
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 17 September 2019
Sec. Perception Science
Volume 13 - 2019 | https://doi.org/10.3389/fnins.2019.00970
This article is part of the Research TopicNeuromodulatory Interventions for PainView all 12 articles
 Inge Timmers1,2,3*
Inge Timmers1,2,3* Jeroen R. de Jong1,4,5
Jeroen R. de Jong1,4,5 Mariëlle Goossens1,6
Mariëlle Goossens1,6 Jeanine A. Verbunt1,4,5
Jeanine A. Verbunt1,4,5 Rob J. Smeets1,7
Rob J. Smeets1,7 Amanda L. Kaas2
Amanda L. Kaas2Exposure in vivo (EXP) is a cognitive-behavioral treatment aimed at reducing pain-related fear in chronic pain, and has proven successful in reducing pain-related disability in patients with chronic low back pain (cLBP). The current longitudinal study aimed to reveal the neural correlates of changes in pain-related fear as a result of EXP. Twenty-three patients with cLBP were included in this study. Patients with cLBP underwent MRI scanning pre-treatment (pre-EXP), post-treatment (post-EXP), and 6 months after end of treatment (FU-EXP). Pain-free controls were scanned at two time points. In the scanner, participants were presented with pictures involving back-related movements, evoking pain-related fear in patients. Pre-treatment, functional MRI revealed increased activation in right posterior insula and increased deactivation in medial prefrontal cortex (mPFC) in patients compared to controls. Post-treatment, patients reported reduced fear and pre-EXP group differences were no longer present. Contrasting pre- to post- and FU-EXP in patients revealed that stimulus-evoked neural responses changed in sensorimotor as well as cognitive/affective brain regions. Lastly, exploratory analyses revealed a tendency toward an association between changes in neural activation and changes in fear ratings, including the hippocampus and temporal lobe (pre- to post-EXP changes), and mPFC and posterior cingulate cortex (pre- to FU-EXP changes). Taken together, we show evidence that neural circuitry for pain-related fear is modulated by EXP, and that changes are associated with self-reported decreases in pain-related fear.
While most of us experience acute low back pain at some point in our lives, some will develop chronic low back pain (cLBP), with persistent pain lasting more than 6 months. An estimated one in five adults is currently in chronic pain, with cLBP being the most common (Breivik et al., 2006) and the world’s leading cause of disability (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; Hartvigsen et al., 2018). It is believed that maladaptive cognitions and emotional responses to pain are important factors for developing and maintaining chronic pain, as described by the fear avoidance model (Vlaeyen et al., 1995b, 2016). This model describes how, if immediate pain control is prioritized, pain-catastrophizing and pain-related fear may lead to pain-hypervigilance and avoidance behavior, and in turn increased functional disabilities. This then may amplify the pain experience and paradoxically increases pain-related fear, creating a vicious cycle. A subgroup of patients with cLBP indeed shows pain-related fears, including fear of movement and/or re-injury (Crombez et al., 1999; Vlaeyen and Crombez, 1999; Camacho-Soto et al., 2012; Thibodeau et al., 2013; Bunzli et al., 2015; Hartvigsen et al., 2018). In fact, pain-related fear is more closely linked to disability than pain intensity (Crombez et al., 1999; Zale et al., 2013).
To specifically target pain-related fears in clinical settings, Exposure in vivo (EXP) was developed. EXP is a cognitive-behavioral treatment based on experimental work showing that exposure to fearful activities and movements, rather than avoiding them, challenges catastrophic pain beliefs and can result in the extinction of fears and maladaptive responses (Vlaeyen et al., 1995a; Meulders and Vlaeyen, 2012). In EXP, movements and activities that are perceived as threatening and fearful are first identified using the pictorial tool The Photographic Series of Daily Activities (PHODA) (Leeuw et al., 2007). Then, the patient is repeatedly exposed to these feared movements and activities, while behavioral experiments are performed to challenge catastrophic expectations and interpretations regarding these movements, activities, and/or sensations. EXP has been applied as treatment for patients with chronic pain and elevated pain-related fear in a variety of settings and different pain conditions, including, but not limited to, non-specific cLBP. Ubiquitously, EXP has been successful in reducing pain-related fears and pain-related disability as compared to no treatment and at least as successful, if not more successful, in comparison to other treatments that are proven effective (Vlaeyen et al., 2001; Boersma et al., 2004; de Jong et al., 2005, 2008, 2012; Leeuw et al., 2008; Woods and Asmundson, 2008; den Hollander et al., 2016; Lalouni et al., 2016; Lopez-de-Uralde-Villanueva et al., 2016; Glombiewski et al., 2018).
It would be expected that EXP specifically impacts the neural circuitry involved in pain-related fear and fear extinction learning. Studies examining pain-related fear have identified altered neural responses in patients with cLBP to viewing and imagining activities/movements associated with pain (Taylor et al., 2015; Meier et al., 2016, 2017) – including increased recruitment of the insula, anterior cingulate cortex (ACC), amygdala, orbitofrontal cortex, striatum (i.e., regions involved in attentional/perceptual as well as affective/reappraisive aspects of pain), and altered crosstalk with the periaqueductal gray (PAG; involved in top-down pain modulation). For fear conditioning and extinction, experimental studies identified a core neural network, including the amygdala, insula, and ACC (Sehlmeyer et al., 2009; Fullana et al., 2016, 2018b). Only few imaging studies investigated fear learning and extinction in the context of pain (Kattoor et al., 2013; Labus et al., 2013; Icenhour et al., 2015), reporting altered neural responses in patients, including in the prefrontal cortex (PFC), ACC, insula, amygdala, hippocampus, PAG and thalamus. Further, results of clinical studies in chronic pain investigating treatment-induced functional brain changes show some overlap with neural changes related to pain-related fear and experimental fear extinction (e.g., implicating the amygdala, mPFC, and PAG) (Baliki et al., 2008; Becerra et al., 2014; Erpelding et al., 2014; Simons et al., 2014). The majority of treatment studies focused on intrinsic brain activity, i.e., in rest and without a specific task (Napadow et al., 2012; Harris et al., 2013; Bosma et al., 2018). The effects of EXP specifically have also only been investigated using resting-state fMRI (Zhu et al., 2018), showing that patients with post-traumatic stress disorder showed enhanced post-treatment resting-state functional connectivity between the amygdala, orbitofrontal cortex, hippocampus and the medial PFC. To date, there have been no studies investigating how (EXP) treatment modulates the circuitry underlying pain-related fear in chronic pain.
Therefore, the current longitudinal fMRI study tested the hypothesis that EXP acts upon the neural circuitry involved in pain-related fear, using a task designed to evoke pain-related fear. We compared patients with cLBP with pain-free volunteers pre- and post-EXP treatment; the cLBP group was also examined 6 months after end of treatment. We evaluated group differences and treatment effects in evoked brain activation. Also, more exploratively, we used (changes in) fear ratings to identify neural correlates specific to (reductions in) pain-related fear. A whole-brain approach was adopted in combination with analyses in a priori defined regions of interest (ROIs) that were considered to be of particular interest due to their involvement in pain-related fear and experimental extinction learning (i.e., amygdala, hippocampus, mPFC, PAG) and/or pain chronification (i.e., mPFC, NAc). We expected (I) pre-treatment group differences in neural circuitry recruited by stimuli evoking pain-related fear, correlated to fear ratings as well as pain-related outcomes in patients; (II) patient-specific pre- to post-treatment changes in regions showing pre-treatment group differences, as well as in other brain regions associated with chronic pain and with extinction (i.e., amygdala, hippocampus, mPFC, NAc, PAG); (III) pre- to post-treatment changes associated with changes in fear and persisting at 6 months follow-up.
This study presents data of a larger study investigating effects of EXP on chronic pain, “BrainEXPain”. BrainEXPain was approved by the Medical Ethical Committee of Maastricht University Hospital/Maastricht University (MUMC+/UM), and the protocol is registered at ClinicalTrials.gov [NCT02347579]. Patient recruitment was done via the department of Rehabilitation Medicine at MUMC+/Adelante rehabilitation center where patients were seen for consultation. If patients were found motivated for rehabilitation treatment and eligible for the multi-disciplinary pain screening program, they were invited by the physiatrist for the study. Recruitment was open between January 2015 and August 2017.
Participants were then contacted by the research team and were screened for in- and exclusion criteria. Informed consent was obtained at study enrollment. Prior to scanning, all participants filled in questionnaires online (Qualtrics, Provo, United States1). The first study visit was scheduled prior to any (information on) treatment (i.e., baseline or pre-EXP). Afterward, patients underwent a multi-disciplinary pain screening and pain education, and started the exposure sessions (if eligible for treatment) – which were all part of standard care. At the end of treatment, patients underwent a post-EXP and a follow-up study visit (6 months after end of treatment; FU-EXP). Healthy controls participated in two study visits, with the time in between these visits matching the patients’ pre- to post-EXP. Participants received €15 per study visit and travel reimbursement for their participation.
Inclusion criteria for patients were age between 18 and 65 years, stable medication,2 experience of non-specific LBP > 6 months, and no other diagnosis explaining the symptoms. Exclusion criteria were claustrophobia, MRI incompatibility (e.g., pacemaker, pregnancy), and severe psychopathology (Symptom Check List-90). Of the 35 patients with cLBP invited by the physiatrist over the 2.5 years inclusion period, 23 patients with cLBP were included in BrainEXPain (8 patients were not interested in participating, 4 patients were MRI incompatible). Of these, three patients dropped out prior to or during the measurement (due to claustrophobia); of two patients the data analyzed here was not acquired due to technical error; three patients were excluded due to extensive motion (see Data Analysis); and one patient was excluded due to lack of any vision-related (occipital) activity (see Data Analysis). The final sample for this study therefore consisted of 14 patients (Table 1). Post-EXP data is available for 10 patients (three did not start EXP, one became MRI incompatible), and FU-EXP data is available for 9 patients (1 was lost to follow-up due to unrelated medical issues).
The patient group was compared to a sample of 14 pain-free healthy volunteers, matched for age, sex and handedness on cohort-level. To match the patient group, 10 controls underwent a second study visit. Controls were recruited through local advertisements. Additional exclusion criteria were: history of a chronic pain syndrome, and seeking treatment for a pain condition in the last 6 months.
Within MUMC+/Adelante, EXP is standard care for patients with cLBP presenting with elevated pain-related fear. No additional restrictions or requirements for EXP were set by BrainEXPain. EXP specifically aims to reduce disability by challenging erroneous interpretations and expectancies about pain (e.g., that pain always indicates harm or that activities cause harm). A detailed description of the exposure-protocol for pain-related fear can be found in Vlaeyen et al. (2012). In brief, EXP always started with identifying movements/activities that are perceived as threatening and fearful, education about treatment rationale and that harm or pain does not mean additional injury (i.e., by discussing MR images of the spine by the treating physiatrist). EXP then continued with repeated exposure to feared movements, activities and/or sensations combined with behavioral experiments to challenge catastrophic interpretations by creating violations of expectancies. Patients were furthermore instructed to keep performing the movements and/or activities they performed during their sessions. EXP typically consists of 16 sessions (although it could be shortened to 8 or extended to 20, per clinicians’ decision), which are guided by a psychologist and either a physical or an occupational therapist. To identify movements and activities that are perceived as threatening and fearful, EXP utilizes The Photographic Series of Daily Activities (PHODA) for the low back (Leeuw et al., 2007). The PHODA consists of photographs depicting back-related movements and activities that are rated based on their perceived harmfulness. See Table 2 for more participant and EXP-related characteristics.
