- 1Department of Biochemistry, College of Medicine, Konyang University, Daejeon, South Korea
- 2Department of Occupational Therapy, Konyang University, Daejeon, South Korea
As the number of older adults increases, the prevalence of dementias, such as Alzheimer’s dementia (AD), vascular dementia, dementia with Lewy bodies, and frontotemporal dementias, also increases. Despite research into pharmacological approaches for treating diverse diseases, there is still no cure. Recently, novel non-pharmacological interventions are attracting attention. Non-pharmacological approaches include cognitive stimulation, alterations in diet, physical activity, and social engagement. Cognitive stimulating activities protect against the negative effects of cognitive decline caused by age-related neurogenerative diseases. Bilingualism is one form of cognitive stimulation that requires multiple aspects of brain activity and has been shown to delay the onset of dementia symptoms in patients by approximately 4–5 years as compared with monolingual patients through cognitive reserve. The purpose of this review was to bilingualism protects against cognitive decline associated with AD and other dementias. We discuss potential underlying neurological mechanisms, including: (1) stimulating adult neurogenesis, (2) enhancing synaptogenesis, (3) strengthening functional connectivity that bilingualism may delay clinical AD symptoms, (4) protecting white matter integrity, and (5) preserving gray matter density.
Introduction
As the elderly population grows, people around the world must overcome familial, economic, and social challenges in order to protect these individuals against age-related cognitive decline. Of particular importance is disease-related cognitive decline due to Alzheimer’s disease (AD) and other types of dementia that destroy the brain network and consciousness (Antoniou et al., 2013). Many preventative strategies and interventions exist to care for those with disease-related cognitive dysfunction (Livingston et al., 2017). However, numerous drugs have failed to lead to improvements, and there is currently no cure (Mangialasche et al., 2010; Anderson et al., 2017). Thus, alternative, non-pharmacological interventions that may protect cognitive function and delay neurodegeneration in healthy older people have been gaining more attention (Klimova et al., 2017).
Interestingly, older people who engage in brain-stimulating activities, such as reading books and playing board games, are less likely to experience memory loss associated with dementia than those who do not engage in these activities (Akbaraly et al., 2009). Cognitive stimulation strengthens the connections between neurons and promotes healthy cognitive aging (Valenzuela and Sachdev, 2006). Cognitive ability includes memory, pattern recognition, concept formation, attention, perception, action, problem solving, and language (Carpenter, 2013). Similarly, bilingualism evokes brain-stimulation because it requires more neural processing than monolingualism (Marian and Shook, 2012). Moreover, the brain functioning of bilingual people is higher than that of monolingual people, and they generally exhibit better performances across a variety of executive control tasks, including the attention network test (Costa et al., 2008), the Simon task (Bialystok et al., 2004), and the Stroop task (Coderre et al., 2013; Grant et al., 2014) than monolingual people. Additionally, studies have revealed that bilingualism is associated with cognitive advantages throughout the life span (Bialystok et al., 2006; Bialystok and Feng, 2009). Since language learning affects a wide range of brain networks, it may be a favorable solution to promote cognitive reserve. Surprisingly, several studies have demonstrated that the cognitive reserve of bilingual people is enhanced as compared with that of monolingual people, and the onset of AD symptoms in bilingual people are delayed as compared with the onset of AD symptoms in monolingual people (Bialystok et al., 2007; Mortimer, 2014; Perani and Abutalebi, 2015; Woumans et al., 2015; Klimova et al., 2017). Indeed, the increased levels of AD-biomarkers (Aβ and tau) in the cerebrospinal fluid of older adults was alleviated in both early and late bilinguals compared with those in monolinguals, and these effect were superior in early bilingual groups (Estanga et al., 2017).
Despite the accumulation of epidemiological evidence that supports the benefits of language experience on cognitive decline, the underlying neurological mechanisms of these benefits remain unknown. In this review, we suggest potential neurological mechanism by which bilingualism delays dementia along with evidence of clinical and structural changes.
Hypotheses of the Neurological Mechanisms Underlying Bilingualism
Adult Neurogenesis
Bilingualism increases the brain activity required to speak two languages (Marian and Shook, 2012; Grady et al., 2015). Sustained exposure to a complicated activity (such as bilingualism) maintains adult neurogenesis at a higher level and improves learning (Kramer et al., 2004). Experience-dependent brain activity provokes the formation of neural connections and structures in order to respond to the demands of managing multiple elements of numerous language systems, such as phonology, semantics, syntax, and grammar (Costa and Sebastian-Galles, 2014). In addition, bilingualism extends to memory tasks (Wodniecka et al., 2010).
There are two neurogenic regions of the adult brain: the subependymal zone of the lateral ventricles and the dentate gyrus (DG) of the hippocampus. The subventricular zone (SVZ) generates the largest number of migratory cells in the adult brain (Goings et al., 2004), and SVZ neuroblasts migrate to the olfactory bulbs (OB) in the adult. Recently, neural stem cells in the adult SVZ have been identified as a potential source of cells for brain restoration (Peterson, 2002). Adult hippocampal neurogenesis occurs throughout life in the subgranular zone of the DG, and evidence suggests that adult-born neurons play a role in brain stimulating activities, such as learning and memory (Kramer et al., 2004; Toda et al., 2019). Adult neurogenesis provokes sustained activity-dependent neural plasticity (Gu et al., 2013), and the relevance of cognitive reserve originates from the prominent role of the hippocampus in higher cognition, such as learning and memory (Kempermann, 2008). Older people who are bilingual perform better on cognitive tasks and have more cognitive reserve than age-matched monolingual people (Bialystok and Feng, 2009). Thus, we provide novel insight that the increasing brain activity through bilingualism may contribute to adult neurogenesis in the brain.
Preclinical studies have reported that granule layer neurons in the DG are produced following brain activity (Hastings and Gould, 1999). Interestingly, unlike other somatic stem cell types, adult neurogenesis is dynamically regulated by activity and experience (Laplagne et al., 2006; Song et al., 2016). Several growth factors are involved in adult neurogenesis, including nerve growth factor (NGF), glial-derived neurotrophic factor (GDNF), and vascular endothelial growth factor (VEGF) (Chen et al., 2005; Frielingsdorf et al., 2007). Cognitive activity may alter the levels of these factors and subsequently change the magnitude of the neurogenic response. VEGF plays a key role in promoting adult neurogenesis (Jin et al., 2002; Licht et al., 2011) by inducing the release of brain-derived neurotropic factor (BDNF) or by acting directly on neuronal precursors through a fetal liver kinase 1 (Flk-1)-dependent mechanism (Schanzer et al., 2004; Nowacka and Obuchowicz, 2013). Additionally, VEGF levels are increased through high intensity hippocampal-dependent cognitive activity (Oh et al., 2012), such as bilingualism. Therefore, bilingualism may contribute to the physiological changes associated with the neurogenesis induced by cognitive activity.
Diseases, such as stroke or ischemia, can induce adult neural progenitor proliferation and the migration of new neurons to sites distal from the injury (Herrera et al., 1999; Parent, 2003; Song et al., 2016). Bilingualism may contribute to cognitive reserve later in life by providing increased neurogenesis and neurons that can travel to relevant circuits. Therefore, the increased brain activity that is associated with bilingualism may be a safeguard against cognitive dysfunction and neuropathology.
Synaptic Integrity and Synaptogenesis
The brain responds to environmental stimuli, cognitive demand, and behavioral experience by functionally and physically changing in structure (Li et al., 2014). This phenomenon is known as neuroplasticity and has been investigated extensively in many areas. Individuals’ experiences with a second language causes changes in brain structure, and these experience-dependent neural changes can also be affected by the intensity and frequency of second language usage (Bialystok, 2007; Li et al., 2014).
Synaptogenesis, the formation of new synapses, is affected by the ability to speak two languages (Calvo et al., 2015), and a previous study demonstrated that the formation of new synapses underlies learning and memory (Geinisman et al., 2001). Learning has also been shown to result in increased axonal growth of granule neurons and synaptogenesis within the hippocampus (Rusakov et al., 1997; Ramirez-Amaya et al., 2001; Prickaerts et al., 2004). Collections of synapses make defined neural circuits that form networks to perform specific functions (Zhang and Ko, 2018), and synapses produced by experience-dependent activities strengthen neural circuitry (Holtmaat and Svoboda, 2009). Bilingualism recruits alternative brain networks to compensate for those that become damaged during aging and dementia (Marian and Shook, 2012), and the efficient utilization of brain networks to enhance brain function during aging increases cognitive reserve (Schroeder and Marian, 2012).
