
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurosci., 26 May 2021
Sec. Neurodevelopment
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.660330
This article is part of the Research TopicAutism Spectrum Disorder within Neurodevelopmental Disorders: Catching Heterogeneity, Specificity and Comorbidity in Clinical Phenotypes and Neurobiological BasesView all 21 articles
 Mirko Uljarević1,2*
Mirko Uljarević1,2* Thomas W. Frazier3
Thomas W. Frazier3 Booil Jo4
Booil Jo4 Jennifer M. Phillips4
Jennifer M. Phillips4 Wesley Billingham5
Wesley Billingham5 Matthew N. Cooper5
Matthew N. Cooper5 Antonio Y. Hardan4
Antonio Y. Hardan4Impairment in social motivation (SM) has been suggested as a key mechanism underlying social communication deficits observed in autism spectrum disorder (ASD). However, the factors accounting for variability in SM remain poorly described and understood. The current study aimed to characterize the relationship between parental and proband SM. Data from 2,759 children with ASD (Mage = 9.03 years, SDage = 3.57, 375 females) and their parents from the Simons Simplex Collection (SSC) project was included in this study. Parental and proband SM was assessed using previously identified item sets from the Social Responsiveness Scale (SRS). Children who had parents with low SM scores (less impairments) showed significantly lower impairments in SM compared to children who had either one or both parents with elevated SM scores. No parent-of-origin effect was identified. No significant interactions were found involving proband sex or intellectual disability (ID) status (presence/absence of ID) with paternal or maternal SM. This study establishes that low SM in children with ASD may be driven, in part, by lower SM in one or both parents. Future investigations should utilize larger family pedigrees, including simplex and multiplex families, evaluate other measures of SM, and include other related, yet distinct constructs, such as social inhibition and anhedonia. This will help to gain finer-grained insights into the factors and mechanisms accounting for individual differences in sociability among typically developing children as well as those with, or at risk, for developing ASD.
Social motivation (SM), or the drive to engage, affiliate, and interact with others, has been proposed as a crucial factor for human adaptation and survival throughout evolution (Boyd et al., 2011; Tomasello et al., 2012). Lack or low levels of SM during very early development has been suggested as a key mechanism behind the subsequent social interaction and communication impairments that characterize autism spectrum disorder (ASD) (Chevallier et al., 2012; Kohls et al., 2012). More specifically, it has been hypothesized that due to low SM, children with ASD are less likely to orient to socially salient stimuli that provide key information for learning and the development and specialization of brain circuits underpinning processes crucial for the ability to successfully navigate the complexities of the social world (Mundy, 1995; Dawson et al., 2005). Although the described causal pathway is yet to be confirmed through longer-term longitudinal studies, several lines of evidence provide some support for the SM theory. Firstly, lack of orienting to, and preference for, visual and auditory social stimuli, have been found during early development (Dawson et al., 1998; Osterling et al., 2002; Klin et al., 2009; Falck-Ytter et al., 2013) and throughout later childhood and adolescence (Klin et al., 2002; Sasson et al., 2011; Chevallier et al., 2015; Wright et al., 2016). Secondly, both structural and functional neuroimaging studies have provided consistent evidence for atypicality in key brain regions within the reward processing circuitry (Scott-Van Zeeland et al., 2010; Delmonte et al., 2012; Herrington et al., 2017; Kohls et al., 2018), although it is still unclear whether noted deficits are constrained to social rewards or extend across other reward types (Clements et al., 2018). Importantly, Naturalistic Developmental Behavioral Interventions such as the Early Start Denver Model (ESDM) (Rogers and Dawson, 2010) and Pivotal Response Treatment (PRT) (Koegel et al., 1999) that focus, among other aspects, on SM as a treatment target, have been shown to be effective in improving a range of skills and domains and to result in the need for fewer services later in life (Cidev et al., 2017; Sandbank et al., 2020).
There is pronounced variability in SM among individuals with ASD, with some individuals lacking social interest and awareness of others or actively avoiding social interactions, and others showing the strong drive to form and sustain friendships and romantic relationships and often experiencing loneliness (Wing and Gould, 1979; Bauminger et al., 2008; Calder et al., 2012; Mendelson et al., 2016; Uljarević et al., 2020a). However, despite the centrality of SM in ASD, the factors accounting for large individual differences in this domain remain poorly characterized and understood. Across a range of neurodevelopmental disorders, even in cases of deleterious de novo mutations, parental traits have been shown to provide a substantial contribution to the phenotypic variability in children’s morphological, behavioral and cognitive characteristics (Hanson et al., 2014; Moreno De Luca et al., 2015; Klaassen et al., 2016; Evans and Uljarević, 2018). Therefore, consideration of SM among parents of children with ASD might provide a potentially promising means for understanding the sources of individual variability in SM among their children.
The presence of the broader autism phenotype (BAP) among parents and family members of individuals with ASD has been recognized since original clinical descriptions by Kanner (1943). Subsequent studies have provided robust empirical evidence that parents of children with ASD tend to show higher levels of difficulties in language, communication, social interaction, and cognition as well as the presence of certain higher-order repetitive behaviors when compared to the general population (Gerdts and Bernier, 2011; Sucksmith et al., 2011). Importantly, evidence of familiality and inter-generational transmission of these traits has also been reported (Virkud et al., 2009; De la Marche et al., 2012; Taylor et al., 2013; Lyall et al., 2014; Uljarević et al., 2016). Both clinical observations by Kanner (1943) and several studies that focused on personality characteristics (e.g., Bolton et al., 1994; Piven et al., 1997; Bailey et al., 1998) have reported traits indicative of lower levels of SM among parents of children with ASD; however, the pattern of relationship between SM in children with ASD and their parents remains largely unexplored. The only exceptions are a study by Sung et al. (2005) that demonstrated high heritability of SM in a sample of 201 families with a child with ASD and a study by Jones et al. (2017) that reported an association between lower levels of parental SM with shorter peak look at faces in their infant children. However, Sung et al. (2005) used the SM subscale of the Broader Autism Phenotype Scale (Dawson et al., 2007) which consists of only two items, therefore providing limited range. Similarly, Jones et al. (2017) used the Social Competence Questionnaire (Sarason et al., 1985) and the Social Avoidance and Distress Scale (Watson and Friend, 1969) that assess social comfort and social anxiety, respectively, rather than directly assessing SM. In addition to measurement limitations, both studies were limited by small sample size.
