
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Neurosci., 23 September 2019
Sec. Neuroendocrine Science
Volume 13 - 2019 | https://doi.org/10.3389/fnins.2019.01008
This article is part of the Research TopicRegulatory Peptides in Neuroscience and Endocrinology: A New Era BeginsView all 17 articles
We here propose a parasympathetic endocrine system (PES) comprised of circulating peptides released from secretory cells in the gut, significantly modulated by vagal projections from the dorsal motor nucleus of the vagus (DMV). While most of these gut peptides mediate well-described satiety and digestive effects that increase parasympathetic control of digestion (Lee et al., 1994; Gutzwiller et al., 1999; Klok et al., 2007), they also have actions that are far-reaching and increase parasympathetic signaling broadly throughout the body. The actions beyond satiety that peptides like somatostatin, cholecystokinin, glucagon-like peptide 1, and vasoactive intestinal peptide have been well-examined, but not in a systematic way. Consideration has been given to the idea that these and other gut-derived peptides are part of an endocrine system has been partially considered (Rehfeld, 2012; Drucker, 2016), but that it is coordinated through parasympathetic control and may act to increase the actions of parasympathetic projections has not been formalized before. Here only gut-derived hormones are included although there are potentially other parasympathetically mediated factors released from other sites like lung and liver (Drucker, 2016). The case for the existence of the PES with the DMV as its integrative controller will be made through examination of an anatomical substrate and evidence of physiological control mechanisms as well as direct examples of PES antagonism of sympathetic signaling in mammals, including humans. The implications for this conceptual understanding of a PES reframe diseases like metabolic syndrome and may help underscore the role of the autonomic nervous system in the associated symptoms.
Although there is evidence that more may participate, four effectors will be considered in this conceptualization: somatostatin (SST), cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), and vasoactive intestinal peptide (VIP). The system as hypothesized is shown in Figure 1 with annotations as to each aspect of the connectivity. While all four are used as neurotransmitters and do not readily cross the blood brain barrier (Banks and Kastin, 1985), their presence in the circulatory system is able to mediate brain function through receptors in the hypothalamus and area postrema (van der Kooy, 1984; Shaffer and Moody, 1986; Breder et al., 1992; Yamamoto et al., 2003; Arora and Anubhuti, 2006), both of which prominently project to the dorsal motor nucleus of the vagus (DMV) (Gray and Magnuson, 1987; Hyde et al., 1996; Zheng et al., 2005). This sets up a pathway by which DMV sensing for circulating peptide levels and their effects may be monitored and controlled. The DMV is capable of modulating secretion of each of these through direct and indirect means utilizing gut postganglionic and myenteric plexus neurons. The work on such vagal influence has not been fully investigated in any one species, so the literature cited here includes work done in rats, guinea pigs, dogs, and humans. VIP is secreted by a subset of secretomotor neurons under the influence of postganglionic parasympathetic neurons directly modulated by vagal efferents (Bitar et al., 1980; Kirchgessner and Gershon, 1989; Yuan et al., 2005). SST is released from D-cells and is also under the influence of postganglionic parasympathetic neurons with vagal efferent projections having a demonstrable influence (Ahrén et al., 1986; Greenberg, 1993; Chisholm and Greenberg, 2002). CCK