
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 08 March 2021
Sec. Neurogenomics
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.600462
This article is part of the Research Topic Genetic and Epigenetic Mechanisms of Parkinson's Disease View all 5 articles
MicroRNAs (miRNAs) and PIWI-interacting RNAs (piRNAs) regulate gene expression and biological processes through specific genetic and epigenetic mechanisms. Recent studies have described a dysregulation of small non-coding RNAs in Parkinson’s disease (PD) tissues but have been limited in scope. Here, we extend these studies by comparing the dysregulation of both miRNAs and piRNAs from transgenic Caenorhabditis elegans (C. elegans) nematodes overexpressing pan-neuronally human α-synuclein wild-type (WT) (HASNWT OX) or mutant (HASNA53T OX). We observed 32 miRNAs and 112 piRNAs dysregulated in HASNA53T OX compared with WT. Genetic crosses of HASNA53T OX PD animal models with tdp-1 null mutants, the C. elegans ortholog of TDP-43, an RNA-binding protein aggregated in frontal temporal lobar degeneration, improved their behavioral deficits and changed the number of dysregulated miRNAs to 11 and piRNAs to none. Neuronal function-related genes T28F4.5, C34F6.1, C05C10.3, camt-1, and F54D10.3 were predicted to be targeted by cel-miR-1018, cel-miR-355-5p (C34F6.1 and C05C10.3), cel-miR-800-3p, and 21ur-1581 accordingly. This study provides a molecular landscape of small non-coding RNA dysregulation in an animal model that provides insight into the epigenetic changes, molecular processes, and interactions that occur during PD-associated neurodegenerative disorders.
MicroRNAs (miRNAs) and PIWI-interacting RNAs (piRNAs) are well conserved small non-coding RNAs 21–23 or 24–31 nucleotides in length, respectively. MiRNAs can regulate gene expression at the transcriptional level by pairing to the 3′ UTR of target mRNAs and inducing cleavage or posttranscriptional gene silencing (Hutvagner and Zamore, 2002; Bartel, 2009). PiRNAs mainly function in the repression of transposons at transcriptional or posttranscriptional levels to safeguard the germline genome (Iwasaki et al., 2015; Ernst et al., 2017). There is increasing interest in the role that miRNAs play in neuronal homeostasis and pathogenesis of neurodegenerative diseases via dysregulation, targeting of key genes, or altering the epigenetic landscape (Tan et al., 2015; Quinlan et al., 2017). The identification of miRNAs dysregulated in neurodegenerative diseases and construction of related animal models may thus aid in delineation of molecular mechanisms underlying their pathologic processes (Juzwik et al., 2019). Moreover, exploitation of these molecular pathways via precision therapy strategies for neurodegenerative diseases is well underway (Junn and Mouradian, 2012; Pereira et al., 2017; Nuzziello et al., 2019). While piRNAs have a well-established role in the protection of the germline genome, they also function in somatic cells including neurons (Lee et al., 2011; Yan et al., 2011). PiRNAs can participate in synaptic plasticity and axon regeneration of neurons (Rajasethupathy et al., 2012; Kim et al., 2018). Hundreds of piRNAs mainly originating from introns were found to be dysregulated in Alzheimer’s disease (AD) patient brains (Roy et al., 2017). In addition, piRNAs were also dysregulated in midbrain neuronal cells differentiated from skin fibroblasts of sporadic Parkinson’s disease (PD) patients similarly to PD postmortem brain samples (Schulze et al., 2018). Furthermore, pathogenic tau induced the reduction of piRNAs in tau-related neurodegenerative diseases such as AD and progressive supranuclear palsy and consequently caused abnormal transposable element activity and subsequently progressive neuronal death (Sun et al., 2018). PiRNAs are thus beginning to be appreciated as pathogenic factors and therapeutic targets for neurodegenerative diseases as well as miRNAs (Wakisaka and Imai, 2019).
α-Synuclein was initially identified as the predominant component in aggregates of Lewy bodies and Lewy neurites in PD patient neurons (Spillantini et al., 1997, 1998). Its mutation and overexpression were subsequently associated with familial PD (Polymeropoulos et al., 1997; Chartier-Harlin et al., 2004). Increasing evidence suggests that α-synuclein is also closely linked to other neurodegenerative diseases including dementia with Lewy bodies (DLB) and multiple system atrophy (MSA), which are termed synucleinopathies together with PD (Wakabayashi et al., 1998; Wong and Krainc, 2017; Visanji et al., 2019). Furthermore, α-synuclein aggregates are found in the abnormal deposition in amyotrophic lateral sclerosis (ALS) and AD frequently (Wilhelmsen et al., 2004; Korff et al., 2013; Takei et al., 2013). Pathologic α-synuclein could impair mitochondria and elevate the reactive oxygen species level, induce endoplasmic reticulum stress, inhibit proteasome activity, and cause cell death via activating caspase-3, caspase-9, and caspase-12 in PD (Smith et al., 2005; Wong and Krainc, 2017). Some studies showed that α-synuclein pre-fibrillar oligomers were likely the toxic species and that α-synuclein fibrils might be protective to neurons (Tanaka et al., 2004; Karpinar et al., 2009; Winner et al., 2011). Other studies showed that α-synuclein fibrils caused more dopaminergic neuron impairment or loss than oligomers due to their ability to disrupt cell membrane permeability (Peters et al., 2012; Taschenberger et al., 2012). Despite many years and a great deal of effort since its discovery, mechanisms underlying the toxicity of α-synuclein in neurodegenerative diseases continue to be developed and defined. As proteopathic intracellular and extracellular α-synuclein is a key protein in various synucleinopathies, its targeting remains a viable and attractive therapeutic strategy (Luk et al., 2012; Recasens et al., 2014; Karpowicz et al., 2019).
The disrupted metabolism of α-synuclein is implicated in its pathogenesis. α-Synuclein A53T mutant protein was more stable than other mutants or wild-type (WT) forms either in vitro or in vivo (Li et al., 2004). The enhanced stability of this α-synuclein A53T protein could then increase its own protein aggregate or fibril formation (Conway et al., 2000; Lee et al., 2002). Subsequently, lysosomal autophagy, which is responsible for WT α-synuclein degradation, was impaired when α-synuclein A53T mutant protein bound the lysosomal membrane receptor, suggesting an intracellular mechanism by which aggregates can begin accumulating (Cuervo et al., 2004). In our previous studies, human α-synuclein A53T overexpressing pan-neuronally (HASNA53T OX) in Caenorhabditis elegans (C. elegans) could impair the locomotion, development, and basal slowing response; induce significant dopaminergic neuron degeneration; extend life span; and cause gene dysregulation. Human WT α-synuclein overexpressing pan-neuronally (HASNWT OX) also impaired dopaminergic neurons, extended life span, and caused differential gene expression, but the locomotion, development, and basal slowing response were not affected to the same extent as HASNA53T OX (Shen et al., 2020). Taken together, the toxicities of HASNWT OX and HASNA53T OX were different, while the underlying molecular process remains unexplored.
In this study, we hypothesized that miRNAs/piRNAs play important roles in the toxicities of HASNWT OX and HASNA53T OX in C. elegans. We previously observed that the deletion of tdp-1, the worm ortholog of human TDP-43 that is aggregated in frontal temporal lobar degeneration and ALS, could improve defects induced by HASNWT/A53T OX in C. elegans (Shen et al., 2020). We further hypothesized that TDP-1 might support HASNWT/A53T OX toxicity via sustaining the related miRNAs/piRNA expression. To test these hypotheses, we performed small RNA sequencing (sRNA-seq) on HASNWT/A53T OX C. elegans and crosses with a tdp-1 knock-out (tdp-1 KO) strains. Here, we identified a large number of differentially expressed miRNAs and piRNAs (DE-miRNAs and DE-piRNAs). The predicted targets of these dysregulated small non-coding RNAs then indicate pathways that support the toxicity observed in these animal models.
C. elegans strain N2 (WT) was obtained from the Caenorhabditis Genetics Center (CGC). Transgenic/mutant C. elegans strains constructed or crossed by our lab were as follows (Lakso et al., 2003; Shen et al., 2020): UM0010 (Pdat–1::GFP, Paex–3::HASNA53T), human α-synuclein A53T overexpressing pan-neuronally (HASNA53T OX); UM0011 (Pdat–1::GFP, Paex–3::HASNWT), human α-synuclein WT overexpressing pan-neuronally (HASNWT OX); UM0012 (Pdat–1::GFP, tdp-1 KO), tdp-1 gene deletion strain; UM0013 (Pdat–1::GFP, Paex–3::HASNA53T, tdp-1 KO), generated from crossing of UM0010 and RB929 (tdp-1 KO); and UM0014 (Pdat–1::GFP, Paex–3::HASNWT, tdp-1 KO), generated from crossing of UM0011 and RB929. The transgenic strains UM0010 and UM0011 had their transgenes integrated by X-ray irradiation and were back-crossed with WT N2 4× times (Vartiainen et al., 2006b).
The C. elegans strains used for sRNA-seq were N2, UM0010, UM0011, UM0012, UM0013, and UM0014. Worms were collected at the L4 stage at 20°C. The total RNAs were isolated by TRIzol (Thermo Fisher Scientific, Waltham, MA, United States) after washing the worms three times with distilled water. Small RNA libraries were prepared according to the instructions of the NEBNext Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs, Ipswich, MA, United States). The quantity and quality of the RNA and its transcribed cDNA were determined by an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, United States). The resulting cDNA libraries were then sequenced in triplicate on the Illumina HiSeq 2500 Platform in a single-end mode (50 nt).
The identification of DE-miRNAs and DE-piRNAs for comparisons was based on six strains assayed with the genotypes, as follows: WT, tdp-1 KO, HASNWT OX, HASNA53T OX, HASNWT OX + tdp-1 KO, and HASNA53T OX + tdp-1 KO. The quality of reads was measured by fastQC, and the raw reads were trimmed using Trimmomatic (Bolger et al., 2014). For the miRNA analysis, trimmed reads were mapped to the genome using bowtie, and the genome sequence (WBcel235/ce11) was downloaded from the Ensembl database (Cunningham et al., 2019). The sam files were then converted to bam files and indexed using SAMtools (Li et al., 2009). The miRNA annotation file was downloaded from miRBase. FeatureCounts was used to measure the read count that mapped to a miRNA. “exactTest” from “edgeR” package was used to identify the DE-miRNAs with the threshold set as p value <0.05 and absolute fold change >2 (Robinson et al., 2010). The pipeline for identification of DE-piRNAs is similar to the identification of DE-miRNAs except that the genome data (WBcel235/ce11) and the piRNA annotation file are downloaded from the National Center for Biotechnology Information (NCBI) database1. Due to the large number of piRNAs, the threshold for the DE-piRNAs was set as p value <0.01, false discovery rate (FDR) <0.1, and absolute fold change >2.
