
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 14 June 2021
Sec. Brain Imaging Methods
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.690705
This article is part of the Research Topic Advanced Imaging and Mapping in Brain Tumors View all 49 articles
 Giuseppe Emmanuele Umana1*
Giuseppe Emmanuele Umana1* Gianluca Scalia2
Gianluca Scalia2 Kaan Yagmurlu3
Kaan Yagmurlu3 Rosalia Mineo4,5
Rosalia Mineo4,5 Simone Di Bella4,5
Simone Di Bella4,5 Matteo Giunta4
Matteo Giunta4 Angelo Spitaleri1
Angelo Spitaleri1 Rosario Maugeri6
Rosario Maugeri6 Francesca Graziano2,6
Francesca Graziano2,6 Marco Fricia1
Marco Fricia1 Giovanni Federico Nicoletti2
Giovanni Federico Nicoletti2 Santino Ottavio Tomasi7
Santino Ottavio Tomasi7 Giuseppe Raudino8
Giuseppe Raudino8 Bipin Chaurasia9
Bipin Chaurasia9 Gianluca Bellocchi10
Gianluca Bellocchi10 Maurizio Salvati11
Maurizio Salvati11 Domenico Gerardo Iacopino6
Domenico Gerardo Iacopino6 Salvatore Cicero1
Salvatore Cicero1 Massimiliano Visocchi12
Massimiliano Visocchi12 Lidia Strigari13
Lidia Strigari13Background: External ventricular drain (EVD) placement is mandatory for several pathologies. The misplacement rate of the EVD varies widely in literature, ranging from 12.3 to 60%. The purpose of this simulation study is to provide preliminary data about the possibility of increasing the safety of one of the most common life-saving procedures in neurosurgery by testing a new device for EVD placement.
Methods: We used a novel guide for positioning the ventricular catheter (patent RM2014A000376). The trajectory was assessed using 25 anonymized head CT scans. The data sets were used to conduct three-dimensional computer-based and combined navigation and augmented reality-based simulations using plaster models. The data set inclusion criteria were volumetric head CT scan, without midline shift, of patients older than 18. Evans’ index was used to quantify the ventricle’s size. We excluded patients with slit ventricles, midline shift, skull fractures, or complex skull malformations. The proximal end of the device was tested on the cadaver.
Results: The cadaveric tests proved that a surgeon could use the device without any external help. The multimodal simulation showed Kakarla grade 1 in all cases but one (grade 2) on both sides, after right and left EVD placement. The mean Evans’ index was 0.28. The geometric principles that explain the device’s efficacy can be summarized by studying the properties of circumference and chord. The contact occurs, for each section considered, at the extreme points of the chord. Its axis, perpendicular to the plane tangent to the spherical surface at the entry point, corresponds to the direction of entry of the catheter guided by the instrument.
Conclusion: According to our multimodal simulation on cadavers, 3D computer-based simulation, 3D plaster modeling, 3D neuronavigation, and augmented reality, the device promises to offer safer and effective EVD placement. Further validation in future clinical studies is recommended.
External ventricular drain (EVD) placement is mandatory for several pathologies, both in emergency and elective surgery. It allows reducing life-threatening intracranial hypertension, thus rapidly improving the patient’s clinical conditions and sometimes saving the patient’s life. The misplacement rate of the ventricular catheters reported in the literature varies widely, ranging from 12.3 to 60% (Saladino et al., 2009; Toma et al., 2009; Park et al., 2011; Bergdal et al., 2013; Chai et al., 2013; Rehman et al., 2013; Phillips et al., 2014), with possible serious complications (e.g., cerebral hematomas, neurological deficits, and seizures) (Woernle et al., 2011; Rosenbaum et al., 2013). This misplacement rate has been considered as the baseline for our results, and we aimed to obtain a lower misplacement rate in a simulation setting. The misplacement rates reported in literature are high, probably due to the use of EVD also in patients affected by midline shift or slit ventricles and in procedures performed by non-neurosurgeons in remote areas with logistic difficulties. Numerous studies have already been published about the reduction of complication after EVD, which is currently a freehand procedure, performed only according to anatomical landmarks that help to introduce the catheter perpendicularly to the burr hole; the perpendicularity is a critical element for the correct positioning of the catheter (Gil et al., 2002; Shimizu et al., 2004; Lee et al., 2008, 2020; Mahan et al., 2013; Patil et al., 2013; Jakola et al., 2014; Siesjö, 2014; Shtaya et al., 2018; Thomale et al., 2018; Wilson et al., 2018; Fuller et al., 2020; Park et al., 2020; Sun et al., 2020; Amoo et al., 2021; Cabrilo et al., 2021; Pishjoo et al., 2021).
One study reported the results in 17 patients, using a guide that allows obtaining perpendicularity (Ghajar, 1985): this study reported obtaining cerebrospinal fluid (CSF) on the first attempt, providing initial evidence of the strong correlation between the perpendicular trajectory offered by their device and the correct positioning of the catheter. In 11 out of 17 patients (64%), the correct positioning at the level of the ipsilateral anterior frontal horn of the ventricle was confirmed by intraoperative fluoroscopy and postoperative CT scan. However, this guide was not suitable for neuronavigation, a useful tool when midline shift is present.
The development of new devices is one of the purest forms of surgical research, but it is rare and challenging, since most of the technical discoveries have already been reported. We highlight the experimental nature of this study, in which we used merged technologies to assess the accuracy and validate the trajectory offered by the device, which allows to immediately obtain the perpendicularity required and, indicated in literature, for correct EVD placement.
The purpose of this study is to provide preliminary data about the possibility of increasing the safety of one of the most common life-saving procedures in neurosurgery by testing a new device for EVD placement.
We used a novel guide for positioning the ventricular catheter (patent RM2014A000376), which is neither invasive nor implanted on the patient, and it does not modify the standard surgical protocol for EVD placement. We used a cadaveric specimen, already used for a cadaver lab during the “First European advanced course on surgical techniques for the obstructive sleep apnea-hypopnea syndrome” in Rome, to develop the device’s proximal end, which is the key: it is fixed to the skull, it does not go beyond the dura mater, and, above all, it has a perpendicular trajectory. Several bilateral, precoronal, and prefrontal tests were performed to improve the device’s insertion into the burr hole. The cadaveric specimen was not used to assess catheter trajectory. The entry points were precoronal: 7–11 cm from the nasion and 3 cm from the midline.
The trajectory was assessed using 25 anonymized head CT scans of patients with a mean Evans’s index of 0.28. The data sets were used to conduct three-dimensional computer-based and augmented reality-based simulations. Data set inclusion criteria were volumetric head CT scan without midline shift of patients older than 18. Evans’ index was used to quantify the ventricle’s size. We excluded patients with slit ventricles, midline shift, skull fractures, or complex skull malformations.
I. Precoronaric burr hole, 11 cm from the nasion and 5–3 cm from midline and dura mater incision.
II. Internal bone spur removal if the bone is very thin, <8 mm.
III. Insert the device inside the burr hole; associate the navigation reference if midline shift is present.
IV. Insert the catheter inside the device, remove the mandrel, and remove the device.
V. Catheter tunneling.
• Step 1: In the standard freehand procedure, the skin incision can be either linear or curvilinear.
• Step 2: A burr hole is placed 10–11 cm from the nasion and 2.5–3 cm on the right of the midline. The burr hole is performed with a 14-mm perforator (Figure 1).
• Step 3: The dura mater is coagulated and cut with a no. 11 blade, and a small corticectomy is performed.
• Step 4: The catheter is introduced for 6 cm from the external cortical bone of the skull, looking for the perpendicularity (90°) compared with the outer skull, having as reference the mid-pupillary line, the external acoustic meatus, and the medial canthus; these are the landmarks for directing the tip of the catheter toward the ipsilateral Monro foramen.
• Step 5: Once the catheter is inserted and CSF is obtained, the catheter is tunneled and connected to the external drainage system.