Experience-dependent alterations modulate BDNF and promote synaptic plasticity (Kang and Schuman, 1995; Figurov et al., 1996; Gold, 2015; Guzman-Velez and Tranel, 2015). GDNF promotes the survival of the dopaminergic frontostriatal circuitry and may be modulated by bilingual experiences (Gold, 2015). VEGF enhances hippocampal-dependent memory by strengthening the neural circuit and increasing neuroplasticity (Cao et al., 2004; Licht et al., 2011) and contributes to cognitive reserve by reducing neuronal loss (Han et al., 2017).
Hence, it could be hypothesized that the bilingual brain responds to multiple language experiences by strengthening synaptogenesis and inducing cognitive reserve. Therefore, we can conclude that language experience can induce structural synaptic plasticity through sufficient brain stimulation and that this is likely to protect against cognitive decline.
Functional Connectivity
In terms of neural connectivity, bilinguals demand the participation and cooperation of multiple brain areas that are responsible for language processing, including Broca’s area and Wernicke’s area (Hickok and Poeppel, 2007; Li et al., 2015). Moreover, studies have reported that the dorsal lateral prefrontal cortex, dorsal anterior cingulate cortex, and subcortical regions are responsible for language control (Crinion et al., 2006; Green and Abutalebi, 2013; Li et al., 2015). These areas do not function independently but interact with other brain areas involved in language processing (Abutalebi et al., 2009; Price, 2010). This functional connectivity can be confirmed by observing how the responses of two brain regions correlate with each other during neuroimaging procedures (Friston et al., 1993; Biswal et al., 1995). Recent neuroimaging studies revealed that increased functional connectivity is associated with bilingualism (Schlegel et al., 2012; Stein et al., 2012; Chai et al., 2016; Bubbico et al., 2019; Qi et al., 2019).
Schlegel et al. (2012) revealed that the connectivity of the organization of white matter (WM) was increased in English speaking students who were learning Chinese using diffusion tensor imaging (DTI) (Schlegel et al., 2012). Additionally, results from functional magnetic resonance imaging (MRI) of adult English speakers learning French for 12 weeks demonstrated that spontaneous reading and lexical retrieval of the secondary language (L2) was related to an intrinsic functional interaction within the language processing area (Chai et al., 2016). Furthermore, the global cognitive and functional connectivity in the brains of elderly Italian speakers were improved after they completed 4-month-long second language programs (Bubbico et al., 2019).
Changes in the functional connectivity in the impaired network were correlated with improvements in executive function (Kelly and Castellanos, 2014). Executive functions include many higher cognitive activities and regulate inhibitory control and switching processes (Stocco et al., 2012). Inhibitory control plays an important role in determining how to perform successful tasks in various work activities (Dowsett and Livesey, 2000). Bilingual people have advanced inhibitory control because they need to simultaneously regulate the activation of two languages (Bialystok et al., 2004; Stocco et al., 2012). This inhibitory control was reported to activate various brain regions, including the dorsolateral prefrontal cortex, medial prefrontal cortex, inferior frontal gyrus, and basal ganglia (Chambers et al., 2009; Christ et al., 2010). Language switching induced activation patterns in the brains of bilingual speakers (Abutalebi et al., 2008; Garbin et al., 2011; Guo et al., 2011). In addition, results from a quantitative meta-analysis revealed that bilingual language switching significantly activated multiple brain regions, including the midline pre-supplementary areas, left inferior frontal gyrus, left middle frontal gyrus, left middle temporal gyrus, right superior temporal gyrus, right precentral gyrus, and bilateral caudate nuclei (Luk et al., 2011b).
These results demonstrate that learning a foreign-language and bilingualism enhance brain functional connectivity. Increases in the functional connectivity between brain regions that are involved in language processing may result in enhanced executive functions. Furthermore, increased functional connectivity may allow for compensation of age- and neurodegenerative-related cognitive declines. Therefore, strengthening functional connectivity through learning a foreign-language or bilingualism may represent an underlying biological mechanism to delay the onset of AD and other types of dementia.
Structural Changes in the Brain Due to Bilingualism
White Matter Integrity
Alterations in neural connectivity are a major pathology of neurodegenerative diseases, especially AD (Palop et al., 2006). The restoration of neural circuits was recently proposed as a strategy for the treatment of AD (Canter et al., 2016). Loss of neural connections is related to widespread network disruption in AD (Daianu et al., 2013), and extensive network deficits cause structural damage to WM (Pievani et al., 2011).
Surprisingly, young bilingual speakers exhibit altered maturation and myelination of WM pathways (Mohades et al., 2015). A study evaluating major WM pathways of elementary school children using magnetic resonance DTI and fractional anisotropy revealed that the mean fractional anisotropy value of the left inferior occipitofrontal fasciculus pathway of bilingual children was significantly enlarged compared with that of monolingual children (Mohades et al., 2015). Using Tract-Based Spatial Statistics analysis, another study showed that the fractional anisotropy values of bilingual people were higher than those of monolingual people in certain WM tracts. Moreover, anatomical brain-imaging studies have demonstrated that adolescents who are bilingual or learning a second language exhibited an increase in WM integrity in the left perisylvian language network (Ferjan Ramirez et al., 2016). These results revealed that learning and using two languages after childhood may have dynamic effects on WM tracts, and this may contribute to maintaining WM integrity later in life (Pliatsikas et al., 2015). Generally, it has been reported that older adults exhibit a decline in WM integrity as result of the gradual process of demyelination (Antoniou et al., 2013). However, bilingual older adults and foreign language learners showed higher WM integrity in the corpus callosum (Luk et al., 2011a, b; Bubbico et al., 2019). In addition, older bilingual speakers show higher WM integrity and stronger anterior/posterior functional connectivity than older monolingual speakers (Luk et al., 2011a).
These results demonstrated that using a second language promotes the integration of global brain areas. Consequently, the slowing down of cognitive functions with age is attenuated in bilingual older adults (Flores and Bronicki, 2017; Bubbico et al., 2019). Additionally, bilingual older adults surpass age-matched monolinguals on executive functioning and attention tasks, such as the Frontal Assessment Battery test (Bubbico et al., 2019), Simon task (Bialystok et al., 2004), and Trail Making Test A-B (Bubbico et al., 2019). These cognitive advantages are associated with neurological correlates, such as maintained WM integrity (Luk et al., 2011a; Antoniou et al., 2013). Bilingualism delays AD symptoms by protecting WM tracks in the frontostriatal and frontoparietal executive control circuitry (Gold, 2015). Thus, superior WM integrity and executive control may act as delaying factors for AD onset through bilingualism-induced cognitive reserve.
Gray Matter Density
Language learning provides an intensive environmental input into the central nervous system that induces structural changes in the brain (Li et al., 2014) and enhances cognitive reserve. During aging, the volume of gray matter (GM) is reduced in the sensorimotor areas, heteromodal association areas, posterior hippocampus, thalamus, and middle cingulate gyrus. However, the volume of GM declines in the precuneus, parahippocampus, and anterior hippocampal regions during the progression of AD. Both aging and AD decrease GM density in the hippocampus and entorhinal cortex (Raji et al., 2009). Bilingualism increases GM density, improves functional connectivity, and preserves brain structure (Li et al., 2014). Several studies have shown that the density of GM in the anterior cingulate cortex (Abutalebi et al., 2012) and basal ganglia, including the left caudate (Zou et al., 2012) and left putamen (Abutalebi et al., 2013), is increased in bilingual people as compared with that in monolingual people (Perani and Abutalebi, 2015).
