
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 17 March 2021
Sec. Neurodevelopment
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.637079
This article is part of the Research TopicAutism Spectrum Disorder within Neurodevelopmental Disorders: Catching Heterogeneity, Specificity and Comorbidity in Clinical Phenotypes and Neurobiological BasesView all 21 articles
 Jierong Chen1*
Jierong Chen1* Zhen Wei1
Zhen Wei1 Chun Liang1
Chun Liang1 Binguang Liu2
Binguang Liu2 Jimin Guo2
Jimin Guo2 Xuejun Kong3
Xuejun Kong3 Minshi Huang1
Minshi Huang1 Ziwen Peng4*
Ziwen Peng4* Guobin Wan1*
Guobin Wan1*Autism spectrum disorder (ASD) is very heterogeneous, particularly in language. Studies have suggested that language impairment is linked to auditory-brainstem dysfunction in ASD. However, not all ASD children have these deficits, which suggests potential subtypes of ASD. We classified ASD children into two subtypes according to their speech-evoked auditory brainstem response (speech-ABR) and explored the neural substrates for possible subtypes. Twenty-nine children with ASD and 25 typically developing (TD) peers were enrolled to undergo speech-ABR testing and structural magnetic resonance imaging (sMRI). There were significant differences between the ASD group and TD group in surface area, cortical volume and cortical thickness. According to speech-ABR results, ASD participants were divided into the ASD-typical (ASD-T) group and ASD-atypical (ASD-A) group. Compared with the ASD-T group, the ASD-A group had a lower score in language of the Gesell Developmental Diagnosis Scale (GDDS), increased left rostral middle frontal gyrus (lRMFG) area and decreased local gyrification index of the right superior temporal gyrus. GDDS-language and surface area of lRMFG were correlated to the wave-A amplitude in ASD. Surface area of lRMFG had an indirect effect on language performance via alteration of the wave-V amplitude. Thus, cortical deficits may impair language ability in children with ASD by causing subcortical dysfunction at preschool age. These evidences support dysfunction of the auditory brainstem as a potential subtype of ASD. Besides, this subtype-based method may be useful for various clinical applications.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder involving deficits in social communication and restricted and repetitive behaviors, with an onset prior to 3 years of age (American Psychiatric Association, 2013). The heterogeneity of individuals with ASD in clinical presentations poses a significant challenge for the interpretability and replicability of research studies (Grzadzinski et al., 2013). Identifying potential subtypes may provide insight into the pathogenesis and/or neurologic mechanism of ASD.
Over the past several decades, researchers have made great efforts to find ways to categorize ASD heterogeneity (Grzadzinski et al., 2013). Classifying individuals with ASD into different groups based on clinical symptoms is one of most widely used methods (Heaton et al., 2008; Deboth and Reynolds, 2017). Besides, numerous studies have used clustering analyses to distinguish different subtypes based on cognitive-behavior and neuropsychology characteristics (Bitsika et al., 2008; Sacco et al., 2012). However, attempts to assign subtypes to individuals with ASD have been largely unsuccessful because distinct, empirically defined subgroups have yet to be identified reliably.
There is an absence of neurobiological evidence for why individuals with ASD present symptoms of varying severity. However, paying attention to the phenotypic behavioral heterogeneity of language in early development is important (Anderson et al., 2007; Pickles et al., 2014). One important reason is that, even though the language trajectories of ASD cases in the first years of life are highly unstable, these trajectories in early childhood are relatively stable and predictive of long-term outcome (Lombardo et al., 2015). Numerous neuroimaging studies have found that abnormalities in the cortex and/or subcortex may be neural mechanisms causing language deficits in ASD (Russo et al., 2008; De Fossé et al., 2010). Nevertheless, the degree of language impairment of individuals with ASD is vastly heterogeneous (Kjelgaard and Tager-Flusberg, 2001). Thus, better understanding of the language-related subtype of ASD in early childhood may help to reveal the neural mechanism that underlies language impairment in such children.
The auditory brainstem response (ABR) is a far-field electric potential measured from the scalp reflecting the electrophysiological activity of the subcortical auditory pathway from the distal portion of the auditory nerve to higher midbrain structures (Skoe et al., 2015). The speech-evoked auditory brainstem response (speech-ABR) is a response to complex sound, and can provide cues as to how temporal and spectral features are preserved in the brainstem (Anderson et al., 2010). Compared with use of questionnaires, the speech-ABR (as an electrophysiological indicator) is more objective, quantitative, non-invasive, precise, and appropriate for varying levels of function. Studies have shown that an abnormal pattern of ABR waveforms in ASD children indicates dysfunction at the subcortical level (Russo et al., 2010a; Miron et al., 2018). More importantly, auditory brainstem function has been linked to language impairment (Banai et al., 2005; Wible et al., 2005; Johnson et al., 2007). However, those results have been contradictory, involving prolongation, shortening, and no abnormalities in the latency and/or amplitude of waveforms (Klin, 1993; Miron et al., 2017). Besides, not all ASD children have subcortical auditory deficits (Russo et al., 2010a), and the exact percentage needs to be obtained by large-sample studies. These findings suggest there are potential auditory subcortical processing-related subtypes of ASD children linked to language impairment.
Functional neuroimaging studies in the past decade have provided evidence that the cortico–subcortical network contributes to auditory, speech and language functions (Dick et al., 2014). According to the theory of corticofugal systems (Winer, 2005), the cortex may provide an inevitable contribution to the subcortex during auditory processing (Yan and Ehret, 2002; Chandrasekaran and Kraus, 2010). Furthermore, numerous studies have found abnormalities in the cortex and subcortex levels of ASD cases, and these abnormalities have been related to language impairment (Brambilla et al., 2003; Bauman and Kemper, 2005). Taken together, those findings suggest the possibility of interactions among the cortex, subcortex, and language in ASD children, but little empirical evidence is available.
In the present study, we subcategorized participants with ASD by the speech-ABR. Then, we used structural magnetic resonance imaging (sMRI) to find the neural substrates for a possible subtype of the subcortical auditory function in ASD. Finally, we explored the interactive relationship among the cortex, subcortex, and language. We hypothesized that: (i) two distinct subtypes in a population of ASD cases aged 3–6 years would have significant differences in language scores; (ii) there would be significant differences in brain structure among ASD subtypes and typically developing (TD) children; and (iii) structural differences of ASD subgroups would interact with the function of the subcortex and language in ASD children (hypothesized model see Supplementary Figure 1).
The study protocol was approved by the Research Ethics Board of Affiliated Shenzhen Maternity & Child Healthcare Hospital (ASMCHH; Shenzhen, China). For ASD children who met the inclusion criteria, relevant researchers informed the parents of the research content, and invited them to participate in the present study. Written informed consent was obtained from the parents of children enrolled in our study.
Twenty-five TD and thirty ASD children between 3 and 6 years of age were recruited from department of Child Psychiatry and Rehabilitation, Affiliated Shenzhen Maternity & Child Healthcare Hospital (Guangdong, China). All ASD participants were diagnosed by child psychiatrists with extensive experience at ASMCHH, and met the criteria for ASD using the Autism Diagnostic Observational Schedule (ADOS) and Diagnostic and Statistical Manual, Fifth Edition (DSM-5) of the American Psychiatric Association (2013). Child psychiatrists recommended these children undergo magnetic resonance imaging (MRI) to exclude organic brain disorders.
