- 1Wake Forest University School of Medicine, Winston-Salem, NC, United States
- 2Department of Neurology, University of Missouri, Columbia, MO, United States
- 3Department of Neurosurgery and Brain Repair, University of South Florida, Tampa, FL, United States
- 4Clinical Nutrition Services, Tampa General Hospital, Tampa, FL, United States
- 5USF Center for Microbiome Research, Microbiomes Institute, University of South Florida, Tampa, FL, United States
- 6Department of Neurology, Atrium Health Wake Forest Baptist, Winston-Salem, NC, United States
In recent years, appreciation for the gut microbiome and its relationship to human health has emerged as a facilitator of maintaining healthy physiology and a contributor to numerous human diseases. The contribution of the microbiome in modulating the gut-brain axis has gained significant attention in recent years, extensively studied in chronic brain injuries such as Epilepsy and Alzheimer’s Disease. Furthermore, there is growing evidence that gut microbiome also contributes to acute brain injuries like stroke(s) and traumatic brain injury. Microbiome-gut-brain communications are bidirectional and involve metabolite production and modulation of immune and neuronal functions. The microbiome plays two distinct roles: it beneficially modulates immune system and neuronal functions; however, abnormalities in the host’s microbiome also exacerbates neuronal damage or delays the recovery from acute injuries. After brain injury, several inflammatory changes, such as the necrosis and apoptosis of neuronal tissue, propagates downward inflammatory signals to disrupt the microbiome homeostasis; however, microbiome dysbiosis impacts the upward signaling to the brain and interferes with recovery in neuronal functions and brain health. Diet is a superlative modulator of microbiome and is known to impact the gut-brain axis, including its influence on acute and neuronal injuries. In this review, we discussed the differential microbiome changes in both acute and chronic brain injuries, as well as the therapeutic importance of modulation by diets and probiotics. We emphasize the mechanistic studies based on animal models and their translational or clinical relationship by reviewing human studies.
Introduction
In recent years, growing attention has been placed on the microbiome playing a role in health and disease. The microbiome (also called “gut flora” and “gut”) can be defined as the presence of abundant living microorganisms (primarily bacterial organisms), their genes and their metabolically produced byproducts residing in the GI tract (Quigley, 2017). The microbiome influences physiology, metabolism, nutrition, and immune function; Specifically, the microbiota impacts host health by producing metabolites that affect physiology of host cells by manipulating cellular and molecular mechanisms (Li et al., 2018). Notable metabolites include short-chain fatty acids (SCFAs), the primarily colonic metabolites believed to be critical in neuronal signaling and function (Morrison and Preston, 2016; Dinan and Cryan, 2017), and trimethylamine n-oxide (TMAO), a metabolite produced by some bacterial strains linked to exacerbating cardiovascular disease and stroke risk (Zhu W. et al., 2021). Butyrate, the most common SCFA, is produced from butyrate-producing bacteria, which includes Clostridium, Eubacterium, and Butyrivibrio genera, and is associated with bacterial proliferation, colonocyte energetic supply, and brain therapeutic potential (Bourassa et al., 2016). A growing body of research is suggesting that the microbiome has a convincing and modulatory role in central nervous system (CNS) function and dysfunction (Yarandi et al., 2016).
The purpose of this article is to review the gut-brain relationship, evidence from both animal and human studies observing changes in microbiome signatures during acute and chronic neurological injury states, and the effect of the diets like the ketogenic diet (KD) in mitigating brain inflammation and supporting brain recovery. This review has the potential of serving as an evidence base to consider the use of microbiome modulators like KD, probiotics, and others in brain recovery and expand its use in acute brain therapy.
The healthy gut
The colonization and character of the human microbiome is established at birth, with rapid development until 2 years old, after which much of the microbial composition is stabilized through adulthood with minor changes through aging (Fouhy et al., 2012). The initial and rapid colonization of the infant gut depends on several factors such as: mode of delivery, feeding type, antibiotic exposure, hospital environment, use of probiotics, and many more (Dominguez-Bello et al., 2010; Fouhy et al., 2012). The composition of the adult microbiome is largely dependent on regional/cultural diet and lifestyle practices (Biagi et al., 2011; Yatsunenko et al., 2012).
In the healthy state, a great amount of variation exists between each individuals’ microbiome profile, however overarching themes of strains exist. Bacterial phyla Firmicutes and Bacteroidetes are suggested to represent the largest proportion of microbiome (Yatsunenko et al., 2012), with microbial diversity maximized with a relative abundance of 80 and 15%, Firmicutes and Bacteroidetes respectively (Manor et al., 2020). Each individual’s enterotype is defined by the prominence of either Prevotella, Bacteroides, or Ruminococcus species (Arumugam et al., 2011). An individual’s enterotype is suggested to be affected by drug use and dietary choices (Arumugam et al., 2011; Wu et al., 2011).
In the healthy aging process, studies have determined a relatively low abundance of Firmicutes, and reduced bacterial diversity in the elderly population (Salazar et al., 2017). Metabolites of bacteria, notably SCFAs, are reduced in older age (Biagi et al., 2010; Salazar et al., 2013). Unfortunately, the elderly state is associated with a pro-inflammatory state (Schiffrin et al., 2010) with higher proportions of gram negative bacteria (Schiffrin et al., 2010) like Enterobacteriaceae and opportunistic bacteria like Proteobacteria, as well changes in the balance of microbes, all of which is linked to dysbiosis (Biagi et al., 2010; Shin et al., 2015).
The composition of the gut microbiome becomes unbalanced, altered, and in a state of “dysbiosis” due to a variety of reasons, including acute infection, acute and chronic disease, poor diet, and excessive antibiotic use (Vangay et al., 2015; Ballway and Song, 2021). Most clinical attempts to correct dysbiosis have been inadequate, but therapeutic utilization of fecal microbiota transplantation (FMT) has delivered successful results (Wang et al., 2019). FMT, often used to treat recurrent Clostridium difficile infections, involves transplanting stool from a healthy individual into another patient’s gastrointestinal (GI) tract to normalize the bacterial composition and correct a dysregulated gut (Surawicz et al., 2013; Gupta and Khanna, 2017; Wang et al., 2019). Long-term safety and efficacy of FMT in elderly and/or immunocompromised patients in treating severe, recurrent Clostridium difficile infections has been studied and proven successful (Kelly et al., 2014; Agrawal M. et al., 2016). Evidence for FMT provides an interesting perspective in disease treatment, especially correcting microbiome dysbiosis to relieve inflammation.
Gut brain axis
At any given instant, there is bidirectional chatter between two brains: our central nervous system and our gastrointestinal system (our gut microbiome). The gut-brain axis (GBA) can be defined as the bidirectional communication between the gut and the CNS that is integrated with multiple signaling pathways, including the enteric nervous system, sympathetic and parasympathetic pathways of the autonomic nervous system, endocrine system, and immunological system (Wang and Wang, 2016). The bidirectional exchange of the GBA in healthy and injured states of the brain is depicted in Figure 1 below.
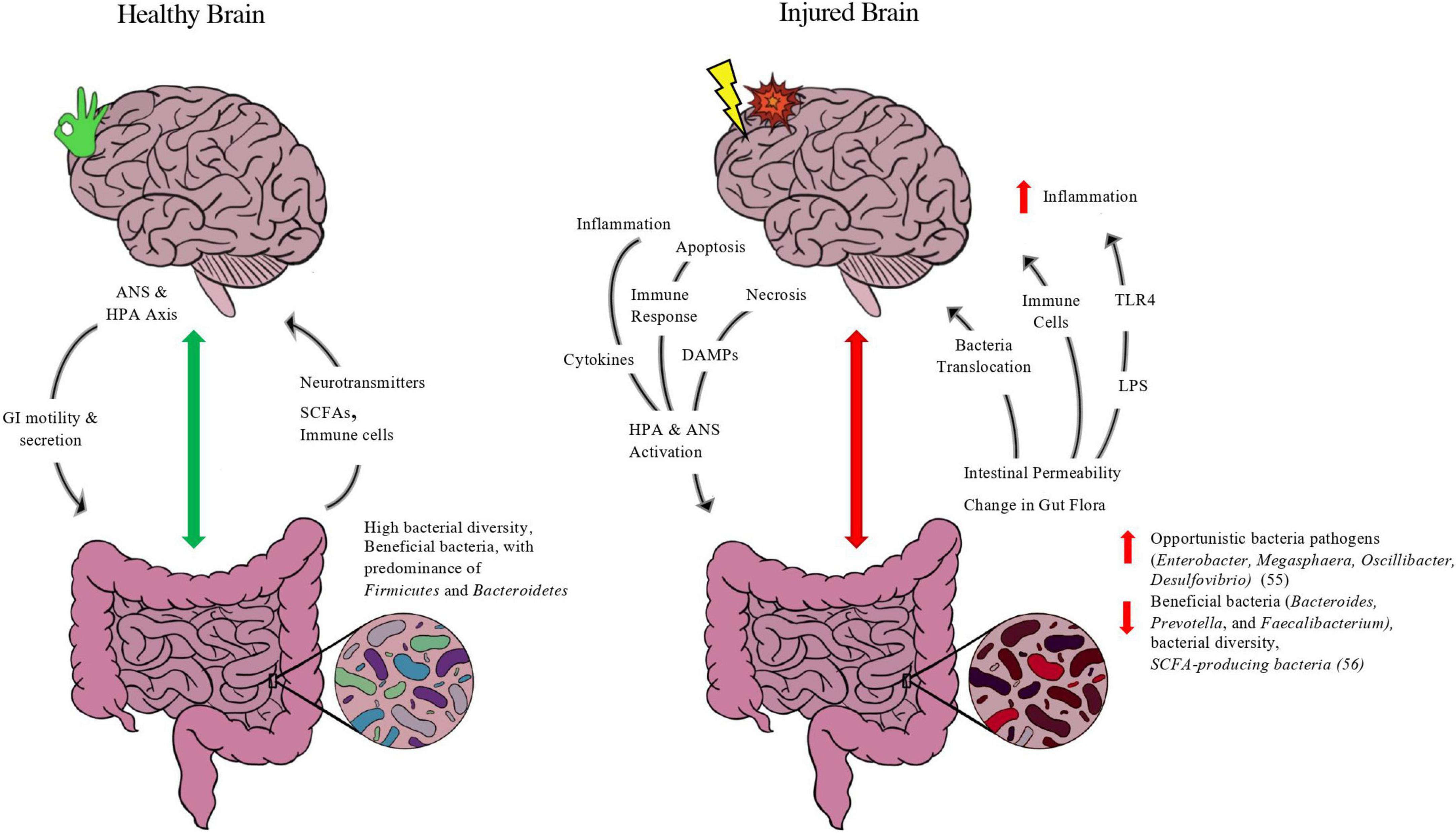
Figure 1. Purported model of gut-brain axis in healthy and injured brain. Depicted in figure is the bidirectional exchange between the brain and the gut in both a healthy state and injured state. In normal functioning, the brain’s neuroendocrine control of the gut through the autonomic nervous system (ANS) and hypothalamus-pituitary-adrenal (HPA) axis via the Vagus Nerve releases molecules such as acetylcholine and corticotropin-releasing hormone (CRH) to modulate enteric functions such as contractility, secretion of digestive enzymes, and immune function. A balanced microbiome with favorable features such as high bacterial diversity and abundance of beneficial taxa of bacteria such as firmicutes and short-chain-fatty-acid (SCFA) producing bacteria, such as Roseburia and Faecalibacterium, will exchange metabolites up the GBA. Gut bacteria produce neurotransmitters, such as serotonin, GABA, and dopamine, and SCFA-producing bacteria release SCFAs, metabolites directly linked to neuronal health and blood-brain-barrier integrity. The gut also is evidenced to modulate brain homeostasis and immune function by its release of macrophages and other white blood cells to aid in immune support. In an injured state, the interplay between the gut-brain is adjusted to meet the energetic changes and inflammation caused by brain injury. After harm is induced by trauma and/or compromised blood supply, the necrotic and injured brain tissue initiates apoptosis and inflammatory pathways that provoke the release of damage associated molecular patterns (DAMPs), cytokines, and other immune cells to trigger the ANS and HPA axis through the vagus nerve as well as sympathetic “stress” neuronal fibers. This injury response by the brain ensues changes to the gut, including microbial composition leading to dysbiosis and the predominance of opportunistic bacteria at the expense of more beneficial taxa, as well as increased gut epithelial barrier permeability. Such changes compromise the integrity of the gut (“leaky gut”), releasing bacteria and its metabolites up the GBA, including lipopolysaccharide (LPS), an endotoxin released from gram-negative bacteria that activates the transmembrane toll-like receptor 4 (TLR4) and initiates an innate immune response that exacerbates neuroinflammation. In addition, immune cells such as macrophages and neutrophils are released from the gut and migrate to the site of injury. While the migration of immune-fighting support can aid in the recovery after brain injury, it often aggravates the brain tissue and leads to delayed parenchymal recovery as well as secondary issues from prolonged inflammation.
Blood brain barrier
The brain, made of billions of neurons and glial cells, maintains connections to our body and gut in a selective and specific manner in a normal state of health. The brain is covered by a highly selective microvasculature called the blood brain barrier (BBB). The BBB is normally impermeable to many large molecules, polar substances, and bacteria through its tight junctions but allows passage of certain molecules through selective transport proteins on the BBB (Ballabh et al., 2004). The BBB can become disrupted by inflammation, such as during CNS hypoxic or injurious events that encourage the delivery of immunologic support to the brain and may contribute to the exacerbation of the CNS injury (Lochhead et al., 2017; Obrenovich, 2018).
During embryonic development of the BBB, the gut microbiome is hypothesized to be a contributor (Clarke et al., 2013) with evidence that the GBA is established from gestation (Braniste et al., 2014). Mice reared germ-free had more permeable BBBs than mice with a healthy microbiome and restoring the microbiome with SCFAs repaired BBB permeability to the levels of healthy pathogen-free mice (Braniste et al., 2014).
Dysregulated gut-brain axis by central nervous system injury
There is crosstalk between the gut and brain along the GBA to maintain homeostasis and healthy functioning. The microbiome produces several key factors that contribute significantly to neuronal and glial cell function and metabolism, such as SCFA, Tryptophan metabolites and neurotransmitters, and vitamins (Fung et al., 2017; Pellegrini et al., 2020). The microbiome also interacts extensively with the peripheral immune system and engages in immune-bacterial interplay that affects the CNS (Pellegrini et al., 2020). Functions of the GI tract are modulated along the GBA, with evidence suggesting that vagal afferents communicate with brainstem nuclei to affect efferent fibers to modulate GI secretion and motility (Filpa et al., 2016).
