- 1Laboratory of Metabolic Medicine, Department of Molecular Pharmacology and Physiology, Morsani College of Medicine, University of South Florida, Tampa, FL, United States
- 2Departments of Pediatrics, Clinical Neurosciences, Physiology and Pharmacology, Alberta Children’s Hospital Research Institute, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 3Division of Pediatric Neurology, Rady Children’s Hospital-San Diego, University of California, San Diego, San Diego, CA, United States
- 4Institute for Human and Machine Cognition, Ocala, FL, United States
The ketogenic diet (KD) is a high-fat, low-carbohydrate treatment for medically intractable epilepsy. One of the hallmark features of the KD is the production of ketone bodies which have long been believed, but not yet proven, to exert direct anti-seizure effects. The prevailing view has been that ketosis is an epiphenomenon during KD treatment, mostly due to clinical observations that blood ketone levels do not correlate well with seizure control. Nevertheless, there is increasing experimental evidence that ketone bodies alone can exert anti-seizure properties through a multiplicity of mechanisms, including but not limited to: (1) activation of inhibitory adenosine and ATP-sensitive potassium channels; (2) enhancement of mitochondrial function and reduction in oxidative stress; (3) attenuation of excitatory neurotransmission; and (4) enhancement of central γ-aminobutyric acid (GABA) synthesis. Other novel actions more recently reported include inhibition of inflammasome assembly and activation of peripheral immune cells, and epigenetic effects by decreasing the activity of histone deacetylases (HDACs). Collectively, the preclinical evidence to date suggests that ketone administration alone might afford anti-seizure benefits for patients with epilepsy. There are, however, pragmatic challenges in administering ketone bodies in humans, but prior concerns may largely be mitigated through the use of ketone esters or balanced ketone electrolyte formulations that can be given orally and induce elevated and sustained hyperketonemia to achieve therapeutic effects.
Key points
- Cellular metabolism plays a key role in the modulation of neuronal excitability.
- The high-fat, low-carbohydrate ketogenic diet (KD) is a validated treatment for persons with epilepsy. and is also effective in preventing seizures in animal models.
- Beta-hydroxybutyrate (βHB) and acetoacetate (AcAc), the ketone bodies that increase during KD treatment, exert anti-seizure effects in animal models of epilepsy and neurometabolic disorders.
- Although human clinical trials are still needed, therapeutic ketosis with ketone esters represents a clinically viable formulation for the potential treatment of epilepsy and other seizure disorders.
Introduction
The traditional paradigm for discovery of new anti-seizure drugs (ASDs, also referred to as antiepileptic drugs or AEDs) has involved the assessment of agents blocking acutely provoked or kindled seizures, and which has led to the development of medications that largely influence cellular membrane-bound ion channels and transporters localized to synapses in the central nervous system (CNS) (Rogawski et al., 2016). More recently, however, it has become clear that metabolic factors – whether substrates or enzymes involved in cellular bioenergetics and metabolism – can profoundly influence neuronal excitability (Rogawski, 2016). Research linking brain metabolic changes to neuronal excitability has been driven by efforts to understand how the high-fat, low-carbohydrate ketogenic diet (KD) exerts its anti-seizure – and potential neuroprotective – effects in persons with epilepsy (Neal et al., 2008; Tanner et al., 2011; Rho and Stafstrom, 2012; Stafstrom and Rho, 2012; Gano et al., 2014; Rogawski et al., 2016).
While the efficacy of the KD in the clinical arena is clearly established (Freeman et al., 1998; Neal et al., 2008, 2009; Lambrechts et al., 2017), the mechanisms underlying its beneficial effects remain incompletely understood. Of the many hypotheses proposed (Rogawski et al., 2016), – a historical and unresolved question – is whether ketone bodies (i.e., β-hydroxybutyrate [βHB], acetoacetate [AcAc] and acetone [ACE]) are direct mediators or whether they are epiphenomena, instead indicative of a shift from glycolysis to fatty acid oxidation. Certainly, the current human clinical data do not yet strongly support the view that ketone bodies possess anti-seizure properties independent of their serving as fuel for ATP production, mostly because clinical and a few experimental studies have shown that blood ketone levels do not correlate directly with seizure control (Gilbert et al., 2000; Thavendiranathan et al., 2000; van Delft et al., 2010; Dallerac et al., 2017), despite increasing evidence in the preclinical literature (Kim et al., 2015; Simeone et al., 2018) and more recent clinical evidence to the contrary (Buchhalter et al., 2017). And all three major ketones (βHB, AcAc and ACE) have been shown to have anti-seizure effects in prior animal studies (Keith, 1931; Rho et al., 2002; Likhodii et al., 2003; Kim et al., 2015). For a comprehensive review of ketone bodies as anti-seizure agents, see Simeone et al. (2018). In this manuscript, we review the literature surrounding exogenous administration of ketogenic agents as a potential anti-seizure therapy. As the field is in its infancy, there is little published clinical data available; therefore, we place particular focus on the scientific rationale and pre-clinical evidence which support the translation of these therapies into currently ongoing and future human trials.
Ketone Metabolism
The pathways involved with ketone body synthesis and metabolism have been firmly established for decades. Fatty acids are converted to acetyl-CoA which then enters the tricarboxylic acid (TCA) cycle. Under conditions where fatty acid levels increase and exceed maximal TCA cycle function, such as during fasting or treatment with the KD, acetyl-CoA is diverted to ketogenesis. Two molecules of acetyl-CoA are used to form acetoacetyl-CoA via acetoacetyl-CoA thiolase. Acetoacetyl-CoA is then condensed with another molecule of acetyl-CoA to form 3-hydroxy-3-methylglutaryl CoA (HMGCoA) in a non-reversible step catalyzed by the rate-limiting enzyme HMG-CoA synthase 2 (HMG-CoAS2). The ketone body AcAc is then produced via the breakdown of HMG-CoA, which releases a molecule of acetyl-CoA. AcAc in turn can either be interconverted to βHB through the βHB-dehydrogenase enzyme or can be spontaneously decarboxylated to acetone and released primarily through the kidneys or lungs. Ketone bodies can then pass through the blood-brain-barrier through monocarboxylic acid transporters (MCTs) and enter the brain interstitial space. After being transported into mitochondria, ketone bodies can be converted back through several enzymatic steps to acetyl-CoA which enters the TCA cycle in neurons or glia to produce ATP. Alternatively, ketone bodies may exert other biological effects such as those described below.
Evidence for the Anti-Seizure Effects of Ketone Bodies
Not surprisingly, given the key hallmark feature of the KD is systemic ketosis, investigators focused on ketone bodies as possible mediators of anti-seizure effects. Indeed, ketone bodies were shown as early as the 1930’s to protect against acutely provoked seizures in rabbits (Keith, 1931, 1932, 1933, 1935), findings that were replicated and expanded decades later in multiple rodent models of seizures and epilepsy (Likhodii and Burnham, 2002; Rho et al., 2002; Likhodii et al., 2003; Minlebaev and Khazipov, 2011; Kim et al., 2015; Yum et al., 2015). Notably, in vivo anti-seizure effects were reported for either BHB, AcAc or ACE. However, the question of whether one or a combination of these ketone bodies affords even greater efficacy has not been answered. Taken together, these and other studies provide compelling evidence that ketone bodies can induce significant anti-seizure effects, and thus one cannot readily dismiss the possibility that these metabolic substrates contribute directly to the clinical effects of the KD.
In contrast to preclinical data referenced above, ketone bodies (when administered in vitro at low millimolar concentrations) were unable to affect either excitatory or inhibitory hippocampal synaptic transmission (Thio et al., 2000) and did not affect voltage-gated sodium channels (Yang et al., 2007) in normal hippocampus, unlike how current anti-seizure drugs are believed to generally work (Rogawski et al., 2016). Notwithstanding these observations, there are two aspects of ketone body action that overlap with synaptic function, but in distinct ways. First, ketone bodies (notably, AcAc) were shown to block neuronal excitability and seizures by inhibiting the presynaptic release of glutamate by modulating vesicular glutamate transporters or VGLUTs (Juge et al., 2010). Second, BHB was shown to alter the aspartate-to-glutamate ratio by driving the aspartate aminotransferase reaction (specifically, by decreasing the transamination of glutamate to aspartate) such that glutamate decarboxylation to GABA is increased (Erecinska et al., 1996; Daikhin and Yudkoff, 1998). The increase in GABA production would then be expected to enhance inhibitory neurotransmission and dampen seizure activity. Despite the rational neurochemical data, more direct evidence for this mechanism has not emerged (Yudkoff et al., 2001; Lund et al., 2009, 2011; Valente-Silva et al., 2015; Zhang et al., 2015), and this GABAergic hypothesis of ketone body action has not been reconciled with the fact that patients with epilepsy who were refractory to GABAergic medications often respond to the KD (Freeman et al., 2006).
