
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 07 February 2022
Sec. Neurodevelopment
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.685372
This article is part of the Research Topic Adolescence and Risk of Psychopathology: Understanding Trajectories and Early Interventions View all 13 articles
 Siyu Ma1†
Siyu Ma1† Yuanli Hu1†
Yuanli Hu1† Yuting Liu2†
Yuting Liu2† Yiwei Pu1†
Yiwei Pu1† Pengcheng Zuo1
Pengcheng Zuo1 Qinghui Hu1
Qinghui Hu1 Zhaocong Yang1
Zhaocong Yang1 Feng Chen1
Feng Chen1 Zongyun Xie1
Zongyun Xie1 Yueshuang Cun1
Yueshuang Cun1 Xiaoxu Liu1
Xiaoxu Liu1 Ming Yang2*
Ming Yang2* Xuming Mo1*
Xuming Mo1*Despite intracardiac malformation correction, children with Tetralogy of Fallot (TOF) may still suffer from brain injury. This cross-sectional study was primarily designed to determine the relationship between blood oxygenation level-dependent (BOLD) signal changes after surgery and cognition in school-aged children with TOF. To evaluate the differences between TOF children (n = 9) and healthy children (n = 9), resting-state functional magnetic resonance imaging (rs-fMRI) and the Wechsler Intelligence Scale for Children–Chinese revised edition (WISC-CR) were conducted in this study. The results showed that TOF children had a lower full-scale intelligence quotient (FSIQ, 95.444 ± 5.354, p = 0.022) and verbal intelligence quotient (VIQ, 92.444 ± 4.708, p = 0.003) than healthy children (FSIQ = 118.500 ± 4.330;VIQ = 124.250 ± 4.404), and that significant differences in regional homogeneity (ReHo) and amplitude of low-frequency fluctuation (ALFF) existed between the two groups. Besides, VIQ had significantly positive correlations with the decreased ALFF value of the middle inferior occipital gyrus (MIOG, beta = 0.908, p = 0.012) after fully adjusting for all covariates. In addition, elevated ReHo values of the left and right precuneus were positively related to ALFF in the MIOG. This study revealed that brain injury substantially influences neural activity and cognition in postoperative TOF children, providing direct evidence of an association between BOLD signal changes and the VIQ and prompting further attention to language development in TOF children.
Tetralogy of Fallot (TOF) is one of the most common cyanotic congenital cardiac malformations (Diaz-Frias and Guillaume, 2020), accounting for approximately 5% of all congenital heart diseases (CHDs) (Apitz et al., 2009). TOF is characterized as a ventricular septal defect (VSD), aortic overriding, right ventricular outflow tract obstruction, and right ventricular hypertrophy (Warnes et al., 2008), and patients with TOF exhibit a decrease in systemic vascular resistance and an increase in pulmonary resistance, which leads to a right-to-left shunt in combination with a VSD and an obvious decrease in saturation (Ho et al., 2018). Less than 1% of patients survive to 40 years old naturally (Ai et al., 2018), but nearly 90% of patients with early diagnosis and surgical treatment will go on to survive (Hickey et al., 2009); however, adverse outcomes can still occur many years after cardiac surgery, including heart failure, arrhythmia, and right ventricular outflow tract reobstruction (Apitz et al., 2009). Notably, brain injury is still a non-negligible issue that persistently negatively influences survivors (Gaynor et al., 2015).
Brain injury among repaired TOF cases has recently been reported, for which children with multiple cerebral domain injuries to areas such as the cortex, basal ganglia, thalamus, and cerebral white matter account for nearly a fifth of cases (Hovels-Gurich et al., 2006; Leonetti et al., 2019). Moreover, many studies have shown that children with cerebral injuries usually have neurodevelopmental disabilities, which may manifest as cognitive impairment, oral dyskinesia, language expression abnormalities, motor delays, or attention deficit/hyperactivity disorder (ADHD) (Marino et al., 2012; Mussatto et al., 2014; Wernovsky and Licht, 2016). Although it is thought that human neurodevelopment is almost complete at the end of the second or third trimester of pregnancy, recent studies have shown that cortical neurogenesis is still active after birth (Morton et al., 2017; Ortinau et al., 2018), and that children with CHDs have delayed brain development (Clouchoux et al., 2013). Thus, early detection and intervention could afford greater neurodevelopmental benefits for children with CHDs. Fortunately, functional magnetic resonance imaging (fMRI) has been applied to identify neurodevelopmental disabilities more accurately (Ma et al., 2020).
Two blood oxygenation level-dependent (BLOD) fMRI signals, regional homogeneity (ReHo) and the amplitude of low-frequency fluctuations (ALFFs) (Zang et al., 2004, 2007), have been widely used to investigate the pathophysiology, diagnosis, and treatment effectiveness of cognitive disorders (Ni et al., 2016; Bak et al., 2018; Gur et al., 2021) and neuropsychiatric disorders, such as depression (Liu et al., 2012), schizophrenia (Guo et al., 2014; Xu et al., 2015), epilepsy (Maneshi et al., 2014), Alzheimer’s disease (Liu et al., 2014; Lyu et al., 2021), and Parkinson’s disease (Helmich et al., 2010; Li et al., 2018; Yue et al., 2020). ReHo reflects regional functional connectivity or synchronization and indicates the regional integration of information processing (Zuo et al., 2013; Jiang et al., 2015). ALFF is a method of monitoring low-frequency brain spontaneous activity and blood oxygen levels, which indicates the spontaneous activity of brain regions (Zang et al., 2007).
However, there was no research on ReHo and ALFF changes in TOF or CHD and the influence of ReHo and ALFF changes on the cognitive abilities of children following TOF repair surgery remains to be elucidated. Our study explored ReHo and ALFF fMRI changes, evaluated the cognitive abilities of TOF children, and further identified the relationship between them. Interestingly, the results revealed that decreased ALFF values in the left middle inferior occipital gyrus are correlated with a lower verbal intelligence quotient (VIQ).