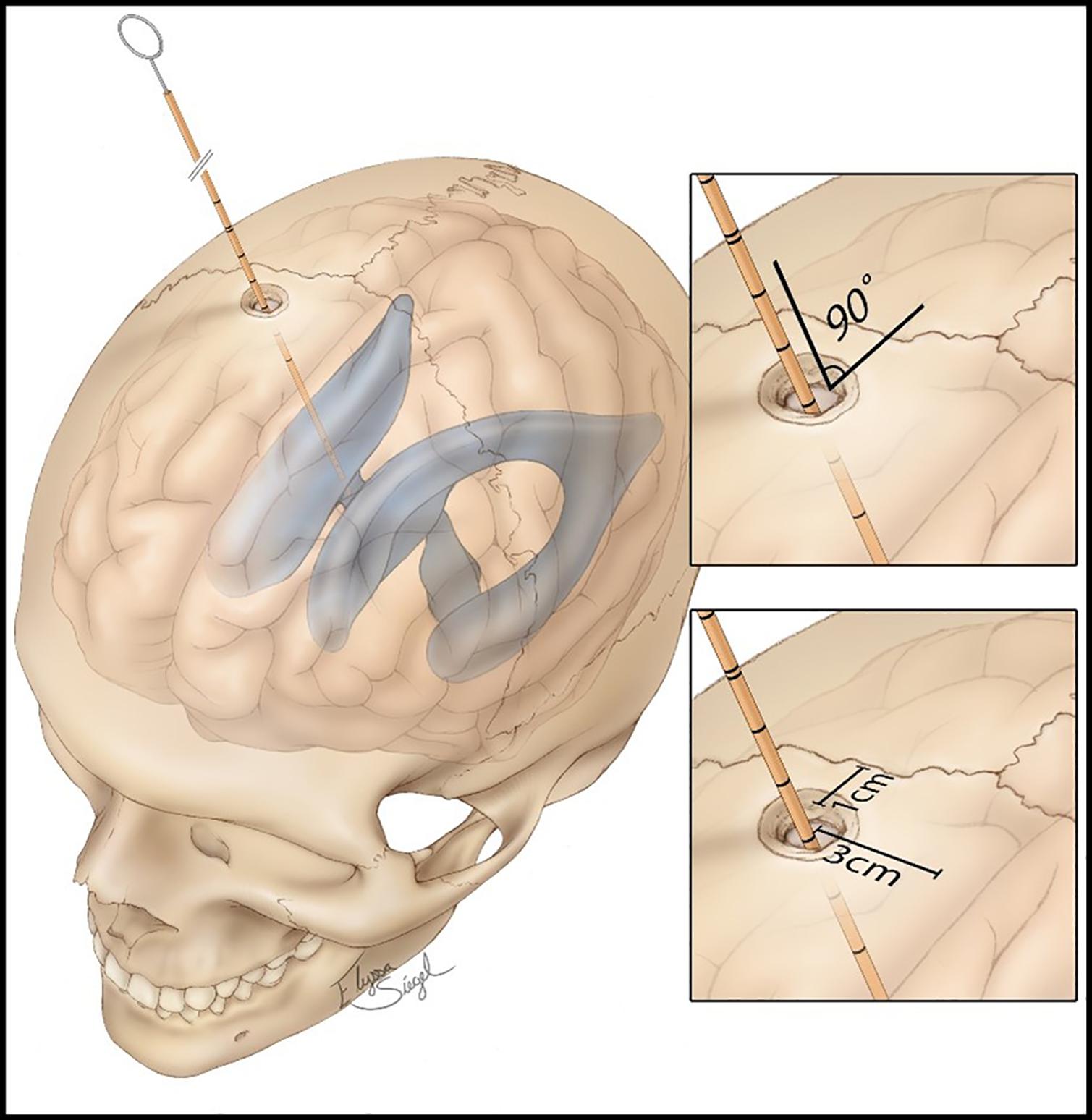
Figure 1. Standard procedure of external ventricular drain (EVD) placement, showing the distances from the coronal suture, midline, and the angle of 90° of the catheter, aiming to perpendicularity. Source: https://assets.neurosurgicalatlas.com/volumes/ATLAS/1-PRINCIPLES_OF_CRANIAL_SURGERY/16-External_ Ventricular_Drain/PCS_ExtIntraventDrain_03.jpg (last accessed: March 30, 2021).
The device’s peculiarity (Figure 2) is identifiable in the bone support base, which allows the fast and non-invasive achievement of the correct trajectory (perpendicular to the curvature of the skull). The distal cylindrical component maintains the trajectory during the insertion and allows interfacing with the neuronavigation, using the navigated stylet or universal reference systems of the common brands. The device can be sterilized multiple times, saving costs. It is semitransparent, allowing to visualize the number indicating the catheter’s length, thus controlling the depth of insertion (Figure 3).
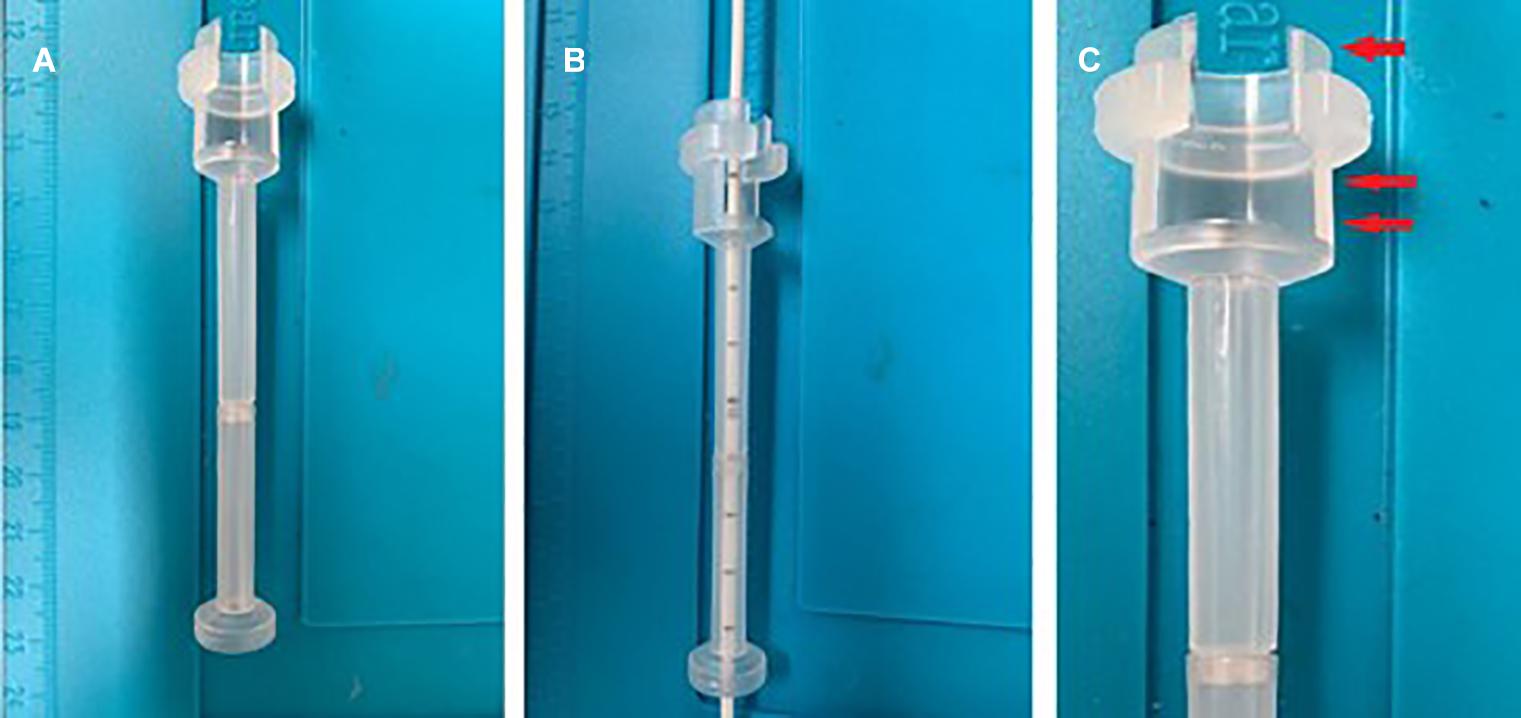
Figure 3. The device is semitransparent, allowing catheter control during insertion (A,B). The catheter’s proximal end (arrow), which helps to fix the device to the skull with the correct trajectory. The proximal end also presents a window to clamp the catheter before removing the mandrel (double arrows) (C).
Twenty-five anonymized head CT scan data sets were uploaded, and 3D head models were obtained. The skull and ventricles were reconstructed. The 3D rendering of the ventricles was highlighted and visualized together with the skull, removing the brain. A 3D representation of the device was created and positioned in Kocher’s point, along its offset and directed proximally, toward the lateral ventricles. The device was positioned perpendicular to the skull by imposing the flat part of the device’s proximal end on the skull’s curvature.