The current study aimed to characterize the relationship between parental and proband SM. It was hypothesized that higher levels of SM impairments in parents would be associated with higher levels of SM impairment in their children with ASD. Given the well established sex differences in SM across normative development and neurodevelopmental disorders, including ASD (Sedgewick et al., 2016; Uljarević et al., 2020b, c), we aimed to explore the possibility of sex-specific transmission of SM. Recent findings suggest that familial risk and heritability may vary depending on the presence or absence of intellectual disability (ID) in probands (Xie et al., 2019), therefore, the familiality pattern of SM depending on the IQ status of the child with ASD was investigated. In this study, parent and proband SM was measured by the SM factor derived in our recent analysis of the Social Responsiveness Scale (SRS-2; Constantino and Gruber, 2005, 2012). The SM factor utilized here was derived in a large sample of N = 27,953 individuals spanning normative and atypical development, including ASD (Uljarević et al., 2020b). We have opted for this specific SRS-2 subscale over the original SM subscale proposed by Constantino and Gruber (2005, 2012) given that the latter was not supported by any of the SRS/SRS-2 factor analytic investigations (e.g., Frazier et al., 2014; Uljarević et al., 2020b). Factor analysis by Frazier et al. (2014) derived a social avoidance factor that included several items related to SM, however, this factor also contained several items that do not readily map onto the construct of SM (e.g., “Expressions on his/her face don’t match what he/she is saying”, and “Is too tense in social situations”). Therefore, to ensure that several distinct constructs are not conflated within a single factor, we have chosen to focus on the SM scale derived in our work given that it was specifically optimized to capture only that specific construct and excluded any other broad/not-related items.
Data was obtained from the Simons Simplex Collection (SSC) project. The SSC consisted of a sample of clinically referred individuals with a diagnosis of ASD but without any other medical conditions and their families. Participants were recruited from 12 university-based sites (Fischbach and Lord, 2010). No age restrictions were applied. Data from 2,759 children with ASD (Mage = 9.03 years, SDage = 3.57, range: 4–18 years; 375 females) and their parents [N = 2,747 fathers (Mage = 42.5 years, SDage = 6.4, range: 22–55 years); N = 2,752 mothers (Mage = 40.4 years, SDage = 5.7, range: 21–58 years)] was included in this study.
This study was approved by the Stanford University Institutional Review Board. All participants or their parent/legal guardian have provided informed consent for participation as part of SSC.
The Social Responsiveness Scale (SRS; Constantino and Gruber, 2005, 2012). The SRS is a 65-item measure designed to index autism trait severity. Each item is rated on a 4-point Likert scale (from 1 = Not True to 4 = Almost Always True) with higher scores indicating higher trait severity/atypicality. Mothers and fathers rated their own traits and behaviors using the adult SRS form, and mothers completed a parent-report version of the SRS for their child with ASD. As noted, in this study we utilized the subscale derived in our previous work (Uljarević et al., 2020b) that contains five items and captures SM. Although originally labeled as Attachment and Affiliation to be aligned with the Research Domain Criteria nomenclature that does not specifically highlight SM as a distinct construct, all five items within this factor map onto the SM construct and do not include attachment-related aspects. In this sample, the SM subscale derived in our recent study (Uljarević et al., 2020b) showed good internal consistency in fathers (α = 0.81) and acceptable internal consistency in mothers (α = 0.74) and children with ASD (α = 0.74). We have chosen a five-item SM factor derived in our previous work over the originally proposed, theoretically derived SRS Social Motivation Scale (Constantino and Gruber, 2005, 2012) which has not been replicated in the subsequent factorizations of the SRS and over the Social Avoidance SRS factor derived by Frazier et al. (2014) given that this factor included several items that do not readily map onto SM (e.g., “Expressions on his/her face don’t match what he/she is saying”, and “Is too tense in social situations”).
Effects of parental SM on children’s SM was firstly investigated by conducting a comparison between children whose mother or father had elevated SM scores. Elevated parental SM score was defined as the top 25th percentile of the score distribution for mothers and fathers, respectively, and the remaining distribution was used as the referent group. Children whose parents both reported low personal SM scores (lower impairment) showed significantly lower impairment in SM compared to children who had either one or both parents with elevated SM scores (Figure 1). A cross-tabulation of these dichotomous SM impairment factors for mothers and fathers resulted in four groups (neither parent with elevated SM scores, only mother with elevated SM scores, only father with elevated SM scores, both parents with elevated SM scores). An analysis of variance (ANOVA) on child SM scores showed a significant difference between these groups, F(3,2743) = 9.01, p < 0.001, and a subsequent Tukey’s post hoc test showed that child had significantly poorer SM when either one or both parents had elevated SM scores. However, child SM was not significantly exasperated when both parents had elevated SM scores compared to just one parent. Please see Figure 1 for the score distribution and Table 1 for a detailed overview of the post hoc comparisons.
