan, I. E. (2017). Aromatherapy massage for neuropathic pain and quality of life in diabetic patients. J. Nurs. Scholarsh. 49, 379–388. doi: 10.1111/jnu.12300
Grace, P. M., Hutchinson, M. R., Bishop, A., Somogyi, A. A., Mayrhofer, G., and Rolan, P. E. (2011). Adoptive transfer of peripheral immune cells potentiates allodynia in a graded chronic constriction injury model of neuropathic pain. Brain Behav. Immun. 25, 503–513. doi: 10.1016/j.bbi.2010.11.018
Grace, P. M., Hutchinson, M. R., Manavis, J., Somogyi, A. A., and Rolan, P. E. (2010). A novel animal model of graded neuropathic pain: Utility to investigate mechanisms of population heterogeneity. J. Neurosci. Methods 193, 47–53. doi: 10.1016/j.jneumeth.2010.08.025
Incontro, S., Díaz-Alonso, J., Iafrati, J., Vieira, M., Asensio, C. S., Sohal, V. S., et al. (2018). The CaMKII/NMDA receptor complex controls hippocampal synaptic transmission by kinase-dependent and independent mechanisms. Nat. Commun. 9:2069. doi: 10.1038/s41467-018-04439-7
Inoue, K., and Tsuda, M. (2018). Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 19, 138–152. doi: 10.1038/nrn.2018.2
Izgu, N., Ozdemir, L., and Bugdayci Basal, F. (2019). Effect of aromatherapy massage on chemotherapy-induced peripheral neuropathic pain and fatigue in patients receiving oxaliplatin: An open label quasi-randomized controlled pilot study. Cancer Nurs. 42, 139–147. doi: 10.1097/NCC.0000000000000577
Jaggi, A. S., Jain, V., and Singh, N. (2011). Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 25, 1–28. doi: 10.1111/j.1472-8206.2009.00801.x
Kissig, M., Shapira, S. N., and Seale, P. (2016). SnapShot: Brown and beige adipose thermogenesis. Cell 166, 258–258. doi: 10.1016/j.cell.2016.06.038
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Li, Y. Z., Miao, R. P., Yu, T. Y., Bai, W. Z., Cui, J. J., Lu, M. Q., et al. (2019). Mild mechanic stimulate on acupoints regulation of CGRP-positive cells and microglia morphology in spinal cord of sciatic nerve injured rats. Front. Integr. Neurosci. 13:58. doi: 10.3389/fnint.2019.00058
Li, Z. Y., Chen, P. Q., Yan, J. T., Yan, X., Yang, X. Y., and Wu, G. C. (2004). Analgesic effect of tender point kneading on neuralgia in rats. Shanghai J. Tradit. Chin. Med. 38, 54–56. doi: 10.16305/j.1007-1334.2004.05.024
Lin, Q., Zhang, H., Zhao, Q., Niu, K., Zhang, G. H., Wang, Y. L., et al. (2013). Biological effect of rolling manipulation on human skeletal muscle cells. Acad. J. Shanghai Univ. Tradit. Chin. Med. 27, 81–84. doi: 10.16306/j.1008-861x.2013.02.022
Liu, W., Lv, Y., and Ren, F. (2018). PI3K/Akt pathway is required for spinal central sensitization in neuropathic pain. Cell. Mol. Neurobiol. 38, 747–755. doi: 10.1007/s10571-017-0541-x
Liu, Z. F., Jiao, Y., Yu, T. Y., Zhang, Y. Q., Liu, D., Guan, Q., et al. (2022). Research progress on analgesic mechanism of Chinese Tuina in recent ten years. Glob. Tradit. Chin. Med. 15, 526–530. doi: 10.3969/j.issn.1674-1749.2022.03.038
Liu, Z. F., Wang, H. R., Yu, T. Y., Jiao, Y., Zhang, Y. Q., Liu, D., et al. (2021). A review on the mechanism of tuina promoting the recovery of peripheral nerve injury. Evid. Based Complement. Alternat. Med. 2021:6652099. doi: 10.1155/2021/6652099
Luo, F., Yang, C., Chen, Y., Shukla, P., Tang, L., Wang, L. X., et al. (2008). Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J. Pharmacol. Exp. Ther. 325, 267–275. doi: 10.1124/jpet.107.132167
Lv, T. T. (2020). Using the RNA-Seq technique to explore the repair mechanism of “Three-Manipulation And Three-Acupoint” on rats with sciatic nerve injury. Ph.D. thesis. Chaoyang: Beijing University of Chinese Medicine.
