
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 26 November 2021
Sec. Brain Imaging Methods
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.766320
This article is part of the Research TopicPositron Emission Tomography (PET) Imaging of Brain Biochemistry: Beyond High-Affinity RadioligandsView all 5 articles
The serotonin 5-HT2C receptor (5-HT2CR) is abundantly expressed throughout the central nervous system, and involved in a variety of neuroendocrine and neurobehavioral processes. The development of a selective radioligand that will enable in vivo imaging and quantification of 5-HT2CR densities represents a significant technological advancement in understanding both the normal function and pathophysiology of the 5-HT2CR. Four 7-halogen-2-phenyl isoindolones (7-F, Cl, Br, I) were synthesized and displayed high affinities for 5-HT2CR and high selectivity over 5-HT2A and 5-HT2B. [11C]7-Chloro-2-[4-methoxy-3-[2-(4-methylpiperidin-1-yl)ethoxy]phenyl]isoindolin-1-one (6) and [11C]7-iodo-2-[4-methoxy-3-[2-(4-methylpiperidin-1-yl)ethoxy]phenyl]isoindolin-1-one (9) were synthesized in high radiochemical yield of 37–44% [n = 10, decay corrected from end of (11C)CH3I synthesis] with high radiochemical purity via O-methylation with [11C]CH3I, respectively. MicroPET imaging studies in male rats with or without 5-HT2C antagonist SB-242084 showed that [11C]6 and [11C]9 display specific bindings to 5-HT2CR in the choroid plexus and hippocampus. In vivo microPET brain imaging studies in rhesus monkeys demonstrated that [11C]6 and [11C]9 exhibit excellent blood-brain barrier penetration. The contrast of bindings to the choroid plexus and hippocampus compared to the cerebellum peaked at 2.7 and 1.6, respectively, for [11C]6, and 3.7 and 2.7, respectively, for [11C]9, which were reduced by administration of a dose of SB-242084. Our results support the candidacy of [11C]6 and [11C]9 for further study as radioligands for in vivo quantitation of 5-HT2C sites by PET.
The serotonin (5-HT) system in the brain is directly involved in regulating various physiological actions and mental states as an important neurotransmission network which control behaviors and physical functions (Jacobs and Azmitia, 1992; Barnes and Sharp, 1999; Carhart-Harris and Nutt, 2017). Dysregulation of serotonergic function and significant alteration in serotonin receptor binding has been increasingly implicated in the pathology of physiological and psychiatric disorders (Messing and Lytle, 1977; Coccaro et al., 1989; Naughton et al., 2000; Stockmeier, 2003). The 5-HT2C receptor (5-HT2CR) is one of 14 5-HT receptor subtypes that binds the endogenous neurotransmitter serotonin. Expression of the 5-HT2CR is widely distributed in the mammalian brain with the highest concentration in the choroid plexus. Sufficient densities for imaging are also identified in the hippocampus, amygdala and hypothalamus, while low densities of 5-HT2CR are found in the cortex and cerebellum (Hoyer et al., 1986; Clemett et al., 2000). The 5-HT2CR has been implicated in mediating the interaction between serotonergic and dopaminergic systems. Substantial preclinical and clinical findings on 5-HT2CR agonists and antagonists demonstrated 5-HT2CR as a potential therapeutic target for the treatment of schizophrenia, anxiety, depression, drug abuse, and Parkinson’s disease (Millan, 2005; Heisler et al., 2007; Rosenzweig-Lipson et al., 2007; Jensen et al., 2010). Its involvement in feeding regulation and energy balance led to the FDA approval of the 5-HT2CR agonist lorcaserin for treatment of obesity.
