
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci., 26 August 2020
Sec. Neurodegeneration
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.00823
Oxidative stress has been suggested to play a key role in multiple sclerosis (MS), but clinical data on oxidative stress markers in MS patients were inconsistent. This study sought to quantitatively summarize the data of oxidative stress markers in the blood and cerebrospinal fluid (CSF) of patients with MS in the literature. We conducted a systematic search of PubMed and Web of Science and included studies if they provided data on the concentrations of oxidative stress markers in the peripheral blood and CSF of MS patients and healthy control (HC) subjects. The systematic search resulted in the inclusion of 31 studies with 2,001 MS patients and 2,212 HC subjects for meta-analysis. Random-effects meta-analysis demonstrated that patients with MS had significantly increased concentrations of blood oxidative stress markers compared with HC subjects for malondialdehyde (MDA; Hedges' g, 2.252; 95% CI, 1.080 to 3.424; p < 0.001) and lipid hydroperoxide by tert-butyl hydroperoxide-initiated chemiluminescence (CL-LOOH; Hedges' g, 0.383; 95% CI, 0.065 to 0.702; p = 0.018). In contrast, concentrations of albumin (Hedges' g, −1.036; CI, −1.679 to −0.394; p = 0.002) were significantly decreased in MS patients when compared with those in HC subjects. However, the other analyzed blood oxidative stress markers did not show significant differences between cases and controls. Furthermore, this meta-analysis showed significant association between CSF MDA and MS (Hedges' g, 3.275; 95% CI, 0.859 to 5.691; p = 0.008). Taken together, our results revealed increased blood and CSF MDA and decreased blood albumin levels in patients with MS, strengthening the clinical evidence of increased oxidative stress in MS.
Multiple sclerosis (MS) is a disease characterized by inflammatory demyelinating lesions in the white matter of the central nervous system, which leads to the impairment of electrical signaling along an axon in the neurons (Hadzovic-Dzuvo et al., 2011). MS usually occurs in adults between the ages of 20 and 45 years, and it has a high disability rate (Goldenberg, 2012; Aboud and Schuster, 2019). The most common symptoms of the disease include spasticity, chronic pain, fatigue, motor and mobility disorders, and cognitive impairment (Aboud and Schuster, 2019). There is no reliable biomarker to predict the location and onset time of the MS, and currently available drugs only provide symptom alleviation (Goldenberg, 2012; Oliveira et al., 2019). Therefore, there is a need to understand the etiology of MS better and subsequently develop more effective drugs for the treatment of the disease.
The development of MS is considered to be an interaction of genetic predisposition, environmental factors, and aberrant immune response, but the precise etiology of the disease remains unknown (Chastain and Miller, 2012). Also, studies have suggested that oxidative stress may play an important role in the pathogenesis of MS (Schreibelt et al., 2007; van Horssen et al., 2011), as one of the common features in the brains of MS patients is the imbalance between oxidants and antioxidants (Wang et al., 2014; Pasquali et al., 2015; Trentini et al., 2017; De Riccardis et al., 2018), with increased concentrations of reactive oxygen species in cerebrospinal fluid (CSF) of MS patients (Acar et al., 2003; Gilgun-Sherki et al., 2004). Furthermore, clinical studies have shown increased oxidative stress in blood of MS patients, including dysregulated malondialdehyde (MDA) (Juybari et al., 2018), superoxide dismutase (SOD) (Tasset et al., 2012), and glutathione (GSH) (Gironi et al., 2014) levels in the patients. However, some studies showed the opposite results between MS patients and controls for SOD (De Riccardis et al., 2018) and GSH (Socha et al., 2014) levels.
Given the inconsistent findings on oxidative stress marker levels in MS patients, it is necessary to review the literature systematically to address this issue. Therefore, here, we performed a systematic search of studies reporting blood and CSF oxidative stress marker levels in patients with MS and quantitatively summarized the oxidative stress marker data with a meta-analytic technique.
The meta-analyses implemented in this study conform to the instructions that are recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (Moher et al., 2009). Furthermore, we followed the methods of (Wei et al., 2018).
A systematic review of peer-reviewed English articles from the databases of PubMed and Web of Science was performed from September 2019 to October 2019. The search term used for the database search was: multiple sclerosis and (oxidative stress or superoxide dismutase or malondialdehyde or glutathione or total antioxidant status or total oxidation state or C-reactive protein or triglycerides or albumin or advanced oxidation protein products or catalase or hydroxyguanosine or uric acid or ceruloplasmin or transferrin or low-density lipoprotein or cholesterol), without year restriction. Clinical studies were included if they reported data on circulating oxidative stress marker concentrations in MS patients and healthy control (HC) subjects. Exclusion criteria were (1) no necessary concentration data; (2) oxidative stress markers were measured in animal models; (3) no HC subjects; (4) samples were overlapping with other studies; (5) in vitro data; (6) patients had serious complications; and (7) individual oxidative stress marker was assessed in <3 studies.