The heatmaps for miRNAs/piRNAs expressed in different strains were drawn as described below. First, all expressed miRNAs/piRNAs were collected. The union set of DE-miRNAs (cutoff was p value <0.05 and absolute fold change >2) and DE-piRNAs (cutoff was p value <0.01, FDR <0.1, and absolute fold change >2) of mutant/transgenic strains compared with the WT strain was obtained. Then, the average read counts of all the miRNAs/piRNAs or the union set in different biological triplicates of each strain and the log2 transformed count-per-million (log2cpm) value were calculated. Finally, the Z-score of the log2cpm value of each miRNA/piRNA was calculated and used to draw the heatmap. The complete linkage method was used for hierarchical clustering analysis, which was performed by the “pheatmap” package. The novel miRNAs were identified with miRDeep2 (Friedlander et al., 2012).
The miRNA target information was downloaded from miRTarBase (Chou et al., 2018), and the piRNA target information was downloaded from piRTarBase (Wu et al., 2019). The Gene Ontology (GO) enrichment analysis in biological process (BP), molecular function (MF), and cellular component (CC) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using “enrichGO” and “enrichKEGG” functions, respectively, from the “clusterProfiler” package (Yu et al., 2012).
The information for the target genes of DE-miRNAs and DE-piRNAs was downloaded from the miRTarBase and piRTarBase using the stringent targeting rules, respectively. The data for transcription factors (TFs), regulating the transcription of miRNAs, were downloaded from the TransmiR database (Tong et al., 2019). The TFs and their regulated genes data were downloaded from the TF2DNA database (Pujato et al., 2014). The differentially expressed genes (DEGs) were obtained from RNA-Seq in our previous work with the criteria of p value <0.05 and absolute fold change >2 (Shen et al., 2020). Based on the interaction information, we constructed the dysregulation networks composed of DE-miRNA and DEG interactions, DE-piRNA and DEG interactions, and networks for TF and DE-miRNA interaction, for each comparison. The network construction was completed by the “igraph” package (Csardi and Nepusz, 2006).
In order to determine whether any DE-miRNA or DE-piRNA targets were associated with a neurodegenerative disease, we downloaded the curated gene–disease associations from the DisGeNET database (Pinero et al., 2020). The human genes associated with the neurodegenerative diseases including PD, AD, MSA, ALS, and Huntington’s disease (HD) were obtained from the DisGeNET database by using the keywords “Parkinson/Alzheimer/multiple system atrophy/amyotrophic lateral sclerosis/Huntington’s disease.” Then, the human homologue analysis for the target genes of the DE-miRNAs and DE-piRNAs in C. elegans was performed by the “homologene” package (Mancarci and French, 2019). Finally, the DE-miRNAs and DE-piRNAs targeting neurodegenerative disease-associated genes were identified by overlapping the homologue genes with the neurodegenerative disease associated genes.
MiRNAs and piRNAs for qRT-PCR were selected from the DE-miRNAs (p < 0.05 and absolute fold change >2) and DE-piRNAs (p < 0.01, FDR < 0.1, and absolute fold change >2) lists generated from the sRNA-seq. The qRT-PCR for target genes was performed on the DEGs (p < 0.05 and absolute fold change >2) generated from our previous RNA-seq (Shen et al., 2020). Primer pairs for small RNAs had 3′ primers supplied by Mir-X miRNA qRT-PCR SYBR Kit (Takara Bio, Mountain View, CA, United States). The 5′ primers and the primer pairs for DEGs were designed and synthesized by BGI (Shenzhen, China) and are listed in Supplementary Table S1. The L4 age-synchronized N2, UM0010, UM0011, UM0012, UM0013, and UM0014 animals were collected (maintained at 20°C). Both total RNAs and small RNAs were isolated and purified by the mirVanaTM miRNA Isolation Kit (Life Technologies, Carlsbad, CA, United States). The target small RNAs were reverse transcribed and amplified by Mir-X miRNA qRT-PCR SYBR Kit (Takara Bio USA, Mountain View, CA, United States) and detected on the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). Target genes were reverse transcribed with RevertAid Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, United States), amplified using SYBR Green (Bio-Rad, Hercules, CA, United States), and detected on the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). Three biological replicates in triplicate technical replicates were performed for each test. U6 and act-1 were used as internal reference for target small RNAs and target genes, respectively, in C. elegans.
A hierarchical clustering analysis of DE-miRNAs was performed to provide a global view on the differences between the strains (Figure 1A). The analysis showed three main groups: 1) HASNA53T OX alone; 2) WT and tdp-1 KO; and 3) HASNWT OX, HASNWT OX + tdp-1 KO, and HASNA53T OX + tdp-1 KO. Hierarchical clustering of all the miRNAs showed a similar clustering profile of HASNA53T OX alone, but differences with WT clustered with HASNWT OX + tdp-1 KO, HASNA53T OX + tdp-1 KO, and HASNWT OX together with tdp-1 KO (Supplementary Figure S1). A hierarchical clustering analysis of DE-piRNAs also showed HASNA53T OX in its own cluster, with WT next, and then the rest of the strains together (Figure 1B). The read counts for miRNAs/piRNAs of different strains are listed in Supplementary Tables S2 and S3. The volcano plots of the miRNAs/piRNAs are shown in Supplementary Figures S2 and S3.
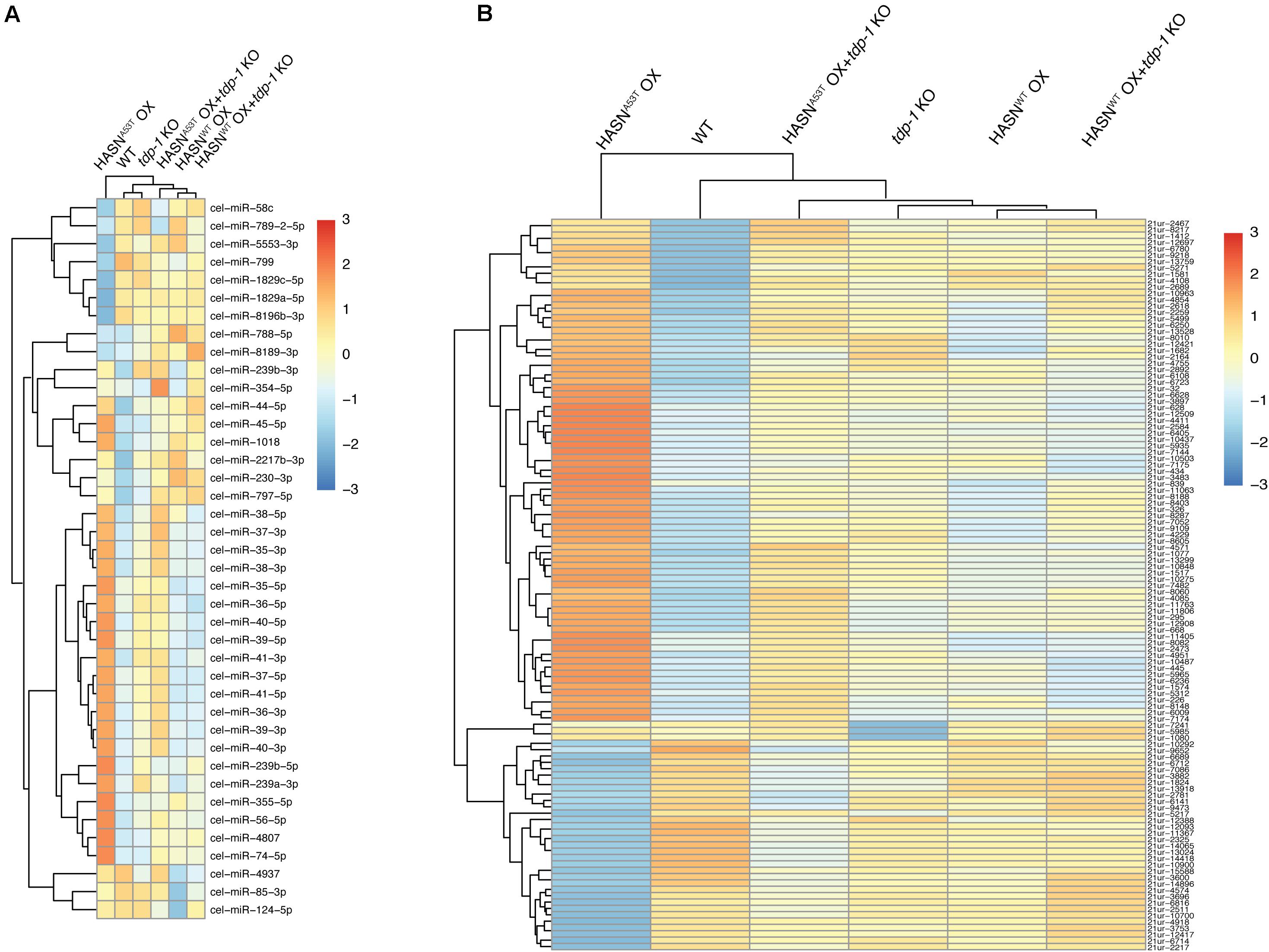
Figure 1. Hierarchical clustering for DE-miRNAs (A) and DE-piRNAs (B) of mutant/transgenic strains compared with WT strain. The cutoff of DE-miRNAs was p < 0.05 and absolute fold change >2; DE-piRNAs were p < 0.01, FDR < 0.1, and absolute fold change >2. The logarithmic scale represents the range of expression values of DE-miRNAs/DE-piRNAs. The top cluster dendrogram indicates the similarity of different strains based on the average expression values of all DE-miRNAs/DE-piRNAs of each strain. The left cluster dendrogram indicates the similarity of different DE-miRNAs/DE-piRNAs based on the average expression values of each DE-miRNA/DE-piRNA of all strains. The right colored bars show the DE-miRNA/DE-piRNA expression values.
In order to characterize the breadth and direction of differential expression between strains, we compared the DE-miRNAs (p < 0.05 and absolute fold change >2) and DE-piRNAs (p < 0.01, FDR < 0.1, and absolute fold change >2). Compared with WT, the expressions of miRNAs (Figure 2A) and piRNAs (Figure 2B) were changed significantly by HASNA53T OX, which displayed 32 DE-miRNAs and 112 DE-piRNAs. Among these, over 2/3 of DE-miRNAs and DE-piRNAs were upregulated. The HASNWT OX strain had far fewer, with eight DE-miRNAs and no DE-piRNAs. A total of 31 miRNAs and 440 piRNAs were differentially expressed when directly comparing HASNA53T OX vs HASNWT OX (Figures 2A,B).
