- 1Department of Otorhinolaryngology, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Anesthesiology, Tianjin Jizhou People’s Hospital, Tianjin, China
- 3Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
- 4Department of Otorhinolaryngology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 5Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, China
- 6Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Tianjin Neurological Institute, Ministry of Education and Tianjin City, Tianjin, China
Hearing loss is a modifiable risk factor for dementia and cognitive decline. However, the association between cognition and hearing acuity at different frequencies is unknown. We aimed to assess the relationships between hearing acuity at different frequencies with global cognitive function and five domains of cognition among a low-income elderly population in northern rural China. A population-based cross-sectional study was conducted to collect basic information from elderly residents aged 60 years and older in rural areas of Tianjin, China from April 2012 to November 2013. Pure tone averages (PTAs) at different frequencies in the ear with better hearing and Mini-Mental State Examination (MMSE) scores were measured, and the relationships between these variables were assessed. A total of 737 residents aged 60 years or more were enrolled in this study, and the prevalence of hearing impairment was 60.7%. After adjusting for sex, age, education, income, smoking, drinking, systolic blood pressure (SBP), total cholesterol (TC), and low-density lipoprotein cholesterol level (LDL-C), MMSE score and immediate recall score were negatively associated with overall PTA (OPTA) at four frequencies (0.5, 1, 2, and 4 kHz), PTA at low frequencies (LPTA; 0.5, 1, and 2 kHz), and PTA at high frequencies (HPTA; 3, 4, and 8 kHz) in the ear with better hearing. Moreover, orientation score was negatively associated with OPTA and LPTA, and the attention and calculation scores were negatively associated with OPTA and HPTA. Each 10-dB increase in OPTA was associated with a MMSE score decrease of 0.464. Each 10-dB increase in LPTA or HPTA was associated with a MMSE score decrease of 0.441 (95% CI: −0.795, −0.086) and 0.351 (95% CI: −0.592, −0.110), respectively. The present study demonstrated significant but weak relationships between OPTA, LPTA, and HPTA with global cognitive function, as defined using MMSE scores; these relationships were independent of age, education, lifestyle factors, and laboratory test values. These results indicated that hearing was associated with cognitive decline among older individuals, who should be screened routinely to identify risk for cognitive decline.
Introduction
Cognitive impairment is the leading cause of disability and a global public health priority for aging populations (Wortmann, 2012; GBD 2017 DALYs and HALE Collaborators, 2018). The World Alzheimer Report estimated there were over 50 million people living with dementia globally, and this number was estimated to increase to more than 152 million by 2050 (Wimo et al., 2013). Moreover, most people with dementia live in developing countries, and the number of people living with dementia in China accounts for approximately 25% of total dementia population worldwide, posing a substantial economic and social burden (GBD 2016 Neurology Collaborators, 2019).
Hearing impairment is the third most prevalent chronic condition in older age (Yueh et al., 2003). According to the Global Burden of Disease study, there were 1.4 billion people living with hearing impairment in 2017, and approximately 90% of people with moderate to severe hearing impairment reside in developing countries (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Hearing impairment has also been recognized as the largest potentially modifiable risk factor for dementia and cognitive decline (Livingston et al., 2017; Loughrey et al., 2018). Numerous epidemiological studies have demonstrated hearing impairment at older age was associated with cognitive decline (Lin et al., 2013; Gurgel et al., 2014; Deal et al., 2017; Pabst et al., 2021; Saji et al., 2021). For example, the Health, Aging and Body Composition Study found that hearing impairment at baseline was related to a 24% increased risk of dementia over a 6-year period (Lin et al., 2013). Hearing loss at older age usually impacts high-frequency hearing long before low-frequency hearing (Panza et al., 2015). Thus, it is important to determine whether hearing loss at high frequencies is associated with impaired cognitive function. However, studies exploring significant association between hearing acuity and cognition have used the pure tone average (PTA) threshold at overall or low frequencies (Bush et al., 2015; Golub et al., 2020). To our knowledge, only one cross-sectional study enrolling 307 elderly demonstrated PTA at low frequencies, but not high frequencies, was related to cognitive performance among older individuals (Mukari et al., 2017). The association between PTA at different frequencies and cognitive function is not as well established.
Moreover, socioeconomic inequality is related to the risk of hearing loss (Emmett and Francis, 2015; Ping et al., 2018), and low income is associated with poor auditory function, with approximately 90% residents living in low- and middle-income countries having moderate to profound hearing impairment (Davis and Hoffman, 2019). There is a high burden of hearing impairment in northern China, where the prevalence of hearing impairment is 49.3% among the low-income rural population aged over 45 years (Yang et al., 2021). Only four studies in China have focused on the mediating role of social isolation, cognitive reserve, and leisure activities between self-reported hearing impairment and cognitive decline (Chen and Lu, 2019; Chen and Zhou, 2020; Gao et al., 2020; Chen, 2021). However, the relationship between cognitive performance and PTA at different frequencies as measured using standardized audiometric tests in an older population has not been reported in China, especially in low-income rural areas.
Thus, the aim of this study was to evaluate the relationships between cognitive function and hearing acuity at different frequencies among a low-income elderly population in northern rural China.