Data of mean oxidative stress marker concentration, standard deviation (SD), and sample size were extracted to calculate the effective size (ES) for meta-analysis. We also extracted data on age, sex, disease duration, BMI, expanded disability status scale, and sampling sources for potential heterogeneity analysis among studies (Supplementary Table 1).
All statistical analysis was achieved by comprehensive meta-analysis software 2. ES was mainly generated by sample size, mean oxidative stress marker concentration, and SD. The standardized mean difference of oxidative stress marker concentration between MS patients and HC subjects was calculated as ES and converted into Hedge's g statistic to provide an unbiased adjusted ES for sample size. A random-effects model was used in the meta-analysis because we assumed that between- and within-study heterogeneity affected the true ES. Sensitivity analysis was performed to test the robustness of the results of meta-analysis, which is achieved by excluding one study at a time to perform a meta-analysis.
Between-study heterogeneity was assessed by the Cochrane Q test and I2 statistic (Qin et al., 2017), and P < 0.10 was considered statistically significant for the Cochrane Q test. The inconsistency across studies was determined by I2 index to evaluate the impact of heterogeneity, and I2 values of 0.25, 0.5, and 0.75 indicate small, moderate, high levels of between-study heterogeneity, respectively. Unrestricted maximum-likelihood random-effects meta-regression analyses of ES were performed to test whether sample size, patient age, and sex (proportion of male individuals), and disease duration had moderating effects on the outcomes of the meta-analysis. Potential publication bias was determined by Egger's test.
P < 0.05 was considered statistically significant in the meta-analysis except for where noted.
The initial search yielded 2,753 records from the PubMed database and 3,002 records from Web of Science. We performed a preliminary screening of the titles and abstracts of the 5,755 records, and 128 articles that were relevant to this study were selected for full-text scrutiny. After full-text scrutiny, 97 studies were excluded because (1) no necessary concentration data (n = 44), (2) oxidative stress markers were measured in animal models (n = 8), (3) no HC subjects (n = 10), (4) samples were overlapping with other studies (n = 4), (5) in vitro data (n = 2); (6) patients had serious complications (n = 8), and (7) individual oxidative stress marker was assessed in <3 studies (n = 21). Therefore, a total of 31 studies (Jimenez-Jimenez et al., 1998; Keles et al., 2001; Ghabaee et al., 2010; Fjeldstad et al., 2011; Hadzovic-Dzuvo et al., 2011; Miller et al., 2011; Tavazzi et al., 2011; Acar et al., 2012; Oliveira et al., 2012, 2017a,b; Tasset et al., 2012; Ashtari et al., 2013; Kirbas et al., 2013; Ljubisavljevic et al., 2013; Aydin et al., 2014, 2015; Gironi et al., 2014; Polachini et al., 2014; Socha et al., 2014, 2017; Wang et al., 2014; Yousefi et al., 2014; Moccia et al., 2015; Bartova et al., 2016; Kallaur et al., 2016; Bystricka et al., 2017; Delgado-Roche et al., 2017; De Riccardis et al., 2018; Juybari et al., 2018; Armon-Omer et al., 2019), including 2,001 MS patients and 2,212 HC subjects, were included in the meta-analysis (Flowchart see Figure 1).
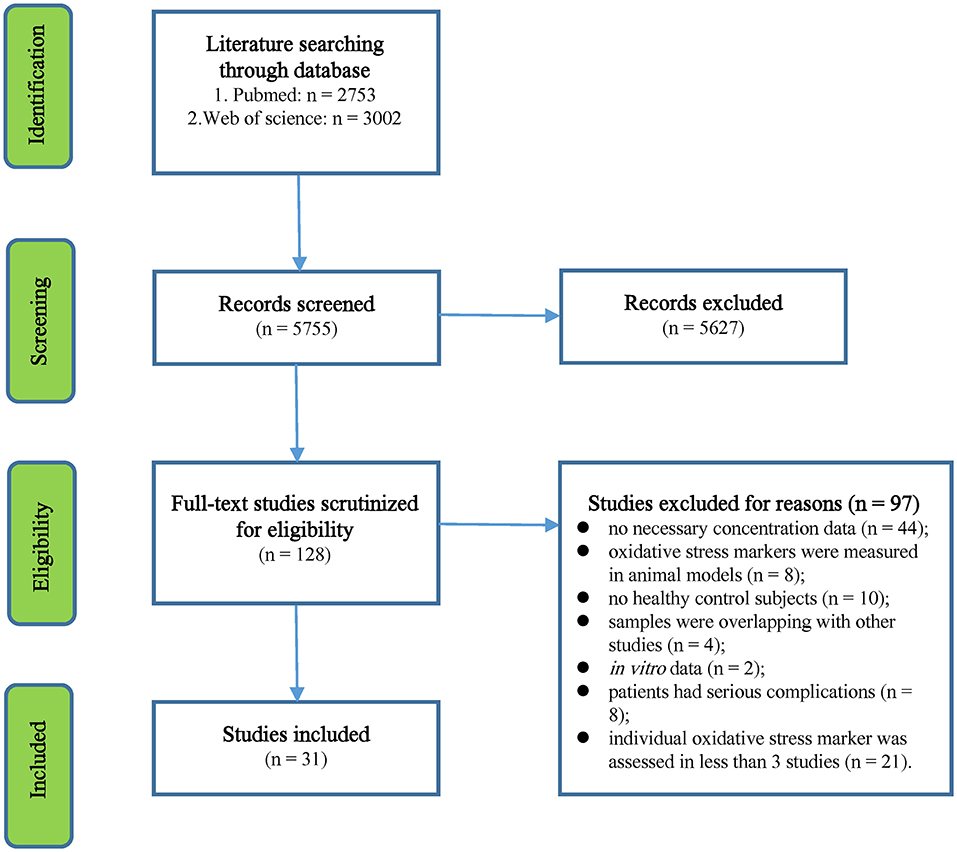
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart of the literature search.