This protocol was designed to virtually simulate the EVD procedure performed with the aid of the novel device in the most realistic way. This device allows the catheter’s insertion in a guided way; mainly, it ensures the catheter to be inserted perpendicular to the skull’s surface, providing a higher success rate. This simulation can be summarized in seven phases:
• Phase 1: Reconstruction of the 3D model. The 3D model of the patient’s bone is created using the Mimics software (Materialize, Leuven Belgium) from the CT images data set (Figure 4A).
• Phase 2: Identification of the catheter entry point. The 3D model just obtained is imported into the Materialize® 3-Matic software. In this virtual-like environment, using a measuring instrument along curved surfaces, we move 100/110 mm, starting from the glabella, along the sagittal plane, and then 30 mm along the coronal plane, thus identifying the entry point of the catheter (Figure 4B).
• Phase 3: Simulation of the burr hole. Performing a Boolean operation of subtraction between solids, the first surgical phase of the external ventricular drain (EDV) placement, represented by the burr hole, is simulated by subtracting a cylinder with a diameter of 14 mm from the 3D model of the skull (Figure 4C).
• Phase 4: Identification of the contact surface. At this point, the surgical procedure involves the positioning of the device inside the hole, orthogonally to the contact surface. To virtually simulate this surgical phase, the instrument/skull contact surface ensemble was isolated, consisting of a crown with an external diameter of 20 mm (coinciding with the outer diameter of the instrument in contact with the skull) and an internal diameter of 14 mm (inner diameter of the burr hole) (Figure 4D).
• Phase 5: Identification of the normal to the contact surface. The plane that best fits the newly isolated surface is then identified. The normal to this plane will specify the device’s direction of insertion (Figure 4E).
• Phase 6: Catheter entry simulation. This phase allows the virtual simulation of the catheter’s insertion. A cylindrical solid with a diameter of 2.7 mm is created, and it is translated along the direction normal to the plane for a length of 60 mm (Figure 4F).
• Phase 7: Verification of correct catheter placement. The cylinder thus positioned is then imported into the Mimics software, where it can be viewed on the patient’s CT images. In this way, it is possible to verify that the cylinder, simulating the catheter, reaches the ipsilateral lateral ventricle (Figure 4G).
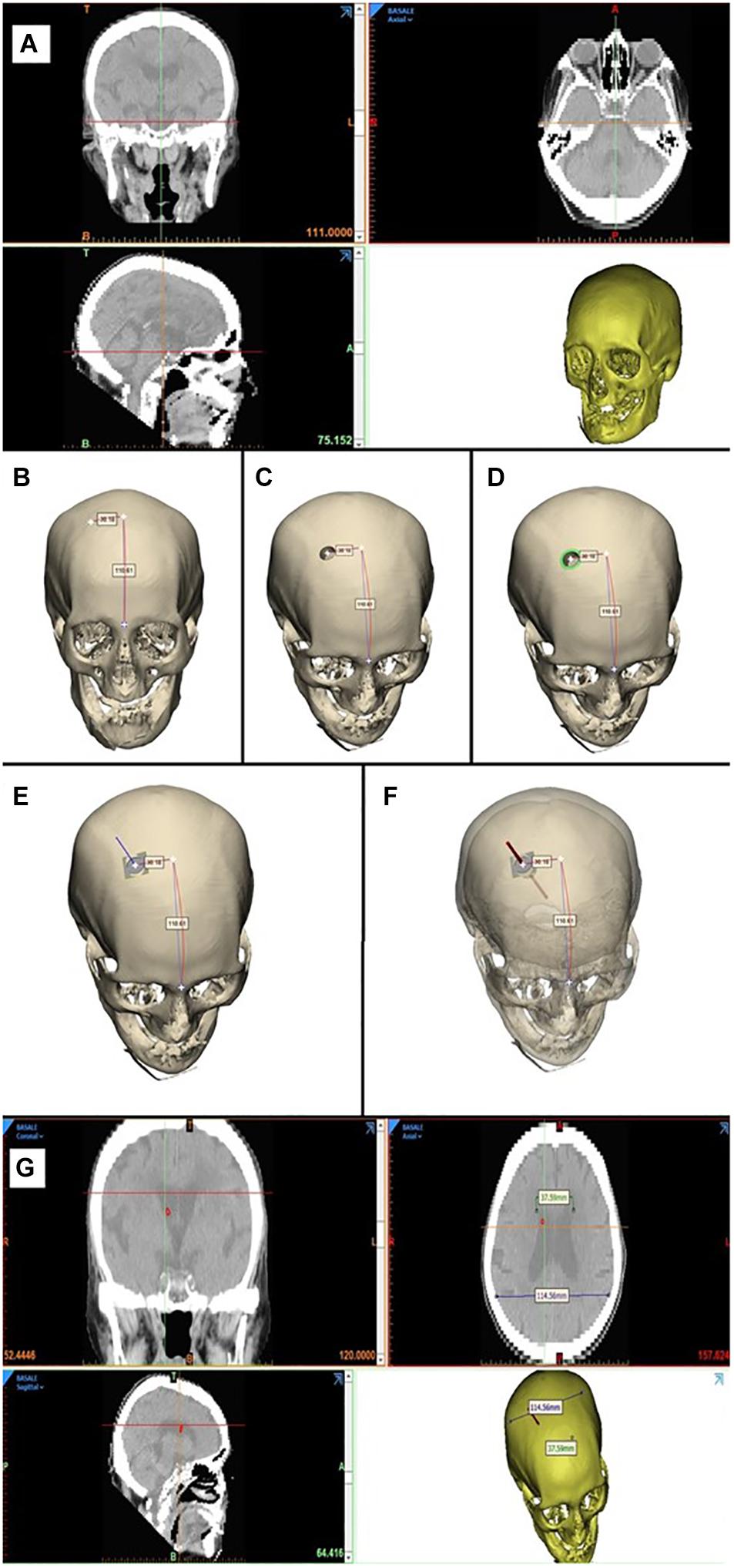
Figure 4. 3D computer-based simulation (A), showing the entry point definition based on anatomical landmarks, to both the right and left, Evans’ evaluation, simulation of the catheter placement (B–F), bilaterally obtaining grade A positioning (G).
Twenty-five plaster models of the patients’ anatomy were created (Fin-Ceramica, Faenza, Italy) and used for combined 3D neuronavigation and augmented reality-based navigation (Figure 5). These plaster models are usually used for cranioplasty planning; thus, their accuracy to the patient’s anatomy is the highest available.