Investigations into the structural plasticity of GM in the left inferior parietal region using voxel-based morphometry have shown that GM density was directly proportional to the proficiency of using a second language and inversely proportional to the age at acquisition of a second language (Mechelli et al., 2004). The MRI results of English-German exchange students revealed that the GM in the left inferior frontal gyrus was increased after they studied Germany for 4 years (Stein et al., 2012). Additionally, adult English speakers who studied Chinese for 4 weeks exhibited increased activation in the left superior parietal lobule and left inferior frontal gyrus region (Qi et al., 2019). Another study investigated the effect of early language exposure on Heschl’s gyrus in bilingual and monolingual groups. They found that Heschl’s gyri were larger in bilingual people than those in monolingual people. They also reported that the GM volume of bilingual people was increased as compared with that of monolingual people (Ressel et al., 2012). Furthermore, a previous study indicated that using a second language increases the cortical thickness in related language areas, including the left middle frontal gyrus, inferior frontal gyrus, and superior temporal gyrus, and the volume of the left hippocampus (Martensson et al., 2012; Klein et al., 2014).
These data suggest that this bilingual-associated increase in GM density plays a role in neural reserve and prevention of cognitive decline in AD and aging.
Limitations and Possibility of Bilingualism for AD Prevention
Although various factors, such as the age and period of secondary language exposure, language proficiency, and usage frequency, are important when evaluating the effectiveness of bilingualism in bilingual individuals, these factors differ from study to study, and some studies do not provide any relevant findings regarding the influence of these factors on bilingualism. These variables make it difficult to integrate and standardize bilingual studies. In addition, social integration and adaptive behaviors may be involved depending on the bilingual learning environment (school, immigration, works, etc.) (Chertkow et al., 2010). Furthermore, the effects of bilingualism can be altered depending on the acquisition order of the mother language (L1) and L2 (Chertkow et al., 2010; Coderre et al., 2013), linguistic similarities between L1 and L2 (Serratrice et al., 2009; Zahodne et al., 2014), and education level of bilingual individuals (Lawton et al., 2015). Moreover, the application of bilingual learning to prevent AD in adulthood involves overcoming multiple hurdles, including motivation, cost, and low frequency of use. Above all, structural and functional changes that occur through learning and acquiring new languages differ between adulthood and childhood. Overall, these points limit the application of bilingualism for the treatment, prevention, or intervention of patients with AD, vascular dementia, dementia with Lewy bodies, and frontotemporal dementias.