Although PET imaging of 5-HTRs has been progressing for almost three decades, the successful radiotracers developed so far for human studies are limited to 5-HT1AR, 5-HT1BR, 5-HT2AR, 5-HT4R and 5-HT6R. Several attempts for the development of 5-HT2CR PET tracers were recently made with limited success (Figure 1). Two potent benzazepine 5-HT2CR agonists, WAY-163909 and Vabicaserin, were labeled with carbon-11. PET imaging evaluation indicated that both [11C]WAY-163909 (Neelamegam et al., 2014) (1) and [11C]Vabicaserin (Neelamegam et al., 2014) (2) exhibited high non-specific binding in baboon brains. [11C]Cimbi-36 (Finnema et al., 2014) (3) was reported as the first agonist suitable for imaging 5-HT2CR in the choroid plexus of the primate brain. However, [11C]Cimbi-36 is also a potent 5-HT2AR agonist with Ki = 0.5 nM for 5HT2AR vs. Ki = 1.7 nM for 5HT2CR. This major shortcoming necessitates blocking 5HT2AR prior to administration of [11C]Cimbi-36 in the quantitative assessment of 5-HT2CR binding in CNS disorders. [18F]4-(3-Fluorophenethoxy)pyrimidine (4) was synthesized and evaluated in the rat brain (Kim et al., 2017). Although [18F]4 exhibited specific binding to 5-HT2CR, its low to moderate ratios of uptake in regions of interest to cerebellum and fast washout from choroid plexus limited its utility as a PET radiotracer for 5-HT2CR. Recently, we evaluated a C-11 labeled pyridyloxypyridyl indole carboxamide derivative, 6-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]1H-indole-3-carboxamide (5) as a 5-HT2CR PET imaging agent (Zeng et al., 2018). MicroPET studies in rhesus monkeys demonstrated that [11C]5 displays a high level of specific binding in the choroid plexus, however, the overall brain uptake and retention of the tracer was low.
As our continuous effort to develop 5-HT2CR-specific PET imaging agents, we selected halogen substituted isoindolones (represented by 6 and 7) as potential target molecules based on the reported excellent in vitro binding profiles (Hamprecht et al., 2007). Chloro- and bromo- isoindolone derivatives (6 and 7) exhibited high affinity for 5-HT2CR and high selectivity over 5-HT2a and 5-HT2b (pKi of 6 for 5-HT2a, 5-Ht2b, and 5-HT2C = 6.1, 6.9, and 8.8; pKi of 7 for 5-HT2a, 5-Ht2b, and 5-HT2C = 6.2, 6.8, and 9.1, respectively). In addition, 6 and 7 showed great brain permeability as measured by brain to blood plasma ratio. We synthesized compounds 6 and 7, and fluoro- and iodo-isoindolone derivatives, 8 and 9, and compared their binding affinity and selectivity at 5-HT2CR. In addition, compounds 6 and 9 were radiolabeled with carbon-11 and PET imaging studies in rats and non-human primates were performed for evaluation as 5-HT2CR PET radioligands.
Compounds 6–9 were synthesized in three steps (Scheme 1), which include alkylation of the phenols with N-(2-chloroethyl)-4-methylpiperidine, reduction of the nitro compounds using Pd/C, and condensation of the resulting phenylamines with the corresponding 6-halogen-2-bromomethylbenzoate. The synthesis of the precursors 10 and 11 followed the same procedure, except that 2-(methoxymethoxy)-5-nitrophenol was used in the alkylation reaction. The MOM protection group was then cleaved with excess p-toluenesulfonic acid to afford the hydroxyphenylisoindolones as the radiolabeling precursors (Scheme 2).
All compounds 6–9 displayed high binding affinities to 5-HT2C with >90% binding at a concentration of 10 μM. The 5-HT2C competition assay with [3H]mesulergine showed that iodo derivative 9 has the highest binding of 1.1 nM, while chloro and bromo derivatives 6 and 7 exhibit similar bindings of 2.2 and 2.9 nM, respectively, which is 2–3 times less than that for 9, and fluoro derivative 8 has the lowest affinity of 28 nM. Compounds 6, 7, and 9 exhibit high binding selectivity over 5-HT2B (80–100 times) and <50% primary bindings at 5-HT2A. In addition, no significant binding was observed in other 5-HT subtypes and D1-D5 receptors (Table 1). We selected 6 and 9 for carbon-11 radiolabelling and PET imaging studies.