Random-effects meta-analysis demonstrated that patients with MS had significantly increased blood oxidative stress marker levels compared with HC subjects for MDA (Hedges' g, 2.252; 95% CI, 1.080 to 3.424; p < 0.001) and lipid hydroperoxide by tert-butyl hydroperoxide-initiated chemiluminescence (CL-LOOH; Hedges' g, 0.383; 95% CI, 0.065–0.702; p = 0.018), as shown in Table 1, Figures 2A,B. In contrast, concentrations of albumin (Hedges' g, −1.036; CI, −1.679 to −0.394; p = 0.002) were significantly lower in MS patients than that of HC subjects (Table 1, Figure 2C). However, blood levels of GSH, SOD, total antioxidant status, total oxidative status, C-reactive protein, copper, cholesterol, uric acid, and advanced oxidation protein product did not show significant differences between MS patients and HC subjects (Table 1).
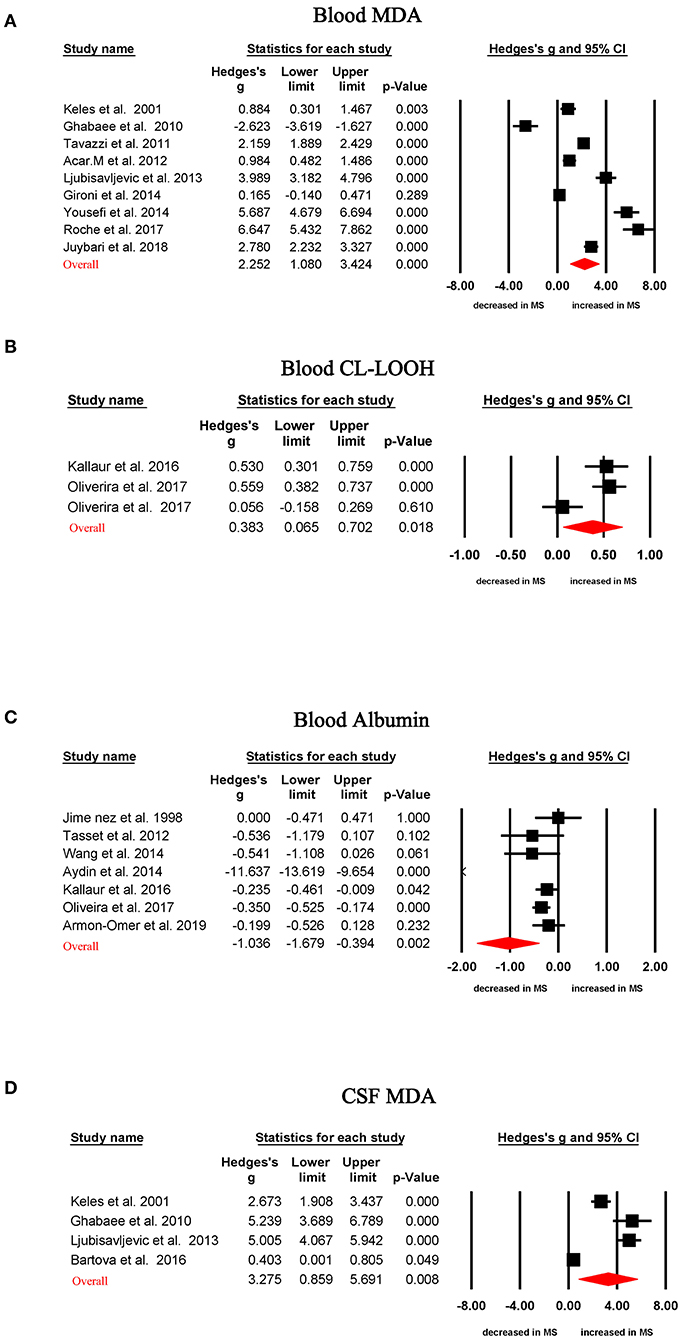
Figure 2. Studies of circulating MDA, CL-LOOH, and albumin in multiple sclerosis. Forest plot displaying random-effects meta-analysis results between blood MDA (A), blood CL-LOOH (B), blood albumin (C), CSF MDA (D), and multiple sclerosis. Standardized mean difference (Hedges g) on the horizontal axis; positive values denote higher in patients with multiple sclerosis; negative values denote higher in healthy control subjects. The diamond denotes pooled effective size (Hedges' g). MDA, malondialdehyde; CL-LOOH, lipid hydroperoxide by tert-butyl hydroperoxide-initiated chemiluminescence; CSF, cerebrospinal fluid.