Shortly after acute brain injury, ischemic tissue will propagate significant neuronal apoptosis, necrosis, and inflammation leading to the release of immune cells, damage associated molecular patterns (DAMPs), and cytokines along the GBA (Li et al., 2020). These down-stream inflammatory mediators lead to the activation of the autonomic nervous system and the hypothalamus-pituitary-adrenal axis (HPA) (Li et al., 2020) which initiate intestinal inflammation through the Vagus nerve (Filpa et al., 2016) and thought to increase intestinal permeability or “leaky gut.” Such that the GBA is a bidirectional relationship, the induced stress of the gut leads to changes in the gut microbiome and release of microbes, endotoxin lipopolysaccharide (LPS), immune cells, and inflammatory mediators like T cell populations (Th1, Th2, Th17, and Treg), and cytokines like tumor necrosis factor-alpha (TNF-α), IL-2, IL-12, IL-17 and interferon-gamma (IFN-γ) that further exacerbate inflammation (Shichita et al., 2009; Arya and Hu, 2018) up the GBA and into the bloodstream to lead to systemic inflammation (Wang and Wang, 2016; Arya and Hu, 2018). The release of LPS, endotoxin found on the outer membrane of gram negative bacteria, activates an innate immune response by binding Toll-like Receptor 4 (TLR4) (Kawai and Akira, 2010) and is linked to exacerbating brain injury, stroke development and neuroinflammation (Macrez et al., 2011; Klimiec et al., 2016; Kurita et al., 2020).
Changes to brain-gut interactions during acute neurological injury
Acute neurological injuries such as ischemic stroke, traumatic brain injury (TBI), and intracerebral hemorrhages (ICH) are considered in this review. They each carry a high morbidity and mortality in the afflicted population. Understanding the brain-gut interactions in these acute injury states could lead to insights about possible new therapeutic interventions.
Ischemic stroke and microbiome
Stroke is the fifth leading cause of morbidity and mortality in the U.S. (Herpich and Rincon, 2020). Ischemic stroke, which, due to compromised blood supply, leads to local brain damage, is followed by an immune and inflammatory response. The ischemic brain tissue releases inflammatory mediators and DAMPs such as nuclear protein high mobility group box protein 1 (HMGB1) and peroxiredoxin family proteins (Shichita et al., 2012) to initiate innate and adaptive immune responses through activation of TLR4 in the brain (Kurita et al., 2020). This is implicated in inducing LPS-mediated inflammation in the gut (Kawai and Akira, 2010) which in turn exacerbates neuroinflammation (Kurita et al., 2020). Cytokines, inflammatory mediators, and inflammatory cells from the gut are suggested to migrate to the injured brain (Wang and Wang, 2016). Several animal studies have identified migration of intestine-derived proinflammatory Th1 and Th17 cells to the injured brain (Benakis et al., 2016; Singh et al., 2016). In addition, as much as 50% of patients suffer from gastrointestinal complications with emerging research suggesting it to be related to a disrupted GBA after stroke (Camara-Lemarroy et al., 2014; Wen and Wong, 2017). Overall, there is strong support that ischemic stroke induces microbiome dysbiosis via top–down processes which affects bottom-up neuroinflammatory signaling from the gut (Tan et al., 2020).
Animal studies
In a rodent MCA occlusion model, post-stroke gut dysbiosis led to changes in bacterial composition, principally a decrease in species diversity and overgrowth of harmful Bacteroidetes and was related to increased gut barrier permeability and reduced gut motility following stroke (Singh et al., 2016). Another study observed a reduction in microbiota species and “stroke specific” changes in bacterial species 3 days after a cerebral artery occlusion (Yamashiro et al., 2017). These changes and preferential overgrowth of particular bacterial species has been hypothesized to be linked to reduced intestinal motility (Yamashiro et al., 2017), autonomic nervous system induced noradrenaline release (Houlden et al., 2016), and increased intestinal permeability (Meddings and Swain, 2000; Caso et al., 2009) inflicted by a post-stroke response. A proof-of-concept study in which healthy mice were transplanted with stroke-affected microbiomes observed increased Th1 and Th17 levels, as well as migration of T-cells to the brain (Yamashiro et al., 2017). Recently, enteric TMAO production has been directly linked to stroke severity by influencing cerebral infarct magnitude and adverse functional outcomes (Zhu W. et al., 2021).
Human studies
Changes in microbial composition has also been noted in human studies, including a study by Yin et al. (2015), that found stroke patients had higher proportions of opportunistic bacterial pathogens such as Enterobacter, Megasphaera, Oscillibacter, and Desulfovibrio, and a reduction in commensal or beneficial genera including Bacteroides, Prevotella, and Faecalibacterium. Similarly, another study found reduced levels of short chain fatty acid (SCFA)-producing bacteria (Roseburia, Bacteroides, Lachnospiraceae, Faecalibacterium, Blautia, and Anaerostipes) and enhanced levels of Lactobacillaceae, Akkermansia, Enterobacteriaceae, and Porphyromonadaceae in those with acute ischemic stroke (Tan et al., 2021). Reductions in SCFAs and SCFA-producing bacteria in stroke is associated with poorer outcomes 90 days after stroke (Tan et al., 2021). In another human study, microbial diversity between cerebral infarction patients and controls were comparable (Li H. et al., 2020); however, in cerebral infarction patients, levels of butyrate producing bacteria (BPB) were reduced while levels of lactic acid bacteria (LAB) increased. The study observed NIH Stroke Scale scores had a negative association with BPB and a positive correlation with LAB (Li H. et al., 2020). Overall, human and mice studies support microbial dysbiosis and exacerbation of inflammation after stroke.
Traumatic brain injury and microbiome
Traumatic brain injury is the leading cause of death and disability in young adults in the western world (Winkler et al., 2016; Pearn et al., 2017) and contributes to nearly one-third of injury related deaths in the United States (Iaccarino et al., 2018). TBI is caused by a mechanical force that induces primary cerebral inflammation, including disrupting the BBB integrity that leads to edema, hemorrhage, hypoxia, cell death, axonal tearing, and gray–white matter disjunction secondary to impact forces (Blennow et al., 2012; Corps et al., 2015). BBB disruption, neural tissue damage, and disturbed autoregulation of cerebral blood flow has been associated with the post-traumatic epilepsy sequelae (Tomkins et al., 2011), increased ICP after TBI (Shahrokhi et al., 2010), and the release of immune “danger” signals (Manson et al., 2012), that can exacerbate neural injury (Corps et al., 2015) and induce systemic immune responses in peripheral organs (Tobin et al., 2014). TBI is also suggested to disturb the GBA by inducing GI mucosal barrier damage, leading to increased gut “leakiness” and mobilization of the defenses, activation of reactive gliosis, and entry of microbes into the CNS (Hanscom et al., 2021). Inflammatory migration to the brain is facilitated by acute BBB breakdown after TBI in humans (Hu and Tao, 2021). The systemic stress response after TBI activates the release of glucocorticoids by the Hypothalamic pituitary adrenal (HPA) axis and catecholamines by the ANS (Rosner et al., 1984; Koiv et al., 1997; Lemke, 2004; McDonald et al., 2020). Interestingly, gut dysbiosis has been reported to disrupt the HPA axis (Farzi et al., 2018), which prompts the question: does a dysregulated gut worsen post-TBI inflammation? The microbiome profile in individuals with TBI and other neurodegenerative diseases compared to normal controls and their respective animal models are vastly different (Gorjifard and Goldszmid, 2016; Ma et al., 2017, 2019; Shen et al., 2017; Simon et al., 2017; Vogt et al., 2017; Grosicki et al., 2018; Kigerl et al., 2018; Treangen et al., 2018; Zhan et al., 2018; Zhu et al., 2018; Zhuang et al., 2018; Nagpal et al., 2019; Rice et al., 2019; Royes and Gomez-Pinilla, 2019; Adriansjach et al., 2020; Urban et al., 2020). Several animal and human studies have studied the relationship between TBI and the microbiome.
Animal studies
In a systematic review by Pathare et al. (2020) seven studies discussing the impact of TBI on the gut microbiome were evaluated, six of which were animal studies. All studies determined a change in microbiome composition, especially an enrichment in Proteobacteria and Firmicutes bacterial populations across all studies. Interestingly, a study that analyzed rodents’ microbiome 2 h after moderate TBI identified that the volume of injured parenchyma correlated positivity with Proteobacteria and negatively with Firmicutes levels (Nicholson et al., 2019). In a more recent study using a mouse model, it was noted that microbiome diversity had time-dependent changes from 1 h to 7 days post-TBI. The study noticed TBI-induced gut inflammation was related to depleted levels of bile acids and suggested that changes to bile acid metabolism may be played by Staphylococcus and Lachnospiraceae (You et al., 2022).
Human studies
A recent human study with 101 moderate-severe TBI patients confirmed the enrichment of Proteobacteria in TBI as seen in previous rodent models, with the largest group being Enterobacteriaceae, as early as 48 h after impact (Mahajan et al., 2021). A study observed the changing microbiome profile of critically injured patients in which 8 of the 12 participants sustained a TBI (Howard et al., 2017). Researchers determined that there was no observed difference in microbial diversity between injured and non-injured patients upon ED admission; however, changes to the microbiome composition were dramatic within 72 h with a reduction of bacterial orders Bacteroidales, Fusobacteriales, and Verrucomicrobiales and enrichment of Clostridiales and Enterococcus in trauma patients (Howard et al., 2017). The increased ratio of pathogenic to commensal bacteria in the gut after trauma is thought to encourage the progression of post-TBI disease severity (Pathare et al., 2020). In a recent case-control study, researchers noted a significant increase in the composition of Enterococcus, Parabacteroides, Akkermansia, and Lachnoclostridium and a reduction in the abundance of Bifidobacterium and Faecalibacterium in TBI patients (Hou Y. et al., 2021). In summary, recent literature is convincing that TBI severity and a dysregulated gut composition are intertwined.
Intracerebral hemorrhages and microbiome
Compared to the above neurological injuries, hemorrhagic strokes or “brain bleeds” have less supporting data regarding their effect on the microbiome. Cerebral hemorrhages include subarachnoid hemorrhages (SAH) from trauma and aneurysmal rupture, intraparenchymal hemorrhage (IPH), subdural hemorrhage (SDH), and epidural hemorrhage (EDH). ICH accounts for 2.8 million deaths per year (Benjamin et al., 2019) and the poor outcome after ICH is due to the secondary injury and neuroinflammation propagated by the primary hematoma (Zhao et al., 2014; Duan et al., 2016; Zhu et al., 2019; Yu et al., 2021b). The detrimental effects of ICH are largely secondary to the induced neuroinflammation which is suspected to be related to proinflammatory T cell production of cytokines and increase in BBB permeability (Arumugam et al., 2005).
Animal studies
To observe the effect of the GBA and microbiome on T cell regulation after ICH, a recent rodent study by Yu et al. (2021b) observed that ICH altered the gut integrity and impaired gut motility, and T cells from the intestines migrated to perihematomal areas and exacerbated ICH neuroinflammation. Interestingly, researchers observed that FMT-treated mice had lower levels of IFN-γ, IL-17, and mRNA expression levels of TNFα 2 weeks following the injury (Yu et al., 2021b), suggesting that the gut microbiome has a strong role in neuroinflammation after ICH. In addition, FMT after ICH significantly alleviated the ICH-induced neurobehavioral deficits and restored gut barrier integrity (Yu et al., 2021b).
Another study using a rat model noted an increase of gut permeability from onset to 7 days following ICH-induction as well as a reduction in IgA and gut barrier tight junction markers (Zhang et al., 2021). The study found that the microflora composition of the ileum and lungs were similar, suggesting the translocation of enteric microflora to the lungs which prompted a pulmonary infection after hemorrhage (Zhang et al., 2021). Moreover, researchers noted that aggravation of the gut permeability promoted the migration of intestinal bacteria and increased the risk of post-injury pneumonia (Zhang et al., 2021). Hemorrhagic transformations (HT) following stroke were analyzed in mice and linked to an elevation in Proteobacteria and Actinobacteria bacteria and reductions in SCFAs. Exacerbation of HT was not seen in mice treated with antibiotics suggesting that susceptibility to HT may be related to changes to the microbiome (Huang et al., 2022).
Subarachnoid hemorrhage (SAH), is most often caused by a ruptured aneurysm (Petridis et al., 2017). In a SAH rodent model, a study found an interesting link between microbiome depletion and aneurysm formation: antibiotic use significantly reduced the risk of aneurysm formation, as well as reduced infiltration of inflammatory markers (Shikata et al., 2019). Overall, the results from recent animal studies support that the outcome as well as potentially the predisposition to cerebrovascular hemorrhages may be related to the microbiome.
Human studies
More recent literature has suggested a link between ICH and the gut. High plasma concentrations of TMAO have been demonstrated to correlate with poor 3-month function outcomes in human ICH patients (Zhai et al., 2021) and low levels of butyrate-producing bacteria was found to be associated with hemorrhagic strokes (Haak et al., 2021). FMT therapy also had efficacy in a human ICH case. A case reported a male patient developing sepsis and multiple organ dysfunction syndrome (MODS) after initial admission for a cerebellar hemorrhage, and successfully underwent a FMT to alleviate MODS (Wei et al., 2016). The FMT successfully corrected the dysbiosis by profoundly enhancing the abundance of Firmicutes and depleting Proteobacteria (Wei et al., 2016). Beyond this data, limited studies have evaluated the acute occurrence of brain bleeds with changes to the microbiome in humans.
Changes to brain–gut interactions during chronic neurological injury
The gut microbiome’s role in the development of chronic neurological disorders such as Alzheimer’s disease, Parkinson’s disease, epilepsy, vascular dementia, and primary brain tumors is discussed in this review. Interestingly, while the mechanisms of GBA disruption in acute and chronic brain injury have distinctions, there are considerable parallels to appreciate.
Alzheimer’s disease and microbiome
Alzheimer’s disease (AD) is a neurodegenerative disease with a state of chronic neuroinflammation and synaptic dysfunction as a primary manifestation (Lepeta et al., 2016; Schain and Kreisl, 2017; Bairamian et al., 2022) and its’ pathogenesis is attributed to several unelucidated comorbid neuropathologies (Soria Lopez et al., 2019).
Recent literature in both rodent and human studies have observed evidence of gut microbiome dysbiosis in advanced AD, suggesting a significant role of the microbiome in the pathogenesis of the AD (Bairamian et al., 2022).
Animal studies
In a rodent pre-clinical model, a role of the microbiome in Abeta amyloid development was suggested (Harach et al., 2017). The sequenced microbiome of Aβ precursor protein (APP) transgenic mice was vastly different than wild-type mice, with significant reductions in Firmicutes, Verrucomicrobia, Proteobacteria, and Actinobacteria and simultaneous increases in Bacteroidetes and Tenericutes phyla (Harach et al., 2017). Moreover, germ-free APP mice have greater increases in Aβ levels after FMT from conventionally reared APP mice compared to FMT from wild-type mice (Harach et al., 2017). Another rodent study links the production of Aβ proteins with the host microbiome (Minter et al., 2016); broad-spectrum antibiotic use shifted the microbial composition of rodents, including enhancing genus Akkermansia and family Lachnospiraceae, reduced cerebral Aβ deposits, and decreased neuro-inflammatory reactive gliosis to Aβ plaques (Minter et al., 2016). More recently, a study using a rodent AD model with gut dysbiosis linked increased expression of gut NLRP3 to the activation of peripheral inflammation and exacerbation of AD neuroinflammation (Shukla et al., 2021), providing a potential mechanism between gut dysbiosis induced AD progression.