All participants passed hearing screenings at birth and had good hearing sensitivity bilaterally (<25 dB HL from 500 to 4,000 Hz). Besides, all participants were screened to ensure that they met the inclusion criteria: (i) met criteria of autistic disorder using both the ADOS and DSM-5 criteria; (ii) age of 3–6 years; (iii) no diseases of the acoustic meatus or hearing disorders; (iv) no history of epilepsy or head injury; and (v) no combined other neurodevelopmental disorders. Age-matched TD participants were screened with questionnaires. Individuals with a family history of any neuropsychiatric disorder (e.g., autism, learning disability, affective disorders, schizophrenia, and epilepsy) were excluded. All parents of participants were informed of the need to add a three-dimensional (3D) scan sequence, speech-ABR, and relevant clinical evaluation.
The ADOS is a semi-structured, standardized interaction-and-observation tool that measures autism symptoms for individuals with possible autism or other pervasive developmental disorders (Lord, 1989). Each module contains standard activities and materials that allow examiners to assess the developmental and language levels of participants (Stacy et al., 2012).
The Childhood Autism Rating Scale (CARS) is a behavior-observation instrument which differentiates people with ASD from those with other developmental disorders (Schopler et al., 1980; Chlebowski et al., 2010). The instrument consists of 15 domains (14 domains assessing behaviors associated with autism and one domain assessing the general impressions of autism) rated on a seven-point scale from “normal” to “severely abnormal.” The total score of all domains is evaluated, which is useful as a continuous measure of autism severity.
The Gesell Developmental Diagnosis Scale (GDDS) can be used to evaluate the developmental status of infants and young children from the age of 0 to 72 months (Westman, 1976; Raheli et al., 2009). GDDS assesses different aspects of developmental (including adaptive, gross motor, fine motor, language, and personal–social) abilities (Meinzen et al., 2010). Each participant was assigned a developmental quotient (DQ). This is the ratio between the developmental age and chronological age in each of the five specific domains. DQ ≥ 76 was considered “normal”; 55–75 denoted “mild retardation”; 40–54 indicated “moderate retardation”; ≤39 reflected “severe retardation.”
The stimulus comprised the five first formants of the syllable /da/, including consonant /d/ and vowel /a/. The /da/ was 40 ms in duration, and synthesized by a Klatt (1980) synthesizer at a rate of 10 kHz. Laterality can affect the speech-ABR, so the stimulus was presented monaurally (right ear) (Hornickel and Skoe, 2009). The stimulus intensity was 80 dB sound pressure level with a presentation rate of 10.9 stimuli/s through headphones. The default stimulus was provided using Biological Marker of Auditory Processing (BioMARK) software (Boise, ID, United States). Recording of the speech-ABR took place in an electrophysiology room at ASMCHH. The Navigator PRO system (Bio-logic Systems, Mundelein, IL, United States) and BioMARK software were used to record the speech-ABR. Three sweeps of 3000 stimuli were recorded and grand-averaged for each participant to improve the signal-to-noise ratio. The parameters used to obtain the speech-ABR have been used in our previous study (Chen et al., 2019).
Biological Marker of Auditory Processing is a testing protocol developed by Auditory Neuroscience Laboratory of Northwestern University (Evanston, IL, USA). The parameters evaluated for the speech-ABR were the: latency of wave-V; latency of wave-A; V/A slope; first formant frequencies; higher frequencies (Billiet and Bellis, 2011; Sanfins et al., 2015). The BioMARK score was obtained as a composite score derived from all five parameters that it assessed. There were different scoring criteria in different age groups. At the age of 3–4 years, a score of ≤3 was considered to be “normal” and a score from 4 to 22 was considered “abnormal.” At the age of 5–12 years, a score of ≤7 was considered “normal” and a score from 8 to 22 was considered “abnormal.” According to whether the speech-ABR was normal or abnormal, ASD participants were divided into the ASD-atypical (ASD-A) group or ASD-typical (ASD-T) group. According to BioMARK scores, ASD participants were divided into the ASD-A group (n = 15) and ASD-T group (n = 15). The BioMARK score was normal for all TD children. The ASD-T, ASD-A, and TD groups were matched on age. Relevant demographic information is shown in Table 1. The grand-average waveforms of the speech-ABR between the two subgroups were illustrated in Figure 1.
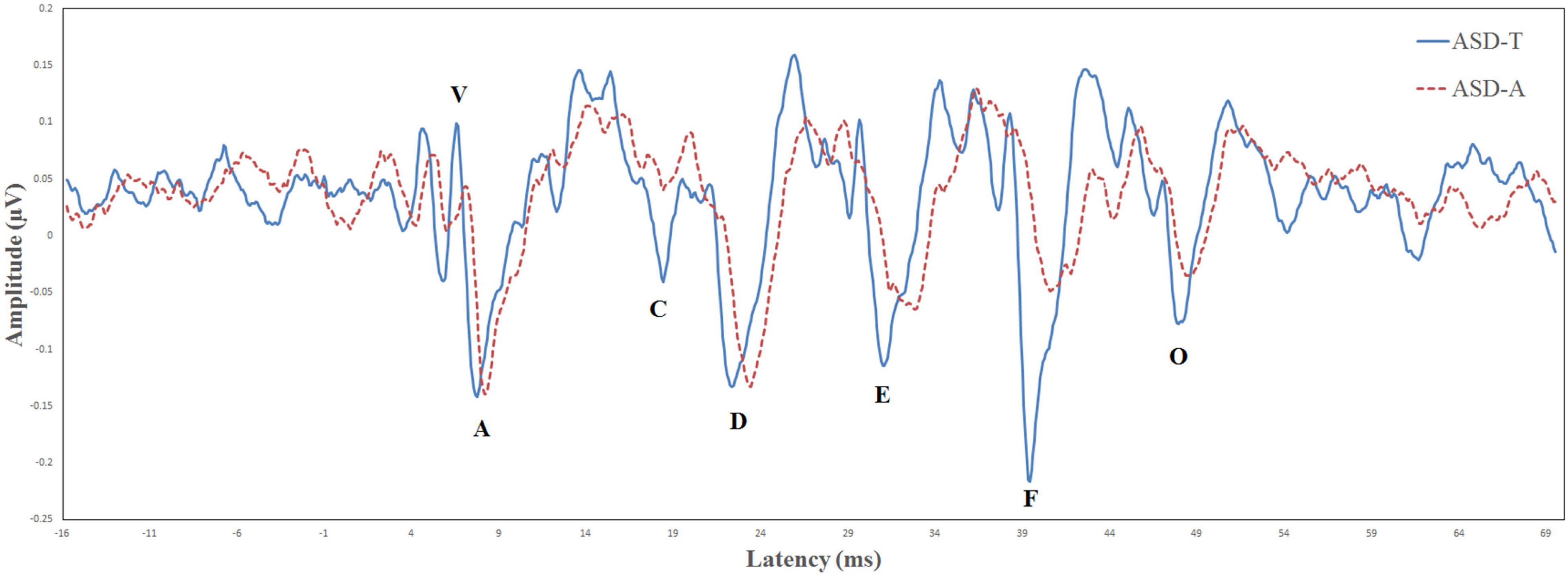
Figure 1. Comparison of the brainstem response to speech in the ASD-T group (solid line) and ASD-A group (dotted line).
For ASD participants who could cooperate to undergo MRI, 10% chloral hydrate (0.75 mL/kg, p.o.) was used for sedation. All ASD children were sedated for MRI according to the sedation protocol set by the Department of Radiology of ASMCHH. The imaging data of TD children were collected during natural sleep at night without use of sedation. High-resolution T1-weighted MRI sequences were obtained on a 1.5-T Magnetom Symphony Maestro Class Syngo MR 2002B scanner (Siemens, Munich, Germany) with echo time = 3.30 ms, repetition time = 10 ms, flip angle = 15°, 180 slices, and in-plane voxel size = 1 mm × 1 mm.