Materials and Methods
Study Population
This population-based, cross-sectional study recruited older individuals from 18 administrative villages in rural areas of Tianjin, China from April 2012 to November 2013 based on the Tianjin Brain Study (Wang et al., 2014; Hu et al., 2016). Owing to the national health policy, all residents aged 60 years and older visit the health center for free physical examinations annually. From this population, all older residents (≥ 60 years old) with previous diagnosis of total hearing loss (over 120 dB) and blindness (best-corrected distance visual acuity < 3/60 or visual field < 10 central degrees) in the better ear/eye were excluded (Martin, 1986; World Health Organization (WHO), 2020).
The study was approved by the ethics committee at the Tianjin Medical University General Hospital, and written informed consent was obtained from all participants.
Risk Factors and Physical Examinations
This study was conducted through face-to-face interviews by trained researchers. Demographic information (including name, sex, date of birth, income, and educational level), individual medical history (including the presence of hypertension, diabetes mellitus, stroke, and coronary heart disease), and lifestyle factors (including smoking, drinking, and exercise) were collected using a pre-designed questionnaire; data regarding exercise was missing for six individuals. The participants were categorized into three age groups (60–64, 65–69, and ≥ 70 years), three educational groups (0–5, 6–8, and ≥ 9 years), and three groups of annual per capita income (< 300 USD, 300–650 USD, and > 650 USD). Smoking was defined as smoking ≥ 1 cigarette daily for more than 1 year. Drinking was defined as drinking > 50 mL of alcohol at least once per week for more than 6 months.
Body height, weight, waist circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were obtained by local general practitioners with the participant wearing thin clothing. The levels of fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were tested in the central laboratory of the Tianjin Ji County People’s Hospital. Hypertension was defined as a SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, the use of antihypertensive drugs, or a history of hypertension. Diabetes was defined as a FBG ≥ 7.0 mmol/L, taking medication for diabetes, or a self-reported history of diabetes. Body mass index (BMI) was calculated as the individual’s weight (kg) divided by the square of the individual’s height (m2) and was classified into four categories (low-weight, < 18.5 kg/m2; normal, 18.5–23.9 kg/m2; overweight, 24.0–27.9 kg/m2; and obese, ≥ 28.0 kg/m2; Zhou, 2002).
Cognitive Impairment
Cognitive function was measured using the Chinese version of the Mini-Mental State Examination (MMSE) owing to its high sensitivity and specificity of screening for cognitive impairment (Li et al., 1989; Canadian Task Force on Preventive Health Care et al., 2016). The diagnostic criteria of cognitive impairment were based on MMSE score according to educational levels. The MMSE is a 30-point scale that assesses five different cognitive domains including orientation, immediate recall, attention and calculation, recall, and language. Cognitive impairment was defined as an MMSE score < 17 points in the illiterate group, < 22 points in the primary school group, and < 26 points in the junior school and above group (Nunes et al., 2010).
Hearing Test
Audiometric assessments in each ear were performed at seven frequencies (0.5, 1, 2, 3, 4, 6, and 8 kHz) in a quiet, soundproof room using the Denmark XETA Audiometer (Xeta EN60645-1 type:3 REF:8-04-12207 GN Otometrics A/S Hoerskaetten 92360 Taastrup DENMARK) and TDH 50P transducer (Telephonics, Huntington, NY). Audiometric thresholds were measured at 5-dB increments in decibels of hearing level (dB HL). Outcome variables reported in this study were overall PTA (OPTA) at four frequencies (0.5, 1, 2, and 4 kHz), PTA at low frequencies (LPTA; 0.5, 1, and 2 kHz), and PTA at high frequencies (HPTA; 3, 4, and 8 kHz) in the ear with better hearing. Hearing impairment was defined as OPTA > 25 dB of the better ear according to the World Health Organization’s definition of impairment [World Health Organization (WHO), 1997]. Participants suspected of having hearing impairment were referred to audiologists for final diagnoses.
Statistical Analysis
Continuous variables (age, BMI, waist circumference, SBP, DBP, FBG, TC, TG, LDL-C, HDL-C, and PTA) are described as means and standard deviations. Categorical variables (binary variables: sex, smoking, drinking, physical exercise, hypertension, diabetes, stroke, hearing impairment, and MMSE group; multi-categorical variables: age, education, income, and BMI groups) are presented as numbers with frequencies. The Student t-test was used to compare MMSE score differences between binary variables; the ANOVA test was used to compare MMSE score differences between multi-categorical variables. Univariate linear analyses were used to evaluate the relationship between each continuous variable and the MMSE score. Multiple linear regression analyses were used to evaluate the relationship between PTA and MMSE score after adjusting for independent variables that were statistically significant in the univariate analyses. The univariate analysis results are shown as unadjusted β-values and 95% confidence intervals (CIs); the multivariate analysis results are shown as adjusted β-values and 95% CIs after adjusting for covariates.
All statistical analyses were performed with SPSS version 19.0 statistical software (SPSS Inc., Chicago, IL, United States), and a two-sided P-value ≤ 0.05 was considered statistically significant.