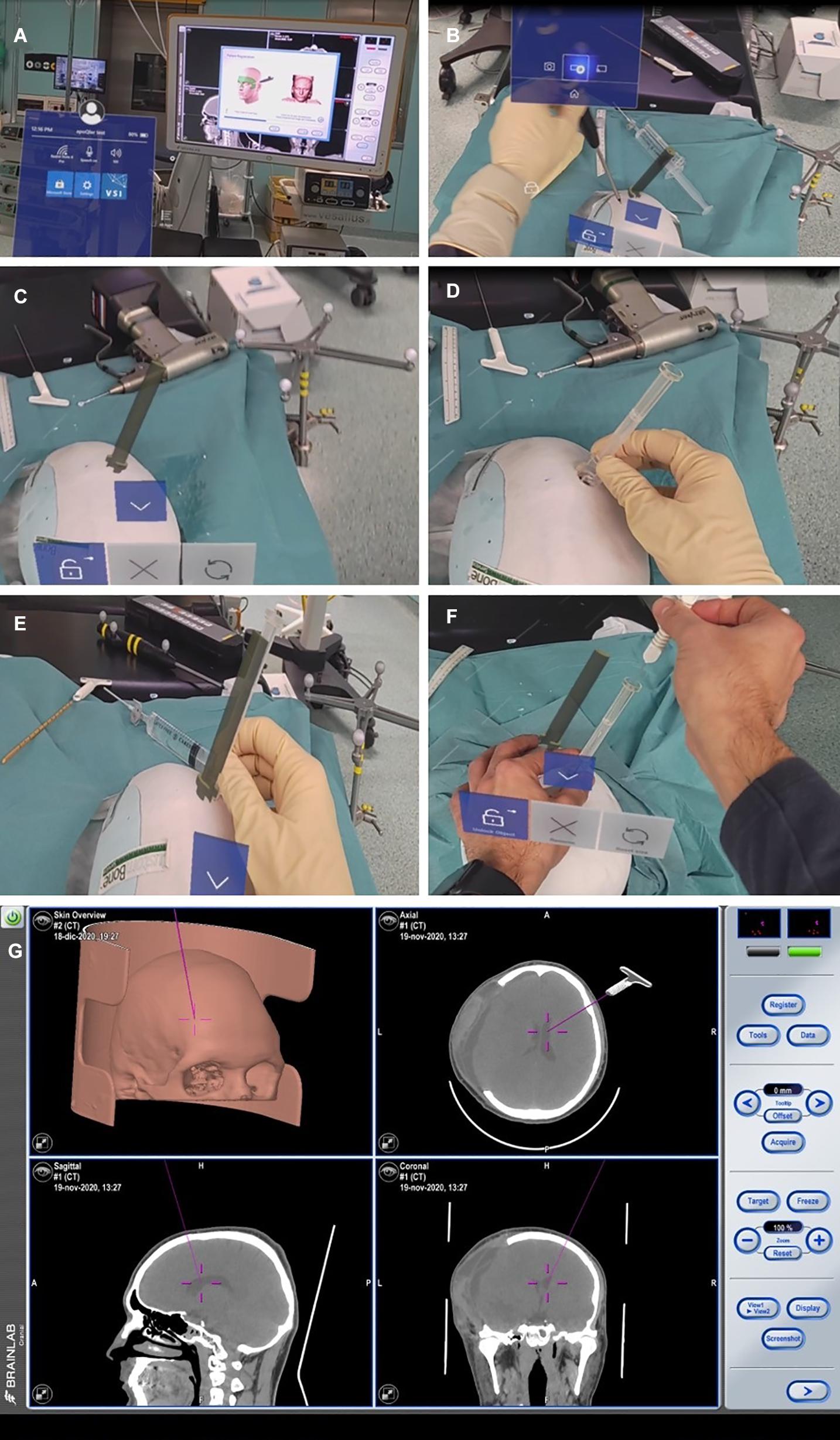
Figure 5. Finceramica plaster model navigation after merging the patient’s CT scan (already treated for post-traumatic craniotomy, but with no midline shift). (A,B) Superficial tracing for navigation registration (BrainLab) of the plaster model. (C) Entry point definition with a holographic representation of the device. (D) Positioning of the real device on the defined entry point. (E) Overlap of the holographic device with the real one, to match the entry point. (F) Introduction of the navigated stylet inside the device. (G) Its representation with the standard navigation.
We used Microsoft’s HoloLens mixed reality glasses and Virtual Surgery Intelligence (VSI) (ApoQlar, Hamburg, Germany). The model can be moved and “edited” in space via gesture control, and speech control can be used to record videos or take pictures. We uploaded the head CT scan data set and STL file of the device to reconstruct the patient’s anatomy and the holographic rendering of the device, respectively, to improve the immersive experience of the simulation (Figure 6).
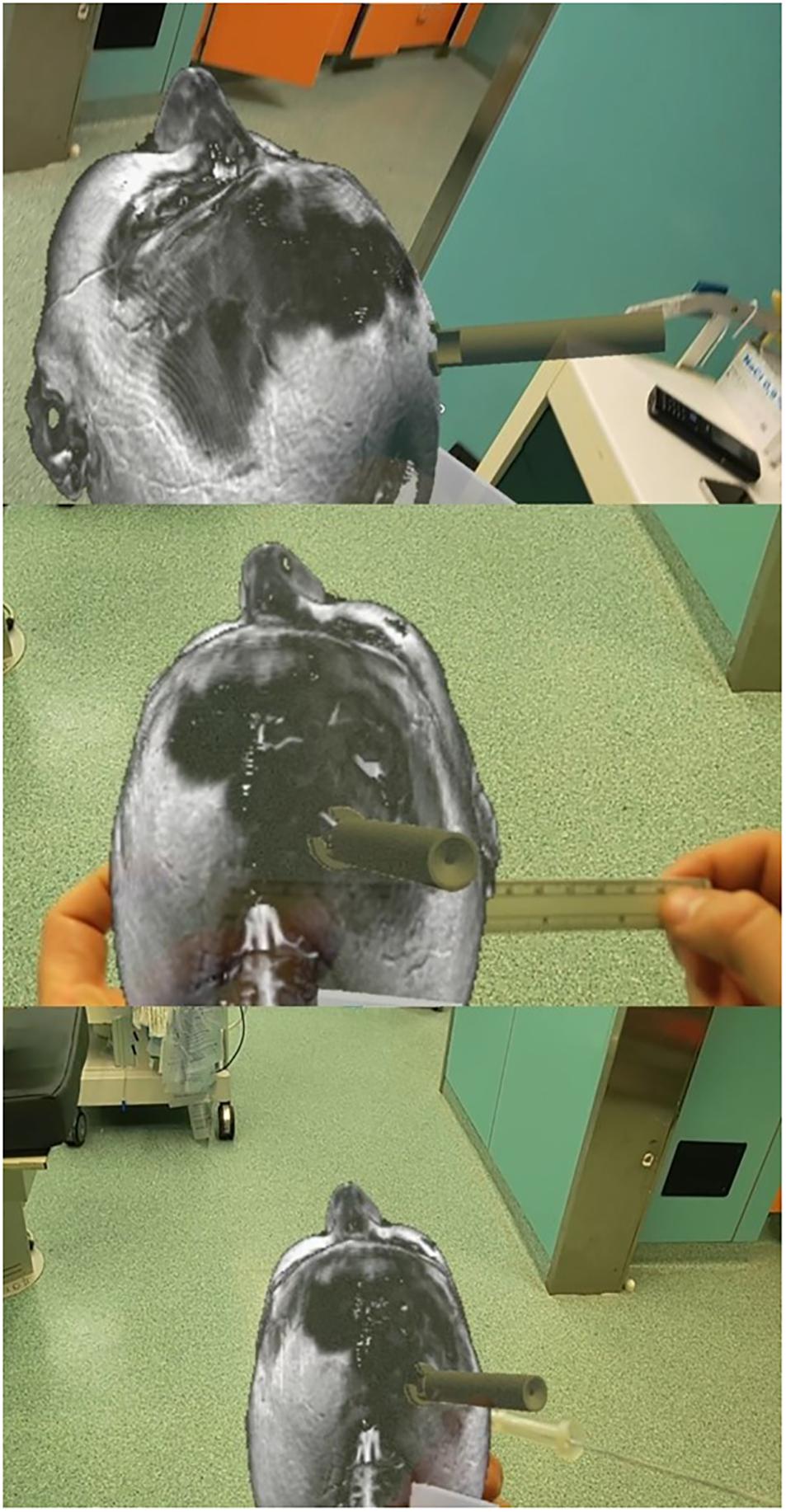
Figure 6. Augmented reality-based simulation (ApoQlar) to locate the entry point. A real ruler was overlapped on the hologram based on both MRI and CT scans; the actual device was overlapped over its holographic reconstruction, and the navigated stylet was introduced to improve familiarity with the procedure and technology.
We conducted a 3D neuronavigation-based simulation by performing a CT scan of the 3D plaster model. The data set obtained was merged with the patient’s head CT scan to allow visualization of the ventricles. The 3D model was then uploaded in BrainLab (Munich, Germany) planning station and used to simulate the introduction of the ventricular catheter in the plaster model, with the device overlapped on its holographic representation, verifying the correct trajectory navigating the catheter. The navigation could be associated with neuronavigation by using a navigated stylet or other navigation tools.
The accuracy was assessed according to the Kakarla classification (Figure 7; Kakarla et al., 2008), which includes three grades (Table 1). The device’s accuracy has been proven to be equal to that of the neuronavigation because we applied the device according to craniometric parameters and using plaster models; furthermore, we applied the navigated stylet to confirm the accuracy of the trajectory provided by the guide. The 3D computer-based navigation should be considered a fine simulation step that geometrically demonstrates the mathematical reasons on which the device’s efficacy is based. The computer did not adjust the trajectory to bias the results and obtain the desired results; instead, it allowed to get the correct measures. The software allows to apply the device perpendicularly to the skull surface and, only after this step, to evaluate its trajectory.