Human studies
In human patients, increases in the abundance of pro-inflammatory taxa like Escherichia/Shigella and decreases in anti-inflammatory taxon E. rectale was observed in the stool of mildly impaired patients and associated with increases in inflammatory cytokines IL-1β, NLRP3, and CXCL2 and brain amyloidosis (Cattaneo et al., 2017). In another study, the bacterial taxonomic makeup of AD revealed lower bacterial diversity and a remarkable shift in bacteria composition, with decreased Firmicutes, increased Bacteroidetes, and decreased Bifidobacterium in those with AD compared to a distinctly different profile in age-matched controls (Vogt et al., 2017). It is thought that the abundance of the pathogenic gram-negative bacteria Bacteroidetes may increase the release of LPS into systemic inflammation, initiating an immune response that exacerbates neuroinflammation in AD (Bairamian et al., 2022).
The study also drew a positive correlation between the severity of microbiome change and the abundance of AD biomarkers in the cerebral spinal fluid (CSF) (Vogt et al., 2017). Another study observed a relation between AD and microbiome alterations, with Faecalibacterium prausnitzii correlating with less cognitive impairment in patients and that the administration of isolated strains of the bacteria improved cognitive functioning in a rodent model (Ueda et al., 2021). Interestingly, different microbiome signatures were observed within those with mild cognitive impairment (MCI); a study correlated levels of Proteobacteria with Aβ-42: Aβ-40 while fecal levels of propionate and butyrate negatively correlated with Aβ-42 (Nagpal et al., 2019). All taken together, the current state of literature is strong in its link between the progression of AD, gut dysbiosis, and the subsequent neuroinflammation, as well as the emerging potential of modulating the microbiome in approaching AD management (Bairamian et al., 2022).
Parkinson’s disease and microbiome
Parkinson’s disease (PD) is a neurodegenerative movement disorder due to the dramatic reduction of dopaminergic neurons in the substantia nigra. Interestingly, the pathological marker of PD, alpha-synuclein aggregates, is first detected in the enteric nervous system prior to the discovery in the brain (Felice et al., 2016), highlighting a role in the GBA in the progression of PD. To this end, researchers have found a suggestive link between PD progression and gut dysbiosis.
Animal studies
Recently, a study found that Osteocalcin (OCN), an osteoblast-derived protein, was successful in reducing dopaminergic cell loss and motor deficits in a PD mouse model through modulation of the gut; OCN depleted Firmicutes and increased abundance of Bacteroidetes, and OCN’s neuroprotective effect is thought to be facilitated by propionate, a SCFA (Hou et al., 2021).
Human studies
Sequencing of PD patients’ fecal samples indicated 77.6% lower levels of Prevotellaceae than the levels in age-matched controls (Scheperjans et al., 2015), significant in that Prevotellaceae is associated with maintaining gut barrier integrity through its role in mucin synthesis (Zhu X. et al., 2021). A high prevalence of Enterobacteriaceae was noted in PD as well as a strong correlation with worse impairments in postural instability and gate performance in PD patients (Scheperjans et al., 2015). Another PD patient study indicated a higher abundance of pro-inflammatory Proteobacteria and lower abundance of commensal Faecalibacterium and butyrate-producing bacteria (Keshavarzian et al., 2015). In short, the microbiome’s influence on the GBA and the pathogenesis of PD is an unfolding topic.
Epilepsy and microbiome
Epilepsy, a chronic neurological condition predisposing patients to frequent seizure production in the brain, estimates 2.4 diagnoses each year (Lum et al., 2020). Seizures consist of disbalanced neuronal excitation by glutamate and are prompted by various neurochemical, ionic, or traumatic triggers (Lum et al., 2020). Currently, anti-epileptic drugs (ADEs) have provided tremendous support for a number of patients with epilepsy, while many others are drug-resistant and have “refractory epilepsy.”
Animal studies
Nearly 15 years ago, a rodent study established a correlation between peripherally stimulated intestinal inflammation and increased neuroinflammation and seizure susceptibility (Riazi et al., 2008). In a more recent rodent model of epilepsy, while intestinal inflammation was associated with ineffective ADE responses and increases in seizure activity, administration of SCFA butyrate had anticonvulsant effects (De Caro et al., 2019). In a study of the microbiome with seizure susceptibility, both rats subjected to chronic stress as well as healthy rats that underwent FMT of stressed mice had accelerated development and prolongation of seizures (Medel-Matus et al., 2018). Animal studies have established a suggestive relationship between dysbiosis and seizure likelihood, and further research has extended to human subjects.
Human studies
Three human subject trials have observed altered gut composition with increased levels of Firmicutes with relation to Bacteroides in patients with refractory epilepsy (Xie et al., 2017; Peng et al., 2018; Lindefeldt et al., 2019). A recent review comparing the three above studies reports that there is not a clear understanding on the alpha diversity change in refractory epilepsy (Lum et al., 2020). Antibiotic use has been associated with changes in seizure risk; a meta-analysis of carbapenem use illustrated an enhanced seizure risk (Cannon et al., 2014) while a combination treatment of macrolides with a penicillin derivative was observed to render a small cohort of patients seizure-free for a period of time (Braakman and van Ingen, 2018). In patients with drug resistant epilepsy, the KD has been known to be therapeutic, and researchers have recently identified a link to the microbiome, which is discussed in the “Ketogenic Diet” section.
Vascular dementia and microbiome
Vascular dementia (VaD), caused by diminished blood supply to the brain due to a compromised cerebrovascular system, is the second most common cause of dementia and leads to a progressive cognitive, functional, and memory impairments (Sahathevan et al., 2012).
Animal studies
Due to the evidence of bidirectional signaling along the GBA, several studies have attempted to better understand the role of the microbiome in the pathogenesis of VaD, but they are limited to rodent studies. A study by Liu J. et al. (2015) was the first to find a modulatory effect on VaD by the microbiome (Sahathevan et al., 2012). Production of butyrate by Clostridium butyricum was shown to have a neuroprotective effect against VaD in a mouse model through improvement of cognitive function, reduced apoptotic rate, and decreased histological changes in the hippocampal CA1 region (Liu J. et al., 2015). In addition, mice models treated with Clostridium butyricum increased levels of Brain-derived neurotrophic factor (BDNF), a potent neural mediator of inflammation and apoptosis (Fanaei et al., 2014). The pathogenesis of VaD is growing in its understanding, and a role by the microbiome is considered but is in need of further study (Mirzaei et al., 2021).
Brain tumors and microbiome
Brain cancers, composed of tumors in the central nervous system, are defined by a chronic state of proliferative growth and angiogenesis. A recent review by Mehrian-Shai et al. (2019) has proposed mechanisms in which the progression of brain cancers can be encouraged by the microbiome and its interplay with host immune function and inflammation, but microbiome studies are mostly limited to a subset of primary brain cancers, gliomas. Glioma, a type of CNS tumor, is the leading cause of brain-related cancer deaths (Weller et al., 2015). While the etiology of gliomas are not clear, recent advances have suggested a relationship between the gut microbiome and its metabolites in facilitating the protective effects of anti-cancer agents (Dono et al., 2022).
Understanding of the microbiome in brain tumors is sparse, and research into microbial changes are limited primarily to animal studies which have recently acknowledged a link between glioma formation and microbiome dysbiosis. In a recent study, Fan et al. (2022) determined that glioma growth changed the gut microbiome, namely by increasing the abundance of Firmicutes and decreasing the abundance of Bacteroidia, in a rodent model (Fan et al., 2022). In addition, researchers observed that gut dysbiosis encouraged glioma growth and downregulation of cerebral Foxp3 expression (Fan et al., 2022), a regulator of regulatory T cell function, which emphasizes a bidirectional influence on the gut and brain through communications along the GBA.
A study of human glioma patients and mouse models found that certain neurotransmitters and SCFAs (butyrate, acetate, and propionate) were reduced with glioma (Dono et al., 2020). Researchers also observed that animal models on temozolomide (TMZ), a chemotherapeutic, did not develop the microbiota alterations associated with glioma (Dono et al., 2020). Another study evaluating the effect of glioma and TMZ on the gut observed that TMZ alleviated the changes seen in glioma-bearing mice; without the effect ofTMZ on the microbiome, fecal samples from glioma-bearing mice and human glioma patients had similar patterns of altered Firmicutes: Bacteroidetes and increased pathogenic Verrucomicrobia phylum and Akkermansia genus compared to control mice and patients (Patrizz et al., 2020). These two studies provide an interesting perspective on how a cytotoxic chemotherapeutic like TMZ has a strong modulatory effect on the microbiome and may provide an alternative mechanism in which TMZ mitigates glioma formation.
Another rodent study administered antibiotics and observed increased glioma growth, altered gut composition, reduced cytotoxic natural killer cells, and altered microglia expression (D’Alessandro et al., 2020). Preliminary pre-clinical studies in glioma-bearing mice have observed that gut microbiome taxa, namely Akkermansia muciniphila, might affect the response of PD-L1, an immune checkpoint (Dono et al., 2022). Akkermansia muciniphila has had evidence in mitigating the PD-L1 response in other non-brain cancers (Sivan et al., 2015; Routy et al., 2018). While still young is the study of glioma and the microbiome, results from several studies provide a plausible relationship between glioma and dysbiosis, potential mechanisms in which drugs like TMZ can affect glioma formation through affecting the microbiome, and a potential effect of microbial organisms such as Akkermansia muciniphila in affecting the progression of cancer.
Amyotrophic lateral sclerosis and microbiome
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that impacts upper and lower motor neurons, compromising functions such as breathing and ultimately leading to death typically within 2 years of disease onset. While some cases of ALS have been linked to genetic mutations such as SOD1, many underlying causes of ALS and how they affect prognosis is not yet elucidated (Chio et al., 2009; Boddy et al., 2021). Attention on the effect of the microbiome on ALS disease progression and severity has been considered in animal and human models (Zhang et al., 2017).
Animal studies
In mouse models, convincing correlations with microbiome health and ALS severity have been made. In a study by Zhang et al. (2017) SOD1G93A mice supplemented with butyrate observed improved survival, enhanced gut barrier integrity and replenishment of butyrate-producing bacteria in the gut. Another study with SOD1G93A mice correlated 11 bacteria with disease severity, such as Ruminococcus torques and Parabacteroides distasonis, and indicated a protective effect with treatment of Akkermansia muciniphila (Blacher et al., 2019). A recent study using C9orf72Harvard mice observed prevalence of Helicobacter spp. in mouse models, and the administration of antibiotics decreased the abundance of Helicobacter spp. as well as the emergence of cytokine storms, neutrophilia, or other inflammatory phenotypes (Burberry et al., 2020).
Human studies
Several human studies noted low Firmicutes/Bacteroidetes ratio in ALS patients (Fang et al., 2016; Rowin et al., 2017; Zeng et al., 2020). Interestingly, a recent study linked an increase F:B ratio and higher bacterial diversity with accelerated disease progression in patients with Motor Neuron Disease (MND) (Ngo et al., 2020). One study also noted significantly greater abundance of harmful organisms within genus Dorea and reductions of beneficial bacteria like Oscillibacter, Anaerostipes, Lachnospiraceae (Fang et al., 2016). In addition, a study noted low microbial diversity in all five ALS patients and reduced levels of beneficial Ruminococcus in majority of ALS patients with reduced F/B levels (Rowin et al., 2017). A longitudinal study observing ALS patients’ microbiomes in between two time points noted significant fluctuations in microbial composition, such as increase in pro-inflammatory Cyanobacteria (Di Gioia et al., 2020). Human studies suggest a strong connection between dysbiosis and ALS and has led to in the study of probiotic supplementation in disease therapy (Di Gioia et al., 2020). While the study of 6-month probiotic supplementation suggested no substantial changes to the microbial composition of ALS patients (Di Gioia et al., 2020), interest in microbiome modulation and ALS disease progression is strong.
Multiple sclerosis and microbiome
Multiple Sclerosis is a neuroinflammatory disease, with acute and chronic exacerbations, that attacks the CNS. Insults lead to inflammatory lesions in the CNS and symptoms of fatigue, vision changes, and motor functions. The etiology of MS has been related to several factors, such as genetics, obesity, viral infections, and more recently, the microbiome (Preiningerova et al., 2022).
Animal studies
The microbiome’s involvement in immune dysregulation and disease development has been studied in the context of relapse-remitting MS for over a decade. In a relapsing-remitting mouse model of spontaneously developing experimental autoimmune encephalomyelitis (EAE), researchers suggest that the initiation of an endogenous immune response is dependent on commensal gut flora (Berer et al., 2011), and the recruitment of autoantibodies rely on the dual role of gut flora and myelin oligodendrocyte glycoprotein (Berer et al., 2011). Additional rat models of EAE suggest a link between microbial dysbiosis with MS progression. Studying colonizing EAE mice with beneficial bacteria has suggested improvement in disease severity; Bacteroides fragilis has been linked to protecting the susceptibility to EAE (Ochoa-Reparaz et al., 2010) and human-derived bacteria Prevotella suppressed the activation of Th1 and Th17 cells and mitigated inflammation in EAE (Mangalam et al., 2017). In contrast, establishment of segmented filamentous bacteria (SFB) in mice guts promotes the inflammatory IL-17 response and worsens EAE (Lee et al., 2011).
Human studies
When comparing the microbiome between identical twins discordant for MS, the MS patients had overall reduced diversity and significant increases in harmful taxa like Akkermansia (Berer et al., 2017). Interestingly, the MS patients’ stools were transplanted into mice and led to a higher incidence of spontaneous brain autoimmunity (Berer et al., 2017). In pediatric patients with relapsing-remitting MS (RRMS), shorter relapse intervals were significantly associated with reduction of Fusobacteria and expansion of Firmicutes (Tremlett et al., 2016). Studies have correlated dysbiosis of the microbiome in RRMS with observed enrichment of harmful genera Psuedomonas, Mycoplana, Haemophilus, Blautia, and Dorea (Chen et al., 2016) and reduction of SCFA-producing bacteria in MS patients (Takewaki et al., 2020). Taken together, several studies note disadvantageous changes to the gut microbiome in patients with MS.
Dietary interventions on brain injury
Probiotic supplements and diet
Probiotics are defined as live strains of selected bacteria (Hill et al., 2014), present in foods such as yogurt, kefir, kombucha, miso, and other fermented foods, that supplement the composition of consumers’ microbiomes. The study of supplementary probiotics’ relation to brain injury is limited, with human studies mainly in the study of drug-resistant epilepsy. In a recent 2018 pilot study, the use of an eight-strain probiotic, including the three genres of Lactobacillus, Bifidobacterium, and Streptococcus, in 45 drug resistant epilepsy patients noted significant improvement in quality of life and 50% reduction in the number of seizures in 28.9% of patients, leading authors to suggest the safe use of probiotics in mitigating seizures (Gomez-Eguilaz et al., 2018). In a study of neonates with rotavirus in a Neonatal Intensive Care Unit (NICU) setting, administration of probiotics, namely Saccharomyces boulardii or Lactobacillus casei, within 24 h of birth reduced the odds ratio of developing seizures while in the NICU (Yeom et al., 2019). Probiotic use in a small cohort of MS patients suggested modest success in decreasing the release of inflammatory cytokines such as intermediate monocytes (CD14highCD16low) and CD80 monocytes, and increased abundance of taxa, notably Lactobacillus, Streptococcus, and Bifidobacterium species (Tankou et al., 2018a). Another study suggested a synergistic effect of probiotics and current MS therapies, with probiotic administration linked to increases in depleted taxa like Lactobacillus and reduced the abundance of MS-associated taxa Akkermansia and Blautia (Tankou et al., 2018b).