At all time-points we assessed: pain intensity using a 0–10 visual analog scale anchored with “no pain at all” and “worst pain imaginable”; pain-related fear using the PHODA short electronic version for low back (Leeuw et al., 2007), and Tampa Scale for Kinesiophobia (TSK; Kori, 1990; Vlaeyen et al., 1995a), Pain Catastrophizing Scale (PCS; Sullivan et al., 1995; Crombez et al., 1999), Pain Disability Index (PDI; Tait et al., 1987; Soer et al., 2013), Physical Activity Rating Scale combined with the Perceived Activity Decline (PARS/PAD; Vercoulen et al., 1997; Verbunt, 2008) questionnaire. Only assessed at baseline as trait measures were: Fear of Pain Questionnaire (PFQ; McNeil and Rainwater, 1998; van Wijk and Hoogstraten, 2006) and State Trait Anxiety Inventory (STAI-Y2; van der Ploeg et al., 1980; Spielberger et al., 1983). In addition, all participants underwent performance testing during all study visits to assess functioning. In the 2 min walking test, participants walked for 2 min on a standardized track and the covered distance was measured in meters. During staircase walking, participants walked a complete staircase (up and down), after which the average time per step was calculated.
In the scanner, the participants were presented with visual stimuli, associated with one of three categories: rest (derived from a web-search – REST), movements and activities perceived as fearful for patients specifically (derived from the extended version of the PHODA, not used in pain assessment and/or treatment – MOVEMENT), or pictures implying bodily damage that may be perceived as fearful in general (derived from IAPS (Lang et al., 1997) and a web-search – MEDICAL). Backgrounds were removed to make the physical properties as similar as possible.
Participants were instructed to carefully look at the pictures and imagine that they were the person in the picture (carrying out the movement or activity, if applicable). After a short delay (see Figure 1 for details), participants were asked to rate how they would feel if they were the person on the picture (indirect assessment of fear). Ratings were done by pressing a button that moved a cursor on a horizontal line presented on the screen (later converted to 0–10 scores). In total, there were 21 trials (7 of each category). Stimuli were presented using Presentation Software (Neurobehavioral Systems Inc.), and were synchronized with MR data acquisition. The total task had a duration of approximately 8 min. The picture imagination task was always performed second, after a resting-state run. The total duration of the scan was approximately 75–90 min (data from other runs will be described elsewhere).
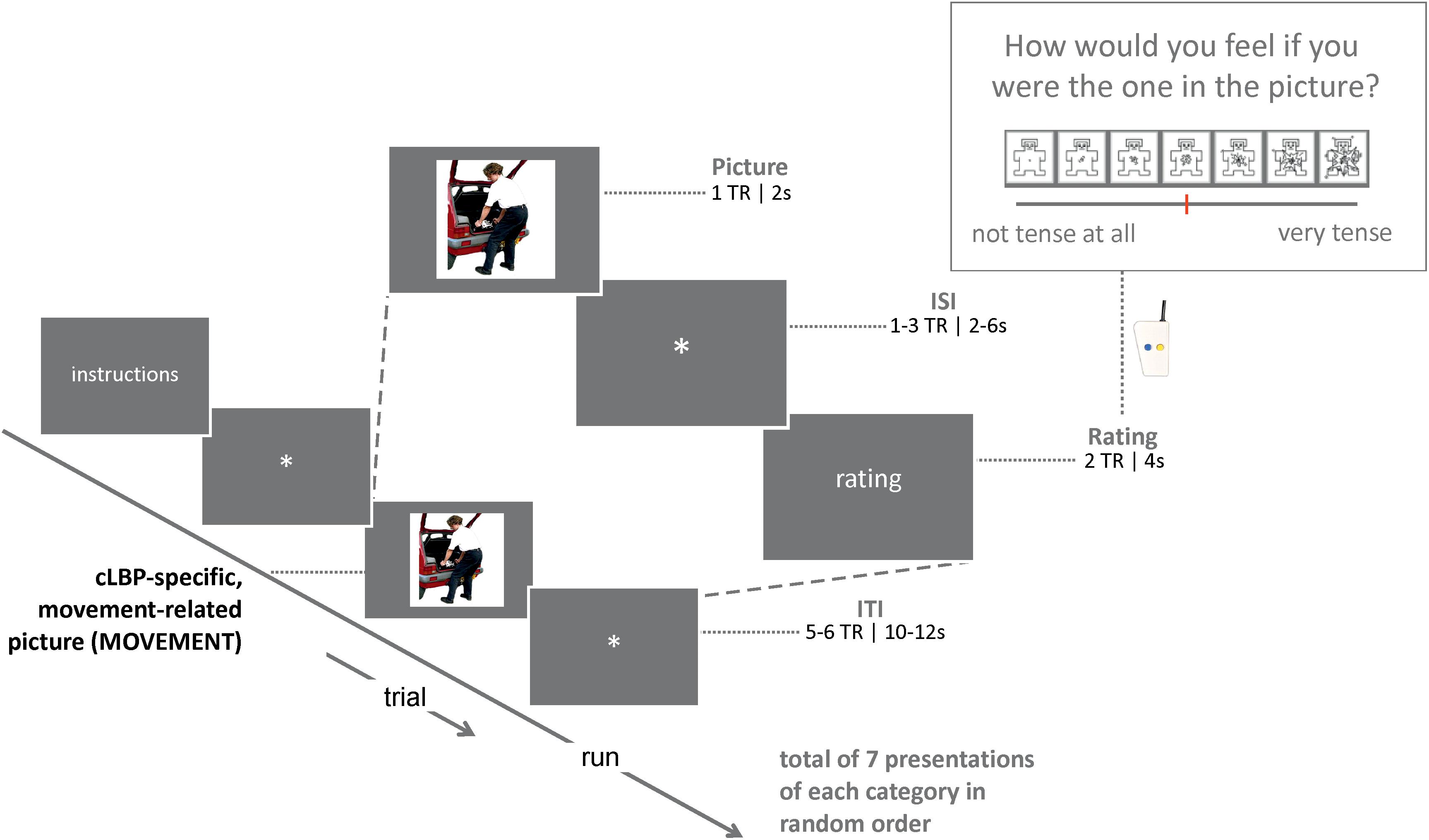
Figure 1. Schematic overview of the design of the picture imagination task, showing the elements plus corresponding timing and a zoom into one example trial; TR, repetition time; ISI, inter-stimulus time; ITI, inter-trial time.
MRI data were collected using a 3 Tesla whole body MRI scanner (Philips Gyroscan Achieva TX) using a 32-channel head coil, at the department of Radiology at MUMC+.
For the functional images, a T2∗-weighted standard echo-planar imaging (EPI) sequence was used to acquire 40 axial slices (3 mm isotropic) covering the entire cortical volume, using the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 25 ms, flip angle = 75°, matrix size = 120 × 240, SENSE factor = 2. In total, 225 functional volumes were collected, of which the first two volumes were dummy volumes that were discarded from subsequent analysis to avoid T1 saturation effects.
T1-weighted anatomical images were acquired using a 3D turbo field echo (TFE) sequence with the following parameters: 170 slices, 1 mm isotropic, TR = 8.1 ms, TE = 3.7 ms, flip angle = 8°, matrix size = 240 × 240.
Questionnaire and performance test data were analyzed using SPSS (version 24). A general linear model (GLM) with Group (patients, controls) as between-subjects (BS) factor was used to examine group differences pre-EXP, as well as post-EXP. In addition, a repeated measures (rmGLM) with Time [pre-EXP, post-EXP, (FU-EXP)] as a within-subjects (WS) factor was used to investigate changes over time.
Group comparisons in in-scanner fear ratings, focusing on MOVEMENT pictures, were evaluated using a rmGLM with Group (patients, controls) as BS factor and Picture Number (7 different Pictures per Category) as WS factor. In addition, the WS factor Time [pre-EXP, post-EXP, (FU-EXP)] was added in a separate analysis.
MRI data analysis was performed using BrainVoyager 3.6 (Brain Innovation, Maastricht, the Netherlands). Pre-processing of the functional data included slice scan time correction, 3D head motion correction, linear trend removal, high-pass filtering (5 cycles per run; corresponding to 0.1 Hz), and spatial smoothing [4 mm using a full-width at half-maximum Gaussian kernel (FWHM)]. Data was then co-registered to the corresponding anatomical image, and normalized to MNI space. The three pictures categories (REST, MOVEMENT, and MEDICAL) plus the delay prior to the rating (i.e., in total 4–8 s) were used as predictors, convolved with the hemodynamic response function (HRF). Additional information on denoising procedures can be found in Supplementary Information.
Whole-brain analyses were run within a mask that excluded the white matter and cerebral spinal fluid, based on the Harvard-Oxford atlases (probability threshold 0.25) (Frazier et al., 2005; Desikan et al., 2006; Makris et al., 2006; Goldstein et al., 2007). To specifically test our hypotheses in brain regions that play important roles in chronic pain and/or fear extinction, additional analyses were run within predefined region-of -interest (ROI) masks. ROIs were defined in bilateral medial frontal cortex (mPFC), bilateral amygdala, bilateral nucleus accumbens (NAc), bilateral hippocampus based on the Harvard-Oxford subcortical atlas (probability threshold 0.25). A ROI corresponding to bilateral PAG was defined by dilating spheres around coordinates from Linnman et al. (2012) [x = 1, y = –29, z = –10 (volume = 1612 mm3, diameter ∼14.5 mm)]. In these ROI masks, FDR correction [q(FDR) < 0.05] and minimum cluster size of 4 voxels (108 mm3) was used for statistical thresholding.
To compare blood-oxygen-level dependent (BOLD) responses across Groups and Times, a univariate random-effects (RFX) analysis with separate subject predictors was run at the first level, after which this data was fed into a second-level RFX analysis where group maps could be estimated and contrasted. FDR correction [q(FDR) < 0.05] was used for map creation. In the whole-brain analysis, an initial threshold of p < 0.001 was used for contrasts across Groups and Times, after which cluster-size thresholding was performed using MonteCarlo simulations (n = 1000) to correct maps at the level of alpha 0.05. The main contrast of interest was MOVEMENT vs. baseline, plus effects of Group and Time herein, as this condition was designed to elicit pain-related fear specifically in the patient group.
Two types of correlation analyses were performed. From regions in which significant Group and Time differences were observed, betas were extracted in order to perform correlation analyses with measures of pain-related outcomes. An additional, explorative, analysis for the patients was to examine correlations between changes in fear ratings and changes in neural activation patterns at a whole-brain level. For this, we used the percentage of change in fear ratings for MOVEMENT pictures (at post- and FU-EXP compared to pre-EXP), and took a less conservative initial cluster-defining threshold of p < 0.005 for the cluster-size thresholding.
Pre-treatment, patients reported significantly higher levels of pain, pain-related fear, catastrophizing and disability compared to controls (Table 3). Groups furthermore differed in trait anxiety, but not in trait fear of pain. Also, patients reported significantly lower levels of physical activity and higher levels of perceived activity decline compared to controls. Lab-assessed performance tests confirmed this: patients covered significantly less distance within 2 min walking, and needed more time to walk stairs, compared to controls.
The in-scanner fear ratings for MOVEMENT pictures showed a significant Group effect [F(1,26) = 188.15, p < 0.001, ηp2 = 0.88, 95% CI = 5.6, 7.5], where patients reported higher fear levels compared to controls (Figure 2 and Supplementary Figure S1 for fear ratings for all Picture Categories). Also, for patients, fear ratings were significantly and strongly correlated with pain-related fear as assessed using the PHODA (r = 0.64, p = 0.01) (Figure 2).