Random-effects meta-analysis showed CSF MDA levels were significantly increased in MS patients when compared with those in HC subjects (Table 1, Figure 2D; Hedges' g, 3.275; 95% CI, 0.859 to 5.691; p = 0.008). However, there were not enough studies to perform a meta-analysis for other CSF oxidative stress markers.
All of the 13 oxidative stress markers (12 in blood and 1 in CSF) analyzed in the meta-analysis showed high levels of between-study heterogeneities (Table 1). Of the oxidative stress markers that were significantly associated with MS, only blood MDA had a relatively large number of studies. We, therefore, tried to perform meta-regression analyses to test whether potential confounders, including age and sex, had moderating effects on blood MDA levels in MS patients, given that information on disease severity and BMI on the patients were limited.
Meta-regression analyses showed that age was positively associated with ES for studies measuring MDA levels in MS patients (Figure 3A, regression coefficient [SE], 0.1861 [0.0436]; 95% CI, 0.1007 to 0.2715; P < 0.001). In contrast, sex was negatively associated (Figure 3B, regression coefficient [SE], −15.0457 [5.3227]; 95% CI, −25.4781 to −4.6133; P = 0.0047) with ES for studies measuring MDA levels in MS patients.
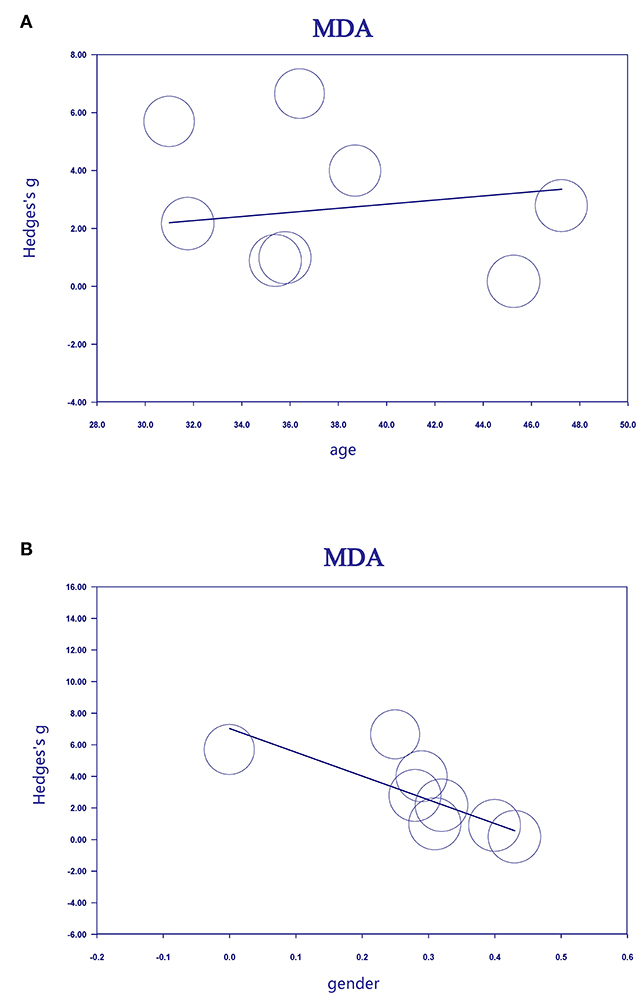
Figure 3. Association between age (A), sex (B), and effective size (Hedges' g) for blood MDA. MDA, malondialdehyde.
Sensitivity analysis showed that no single study affected the significant difference in blood MDA levels between MS patients and HC subjects.
The Egger's test suggested that there was no significant risk of publication bias for the included studies analyzing MDA levels (Table 1).
To the best of our knowledge, this is the first systematic review and meta-analysis on peripheral blood and CSF oxidative stress marker levels in patients with MS. The meta-analysis included 31 articles with 2,001 MS patients and 2,212 HC subjects and reported that CL-LOOH and MDA levels were significantly increased in patients with MS when compared with those in controls. Furthermore, CSF MDA levels were found to be elevated in MS patients. In contrast, albumin levels were significantly decreased in patients with MS when compared with those in controls. Sensitivity analysis demonstrated that the significant association of MS with MDA was not influenced by any single study, suggesting the robustness of the outcomes of the meta-analysis. Although results from previous studies were inconsistent for oxidative stress marker data in MS, the present meta-analysis provides strong clinical evidence of increased oxidative stress in patients with MS. Our study thus clarified the circulating oxidative stress marker profile in patients with MS and may enhance our understanding of the etiology of MS.
