- 1Institutes of Biomedical Science and Children's Hospital, Fudan University, Shanghai, China
- 2Henan Key Laboratory of Child Brain Injury, Department of Pediatrics, The 3rd Affiliated Hospital of Zhengzhou University and Institute of Neuroscience, Zhengzhou, China
- 3Department of Pediatrics, Qilu Hospital of Shandong University, Jinan, China
- 4Department of Pediatrics, Children's Hospital of Zhengzhou University and Henan Children's Hospital, Zhengzhou, China
- 5Child Rehabilitation Center, The 3rd Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 6Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden
- 7Center for Brain Repair and Rehabilitation, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 8Shanghai Center for Women and Children's Health, Shanghai, China
Background: Cerebral palsy (CP) is a syndrome of non-progressive motor dysfunction caused by early brain development injury. Recent evidence has shown that immunological abnormalities are associated with an increased risk of CP.
Methods: We recruited 782 children with CP as the case group and 770 healthy children as the control group. The association between IL-23R single nucleotide polymorphisms (SNPs; namely, rs10889657, rs6682925, rs1884444, rs17375018, rs1004819, rs11805303, and rs10889677) and CP was studied by using a case–control method and SHEsis online software. Subgroup analysis based on complications and clinical subtypes was also carried out.
Results: There were differences in the allele and genotype frequencies between CP cases and controls at the rs11805303 and rs10889677 SNPs (Pallele = 0.014 and 0.048, respectively; Pgenotype = 0.023 and 0.008, respectively), and the difference in genotype frequency of rs10889677 remained significant after Bonferroni correction (Pgenotype = 0.048). Subgroup analysis revealed a more significant association of rs10889677 with CP accompanied by global developmental delay (Pgenotype = 0.024 after correction) and neonatal encephalopathy (Pgenotype = 0.024 after correction).
Conclusion: The present results showed a significant association between IL-23R and CP, suggesting that IL-23R may play a potential role in CP pathogenesis.
Introduction
Cerebral palsy (CP) is a central motor disorder syndrome that manifests with abnormal muscle tension and motor function (Cheng et al., 2011; Wu et al., 2011). Individuals with CP often exhibit sensory, perceptual, cognitive, communication, and behavioral disorders, as well as epilepsy and secondary musculoskeletal problems (Beliakoff and Sun, 2006). Although perinatal medicine has developed rapidly in recent years, epidemiological studies have shown that the incidence of CP has remained stable at 2–3.5 children out of every 1,000 children (Pennington et al., 2013). CP has been a prominent disease among children's disabilities for a long time due to a lack of effective treatments (Tator et al., 2007; Tatla et al., 2013). CP seriously affects the quality of life of individuals and also brings a heavy financial burden to families and society (Duval et al., 2003; Yunus and Lima, 2009). The identification of its etiology and pathogenesis is essential to the prevention and control of CP.
CP is caused by non-progressive brain damage during the development of the fetus or infant, which can be divided into congenital and acquired damage. Congenital non-progressive injuries are caused by prenatal developmental defects, such as genetic defects, developmental defects, malformations, intrauterine infections, etc. Acquired injuries are caused by postpartum acquired factors, such as premature labor, asphyxia, hypoxic–ischemic encephalopathy (HIE), low birth weight (LOW), and pathogenic jaundice (Sachdev et al., 2001; Jacobsson and Hagberg, 2004). Increasing evidence now indicates that genetic factors are likely to play an important role in CP pathogenesis. In general, CP is regarded as the result of the combined effects of multiple genes and environmental factors. Moreover, CP has been reported to have multiple susceptibility genes, including IL-6, NOS1, OLIG2, ATG5, and ATG7 (Xu et al., 2017; Yu et al., 2018; Sun et al., 2019; Xia et al., 2019).
A great deal of evidence suggests that neuroinflammation has been found to participate in, modulate, and even induce the pathological process of immature brain injury and various cytokines have been associated with CP and neurodevelopmental disability. Previous studies have found that immature brain injury induced by secondary inflammation is one of the important pathological mechanisms of hypoxic–ischemic brain injury (Elovitz et al., 2011; Albertsson et al., 2014; Marshall and Plotkin, 2019). Abnormal activation of cytokines can cause brain damage, and fetuses are more likely to produce inflammatory reactions or brain damage after being affected by inflammatory cytokines due to immature brain development, thus further affecting the normal development of the brain. At present, many inflammatory cytokines have been reported to be significantly associated with CP or neurodevelopmental disorders, such as IL-6, IL-8, IL-10, and IL-17 (Strle et al., 2001; Chiricozzi et al., 2011; Chen et al., 2013; Magalhaes et al., 2019).
Interleukin-23 (IL-23), also known as p19, is a member of the IL-12 heterodimer cytokine family (Oppmann et al., 2000), which is mainly produced by activated dendritic cells, macrophages, and monocytes. IL-23 plays an important role in the regulation of tissue homeostasis and congenital or adaptive immunity. IL-23 is involved in the pathogenesis of many chronic inflammatory diseases, such as psoriasis, arthritis, inflammatory bowel disease, and multiple sclerosis (MS). Drugs targeting IL-23 have been used in clinical research regarding immunologic diseases (Wiekowski et al., 2001; Schon and Erpenbeck, 2018). IL-23 binds to the IL-23 receptor (IL-23R) through its N-terminal immunoglobulin domain, which activates downstream signaling pathways and exerts biological functions. Human IL-23 receptors (IL-23R) are mainly expressed in activated memory T cells, natural killer (NK) cells, and intrinsic immune cells (ILCs). Its extracellular domain contains a signal sequence, one N-terminal Ig-like domain, and two cytokine receptor domains. In a genome-wide association study in 2006, IL-23R was significantly associated with Crohn's disease, an inflammatory bowel disease. The A allele of rs11209026, a low-frequency IL-23R variant, was negatively correlated with Crohn's disease (Bloch et al., 2018). At the same time, some studies have shown that IL-23R mutation is significantly correlated with inflammatory demyelinating diseases, such as MS (Li et al., 2016).