The central challenge within the field of diet-based treatments for epilepsy has been to demonstrate clear causal links between cellular metabolism and plasmalemmal membrane excitability. A strong candidate molecular target was discussed nearly 20 years ago, i.e., ATP-sensitive potassium channels that, when activated by reduced ATP-to-ADP ratios, cause membrane hyperpolarization (Schwartzkroin, 1999). Using brain slices from normal and genetically engineered mice, Yellen and colleagues (Ma et al., 2007) showed that ketone bodies decreased the spontaneous firing of GABAergic interneurons in the substantia nigra pars reticulata (which is a known subcortical modulator of seizure propagation in the brain). Moreover, they demonstrated that this action required KATP channels and GABAB receptors (Ma et al., 2007). However, it remains unclear whether KATP channels can be directly activated by ketone bodies, as other investigators have shown that both the KD and ketone bodies increase cellular levels of ATP, which would inhibit KATP channel opening (DeVivo et al., 1978; Bough et al., 2006; Kim et al., 2010). One potential mechanism reconciling these discrepant observations was provided by Kawamura et al. (2010) a few years later. These investigators showed that under low-glucose conditions (observed during KD treatment), ATP efflux from pyramidal neurons in CA3 hippocampus leads to conversion of ATP to adenosine by ectonucleotidase enzymes and subsequent activation of inhibitory adenosine receptors (A1Rs) which are coupled to KATP channel activation (Kawamura et al., 2010).
In more recent years, other novel targets for ketone body action have been reported. Kim et al. (2015) reported that BHB blocks spontaneous recurrent seizures in the Kcna1-null mouse model of epilepsy, and does so by inhibiting mitochondrial permeability transition – a critical death switch for the cell (Izzo et al., 2016). Further, while other studies have revealed ever increasing complexity of ketone body action on biological targets, they involved non-epileptic and/or extra-CNS tissues. Among the most intriguing are the following: (1) systemic anti-inflammatory effects induced by BHB via inhibition of nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome assembly (Youm et al., 2015); (2) neuroprotective and anti-inflammatory effects of BHB through an interaction with the hydroxycarboxylic acid 2 (HCA2) receptor (Rahman et al., 2014); and (3) inhibition of histone deacetylases (HDACs) and anti-oxidant effects in renal tissue by BHB (Shimazu et al., 2013). All of these mechanisms, although incompletely understood in the context of epileptic brain, expand the biological profile of BHB and provide further evidence that a strategy based on ketone body administration or inducing prominent ketosis might yield significant and measurable anti-seizure effects in the clinical setting.
History and Pragmatic Challenges of Implementing Therapeutic Ketosis – Rationale for Ketone Esters
Administering the KD to implement therapeutic ketosis for seizure disorders is not without its challenges. The restrictive and precise macronutrient composition required to maintain and sustain nutritional ketosis can be difficult to implement. So while fundamental research may be spurred by the intrinsic curiosity and appeal of understanding how a dietary treatment can control epileptic seizures, a longstanding goal has been to determine whether a “KD in a pill” could be developed (Rho and Sankar, 2008). Indeed, investigators have sought ways to circumvent conventional means to administer the KD, one through more liberal and less restrictive diets such as the modified Atkins diet (Eric Kossoff et al., 2016) or the Low-Glycemic Index Therapy (LGIT) (Muzykewicz et al., 2009). Experimentally, other researchers have focused on ketone bodies and pragmatic formulations that could eventually be administered to humans to safely induce a dose-dependent and therapeutic hyperketonemia (Veech, 2004; D’Agostino et al., 2013; Hashim and VanItallie, 2014).
The idea of administering a ketogenic agent to induce and sustain therapeutic ketosis for parenteral and oral nutrition has been around for decades (Miller and Dymsza, 1967). Researchers in the 1950s at Massachusetts Institute of Technology, in collaboration with the Air Force Research Laboratory (AFRL), focused their efforts on high energy-dense compounds that had the greatest nutritional potential for long-duration manned spaceflight (Bornmann, 1954). Numerous agents were tested, but the ketogenic compound R,S-1,3-butanediol (BD; also known as R,S-1,3-butylene glycol) was selected as the most promising energy source, leading to further studies to determine its safety, stability, and potential as a food additive and preservative (Dymsza, 1975). Data were collected on rodents, dogs, pigs, and humans given this ketogenic compound, and although it induced hypoglycemia concomitant with ketonemia, it was deemed remarkably safe. R,S-1,3-butanediol met the criteria needed for the optimal synthetic “space food”, but the “unpleasant taste problem” and lack of FDA approval prevented its use for military or space flight applications.
Despite the palatability challenges, investigators remained intrigued with the potential applications of BD given its metabolic characteristics that mimicked fasting – mild hypoglycemia and safe and predictable hyperketonemia. When ingested orally, BD is metabolized by the liver via alcohol dehydrogenase (ADH) to β-hydroxybutyraldehyde, which is then rapidly oxidized to BHB by aldehyde dehydrogenase (Tate et al., 1971). BD contributes approximately 6 kcal/gm of energy and can produce dose-dependent millimolar concentrations of ketones in the blood at a ratio of 6:1 of BHB to AcAc (Tobin et al., 1972; Desrochers et al., 1992; D’Agostino et al., 2013). Published studies and a number of unpublished reports pertaining to the nutritional and metabolic effects of BD, including a human clinical study feeding study (young male and female subjects given 250 mg/kg body weight per day in bread for four separate 7-day periods), reported a blood glucose lowering effect as well (12% lower relative to controls) (Tobin et al., 1975). This was presumably due to a redox shift in the liver suppressing gluconeogenesis (Ciraolo and Previs, 1995). Although the mild hypoglycemic effect was a potential concern, extensive toxicology studies concluded that BD is safe with very few adverse health effects in animals and humans (Scala and Paynter, 1967; Dymsza, 1975; Hess et al., 1981). Consequently, it was given the status of being Generally Recognized As Safe (GRAS) in May 1997 by the FDA (Docket No. 87G-0351).
The early extensive safety and feasibility studies of BD, its FDA GRAS status, and high stability (i.e., shelf-life) inspired chemists and researchers to use BD as a backbone for synthesizing ketone esters (Brunengraber, 1997; Veech et al., 2001). Chemical synthesis by adding ketones (either BHB or ACAC) to this ketogenic diol through transesterification makes the resulting ketone esters the most energy-dense ketogenic supplements on a per gram basis. In addition to BD-derived ketone esters, there also exist glycerol-derived ketone esters of BHB. The diol BD and triol glycerol contain two or three hydroxyl groups, respectively, and through transesterification, these functional groups can pair with ketone bodies to make mono-esters, di-esters, or such as in the case of glycerol, a tri-ester compound known as glyceryl-tris-3-hydroxybutyrate (Hashim and VanItallie, 2014). Although deriving ketone esters utilizing glycerol as a backbone is feasible (Birkhahn and Border, 1978), the simultaneous elevation of glucose (glycerol is a gluconeogenic precursor) upon hydrolysis and subsequent increase in glycolysis can be unfavorable in the context of inducing anti-seizure effects. The advantage of BD as a backbone is that it delivers ketones upon esterase hydrolysis (in both gut and liver) and also metabolizes completely to BHB to further elevate and sustain ketosis in a predictable manner. Furthermore, dietary interventions that reduce glucose availability, Muzykewicz et al. (2009) and drugs targeting glycolysis such as 2-deoxyglucose (2-DG), Stafstrom et al. (2009) induce anti-seizure effects independent of ketone elevation, so mild hypoglycemia as a “side-effect” is theoretically advantageous for choosing ketogenic supplements that can be effective in controlling epileptic seizures.
The BD-derived ketone esters have been shown to induce a dose-dependent hyperketonemia (1–7 mM) in mice, rats, dogs, pigs, and humans (Desrochers et al., 1995; Clarke et al., 2012; Pascual et al., 2014; Newport et al., 2015). The emerging data indicate that these compounds produce no negative health effects when given acutely or chronically, aside from an aversive taste and the potential for dose-dependent gastrointestinal side effects. There are a growing number of promising metabolic alternatives to ketone esters that have improved or neutral taste and are considerably less expensive to produce. Emerging ketogenic supplements and formulas are being evaluated for their therapeutic efficacy (Borges and Sonnewald, 2012; Kesl et al., 2016) and their anti-seizure potential is discussed below (Table 1).