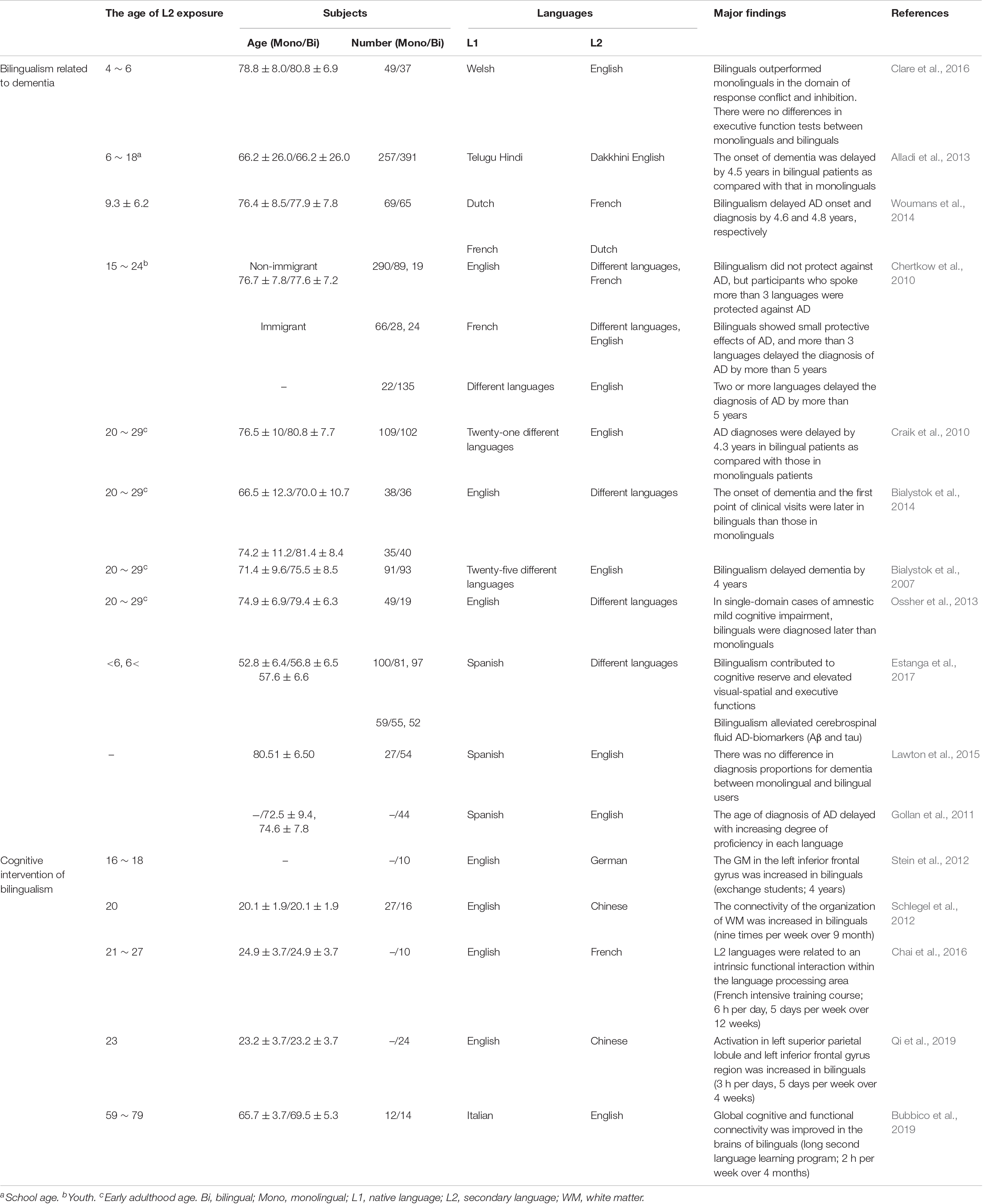
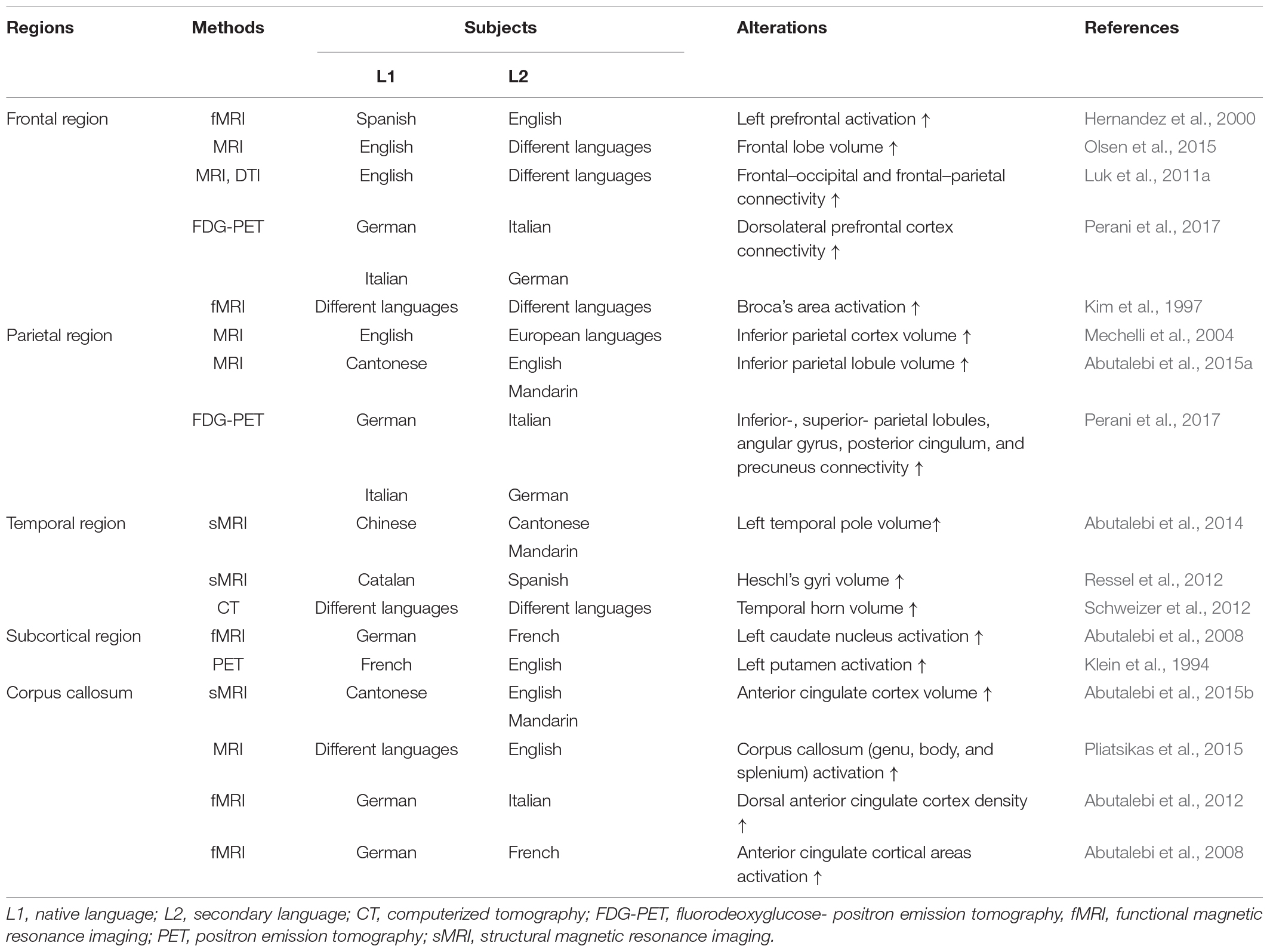
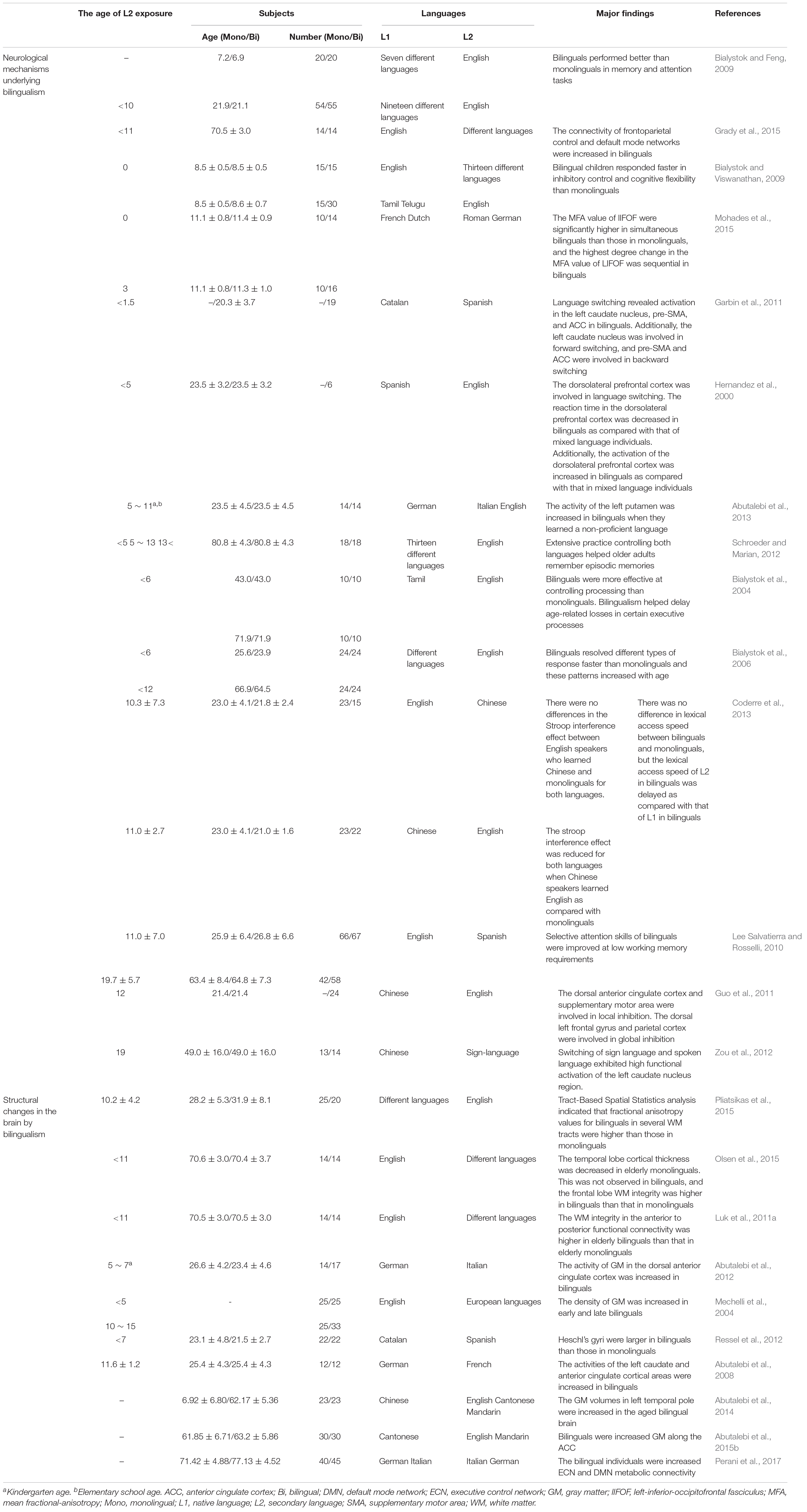
However, several studies reported that bilingualism delayed the onset of dementia and also revealed that the age at which a person was exposed to a second language was not limited to adulthood or childhood (Table 1). In addition, bilingualism can induce changes in brain plasticity and functional connectivity if the second language is learned and used throughout the lifetime or if it has only been used for 1–9 months (Schlegel et al., 2012; Chai et al., 2016; Qi et al., 2019). Furthermore, 4 months of learning a second language improved the functional connective and cognitive function in elderly people with normal cognitive function (Bubbico et al., 2019). Additionally, Briellmann et al. (2004) reported that the activation of the whole brain due to bilingualism was not correlated with language learning age, and there was no difference in brain activation between L1 and L2 use in multilingual individuals (Briellmann et al., 2004). Although the application of bilingual learning to interventional or therapeutic purposes for AD and MCI patients is limited, these findings suggest that bilingual learning can increase functional connectivity and cognitive reserve through neurological mechanisms that occur during short- or long-term secondary language exposure in childhood and adulthood (Table 1). In particular, brain alterations in bilinguals, evidenced by radiological images such as CT, MRI, and PET, support these findings (Table 2). Most importantly, studies that have revealed that bilingualism delayed dementia in both childhood and adulthood suggest that bilingual learning may prevent different types of dementia, such as dementia associated with AD.
Conclusion
In this review, we have outlined possible neurological mechanisms that underly the effects of bilingualism on cognitive function and decline. Furthermore, we have integrated studies of dementia delay in bilinguals and summarized evidences for brain alterations in bilingualism. Specifically, we suggested that (1) increased adult neurogenesis, (2) strengthened synaptogenesis, and (3) enhanced functional connectivity may underly the benefits of language experience on cognitive decline. In addition, this review provided evidence for bilingual-induced brain structure conservation, (4) including enhanced WM integrity, and (5) GM density, from age and neurodegenerative related alterations (Figure 1 and Table 3).