and GLP-1 secretion occurs under direct modulation by enteric neurons, which themselves have their influence from the postganglionic parasympathetic neurons. There is evidence that GLP-1 secretion can occur as a direct consequence of vagal efferent activity (Rocca and Brubaker, 1999). Although no such linear pathway has been described for CCK release as a result of vagal efferent activity, there is molecular and anatomical evidence that an indirect pathway exists. This pathway contains enteric secretomotor neurons that produce CCK and interactions with enteric neurons and possibly postganglionic neurons.
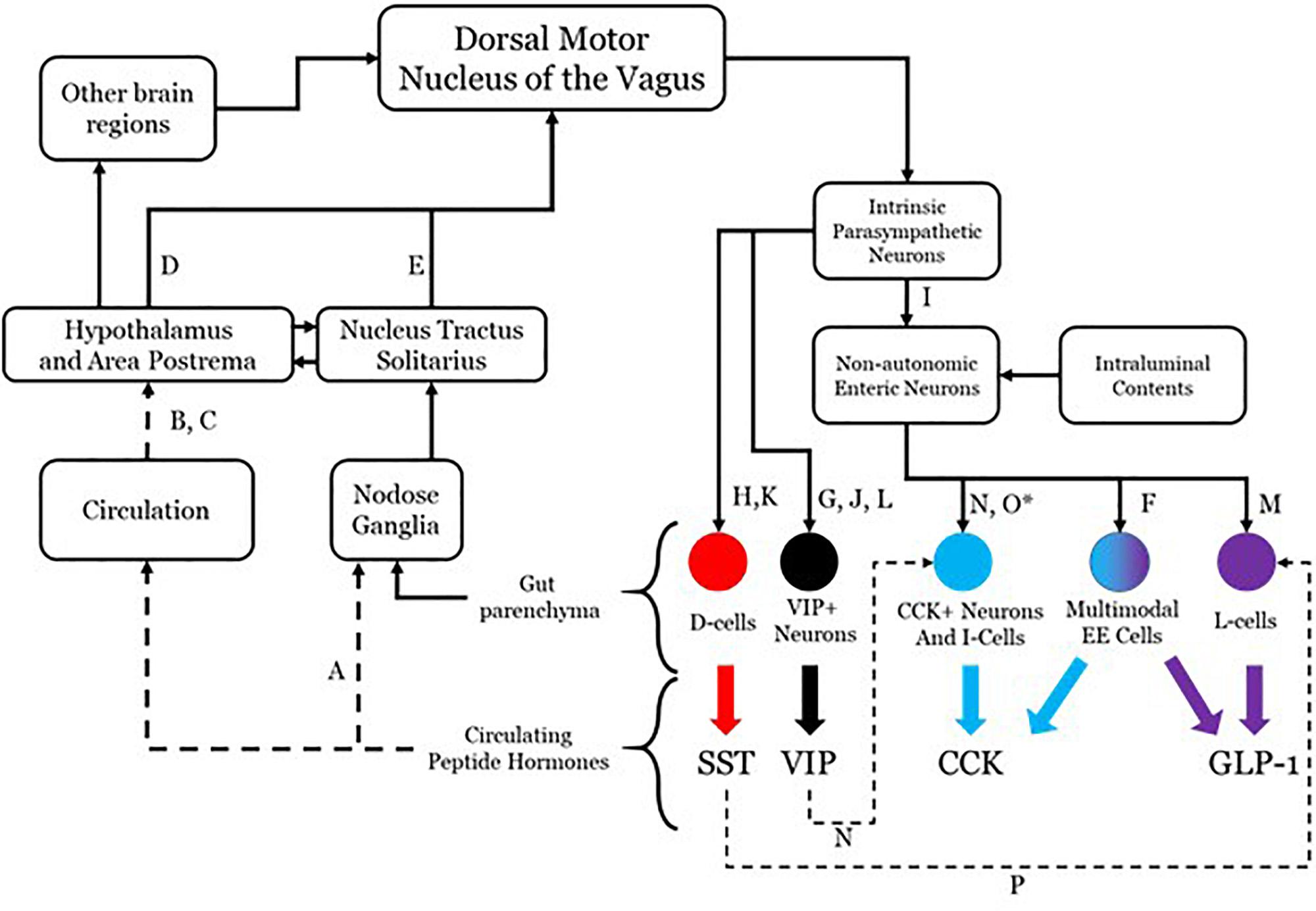
Figure 1. Anatomical connectivity of the proposed parasympathetic endocrine system. Each solid arrow represents direct synaptic neuronal connections and dashed lines represent humoral or indirect influence, but with documented influence. The afferent limb is shown on the left and the efferent limb shown on the right. The letters in the connectivity map identify examples of literature that supports the existence of the specific connection. A, Dockray (2013); B, Dockray (2012); C, Sandoval (2008); D, ter Horst (1984); E, Sawchenko (1983); F, Egerod (2012); G, Bitar et al. (1980); H, Greenberg (1993); I, Hayakawa (2006); J, Yuan et al. (2005); K, Ahrén et al. (1986); L, Kirchgessner and Gershon (1989); M, Rocca and Brubaker (1999); N, Furness (2000); O, Liddle (2000) (∗permissive in human); P, Chisholm and Greenberg (2002).
The DMV efferent projections are capable of modulating the release of several peptides in the gut, including the four enumerated above. Each of these four peptides are released through a complex network of local (gut sensing) and central (vagal) mechanisms. It is possible that the release of all gut peptides, including but not limited to the four discussed here, results from generic vagal efferent activity [release of acetylcholine (Ach)]. There is even evidence that changing the firing frequency can modulate peptide release of DMV efferents, which itself may be influenced by the transcriptional milieu of ion channels (Guzman et al., 1979; Nishi et al., 1985). DMV efferent neurons have the potential to express several other transmitters and peptides, the effects of which are still incompletely understood, especially with regard to local effects on signaling of gut projections.
The proposed parasympathetic endocrine system (PES) counterbalances the sympathetic nervous system broadly, not just with regard to digestion and orexigenic behavior. As the role of the four peptides examined here in digestion and satiety has been well-described (e.g., Arora and Anubhuti, 2006), we here focus on the other aspects of their control over visceral functions as they pertain to sympathetic antagonism. First, it is helpful to recall what the canonical effects of the sympathetic nervous system are. Sympathetic activation causes pupillary dilation, increased rate and contractility in the heart, bronchial dilation in the lungs, constriction of blood vessels generally, fluid retention and Na+ reuptake in kidney, urinary bladder relaxation, and activation of sweat glands, and is generally proinflammatory (Grebe et al., 2010; Kreibig, 2010). While not antagonistic to sympathetic activity, several PES peptides have the parasympathetic-like activity of enhancing erection induction, as will be discussed. What follows is evidence for sympathetic balancing in mammals from each of the PES effectors under consideration with these effects being summarized in Figure 2.
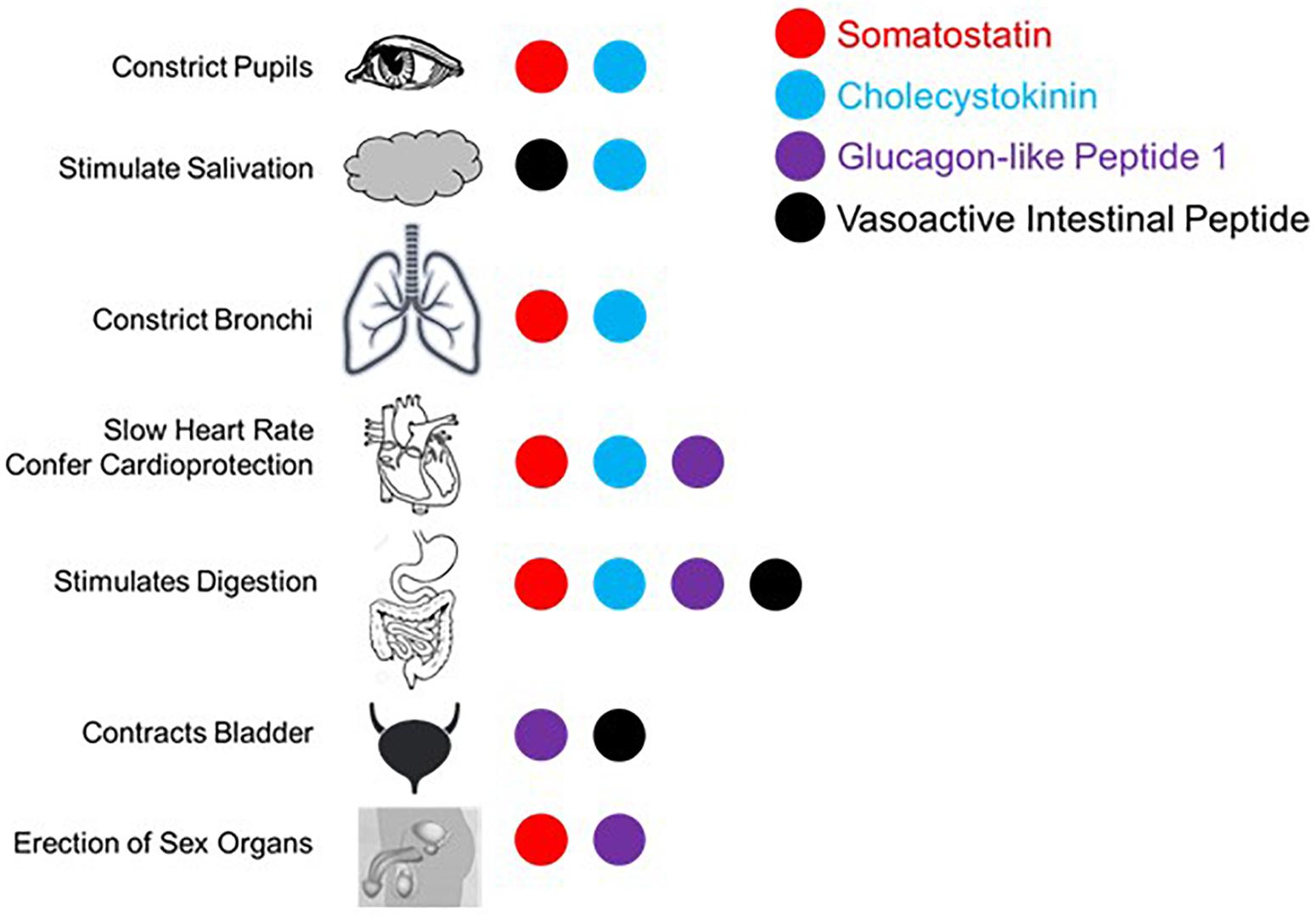
Figure 2. Summary of published effects of four selected gut peptides and their effects in augmenting parasympathetic function canonically effected via the vagus nerve and/or sacral parasympathetic projections.
