
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci., 25 October 2021
Sec. Neuropharmacology
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.757505
This article is part of the Research TopicUpdate and Future Perspectives in Aneurysmal Subarachnoid HemorrhageView all 6 articles
 Tao Liu1†
Tao Liu1† Shiyu Zhong1†
Shiyu Zhong1† Qingqing Zhai2
Qingqing Zhai2 Xudong Zhang1
Xudong Zhang1 Huiquan Jing3
Huiquan Jing3 Kunhang Li1
Kunhang Li1 Shengyu Liu1
Shengyu Liu1 Shuo Han1
Shuo Han1 Lishuai Li1
Lishuai Li1 Xin Shi4,5*
Xin Shi4,5* Yijun Bao1*
Yijun Bao1*Statins are used in clinical practice to prevent from complications such as cerebral vasospasm (CVS) after aneurysmal subarachnoid hemorrhage (aSAH). However, the efficacy and safety of statins are still controversial due to insufficient evidence from randomized controlled trials and inconsistent results of the existing studies. This meta-analysis aimed to systematically review the latest evidence on the time window and complications of statins in aSAH. The randomized controlled trials in the databases of The Cochrane Library, PubMed, Web of Science, Embase, CNKI, and Wanfang from January 2005 to April 2021 were searched and analyzed systematically. Data analysis was performed using Stata version 16.0. The fixed-effects model (M-H method) with effect size risk ratio (RR) was used for subgroups with homogeneity, and the random-effects model (D-L method) with effect size odds ratio (OR) was used for subgroups with heterogeneity. The primary outcomes were poor neurological prognosis and all-cause mortality, and the secondary outcomes were cerebral vasospasm (CVS) and statin-related complications. This study was registered with PROSPERO (International Prospective Register of Systematic Reviews; CRD42021247376). Nine studies comprising 1,464 patients were included. The Jadad score of the patients was 5–7. Meta-analysis showed that poor neurological prognosis was reduced in patients who took oral statins for 14 days (RR, 0.73 [0.55–0.97]; I2 = 0%). Surprisingly, the continuous use of statins for 21 days had no significant effect on neurological prognosis (RR, 1.04 [0.89–1.23]; I2 = 17%). Statins reduced CVS (OR, 0.51 [0.36–0.71]; I2 = 0%) but increased bacteremia (OR, 1.38 [1.01–1.89]; I2 = 0%). In conclusion, a short treatment course of statins over 2 weeks may improve neurological prognosis. Statins were associated with reduced CVS. Based on the pathophysiological characteristics of CVS and the evaluation of prognosis, 2 weeks could be the optimal time window for statin treatment in aSAH, although bacteremia may increase.
The annual rate of aneurysmal subarachnoid hemorrhage (aSAH) is 10/100,000 person-years, accounting for Keywords: Statins, Aneurysmal, Subarachnoid Hemorrhage, Outcome, complications, Meta-analysis 4–5% of all strokes, with high mortality and disability rates (Andersen et al., 2019). The incidence of aSAH accounts for ~80% of the cases of non-traumatic subarachnoid hemorrhage (which also includes arteriovenous malformation etc.) (D'Souza, 2015). Andersen et al. (2019) mentioned that patients who suffered from aSAH had a 1-year mortality of 50%, and nearly half of the surviving patients lived with cognitive and functional limitations. Even with early aneurysm clipping or interventional therapy, the 1-year mortality with a Glasgow Coma Scale (GCS) of 3–5 was 65.8% (Lashkarivand et al., 2020). In China, the cumulative 12-month mortality with aSAH was reported to be 24.6%, and aSAH accounted for ~77.4% of all causes of SAH (Bian et al., 2012). Therefore, it is crucial for neurologists to employ a safe and reliable pharmacological therapy for patients with aSAH.
The American Heart Association/American Stroke Association guidelines regarding 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) for aSAH are inconclusive, and potential values of statins in aSAH should still be explored (Connolly et al., 2012; Juif et al., 2020). The results of many trials are still controversial owing to the insufficient evidence from randomized controlled trials (RCTs) and the inconsistent results of the existing studies (Liu et al., 2013; Lei et al., 2014; Lizza et al., 2014; Sikora Newsome et al., 2015; Akhigbe et al., 2017; Shen et al., 2017). In addition, some complications have been reported in patients with aSAH during statin therapy, such as bacteremia, epilepsy, elevated transaminase, and rhabdomyolysis (Magulick et al., 2014; Guo et al., 2015; Sikora Newsome et al., 2015; Berent et al., 2019; Nikolic et al., 2020). These complications might affect outcomes in patients with aSAH treated with statins. In previous studies, the usual treatment duration was 2 or 3 weeks. Statins are believed to relieve cerebral vasospasm (CVS) (Siasios et al., 2013; Lizza et al., 2014; Sikora Newsome et al., 2015). The high-risk period of CVS is 3–14 days after aSAH, with a peak at 6–8 days, and symptomatic CVS usually disappears after 12 days of aSAH (De Backer, 2016; Berent et al., 2019). Therefore, 2 weeks could be a more reasonable treatment duration. Following a literature update, the RCTs of statins were selected for systematic review and meta-analysis, including recently published trials on the efficacy and safety of statins in aSAH.