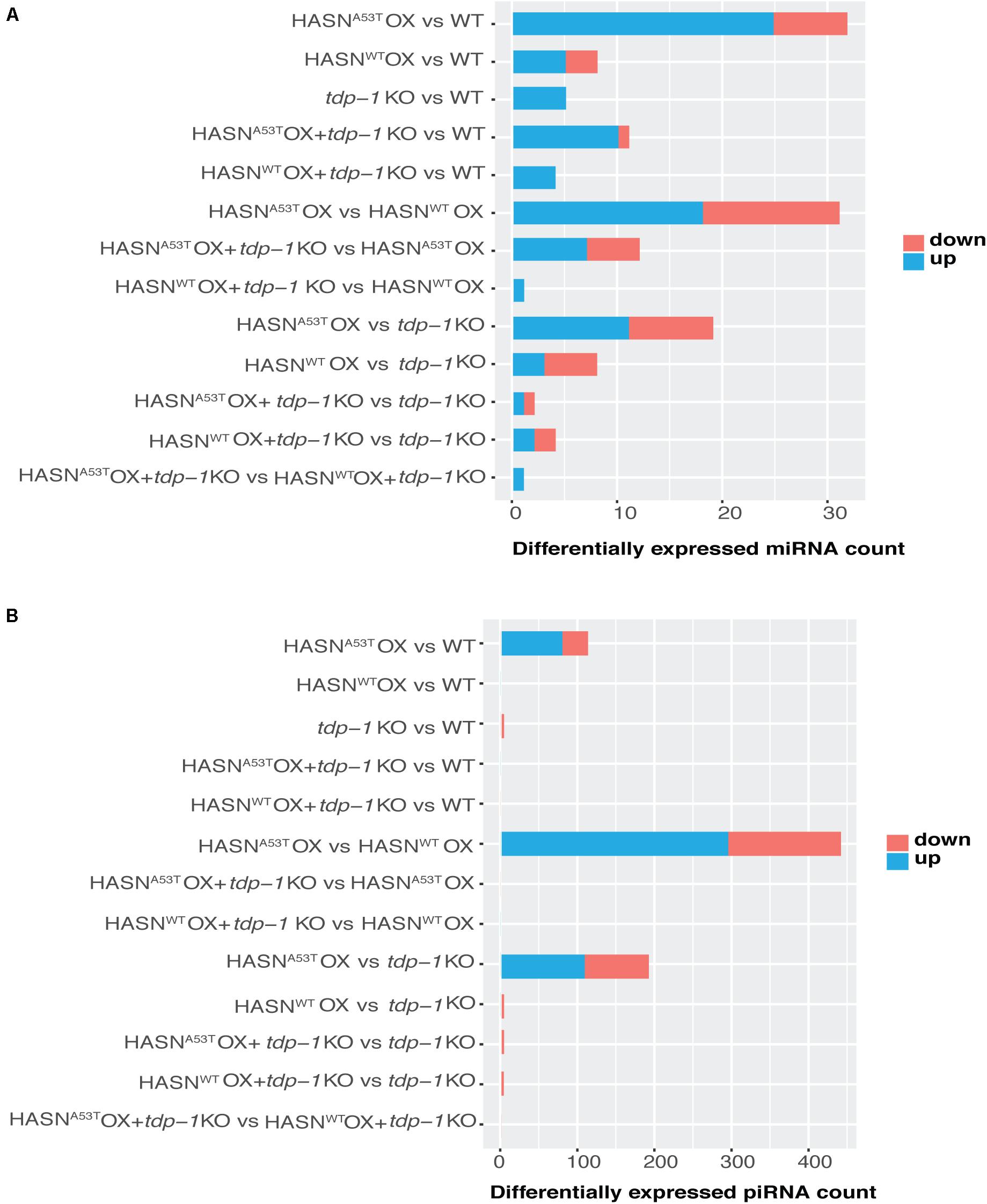
Figure 2. Counts of DE-miRNAs (A) and DE-piRNAs (B) generated from different comparisons. The cutoff of DE-miRNAs was p < 0.05 and absolute fold change > 2; that of DE-piRNAs was p < 0.01, FDR < 0.1, and absolute fold change > 2. The vertical lists show the comparisons. Horizontal bars indicate the DE-miRNAs/DE-piRNAs counts based on the bottom scales. Blue represents upregulated, and red represents downregulated.
We then analyzed the effect of the tdp-1 KO. The loss of tdp-1 caused a dramatic decrease in the number of DE-miRNAs and DE-piRNAs in HASNA53T OX strain going from 32 to 11 and 112 to 0, respectively (Figures 2A,B). However, no such apparent difference was observed in HASNWT OX + tdp-1 KO strain compared with WT strain. HASNA53T OX + tdp-1 KO vs HASNWT OX + tdp-1 KO directly also resulted in fewer DE-miRNAs/DE-piRNAs going from 31 to 1 and 440 to 0, respectively (Figures 2A,B). These results demonstrate a clear effect of tdp-1 KO to diminish the number of DE-miRNAs/DE-piRNAs between HASNA53T OX and WT, and between HASNA53T OX and HASNWT OX. The DE-miRNAs and DE-piRNAs for the different comparisons are listed in Supplementary Tables S4 and S5. In addition, we also listed DE-piRNAs with p value < 0.01 and absolute fold change >2 in Supplementary Table S6 for reference.
To identify the functions of DE-miRNAs targets, we performed the GO and KEGG enrichment analysis from the comparisons: HASNA53T OX vs WT, HASNWT OX vs WT, HASNA53T OX vs HASNWT OX, tdp-1 KO vs WT, HASNA53T OX + tdp-1 KO vs HASNA53T OX, and HASNWT OX + tdp-1 KO vs HASNWT OX. All the enrichment analysis results we obtained are shown in Figure 3. Upregulated miRNAs comparing HASNA53T OX vs WT identified targets enriched for energy generation (carbon metabolism), biosynthesis of amino acids, drug metabolism, mitochondrial membrane, mitochondrial protein complex, and oxidative phosphorylation categories (Figure 3A). For down-/upregulated miRNAs comparing HASNWT OX vs WT, the ATPase complex, ATPase coupled ion transmembrane transporter activity and intramolecular oxidoreductase activity, protein localization to endoplasmic reticulum, protein export, and amino sugar and nucleotide sugar metabolism were identified (Figures 3B,C). For comparisons of HASNA53T OX vs HASNWT OX, energy generation and amino acid metabolism were enriched (Figures 3D,E). Taken together, the functions of target genes of DE-miRNAs induced by HASNA53T OX were different from those induced by HASNWT OX, particularly in energy generation and amino acid metabolism. Furthermore, energy generation and biosynthesis of amino acids were enriched not only for the upregulated miRNAs comparing HASNA53T OX vs WT but also for the downregulated miRNAs comparing HASNA53T OX + tdp-1 KO vs HASNA53T OX (Figure 3F). This suggested that the expression of miRNAs associated with energy generation and amino acid metabolism might be supported by TDP-1 under the condition of HASNA53T OX.
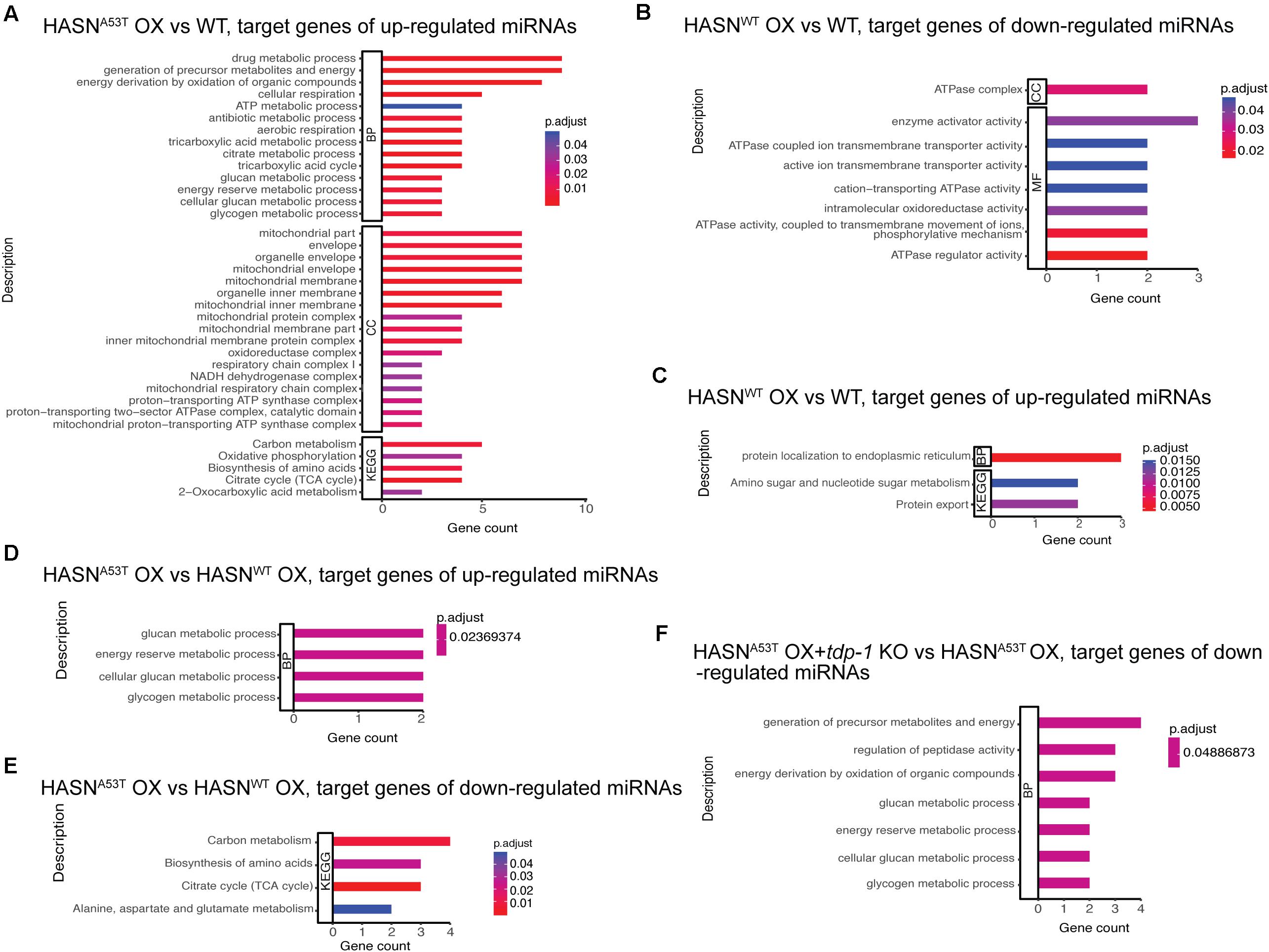
Figure 3. (A–F) GO and KEGG enrichment analysis for the target genes of DE-miRNAs from different comparisons. The cutoff of DE-miRNAs was p < 0.05 and absolute fold change >2. The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Similar to the analysis of DE-miRNAs, we performed GO and KEGG enrichment analysis for the target genes of DE-piRNAs. All of the significant enrichment analysis results we obtained are shown in Figures 4A,B. The genes targeted by upregulated piRNAs in HASNA53T OX vs WT were enriched in post-embryonic development, regulation of locomotion and localization, cell surface receptor signaling pathway, response to stimulus, DNA conformation change and packaging, plasma membrane, RNA transport, axon regeneration, ATPase activity, and hydrolase activity (Figure 4A). The target genes of downregulated piRNAs for comparison of tdp-1 KO vs WT were mainly enriched in proteasome assembly (target genes were D2045.2/H04D03.3, which were predicted to be targeted by 21ur-5985); mitophagy was also enriched with fzo-1 gene (ortholog of human mitofusin 1, and mitofusin 2, involved in mitochondrial fusion), predicted to be targeted by 21ur-1080 (Figure 4B). Based on these results, the expression of piRNAs associated with the neuronal functions, such as regulation of locomotion and localization, axon regeneration, and hydrolase activity, might be induced by HASNA53T OX and might cause a series of neuronal toxicities. tdp-1 deletion caused piRNAs involved in proteasome assembly to be downregulated, which might promote the degradation of HASNWT/A53T when tdp-1 KO was crossed into HASNWT/A53T OX strains. In addition, there were many differences between HASNA53T OX and HASNWT OX according to the enrichment analysis (Supplementary Figure S4). Post-embryonic development, chromosome organization, locomotion, DNA repair, endocytosis, autophagy, lysosome, synapse, axon regeneration, supramolecular complex, hydrolase activity, MAPK signaling pathway, mRNA surveillance pathway, mTOR signaling pathway, calcium signaling pathway, and longevity regulating pathway enrichments suggest that the piRNAs induced by HASNA53T OX are distinct from HASNWT OX.