The use of probiotic supplementation has been studied in stress-induced animal models and has some evidence in mitigating symptoms of anxiety and depression through various unelucidated potential mechanisms, including microbial composition alterations, reduction in cytokines, increase in brain-derived neurotrophic factor, and modulating inflammatory immune pathways (Liu X. et al., 2015). Probiotic supplementation in stroke was recently reviewed (Wu and Chiou, 2021), with studies limited to animal models that suggested beneficial protection and improved ischemia with probiotic use, such as a study that observed mitigation of infarct volume and neuronal cell death with Lactobacillus amylovorus DSM 16698T (ILA) supplementation (Wanchao et al., 2018) and another study that noted significant reduction in tumor necrosis factor-alpha level with Bifidobacterium breve, Lactobacillus casei, Lactobacillus bulgaricus, and Lactobacillus acidophilus supplementation (Akhoundzadeh et al., 2018). Supplementation of Lactobacillus spp. to a rodent model of MS mitigated neuroinflammation and correlated to IL-10’s production of regulatory T cells (Lavasani et al., 2010).
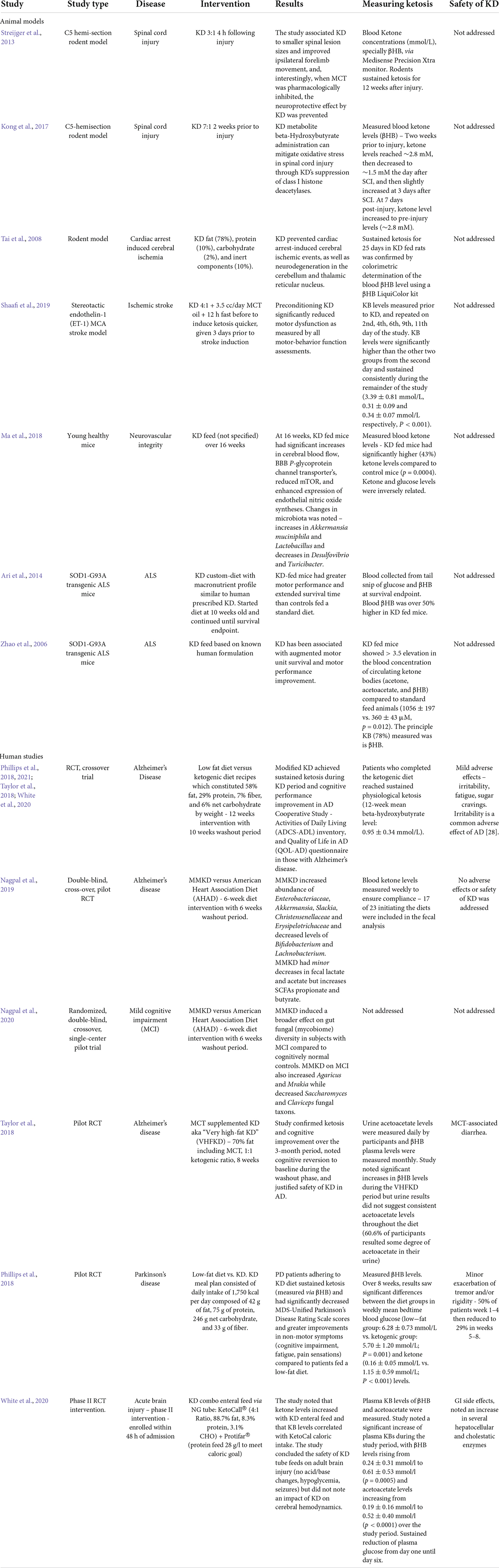
A recent review on the Mediterranean diet addressed several human studies that considered fish intake as neuroprotective against dementia and cognitive decline, as well as several human studies that correlated lower stroke risk with fish intake (Roman et al., 2019). Researchers related the mechanisms behind the observed health benefits to an increase of plasma omega-3 fatty acids after fish consumption (Roman et al., 2019). Regarding diet and acute brain injury, whole grain consumption through cold breakfast cereal and bran has been linked to significantly lower ischemic stroke incidence in two cohort studies (Juan et al., 2017). The effect of different diets on the outcomes in acute brain injury are described in Table 1. The therapeutic potential of a specific dietary intervention is particularly promising in the realm of brain injury: the ketogenic diet.
Ketogenic diet
The KD is defined as a high-fat, adequate-protein, low-carb diet (Pondel et al., 2020). The KD originates from science’s understanding of ketogenesis, the process in which ketone bodies (KBs) are generated from fatty acid metabolism from acetyl-CoA in the liver. KBs include acetoacetate (ACA), D(-)3-hydroxybutyrate (D-βHB, β-HB), and acetone and the former two are shuttled into the citric acid cycle to generate ATP. Increased levels of ketones are able to “spare” glucose from being used in energetic tasks, and this action of KD has been implicated in underlying the therapeutic potential of KD on the brain (Zilberter and Zilberter, 2020).
Ketogenic diet relation to the brain
The brain is a highly metabolic instrument, and while glucose is its primary energy source, during times of starvation, ketone bodies fulfill up to 70% of the brain’s metabolic requirements (Owen et al., 1967). Plasma levels of KBs during prolonged fasting states range from 5.8 to 9.7 mM/l (Owen et al., 1967). The cerebral ability of ketone uptake is dependent on the plasma concentration of KBs and the level of monocarboxylic acid transporters (MCT) on the BBB (White and Venkatesh, 2011). It has been proposed that, while the adult brain is thought to have low levels of microvascular MCT expression on the BBB (Vannucci and Simpson, 2003), high levels of plasma KB correlate with high uptake of KBs in the brain. Therefore, MCT expression is suggested to be concentration-dependent on KBs (Hawkins et al., 1971; Leino et al., 2001; Vannucci and Simpson, 2003), evident in a study (Bentourkia et al., 2009) observing up to eight-fold increases in cerebral KB uptake during ketosis states. Ketones alone cannot adequately maintain the cerebral energetic needs so supplementation with proteins and minimal carbohydrates is often accompanied with a KD (White and Venkatesh, 2011).
Role of ketogenic diet in brain injury
The KD has been proposed to be beneficial and neuroprotective in chronic brain injury (Prins, 2008). The use of KD in providing alternative fuel to the brain during injury has been considered for decades; in a state of low cerebral blood flow, the energetic supply of glucose is compromised, and its metabolism is affected (O’Connell et al., 2005; Prins et al., 2013), which in turn compromises the metabolic needs of the brain. Exogenous supply of ketones can fulfill the energetic demands, especially considering the production and maintenance of ketones as well as its associated ATP production is suggested to be more efficient than glucose (Prins et al., 2004; Prins, 2008). Moreover, there is suggestive evidence in mouse models that the injured brain prefers ketone metabolism, as studies have observed increases in MCT2 (neuronal MCTs) transporters 6 and 24 h after TBI [178] and increases in MCT1 transporters within 24 h of ischemic injury (Tseng et al., 2003; Zhang et al., 2005). Recent studies have also highlighted that adequate and/or over-expression of MCTs likely has a role in alleviating poor stroke outcomes (Wang et al., 2011; Yu et al., 2021a). The temporal shift from glucose metabolism to ketone metabolism is unclear. Several studies (Zhang et al., 2013; Ma and Suzuki, 2019) on nutritional ketosis suggest that the shift from glucose metabolism to ketone metabolism takes time, but how long is unclear. Nonetheless, there is growing evidence to suggest that in an injured, energetically deprived state, cerebrum metabolism will switch to ketone metabolism to meet energetic demands.
Inflammation is a hallmark in exacerbating neural disease and/or injury. Neuroinflammation is associated with the following changes in the brain: microglia activation, increased circulation of inflammatory markers such as chemokines, cytokines (such as IL-1β, IL-6) and necrosis factor (TNF), peripheral cell recruitment, and local tissue damage (O’Callaghan et al., 2008; Tremblay et al., 2011; Estes and McAllister, 2014; Woodburn et al., 2021). Means to mitigate the inflammation propagated by primary brain injury is vital in avoiding poor outcomes after brain injury, and KD’s role in doing so is depicted in Figure 2. Mechanisms of KD’s neuroprotective and anti-inflammatory affect has been proposed by Gough et al. (2021) to be of several means, including: KBs serving as alternative fuel for brain metabolism and thereby restoring energetic needs of the brain, induction of anti-inflammatory signaling pathways such as suppressing the NLRP3 inflammasome, reduction of oxidative stress by increasing the histone acetylation of FoxP3 leading to the reduced release of reactive oxygen species, and alterations to the gut microbiome. The KD and its production of ketones have been linked to mitigating acute and chronic neuroinflammation and injury.
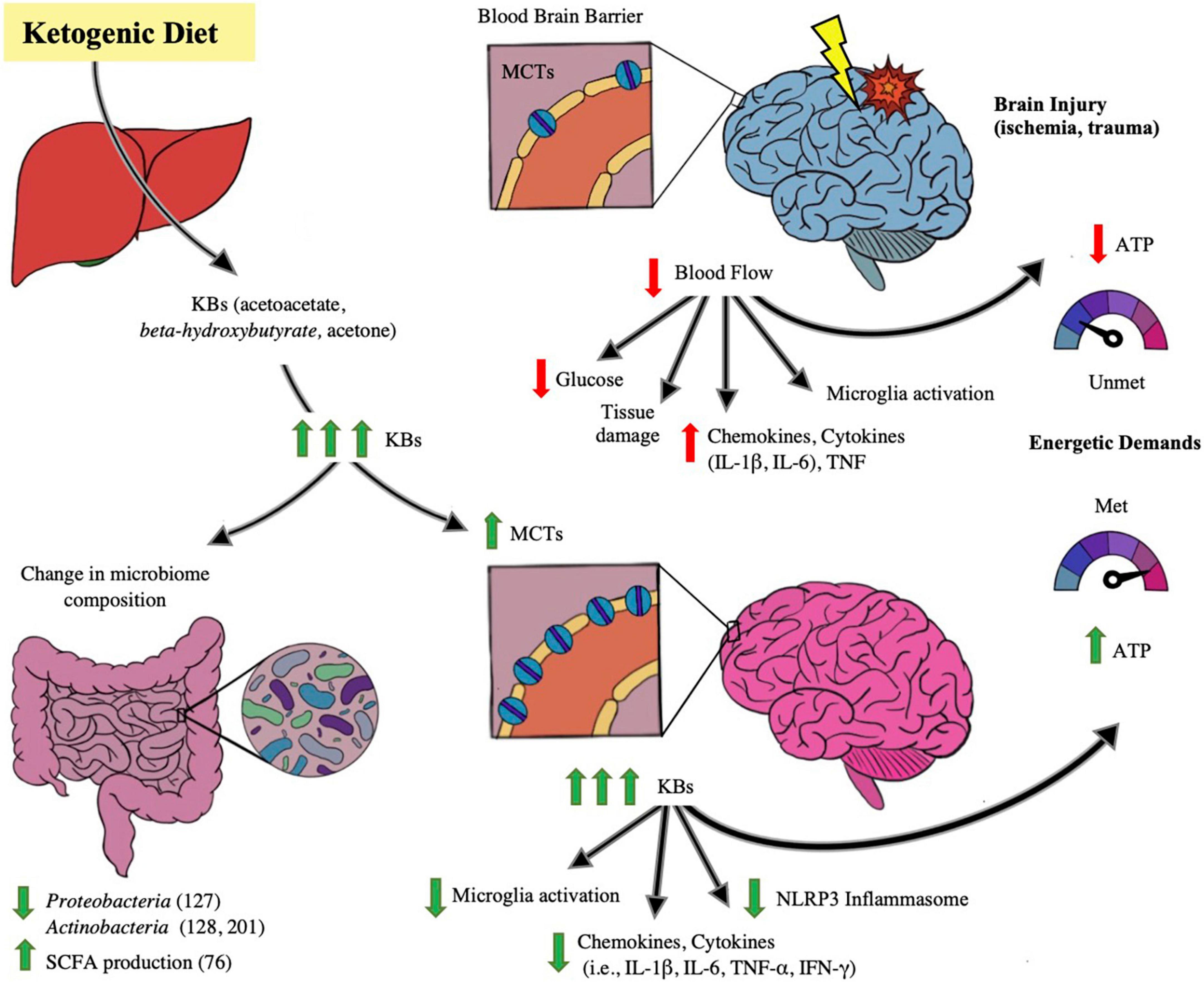
Figure 2. Impact of ketogenic diet ameliorating abnormalities in microbiome-gut-brain axis. The figure illustrates the effect of the ketogenic diet (KD) on alleviating brain injury and modulating the microbiome. With ischemic and traumatic acute brain injury, the energetic demands of the brain are compromised due to injury-provoked low cerebral blood flow and hence glucose supply to the brain- leading to lower production of ATP, exacerbation of brain parenchymal damage and propagating neuroinflammation, including microglial macrophage-like activity and cytokine (i.e., IL-1β, IL-6, TNF) release. Also depicted in the injured brain state is a baseline number of monocarboxylic acid transporters (MCTs) on the blood brain barrier (BBB), receptors that determine cerebral uptake of ketone bodies (KB). When KD is ingested and converted to its component ketone bodies (KB) through liver metabolism, the KBs lead to changes in the brain as well as the gut microbiome. In the microbiome, several studies note beneficial changes in taxa in both animal and human models, including a decrease in Proteobacteria (Medel-Matus et al., 2018), Actinobacteria (De Caro et al., 2019; Zilberter and Zilberter, 2020), and a rise in SCFA levels (Nagpal et al., 2019). In the brain, with increases of systemic KB supply, there is increase of synthesis of MCT receptors on the BBB, leading to enhanced cerebral uptake of KBs. KBs have been proposed to act as efficient alternative cerebral fuel, reinstating the energetic demands of the brain by increasing ATP production, as well as mitigating neuroinflammation by decreasing the pro-inflammatory activation of NLRP3 Inflammasome, and decreasing microglial activation and cytokine and chemokine release. There is growing evidence that KB has the potential of improving the injured and diseased brain states.
Ketogenic diet interventions in chronic neurologic disorders
The use of KD and its induced state of ketosis to treat neurological disease dates back to over a century ago, where it was implemented as a treatment for pediatric epilepsy at the Mayo Clinic in 1921 (Wheless, 2008). Since then, research on the KD diets in relation to disease states have greatly increased in the last two decades. The efficacy and safety of achieving a state of ketosis in the study of neuronal injury in both animal and human trials is discussed in Table 2.