Evidence for the Efficacy of Ketogenic Agent-Based Therapies for Epilepsy
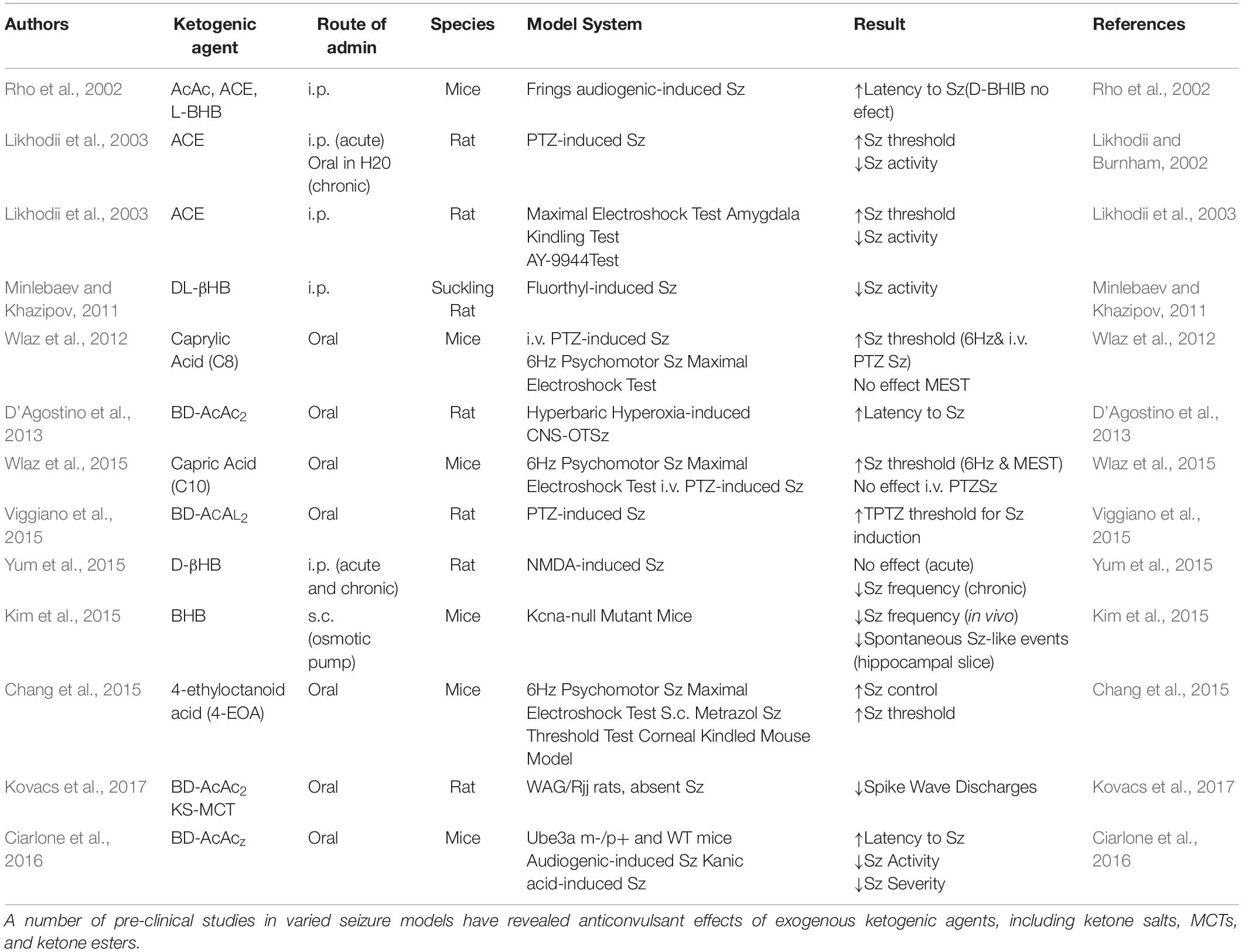
The science and clinical applications of therapeutic ketosis for neurological applications is growing rapidly, but work evaluating exogenous ketogenic agents remains largely in the pre-clinical space (Stafstrom and Rho, 2012). In addition to ketone esters, there are numerous alternative sources of ketones and ketogenic precursors being developed and shown to produce dose-dependent elevations in blood BHB and AcAc in animals, human case report and pilot studies (Puchowicz et al., 2000; Clarke et al., 2012; D’Agostino et al., 2013; Kesl et al., 2016). Ketone supplemental therapies allow for a calculated, rapid induction and maintenance of physiologic ketosis that mimics levels associated with KD treatment for epileptic seizures (Figure 1). In humans it is likely that 2–3 doses/day would be needed to maintain therapeutic hyperketonemia. Ketone supplementation also appears to fundamentally shift metabolic physiology and fuel utilization (Cox et al., 2016), so its potential for supporting physical endurance and military applications is emerging. Since metabolic shifts can affect so many cellular and molecular processes simultaneously, it is not surprising that there is a growing list of mechanisms that have been implicated for exogenous ketones, as previously discussed. However, efficacy may vary depending on the model and endpoints utilized, as well as the physicochemical and pharmacological properties of the individual ketogenic agents and formulations.
Figure 1. Exogenous ketogenic agents induce therapeutic ketosis within hours of oral ingestion. (A–C) Blood ketones following intragastric administration (time 0) of water, R,S-1,3-butanediol acetoacetate diester (BD-AcAc2), and 1,3-butanediol (BD) in Sprague Dawley rats. (A) β-hydroxybutyrate (βHB) level was elevated compared with control after either ketogenic compound (P < 0.001). (B) Acetoacetate (AcAc) level was increased significantly by BD-AcAc2 (P < 0.001) compared with water or BD. (C) Acetone level increased significantly more after treatment with BD-AcAc2 (P < 0.001). (D) Blood ketones (R-BHB only) following intragastric administration (time 0) of BD-AcAc2 in male human (n = 1). Figure adapted with permission from D’Agostino et al. (2013), American Physiological Society. ∗Significant difference from control and #significant difference from BD.
Ketone Esters
Currently, it appears that certain ketone esters hold great anti-seizure potential based upon previous work and on their pharmacokinetic profiles, although the current limitation is that research to date has largely been performed in pre-clinical animal models. When ketone esters are administered, gastric esterases liberate ketones (BHB or AcAc) as a free acid from a backbone molecule. This varies depending upon the specific formulation, but a ketogenic precursor such as BD would be ideal. As previously discussed, BD is subsequently metabolized by the liver to produce BHB (D’Agostino et al., 2013). Thus, the ketone esters currently available are unique in that they can directly elevate ketones and supply ketogenic precursors that can favorably change metabolic parameters like the glucose-ketone index (GKI) (Meidenbauer et al., 2015). Additionally, synthetically derived ketone esters are currently the most potent form of exogenous ketones available, but their potency also necessitates a thorough investigation of their long-term safety and toxicity which is currently lacking. When given at tolerable doses, the potential for side-effects like ketoacidosis are theoretically possible, so human studies are needed to assess at what dose and time interval exogenous ketones should be administered.
The first ketone esters appeared in the late 1970s. Birkhahn and colleagues synthesized a monoester of glycerol and AcAc (monoacetoacetin) for parenteral nutrition. These studies demonstrated that monoacetoacetin induced hyperketonemia comparable to fasted rats at a high dose of 50 g/kg per day (Birkhahn et al., 1977, 1979; Birkhahn and Border, 1978). In an attempt to increase the caloric density of monoacetoacetin, they synthesized both a monoester and triester of glycerol and BHB. Later, Desrochers and colleagues synthesized monoesters and diesters of AcAc with BD, and these had distinctly different pharmacokinetic profiles – i.e., they elevated both AcAc and BHB (Desrochers et al., 1992, 1995). Pigs given oral boluses of the ketone ester R,S-1,3-butanediol acetoacetate diester (BD-AcAc2), at 15% of the daily caloric requirement exhibited a peak total ketone level of 5 mM within 30 min, before slowly returning to baseline after several hours (Desrochers et al., 1995). No deleterious side-effects were observed through intragastric or high-dose IV administration, including an absence of pathological hypoglycemia and acidosis. These and other ketone esters have demonstrated an ability to induce a dose-dependent hyperketonemia (1–7 mM) in mice, rats, dogs, and humans (Figure 1; Ciraolo and Previs, 1995; Sylvain et al., 1995; Brunengraber, 1997; Puchowicz et al., 2000; Srivastava et al., 2012). In a 28-day study, a daily intragastric gavage (5 gm/kg body weight) of BD-AcAc2 induced significantly elevated blood ketone levels and significantly reduced blood glucose levels without significantly altering blood triglyceride or lipoprotein levels (Kesl et al., 2015). In a 15-week chronic feeding study, the BD-AcAc2 was administered to Sprague Dawley rats in low-dose (10 gm/kg/day) (LKE) and high-dose (25 g/kg/day) (HKE) ad libitum protocols. Serum clinical chemistry of both LKE and HKE did not reveal any alterations in markers of kidney and liver function compared to rats fed standard chow (Poff et al., 2016), suggesting that chronic high-dose feeding was without overt toxicity. Similarly, Clarke et al. (2012) demonstrated the safety of a BD-backed BHB monoester in rats and humans, and this has also been documented in a recent case study of Alzheimer’s disease, where the subject consumed a relatively high dose (20–30 g) thrice daily over 20 months (Srivastava et al., 2012; Newport et al., 2015).
The anti-seizure effects of ketone esters were first reported in a unique seizure model which uses hyperbaric hyperoxia (HBO) to reliably induce epileptic-like (i.e., tonic-clonic) seizures in normal rats, a condition known as CNS oxygen toxicity (CNS-OT). The rats in this study were eating standard rodent chow with abundant carbohydrate (>60%) before induced into hyperketonemia. A single oral dose of the ketone ester BD-AcAc2 induced rapid (within 30 min) and sustained (>4 h) ketosis (>3 mM BHB and >3 mM AcAc, 0.5 mM ACE) and prolonged the latency to seizures by 574% (Figure 1) (D’Agostino et al., 2013). Elevations in AcAc and ACE levels were necessary for producing the anti-seizure effects in this particular model of tonic-clonic seizures. BD alone (not in ester form) elevated blood BHB levels (>5 mM) but did not significantly alter AcAc or ACE levels, nor did it prolong the latency to seizure induction. This encouraging response prompted preliminary investigations into preventing or delaying seizures with ketogenic supplements in a variety of transgenic rodent and chemical-induced seizure models.
Pentylenetetrazol (PTZ) is a GABAA receptor antagonist and epileptogenic agent that is used to induce seizures in rodents for preclinical development of anti-seizure therapies. In a study by Coppola and colleagues, the dosage threshold for seizure induction by PTZ was assessed in control (water) and KE-treated rats (Viggiano et al., 2015). A single oral dose (4 gm/kg body weight) of BD-AcAc2 elevated blood BHB to 2.7 mM and increased the threshold of PTZ-evoked seizures from 122 ± 6 mg/kg to 140 ± 11 mg/kg. Although AcAc was not measured in this study, the KE was BD-AcAc2 which produces an approximate 1:1 ratio of BHB and AcAc in the blood. Thus, it can be assumed based on PK data (D’Agostino et al., 2013) that the concentration of AcAc was >2 mM.