In this study, a systematic review and meta-analysis of RCTs of statins for treating aSAH was performed, and the efficacy and safety of statins treatment were evaluated. Based on the pathological characteristics of CVS and possible complications, we hypothesized that a short-course administration of statins might be more reasonable than a longer course.
We carried out a systematic literature search of articles comparing statins with placebo in patients with aSAH using the keywords [“Statin” and “aneurysmal subarachnoid hemorrhage”] and their synonyms from January 2005 to April 2021 through The Cochrane Library, PubMed, Web of Science, Embase, China National Knowledge Internet (CNKI), and Wanfang. The records were systematically evaluated using the inclusion and exclusion criteria (see below). In the initial search, the discrepancies were resolved by two researchers (TL and SZ). The search strategy is presented in Supplementary Table 1.
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the literature search strategy is presented in Figure 1 (Moher et al., 2009). This study was registered with PROSPERO (CRD42021247376).
The selected studies met the following inclusion criteria: the study type was RCT; the study subjects met diagnostic criteria for aSAH; articles in Chinese or English; statins used as treatment modality and compared with placebo. Exclusion criteria were duplicate publication, animal studies, unavailable full text, and no clear outcome or efficacy evaluation criteria.
Two independent researchers (YB and XS) performed the data extraction and quality assessment using a standardized extraction method, including authors, study design, intervention duration, year of publication, sample size, sex, outcome, and follow-up time.
The primary outcomes were poor neurological prognosis and all-cause mortality. Poor neurological prognosis was defined as a modified Rankin scale (mRS) score of 3–6 or a Glasgow Outcome Score (GOS) of 1–3 (Jennett and Bond, 1975; van Swieten et al., 1988). The secondary outcomes were CVS and statin-related complications, such as bacteremia, epilepsy, elevated transaminase, and rhabdomyolysis.
Stata 16.0 (StataCorp LP) and RevMan version 5.3 software (Cochrane Collaboration) were used for literature analysis. An inconsistent index (I2) test, which ranges from 0 to 100%, was used to assess homogeneity among the studies, and both P ≥ 0.1 and I2 ≤ 50% were considered to indicate no heterogeneity.
If the studies were homogeneous, the fixed-effects model (M-H method) with the effect size risk ratio (RR, 95% CI) was used. If the homogeneity condition was not met, a funnel plot, Labbé plot, and sensitivity analysis were used to determine the heterogeneity. Moreover, relevant literature with an excessive impact on model stability was excluded and re-analyzed. If a significant heterogeneity was observed among the studies without distinct reasons, the statistical analysis was performed using the random-effects model (D-L method) with the effect size odds ratio (OR, 95% CI). The chi-square test was used to analyze the results of each study, and differences were considered statistically significant when P ≤ 0.05.
According to the retrieval strategy, 230 articles in English and 87 in Chinese were collected from the databases (Figure 1). After eliminating duplicate articles, those with irrelevant prognosis indicators, reviews, meeting abstracts, and articles describing animal experiments, 14 articles remained. After assessing the 14 full texts for eligibility, we excluded five publications because of lack of controls (one paper) or because they were not RCT studies (four papers). Finally, nine articles were included (Lynch et al., 2005; Tseng et al., 2006; Chou et al., 2008; Vergouwen et al., 2009; Garg et al., 2013; Kirkpatrick et al., 2014; Diringer et al., 2016; Naraoka et al., 2018; Chen et al., 2020). The baseline characteristics of the included trials are summarized in Table 1.