From November 2015 to June 2016, 9 school-aged children with TOF after repair surgery were validated as participants, and 9 healthy children (HC), identified as having no cardiovascular or nervous system diseases and matched with the TOF children by age, sex, and education, were enrolled as the control group. All TOF children underwent correction surgery at Children’s Hospital of Nanjing Medical University and were identified to be free of central nervous system diseases or hereditary syndromes, such as craniocerebral trauma, cerebral tumors, or Down syndrome. Informed consent was obtained from all the participants’ legal guardians. All children were right handed and had no known contraindications to MRI, including claustrophobia and implanted pacemakers. Finally, all 18 children underwent resting-state fMRI (rs-fMRI) examination. Additionally, all data met the standards for further analysis: All participants completed all testing tasks of Wechsler Intelligence Scale. The results need not to be calculated by alternative subtests. All children were requested to keep their eyes closed and avoid sleeping, thinking about anything, or any head motion as far as possible (less than 1 mm of translation or 1° of rotation) during the MRI scanning.
The Wechsler Intelligence Scale for Children–Chinese revised edition (WISC-CR) was used to evaluate the cognitive abilities of the subjects. The WISC is an authoritative intelligence scale to assess the cognitive ability of children (Marino et al., 2012). Based on the Chinese population, the WISC-CR was adaptive for Chinese children aged 6–16, and the test contains 12 domains, including analogies, common sense, arithmetic, comprehension, vocabulary, digit span, picture arrangement, missing picture completion, block design, decoding, object collocation, and mazes (of these, digit span and mazes are optional). According to the operating manual and adjusted for the subject’s age, the subjects’ scores were calculated. The VIQ depended on the first six items, and the performance intelligence quotient (PIQ) depended on the last six items. Finally, the full-scale intelligence quotient (FSIQ) was measured.
Functional MRI scans were performed on all subjects using a 1.5T MRI scanner (Siemens MAGNETOM Trio, Erlangen, Germany) at the Radiology Department of our hospital. Earplugs and foam were used to decrease scanning noise and head motion, respectively. All subjects were not anesthetized, and were required to keep their eyes closed and avoid sleeping, thinking about anything, or any head motion as far as possible during the scanning. In total, 176 high spatial resolution T1-weighted structural images were acquired using a magnetic prepared gradient echo (GE) sequence [repetition time (TR) = 1,940 ms, echo time (TE) = 3.08 ms, field of view (FOV) = 250 mm × 250 mm, matrix = 256 × 256, slice thickness = 1 mm; flip angle = 15°], and 180 functional images by using an echo-planar imaging sequence sensitive to BOLD contrast (TR = 2,000 ms, TE = 25 ms, FOV = 240 mm × 240 mm, matrix = 64 × 64, slice thickness = 4 mm, flip angle = 90°). Twenty-two fluid attenuated inversion recovery images were used to screen for structural brain lesions by two radiology chief physicians (TR = 8,000 ms, TE = 92 ms, FOV = 220 mm × 220 mm, matrix = 512 × 464, slice thickness = 5 mm).
Preprocessing was carried out by applying DPARSFA using SPM8.1
We underwent preprocessing as follows:
(1) We have collected 180 points by BOLD in this study and removed the first 10 time points to reduce the influence of MRI magnetic field instability and noise during the initial scan.
(2) Slice-timing correction: we carried out slice-timing correction for the remaining time points make different layers within a TR equivalent to the same time acquisition.
(3) Head movements correction: This included translation and rotation in 3D space. Considering the long scan time and the influence of magnetic resonance noise, the purpose of head movement correction was to eliminate the tiny head movement caused by respiration, heartbeat, and other physiological factors. Subjects would be removed from this study when their head movement of x, y, or z axis were more than 1 mm of translation or 1° of rotation.
(4) Spatial registration: All MRI images were standardized to the same reference space (standard anatomical template for the head, Montreal Neurological Institute, Canada) because of the differences in brain morphology among different subjects.
(5) Linear detrending: The standardized data were then processed to remove linear trends to remove physiological linear drift, noise caused by head movement, and instability of the machine.
(6) Low-frequency filtering. A frequency band of 0.01–0.08 Hz was considered valuable physiological signals and used to filter out low-frequency drift.
(7) Spatial smoothing. A Gaussian kernel function with a full width at half maximum (FWHM) of 4 × 4 × 4 mm3 was used to perform spatial smoothing of fMRI images.
The preprocessed fMRI data were used for the ReHo analysis of all subjects. Kendall’s coefficient concordance (KCC) was used as an indicator to measure the similarity between the time series of a voxel and those of its adjacent neighbors in the ReHo analysis. We considered 27 individual voxels as a whole and acquired ReHo maps for each participant by calculating the KCC value between each individual voxel and the other 26 adjacent voxels. ReHo images were smoothed with an isotropic Gaussian kernel of 4 mm full-width half-maximum (FWHM) to reduce space noise. The brain was divided into different regions of interest (ROI), and the mean ALFF value of each ROI was calculated with the Resting-State fMRI Data Analysis Toolkit (REST version 1.8)2 (Song et al., 2011). ReHo and ALFF differences between the children with TOF and the HC were analyzed with a two-sample t-test in REST software. AlphaSim was calibrated with P < 0.01 and a cluster size = 23 voxels in the ReHo analysis. AlphaSim was calibrated with P < 0.05 and a voxel size > 65 in the ALFF analysis.
SPSS 20.0 (IBM Corp., Armonk, NY, United States) was used to perform the statistical analyses in the study. We present continuous variable data as the mean ± SE in Table 1. Differences between the children with TOF and the HC were calculated by two-sample t-tests. Correlations between the ALFF value, ReHo value, intelligence quotient (IQ), and related covariates were investigated by using single and multiple linear regression analyses. Statistical significance was considered when P < 0.05.
The demographic characteristics of the TOF and HC groups are shown in Table 1. The summarized hospital information of the TOF children is also shown. No significant differences were observed for age, sex, education, or household income. Additionally, children with TOF had lower VIQ (92.444 ± 4.708, P = 0.003) and FSIQ (95.444 ± 5.354, P = 0.022) scores than the HC.
Differences in ReHo and ALFF are separately shown in Tables 2, 3, respectively. Compared with the HC group, ReHo values were increased in the right brainstem, right middle occipital gyrus, right inferior parietal gyrus, right precuneus, and left precuneus of the TOF group, but reduced in the right posterior lobe of the cerebellum and right inferior temporal gyrus (Table 2 and Figure 1). In addition, TOF children had higher ALFF values of the left medial prefrontal cortex, left cingulum and right parahippocampal gyrus, and lower ALFF values of the left cerebellum, left middle inferior occipital gyrus (MIOG.L), left inferior occipital gyrus, and right cerebellum (Table 3 and Figure 2).