Based on the above information, we speculate that IL-23R may be associated with susceptibility to CP, but no relevant studies have been reported thus far. Then, we used a case–control study to explore the possible association of IL-23R with CP, which will provide genetic evidence for evaluating the role of IL-23R in the etiology of CP and its related potential mechanisms.
Materials and Methods
Participants
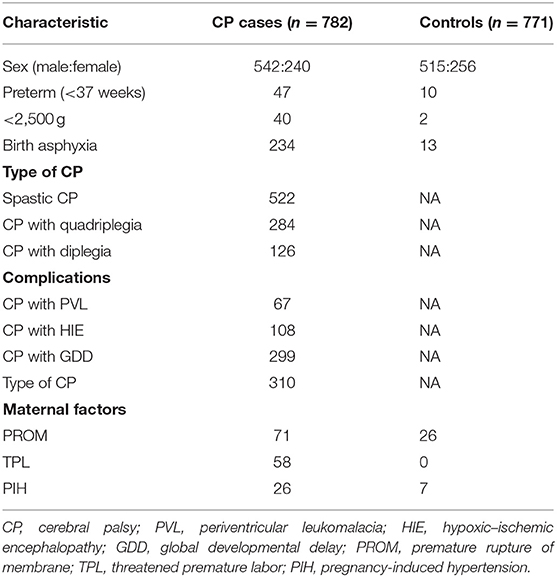
In this study, 782 children with CP and 771 healthy controls were recruited from the centers for CP rehabilitation and Child Healthcare Departments in the Third Affiliated Hospital of Zhengzhou University, Zhengzhou Children's Hospital. This study was approved by the ethics committee of Zhengzhou University. The guardians of these participants provided written informed consent. The case group comprised 542 males (69.3%) and 240 females (30.7%), and the mean age was 18.5 ± 15.4 months. The control group comprised 771 healthy children, including 515 males (66.8%) and 256 females (33.2%), and the mean age was 19.3 ± 16.8 months (Table 1).
CP Diagnosis, Classification, and Exclusion Criteria
In the case group, we excluded children diagnosed with congenital metabolic diseases and myopathy as well as children with a family history of nervous system diseases. Pediatric rehabilitation specialists confirmed the CP diagnosis using standard criteria related to non-progressive disorders of movement control and posture (Rosenbaum et al., 2007). All participants received a detailed clinical evaluation with comprehensive pre-test counseling.
The available clinical information included demographic variables, such as sex, gestational age, mode of delivery, singletons, and twins, as well as the known risk factors [such as pregnancy-induced hypertension (PIH), perinatal asphyxia, and threatened premature labor], CP complications [such as global developmental delay (GDD)], and neonatal complications (such as HIE).
GDD diagnosis is limited to individuals under the age of 5 years old when the clinical severity level cannot be reliably assessed during early childhood. GDD is diagnosed when an individual fails to meet the expected developmental milestones in several areas of intellectual functioning and applies to individuals who are unable to undergo systematic assessments of intellectual functioning, including children who are too young to participate in standardized testing. Neonatal encephalopathy (NE) is a clinical syndrome that includes HIE, intracranial hemorrhage, various metabolic disorders, neurodegenerative diseases, and so on; its diagnosis requires at least two senior neonatologists.
Genotyping and Statistical Analysis
Peripheral blood samples were obtained from the subjects for genomic DNA extraction. According to the single nucleotide polymorphism (SNP) location in IL-23R, a minor allele frequency (MAF) >0.1, and potential function, we selected seven SNPs (rs10889657, rs6682925, rs1884444, rs17375018, rs1004819, rs11805303, and rs10889677, Figure 1) as candidates and genotyped them by the MassARRAY system. Shanghai Perchant Biotechnology Co., Ltd. synthesized primers and probes.
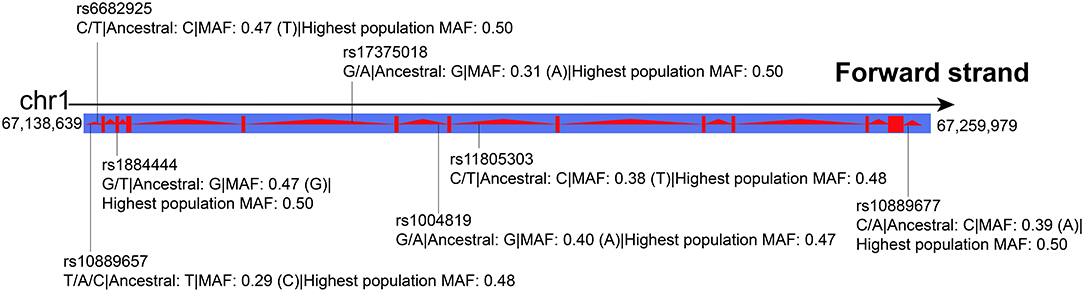
Figure 1. SNPs in IL-23R. Highest population minor allele frequency: highest minor allele frequency observed in any population including 1,000 Genomes Phase 3, ESP, and genomAD.
We performed statistical analysis with SHEsis, an online program (http://analysis.bio-x.cn/) that can test Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) and calculate allele frequencies, genotype frequencies, and haplotype frequencies for each SNP locus in the case group and control group. The P values were two-tailed, and we considered P < 0.05 to be significant. We also calculated the odds ratio (OR) and its 95% confidence interval (CI). We employed the Bonferroni correction to account for multiple testing on each individual SNP and haplotype. We used the G*power 3.1 software to evaluate the statistical efficacy.