Recent research has shifted attention on the microbiome and how it can be connected to the neuroprotective effects of KD in brain injury and disease. An interesting study by Olson et al. (2018) observed KD protective effects against seizures; KD-fed mice subjected to microbiome depletion through antibiotic use or germ-free rearing saw increases in seizure frequency—no longer experiencing the protective effect of KD. When antibiotics were discontinued, the microbiome was restored and showed a reduced alpha diversity and enhanced KD associated Akkermansia and Parabacteroides that, combined, restored the KD defense against seizures. Furthermore, the study demonstrated the same KD-associated seizure effect in control mice that underwent a FMT from KD-fed mice (Olson et al., 2018). The study also noticed a correlation with KD-induced seizure protection on a systemic level such as increased GABA:glutamate hippocampal levels and decreased systemic gamma-glutamylated amino acids (Olson et al., 2018). This study was novel in its suggestion of microbes playing a role in influencing hippocampal excitability through regulation of peripheral metabolites with KD.
Several human studies have correlated microbial changes with KD and seizure alleviation. A study on children with drug-resistant epilepsy (DRE) saw, within 1 week of KD diet initiation, KD-associated changes to the microbiome included decreased alpha diversity, reduced composition of Proteobacteria, and enhanced composition of beneficial bacteria such as the phylum Bacteroidetes (Xie et al., 2017). Another study noted that 3-month KD use decreased levels of Actinobacteria and Bifidobacterium and increased levels of Proteobacteria (Lindefeldt et al., 2019) while another study observed that levels of Actinobacteria and Firmicutes were reduced relative to Bacteroides after 6 months of KD treatment in children with DRE (Zhang et al., 2018). A study by Zhang et al. (2018) saw variable responses to KD and correlated it to microbiome compositions; Interestingly, those that did not respond favorably to the KD had enhanced levels of Alistipes, Clostridiales, Lachnospiraceae, Ruminococcaceae, and Rikenellaceae compared to patients that responded well to KD (Zhang et al., 2018). A recent study observed a higher ketogenic ratio and diet compliance in DRE adult patients supplemented an Adkins diet with KetoCal®; however, the study did not notice augmented seizure reduction (McDonald et al., 2018). The effect of KD on adults with DRE is currently not clear (Martin-McGill et al., 2020) and warrants more study.
Beyond epilepsy, KD has been suggested to be therapeutic in mitigating neuroinflammatory processes. In a rodent model of Parkinson’s disease, KD was suggested to reduce microglia activation and suppress inflammatory factors such as IL-1β, IL-6, and TNF-α (Yang and Cheng, 2010). KD therapeutic use in human PD has suggested safety and efficacy, with a recent pilot RCT observing greater improvements in non-motor symptoms such as fatigue and pain sensation in those fed the KD diet for 8 weeks compared to a low-fat diet (Phillips et al., 2018). The therapeutic tie between the microbiome and KD in PD has not be described. In ALS, KD has been associated with augmented motor unit survival, motor performance improvement, and extended survival time in rodent models (Zhao et al., 2006; Ari et al., 2014).
The role of KD in Multiple Sclerosis has been described in rodent and human modals. In a rodent model of MS, KD was associated with the downregulation of CD4 + cells, microglia, and expression of cytokines (IL-1β, IL-6, TNF-α, IL-12, IL-17) and chemokines (IFN-γ, MCP-1, MIP-1α, MIP-1β) (Kim et al., 2012). KD has been implicated in providing therapeutic potential in MS rodent models, such as the potential of axonal regeneration (Storoni and Plant, 2015), however clinical evidence in human trials is lacking (Bahr et al., 2020). In a human study of 25 MS patients, quantification of microbiome signatures defined significant reductions in colonic bacteria, namely Roseburia, Bacteroides, and Faecalibacterium prausnitzii (Swidsinski et al., 2017); however, with KD, microbiome abundance and diversity improved. In KD-fed MS patients the study observed increases in colonic composition at week 12, and improved diversity and abundance values (except for Akkermansia) comparable to healthy controls by week 24 (Swidsinski et al., 2017). In a more recent study on the use of long-term (18 months) KD therapy, the authors cited limited clinical evidence of KD improving MS disease severity (Bahr et al., 2020). Further study of KD therapy for MS disease severity as well as microbiome improvement is warranted.
A recent study on mild cognitively impaired (MCI) patients attributed the modified-KD to changes in the microbiome signatures and increases in SCFA production (Nagpal et al., 2019). Bacterial composition in KD-fed MCI patients was noteworthy for increases in Enterobacteriaceae, Akkermansia, Slackia, Christensenellaceae, and Erysipelotrichaceae while reductions in abundance of Bifidobacterium and Lachnobacterium were noted. Researchers linked particular microbiome changes to improved AD biomarkers, such as amyloid beta and tau proteins, in the CSF (Nagpal et al., 2019). A recent crossover study with AD patients noted the safety and efficacy of KD in inducing sustained ketosis and leading to cognitive performance improvement (Phillips et al., 2021). The safety of using KD in AD patients was further described, with another study utilizing a KD supplemented with medium-chain triglycerides (MCTs) noting MCT-associated diarrhea in 50% of patients, but reporting no changes in blood glucose levels, insulin, liver function tests, renal functioning, electrolyte balances, body mass index, bone mass or EKG, concluding the safety of this high-fat diet in AD for at least 3 months (Taylor et al., 2018). Another recent study observed the effect of KD on neurovascular integrity and its relationship with microbiome changes in mice (Ma et al., 2018). The study noted changes in microbiota composition which included increased abundance of beneficial Akkermansia muciniphila and Lactobacillus and decreased abundance of inflammatory Desulfovibrio and Turicibacter. In addition, after 16 weeks of KD feed, mice had significant increases in cerebral blood flow and P-glycoprotein channel transporters on the BBB to encourage the clearance of Amyloid beta proteins (Ma et al., 2018). The study of KD in healing chronic brain injury states is promising in animal models and has been extended for therapeutic use in humans. Studies in chronic injury states have provided a basis of efficacy and safety of KD and have encouraged study into the interplay of acute brain injury and the microbiome.
Ketogenic diet interventions in acute brain injuries
While limited, the study of KD and its component KBs in neuroinflammation is growing. Several studies have suggested a significant role by the ketone body β-HB in mitigating neuroinflammation. In an animal model looking at the acute response of KD on stress, it was noted β-HB caused the acute production of mitochondrial H2O2 and 4-hydroxy-2-nonenal (4-HNE), suggestive of activating the Nrf2 pathway, the primary cellular response pathway (Milder et al., 2010). Similarly, a study isolated β-HB in its direct role in preventing the NLRP3 inflammasome, which in turn suppressed the release of inflammatory cytokines IL-1β and IL-18 by caspase-1 in human monocytes (Youm et al., 2015). Suppression of NLRP3 inflammasome is considered significant and, in recent years, has gained attention in being utilized as a target in mitigating neuroinflammatory disease (Shao et al., 2018). More recently, in a fish model, β-HB was observed to inhibit LPS-induced inflammation seemingly by up-regulating anti-inflammatory genes such as NF-κBIA (Qiao et al., 2020).
A few animal models investigate the KD effect on acute CNS injury. While traumatic spinal cord injury (SCI) is not addressed in this review due to the scope of brain injury, there are several parallels to the pathophysiology of TBI with SCI (Jogia and Ruitenberg, 2020). In rodent SCI models, the KD intervention was associated with smaller spinal lesion sizes and improved ipsilateral forelimb movement (Streijger et al., 2013) and reduction of oxidative stress (Kong et al., 2017). In stroke models, KD-preconditioning significantly reduced motor dysfunction after stroke (Shaafi et al., 2019). Such promise in improved stroke outcomes has prompted study in human studies.
Human studies of KD and acute brain injury are very limited. Recently, a Phase II randomized control trial was done in the setting of acute brain injury with KD enteral administration (White et al., 2020). The study included patients with ischemic stroke, subarachnoid hemorrhage, and TBI and noted that ketone levels increased with KD enteral feed and that KB levels correlated with KetoCal® caloric intake, but no impact of KD on cerebral hemodynamics was observed (White et al., 2020). The study concluded the safety of KD tube feeds on adult brain injury, with no acid/base changes, hypoglycemic emergencies, or seizure development (White et al., 2020). KetoCal® and KetoVie® are two commercially available formulas for dietary medical management of DRE epilepsy treatment and other KD-benefiting conditions, with both products consisting of a liquid diet with a 4:1 ketogenic ratio formula. Long-term compliance with KD can be challenging considering patients may opt to deter from the regimented diet for more appetizing foods. In the realm of acute brain injury, the use of Ketogenic feeds can be very promising, considering issues with compliance can be regulated in a hospital setting. While the studies mentioned prior noted effects of KD on chronic brain injury and correlated them with microbiome changes, studies in the context of KD therapy for acute brain injury do not cite any microbiome observations. Given the infancy of the study of the KD-microbiome connection, especially in the context of acute brain injury, such a gap is expected but warrants further trial.
Conclusion/future directions
Taken together, the microbiome is affected by neurodegenerative conditions as well as brain injury states. While the specific changes to the microbiomes of patients suffering different neurological disorders are varied, a common theme is presented, in both acute and chronic brain injury: pathogenic and opportunistic bacteria like Enterobacteriaceae and Proteobacteria are enhanced why mutualistic and beneficial taxa such as Faecalibacterium and SCFA-producing bacteria are relatively depleted. It can be argued that the presence of gut dysbiosis and abundance of opportunistic bacteria is linked to the exaggeration of neuroinflammation, delay in functional recovery, and aggravation of tissue damage. With our growing understanding of the link between brain injury and dysbiosis, the KD provides a promising opportunity in meeting the unmet metabolic, energetic, and immunologic demands of the brain in an acute and chronic state of injury and inflammation.
The safety and efficacy of KD in human subjects has been extensively studied and confirmed in chronic neurological disorders (Walczyk and Wick, 2017; Gough et al., 2021; Yurista et al., 2021; Pietrzak et al., 2022; Zhu et al., 2022). While safety in chronic use has been established and confirmed with some acute injury studies, there is a relative lack in understanding the therapeutic potential of KD in acute injury states such as ischemic stroke, cerebral hemorrhages, and TBI. Moreover, the impact of KD on the microbiome and how it interacts with the pathogenesis and/or outcomes of various neurological disorders warrants better understanding. Substantial efforts should be made in order to elucidate the potential of high fat diets like KD in modulating the gut microbiome and lending itself to the establishment of new therapeutic targets.
Author contributions
MK was involved in the literature search of the current state of science to be discussed in the review as well as consolidating ideas and writing up the greater majority of the review, created the tables and as well as the original artwork of the figures. AKS was MK’s primary mentor along the review writing process, guided the direction of MK’s research, provided frequent feedback, created the format and flow of the article, and edited multiple draft phases. NA provided meaningful edits in the draft phases of the review and contributed some information into the review. HY directed the formatting and scope of the review and provided meaningful feedback in the writing process. SJ, JG, KD, and BH provided meaningful feedback during the editing phase. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adriansjach, J., Baum, S. T., Lefkowitz, E. J., Van Der Pol, W. J., Buford, T. W., and Colman, R. J. (2020). Age-related differences in the gut microbiome of Rhesus macaques. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1293–1298. doi: 10.1093/gerona/glaa048
Agrawal, M., Aroniadis, O. C., Brandt, L. J., Kelly, C., Freeman, S., Surawicz, C., et al. (2016). The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated clostridium difficile infection in 146 elderly individuals. J. Clin. Gastroenterol. 50, 403–407. doi: 10.1097/MCG.0000000000000410
Agrawal, R., Noble, E., Vergnes, L., Ying, Z., Reue, K., and Gomez-Pinilla, F. (2016). Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J. Cereb. Blood Flow Metab. 36, 941–953. doi: 10.1177/0271678X15606719
Akhoundzadeh, K., Vakili, A., Shadnoush, M., and Sadeghzadeh, J. (2018). Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J. Med. Sci. 43, 32–40.