Results
Demographic Characteristics
A total of 737 residents aged more than 60 years (mean age, 68.95 years) were enrolled in this study, including 324 men (44.0%; mean age, 69.65 years) and 413 women (56.0%; mean age, 68.39 years). In this rural population, the prevalence of hearing impairment was 60.7% overall, 64.2% in men, and 57.9% in women. The mean OPTA of all residents was 30.27 dB HL, with 28.99 dB in LPTA, and 35.44 dB in HPTA. The average education level of the participants was low: 41.7% were illiterate. Moreover, 733 (99.5%) participants had annual per capita incomes of < 650 USD (Table 1).
Factors Associated With MMSE Score in the Univariate Analysis
The MMSE score was higher among participants with female sex, older age, high education, high income, smoking, and drinking, compared with other groups (all, P < 0.001). Compared with individuals with normal hearing, MMSE score of those with hearing impairment did not approached statistical significance (P = 0.872; Table 2).
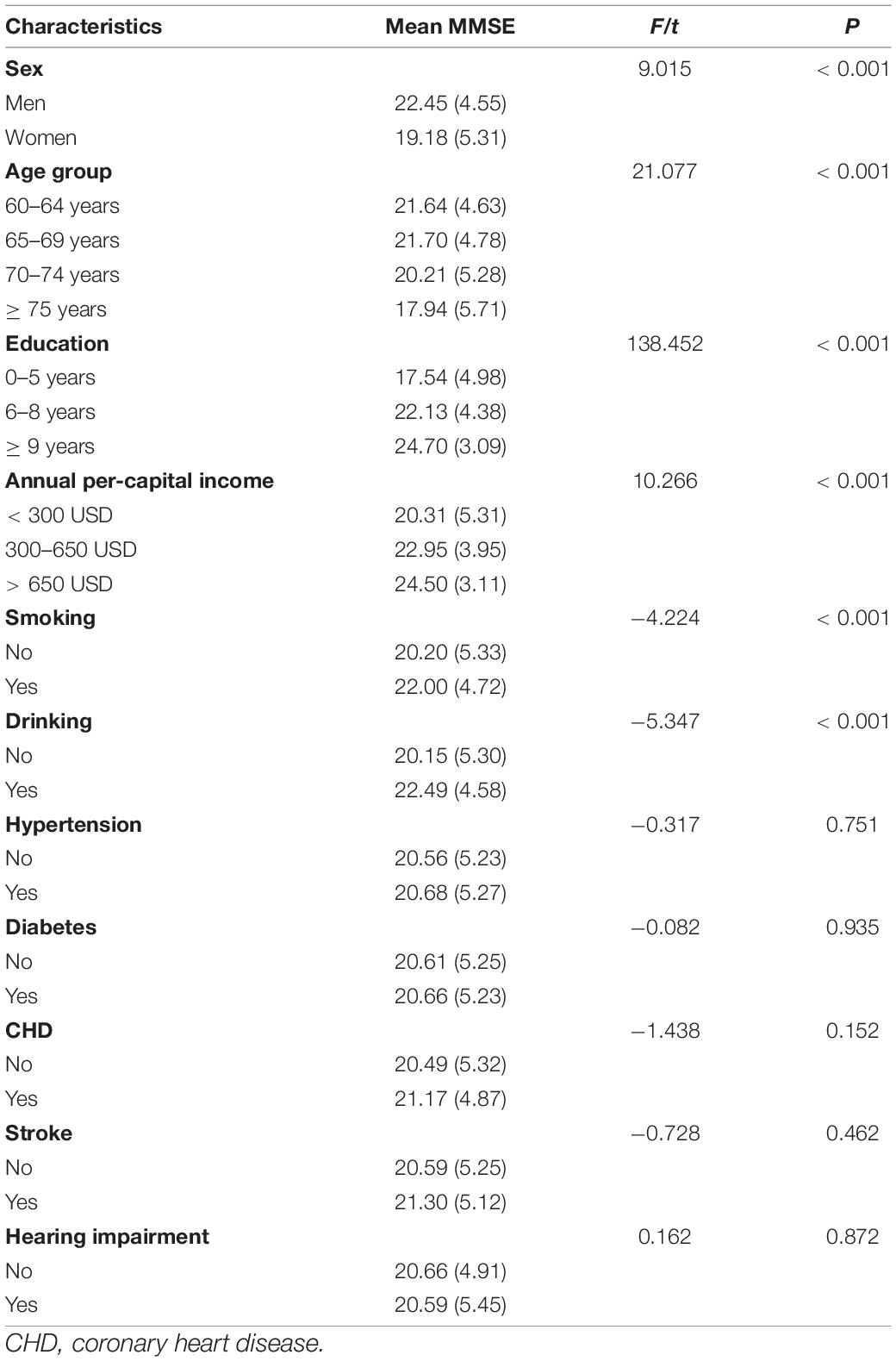
Table 2. Differences in mean MMSE score, according to demographic characteristics and risk factors groups.
In the linear regression analysis, MMSE score was negatively associated with SBP, TC, and LDL-C (all, P < 0.05; Table 3).
Association of MMSE Score and Its Domains With PTA in the Univariate Analysis
Linear regression analysis showed that MMSE score and its domains, immediate recall, and attention and calculation, were negatively correlated with OPTA, LPTA, and HPTA (all, P < 0.05). Orientation was negatively correlated with OPTA and LPTA in the univariate analysis (Table 4).