Results
Overall Analysis
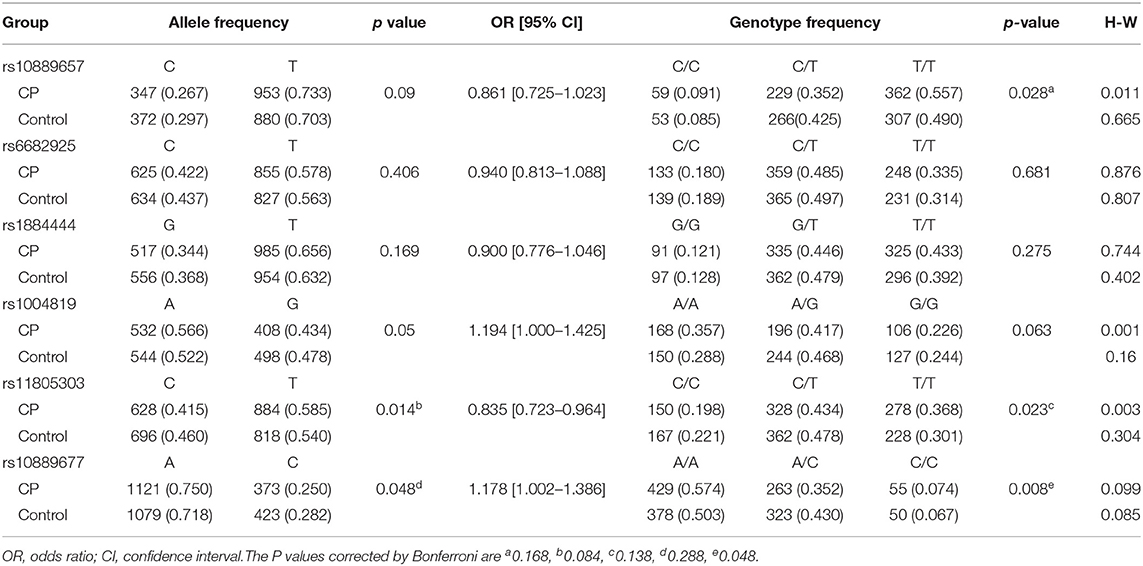
By performing power calculations, the sample size utilized in the present study has >85% power to detect a significant association (α < 0.05) when using an effect size index of 0.1. The genotype distributions of rs17375018 among control subjects showed a significant deviation from HWE (P = 0.011); therefore, we only analyzed the remaining six SNPs, namely, rs10889657, rs6682925, rs1884444, rs1004819, rs11805303, and rs10889677. There were two linked LD blocks with coefficient D′ value more than 0.8 (Figure 2). Block 1 included rs10889657, rs6682925, and rs1884444, whereas block 2 comprised rs1004819, rs11805303, and rs10889677.
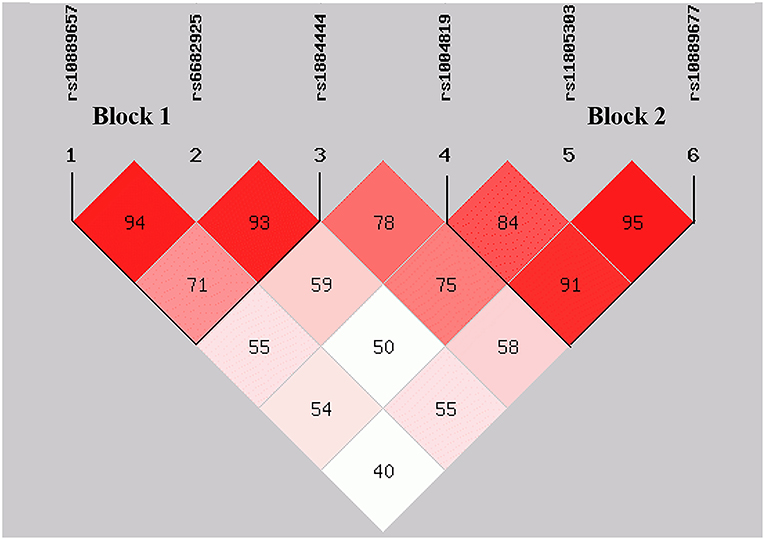
Figure 2. Distribution of blocks defined by linkage disequilibrium scores of six SNPs in IL-23R. The data indicate D′ values.
For all subjects, the allele frequencies of rs11805303 (P = 0.014) and rs10889677 (P = 0.048) and the genotype frequencies of rs10889657 (P = 0.028), rs11805303 (P = 0.023), and rs10889677 (P = 0.008) were significantly different between CP patients and controls. After Bonferroni correction, only the rs10889677 AA genotype frequency was significantly more enriched in CP children than in controls (OR = 1.178, 95% CI = 1.002–1.386, Pc = 0.048) (Table 2).
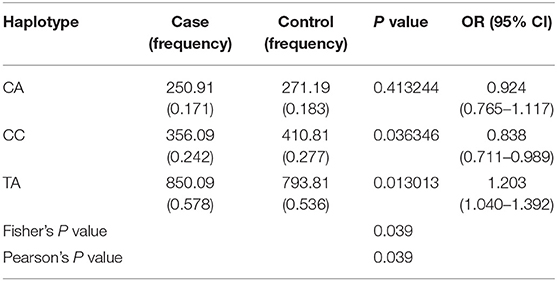
Haplotype analysis is a powerful strategy to determine whether or not the above-mentioned CP-associated SNPs have a greater effect when analyzed together. Hence, we performed a haplotype analysis of rs11805303 and rs10889677 SNPs. The haplotypes CC (P = 0.036) and TA (P = 0.013) presented a significant association with CP; the positive association of TA with CP was significant even after Bonferroni correction (P = 0.039). Furthermore, there was a statistically significant global effect of the haplotype (P = 0.039) (Table 3).
Subgroup Analysis
CP is a highly heterogeneous condition that likely has multiple etiologies. It is reasonable to speculate that the broad clinical spectrum of CP can be at least partially attributed to considerable genetic heterogeneity. Therefore, we conducted subgroup analysis according to clinical phenotypes. There was a significant association of CP + GDD with rs10889657, rs1004819, rs11805303, and rs10889677, and the association of rs10889677 met the Bonferroni correction cut-off for multiple testing (OR = 1.293, 95% CI = 1.034–1.61, P = 0.024, Table 4).