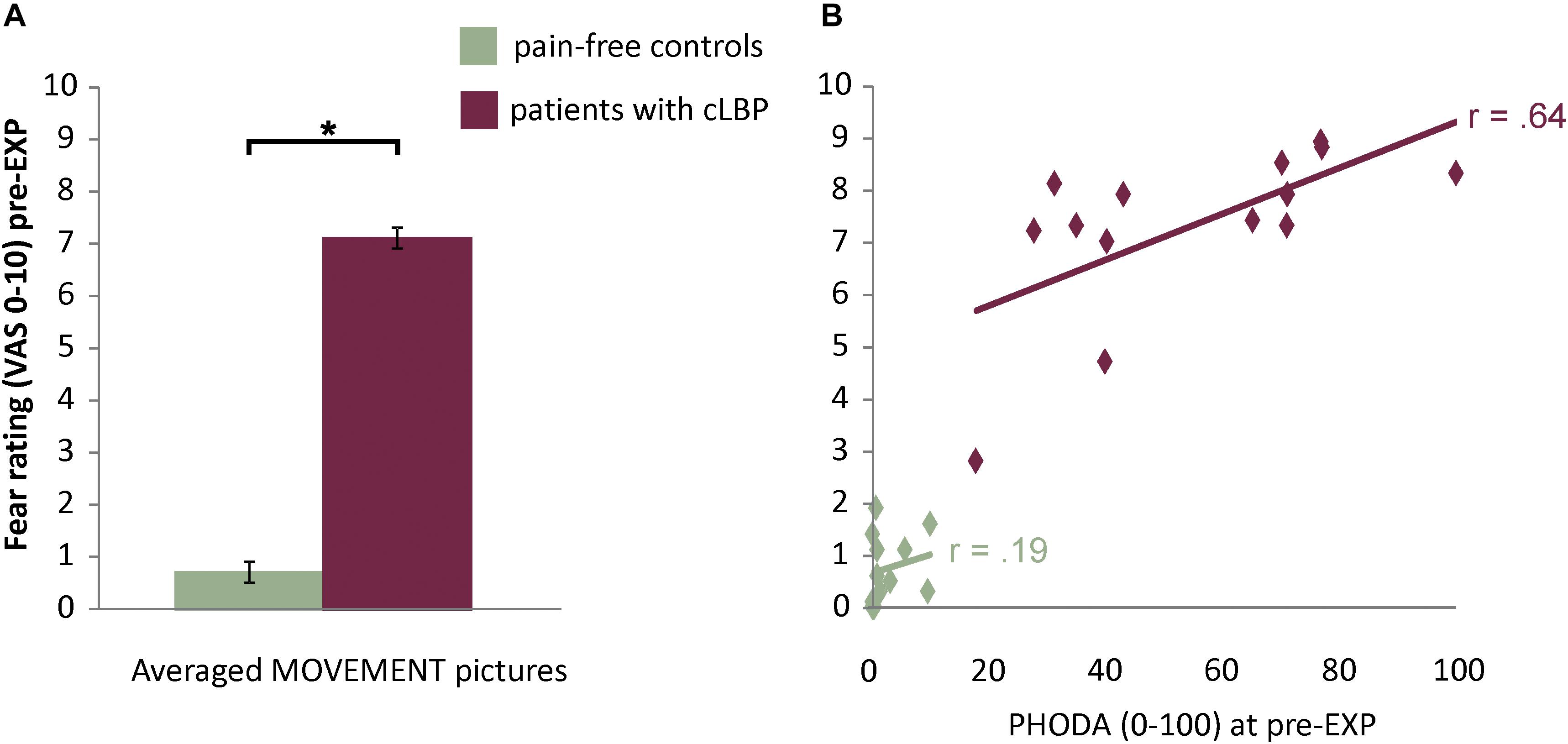
Figure 2. (A) Fear ratings for the MOVEMENT pictures. Presented are means and standard errors for each group. Horizontal lines and asterisks indicate significant effects (∗p < 0.05). (B) Correlation between fear ratings for MOVEMENT pictures and pain-related fear as assessed using the PHODA.
Figure 3 shows activation maps for the MOVEMENT pictures, per Group (see Supplementary Figure S2 for activation maps of all Picture Categories). Overall, the MOVEMENT pictures elicited activation in a similar network in patients and controls.
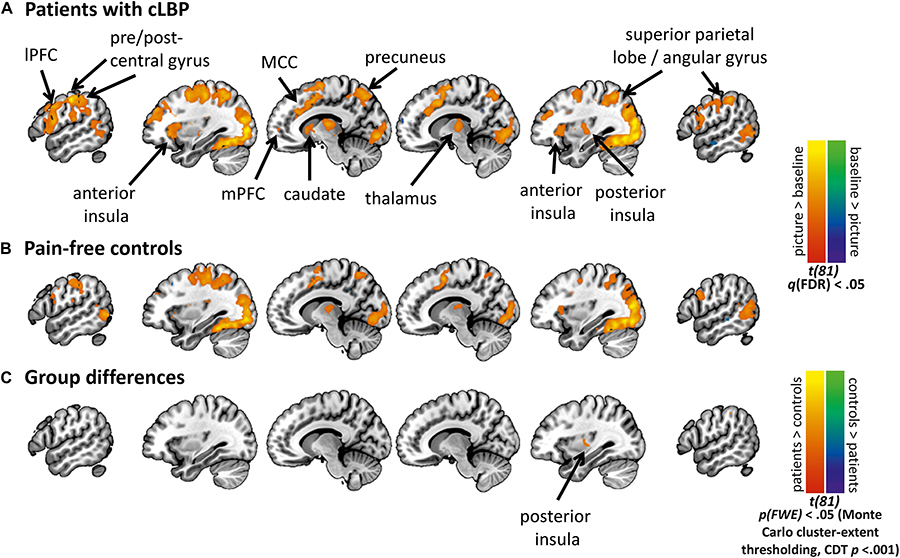
Figure 3. Activation maps for the MOVEMENT Picture Category at pre-EXP, per group. Statistical maps are presented showing the neural activation of the MOVEMENT category relative to baseline for (A) Patients with cLBP, and (B) Pain-free volunteers, (C) Group differences in MOVEMENT condition. Cluster-level correction using p < 0.001 as initial threshold. CDT, cluster-defining threshold. lPFC, lateral prefrontal cortex; mPFC, medial prefrontal cortex; MCC, mid-cingulate cortex.
The whole-brain analysis showed a significant group difference in the right posterior insula (MNI x = 33, y = −10, z = 10, k cluster size = 206 mm3), with patients showed increased BOLD activation compared to controls (Figures 3, 4A). The masked analyses in the pre-defined ROIs additionally showed a difference in mPFC (MNI x = 0, y = 41, z = −11, k = 4 mm3), with patients showing increased BOLD deactivation compared to controls (Figure 4A, Supplementary Figure S2, and Supplementary Tables S1, S2).
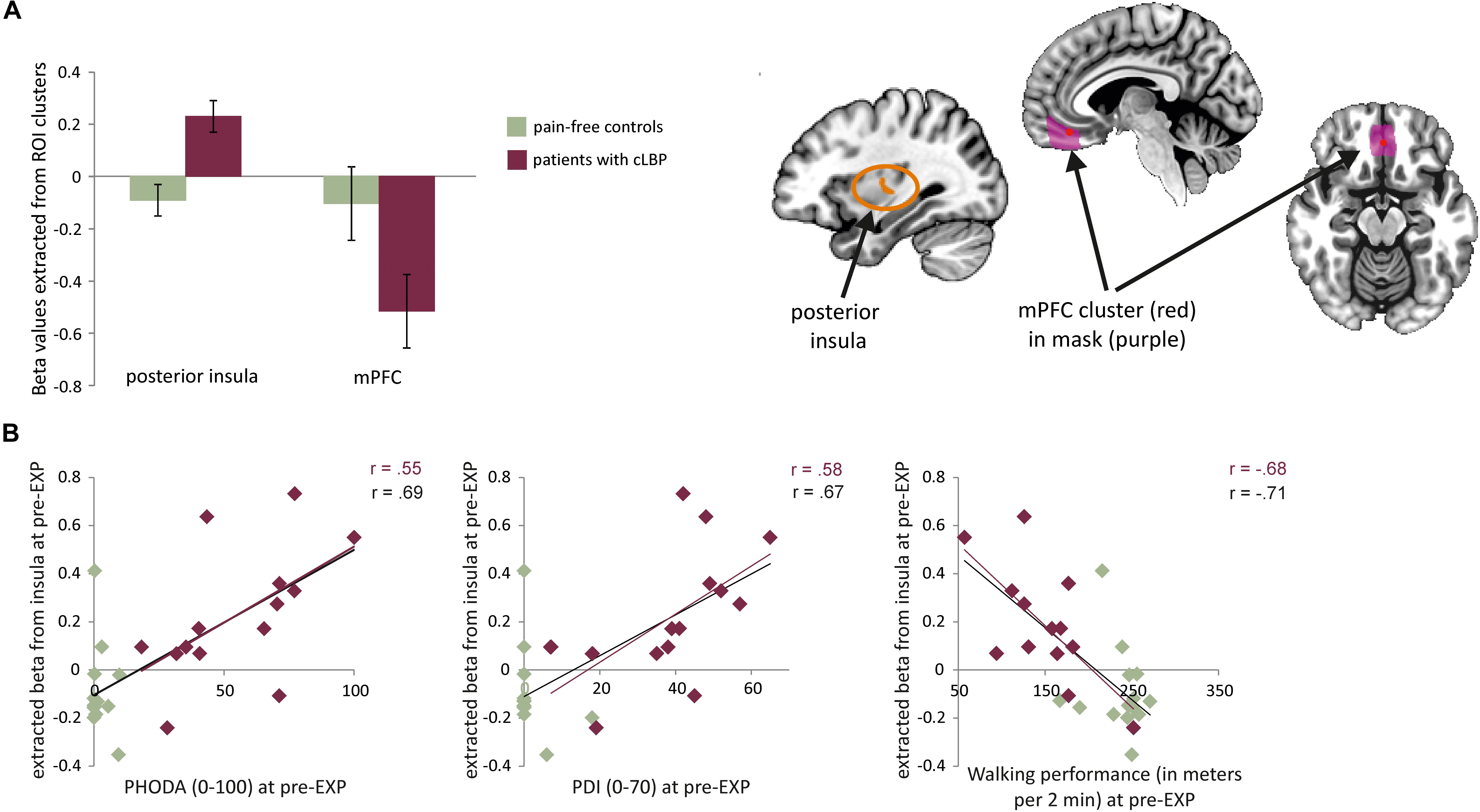
Figure 4. (A) Left: average beta values and standard errors for the MOVEMENT vs. baseline contrast for each group at pre-EXP, extracted from the two areas showing group differences. Right: Depiction of the location of the identified clusters. (B) Correlations between the posterior insula activation (beta value) and pain-related variables (self-reported and performance tasks). Note that the trendlines and magnitude of the correlations are shown for both the whole group (black) as well as the patient group only (red). mPFC, medial prefrontal cortex; PDI, pain disability index.
Correlation analyses were performed using betas extracted from the right posterior insula and mPFC (i.e., averaged across all voxels in the cluster). When investigating the entire sample, both the activation in the posterior insula and mPFC was correlated to pain intensity and pain-related fear. Activation in the posterior insula was furthermore correlated to pain catastrophizing, pain disability, and both performance tests (Table 4). When zooming into the patient group, activation during MOVEMENT pictures in the posterior insula was positively correlated with pain-related fear, pain disability and both performance tasks while activation in mPFC did not correlate with any of the variables (Table 4). For the posterior insula, correlations reflected that increased neural activation was related to increased levels of fear, disability and worse performance (Figure 4B). For the mPFC, the correlations were negative and reflected that decreased neural activation was related to increased levels of fear.