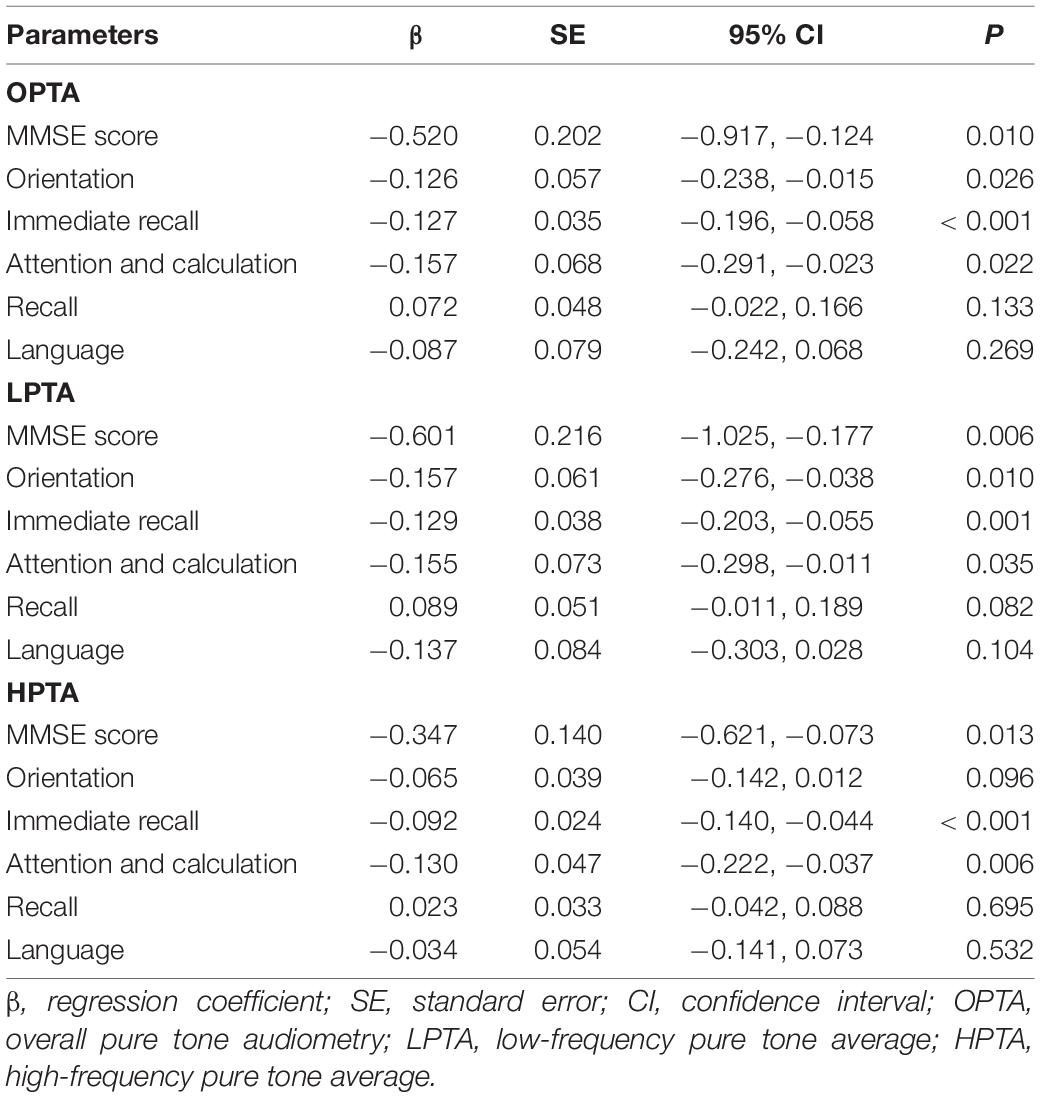
Table 4. Association of MMSE and its domains with pure tone average in the linear regression analysis.
Association of MMSE Score and Its Domains With PTA in the Multiple Linear Regression Analysis
Mini-Mental State Examination score and immediate recall score were negatively associated with OPTA, LPTA, and HPTA in multiple linear regression analyses after adjusting for sex, age, education, income, smoking, drinking, SBP, TC, and LDL-C (all, P < 0.05). Moreover, orientation score was negatively associated with OPTA and LPTA (all, P < 0.05), and attention and calculation score were negatively associated with OPTA and HPTA after adjusting for sex, age, education, income, smoking, drinking, SBP, TC, and LDL-C (all, P < 0.05; Table 5). The R2 value in linear regression was similar between LPTA and HPTA (adjusted R2 0.332 vs. 0.334).