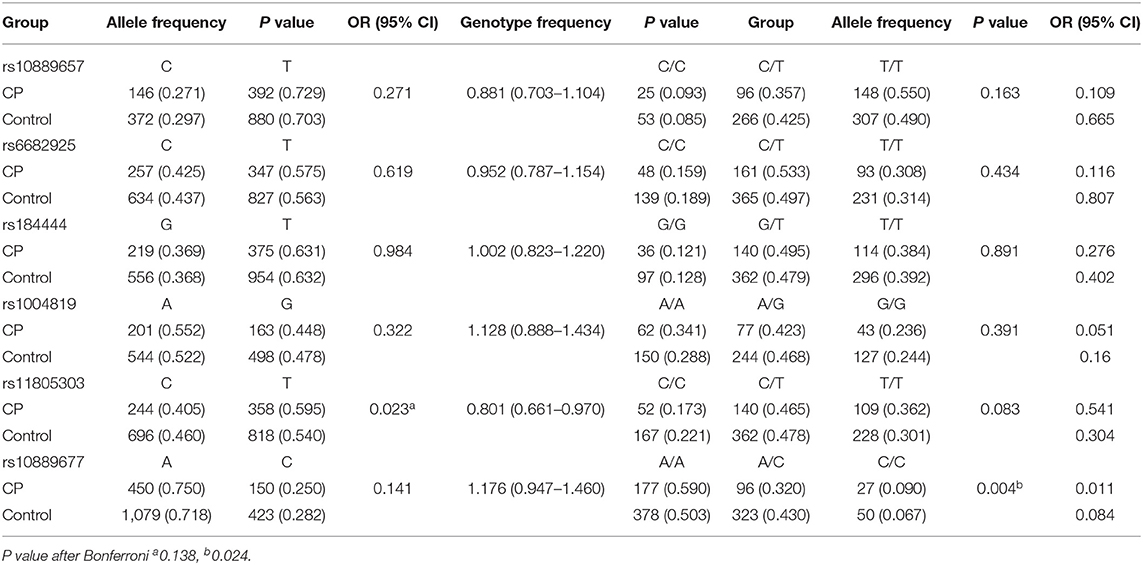
Furthermore, we analyzed the associations between IL-23R and CP subtypes with various risk factors. There were significant differences in the rs11805303 allele frequency and the rs10889677 genotype frequency between controls and CP patients with NE; the association of rs10889677 with CP + NE remained significant even after Bonferroni correction (OR = 1.176, 95% CI = 0.947–1.460, P = 0.024, Table 5). There were no significant differences in either allele or genotype frequencies of the other SNPs in the other CP subgroups defined by clinical phenotypes and available risk factors (Supplementary Table 1).
Discussion
CP is a heterogeneous condition with multiple causes (Badawi and Keogh, 2013). The etiology in an individual patient is often multifactorial. These known CP causes, such as periventricular leukomalacia (PVL), NE, infarct, and premature delivery, account for only a minority of the total cases (Cowan et al., 2003; Yildiz et al., 2012; Colver et al., 2014; Chang et al., 2015). A single severe adverse event can be sufficient to cause CP, but much more often it is not a single cause, rather multiple concurrent risk factors that precede CP (Hankins and Speer, 2003; Djukic et al., 2009). Secondary neuroinflammation is associated with many CP risk factors. Findings from animal and clinical studies suggested that persistent neuroinflammation might prevent regeneration or exacerbate brain damage (Elovitz et al., 2011). Altered inflammation is one of the common causes of CP (Chiricozzi et al., 2011; Chen et al., 2013; Xia et al., 2018). Although the exact cause of CP is largely unknown, it is thought to be due to a combination of an altered fetal inflammatory response and primary brain damages.
Abnormal inflammation is one of the important pathological causes of immature brain injury (Djukic et al., 2009; Du et al., 2011), which is involved in the pathogenesis of central nervous system diseases, such as epilepsy, Parkinson's disease, cerebral ischemia, and hemorrhage, and may easily lead to secondary brain insult. During cerebral ischemia–reperfusion injury, inflammatory cells, such as microglia, astrocytes, and leukocytes, are activated. The activated inflammatory cells synthesize and secrete inflammatory mediators, phenomena that, in turn, further activate inflammatory cells and aggravate brain injury (Moon et al., 2009; Albertsson et al., 2018).
Some inflammatory cytokines are involved in immune responses, which cause brain injury through an inflammatory mechanism (McAdams and Juul, 2012). Several inflammatory cytokines have been identified as being involved in CP or other neurodevelopmental disorders, such as IL-6, IL-8, and IL-17 (Chiricozzi et al., 2011; Chen et al., 2013). Our previous studies found that rs1800795 (G174C), located in the IL-6 promoter region, was significantly associated with CP, and that the risk of spastic hemiplegia and quadriplegia in carriers of the rs1800795 C allele also significantly increased (Djukic et al., 2009; Wu et al., 2009). Central nervous system inflammation is often characterized by microglia activation, and active microglia will mediate neurotoxicity by secreting inflammatory cytokines, proteins, or other bioactive substances, resulting in secondary brain injury (Tang and Le, 2016).
A recent study demonstrated that inflammatory factors are related to microglia activation (Zhao et al., 2020). IL-23 mainly acts as a pro-inflammatory cytokine and has potential anti-tumor and anti-infection effects. IL-23R can activate Janus kinase (JAK). IL-23 bound IL-23R can active downstream JAKs and phosphorylate the signal transducer and activator of transcription (STAT) binding site in the intracellular region of the receptor. STAT dimerizes and is phosphorylated by JAKs. The phosphorylated STAT dimers enter the nucleus and act on downstream target genes. IL-23R has been identified to associate with multiple diseases, including alopecia areata (rs10889677) and nephropathy (rs10805303) (Figure 3) (Safrany et al., 2011; Yu et al., 2012; Huang et al., 2016; Qin et al., 2016; Poomarimuthu et al., 2018; Sode et al., 2018; Zhong et al., 2018; Kramer et al., 2019; Loures et al., 2019; Ruyssen-Witrand et al., 2019; Tabatabaei-Panah et al., 2020).