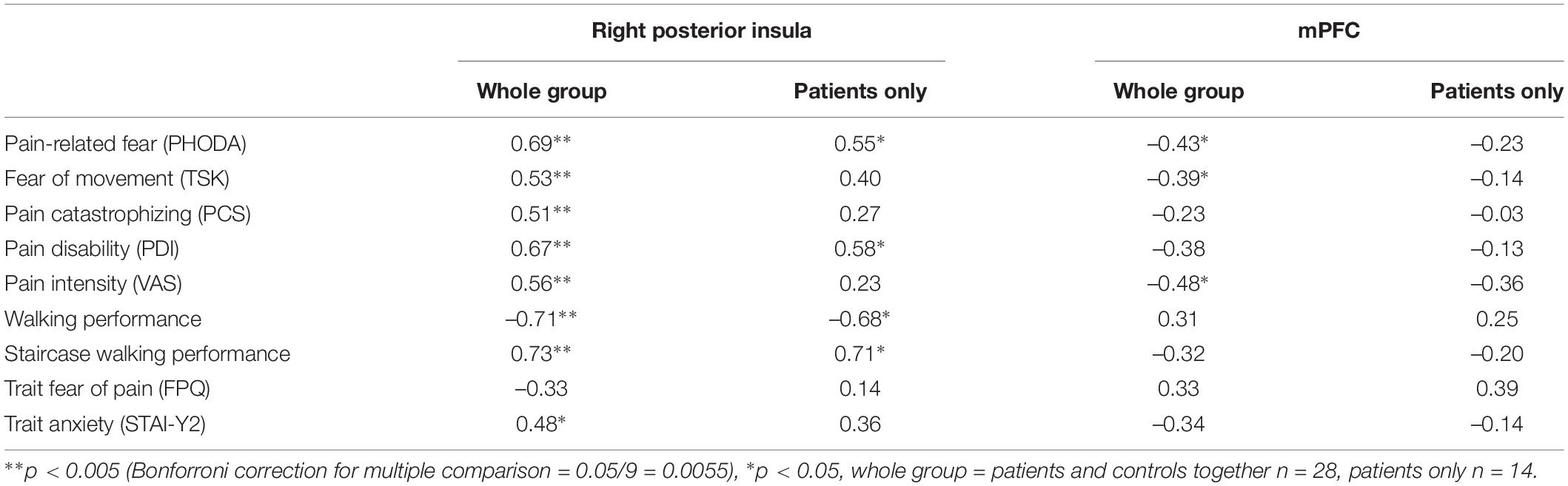
Table 4. Correlations at pre-EXP between pain-related variables and activation in the regions displaying a group difference.
Patients showed main effects of Time for pain-related fear, pain-related disability, perceived activity decline, and the performance tests (Table 5). There were no main effects for pain intensity, pain catastrophizing and self-reported physical activity, although these measures generally showed a decrease, and showed clinically relevant reductions (defined as reduction of 30% or more compared to baseline) in 60, 60, and 40% of patients in these domains, respectively, from pre- to post-EXP (Table 5).
Controls did not show any effects of Time (all p’s > 0.05).
Post-EXP, groups did not differ anymore in fear of movement, pain catastrophizing, self-reported physical activity, and staircase walking. Patients still reported higher pain intensity and pain-related disability compared to controls, and performed significantly worse on the 2 min walking test (Table 6).
There was a significant effect of Time for fear ratings for the MOVEMENT pictures [F(1.56, 12.44) = 24.76, p < 001, ηp2 = 0.76], with a significant decrease in ratings between pre- and post-EXP (p-corr < 0.001, 95% CI = –7.0, –3.2) and between pre- and FU-EXP (p-corr = 0.006, 95% CI = –7.2, –1.4), but no difference between post-EXP and FU-EXP (p-corr = 0.81, 95% CI = –1.3, 2.9) (Figure 5 and Supplementary Figure S3 ratings across all Picture Categories).
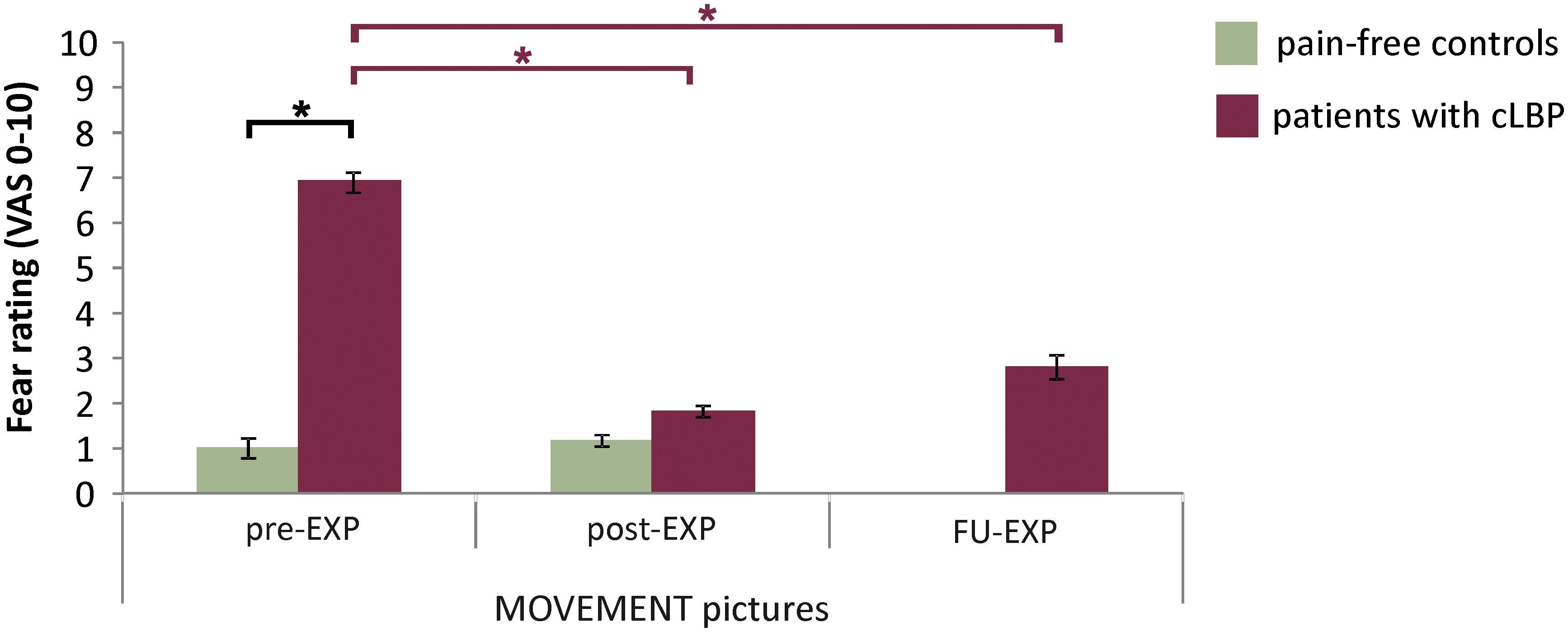
Figure 5. EXP treatment-induced changes in fear ratings. Presented are the means and standard errors for the MOVEMENT pictures for each group across time. Horizontal lines and asterisks indicate significant effects (∗p < 0.05): group effects are shown in black, while simple effects of Session, separate per group are shown in color (red for patients with cLBP; there were no significant Session effects for controls).
There was no significant effect of Time [F(1, 9) = 0.31, p = 0.59, ηp2 = 0.03].
There was a significant Time x Group interaction [F(1, 18) = 55.20, p < 0.001, ηp2 = 0.78]. Simple effects per time point showed that at post-EXP, there was no longer a Group difference [F(1, 18) = 1.12, p = 0.30, ηp2 = 0.06, 95% CI = –1.9, 0.6].
The effect of Time was investigated in the clusters showing a group difference pre-treatment (extracted betas from right posterior insula and mPFC clusters) as well as in a whole-brain analysis and in the predefined ROI masks.
The posterior insula cluster showed a main effect of Time [F(1.8, 14.8) = 4.06, p = 0.04, ηp2 = 0.34], explained by a linearly decreasing response to MOVEMENT pictures over Time [F(1, 8) = 7.02, p = 0.03, ηp2 = 0.40]. The mPFC only showed a marginally significant main effect of Time [F2.0, 158 = 3.25, p = 0.07, ηp2 = 0.29], explained by linearly increasing response to MOVEMENT pictures over Time [F(1, 8) = 8.7878, p = 0.02, ηp2 = 0.41] (see Figure 6).
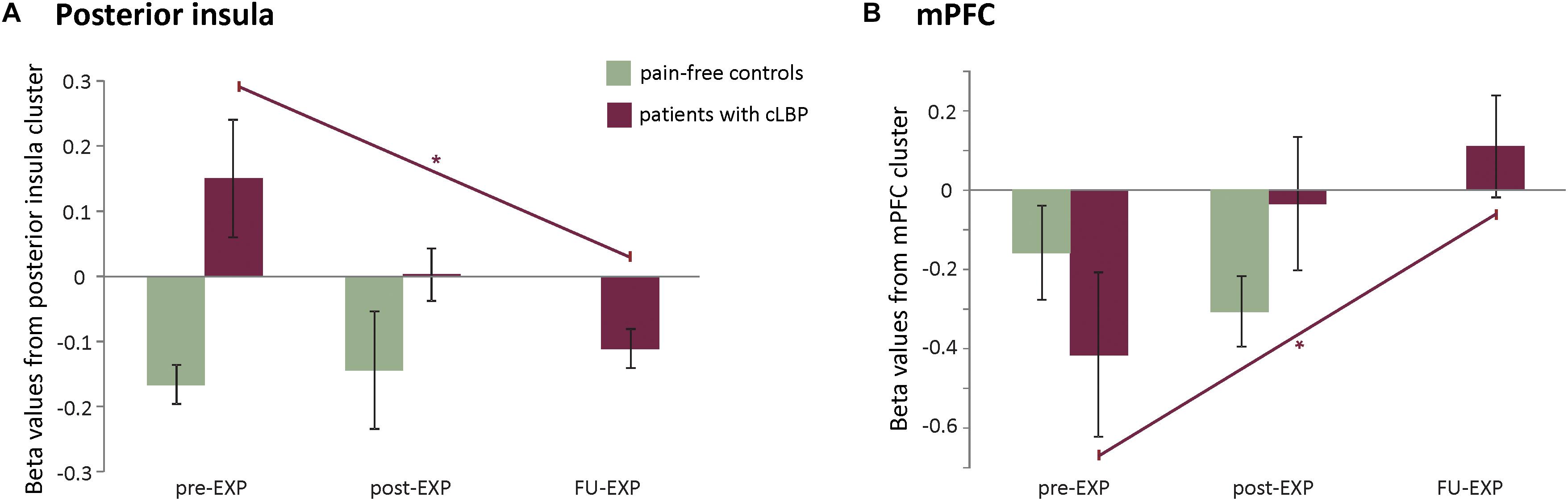
Figure 6. EXP treatment-induced effects in neural activation to MOVEMENT pictures in the posterior insula (A) and mPFC (B). Plotted are averaged beta values and standard errors per time point and per group. Purple lines and asterisks indicate the significant linear effects over Time in patients. ∗p < 0.05.