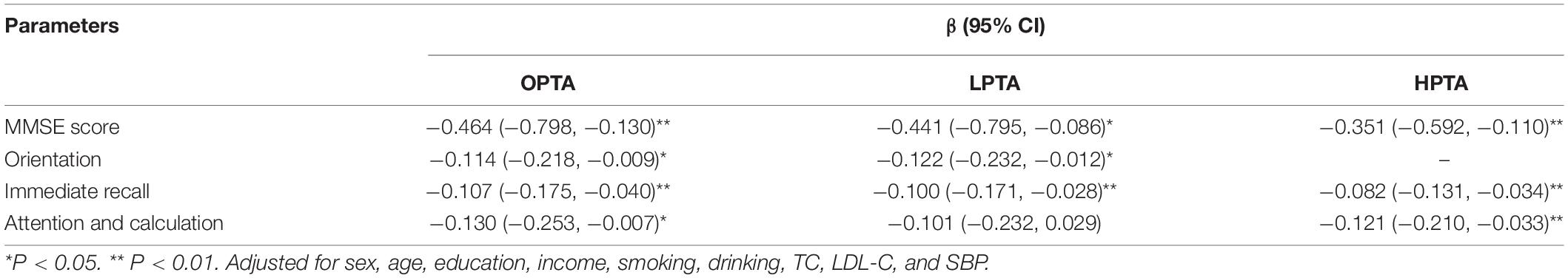
Table 5. Association of MMSE and its domains with pure tone average in the multiple linear analysis.
Discussion
This study evaluated the associations between peripheral hearing with global cognitive function and five domains of cognition among low-income elderly individuals in northern rural China. The prevalence of hearing impairment was 60.7% in this low-income rural population. LPTA and HPTA were negatively and independently related to MMSE score and its domains; this association was independent of age, education, lifestyle factors, and laboratory test values. In multiple linear regression analysis, both LPTA and HPTA accounted for a minimal proportion variance of MMSE score after adjusting for other covariates. There was a 0.441-point and 0.351-point MMSE score decrease associated with each 10-dB increase in LPTA and HPTA, respectively.
Hearing loss is prevalent among older adults and associated with a high prevalence of cognitive decline, apathy, and poor functional status (Sugawara et al., 2011; Miyake et al., 2020). In the present study, four-frequency PTA in the ear with better hearing was an independent risk factor for global cognitive status (MMSE score) and its domains; these findings are consistent with most previous studies. For instance, a longitudinal community-dwelling study found that hearing loss was related to accelerated cognitive decline and dementia among older adults, and individuals with hearing impairment at baseline was related to a 24% increased risk of dementia after a 6-year follow-up (Lin et al., 2013). Two American epidemiologic studies also found an independent association between cognitive performance and subclinical hearing loss; there was a 0.97-point decrease in the Digit Symbol Substitution Test score associated with a 10-dB increase of the PTA (Golub et al., 2020). Furthermore, a systematic review and meta-analysis demonstrated that hearing impairment was associated with a decline of global cognition, cognitive domains of executive function, and episodic memory, as well as increased risk of incident dementia and cognitive impairment (Loughrey et al., 2018). Other studies found stronger associations between hearing decline and lower episodic memory levels (Maharani et al., 2018b; Guglielmi et al., 2020). As a proxy measurement for episodic memory, immediate recall was strongly associated with worse hearing acuity in present study. Moreover, hearing aids use helps to slow down cognitive decline and improve functional status of older individuals (Maharani et al., 2018a; Sarant et al., 2020). However, a prospective cohort study in four American metropolitan areas demonstrated that vision, but not hearing impairment, was associated with cognitive decline (Lin et al., 2004). Another longitudinal study found that hearing loss did not accelerate cognitive decline over time after adjusting for the non-linear effects of age (Croll et al., 2021). In the present study, PTA was negatively and independently related to MMSE score and its domains independent of age, education, lifestyle factors, and laboratory test values.
Additionally, we found an independent association of both LPTA and HPTA with MMSE score. To our knowledge, only one study has assessed the association between PTA of different frequencies and cognitive performance; this prior study reported that LPTA, but not HPTA, was significantly and independently related to the MMSE score (Mukari et al., 2017). Studies have consistently confirmed that PTA is highly correlated with speech recognition (Coren and Hakstian, 1994; Vermiglio et al., 2012). In addition, a cross-section study demonstrated that PTA at low frequency exhibited the highest effect on speech recognition threshold compared to PTA at full range and high frequencies (Coren and Hakstian, 1994). High LPTA was more associated with poor speech recognition, which resulted in difficulty in communicating and maintaining interpersonal relationships (Lindenberger and Baltes, 1994; Maharani et al., 2019). These reasons will further cause social isolation, loneliness, and cognitive decline.
Three hypotheses have been proposed to explain the association between hearing and cognitive function (Wayne and Johnsrude, 2015; Uchida et al., 2019). In the cognitive load hypothesis, auditory signals are degraded among individuals with hearing loss (Lavie, 1995). Consequently, greater cognitive resource is required to understand speech, which affects other cognitive tasks and results in cognitive reserve depletion (Tun et al., 2009). Excessive cognitive load in daily life would cause neurodegeneration and structural changes in the brain, which subsequently impairs cognitive function (Martini et al., 2014). In addition, according to the common cause hypothesis, hearing impairment usually occurs simultaneously with cognitive decline at older ages; both hearing impairment and cognitive decline are results of neuropathological cause without direction of causality (Stahl, 2017). Finally, the sensory deprivation hypothesis suggests that sensory impairment, like hearing and vision impairment, could prevent older adults from communicating, resulting in social isolation, loneliness, and poor cognitive status (Rutherford et al., 2018). Some studies have reported the mediating effect of social isolation and loneliness between hearing and cognition (Rutherford et al., 2018; Maharani et al., 2019).