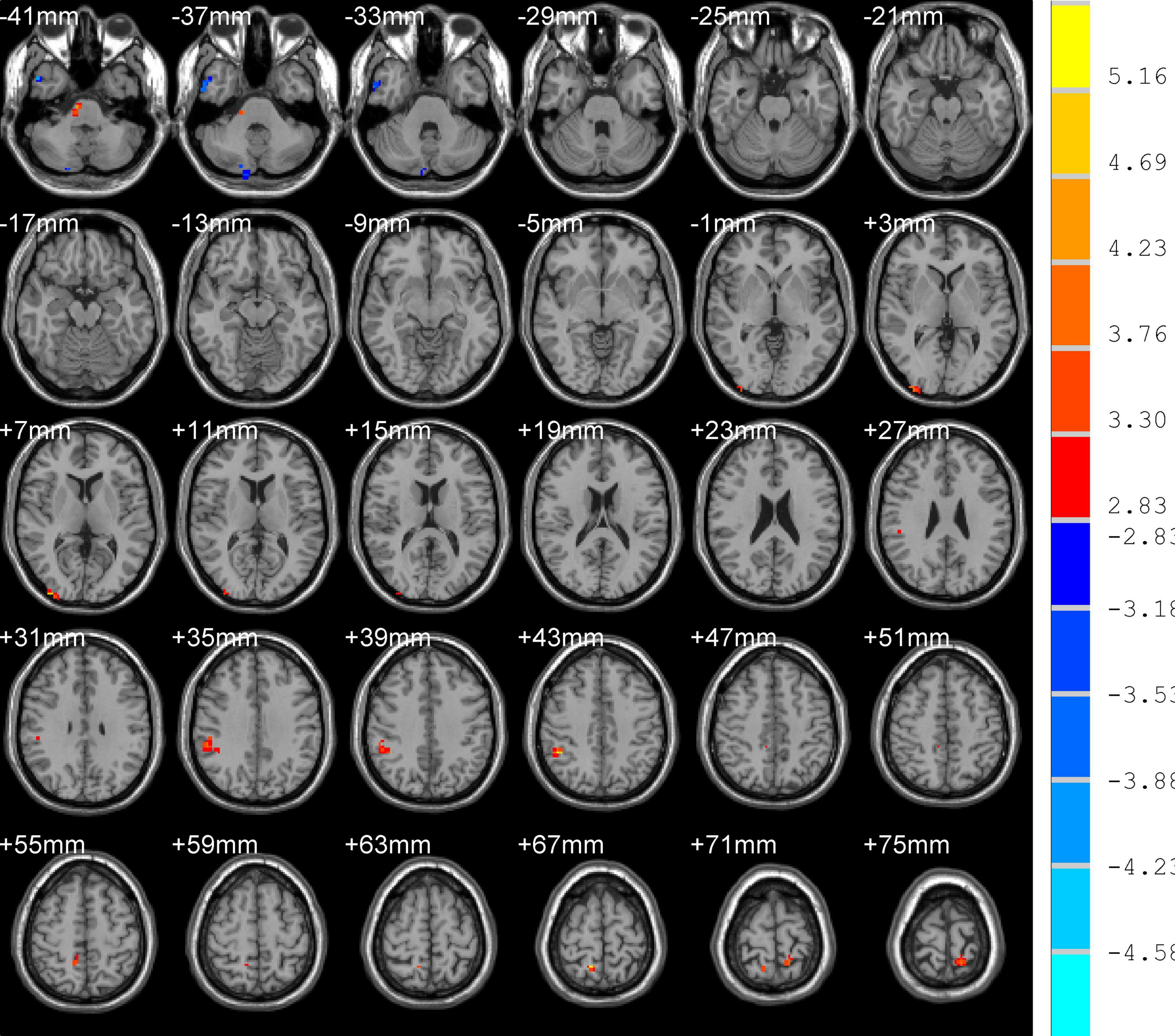
Figure 1. Compared with HC, TOF children showed increased ReHo values in the right brainstem, right middle occipital gyrus, right inferior parietal gyrus, right precuneus, and left precuneus, and decreased ReHo values in the right posterior lobe of the cerebellum and right inferior temporal gyrus. AlphaSim correction with P < 0.01; cluster size > 23.