Considering the two different treatment courses (14 and 21 days), a subgroup analysis was conducted (Figure 2). Since the heterogeneity was not significant in either group (I2 = 0.0%, P = 0.84; I2 = 17%, P = 0.30), a fixed-effects model (M-H method) was used for the analysis. The results showed that taking statins continuously for 14 days reduced the incidence of poor neurological prognosis (RR, 0.73 [0.55–0.97]; I2 = 0%; Figure 2A). The treatment course of 21 days showed no statistically significant effect on neurological prognosis (RR, 1.04 [0.89–1.23]; I2 = 17%; Figure 2B). All-cause mortality was evaluated in six RCTs (Chou et al., 2008; Vergouwen et al., 2009; Garg et al., 2013; Kirkpatrick et al., 2014; Diringer et al., 2016; Chen et al., 2020). Pooled analysis found that statins had no effect on mortality (RR, 0.98 [0.68–1.41]; I2 = 0%; Figure 2C).

Figure 2. Forest plot comparing the poor neurological prognosis for the course of 14 (A) and 21 days (B) respectively and all-cause mortality (C) between statin and placebo groups.
Seven publications reported CVS and statin-related complications, such as elevated transaminase, bacteremia, rhabdomyolysis, and epilepsy (Lynch et al., 2005; Tseng et al., 2006; Chou et al., 2008; Vergouwen et al., 2009; Kirkpatrick et al., 2014; Naraoka et al., 2018; Chen et al., 2020). This meta-analysis indicated that complications were not significantly different between the statin and control groups (OR, 0.78 [0.54–1.13]; I2 = 47%; Figure 3), but there was a relatively high heterogeneity. Subgroup analysis revealed a statistically significant difference in the rate of CVS events, suggesting that statins might prevent the occurrence of CVS (OR, 0.51 [0.36–0.71]; I2 = 0%; Figure 3A). Statins were associated with increased bacteremia (OR, 1.38 [1.01–1.89]; I2 = 0%; Figure 3C). The risks for elevated transaminase (OR, 0.73 [0.40, 1.13]; I2 = 0%; Figure 3B), epilepsy (OR, 0.75 [0.29, 1.92]; Figure 3D), and rhabdomyolysis (OR, 4.21 [0.45–39.25]; I2 = 0%; Figure 3E) were similar in both the statin and placebo groups.
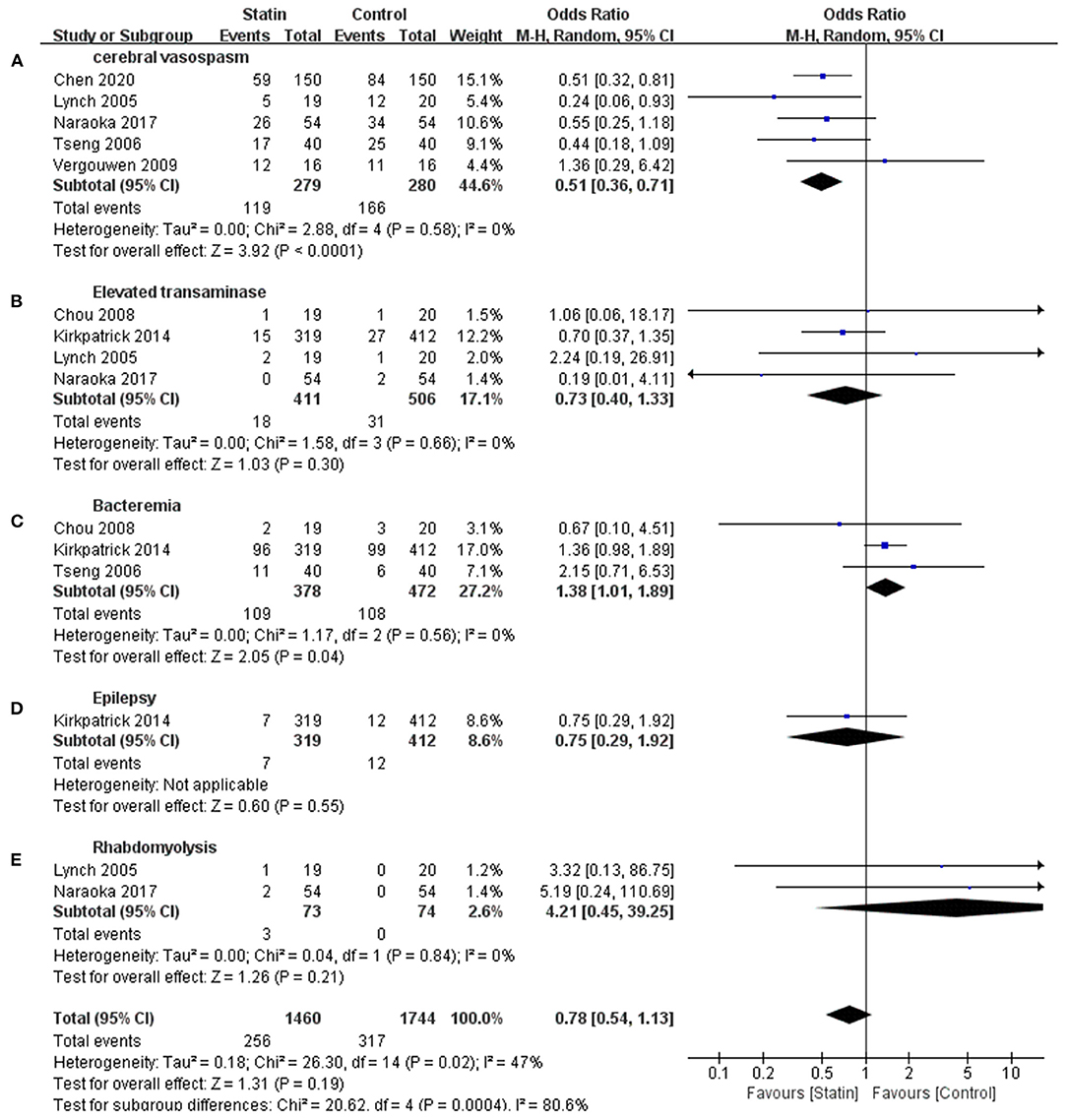
Figure 3. Meta-analysis of complications for statin vs. placebo. (A) Lower cerebral vasospasm rate in statin group was demonstrated. (C) Increased bacteremia was shown in statin group. (B,D,E) Complications events including elevated transaminase, rhabdomyolysis, and epilepsy were similar in both groups.
We used the Jadad score to quantitatively assess the quality of the included studies. Nine studies had a Jadad score of 5–7 points, indicating high quality (Table 2). Regression-based Harbord's and Egger's tests were not statistically significant for either primary or secondary outcomes (Table 3).