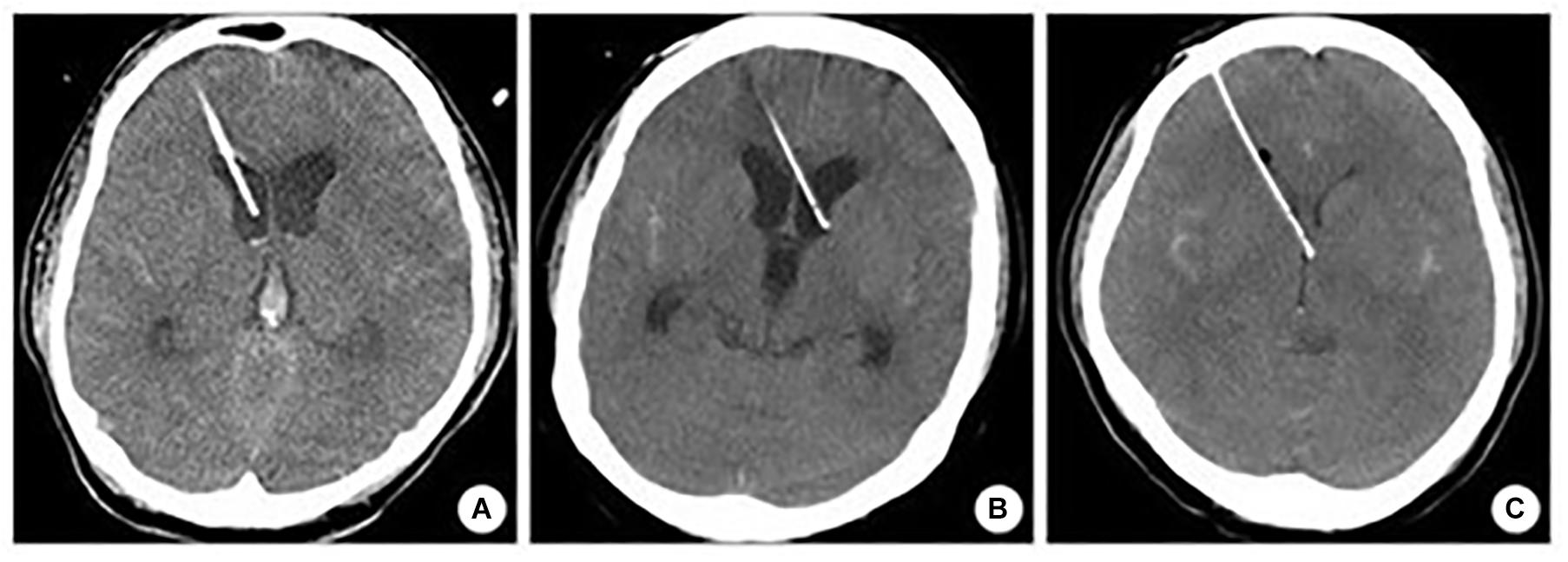
Figure 7. Kakarla grading system. (A) Homolateral lateral ventricle positioning. (B) Contralateral lateral ventricle positioning. (C) Misplacement.
We suggest considering this computer-based simulation step as the finest and most objective way to test the device in a simulation setting. In daily life, the head’s position can affect the accuracy of the EVD placement; thus, a guide that is of aid to get perpendicularity independently from the head position would be of great benefit.
The trajectory was assessed using an anonymized head CT scan of 25 patients (14 males and 11 females) with a median age of 71 (range 36–90) years. The mean of the Evans’ index was 0.28 (±0.03 standard deviation); see Table 2. The catheter tip distribution was calculated in 23/25 and 24/25 cases in the right and left hemispheres, respectively. The calculation was not performed in patients with a tumoral mass in the right hemisphere, and, in one case, the CT images did not include the entire skull.
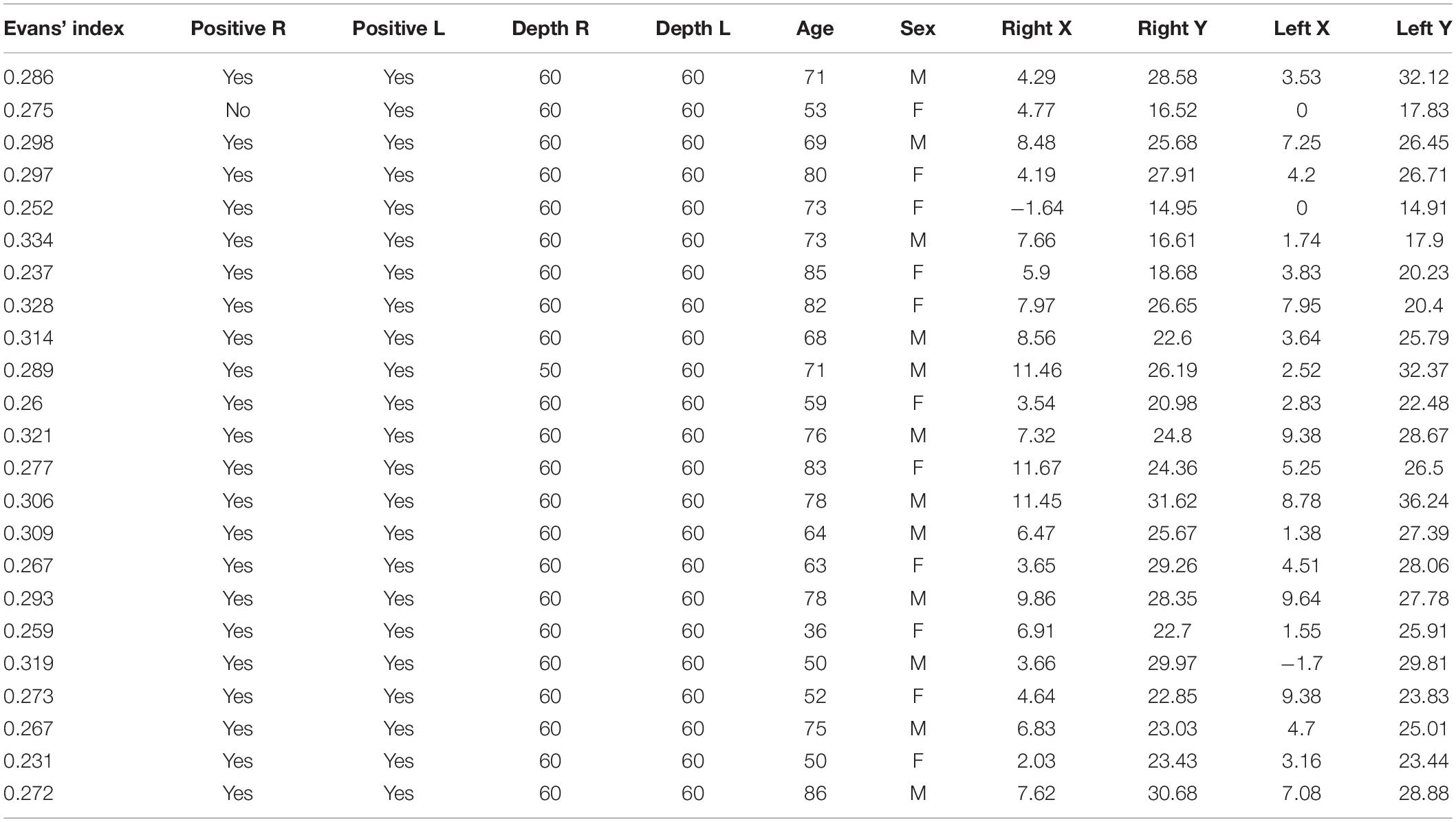
Table 2. Computer-based simulation with report of Evans’ index, depth in both sides, and data analysis.
The catheter tip distribution in the craniocaudal (CC) and in the latero-lateral (LL) position, calculated in our cohort using the computer-based simulation in the left and right brain hemispheres, is shown in Figure 8.