In general, oxidative stress is caused by an imbalance between free radical production and antioxidant defensive system (Supplementary Figure 1). Increased free radicals, including reactive oxygen species and reactive nitrogen species, can lead to lipid and protein damages through peroxidation and nitration processes (Adamczyk and Adamczyk-Sowa, 2016). Both MDA and CL-LOOH are considered to be markers for lipid damage in the body, and MDA can cause cross-linking polymerization of proteins, nucleic acids, and other life macromolecules, leading to cytotoxicity. Our meta-analysis showed blood MDA, and CL-LOOH levels were significantly increased in patients with MS, suggesting that lipid damage in patients with MS may contribute to neurodegeneration in the disease. These results are consistent with a previous study suggesting that lipophilic antioxidant deficiency in the blood of MS patients contributed to the reduced reparative demyelinating processes and promoted neurodegeneration (Kuracka et al., 2014).
Although the meta-analysis provided strong clinical evidence of increased oxidative stress in patients with MS, whether oxidative stress is involved in the pathogenesis of MS is unclear. However, the hypothesis that oxidative stress contributes to the progress of MS is plausible, given the considering evidence showing the beneficial effects of antioxidants on MS patients (Adamczyk and Adamczyk-Sowa, 2016). Indeed, one study reported that melatonin reduced oxidative stress, included increased MDA levels, and increased SOD and GSH peroxidase activities, suggesting a positive effect of melatonin on the progression of the severe form of MS (Miller et al., 2013). Additionally, a randomized, double-blinded, placebo-controlled trial showed that supplementation of coenzyme Q10 in relapsing–remitting MS patients increased plasma SOD activity and reduced MDA levels in the patients (Sanoobar et al., 2013). Direct evidence of functional involvement of oxidative stress in MS pathogenesis comes from animal models of MS. Ghaffari et al. utilized a toxic model of MS by intrahippocampal injection of ethidium bromide in rats and showed that saffron extract treatment improved learning and memory in the experimental model of MS. The improved cognition by saffron extract treatment in animals was accompanied by the restoration of antioxidant status, including reduced MDA levels in the hippocampus (Ghaffari et al., 2015). In another animal model of MS, Wang et al. used a natural, endogenous antioxidant-α-lipoic acid to treat experimental autoimmune encephalomyelitis mice and showed that α-lipoic reduced disease severity by inhibition of infiltration of inflammatory cells into the central nervous system (Wang et al., 2013). Therefore, these results, together with the data from the present meta-analysis, suggest that oxidative stress is a promising target for MS treatment.
In addition to the increased oxidative stress found in MS patients, oxidative stress is suggested to be involved in other neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). Although clinical data on oxidative stress markers levels in patients with AD, PD, and ALS were inconsistent in the literature, previous meta-analyses demonstrated patients with AD (Schrag et al., 2013), PD (Wei et al., 2018), and ALS (Wang et al., 2019) were accompanied by increased peripheral blood MDA levels, similar to the heightened blood MDA levels in MS patients found in the present meta-analysis. These results suggest that lipid damage might be a common phenomenon for neurodegenerative diseases. However, a limitation of this meta-analysis is that most oxidative stress markers were assessed in a small number of studies; these include SOD and GSH, which make it difficult to observe significant associations of MS with these oxidative stress markers, therefore, precludes us from comparing MS with other neurodegenerative diseases for most of the oxidative stress markers.
The second limitation of the meta-analysis is that only a few CSF studies were included; therefore, it is difficult to compare blood with CSF on oxidative stress marker levels. However, the meta-analysis found that blood and CSF MDA levels were consistently increased in MS patients. Therefore, we hypothesize good parallels between central and peripheral on the changes of oxidative stress marker levels in MS patients, and future studies are necessary to confirm this hypothesis. Third, due to the limited number of studies analyzing oxidative stress marker levels in different MS subtypes, whether oxidative stress marker levels were differentially changed in different disease stages is unclear, and further longitudinal studies are required to address the issue. The last limitation is that we only include English articles in the meta-analysis, which may create potential publication bias. However, given the small number of non-English articles in this field, non-English articles are not likely to significantly affect the outcomes of the meta-analysis.
The data used to support the findings of this study are included in the article.
YC and SS conceived and designed the study. S-YZ and L-NG collected the data. S-YZ, L-NG, Y-YL, SS, and YC analyzed and interpreted the data. S-YZ drafted the manuscript with critical revisions from all the authors.