More recently, the anti-seizure effect of ketone ester treatment has been evaluated in other Sz models. For example, it has been demonstrated that intragastric administration (gavage) of ketone supplements, such as KE, decreased the absence epileptic activity (spike-wave discharges: SWDs) in WAG/Rij rats (Kovacs et al., 2017). The increase in BHB may exert its therapeutic effects on neurological diseases via modulation of inflammatory systems (Newman and Verdin, 2014; Youm et al., 2015; Yamanashi et al., 2017), which are implicated in the pathophysiology of absence epilepsy (Kovacs et al., 2006, 2011; Tolmacheva et al., 2012; Russo et al., 2014). For example, BHB decreased the expression of NLRP3, ASC, caspase-1 and IL-1β (Bae et al., 2016), attenuated release of IL-1β in human monocytes (Youm et al., 2015) and mitigated stress-induced increase in TNF-α and IL-1β in the hippocampus (Yamanashi et al., 2017). Moreover, BHB attenuates the LPS-evoked increase in IL-1β and TNF-α level, as well as LPS-generated increase in COX-2, IL-1β, and TNF-α mRNA expression in BV-2 cells, likely via inhibition of NF-κB signaling (Fu et al., 2014). It was also demonstrated that BHB may decrease inflammatory processes (e.g., expression of COX and IL-1β) via its G-protein-coupled receptor 109A (GPR109A), which evoked inhibitory influence on NF-κB signaling pathway in microglial cells (Fu et al., 2015; Graff et al., 2016). Thus, ketosis suppresses inflammatory signaling (e.g., NLRP3/TLR4/IL-1R/NF-κB) signaling pathways and proinflammatory cytokines/enzymes (e.g., IL-1β and COX-2) that are linked pathophysiologically to epilepsy and other seizure disorders. Interestingly, BHB induced suppression of inflammation was independent of TCA cycle oxidation, and is thus independent of its function as an energy metabolite. Furthermore, the anti-inflammatory changes associated with BHB were not dependent on AMPK signaling, reactive oxygen species (ROS), glycolytic inhibition, UCP, or SIRT2 signaling, further validating its function as a signaling metabolite with potential anti-seizure function. Indeed, other research has shown that inhibition of the NLRP3 inflammasome mitigates the severity of numerous inflammatory diseases, including atherosclerosis, type 2 diabetes, Alzheimer’s disease, and gout, among others (Martinon et al., 2006; Duewell et al., 2010; Vandanmagsar et al., 2011; Heneka et al., 2013; Youm et al., 2013, 2015). It is also well established that proinflammatory mediators evoke epileptogenic and ictogenic properties following traumatic brain injury (Webster et al., 2017), and thus ketogenic supplementation like BD-AcAc2 that target these inflammatory pathways hold potential for treating post traumatic epilepsy, especially penetrating brain injuries where neuroinflammation is thought to trigger seizure occurrence.
In addition to classical seizure models, the seizure-prone Ube3a m-/p+ mouse model of Angelman Syndrome was studied by supplementing BD-AcAc2 in the food ad libitum for 8 weeks (Ciarlone et al., 2016). The KE therapy improved motor coordination, learning and memory, and synaptic plasticity and in AS mice, as well as suppressed Sz frequency and severity. The kainic acid-induced mouse seizure model was also studied. KE increased latency to Sz, decreased Sz activity, and decreased Sz severity. Interestingly, the KE altered brain amino acid metabolism in AS treated animals by increasing levels of glutamic acid decarboxylase (GAD) 65 and 67 (Ciarlone et al., 2016), thus shifting the neuropharmacology of the brain to favor a higher GABA/glutamate ratio. These pre-clinical findings suggested that KE supplementation produces sustained ketosis and ameliorates many symptoms of AS, including seizure activity. Pre-clinical animal studies with exogenous ketone supplementation therapy have inspired human clinical trials in patients with Angelman syndrome (ClinicalTrials.gov Identifier: NCT03644693) and a wide variety of other neurological and metabolic disorders (e.g., NCT03659825, NCT03531554, NCT03226197, NCT03011203, NCT03889210 NCT03878225).
Medium Chain Triglycerides
Ketogenic fatty acids such medium-chain triglycerides (MCTs) have been a therapy for intractable childhood epilepsy since the early 1970s (Huttenlocher et al., 1971). MCTs are rapidly absorbed, energy dense (8.3 calories/gram), water-miscible, tasteless, and have a much greater ketogenic potential than long chain fatty acids, Huttenlocher et al. (1971) making them an ideal alternative fat source for the KD. Commercial MCT oil is comprised primarily of caprylic acid (C8:0, octanoic acid) and capric acid (C10:0, decanoic acid), and these are absorbed directly into the bloodstream via the hepatic portal vein without the need for bile or pancreatic enzymes for degradation. MCT-induced ketosis (up to 1 mM βHB) occurs independent of carbohydrate or protein consumption, but is currently limited in clinical usage due to gastrointestinal (GI) side effects associated with the dose needed to produce therapeutic ketosis (approximately 40 g/day) (Huttenlocher, 1976). Similarly, the original MCT-based KD allowed 60% of its energy from MCTs, but the reported GI distress in some children (Huttenlocher, 1976; Trauner, 1985; Sills et al., 1986; Mak et al., 1999) lead to a modified MCT-based (30%) KD that induced lower levels of ketosis (Neal et al., 2009).
Several pre-clinical studies have demonstrated that specific MCTs (e.g., C10:0) may have anti-seizure properties through a mechanism of action independent of ketone metabolism and signaling (Chang et al., 2015; Augustin et al., 2018). Oral administration of 4-ethyloctanoic acid (4-EOA) increased Sz control and Sz threshold in several murine Sz models, including the 6 Hz psychomotor Sz model, the maximal electroshock test (MEST), the s.c. metrazol Sz threshold test, and the corneal kindled mouse model (Chang et al., 2015). Capric acid (C10 MCFA) increased Sz threshold in the 6 Hz psychomotor and MEST Sz models, but did not affect outcome in i.v. PTZ-induced Sz (Wlaz et al., 2015). And caprylic acid (C8 MCFA) increased Sz threshold in the 6 Hz psychomotor and i.v. PTZ-induced Sz models, but not in the MEST model (Wlaz et al., 2012).
The addition of MCTs to ketone esters or ketone salts may offer a novel way to improve or further augment their anti-seizure/neuroprotective potential (Ari et al., 2018). A combination of BHB salts and MCT oil has been administered in ratios of 1:1 to 1:2 mixtures. Formulating in this way allows for rapid and sustained elevation of ketosis by delivering exogenous ketones while simultaneously stimulating endogenous ketogenesis with MCTs. In addition, the combination formulation allows for a lower dosing of the components as compared to administering the individual supplements, thus reducing potential for side effects (gastric hyperosmolality) and resulting in a distinct blood ketone profile that is sustained over a longer period of time (D’Agostino et al., 2015). In a 28-day study in rats, the combination of MCT with a 50% Na+/K+ βHB salt mixture (in 1:1 solution) significantly elevated and sustained blood ketone levels and reduced blood glucose levels in a dose-dependent manner (Kesl et al., 2016). In a 15-week study, Sprague Dawley rats were administered a 1:1 mixture of Na+/Ca+2 ketone salt + MCT oil (20% by weight which resulted in approximately ∼25 g/kg/day) in their food fed ad libitum. The combination-supplemented rats had significantly sustained and elevated blood ketone levels at weeks 3, 4, 8, 10, and 13 (Kesl et al., 2014). Exogenous ketone supplements have typically been studied as a single stand-alone supplement, but the unique combination MCT added to ketone salts or ketone esters appears to have pharmacokinetic advantages and favorable behavior effects (Ari et al., 2016; Kesl et al., 2016). Formulating specific supplements will likely enhance the tolerability, absorption, peak and sustained levels of ketones in the blood, which may also translate to greater therapeutic potency and anti-seizure efficacy (Kovacs et al., 2017; Ari et al., 2018).