Somatostatin and neuronostatin, a closely related peptide derived from the same mRNA transcript, mediate broad sympathetic antagonism and parasympathetic potentiating effects. In the heart, SST reduces contractility in an Ach-dependent fashion (Franco-Cereceda et al., 1987; Yoshikawa et al., 1996) and directly antagonizes sympathetic adrenergic beta receptors (Murray et al., 2001). Neurostatin also diminishes contractility and has the net effect of reducing blood pressure when in circulation (Samson et al., 2008; Vainio et al., 2012). Central administration of SST causes apnea (Yamamoto et al., 1988) and local levels in the lungs result in bronchoconstriction (Barrios et al., 1987). SST also has broad anti-inflammatory effects (Hofland et al., 1999; Krantic, 2000; ten Bokum et al., 2000). In penile tissue, SST potentiates the effects of Ach induction of erection (Hedlund and Andersson, 1985). Even in the eye, SST alone causes minor pupillary constriction (Bito et al., 1982). Along with the effects mentioned above, SST is able to mimic the effects of parasympathetically mediated ischemic preconditioning in generating cardioprotection (Wang et al., 2005).
The cardiovascular effects of CCK (Lovick, 2009) include reduction of heart rate (Kaczyńska and Szereda-Przestaszewska, 2015). CCK is also a vasodilator, acting locally (Sánchez-Fernández et al., 2004) or directly antagonizing neurons in rostral ventrolateral medulla (RVLM) and caudal ventrolateral medulla (CVLM) that selectively mediate vasoconstriction (Sartor and Verberne, 2002, 2006). Systemically administered CCK causes dyspnea with large enough doses in adults (Bradwejn et al., 1998) and can even induce panic attack like breathing patterns (Shlik et al., 1997). In the kidney, CCK decreases Na+ reuptake and reduces vascular resistance (von Schrenck et al., 2000) along with decreasing renal inflammation (Miyamoto et al., 2012). It has more broad anti-inflammatory effects via enhancement of vagal signaling (Luyer et al., 2005). CCK causes pupillary constriction, but only in primates and humans (Bill et al., 1990).
In the lungs, GLP-1 stimulates macromolecule secretion, mimicking the effects of Ach, and increases pulmonary blood flow (Richter et al., 1993). In the kidney, GLP-1 inhibits Na+ reuptake, thus acting as a diuretic (Okerson and Chilton, 2012) and also induces diuresis in the bladder through contraction (Ahrén, 2004). Like SST, GLP-1 also enhances erectile function via direct binding of receptors in erectile tissue (Giagulli et al., 2015). GLP-1 has also been shown to mediate a robust cardioprotective response (Ban, 2010; Basalay et al., 2016).
Like the other peptides, VIP is a potent vasodilator capable of decreasing heart rate and conduction velocity (Henning and Sawmiller, 2001). Interestingly, there are more VIP receptors in the right ventricle compared with the left, although the functional implications of this is not well understood (Henning and Sawmiller, 2001). In the kidney, VIP increases Na+ excretion (Rosa et al., 1985) and can induce erection or vaginal lubrication (Sjöstrand et al., 1981; Ottesen et al., 1984, 1987; Hedlund and Andersson, 1985). There are several ways in which VIP mediates anti-inflammatory effects (Pozo et al., 2000; Ganea and Delgado, 2002; Delgado et al., 2004): inhibits mast cell degranulation (Tunçel et al., 2000), decreases lymphocyte proliferation in Peyer’s patches (Stanisz et al., 1986), induces Treg and regulatory dendritic cell expansion (Chorny et al., 2005), and is generally immunosuppressive in aqueous humor (Taylor et al., 1994). In contrast to the other peptides considered here, VIP is sympathomimetic in the lungs, antagonizing bronchoconstriction (Barnes and Dixon, 1984). On the balance, VIP has parasympathetic-like activity in spite of the respiratory function described here.
There are likely more effectors of the PES than the four peptides explored above with partial or complete sympathetic antagonism. What makes this idea more than a collection of peptide functions is that it is coordinated by DMV projections. Reconsideration of disease processes in this context as a system may provide a foundation for new treatment approaches. Autonomic dysfunction accompanies many metabolic syndromes, including obesity and type II diabetes. There therefore may be some aspects of metabolic syndrome that are mediated, or at least modulated by autonomic functions via this parasympathetic endocrine system.
The diagnostic criteria for metabolic syndrome includes a combination of at least three of the following: abdominal obesity, hypertension, hyperglycemia, increased triglycerides, and decreased high-density lipoprotein (HDL) cholesterol (Alberti et al., 2005; Opie, 2007). The four peptides highlighted in this review each contribute to negating the effects of metabolic syndrome across these five criteria as shown in Figure 3, the underlying network being based largely on the work of Eckel et al. (2005). Abdominal obesity can be reduced/prevented through circulating SST (Lustig et al., 1999; Boehm and Lustig, 2002; Boehm, 2003), CCK (Pi-Sunyer et al., 1982; Matson and Ritter, 1999; Neary and Batterham, 2009), or GLP-1 (Näslund et al., 1999; Day et al., 2009; Astrup et al., 2012). Hypertension can be ameliorated by all four peptides focused upon here; SST (Rosenthal et al., 1977; Carretta et al., 1989), CCK (Kagebayashi et al., 2012), GLP-1 (Wang et al., 2013; Katout et al., 2014), and VIP (Gao et al., 1994). All four peptides contribute to lowering fasting plasma glucose through a variety of mechanisms including stimulation of insulin secretion and inhibition of glucagon secretion; SST (Gerich et al., 1974, 1975; Wahren, 1976; Vergès, 2017), CCK (Cheung et al., 2009; Irwin et al., 2015), GLP-1 (Nauck et al., 1993; Toft-Nielsen et al., 1999; Irwin et al., 2015), and VIP (Kato et al., 1994). Effects on triglycerides and HDL cholesterol have less evidence to date, yet it has been demonstrated that GLP-1 can lower triglycerides (Qin et al., 2005; Meier et al., 2006) with CCK recently shown to have similar effects on absorption in a preclinical model (Plaza et al., 2018). Of the four, only GLP-1 can increase HDL cholesterol, although it appears to be mediated through a variety of other mechanisms as opposed to a direct effect on the production and processing of cholesterol (Ponzani et al., 2016).