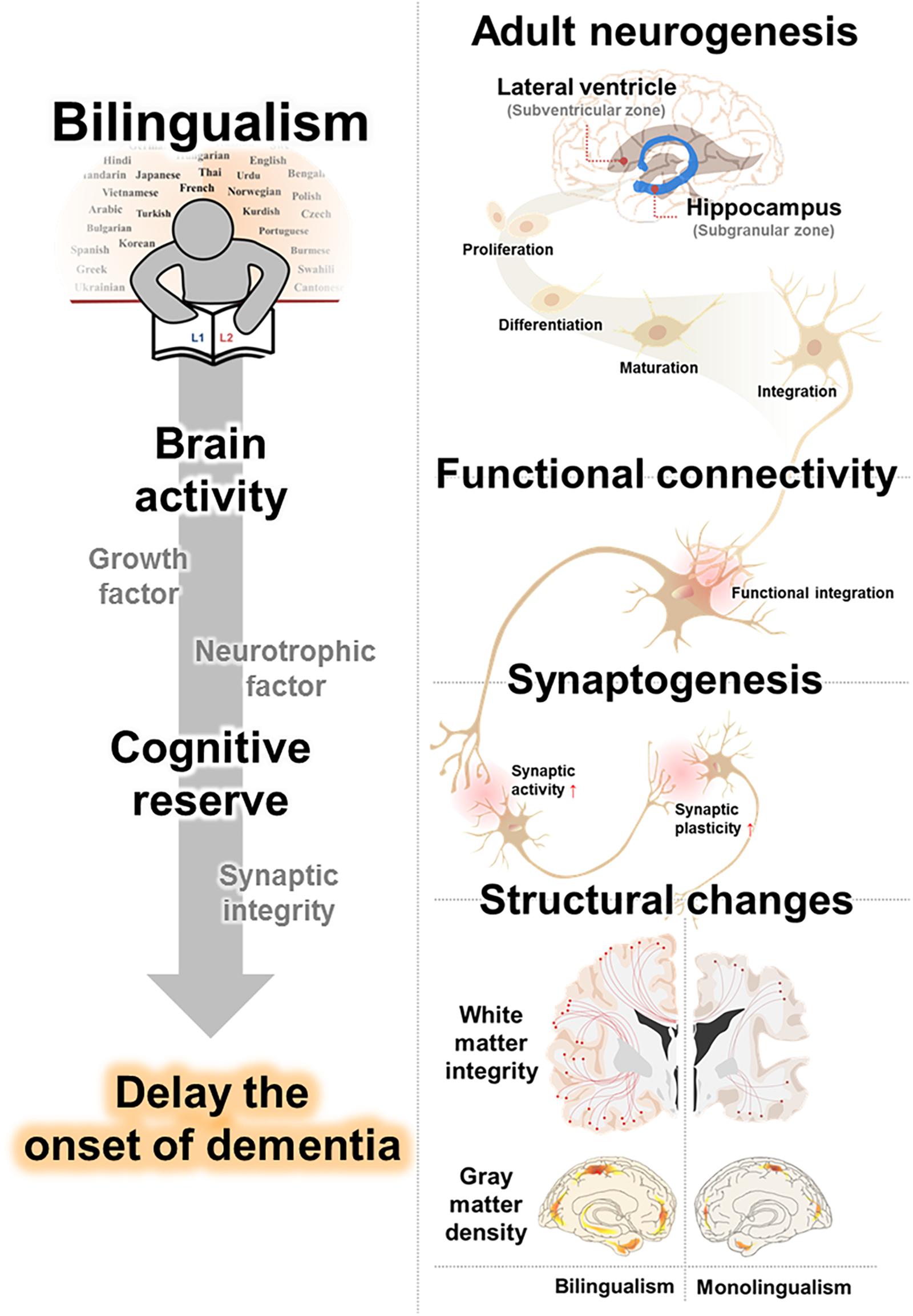
Figure 1. Proposed clausal mechanisms underlying bilingualism-induced delay of dementia. Cognitive reserve from the benefits of language experience on cognitive decline is caused by an increased adult neurogenesis, strengthened synaptogenesis, and enhanced functional connectivity. Bilinguals, accompanied with changes in brain structure, including white matter integrity and gray matter density, delay the onset of dementia.
Such a scientific inquiry would reveal if foreign language learning contributes to cognitive reserve and promotes healthy cognitive aging. However, bilingualism studies are difficult to standardize and change depending on variables, including like that the learning environment, order of acquisition of L1 and L2, and the linguistic similarities between L1 and L2. Nevertheless, bilingualism delays brain damage caused by AD and other dementias in both childhood and adulthood and indicated the potential for cognitive intervention (Table 1). In addition, the substantial brain structures and activation regions also altered in bilinguals (Table 3). Therefore, bilingualism may be considered as part of cognitive multiple interventions for patients with dementia. In conclusion, bilingualism may be a precautionary measure that can be used to has a potential role in delaying the onset and progression of neurodegenerative dementia, including dementia associated with AD.
Author Contributions
All authors had full access to all the data in the study, took responsibility for the integrity of the data and accuracy of the analysis, contributed to the manuscript revision, and read and approved the submitted version. MM and D-HY conceived the study and acquired the funding. SK, HK, and YN performed the methodology. HK, SJ, and YN investigated the study. SJ, D-HY, and YN provided the resources. SK and HK wrote the original draft of the manuscript. SK, SJ, and YN wrote, reviewed, and edited the manuscript. SK and SJ visualized the study. MM supervised the study.
Funding
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1D1A3B07041059 to MM and NRF-2019R1G1A1004010 to D-HY) and the Cooperative Research Program for Agriculture Science and Technology Development (Project Nos. PJ01319901 and PJ01428603), Rural Development Administration, South Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abutalebi, J., Annoni, J. M., Zimine, I., Pegna, A. J., Seghier, M. L., Lee-Jahnke, H., et al. (2008). Language control and lexical competition in bilinguals: an event-related FMRI study. Cereb. Cortex 18, 1496–1505. doi: 10.1093/cercor/bhm182
Abutalebi, J., Canini, M., Della Rosa, P. A., Green, D. W., and Weekes, B. S. (2015a). The neuroprotective effects of bilingualism upon the inferior parietal lobule: a structural neuroimaging study in aging Chinese bilinguals. J. Neurolinguistics 33, 3–13. doi: 10.1016/j.jneuroling.2014.09.008
Abutalebi, J., Canini, M., Della Rosa, P. A., Sheung, L. P., Green, D. W., and Weekes, B. S. (2014). Bilingualism protects anterior temporal lobe integrity in aging. Neurobiol. Aging 35, 2126–2133. doi: 10.1016/j.neurobiolaging.2014.03.010
Abutalebi, J., Della Rosa, P. A., Gonzaga, A. K., Keim, R., Costa, A., and Perani, D. (2013). The role of the left putamen in multilingual language production. Brain Lang. 125, 307–315. doi: 10.1016/j.bandl.2012.03.009
Abutalebi, J., Della Rosa, P. A., Green, D. W., Hernandez, M., Scifo, P., Keim, R., et al. (2012). Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb. Cortex 22, 2076–2086. doi: 10.1093/cercor/bhr287
Abutalebi, J., Guidi, L., Borsa, V., Canini, M., Della Rosa, P. A., Parris, B. A., et al. (2015b). Bilingualism provides a neural reserve for aging populations. Neuropsychologia 69, 201–210. doi: 10.1016/j.neuropsychologia.2015.01.040
Abutalebi, J., Rosa, P. A., Tettamanti, M., Green, D. W., and Cappa, S. F. (2009). Bilingual aphasia and language control: a follow-up fMRI and intrinsic connectivity study. Brain Lang. 109, 141–156. doi: 10.1016/j.bandl.2009.03.003
Akbaraly, T. N., Portet, F., Fustinoni, S., Dartigues, J. F., Artero, S., Rouaud, O., et al. (2009). Leisure activities and the risk of dementia in the elderly: results from the three-city study. Neurology 73, 854–861. doi: 10.1212/WNL.0b013e3181b7849b
Alladi, S., Bak, T. H., Duggirala, V., Surampudi, B., Shailaja, M., Shukla, A. K., et al. (2013). Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology 81, 1938–1944. doi: 10.1212/01.wnl.0000436620.33155.a4
Anderson, R. M., Hadjichrysanthou, C., Evans, S., and Wong, M. M. (2017). Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 390, 2327–2329. doi: 10.1016/s0140-6736(17)32399-1
Antoniou, M., Gunasekera, G. M., and Wong, P. C. (2013). Foreign language training as cognitive therapy for age-related cognitive decline: a hypothesis for future research. Neurosci. Biobehav. Rev. 37, 2689–2698. doi: 10.1016/j.neubiorev.2013.09.004
Bialystok, E. (2007). Cognitive effects of bilingualism: how linguistic experience leads to cognitive change. Int. J. Biling. Educ. Biling. 10, 210–223. doi: 10.2167/beb441.0
Bialystok, E., Craik, F. I., and Freedman, M. (2007). Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia 45, 459–464. doi: 10.1016/j.neuropsychologia.2006.10.009
Bialystok, E., Craik, F. I., Klein, R., and Viswanathan, M. (2004). Bilingualism, aging, and cognitive control: evidence from the Simon task. Psychol. Aging 19, 290–303. doi: 10.1037/0882-7974.19.2.290
Bialystok, E., Craik, F. I., and Ryan, J. (2006). Executive control in a modified antisaccade task: effects of aging and bilingualism. J. Exp. Psychol. Learn. Mem. Cogn. 32, 1341–1354. doi: 10.1037/0278-7393.32.6.1341
Bialystok, E., Craik, F. I. M., Binns, M. A., Ossher, L., and Freedman, M. (2014). Effects of bilingualism on the age of onset and progression of MCI and AD: evidence from executive function tests. Neuropsychology 28, 290–304. doi: 10.1037/neu0000023
Bialystok, E., and Feng, X. (2009). Language proficiency and executive control in proactive interference: evidence from monolingual and bilingual children and adults. Brain Lang. 109, 93–100. doi: 10.1016/j.bandl.2008.09.001