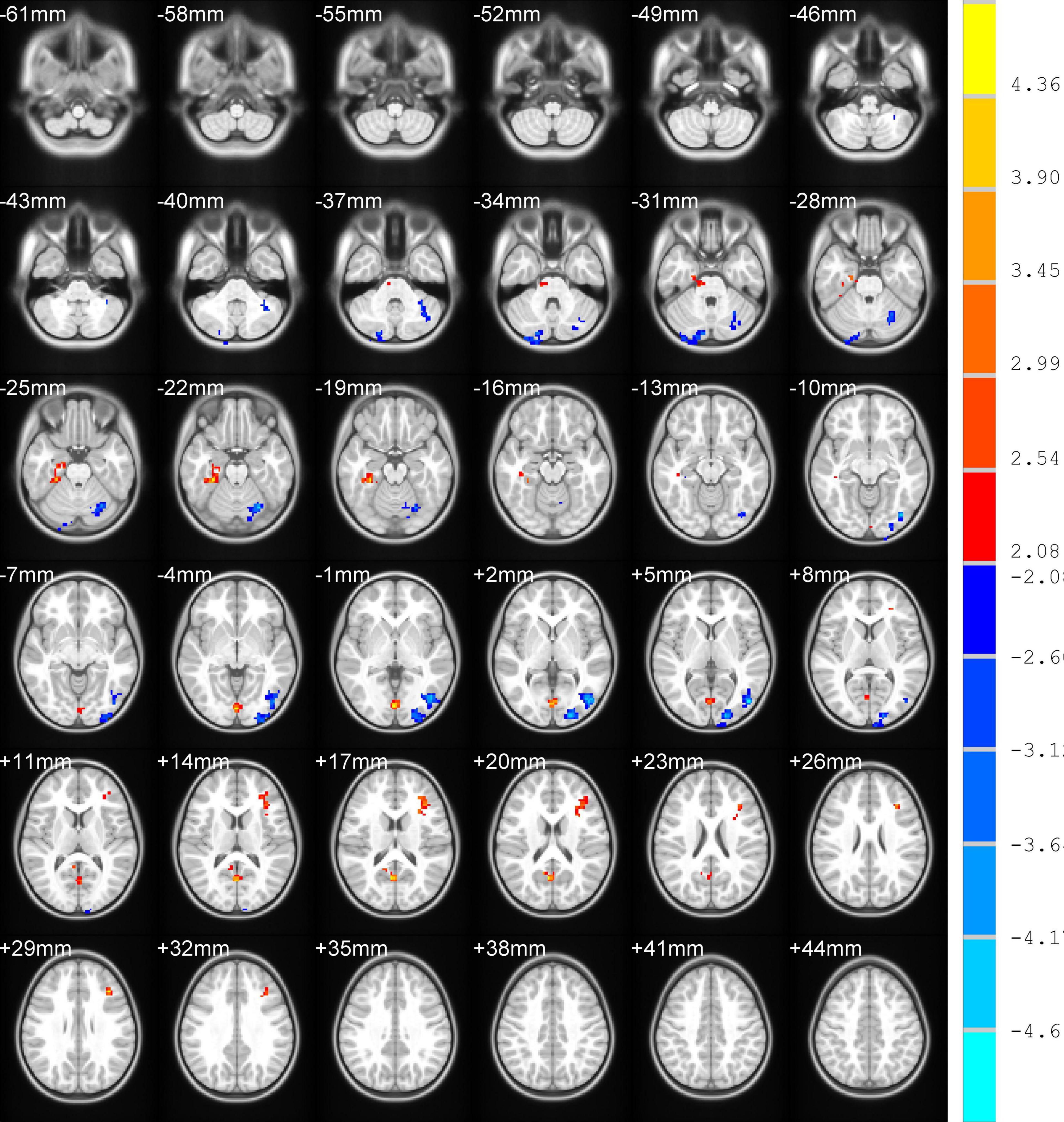
Figure 2. Compared with HC, TOF children had higher ALFF values in the left medial prefrontal cortex, left cingulum, and right parahippocampal gyrus, but lower ALFF values in the bilateral cerebellum, left middle inferior occipital gyrus, and left inferior occipital gyrus. AlphaSim correction with P < 0.05; cluster size > 65.
After analyzing the Pearson correlations between the demographic variables and ReHo or ALFF changes in TOF children, age at surgery, postoperative time, preoperative saturation of pulse oxygen (SpO2), preoperative systolic blood pressure (SBP), cardiopulmonary bypass (CBP) time, and aortic occlusion (AO) time were found to be related to ReHo changes (Supplementary Table 1), and preoperative SpO2, CBP time, and AO time were related to ALFF changes (Supplementary Table 2).
The correlations between VIQ, FSIQ, and ReHo or ALFF changes were further determined by multiple linear regression (Table 4 and Supplementary Table 3). The results showed that only ALFF changes in the MIOG.L were positively associated with VIQ (beta = 0.908, P = 0.012) after adjusting for all covariates (model 3). Additionally, Table 5 shows that ReHo changes in the right precuneus (PCUN.R) and left precuneus (PCUN.L) were positively associated with ALFF changes in the MIOG.L.
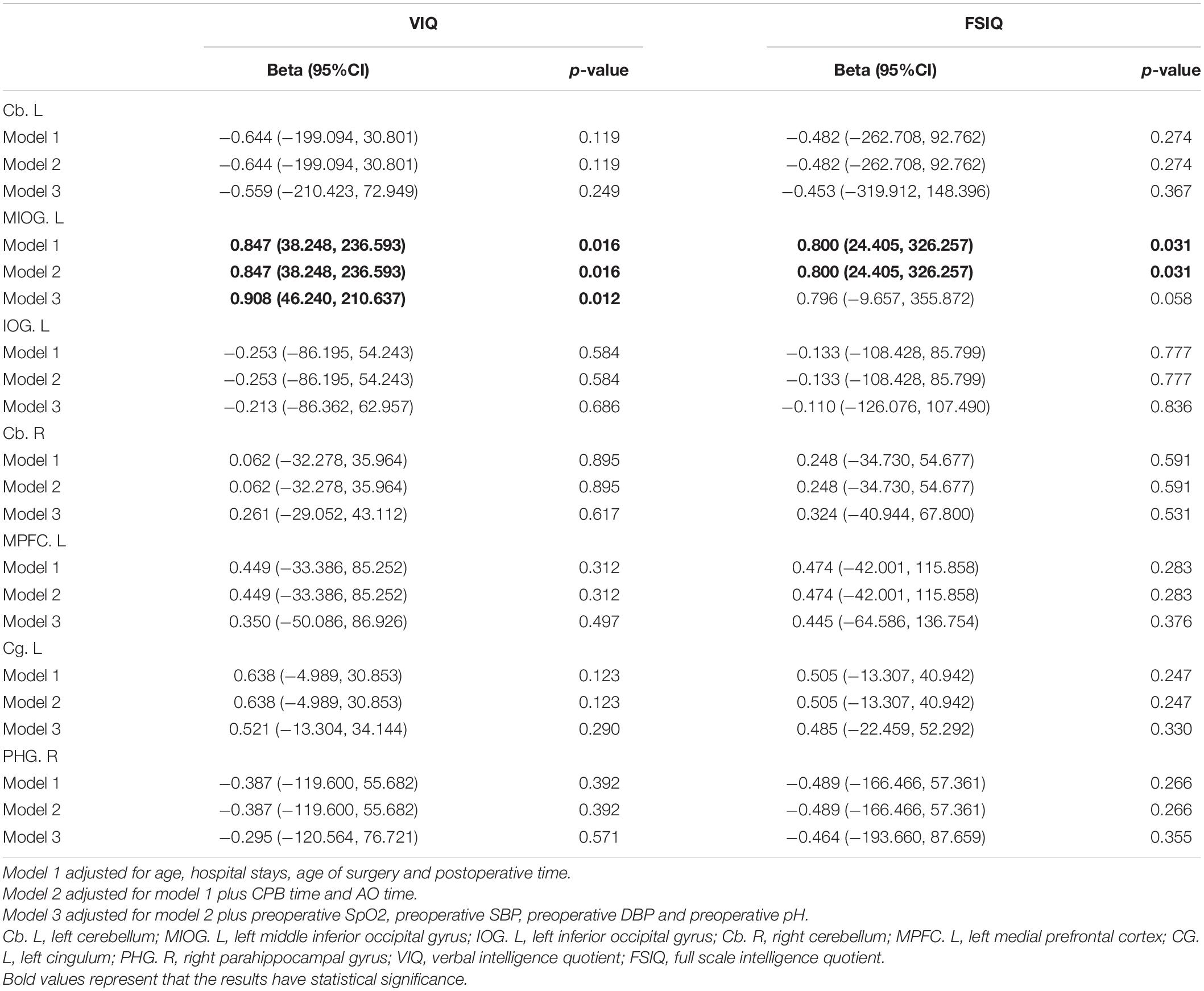
Table 4. Multivariable association of cerebral amplitude of low frequency fluctuations changings and cognitive abilities in TOF postoperative children.