Ketone Salts
The recent commercialization of ketone salt supplements has fueled interest in these formulations for general health and wellness, but their clinical efficacy for seizure disorders remains largely unknown. Originally, researchers attempted to administer oral BHB or AcAc in their free acid forms; however, this was prohibitively expensive and ineffective. Subsequently, it was suggested to buffer the free acid of BHB with sodium, but it was feared that sodium overload would occur at therapeutic levels of ketosis. Furthermore, the existing data does not support elevating BHB alone will effectively prevent seizures in animal models (Bough and Rho, 2007). A study showed that oral administration D,LBHB (racemic BHB) treatment for multiple acyl-CoA dehydrogenase deficiency (MADD) was remarkably therapeutic for cerebral and cardiac complication in doses from 80 to 900 mg/kg/day (BHB levels 0.19-0.36 mM) in children with the disease (Hove et al., 2003). Similarly, a successful treatment of severe cardiomyopathy in a pediatric patient with glycogen storage disease type III with the KD and racemic ketone (D/L-BHB) sodium salts was achieved (Valayannopoulos et al., 2011). Although these results are compelling, these protocols would require ingesting between 5.6 and 6.3 g sodium/day for a 70 kg man. Considering the potential safety effects of such a large sodium load, the costs of the administration of Na+/βHB salts to achieve ketosis made this approach unrealistic (Veech, 2004). Since any physiological electrolyte (Na+, K+, Ca2+, Mg2+) readily ionically bonds with BHB, it was determined that a balanced ketone electrolyte formulation would be safer and more feasible for sustaining therapeutic ketosis. Over the last few years chemists have synthesized these balanced ketone electrolyte formulations and numerous studies have been published in animal models (Kephart et al., 2017) and humans (Stubbs et al., 2017, 2018a). A few pre-clinical studies have evaluated the anti-convulsant effects of ketone salts delivered exogenously by i.p. injection (Figure 1). D-BHB i.p. increased latency to Sz in the Frings audiogenic-induced Sz mouse model (Rho et al., 2002). I.p. D/L-BHB decreased Sz activity in suckling rats given flurothyl (Minlebaev and Khazipov, 2011), and chronic, but not acute, i.p. administration of D-BHB decreased Sz frequency in NMDA-induced rat Sz model (Yum et al., 2015). Similarly, s.c. delivery of BHB via osmotic pump decreased Sz frequency in vivo, and decreased spontaneous Sz-like events (SLE) in hippocampal slices, from Kcna1-null mutant mice (Kim et al., 2015). At the time of this writing, millions of doses of commercially available ketone salt products have been purchased and consumed, and no severe adverse reactions have been reported on the FDA website. Widespread use of these products, and better formulations for palatability and tolerability, may help to advance their clinical acceptance and implementation as a means to induce and sustain therapeutic ketosis. Regardless, significant clinical evaluation of the safety and efficacy of chronic ketone salt consumption for seizure disorders has yet to be published.
Limitations
There are a number of studies that highlight the limitations to ketone salt and ketone esters that are available commercially or for research applications. These limitations are primarily due to gastrointestinal symptoms associated with aversive taste or osmotic load in the GI tract (Leckey et al., 2017; Fischer et al., 2018). Future studies need to assure that the ketone supplement formulations are well tolerated and provide an ideal pharmacokinetic profile of sustained ketone elevation before such supplements are evaluated in humans (Stubbs et al., 2018b). Of relevance to this review, it is important to highlight that while pre-clinical studies have demonstrated that ketone supplements offer promising anti-convulsant effects in a variety of animal models, very little work to date has been published evaluating their potential anti-seizure efficacy or utility in humans.
Historically, issues of palatability and tolerability have limited the clinical investigation of exogenous ketone supplements. More recently, commercialization of ketone salt and MCT oil products have resulted in formulations that are pleasant to the taste and unlikely to elicit significant gastrointestinal distress unless overconsumed. For more potent formulations, such as ketone esters, it has proven more difficult to mask these flavoring issues and GI effects. In fact, in a recent study evaluating the potential utility of BD-AcAc2 as an ergogenic agent in cyclists, performance was impaired in the group receiving the ketone ester (Leckey et al., 2017). However, all of the study participants experienced notable gastrointestinal discomfort from consuming the supplement, confounding interpretation of the results (Stubbs et al., 2018b). Still, some ketone ester formulations have overcome these major obstacles, resulting in commercially viable products with a much improved taste and GI effect profile, such as the beta-hydroxybutyrate monoester (Stubbs et al., 2018a). Ongoing efforts to optimize other ketone esters such as BD-AcAc2 is promising, and likely to result in a similar commercialized product soon. Combining multiple ketogenic agents in a controlled-release formulation appears to be a promising direction (Meidenbauer et al., 2015).
Another major issue that will need to be addressed to move ketogenic agents into the clinic is establishing an understanding of their appropriate method of administration and dosing regimen. As described, different formulations of ketone supplements elicit markedly different pharmacokinetic profiles, with variable concentrations and durations of blood BHB and/or AcAc produced. Currently, a single dose of most commercially available ketone salt formulations can elevate blood BHB by approximately 1 mM for 1–3 h (O’Malley et al., 2017). If sustained ketosis is required for therapeutic effects, numerous daily doses of these agents would be needed, which may prove to be logistically difficult. Furthermore, as ketone salt formulations often contain large quantities of electrolytes, namely sodium, frequent dosing may present challenges in complying with the recommended maximum daily intake guidelines for these minerals. MCT oil alone can elevate blood ketones modestly (∼0.5 mM) (Courchesne-Loyer et al., 2015), but can also produce significant GI distress at large or frequent doses, especially in naïve patients. Pre-clinical work in rats suggest that adding MCTs to a ketone salt formulation may provide a method to improve the sustained elevation of ketosis with less side effects (Kesl et al., 2016), and therefore may provide a viable option for clinical use. Ketone esters appear to be able to elevate blood ketones to higher concentrations and for longer periods of time than any other currently available ketone formulation (Stubbs et al., 2017), but carry with them a greater need for safety testing and a higher risk of inducing hyperketonemia if overconsumed. In addition to these obstacles, the background diet of the patient would need to be considered, as it may affect the clinical profile of exogenous ketone therapy. If an individual is consuming a KD, exogenous ketone supplements may increase levels of ketosis overall, but also could potentially reduce rates of endogenous fatty acid oxidation and ketogenesis which may play a role in the diet’s therapeutic efficacy. Thus, these and other details regarding dosing protocols will need to be established for individual clinical applications. We expect that optimal protocols will depend on the type of ketone formulation being utilized and the specific condition being treated, similar to the use of any pharmaceutical agent. Efforts to optimize the composition and delivery of exogenous ketone supplements are ongoing – such as efforts to improve palatability, reduce GI side effects, and prolong sustained ketosis – and will likely improve tolerability and utility of these agents with time.
Conclusion
Considering the multifaceted therapeutic effects and success of the KD for seizure disorders, the goal of many ketone supplement researchers has often been described as creating “the KD in a pill.” As such, exogenous ketone supplements are being developed as an alternative or adjuvant method of inducing therapeutic ketosis without the need for a strict dietary regimen. Considering the promising results of the recent pre-clinical studies described here, along with advancements in optimizing ketone supplement formulations, it is possible that many of the seizure conditions which are known to benefit from the KD could receive some benefit from exogenous ketone supplementation by elevating blood ketones and lowering blood glucose. Importantly, if ketone supplements prove safe and efficacious in human trials, they may provide a tool for achieving ketosis in patients who are unable, unwilling, or uninterested in consuming a classic KD, modified Atkins diet, or LGIT. Ketone supplementation may also help circumvent some of the difficulties associated with dietary therapy, as it allows for a rapid dose-dependent induction of ketosis, which can be sustained with prolonged consumption and monitored precisely with commercially available technologies (e.g., blood ketone meters). Simultaneously, it could provide patients with the opportunity to reap the benefits of therapeutic ketosis without the practical and social difficulties of a highly restrictive diet. Moreover, these agents may represent a means to further enhance or optimize existing ketogenic therapies by supplying a form of non-glycemic calories that improves parameters (e.g., GKI) that are associated with therapeutic benefits.
Research on the potential applications of ketone supplementation is rapidly growing, and there are currently several registered clinical trials evaluating their safety and efficacy in a variety of conditions, including healthy adults, athletes, and patients with various diseases including Alzheimer’s, Parkinson’s, Type 2 Diabetes Mellitus, and more1. Encouragingly, clinical studies evaluating these agents in seizure disorders are beginning to emerge. An ongoing trial in Angelman syndrome – a genetic neurodevelopmental disorder characterized intellectual and developmental disability and seizures – is evaluating the use of a fat-based nutritional formulation containing exogenous ketones to support nutritional needs of this patient population (NCT03644693). As a secondary outcome measure, the investigators will also be tracking changes in EEG and seizure activity. Anecdotal reports of individuals consuming commercially available ketone supplements have suggested that some individuals experience a subjective improvement in seizure activity with their use, despite the fact that some of the more potent formulations, such as BD-AcAc2, are not yet commercially available. Regardless, it is important to highlight that there is a lack of published clinical work demonstrating efficacy of such agents in patients with seizure disorders, and the relationship between blood ketone elevation and the protective effects of ketosis on seizures is unclear. Thus, further research is needed to fully investigate the molecular mechanisms, clinical utility, and feasibility of exogenous ketone supplements as a method of inducing therapeutic ketosis for managing seizures.
Disclosure
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. JR has served as a paid consultant for Accera Pharma, Xenon Pharmaceuticals, Danone Nutricia, and Ajinomoto United States. DD’A and AP receive travel reimbursement and honoraria for speaking at scientific and clinical conferences. AP has served as a paid consultant for Pruvit Ventures, LLC.
Author Contributions
AP, JR, and DD’A contributed to the literature review, figure and table design, and writing of the manuscript. All authors read and approved the submitted version.
Funding
DD’A and AP research has been supported by the U.S. Office of Naval Research, Disruptive Nutrition, the Glucose Transporter Type-1 Deficiency (GLUT1D) Syndrome Foundation, and the Epigenix Foundation. JR research has been supported by the Canadian Institutes of Health Research, the Alberta Children’s Hospital Research Institute, and the Hotchkiss Brain Institute.