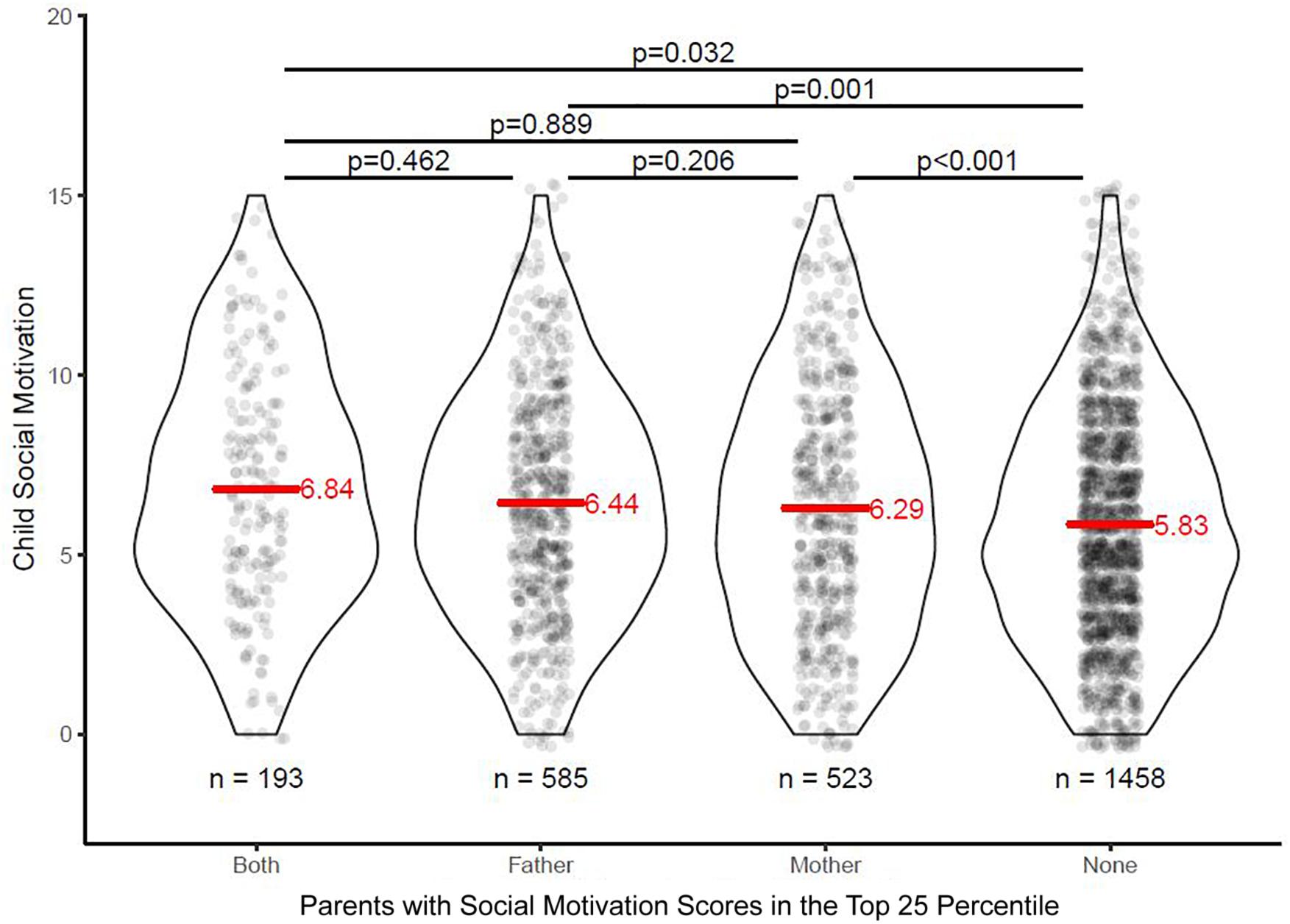
Figure 1. Children’s social motivation scores as a function of parental social motivation status. Both, both mother and father had SM scores in the top 25 percentile of the respective score distribution; neither, neither mother or father had SM scores in the top 25 percentile of the respective score distribution; SM, social motivation.
Next, a linear regression model was used to investigate the relationship between SM scores of parents and their child with ASD (Figure 2). An increase of 1 unit in mother SM score was significantly associated with a small increase (0.12; 95% CI: 0.07, 0.17; p < 0.001; Model 1, Table 2) in child SM, and the same 1 unit increase in father SM was significantly associated with a similarly small increase (0.09; 95% CI: 0.05, 0.13; p < 0.001; Model 2 in Table 2) in child SM. A multivariate regression model was then fitted with child SM as the outcome and both mother and father SM included in the model with an interaction term (Model 3, Table 2). The interaction term was non-significant and therefore dropped from the final model, which showed a cumulative effect of maternal SM (0.12; 95% CI: 0.07, 0.18; p < 0.001) and paternal SM (0.09; 95% CI: 0.05, 0.13; p < 0.001; Model 4, Table 2) on child SM. Full regression models are presented in Table 2.