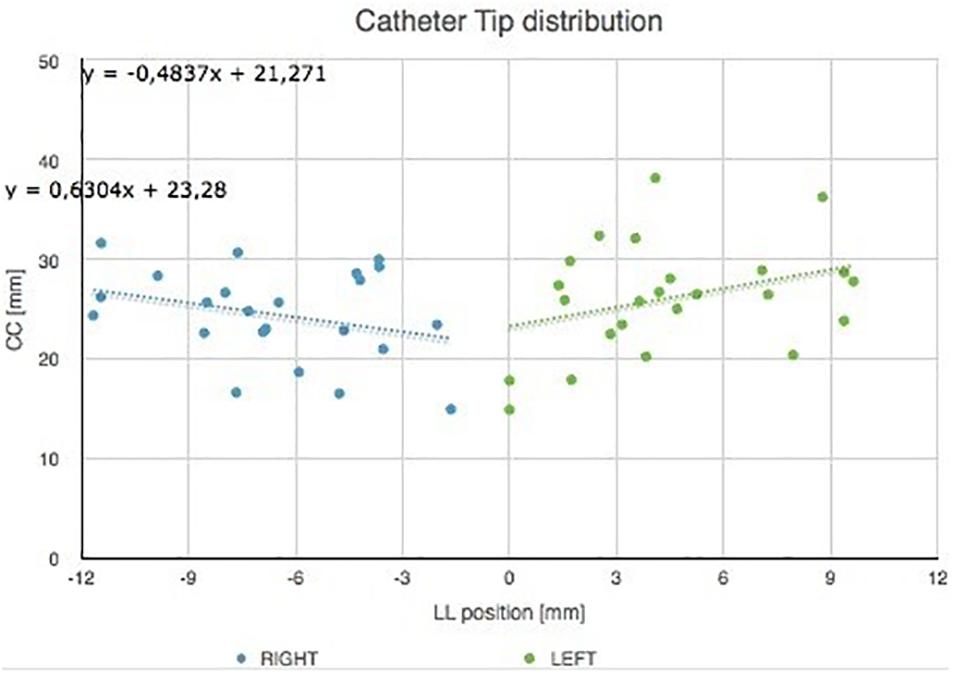
Figure 8. Catheter tip distribution in the craniocaudal (CC) and in the latero-lateral (LL) position in the left and right brain hemispheres, calculated in our cohort using the augmented reality tool. The trend lines of catheter tip position in the right and left lobes are indicated.
Figure 9 shows catheter tip distribution against patients’ sex/gender, highlighting, as expected, that CC position is lower for female than male patients due to skull shape and anatomical characteristics (t-test, p = 0.0003).
The cadaveric tests proved that any surgeon could use the device without any external help because it shows a good fixation inside the burr hole. The internal bone border created by the burr hole perforator, close to the dura, should be removed with a Kerrison rongeur to obtain the complete insertion of the proximal end of the device. The burr hole should have a minimum thickness of 5 mm for optimal insertion of the device. Thus, only in the case of a thick skull is it possible not to remove the inner skull layer and directly use the device.
The multimodal simulation performed with 3D neuronavigation and computer-based simulation showed Kakarla grade 1 in both sides after right and left EVD placements, in all cases but one, classified as grade 2. We considered only Kocher’s point, 11 cm from the nasion and 3 cm from the midline. The simulation with augmented reality, associated with 3D navigation using plaster skulls, demonstrated that the device positioned with the aid of mixed reality allowed an excellent entry point definition, as confirmed by the 3D navigation. The mean Evans’ index was 0.28 (Table 2).
The contact between the trocar and the skull occurs on a crown of points small enough to be assimilated to a circumference. Therefore, in order to study the geometric characteristics at the interface, we can analyze what happens in a generic section, as shown in Figure 10.
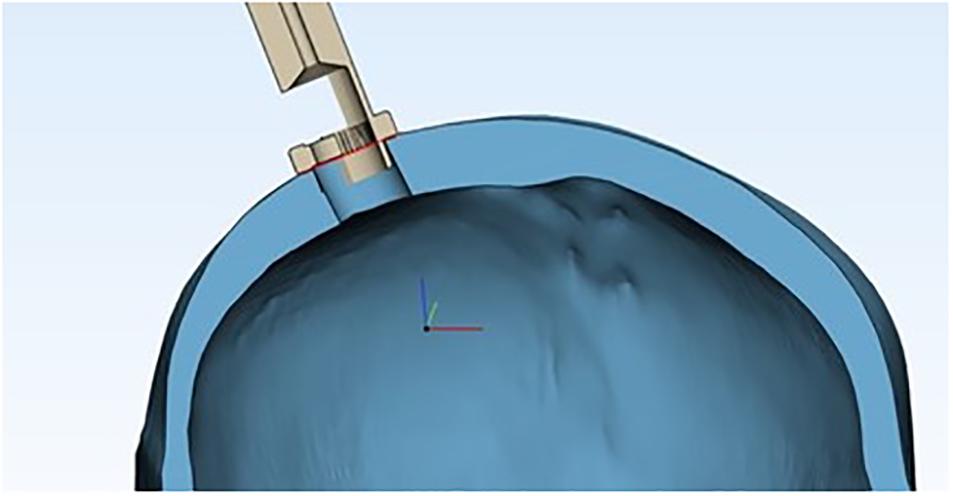
Figure 10. Cross section of the device’s proximal end inside the burr hole, showing the skull’s geometric properties, assuming the skull as a sphere and the burr hole as a chord.
The problem thus leads back to studying the geometric properties of circumference and chord (Figure 11).
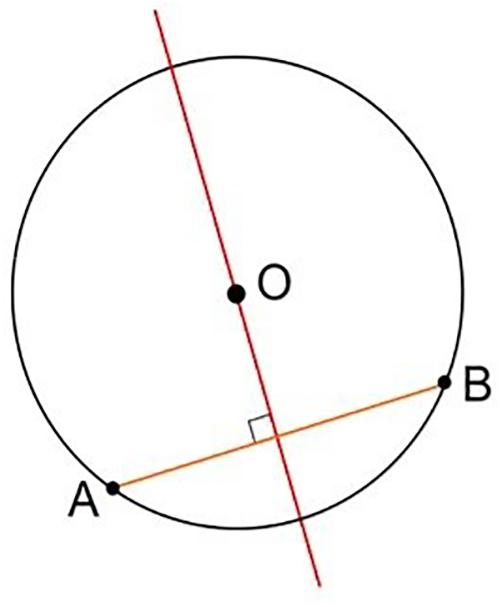
Figure 11. Geometric representation of the perpendicularity obtained, considering the plane as tangent to the spherical surface at the entry point.
Contact occurs, for each section considered, at the extreme points of the chord (A and B in Figure 11). Its axis, perpendicular to the plane tangent to the spherical surface at the entry point, corresponds to the direction of entry of the catheter guided by the instrument (Figure 12).
International recommendations for EVD placement suggest performing another burr hole if no CSF is obtained after more than three attempts (Greenberg, 2019), indirectly confirming that EVD misplacement is a risk that everybody will experience during their professional lives. The incidence of misplacement of the ventricular catheters reported in the literature is high, ranging from 12.3 to 60% according to the statistics (Saladino et al., 2009; Toma et al., 2009; Park et al., 2011; Bergdal et al., 2013; Chai et al., 2013; Rehman et al., 2013; Phillips et al., 2014), with possible associated complications (cerebral hematomas, neurological deficits, and seizures), which can dramatically worsen a patient’s clinical conditions (Woernle et al., 2011; Rosenbaum et al., 2013). Of notice, using the freehand technique, the only indirect sign of ventricular reach is the CSF drained from the distal catheter end, which does not give any information on the catheter’s trajectory.
Traversing brain parenchyma means creating damage from the cortex to the ventricle, with the risk of injury to cranial nerves, vessels, basal nuclei, or even the brainstem in case of mispositioning (Chai et al., 2013; Rosenbaum et al., 2013). Repeated attempts or even performing a second, contralateral, burr hole exponentially increases the risk of damage to eloquent structures.
We highlight the experimental nature of this study, in which we used merged technologies to assess the accuracy of the device. The use of the guide, together with the navigation, offered the possibility to assess the accuracy of the trajectory offered by the device, since CSF drain was not possible in a simulation setting.
The present novel device aims to obtain the perpendicularity relative to the bone surface, thus reducing EVD misplacement rate without modifying the standard EVD placement method.
Once the device is introduced into the skull, the catheter will follow a perpendicular route to the ventricle without further trajectory adjustments, thus allowing EVD placement independently from the head’s position. The device’s utility includes the possibility to perform ventricular catheter placement with the same head rotation as used for ventriculoperitoneal shunt placement.