Following visual assessment of MRI data and output from the FreeSurfer image analysis suite1, the raw data from one ASD participant which were of insufficient quality were excluded. Hence, the final analysis comprised data from 29 ASD cases and 25 TD participants.
T1-weighted images were processed using FreeSurfer v6.0.0. Cortical reconstruction for each participant was examined slice-by-slice to identify inaccuracies in surface placement. Inaccuracies were corrected or excluded by a single experienced FreeSurfer user. The details of these procedures are described elsewhere (Wallace et al., 2013). Surface area, cortical thickness, and cortical volume were measured automatically at each FreeSurfer surface vertex. The local gyrification index is a 3D surface-based measure of the degree of cortical folding (which is the ratio of the cortical surface area within the sulcal folds relative to the amount of cortex on the outer visible cortex). The local gyrification index was measured using an added flag to the FreeSurfer reconstruction-processing stream (Schaer et al., 2008).
Group differences in the latency and amplitude of speech-ABR were assessed using one-way analysis of variance (ANOVA) with the diagnostic group as a three-level (ASD-A, ASD-T and TD) factor adjusted for multiple comparisons. If the main effect of the group was significant, post hoc pairwise comparisons were conducted using the Bonferroni correction. The complete characterization and compare of speech-ABR among three group were reported in Table 2. A between-group analysis using a two-sample t-test was undertaken to detect differences in scores for the ADOS, CARS, GDDS, and speech-ABR between the ASD-A group and ASD-T group. A two-step general linear model was used to estimate the difference in surface area, cortical thickness, cortical volume and the local gyrification index between two groups, including ASD and TD, ASD-A and TD, ASD-T and TD, and ASD-A and ASD-T. Surface area, cortical thickness, and cortical volume were smoothed using a 10-mm full-width at half maximum (FWHM) 2D Gaussian kernel. The local gyrification index measure is intrinsically smooth, so data were smoothed at 5-mm FWHM. To provide stringent criteria to minimize false-positive results, all analyses were set at p < 0.01 (corrected for multiple comparisons using Monte Carlo stimulations) (Hagler et al., 2006). Clusters identified in the Monte Carlo stimulations were used as regions of interest which the value of the brain structure was extracted from. A Fisher transformation was applied to improve the normality of the correlation coefficient (Lowe et al., 1998). Based on all ASD participants having different degrees of language impairment, Pearson correlations were undertaken in all ASD participants to investigate the relationship among brain structure, speech-ABR waveforms, and clinical assessments.
According to the result of correlation analyses, we further explored whether the auditory brainstem function moderated the relationship between the brain structure and language ability of all ASD participants controlled for age and sex. The PROCESS macro program within SPSS (IBM, Armonk, NY, United States) designed by Hayes (2013) was used to measure the mediating or moderating effect. Within PROCESS, “model 4” was selected and the confidence interval was set to 95%. In the moderation model, the surface area of the left rostral middle frontal gyrus (lRMFG) was entered as the predictor (X), language score of the GDDS as the outcome (Y), and the amplitude of wave-V as the moderator (M). Statistical tests were evaluated at p < 0.05 (two-tailed).
As shown in Table 3, ASD subgroups did not differ in scores for the ADOS or CARS (Table 3). Two subgroups of ASD participants were mildly retarded and did not differ in gross-motor, fine-motor, or adaptive functions. In GDDS-language, the score of the ASD-A group was lower than that of the ASD-N group (t = 2.425, p = 0.025).
There were significant (cluster-corrected p < 0.01) differences between the TD group and ASD group for cortical thickness, cortical volume and surface area (Supplementary Table 1 and Figure 2). Compared with the TD group, the ASD-T group and ASD-A group showed a significant difference in surface area, cortical volume, cortical thickness, and the local gyrification index (Table 4). Surface area of lRMFG in ASD-A group was larger than that in the ASD-T group and TD group (Figure 2 and Table 4). The local gyrification index of the right superior temporal gyrus (rSTG) in the ASD-A group was decreased significantly compared with that in the ASD-T group (Figure 3 and Table 4).
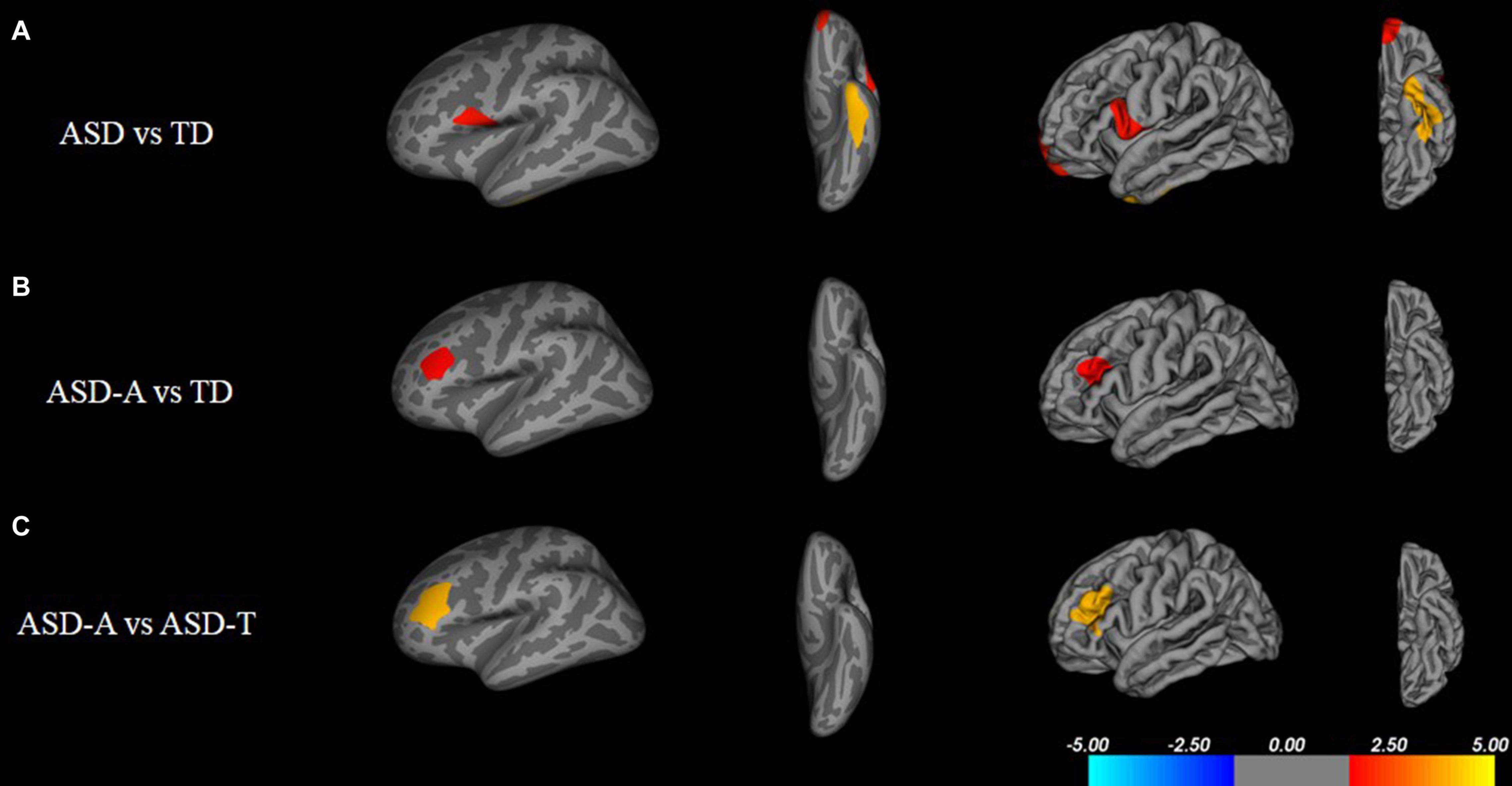
Figure 2. Inflated and pial surface maps (dark gray, sulci; light gray, gyri) of the left hemispheres showing increasing surface area in (A) fusiform, frontal pole and pars opercularis in the ASD group compared with that in the TD group, (B,C) rostral middle frontal gyrus in the ASD-A group compared with that in the TD group and ASD-T group. Significance threshold was set at p < 0.01 (cluster-corrected).