Nine studies in English were included in our research, and no literature from Chinese databases met the inclusion criteria. All patients were randomly assigned to the experimental and control groups, with the study population ranging from 25 to 803. We defined mRS ≤ 2 or GOS ≥ 4 as favorable prognosis, and a mRS score of 3–6 or GOS of 1–3 as poor prognosis. Our research aimed to evaluate the efficacy and safety of statins in patients with aSAH.
This meta-analysis indicated that a short treatment course of statins over a period of 2 weeks may improve the neurological prognosis and that statins had no favorable effect on neurological prognosis when administered continuously for 21 days. Statins were associated with reduced CVS but they also increased bacteremia.
Statins are the most commonly used lipid-lowering drugs (Lin et al., 2015). Previous studies have indicated that statins increase serum cholesterol clearance by competitively inhibiting endogenous cholesterol synthesis of the rate-limiting enzyme HMG-CoA reductase. Meanwhile, some trials have found that statins have a positive effect on the prognosis of aSAH (McGirt et al., 2002; Ferro et al., 2008).
It has been demonstrated that high lipid levels increase the risk of poor prognosis for aSAH, possibly because high lipid levels promote aneurysm wall inflammation and induce vascular smooth muscle cell death (Lindbohm et al., 2018). In general, vascular smooth muscle cells can repair damaged aneurysm walls and delay the progression of inflammation. Additionally, the effect of statins on prognosis may also be independent of the mechanism of lowering lipid levels (Tada et al., 2011; Rowland et al., 2012; Woo et al., 2015). Statins are known to inhibit the migration and proliferation of white blood cells into the blood vessels, activate cytokines, upregulate the expression and activity of endothelial nitric oxide synthase, improve endothelial reactivity, increase cerebral blood flow, and exert antioxidant effects. It is therefore not surprising that the synergistic effects of multiple mechanisms enhance the prognosis of patients with aSAH (Kotlega et al., 2015; Arati et al., 2019).
Considering the two different treatment courses (14 and 21 days), subgroup analysis indicated that a short-course treatment (14 days) had a positive effect on neurological outcomes. Surprisingly, long-course treatment (21 days) of statins did not improve the prognosis. This finding needs further clarification with a larger sample of RCTs. However, more attention should be paid to the course of statin treatment for aSAH.
In terms of safety, our study showed no statistically significant difference in mortality after statin intervention. Shen et al. suggested that statins were not associated with mortality in patients with aSAH and that this condition might be exacerbated by risk factors other than CVS, such as ischemic cerebral infarction (Shen et al., 2019). Another study suggested that statins might be associated with the risk of hemorrhagic stroke (Shen et al., 2017).