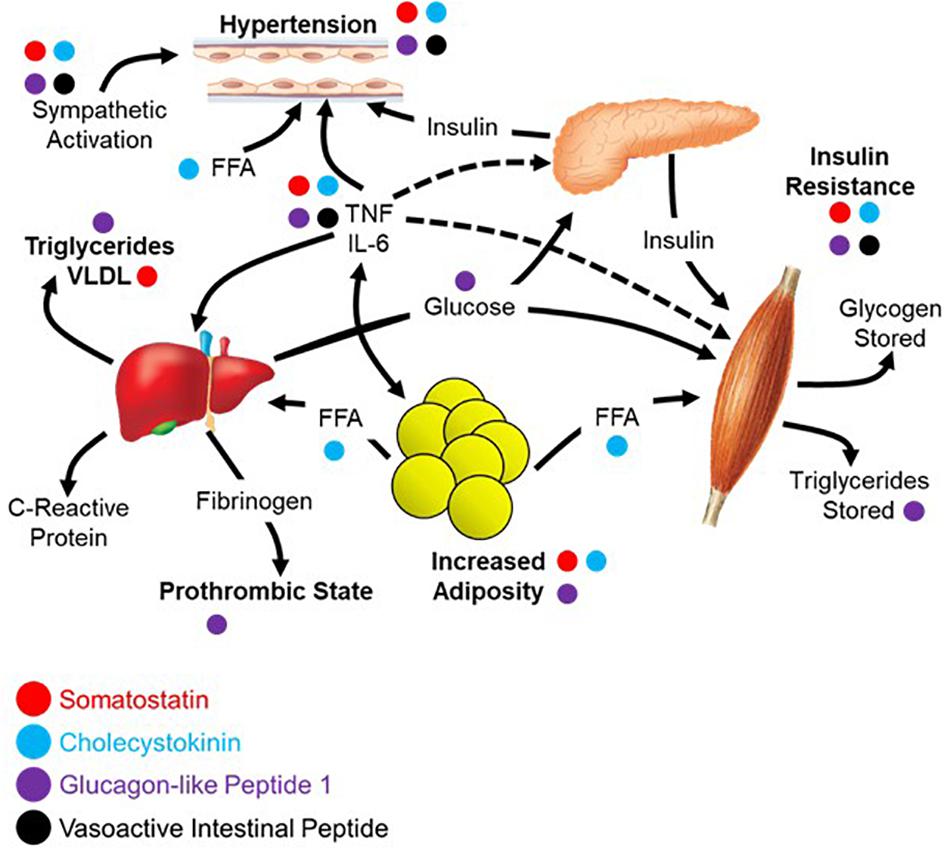
Figure 3. Parasympathetic endocrine effects on multi-organ network interactions that give rise to metabolic syndrome. Aspects of metabolic syndrome that are inhibited by the four selected gut peptides as was summarized in the text of the section “Reconsidering Metabolic Syndrome.” The relationships between different aspects of metabolic syndrome are recreated from the work of Eckel et al. (2005).
Apart from the direct symptoms of metabolic syndrome, there is a broad sympathetic dominance across multiple organ systems (Tentolouris et al., 2006, 2008). This includes cardiovascular problems like resting tachycardia, reduced heart rate variability, and decreased baroreflex sensitivity (Garruti et al., 2012; Verrotti et al., 2014). Metabolic syndrome causes erectile dysfunction with a high incidence of comorbidity (Gündüz et al., 2004; Bal et al., 2007). Also in metabolic syndrome, there is a markedly decreased release of multiple gut peptides (Verrotti et al., 2014). It is possible that the diminished gut peptide release contributes to the dysautonomia and sympathetic dominance through absence of antagonism. This may be due to physical changes in the gut or perturbations in the sensory mechanisms that otherwise mediate their release. If this is the case, it might be possible to replace these peptides and treat the autonomic symptoms. There is an example of this in a clinical trial showing that the use of SST analog treats diarrhea and orthostatic hypotension in patients with diabetes (Dudl et al., 1987). Moving forward, it may be helpful to assay for circulating peptide levels and use them either a biomarkers of disease or as indications to initiate peptide replacement therapy. It is likely that replacing the milieu of peptides rather than one or the other will be required for maximal clinical benefit.
The requirements of the PES laid out here were that it has a central controller, broadly counterbalances sympathetic effects, and can help explain disease pathology. The evidence provided suggests that there is a parasympathetic endocrine system that is coordinated by the DMV. The power lent by this concept derives from the network coordination of the four peptides discussed here and many additional circulating gut factors at the level of the DMV where there can be integration of peripheral and central neuronal inputs and orchestration of multiple gut endocrine activities. This also enables identification via disease markers and interventions aimed at treating a network of factors at the central or peripheral level. The conceptualization of physiology laid out here goes beyond the boundaries of traditional medical specialties like gastroenterology, neurology, or cardiology and instead requires a systems approach to health and medicine. As the ability to deal with human health in a more comprehensive and complete way matures in the era of big data, clinicians must be ready to incorporate the growing complexity of the body as a system in the design and implementation of therapeutic intervention.
JG developed the main concepts and was the primary writer of the manuscript. JS provided the significant editorial contributions.