The device’s proximal end shows a notch that prevents excessive insertion, thus avoiding parenchymal lesion. Still, the essential function is represented by its geometric characteristic of a plane superimposed on the skull, which can be assimilated to a sphere, thus reproducing the tangent of a sphere, which in turn helps to achieve the needed perpendicularity. The critical point of the procedure is the correct selection of the burr hole, which must be performed at the desired location, 11 cm from the nasion and 3 cm from midline; of course, a wrong burr hole placement will alter the entire procedure, as what happens in the standard freehand technique. The novel guide is entirely non-invasive, so it does not add any injury to the patient. The burr hole angle does not affect the trajectory because the device works on the skull’s surface, and the internal bone border can be removed.
This preliminary, non-invasive investigation is expected to improve the learning curves of these procedures and decrease the rates of misplacement of the ventricular catheters by adopting a patient-specific approach. The process can be performed with no additional dose of ionizing radiation, just by using the conventional imaging acquired before the surgical procedure. The device can be used on its own but is neuronavigation-ready. Navigation is handy in the case of midline shifts, in which the normal anatomy is altered and it is not possible to use the typical anatomical landmarks and in the case of “slit ventricles,” in which the target is challenging to reach (Gil et al., 2002; Mahan et al., 2013; Jakola et al., 2014; Thomale et al., 2018). If used on a patient with no midline shift, the device is as accurate as navigation, which is then unnecessary, thus saving costs (Chai et al., 2013).
The low economic impact is of great importance since this device could be particularly useful in low- and middle-income countries (LMICs), in which EVD placement is performed by non-neurosurgeons, mostly general surgeons, in remote areas where referral to neurosurgeons is time-consuming or impossible or the patients’ clinical conditions are worsening rapidly (Robertson et al., 2019a,b,c; Fuller et al., 2020). The aid of a simple guide for EVD placement could be of great benefit in those contexts, helping surgeons and improving outcomes. Given the high incidence of mispositioning among the neurosurgical statistics reported in the literature (Saladino et al., 2009; Toma et al., 2009; Park et al., 2011; Bergdal et al., 2013; Chai et al., 2013; Rehman et al., 2013; Phillips et al., 2014), the incidence of mispositioning in LMICs could be expected to be higher. The possibility of using a device that offers similar accuracy to neuronavigation could be of great benefit for LMICs, where expensive tools for neuronavigation are not available and non-neurosurgeons often perform EVD procedures in remote areas.
We did not consider midline shift because it includes an extensive range of conditions, causing the data to be non-homogeneous, and it is out of the interest of the study, which is focused on the assessment of the possibility to get the perpendicularity in the majority of cases in which EVD is required, i.e., patients affected by hydrocephalus, with no midline shift. Patients who require EVD placement should be distinguished from those requiring other surgeries, like patients affected by midline shift, in whom a craniotomy is usually needed. All this would have included biases and methodological inaccuracies that are not desirable and are not the core of this simulation study.
To obtain reliable results, we performed multimodal simulation by using the most advanced tools available. Plaster models of the skull were created to verify the accuracy of entry point definition by merging craniometric measures, augmented holographic reality reconstruction of the device, and 3D navigation. The latter was also used to assess the target by using a navigated stylet through the device, positioned inside the burr hole created in the plaster model. Augmented reality was also used to simulate the procedure using a holographic representation of both the skull and the device, thus improving familiarity with the simulation process and optimizing the device use. Of the utmost importance, the computer-based simulation using engineering software allows us to demonstrate the device’s accuracy mathematically.
According to our preliminary simulation study, the guide is a reliable device that deserves further investigation in larger cohorts and clinical settings. Considering a misplacement rate, reported in literature, of 12–60%, our results suggest that the device could improve safety and reduce the misplacement rate. Of course, clinical tests will provide more realistic data about its strength and limitations.
This study has been endorsed by the International Society of Reconstructive Neurosurgery (ISRN), and it has been proposed and accepted by five centers in Europe (Cannizzaro Hospital, Catania, Italy; Christian Doppler-Klinik – Universitätsklinikum der PMU, Salzburg, Austria; Fondazione Policlinico Universitario A. Gemelli IRCCS, Catholic University, Rome, Italy; Tor Vergata University Medical School, Rome, Italy; and Highly Specialized Hospital of National Importance Garibaldi, Catania, Italy), which could be further extended if needed.
Multimodal simulation offers unheard-of opportunities, allowing professionals to experience surgical procedures in a virtual setting that replicates, with high efficacy, the actual clinical practice, but with the further advantage of quantitatively analyzing all the steps, with the final aim to favor a safer and effective quality of care. Even in future clinical studies that we will promote, the association with neuronavigation will offer greater safety in EVD placement with this novel device.
Only after getting enough volume of data confirming the safety of EVD placement with this device would the procedure be performed only device-based with reasonable safety and efficacy. Again, this is a simulation, an experimental study to validate the trajectory offered by the device and its property to get the perpendicularity required immediately, indicated in literature to achieve the correct EVD placement.
This study provides encouraging results on a simple, cheap device for EVD placement. Our multimodal simulation, through cadaveric, 3D computer-based simulation, 3D plaster modeling, 3D neuronavigation, and augmented reality, shows that the device could offer a safer and more effective EVD placement. Our results should be validated in a multicenter clinical study, based on the encouraging data obtained in the present simulation study.
Number RM2014A000376: Guide for the positioning of cerebral catheters in neuronavigation, for the monitoring of intracranial pressure, CSF drainage in the case of sub-arachnoid hemorrhage or hydrocephalus; possible extension of drainage of abscesses and cerebral hematoma evacuation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
GU: conceptualization, writing—original draft preparation, supervision, and project administration. GU and LS: methodology. GS: methodology, form analysis, and writing—review and editing. RMi, SD, MG, and AS: software. SC, MV, DI, and GB: validation. GU, GS, LS, and KY: formal analysis and writing—review and editing. GU, MF, ST, GN, GR, and FG: investigation. GU, RMi, SD, and MG: resources. RMi, SD, and MG: data curation. GU, MS, and RMa: visualization. LS: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Authors RMi, SD, and MG are employed by the company MtOrtho which does not produce the device. They simply used the software to obtain calculations.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thank Profs. M. F. Fraioli and F. G. Garaci for their support in the preliminary steps. A special thanks to ApoQlar (Sirko Pelzl and Liliana Duarte) and MtOrtho (Gianluca Tropea) for their technical support for augmented reality and 3D computer reconstruction simulations, respectively. Thanks to Lorenzo Falcolini and Federico Righetti, who provided the prototypes of the device for testing.
Amoo, M., Henry, J., and Javadpour, M. (2021). Common trajectories for freehand frontal ventriculostomy: a systematic review. World Neurosurg. 146, 292–297. doi: 10.1016/j.wneu.2020.11.065
Bergdal, O., Springborg, J. B., Holst, A. V., Hauerberg, J., Way, S., Breum, P., et al. (2013). Accuracy of tunnelated vs. bolt-connected external ventricular drains. Clin. Neurol. Neurosurg. 115, 1972–1975. doi: 10.1016/j.clineuro.2013.05.026
Cabrilo, I., Craven, C. L., Dasgupta, D., Reddy, U., and Toma, A. K. (2021). Accuracy of bolt external ventricular drain insertion by neurosurgeons of different experience. Acta Neurochir. (Wien) 163, 1121–1126. doi: 10.1007/s00701-021-04712-7
Chai, F. Y., Farizal, F., and Jegan, T. (2013). Coma due to malplaced external ventricular drain. Turk Neurosurg. 23, 561–563. doi: 10.5137/1019-5149.JTN.5724-12.1
Fuller, A. T., Barkley, A., Du, R., Elahi, C., MScGH, Tafreshi, A. R., et al. (2020). Global neurosurgery: a scoping review detailing the current state of international neurosurgical outreach. J. Neurosurg. [Online ahead of pring] 1–9. doi: 10.3171/2020.2.JNS192517
Ghajar, J. B. (1985). A guide for ventricular catheter placement. Technical note. J. Neurosurg. 63, 985–986. doi: 10.3171/jns.1985.63.6.0985
Gil, Z., Siomin, V., Beni-Adani, L., Sira, B., and Constantini, S. (2002). Ventricular catheter placement in children with hydrocephalus and small ventricles: the use of a frameless neuronavigation system. Childs Nerv. Syst. 18, 26–29. doi: 10.1007/s00381-001-0550-3
Greenberg, M. S. (2019). Handbook of Neurosurgery, 9th Edn. New York, NY: Thieme Medical Publishers Inc.