CVS is a common complication in patients with aSAH that occurs 4–14 days after bleeding (Smetana et al., 2020). It can lead to severe cerebral infarction and is the leading cause of disability and death in patients, with an incidence of 50–70% (Weir et al., 1978). Shen et al. suggested that statins might prevent CVS, and our results confirmed this conclusion (Shen et al., 2019). Statins can inhibit inflammatory chemokine expression, leukocyte migration, adhesion, and monocyte/macrophage proliferation and facilitate T-helper 2 cell polarization and regulatory T-cell expansion, leading to an increase in gram-negative bacilli infection. Additionally, statins can also alter the metabolic rate of certain antibiotics (i.e., azole antifungals), indirectly causing an increase in bacteremia (Seo et al., 2015). In our study, statins were found to increase bacteremia, but not the level of transaminase or the severity of epilepsy or rhabdomyolysis. Although the incidence of bacteremia increased, statins improved the overall prognosis. These results indicated that the benefit from a 2-week treatment of statin outweighed the detrimental effects. In a retrospective study, statins were found to be associated with an increased risk of pneumonia and other infections (Magulick et al., 2014). A slight increase in bacteremia was found on day 21 of statin treatment compared with day 14, although the statistical difference was not significant (29 vs. 27.5%, P = 0.844).
Statins are hepatotoxic, causing elevated transaminase levels in ~3% of patients with aSAH, but they rarely cause clinical symptoms (Russo et al., 2014; Benes et al., 2016). In our study, elevated transaminase levels in patients were not statistically significant due to the limited sample size. Rhabdomyolysis is a rare complication associated with statins, and its mechanism is still unclear (Simons et al., 2015). Rhabdomyolysis was reported in only two trials included in our study, and the insufficient sample size might be the reason for the lack of significant differences or a strong conclusion in this regard.
Statins differ in their pharmacological characteristics. For example, pravastatin is hydrophilic and has a half-life of 1–3 h, which is significantly shorter than that of atorvastatin and simvastatin. Thus, it hardly crosses the blood-brain barrier. Atorvastatin and simvastatin are lipophilic drugs with significant side effects, including muscle symptoms. Therefore, there might be differences in clinical outcomes among several statins (Sirtori, 2014). In our meta-analysis, only one study focused on epilepsy, and the risk of epilepsy was similar in both the statin and placebo groups (Kirkpatrick et al., 2014). In the included studies using a lower dose of simvastatin (40 mg/day) epilepsy was not observed, while Lin et al. indicated that a high-dose treatment of statins such as atorvastatin and rosuvastatin leads to epilepsy (Lin et al., 2018). Further investigations of epilepsy in patients on statin treatment are warranted in the future.
This meta-analysis had several strengths. First, we searched different databases to identify relevant RCTs. Further, our study provided a comprehensive overview of the efficacy and safety of statin administration in aSAH subjects. Moreover, by analyzing the data of all the included RCTs on poor neurological prognosis, all-cause mortality, and statin-related complications such as CVS, bacteremia, epilepsy, elevated transaminase, and rhabdomyolysis, we further confirmed that the use of statins is related to the incidence of CVS and bacteremia.
Nevertheless, our study has some limitations. Our broad inclusion criteria led to a heterogeneous population in terms of the length of statin treatment, intervention dose, and disease severity, which might have led to potential bias in the evaluation of the efficacy and safety of statins. In addition, the sample size of some studies was small. There might be an overrepresentation of recently published papers by Chen et al. since it represented nearly a fifth of the included population in our meta-analysis (Chen et al., 2020). Furthermore, because different prognostic indicators (mRS and GOS) were used, there was some uncertainty regarding the estimates. Finally, there was high heterogeneity in the integrated analysis of complications due to insufficient monitoring of complications in various studies.
This meta-analysis indicated that a short treatment course of statins with a duration of 2 weeks may improve neurological prognosis. Statins were associated with reduced CVS. Although statins increased bacteremia, 2 weeks could be the optimal time window for statin treatment in aSAH according to the pathophysiological characteristics of CVS and the evaluation of prognosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. TL, SZ, XZ, HJ, and YB concept and design. TL, SZ, YB, and QZ acquisition, analysis, interpretation of data, and statistical analysis. TL, SZ, KL, SH, LL, QZ, XS, and YB critical revision of the manuscript for important intellectual content. QZ, XS, and YB supervision. All authors contributed to the article and approved the submitted version.