Ari, C., Poff, A. M., Held, H. E., Landon, C. S., Goldhagen, C. R., Mavromates, N., et al. (2014). Metabolic therapy with deanna protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS One 9:e103526. doi: 10.1371/journal.pone.0103526
Arumugam, M., Raes, J., Pelletier, E., Le Paslier, D., Yamada, T., Mende, D. R., et al. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. doi: 10.1038/nature09944
Arumugam, T. V., Granger, D. N., and Mattson, M. P. (2005). Stroke and T-cells. Neuromol. Med. 7, 229–242. doi: 10.1385/NMM:7:3:229
Arya, A. K., and Hu, B. (2018). Brain-gut axis after stroke. Brain Circ. 4, 165–173. doi: 10.4103/bc.bc_32_18
Aune, D., Giovannucci, E., Boffetta, P., Fadnes, L. T., Keum, N., Norat, T., et al. (2017). Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 46, 1029–1056. doi: 10.1093/ije/dyw319
Bahr, L. S., Bock, M., Liebscher, D., Bellmann-Strobl, J., Franz, L., Pruss, A., et al. (2020). Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): Protocol of a randomized controlled study. Trials 21:3. doi: 10.1186/s13063-019-3928-9
Bairamian, D., Sha, S., Rolhion, N., Sokol, H., Dorothee, G., Lemere, C. A., et al. (2022). Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 17:19. doi: 10.1186/s13024-022-00522-2
Ballabh, P., Braun, A., and Nedergaard, M. (2004). The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. doi: 10.1016/j.nbd.2003.12.016
Ballway, J. W., and Song, B. J. (2021). Translational approaches with antioxidant phytochemicals against alcohol-mediated oxidative stress, gut dysbiosis, intestinal barrier dysfunction, and fatty liver disease. Antioxidants 10:384. doi: 10.3390/antiox10030384
Benakis, C., Brea, D., Caballero, S., Faraco, G., Moore, J., Murphy, M., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 22, 516–523. doi: 10.1038/nm.4068
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 139, e56–e528. doi: 10.1161/CIR.0000000000000659
Bentourkia, M., Tremblay, S., Pifferi, F., Rousseau, J., Lecomte, R., and Cunnane, S. (2009). PET study of 11C-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am. J. Physiol. Endocrinol. Metab. 296, E796–E801. doi: 10.1152/ajpendo.90644.2008
Berer, K., Gerdes, L. A., Cekanaviciute, E., Jia, X., Xiao, L., Xia, Z., et al. (2017). Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 10719–10724. doi: 10.1073/pnas.1711233114
Berer, K., Mues, M., Koutrolos, M., Rasbi, Z. A., Boziki, M., Johner, C., et al. (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. doi: 10.1038/nature10554
Biagi, E., Candela, M., Franceschi, C., and Brigidi, P. (2011). The aging gut microbiota: New perspectives. Ageing Res. Rev. 10, 428–429. doi: 10.1016/j.arr.2011.03.004
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. doi: 10.1371/journal.pone.0010667
Blacher, E., Bashiardes, S., Shapiro, H., Rothschild, D., Mor, U., Dori-Bachash, M., et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. doi: 10.1038/s41586-019-1443-5
Blennow, K., Hardy, J., and Zetterberg, H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899. doi: 10.1016/j.neuron.2012.11.021
Boddy, S. L., Giovannelli, I., Sassani, M., Cooper-Knock, J., Snyder, M. P., Segal, E., et al. (2021). The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 19:13. doi: 10.1186/s12916-020-01885-3
Bourassa, M. W., Alim, I., Bultman, S. J., and Ratan, R. R. (2016). Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 625, 56–63. doi: 10.1016/j.neulet.2016.02.009
Braakman, H. M. H., and van Ingen, J. (2018). Can epilepsy be treated by antibiotics? J. Neurol. 265, 1934–1936. doi: 10.1007/s00415-018-8943-3
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Burberry, A., Wells, M. F., Limone, F., Couto, A., Smith, K. S., Keaney, J., et al. (2020). C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582, 89–94. doi: 10.1038/s41586-020-2288-7
Camara-Lemarroy, C. R., Ibarra-Yruegas, B. E., and Gongora-Rivera, F. (2014). Gastrointestinal complications after ischemic stroke. J. Neurol. Sci. 346, 20–25. doi: 10.1016/j.jns.2014.08.027
Cannon, J. P., Lee, T. A., Clark, N. M., Setlak, P., and Grim, S. A. (2014). The risk of seizures among the carbapenems: A meta-analysis. J. Antimicrob. Chemother. 69, 2043–2055. doi: 10.1093/jac/dku111
Caso, J. R., Hurtado, O., Pereira, M. P., Garcia-Bueno, B., Menchen, L., Alou, L., et al. (2009). Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R979–R985. doi: 10.1152/ajpregu.90825.2008
Cattaneo, A., Cattane, N., Galluzzi, S., Provasi, S., Lopizzo, N., Festari, C., et al. (2017). Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. doi: 10.1016/j.neurobiolaging.2016.08.019
Chen, J., Chia, N., Kalari, K. R., Yao, J. Z., Novotna, M., Paz Soldan, M. M., et al. (2016). Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6:28484. doi: 10.1038/srep28484
Chio, A., Logroscino, G., Hardiman, O., Swingler, R., Mitchell, D., Beghi, E., et al. (2009). Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 10, 310–323. doi: 10.3109/17482960802566824
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., et al. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673. doi: 10.1038/mp.2012.77
Corps, K. N., Roth, T. L., and McGavern, D. B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362. doi: 10.1001/jamaneurol.2014.3558
D’Alessandro, G., Antonangeli, F., Marrocco, F., Porzia, A., Lauro, C., Santoni, A., et al. (2020). Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 50, 705–711. doi: 10.1002/eji.201948354
De Caro, C., Leo, A., Nesci, V., Ghelardini, C., di Cesare Mannelli, L., Striano, P., et al. (2019). Intestinal inflammation increases convulsant activity and reduces antiepileptic drug efficacy in a mouse model of epilepsy. Sci. Rep. 9:13983. doi: 10.1038/s41598-019-50542-0
Di Gioia, D., Bozzi Cionci, N., Baffoni, L., Amoruso, A., Pane, M., Mogna, L., et al. (2020). A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 18:153. doi: 10.1186/s12916-020-01607-9
Dinan, T. G., and Cryan, J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89. doi: 10.1016/j.gtc.2016.09.007
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., et al. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U.S.A. 107, 11971–11975. doi: 10.1073/pnas.1002601107
Dono, A., Nickles, J., Rodriguez-Armendariz, A. G., McFarland, B. C., Ajami, N. J., Ballester, L. Y., et al. (2022). Glioma and the gut-brain axis: Opportunities and future perspectives. Neurooncol. Adv. 4:vdac054. doi: 10.1093/noajnl/vdac054
Dono, A., Patrizz, A., McCormack, R. M., Putluri, N., Ganesh, B. P., Kaur, B., et al. (2020). Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncol. 9:CNS57. doi: 10.2217/cns-2020-0007
Duan, X., Wen, Z., Shen, H., Shen, M., and Chen, G. (2016). Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid. Med. Cell. Longev. 2016:1203285. doi: 10.1155/2016/1203285
Estes, M. L., and McAllister, A. K. (2014). Alterations in immune cells and mediators in the brain: It’s not always neuroinflammation! Brain Pathol. 24, 623–630. doi: 10.1111/bpa.12198
Estruch, R., Ros, E., Salas-Salvado, J., Covas, M. I., Corella, D., Aros, F., et al. (2018). Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378:e34. doi: 10.1056/NEJMoa1800389
Fan, Y., Su, Q., Chen, J., Wang, Y., and He, S. (2022). Gut microbiome alterations affect glioma development and Foxp3 expression in tumor microenvironment in mice. Front. Oncol. 12:836953. doi: 10.3389/fonc.2022.836953
Fanaei, H., Karimian, S. M., Sadeghipour, H. R., Hassanzade, G., Kasaeian, A., Attari, F., et al. (2014). Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 1558, 74–83. doi: 10.1016/j.brainres.2014.02.028
Fang, X., Wang, X., Yang, S., Meng, F., Wang, X., Wei, H., et al. (2016). Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front. Microbiol. 7:1479. doi: 10.3389/fmicb.2016.01479
Farzi, A., Frohlich, E. E., and Holzer, P. (2018). Gut microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22. doi: 10.1007/s13311-017-0600-5
Felice, V. D., Quigley, E. M., Sullivan, A. M., O’Keeffe, G. W., and O’Mahony, S. M. (2016). Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Parkinsonism Relat. Disord. 27, 1–8. doi: 10.1016/j.parkreldis.2016.03.012
Filpa, V., Moro, E., Protasoni, M., Crema, F., Frigo, G., and Giaroni, C. (2016). Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology 111, 14–33. doi: 10.1016/j.neuropharm.2016.08.024
Fouhy, F., Ross, R. P., Fitzgerald, G. F., Stanton, C., and Cotter, P. D. (2012). Composition of the early intestinal microbiota: Knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 3, 203–220. doi: 10.4161/gmic.20169
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Gomez-Eguilaz, M., Ramon-Trapero, J. L., Perez-Martinez, L., and Blanco, J. R. (2018). The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: A pilot study. Benef. Microbes 9, 875–881. doi: 10.3920/BM2018.0018
Gorjifard, S., and Goldszmid, R. S. (2016). Microbiota-myeloid cell crosstalk beyond the gut. J. Leukoc. Biol. 100, 865–879. doi: 10.1189/jlb.3RI0516-222R
Gough, S. M., Casella, A., Ortega, K. J., and Hackam, A. S. (2021). Neuroprotection by the ketogenic diet: Evidence and controversies. Front. Nutr. 8:782657. doi: 10.3389/fnut.2021.782657
Grosicki, G. J., Fielding, R. A., and Lustgarten, M. S. (2018). Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 102, 433–442. doi: 10.1007/s00223-017-0345-5
Gupta, A., and Khanna, S. (2017). Fecal microbiota transplantation. JAMA 318:102. doi: 10.1001/jama.2017.6466
Haak, B. W., Westendorp, W. F., van Engelen, T. S. R., Brands, X., Brouwer, M. C., Vermeij, J. D., et al. (2021). Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: A prospective case-control study. Transl. Stroke Res. 12, 581–592. doi: 10.1007/s12975-020-00863-4
Hanscom, M., Loane, D. J., and Shea-Donohue, T. (2021). Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Invest. 131:e143777. doi: 10.1172/JCI143777
Harach, T., Marungruang, N., Duthilleul, N., Cheatham, V., Mc Coy, K. D., Frisoni, G., et al. (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7:41802. doi: 10.1038/srep46856
Hawkins, R. A., Williamson, D. H., and Krebs, H. A. (1971). Ketone-body utilization by adult and suckling rat brain in vivo. Biochem. J. 122, 13–18. doi: 10.1042/bj1220013
Herpich, F., and Rincon, F. (2020). Management of acute ischemic stroke. Crit. Care Med. 48, 1654–1663. doi: 10.1097/CCM.0000000000004597
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hoane, M. R., Swan, A. A., and Heck, S. E. (2011). The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav. Brain Res. 223, 119–124. doi: 10.1016/j.bbr.2011.04.028
Hou, Y., Xu, L., Song, S., Fan, W., Wu, Q., Tong, X., et al. (2021). Oral administration of brain protein combined with probiotics induces immune tolerance through the tryptophan pathway. Front. Mol. Neurosci. 14:634631. doi: 10.3389/fnmol.2021.634631
Hou, Y. F., Shan, C., Zhuang, S. Y., Zhuang, Q. Q., Ghosh, A., Zhu, K. C., et al. (2021). Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 9:34. doi: 10.1186/s40168-020-00988-6
Houlden, A., Goldrick, M., Brough, D., Vizi, E. S., Lenart, N., Martinecz, B., et al. (2016). Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 57, 10–20. doi: 10.1016/j.bbi.2016.04.003
Howard, B. M., Kornblith, L. Z., Christie, S. A., Conroy, A. S., Nelson, M. F., Campion, E. M., et al. (2017). Characterizing the gut microbiome in trauma: Significant changes in microbial diversity occur early after severe injury. Trauma Surg. Acute Care Open 2:e000108. doi: 10.1136/tsaco-2017-000108
Hu, Y., and Tao, W. (2021). Microenvironmental variations after blood-brain barrier breakdown in traumatic brain injury. Front. Mol. Neurosci. 14:750810. doi: 10.3389/fnmol.2021.750810
Huang, Q., Di, L., Yu, F., Feng, X., Liu, Z., Wei, M., et al. (2022). Alterations in the gut microbiome with hemorrhagic transformation in experimental stroke. CNS Neurosci. Ther. 28, 77–91. doi: 10.1111/cns.13736
Iaccarino, C., Carretta, A., Nicolosi, F., and Morselli, C. (2018). Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 62, 535–541. doi: 10.23736/S0390-5616.18.04532-0
Jogia, T., and Ruitenberg, M. J. (2020). Traumatic spinal cord injury and the gut microbiota: Current insights and future challenges. Front. Immunol. 11:704. doi: 10.3389/fimmu.2020.00704
Juan, J., Liu, G., Willett, W. C., Hu, F. B., Rexrode, K. M., and Sun, Q. (2017). Whole grain consumption and risk of ischemic stroke: Results from 2 prospective cohort studies. Stroke 48, 3203–3209. doi: 10.1161/STROKEAHA.117.018979
Kawai, T., and Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 11, 373–384. doi: 10.1038/ni.1863
Kelly, C. R., Ihunnah, C., Fischer, M., Khoruts, A., Surawicz, C., Afzali, A., et al. (2014). Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 109, 1065–1071. doi: 10.1038/ajg.2014.133
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kigerl, K. A., Mostacada, K., and Popovich, P. G. (2018). Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics 15, 60–67. doi: 10.1007/s13311-017-0583-2
Kim, D. Y., Hao, J., Liu, R., Turner, G., Shi, F. D., and Rho, J. M. (2012). Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One 7:e35476. doi: 10.1371/journal.pone.0035476
Klimiec, E., Pera, J., Chrzanowska-Wasko, J., Golenia, A., Slowik, A., and Dziedzic, T. (2016). Plasma endotoxin activity rises during ischemic stroke and is associated with worse short-term outcome. J. Neuroimmunol. 297, 76–80. doi: 10.1016/j.jneuroim.2016.05.006
Koiv, L., Merisalu, E., Zilmer, K., Tomberg, T., and Kaasik, A. E. (1997). Changes of sympatho-adrenal and hypothalamo-pituitary-adrenocortical system in patients with head injury. Acta Neurol. Scand. 96, 52–58. doi: 10.1111/j.1600-0404.1997.tb00238.x
Kong, G., Huang, Z., Ji, W., Wang, X., Liu, J., Wu, X., et al. (2017). The ketone metabolite beta-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J. Neurotrauma 34, 2645–2655. doi: 10.1089/neu.2017.5192
Kurita, N., Yamashiro, K., Kuroki, T., Tanaka, R., Urabe, T., Ueno, Y., et al. (2020). Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 40, 2505–2520. doi: 10.1177/0271678X19899577
Lavasani, S., Dzhambazov, B., Nouri, M., Fak, F., Buske, S., Molin, G., et al. (2010). A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5:e9009. doi: 10.1371/journal.pone.0009009
Lee, Y. K., Menezes, J. S., Umesaki, Y., and Mazmanian, S. K. (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 108, (Suppl. 1), 4615–4622. doi: 10.1073/pnas.1000082107
Leino, R. L., Gerhart, D. Z., Duelli, R., Enerson, B. E., and Drewes, L. R. (2001). Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 38, 519–527. doi: 10.1016/S0197-0186(00)00102-9
Lemke, D. M. (2004). Riding out the storm: Sympathetic storming after traumatic brain injury. J. Neurosci. Nurs. 36, 4–9. doi: 10.1097/01376517-200402000-00002
Lepeta, K., Lourenco, M. V., Schweitzer, B. C., Martino Adami, P. V., Banerjee, P., Catuara-Solarz, S., et al. (2016). Synaptopathies: Synaptic dysfunction in neurological disorders - A review from students to students. J. Neurochem. 138, 785–805. doi: 10.1111/jnc.13713
Li, H., Zhang, X., Pan, D., Liu, Y., Yan, X., Tang, Y., et al. (2020). Dysbiosis characteristics of gut microbiota in cerebral infarction patients. Transl. Neurosci. 11, 124–133. doi: 10.1515/tnsci-2020-0117
Li, X. J., You, X. Y., Wang, C. Y., Li, X. L., Sheng, Y. Y., Zhuang, P. W., et al. (2020). Bidirectional brain-gut-microbiota axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci. Ther. 26, 783–790. doi: 10.1111/cns.13401
Li, Z., Quan, G., Jiang, X., Yang, Y., Ding, X., Zhang, D., et al. (2018). Effects of metabolites derived from gut microbiota and hosts on pathogens. Front. Cell. Infect. Microbiol. 8:314. doi: 10.3389/fcimb.2018.00314