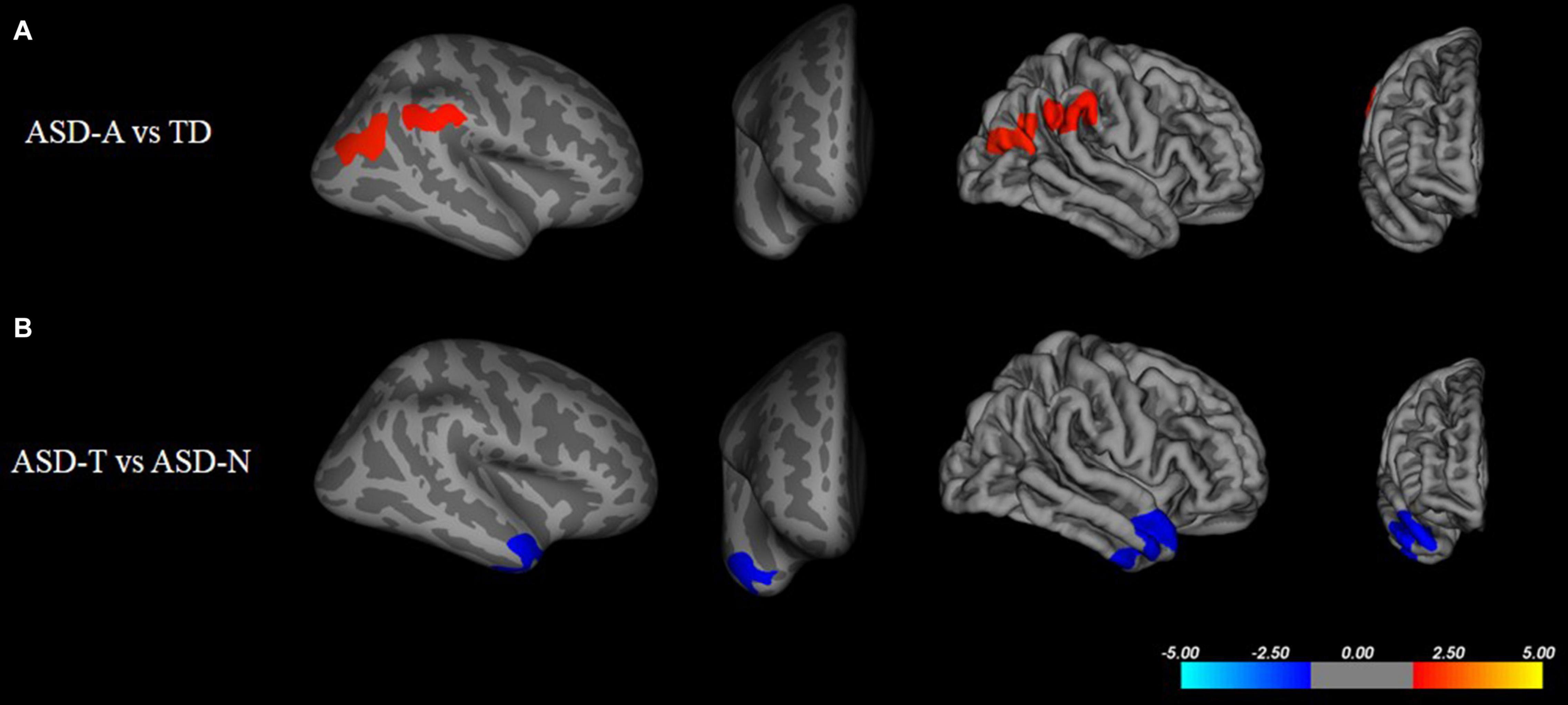
Figure 3. Inflated and pial surface maps (dark gray, sulci; light gray, gyri) of the right hemispheres showing a decreasing local gyrification index in (A) inferior parietal and supramarginal areas in the TD group compared with that in the ASD-A group, (B) superior temporal gyrus (STG) in the ASD-A group compared with that in the ASD-T group. Significance threshold was set at p < 0.01 (cluster-corrected).
We also analyzed the potential link among the speech-ABR, brain-structural and clinical-assessment data. Surface area of lRMFG was related to the latency of waves V and A, and amplitude of wave-V (Table 5). Local gyrification index of rSTG was related to the latency of waves V and A. GDDS-language scores were related to the amplitude of wave-V and latency of wave-A.
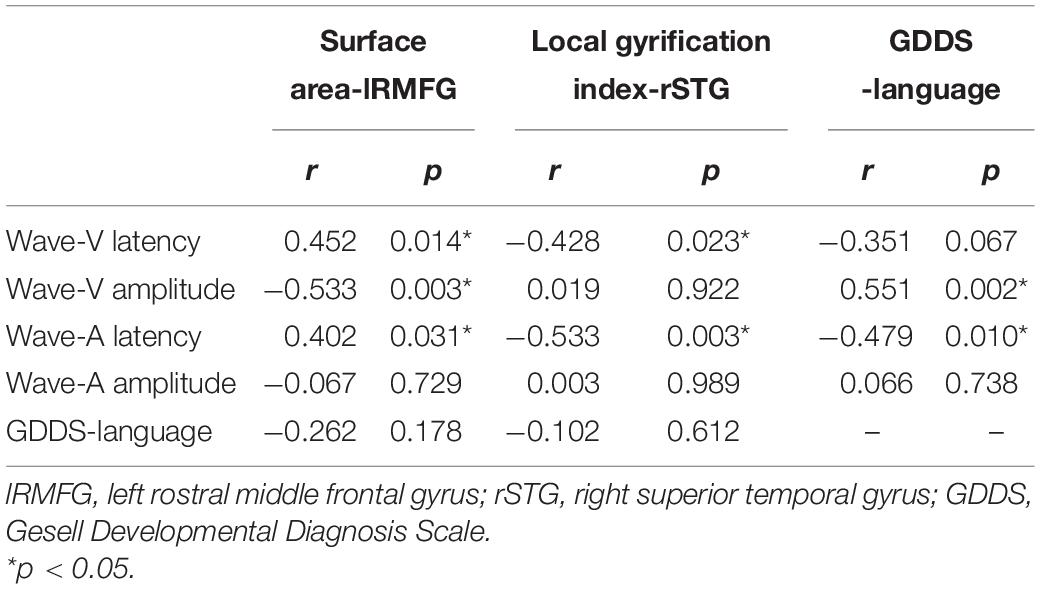
Table 5. Association between speech-ABR waveforms and brain structure and GDDS-language characteristics in ASD participants.