This project was sponsored by Liaoning Provincial Natural Science Foundation (2020-MS-155), China Medical University novel coronavirus pneumonia prevention and control research project (No. 2020-12-11), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2013-1792), and National Science Foundation of China (72074104). The researchers are grateful for the support of several organizations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.757505/full#supplementary-material
Akhigbe, T., Zolnourian, A., and Bulters, D. (2017). Cholesterol-reducing agents for treatment of aneurysmal subarachnoid hemorrhage: systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 101, 476–485. doi: 10.1016/j.wneu.2017.01.125
Andersen, C. R., Fitzgerald, E., Delaney, A., and Finfer, S. (2019). A systematic review of outcome measures employed in aneurysmal subarachnoid hemorrhage (aSAH) clinical research. Neurocrit. Care 30, 534–541. doi: 10.1007/s12028-018-0566-0
Arati, S., Chetan, G. K., Sibin, M. K., Bhat, D. I., Vazhayil, V., and Narasingarao, K. V. L. (2019). Prognostic significance of factor XIIIA promoter methylation status in aneurysmal subarachnoid haemorrhage (aSAH). BMC Cardiovasc. Disord. 19:170. doi: 10.1186/s12872-019-1146-8
Benes, L. B., Bassi, N. S., and Davidson, M. H. (2016). The risk of hepatotoxicity, new onset diabetes and rhabdomyolysis in the era of high-intensity statin therapy: does statin type matter? Prog. Cardiovasc. Dis. 59, 145–152. doi: 10.1016/j.pcad.2016.08.001
Berent, T., Berent, R., Steiner, S., and Sinzinger, H. (2019). Statin-induced muscular side effects at rest and exercise - An anatomical mapping. Atheroscler. Suppl. 40, 73–78. doi: 10.1016/j.atherosclerosissup.2019.08.026
Bian, L. H., Liu, Y. F., Nichols, L. T., Wang, C. X., Wang, Y. L., Liu, G. F., et al. (2012). Epidemiology of subarachnoid hemorrhage, patterns of management, and outcomes in China: a hospital-based multicenter prospective study. CNS Neurosci. Ther. 18, 895–902. doi: 10.1111/cns.12001
Chen, J., Li, M., Zhu, X., Chen, L., Yang, S., Zhang, C., et al. (2020). Atorvastatin reduces cerebral vasospasm and infarction after aneurysmal subarachnoid hemorrhage in elderly Chinese adults. Aging 12, 2939–2951. doi: 10.18632/aging.102788
Chou, S. H., Smith, E. E., Badjatia, N., Nogueira, R. G., Sims, J. R., Ogilvy, C. S., et al. (2008). A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke 39, 2891–2893. doi: 10.1161/strokeaha.107.505875
Connolly, E. S. Jr., Rabinstein, A. A., Carhuapoma, J. R., Derdeyn, C. P., Dion, J., Higashida, R. T., et al. (2012). Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43, 1711–1737. doi: 10.1161/STR.0b013e3182587839
De Backer, A. (2016). Handbook of neurosurgery, 8th edition. Acta Chir. Belg. 116:269. doi: 10.1080/00015458.2016.1229422
Diringer, M. N., Dhar, R., Scalfani, M., Zazulia, A. R., Chicoine, M., Powers, W. J., et al. (2016). Effect of high-dose simvastatin on cerebral blood flow and static autoregulation in subarachnoid hemorrhage. Neurocrit. Care 25, 56–63. doi: 10.1007/s12028-015-0233-7
D'Souza, S. (2015). Aneurysmal subarachnoid hemorrhage. J. Neurosurg. Anesthesiol. 27, 222–240. doi: 10.1097/ana.0000000000000130
Ferro, J. M., Canhão, P., and Peralta, R. (2008). Update on subarachnoid haemorrhage. J. Neurol. 255, 465–479. doi: 10.1007/s00415-008-0606-3
Garg, K., Sinha, S., Kale, S. S., Chandra, P. S., Suri, A., Singh, M. M., et al. (2013). Role of simvastatin in prevention of vasospasm and improving functional outcome after aneurysmal sub-arachnoid hemorrhage: a prospective, randomized, double-blind, placebo-controlled pilot trial. Br. J. Neurosurg. 27, 181–186. doi: 10.3109/02688697.2012.757293
Guo, J., Guo, J., Li, J., Zhou, M., Qin, F., Zhang, S., et al. (2015). Statin treatment reduces the risk of poststroke seizures. Neurology 85, 701–707. doi: 10.1212/wnl.0000000000001814
Jennett, B., and Bond, M. (1975). Assessment of outcome after severe brain damage. Lancet 1, 480–484. doi: 10.1016/s0140-6736(75)92830-5