Lindefeldt, M., Eng, A., Darban, H., Bjerkner, A., Zetterstrom, C. K., Allander, T., et al. (2019). The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5:5. doi: 10.1038/s41522-018-0073-2
Liu, J., Sun, J., Wang, F., Yu, X., Ling, Z., Li, H., et al. (2015). Neuroprotective effects of clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res. Int. 2015:412946. doi: 10.1155/2015/412946
Liu, X., Cao, S., and Zhang, X. (2015). Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 63, 7885–7895. doi: 10.1021/acs.jafc.5b02404
Lochhead, J. J., Ronaldson, P. T., and Davis, T. P. (2017). Hypoxic stress and inflammatory pain disrupt blood-brain barrier tight junctions: Implications for drug delivery to the central nervous system. AAPS J. 19, 910–920. doi: 10.1208/s12248-017-0076-6
Lum, G. R., Olson, C. A., and Hsiao, E. Y. (2020). Emerging roles for the intestinal microbiome in epilepsy. Neurobiol. Dis. 135:104576. doi: 10.1016/j.nbd.2019.104576
Ma, D., Wang, A. C., Parikh, I., Green, S. J., Hoffman, J. D., Chlipala, G., et al. (2018). Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 8:6670. doi: 10.1038/s41598-018-25190-5
Ma, E. L., Smith, A. D., Desai, N., Cheung, L., Hanscom, M., Stoica, B. A., et al. (2017). Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 66, 56–69. doi: 10.1016/j.bbi.2017.06.018
Ma, Q., Xing, C., Long, W., Wang, H. Y., Liu, Q., and Wang, R. F. (2019). Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 16:53. doi: 10.1186/s12974-019-1434-3
Ma, S., and Suzuki, K. (2019). Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention: A narrative review. Sports 7:40. doi: 10.3390/sports7020040
Macrez, R., Ali, C., Toutirais, O., Le Mauff, B., Defer, G., Dirnagl, U., et al. (2011). Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480. doi: 10.1016/S1474-4422(11)70066-7
Mahajan, C., Khurana, S., Kapoor, I., Sokhal, S., Kumar, S., Prabhakar, H., et al. (2021). Characteristics of gut microbiome after traumatic brain injury. J. Neurosurg. Anesthesiol. [Epub ahead of print]. doi: 10.1097/ANA.0000000000000789
Mangalam, A., Shahi, S. K., Luckey, D., Karau, M., Marietta, E., Luo, N., et al. (2017). Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 20, 1269–1277. doi: 10.1016/j.celrep.2017.07.031
Manor, O., Dai, C. L., Kornilov, S. A., Smith, B., Price, N. D., Lovejoy, J. C., et al. (2020). Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 11:5206. doi: 10.1038/s41467-020-18871-1
Manson, J., Thiemermann, C., and Brohi, K. (2012). Trauma alarmins as activators of damage-induced inflammation. Br. J. Surg. 99, (Suppl. 1), 12–20. doi: 10.1002/bjs.7717
Martin-McGill, K. J., Bresnahan, R., Levy, R. G., and Cooper, P. N. (2020). Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 6:CD001903. doi: 10.1002/14651858.CD001903.pub5
Matt, S. M., Allen, J. M., Lawson, M. A., Mailing, L. J., Woods, J. A., and Johnson, R. W. (2018). Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 9:1832. doi: 10.3389/fimmu.2018.01832
McDonald, S. J., Sharkey, J. M., Sun, M., Kaukas, L. M., Shultz, S. R., Turner, R. J., et al. (2020). Beyond the brain: Peripheral interactions after traumatic brain injury. J. Neurotrauma 37, 770–781. doi: 10.1089/neu.2019.6885
McDonald, T. J. W., Henry-Barron, B. J., Felton, E. A., Gutierrez, E. G., Barnett, J., Fisher, R., et al. (2018). Improving compliance in adults with epilepsy on a modified Atkins diet: A randomized trial. Seizure 60, 132–138. doi: 10.1016/j.seizure.2018.06.019
Meddings, J. B., and Swain, M. G. (2000). Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 119, 1019–1028. doi: 10.1053/gast.2000.18152
Medel-Matus, J. S., Shin, D., Dorfman, E., Sankar, R., and Mazarati, A. (2018). Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open 3, 290–294. doi: 10.1002/epi4.12114
Mehrian-Shai, R., Reichardt, J. K. V., Harris, C. C., and Toren, A. (2019). The gut-brain axis, paving the way to brain cancer. Trends Cancer 5, 200–207. doi: 10.1016/j.trecan.2019.02.008
Milder, J. B., Liang, L. P., and Patel, M. (2010). Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 40, 238–244. doi: 10.1016/j.nbd.2010.05.030
Minter, M. R., Zhang, C., Leone, V., Ringus, D. L., Zhang, X., Oyler-Castrillo, P., et al. (2016). Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 6:30028. doi: 10.1038/srep30028
Mirzaei, R., Bouzari, B., Hosseini-Fard, S. R., Mazaheri, M., Ahmadyousefi, Y., Abdi, M., et al. (2021). Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 139:111661. doi: 10.1016/j.biopha.2021.111661
Morrison, D. J., and Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200. doi: 10.1080/19490976.2015.1134082
Nagpal, R., Neth, B. J., Wang, S., Craft, S., and Yadav, H. (2019). Modified mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. doi: 10.1016/j.ebiom.2019.08.032
Nagpal, R., Neth, B. J., Wang, S., Mishra, S. P., Craft, S., and Yadav, H. (2020). Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 59:102950. doi: 10.1016/j.ebiom.2020.102950
Ngo, S. T., Restuadi, R., McCrae, A. F., Van Eijk, R. P., Garton, F., Henderson, R. D., et al. (2020). Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 549–562. doi: 10.1080/21678421.2020.1772825
Nicholson, S. E., Watts, L. T., Burmeister, D. M., Merrill, D., Scroggins, S., Zou, Y., et al. (2019). Moderate traumatic brain injury alters the gastrointestinal microbiome in a time-dependent manner. Shock 52, 240–248. doi: 10.1097/SHK.0000000000001211
Obrenovich, M. E. M. (2018). Leaky gut, leaky brain? Microorganisms 6:107. doi: 10.3390/microorganisms6040107
O’Callaghan, J. P., Sriram, K., and Miller, D. B. (2008). Defining “neuroinflammation”. Ann. N. Y. Acad. Sci. 1139, 318–330. doi: 10.1196/annals.1432.032
Ochoa-Reparaz, J., Mielcarz, D. W., Ditrio, L. E., Burroughs, A. R., Begum-Haque, S., Dasgupta, S., et al. (2010). Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108. doi: 10.4049/jimmunol.1001443
O’Connell, M. T., Seal, A., Nortje, J., Al-Rawi, P. G., Coles, J. P., Fryer, T. D., et al. (2005). Glucose metabolism in traumatic brain injury: A combined microdialysis and [18F]-2-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) study. Acta Neurochir. Suppl. 95, 165–168. doi: 10.1007/3-211-32318-X_35
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741.e13. doi: 10.1016/j.cell.2018.04.027
Owen, O. E., Morgan, A. P., Kemp, H. G., Sullivan, J. M., Herrera, M. G., and Cahill, G. F. Jr. (1967). Brain metabolism during fasting. J. Clin. Invest. 46, 1589–1595. doi: 10.1172/JCI105650
Pathare, N., Sushilkumar, S., Haley, L., Jain, S., and Osier, N. N. (2020). The impact of traumatic brain injury on microbiome composition: A systematic review. Biol. Res. Nurs. 22, 495–505. doi: 10.1177/1099800420943961
Patrizz, A., Dono, A., Zorofchian, S., Hines, G., Takayasu, T., Husein, N., et al. (2020). Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 10:21002. doi: 10.1038/s41598-020-77919-w
Pearn, M. L., Niesman, I. R., Egawa, J., Sawada, A., Almenar-Queralt, A., Shah, S. B., et al. (2017). Pathophysiology associated with traumatic brain injury: Current treatments and potential novel therapeutics. Cell. Mol. Neurobiol. 37, 571–585. doi: 10.1007/s10571-016-0400-1
Pellegrini, C., Antonioli, L., Calderone, V., Colucci, R., Fornai, M., and Blandizzi, C. (2020). Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog. Neurobiol. 191:101806. doi: 10.1016/j.pneurobio.2020.101806
Peng, A., Qiu, X., Lai, W., Li, W., Zhang, L., Zhu, X., et al. (2018). Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 147, 102–107. doi: 10.1016/j.eplepsyres.2018.09.013
Petridis, A. K., Kamp, M. A., Cornelius, J. F., Beez, T., Beseoglu, K., Turowski, B., et al. (2017). Aneurysmal Subarachnoid Hemorrhage. Dtsch. Arztebl. Int. 114, 226–236. doi: 10.3238/arztebl.2017.0226
Phillips, M. C. L., Deprez, L. M., Mortimer, G. M. N., Murtagh, D. K. J., McCoy, S., Mylchreest, R., et al. (2021). Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 13:51. doi: 10.1186/s13195-021-00783-x
Phillips, M. C. L., Murtagh, D. K. J., Gilbertson, L. J., Asztely, F. J. S., and Lynch, C. D. P. (2018). Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 33, 1306–1314. doi: 10.1002/mds.27390
Pietrzak, D., Kasperek, K., Rekawek, P., and Piatkowska-Chmiel, I. (2022). The therapeutic role of ketogenic diet in neurological disorders. Nutrients 14:1952. doi: 10.3390/nu14091952
Pondel, N., Liskiewicz, D., and Liskiewicz, A. (2020). Ketogenic diet - mechanism of action and perspectives for the use in the therapy: Data from clinical studies. Postepy Biochem. 66, 270–286.
Preiningerova, J. L., Jiraskova Zakostelska, Z., Srinivasan, A., Ticha, V., Kovarova, I., Kleinova, P., et al. (2022). Multiple Sclerosis and Microbiome. Biomolecules 12:433. doi: 10.3390/biom12030433
Prins, M., Greco, T., Alexander, D., and Giza, C. C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis. Model Mech. 6, 1307–1315. doi: 10.1242/dmm.011585
Prins, M. L. (2008). Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 28, 1–16. doi: 10.1038/sj.jcbfm.9600543
Prins, M. L., Lee, S. M., Fujima, L. S., and Hovda, D. A. (2004). Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J. Neurochem. 90, 666–672. doi: 10.1111/j.1471-4159.2004.02542.x
Puig, K. L., Floden, A. M., Adhikari, R., Golovko, M. Y., and Combs, C. K. (2012). Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One 7:e30378. doi: 10.1371/journal.pone.0030378
Qiao, G., Lv, T., Zhang, M., Chen, P., Sun, Q., Zhang, J., et al. (2020). beta-hydroxybutyrate (beta-HB) exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated macrophages in Liza haematocheila. Fish Shellfish Immunol. 107(Pt B), 444–451. doi: 10.1016/j.fsi.2020.11.005
Quigley, E. M. M. (2017). Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17:94. doi: 10.1007/s11910-017-0802-6
Riazi, K., Galic, M. A., Kuzmiski, J. B., Ho, W., Sharkey, K. A., and Pittman, Q. J. (2008). Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. U.S.A. 105, 17151–17156. doi: 10.1073/pnas.0806682105
Rice, M. W., Pandya, J. D., and Shear, D. A. (2019). Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front. Neurol. 10:875. doi: 10.3389/fneur.2019.00875
Roman, G. C., Jackson, R. E., Gadhia, R., Roman, A. N., and Reis, J. (2019). Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 175, 724–741. doi: 10.1016/j.neurol.2019.08.005
Rosner, M. J., Newsome, H. H., and Becker, D. P. (1984). Mechanical brain injury: The sympathoadrenal response. J. Neurosurg. 61, 76–86. doi: 10.3171/jns.1984.61.1.0076
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillere, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97. doi: 10.1126/science.aan3706
Rowin, J., Xia, Y., Jung, B., and Sun, J. (2017). Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 5:e13443. doi: 10.14814/phy2.13443
Royes, L. F. F., and Gomez-Pinilla, F. (2019). Making sense of gut feelings in the traumatic brain injury pathogenesis. Neurosci. Biobehav. Rev. 102, 345–361. doi: 10.1016/j.neubiorev.2019.05.012
Sahathevan, R., Brodtmann, A., and Donnan, G. A. (2012). Dementia, stroke, and vascular risk factors; a review. Int. J. Stroke 7, 61–73. doi: 10.1111/j.1747-4949.2011.00731.x
Salazar, N., Lopez, P., Valdes, L., Margolles, A., Suarez, A., Patterson, A. M., et al. (2013). Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J. Am. Coll. Nutr. 32, 399–406. doi: 10.1080/07315724.2013.827047
Salazar, N., Valdes-Varela, L., Gonzalez, S., Gueimonde, M., and de Los Reyes-Gavilan, C. G. (2017). Nutrition and the gut microbiome in the elderly. Gut Microbes 8, 82–97. doi: 10.1080/19490976.2016.1256525
Schain, M., and Kreisl, W. C. (2017). Neuroinflammation in neurodegenerative disorders-a review. Curr. Neurol. Neurosci. Rep. 17:25. doi: 10.1007/s11910-017-0733-2
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015). Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Schiffrin, E. J., Morley, J. E., Donnet-Hughes, A., and Guigoz, Y. (2010). The inflammatory status of the elderly: The intestinal contribution. Mutat. Res. 690, 50–56. doi: 10.1016/j.mrfmmm.2009.07.011
Shaafi, S., Sharifi-Bonab, M., Ghaemian, N., Mokhtarkhani, M., and Akbari, H. (2019). Early motor-behavioral outcome of ischemic stroke with ketogenic diet preconditioning: Interventional animal study. J. Stroke Cerebrovasc. Dis. 28, 1032–1039. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.024
Shahrokhi, N., Khaksari, M., Soltani, Z., Mahmoodi, M., and Nakhaee, N. (2010). Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can. J. Physiol. Pharmacol. 88, 414–421. doi: 10.1139/Y09-126
Shao, B. Z., Cao, Q., and Liu, C. (2018). Targeting NLRP3 inflammasome in the treatment of CNS diseases. Front. Mol. Neurosci. 11:320. doi: 10.3389/fnmol.2018.00320
Shen, L., Liu, L., and Ji, H. F. (2017). Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimers Dis. 56, 385–390. doi: 10.3233/JAD-160884
Sherman, M., Liu, M. M., Birnbaum, S., Wolf, S. E., Minei, J. P., and Gatson, J. W. (2016). Adult obese mice suffer from chronic secondary brain injury after mild TBI. J. Neuroinflammation 13:171. doi: 10.1186/s12974-016-0641-4
Shichita, T., Hasegawa, E., Kimura, A., Morita, R., Sakaguchi, R., Takada, I., et al. (2012). Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 18, 911–917. doi: 10.1038/nm.2749
Shichita, T., Sugiyama, Y., Ooboshi, H., Sugimori, H., Nakagawa, R., Takada, I., et al. (2009). Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950. doi: 10.1038/nm.1999
Shikata, F., Shimada, K., Sato, H., Ikedo, T., Kuwabara, A., Furukawa, H., et al. (2019). Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension 73, 491–496. doi: 10.1161/HYPERTENSIONAHA.118.11804
Shin, N. R., Whon, T. W., and Bae, J. W. (2015). Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503. doi: 10.1016/j.tibtech.2015.06.011
Shukla, P. K., Delotterie, D. F., Xiao, J., Pierre, J. F., Rao, R., McDonald, M. P., et al. (2021). Alterations in the gut-microbial-inflammasome-brain axis in a mouse model of Alzheimer’s disease. Cells 10:779. doi: 10.3390/cells10040779
Simon, D. W., McGeachy, M. J., Bayir, H., Clark, R. S., Loane, D. J., and Kochanek, P. M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191. doi: 10.1038/nrneurol.2017.13
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-Michaels, K., Earley, Z. M., et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089. doi: 10.1126/science.aac4255
Soria Lopez, J. A., Gonzalez, H. M., and Leger, G. C. (2019). Alzheimer’s disease. Handb. Clin. Neurol. 167, 231–255. doi: 10.1016/B978-0-12-804766-8.00013-3
Storoni, M., and Plant, G. T. (2015). The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult. Scler. Int. 2015:681289. doi: 10.1155/2015/681289
Streijger, F., Plunet, W. T., Lee, J. H., Liu, J., Lam, C. K., Park, S., et al. (2013). Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PLoS One 8:e78765. doi: 10.1371/journal.pone.0078765
Surawicz, C. M., Brandt, L. J., Binion, D. G., Ananthakrishnan, A. N., Curry, S. R., Gilligan, P. H., et al. (2013). Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 108, 478–498; quiz499. doi: 10.1038/ajg.2013.4
Swidsinski, A., Dorffel, Y., Loening-Baucke, V., Gille, C., Goktas, O., Reisshauer, A., et al. (2017). Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 8:1141. doi: 10.3389/fmicb.2017.01141
Tai, K. K., Nguyen, N., Pham, L., and Truong, D. D. (2008). Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J. Neural Transm 115, 1011–1017. doi: 10.1007/s00702-008-0050-7
Takewaki, D., Suda, W., Sato, W., Takayasu, L., Kumar, N., Kimura, K., et al. (2020). Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 117, 22402–22412. doi: 10.1073/pnas.2011703117
Tan, B. Y. Q., Paliwal, P. R., and Sharma, V. K. (2020). Gut microbiota and stroke. Ann. Indian Acad. Neurol. 23, 155–158.