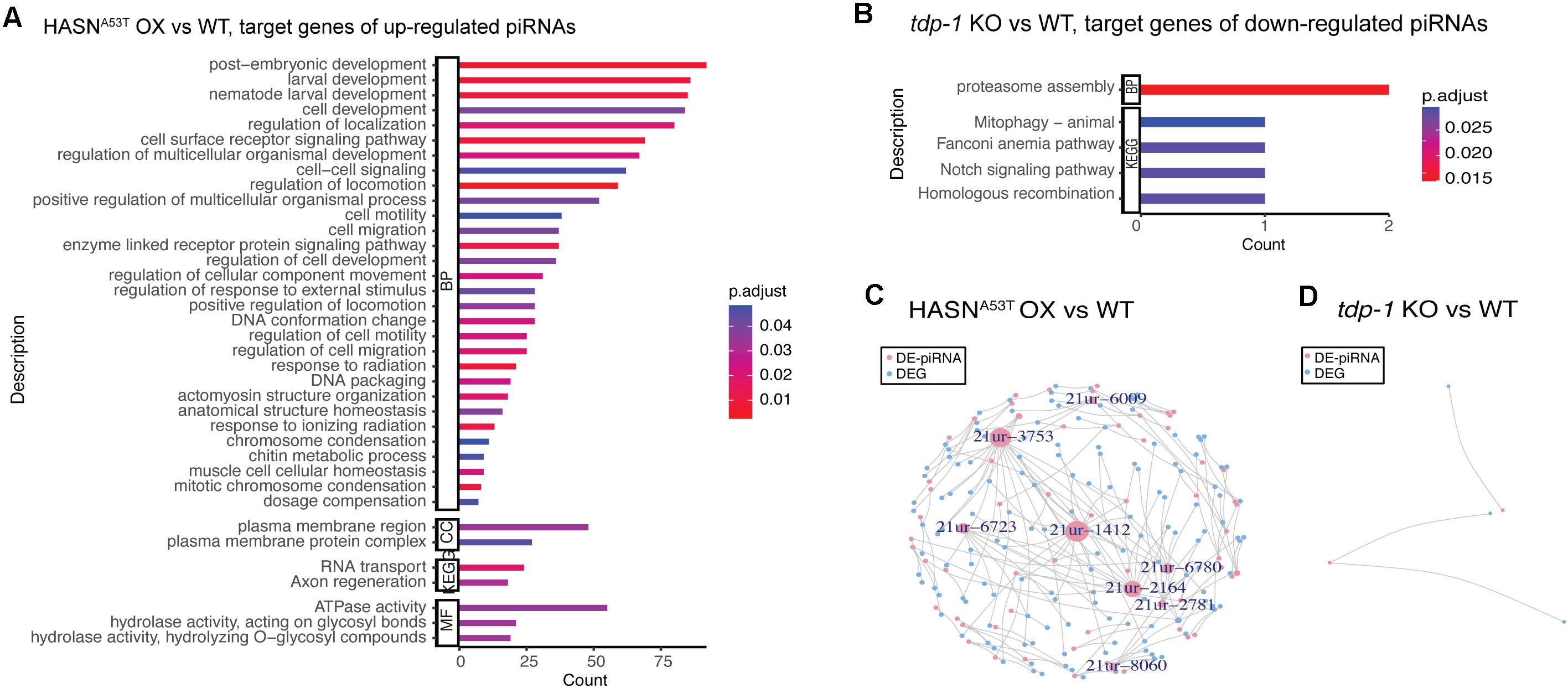
Figure 4. GO and KEGG enrichment analysis for the target genes of DE-piRNAs (A,B) and network of DE-piRNAs and DEGs (C,D). The cutoff of DE-piRNAs was p < 0.01, FDR < 0.1, and absolute fold change >2. (A,B) The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes. (C,D) Pink nodes represent DE-piRNAs. Blue nodes represent DEGs. Nodes labeled have a node degree ≥5. The node size represents the node degree.
To view the potential functional consequence of DE-piRNAs with a more relaxed criteria, we also performed GO and KEGG enrichment analysis for the target genes of DE-piRNAs with p value < 0.01 and absolute fold change > 2. More clusters were obtained compared with the previous stricter criteria of FDR < 0.1, p value < 0.01, and absolute fold change > 2 (Supplementary Figures S5–S7). For the comparison of HASNA53T OX vs WT, more clusters associated with neurons were found from the upregulated DE-piRNAs: neurogenesis, neuron differentiation and development, regulation of nervous system development, and calcium signaling pathway. In addition, plasma membrane, endosome membrane, basement membrane, endocytosis, and autophagy were also enriched, which might be closely associated with the impairment of membranes induced by HASNA53T OX (Supplementary Figure S5A), while supramolecular complex/polymer/fiber and hydrolase activity was found enriched for downregulated DE-piRNAs of comparison of HASNA53T OX vs WT as well as regulation of nervous system development and regulation of axon guidance (Supplementary Figure S5B). Similar to the comparison of HASNA53T OX vs WT, the target genes of DE-piRNAs of comparison of HASNWT OX vs WT were mainly enriched in cytoskeleton (supramolecular complex), neuron projection cytoplasm, hydrolase activity, and ATPase activity (Supplementary Figures S6A,B). Regulation of locomotion, RNAi, myofibril, hydrolase activity, chromosome, homologous recombination, and protein dimerization activity were enriched for target genes of DE-piRNAs from the comparison of tdp-1 KO vs WT (Supplementary Figures S7A,B). After the tdp-1 KO was genetically crossed into the HASNA53T OX strain, piRNAs involved in chromosome organization, morphogenesis, positive regulation of locomotion, muscle development, chromatin remodeling, supramolecular complex/polymer/fiber, synapse, bounding membrane of organelle, ErbB signaling pathway, MAPK signaling pathway, and calcium signaling pathway were dysregulated (Supplementary Figures S7C,D). Morphogenesis, supramolecular complex/polymer/fiber, hydrolase activity, helicase activity, ATPase activity, and cytoskeletal protein binding were enriched for DE-piRNAs of comparison of HASNWT OX + tdp-1 KO vs HASNWT OX (Supplementary Figures S7E,F). Based on these results, piRNAs might contribute to the toxicities of HASNA53T/WT OX on the nervous system, organelles membrane, and supramolecular complex. At the nuclear level, piRNAs might be involved in chromosome organization, chromatin remodeling, morphogenesis, locomotion, supramolecular complex/polymer/fiber, synapse, and calcium signaling pathway.
To further evaluate the roles of DE-miRNAs/DE-piRNAs in neurodegenerative diseases, we explored the correlation between DE-miRNAs/DE-piRNAs target genes and human neurodegenerative diseases including AD, ALS, PD, MSA, and HD based on the gene–disease associations from the DisGeNET database. PD-associated gene K07H8.2, SLC41A1 ortholog in human, was predicted to be targeted by three DE-miRNAs: cel-miR-1018, cel-miR-230-3p, and cel-miR-797-5p. tomm-40, TOMM40 ortholog in human and related to AD, was predicted to be targeted by cel-miR-85-3p (Supplementary Table S7). Many DE-piRNAs from the comparison of HASNA53T OX vs WT were found to be associated with PD including 21ur-10848, 21ur-11806, 21ur-295, 21ur-1412, 21ur-10487, 21ur-839, and 21ur-2781. Their predicted target genes were gba-1 (GBA in human), pgp-1 (ABCB1 in human), T08G11.1 (VPS13A in human), rab-39 (RAB39B in human), tpa-1 (PRKCD in human), taf-1 (TAF1 in human), dop-3 (DRD2 in human), and rme-8 (DNAJC13 in human). Among them, 21ur-1412 was predicted to target three genes related to neurodegenerative diseases PD or ALS, while 21ur-10487, 21ur-839, and 21ur-2781 were predicted to target two genes related to neurodegenerative diseases PD or AD (Supplementary Table S7).