Juif, P. E., Dingemanse, J., and Ufer, M. (2020). Clinical pharmacology of clazosentan, a selective endothelin a receptor antagonist for the prevention and treatment of aSAH-related cerebral vasospasm. Front. Pharmacol. 11:628956. doi: 10.3389/fphar.2020.628956
Kirkpatrick, P. J., Turner, C. L., Smith, C., Hutchinson, P. J., and Murray, G. D. (2014). Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 13, 666–675. doi: 10.1016/s1474-4422(14)70084-5
Kotlega, D., Gołab-Janowska, M., Masztalewicz, M., Ciećwiez, S., and Nowacki, P. (2015). Potential role of statins in the intracerebral hemorrhage and subarachnoid hemorrhage. Neurol. Neurochir. Pol. 49, 322–328. doi: 10.1016/j.pjnns.2015.07.007
Lashkarivand, A., Sorteberg, W., Rosseland, L. A., and Sorteberg, A. (2020). Survival and outcome in patients with aneurysmal subarachnoid hemorrhage in Glasgow coma score 3-5. Acta Neurochir. 162, 533–544. doi: 10.1007/s00701-019-04190-y
Lei, C., Wu, B., Liu, M., and Chen, Y. (2014). Association between statin use and intracerebral hemorrhage: a systematic review and meta-analysis. Eur. J. Neurol. 21, 192–198. doi: 10.1111/ene.12273
Lin, H. W., Ho, Y. F., and Lin, F. J. (2018). Statin use associated with lower risk of epilepsy after intracranial haemorrhage: A population-based cohort study. Br. J. Clin. Pharmacol. 84, 1970–1979. doi: 10.1111/bcp.13626
Lin, S. H., Huang, K. J., Weng, C. F., and Shiuan, D. (2015). Exploration of natural product ingredients as inhibitors of human HMG-CoA reductase through structure-based virtual screening. Drug Des. Devel. Ther. 9, 3313–3324. doi: 10.2147/dddt.S84641
Lindbohm, J., Korja, M., Jousilahti, P., Salomaa, V., and Kaprio, J. (2018). Adverse lipid profile elevates risk for subarachnoid hemorrhage: A prospective population-based cohort study. Atherosclerosis 274, 112–119. doi: 10.1016/j.atherosclerosis.2018.05.011
Liu, Z., Liu, L., Zhang, Z., Chen, Z., and Zhao, B. (2013). Cholesterol-reducing agents for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 4:Cd008184. doi: 10.1002/14651858.CD008184.pub2
Lizza, B. D., Kosteva, A., Maas, M. B., Rosenberg, N. F., Liotta, E., Guth, J., et al. (2014). Preadmission statin use does not improve functional outcomes or prevent delayed ischemic events in patients with spontaneous subarachnoid hemorrhage. Pharmacotherapy 34, 811–817. doi: 10.1002/phar.1436
Lynch, J. R., Wang, H., McGirt, M. J., Floyd, J., Friedman, A. H., Coon, A. L., et al. (2005). Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke 36, 2024–2026. doi: 10.1161/01.Str.0000177879.11607.10
Magulick, J. P., Frei, C. R., Ali, S. K., Mortensen, E. M., Pugh, M. J., Oramasionwu, C. U., et al. (2014). The effect of statin therapy on the incidence of infections: a retrospective cohort analysis. Am. J. Med. Sci. 347, 211–216. doi: 10.1097/MAJ.0b013e31828318e2
McGirt, M. J., Lynch, J. R., Parra, A., Sheng, H., Pearlstein, R. D., Laskowitz, D. T., et al. (2002). Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke 33, 2950–2956. doi: 10.1161/01.str.0000038986.68044.39
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Naraoka, M., Matsuda, N., Shimamura, N., Asano, K., Akasaka, K., Takemura, A., et al. (2018). Long-acting statin for aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled trial. J. Cereb. Blood Flow Metab. 38, 1190–1198. doi: 10.1177/0271678x17724682
Nikolic, D., Banach, M., Chianetta, R., Luzzu, L. M., Pantea Stoian, A., Diaconu, C. C., et al. (2020). An overview of statin-induced myopathy and perspectives for the future. Expert Opin. Drug Saf. 19, 601–615. doi: 10.1080/14740338.2020.1747431
Rowland, M. J., Hadjipavlou, G., Kelly, M., Westbrook, J., and Pattinson, K. T. (2012). Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br. J. Anaesth. 109, 315–329. doi: 10.1093/bja/aes264