This study was supported by the National Science Foundation of China (81703492), the Beijing Natural Science Foundation (7182092), the Minzu University Research Fund (2018CXTD03), and the MUC 111 project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2020.00823/full#supplementary-material
Aboud, T., and Schuster, N. M. (2019). Pain management in multiple sclerosis: a review of available treatment options. Curr. Treat. Options Neurol. 21:62. doi: 10.1007/s11940-019-0601-2
Acar, A., Ugur Cevik, M., Evliyaoglu, O., Uzar, E., Tamam, Y., Arikanoglu, A., et al. (2012). Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol. Belg. 112, 275–280. doi: 10.1007/s13760-012-0059-4
Acar, G., Idiman, F., Idiman, E., Kirkali, G., Cakmakci, H., and Özakbas, S. (2003). Nitric oxide as an activity marker in multiple sclerosis. J. Neurol. 250, 588–592. doi: 10.1007/s00415-003-1041-0
Adamczyk, B., and Adamczyk-Sowa, M. (2016). New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid. Med. Cell. Longev. 2016:1973834. doi: 10.1155/2016/1973834
Armon-Omer, A., Waldman, C., Simaan, N., Neuman, H., Tamir, S., and Shahien, R. (2019). New insights on the nutrition status and antioxidant capacity in multiple sclerosis patients. Nutrients. 11:427. doi: 10.3390/nu11020427
Ashtari, F., Bahar, M., Aghaei, M., and Zahed, A. (2013). Serum uric acid level in patients with relapsing-remitting multiple sclerosis. J. Clin. Neurosci. 20, 676–678. doi: 10.1016/j.jocn.2012.05.054
Aydin, O., Ellidag, H. Y., Eren, E., Kurtulus, F., Yaman, A., and Yilmaz, N. (2014). Ischemia modified albumin is an indicator of oxidative stress in multiple sclerosis. Biochem. Med. (Zagreb). 24, 383–9. doi: 10.11613/BM.2014.041
Aydin, O., Kurtulus, F., Eren, E., Ellidag, H. Y., Yilmaz, N., and Yaman, A. (2015). Balanced oxidative stress index in spite of decreased uric acid levels in multiple sclerosis patients. Neurochem J. 9, 153–158. doi: 10.1134/S1819712415020026
Bartova, R., Petrlenicova, D., Oresanska, K., Prochazkova, L., Liska, B., Turecky, L., et al. (2016). Changes in levels of oxidative stress markers and some neuronal enzyme activities in cerebrospinal fluid of multiple sclerosis patients. Neuro Endocrinol. Lett. 37, 102–106.
Bystricka, Z., Laubertova, L., Durfinova, M., and Paduchova, Z. (2017). Methionine metabolism and multiple sclerosis. Biomarkers 22, 747–754. doi: 10.1080/1354750X.2017.1334153
Chastain, E. M., and Miller, S. D. (2012). Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol. Rev. 245, 227–238. doi: 10.1111/j.1600-065X.2011.01076.x
De Riccardis, L., Buccolieri, A., Muci, M., Pitotti, E., De Robertis, F., Trianni, G., et al. (2018). Copper and ceruloplasmin dyshomeostasis in serum and cerebrospinal fluid of multiple sclerosis subjects. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1828–1838. doi: 10.1016/j.bbadis.2018.03.007
Delgado-Roche, L., Riera-Romo, M., Mesta, F., Hernandez-Matos, Y., Barrios, J. M., Martinez-Sanchez, G., et al. (2017). Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur. J. Pharmacol. 811, 148–154. doi: 10.1016/j.ejphar.2017.06.017
Fjeldstad, A. S., McDaniel, J., Ives, S. J., Witman, M. A. H., Zhao, J., Rose, J. W., et al. (2011). Oxidative stress and flow-mediated dilation during incremental handgrip exercise in multiple sclerosis. Med. Sci. Sport Exer. 43:744. doi: 10.1249/01.MSS.0000402069.76599.af
Ghabaee, M., Jabedari, B., Al, E. E. N., Ghaffarpour, M., and Asadi, F. (2010). Serum and cerebrospinal fluid antioxidant activity and lipid peroxidation in Guillain-Barre syndrome and multiple sclerosis patients. Int. J. Neurosci. 120, 301–304. doi: 10.3109/00207451003695690
Ghaffari, S., Hatami, H., and Dehghan, G. (2015). Saffron ethanolic extract attenuates oxidative stress, spatial learning, and memory impairments induced by local injection of ethidium bromide. Res. Pharm. Sci. 10, 222–232.