We inspected the overlaps between the predicted target genes of DE-miRNAs (p value <0.05 and absolute fold change >2), DE-piRNAs (p value <0.01, absolute fold change >2, and FDR < 0.1), and the DEGs generated from the RNA-Seq of the same strains in our previous work with the cutoff of p value <0.05 and absolute fold change >2 (Shen et al., 2020). From the list of DE-miRNA/DE-piRNA target genes (Supplementary Tables S8, S9), the DE-miRNAs targeting the DEGs related to neuronal functions: autophagy, amino acid degradation, mitochondrion, and transcription activator were identified and listed in Table 1. Among these, cel-miR-1018, upregulated in the comparison of HASNA53T OX vs WT, had its target DEG T28F4.5 (linked to apoptotic signaling pathway and autophagy) downregulated. Cel-miR-355-5p, upregulated in the comparison of HASNA53T OX vs WT and downregulated in the comparison of HASNA53T OX + tdp-1 KO vs HASNA53T OX, had its target DEGs C34F6.1 and C05C10.3 down-/upregulated accordingly. Cel-miR-800-3p, upregulated in the comparison of HASNA53T OX + tdp-1 KO vs HASNA53T OX, had its target DEG camt-1 downregulated. PiRNA 21ur-1581, upregulated in the comparison of HASNA53T OX vs WT, had its target DEG F54D10.3, which is related to dopaminergic neuron, downregulated. In addition, F54D10.3 was also confirmed as a target of piRNA by CLASH (crosslinking, ligation, and sequencing of hybrids) data (Shen et al., 2018) from piRTarbase. Based on these results, cel-miR-1018, cel-miR-355-5p, cel-miR-800-3p, 21ur-1581, and their targets T28F4.5, C34F6.1, F54D10.3, C05C10.3, and camt-1 were suggested to be associated in the neurotoxicity of HASNA53T OX.
To explore the TF–miRNA–gene regulatory pathways involved in the toxicity of HASNA53T/WT OX with or without tdp-1 in C. elegans, we constructed the networks of TFs, DE-miRNAs, and DEGs. We obtained networks for only two comparisons: HASNA53T OX vs WT and HASNA53T OX + tdp-1 KO vs HASNA53T OX (Figure 5). The nodes indicated in Figures 5A,B are those with a node degree >3. As shown in Figures 5A,B, blmp-1 regulated the most DE-miRNAs and DEGs in both comparisons. Efl-1, hlh-30, pha-4, and dpy-27 were also linked to many DE-miRNAs and DEGs for the comparison of HASNA53T OX vs WT. Cel-miR-355-5p was the only miRNA identified for both comparisons with upregulation in HASNA53T OX vs WT (2.95 ± 0.43, p = 9.71e−5) and downregulation in HASNA53T OX + tdp-1 KO vs HASNA53T OX (0.34 ± 0.03, p = 4.64e−4). All the elements in the networks are listed in Supplementary Table S10.
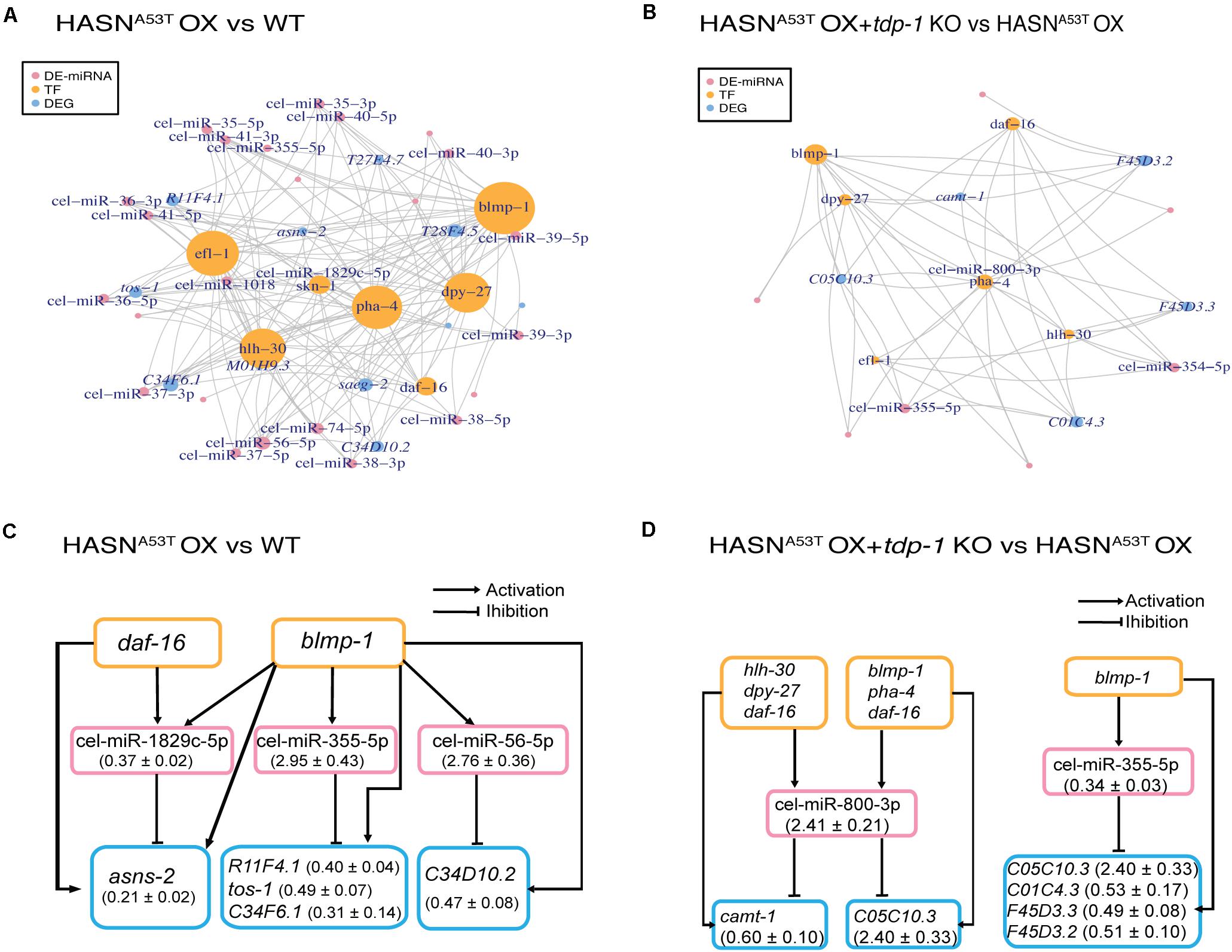
Figure 5. Networks of DE-miRNAs, DEGs (differentially expressed genes), and TFs (transcription factors). We obtained networks for only two comparisons: HASNA53T OX vs WT and HASNA53T OX + tdp-1 KO vs HASNA53T OX. DEGs were generated from the RNA-Seq of the same strains in our previous work with the cutoff of p value <0.05 and absolute fold change >2 (Shen et al., 2020). (A,B) Pink nodes represent DE-miRNAs. Orange nodes represent TFs. Blue nodes represent DEGs. The node size represents the node degree. Nodes labeled are the ones with a node degree >3. TFs were written as a vertical word in order to distinguish it from DEGs in these figures. (C,D) The pink rectangles indicate DE-miRNAs. The orange rectangles indicate TFs. The blue rectangles indicate DEGs. Fold change (mean ± SEM) of DE-miRNAs and DEGs are indicated in the parentheses.
Regulatory loops and pathways of TFs, DE-miRNAs, and DEGs were extracted from the networks in Figures 5A,B and displayed in Figures 5C,D and Supplementary Table S11. For comparison of HASNA53T OX vs WT, six regulatory loops were built on two TFs, three DE-miRNAs, and five DEGs (Figure 5C). Based on the fold changes of DE-miRNAs and DEGs, blmp-1 may have inhibited DEG R11F4.1, tos-1, C34F6.1, and C34D10.2 expressions indirectly via activating cel-miR-355-5p and cel-miR-56-5p expressions in HASNA53T OX. Furthermore, the TF-miRNA co-regulatory pair of blmp-1 and cel-miR-355-5p was also involved in HASNA53T OX + tdp-1 KO vs HASNA53T OX (Figure 5D). Accordingly, based on the fold changes, C05C10.3 might be downregulated by this co-regulatory pair in HASNA53T OX compared with HASNA53T OX + tdp-1 KO, while hlh-30, dpy-27, daf-16, blmp-1, and pha-4 might inhibit camt-1 expression via activating cel-miR-800-3p expression in HASNA53T OX + tdp-1 KO compared with HASNA53T OX (Figure 5D). All of the TF–miRNA–gene regulatory pathways of these two comparisons with or without loops are listed row by row in Supplementary Table S11. The related lists for DE-miRNAs targeting DEGs, and TFs targeting DE-miRNAs are shown in Supplementary Tables S8 and S12 separately.
Due to the lack of a database for the interactions between TFs and piRNAs, we were not able to construct regulatory networks of TF–piRNA–gene. We constructed regulatory networks for DE-piRNAs and their target DEGs only (Figures 4C,D). As the cutoff we used for DE-piRNAs was p <0.01, FDR < 0.1, and absolute fold change >2, only comparisons HASNA53T OX vs WT and tdp-1 KO vs WT had results. Based on the ones exhibited in Figure 4C with node degree ≥5, there were eight DE-piRNAs – 21ur-6009, 21ur-3753, 21ur-6723, 21ur-1412, 21ur-6780, 21ur-2164, 21ur-2781, and 21ur-8060, targeting five DEGs or more. These piRNAs might play important roles in the toxicity of HASNA53T OX. All of the DE-piRNAs targeting DEGs are listed in Supplementary Table S9.
miRDeep2 analysis identified a total of 293 novel miRNAs with significant randfold p values of which 34 novel miRNAs had a cutoff of miRDeep2 score ≥10 from all the sRNA-seq samples. The information for these novel miRNAs is listed in Supplementary Table S13.
To validate the expressions of DE-miRNAs/DE-piRNAs and DEGs, we performed qRT-PCR. The DE-miRNAs/DE-piRNAs and DEGs chose for qRT-PCR were mainly related to neuronal functions. The qRT-PCR results were compared with the sRNA-seq result of this study and the RNA-seq result in our previous study (Shen et al., 2020) and are shown in Table 2. The qRT-PCR results were consistent with our sRNA-seq/RNA-seq results, such as cel-miR-355-5p and its target DEGs. The expression of cel-miR-355-5p in comparison of HASNA53T OX + tdp-1 KO vs HASNA53T OX was downregulated. Its corresponding target DEG C05C10.3 was upregulated, while its expression in comparison of HASNA53T OX vs WT was upregulated and its corresponding target DEG C34F6.1 (serine-type endopeptidase inhibitor) was downregulated.
In this study, we observed that the miRNA/piRNA expression was broadly and abundantly altered by HASNA53T OX, but not HASNWT OX. TDP-1 likely supported the alterations by HASNA53T OX since genetic crosses into tdp-1 null mutants massively reduced the changes. Functional annotations for the target genes of DE-miRNAs/DE-piRNAs showed their effects on neuronal development and function. The relationship between the target genes and neurodegenerative diseases was also explored. The networks of TFs, DE-miRNAs, and DEGs, or DE-piRNAs and DEGs, predicted potential TF–miRNA–gene regulatory pathways and piRNA–gene regulatory pairs, which might play important roles in HASNA53T OX toxicity.