This was a population-based real-world study. Although studies have shown that hearing impairment increases the risk of cognitive decline, the relationship between hearing acuity and cognition remains inconclusive, especially in studies of large-scale low-income people. Moreover, many factors including age, sex, education, income, blood pressure, serum lipids, diabetes, smoking, and drinking can affect cognitive function (Yaffe et al., 2021). In the present study, both LPTA and HPTA accounted for a minimal proportion variance of the MMSE score; this association was independent of age, education, lifestyle factors, and laboratory test values. Moreover, due to earlier hearing loss at high frequencies, it is of great importance to discover and manage hearing loss to reduce risk for cognitive decline on the early stage.
There are several limitations in this study. First, cognitive function was evaluated using MMSE scores rather than a cognitive test battery; therefore, cognitive domain deficit could not be further diagnosed. Second, the speech-in-noise test could better simulate communication environments of daily living. The Mandarin Quick Speech-in-Noise test (M-Quick SIN) is quick and reliable with high clinical feasibility (Zhou et al., 2014) in population-based study. As M-Quick SIN was not established until 2014, it was not included in the present study. In the future, we plan to conduct a study including the speech-in-noise test. Third, the study population was from a low-income, low-education, rural population in northern China, thus its representativeness and generalizability are limited. Fourth, other confounding factors, including APOE4 genotype and diet, are important factors for cognitive decline and were not excluded in this study (Davies et al., 2018; Kivipelto et al., 2018). Fifth, asymmetrical hearing can be detrimental to cognitive function (Brännström et al., 2018) but was not included in the present study. Our follow-up research will further focus on asymmetrical hearing. Last, this was a cross-sectional study, and therefore causal relationships could not be identified.
Conclusion
The present study demonstrated significant but weak relationships between OPTA, LPTA, and HPTA with global cognitive function, as defined using MMSE scores, independent of age, education, lifestyle factors, and laboratory test values. These results indicate that hearing was associated with cognitive decline among older individuals, who should be screened routinely to identify risk for cognitive decline.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Tianjin Medical University General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DY, XL, and XN were involved in the conception and design of the study, data interpretation, and critically reviewed the manuscript. YX, YL, DG, XZ, HG, HC, XL, JZ, JT, and DY were involved in the data collection, case diagnosis, and confirmation for this manuscript. YX, YL, DG, and XZ were involved in the manuscript drafting and revision. JW was involved in the data analysis for this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all participants of the Tianjin Brain Study and local medical care professionals for their valuable contributions.
References
Brännström, K. J., Karlsson, E., Waechter, S., and Kastberg, T. (2018). Extended high-frequency pure tone hearing thresholds and core executive functions. Int. J. Audiol. 57, 639–645. doi: 10.1080/14992027.2018.1475755
Bush, A., Lister, J. J., Lin, F. R., Betz, J., and Edwards, J. D. (2015). Peripheral hearing and cognition: evidence from the staying keen in later life (skill) study. Ear Hear. 36, 395–407. doi: 10.1097/aud.0000000000000142
Canadian Task Force on Preventive Health Care, Pottie, K., Rahal, R., Jaramillo, A., Birtwhistle, R., Thombs, B. D., et al. (2016). Recommendations on screening for cognitive impairment in older adults. CMAJ 188, 37–46.
Chen, L. (2021). Self-reported hearing difficulty increases 3-year risk of incident cognitive impairment: the role of leisure activities and psychological resilience. Int. J. Geriatr. Psychiatry. 36, 1197–1203. doi: 10.1002/gps.5511
Chen, L., and Lu, B. (2019). Cognitive reserve regulates the association between hearing difficulties and incident cognitive impairment evidence from a longitudinal study in China. Int. Psychogeriatr. 32, 635–643. doi: 10.1017/S1041610219001662
Chen, L., and Zhou, R. (2020). Does self-reported hearing difficulty decrease older adults’ cognitive and physical functioning? The mediating role of social isolation. Maturitas 141, 53–58. doi: 10.1016/j.maturitas.2020.06.011
Coren, S., and Hakstian, A. R. (1994). Predicting speech recognition thresholds from pure tone hearing thresholds. Percept. Mot. Skills 79, 1003–1008. doi: 10.2466/pms.1994.79.2.1003
Croll, P. H., Vinke, E. J., Armstrong, N. M., Licher, S., Vernooij, M. W., Baatenburg de Jong, R. J., et al. (2021). Hearing loss and cognitive decline in the general population: a prospective cohort study. J. Neurol. 268, 860–871. doi: 10.1007/s00415-020-10208-8