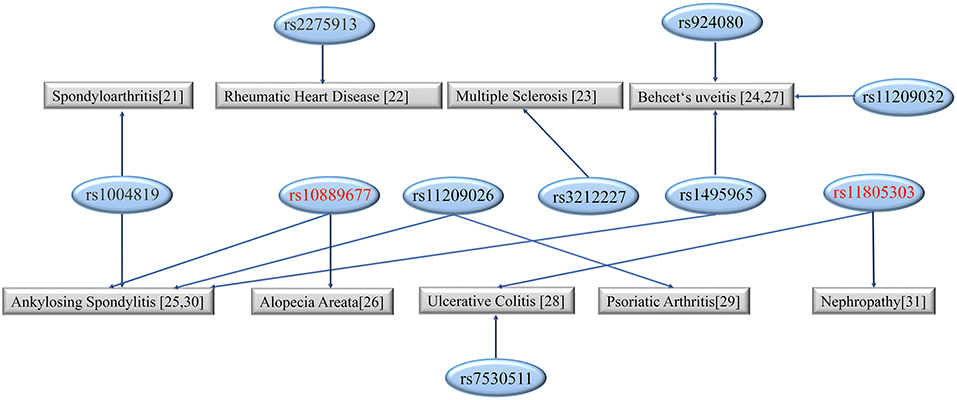
Figure 3. The diseases linked to different sites of IL-23R. The SNPs studied in the present study are marked in red.
Our results showed that IL-23R rs10889677 increases susceptibility to CP at the overall level and in some subgroups. These findings suggest that IL-23R is a potential susceptibility gene for CP. Furthermore, studies have shown that rs10889677 is related with different diseases in different races, such as Crohn's disease in both Jewish and non-Jewish populations, ankylosing spondylitis in Caucasian patients, Graves' disease in North Americans, and ulcerative colitis in Europeans (Duerr et al., 2006; Wellcome Trust Case Control et al., 2007; Brown, 2008; Huber et al., 2008; Silverberg et al., 2009). Therefore, we have sufficient reason to assume the positive association of IL-23R gene with the CP etiology. Whether IL-23R, as a gene associated with inflammatory bowel disease, can also affect the brain development of children by affecting intestinal flora remains to be seen.
Given that TNF-α and IL-6 are significantly increased after brain injury (Leviton and Dammann, 2004; Xie et al., 2014), we hypothesize that IL-23R, like other inflammatory factors TNF-α and IL-6, may cause brain damage and lead to CP through the following steps. (1) Increased inflammatory cytokines promote the release of nitric oxide synthase and free radicals and excitatory amino acids, which have toxic effects on neurons, especially developing brain tissue. (2) They will trigger a systemic inflammatory response that leads to brain damage when undergoing intrauterine infection. (3) Endothelial cell damage can cause thrombosis. Inflammatory factors will activate platelets, lead to their aggregation, activate coagulation factors, and damage white matter neurons. (4) The damage will increase the permeability of the blood–brain barrier, thereby allowing peripheral bacteria and inflammatory factors to enter the brain, aggravating brain damage. (5) They promote the release of prostaglandins and other substances, resulting in pregnancy's advance labor, leading to an increased risk of CP.
Our study has some limitations. First, this is a study based on a single gene for susceptibility to CP. Given the genetic heterogeneity and gene–gene interaction involved in the CP etiology, other candidate genes that are part of the IL-23R signaling pathway need to be analyzed together. Second, we were unable to measure IL-23R protein expression in the brains of the subjects in the current study; future studies are encouraged to examine the inflammatory cytokine alteration in the brain. Third, although our study demonstrated an association between the IL-23R rs10889677 SNP and CP, further functional and replicated studies are necessary to verify the association of IL-23R with CP, which is of great significance to identify the CP etiology and pathogenesis.
In summary, a significant association between IL-23R and CP was firstly detected in Han Chinese, suggesting that the IL-23R gene has a significant effect on the risk of CP, especially in subjects with GDD or NE. The inflammatory response and cytokine cascade are likely to play a role in the occurrence and development of CP. This result needs to be further validated with well-designed studies with large sample sizes and in other populations. We should also pay attention to the possibility of increased risk of CP if the fetus is found to carry the IL-23R risk genotypes before or after delivery.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhengzhou University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
QX and CZ conceived and designed the study. YX, JS, LX, QS, CG, and DZ recruited subjects and sorted out clinical information. YW and DB performed all of the laboratory work. YW and YF performed all data and statistical analyses. YF drafted the manuscript, and QX, CZ, XW, YW, and YX revised the manuscript critically for important intellectual content. YQ and JS provided data, developed models, reviewed results, and provided guidance on the methods. All authors contributed and critically reviewed the final version of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Plan for Stem Cell and Transformation Research (2017YFA0104202), the Department of Science and Technology of Henan Province, China (171100310200), the Health Department of Henan Province (SB201901055), the Shanghai Municipal Commission of Science and Technology Research Project (19JC1411000), the Capacity-Building Planning Program for Shanghai Women and Children's Health Service, the collaborative innovation center project construction for Shanghai women and children's health, the Fourth Round of the Shanghai Three-year Action Plan on Public Health Discipline and Talent Program: Women and Children's Health (No. 15GWZK0401), the Swedish Research Council (2015-04780, 2018-02667), the Swedish Governmental grants to scientists working in health care (ALFGBG-717791), the Swedish Brain Foundation (FO2018-0034), the VINNMER-Marie Curie (VINNOVA, 2015-04780), and the National Natural Science Foundation of China (81801499).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HB-P declared a past co-authorship with the authors XW and CZ to the handling editor.