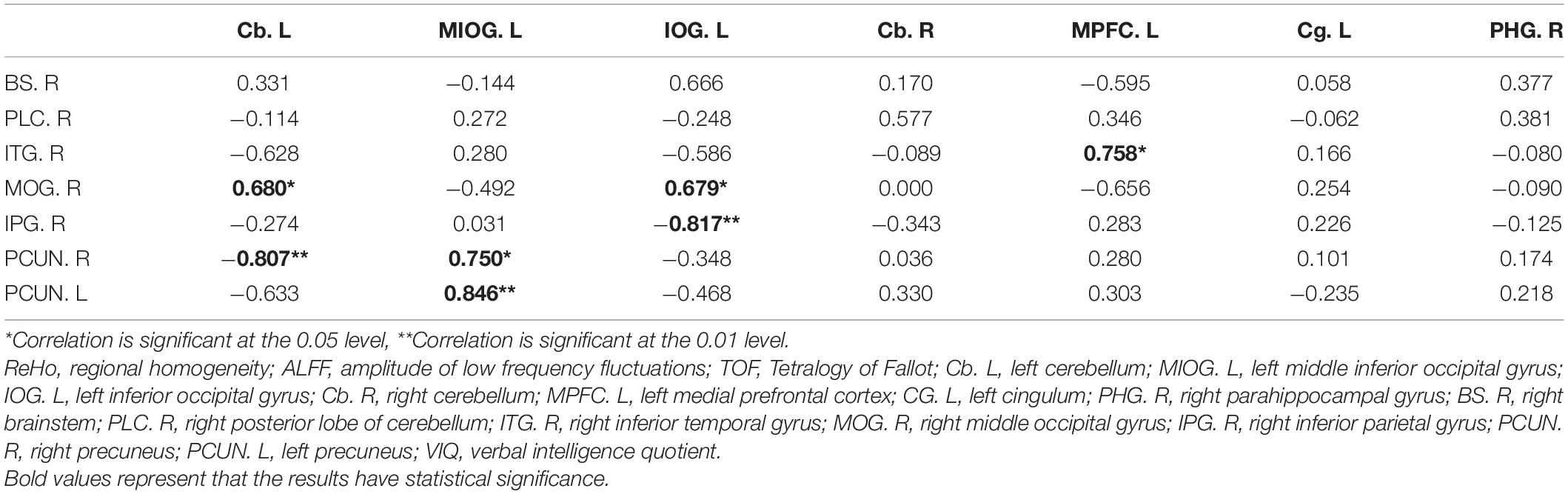
Table 5. Pearson correlation between cerebral ReHo changings and cerebral ALFF changings in TOF group.
Our cross-sectional study was the first to identify positive correlations between decreased ALFF values in the MIOG.L and a lower VIQ in postoperative TOF children at school age. In addition, abnormal ReHo values in the PCUN.L and PCUN.R were also positively related to ALFF values in the MIOG.L, which may compensate for the VIQ caused by low ALFF values in MIOG.L.
ALFF and ReHo, two BOLD signals, can indirectly reflect brain function (Golestani et al., 2017). Our previous study focused on the effects of structural alteration on the cognitive abilities of TOF children, indicating positive correlations between decreased cortical thickness and a low VIQ (Ma et al., 2020). Considering decreased oxygen consumption and oxygen delivery in the brain before surgery (Sun et al., 2015), close attention to neuronal activity changes should be paid in children with TOF. However, few studies have investigated the relationship between neuronal activity and cognition in TOF children. Herein, based on units of neurons, the association between neuronal activity and cognition was determined in this study, and combined with the existing research, the underlying mechanisms of a low VIQ induced by abnormal ReHo and ALFF values were also explored.
The results revealed that TOF children with lower ALFF values in the MIOG.L had a lower VIQ, indicating that the MIOG.L plays a critical role in language development. Generally, the MIOG.L is recognized as a visual processing region; however, it is also important to speech perception, including semantic and syntactic processing, language processing strategy, semantic representation, object identification, and generalization (Pigdon et al., 2019; Stroh et al., 2019; Victoria et al., 2019; Fairhall, 2020; Liu et al., 2020; Maffei et al., 2020). Different from the traditional understanding of visual regions, some studies have shown that visual processing also promotes early language development (Teinonen et al., 2008). Recently, left occipital regions were demonstrated as a visual input to language areas, which might manifest as pure alexia (without agraphia) when affected by ischemia (Sheetal et al., 2019). Additionally, a study on children with developmental language disorders showed that increased left inferior occipital volume may compensate for language regions (Pigdon et al., 2019). In addition, occipital regions can couple with other brain regions participating in many forms of verbal processing. When syntactic processing occurs, the left middle occipital regions along with the right supramarginal gyrus (SMG) are activated (Stroh et al., 2019). Moreover, population-based studies have shown that occipitotemporal (OT) regions play a crucial role in transforming visual symbols into meanings and sounds (Taylor et al., 2019; Zhou et al., 2019) and that semantic information strengthens the connection between OT regions and the left ventral inferior frontal gyrus (Wang J. et al., 2019).