Gilgun-Sherki, Y., Melamed, E., and Offen, D. (2004). The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J. Neurol. 251, 261–268. doi: 10.1007/s00415-004-0348-9
Gironi, M., Borgiani, B., Mariani, E., Cursano, C., Mendozzi, L., Cavarretta, R., et al. (2014). Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J. Immunol. Res. 2014:961863. doi: 10.1155/2014/961863
Hadzovic-Dzuvo, A., Lepara, O., Valjevac, A., Avdagic, N., Hasic, S., Kiseljakovic, E., et al. (2011). Serum total antioxidant capacity in patients with multiple sclerosis. Bosn. J. Basic Med. Sci. 11, 33–36. doi: 10.17305/bjbms.2011.2620
Jimenez-Jimenez, F. J., de Bustos, F., Molina, J. A., de Andres, C., Gasalla, T., Orti-Pareja, M., et al. (1998). Cerebrospinal fluid levels of alpha-tocopherol in patients with multiple sclerosis. Neurosci. Lett. 249, 65–67. doi: 10.1016/S0304-3940(98)00370-X
Juybari, K. B., Ebrahimi, G., Momeni Moghaddam, M. A., Asadikaram, G., Torkzadeh-Mahani, M., Akbari, M., et al. (2018). Evaluation of serum arsenic and its effects on antioxidant alterations in relapsing-remitting multiple sclerosis patients. Mult. Scler. Relat. Disord. 19, 79–84. doi: 10.1016/j.msard.2017.11.010
Kallaur, A. P., Lopes, J., Oliveira, S. R., Simao, A. N., Reiche, E. M., de Almeida, E. R., et al. (2016). Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple sclerosis: increased peripheral inflammation but less acute neuroinflammation. Mol. Neurobiol. 53, 5191–5202. doi: 10.1007/s12035-015-9443-4
Keles, M. S., Taysi, S., Sen, N., Aksoy, H., and Akcay, F. (2001). Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 28, 141–143. doi: 10.1017/S0317167100052823
Kirbas, A., Kirbas, S., Anlar, O., Efe, H., and Yilmaz, A. (2013). Serum paraoxonase and arylesterase activity and oxidative status in patients with multiple sclerosis. J. Clin. Neurosci. 20, 1106–1109. doi: 10.1016/j.jocn.2012.09.020
Kuracka, L., Kalnovicova, T., Kucharska, J., and Turcani, P. (2014). Multiple sclerosis: evaluation of purine nucleotide metabolism in central nervous system in association with serum levels of selected fat-soluble antioxidants. Mult. Scler. Int. 2014:759808. doi: 10.1155/2014/759808
Ljubisavljevic, S., Stojanovic, I., Vojinovic, S., Stojanov, D., Stojanovic, S., Kocic, G., et al. (2013). Cerebrospinal fluid and plasma oxidative stress biomarkers in different clinical phenotypes of neuroinflammatory acute attacks. Conceptual accession: from fundamental to clinic. Cell Mol. Neurobiol. 33, 767–777. doi: 10.1007/s10571-013-9944-5
Miller, E., Mrowicka, M., Saluk-Juszczak, J., and Ireneusz, M. (2011). The level of isoprostanes as a non-invasive marker for in vivo lipid peroxidation in secondary progressive multiple sclerosis. Neurochem. Res. 36, 1012–1016. doi: 10.1007/s11064-011-0442-1
Miller, E., Walczak, A., Majsterek, I., and Kedziora, J. (2013). Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J. Neuroimmunol. 257, 97–101. doi: 10.1016/j.jneuroim.2013.02.012
Moccia, M., Lanzillo, R., Palladino, R., Russo, C., Carotenuto, A., Massarelli, M., et al. (2015). Uric acid: a potential biomarker of multiple sclerosis and of its disability. Clin. Chem. Lab. Med. 53, 753–759. doi: 10.1515/cclm-2014-0744
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Oliveira, A. G., Goncalves, M., and Ferreira, H., N. M. N. (2019). Growing evidence supporting the use of mesenchymal stem cell therapies in multiple sclerosis: a systematic review. Mult. Scler. Relat. Disord. 38:101860. doi: 10.1016/j.msard.2019.101860
Oliveira, S. R., Kallaur, A. P., Reiche, E. M. V., Kaimen-Maciel, D. R., Panis, C., Lozovoy, M. A. B., et al. (2017a). Albumin and protein oxidation are predictors that differentiate relapsing-remitting from progressive clinical forms of multiple sclerosis. Mol. Neurobiol. 54, 2961–2968. doi: 10.1007/s12035-016-9860-z
Oliveira, S. R., Kallaur, A. P., Simao, A. N., Morimoto, H. K., Lopes, J., Panis, C., et al. (2012). Oxidative stress in multiple sclerosis patients in clinical remission: association with the expanded disability status scale. J. Neurol. Sci. 321, 49–53. doi: 10.1016/j.jns.2012.07.045
Oliveira, S. R., Simao, A. N. C., Alfieri, D. F., Flauzino, T., Kallaur, A. P., Mezzaroba, L., et al. (2017b). Vitamin D deficiency is associated with disability and disease progression in multiple sclerosis patients independently of oxidative and nitrosative stress. J. Neurol. Sci. 381, 213–219. doi: 10.1136/lupus-2017-000215.77