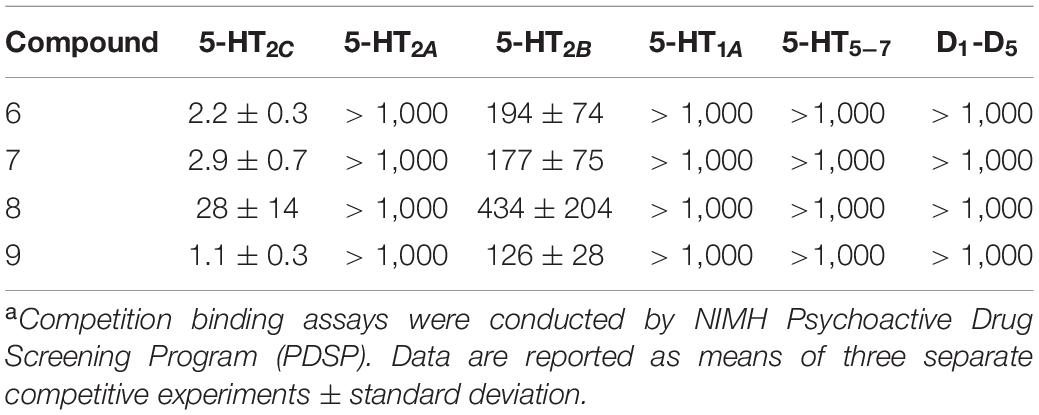
Table 1. Binding affinities of 7-halogen isoindolones at serotonin (5-HT1A-5-HT7) and dopamine (D1-D5) receptors (Ki, nM)a.
[11C]6 and [11C]9 were prepared via O-methylation of 10 and 11 with [11C]CH3I in the presence of 0.1 M Bu4NOH in DMF followed by HPLC purification, respectively. [11C]6 and [11C]9 were obtained in an average radiochemical yield of 37–44 ± 4% (n = 10, decay corrected) with ≥97% radiochemical and chemical purity. The total synthesis time was 50 ± 5 min end of bombardment and the specific activity were in the range of 0.5–1.2 Ci/μmol at time of injection. The lipophilicities of 6 and 9 (octanol/phosphate buffer partition) were measured. The log P7.4 values of [11C]6 and [11C]9 are 2.81 and 2.89, respectively, which are in the optimal range (1.0–3.0) for compounds expected to enter the brain readily.
[11C]6 and [11C]9 were intravenously administered to Sprague–Dawley rats and brain images were acquired with a Siemens Inveon microPET/CT imaging system, respectively (Figure 2). The regional uptake of [11C]6 and [11C]9 within the brain was observed in the choroid plexus and hippocampus, whereas retention in all other brain regions was low. The time-activity curves generated from imaging data showed that ratios of uptake in choroid plexus and hippocampus to that in cerebellum peaked at 1.8 and 1.2 for [11C]6, and 1.9 and 1.3 for [11C]9, respectively. Pretreating the rats with a dose of 0.1 mg/kg of SB-242084, a 5-HT2CR antagonist (Ki at 5-HT2CR = 3.6 nM), prior to injection resulted in a reduction of radioactivity uptake in choroid plexus and hippocampus to the same level as of the cerebellum, suggesting that the uptake of [11C]6 and [11C]9 in choroid plexus and hippocampus reflected specific binding.