Table 6 presents the results of the mediation model. Figure 4 shows the regression coefficient for each pathway from surface area of lRMFG to language-GDDS in the mediation model. There was a significant negative association between surface area of lRMFG and the wave-V amplitude (β = −0.514, p = 0.006). There was a significant positive association between the wave-V amplitude and GDDS-language (β = 0.566, p = 0.008). The bootstrap procedure revealed significant indirect effects between surface area of lRMFG and GDDS-language controlled for age and sex [β = −0.013, SE = 0.007, BC 95%CI (−0.028, −0.002)].
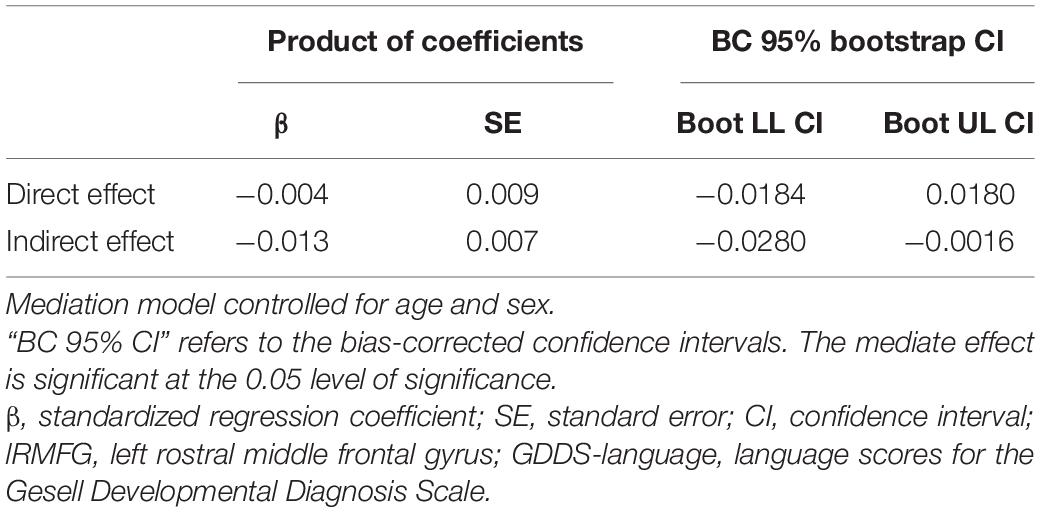
Table 6. Direct and indirect effects between surface area of lRMFG and GDDS-language mediated by the amplitude of wave-V.
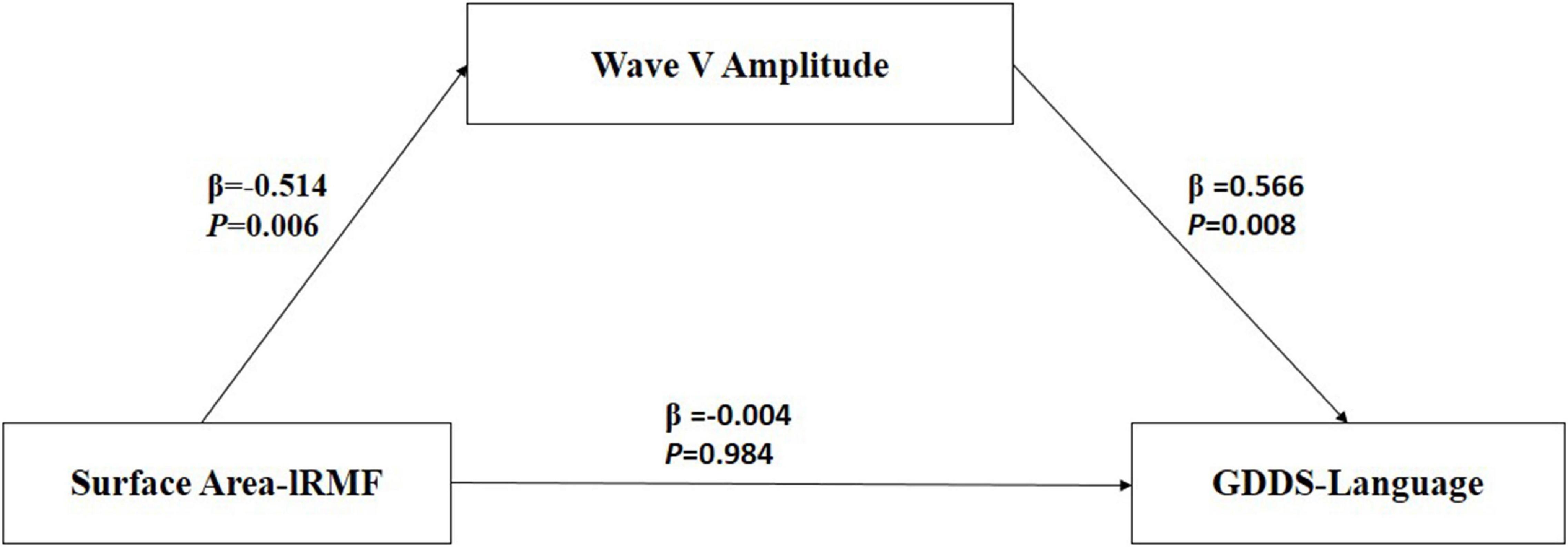
Figure 4. Mediation model among surface area-lRMFG (predictor), Wave-V amplitude (mediation), and GDDS-language (outcome). β, standardized regression coefficient. Surface area-lRMFG, surface area of left rostral middle frontal gyrus; GDDS-language, language scores of the Gesell Developmental Diagnosis Scale.
This is the first study to classify ASD children into two subtypes based on function of auditory brainstem and to discover neural substrates for the possible subtypes.
Our study elicited four main findings. First, the language score of the ASD-A group was lower than that of the ASD-T group. Second, compared with the TD group, the ASD subgroups showed significant differences in brain structure. Surface area of lRMFG in the ASD-A group was larger, and the local gyrification index of rSTG in the ASD-A group was decreased significantly, compared with those in the ASD-T group. Third, GDDS-language and surface area of lRMFG were correlated to the wave-A amplitude in ASD. Finally, analysis revealed surface area of lRMFG to have an indirect effect on language via the wave-V amplitude in ASD children.
Autism spectrum disorder subgroups defined in the present study showed a significant difference in the speech-ABR at preschool age. The ASD-T group exhibited age-appropriate subcortical auditory processing function. In contrast, the ASD-A group showed the latency of speech-ABR waves to be longer and/or the amplitude to be smaller compared with those in the TD group and ASD-T group. Besides, ASD subtypes showed a significant difference in language but not in other aspects or symptom severity. Consistent with our previous research showing that the speech-ABR is related to language ability (Chen et al., 2019), children with an efficient speech-ABR had better language ability. Our study indicated that the speech-ABR could be a clinical-assessment tool to predict the language ability of ASD children at preschool age. Combined with findings that early language ability is an important predictive factor for later outcomes of ASD (Szatmari et al., 2010; Tager-Flusberg and Kasari, 2013), ASD children with a normal speech-ABR may have a better outcome compared with abnormal ones. Taken together, these data suggest that auditory brainstem function not only has a crucial role in language, but also in the long-term outcome of ASD children.
We found differences in brain structure not only between TD children and ASD children, but also among ASD subtypes. Consistent with other studies, we found an aberrant cortical structure in ASD children, including cortical thickness, surface area, and cortical volume (Blackmon et al., 2016; Patriquin et al., 2016). Besides, differences in the local gyrification index were observed only between ASD subtypes and TD, and not ASD and TD. Combined with contradictory results in the neuroimaging of ASD (Pua et al., 2017), ASD may be composed of different subtypes which exist differences in brain anatomy. Hence, comparison of ASD and TD before correct differentiation of subtypes will lead to unstable and unrepeatable research results.