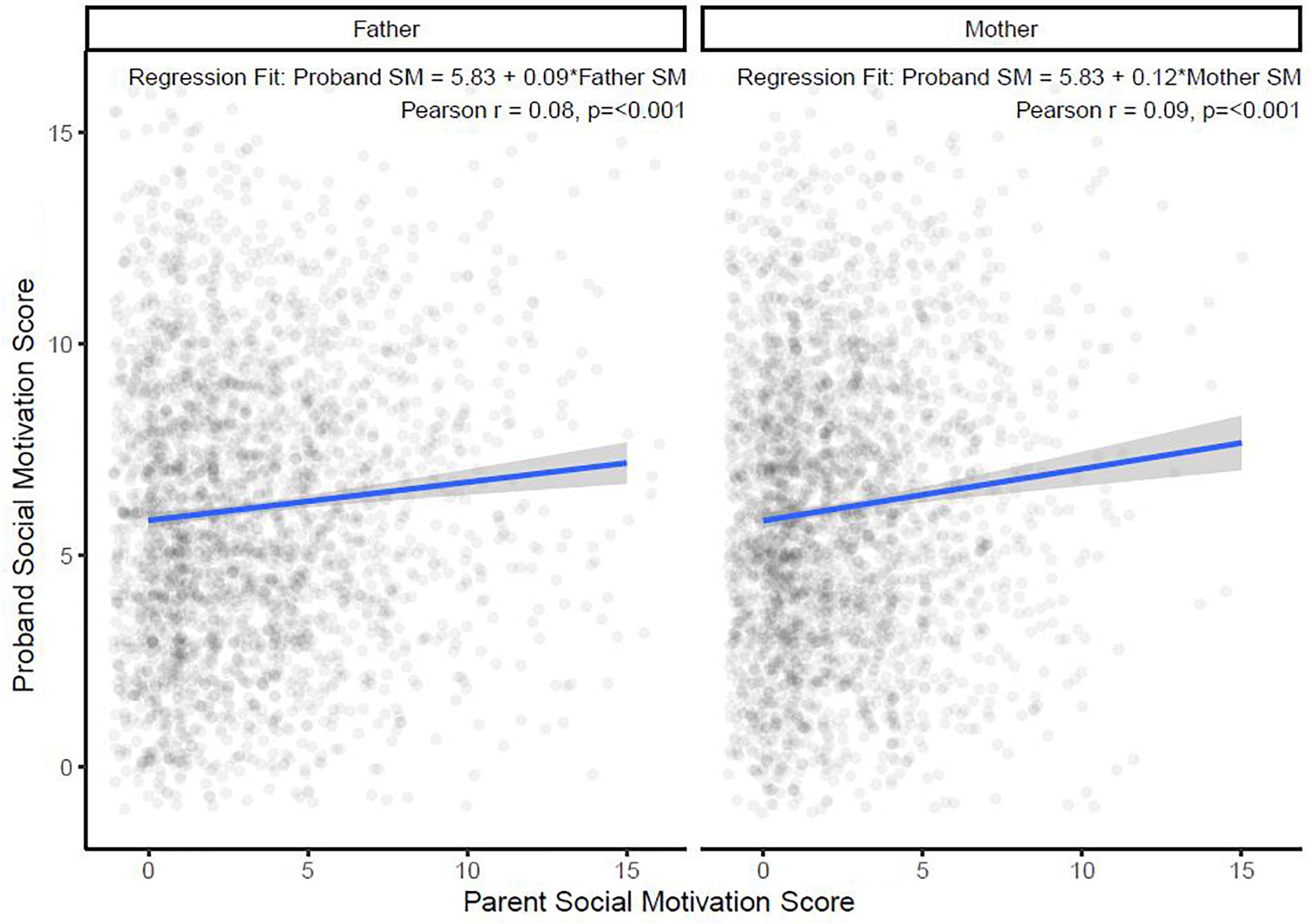
Figure 2. Relationship between paternal and maternal social motivation scores with autistic children’s with social motivation scores. SM, social motivation.
Further multiple regression models were used to examine whether the effect of each parent’s SM on the child’s SM depended on the child’s sex [Figure 3; models 5 (effect of maternal SM) and 6 (effect of paternal SM) in Table 2] and/or on the presence/absence of ID in the child [Figure 4; models 7 (effect of maternal SM) and 8 (effect of paternal SM) in Table 2]. No significant sex interaction with paternal or maternal SM was found. The observed paternal effect on a male child was over threefold higher than on a female child, however, it was not statistically significant. No significant IQ interaction with paternal or maternal SM was found. A final multiple regression model was run with mother SM, father SM, child sex, and child ID as covariates (Model 9, Table 2). Mother SM (0.13; 95% CI: 0.08, 0.18; p < 0.001), father SM (0.11; 95% CI: 0.07, 0.15; p < 0.001) and ID (−1.14; 95% CI: −1.41, −0.88, p < 0.001) were all significant predictors of child SM, while sex (male: 0.02, 95% CI: −0.34, 0.38, p = 0.9) was not significant as a predictor. Full regression models are presented in Table 2.
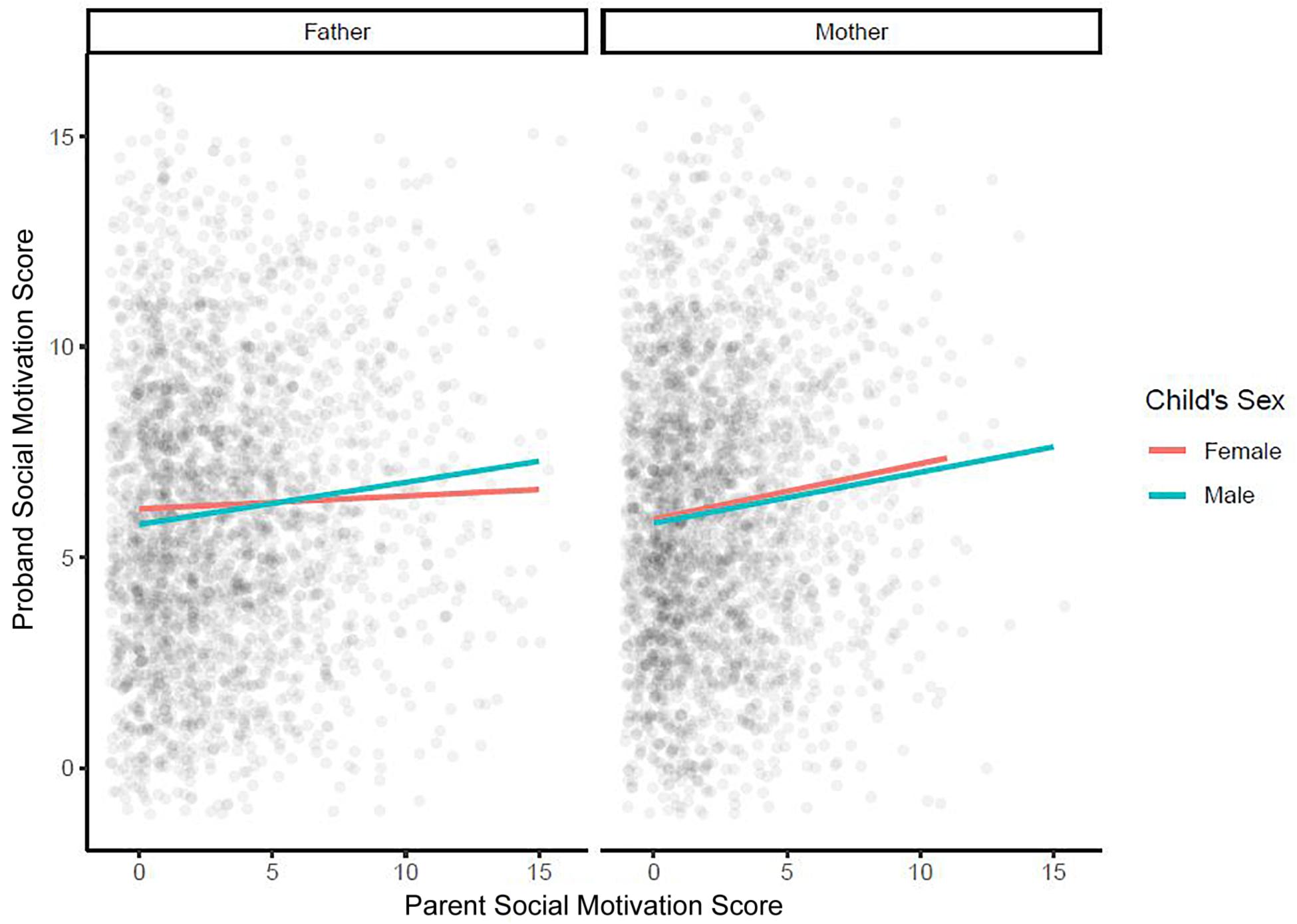
Figure 3. Multiple regression model examining whether the effect of each parent’s social motivation on a child’s social motivation depends on a child’s sex. SM, social motivation.