The whole-brain analyses showed a decrease in right post-central/supramarginal gyrus and pre-central gyrus, and an increase in activity in the precuneus from pre- to post-treatment (Figure 7 and Table 7). Comparing pre-treatment to 6 months follow-up, the right angular/inferior parietal lobe, right post-central, right middle frontal/dorsolateral PFC, right inferior frontal/ventrolateral PFC as well as left middle frontal gyrus showed a significant decrease in activation. Lastly, from post-treatment to 6 months follow-up, the right posterior cingulate cortex showed an additional decrease in activation. When evaluating the effect of Time in the predefined ROIs, there was a significant decrease from pre- to FU-EXP in the NAc (Table 7), but not in the other ROIs.
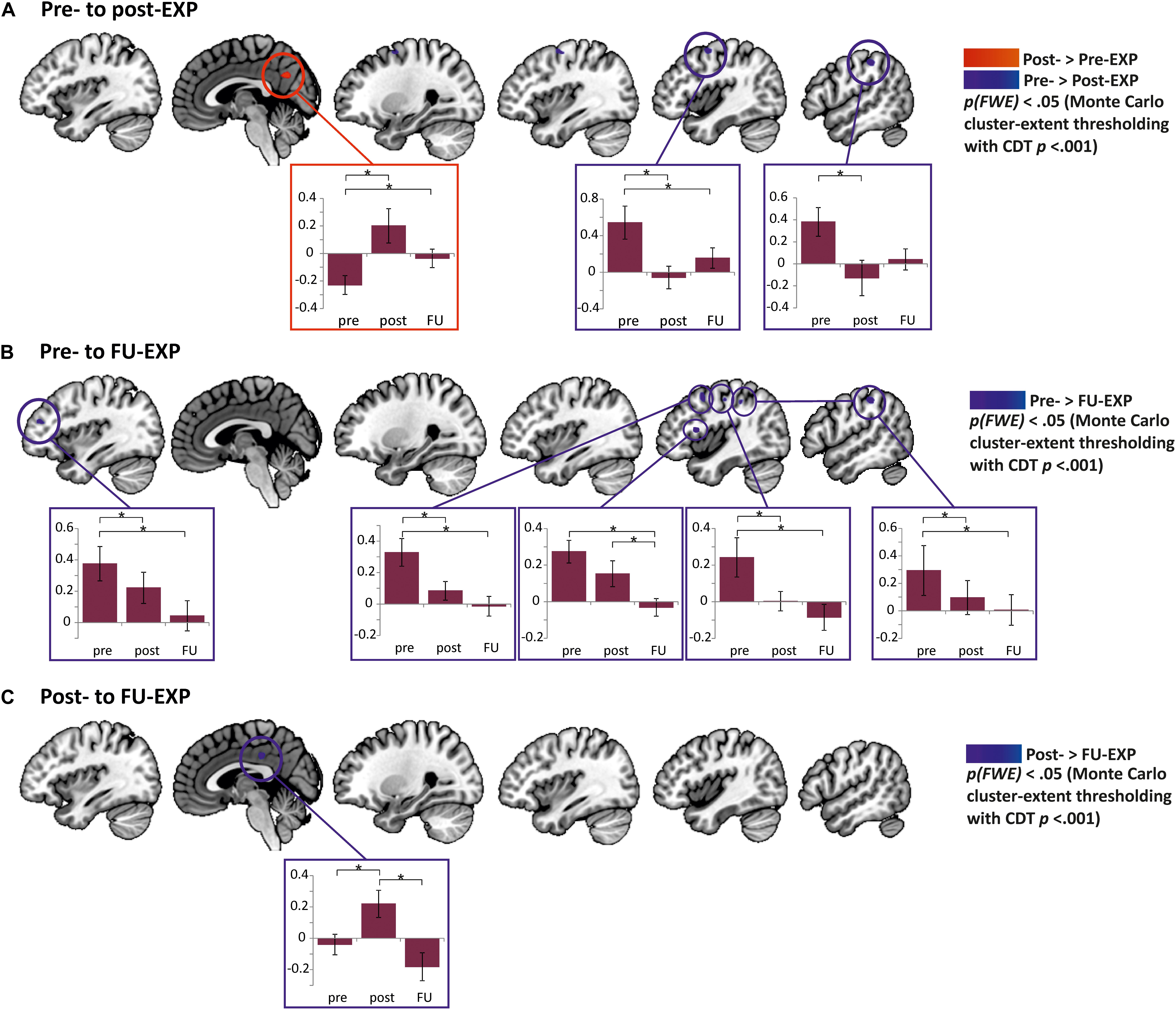
Figure 7. Clusters of EXP treatment-induced changes in neural activation to MOVEMENT pictures in patients with cLBP. (A) Differences from pre- to post-EXP were observed in precuneus (increase, red) as well as precentral gyrus and postcentral gyrus/supramarginal gyrus (from left to right; both decreases, blue). In the boxes, the extracted betas from the corresponding cluster are presented seperately for pre-, post- and FU-EXP. Significant differences across Sessions are highlighted by an asterisk (p < 0.05). (B) Differences from pre- to FU-EXP along with corresponding beta plots. Significant changes were observed in left middle frontal gyrus, right middle frontal/dorsolateral PFC, right inferior frontal/ventrolateral PFC, right postcentral gyrus, and right angular/inferior parietal lobe (from left to right, all decreases, blue). (C) Differences from post- to FU-EXP changes along with corresponding beta plots. A significant difference was found in the posterior cingulate cortex (PCC, decrease, blue). Cluster-level correction using p < 0.001 as initial threshold. Presented in the boxes are means and standard errors. CDT, cluster-defining threshold.
There were no effects of Time in the posterior insula and mPFC cluster. In controls, the whole-brain analysis revealed a change in two regions that do not overlap with the regions identified in patients (Supplementary Table S4). None of the predefined ROIs showed an effect of Time.
Post-treatment, no group differences were present anymore in the whole-brain analysis (also not when being less conservative with an initial threshold of p < 0.005 for cluster-size thresholding). None of the predefined ROIs showed a group difference post-EXP. In addition, when performing a Group comparison of the extracted betas from these ROIs, no group difference was identified at post-EXP [posterior insula: F(1, 18) = 2.58, p = 0.13, ηp2 = 0.13, 95% CI = −0.38, 0.05; mPFC: F(1, 18) = 2.11, p = 0.16, ηp2 = 0.11, 95% CI = −0.12, 0.63].
We explored whether changes in fear ratings for the MOVEMENT pictures from pre- to post-treatment were associated with specific changes in BOLD activation from pre- to post-treatment in patients. We found indications that a decrease in fear ratings from pre- to post-treatment was correlated to an increase of neural activation in the right hippocampus (MNI x = 30, y = –22, z = –17, k = 396 mm3) and the left temporal pole (MNI x = –42, y = 14, z = –20, k = 568 mm3) (see Figure 8). When extracting beta values, we found that the increase in BOLD activation in both regions was also related to decreases in pain-related fear from pre- to post-EXP (PHODA; left hippocampus: r = –0.82, p = 0.003, temporal pole: r = –0.89, p = 0.001) and to decreases in pain-related disability from pre- to post-EXP (PDI; left hippocampus: r = –0.78, p = 0.007, temporal pole: r = –0.71, p = 0.02).
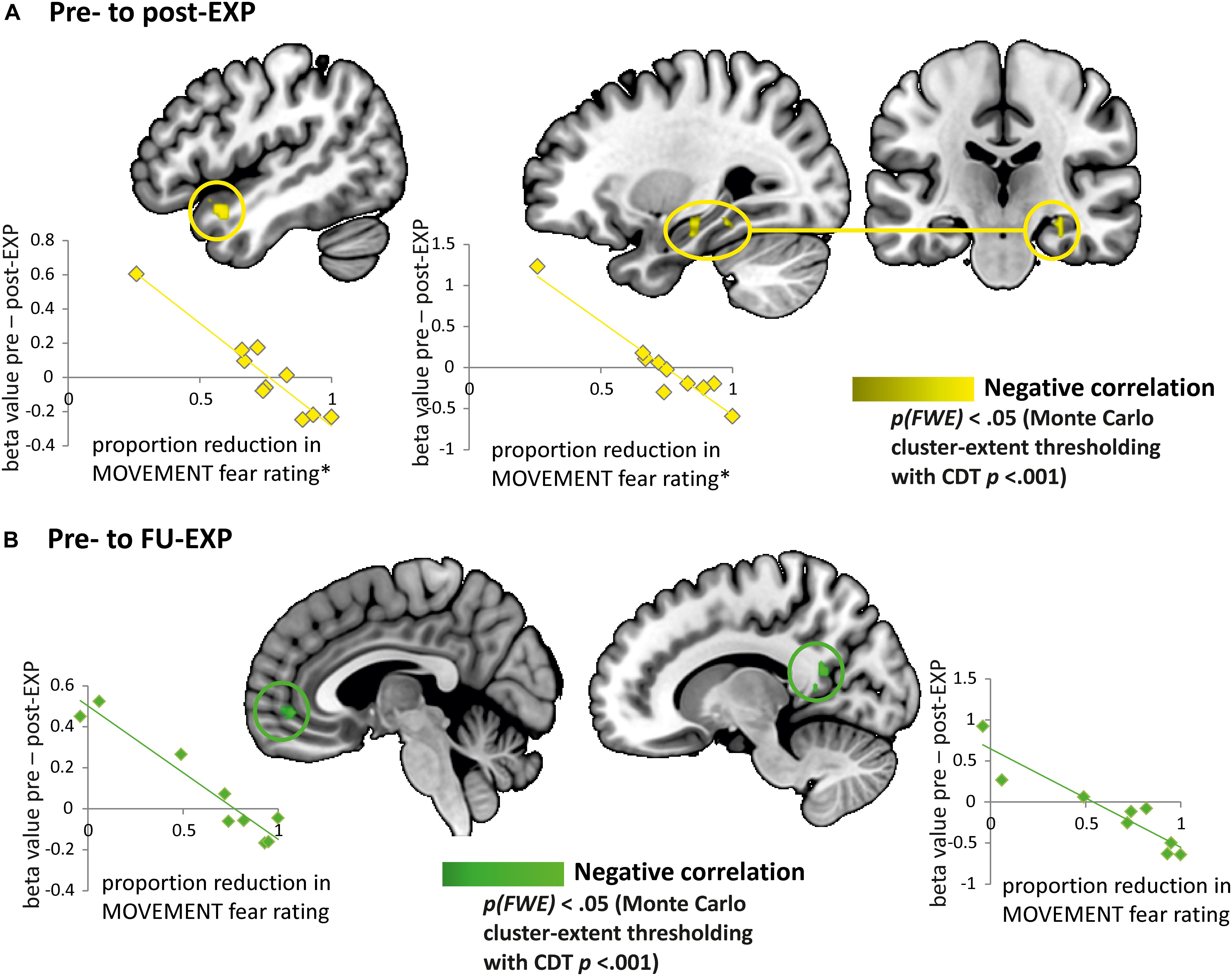
Figure 8. Explorative analyses: EXP treatment-induced changes in fear correlate with changes in neural responses to pain-related fear. (A) Brain regions showing a correlation between change in fear rating and change in neural activation (beta value) from pre- to post-EXP, corresponding to the left temporal pole and the left hippocampus (yellow). The scatterplots present the correlations between the change in neural activation in temporal pole (left) and hippocampus (right) with the proportion of reduction in fear ratings from pre- to post-EXP. ∗Note that the correlations were evaluated with and without the outlier (i.e., the individual with the lowest reduction in MOVEMENT fear rating). The outlier was not influential, as the correlations were still highly significant. (B) Brain regions showing a correlation between change in fear rating and change in neural activation (beta value) from pre- to FU-EXP, corresponding to the ventromedial prefrontal cortex (vmPFC)/anterior cingulate cortex (ACC) and the posterior cingulate cortex (PCC) (green). The scatterplots present the correlations between the change in neural activation in vmPFC (left) and PCC (right) with the proportion of reduction in fear ratings from pre- to FU-EXP. Cluster-level correction using p < 0.005 as initial cluster-defining threshold (CDT).
