
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 26 September 2019
Sec. Neuroprosthetics
Volume 13 - 2019 | https://doi.org/10.3389/fnins.2019.00901
This article is part of the Research TopicElectrocorticographic Brain-Computer InterfacesView all 17 articles
 Johannes Gruenwald1,2*
Johannes Gruenwald1,2* Andrei Znobishchev3
Andrei Znobishchev3 Christoph Kapeller1
Christoph Kapeller1 Kyousuke Kamada4
Kyousuke Kamada4 Josef Scharinger2
Josef Scharinger2 Christoph Guger1,5
Christoph Guger1,5Invasive brain-computer interfaces yield remarkable performance in a multitude of applications. For classification experiments, high-gamma bandpower features and linear discriminant analysis (LDA) are commonly used due to simplicity and robustness. However, LDA is inherently static and not suited to account for transient information that is typically present in high-gamma features. To resolve this issue, we here present an extension of LDA to the time-variant feature space. We call this method time-variant linear discriminant analysis (TVLDA). It intrinsically provides a feature reduction stage, which makes external approaches thereto obsolete, such as feature selection techniques or common spatial patterns (CSPs). As well, we propose a time-domain whitening stage which equalizes the pronounced 1/f-shape of the typical brain-wave spectrum. We evaluated our proposed architecture based on recordings from 15 epilepsy patients with temporarily implanted subdural grids, who participated in additional research experiments besides clinical treatment. The experiments featured two different motor tasks involving three high-level gestures and individual finger movement. We used log-transformed bandpower features from the high-gamma band (50–300 Hz, excluding power-line harmonics) for classification. On average, whitening improved the classification performance by about 11%. On whitened data, TVLDA outperformed LDA with feature selection by 11.8%, LDA with CSPs by 13.9%, and regularized LDA with vectorized features by 16.4%. At the same time, TVLDA only required one or two internal features to achieve this. TVLDA provides stable results even if very few trials are available. It is easy to implement, fully automatic and deterministic. Due to its low complexity, TVLDA is suited for real-time brain-computer interfaces. Training is done in less than a second. TVLDA performed particularly well in experiments with data from high-density electrode arrays. For example, the three high-level gestures were correctly identified at a rate of 99% over all subjects. Similarly, the decoding accuracy of individual fingers was 96% on average over all subjects. To our knowledge, these mean accuracies are the highest ever reported for three-class and five-class motor-control BCIs.
A brain-computer interface (BCI) establishes a communication pathway from a person's mind to the environment via brain activity alone (Wolpaw et al., 2002; Wolpaw and Wolpaw, 2012). BCIs operate on brain waves that are usually recorded from the electroencephalogram (EEG), the electrocorticogram (ECoG), or depth electrodes. Many types of task-related information (or features) can be extracted from brain waves, depending on the specific experimental protocol and expected neurophysiological activation pattern. Prominent examples include event-related potentials (ERP) (Blankertz et al., 2011), steady-state evoked potentials (SSEP) (Prueckl and Guger, 2009), event-related (de-)synchronization (Pfurtscheller and Lopes da Silva, 1999), and high-gamma activation (Miller et al., 2009; Kapeller et al., 2018). The latter refers to power changes in frequencies above 50 Hz, which can only be computed from invasively recorded data, such as from ECoG or depth electrodes. Invasive BCIs that operate on high-gamma based features have gained considerable attention and are subject to intensive research. For example, this encompasses real-time passive functional mapping in the course of surgery planning (Ogawa et al., 2014), visual categorization tasks (Kapeller et al., 2018), or the development of BCI prototypes for prosthetic limb, hand, or finger control (Shenoy et al., 2007; Kubánek et al., 2009; Onaran et al., 2011; Yanagisawa et al., 2011; Pistohl et al., 2012; Chestek et al., 2013; Kapeller et al., 2014; Xie et al., 2015; Bleichner et al., 2016; Hotson et al., 2016; Branco et al., 2017; Jiang et al., 2017; Li et al., 2017; Pan et al., 2018).
It is known that high-gamma based invasive BCIs can yield very high classification accuracies, depending on the complexity of the experiment, the electrode location and density, and the processing methods and parameters. For example, Yanagisawa et al. (2011) classified hand movement vs. rest in one patient with an accuracy of 79.6%. Pistohl et al. (2012) achieved an average accuracy of 87.8% over three subjects for two different types of grasp movements. In another study, two high-level hand gestures were correctly identified at an average rate of 95.5% over four subjects (Xie et al., 2015). All these results entailed standard ECoG grids. Performance increases considerably when high-density electrode arrays are employed. Among others, this was shown by Jiang et al. (2017), who achieved 100% accuracy for two subjects in a two-class experiment involving hand gestures.