Conflict of Interest
International Patent # PCT/US2014/031237, University of South Florida, DD’A, S. Kesl, P. Arnold, “Compositions and Methods for Producing Elevated and Sustained Ketosis.” USF Ref. No: 16B128 (provisional patent); C. Ari, DD’A, J. B. Dean. Technology Title: “Delaying latency to seizure by combinations of ketone supplements.” DD’A is co-owner of the company Ketone Technologies, LLC, providing scientific consulting and public speaking engagements about ketogenic therapies. The company obtained an option agreement from the University of South Florida on the non-provisional patent No. 62/310,302 “Methods of increasing latency of anesthetic induction using ketone supplementation.” These interests have been reviewed and managed by the University in accordance with its Institutional and Individual Conflict of Interest policies. AP is a co-founder and owner of Metabolic Health Initiative, LLC, a company that provides educational content and seminars related to ketogenic and metabolic therapies. AP is also a co-owner of Poff Medical Consulting & Communications, LLC, a scientific consulting company. JR is a co-founder and shareholder of Path Therapeutics, Inc., based on Calgary, AB, Canada.
Footnotes
References
Ari, C., Koutnik, A. P., DeBlasi, J., Landon, C., Rogers, C. Q., Vallas, J., et al. (2018). Delaying latency to hyperbaric oxygen-induced CNS oxygen toxicity seizures by combinations of exogenous ketone supplements Physiol. Rep. 7:e13961. doi: 10.14814/phy2.13961
Ari, C., Kovács, Z., Juhasz, G., Murdun, C., Goldhagen, C., Koutnik, A., et al. (2016). Exogenous ketone supplements reduce anxiety-related behavior in sprague dawley and wistar albino Glaxo/Rijswijk rats. Front. Mol. Neurosci. 9:137. doi: 10.3389/fnmol.2016.00137
Augustin, K., Khabbush, A., Williams, S., Eaton, S., Orford, M., Cross, J. H., et al. (2018). Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 17, 84–93. doi: 10.1016/s1474-4422(17)30408-8
Bae, H. R., Kim, D. H., Park, M. H., Lee, B., Kim, M. J., Lee, E. K., et al. (2016). beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7, 66444–66454.
Birkhahn, R. H., and Border, J. R. (1978). Intravenous feeding of the rat with short chain fatty acid esters. II. Monoacetoacetin. Am. J. Clin. Nutr. 31, 436–441. doi: 10.1093/ajcn/31.3.436
Birkhahn, R. H., McMenamy, R. H., and Border, J. R. (1977). Intravenous feeding of the rat with short chain fatty acid esters. I. Glycerol monobutyrate. Am. J. Clin. Nutr. 30, 2078–2082. doi: 10.1093/ajcn/30.12.2078
Birkhahn, R. H., McMenamy, R. H., and Border, J. R. (1979). Monoglyceryl acetoacetate: a ketone body-carbohydrate substrate for parenteral feeding of the rat. J. Nutr. 109, 1168–1174. doi: 10.1093/jn/109.7.1168
Borges, K., and Sonnewald, U. (2012). Triheptanoin–a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment? Epilepsy Res. 100, 239–244. doi: 10.1016/j.eplepsyres.2011.05.023
Bornmann, G. (1954). Basic effects of the glycols and their toxicological significance. II. Arzneimittelforschung 4, 710–715.
Bough, K. J., and Rho, J. M. (2007). Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48, 43–58.
Bough, K. J., Wetherington, J., Hassel, B., Pare, J. F., Gawryluk, J. W., Greene, J. G., et al. (2006). Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 60, 223–235. doi: 10.1002/ana.20899
Brunengraber, H. (1997). Potential of ketone body esters for parenteral and oral nutrition. Nutrition 13, 233–235. doi: 10.1016/s0899-9007(96)00409-1
Buchhalter, J. R., D’Alfonso, S., Connolly, M., Fung, E., Michoulas, A., Sinasac, D., et al. (2017). The relationship between d-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open 2, 317–321. doi: 10.1002/epi4.12058
Chang, P., Zuckermann, A. M. E., Williams, S., Close, A. J., Cano-Jaimez, M., McEvoy, J. P., et al. (2015). Seizure control by derivatives of medium chain fatty acids associated with the ketogenic diet show novel branching-point structure for enhanced potency. J. Pharmacol. Exp. Ther. 352, 43–52. doi: 10.1124/jpet.114.218768
Ciarlone, S. L., Grieco, J. C., D’Agostino, D. P., and Weeber, E. J. (2016). Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol. Dis. 96, 38–46. doi: 10.1016/j.nbd.2016.08.002
Ciraolo, S., and Previs, S. (1995). Model of extreme hypoglycemia in dogs made ketotic with (R, S)-1, 3-butanediol acetoacetate esters. Am. J. Physiol. 269(1 Pt 1), E67–E75.
Clarke, K., Tchabanenko, K., Pawlosky, R., Carter, E., Todd King, M., Musa-Veloso, K., et al. (2012). Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 63, 401–408. doi: 10.1016/j.yrtph.2012.04.008
Courchesne-Loyer, A., St-Pierre, V., Hennebelle, M., Castellano, C. A., Fortier, M., Tessier, D., et al. (2015). Ketogenic response to cotreatment with bezafibrate and medium chain triacylglycerols in healthy humans. Nutrition 31, 1255–1259. doi: 10.1016/j.nut.2015.05.015
Cox, P. J., Kirk, T., Ashmore, T., Willerton, K., Evans, R., Smith, A., et al. (2016). Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 24, 256–268. doi: 10.1016/j.cmet.2016.07.010
D’Agostino, D., Arnold, P., and Kesl, S. (2015). Compositions and Methods for Producing Elevated and Sustained Ketosis. International Patent WO2014153416A1.
D’Agostino, D. P., Pilla, R., Held, H. E., Landon, C. S., Puchowicz, M., Brunengraber, H., et al. (2013). Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. 304, R829–R836.
Daikhin, Y., and Yudkoff, M. (1998). Ketone bodies and brain glutamate and GABA metabolism. Dev. Neurosci. 20, 358–364. doi: 10.1159/000017331
Dallerac, G., Moulard, J., Benoist, J. F., Rouach, S., Auvin, S., Guilbot, A., et al. (2017). Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci. Rep. 7:5496.
Desrochers, S., David, F., Garneau, M., Jette, M., and Brunengraber, H. (1992). Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem. J. 285(Pt 2), 647–653. doi: 10.1042/bj2850647
Desrochers, S., Dubreuil, P., Brunet, J., Jette, M., David, F., Landau, B. R., et al. (1995). Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am. J. Physiol. 268, E660–E667.
DeVivo, D. C., Leckie, M. P., Ferrendelli, J. S., and McDougal, D. B. Jr. (1978). Chronic ketosis and cerebral metabolism. Ann. Neurol. 3, 331–337. doi: 10.1002/ana.410030410
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361. doi: 10.1038/nature08938
Dymsza, H. A. (1975). Nutritional application and implication of 1,3-butanediol. Fed. Proc. 34, 2167–2170.
Erecinska, M., Nelson, D., Daikhin, Y., and Yudkoff, M. (1996). Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 67, 2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x
Eric Kossoff, H., Doerrer, S., Cervenka, M., and Henry, B. (2016). The Ketogenic and Modified Atkins Diets: Treatments for Epilepsy and Other Disorders. Cham: Springer Publishing Company.
Fischer, T., Och, U., Klawon, I., Och, T., Gruneberg, M., Fobker, M., et al. (2018). Effect of a Sodium and Calcium DL-beta-Hydroxybutyrate salt in healthy adults. J. Nutr. Metab. 2018:9812806.
Freeman, J., Veggiotti, P., Lanzi, G., Tagliabue, A., and Perucca, E., and Institute of Neurology IRCCS C. Mondino Foundation, (2006). The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 68, 145–180. doi: 10.1016/j.eplepsyres.2005.10.003
Freeman, J. M., Vining, E. P., Pillas, D. J., Pyzik, P. L., Casey, J. C., and Kelly, L. M. (1998). The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics 102, 1358–1363. doi: 10.1542/peds.102.6.1358
Fu, S. P., Li, S. N., Wang, J. F., Li, Y., Xie, S. S., Xue, W. J., et al. (2014). BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediators Inflamm. 2014:983401.
Fu, S. P., Wang, J. F., Xue, W. J., Liu, H. M., Liu, B. R., Zeng, Y. L., et al. (2015). Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation 12:9. doi: 10.1186/s12974-014-0230-3
Gano, L. B., Patel, M., and Rho, J. M. (2014). Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 55, 2211–2228. doi: 10.1194/jlr.r048975
Gilbert, D. L., Pyzik, P. L., and Freeman, J. M. (2000). The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J. Child Neurol. 15, 787–790. doi: 10.1177/088307380001501203
Graff, E. C., Fang, H., Wanders, D., and Judd, R. L. (2016). Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism 65, 102–113. doi: 10.1016/j.metabol.2015.10.001
Hashim, S. A., and VanItallie, T. B. (2014). Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J. Lipid Res. 55, 1818–1826. doi: 10.1194/jlr.r046599
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. doi: 10.1038/nature11729
Hess, F. G. Jr., Cox, G. E., Bailey, D. E., Parent, R. A., and Becci, P. J. (1981). Reproduction and teratology study of 1,3-butanediol in rats. J. Appl. Toxicol. 1, 202–209. doi: 10.1002/jat.2550010404
Hove, J. L. K., Grünewald, S., Jaeken, J., and Demaerel, P. (2003). D, L-3-hydroxybutyrate treatment of multiple acyl-CoA dehydrogenase deficiency (MADD). Lancet 361, 1433–1435. doi: 10.1016/s0140-6736(03)13105-4
Huttenlocher, P. R. (1976). Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 10, 536–540. doi: 10.1203/00006450-197605000-00006
Huttenlocher, P. R., Wilbourn, A. J., and Signore, J. M. (1971). Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 21, 1097–1103.