Our results also showed that decreased ALFF values in the MIOG.L are positively correlated with increased ReHo values in the PCUN.L and PCUN.R, suggesting that elevated neuronal connections in the PCUN.L and PCUN.R may compensate for the low VIQ induced by inactive spontaneous activity in the MIOG.L. The PCUN, an associative region, is a part of the posteromedial parietal cortex, which engages in visuospatial imagery, episodic memory retrieval, self-processing, and consciousness by associating with many brain regions, including the thalamus, posterior cingulate, supplementary motor area, and dorsal premotor area (Cavanna and Trimble, 2006; Cunningham et al., 2017; Wang Z. et al., 2019). In addition, the parietal cortex, along with the temporal cortex and occipital cortex, connects to the temporoparietooccipital cortex (TPO), which is a highly associative cortical network and is involved in the integration of somatosensory, auditory, and visual information (Leichnetz, 2001). Additionally, many studies have reported that the PCUN is related to semantic processing and phonologic processing (Kjaer et al., 2001; Coslett and Schwartz, 2018). Similarly, our previous study suggested that the PCUN may connect with the temporal lobe via the temporoparietal junction (TPJ) and influence VIQ in TOF children (Ma et al., 2020). Thus, based on our results and those of previous studies, we speculate that increased neuronal connections in the PCUN.L and PCUN.R play a compensatory role in low VIQ induced by decreased ALFFs in the MIOG.L.
However, some limitations still exist in our study. First, our research should have recruited more participants to increase the reliability and generalizability of the results. Moreover, we evaluated the cognitive ability of TOF children after surgery at school age, and the specific timing of the start and maintenance of ReHo and ALFF changes is still unclear. Those children require continuous follow up. Furthermore, though recent study on 7–11 years old children who were conducted general anesthesia under 3 has shown that the exposure to general anesthesia in early childhood was not markedly related to the reduced intelligence in later stage (Schuttler et al., 2021), anesthesia was still demonstrated to be a non-negligible factor of cognitive levels in many studies (Pang et al., 2021; Shen et al., 2021; Wu and Zhu, 2021). However, we did not analyze the effect of anesthesia on cognition because of lots of anesthesia data missed. Additionally, children who were suspected of having neurodevelopmental disorders according to their guardians were more likely to be enrolled in this study, which may result in certain biases. Finally, this study was a cross-sectional study and cannot be used to determine the causal relationship between fMRI changes and cognition.
In summary, compared with HC, postoperative children with TOF had a lower VIQ at school age, which was positively related to decreased ALFF values of the MIOG.L. The results revealed that preoperative brain injury in TOF children, even at school age, have persistent negative effects on neurons, especially in the MIOG.L. The possible mechanisms may be delayed language development caused by inactive spontaneous neural activity in the MIOG.L. Fortunately, these changes might be compensated by increased neural connections in the PCUN.L and PCUN.R. Therefore, it is important to pay close attention to the language development of children with TOF. Cognitive ability evaluations, especially verbal cognition, should be included in conventional postoperative reviews and follow-ups.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Children’s Hospital of Nanjing Medical University ethics committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
XM and MY designed the study protocol and proofread the manuscript. YH, YL, and SM collected the information and performed the data analysis under the close supervision of PZ, YP, ZY, FC, ZX, YC, and XL. SM drafted the manuscript. YP provided the help of technology. QH took charge of the modification of language. All authors contributed to the article and approved the submitted version.
This project was supported by the China Postdoctoral Science Foundation (2019M651904), the Medical Science and Technology Development Foundation, the Nanjing Municipality Health Bureau (ZKX19039), the National Key Research and Development Program of China (2016YFC1101001, 2017YFC1308105), the National Natural Science Foundation of China (81970265, 82000303), the Natural Science Foundation of Jiangsu Province (BK20180144), the Jiangsu Commission of Health Project (LGY2019009), and the Nanjing Science and Technology Development Project (2019060007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer Y-CC declared a shared affiliation, though no other collaboration, with the authors to the handling Editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.685372/full#supplementary-material
Ai, X., Ye, Z., Li, W., Zheng, J., You, C., and Xu, J. (2018). Intracerebral hemorrhage in an adult patient with Tetralogy of Fallot:case report and review of the literature. Medicine 97:e11733. doi: 10.1097/MD.0000000000011733
Apitz, C., Webb, G. D., and Redington, A. N. (2009). Tetralogy of Fallot. Lancet 374, 1462–1471. doi: 10.1016/S0140-6736(09)60657-7
Bak, Y., Jun, S., Choi, J. Y., Lee, Y., Lee, S. K., Han, S., et al. (2018). Altered intrinsic local activity and cognitive dysfunction in HIV patients:a resting-state fMRI study. PLoS One 13:e0207146. doi: 10.1371/journal.pone.0207146
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Clouchoux, C., du Plessis, A. J., Bouyssi-Kobar, M., Tworetzky, W., McElhinney, D. B., Brown, D. W., et al. (2013). Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 23, 2932–2943. doi: 10.1093/cercor/bhs281
Coslett, H. B., and Schwartz, M. F. (2018). The parietal lobe and language. Handb. Clin. Neurol. 151, 365–375. doi: 10.1016/B978-0-444-63622-5.00018-8
Cunningham, S. I., Tomasi, D., and Volkow, N. D. (2017). Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum. Brain Mapp. 38, 938–956. doi: 10.1002/hbm.23429
Diaz-Frias, J., and Guillaume, M. (2020). Tetralogy of Fallot. In: StatPearls. Treasure Island, FL: StatPearls Publishing.