Three or more different hand gestures can also be identified by high-gamma based BCIs. Yanagisawa et al. (2011) reported 68.3% in one subject for three different hand postures. For the same experiment, Kapeller et al. (2014) published accuracies up to 95.9% averaged over two subjects, and Li et al. (2017) scored on average 80.0% in three subjects. In a similar setup, Xie et al. (2015) obtained correct classification rates of 92.7% over three hand gestures in four subjects on average. Whereas, these experiments were obtained from standard ECoG grids, several studies with hand posture classification were conducted with subjects having high-density grids implanted. Using both high-density and standard electrode arrays, Chestek et al. (2013) conducted experiments to discriminate four hand postures and rest at an accuracy rate of 77.7% on average over three subjects. Using only high-density grids, Pan et al. (2018) reported up to 90% for three different hand gestures over 5 subjects. Involving four different hand gestures, Bleichner et al. (2016) achieved 85.5% accuracy over two subjects, and Branco et al. (2017) attained 85.0% over five subjects.
Many efforts have also gone into decoding individual finger movements. Using standard ECoG grids, Shenoy et al. (2007) achieved an average accuracy of 77.0% for classifying each of the five fingers over six subjects. Kubánek et al. (2009) reported 80.3% over five subjects for the same experiment, whereas Onaran et al. (2011) got 86.3% over three subjects. Four fingers vs. rest were correctly decoded at a rate of 79.3% in three subjects by Chestek et al. (2013). One subject with high-density electrodes implanted achieved 96.5% accuracy for each finger in a study conducted by Hotson et al. (2016).
Table 1 summarizes these results and provides a comprehensive overview of the state of the art.
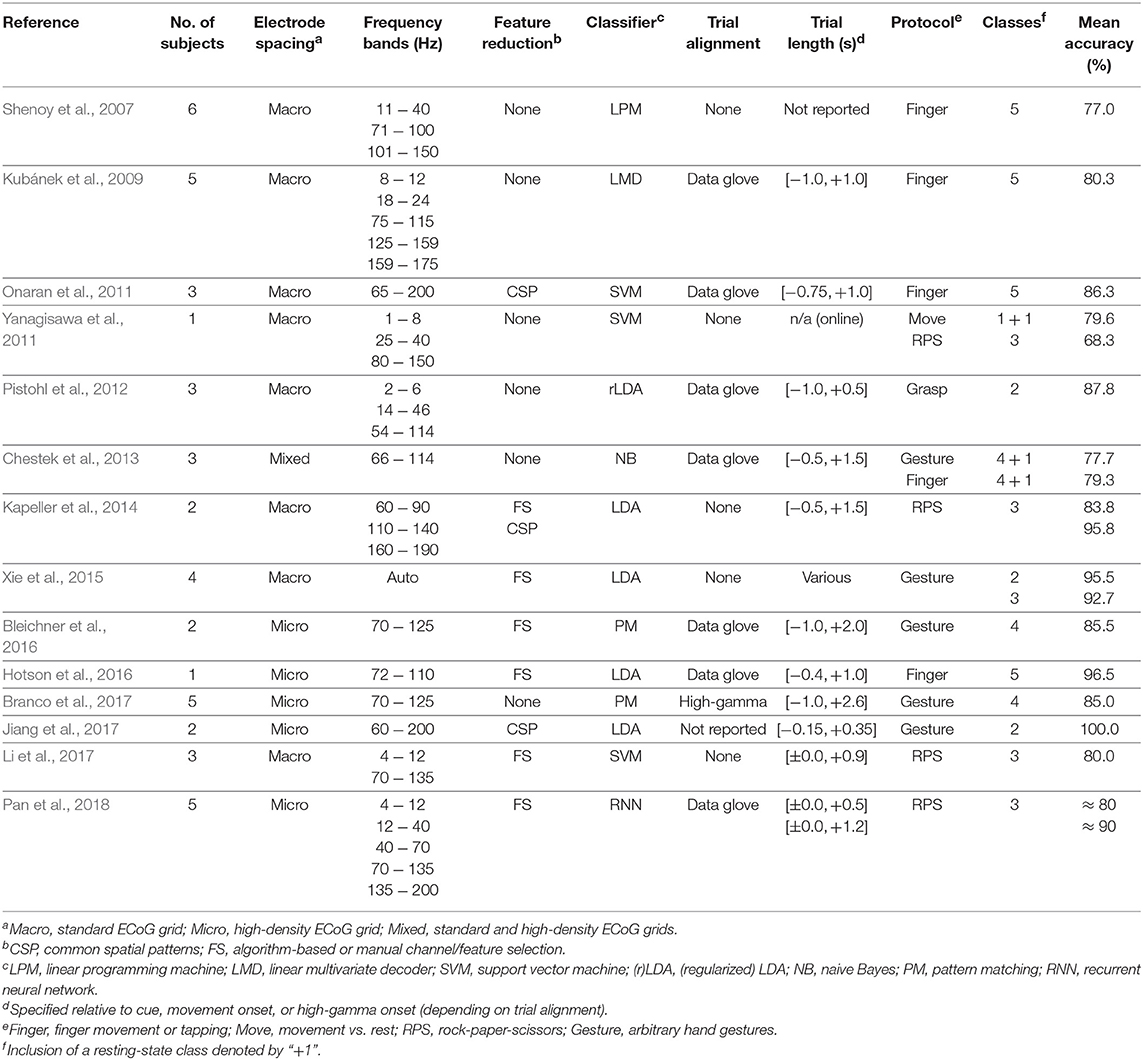
Table 1. State-of-the art summary of hand-motor decoding experiments involving high-gamma based invasive BCIs.