Izzo, V., Bravo-San Pedro, J. M., Sica, V., Kroemer, G., and Galluzzi, L. (2016). Mitochondrial permeability transition: new findings and persisting uncertainties. Trends Cell Biol. 26, 655–667. doi: 10.1016/j.tcb.2016.04.006
Juge, N., Gray, J. A., Omote, H., Miyaji, T., Inoue, T., Hara, C., et al. (2010). Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112. doi: 10.1016/j.neuron.2010.09.002
Kawamura, M. Jr., Ruskin, D. N., and Masino, S. A. (2010). Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 30, 3886–3895. doi: 10.1523/jneurosci.0055-10.2010
Keith, H. (1931). The effect of various factors on experimentally produced convulsions. Am. J. Dis. Child 41, 532–543.
Keith, H. (1932). Further studies of the control of experimentally produced convulsions. Pharmacol. Exp. Ther. 44, 449–455.
Keith, H. (1933). Factors influencing experimentally produced convulsions. Arch. Neurol. Psychiatry 29, 148–154.
Keith, H. (1935). Experimental convulsions induced by administration of thujone. Arch. Neurol. Psychiatry 34, 1022–1040.
Kephart, W. C., Mumford, P. W., Mao, X., Romero, M. A., Hyatt, H. W., Zhang, Y., et al. (2017). The 1-Week and 8-Month effects of a ketogenic diet or ketone salt supplementation on multi-organ markers of oxidative stress and mitochondrial function in rats. Nutrients 9:1019. doi: 10.3390/nu9091019
Kesl, S., Poff, A., Ward, N., Fiorelli, T., Ari, C., and D’Agostino, D. (2014). Methods of Sustaining Dietary Ketosis in Sprague-Dawley Rats. San Diego, CA: Federation of the American Societies for Experimental Biology.
Kesl, S. L., Poff, A., Ward, N., Fiorelli, T., Ari, C., Van Putten, A., et al. (2015). Effects of oral ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in sprague-drawley rats. Nutr. Metab. 13:9.
Kesl, S. L., Poff, A. M., Ward, N. P., Fiorelli, T. N., Ari, C., Van Putten, A. J., et al. (2016). Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr. Metab. 13:9.
Kim, D. Y., Simeone, K. A., Simeone, T. A., Pandya, J. D., Wilke, J. C., Ahn, Y., et al. (2015). Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 78, 77–87. doi: 10.1002/ana.24424
Kim, D. Y., Vallejo, J., and Rho, J. M. (2010). Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 114, 130–141.
Kovacs, Z., Czurko, A., Kekesi, K. A., and Juhasz, G. (2011). Intracerebroventricularly administered lipopolysaccharide enhances spike-wave discharges in freely moving WAG/Rij rats. Brain Res. Bull. 85, 410–416. doi: 10.1016/j.brainresbull.2011.05.003
Kovacs, Z., D’Agostino, D. P., Dobolyi, A., and Ari, C. (2017). Adenosine A1 receptor antagonism abolished the anti-seizure effects of exogenous ketone supplementation in wistar albino glaxo rijswijk rats. Front. Mol. Neurosci. 10:235. doi: 10.3389/fnmol.2017.00235
Kovacs, Z., Kekesi, K. A., Szilagyi, N., Abraham, I., Szekacs, D., Kiraly, N., et al. (2006). Facilitation of spike-wave discharge activity by lipopolysaccharides in Wistar Albino Glaxo/Rijswijk rats. Neuroscience 140, 731–742. doi: 10.1016/j.neuroscience.2006.02.023
Lambrechts, D., de Kinderen, R. J. A., Vles, J. S. H., de Louw, A. J., Aldenkamp, A. P., and Majoie, H. J. M. (2017). A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol. Scand. 135, 231–239. doi: 10.1111/ane.12592
Leckey, J. J., Ross, M. L., Quod, M., Hawley, J. A., and Burke, L. M. (2017). Ketone diester ingestion impairs time-trial performance in professional cyclists. Front. Physiol. 8:806. doi: 10.3389/fphys.2017.00806
Likhodii, S. S., and Burnham, W. M. (2002). Ketogenic diet: does acetone stop seizures? Med. Sci. Monit. 8, HY19–HY24.
Likhodii, S. S., Serbanescu, I., Cortez, M. A., Murphy, P., Snead, O. C.III, and Burnham, W. M. (2003). Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 54, 219–226. doi: 10.1002/ana.10634
Lund, T. M., Obel, L. F., Risa, O., and Sonnewald, U. (2011). beta-Hydroxybutyrate is the preferred substrate for GABA and glutamate synthesis while glucose is indispensable during depolarization in cultured GABAergic neurons. Neurochem. Int. 59, 309–318. doi: 10.1016/j.neuint.2011.06.002
Lund, T. M., Risa, O., Sonnewald, U., Schousboe, A., and Waagepetersen, H. S. (2009). Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured neurons. J. Neurochem. 110, 80–91. doi: 10.1111/j.1471-4159.2009.06115.x
Ma, W., Berg, J., and Yellen, G. (2007). Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J. Neurosci. 27, 3618–3625. doi: 10.1523/jneurosci.0132-07.2007
Mak, S. C., Chi, C. S., and Wan, C. J. (1999). Clinical experience of ketogenic diet on children with refractory epilepsy. Acta Paediatr. Taiwan 40, 97–100.
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A., and Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. doi: 10.1038/nature04516
Meidenbauer, J. J., Mukherjee, P., and Seyfried, T. N. (2015). The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr. Metab. 12:12. doi: 10.1186/s12986-015-0009-2
Miller, S. A., and Dymsza, H. A. (1967). Utilization by the rat of 1,3-butanediol as a synthetic source of dietary energy. J. Nutr. 91, 79–88. doi: 10.1093/jn/91.1.79
Minlebaev, M., and Khazipov, R. (2011). Antiepileptic effects of endogenous beta-hydroxybutyrate in suckling infant rats. Epilepsy Res. 95, 100–109. doi: 10.1016/j.eplepsyres.2011.03.003
Muzykewicz, D. A., Lyczkowski, D. A., Memon, N., Conant, K. D., Pfeifer, H. H., and Thiele, E. A. (2009). Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia 50, 1118–1126. doi: 10.1111/j.1528-1167.2008.01959.x
Neal, E. G., Chaffe, H., Schwartz, R. H., Lawson, M. S., Edwards, N., Fitzsimmons, G., et al. (2008). The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 7, 500–506. doi: 10.1016/s1474-4422(08)70092-9
Neal, E. G., Chaffe, H., Schwartz, R. H., Lawson, M. S., Edwards, N., Fitzsimmons, G., et al. (2009). A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 50, 1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x
Newman, J. C., and Verdin, E. (2014). Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 25, 42–52. doi: 10.1016/j.tem.2013.09.002
Newport, M. T., VanItallie, T. B., Kashiwaya, Y., King, M. T., and Veech, R. L. (2015). A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 11, 99–103. doi: 10.1016/j.jalz.2014.01.006
O’Malley, T., Myette-Cote, E., Durrer, C., and Little, J. P. (2017). Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl. Physiol. Nutr. Metab. 42, 1031–1035. doi: 10.1139/apnm-2016-0641
Pascual, J. M., Liu, P., Mao, D., Kelly, D. I., Hernandez, A., Sheng, M., et al. (2014). Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement. JAMA Neurol. 71, 1255–1265.
Poff, A., Kesl, S., Ward, N., and D’Agostino, D. (2016). “Metabolic effects of exogenous ketone supplementation – an alternative or adjuvant to the ketogenic diet as a cancer therapy?,” in Keystone Symposia - New Frontiers in Tumor Metabolism, Banff, AB.
Puchowicz, M. A., Smith, C. L., Bomont, C., Koshy, J., David, F., and Brunengraber, H. (2000). Dog model of therapeutic ketosis induced by oral administration of R,S-1,3-butanediol diacetoacetate. J. Nutr. Biochem. 11, 281–287. doi: 10.1016/s0955-2863(00)00079-6
Rahman, M., Muhammad, S., Khan, M. A., Chen, H., Ridder, D. A., Muller-Fielitz, H., et al. (2014). The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 5:3944.