The phenotypic differences between HASNA53T and HASNWT in humans are well established. The A53T mutation was first identified in a pedigree displaying early onset PD in a dominant mode of inheritance pattern (Polymeropoulos et al., 1997). Subsequent studies in model systems including our own show more severe phenotypes in animals with this specific mutation (Giasson et al., 2002; Gispert et al., 2003). HASNA53T OX C. elegans animals had significant deficits including uncoordinated locomotion, impaired dopaminergic neurons, development and basal slowing response, and lengthened life span due to dietary restriction (Lakso et al., 2003; Vartiainen et al., 2006a; Shen et al., 2020). HASNWT OX was similar to HASNA53T OX in dopaminergic neuron loss and lengthened life span, but not in locomotion, development, and basal slowing response. There also existed differences in functional enrichments of their DEGs mainly in fatty acid and amino acid metabolism, immune response, endoplasmic reticulum unfolded protein response, posttranscriptional regulation of gene expression, enzyme activator activity, longevity regulating pathway, and the FoxO signaling pathway. In the present study, the target genes of DE-miRNAs/DE-piRNAs of HASNA53T OX vs HASNWT OX were mainly clustered in energy generation, amino acid metabolism, post-embryonic development, chromosome organization, locomotion, DNA repair, endocytosis, autophagy, lysosome, synapse, axon regeneration, supramolecular complex, hydrolase activity, MAPK signaling pathway, mRNA surveillance pathway, mTOR signaling pathway, calcium signaling pathway, and longevity regulating pathway. Hence, the differential effects of HASNA53T OX and HASNWT OX in C. elegans were also reflected by miRNA/piRNA expressions.
Although there are many different animal models constructed for PD research, those with α-synuclein overexpression in C. elegans have some distinctive advantages such as flexible genetic manipulation, a short life span, and a transparent body to facilitate the observation of specific phenotypes. Moreover, the α-synuclein overexpression C. elegans models can recapitulate some important neurochemical features of PD, such as the aggregation of α-synuclein and the degeneration of dopaminergic neurons (Gaeta et al., 2019). Many studies have furthermore shown promising translational outcomes in PD models. Ypt1p/Rab1 was screened in yeast as a suppressor of α-synuclein toxicity, which blocked vesicle trafficking between ER and Golgi. Its neuroprotective effect was then confirmed in animals including Drosophila, C. elegans, and neuronal cells from rat midbrain (Cooper et al., 2006). Proteins glucose-6-phosphate isomerase (GPI), VPS41 (involved in lysosomal biogenesis and Golgi-vacuole trafficking), and cathepsin D were identified in a screen as a protector against α-synuclein-mediated dopaminergic neuronal degeneration by RNAi in C. elegans and then verified in Drosophila, mice, and human neuroblastoma cells (Hamamichi et al., 2008; Qiao et al., 2008; Ruan et al., 2010; Knight et al., 2014). The chemical compound N-aryl benzimidazole (NAB) was obtained from a screen of α-synuclein overexpressing yeast cells and was also found to protect dopaminergic neurons from α-synuclein-mediated neurodegeneration in C. elegans, rat neurons, and human cells (Qiao et al., 2008). Together, these studies suggest that model organisms can be valuable tools in the translation for treatment of PD.
While our results indicate widespread changes in transcription involving hundreds of genes, it is possible that only a few genes or a single gene is important to facilitate the phenotypes observed. As an example, the PD-related gene K07H8.2 was predicted to be targeted by cel-miR-1018 in the comparison of HASNA53T OX vs WT and by cel-miR-230-3p and cel-miR-797-5p in the comparison of HASNWT OX vs WT. K07H8.2 was predicted to be targeted by different miRNAs in these two different types of HASN overexpression C. elegans, suggesting that it might be an important target in PD and related synucleinopathies. SLC41A1, a human ortholog of K07H8.2, is a Na+/Mg2+ exchanger responsible for the homeostasis of magnesium. Enhancement or loss of Mg2+-efflux induced by the dysfunction of SLC41A1 was found in PD patients and might trigger the following neurodegeneration (Kolisek et al., 2013; Lin et al., 2014; Wang et al., 2015). Our result is consistent with those of previous human studies.
An example of a single piRNA that could potentially have a significant impact is 21ur-10848, which was found to be upregulated (fold change = 4.15 ± 1.18, FDR = 0.014) in HASNA53T OX compared with WT. Its predicted target gene is gba-1, an ortholog of human GBA (glucocerebrosidase, a lysosomal enzyme). GBA gene mutations are responsible for not only Gaucher’s disease (GD) and DLB but also PD (O’Regan et al., 2017; Gegg and Schapira, 2018; Riboldi and Di Fonzo, 2019). Its dysfunction causes metabolic imbalances of α-synuclein and other glucocerebrosidase substrates such as glucosylceramide.
We observed that tdp-1 could influence dysregulation of miRNAs/piRNAs. TDP-43/TDP-1 has multiple functions in shaping the transcriptome including regulation of DNA transcription; splicing of pre-mRNA; translation, stabilization, and translocation of mRNA; and biogenesis of miRNA (Buratti and Baralle, 2012; Kawahara and Mieda-Sato, 2012). It also interacts with other proteins and regulates protein homeostasis via activating the TFs FOXOs (Buratti and Baralle, 2012; Zhang et al., 2014). The tdp-1 product TDP-1 could support dysregulation of miRNAs through a number of direct or indirect mechanisms. A direct mechanism would be to control miRNA biogenesis via binding C. elegans orthologs of Drosha and/or Dicer (Kawahara and Mieda-Sato, 2012). Alternatively, it could bind miRNAs directly, although this binding is selective for specific miRNAs (Buratti et al., 2010; Paez-Colasante et al., 2020). Finally, it could act upstream during transcription of primary miRNAs as suggested by CHIP-seq studies, which show TDP-43 associated with miRNA transcription sites (Fan et al., 2014). Since TDP-43 globally regulates miRNA levels via these different mechanisms, and we observed many individual miRNA dysregulations, it is likely that one or more of these mechanisms take place. The regulatory mechanism of piRNAs by TDP-43 is more obscure. PiRNAs may be transcribed as primary piRNAs by noncanonical pol II actions or produced via transcription of transposons prior to amplification (Huang et al., 2017). The role of TDP-43 in any of these piRNA biogenesis pathways has not been studied deeply. A CHIP-seq study demonstrating TDP-43 binding at distinct piRNA loci suggests a pre-transcription control mechanism; however, that other roles similar to how miRNAs are regulated may also be possible (Fan et al., 2014), while tdp-1 KO decreased the expression of α-synuclein according to our previous study (Shen et al., 2020). The decrease of dysregulated small RNAs might also be indirect due to the decreased expression of α-synuclein.
In this study, the target genes of TF–miRNA–gene regulatory loops, generated from both comparisons of HASNA53T OX vs WT and HASNA53T OX + tdp-1 KO vs HASNA53T OX, mainly functioned in amino acid metabolism (C34F6.1, asns-2, and C05C10.3), kinase activity (R11F4.1 and C01C4.3), and transcription activation (camt-1). A TF–miRNA–gene forward and feedback regulation system might thus play a role in the toxicity of HASNA53T OX. Whether piRNAs can form regulatory loops were not possible for us to determine in this study, as we were not able to directly link miRNAs and piRNAs in our network analysis. They may work cooperatively to regulate gene expression; however, there does not seem to be a great deal of overlap between miRNA and piRNA targets. Moreover, the role of transposons, the more well-established targets of piRNAs, in neurodegeneration is currently obscure. From the current study, it appears that miRNAs and piRNAs, though greatly dysregulated in our PD model, may independently regulate specific targets that together contribute to the observed neurodegeneration and behavioral phenotypes.
PiRNA works mainly in germline cells to protect the germline genome. As we used the whole body of the worm to perform the sRNA-seq, the dysregulated piRNAs in this study showed many target enrichment clusters related to post-embryonic development. However, some enrichments, which were related to neuronal toxicities, were also clustered for the targets of DE-piRNAs from the comparison of HASNA53T OX vs WT, including regulation of nervous system development, axon regeneration, endocytosis, autophagy, hydrolase activity, and calcium signaling pathway. These results suggest the possibility of piRNA involvement in HASNA53T OX neuronal toxicity. A more precise relationship between piRNAs, germline cells, and neurodegeneration would require additional studies.
This study profiled miRNAs and piRNAs for both HASNA53T OX and HASNWT OX based on the next-generation sequencing (NGS) techniques, which are sensitive and high-throughput (Behjati and Tarpey, 2013). We demonstrate abundant dysregulation, which is more predominant in HASNA53T OX strains and also might depend upon tdp-1 either directly through its own actions or indirectly through regulating α-synuclein or other neurodegeneration-related gene expressions. The dysregulated miRNAs/piRNAs and targets provide us with perturbed pathways and regulatory loops that together contribute to PD. Although we did not find evidence of cooperativity in miRNA and piRNA dysregulation, we did observe that their individual targets may independently contribute to PD and other neurodegeneration phenotypes. Additional layers of epigenetic complexity may also occur from other marks such as those from histones or in methylated DNA not studied here. These data thus provide insight into epigenetic mechanisms in PD that might be beneficial in development of potential therapies and diagnostic biomarkers for PD and related synucleinopathies.
The sRNA-seq raw data is available at SRA database under BioProject accession number, PRJNA649048. The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
LS designed and carried out the experiments, performed some of the sRNA-seq analysis, analyzed the data, and wrote the manuscript. CW performed most of the sRNA-seq analysis. LC performed some of the sRNA-seq analysis. GW conceived and supervised the project and edited the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Some of the strains used in this study were obtained from the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This study was supported by MYRG2017-00123 to GW.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.600462/full#supplementary-material
Supplementary Figure 1 | Hierarchical clustering of all miRNAs from strains with genotypes: WT, tdp-1 KO, and HASNWT/A53T OX with or without tdp-1 gene. Hierarchical clustering was performed by complete linkage method with Euclidean distance. Logarithmic scale represents the range of expression values of miRNAs. The top cluster dendrogram indicates the similarity of different strains based on the expression values of all miRNAs of each strain. The left cluster dendrogram indicates the similarity of miRNAs based on the expression values of each miRNA of different strains. The right colored bars show the miRNAs expression values.
Supplementary Figure 2 | (A–M) Volcano plots of miRNAs of different comparisons. The volcano plots were drawn by “plot” function and colored by “points” function of “graphics” package. The green nodes represent the miRNAs with p value <0.05 and absolute fold change >2. The orange nodes represent the miRNAs with absolute fold change >2 but p value ≥0.05. The red nodes represent the miRNAs with p value <0.05 but absolute fold change ≤2. The black nodes represent the miRNAs with p value ≥0.05 and absolute fold change ≤2.