Lv, T. T., Mo, Y. J., Yu, T. Y., Shao, S., Lu, M. Q., Luo, Y. T., et al. (2020a). Using RNA-Seq to Explore the repair mechanism of the three methods and three-acupoint technique on drgs in sciatic nerve injured rats. Pain Res. Manag. 2020:7531409. doi: 10.1155/2020/7531409
Lv, T. T., Mo, Y. J., Yu, T. Y., Zhang, Y. M., Shao, S., Luo, Y. T., et al. (2020b). An investigation into the rehabilitative mechanism of tuina in the treatment of sciatic nerve injury. Evid. Based Complement. Alternat. Med. 2020:5859298. doi: 10.1155/2020/5859298
Ma, C., Yao, B. B., Yu, T. Y., Tao, Y. H., Lu, M. Q., Jia, W. D., et al. (2017). Effects of BoFa on expression of IL-6 and Socs3 in spinal cord in CCI rats. J. Nanjing Univ. Tradit. Chin. Med. 33, 399–402. doi: 10.14148/j.issn.1672-0482.2017.0399
MacDonald, D. I., Luiz, A. P., Iseppon, F., Millet, Q., Emery, E. C., and Wood, J. N. (2021). Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain 144, 1711–1726. doi: 10.1093/brain/awab086
Mills, E. L., Pierce, K. A., Jedrychowski, M. P., Garrity, R., Winther, S., Vidoni, S., et al. (2018). Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106. doi: 10.1038/s41586-018-0353-2
Qing, L. X., An, Y., Liu, C. X., Zhai, J. X., Feng, L., Liu, X. Z., et al. (2022). effects of nine-step tuina in eight minutes on ultrasound perfusion imaging of rectus femoris in women with knee osteoarthritis. J. Tradit. Chin. Med. 63, 851–855. doi: 10.13288/j.11-2166/r.2022.09.011
Rivaz, M., Rahpeima, M., Khademian, Z., and Dabbaghmanesh, M. H. (2021). The effects of aromatherapy massage with lavender essential oil on neuropathic pain and quality of life in diabetic patients: A randomized clinical trial. Complement. Ther. Clin. Pract. 44:101430. doi: 10.1016/j.ctcp.2021.101430
Schaefer, C., Sadosky, A., Mann, R., Daniel, S., Parsons, B., Tuchman, M., et al. (2014). Pain severity and the economic burden of neuropathic pain in the United States: BEAT neuropathic pain observational study. Clinicoecon. Outcomes Res. 6, 483–496. doi: 10.2147/CEOR.S63323
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 160, 53–59. doi: 10.1097/j.pain.0000000000001365
Shen, S., Park, J. W., Lu, Z. X., Lin, L., Henry, M. D., Wu, Y. N., et al. (2014). rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. U.S.A. 111, 5593–5601. doi: 10.1073/pnas.1419161111
Shepherd, A. J., Mickle, A. D., Golden, J. P., Mack, M. R., Halabi, C. M., de Kloet, A. D., et al. (2018). Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc. Natl. Acad. Sci. U.S.A. 115, 8057–8066. doi: 10.1073/pnas.1721815115
Simeoli, R., Montague, K., Jones, H. R., Castaldi, L., Chambers, D., Kelleher, J. H., et al. (2017). Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 8:1778. doi: 10.1038/s41467-017-01841-5
Talaei, S. A., Banafshe, H. R., Moravveji, A., Shabani, M., Tehrani, S. S., and Abed, A. (2022). Anti-nociceptive effect of black seed oil on an animal model of chronic constriction injury. Res. Pharm. Sci. 17, 383–391. doi: 10.4103/1735-5362.350239
Vinel, C., Lukjanenko, L., Batut, A., Deleruyelle, S., Pradère, J. P., Le Gonidec, S., et al. (2018). The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24, 1360–1371. doi: 10.1038/s41591-018-0131-6
Wang, Q., Lin, J., Yang, P., Liang, Y., Lu, D., Wang, K., et al. (2020). Effect of massage on the TLR4 signalling pathway in rats with neuropathic pain. Pain Res. Manag. 2020:8309745. doi: 10.1155/2020/8309745
Yang, H. W., Hu, X. D., Zhang, H. M., Xin, W. J., Li, M. T., Zhang, T., et al. (2004). Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J. Neurophysiol. 91, 1122–1133. doi: 10.1152/jn.00735.2003
Keywords: neuropathic pain, tuina, RNA sequencing, dorsal root ganglia, spinal dorsal horn, analgesia
Citation: Wang H, Liu Z, Yu T, Zhang Y, Xu Y, Jiao Y, Guan Q and Liu D (2022) Exploring the mechanism of immediate analgesic effect of 1-time tuina intervention in minor chronic constriction injury rats using RNA-seq. Front. Neurosci. 16:1007432. doi: 10.3389/fnins.2022.1007432
Received: 30 July 2022; Accepted: 06 September 2022;
Published: 04 October 2022.
Edited by:
Min Fang, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Zhiwei Wu, Shanghai University of Traditional Chinese Medicine, ChinaQimiao Hu, Zhejiang Chinese Medical University, China
Copyright © 2022 Wang, Liu, Yu, Zhang, Xu, Jiao, Guan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyuan Yu, eXV0aWFueXVhbkBzaW5hLmNvbQ==; Zhifeng Liu, bGl1emhpZmVuZzA2MTZAMTI2LmNvbQ==
 Hourong Wang
Hourong Wang Zhifeng Liu
Zhifeng Liu Tianyuan Yu
Tianyuan Yu Yingqi Zhang1
Yingqi Zhang1 Yi Jiao
Yi Jiao