The decrease in fear ratings from pre- to FU-EXP was furthermore related to an increase in right PCC (MNI x = 6, y = –55, z = 10, k = 407 mm3) and mPFC (MNI x = 0, y = 47, z = –5, k = 564 mm3). The right PCC betas additionally showed significant correlations to decreases in pain-related fear from pre- to FU-EXP (PHODA; r = –0.88, p = 0.002). Figure 8 shows these relations in more detail.
None of these clusters showed a main effect of Time [hippocampus: F(2.0, 15.7) = 0.35, p = 0.71, ηp2 = 0.04; temporal pole: F(1.6, 12.5) = 0.03, p = 0.94, ηp2 = 0.004; PCC: F(1.7, 13.9) = 2.87, p = 0.10, ηp2 = 0.26; mPFC: F(1.5, 12.1) = 0.29, p = 0.69, ηp2 = 0.04].
We provide the first evidence that clinical improvements following EXP in patients with cLBP are mirrored by changes in the neural circuitry for pain-related fear, the main target of EXP. Pre-treatment, we identified group differences in in-scanner fear ratings and neural responses to pictures of back-specific movements: compared to pain-free controls, patients with cLBP showed increased activation in the right posterior insula and increased deactivation in mPFC. Post-treatment, group differences were no longer present, and the process of change continued in patients at 6 months follow-up. Apart from general changes across treatment in lateral PFC, PCC, precuneus, NAc, and pre- and post-central gyrus, patients showed neural changes specifically related to changes in in-scanner fear ratings in the temporal pole, mPFC, PCC, and hippocampus. Pain-free volunteers did not show this, indicating that these changes cannot be attributed to general habituation effects. Hence, we provide evidence for treatment-induced neural changes in chronic pain that are specific to and correlate with improvements in self-reported fear.
As expected, after EXP treatment, pain-related fear and disability significantly decreased while the patient’s performance (i.e., walking and stair case walking) improved significantly. Changes were maintained, or in some cases even more pronounced, 6 months after the end of treatment. We did not observe a significant effect of EXP on pain intensity, which is not uncommon nor unexpected. EXP focuses on reducing pain-related disabilities and reducing pain intensity is no explicit aim. Some studies, however, have observed significant improvements in pain intensity on a group level (den Hollander et al., 2016; Glombiewski et al., 2018), and also in the current study we observed improvements in some patients (i.e., clinically meaningful reduction in 60% of the patients). In future studies, it would be interesting to examine why some people respond with a reduction in pain intensity, while others do not. The lack of effect on pain catastrophizing is surprising though and not expected, given previous studies (see e.g., Leeuw et al., 2008; den Hollander et al., 2016; Lopez-de-Uralde-Villanueva et al., 2016) and the focus of EXP on disconfirming negative beliefs (Vlaeyen et al., 2012; den Hollander et al., 2015). Also for pain catastrophizing, however, we did observe a reduction on average as well as clinically meaningful reductions in 60% of patients (pre to post-EXP), suggesting that there was an effect which did not reach significance due to a relatively small sample size.
We identified two brain regions showing a group difference in neural responses to pain-related fear. In the right posterior insula and mPFC, patients with cLBP showed altered neural activation compared to controls in response to our fear-evoking task. Focusing on pain-related fear, previous studies have demonstrated increased activation in the insula, as well as in other in regions including the ACC, superior parietal cortex, amygdala, orbitofrontal cortex, and striatum in patients compared to controls (Taylor et al., 2015; Meier et al., 2016). A potential explanation for the difference in extent of findings is our more stringent statistical thresholding (Woo et al., 2014) (i.e., with less stringent parameters, additional brain regions showed group differences; and when taking the picture categories together, a multitude of regions differed across groups, including ACC, superior parietal cortex and striatum, see Supplementary Information). Previous work related activation in insula, amygdala and several other regions to the amount of pain-related fear (Meier et al., 2016). Here, we extend these findings by showing that increased posterior insula activation is furthermore related to pain-related disability and actual physical performance (i.e., walking). In addition, its response was parametrically modulated by in-scanner fear ratings (Supplementary Information), further strengthening its specific involvement in pain-related fear. The insula is a core region involved in fear learning (Sehlmeyer et al., 2009; Fullana et al., 2016, 2018b), although loci are typically more anterior. The posterior insula, in contrast, has been associated with interoceptive integration (Craig, 2002), sensory aspects of pain/nociception (Garcia-Larrea and Peyron, 2013; Wager et al., 2013; Segerdahl et al., 2015), and experimental rather than clinical pain (Schweinhardt and Bushnell, 2010). This fits with abundant connections between posterior insula and somatosensory cortex (SI/SII; Wiech et al., 2014). Our finding that posterior insula activation was modulated by fear ratings, however, indicates additional involvement in pain-related fear, possibly due to a top-down modulatory effect of fear on this more sensory region.
The mPFC, and more specifically its ventromedial part (vmPFC), is also a core region involved in fear acquisition and extinction (Sehlmeyer et al., 2009), and general emotion regulation (Sotres-Bayon et al., 2006; Hartley and Phelps, 2010). mPFC involvement in pain and chronic pain is furthermore extensive (Ong et al., 2018). Our finding that mPFC showed a decreased (i.e., increased deactivation) response to fear-evoking stimuli in patients could point toward altered inhibitory control, and reduced ability to modulate or self-regulate pain (Tracey, 2010; Woo et al., 2015; Ong et al., 2018). To our surprise, amygdala activation to feared stimuli was not different across groups. Previous studies consistently reported the amygdala as a brain area of interest in (chronic) pain (see e.g., Simons et al., 2012) and fear or more generally threat (LeDoux, 1993). It may be that functional connectivity rather than neural activation distinguishes patients from controls. This will have to be explored in further analyses.
The increased posterior insula response to our stimuli in patients pre-treatment was reduced over the course of EXP, as was the increased mPFC deactivation. Importantly, we no longer observed group differences post-treatment. This is in accordance with normalizations observed in fear ratings as well as in most clinical measures. Treatment effects were still present or even increased at 6 months follow-up, suggesting generalization to daily life. This is in accordance with a recent RCT in complex regional pain syndrome, where EXP effect sizes were larger at 6 months follow up compared to post-treatment (den Hollander et al., 2016).
Furthermore, several brain regions showed changes in neural responses across treatment, including pre- and post-central gyrus/supramarginal gyrus, precuneus, lateral PFC, and NAc. In pre- and post-central gyrus/supramarginal gyrus, we observed decreases from pre- to post-EXP and from pre-EXP to follow-up. Recruitment of these areas associated with motor control, sensory properties of somatosensory stimuli (Peyron et al., 2000), as well as sensorimotor imagery (McNorgan, 2012; Hetu et al., 2013) was expected, as participants were imagining performing movements and activities depicted in the stimuli. Functional changes in sensorimotor regions have previously been identified in chronic pain (Flodin et al., 2014; Kregel et al., 2015). The changes over time we observed may reflect normalizations in sensorimotor neurocircuitry, and along similar lines it may also reflect changes in physical performance that go alongside with EXP, as an indirect result of reducing pain-related fear. The precuneus, on the other hand, showed increased activation over the course of treatment. The precuneus is part of the default-mode network (DMN), involved in interoception, mentalizing, integrating information more than processing it (Cavanna and Trimble, 2006). Its activation has been negatively correlated to pain sensitivity, without contributing to the actual neural representation of pain (Goffaux et al., 2014), the direction of which is in line with our findings. Interestingly, in fibromyalgia, abnormalities in connectivity between the insula (including posterior part) and the DMN have been observed (Napadow et al., 2010), and changes herein and in posterior insula glutamate levels have been observed following treatment-induced pain reductions (Napadow et al., 2012; Harris et al., 2013). Two prefrontal clusters, one in dorsal, one in ventral lateral PFC and a subcortical NAc cluster showed decreased activation from pre-EXP to 6 months follow-up. The NAc is a major reward center of the brain, and has been implicated in the regulation of pain (Woo et al., 2015) and in the chronification of pain (Baliki et al., 2012; Borsook et al., 2016). It is also associated with experiencing pain in the chronic phase (Hashmi et al., 2013), representing its motivational value. Our finding indicates that EXP also induces changes in the motivational component of pain and associated pain-related cues (e.g., reduced motivational salience of the back-related pictures following EXP). The dlPFC is also involved in the regulation of pain (Lorenz et al., 2003; Seminowicz and Moayedi, 2017), and abnormally increased activation has been observed in chronic pain (Seminowicz and Moayedi, 2017). Interestingly, following treatment, activation in the dlPFC during a cognitively demanding task as well as increases in cortical thickness were normalized (Seminowicz et al., 2011). In contrast, the vlPFC has been associated with affective/motivational processing, and control of goal-directed behavior (Taylor et al., 2004; Sakagami and Pan, 2007). It has extensive connections with orbitofrontal cortex and subcortical areas such as the amygdala, and also interacts with motor regions to orient attention (Corbetta and Shulman, 2002). Neural changes in this region to pain stimuli have been observed following CBT in fibromyalgia, but in opposite directions (Jensen et al., 2012). Importantly, additional analyses show that such changes did not occur in controls (Supplementary Information), suggesting that these time-dependent changes are not due to general habituation effects, but instead specific to the patient group and likely attributable to treatment.
We explored whether fear reduction was associated with specific changes in neural activation to our stimuli. In these explorative analyses, we found indications that pre- to post-EXP decreases in fear ratings were associated with neural activation increases in right hippocampus and left temporal pole. Decreased ratings from pre-EXP to follow-up were associated with increases in the mPFC and PCC. The mPFC, PCC, and hippocampus are associated with fear extinction (Sehlmeyer et al., 2009). Reduced hippocampal volumes and abnormal hippocampal connectivity have been reported in chronic pain (Mutso et al., 2012, 2013). Treatment-induced increases in mPFC neural activation in relation to decreases in fear is in agreement with increased inhibitory control occurring during fear extinction. Cautiously, our findings suggest that extinction during EXP may reflect similar working mechanisms as observed during experimental extinction studies. Noted, the initial cluster-defining statistical threshold (CDT) for cluster-size thresholding was slightly less conservative (p < 0.005), which we consider fair given the additional constraints of the analysis. Also note that these regions did not show main effects across treatment, suggesting individual rather than group-level differences. Future analyses will have to investigate whether there are functional connectivity alterations between mPFC and amygdala, which would be the hypothesized mechanism of extinction (Phelps et al., 2004; but also see Fullana et al., 2018a; Morriss et al., 2018).
Our findings should be interpreted in light of its limitations. First, there was no control treatment, hence we cannot infer that neural changes are specific to EXP. Though, our pain-free control group did control for effects of practice and time. And as we focused on pain-related fear -the main target of EXP-, related findings to within-session fear ratings as well as to clinical assessments of pain-related outcomes, this adds to the specificity of our findings. Second, the focus here is on the MOVEMENT category, because it is most relevant for our patient group, but also for simplicity reasons. Not all findings were specific to this category (e.g., the other two categories also showed pre-EXP posterior insula differences). However, most importantly, time-dependent changes in these regions were specific to this category (Supplementary Information). Finally, the relatively small sample size may have comprimised our statistical power, and motivated us to focus on the whole-brain correlation analysis only (i.e., no other correlations with changes over time), limiting the generalizability of our findings. Several participants could not be included in our analyses or were lost to follow-up, partly because our study was conducted amidst clinical standard care (e.g., the patient and/or clinical team decided not to start EXP), and partly due to the challenges of conducting MRI research in clinical pain populations. Despite that, we show strong data of group differences as well as changes across time, all surviving stringent statistical testing. Larger samples will be needed to reproduce the current findings, and to extend to models predicting treatment responses.