This work was made possible by grants from the National Institute of Health (5U01HL133360-02 and 3OT2OD023848).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ahrén, B. (2004). GLP-1 and extra-islet effects. Horm. Metab. Res. 36, 842–845. doi: 10.1055/s-2004-826173
Ahrén, B., Paquette, T. L., and Taborsky, G. J. (1986). Effect and mechanism of vagal nerve stimulation on somatostatin secretion in dogs. Am. J. Physiol. 250, E212–E217. doi: 10.1152/ajpendo.1986.250.2.E212
Alberti, K. G., Zimmet, P., Shaw, J., and IDF Epidemiology Task Force Consensus Group, (2005). The metabolic syndrome–a new worldwide definition. Lancet 366, 1059–1062.
Arora, S., and Anubhuti (2006). Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 40, 375–401. doi: 10.1016/j.npep.2006.07.001
Astrup, A., Carraro, R., Finer, N., Harper, A., Kunesova, M., Lean, M. E. J., et al. 1807 Investigators (2012). Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 36, 843–854. doi: 10.1038/ijo.2011.158
Bal, K., Oder, M., Sahin, A. S., Karataş, C. T., Demir, O., Can, E., et al. (2007). Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology 69, 356–360. doi: 10.1016/j.urology.2006.09.057
Ban, K. (2010). Mechanisms Underlying Cardioprotective Effects of Glucagon like Peptide-1 in Ischemia-reperfusion Injury. Ph. D Thesis, University of Toronto: Toronto, ON.
Banks, W. A., and Kastin, A. J. (1985). Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res. Bull. 15, 287–292. doi: 10.1016/0361-9230(85)90153-4
Barnes, P. J., and Dixon, C. M. (1984). The effect of inhaled vasoactive intestinal peptide on bronchial reactivity to histamine in humans. Am. Rev. Respir. Dis. 130, 162–166. doi: 10.1164/arrd.1984.130.2.162
Barrios, V., Fernandez Iriarte, M. C., Morcillo, E., Prieto, J. C., and Arilla, E. (1987). Effect of sensitization on somatostatin concentration and binding in cytosol from guinea pig airways. Regul. Peptides 19, 161–168. doi: 10.1016/0167-0115(87)90273-4
Basalay, M. V., Mastitskaya, S., Mrochek, A., Ackland, G. L., Del Arroyo, A. G., Sanchez, J., et al. (2016). Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc. Res. 112, 669–676. doi: 10.1093/cvr/cvw216
Bill, A., Andersson, S. E., and Almegård, B. (1990). Cholecystokinin causes contraction of the pupillary sphincter in monkeys but not in cats, rabbits, rats and guinea-pigs: antagonism by lorglumide. Acta Physiol. Scand. 138, 479–485. doi: 10.1111/j.1748-1716.1990.tb08875.x
Bitar, K. N., Said, S. I., Weir, G. C., Saffouri, B., and Makhlouf, G. M. (1980). Neural release of vasoactive intestinal peptide from the gut. Gastroenterology 79, 1288–1294. doi: 10.1016/0016-5085(80)90927-0
Bito, L. Z., Nichols, R. R., and Baroody, R. A. (1982). A comparison of the miotic and inflammatory effects of biologically active polypeptides and prostaglandin E2 on the rabbit eye. Exp. Eye Res. 34, 325–337. doi: 10.1016/0014-4835(82)90081-1
Boehm, B. O. (2003). The therapeutic potential of somatostatin receptor ligands in the treatment of obesity and diabetes. Expert Opin. Investig. Drugs 12, 1501–1509. doi: 10.1517/eoid.12.9.1501.21813
Boehm, B. O., and Lustig, R. H. (2002). Use of somatostatin receptor ligands in obesity and diabetic complications. Best Pract. Res. Clin. Gastroenterol. 16, 493–509. doi: 10.1053/bega.2002.0320
Bradwejn, J., LeGrand, J. M., Koszycki, D., Bates, J. H., and Bourin, M. (1998). Effects of cholecystokinin tetrapeptide on respiratory function in healthy volunteers. Am. J. Psychiatry 155, 280–282.
Breder, C. D., Yamada, Y., Yasuda, K., Seino, S., Saper, C. B., and Bell, G. I. (1992). Differential expression of somatostatin receptor subtypes in brain. J. Neurosci. 12, 3920–3934. doi: 10.1523/jneurosci.12-10-03920.1992
Carretta, R., Fabris, B., Fischetti, F., Costantini, M., De Biasi, F., Muiesan, S., et al. (1989). Reduction of blood pressure in obese hyperinsulinaemic hypertensive patients during somatostatin infusion. J. Hypertens. Suppl. 7, S196–S197.
Cheung, G. W. C., Kokorovic, A., Lam, C. K. L., Chari, M., and Lam, T. K. T. (2009). Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 10, 99–109. doi: 10.1016/j.cmet.2009.07.005
Chisholm, C., and Greenberg, G. R. (2002). Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am. J. Physiol. Endocrinol. Metab. 283, E311–E317. doi: 10.1152/ajpendo.00434.2001
Chorny, A., Gonzalez-Rey, E., Fernandez-Martin, A., Pozo, D., Ganea, D., and Delgado, M. (2005). Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc. Natl. Acad. Sci. U.S.A. 102, 13562–13567. doi: 10.1073/pnas.0504484102
Day, J. W., Ottaway, N., Patterson, J. T., Gelfanov, V., Smiley, D., Gidda, J., et al. (2009). A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757. doi: 10.1038/nchembio.209
Delgado, M., Pozo, D., and Ganea, D. (2004). The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 56, 249–290. doi: 10.1124/pr.56.2.7
Dockray, G. J. (2012). Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 19, 8–12. doi: 10.1097/MED.0b013e32834eb77d
Dockray, G. J. (2013). Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 13, 954–958. doi: 10.1016/j.coph.2013.09.007
Drucker, D. J. (2016). Evolving concepts and translational relevance of enteroendocrine cell biology. J. Clin. Endocrinol. Metab. 101, 778–786. doi: 10.1210/jc.2015-3449
Dudl, R. J., Anderson, D. S., Forsythe, A. B., Ziegler, M. G., and O’Dorisio, T. M. (1987). Treatment of diabetic diarrhea and orthostatic hypotension with somatostatin analogue SMS 201-995. Am. J. Med. 83, 584–588. doi: 10.1016/0002-9343(87)90777-7
Eckel, R. H., Grundy, S. M., and Zimmet, P. Z. (2005). The metabolic syndrome. Lancet 365, 1415–1428. doi: 10.1016/S0140-6736(05)66378-7
Egerod, K. L., Engelstoft, M. S., Grunddal, K. V., Nøhr, M. K., Secher, A., Sakata, I., et al. (2012). A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782–5795. doi: 10.1210/en.2012-1595
Furness, J. B. (2000). Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 81, 87–96. doi: 10.1016/S0165-1838(00)00127-2
Franco-Cereceda, A., Bengtsson, L., and Lundberg, J. M. (1987). Inotropic effects of calcitonin gene-related peptide, vasoactive intestinal polypeptide and somatostatin on the human right atrium in vitro. Eur. J. Pharmacol 134, 69–76. doi: 10.1016/0014-2999(87)90132-4
Ganea, D., and Delgado, M. (2002). Vasoactive intestinal peptide (VIP)and pituitary adenylate cyclase-activating polypeptide (PACAP)as modulators of both innate and adaptive immunity. Critic. Rev. Oral Biol. Med. 13, 229–237.