A variety of classifiers for both offline and real-time BCIs exist. Besides linear programming machines (Shenoy et al., 2007), Bayesian approaches (Chestek et al., 2013), pattern matching (Bleichner et al., 2016; Branco et al., 2017; Kapeller et al., 2018), neural networks (Pan et al., 2018), and support vector machines (Onaran et al., 2011; Yanagisawa et al., 2011; Li et al., 2017), linear discriminant analysis (LDA) is widely used for both non-invasive and invasive BCI and all types of features (Bostanov, 2004; Scherer et al., 2004; Blankertz et al., 2008, 2011; Hoffmann et al., 2008; Prueckl and Guger, 2009; Onaran et al., 2011; Yanagisawa et al., 2011; Pistohl et al., 2012; Kapeller et al., 2014; Xu et al., 2014; Lotte et al., 2015; Xie et al., 2015; Hotson et al., 2016; Gruenwald et al., 2017a; Jiang et al., 2017; Li et al., 2017). LDA is robust, has low complexity due to linearity and performs well in line with more sophisticated methods (Garrett et al., 2003; Lee et al., 2005; Lotte et al., 2007).
If the dimension of the feature space is high, a spatial filter must be employed to reduce the number of features and to prevent the classifier from overfitting. The most straightforward approach is feature selection, either manual from a-priori data inspection or automatized via statistical algorithms (Kapeller et al., 2014; Xie et al., 2015; Bleichner et al., 2016; Hotson et al., 2016; Li et al., 2017; Pan et al., 2018). Another approach for feature reduction in invasive and non-invasive bandpower-based BCIs is common spatial patterns (CSPs), a linear projection scheme that optimizes class separation within a pre-defined window (Blankertz et al., 2008; Onaran et al., 2011; Wu et al., 2013; Kapeller et al., 2014, 2018; Lotte et al., 2015; Gruenwald et al., 2017a).
To underline the popularity of the aforementioned methods, 5 out of 14 setups as listed in Table 1 utilize LDA while scoring top results, and all feature reduction approaches (9 out of 15) are either selection-based or CSP-based.
Despite their striking advantages, all of the three outlined techniques (LDA, CSP, and feature selection) suffer from substantial drawbacks.
First of all, LDA is inherently static, since it is designed to operate on two multidimensional point clouds. However, the trials of (synchronous) BCIs are usually given as spatiotemporal feature matrices that also contain transient information. This transient information cannot be exploited by LDA in a straightforward manner. Sometimes, it is feasible to vectorize the feature matrices and apply LDA on the resulting vectors. This approach however inflates the dimension of the feature space dramatically. It therefore requires a large amount of trials to maintain statistical robustness, which are only available in particular BCI protocols (such as in P300-based experiments; Hoffmann et al., 2008). If the statistics are too weak for this approach, a regularized version of LDA may be used. In the current context of invasive BCIs for motor control, this approach was followed by Li et al. (2017), whose feature space was spanned by the vectorized power samples from the time × frequency × channel cube. Another attempt to explicitly account for feature transients was pursued by Pan et al. (2018), who employed recursive neural networks. In general, however, LDA is usually employed such that it is applied to the features at a given point in time within the trial that promises to yield good performance. In turn, this creates the challenge of robustly identifying this time point. Furthermore, the features are usually temporally smoothed to enhance performance—the appropriate smoothing level must thus be found empirically as well.