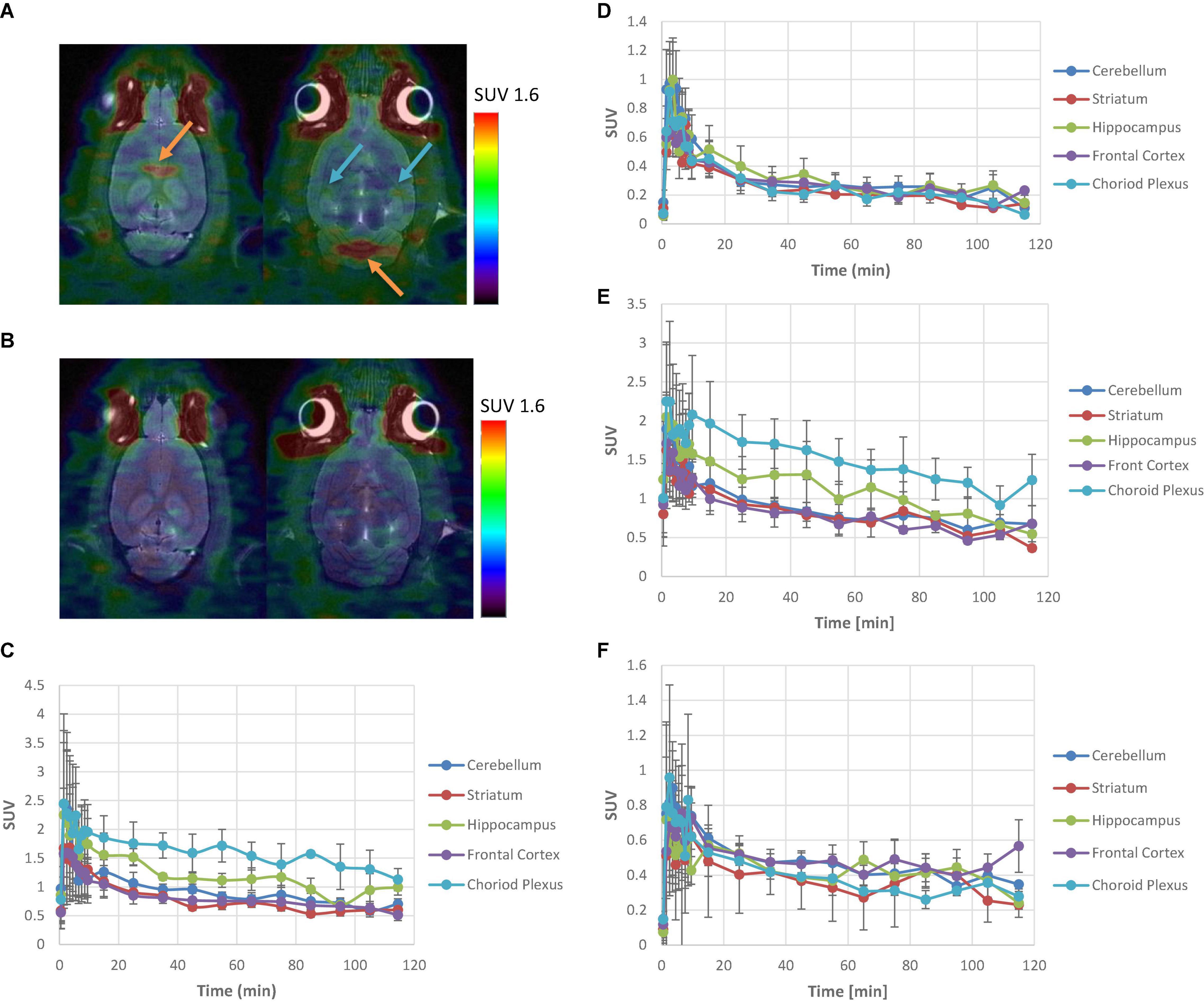
Figure 2. PET analysis of [11C]6 and [11C]9 in Sprague–Dawley rats. (A) Summed images of [11C]6 PET/CT data from 10 to 120 min p.i. at baseline. Orange arrows depict choroid plexus; Blue arrows depict hippocampus. (B) Summed images of [11C]6 PET/CT data from 10 to 120 min p.i. after pretreatment with 0.1 mg/kg of SB-242084. (C) Mean (n = 3) time-activity curves for brain regions of interest of [11C]6 at baseline with standard error. (D) Mean (n = 3) time-activity curves for brain regions of interest of [11C]6 after pretreatment with 0.1 mg/kg of SB-242084 with standard error. (E) Mean (n = 3) time-activity curves for brain regions of interest of [11C]9 at baseline with standard error. (F) Mean (n = 3) time-activity curves for brain regions of interest of [11C]9 after pretreatment with 0.1 mg/kg of SB-242084 with standard error.
[11C]6 and [11C]9 were further evaluated in rhesus monkeys using a Siemens MicroPET Focus 220 scanner (Figure 3). Contrary to the relatively low uptakes in rat brain, [11C]6 and [11C]9 exhibited excellent brain blood barrier penetration in the monkey. The regional uptake of [11C]6 showed high uptake in the choroid plexus and hippocampus with low uptake in the cerebellum. The corresponding time-activity curves (TACs) of [11C]6 showed that the radioactivity uptake in the choroid plexus peaked between 20–30 min after injection with a mean SUV value of 5.6, and the peak uptakes in the hippocampus, amygdala, frontal cortex, and cerebellum were achieved at 9.5–18.5 min postinjection. Ratios of uptake in choroid plexus, hippocampus, amygdala, frontal cortex to that in cerebellum peaked at 2.7, 1.6, 1.3, and 1.1, respectively.