Acknowledgments
We thank all participants involved in this study and all authors.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2020.590098/full#supplementary-material
References
Albertsson, A. M., Bi, D., Duan, L., Zhang, X., Leavenworth, J. W., Qiao, L., et al. (2014). The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J. Neuroinflammation 11:153. doi: 10.1186/s12974-014-0153-z
Albertsson, A. M., Zhang, X., Vontell, R., Bi, D., Bronson, R. T., Supramaniam, V., et al. (2018). gammadelta T Cells Contribute to injury in the developing brain. Am. J. Pathol. 188, 757–767. doi: 10.1016/j.ajpath.2017.11.012
Badawi, N., and Keogh, J. M. (2013). Causal pathways in cerebral palsy. J. Paediatr. Child Health 49, 5–8. doi: 10.1111/jpc.12068
Beliakoff, J., and Sun, Z. (2006). Zimp7 and Zimp10, two novel PIAS-like proteins, function as androgen receptor coregulators. Nucl. Recept. Signal 4:e017. doi: 10.1621/nrs.04017
Bloch, Y., Bouchareychas, L., Merceron, R., Skladanowska, K., Van den Bossche, L., Detry, S., et al. (2018). Structural activation of pro-inflammatory human Cytokine IL-23 by Cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rbeta1. Immunity 48, 45–58 e46. doi: 10.1016/j.immuni.2017.12.008
Brown, M. A. (2008). Breakthroughs in genetic studies of ankylosing spondylitis. Rheumatology 47, 132–137. doi: 10.1093/rheumatology/kem269
Chang, M. J., Ma, H. I., and Lu, T. H. (2015). Estimating the prevalence of cerebral palsy in Taiwan: a comparison of different case definitions. Res. Dev. Disabil. 36C, 207–212. doi: 10.1016/j.ridd.2014.10.001
Chen, M., Li, T., Lin, S., Bi, D., Zhu, D., Shang, Q., et al. (2013). Association of interleukin 6 gene polymorphisms with genetic susceptibilities to spastic tetraplegia in males: a case-control study. Cytokine 61, 826–830. doi: 10.1016/j.cyto.2013.01.011
Cheng, X., Li, T., Wang, H., Zhu, D., Ma, C., Ma, B., et al. (2011). Methylenetetrahydrofolate reductase gene polymorphisms and cerebral palsy in Chinese infants. J. Hum. Genet. 56, 17–21. doi: 10.1038/jhg.2010.127
Chiricozzi, A., Guttman-Yassky, E., Suarez-Farinas, M., Nograles, K. E., Tian, S., Cardinale, I., et al. (2011). Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687. doi: 10.1038/jid.2010.340
Colver, A., Fairhurst, C., and Pharoah, P. O. (2014). Cerebral palsy. Lancet 383, 1240–1249. doi: 10.1016/S0140-6736(13)61835-8
Cowan, F., Rutherford, M., Groenendaal, F., Eken, P., Mercuri, E., Bydder, G. M., et al. (2003). Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361, 736–742. doi: 10.1016/S0140-6736(03)12658-X
Djukic, M., Gibson, C. S., Maclennan, A. H., Goldwater, P. N., Haan, E. A., McMichael, G., et al. (2009). Genetic susceptibility to viral exposure may increase the risk of cerebral palsy. Aust. N. Z. J. Obstet. Gynaecol. 49, 247–253. doi: 10.1111/j.1479-828X.2009.00999.x
Du, X., Fleiss, B., Li, H., D'Angelo, B., Sun, Y., Zhu, C., et al. (2011). Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS ONE 6:e19583. doi: 10.1371/journal.pone.0019583
Duerr, R. H., Taylor, K. D., Brant, S. R., Rioux, J. D., Silverberg, M. S., Daly, M. J., et al. (2006). A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463. doi: 10.1126/science.1135245
Duval, D., Duval, G., Kedinger, C., Poch, O., and Boeuf, H. (2003). The ‘PINIT' motif, of a newly identified conserved domain of the PIAS protein family, is essential for nuclear retention of PIAS3L. FEBS Lett. 554, 111–118. doi: 10.1016/S0014-5793(03)01116-5
Elovitz, M. A., Brown, A. G., Breen, K., Anton, L., Maubert, M., and Burd, I. (2011). Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 29, 663–671. doi: 10.1016/j.ijdevneu.2011.02.011
Hankins, G. D., and Speer, M. (2003). Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet. Gynecol. 102, 628–636. doi: 10.1016/S0029-7844(03)00574-X
Huang, J., Yang, Y., Zhou, F., Liang, Z., Kang, M., Kuang, Y., et al. (2016). Meta-analysis of the IL23R and IL12B polymorphisms in multiple sclerosis. Int. J. Neurosci. 126, 205–212. doi: 10.3109/00207454.2015.1007508
Huber, A. K., Jacobson, E. M., Jazdzewski, K., Concepcion, E. S., and Tomer, Y. (2008). Interleukin (IL)-23 receptor is a major susceptibility gene for Graves' ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J. Clin. Endocrinol. Metab. 93, 1077–1081. doi: 10.1210/jc.2007-2190
Jacobsson, B., and Hagberg, G. (2004). Antenatal risk factors for cerebral palsy. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 425–436. doi: 10.1016/j.bpobgyn.2004.02.011
Kramer, M., Hasanreisoglu, M., Weiss, S., Kumova, D., Schaap-Fogler, M., Guntekin-Ergun, S., et al. (2019). Single-nucleotide polymorphisms in IL23R-IL12RB2 (rs1495965) are highly prevalent in patients with Behcet's Uveitis and vary between populations. Ocul. Immunol. Inflamm. 27, 766–773. doi: 10.1080/09273948.2018.1467463