We show the first evidence that clinical improvements in chronic pain following EXP treatment are mirrored by changes in pain-related fear neural circuitry. Group differences identified prior to treatment were no longer present after treatment. Time-dependent effects in patients continued up to 6 months after the end of EXP, and involved regions implicated in cognitive/affective, motivational as well as sensory aspects related to pain. This suggests that the effects of EXP are long-term and go above and beyond modulating fear circuitry. Lastly, explorative analyses found indications that brain regions implicated in fear extinction -including the hippocampus, PCC and mPFC- changed their neural response proportionate to the change in self-reported fear, suggesting that extinction during EXP may reflect similar working mechanisms as extinction in experimental settings. Taken together, our findings show that neural circuitry for pain-related fear is modulated by EXP, and that changes are associated with self-reported improvements in pain-related fear.
The datasets generated for this study are available on reasonable request to the corresponding author.
This study involving human participants was reviewed and approved by the Medical Ethical Committee of Maastricht University Hospital/Maastricht University (MUMC+/UM). All participants provided their written informed consent to participate in this study.
IT, JJ, MG, JV, RS, and AK contributed to the conception and design of the study. IT and AK acquired the data and contributed to the data analysis plan. IT performed the data analysis and wrote the manuscript. All authors contributed to the manuscript, and read and approved the final version.
This work was supported by a grant from the Health Foundation Limburg (Stichting Sint Annadal, Maastricht), Board of Directors of Maastricht University Medical Center (MUMC+), and Esperance Foundation (Stichting Esperance).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all participants for their time and effort. We would also like to thank the staff of the Department of Rehabilitation Medicine, and in particular Daniëlle Wijnants, and Department of Radiology of MUMC+/Adelante for their help in (coordinating) the study visits and support with scanning, respectively, and Emma Biggs, Judith Eck, and Johan Vlaeyen for fruitful discussions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2019.00970/full#supplementary-material
Baliki, M. N., Geha, P. Y., Jabakhanji, R., Harden, N., Schnitzer, T. J., and Apkarian, A. V. (2008). A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol. Pain 4:47. doi: 10.1186/1744-8069-4-47
Baliki, M. N., Petre, B., Torbey, S., Herrmann, K. M., Huang, L., Schnitzer, T. J., et al. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 15, 1117–1119. doi: 10.1038/nn.3153
Becerra, L., Sava, S., Simons, L. E., Drosos, A. M., Sethna, N., Berde, C., et al. (2014). Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 6, 347–369. doi: 10.1016/j.nicl.2014.07.012
Boersma, K., Linton, S., Overmeer, T., Jansson, M., Vlaeyen, J., and De Jong, J. (2004). Lowering fear-avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain 108, 8–16. doi: 10.1016/j.pain.2003.03.001
Borsook, D., Linnman, C., Faria, V., Strassman, A. M., Becerra, L., and Elman, I. (2016). Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 68, 282–297. doi: 10.1016/j.neubiorev.2016.05.033
Bosma, R. L., Cheng, J. C., Rogachov, A., Kim, J. A., Hemington, K. S., Osborne, N. R., et al. (2018). Brain dynamics and temporal summation of pain predicts neuropathic pain relief from ketamine infusion. Anesthesiology 129, 1015–1024. doi: 10.1097/ALN.0000000000002417
Breivik, H., Collett, B., Ventafridda, V., Cohen, R., and Gallacher, D. (2006). Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333.
Bunzli, S., Smith, A., Schutze, R., and O’sullivan, P. (2015). Beliefs underlying pain-related fear and how they evolve: a qualitative investigation in people with chronic back pain and high pain-related fear. BMJ Open 5:e008847. doi: 10.1136/bmjopen-2015-008847
Camacho-Soto, A., Sowa, G. A., Perera, S., and Weiner, D. K. (2012). Fear avoidance beliefs predict disability in older adults with chronic low back pain. PM R 4, 493–497. doi: 10.1016/j.pmrj.2012.01.017
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. doi: 10.1038/nrn755
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Crombez, G., Vlaeyen, J. W. S., Heuts, P. H., and Lysens, R. (1999). Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain 80, 329–339. doi: 10.1016/s0304-3959(98)00229-2
de Jong, J. R., Vangronsveld, K., Peters, M. L., Goossens, M. E. J. B., Onghena, P., Bulté, I., et al. (2008). Reduction of pain-related fear and disability in post-traumatic neck pain: a replicated single-case experimental study of exposure in vivo. J. Pain 9, 1123–1134. doi: 10.1016/j.jpain.2008.06
de Jong, J. R., Vlaeyen, J. W. S., Onghena, P., Goossens, M. E. J. B., Geilen, M., and Mulder, H. (2005). Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin. J. Pain 21, 9–17. doi: 10.1097/00002508-200501000-00002
de Jong, J. R., Vlaeyen, J. W. S., Van Eijsden, M., Loo, C., and Onghena, P. (2012). Reduction of pain-related fear and increased function and participation in work-related upper extremity pain (WRUEP): effects of exposure in vivo. Pain 153, 2109–2118. doi: 10.1016/j.pain.2012.07.001
den Hollander, M., Goossens, M. E. J. B., De Jong, J. R., Ruijgrok, J., Oosterhof, J., Onghena, P., et al. (2016). Expose or protect? A randomized controlled trial of exposure in vivo versus physiotherapy in patients with complex regional pain syndrome type 1. Pain 157, 2318–2329. doi: 10.1097/j.pain.0000000000000651
den Hollander, M., Meulders, A., Jakobs, M., and Vlaeyen, J. W. (2015). The effect of threat information on acquisition, extinction, and reinstatement of experimentally conditioned fear of movement-related pain. Pain Med. 16, 2302–2315. doi: 10.1111/pme.12836
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Erpelding, N., Simons, L., Lebel, A., Serrano, P., Pielech, M., Prabhu, S., et al. (2014). Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct. Funct. 221, 1095–1111. doi: 10.1007/s00429-014-0957-8
Flodin, P., Martinsen, S., Lofgren, M., Bileviciute-Ljungar, I., Kosek, E., and Fransson, P. (2014). Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain Connect. 4, 587–594. doi: 10.1089/brain.2014.0274
Frazier, J. A., Chiu, S., Breeze, J. L., Makris, N., Lange, N., Kennedy, D. N., et al. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 162, 1256–1265. doi: 10.1176/appi.ajp.162.7.1256
Fullana, M. A., Albajes-Eizagirre, A., Soriano-Mas, C., Vervliet, B., Cardoner, N., Benet, O., et al. (2018a). Amygdala where art thou? Neurosci. Biobehav. Rev. 102, 430–431.
Fullana, M. A., Albajes-Eizagirre, A., Soriano-Mas, C., Vervliet, B., Cardoner, N., Benet, O., et al. (2018b). Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 88, 16–25. doi: 10.1016/j.neubiorev.2018.03.002
Fullana, M. A., Harrison, B. J., Soriano-Mas, C., Vervliet, B., Cardoner, N., Avila-Parcet, A., et al. (2016). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol. Psychiatry 21, 500–508. doi: 10.1038/mp.2015.88
Garcia-Larrea, L., and Peyron, R. (2013). Pain matrices and neuropathic pain matrices: a review. Pain 154(Suppl.), S29–S43. doi: 10.1016/j.pain.2013.09.001
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 388, 1545–1602. doi: 10.1016/S0140-6736(16)31678-6
Glombiewski, J. A., Holzapfel, S., Riecke, J., Vlaeyen, J. W. S., De Jong, J., Lemmer, G., et al. (2018). Exposure and CBT for chronic back pain: an RCT on differential efficacy and optimal length of treatment. J. Consult. Clin. Psychol. 86, 533–545. doi: 10.1037/ccp0000298
Goffaux, P., Girard-Tremblay, L., Marchand, S., Daigle, K., and Whittingstall, K. (2014). Individual differences in pain sensitivity vary as a function of precuneus reactivity. Brain Topogr. 27, 366–374. doi: 10.1007/s10548-013-0291-0
Goldstein, J. M., Seidman, L. J., Makris, N., Ahern, T., O’brien, L. M., Caviness, V. S., et al. (2007). Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol. Psychiatry 61, 935–945. doi: 10.1016/j.biopsych.2006.06.027
Harris, R. E., Napadow, V., Huggins, J. P., Pauer, L., Kim, J., Hampson, J., et al. (2013). Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 119, 1453–1464. doi: 10.1097/ALN.0000000000000017
Hartley, C. A., and Phelps, E. A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35, 136–146. doi: 10.1038/npp.2009.121
Hartvigsen, J., Hancock, M. J., Kongsted, A., Louw, Q., Ferreira, M. L., Genevay, S., et al. (2018). What low back pain is and why we need to pay attention. Lancet 391, 2356–2367. doi: 10.1016/S0140-6736(18)30480-X
Hashmi, J. A., Baliki, M. N., Huang, L., Baria, A. T., Torbey, S., Hermann, K. M., et al. (2013). Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136, 2751–2768. doi: 10.1093/brain/awt211
Hetu, S., Gregoire, M., Saimpont, A., Coll, M. P., Eugene, F., Michon, P. E., et al. (2013). The neural network of motor imagery: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37, 930–949. doi: 10.1016/j.neubiorev.2013.03.017
Icenhour, A., Langhorst, J., Benson, S., Schlamann, M., Hampel, S., Engler, H., et al. (2015). Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol. Motil. 27, 114–127. doi: 10.1111/nmo.12489
Jensen, K. B., Kosek, E., Wicksell, R., Kemani, M., Olsson, G., Merle, J. V., et al. (2012). Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153, 1495–1503. doi: 10.1016/j.pain.2012.04.010
Kattoor, J., Gizewski, E. R., Kotsis, V., Benson, S., Gramsch, C., Theysohn, N., et al. (2013). Fear conditioning in an abdominal pain model: neural responses during associative learning and extinction in healthy subjects. PLoS One 8:e51149. doi: 10.1371/journal.pone.0051149
Kregel, J., Meeus, M., Malfliet, A., Dolphens, M., Danneels, L., Nijs, J., et al. (2015). Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin. Arthritis Rheum. 45, 229–237. doi: 10.1016/j.semarthrit.2015.05.002
Labus, J. S., Hubbard, C. S., Bueller, J., Ebrat, B., Tillisch, K., Chen, M., et al. (2013). Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology 145, e1–e3. doi: 10.1053/j.gastro.2013.08.016
Lalouni, M., Olen, O., Bonnert, M., Hedman, E., Serlachius, E., and Ljotsson, B. (2016). Exposure-Based cognitive behavior therapy for children with abdominal pain: a pilot trial. PLoS One 11:e0164647. doi: 10.1371/journal.pone.0164647
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, 39–58.
LeDoux, J. E. (1993). Emotional memory: in search of systems and synapses. Ann. N. Y. Acad. Sci. 702, 149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x
Leeuw, M., Goossens, M. E., Van Breukelen, G. J., Boersma, K., and Vlaeyen, J. W. (2007). Measuring perceived harmfulness of physical activities in patients with chronic low back pain: the Photograph Series of Daily Activities–short electronic version. J. Pain 8, 840–849. doi: 10.1016/j.jpain.2007.05.013
Leeuw, M., Goossens, M. E. J. B., Van Breukelen, G. J. P., De Jong, J. R., Heuts, P. H. T. G., Smeets, R. J. E. M., et al. (2008). Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain 138, 192–207. doi: 10.1016/j.pain.2007.12.009
Linnman, C., Moulton, E. A., Barmettler, G., Becerra, L., and Borsook, D. (2012). Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 60, 505–522. doi: 10.1016/j.neuroimage.2011.11.095
Lopez-de-Uralde-Villanueva, I., Munoz-Garcia, D., Gil-Martinez, A., Pardo-Montero, J., Munoz-Plata, R., Angulo-Diaz-Parreno, S., et al. (2016). A systematic review and meta-analysis on the effectiveness of graded activity and graded exposure for chronic nonspecific low back pain. Pain Med. 17, 172–188.