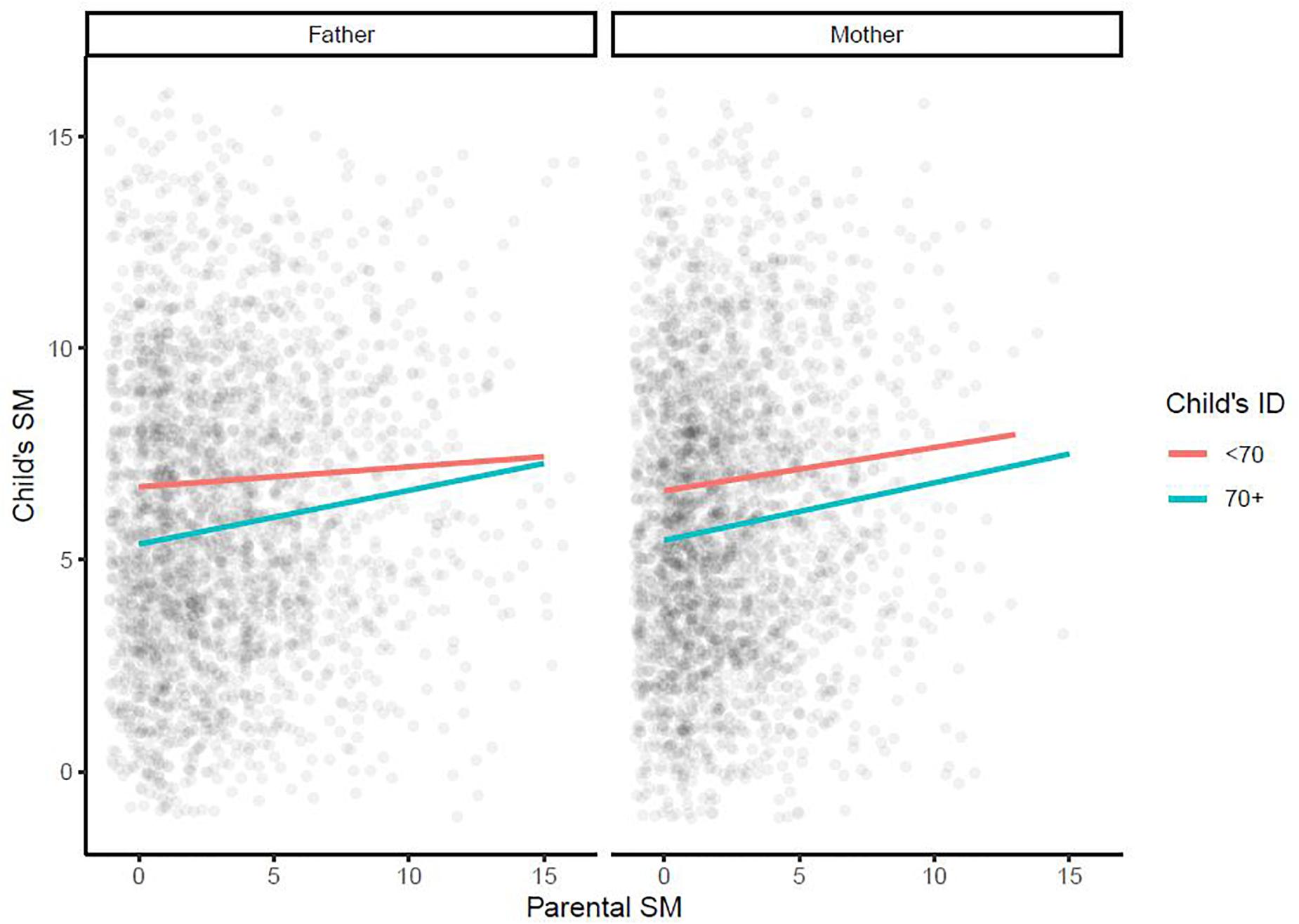
Figure 4. Multiple regression model examining whether the effect of each parent’s social motivation on a child’s social motivation depends on the presence/absence of intellectual disability in children. ID, intellectual disability; SM, social motivation.