Moreover, we found a significant difference in brain structure between ASD subtypes, which may provide neuroimaging evidence for subtype classification. We found a larger regional surface area (lRMFG) in the ASD-A group compared with that in the ASD-T group and TD group. This result indicated that an increase in surface area of lRMFG was a characteristic structural change in the ASD-A subtype. The RMFG, as part of the dorsolateral prefrontal cortex (DLPFC), is related to the function of language, as discovered in various lesion studies (Chapman et al., 1992, 1998). Patients with left-DLPFC lesions have been found to use fewer complex sentences and to perseverate on the first proposition (Carl et al., 2012). These symptoms can also occur in some ASD children who use simple sentences and “chatter” on a topic. Besides, a lower local gyrification index of the right STG was found in the ASD-A group. The traditional view is that the STG is involved primarily in auditory processing, including language (Gernsbacher and Kaschak, 2003; Martin, 2003). However, recent studies have reported that the STG is not only involved in auditory processing, but is also implicated in social cognition (Bigler et al., 2007; Ethofer et al., 2013). Taken together, the differences in brain structure between ASD subtypes are involved in the cortex related to auditory and language, but also in the high-order cortex related to society. The differences in altered subtypes in the brain may be one of the important reasons for ASD heterogeneity.
Further analyses revealed that surface area of lRMFG had an indirect effect on language by altering the wave-V amplitude in ASD children. Combined with the previous findings showing that the neural generators of waves V and A may be the inferior colliculus (Song et al., 2008), one parsimonious explanation is that the lRMFG targeting the inferior colliculus mediates the language of ASD children through corticofugal neurons. As a crucial mechanism of neuromodulation, physiological studies have demonstrated that these descending pathways can affect several aspects of subcortical function (Villa et al., 1991; Diamond et al., 1992; Ma and Suga, 2001). Studies have demonstrated language impairment to be related to cortical and/or subcortical dysfunctions in ASD children (Groen et al., 2008; Russo et al., 2010a). Our findings suggest that cortical deficits may impair language ability in children with ASD by causing subcortical dysfunction at preschool age.
Using subtypes may be the most useful method for specific intervention in ASD. Experience-dependent mechanisms drive the plasticity of the auditory brainstem, which can be improved by auditory training (Hayes et al., 2003; Russo et al., 2005) and language experience (Krishnan et al., 2004, 2005). Skoe et al. (2015) proposed the corticofugal system nears maturation stabilization around the age of 8–11 years. Therefore, we postulate that ASD children with an abnormal speech-ABR should undergo related auditory and language training as soon as possible, especially at preschool age. The Fast ForWord language-training program has been found to improve auditory-brainstem and cortical responses in ASD children (Russo et al., 2010b). Few intervention studies on ASD have been done. However, based on behavioral and genetic similarities (Herbert and Kenet, 2007; Smith, 2007) in language impairment between ASD and language-based learning disorders (Rapin and Dunn, 2003; Cardy et al., 2005), intervention programs of language-based learning disorders to improve auditory-brainstem function could be conducted in ASD children with an abnormal speech-ABR. In addition, the subtype-based method may be useful as a prognostic biomarker in ASD children. A stable ABR is associated with heightened language abilities (Hornickel and Kraus, 2013). Previously, we found the auditory brainstem to be impaired and immature in preschool children with ASD (Chen et al., 2019). We speculated the ASD children with an abnormal speech-ABR indicates impairment of the related brain region and poor language ability, which may lead to a poor prognosis. In the future, we will examine if an abnormal speech-ABR is associated with a worse long-term outcome compared with normal ones. Overall, ASD children should undergo a speech-ABR after 3 years of age for use as a subtype and prognostic biomarker, and a subtype-specific intervention should be employed for a better outcome.
In interpreting our results, some limitations should be considered. First, our study had a relatively small sample size. There were only two female participants in this study, and both were in the ASD-A. This study does not exclude the potential of gender factors for subtypes. Our future studies will recruit more children with ASD who meet the criteria for an appropriate gender ratio. Second, MRI was done on a 1.5-T system, but several recent studies have used 3-T MRI scanners. MRI scanners working at 3 T have a higher field strength and increased signal-to-noise ratio compared with those using a 1.5-T MRI scanner. In future work, we will use a 3.0-T MRI scanner to acquire higher spatial resolution data. Finally, intelligence and language ability are highly correlated. Although there were no significant differences in overall developmental levels between the two subtypes. It is possible that there were significant differences in intelligence levels. Our future studies will investigate whether intellectual factors affect subcortical auditory processing in ASD.
This is first study to distinguish ASD children by auditory brainstem function and to explore neural evidence for identification of potential subtypes. There were significant differences between the ASD group and TD group in surface area, cortical volume, and cortical thickness. Compared with the ASD-T group, the ASD-A group showed a lower score in GDDS-language, increased surface area of lRMFG, and decreased local gyrification index of rSTG. GDDS-language and surface area of lRMFG were correlated with the wave-A amplitude. Surface area of lRMFG had an indirect effect on the performance of language by altering the wave-V amplitude. Thus, cortical deficits may impair language ability in children with ASD by causing subcortical dysfunction at preschool age. These evidences support dysfunction of the auditory brainstem as a potential subtype of ASD. Besides, this subtype-based method may be useful for various clinical applications (e.g., prognosis and subtype-specific intervention).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Research Ethics Board of Affiliated Shenzhen Maternity & Child Healthcare Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JC, ZP, and GW conceived the study, prepared the data, analyzed the data, and drafted, and revised the manuscript. BL, JG, and MH helped to analyze the data and helped to draft the manuscript. XK and CL participated in the study design and helped to draft and revise the manuscript. All authors approved the final version of the manuscript.
This study was supported by the Key-Area Research and Development Program of Guangdong Province (2019B030335001), the Shenzhen Key Medical Discipline Construction Fund (SZXK071), Sanming Project of Medicine of Shenzhen (SZSM201612079), National Natural Science Foundation of China (31871113 and 31900770), and Guangdong Key Project in development of new tools for diagnosis and treatment of autism (2018B030335001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the children and their families for their participation to make this research possible.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.637079/full#supplementary-material
Supplementary Figure1 | Hypothesized model among cortex, subcortex, and language in ASD preschool children.
Supplementary Table 1 | Cluster of significant differences in cortical morphometry between the TD group and ASD group.
ASD, autism spectrum disorder; ABR, auditory brainstem response; speech-ABR, speech-evoked auditory brainstem response; MRI, magnetic resonance imaging; ADOS, Autism Diagnostic Observational Schedule; CARS, Childhood Autism Rating Scale; GDDS, Gesell Developmental Diagnosis Scale; DQ, developmental quotient; BioMARK, Biological Marker of Auditory Processing; lRMFG, left rostral middle frontal gyrus; rSTG, right superior temporal gyrus.
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Pshychartic Association.