Fairhall, S. L. (2020). Cross Recruitment of Domain-Selective Cortical Representations Enables Flexible Semantic Knowledge. J. Neurosci. 40, 3096–3103. doi: 10.1523/JNEUROSCI.2224-19.2020
Gaynor, J. W., Stopp, C., Wypij, D., Andropoulos, D. B., Atallah, J., Atz, A. M., et al. (2015). Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 135, 816–825. doi: 10.1542/peds.2014-3825
Golestani, A. M., Kwinta, J. B., Khatamian, Y. B., and Chen, J. J. (2017). The Effect of Low-Frequency Physiological Correction on the Reproducibility and Specificity of Resting-State fMRI Metrics: functional Connectivity. ALFF ReHo. Front. Neurosci. 11:546. doi: 10.3389/fnins.2017.00546
Guo, W., Su, Q., Yao, D., Jiang, J., Zhang, J., Zhang, Z., et al. (2014). Decreased regional activity of default-mode network in unaffected siblings of schizophrenia patients at rest. Eur. Neuropsychopharmacol. 24, 545–552. doi: 10.1016/j.euroneuro.2014.01.004
Gur, R. C., Butler, E. R., Moore, T. M., Rosen, A. F. G., Ruparel, K., Satterthwaite, T. D., et al. (2021). Structural and Functional Brain Parameters Related to Cognitive Performance Across Development: replication and Extension of the Parieto-Frontal Integration Theory in a Single Sample. Cereb. Cortex 31, 1444–1463. doi: 10.1093/cercor/bhaa282
Helmich, R. C., Derikx, L. C., Bakker, M., Scheeringa, R., Bloem, B. R., and Toni, I. (2010). Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb. Cortex 20, 1175–1186. doi: 10.1093/cercor/bhp178
Hickey, E. J., Veldtman, G., Bradley, T. J., Gengsakul, A., Manlhiot, C., Williams, W. G., et al. (2009). Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur. J. Cardiothorac. Surg. 35, 156–164. discussion 164. doi: 10.1016/j.ejcts.2008.06.050
Ho, A. B., Bharucha, T., Jones, E., Thuraisingham, J., Kaarne, M., and Viola, N. (2018). Primary surgical repair of tetralogy of Fallot at under three months of age. Asian. Cardiovasc. Thorac. Ann. 26, 529–534. doi: 10.1177/0218492318803037
Hovels-Gurich, H. H., Konrad, K., Skorzenski, D., Nacken, C., Minkenberg, R., Messmer, B. J., et al. (2006). Long-term neurodevelopmental outcome and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann. Thorac. Surg. 81, 958–966. doi: 10.1016/j.athoracsur.2005.09.010
Jiang, L., Xu, T., He, Y., Hou, X. H., Wang, J., Cao, X. Y., et al. (2015). Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct. Funct. 220, 2485–2507. doi: 10.1007/s00429-014-0795-8
Kjaer, T. W., Nowak, M., Kjaer, K. W., Lou, A. R., and Lou, H. C. (2001). Precuneus-prefrontal activity during awareness of visual verbal stimuli. Conscious. Cogn. 10, 356–365. doi: 10.1006/ccog.2001.0509
Leichnetz, G. R. (2001). Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat. Rec. 263, 215–236. doi: 10.1002/ar.1082
Leonetti, C., Back, S. A., Gallo, V., and Ishibashi, N. (2019). Cortical Dysmaturation in Congenital Heart Disease. Trends Neurosci. 42, 192–204. doi: 10.1016/j.tins.2018.12.003
Li, Z., Chen, J., Cheng, J., Huang, S., Hu, Y., Wu, Y., et al. (2018). Acupuncture Modulates the Cerebello-Thalamo-Cortical Circuit and Cognitive Brain Regions in Patients of Parkinson’s Disease With Tremor. Front. Aging Neurosci. 10:206. doi: 10.3389/fnagi.2018.00206
Liu, C. H., Li, F., Li, S. F., Wang, Y. J., Tie, C. L., Wu, H. Y., et al. (2012). Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 203, 175–179. doi: 10.1016/j.pscychresns.2012.02.007
Liu, X., Wang, S., Zhang, X., Wang, Z., Tian, X., and He, Y. (2014). Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. J. Alzheimers Dis. 40, 387–397. doi: 10.3233/JAD-131322
Liu, X., Wu, Q., Ying, K., Li, A., Sun, Y., and Mei, L. (2020). Functional laterality of the anterior and posterior occipitotemporal cortex is affected by language experience and processing strategy, respectively. Neuropsychologia 137:107301. doi: 10.1016/j.neuropsychologia.2019.107301
Lyu, D., Li, T., and Lyu, X. (2021). Resting-state functional reorganisation in Alzheimer’s disease and amnestic mild cognitive impairment: protocol for a systematic review and meta-analysis. BMJ Open 11:e049798. doi: 10.1136/bmjopen-2021-049798
Ma, S., Li, Y., Liu, Y., Xu, C., Li, H., Yao, Q., et al. (2020). Changes in Cortical Thickness Are Associated With Cognitive Ability in Postoperative School-Aged Children With Tetralogy of Fallot. Front. Neurol. 11:691. doi: 10.3389/fneur.2020.00691
Maffei, V., Indovina, I., Mazzarella, E., Giusti, M. A., Macaluso, E., Lacquaniti, F., et al. (2020). Sensitivity of occipito-temporal cortex, premotor and Broca’s areas to visible speech gestures in a familiar language. PLoS One 15:e0234695. doi: 10.1371/journal.pone.0234695
Maneshi, M., Vahdat, S., Fahoum, F., Grova, C., and Gotman, J. (2014). Specific resting-state brain networks in mesial temporal lobe epilepsy. Front. Neurol. 5:127. doi: 10.3389/fneur.2014.00127
Marino, B. S., Lipkin, P. H., Newburger, J. W., Peacock, G., Gerdes, M., Gaynor, J. W., et al. (2012). Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 126, 1143–1172. doi: 10.1161/CIR.0b013e318265ee8a
Morton, P. D., Korotcova, L., Lewis, B. K., Bhuvanendran, S., Ramachandra, S. D., Zurakowski, D., et al. (2017). Abnormal neurogenesis and cortical growth in congenital heart disease. Sci. Transl. Med. 9:eaah7029. doi: 10.1126/scitranslmed.aah7029
Mussatto, K. A., Hoffmann, R. G., Hoffman, G. M., Tweddell, J. S., Bear, L., Cao, Y., et al. (2014). Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics 133, e570–e577. doi: 10.1542/peds.2013-2309
Ni, L., Liu, R., Yin, Z., Zhao, H., Nedelska, Z., Hort, J., et al. (2016). Aberrant Spontaneous Brain Activity in Patients with Mild Cognitive Impairment and concomitant Lacunar Infarction:a Resting-State Functional MRI Study. J. Alzheimers Dis. 50, 1243–1254. doi: 10.3233/JAD-150622
Ortinau, C. M., Mangin-Heimos, K., Moen, J., Alexopoulos, D., Inder, T. E., Gholipour, A., et al. (2018). Prenatal to postnatal trajectory of brain growth in complex congenital heart disease. Neuroimage Clin. 20, 913–922. doi: 10.1016/j.nicl.2018.09.029
Pang, Q. Y., Duan, L. P., Jiang, Y., and Liu, H. L. (2021). Effects of inhalation and propofol anaesthesia on postoperative cognitive dysfunction in elderly noncardiac surgical patients:a systematic review and meta-analysis. Medicine 100:e27668. doi: 10.1097/MD.0000000000027668
Pigdon, L., Willmott, C., Reilly, S., Conti-Ramsden, G., Gaser, C., Connelly, A., et al. (2019). Grey matter volume in developmental speech and language disorder. Brain Struct. Funct. 224, 3387–3398. doi: 10.1007/s00429-019-01978-7
Sheetal, S., Mathew, R., and Byju, P. (2019). Alexia without Agraphia-report of Five Cases and Review of Literature. J. Assoc. Physicians India 67, 78–80.