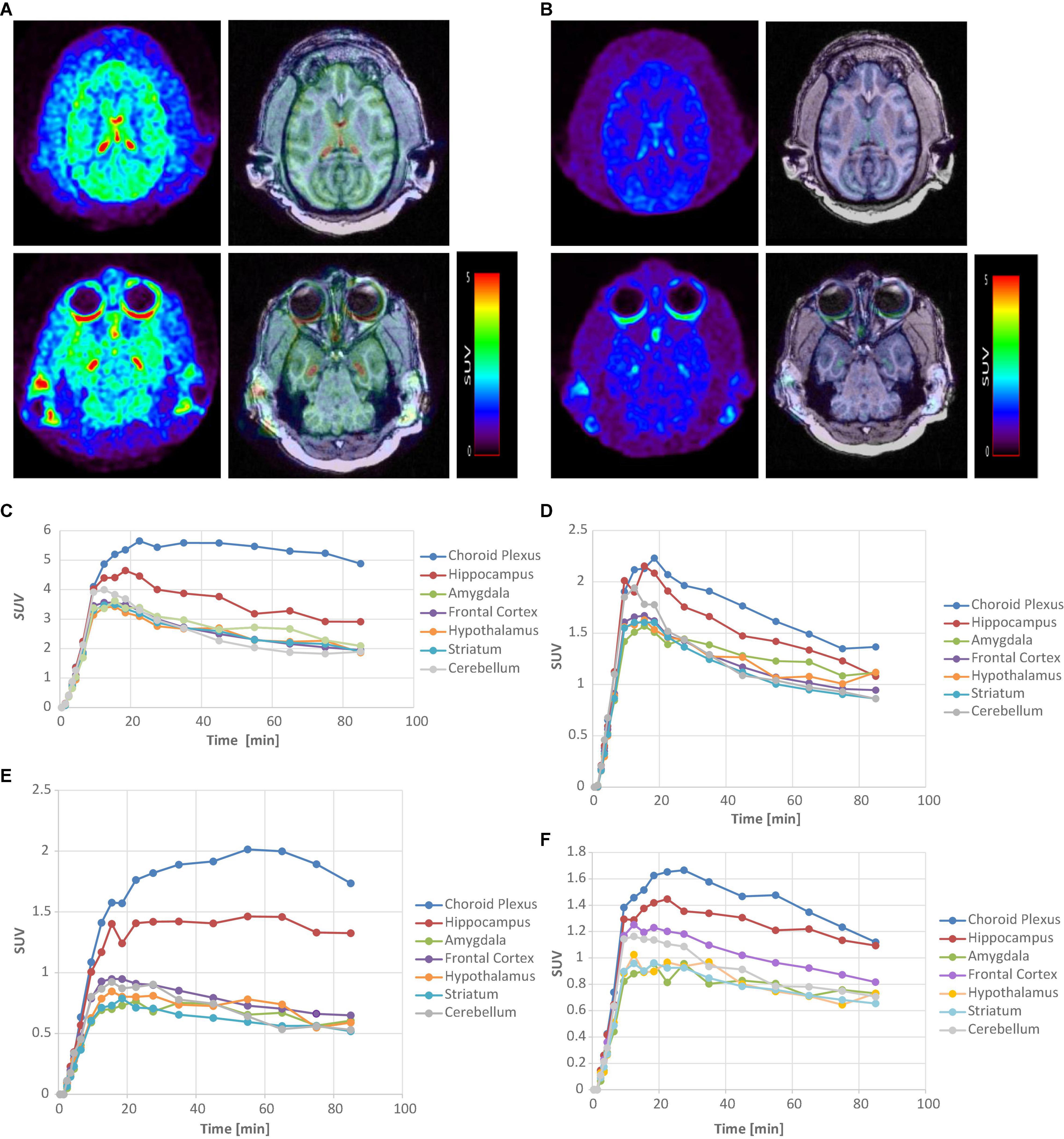
Figure 3. PET-MRI analysis of [11C]6 and [11C]9 in rhesus monkey. (A) MicroPET images of [11C]6 (left) and corresponding fused PET/MRI images summed over all time at baseline. (B) MicroPET images [11C]6 (left) and corresponding fused PET/MRI images summed over all time after pretreatment with 0.1 mg/kg of SB-242084. (C) Time-activity curves of [11C]6 for brain regions of interest at baseline study. (D) Time-activity curves of [11C]6 for brain regions after pretreatment with SB-242084. (E) Time-activity curves of [11C]9 for brain regions of interest at baseline study. (F) Time-activity curves of [11C]9 for brain regions after pretreatment with SB-242084.