Pasquali, L., Pecori, C., Chico, L., Iudice, A., Siciliano, G., and Bonuccelli, U. (2015). Relation between plasmatic and cerebrospinal fluid oxidative stress biomarkers and intrathecal Ig synthesis in Multiple Sclerosis patients. J. Neuroimmunol. 283, 39–42. doi: 10.1016/j.jneuroim.2015.04.010
Polachini, C. R., Spanevello, R. M., Casali, E. A., Zanini, D., Pereira, L. B., Martins, C. C., et al. (2014). Alterations in the cholinesterase and adenosine deaminase activities and inflammation biomarker levels in patients with multiple sclerosis. Neuroscience. 266, 266–274. doi: 10.1016/j.neuroscience.2014.01.048
Qin, X. Y., Wu, H. T., Cao, C., Loh, Y. P., and Cheng, Y. (2017). A meta-analysis of peripheral blood nerve growth factor levels in patients with schizophrenia. Mol. Psychiatry. 22, 1306–1312. doi: 10.1038/mp.2016.235
Sanoobar, M., Eghtesadi, S., Azimi, A., Khalili, M., Jazayeri, S., and Reza Gohari, M. (2013). Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int. J. Neurosci. 123, 776–782. doi: 10.3109/00207454.2013.801844
Schrag, M., Mueller, C., Zabel, M., Crofton, A., Kirsch, W. M., Ghribi, O., et al. (2013). Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: a meta-analysis. Neurobiol. Dis. 59, 100–10. doi: 10.1016/j.nbd.2013.07.005
Schreibelt, G., van Horssen, J., van Rossum, S., Dijkstra, C. D., Drukarch, B., and de Vries, H. E. (2007). Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 56, 322–330. doi: 10.1016/j.brainresrev.2007.07.005
Socha, K., Karpinska, E., Kochanowicz, J., Soroczynska, J., Jakoniuk, M., Wilkiel, M., et al. (2017). Dietary habits; concentration of copper, zinc, and Cu-to-Zn ratio in serum and ability status of patients with relapsing-remitting multiple sclerosis. Nutrition 39–40, 76–81. doi: 10.1016/j.nut.2017.03.009
Socha, K., Kochanowicz, J., Karpinska, E., Soroczynska, J., Jakoniuk, M., Mariak, Z., et al. (2014). Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 13:62. doi: 10.1186/1475-2891-13-62
Tasset, I., Aguera, E., Sanchez-Lopez, F., Feijoo, M., Giraldo, A. I., Cruz, A. H., et al. (2012). Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin. Biochem. 45, 440–444. doi: 10.1016/j.clinbiochem.2012.01.023
Tavazzi, B., Batocchi, A. P., Amorini, A. M., Nociti, V., D'Urso, S., Longo, S., et al. (2011). Serum metabolic profile in multiple sclerosis patients. Mult. Scler. Int. 2011:167156. doi: 10.1155/2011/167156
Trentini, A., Castellazzi, M., Romani, A., Squerzanti, M., Baldi, E., Caniatti, M. L., et al. (2017). Evaluation of total, ceruloplasmin-associated and type II ferroxidase activities in serum and cerebrospinal fluid of multiple sclerosis patients. J. Neurol. Sci. 377, 133–136. doi: 10.1016/j.jns.2017.04.021
van Horssen, J., Witte, M. E., Schreibelt, G., and de Vries, H. E. (2011). Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta. 1812, 141–150. doi: 10.1016/j.bbadis.2010.06.011
Wang, K. C., Tsai, C. P., Lee, C. L., Chen, S. Y., Lin, G. J., Yen, M. H., et al. (2013). alpha-Lipoic acid enhances endogenous peroxisome-proliferator-activated receptor-gamma to ameliorate experimental autoimmune encephalomyelitis in mice. Clin. Sci. 125, 329–340. doi: 10.1042/CS20120560
Wang, P., Xie, K., Wang, C., and Bi, J. (2014). Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 72, 249–254. doi: 10.1159/000363515
Wang, Z., Bai, Z., Qin, X., and Cheng, Y. (2019). Aberrations in oxidative stress markers in amyotrophic lateral sclerosis: a systematic review and meta-analysis. Oxid. Med. Cell. Longev. 2019:1712323. doi: 10.1155/2019/1712323
Wei, Z., Li, X., Li, X., Liu, Q., and Cheng, Y. (2018). Oxidative stress in parkinson's disease: a systematic review and meta-analysis. Front. Mol. Neurosci. 11:236. doi: 10.3389/fnmol.2018.00236
Keywords: oxidative stress, multiple sclerosis, peripheral blood, cerebrospinal fluid, meta-analysis
Citation: Zhang S-Y, Gui L-N, Liu Y-Y, Shi S and Cheng Y (2020) Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 14:823. doi: 10.3389/fnins.2020.00823
Received: 11 January 2020; Accepted: 14 July 2020;
Published: 26 August 2020.
Edited by:
Mark P. Burns, Georgetown University, United StatesReviewed by:
Ghulam Md Ashraf, King Abdulaziz University, Saudi ArabiaCopyright © 2020 Zhang, Gui, Liu, Shi and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Cheng, eW9uZ2NoZW5nQG11Yy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.