Schuttler, C., Munster, T., Gall, C., Trollmann, R., and Schuttler, J. (2021). General Anesthesia in the First 36 Months of Life-a Study of Cognitive Performance in Children Aged 7-11 Years (ANFOLKI-36). Dtsch. Arztebl. Int. doi: 10.3238/arztebl.m2021.0355 [Epub Online ahead of print].
Shen, Y., Zhou, T., Liu, X., Liu, Y., Li, Y., Zeng, D., et al. (2021). Sevoflurane-Induced miR-211-5p Promotes Neuronal Apoptosis by Inhibiting Efemp2. ASN Neuro. 13:17590914211035036. doi: 10.1177/17590914211035036
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. doi: 10.1371/journal.pone.0025031
Stroh, A. L., Rosler, F., Dormal, G., Salden, U., Skotara, N., Hanel-Faulhaber, B., et al. (2019). Neural correlates of semantic and syntactic processing in German Sign Language. Neuroimage 200, 231–241. doi: 10.1016/j.neuroimage.2019.06.025
Sun, L., Macgowan, C. K., Sled, J. G., Yoo, S. J., Manlhiot, C., Porayette, P., et al. (2015). Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 131, 1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051
Taylor, J. S. H., Davis, M. H., and Rastle, K. (2019). Mapping visual symbols onto spoken language along the ventral visual stream. Proc. Natl. Acad. Sci. U S A 116, 17723–17728. doi: 10.1073/pnas.1818575116
Teinonen, T., Aslin, R. N., Alku, P., and Csibra, G. (2008). Visual speech contributes to phonetic learning in 6-month-old infants. Cognition 108, 850–855. doi: 10.1016/j.cognition.2008.05.009
Victoria, L. W., Pyles, J. A., and Tarr, M. J. (2019). The relative contributions of visual and semantic information in the neural representation of object categories. Brain Behav. 9:e01373. doi: 10.1002/brb3.1373
Wang, J., Deng, Y., and Booth, J. R. (2019). Automatic semantic influence on early visual word recognition in the ventral occipito-temporal cortex. Neuropsychologia 133:107188. doi: 10.1016/j.neuropsychologia.2019.107188
Wang, Z., Fei, L., Sun, Y., Li, J., Wang, F., and Lu, Z. (2019). The role of the precuneus and posterior cingulate cortex in the neural routes to action. Comput. Assist. Surg. 24, 113–120. doi: 10.1080/24699322.2018.1557903
Warnes, C. A., Williams, R. G., Bashore, T. M., Child, J. S., Connolly, H. M., Dearani, J. A., et al. (2008). ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52, e143–e263. doi: 10.1016/j.jacc.2008.10.001
Wernovsky, G., and Licht, D. J. (2016). Neurodevelopmental Outcomes in Children With Congenital Heart Disease-What Can We Impact? Pediatr. Crit. Care Med. 17, S232–S242. doi: 10.1097/PCC.0000000000000800
Wu, Z., and Zhu, Y. (2021). Comparison of the Effects of Epidural Anesthesia and General Anesthesia on Perioperative Cognitive Function and Deep Vein Thrombosis in Patients Undergoing Total Knee Arthroplasty. Evid. Based Complement Alternat. Med. 2021:1565067. doi: 10.1155/2021/1565067
Xu, Y., Zhuo, C., Qin, W., Zhu, J., and Yu, C. (2015). Altered Spontaneous Brain Activity in Schizophrenia:a Meta-Analysis and a Large-Sample Study. Biomed. Res. Int. 2015:204628. doi: 10.1155/2015/204628
Yue, Y., Jiang, Y., Shen, T., Pu, J., Lai, H. Y., and Zhang, B. (2020). ALFF and ReHo Mapping Reveals Different Functional Patterns in Early- and Late-Onset Parkinson’s Disease. Front. Neurosci. 14:141. doi: 10.3389/fnins.2020.00141
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zhou, Z., Vilis, T., and Strother, L. (2019). Functionally Separable Font-invariant and Font-sensitive Neural Populations in Occipitotemporal Cortex. J. Cogn. Neurosci. 31, 1018–1029. doi: 10.1162/jocn_a_01408
Keywords: Tetralogy of Fallot, ALFF, ReHo, cognition, VIQ, brain injury
Citation: Ma S, Hu Y, Liu Y, Pu Y, Zuo P, Hu Q, Yang Z, Chen F, Xie Z, Cun Y, Liu X, Yang M and Mo X (2022) The Effect of Abnormal Regional Homogeneity and Spontaneous Low-Frequency Brain Activity on Lower Cognitive Ability: A Cross-Sectional Study on Postoperative Children With Tetralogy of Fallot. Front. Neurosci. 15:685372. doi: 10.3389/fnins.2021.685372
Received: 25 March 2021; Accepted: 13 December 2021;
Published: 07 February 2022.
Edited by:
Alessia Mastrodonato, Columbia University, United StatesReviewed by:
Yu-Chen Chen, Nanjing Medical University, ChinaCopyright © 2022 Ma, Hu, Liu, Pu, Zuo, Hu, Yang, Chen, Xie, Cun, Liu, Yang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Yang, eWFuZ21pbmcxOTcxMDIxN0AxNjMuY29t; Xuming Mo, bW9oc3VtaW5nMTVAbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.