Similarly, [11C]9 showed high uptake in the choroid plexus and hippocampus, with low uptake in the cerebellum in the baseline study. The TACs of [11C]9 indicated that the peak uptake of radioactivity in the choroid plexus and hippocampus were achieved 25–55 min after injection, and slowly washed out during the 90 min-course of PET study. The slower binding kinetics exhibited by [11C]9 in comparison to [11C]6 may reflect a higher binding affinity of [11C]9 to the 5-HT2CR. Although [11C]9 showed lower uptakes in the choroid plexus and hippocampus than [11C]6, with SUV values of 2.01 and 1.46 vs. 5.6 and 4.6, respectively, the ratio of uptake in the choroid plexus and hippocampus compared to the cerebellum peaked higher than [11C]6 at 3.7 and 2.7, respectively.
To test specific binding of [11C]6 and [11C]9 to the choroid plexus and hippocampus, an in vivo microPET blocking study was performed with 0.1 mg/kg SB-242084 30 min prior to injection, respectively. Unfortunately, due to the unexpected low solubility of SB-242084 in the injection form (10% ethanol in saline), the administered SB-242084 in soluble form to the monkeys was likely much less than the target 0.1 mg/kg. As a result, the uptake of [11C]6 and [11C]9 in the choroid plexus and hippocampus was still present. However, a reduction in the uptake and faster washout from the choroid plexus and hippocampus was observed compared to the baseline studies shown in Figures 3D,F. Combined with the blocking study results on rats, these results strongly suggest that PET images acquired with [11C]6 and [11C]9 represent the specific binding to 5-HT2CR.
In summary, two 5-HT2C ligands, 7-chloro-2-[4-methoxy-3-[2-(4-methylpiperidin-1-yl)ethoxy]phenyl]isoindolin-1-one (6) and 7-iodo-2-[4-methoxy-3-[2-(4-methylpiperidin-1-yl)ethoxy]phenyl]isoindolin-1-one (9) have been synthesized, radiolabeled with carbon-11, and evaluated in rats and non-human primates. Competition binding assays demonstrated that (6) and (9) possess high 5-HT2C binding affinity and high selectivity over 5-HT2A and 5-HT2B. In vivo microPET imaging studies in rats showed that [11C]6 and [11C]9 display specific binding to 5-HT2CR in the choroid plexus and hippocampus. In vivo microPET imaging studies in monkeys demonstrated that [11C]6 and [11C]9 exhibited high brain uptake with specific binding to the choroid plexus and hippocampus. The high uptakes in the choroid plexus and hippocampus to cerebellar ratios in non-human primates strongly support the candidacy of [11C]6 and [11C]9 for further study as radioligands for in vivo quantitation of 5-HT2C sites by PET.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC), Emory University.
FZ and MG contributed to the design of the study. FZ performed the synthesis and radiolabelling. RV and JM contributed to the radiolabeling. JN performed the imaging process. All authors contributed to manuscript revision, read, and approved the submitted version.
This project was funded by a grant from the National Institute of Health (1R21MH108928).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Barnes, N. M., and Sharp, T. A. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. doi: 10.1016/s0028-3908(99)00010-6
Carhart-Harris, R. L., and Nutt, D. J. (2017). Serotonin and brain function: a tale of two receptors. J. Psychopharmacol. 31, 1091–1120. doi: 10.1177/0269881117725915
Clemett, D. A., Punhani, T., Duxon, M. S., Blackburn, T. P., and Fone, K. C. F. (2000). Immunohistochemical localization of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39, 123–132. doi: 10.1016/s0028-3908(99)00086-6
Coccaro, E. F., Siever, L. J., Klar, H. M., Maurer, G., Cochrane, K., Cooper, T. B., et al. (1989). Serotoninergic studies in patients with affective and personality disorders. Arch. Gen. Psychiatry 46, 587–599.