Supplementary Figure 3 | (A–M) Volcano plots of piRNAs from different comparisons. The volcano plots were drawn by “plot” function and colored by “points” function of “graphics” package. The green nodes represent the piRNAs with FDR < 0.1 and absolute fold change >2. The orange nodes represent the piRNAs with absolute fold change >2 but FDR ≥ 0.1. The red nodes represent the piRNAs with FDR < 0.1 but absolute fold change ≤2. The black nodes represent the piRNAs with FDR ≥ 0.1 and absolute fold change ≤2.
Supplementary Figure 4 | (A,B) GO and KEGG enrichment analysis for the target genes of DE-piRNAs (p < 0.01, FDR < 0.1, and absolute fold change >2) from the comparison of HASNA53T OX vs HASNWT OX. The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Supplementary Figure 5 | (A,B) GO and KEGG enrichment analysis for the target genes of DE-piRNAs (p < 0.01 and absolute fold change >2) from the comparison of HASNA53T OX vs WT. The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Supplementary Figure 6 | (A,B) GO and KEGG enrichment analysis for the target genes of DE-piRNAs (p < 0.01 and absolute fold change >2) from the comparison of HASNWT OX vs WT. The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Supplementary Figure 7 | (A–F) GO and KEGG enrichment analysis for the target genes of DE-piRNAs (p < 0.01 and absolute fold change > 2) from the comparisons of tdp-1 KO vs WT, HASNA53T OX + tdp-1 KO vs HASNA53T OX, HASNWT OX + tdp-1 KO vs HASNWT OX. The bar length represents the counts of target genes corresponding to the vertical terms. The bar color represents the p-adjust value of each term. BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
AD, Alzheimer’s disease; A β, β-amyloid; ALS, amyotrophic lateral sclerosis; BP, biological process; CC, cellular component; C. elegans, Caenorhabditis elegans; CLASH, crosslinking ligation and sequencing of hybrids; DEG(s), differentially expressed gene(s); DLB, dementia with Lewy bodies; DE-miRNA, differentially expressed miRNA; DE-piRNA, differentially expressed piRNA; GO, Gene Ontology; NGS, next-generation sequencing; HASNA53T OX, human α-synuclein A53T mutant overexpressing pan-neuronally; HASNWT OX, human wild type α-synuclein overexpressing pan-neuronally; HASNWT/A53T OX, human α-synuclein (WT or A53T) overexpressing pan-neuronally; HD, Huntington’s disease; KEGG, Kyoto Encyclopedia of Genes and Genomes; MF, molecular function; miRNA, microRNA; MSA, multiple system atrophy; PD, Parkinson’s disease; piRNA, PIWI-interacting RNA; sRNA-seq, small RNA sequencing; TarG, target gene; tdp-1 KO, tdp-1 knock out; TDP-43, Tar-DNA binding protein 43; TF(s), transcription factor(s); WT, wild type.
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. doi: 10.1016/j.cell.2009.01.002
Behjati, S., and Tarpey, P. S. (2013). What is next generation sequencing? Arch. Dis. Child Educ. Pract. Ed. 98, 236–238. doi: 10.1136/archdischild-2013-304340
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Buratti, E., and Baralle, F. E. (2012). TDP-43: gumming up neurons through protein-protein and protein-RNA interactions. Trends Biochem. Sci. 37, 237–247. doi: 10.1016/j.tibs.2012.03.003
Buratti, E., De Conti, L., Stuani, C., Romano, M., Baralle, M., and Baralle, F. (2010). Nuclear factor TDP-43 can affect selected microRNA levels. FEBS J. 277, 2268–2281. doi: 10.1111/j.1742-4658.2010.07643.x
Chartier-Harlin, M. C., Kachergus, J., Roumier, C., Mouroux, V., Douay, X., Lincoln, S., et al. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169. doi: 10.1016/S0140-6736(04)17103-1
Chou, C. H., Shrestha, S., Yang, C. D., Chang, N. W., Lin, Y. L., Liao, K. W., et al. (2018). miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 46, D296–D302. doi: 10.1093/nar/gkx1067
Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Williamson, R. E., and Lansbury, P. T. Jr. (2000). Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576. doi: 10.1073/pnas.97.2.571
Cooper, A. A., Gitler, A. D., Cashikar, A., Haynes, C. M., Hill, K. J., Bhullar, B., et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313, 324–328. doi: 10.1126/science.1129462
Csardi, G., and Nepusz, T. (2006). The igraph software package for complex network research. InterJ. Complex Syst. 1695:1695.
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T., and Sulzer, D. (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295. doi: 10.1126/science.1101738
Cunningham, F., Achuthan, P., Akanni, W., Allen, J., Amode, M. R., Armean, I. M., et al. (2019). Ensembl 2019. Nucleic Acids Res. 47, D745–D751. doi: 10.1093/nar/gky1113
Ernst, C., Odom, D. T., and Kutter, C. (2017). The emergence of piRNAs against transposon invasion to preserve mammalian genome integrity. Nat. Commun. 8:1411. doi: 10.1038/s41467-017-01049-7
Fan, Z., Chen, X., and Chen, R. (2014). Transcriptome-wide analysis of TDP-43 binding small RNAs identifies miR-NID1 (miR-8485), a novel miRNA that represses NRXN1 expression. Genomics 103, 76–82. doi: 10.1016/j.ygeno.2013.06.006
Friedlander, M. R., Mackowiak, S. D., Li, N., Chen, W., and Rajewsky, N. (2012). miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40, 37–52. doi: 10.1093/nar/gkr688
Gaeta, A. L., Caldwell, K. A., and Caldwell, G. A. (2019). Found in translation: the utility of C. elegans alpha-synuclein models of Parkinson’s Disease. Brain. Sci. 9:73. doi: 10.3390/brainsci9040073
Gegg, M. E., and Schapira, A. H. V. (2018). The role of glucocerebrosidase in Parkinson disease pathogenesis. FEBS J. 285, 3591–3603. doi: 10.1111/febs.14393
Giasson, B. I., Duda, J. E., Quinn, S. M., Zhang, B., Trojanowski, J. Q., and Lee, V. M. (2002). Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34, 521–533. doi: 10.1016/s0896-6273(02)00682-7
Gispert, S., Del Turco, D., Garrett, L., Chen, A., Bernard, D. J., Hamm-Clement, J., et al. (2003). Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol. Cell. Neurosci. 24, 419–429. doi: 10.1016/s1044-7431(03)00198-2
Hamamichi, S., Rivas, R. N., Knight, A. L., Cao, S., Caldwell, K. A., and Caldwell, G. A. (2008). Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc. Natl. Acad. Sci. U.S.A. 105, 728–733. doi: 10.1073/pnas.0711018105
Huang, X., Fejes Toth, K., and Aravin, A. A. (2017). piRNA biogenesis in Drosophila melanogaster. Trends Genet. 33, 882–894. doi: 10.1016/j.tig.2017.09.002
Hutvagner, G., and Zamore, P. D. (2002). A microRNA in a multiple-turnover RNAi enzyme complex. Science 297, 2056–2060. doi: 10.1126/science.1073827
Iwasaki, Y. W., Siomi, M. C., and Siomi, H. (2015). PIWI-interacting RNA: its biogenesis and functions. Annu. Rev. Biochem. 84, 405–433. doi: 10.1146/annurev-biochem-060614-034258
Junn, E., and Mouradian, M. M. (2012). MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol. Ther. 133, 142–150. doi: 10.1016/j.pharmthera.2011.10.002
Juzwik, C. A., S Drake, S., Zhang, Y., Paradis-Isler, N., Sylvester, A., Amar-Zifkin, A., et al. (2019). microRNA dysregulation in neurodegenerative diseases: a systematic review. Prog. Neurobiol. 182:101664. doi: 10.1016/j.pneurobio.2019.101664
Karpinar, D. P., Balija, M. B. G., Kugler, S., Opazo, F., Rezaei-Ghaleh, N., Wender, N., et al. (2009). Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 28, 3256–3268. doi: 10.1038/emboj.2009.257
Karpowicz, R. J. Jr., Trojanowski, J. Q., and Lee, V. M. (2019). Transmission of alpha-synuclein seeds in neurodegenerative disease: recent developments. Lab. Invest. 99, 971–981. doi: 10.1038/s41374-019-0195-z
Kawahara, Y., and Mieda-Sato, A. (2012). TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc. Natl. Acad. Sci. U.S.A. 109, 3347–3352. doi: 10.1073/pnas.1112427109
Kim, K. W., Tang, N. H., Andrusiak, M. G., Wu, Z., Chisholm, A. D., and Jin, Y. (2018). A neuronal piRNA pathway inhibits axon regeneration in C. elegans. Neuron 97, 511–519.e6. doi: 10.1016/j.neuron.2018.01.014
Knight, A. L., Yan, X., Hamamichi, S., Ajjuri, R. R., Mazzulli, J. R., Zhang, M. W., et al. (2014). The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab. 20, 145–157. doi: 10.1016/j.cmet.2014.04.017
Kolisek, M., Sponder, G., Mastrototaro, L., Smorodchenko, A., Launay, P., Vormann, J., et al. (2013). Substitution p.A350V in Na(+)/Mg(2)(+) exchanger SLC41A1, potentially associated with Parkinson’s disease, is a gain-of-function mutation. PLoS One 8:e71096. doi: 10.1371/journal.pone.0071096
Korff, A., Liu, C., Ginghina, C., Shi, M., and Zhang, J. Alzheimer’s Disease Neuroimaging Initiative (2013). Alpha-synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J. Alzheimers Dis. 36, 679–688. doi: 10.3233/JAD-130458
Lakso, M., Vartiainen, S., Moilanen, A. M., Sirvio, J., Thomas, J. H., Nass, R., et al. (2003). Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J. Neurochem. 86, 165–172. doi: 10.1046/j.1471-4159.2003.01809.x
Lee, E. J., Banerjee, S., Zhou, H., Jammalamadaka, A., Arcila, M., Manjunath, B. S., et al. (2011). Identification of piRNAs in the central nervous system. RNA 17, 1090–1099. doi: 10.1261/rna.2565011
Lee, M. K., Stirling, W., Xu, Y., Xu, X., Qui, D., Mandir, A. S., et al. (2002). Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 –> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 99, 8968–8973. doi: 10.1073/pnas.132197599
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, W., Lesuisse, C., Xu, Y., Troncoso, J. C., Price, D. L., and Lee, M. K. (2004). Stabilization of alpha-synuclein protein with aging and familial Parkinson’s disease-linked A53T mutation. J. Neurosci. 24, 7400–7409. doi: 10.1523/JNEUROSCI.1370-04.2004
Lin, C. H., Wu, Y. R., Chen, W. L., Wang, H. C., Lee, C. M., Lee-Chen, G. J., et al. (2014). Variant R244H in Na+/Mg2+ exchanger SLC41A1 in Taiwanese Parkinson’s disease is associated with loss of Mg2+ efflux function. Parkinsonism Relat. Disord. 20, 600–603. doi: 10.1016/j.parkreldis.2014.02.027
Luk, K. C., Kehm, V., Carroll, J., Zhang, B., O’brien, P., Trojanowski, J. Q., et al. (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. doi: 10.1126/science.1227157
Mancarci, O., and French, L. (2019). homologene: Quick Access to Homologene and Gene Annotation Updates.