Anderson, D. K., Catherine, L., Susan, R., Dilavore, P. S., Cory, S., Audrey, T., et al. (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. J. Consult. Clin. Psychol. 75:594. doi: 10.1037/0022-006X.75.4.594
Anderson, S., Skoe, E., Chandrasekaran, B., and Kraus, N. (2010). Neural timing is linked to speech perception in noise. J. Neurosci. 30, 4922–4926. doi: 10.1523/jneurosci.0107-10.2010
Banai, K., Nicol, T., Zecker, S. G., and Kraus, N. (2005). Brainstem timing: implications for cortical processing and literacy. J. Neurosci. 25, 9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005
Bauman, M. L., and Kemper, T. L. (2005). Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 23, 183–187. doi: 10.1016/j.ijdevneu.2004.09.006
Bigler, E. D., Mortensen, S., Neeley, E. S., Ozonoff, S., Krasny, L., Johnson, M., et al. (2007). Superior temporal gyrus, language function, and autism. Dev. Neuropsychol. 31, 217–238. doi: 10.1080/87565640701190841
Billiet, C. R., and Bellis, T. J. (2011). The relationship between brainstem temporal processing and performance on tests of central auditory function in children with reading disorders. J. Speech Lang. Hear. Res. 54, 228–242. doi: 10.1044/1092-4388(2010/09-0239)
Bitsika, V., Sharpley, C. F., and Orapeleng, S. (2008). An exploratory analysis of the use of cognitive, adaptive and behavioural indices for cluster analysis of ASD subgroups. J. Intell. Disabil. Res. 52, 973–985. doi: 10.1111/j.1365-2788.2008.01123.x
Blackmon, K., Ben-Avi, E., Wang, X., Pardoe, H. R., Di Martino, A., Halgren, E., et al. (2016). Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. Neuroimage Clin. 10, 36–45. doi: 10.1016/j.nicl.2015.10.017
Brambilla, P., Hardan, A., di Nemi, S. U., Perez, J., Soares, J. C., and Barale, F. (2003). Brain anatomy and development in autism: review of structural MRI studies. Brain Res. Bull. 61, 557–569. doi: 10.1016/j.brainresbull.2003.06.001
Cardy, J. E. O., Flagg, E. J., Roberts, W., Brian, J., and Roberts, T. P. J. N. (2005). Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport 16, 329–332. doi: 10.1097/00001756-200503150-00005
Carl, C., Karen, L., Jennifer, M., Frank, K., and Jordan, G. J. N. (2012). Discourse production following injury to the dorsolateral prefrontal cortex. Neuropsychologia 50, 3564–3572. doi: 10.1016/j.neuropsychologia.2012.09.005
Chandrasekaran, B., and Kraus, N. (2010). The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology 47, 236–246. doi: 10.1111/j.1469-8986.2009.00928.x
Chapman, S. B., Culhane, K. A., Levin, H. S., Harward, H., Mendelsohn, D., Ewing-Cobbs, L., et al. (1992). Narrative discourse after closed head injury in children and adolescents. Brain Lang. 43, 42–65. doi: 10.1016/0093-934x(92)90020-f
Chapman, S. B., Levin, H. S., Wanek, A., Weyrauch, J., and Kufera, J. (1998). Discourse after closed head injury in young children. Brain Lang. 61, 420–449. doi: 10.1006/brln.1997.1885
Chen, J., Liang, C., Wei, Z., Cui, Z., Kong, X., Dong, C. J., et al. (2019). Atypical longitudinal development of speech-evoked auditory brainstem response in preschool children with autism spectrum disorders. Autism Res. 12, 1022–1031. doi: 10.1002/aur.2110
Chlebowski, C., Green, J. A., Barton, M. L., and Fein, D. (2010). Using the childhood autism rating scale to diagnose autism spectrum disorders. J. Autism Dev. Disord. 40, 787–799.
De Fossé, L., Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S., Mcgrath, L., et al. (2010). Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 56, 757–766. doi: 10.1002/ana.20275
Deboth, K. K., and Reynolds, S. (2017). A systematic review of sensory-based autism subtypes. Res. Autism Spectrum Disord. 36, 44–56. doi: 10.1016/j.rasd.2017.01.005
Diamond, M. E., Armstrong-James, M., Budway, M. J., and Ebner, F. F. (1992). Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J. Comp. Neurol. 319, 66–84. doi: 10.1002/cne.903190108
Dick, A. S., Bernal, B., and Tremblay, P. (2014). The language connectome: new pathways, new concepts. Neurosci. 20, 453–467. doi: 10.1177/1073858413513502
Ethofer, T., Bretscher, J., Wiethoff, S., Bisch, J., Schlipf, S., Wildgruber, D., et al. (2013). Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. Neuroimage 76, 45–56. doi: 10.1016/j.neuroimage.2013.02.064
Gernsbacher, M. A., and Kaschak, M. P. (2003). Neuroimaging studies of language production and comprehension. Annu. Rev. Psychol. 54, 91–114. doi: 10.1146/annurev.psych.54.101601.145128
Groen, W. B., Zwiers, M. P., van der Gaag, R. J., and Buitelaar, J. K. (2008). The phenotype and neural correlates of language in autism: an integrative review. Neurosci. Biobehav. Rev. 32, 1416–1425. doi: 10.1016/j.neubiorev.2008.05.008
Grzadzinski, R., Huerta, M., and Lord, C. (2013). DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol. Autism 4, 1–6. doi: 10.1186/2040-2392-4-12
Hagler, D. J. Jr., Saygin, A. P., and Sereno, M. I. (2006). Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33, 1093–1103. doi: 10.1016/j.neuroimage.2006.07.036
Hayes, A. F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis. New York, NY: Guilford Press, 335–337.
Hayes, E. A., Warrier, C. M., Nicol, T. G., Zecker, S. G., and Kraus, N. (2003). Neural plasticity following auditory training in children with learning problems. Clin. Neurophysiol. 114, 673–684. doi: 10.1016/s1388-2457(02)00414-5
Heaton, P., Williams, K., Cummins, O., and Happe, F. (2008). Autism and pitch processing splinter skills: a group and subgroup analysis. Autism 12, 203–219. doi: 10.1177/1362361307085270
Herbert, M. R., and Kenet, T. (2007). Brain abnormalities in language disorders and in autism. Pediatr. Clin. North Am. 54, 563–583. doi: 10.1016/j.pcl.2007.02.007
Hornickel, J., and Kraus, N. (2013). Unstable representation of sound: a biological marker of dyslexia. J. Neurosci. 33, 3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013
Hornickel, J., and Skoe, E. N. (2009). Subcortical laterality of speech encoding. Audiol. Neurotol. 14, 198–207. doi: 10.1159/000188533
Johnson, K. L., Nicol, T. G., Zecker, S. G., and Kraus, N. (2007). Auditory brainstem correlates of perceptual timing deficits. J. Cogn. Neurosci. 19, 376–385. doi: 10.1162/jocn.2007.19.3.376
Kjelgaard, M. M., and Tager-Flusberg, H. (2001). An investigation of language impairment in autism: implications for genetic subgroups. Lang. Cogn. Process. 16, 287–308. doi: 10.1080/01690960042000058
Klatt, D. (1980). Software for cascade/parallel formant synthesizer. J. Acoustical Soc. Am., 67, 971–975. doi: 10.1121/1.383940
Klin, A. (1993). Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J. Autism Dev. Disord. 23, 15–35. doi: 10.1007/BF01066416
Krishnan, A., Xu, Y., Gandour, J., and Cariani, P. (2005). Encoding of pitch in the human brainstem is sensitive to language experience. Brain Res. Cogn. Brain Res. 25, 161–168. doi: 10.1016/j.cogbrainres.2005.05.004
Krishnan, A., Xu, Y., Gandour, J. T., and Cariani, P. A. (2004). Human frequency-following response: representation of pitch contours in Chinese tones. Hear. Res. 189, 1–12. doi: 10.1016/S0378-5955(03)00402-7
Lombardo, M. V., Pierce, K., Eyler, L. T., Barnes, C. C., Ahrens-Barbeau, C., Solso, S., et al. (2015). Different functional neural substrates for good and poor language outcome in autism. Neuron 86, 567–577. doi: 10.1016/j.neuron.2015.03.023
Lord, C. O. (1989). Austism diagnostic observation schedule: a standardized observation of communicative and social behavior. J. Autism Dev. Disord. 19:185. doi: 10.1007/BF02211841
Lowe, M. J., Mock, B. J., and Sorenson, J. A. (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 7, 119–132. doi: 10.1006/nimg.1997.0315
Ma, X., and Suga, N. (2001). Plasticity of bat’s central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J. Neurophysiol. 85, 1078–1087. doi: 10.1152/jn.2001.85.3.1078
Martin, R. C. (2003). Language processing: functional organization and neuroanatomical basis. Annu. Rev. Psychol. 54, 55–89. doi: 10.1146/annurev.psych.54.101601.145201
Meinzen, D. J., Wiley, S. S., and Choo, D. I. (2010). Language performance in children with cochlear implants and additional disabilities. Laryngoscope 120, 405–413. doi: 10.1002/lary.20728
Miron, O., Beam, A. L., and Kohane, I. S. (2018). Auditory brainstem response in infants and children with autism spectrum disorder: a meta-analysis of wave V. Autism Res. 11, 355–363.