Rho, J. M., Anderson, G. D., Donevan, S. D., and White, H. S. (2002). Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia 43, 358–361. doi: 10.1046/j.1528-1157.2002.47901.x
Rho, J. M., and Sankar, R. (2008). The ketogenic diet in a pill: is this possible? Epilepsia 49(Suppl. 8), 127–133. doi: 10.1111/j.1528-1167.2008.01857.x
Rho, J. M., and Stafstrom, C. E. (2012). The ketogenic diet: what has the science taught us? Epilepsy Res. 100, 210–217. doi: 10.1016/j.eplepsyres.2011.05.021
Rogawski, M. A. (2016). A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks AMPA receptors. Brain 139, 306–309. doi: 10.1093/brain/awv369
Rogawski, M. A., Loscher, W., and Rho, J. M. (2016). Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb. Perspect. Med. 6:a022780. doi: 10.1101/cshperspect.a022780
Russo, E., Andreozzi, F., Iuliano, R., Dattilo, V., Procopio, T., Fiume, G., et al. (2014). Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav. Immun. 42, 157–168. doi: 10.1016/j.bbi.2014.06.016
Scala, R. A., and Paynter, O. E. (1967). Chronic oral toxicity of 1,3-butanediol. Toxicol. Appl. Pharmacol. 10, 160–164. doi: 10.1016/0041-008x(67)90137-8
Schwartzkroin, P. A. (1999). Mechanisms underlying the anti-epileptic efficacy of the ketogenic diet. Epilepsy Res. 37, 171–180. doi: 10.1016/s0920-1211(99)00069-8
Shimazu, T., Hirschey, M. D., Newman, J., He, W., Shirakawa, K., Le Moan, N., et al. (2013). Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214. doi: 10.1126/science.1227166
Sills, M. A., Forsythe, W. I., Haidukewych, D., MacDonald, A., and Robinson, M. (1986). The medium chain triglyceride diet and intractable epilepsy. Arch. Dis. Child. 61, 1168–1172. doi: 10.1136/adc.61.12.1168
Simeone, T. A., Simeone, K. A., Stafstrom, C. E., and Rho, J. M. (2018). Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133, 233–241. doi: 10.1016/j.neuropharm.2018.01.011
Srivastava, S., Kashiwaya, Y., King, M., Baxa, U., Tam, J., Niu, G., et al. (2012). Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26, 2351–2362. doi: 10.1096/fj.11-200410
Stafstrom, C. E., Ockuly, J. C., Murphree, L., Valley, M. T., Roopra, A., and Sutula, T. P. (2009). Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann. Neurol. 65, 435–447. doi: 10.1002/ana.21603
Stafstrom, C. E., and Rho, J. M. (2012). The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 3:59. doi: 10.3389/fphar.2012.00059
Stubbs, B. J., Cox, P. J., Evans, R. D., Cyranka, M., Clarke, K., and de Wet, H. (2018a). A ketone ester drink lowers human ghrelin and appetite. Obesity 26, 269–273. doi: 10.1002/oby.22051
Stubbs, B. J., Cox, P. J., Evans, R. D., Santer, P., Miller, J. J., Faull, O. K., et al. (2017). On the metabolism of exogenous ketones in humans. Front. Physiol. 8:848. doi: 10.3389/fphys.2017.00848
Stubbs, B. J., Koutnik, A. P., Poff, A. M., Ford, K. M., and D’Agostino, D. P. (2018b). Commentary: ketone diester ingestion impairs time-trial performance in professional cyclists. Front. Physiol. 9:279. doi: 10.3389/fphys.2018.00279
Sylvain, D., Khadijah, Q., Hermann, D., Pascal, D., Catherine, B., France, D., et al. (1995). R,S-1,3-butanediol acetoacetate esters, potential alternates to lipid emulsions for total parenteral nutrition. J. Nutr. Biochem. 6, 111–118. doi: 10.1016/0955-2863(94)00011-a
Tanner, G. R., Lutas, A., Martinez-Francois, J. R., and Yellen, G. (2011). Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. J. Neurosci. 31, 8689–8696. doi: 10.1523/jneurosci.5951-10.2011
Tate, R. L., Mehlman, M. A., and Tobin, R. B. (1971). Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J. Nutr. 101, 1719–1726. doi: 10.1093/jn/101.12.1719
Thavendiranathan, P., Mendonca, A., Dell, C., Likhodii, S. S., Musa, K., Iracleous, C., et al. (2000). The MCT ketogenic diet: effects on animal seizure models. Exp. Neurol. 161, 696–703. doi: 10.1006/exnr.1999.7298
Thio, L. L., Wong, M., and Yamada, K. A. (2000). Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology 54, 325–331.
Tobin, R. B., Mehlman, M. A., Kies, C., Fox, H. M., and Soeldner, J. S. (1975). Nutritional and metabolic studies in humans with 1,3-butanediol. Fed. Proc. 34, 2171–2176.
Tobin, R. B., Mehlman, M. A., and Parker, M. (1972). Effect of 1,3-butanediol and propionic acid on blood ketones, lipids and metal ions in rats. J. Nutr. 102, 1001–1008. doi: 10.1093/jn/102.8.1001
Tolmacheva, E. A., Oitzl, M. S., and van Luijtelaar, G. (2012). Stress, glucocorticoids and absences in a genetic epilepsy model. Horm. Behav. 61, 706–710. doi: 10.1016/j.yhbeh.2012.03.004
Trauner, D. A. (1985). Medium-chain triglyceride (MCT) diet in intractable seizure disorders. Neurology 35, 237–238.
Valayannopoulos, V., Bajolle, F., Arnoux, J. B., Dubois, S., Sannier, N., Baussan, C., et al. (2011). Successful treatment of severe cardiomyopathy in glycogen storage disease type III With D,L-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatr. Res. 70, 638–641. doi: 10.1203/pdr.0b013e318232154f
Valente-Silva, P., Lemos, C., Kofalvi, A., Cunha, R. A., and Jones, J. G. (2015). Ketone bodies effectively compete with glucose for neuronal acetyl-CoA generation in rat hippocampal slices. NMR Biomed. 28, 1111–1116. doi: 10.1002/nbm.3355
van Delft, R., Lambrechts, D., Verschuure, P., Hulsman, J., and Majoie, M. (2010). Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure 19, 36–39. doi: 10.1016/j.seizure.2009.10.009
Vandanmagsar, B., Youm, Y. H., Ravussin, A., Galgani, J. E., Stadler, K., Mynatt, R. L., et al. (2011). The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188. doi: 10.1038/nm.2279
Veech, R. L. (2004). The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 70, 309–319. doi: 10.1016/j.plefa.2003.09.007
Veech, R. L., Chance, B., Kashiwaya, Y., Lardy, H. A., and Cahill, G. F. Jr. (2001). Ketone bodies, potential therapeutic uses. IUBMB Life 51, 241–247. doi: 10.1080/152165401753311780
Viggiano, A., Pilla, R., Arnold, P., Monda, M., D’Agostino, D., and Coppola, G. (2015). Anticonvulsant properties of an oral ketone ester in a pentylenetetrazole-model of seizure. Brain Res. 1618, 50–54. doi: 10.1016/j.brainres.2015.05.023
Webster, K. M., Sun, M., Crack, P., O’Brien, T. J., Shultz, S. R., and Semple, B. D. (2017). Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation 14:10.
Wlaz, P., Socala, K., Nieoczym, D., Luszczki, J. J., Zarnowska, I., Zarnowski, T., et al. (2012). Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 62, 1882–1889. doi: 10.1016/j.neuropharm.2011.12.015
Wlaz, P., Socala, K., Nieoczym, D., Zarnowski, T., Zarnowska, I., Czuczwar, S. J., et al. (2015). Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 57, 110–116. doi: 10.1016/j.pnpbp.2014.10.013
Yamanashi, T., Iwata, M., Kamiya, N., Tsunetomi, K., Kajitani, N., Wada, N., et al. (2017). Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 7:7677.
Yang, L., Zhao, J., Milutinovic, P. S., Brosnan, R. J., Eger, E. I. II, and Sonner, J. M. (2007). Anesthetic properties of the ketone bodies beta-hydroxybutyric acid and acetone. Anesth. Analg. 105, 673–679. doi: 10.1213/01.ane.0000278127.68312.dc
Youm, Y. H., Grant, R. W., McCabe, L. R., Albarado, D. C., Nguyen, K. Y., Ravussin, A., et al. (2013). Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532. doi: 10.1016/j.cmet.2013.09.010
Youm, Y. H., Nguyen, K. Y., Grant, R. W., Goldberg, E. L., Bodogai, M., Kim, D., et al. (2015). The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269. doi: 10.1038/nm.3804
Yudkoff, M., Daikhin, Y., Nissim, I., Lazarow, A., and Nissim, I. (2001). Brain amino acid metabolism and ketosis. J. Neurosci. Res. 66, 272–281. doi: 10.1002/jnr.1221
Yum, M. S., Lee, M., Woo, D. C., Kim, D. W., Ko, T. S., and Velisek, L. (2015). beta-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 117, 125–132. doi: 10.1016/j.eplepsyres.2015.08.005
Keywords: ketogenic diet, metabolic therapy, beta-hydroxybutyrate, acetoacetate, ketosis, exogenous ketones, ketone esters, epilepsy
Citation: Poff AM, Rho JM and D’Agostino DP (2019) Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives. Front. Neurosci. 13:1041. doi: 10.3389/fnins.2019.01041
Received: 27 November 2018; Accepted: 13 September 2019;
Published: 15 October 2019.
Edited by:
Rubem C. A. Guedes, Federal University of Pernambuco, BrazilReviewed by:
Stéphane Auvin, Hôpital Robert-Debré, FranceGiangennaro Coppola, University of Salerno, Italy
Copyright © 2019 Poff, Rho and D’Agostino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela M. Poff, YWJlbm5ldHRAaGVhbHRoLnVzZi5lZHU=; Dominic P. D’Agostino, ZGRhZ29zdGlAaGVhbHRoLnVzZi5lZHU=
 Angela M. Poff
Angela M. Poff Jong M. Rho
Jong M. Rho Dominic P. D’Agostino
Dominic P. D’Agostino