Bialystok, E., and Viswanathan, M. (2009). Components of executive control with advantages for bilingual children in two cultures. Cognition 112, 494–500. doi: 10.1016/j.cognition.2009.06.014
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Briellmann, R. S., Saling, M. M., Connell, A. B., Waites, A. B., Abbott, D. F., and Jackson, G. D. (2004). A high-field functional MRI study of quadri-lingual subjects. Brain Lang. 89, 531–542. doi: 10.1016/j.bandl.2004.01.008
Bubbico, G., Chiacchiaretta, P., Parenti, M., Di Marco, M., Panara, V., Sepede, G., et al. (2019). Effects of second language learning on the plastic aging brain: functional connectivity, cognitive decline, and reorganization. Front. Neurosci. 13:423. doi: 10.3389/fnins.2019.00423
Calvo, N., Garcia, A. M., Manoiloff, L., and Ibanez, A. (2015). Bilingualism and cognitive reserve: a critical overview and a plea for methodological innovations. Front. Aging Neurosci. 7:249. doi: 10.3389/fnagi.2015.00249
Canter, R. G., Penney, J., and Tsai, L. H. (2016). The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539, 187–196. doi: 10.1038/nature20412
Cao, L., Jiao, X., Zuzga, D. S., Liu, Y., Fong, D. M., Young, D., et al. (2004). VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36, 827–835. doi: 10.1038/ng1395
Carpenter, D. O. (2013). Effects of Persistent and Bioactive Organic Pollutants on Human Health. Hoboken, NJ: Wiley.
Chai, X. J., Berken, J. A., Barbeau, E. B., Soles, J., Callahan, M., Chen, J. K., et al. (2016). Intrinsic functional connectivity in the adult brain and success in second-language learning. J. Neurosci. 36, 755–761. doi: 10.1523/JNEUROSCI.2234-15.2016
Chambers, C. D., Garavan, H., and Bellgrove, M. A. (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 33, 631–646. doi: 10.1016/j.neubiorev.2008.08.016
Chen, Y., Ai, Y., Slevin, J. R., Maley, B. E., and Gash, D. M. (2005). Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp. Neurol. 196, 87–95. doi: 10.1016/j.expneurol.2005.07.010
Chertkow, H., Whitehead, V., Phillips, N., Wolfson, C., Atherton, J., and Bergman, H. (2010). Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: evidence from a bilingual community. Alzheimer Dis. Assoc. Disord. 24, 118–125. doi: 10.1097/WAD.0b013e3181ca1221
Christ, S. E., Huijbregts, S. C., De Sonneville, L. M., and White, D. A. (2010). Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol. Genet. Metab. 99(Suppl. 1), S22–S32. doi: 10.1016/j.ymgme.2009.10.007
Clare, L., Whitaker, C. J., Craik, F. I., Bialystok, E., Martyr, A., Martin-Forbes, P. A., et al. (2016). Bilingualism, executive control, and age at diagnosis among people with early-stage Alzheimer’s disease in Wales. J. Neuropsychol. 10, 163–185. doi: 10.1111/jnp.12061
Coderre, E. L., Wj, V. H., and Conklin, K. (2013). The timing and magnitude of Stroop interference and facilitation in monolinguals and bilinguals. Biling 16, 420–441. doi: 10.1017/s1366728912000405
Costa, A., Hernandez, M., and Sebastian-Galles, N. (2008). Bilingualism aids conflict resolution: evidence from the ANT task. Cognition 106, 59–86. doi: 10.1016/j.cognition.2006.12.013
Costa, A., and Sebastian-Galles, N. (2014). How does the bilingual experience sculpt the brain? Nat. Rev. Neurosci. 15, 336–345. doi: 10.1038/nrn3709
Craik, F. I., Bialystok, E., and Freedman, M. (2010). Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology 75, 1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c
Crinion, J., Turner, R., Grogan, A., Hanakawa, T., Noppeney, U., Devlin, J. T., et al. (2006). Language control in the bilingual brain. Science 312, 1537–1540. doi: 10.1126/science.1127761
Daianu, M., Jahanshad, N., Nir, T. M., Toga, A. W., Jack, CR Jr, Weiner, M. W., et al. (2013). Breakdown of brain connectivity between normal aging and Alzheimer’s disease: a structural k-core network analysis. Brain Connect. 3, 407–422. doi: 10.1089/brain.2012.0137
Dowsett, S. M., and Livesey, D. J. (2000). The development of inhibitory control in preschool children: effects of “executive skills” training. Dev. Psychobiol. 36, 161–174. doi: 10.1002/(sici)1098-2302(200003)36:2<161::aid-dev7>3.0.co;2-0
Estanga, A., Ecay-Torres, M., Ibañez, A., Izagirre, A., Villanua, J., Garcia-Sebastian, M., et al. (2017). Beneficial effect of bilingualism on Alzheimer’s disease CSF biomarkers and cognition. Neurobiol. Aging 50, 144–151. doi: 10.1016/j.neurobiolaging.2016.10.013
Ferjan Ramirez, N., Leonard, M. K., Davenport, T. S., Torres, C., Halgren, E., and Mayberry, R. I. (2016). Neural language processing in adolescent first-language learners: longitudinal case studies in American sign language. Cereb. Cortex 26, 1015–1026. doi: 10.1093/cercor/bhu273
Figurov, A., Pozzo-Miller, L. D., Olafsson, P., Wang, T., and Lu, B. (1996). Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709. doi: 10.1038/381706a0
Flores, S., and Bronicki, R. A. (2017). Fluid management after congenital cardiac surgery: the good, the bad, and the indifferent. Pediatr. Crit. Care Med. 18, 718–719. doi: 10.1097/pcc.0000000000001172
Frielingsdorf, H., Simpson, D. R., Thal, L. J., and Pizzo, D. P. (2007). Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol. Dis. 26, 47–55. doi: 10.1016/j.nbd.2006.11.015
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. doi: 10.1038/jcbfm.1993.4
Garbin, G., Costa, A., Sanjuan, A., Forn, C., Rodriguez-Pujadas, A., Ventura, N., et al. (2011). Neural bases of language switching in high and early proficient bilinguals. Brain Lang. 119, 129–135. doi: 10.1016/j.bandl.2011.03.011
Geinisman, Y., Berry, R. W., Disterhoft, J. F., Power, J. M., and Van Der Zee, E. A. (2001). Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 21, 5568–5573. doi: 10.1523/jneurosci.21-15-05568.2001
Goings, G. E., Sahni, V., and Szele, F. G. (2004). Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996, 213–226. doi: 10.1016/j.brainres.2003.10.034
Gold, B. T. (2015). Lifelong bilingualism and neural reserve against Alzheimer’s disease: a review of findings and potential mechanisms. Behav. Brain Res. 281, 9–15. doi: 10.1016/j.bbr.2014.12.006