Lorenz, J., Minoshima, S., and Casey, K. L. (2003). Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091. doi: 10.1093/brain/awg102
Makris, N., Goldstein, J. M., Kennedy, D., Hodge, S. M., Caviness, V. S., Faraone, S. V., et al. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 83, 155–171. doi: 10.1016/j.schres.2005.11.020
McNeil, D. W., and Rainwater, A. J. (1998). Development of the fear of pain questionnaire-III. J. Behav. Med. 21, 389–410.
McNorgan, C. (2012). A meta-analytic review of multisensory imagery identifies the neural correlates of modality-specific and modality-general imagery. Front. Hum. Neurosci. 6:285. doi: 10.3389/fnhum.2012.00285
Meier, M. L., Stampfli, P., Humphreys, B. K., Vrana, A., Seifritz, E., and Schweinhardt, P. (2017). The impact of pain-related fear on neural pathways of main modulation in chronic low back pain. Pain Rep. 2:e601. doi: 10.1097/PR9.0000000000000601
Meier, M. L., Stampfli, P., Vrana, A., Humphreys, B. K., Seifritz, E., and Hotz-Boendermaker, S. (2016). Neural correlates of fear of movement in patients with chronic low Back Pain vs. Pain-Free individuals. Front. Hum. Neurosci. 10:386. doi: 10.3389/fnhum.2016.00386
Meulders, A., and Vlaeyen, J. W. (2012). Reduction of fear of movement-related pain and pain-related anxiety: an associative learning approach using a voluntary movement paradigm. Pain 153, 1504–1513. doi: 10.1016/j.pain.2012.04.013
Morriss, J., Hoare, S., and Van Reekum, C. M. (2018). It’s time: a commentary on fear extinction in the human brain using fMRI. Neurosci. Biobehav. Rev. 94, 321–322. doi: 10.1016/j.neubiorev.2018.06.025
Mutso, A. A., Petre, B., Huang, L., Baliki, M. N., Torbey, S., Herrmann, K. M., et al. (2013). Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 111, 1065–1076. doi: 10.1152/jn.00611.2013
Mutso, A. A., Radzicki, D., Baliki, M. N., Huang, L., Banisadr, G., Centeno, M. V., et al. (2012). Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 32, 5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012
Napadow, V., Kim, J., Clauw, D. J., and Harris, R. E. (2012). Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 64, 2398–2403. doi: 10.1002/art.34412
Napadow, V., Lacount, L., Park, K., As-Sanie, S., Clauw, D. J., and Harris, R. E. (2010). Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62, 2545–2555. doi: 10.1002/art.27497
Ong, W. Y., Stohler, C. S., and Herr, D. R. (2018). Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 56, 1137–1166. doi: 10.1007/s12035-018-1130-9
Peyron, R., Laurent, B., and Garcia-Larrea, L. (2000). Functional imaging of brain responses to pain: a review and meta-analysis. Neuropsychol. Clin. 30, 263–288. doi: 10.1016/s0987-7053(00)00227-6
Phelps, E. A., Delgado, M. R., Nearing, K. I., and Ledoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905.
Sakagami, M., and Pan, X. (2007). Functional role of the ventrolateral prefrontal cortex in decision making. Curr. Opin. Neurobiol. 17, 228–233. doi: 10.1016/j.conb.2007.02.008
Schweinhardt, P., and Bushnell, M. C. (2010). Pain imaging in health and disease — how far have we come ? J. Clin. Investig. 120, 3788–3797. doi: 10.1172/JCI43498
Segerdahl, A. R., Mezue, M., Okell, T. W., Farrar, J. T., and Tracey, I. (2015). The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci. 18, 499–500. doi: 10.1038/nn.3969
Sehlmeyer, C., Schoning, S., Zwitserlood, P., Pfleiderer, B., Kircher, T., Arolt, V., et al. (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 4:e5865. doi: 10.1371/journal.pone.0005865
Seminowicz, D., Wideman, T. H., Naso, L., Hatami-Khoroushahi, Z., Fallatah, S., Ware, M., et al. (2011). Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 31, 7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011
Seminowicz, D. A., and Moayedi, M. (2017). The dorsolateral prefrontal cortex in acute and chronic pain. J. Pain 18, 1027–1035. doi: 10.1016/j.jpain.2017.03.008
Simons, L. E., Moulton, E. A., Linnman, C., Carpino, E., Becerra, L., and Borsook, D. (2012). The human amygdala and pain: evidence from neuroimaging. Hum. Brain Mapp. 35, 527–538. doi: 10.1002/hbm.22199
Simons, L. E., Pielech, M., Erpelding, N., Linnman, C., Moulton, E., Sava, S., et al. (2014). The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 155, 1727–1742. doi: 10.1016/j.pain.2014.05.023
Soer, R., Koke, A. J., Vroomen, P. C., Stegeman, P., Smeets, R. J., Coppes, M. H., et al. (2013). Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine 38, E562–E568. doi: 10.1097/BRS.0b013e31828af21f
Sotres-Bayon, F., Cain, C. K., and Ledoux, J. E. (2006). Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry 60, 329–336. doi: 10.1016/j.biopsych.2005.10.012
Spielberger, C. D., Gorsuch, R. L., Lushene, P. R., Vagg, P. R., and Jacobs, A. G. (1983). Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press.
Sullivan, M. J., Bishop, S. R., and Pivik, J. (1995). The pain catastrophizing scale: development and validation. Psychol. Assess. 7, 524–532. doi: 10.1037//1040-3590.7.4.524
Tait, R. C., Pollard, C. A., Margolis, R. B., Duckro, P. N., and Krause, S. J. (1987). The pain disability index: psychometric and validity data. Arch. Phys. Med. Rehabil. 68, 438–441.
Taylor, A. M., Harris, A. D., Varnava, A., Phillips, R., Taylor, J. O., Hughes, O., et al. (2015). A functional magnetic resonance imaging study to investigate the utility of a picture imagination task in investigating neural responses in patients with chronic musculoskeletal pain to daily physical activity photographs. PLoS One 10:e0141133. doi: 10.1371/journal.pone.0141133
Taylor, S. F., Welsh, R. C., Wager, T. D., Phan, K. L., Fitzgerald, K. D., and Gehring, W. J. (2004). A functional neuroimaging study of motivation and executive function. Neuroimage 21, 1045–1054. doi: 10.1016/j.neuroimage.2003.10.032
Thibodeau, M. A., Fetzner, M. G., Carleton, R. N., Kachur, S. S., and Asmundson, G. J. (2013). Fear of injury predicts self-reported and behavioral impairment in patients with chronic low back pain. J. Pain 14, 172–181. doi: 10.1016/j.jpain.2012.10.014
Tracey, I. (2010). Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 16, 1277–1283. doi: 10.1038/nm.2229
van der Ploeg, H. M., Defares, P. B., Spielberger, C. D., Defares, P. B., and Spielberger, C. D. (1980). Handleiding bij de Zelf-Beoordelings Vragenlijst ZBV: een nederlandstalige bewerking van de Spielberger State-trait Anxiety Inventory STAI-DY. [Manual for the Self-Assessment Questionnaire ZBV: a Dutch-language adaptation of the Spielberger State-Trait Anxiety Inventory STAI-DY.]. Lisse: Swets & Zeitlinger.
van Wijk, A. J., and Hoogstraten, J. (2006). Dutch translation of the fear of pain questionnaire: factor structure, reliability and validity. Eur. J. Pain 10, 479–486.
Verbunt, J. A. (2008). Reliability and validity of the PAD questionnaire: a measure to assess pain-related decline in physical activity. J. Rehabil. Med. 40, 9–14. doi: 10.2340/16501977-0126
Vercoulen, J. H., Bazelmans, E., Swanink, C. M., Fennis, J. F., Galama, J. M., Jongen, P. J., et al. (1997). Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J. Psychiatr. Res. 31, 661–673.
Vlaeyen, J. W., Crombez, G., and Linton, S. J. (2016). The fear-avoidance model of pain. Pain 157, 1588–1589. doi: 10.1097/j.pain.0000000000000574
Vlaeyen, J. W., De Jong, J., Geilen, M., Heuts, P. H., and Van Breukelen, G. (2001). Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav. Res. Ther. 39, 151–166. doi: 10.1016/s0005-7967(99)00174-6
Vlaeyen, J. W., Kole-Snijders, A. M., Boeren, R. G., and Van Eek, H. (1995a). Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain 62, 363–372. doi: 10.1016/0304-3959(94)00279-n
Vlaeyen, J. W. S., Kole-Snijders, A. M., Rotteveel, A. M., Ruesink, R., and Heuts, P. H. (1995b). The role of fear of movement/(re)injury in pain disability. J. Occup. Rehabil. 5, 235–252. doi: 10.1007/bf02109988
Vlaeyen, J. W., Morley, S. J., Linton, S. J., Boersma, K., and De Jong, J. (2012). Pain-related Fear: Exposure-Based Treatment for Chronic Pain. Seattle, WA: IASP Press.
Vlaeyen, J. W. S., and Crombez, G. (1999). Fear of movement/(re)injury, avoidance and pain disability in chronic low back pain patients. Man. Ther. 4, 187–195. doi: 10.1054/math.1999.0199
Wager, T. D., Atlas, L. Y., Lindquist, M. A., Roy, M., Woo, C.-W., and Kross, E. (2013). An fMRI-based neurologic signature of physical pain. New Engl. J. Med. 368, 1388–1397. doi: 10.1056/NEJMoa1204471
Wiech, K., Jbabdi, S., Lin, C. S., Andersson, J., and Tracey, I. (2014). Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain 155, 2047–2055. doi: 10.1016/j.pain.2014.07.009
Woo, C. W., Krishnan, A., and Wager, T. D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419. doi: 10.1016/j.neuroimage.2013.12.058
Woo, C. W., Roy, M., Buhle, J. T., and Wager, T. D. (2015). Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 13:e1002036. doi: 10.1371/journal.pbio.1002036
Woods, M. P., and Asmundson, G. J. (2008). Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain 136, 271–280. doi: 10.1016/j.pain.2007.06.037
Zale, E. L., Lange, K. L., Fields, S. A., and Ditre, J. W. (2013). The relation between pain-related fear and disability: a meta-analysis. J. Pain 14, 1019–1030. doi: 10.1016/j.jpain.2013.05.005
Keywords: chronic pain, exposure in vivo, neuroimaging, pain-related fear, rehabilitation, chronic low back pain
Citation: Timmers I, de Jong JR, Goossens M, Verbunt JA, Smeets RJ and Kaas AL (2019) Exposure in vivo Induced Changes in Neural Circuitry for Pain-Related Fear: A Longitudinal fMRI Study in Chronic Low Back Pain. Front. Neurosci. 13:970. doi: 10.3389/fnins.2019.00970
Received: 30 May 2019; Accepted: 29 August 2019;
Published: 17 September 2019.
Edited by:
Hannah Madaleine Hobson, University of Greenwich, United KingdomReviewed by:
Marcus Grueschow, University of Zurich, SwitzerlandCopyright © 2019 Timmers, de Jong, Goossens, Verbunt, Smeets and Kaas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inge Timmers, aXRpbW1lcnNAc3RhbmZvcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.