Leviton, A., and Dammann, O. (2004). Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr. Res. 55, 541–545. doi: 10.1203/01.PDR.0000121197.24154.82
Li, F. F., Zhu, X. D., Yan, P., Jin, M. H., Yue, H., Zhang, Q., et al. (2016). Characterization of variations in IL23A and IL23R genes: possible roles in multiple sclerosis and other neuroinflammatory demyelinating diseases. Aging 8, 2734–2746. doi: 10.18632/aging.101058
Loures, M. A. R., Alves, H. V., de Moraes, A. G., Santos, T. D. S., Lara, F. F., Neves, J. S. F., et al. (2019). Association of TNF, IL12, and IL23 gene polymorphisms and psoriatic arthritis: meta-analysis. Expert Rev. Clin. Immunol 15, 303–313. doi: 10.1080/1744666X.2019.1564039
Magalhaes, R. C., Moreira, J. M., Lauar, A. O., da Silva, A. A. S., and Teixeira, A. L.. (2019). Inflammatory biomarkers in children with cerebral palsy: a systematic review. Res. Dev. Disabil. 95:103508. doi: 10.1016/j.ridd.2019.103508
Marshall, H. S., and Plotkin, S. (2019). The changing epidemiology of mumps in a high vaccination era. Lancet Infect. Dis. 19, 118–119. doi: 10.1016/S1473-3099(18)30541-3
McAdams, R. M., and Juul, S. E. (2012). The role of cytokines and inflammatory cells in perinatal brain injury. Neurol. Res. Int. 2012:561494. doi: 10.1155/2012/561494
Moon, J. B., Lee, C. H., Park, C. W., Cho, J. H., Hwang, I. K., Yoo, K. Y., et al. (2009). Neuronal degeneration and microglial activation in the ischemic dentate gyrus of the gerbil. J. Vet. Med. Sci. 71, 1381–1386. doi: 10.1292/jvms.001381
Oppmann, B., Lesley, R., Blom, B., Timans, J. C., Xu, Y., Hunte, B., et al. (2000). Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725. doi: 10.1016/S1074-7613(00)00070-4
Pennington, L., Virella, D., Mjoen, T., da Graca Andrada, M., Murray, J., Colver, A., et al. (2013). Development of The Viking Speech Scale to classify the speech of children with cerebral palsy. Res. Dev. Disabil. 34, 3202–3210. doi: 10.1016/j.ridd.2013.06.035
Poomarimuthu, M., Elango, S., Solomon, P. R., Soundrapandian, S., and Mariakuttikan, J. (2018). Association of IL17 and IL23R gene polymorphisms with rheumatic heart disease in South Indian population. Immunol. Invest. 47, 754–764. doi: 10.1080/08820139.2018.1493053
Qin, X., Xu, J., Wu, Z., Sun, F., Chen, H., Zheng, W., et al. (2016). Association study of rs924080 and rs11209032 polymorphisms of IL23R-IL12RB2 in a Northern Chinese Han population with Behcet's disease. Hum. Immunol. 77, 1284–1290. doi: 10.1016/j.humimm.2016.09.006
Rosenbaum, P., Paneth, N., Leviton, A., Goldstein, M., Bax, M., Damiano, D., et al. (2007). A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 109, 8–14. doi: 10.1111/j.1469-8749.2007.tb12610.x
Ruyssen-Witrand, A., Luxembourger, C., Cantagrel, A., Nigon, D., Claudepierre, P., Degboe, Y., et al. (2019). Association between IL23R and ERAP1 polymorphisms and sacroiliac or spinal MRI inflammation in spondyloarthritis: DESIR cohort data. Arthritis Res. Ther. 21:22. doi: 10.1186/s13075-018-1807-5
Sachdev, S., Bruhn, L., Sieber, H., Pichler, A., Melchior, F., and Grosschedl, R. (2001). PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. 15, 3088–3103. doi: 10.1101/gad.944801
Safrany, E., Szell, M., Csongei, V., Jaromi, L., Sipeky, C., Szabo, T., et al. (2011). Polymorphisms of the IL23R gene are associated with psoriasis but not with immunoglobulin A nephropathy in a Hungarian population. Inflammation 34, 603–608. doi: 10.1007/s10753-010-9268-2
Schon, M. P., and Erpenbeck, L. (2018). The Interleukin-23/Interleukin-17 axis links adaptive and innate immunity in psoriasis. Front. Immunol. 9:1323. doi: 10.3389/fimmu.2018.01323
Silverberg, M. S., Cho, J. H., Rioux, J. D., McGovern, D. P., Wu, J., Annese, V., et al. (2009). Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat. Genet. 41, 216–220. doi: 10.1038/ng.275
Sode, J., Bank, S., Vogel, U., Andersen, P. S., Sorensen, S. B., Bojesen, A. B., et al. (2018). Genetically determined high activities of the TNF-alpha, IL23/IL17, and NFkB pathways were associated with increased risk of ankylosing spondylitis. BMC Med. Genet. 19:165. doi: 10.1186/s12881-018-0680-z
Strle, K., Zhou, J. H., Shen, W. H., Broussard, S. R., Johnson, R. W., Freund, G. G., et al. (2001). Interleukin-10 in the brain. Crit. Rev. Immunol. 21, 427–449. doi: 10.1615/CritRevImmunol.v21.i5.20
Sun, L., Xia, L., Wang, M., Zhu, D., Wang, Y., Bi, D., et al. (2019). Variants of the OLIG2 gene are associated with cerebral palsy in Chinese Han infants with hypoxic-ischemic encephalopathy. Neuromolecular. Med. 21, 75–84. doi: 10.1007/s12017-018-8510-1
Tabatabaei-Panah, P. S., Moravvej, H., Delpasand, S., Jafari, M., Sepehri, S., Abgoon, R., et al. (2020). IL12B and IL23R polymorphisms are associated with alopecia areata. Genes Immun. 21, 203–210. doi: 10.1038/s41435-020-0100-1