Gao, X., Noda, Y., Rubinstein, I., and Paul, S. (1994). Vasoactive intestinal peptide encapsulated in liposomes: effects on systemic arterial blood pressure. Life Sci. 54, L247–L252.
Garruti, G., Giampetruzzi, F., Vita, M. G., Pellegrini, F., Lagioia, P., Stefanelli, G., et al. (2012). Links between metabolic syndrome and cardiovascular autonomic dysfunction. Exp. Diabetes Res. 2012:615835. doi: 10.1155/2012/615835
Gerich, J. E., Lorenzi, M., Hane, S., Gustafson, G., Guillemin, R., and Forsham, P. H. (1975). Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Metab. Clin. Exp. 24, 175–182. doi: 10.1016/0026-0495(75)90018-9
Gerich, J. E., Lorenzi, M., Schneider, V., Karam, J. H., Rivier, J., Guillemin, R., et al. (1974). Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N. Engl. J. Med. 291, 544–547. doi: 10.1056/nejm197409122911102
Giagulli, V. A., Carbone, M. D., Ramunni, M. I., Licchelli, B., De Pergola, G., Sabbà, C., et al. (2015). Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 3, 1094–1103. doi: 10.1111/andr.12099
Gray, T. S., and Magnuson, D. J. (1987). Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J. Comp. Neurol. 262, 365–374. doi: 10.1002/cne.902620304
Grebe, K. M., Takeda, K., Hickman, H. D., Bailey, A. L., Embry, A. C., Bennink, J. R., et al. (2010). Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. 184, 540–544. doi: 10.4049/jimmunol.0903395
Greenberg, G. R. (1993). Differential neural regulation of circulating somatostatin-14 and somatostatin-28 in conscious dogs. Am. J. Physiol. 264, G902–G909. doi: 10.1152/ajpgi.1993.264.5.G902
Gündüz, M. I., Gümüs, B. H., and Sekuri, C. (2004). Relationship between metabolic syndrome and erectile dysfunction. Asian J. Androl. 6, 355–358.
Gutzwiller, J.-P., Drewe, J., Göke, B., Schmidt, H., Rohrer, B., Lareida, J., et al. (1999). Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276, R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541
Guzman, S., Chayvialle, J. A., Banks, W. A., Rayford, P. L., and Thompson, J. C. (1979). Effect of vagal stimulation on pancreatic secretion and on blood levels of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide, and somatostatin. Surgery 86, 329–336.
Hayakawa, T., Kuwahara, S., Maeda, S., Tanaka, K., and Seki, M. (2006). Direct synaptic contacts on the myenteric ganglia of the rat stomach from the dorsal motor nucleus of the vagus. J. Comp. Neurol. 498, 352–362. doi: 10.1002/cne.21069
Hedlund, H., and Andersson, K. E. (1985). Effects of some peptides on isolated human penile erectile tissue and cavernous artery. Acta Physiol. Scand. 124, 413–419. doi: 10.1111/j.1748-1716.1985.tb07677.x
Henning, R. J., and Sawmiller, D. R. (2001). Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc. Res. 49, 27–37. doi: 10.1016/s0008-6363(00)00229-7
Hofland, L. J., van Hagen, P. M., and Lamberts, S. W. (1999). Functional role of somatostatin receptors in neuroendocrine and immune cells. Ann. Med. 31(Suppl. 2), 23–27.
Hyde, T. M., Knable, M. B., and Murray, A. M. (1996). Distribution of dopamine D1-D4 receptor subtypes in human dorsal vagal complex. Synapse 24, 224–232. doi: 10.1002/(sici)1098-2396(199611)24:3<224::aid-syn4>3.0.co;2-g
Irwin, N., Pathak, V., and Flatt, P. R. (2015). A Novel CCK-8/GLP-1 hybrid peptide exhibiting prominent insulinotropic, glucose-lowering, and satiety actions with significant therapeutic potential in high-fat-fed Mice. Diabetes Metab. Res. Rev 64, 2996–3009. doi: 10.2337/db15-0220
Kaczyńska, K., and Szereda-Przestaszewska, M. (2015). Contribution of CCK1 receptors to cardiovascular and respiratory effects of cholecystokinin in anesthetized rats. Neuropeptides 54, 29–34. doi: 10.1016/j.npep.2015.08.006
Kagebayashi, T., Kontani, N., Yamada, Y., Mizushige, T., Arai, T., Kino, K., et al. (2012). Novel CCK-dependent vasorelaxing dipeptide, Arg-Phe, decreases blood pressure and food intake in rodents. Mol. Nutr. Food Res. 56, 1456–1463. doi: 10.1002/mnfr.201200168
Kato, I., Suzuki, Y., Akabane, A., Yonekura, H., Tanaka, O., Kondo, H., et al. (1994). Transgenic mice overexpressing human vasoactive intestinal peptide (VIP) gene in pancreatic beta cells. Evidence for improved glucose tolerance and enhanced insulin secretion by VIP and PHM-27 in vivo. J. Biol. Chem. 269, 21223–21228.