Tan, C., Wu, Q., Wang, H., Gao, X., Xu, R., Cui, Z., et al. (2021). Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J. Parenter. Enteral Nutr. 45, 518–529. doi: 10.1002/jpen.1861
Tankou, S. K., Regev, K., Healy, B. C., Cox, L. M., Tjon, E., Kivisakk, P., et al. (2018a). Investigation of probiotics in multiple sclerosis. Mult. Scler. 24, 58–63. doi: 10.1177/1352458517737390
Tankou, S. K., Regev, K., Healy, B. C., Tjon, E., Laghi, L., Cox, L. M., et al. (2018b). A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 83, 1147–1161. doi: 10.1002/ana.25244
Taylor, M. K., Sullivan, D. K., Mahnken, J. D., Burns, J. M., and Swerdlow, R. H. (2018). Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement. 4, 28–36. doi: 10.1016/j.trci.2017.11.002
Tobin, R. P., Mukherjee, S., Kain, J. M., Rogers, S. K., Henderson, S. K., Motal, H. L., et al. (2014). Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol. Commun. 2:143. doi: 10.1186/s40478-014-0143-5
Tomkins, O., Feintuch, A., Benifla, M., Cohen, A., Friedman, A., and Shelef, I. (2011). Blood-brain barrier breakdown following traumatic brain injury: A possible role in posttraumatic epilepsy. Cardiovasc. Psychiatry Neurol. 2011:765923. doi: 10.1155/2011/765923
Treangen, T. J., Wagner, J., Burns, M. P., and Villapol, S. (2018). Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front. Immunol. 9:2757. doi: 10.3389/fimmu.2018.02757
Tremblay, M. E., Stevens, B., Sierra, A., Wake, H., Bessis, A., and Nimmerjahn, A. (2011). The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011
Tremlett, H., Fadrosh, D. W., Faruqi, A. A., Hart, J., Roalstad, S., Graves, J., et al. (2016). Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J. Neurol. Sci. 363, 153–157. doi: 10.1016/j.jns.2016.02.042
Tseng, M. T., Chan, S. A., and Schurr, A. (2003). Ischemia-induced changes in monocarboxylate transporter 1 reactive cells in rat hippocampus. Neurol. Res. 25, 83–86. doi: 10.1179/016164103101200978
Ueda, A., Shinkai, S., Shiroma, H., Taniguchi, Y., Tsuchida, S., Kariya, T., et al. (2021). Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep. Med. 2:100398. doi: 10.1016/j.xcrm.2021.100398
Urban, R. J., Pyles, R. B., Stewart, C. J., Ajami, N., Randolph, K. M., Durham, W. J., et al. (2020). Altered fecal microbiome years after traumatic brain injury. J. Neurotrauma 37, 1037–1051. doi: 10.1089/neu.2019.6688
Vangay, P., Ward, T., Gerber, J. S., and Knights, D. (2015). Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17, 553–564. doi: 10.1016/j.chom.2015.04.006
Vannucci, S. J., and Simpson, I. A. (2003). Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 285, E1127–E1134. doi: 10.1152/ajpendo.00187.2003
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Walczyk, T., and Wick, J. Y. (2017). The ketogenic diet: Making a comeback. Consult Pharm. 32, 388–396. doi: 10.4140/TCP.n.2017.388
Wanchao, S., Chen, M., Zhiguo, S., Futang, X., and Mengmeng, S. (2018). Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz. J. Med. Biol. Res. 51:e7172. doi: 10.1590/1414-431x20187172
Wang, H. X., and Wang, Y. P. (2016). Gut microbiota-brain axis. Chin. Med. J. 129, 2373–2380. doi: 10.4103/0366-6999.190667
Wang, J. W., Kuo, C. H., Kuo, F. C., Wang, Y. K., Hsu, W. H., Yu, F. J., et al. (2019). Fecal microbiota transplantation: Review and update. J. Formos Med. Assoc. 118, (Suppl. 1), S23–S31. doi: 10.1016/j.jfma.2018.08.011
Wang, Y., Guo, S. Z., Bonen, A., Li, R. C., Kheirandish-Gozal, L., Zhang, S. X., et al. (2011). Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J. Neurosci. 31, 10241–10248. doi: 10.1523/JNEUROSCI.1462-11.2011
Wei, Y., Yang, J., Wang, J., Yang, Y., Huang, J., Gong, H., et al. (2016). Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit. Care 20:332. doi: 10.1186/s13054-016-1491-2
Weller, M., Wick, W., Aldape, K., Brada, M., Berger, M., Pfister, S. M., et al. (2015). Glioma. Nat. Rev. Dis. Primers 1:15017. doi: 10.1038/nrdp.2015.17
Wen, S. W., and Wong, C. H. Y. (2017). An unexplored brain-gut microbiota axis in stroke. Gut Microbes 8, 601–606. doi: 10.1080/19490976.2017.1344809
Wheless, J. W. (2008). History of the ketogenic diet. Epilepsia 49, (Suppl. 8), 3–5. doi: 10.1111/j.1528-1167.2008.01821.x
White, H., and Venkatesh, B. (2011). Clinical review: Ketones and brain injury. Crit. Care 15, 219. doi: 10.1186/cc10020
White, H., Venkatesh, B., Jones, M., Kruger, P. S., Walsham, J., and Fuentes, H. (2020). Inducing ketogenesis via an enteral formulation in patients with acute brain injury:a phase II study. Neurol. Res. 42, 275–285. doi: 10.1080/01616412.2019.1709743
Winkler, E. A., Minter, D., Yue, J. K., and Manley, G. T. (2016). Cerebral edema in traumatic brain injury: Pathophysiology and prospective therapeutic targets. Neurosurg. Clin. N. Am. 27, 473–488. doi: 10.1016/j.nec.2016.05.008
Woodburn, S. C., Bollinger, J. L., and Wohleb, E. S. (2021). The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 18:258. doi: 10.1186/s12974-021-02309-6
Wu, A., Molteni, R., Ying, Z., and Gomez-Pinilla, F. (2003). A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience 119, 365–375. doi: 10.1016/S0306-4522(03)00154-4
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y. Y., Keilbaugh, S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. doi: 10.1126/science.1208344
Wu, H., and Chiou, J. (2021). Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients 13:2878. doi: 10.3390/nu13082878
Xie, G., Zhou, Q., Qiu, C. Z., Dai, W. K., Wang, H. P., Li, Y. H., et al. (2017). Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 23, 6164–6171. doi: 10.3748/wjg.v23.i33.6164
Yamashiro, K., Tanaka, R., Urabe, T., Ueno, Y., Yamashiro, Y., Nomoto, K., et al. (2017). Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One 12:e0171521. doi: 10.1371/journal.pone.0171521
Yang, X., and Cheng, B. (2010). Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 42, 145–153. doi: 10.1007/s12031-010-9336-y
Yarandi, S. S., Peterson, D. A., Treisman, G. J., Moran, T. H., and Pasricha, P. J. (2016). Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 22, 201–212. doi: 10.5056/jnm15146
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. doi: 10.1038/nature11053
Yeom, J. S., Park, J. S., Kim, Y. S., Kim, R. B., Choi, D. S., Chung, J. Y., et al. (2019). Neonatal seizures and white matter injury: Role of rotavirus infection and probiotics. Brain Dev. 41, 19–28. doi: 10.1016/j.braindev.2018.07.001
Yin, J., Liao, S. X., He, Y., Wang, S., Xia, G. H., Liu, F. T., et al. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 4:e002699. doi: 10.1161/JAHA.115.002699
You, W., Zhu, Y., Wei, A., Du, J., Wang, Y., Zheng, P., et al. (2022). Traumatic brain injury induces gastrointestinal dysfunction and dysbiosis of gut microbiota accompanied by alterations of bile acid profile. J. Neurotrauma 39, 227–237. doi: 10.1089/neu.2020.7526
Youm, Y. H., Nguyen, K. Y., Grant, R. W., Goldberg, E. L., Bodogai, M., Kim, D., et al. (2015). The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269. doi: 10.1038/nm.3804
Yu, X., Zhang, R., Wei, C., Gao, Y., Yu, Y., Wang, L., et al. (2021a). MCT2 overexpression promotes recovery of cognitive function by increasing mitochondrial biogenesis in a rat model of stroke. Anim. Cells Syst. 25, 93–101. doi: 10.1080/19768354.2021.1915379
Yu, X., Zhou, G., Shao, B., Zhou, H., Xu, C., Yan, F., et al. (2021b). Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice. Front. Microbiol. 12:647304. doi: 10.3389/fmicb.2021.647304
Yue, Q., Cai, M., Xiao, B., Zhan, Q., and Zeng, C. A. (2021). High-tryptophan diet reduces seizure-induced respiratory arrest and alters the gut microbiota in DBA/1 mice. Front. Neurol. 12:762323. doi: 10.3389/fneur.2021.762323
Yurista, S. R., Chong, C. R., Badimon, J. J., Kelly, D. P., de Boer, R. A., and Westenbrink, B. D. (2021). Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 77, 1660–1669. doi: 10.1016/j.jacc.2020.12.065
Zeng, Q., Shen, J., Chen, K., Zhou, J., Liao, Q., Lu, K., et al. (2020). The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 10:12998. doi: 10.1038/s41598-020-69845-8
Zhai, Q., Sun, T., Sun, C., Yan, L., Wang, X., Wang, Y., et al. (2021). High plasma levels of trimethylamine N-oxide are associated with poor outcome in intracerebral hemorrhage patients. Neurol. Sci. 42, 1009–1016. doi: 10.1007/s10072-020-04618-9
Zhan, X., Stamova, B., and Sharp, F. R. (2018). Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: A review. Front. Aging Neurosci. 10:42. doi: 10.3389/fnagi.2018.00042
Zhang, F., Vannucci, S. J., Philp, N. J., and Simpson, I. A. (2005). Monocarboxylate transporter expression in the spontaneous hypertensive rat: Effect of stroke. J. Neurosci. Res. 79, 139–145. doi: 10.1002/jnr.20312
Zhang, H., Huang, Y., Li, X., Han, X., Hu, J., Wang, B., et al. (2021). Dynamic process of secondary pulmonary infection in mice with intracerebral hemorrhage. Front. Immunol. 12:767155. doi: 10.3389/fimmu.2021.767155
Zhang, Y., Kuang, Y., Xu, K., Harris, D., Lee, Z., LaManna, J., et al. (2013). Ketosis proportionately spares glucose utilization in brain. J. Cereb. Blood Flow Metab. 33, 1307–1311. doi: 10.1038/jcbfm.2013.87
Zhang, Y., Zhou, S., Zhou, Y., Yu, L., Zhang, L., and Wang, Y. (2018). Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 145, 163–168. doi: 10.1016/j.eplepsyres.2018.06.015
Zhang, Y. G., Wu, S., Yi, J., Xia, Y., Jin, D., Zhou, J., et al. (2017). Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 39, 322–336. doi: 10.1016/j.clinthera.2016.12.014
Zhao, X., Sun, G., Zhang, H., Ting, S. M., Song, S., Gonzales, N., et al. (2014). Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Transl. Stroke Res. 5, 554–561. doi: 10.1007/s12975-014-0341-2
Zhao, Z., Lange, D. J., Voustianiouk, A., MacGrogan, D., Ho, L., Suh, J., et al. (2006). A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 7:29. doi: 10.1186/1471-2202-7-29
Zhu, C. S., Grandhi, R., Patterson, T. T., and Nicholson, S. E. (2018). A review of traumatic brain injury and the gut microbiome: Insights into novel mechanisms of secondary brain injury and promising targets for neuroprotection. Brain Sci. 8:113. doi: 10.3390/brainsci8060113
Zhu, H., Bi, D., Zhang, Y., Kong, C., Du, J., Wu, X., et al. (2022). Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 7:11. doi: 10.1038/s41392-021-00831-w
Zhu, H., Wang, Z., Yu, J., Yang, X., He, F., Liu, Z., et al. (2019). Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 178:101610. doi: 10.1016/j.pneurobio.2019.03.003
Zhu, W., Romano, K. A., Li, L., Buffa, J. A., Sangwan, N., Prakash, P., et al. (2021). Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 29, 1199–1208.e5. doi: 10.1016/j.chom.2021.05.002
Zhu, X., Li, B., Lou, P., Dai, T., Chen, Y., Zhuge, A., et al. (2021). The relationship between the gut microbiome and neurodegenerative diseases. Neurosci. Bull. 37, 1510–1522. doi: 10.1007/s12264-021-00730-8
Zhuang, Z. Q., Shen, L. L., Li, W. W., Fu, X., Zeng, F., Gui, L., et al. (2018). Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimers Dis. 63, 1337–1346. doi: 10.3233/JAD-180176
Keywords: microbiome, ketogenic diet, acute brain injury, chronic neurological disorders, stroke, neurodegeneration, probiotics
Citation: Krakovski MA, Arora N, Jain S, Glover J, Dombrowski K, Hernandez B, Yadav H and Sarma AK (2022) Diet-microbiome-gut-brain nexus in acute and chronic brain injury. Front. Neurosci. 16:1002266. doi: 10.3389/fnins.2022.1002266
Received: 25 July 2022; Accepted: 29 August 2022;
Published: 16 September 2022.
Edited by:
Hongjin Wu, Boao International Hospital, ChinaReviewed by:
Sylwia Terpilowska, Jan Kochanowski University, PolandNeetu Tyagi, University of Louisville, United States
Copyright © 2022 Krakovski, Arora, Jain, Glover, Dombrowski, Hernandez, Yadav and Sarma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hariom Yadav, aHlhZGF2QHVzZi5lZHU=; Anand Karthik Sarma, YXNhcm1hQHdha2VoZWFsdGguZWR1
 Maria Alexander Krakovski
Maria Alexander Krakovski Niraj Arora
Niraj Arora Shalini Jain
Shalini Jain Jennifer Glover3
Jennifer Glover3 Beverly Hernandez
Beverly Hernandez Hariom Yadav
Hariom Yadav Anand Karthik Sarma
Anand Karthik Sarma