Tang, Y., and Le, W. (2016). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. doi: 10.1007/s12035-014-9070-5
Tatla, S. K., Sauve, K., Virji-Babul, N., Holsti, L., Butler, C., and Van Der Loos, H. F. (2013). Evidence for outcomes of motivational rehabilitation interventions for children and adolescents with cerebral palsy: an American Academy for Cerebral Palsy and Developmental Medicine systematic review. Dev. Med. Child Neurol. 55, 593–601. doi: 10.1111/dmcn.12147
Tator, C., Bray, G., and Morin, D. (2007). The CBANCH report–the burden of neurological diseases, disorders, and injuries in Canada. Can. J. Neurol. Sci. 34, 268–269. doi: 10.1017/S0317167100006673
Wellcome Trust Case Control, C., Australo-Anglo-American Spondylitis, C., Burton, P. R., Clayton, D. G., Cardon, L. R., Craddock, N., et al. (2007). Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337. doi: 10.1038/ng.2007.17
Wiekowski, M. T., Leach, M. W., Evans, E. W., Sullivan, L., Chen, S. C., Vassileva, G., et al. (2001). Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166, 7563–7570. doi: 10.4049/jimmunol.166.12.7563
Wu, Y. W., Croen, L. A., Torres, A. R., Van De Water, J., Grether, J. K., and Hsu, N. N. (2009). Interleukin-6 genotype and risk for cerebral palsy in term and near-term infants. Ann. Neurol. 66, 663–670. doi: 10.1002/ana.21766
Wu, Y. W., Croen, L. A., Vanderwerf, A., Gelfand, A. A., and Torres, A. R. (2011). Candidate genes and risk for CP: a population-based study. Pediatr. Res. 70, 642–646. doi: 10.1203/PDR.0b013e31823240dd
Xia, L., Chen, M., Bi, D., Song, J., Zhang, X., Wang, Y., et al. (2018). Combined analysis of Interleukin-10 gene polymorphisms and protein expression in children with cerebral palsy. Front. Neurol. 9:182. doi: 10.3389/fneur.2018.00182
Xia, L., Xu, J., Song, J., Xu, Y., Zhang, B., Gao, C., et al. (2019). Autophagy-related gene 7 polymorphisms and cerebral palsy in Chinese infants. Front. Cell Neurosci. 13:494. doi: 10.3389/fncel.2019.00494
Xie, C., Zhou, K., Wang, X., Blomgren, K., and Zhu, C. (2014). Therapeutic benefits of delayed lithium administration in the neonatal rat after cerebral hypoxia-ischemia. PLoS ONE 9:e107192. doi: 10.1371/journal.pone.0107192
Xu, J., Xia, L., Shang, Q., Du, J., Zhu, D., Wang, Y., et al. (2017). A variant of the autophagy-related 5 gene is associated with child cerebral palsy. Front. Cell Neurosci. 11:407. doi: 10.3389/fncel.2017.00407
Yildiz, E. P., Tatli, B., Ekici, B., Eraslan, E., Aydinli, N., Caliskan, M., et al. (2012). Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr. Neurol. 47, 186–192. doi: 10.1016/j.pediatrneurol.2012.05.015
Yu, P., Shen, F., Zhang, X., Cao, R., Zhao, X., Liu, P., et al. (2012). Association of single nucleotide polymorphisms of IL23R and IL17 with ulcerative colitis risk in a Chinese Han population. PLoS ONE 7:e44380. doi: 10.1371/journal.pone.0044380
Yu, T., Xia, L., Bi, D., Wang, Y., Shang, Q., Zhu, D., et al. (2018). Association of NOS1 gene polymorphisms with cerebral palsy in a Han Chinese population: a case-control study. BMC Med Genomics 11:56. doi: 10.1186/s12920-018-0374-6
Yunus, A. A., and Lima, C. D. (2009). Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA. Mol. Cell 35, 669–682. doi: 10.1016/j.molcel.2009.07.013
Zhao, Y., Gan, Y., Xu, G., Yin, G., and Liu, D. (2020). MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization. Neurochem. Res. 45, 1180–1190. doi: 10.1007/s11064-020-02998-0
Keywords: cerebral palsy, inflammatory cytokines, interleukin, gene polymorphism, IL23R
Citation: Wang Y, Xu Y, Fan Y, Bi D, Song J, Xia L, Shang Q, Gao C, Zhang X, Zhu D, Qiao Y, Su Y, Wang X, Zhu C and Xing Q (2020) The Association Study of IL-23R Polymorphisms With Cerebral Palsy in Chinese Population. Front. Neurosci. 14:590098. doi: 10.3389/fnins.2020.590098
Received: 31 July 2020; Accepted: 14 October 2020;
Published: 25 November 2020.
Edited by:
Patricia Pelufo Silveira, McGill University, CanadaReviewed by:
Hilla Ben-Pazi, Shaare Zedek Medical Center, IsraelClaudia Vianna Maurer-Morelli, State University of Campinas, Brazil
Copyright © 2020 Wang, Xu, Fan, Bi, Song, Xia, Shang, Gao, Zhang, Zhu, Qiao, Su, Wang, Zhu and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changlian Zhu, emh1Y0B6enUuZWR1LmNu; Q2hhbmdsaWFuLnpodUBuZXVyby5ndS5zZQ==; Qinghe Xing, eGluZ3FpbmdoZUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yangong Wang
Yangong Wang Yiran Xu
Yiran Xu Yangyi Fan
Yangyi Fan Dan Bi
Dan Bi Juan Song
Juan Song Lei Xia
Lei Xia Qing Shang
Qing Shang Chao Gao
Chao Gao Xiaoli Zhang
Xiaoli Zhang Dengna Zhu
Dengna Zhu Yimeng Qiao
Yimeng Qiao Yu Su
Yu Su Xiaoyang Wang
Xiaoyang Wang Changlian Zhu
Changlian Zhu Qinghe Xing
Qinghe Xing