Katout, M., Zhu, H., Rutsky, J., Shah, P., Brook, R. D., Zhong, J., et al. (2014). Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am. J. Hypertens. 27, 130–139. doi: 10.1093/ajh/hpt196
Kirchgessner, A. L., and Gershon, M. D. (1989). Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J. Comp. Neurol. 285, 38–53. doi: 10.1002/cne.902850105
Klok, M. D., Jakobsdottir, S., and Drent, M. L. (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. 8, 21–34. doi: 10.1111/j.1467-789x.2006.00270.x
Krantic, S. (2000). Peptides as regulators of the immune system: emphasis on somatostatin. Peptides 21, 1941–1964. doi: 10.1016/s0196-9781(00)00347-8
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421. doi: 10.1016/j.biopsycho.2010.03.010
Lee, M. C., Schiffman, S. S., and Pappas, T. N. (1994). Role of neuropeptides in the regulation of feeding behavior: a review of cholecystokinin, bombesin, neuropeptide Y, and galanin. Neurosci. Biobehav. Rev. 18, 313–323. doi: 10.1016/0149-7634(94)90045-0
Liddle, R. A. (2000). Regulation of cholecystokinin secretion in humans. J. Gastroenterol. 35, 181–187. doi: 10.1007/s005350050328
Lovick, T. A. (2009). CCK as a modulator of cardiovascular function. J. Chem. Neuroanat. 38, 176–184. doi: 10.1016/j.jchemneu.2009.06.007
Lustig, R. H., Rose, S. R., Burghen, G. A., Velasquez-Mieyer, P., Broome, D. C., Smith, K., et al. (1999). Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J. Pediatr. 135(2 Pt 1), 162–168. doi: 10.1016/s0022-3476(99)70017-x
Luyer, M. D., Greve, J. W. M., Hadfoune, M., Jacobs, J. A., Dejong, C. H., and Buurman, W. A. (2005). Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 202, 1023–1029. doi: 10.1084/jem.20042397
Matson, C. A., and Ritter, R. C. (1999). Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276, R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038
Meier, J. J., Gethmann, A., Götze, O., Gallwitz, B., Holst, J. J., Schmidt, W. E., et al. (2006). Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 49, 452–458. doi: 10.1007/s00125-005-0126-y
Miyamoto, S., Shikata, K., Miyasaka, K., Okada, S., Sasaki, M., Kodera, R., et al. (2012). Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: anti-inflammatory effect of cholecystokinin. Diabetes Metab. Res. Rev. 61, 897–907. doi: 10.2337/db11-0402
Murray, F., Bell, D., Kelso, E. J., Millar, B. C., and McDermott, B. J. (2001). Positive and negative contractile effects of somatostatin-14 on rat ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 37, 324–332. doi: 10.1097/00005344-200103000-00011
Näslund, E., Barkeling, B., King, N., Gutniak, M., Blundell, J. E., Holst, J. J., et al. (1999). Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. Relat. Metab. Disord. 23, 304–311. doi: 10.1038/sj.ijo.0800818
Nauck, M. A., Kleine, N., Orskov, C., Holst, J. J., Willms, B., and Creutzfeldt, W. (1993). Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744. doi: 10.1007/bf00401145
Neary, M. T., and Batterham, R. L. (2009). Gut hormones: implications for the treatment of obesity. Pharmacol. Ther. 124, 44–56. doi: 10.1016/j.pharmthera.2009.06.005
Nishi, S., Seino, Y., Takemura, J., Ishida, H., Seno, M., Chiba, T., et al. (1985). Vagal regulation of GRP, gastric somatostatin, and gastrin secretion in vitro. Am. J. Physiol. 248(4 Pt 1), E425–E431.
Okerson, T., and Chilton, R. J. (2012). The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc. Ther. 30, e146–e155. doi: 10.1111/j.1755-5922.2010.00256.x
Ottesen, B., Pedersen, B., Nielsen, J., Dalgaard, D., Wagner, G., and Fahrenkrug, J. (1987). Vasoactive intestinal polypeptide (VIP) provokes vaginal lubrication in normal women. Peptides 8, 797–800. doi: 10.1016/0196-9781(87)90061-1
Ottesen, B., Wagner, G., Virag, R., and Fahrenkrug, J. (1984). Penile erection: possible role for vasoactive intestinal polypeptide as a neurotransmitter. Br. Med. J. 288, 9–11. doi: 10.1136/bmj.288.6410.9
Pi-Sunyer, X., Kissileff, H. R., Thornton, J., and Smith, G. P. (1982). C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol. Behav. 29, 627–630. doi: 10.1016/0031-9384(82)90230-x
Plaza, A., Merino, B., Cano, V., Domínguez, G., Pérez-Castells, J., Fernández-Alfonso, M. S., et al. (2018). Cholecystokinin is involved in triglyceride fatty acid uptake by rat adipose tissue. J. Endocrinol. 236, 137–150. doi: 10.1530/JOE-17-0580
Ponzani, P., Scardapane, M., Nicolucci, A., and Rossi, M. C. (2016). Effectiveness and safety of liraglutide after three years of treatment. Minerva Endocrinol. 41, 35–42.
Pozo, D., Delgado, M., Martínez, C., Guerrero, J. M., Leceta, J., Gomariz, R. P., et al. (2000). Immunobiology of vasoactive intestinal peptide (VIP). Immunol. Today 21, 7–11. doi: 10.1016/s0167-5699(99)01525-x
Qin, X., Shen, H., Liu, M., Yang, Q., Zheng, S., Sabo, M., et al. (2005). GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G943–G949.
Rehfeld, J. F. (2012). Beginnings: a reflection on the history of gastrointestinal endocrinology. Regul. Peptides 177(Suppl.), S1–S5. doi: 10.1016/j.regpep.2012.05.087
Richter, G., Feddersen, O., Wagner, U., Barth, P., Göke, R., and Göke, B. (1993). GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. 265(4 Pt 1), L374–L381.
Rocca, A. S., and Brubaker, P. L. (1999). Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140, 1687–1694. doi: 10.1210/endo.140.4.6643
Rosa, R. M., Silva, P., Stoff, J. S., and Epstein, F. H. (1985). Effect of vasoactive intestinal peptide on isolated perfused rat kidney. Am. J. Physiol. 249(5 Pt 1), E494–E497.
Rosenthal, J., Escobar-Jimenez, F., and Raptis, S. (1977). Prevention by somatostatin of rise in blood pressure and plasma renin mediated by beta-receptor stimulation. Clin. Endocrinol. 6, 455–462. doi: 10.1111/j.1365-2265.1977.tb03329.x
Samson, W. K., Zhang, J. V., Avsian-Kretchmer, O., Cui, K., Yosten, G. L. C., Klein, C., et al. (2008). Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J. Biol. Chem. 283, 31949–31959. doi: 10.1074/jbc.M804784200
Sánchez-Fernández, C., González, M. C., Beart, P. M., Mercer, L. D., Ruiz-Gayo, M., and Fernández-Alfonso, M. S. (2004). A novel role for cholecystokinin: regulation of mesenteric vascular resistance. Regul. Peptides 121, 145–153. doi: 10.1016/j.regpep.2004.04.018
Sandoval, D. (2008). CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol. Behav. 94, 670–674. doi: 10.1016/j.physbeh.2008.04.018
Sartor, D. M., and Verberne, A. J. M. (2002). Cholecystokinin selectively affects presympathetic vasomotor neurons and sympathetic vasomotor outflow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1174–R1184.