Gollan, T. H., Salmon, D. P., Montoya, R. I., and Galasko, D. R. (2011). Degree of bilingualism predicts age of diagnosis of Alzheimer’s disease in low-education but not in highly educated Hispanics. Neuropsychologia 49, 3826–3830. doi: 10.1016/j.neuropsychologia.2011.09.041
Grady, C. L., Luk, G., Craik, F. I., and Bialystok, E. (2015). Brain network activity in monolingual and bilingual older adults. Neuropsychologia 66, 170–181. doi: 10.1016/j.neuropsychologia.2014.10.042
Grant, A., Dennis, N. A., and Li, P. (2014). Cognitive control, cognitive reserve, and memory in the aging bilingual brain. Front. Psychol. 5:1401. doi: 10.3389/fpsyg.2014.01401
Green, D. W., and Abutalebi, J. (2013). Language control in bilinguals: the adaptive control hypothesis. J. Cogn. Psychol. 25, 515–530. doi: 10.1080/20445911.2013.796377
Gu, Y., Janoschka, S., and Ge, S. (2013). Neurogenesis and hippocampal plasticity in adult brain. Curr. Top. Behav. Neurosci. 15, 31–48. doi: 10.1007/7854_2012_217
Guo, T., Liu, H., Misra, M., and Kroll, J. F. (2011). Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. Neuroimage 56, 2300–2309. doi: 10.1016/j.neuroimage.2011.03.049
Guzman-Velez, E., and Tranel, D. (2015). Does bilingualism contribute to cognitive reserve? Cognitive and neural perspectives. Neuropsychology 29, 139–150. doi: 10.1037/neu0000105
Han, W., Song, X., He, R., Li, T., Cheng, L., Xie, L., et al. (2017). VEGF regulates hippocampal neurogenesis and reverses cognitive deficits in immature rats after status epilepticus through the VEGF R2 signaling pathway. Epilepsy Behav. 68, 159–167. doi: 10.1016/j.yebeh.2016.12.007
Hastings, N. B., and Gould, E. (1999). Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 413, 146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b
Hernandez, A. E., Martinez, A., and Kohnert, K. (2000). In search of the language switch: an fMRI study of picture naming in Spanish-English bilinguals. Brain Lang. 73, 421–431. doi: 10.1006/brln.1999.2278
Herrera, D. G., Garcia-Verdugo, J. M., and Alvarez-Buylla, A. (1999). Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 46, 867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z
Hickok, G., and Poeppel, D. (2007). The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402. doi: 10.1038/nrn2113
Holtmaat, A., and Svoboda, K. (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658. doi: 10.1038/nrn2699
Jin, K., Zhu, Y., Sun, Y., Mao, X. O., Xie, L., and Greenberg, D. A. (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 99, 11946–11950. doi: 10.1073/pnas.182296499
Kang, H., and Schuman, E. M. (1995). Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662. doi: 10.1126/science.7886457
Kelly, C., and Castellanos, F. X. (2014). Strengthening connections: functional connectivity and brain plasticity. Neuropsychol. Rev. 24, 63–76. doi: 10.1007/s11065-014-9252-y
Kempermann, G. (2008). The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 31, 163–169. doi: 10.1016/j.tins.2008.01.002
Kim, K. H. S., Relkin, N. R., Lee, K.-M., and Hirsch, J. (1997). Distinct cortical areas associated with native and second languages. Nature 388, 171–174. doi: 10.1038/40623
Klein, D., Mok, K., Chen, J. K., and Watkins, K. E. (2014). Age of language learning shapes brain structure: a cortical thickness study of bilingual and monolingual individuals. Brain Lang. 131, 20–24. doi: 10.1016/j.bandl.2013.05.014
Klein, D., Zatorre, R., Milner, B., Meyer, E., and Evans, A. (1994). Left putaminal activation when speaking a second language: evidence from PET. Neuroreport 5, 2295–2297. doi: 10.1097/00001756-199411000-00022
Klimova, B., Valis, M., and Kuca, K. (2017). Bilingualism as a strategy to delay the onset of Alzheimer’s disease. Clin. Interv. Aging 12, 1731–1737. doi: 10.2147/CIA.S145397
Kramer, A. F., Bherer, L., Colcombe, S. J., Dong, W., and Greenough, W. T. (2004). Environmental influences on cognitive and brain plasticity during aging. J. Gerontol. A Biol. Sci. Med. Sci. 59, M940–M957.
Laplagne, D. A., Esposito, M. S., Piatti, V. C., Morgenstern, N. A., Zhao, C., Van Praag, H., et al. (2006). Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4:e409. doi: 10.1371/journal.pbio.0040409
Lawton, D. M., Gasquoine, P. G., and Weimer, A. A. (2015). Age of dementia diagnosis in community dwelling bilingual and monolingual Hispanic Americans. Cortex 66, 141–145. doi: 10.1016/j.cortex.2014.11.017
Lee Salvatierra, J., and Rosselli, M. (2010). The effect of bilingualism and age on inhibitory control. Int. J. Biling. 15, 26–37. doi: 10.1177/1367006910371021
Li, L., Abutalebi, J., Zou, L., Yan, X., Liu, L., Feng, X., et al. (2015). Bilingualism alters brain functional connectivity between “control” regions and “language” regions: evidence from bimodal bilinguals. Neuropsychologia 71, 236–247. doi: 10.1016/j.neuropsychologia.2015.04.007
Li, P., Legault, J., and Litcofsky, K. A. (2014). Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex 58, 301–324. doi: 10.1016/j.cortex.2014.05.001
Licht, T., Goshen, I., Avital, A., Kreisel, T., Zubedat, S., Eavri, R., et al. (2011). Reversible modulations of neuronal plasticity by VEGF. Proc. Natl. Acad. Sci. U.S.A. 108, 5081–5086. doi: 10.1073/pnas.1007640108
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734.
Luk, G., Bialystok, E., Craik, F. I., and Grady, C. L. (2011a). Lifelong bilingualism maintains white matter integrity in older adults. J. Neurosci. 31, 16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011
Luk, G., Green, D. W., Abutalebi, J., and Grady, C. (2011b). Cognitive control for language switching in bilinguals: a quantitative meta-analysis of functional neuroimaging studies. Lang. Cogn. Process. 27, 1479–1488. doi: 10.1080/01690965.2011.613209
Mangialasche, F., Solomon, A., Winblad, B., Mecocci, P., and Kivipelto, M. (2010). Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 9, 702–716.
Martensson, J., Eriksson, J., Bodammer, N. C., Lindgren, M., Johansson, M., Nyberg, L., et al. (2012). Growth of language-related brain areas after foreign language learning. Neuroimage 63, 240–244. doi: 10.1016/j.neuroimage.2012.06.043
Mechelli, A., Crinion, J. T., Noppeney, U., O’doherty, J., Ashburner, J., Frackowiak, R. S., et al. (2004). Neurolinguistics: structural plasticity in the bilingual brain. Nature 431:757.