Nuzziello, N., Ciaccia, L., and Liguori, M. (2019). Precision medicine in neurodegenerative diseases: some promising tips coming from the microRNAs’ world. Cells 9:75. doi: 10.3390/cells9010075
O’Regan, G., Desouza, R. M., Balestrino, R., and Schapira, A. H. (2017). Glucocerebrosidase mutations in Parkinson disease. J. Parkinsons Dis. 7, 411–422. doi: 10.3233/JPD-171092
Paez-Colasante, X., Figueroa-Romero, C., Rumora, A. E., Hur, J., Mendelson, F. E., Hayes, J. M., et al. (2020). Cytoplasmic TDP43 binds microRNAs: new disease targets in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 14:117. doi: 10.3389/fncel.2020.00117
Pereira, P., Queiroz, J. A., Figueiras, A., and Sousa, F. (2017). Current progress on microRNAs-based therapeutics in neurodegenerative diseases. Wiley Interdiscip. Rev. RNA 8:e1409. doi: 10.1002/wrna.1409
Peters, O. M., Millership, S., Shelkovnikova, T. A., Soto, I., Keeling, L., Hann, A., et al. (2012). Selective pattern of motor system damage in gamma-synuclein transgenic mice mirrors the respective pathology in amyotrophic lateral sclerosis. Neurobiol. Dis. 48, 124–131. doi: 10.1016/j.nbd.2012.06.016
Pinero, J., Ramirez-Anguita, J. M., Sauch-Pitarch, J., Ronzano, F., Centeno, E., Sanz, F., et al. (2020). The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–D855. doi: 10.1093/nar/gkz1021
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. doi: 10.1126/science.276.5321.2045
Pujato, M., Kieken, F., Skiles, A. A., Tapinos, N., and Fiser, A. (2014). Prediction of DNA binding motifs from 3D models of transcription factors; identifying TLX3 regulated genes. Nucleic Acids Res. 42, 13500–13512. doi: 10.1093/nar/gku1228
Qiao, L., Hamamichi, S., Caldwell, K. A., Caldwell, G. A., Yacoubian, T. A., Wilson, S., et al. (2008). Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol. Brain 1:17. doi: 10.1186/1756-6606-1-17
Quinlan, S., Kenny, A., Medina, M., Engel, T., and Jimenez-Mateos, E. M. (2017). MicroRNAs in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 334, 309–343. doi: 10.1016/bs.ircmb.2017.04.002
Rajasethupathy, P., Antonov, I., Sheridan, R., Frey, S., Sander, C., Tuschl, T., et al. (2012). A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 149, 693–707. doi: 10.1016/j.cell.2012.02.057
Recasens, A., Dehay, B., Bove, J., Carballo-Carbajal, I., Dovero, S., Perez-Villalba, A., et al. (2014). Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 75, 351–362. doi: 10.1002/ana.24066
Riboldi, G. M., and Di Fonzo, A. B. (2019). GBA, Gaucher disease, and Parkinson’s disease: from genetic to clinic to new therapeutic approaches. Cells 8:364. doi: 10.3390/cells8040364
Robinson, M. D., Mccarthy, D. J., and Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. doi: 10.1093/bioinformatics/btp616
Roy, J., Sarkar, A., Parida, S., Ghosh, Z., and Mallick, B. (2017). Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’s disease and their probable role in pathogenesis. Mol. Biosyst. 13, 565–576. doi: 10.1039/c6mb00699j
Ruan, Q., Harrington, A. J., Caldwell, K. A., Caldwell, G. A., and Standaert, D. G. (2010). VPS41, a protein involved in lysosomal trafficking, is protective in Caenorhabditis elegans and mammalian cellular models of Parkinson’s disease. Neurobiol. Dis. 37, 330–338. doi: 10.1016/j.nbd.2009.10.011
Schulze, M., Sommer, A., Plotz, S., Farrell, M., Winner, B., Grosch, J., et al. (2018). Sporadic Parkinson’s disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of piRNAs. Acta Neuropathol. Commun. 6:58. doi: 10.1186/s40478-018-0561-x
Shen, E. Z., Chen, H., Ozturk, A. R., Tu, S., Shirayama, M., Tang, W., et al. (2018). Identification of piRNA binding sites reveals the argonaute regulatory landscape of the C. elegans germline. Cell 172, 937–951.e18. doi: 10.1016/j.cell.2018.02.002
Shen, L., Wang, C., Chen, L., Leung, K. L., Lo, E., Lakso, M., et al. (2020). TDP-1/TDP-43 potentiates human alpha-Synuclein (HASN) neurodegeneration in Caenorhabditis elegans. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165876. doi: 10.1016/j.bbadis.2020.165876
Smith, W. W., Jiang, H., Pei, Z., Tanaka, Y., Morita, H., Sawa, A., et al. (2005). Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum. Mol. Genet. 14, 3801–3811. doi: 10.1093/hmg/ddi396
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998). Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473. doi: 10.1073/pnas.95.11.6469
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. doi: 10.1038/42166
Sun, W., Samimi, H., Gamez, M., Zare, H., and Frost, B. (2018). Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 21, 1038–1048. doi: 10.1038/s41593-018-0194-1
Takei, Y., Oguchi, K., Koshihara, H., Hineno, A., Nakamura, A., and Ohara, S. (2013). Alpha-synuclein coaggregation in familial amyotrophic lateral sclerosis with SOD1 gene mutation. Hum. Pathol. 44, 1171–1176. doi: 10.1016/j.humpath.2012.10.024
Tan, L., Yu, J. T., and Tan, L. (2015). Causes and consequences of MicroRNA dysregulation in neurodegenerative diseases. Mol. Neurobiol. 51, 1249–1262. doi: 10.1007/s12035-014-8803-9
Tanaka, M., Kim, Y. M., Lee, G., Junn, E., Iwatsubo, T., and Mouradian, M. M. (2004). Aggresomes formed by α-synuclein and synphilin-1 are cytoprotective. J. Biol. Chem. 279, 4625–4631. doi: 10.1074/jbc.M310994200
Taschenberger, G., Garrido, M., Tereshchenko, Y., Bahr, M., Zweckstetter, M., and Kugler, S. (2012). Aggregation of alphaSynuclein promotes progressive in vivo neurotoxicity in adult rat dopaminergic neurons. Acta Neuropathol. 123, 671–683. doi: 10.1007/s00401-011-0926-8
Tong, Z., Cui, Q., Wang, J., and Zhou, Y. (2019). TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 47, D253–D258. doi: 10.1093/nar/gky1023
Vartiainen, S., Aarnio, V., Lakso, M., and Wong, G. (2006a). Increased lifespan in transgenic Caenorhabditis elegans overexpressing human alpha-synuclein. Exp. Gerontol. 41, 871–876. doi: 10.1016/j.exger.2006.05.005
Vartiainen, S., Pehkonen, P., Lakso, M., Nass, R., and Wong, G. (2006b). Identification of gene expression changes in transgenic C. elegans overexpressing human alpha-synuclein. Neurobiol. Dis. 22, 477–486. doi: 10.1016/j.nbd.2005.12.021
Visanji, N. P., Lang, A. E., and Kovacs, G. G. (2019). Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl. Neurodegener. 8:28. doi: 10.1186/s40035-019-0172-x
Wakabayashi, K., Yoshimoto, M., Tsuji, S., and Takahashi, H. (1998). Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 249, 180–182. doi: 10.1016/s0304-3940(98)00407-8
Wakisaka, K. T., and Imai, Y. (2019). The dawn of pirna research in various neuronal disorders. Front. Biosci. 24:1440–1451. doi: 10.2741/4789
Wang, L., Cheng, L., Li, N. N., Yu, W. J., Sun, X. Y., and Peng, R. (2015). Genetic analysis of SLC41A1 in Chinese Parkinson’s disease patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168, 706–711. doi: 10.1002/ajmg.b.32365
Wilhelmsen, K. C., Forman, M. S., Rosen, H. J., Alving, L. I., Goldman, J., Feiger, J., et al. (2004). 17q-linked frontotemporal dementia-amyotrophic lateral sclerosis without tau mutations with tau and alpha-synuclein inclusions. Arch. Neurol. 61, 398–406. doi: 10.1001/archneur.61.3.398
Winner, B., Jappelli, R., Maji, S. K., Desplats, P. A., Boyer, L., Aigner, S., et al. (2011). In vivo demonstration that -synuclein oligomers are toxic. Proc. Natl. Acad. Sci. U.S.A. 108, 4194–4199. doi: 10.1073/pnas.1100976108
Wong, Y. C., and Krainc, D. (2017). Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat. Med. 23, 1–13. doi: 10.1038/nm.4269
Wu, W. S., Brown, J. S., Chen, T. T., Chu, T. H., Huang, W. C., Tu, S., et al. (2019). piRTarBase: a database of piRNA targeting sites and their roles in gene regulation. Nucleic Acids Res. 47, D181–D187. doi: 10.1093/nar/gky956
Yan, Z., Hu, H. Y., Jiang, X., Maierhofer, V., Neb, E., He, L., et al. (2011). Widespread expression of piRNA-like molecules in somatic tissues. Nucleic Acids Res. 39, 6596–6607. doi: 10.1093/nar/gkr298
Yu, G., Wang, L. G., Han, Y., and He, Q. Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287. doi: 10.1089/omi.2011.0118
Keywords: microRNA, PIWI-interacting RNA, neurodegenerative disease, synucleinopathies, Caenorhabditis elegans
Citation: Shen L, Wang C, Chen L and Wong G (2021) Dysregulation of MicroRNAs and PIWI-Interacting RNAs in a Caenorhabditis elegans Parkinson’s Disease Model Overexpressing Human α-Synuclein and Influence of tdp-1. Front. Neurosci. 15:600462. doi: 10.3389/fnins.2021.600462
Received: 02 September 2020; Accepted: 27 January 2021;
Published: 08 March 2021.
Edited by:
Song Li, Dalian Medical University, ChinaReviewed by:
Guy A. Caldwell, University of Alabama, United StatesCopyright © 2021 Shen, Wang, Chen and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Garry Wong, Z2Fycnlnd29uZ0B1bS5lZHUubW8=; R2FycnlHV29uZ0B1bWFjLm1v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.