Davies, G., Lam, M., Harris, S. E., Trampush, J. W., Luciano, M., Hill, W. D., et al. (2018). Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9:2098. doi: 10.1038/s41467-018-04362-x
Davis, A. C., and Hoffman, H. J. (2019). Hearing loss: rising prevalence and impact. Bull. World Health Organ. 97, 646A–646A. doi: 10.2471/BLT.19.224683
Deal, J. A., Betz, J., Yaffe, K., Harris, T., Purchase-Helzner, E., Satterfield, S., et al. (2017). Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 703–709. doi: 10.1093/gerona/glw069
Emmett, S. D., and Francis, H. W. (2015). The socioeconomic impact of hearing loss in U.S. adults. Otol. Neurotol. 36, 545–550. doi: 10.1097/mao.0000000000000562
Gao, J., Armstrong, N. M., Deal, J. A., Lin, F. R., and He, P. (2020). Hearing loss and cognitive function among Chinese older adults: the role of participation in leisure activities. BMC Geriatr 20:215. doi: 10.1186/s12877-020-01615-7
GBD 2016 Neurology Collaborators (2019). Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480. doi: 10.1016/S1474-4422(18)30499-X
GBD 2017 DALYs and HALE Collaborators (2018). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922. doi: 10.1016/S0140-6736(18)32335-3
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Golub, J. S., Brickman, A. M., Ciarleglio, A. J., Schupf, N., and Luchsinger, J. A. (2020). Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol. Head Neck Surg. 146, 57–67. doi: 10.1001/jamaoto.2019.3375
Guglielmi, V., Marra, C., Picciotti, P. M., Masone Iacobucci, G., Giovannini, S., Quaranta, D., et al. (2020). Does hearing loss in the elderly individuals conform to impairment of specific cognitive domains. J. Geriatr. Psychiatry Neurol. 33, 231–240. doi: 10.1177/0891988719874117
Gurgel, R. K., Ward, P. D., Schwartz, S., Norton, M. C., Foster, N. L., and Tschanz, J. T. (2014). Relationship of hearing loss and dementia: a prospective, population-based study. Otol. Neurotol. 35, 775–781. doi: 10.1097/mao.0000000000000313
Hu, Y., Zhao, L., Zhang, H., Yu, X., Wang, Z., Ye, Z., et al. (2016). Sex differences in the recurrence rate and risk factors for primary giant cell tumors around the knee in China. Sci. Rep. 6:28173. doi: 10.1038/srep28173
Kivipelto, M., Mangialasche, F., and Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666. doi: 10.1038/s41582-018-0070-3
Lavie, N. (1995). Perceptual load as a necessary condition for selective attention. J. Exp. Psychol. Hum. Percept. Perform. 21, 451–468. doi: 10.1037/0096-1523.21.3.451
Li, G., Shen, Y., Chen, C., Li, S., Zhang, W., and Liu, M. (1989). Mini Mental State Examination (MMSE) in different population test study. Chin. Mental Health J. 4, 148–151.
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Lin, M. Y., Gutierrez, P. R., Stone, K. L., Yaffe, K., Ensrud, K. E., Fink, H. A., et al. (2004). Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J. Am. Geriatr. Soc. 52, 1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x
Lindenberger, U., and Baltes, P. B. (1994). Sensory functioning and intelligence in old age: a strong connection. Psychol. Aging 9, 339–355. doi: 10.1037/0882-7974.9.3.339
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., and Lawlor, B. A. (2018). Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 144, 115–126. doi: 10.1001/jamaoto.2017.2513
Maharani, A., Dawes, P., Nazroo, J., Tampubolon, G., Pendleton, N., and Sense-COG WP1 Group (2018a). Longitudinal relationship between hearing aid use and cognitive function in older Americans. J. Am. Geriatr. Soc. 66, 1130–1136. doi: 10.1111/jgs.15363
Maharani, A., Dawes, P., Nazroo, J., Tampubolon, G., Pendleton, N., and Sense-COG WP1 Group (2018b). Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing 47, 575–581. doi: 10.1093/ageing/afy061
Maharani, A., Pendleton, N., and Leroi, I. (2019). Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English longitudinal study on ageing. Am. J. Geriatr. Psychiatry 27, 1348–1356. doi: 10.1016/j.jagp.2019.07.010
Martin, M. C. (1986). Total deafness: the need and possibility for a working definition. Br. J. Audiol. 20, 85–88. doi: 10.3109/03005368609079000
Martini, A., Castiglione, A., Bovo, R., Vallesi, A., and Gabelli, C. (2014). Aging, cognitive load, dementia and hearing loss. Audiol. Neurootol. 19(Suppl. 1), 2–5. doi: 10.1159/000371593
Miyake, Y., Tanaka, K., Senba, H., Ogawa, S., Suzuki, H., Fujiwara, Y., et al. (2020). Hearing impairment and prevalence of mild cognitive impairment in Japan: baseline data from the Aidai Cohort Study in Yawatahama and Uchiko. Ear Hear. 41, 254–258. doi: 10.1097/aud.0000000000000773
Mukari, S., Ishak, W. S., Maamor, N., and Wan Hashim, W. F. (2017). A preliminary study investigating the association between hearing acuity and a screening cognitive tool. Ann. Otol. Rhinol. Laryngol. 126, 697–705. doi: 10.1177/0003489417727547
Nunes, B., Silva, R. D., Cruz, V. T., Roriz, J. M., Pais, J., and Silva, M. C. (2010). Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 10:42. doi: 10.1186/1471-2377-10-42