Miron, O., Beam, A. L., Kohane, I. S., and Miron, O. (2017). Auditory brainstem response in infants and children with autism: a meta-analysis. Autism Res. 11, 355–363. doi: 10.1002/aur.1886
Patriquin, M. A., Deramus, T., Libero, L. E., Laird, A., and Kana, R. K. (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 37, 3957–3978. doi: 10.1002/hbm.23288
Pickles, A., Anderson, D. K., and Lord, C. (2014). Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J. Child Psychol. Psychiatry 55, 1354–1362. doi: 10.1111/jcpp.12269
Pua, E. P. K., Bowden, S. C., and Seal, M. L. (2017). Autism spectrum disorders: neuroimaging findings from systematic reviews. Res. Autism Spectrum Disord. 34, 28–33. doi: 10.1016/j.rasd.2016.11.005
Raheli, D., Gustavo, M., Liat, B. S., Dorit, L., Pick, C. G., and Tally, L. S. (2009). Developmental outcome of children with enlargement of the cisterna magna identified in utero. J. Child Neurol. 24, 1486–1492. doi: 10.1177/0883073808331358
Rapin, I., and Dunn, M. (2003). Update on the language disorders of individuals on the autistic spectrum. Brain Dev. 25, 166–172. doi: 10.1016/s0387-7604(02)00191-2
Russo, N., Nicol, T., Trommer, B., Zecker, S., and Kraus, N. (2010a). Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Dev. Sci. 12, 557–567. doi: 10.1111/j.1467-7687.2008.00790.x
Russo, N. M., Hornickel, J., Nicol, T., Zecker, S., and Kraus, N. (2010b). Biological changes in auditory function following training in children with autism spectrum disorders. Behav. Brain Funct. 6:60. doi: 10.1186/1744-9081-6-60
Russo, N. M., Nicol, T. G., Zecker, S. G., Hayes, E. A., and Kraus, N. (2005). Auditory training improves neural timing in the human brainstem. J. Behav. Brain Res. 156, 95–103. doi: 10.1016/j.bbr.2004.05.012
Russo, N. M., Skoe, E., Trommer, B., Nicol, T., Zecker, S., Bradlow, A., et al. (2008). Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clin. Neurophysiol. Offic. J. Int. Feder. Clin. Neurophysiol. 119, 1720–1731. doi: 10.1016/j.clinph.2008.01.108
Sacco, R., Lenti, C., Saccani, M., Curatolo, P., Manzi, B., Bravaccio, C., et al. (2012). Cluster analysis of autistic patients based on principal pathogenetic components. Autism Res. 5, 137–147. doi: 10.1002/aur.1226
Sanfins, M. D., Borges, L. R., Ubiali, T., and Colella-Santos, M. F. (2015). Speech auditory brainstem response (speech ABR) in the differential diagnosis of scholastic difficulties. Braz. J. Otorhinolaryngol. 83, 31–40.
Schaer, M., Cuadra, M. B., Tamarit, L., Lazeyras, F., Eliez, S., and Thiran, J. P. (2008). A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging 27, 161–170. doi: 10.1109/TMI.2007.903576
Schopler, E., Reichler, R. J., Devellis, R. F., and Daly, K. (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 10, 91–103. doi: 10.1007/BF02408436
Skoe, E., Krizman, J., Anderson, S., and Kraus, N. (2015). Stability and plasticity of auditory brainstem function across the lifespan. Cereb. Cortex 25:1415. doi: 10.1093/cercor/bht311
Smith, S. D. (2007). Genes, language development, and language disorders. Ment. Retard Dev. Disabil. Res. Rev. 13, 96–105. doi: 10.1002/mrdd.20135
Song, J. H., Banai, K., and Kraus, N. (2008). Brainstem timing deficits in children with learning impairment may result from corticofugal origins. Audiol. Neurotol. 13, 335–344. doi: 10.1159/000132689
Stacy, S., Cristan, F., Audrey, T., Lisa, J., David, B., and Christine, G. (2012). The ADOS calibrated severity score: relationship to phenotypic variables and stability over time. Autism Res. 5, 267–276. doi: 10.1002/aur.1238
Szatmari, P., Bryson, S. E., Boyle, M. H., Streiner, D. L., and Duku, E. (2010). Predictors of outcome among high functioning children with autism and Asperger syndrome. J. Child Psychol. Psychiatry 44, 520–528. doi: 10.1111/1469-7610.00141
Tager-Flusberg, H., and Kasari, C. (2013). Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Res. 6, 468–478. doi: 10.1002/aur.1329
Villa, A. E., Rouiller, E. M., Simm, G. M., Zurita, P., de Ribaupierre, Y., and de Ribaupierre, F. (1991). Corticofugal modulation of the information processing in the auditory thalamus of the cat. Exp. Brain Res. 86, 506–517. doi: 10.1007/bf00230524
Wallace, G. L., Briana, R., Nathan, D., Lauren, K., Giedd, J. N., and Alex, M. (2013). Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain J. Neurol. 136, 1956–1967. doi: 10.1093/brain/awt106
Westman, J. C. (1976). Gesell and amatruda’s developmental diagnosis: the evaluation and management of normal and abnormal neuropsychologic development in infancy and early childhood. Am. J. Psychiatry 133:351. doi: 10.1176/ajp.133.3.351
Wible, B., Nicol, T., and Kraus, N. (2005). Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain 128, 417–423. doi: 10.1093/brain/awh367
Winer, J. A. (2005). Decoding the auditory corticofugal systems. Hear. Res. 207, 1–9. doi: 10.1016/j.heares.2005.06.007
Keywords: autism spectrum disorder, subtype, speech-ABR, neuroimaging, mediation, neurophysiology
Citation: Chen J, Wei Z, Liang C, Liu B, Guo J, Kong X, Huang M, Peng Z and Wan G (2021) Dysfunction of the Auditory Brainstem as a Neurophysiology Subtype of Autism Spectrum Disorder. Front. Neurosci. 15:637079. doi: 10.3389/fnins.2021.637079
Received: 02 December 2020; Accepted: 19 February 2021;
Published: 17 March 2021.
Edited by:
Francesco Craig, University of Calabria, ItalyReviewed by:
Andrea De Giacomo, University of Bari Aldo Moro, ItalyCopyright © 2021 Chen, Wei, Liang, Liu, Guo, Kong, Huang, Peng and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guobin Wan, Z2J3MTk3OEBhbGl5dW4uY29t; Ziwen Peng, cGVuZ3p3QGVtYWlsLnN6dS5lZHUuY24=; Jierong Chen, bGVvYW5keG1AZm94bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.