Jakola, A. S., Reinertsen, I., Selbekk, T., Solheim, O., Lindseth, F., Gulati, S., et al. (2014). Three-dimensional ultrasound-guided placement of ventricular catheters. World Neurosurg. 82, 536.e5–539.e5. doi: 10.1016/j.wneu.2013.08.021
Kakarla, U. K., Kim, L. J., Chang, S. W., Theodore, N., and Spetzler, R. F. (2008). Safety and accuracy of bedside external ventricular drain placement. Neurosurgery 63(Suppl. 1) ONS162–ONS166, discussion ONS166-ONS167. doi: 10.1227/01.neu.0000335031.23521.d0<pmid<
Lee, C. K., Tay, L. L., Ng, W. H., Ng, I., and Ang, B. T. (2008). Optimization of ventricular catheter placement via posterior approaches: a virtual reality simulation study. Surg. Neurol. 70, 274–277. doi: 10.1016/j.surneu.2007.07.020 discussion 277-8,
Lee, K. S., Zhang, J. J. Y., Bolem, N., Leong, M. L., Goh, C. P., Hassan, R., et al. (2020). Freehand insertion of external ventricular drainage catheter: evaluation of accuracy in a single center. Asian J. Neurosurg. 15, 45–50. doi: 10.4103/ajns.AJNS_292_19
Mahan, M., Spetzler, R. F., and Nakaji, P. (2013). Electromagnetic stereotactic navigation for external ventricular drain placement in the intensive care unit. J. Clin. Neurosci. 20, 1718–1722. doi: 10.1016/j.jocn.2013.03.005
Park, B., Han, S., Byoun, H. S., Han, S., Choi, S. W., and Lim, J. (2020). The assessment of geometric reliability of conventional trajectory of ventriculostomy in a three dimensional virtual model and proposal of a new trajectory. Neurol. Med. Chir. (Tokyo) 60, 264–270. doi: 10.2176/nmc.oa.2019-0304
Park, Y. G., Woo, H. J., Kim, E., and Park, J. (2011). Accuracy and safety of bedside external ventricular drain placement at two different cranial sites : kocher’s point versus forehead. J. Korean Neurosurg. Soc. 50, 317–321. doi: 10.3340/jkns.2011.50.4.317
Patil, V., Lacson, R., Vosburgh, K. G., Wong, J. M., Prevedello, L., Andriole, K., et al. (2013). Factors associated with external ventricular drain placement accuracy: data from an electronic health record repository. Acta Neurochir. (Wien) 155, 1773–1779. doi: 10.1007/s00701-013-1769-y
Phillips, S. B., Delly, F., Nelson, C., and Krishnamurthy, S. (2014). Bedside external ventricular drain placement: can multiple passes be predicted on the computed tomography scan before the procedure? World Neurosurg. 82, 739–744. doi: 10.1016/j.wneu.2013.08.030
Pishjoo, M., Khatibi, K., Etemadrezaie, H., Zabihyan, S., Ganjeifar, B., Safdari, M., et al. (2021). Determinants of accuracy of freehand external ventricular drain placement by neurosurgical trainees. Acta Neurochir. (Wien) 163, 1113–1119. doi: 10.1007/s00701-020-04671-5
Rehman, T., Rehman, A., Ali, R., Rehman, A., Bashir, H., Ahmed Bhimani, S., et al. (2013). A radiographic analysis of ventricular trajectories. World Neurosurg. 80, 173–178. doi: 10.1016/j.wneu.2012.12.012
Robertson, F. C., Briones, R., Mekary, R. A., Baticulon, R. E., Jimenez, M. A., Leather, A. J. M., et al. (2019a). Task-sharing for emergency neurosurgery: a retrospective cohort study in the Philippines. World Neurosurg. X 6:100058. doi: 10.1016/j.wnsx.2019.100058
Robertson, F. C., Esene, I. N., Kolias, A. G., Kamalo, P., Fieggen, G., Gormley, W. B., et al. (2019b). Task-shifting and task-sharing in neurosurgery: an international survey of current practices in low- and middle-income countries. World Neurosurg. X 6:100059. doi: 10.1016/j.wnsx.2019.100059
Robertson, F. C., Esene, I. N., Kolias, A. G., Khan, T., Rosseau, G., Gormley, W. B., et al. (2019c). Global perspectives on task shifting and task sharing in neurosurgery. World Neurosurg. X 6:100060. doi: 10.1016/j.wnsx.2019.100060
Rosenbaum, B. P., Wheeler, A. M., and Krishnaney, A. A. (2013). External ventricular drain placement causing upgaze palsy: case report. Clin. Neurol. Neurosurg. 115, 1514–1516. doi: 10.1016/j.clineuro.2012.12.008
Saladino, A., White, J. B., Wijdicks, E. F., and Lanzino, G. (2009). Malplacement of ventricular catheters by neurosurgeons: a single institution experience. Neurocrit. Care 10, 248–252. doi: 10.1007/s12028-008-9154-z
Shimizu, S., Tanaka, R., Iida, H., and Fujii, K. (2004). Manual occipital ventricular puncture for cerebrospinal fluid shunt surgery: can aiming be standardized? Neurol. Med. Chir. (Tokyo) 44, 353–357. discussion 358, doi: 10.2176/nmc.44.353
Shtaya, A., Roach, J., Sadek, A. R., Gaastra, B., Hempenstall, J., and Bulters, D. (2018). Image guidance and improved accuracy of external ventricular drain tip position particularly in patients with small ventricles. J. Neurosurg. [Online ahead of print] 1–6. doi: 10.3171/2017.11.JNS171892
Siesjö, P. (2014). The enigma of external ventricular drain placement. World Neurosurg. 82, 597–598. doi: 10.1016/j.wneu.2013.10.056
Sun, Z., Wu, L., Liu, Z., Zhong, W., Kou, Z., and Liu, J. (2020). Optimizing accuracy of freehand cannulation of the ipsilateral ventricle for intracranial pressure monitoring in patients with brain trauma. Quant. Imaging Med. Surg. 10, 2144–2156. doi: 10.21037/qims-20-128
Thomale, U. W., Schaumann, A., Stockhammer, F., Giese, H., Schuster, D., Kästner, S., et al. (2018). GAVCA Study: randomized, multicenter trial to evaluate the quality of ventricular catheter placement with a mobile health assisted guidance technique. Neurosurgery 83, 252–262. doi: 10.1093/neuros/nyx420
Toma, A. K., Camp, S., Watkins, L. D., Grieve, J., and Kitchen, N. D. (2009). External ventricular drain insertion accuracy: is there a need for change in practice? Neurosurgery 65, 1197–1200, discussion 1200-1201 doi: 10.1227/01.NEU.0000356973.39913.0B
Wilson, M. P., O’Kelly, C., Jack, A. S., and Rempel, J. (2018). Utilizing preprocedural CT scans to identify patients at risk for suboptimal external ventricular drain placement with the freehand insertion technique. J. Neurosurg. [Online ahead of print] 1–7. doi: 10.3171/2018.1.JNS172839
Keywords: hydrocephalus, shunt, ventricle, cerebral hypertension, ventricular catheter
Citation: Umana GE, Scalia G, Yagmurlu K, Mineo R, Di Bella S, Giunta M, Spitaleri A, Maugeri R, Graziano F, Fricia M, Nicoletti GF, Tomasi SO, Raudino G, Chaurasia B, Bellocchi G, Salvati M, Iacopino DG, Cicero S, Visocchi M and Strigari L (2021) Multimodal Simulation of a Novel Device for a Safe and Effective External Ventricular Drain Placement. Front. Neurosci. 15:690705. doi: 10.3389/fnins.2021.690705
Received: 03 April 2021; Accepted: 04 May 2021;
Published: 14 June 2021.
Edited by:
Sandro M. Krieg, Technical University of Munich, GermanyReviewed by:
Camilo Molina, Washington University School of Medicine, United StatesCopyright © 2021 Umana, Scalia, Yagmurlu, Mineo, Di Bella, Giunta, Spitaleri, Maugeri, Graziano, Fricia, Nicoletti, Tomasi, Raudino, Chaurasia, Bellocchi, Salvati, Iacopino, Cicero, Visocchi and Strigari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Emmanuele Umana, dW1hbmEubmNoQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.