Sartor, D. M., and Verberne, A. J. M. (2006). The sympathoinhibitory effects of systemic cholecystokinin are dependent on neurons in the caudal ventrolateral medulla in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1390–R1398.
Sawchenko, P. E. (1983). Central connections of the sensory and motor nuclei of the vagus nerve. J. Auton. Nerv. Syst. 9, 13–26. doi: 10.1016/0165-1838(83)90129-7
Shaffer, M. M., and Moody, T. W. (1986). Autoradiographic visualization of CNS receptors for vasoactive intestinal peptide. Peptides 7, 283–288. doi: 10.1016/0196-9781(86)90226-3
Shlik, J., Vasar, V., Aluoja, A., Kingisepp, P. H., Jagomägi, K., Vasar, E., et al. (1997). The effect of cholecystokinin tetrapeptide on respiratory resistance in healthy volunteers. Biol. Psychiatry 42, 206–212. doi: 10.1016/s0006-3223(96)00334-4
Sjöstrand, N. O., Klinge, E., and Himberg, J. J. (1981). Effects of VIP and other putative neurotransmitters on smooth muscle effectors of penile erection. Acta Physiol. Scand. 113, 403–405. doi: 10.1111/j.1748-1716.1981.tb06914.x
Stanisz, A. M., Befus, D., and Bienenstock, J. (1986). Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer’s patches, mesenteric lymph nodes, and spleen. J. Immunol. 136, 152–156.
Taylor, A. W., Streilein, J. W., and Cousins, S. W. (1994). Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 153, 1080–1086.
ten Bokum, A. M., Hofland, L. J., and van Hagen, P. M. (2000). Somatostatin and somatostatin receptors in the immune system: a review. Eur. Cytokine Netw. 11, 161–176.
ter Horst, G. J., Luiten, P. G. M., and Kuipers, F. (1984). Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J. Auton. Nerv. Syst. 11, 59–75. doi: 10.1016/0165-1838(84)90008-0
Tentolouris, N., Argyrakopoulou, G., and Katsilambros, N. (2008). Perturbed autonomic nervous system function in metabolic syndrome. Neuromol. Med. 10, 169–178. doi: 10.1007/s12017-008-8022-5
Tentolouris, N., Liatis, S., and Katsilambros, N. (2006). Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 1083, 129–152. doi: 10.1196/annals.1367.010
Toft-Nielsen, M. B., Madsbad, S., and Holst, J. J. (1999). Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22, 1137–1143. doi: 10.2337/diacare.22.7.1137
Tunçel, N., Töre, F., Şahintürk, V., Ak, D., and Tunçel, M. (2000). Vasoactive intestinal peptide inhibits degranulation and changes granular content of mast cells: a potential therapeutic strategy in controlling septic shock. Peptides 21, 81–89. doi: 10.1016/s0196-9781(99)00177-1
Vainio, L., Perjes, A., Ryti, N., Magga, J., Alakoski, T., Serpi, R., et al. (2012). Neuronostatin, a novel peptide encoded by somatostatin gene, regulates cardiac contractile function and cardiomyocyte survival. J. Biol. Chem. 287, 4572–4580. doi: 10.1074/jbc.M111.289215
van der Kooy, D. (1984). Area postrema: site where cholecystokinin acts to decrease food intake. Brain Res. 295, 345–347. doi: 10.1016/0006-8993(84)90982-x
Vergès, B. (2017). Effects of anti-somatostatin agents on glucose metabolism. Diabetes Metab. 43, 411–415. doi: 10.1016/j.diabet.2017.05.003
Verrotti, A., Prezioso, G., Scattoni, R., and Chiarelli, F. (2014). Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. 5:205. doi: 10.3389/fendo.2014.00205
von Schrenck, T., Ahrens, M., de Weerth, A., Bobrowski, C., Wolf, G., Jonas, L., et al. (2000). CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney Int. 58, 995–1003. doi: 10.1046/j.1523-1755.2000.00257.x
Wahren, J. (1976). Influence of somatostatin on carbohydrate disposal and absorption in diabetes mellitus. Lancet 2, 1213–1216. doi: 10.1016/s0140-6736(76)91142-9
Wang, B., Zhong, J., Lin, H., Zhao, Z., Yan, Z., He, H., et al. (2013). Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes. Metab. 15, 737–749. doi: 10.1111/dom.12085
Wang, T.-L., Huang, Y.-H., and Chang, H. (2005). Somatostatin analogue mimics acute ischemic preconditioning in a rat model of myocardial infarction. J. Cardiovasc. Pharmacol. 45, 327–332. doi: 10.1097/01.fjc.0000156823.35210.21
Yamamoto, H., Kishi, T., Lee, C. E., Choi, B. J., Fang, H., Hollenberg, A. N., et al. (2003). Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J. Neurosci. 23, 2939–2946. doi: 10.1523/jneurosci.23-07-02939.2003
Yamamoto, Y., Runold, M., Prabhakar, N., Pantaleo, T., and Lagercrantz, H. (1988). Somatostatin in the control of respiration. Acta Physiol. Scand. 134, 529–533. doi: 10.1111/j.1365-201x.1988.tb10631.x
Yoshikawa, T., Port, J. D., Asano, K., Chidiak, P., Bouvier, M., Dutcher, D., et al. (1996). Cardiac adrenergic receptor effects of carvedilol. Eur. Heart J. 17(Suppl. B), 8–16. doi: 10.1093/eurheartj/17.suppl_b.8
Yuan, P.-Q., Kimura, H., Million, M., Bellier, J.-P., Wang, L., Ohning, G. V., et al. (2005). Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides 26, 653–664. doi: 10.1016/j.peptides.2004.11.015
Keywords: parasympathetic, autonomic, endocrine, gut, vagus
Citation: Gorky J and Schwaber J (2019) Conceptualization of a Parasympathetic Endocrine System. Front. Neurosci. 13:1008. doi: 10.3389/fnins.2019.01008
Received: 09 March 2019; Accepted: 05 September 2019;
Published: 23 September 2019.
Edited by:
Lee E. Eiden, National Institutes of Health (NIH), United StatesReviewed by:
Limei Zhang, National Autonomous University of Mexico, MexicoCopyright © 2019 Gorky and Schwaber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Gorky, am9uYXRoYW4uZ29ya3lAamVmZmVyc29uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.