Finnema, S. J., Stepanov, V., Etrup, A., Nakao, R., Amini, N., Svedberg, M., et al. (2014). Characterization of [11C]Cimbi-36 as an agonist PET radioligand for the 5-HT2A and 5-HT2C receptors in the non-human primate brain. Neuroimage 84, 342–353. doi: 10.1016/j.neuroimage.2013.08.035
Hamprecht, D., Micheli, F., Tedesco, G., Checchia, A., Donati, D., Petrone, M., et al. (2007). Isoindolone derivatives, a new class of 5-HT2C antagonists: synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 17, 428–433. doi: 10.1016/j.bmcl.2006.10.029
Heisler, L. K., Zhou, L., Bajwa, P., Hsu, J., and Tecott, L. H. (2007). Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 6, 491–496. doi: 10.1111/j.1601-183x.2007.00316.x
Hoyer, D., Pazos, A., Probst, A., and Palacios, J. M. (1986). Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 376, 97–107. doi: 10.1016/0006-8993(86)90903-0
Jacobs, B. L., and Azmitia, E. C. (1992). Structure and Function of the Brain Serotonin System. Physiol. Rev. 72, 165–229. doi: 10.1152/physrev.1992.72.1.165
Jensen, N. H., Cremers, T. I., and Sotty, F. (2010). Therapeutic potential of 5-HT2C receptor ligands. Sci. World J. 10, 1870–1885.
Kim, J., Moon, B. S., Lee, B. C., Lee, H., Kim, H., Choo, H., et al. (2017). A potential PET radiotracer for the 5-HT2C receptors: synthesis and in Vivo evaluation of 4-(3-[18F]fluorophenethoxy)pyrimidine. ACS Chem. Neuosci. 8, 996–1003.
Messing, R. B., and Lytle, L. D. (1977). Serotonin-containing neurons: their possible role in pain and analgesia. Pain 21, 1–21. doi: 10.1016/0304-3959(77)90083-5
Millan, M. J. (2005). Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie 60, 441–460. doi: 10.2515/therapie:2005065
Naughton, M., Mulrooney, J. B., and Leonard, B. E. (2000). A review of the role of serotonin receptors in psychiatric disorders. Hum. Psychopharmacol. 15, 397–415. doi: 10.1002/1099-1077(200008)15:6<397::aid-hup212>3.0.co;2-l
Neelamegam, R., Hellenbrand, T., Schroeder, F. A., Wang, C., and Hooker, J. M. (2014). Imaging evaluation of 5HT2C agonists, [11C]WAY-163909 and [11C]Vabicaserin, formed by Pictet-Spengler cyclization. J. Med. Chem. 57, 1488–1494.
Rosenzweig-Lipson, S., Dunlop, J., and Marquis, K. L. (2007). 5-HT2C Receptor agonists as an innovative approach for psychiatric disorders. Drug News Perspect. 20, 565–571. doi: 10.1358/dnp.2007.20.9.1162244
Stockmeier, C. A. (2003). Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 37, 357–373.
Keywords: PET radioligand, 5-HT2C receptor, in vivo, brain imaging, carbon-11
Citation: Zeng F, Nye JA, Voll RJ, Mun J and Goodman MM (2021) Synthesis and Evaluation of [11 C]7-Halogen-2-Phenyl Isoindolone Derivatives: Potential PET Radioligands for in vivo Imaging of 5-HT2C Receptors. Front. Neurosci. 15:766320. doi: 10.3389/fnins.2021.766320
Received: 28 August 2021; Accepted: 03 November 2021;
Published: 26 November 2021.
Edited by:
Neil Vasdev, Center for Addiction and Mental Health (CAMH), CanadaReviewed by:
Luc Maroteaux, INSERM U839 Institut du Fer à Moulin (IFM), FranceCopyright © 2021 Zeng, Nye, Voll, Mun and Goodman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanxing Zeng, ZnplbmdAZW1vcnkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.