The current study aimed to examine the familiality of SM by exploring the link between parental and proband the social responsiveness scale (SRS-2) SM scores. Our analysis demonstrated that low levels of paternal and maternal SM were associated with a significant deficit in SM in children with ASD. Importantly, these effects were independent and cumulative, and no parent-of-origin effect was found. This finding is in line with two previous studies that have investigated familiality and heritability of SM in small samples of families of children with ASD (Sung et al., 2005) and those with typically developing youth (Jones et al., 2017). While indications for potential sex-specific transmission of SM were observed as paternal effect on a male child with ASD was over three-fold higher than the effect on a female child with ASD, this effect was no statistically significant and these findings should therefore be interpreted as very preliminary and warrant further replication.
The present study used the SSC data which is a relatively large and well-characterized sample of mother-father-child with ASD triads. In contrast to previous studies by Sung et al. (2005) and Jones et al. (2017) who used a two-item subscale and constructs of social discomfort and anxiety to capture SM, respectively, our investigation utilized SM items derived from the SRS in our recent SRS factorization (Uljarević et al., 2020b). The SM scale used here had good conceptual clarity as it encompasses only items directly relating to the drive for social approach/to interact socially. However, the findings reported here should also be considered in light of several limitations. Firstly, we relied on a questionnaire measure of SM and therefore a potential impact of the common method variance will need to be considered. This is particularly relevant in the light of the findings by De la Marche et al. (2015) and Jones et al. (2017) that emphasize potential method-specific (questionnaire versus more objective assessments and experimental protocols) pattern of findings in the studies of similar design as ours. Therefore, it will be crucial to replicate and further refine findings reported here by utilizing multi-method assessment protocols. Secondly, the sample used here only included simplex families and did not include a general population sample. Given the suggestions that etiologic mechanisms operating within simplex and multiplex families might be somewhat distinct (Virkud et al., 2009; Lyall et al., 2014), it will be important for future studies to better characterize the pattern of transmission of SM depending on simplex versus multiplex status and whether any potential specificities would emerge when compared to the transmission pattern in the general population. Thirdly, although SSC database afforded a significantly larger sample size for female participants. However, given the well established over-representation of ASD in males, the sample used in this study was nevertheless heavily skewed toward male participants, which could have impacted the ability to detect some of the more nuanced sex-specific effects. Therefore, it will be important for future studies to further investigate the possibility of sex-specific transmission of SM.
Importantly, SM is a complex construct and has been suggested to encompass a range of inter-related elements including social orienting, seeking enjoyment in social interactions, and behaviors and actions aimed at maintaining social bonds (Chevallier et al., 2012). The SM scale used here only captures the seeking/enjoyment element, and it is not clear whether the familiality pattern would be continuous with the social orienting and maintenance elements, or whether potential discontinuities might arise. Despite the centrality of the SM construct in ASD, there is a paucity of instruments that can effectively and comprehensively capture individual differences in SM in a sensitive and quantitative manner. The recently developed Stanford Social Dimensions Scale (SSDS) (Phillips et al., 2019) has been specifically designed to capture a broad spectrum of traits and behaviors indicative of the seeking/linking and maintenance components described by Chevallier et al. (2012) and shows promising psychometric properties and ability to capture individual differences in distinct SM subdomains in children and adolescents with ASD (Uljarević et al., 2020a). It will therefore be crucial for future investigations to incorporate the SSDS and other scales capturing related, yet distinct constructs such as social inhibition and anhedonia, to gain an in-depth insight into the factors and mechanisms accounting for the individual differences in key determinants of sociability among children with, and at risk, for developing ASD.
Publicly available datasets were analyzed in this study. This data can be found here: www.sfari.org.
The studies involving human participants were reviewed and approved by the Stanford University Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
MU, TF, and AH designed the study. MU, WB, and MC analyzed the data. MU, TF, BJ, JP, WB, and AH wrote the manuscript. All authors reviewed the manuscript and approved the final version.
This study was supported by the R03MH111846-01 National Institute of Mental Health grant (AH and BJ) by the National Institute of Mental Health. MU was currently supported by the Australian Research Council Discovery Early Career Researcher Award (DE180100632).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bailey, A., Palferman, S., Heavey, L., and Le Couteur, A. (1998). Autism: the phenotype in relatives. J. Autism Dev. Disord. 28, 369–392.
Bauminger, N., Solomon, M., Aviezer, A., Heung, K., Gazit, L., Brown, J., et al. (2008). Friendship manifestations, dyadic qualities of friendship and friendship perception in high-functioning preadolescents with autism spectrum disorder. J. Abnorm. Child Psychol. 36, 135–150.
Bolton, P., Macdonald, H., Pickles, A., Rios, P., Goode, S., Crowson, M., et al. (1994). A case-control family history study of autism. J. Child. Psychol. Psychiatry 35, 877–900.
Boyd, R., Richerson, P. J., and Henrich, J. (2011). The cultural niche: why social learning is essential for human adaptation. PNAS 108, 10918–10925. doi: 10.1073/pnas.1100290108
Calder, L., Hill, V., and Pellicano, E. (2012). ‘Sometimes I want to play by myself’: understanding what friendship means to children with autism in mainstream primary schools. Autism 17, 296–316. doi: 10.1177/1362361312467866
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., and Schultz, R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239.
Chevallier, C., Parish-Morris, J., McVey, A., Rump, K. M., Sasson, N. J., Herrington, J. D., et al. (2015). Measuring social attention and motivation in Autism Spectrum Disorder using eye-tracking: stimulus type matters. Autism Res. 8, 620–628. doi: 10.1002/aur.1479
Cidev, Z., Munson, J., Estes, A., Dawson, G., Rogers, S., and Mandell, D. (2017). Cost offset associated with early start denver model for children with autism. J. Am. Acad. Child Adolesc. Psychiatry 56, 777–783. doi: 10.1016/j.jaac.2017.06.007
Clements, C. C., Zoltowski, A. R., Yankowitz, L. D., Yerys, B. E., Schultz, R. T., and Herrington, J. D. (2018). Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75, 797–808. doi: 10.1001/jamapsychiatry.2018.1100
Constantino, J. N., and Gruber, C. P. (2005). The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services.