Pabst, A., Bär, J., Röhr, S., Löbner, M., Kleineidam, L., Heser, K., et al. (2021). Do self-reported hearing and visual impairments predict longitudinal dementia in older adults. J. Am. Geriatr. Soc. 69, 1519–1528. doi: 10.1111/jgs.17074
Panza, F., Solfrizzi, V., and Logroscino, G. (2015). Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 11, 166–175. doi: 10.1038/nrneurol.2015.12
Ping, H., Luo, Y., Hu, X., Rui, G., Xu, W., Zheng, X., et al. (2018). Association of socioeconomic status with hearing loss in Chinese working-aged adults: a population-based study. PLoS One 13:e0195227. doi: 10.1371/journal.pone.0195227
Rutherford, B. R., Brewster, K., Golub, J. S., Kim, A. H., and Roose, S. P. (2018). Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatry 175, 215–224. doi: 10.1176/appi.ajp.2017.17040423
Saji, N., Makizako, H., Suzuki, H., Nakai, Y., Tabira, T., Obuchi, S., et al. (2021). Hearing impairment is associated with cognitive function in community-dwelling older adults: a cross-sectional study. Arch. Gerontol. Geriatr. 93:104302. doi: 10.1016/j.archger.2020.104302
Sarant, J., Harris, D., Busby, P., Maruff, P., Schembri, A., Lemke, U., et al. (2020). The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function. J. Clin. Med. 9:254. doi: 10.3390/jcm9010254
Stahl, S. M. (2017). Does treating hearing loss prevent or slow the progress of dementia? Hearing is not all in the ears, but who’s listening. CNS Spectr. 22, 247–250. doi: 10.1017/s1092852917000268
Sugawara, N., Sasaki, A., Yasui-Furukori, N., Kakehata, S., Umeda, T., Namba, A., et al. (2011). Hearing impairment and cognitive function among a community-dwelling population in Japan. Ann. Gen. Psychiatry 10:27. doi: 10.1186/1744-859x-10-27
Tun, P. A., McCoy, S., and Wingfield, A. (2009). Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 24, 761–766. doi: 10.1037/a0014802
Uchida, Y., Sugiura, S., Nishita, Y., Saji, N., Sone, M., and Ueda, H. (2019). Age-related hearing loss and cognitive decline–the potential mechanisms linking the two. Auris Nasus Larynx 46, 1–9. doi: 10.1016/j.anl.2018.08.010
Vermiglio, A. J., Soli, S. D., Freed, D. J., and Fisher, L. M. (2012). The relationship between high-frequency pure-tone hearing loss, hearing in noise test (HINT) thresholds, and the articulation index. J. Am. Acad. Audiol. 23, 779–788. doi: 10.3766/jaaa.23.10.4
Wang, J., Ning, X., Yang, L., Tu, J., Gu, H., Zhan, C., et al. (2014). Sex differences in trends of incidence and mortality of first-ever stroke in rural Tianjin, China, from 1992 to 2012. Stroke 45, 1626–1631. doi: 10.1161/strokeaha.113.003899
Wayne, R. V., and Johnsrude, I. S. (2015). A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res. Rev. 23(Pt B), 154–166. doi: 10.1016/j.arr.2015.06.002
Wimo, A., Jönsson, L., Bond, J., Prince, M., Winblad, B., and Alzheimer Disease International (2013). The worldwide economic impact of dementia 2010. Alzheimers Dement. 9, 1.e–11.e. doi: 10.1016/j.jalz.2012.11.006
World Health Organization (WHO) (1997). Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment. Geneva: WHO.
Wortmann, M. (2012). Dementia: a global health priority–highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 4:40. doi: 10.1186/alzrt143
Yaffe, K., Vittinghoff, E., Hoang, T., Matthews, K., Golden, S. H., and Zeki Al Hazzouri, A. (2021). Cardiovascular risk factors across the life course and cognitive decline: a pooled cohort study. Neurology 96, e2212–e2219. doi: 10.1212/WNL.0000000000011747
Yang, D., Liu, J., Yang, Q., Lin, Q., Zhang, X., Wang, M., et al. (2021). Hearing impairment prevalence and risk factors among adults in rural China: a population-based cross-sectional study. Postgrad. Med. 133, 369–376. doi: 10.1080/00325481.2020.1855852
Yueh, B., Shapiro, N., MacLean, C. H., and Shekelle, P. G. (2003). Screening and management of adult hearing loss in primary care: scientific review. JAMA 289, 1976–1985. doi: 10.1001/jama.289.15.1976
Zhou, B. F. (2002). Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed. Environ. Sci. 15, 245–252.
Keywords: cognitive function, hearing loss, pure tone average, low-frequency pure tone average, aging
Citation: Xu Y, Li Y, Guo D, Zhang X, Guo H, Cao H, Li X, Zhang J, Tu J, Wang J, Ning X and Yang D (2021) Association of Hearing Acuity and Cognitive Function Among a Low-Income Elderly Population in Rural China: A Population-Based Cross-Sectional Study. Front. Neurosci. 15:704871. doi: 10.3389/fnins.2021.704871
Received: 04 May 2021; Accepted: 15 July 2021;
Published: 16 August 2021.
Edited by:
Shaowen Bao, University of Arizona, United StatesReviewed by:
David R. Moore, Cincinnati Children’s Hospital Medical Center, United StatesYihsin Tai, Ball State University, United States
Copyright © 2021 Xu, Li, Guo, Zhang, Guo, Cao, Li, Zhang, Tu, Wang, Ning and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Yang, entyd@sohu.com; Xianjia Ning, xning@tmu.edu.cn; Xin Li, entlixin@sina.com
†These authors have contributed equally to this work
 Yi Xu1†
Yi Xu1†