Russo, M. W., Hoofnagle, J. H., Gu, J., Fontana, R. J., Barnhart, H., Kleiner, D. E., et al. (2014). Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology 60, 679–686. doi: 10.1002/hep.27157
Seo, S., Boeckh, M., Storer, B. E., Schubert, M. M., Rotta, M., Sandmaier, B. M., et al. (2015). The association between donor and recipient statin use and infections after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 50, 444–448. doi: 10.1038/bmt.2014.279
Shen, J., Huang, K. Y., Zhu, Y., Pan, J. W., Jiang, H., Weng, Y. X., et al. (2017). Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J. Neurosurg. 127, 291–301. doi: 10.3171/2016.5.Jns152900
Shen, J., Shen, J., Zhu, K., Zhou, H., Tian, H., and Yu, G. (2019). Efficacy of statins in cerebral vasospasm, mortality, and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 131, e65–e73. doi: 10.1016/j.wneu.2019.07.016
Siasios, I., Kapsalaki, E. Z., and Fountas, K. N. (2013). Cerebral vasospasm pharmacological treatment: an update. Neurol. Res. Int. 2013:571328. doi: 10.1155/2013/571328
Sikora Newsome, A., Casciere, B. C., Jordan, J. D., Rhoney, D. H., Sullivan, K. A., Morbitzer, K. A., et al. (2015). The role of statin therapy in hemorrhagic stroke. Pharmacotherapy 35, 1152–1163. doi: 10.1002/phar.1674
Simons, J. E., Holbrook, A. M., and Don-Wauchope, A. C. (2015). Successful reintroduction of statin therapy after statin-associated rhabdomyolysis. J. Clin. Lipidol. 9, 594–596. doi: 10.1016/j.jacl.2015.03.005
Sirtori, C. R. (2014). The pharmacology of statins. Pharmacol. Res. 88, 3–11. doi: 10.1016/j.phrs.2014.03.002
Smetana, K. S., Buschur, P. L., Owusu-Guha, J., and May, C. C. (2020). Pharmacologic management of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Crit. Care Nurs. Q. 43, 138–156. doi: 10.1097/cnq.0000000000000299
Tada, Y., Kitazato, K. T., Yagi, K., Shimada, K., Matsushita, N., Kinouchi, T., et al. (2011). Statins promote the growth of experimentally induced cerebral aneurysms in estrogen-deficient rats. Stroke 42, 2286–2293. doi: 10.1161/strokeaha.110.608034
Tseng, M. Y., Czosnyka, M., Richards, H., Pickard, J. D., and Kirkpatrick, P. J. (2006). Effects of acute treatment with statins on cerebral autoregulation in patients after aneurysmal subarachnoid hemorrhage. Neurosurg. Focus 21:E10. doi: 10.3171/foc.2006.21.3.10
van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J., and van Gijn, J. (1988). Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19, 604–607. doi: 10.1161/01.str.19.5.604
Vergouwen, M. D., Meijers, J. C., Geskus, R. B., Coert, B. A., Horn, J., Stroes, E. S., et al. (2009). Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J. Cereb. Blood Flow Metab. 29, 1444–1453. doi: 10.1038/jcbfm.2009.59
Weir, B., Grace, M., Hansen, J., and Rothberg, C. (1978). Time course of vasospasm in man. J. Neurosurg. 48, 173–178. doi: 10.3171/jns.1978.48.2.0173
Woo, S. W., Kim, J. H., Kang, H. I., Kim, D. R., Moon, B. G., and Kim, J. S. (2015). High-dose simvastatin is effective in preventing cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a prospective cohort study in Korean patients. J. Korean Neurosurg. Soc. 58, 328–333. doi: 10.3340/jkns.2015.58.4.328
Keywords: statins, aneurysmal, subarachnoid hemorrhage, outcome, complications, meta-analysis
Citation: Liu T, Zhong S, Zhai Q, Zhang X, Jing H, Li K, Liu S, Han S, Li L, Shi X and Bao Y (2021) Optimal Course of Statins for Patients With Aneurysmal Subarachnoid Hemorrhage: Is Longer Treatment Better? A Meta-Analysis of Randomized Controlled Trials. Front. Neurosci. 15:757505. doi: 10.3389/fnins.2021.757505
Received: 12 August 2021; Accepted: 20 September 2021;
Published: 25 October 2021.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Shafqat Rasul Chaudhry, Shifa Tameer-e-Millat University, PakistanCopyright © 2021 Liu, Zhong, Zhai, Zhang, Jing, Li, Liu, Han, Li, Shi and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijun Bao, eWpiYW9AY211LmVkdS5jbg==; Xin Shi, eC5zaGlAbW11LmFjLnVr
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.