Constantino, J. N., and Gruber, C. P. (2012). Social Responsive Scale Manual (2nd Edition, Srs-2). Los Angeles, CA: Western Psychological Services.
Dawson, G., Estes, A., Munson, J., Schellenberg, G., Bernier, R., and Abbott, R. J. (2007). Quantitative assessment of autism symptom-related traits in probands and parents: broader phenotype autism symptom scale. J. Autism. Dev. Disord. 37, 523–536. doi: 10.1007/s10803-006-0182-2
Dawson, G., Meltzoff, A., Osterling, J., Rinaldi, J., and Brown, E. (1998). Children with autism fail to orient to naturally occurring social stimuli. J. Autism Dev. Disord. 28, 479–485.
Dawson, G., Webb, S. J., and McPartland, J. (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424. doi: 10.1207/s15326942dn2703_6
De la Marche, W., Noens, I., Kuppens, S., Split, J. L., Boets, B., and Steyaert, J. (2015). Measuring quantitative autism traits in families: informant effect or intergenerational transmission? Eur. Child Adolesc. Psychiatry 24, 385–395. doi: 10.1007/s00787-014-0586-z
De la Marche, W., Noens, I., Luts, J., Scholte, E., Van Huffel, S., and Steyaert, J. (2012). Quantitative autism traits in first degree relatives: evidence for the broader autism phenotype in fathers, but not in mothers and siblings. Autism 16, 247–260. doi: 10.1177/1362361311421776
Delmonte, S., Balsters, J. H., McGrath, J., Fitzgerald, J., Brennan, S., Fagan, A. J., et al. (2012). Social and monetary reward processing in autism spectrum disorders. Mol. Autism 3:7. doi: 10.1186/2040-2392-3-7
Evans, D. W., and Uljarević, M. (2018). Parental education accounts for variability in the IQs of probands with Down syndrome: a longitudinal study. Am. J. Med. Genet. 176, 29–33. doi: 10.1002/ajmg.a.38519
Falck-Ytter, T., Rehnberg, E., and Bölte, S. (2013). Lack of visual orienting to biological motion and audiovisual synchrony in 3-year-olds with autism. PLoS One. 8:e68816. doi: 10.1371/journal.pone.0068816
Fischbach, G. D., and Lord, C. (2010). The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron 68, 192–195. doi: 10.1016/j.neuron.2010.10.006
Frazier, T. W., Ratliff, K. R., Gruber, C., Zhang, Y., Law, P. A., and Constantino, J. N. (2014). Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism 18, 31–44. doi: 10.1177/1362361313500382
Gerdts, J., and Bernier, R. (2011). The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Res. Treat. 2011:545901.
Hanson, E., Bernier, R., Porche, K., Goin-Kochel, R. P., Snyder, L. G., Snow-Gallagher, A., et al. (2014). The cognitive and behavioral phenotype of the 16p11.2 deletion. Biol. Psychiatry 77, 785–793.
Herrington, J. D., Maddox, B. B., Kerns, C. M., Rump, K., Worley, J. A., Bush, J. C., et al. (2017). Amygdala volume differences in autism spectrum disorder are related to anxiety. J. Autism Dev. Disord. 47, 3628–3691.
Jones, E. J. H., Venema, K., Earl, R. K., Lowy, R., and Webb, S. J. (2017). Infant social attention: an endophenotype of ASD-related traits? J. Child Psychol. Psyc. 58, 270–281. doi: 10.1111/jcpp.12650
Klaassen, P., Duijff, A., de, V., Beemer, F., Sinnema, G., Breetvelt, E., et al. (2016). Explaining the variable penetrance of CNVs: parental intelligence modulates expression of intellectual impairment caused by the 22q11.2 deletion. Am. J. Med. Genet. 171, 790–796. doi: 10.1002/ajmg.b.32441
Klin, A., Jones, W., Schultz, R., Volkmar, F., and Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry 59, 809–816. doi: 10.1001/archpsyc.59.9.809
Klin, A., Lin, D., Gorrindo, P., Ramsay, G., and Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459, 257–261. doi: 10.1038/nature07868
Koegel, L. K., Koegel, R. L., Harrower, J. K., and Carter, C. M. (1999). Pivotal response intervention I: overview of approach. Res. Pract. Pers. Sev. Disabil. 24, 174–185. doi: 10.2511/rpsd.24.3.174
Kohls, G., Antezana, L., Mosner, M. G., Schultz, R. T., and Yerys, B. E. (2018). Altered reward system reactivity for personalized circumscribed interests in autism. Mol. Autism 9:9.
Kohls, G., Chevallier, C., Troiani, V., and Schultz, R. T. (2012). Social wanting dysfunction in autism: neurobiological underpinnings and treatment implications. J. Neurodev. Disord. 4:1.
Lyall, K., Constantino, J. N., Weisskopf, M. G., Roberts, A. L., Ascherio, A., and Santangelo, S. L. (2014). Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry 71, 936–942. doi: 10.1001/jamapsychiatry.2014.476
Mendelson, J. L., Gates, J. A., and Lerner, M. D. (2016). Friendship in school-age boys with autism spectrum disorders: a meta-analytic summary and developmental, process-based model. Psychol. Bull. 142, 601–622. doi: 10.1037/bul0000041
Moreno De Luca, A., Evans, D. W., Boomer, K., Hanson, E., Bernier, R., et al. (2015). Parental cognitive, behavioral and motor profiles impact the neurodevelopmental profile of individuals with de novo mutations. JAMA Psychiatry 72, 119–126. doi: 10.1001/jamapsychiatry.2014.2147
Mundy, P. (1995). Joint attention and social-emotional approach behavior in children with autism. Dev. Psychopathol. 7, 63–82. doi: 10.1017/s0954579400006349
Osterling, J., Dawson, G., and Munson, J. (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev. Psychopathol. 14, 239–251. doi: 10.1017/s0954579402002031
Phillips, J. M., Uljarević, M., Schuck, R. K., Schapp, S., Solomon, E. M., Salzman, E., et al. (2019). Development of the Stanford Social Dimensions Scale: initial validation in autism spectrum disorder and in neurotypicals. Mol. Autism 10, 1–16.