Mohades, S. G., Van Schuerbeek, P., Rosseel, Y., Van De Craen, P., Luypaert, R., and Baeken, C. (2015). White-matter development is different in bilingual and monolingual children: a longitudinal DTI study. PLoS One 10:e0117968. doi: 10.1371/journal.pone.0117968
Mortimer, J. A. (2014). Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology 82:1936. doi: 10.1212/WNL.0000000000000400
Nowacka, M., and Obuchowicz, E. (2013). BDNF and VEGF in the pathogenesis of stress-induced affective diseases: an insight from experimental studies. Pharmacol. Rep. 65, 535–546. doi: 10.1016/s1734-1140(13)71031-4
Oh, D. H., Kim, B. W., Choi, M., Lee, G., Choi, J. S., and Hyeon, S. (2012). Changes in vascular endothelial growth factor (VEGF) induced by the Morris water maze task. Mol. Cells 33, 295–300. doi: 10.1007/s10059-012-2254-9
Olsen, R. K., Pangelinan, M. M., Bogulski, C., Chakravarty, M. M., Luk, G., Grady, C. L., et al. (2015). The effect of lifelong bilingualism on regional grey and white matter volume. Brain Res. 1612, 128–139. doi: 10.1016/j.brainres.2015.02.034
Ossher, L., Bialystok, E., Craik, F. I., Murphy, K. J., and Troyer, A. K. (2013). The effect of bilingualism on amnestic mild cognitive impairment. J. Gerontol. B Psychol. Sci. Soc. Sci. 68, 8–12. doi: 10.1093/geronb/gbs038
Palop, J. J., Chin, J., and Mucke, L. (2006). A network dysfunction perspective on neurodegenerative diseases. Nature 443, 768–773. doi: 10.1038/nature05289
Parent, J. M. (2003). Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist 9, 261–272. doi: 10.1177/1073858403252680
Perani, D., and Abutalebi, J. (2015). Bilingualism, dementia, cognitive and neural reserve. Curr. Opin. Neurol. 28, 618–625. doi: 10.1097/WCO.0000000000000267
Perani, D., Farsad, M., Ballarini, T., Lubian, F., Malpetti, M., Fracchetti, A., et al. (2017). The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer’s dementia. Proc. Natl. Acad. Sci. U. S. A. 114, 1690–1695. doi: 10.1073/pnas.1610909114
Peterson, D. A. (2002). Stem cells in brain plasticity and repair. Curr. Opin. Pharmacol. 2, 34–42. doi: 10.1016/s1471-4892(01)00118-7
Pievani, M., De Haan, W., Wu, T., Seeley, W. W., and Frisoni, G. B. (2011). Functional network disruption in the degenerative dementias. Lancet Neurol. 10, 829–843. doi: 10.1016/S1474-4422(11)70158-2
Pliatsikas, C., Moschopoulou, E., and Saddy, J. D. (2015). The effects of bilingualism on the white matter structure of the brain. Proc. Natl. Acad. Sci. U.S.A. 112, 1334–1337. doi: 10.1073/pnas.1414183112
Price, C. J. (2010). The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 1191, 62–88. doi: 10.1111/j.1749-6632.2010.05444.x
Prickaerts, J., Koopmans, G., Blokland, A., and Scheepens, A. (2004). Learning and adult neurogenesis: survival with or without proliferation? Neurobiol. Learn. Mem. 81, 1–11. doi: 10.1016/j.nlm.2003.09.001
Qi, Z., Han, M., Wang, Y., De Los Angeles, C., Liu, Q., Garel, K., et al. (2019). Speech processing and plasticity in the right hemisphere predict variation in adult foreign language learning. Neuroimage 192, 76–87. doi: 10.1016/j.neuroimage.2019.03.008
Raji, C. A., Lopez, O. L., Kuller, L. H., Carmichael, O. T., and Becker, J. T. (2009). Age, Alzheimer disease, and brain structure. Neurology 73, 1899–1905. doi: 10.1212/WNL.0b013e3181c3f293
Ramirez-Amaya, V., Balderas, I., Sandoval, J., Escobar, M. L., and Bermudez-Rattoni, F. (2001). Spatial long-term memory is related to mossy fiber synaptogenesis. J. Neurosci. 21, 7340–7348. doi: 10.1523/jneurosci.21-18-07340.2001
Ressel, V., Pallier, C., Ventura-Campos, N., Diaz, B., Roessler, A., Avila, C., et al. (2012). An effect of bilingualism on the auditory cortex. J. Neurosci. 32, 16597–16601. doi: 10.1523/JNEUROSCI.1996-12.2012
Rusakov, D. A., Davies, H. A., Harrison, E., Diana, G., Richter-Levin, G., Bliss, T. V., et al. (1997). Ultrastructural synaptic correlates of spatial learning in rat hippocampus. Neuroscience 80, 69–77. doi: 10.1016/s0306-4522(97)00125-5
Schanzer, A., Wachs, F. P., Wilhelm, D., Acker, T., Cooper-Kuhn, C., Beck, H., et al. (2004). Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 14, 237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x
Schlegel, A. A., Rudelson, J. J., and Tse, P. U. (2012). White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 24, 1664–1670. doi: 10.1162/jocn_a_00240
Schroeder, S. R., and Marian, V. (2012). A bilingual advantage for episodic memory in older adults. J. Cogn. Psychol. 24, 591–601. doi: 10.1080/20445911.2012.669367
Schweizer, T. A., Ware, J., Fischer, C. E., Craik, F. I. M., and Bialystok, E. (2012). Bilingualism as a contributor to cognitive reserve: evidence from brain atrophy in Alzheimer’s disease. Cortex 48, 991–996. doi: 10.1016/j.cortex.2011.04.009
Serratrice, L., Sorace, A., Filiaci, F., and Baldo, M. (2009). Bilingual children’s sensitivity to specificity and genericity: evidence from metalinguistic awareness. Biling. Lang. Cogn. 12, 239–257. doi: 10.1017/s1366728909004027
Song, J., Olsen, R. H., Sun, J., Ming, G. L., and Song, H. (2016). Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb. Perspect. Biol. 8:a018937. doi: 10.1101/cshperspect.a018937
Stein, M., Federspiel, A., Koenig, T., Wirth, M., Strik, W., Wiest, R., et al. (2012). Structural plasticity in the language system related to increased second language proficiency. Cortex 48, 458–465. doi: 10.1016/j.cortex.2010.10.007
Stocco, A., Yamasaki, B., Natalenko, R., and Prat, C. S. (2012). Bilingual brain training: a neurobiological framework of how bilingual experience improves executive function. Int. J. Biling. 18, 67–92. doi: 10.1177/1367006912456617
Toda, T., Parylak, S. L., Linker, S. B., and Gage, F. H. (2019). The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87. doi: 10.1038/s41380-018-0036-2
Valenzuela, M. J., and Sachdev, P. (2006). Brain reserve and dementia: a systematic review. Psychol. Med. 36, 441–454. doi: 10.1017/s0033291705006264
Wodniecka, Z., Craik, F. I. M., Luo, L., and Bialystok, E. (2010). Does bilingualism help memory? Competing effects of verbal ability and executive control. Int. J. Biling. Educ. Biling. 13, 575–595. doi: 10.1080/13670050.2010.488287
Woumans, E., Santens, P., Sieben, A., Versijpt, J., Stevens, M., and Duyck, W. (2015). Bilingualism delays clinical manifestation of Alzheimer’s disease. Biling. Lang. Cogn. 18, 568–574. doi: 10.1017/s136672891400087x
Woumans, E. V. Y., Santens, P., Sieben, A., Versijpt, J., Stevens, M., and Duyck, W. (2014). Bilingualism delays clinical manifestation of Alzheimer’s disease. Biling. Lang. Cogn. 18, 568–574. doi: 10.1017/s136672891400087x
Zahodne, L. B., Schofield, P. W., Farrell, M. T., Stern, Y., and Manly, J. J. (2014). Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology 28, 238–246. doi: 10.1037/neu0000014
Zhang, C., and Ko, J. (2018). Editorial: synaptic assembly and neural circuit development. Front. Synaptic Neurosci. 10:30. doi: 10.3389/fnsyn.2018.0003
Keywords: Alzheimer’s disease, dementia, bilingualism, brain connectivity, cognitive reserve
Citation: Kim S, Jeon SG, Nam Y, Kim Hs, Yoo D-H and Moon M (2019) Bilingualism for Dementia: Neurological Mechanisms Associated With Functional and Structural Changes in the Brain. Front. Neurosci. 13:1224. doi: 10.3389/fnins.2019.01224
Received: 24 July 2019; Accepted: 29 October 2019;
Published: 14 November 2019.
Edited by:
Xuping Li, Houston Methodist Research Institute, United StatesReviewed by:
Foteini Christidi, National and Kapodistrian University of Athens, GreeceAndrea Pilotto, University of Brescia, Italy
Copyright © 2019 Kim, Jeon, Nam, Kim, Yoo and Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doo-Han Yoo, Z2xvdmlhQGtvbnlhbmcuYWMua3I=; Minho Moon, aG9taW5tb29uQGtvbnlhbmcuYWMua3I=; aG9taW5tb29uQGRhdW0ubmV0
†These authors have contributed equally to this work
 Sujin Kim1†
Sujin Kim1† Hyeon soo Kim
Hyeon soo Kim Doo-Han Yoo
Doo-Han Yoo Minho Moon
Minho Moon