Piven, J., Palmer, P., Landa, R., Santangelo, S., Jacobi, D., and Childress, D. (1997). Personality and language characteristics in parents from multiple-incidence autism families. Am. J. Med. Genet. 74, 398–411. doi: 10.1002/(sici)1096-8628(19970725)74:4<398::aid-ajmg11>3.0.co;2-d
Rogers, S. J., and Dawson, G. (2010). Early Start Denver Model For Young Children With Autism: Promoting Language, Learning, And Engagement. New York, NY: Guilford Press.
Sandbank, M., Bottema-Beutel, K., Crowley, S., Cassidy, M., Dunham, K., Feldman, J. I., et al. (2020). Project AIM: autism intervention meta-analysis for studies of young children. Psychol. Bull. 146, 1–29. doi: 10.1037/bul0000215
Sarason, B. R., Sarason, I. G., Anthony, T., and Basham, R. B. (1985). Concomitants of social support: social skills, physical attractiveness, and gender. J. Pers. Soc. Psychol. 49, 469–480. doi: 10.1037/0022-3514.49.2.469
Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., and Bodfish, J. W. (2011). Brief report: circumscribed attention in young children with autism. J. Autism Dev. Disord. 41, 242–247. doi: 10.1007/s10803-010-1038-3
Scott-Van Zeeland, A. A., Dapretto, M., Ghahremani, D. G., Poldrack, R. A., and Bookheimer, S. Y. (2010). Reward processing in autism. Autism Res. 3, 53–67.
Sedgewick, F., Hill, V., Yates, R., Pickering, L., and Pellicano, E. (2016). Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. J. Autism Dev. Disord. 46, 1297–1306. doi: 10.1007/s10803-015-2669-1
Sucksmith, E., Roth, R. A., and Hoekstra, A. (2011). Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century. Neuropsychol. Rev. 21, 360–389. doi: 10.1007/s11065-011-9183-9
Sung, Y. J., Dawson, G., Munson, J., Estes, A., Schellenberg, G. D., and Wijsman, E. M. (2005). Genetic investigation of quantitative traits related to autism: use of multivariate polygenic models with ascertainment adjustment. Am. J. Hum. Genet. 76, 68–81. doi: 10.1086/426951
Taylor, L., Maybery, M., Wray, J., Ravine, D., Hunt, A., and Whitehouse, A. (2013). Brief report: do the nature of communication impairments in autism spectrum disorders relate to the broader autism phenotype in parents? J. Autism Dev. Disord. 43, 2984–2989. doi: 10.1007/s10803-013-1838-3
Tomasello, M., Melis, A. P., Tennie, C., Wyman, E., and Herrmann, E. (2012). Two key steps in the evolution of cooperation: the interdependence hypothesis. Curr. Anthropol. 53, 673–692. doi: 10.1086/668207
Uljarević, M., Evans, D. W., Alvares, G. A., and Whitehouse, A. J. O. (2016). Short report: relationship between restricted and repetitive behaviours in children with autism spectrum disorder and their parents. Mol. Autism 7:29.
Uljarević, M., Phillips, J. M., Schuck, R. K., Schapp, S., Solomon, E. M., Salzman, E., et al. (2020a). Exploring Social Subtypes in Autism Spectrum Disorder: a Preliminary Study. Autism Res. 13, 1335–1342. doi: 10.1002/aur.2294
Uljarević, M., Frazier, T. W., Phillips, J. M., Jo, B., Littlefield, S., and Hardan, A. Y. (2020b). Mapping the research domain criteria social processes constructs to the social responsiveness scale. J. Am. Acad. Child Adolesc. Psychiatry 59, 1252–1263. doi: 10.1016/j.jaac.2019.07.938
Uljarević, M., Frazier, T. W., Phillips, J. W., Jo, B., Littlefield, S., and Hardan, A. Y. (2020c). Quantifying Research Domain Criteria Social Communication Sub-Constructs using the Social Communication Questionnaire in youth. J. Clin. Child Adolesc. Psychol. 1–11. doi: 10.1080/15374416.2019.1669156 [Epub Online ahead of print].
Virkud, Y. V., Todd, R. D., Abbacchi, A. M., Zhang, Y., and Constantino, J. N. (2009). Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B, 328–334. doi: 10.1002/ajmg.b.30810
Watson, D., and Friend, R. (1969). Measurement of social evaluative anxiety. J. Consult. Clin. Psychol. 33, 448–457. doi: 10.1037/h0027806
Wing, L., and Gould, J. (1979). Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 9, 11–29. doi: 10.1007/bf01531288
Wright, K., Kelley, E., and Poulin-Dubois, D. (2016). Biological motion and the animate–inanimate distinction in children with high-functioning Autism Spectrum Disorder. Res. Autism Spectr. Disord. 25, 1–11.
Keywords: social motivation, familiality, broader autism phenotype, autism spectral disorder, heterogeneity
Citation: Uljarević M, Frazier TW, Jo B, Phillips JM, Billingham W, Cooper MN and Hardan AY (2021) Relationship Between Social Motivation in Children With Autism Spectrum Disorder and Their Parents. Front. Neurosci. 15:660330. doi: 10.3389/fnins.2021.660330
Received: 29 January 2021; Accepted: 14 April 2021;
Published: 26 May 2021.
Edited by:
Brian Lee, Drexel University, United StatesReviewed by:
Hsiao-Tuan Chao, Baylor College of Medicine, United StatesCopyright © 2021 Uljarević, Frazier, Jo, Phillips, Billingham, Cooper and Hardan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirko Uljarević, bWlya28udWxqYXJldmljQHVuaW1lbGIuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.