To reduce the dimension of the feature space, feature selection is straightforward and seems to deliver satisfying performance. However, the nature of selecting a feature entirely dismisses information in unselected features. Moreover, feature selection is unable to combine joint information from coherent features, leading to redundancy in the final feature set. The search for the optimal set of features may be computationally demanding, if statistically robust results should be obtained. In any case, not only identifying the features themselves, but also determining the appropriate number of features is an additional degree of freedom of this method that must be properly taken care of.
In contrast to feature selection, CSPs inherently overcome the two main issues of feature selection, such as information redundancy and feature dismissal. However, finding the optimal location and size of the CSP window may be challenging and requires manual intervention. To the best of our knowledge, no automatized approaches thereto exist. As well, the optimal number of features to be selected for optimal performance needs to be determined in advance.
In view of the shortcomings of state-of-the art methods delineated above, we here present a novel classification method for machine-learning systems with spatiotemporal features in general, and for high-gamma based invasive BCIs for motor control in particular. Our method extends LDA such that it accounts for the time-varying nature of features, we thus name it time-variant linear discriminant analysis (TVLDA). Since TVLDA is applied to one trial as a whole, it avoids the need of estimating the optimal time point for classification as was necessary for LDA. We will also describe an intrinsic property of TVLDA that allows for straightforward and powerful feature reduction via principal component analysis (PCA). Additionally, we investigate the impact of a simple time-domain spectral whitening stage during preprocessing. The resulting system is still linear and of low complexity, which enables it for future real-time experiments. We quantitatively assess and compare the performance of our method by means of recordings from 15 subjects with temporarily implanted ECoG electrodes.
In the original study conducted for this publication, we evaluated data from six epilepsy patients undergoing surgical treatment at Asahikawa Medical University, Asahikawa, Japan. For surgery planning, the patients had a variety of ECoG grids of different types, sizes, and channel counts implanted over the course of several weeks. Besides the standard clinical procedure, all of them volunteered to participate in additional research experiments. The study was approved by the institutional review board of Asahikawa Medical University and received certificate number 245 in June 2012. Written informed consent was obtained from each patient before participating in the study.
Table 2 summarizes the most important data and recordings from the patients, which we subsequently refer to as subjects S1 through S6. Their ages ranged between 17 and 37 years at the day of electrode implantation. S1 and S4 (one third) are female, whereas S2, S3, S5, and S6 are male. S4 is the only left-handed subject. Covered hemispheres are left for S3 and S4 and right for the others.
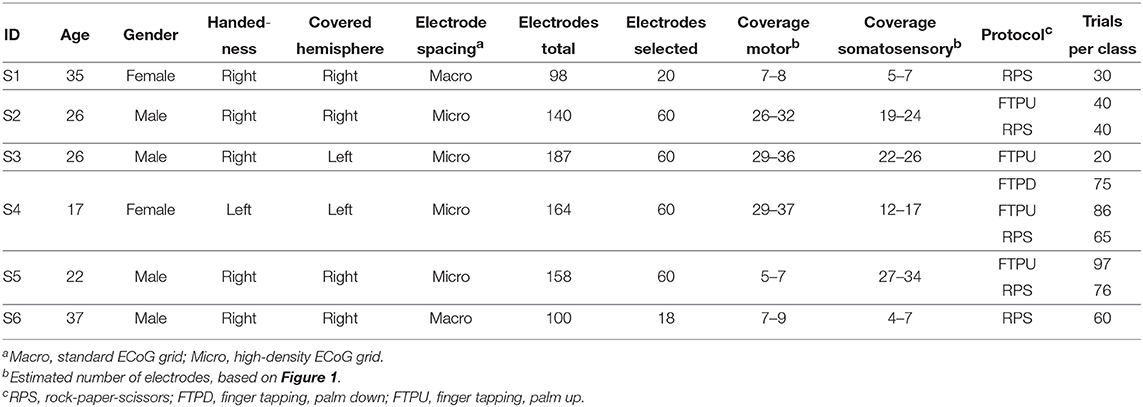
Table 2. Subjects S1–S6 and experiment overview of the original study conducted in Asahikawa, Japan.
From the total number of implanted ECoG grids, we only used the ones covering sensorimotor areas for further evaluation. These were standard 20-channel grids (Unique Medical Co., Ltd., Tokyo, Japan; diameter 3 mm, spacing 10 mm, geometry 4 × 5) for S1 and S6, and 60-channel high-density ECoG grids (Unique Medical Co., Ltd.; diameter 1.5 mm, spacing 5 mm, geometry 6 × 10) for the others. Based on MRI and CT scans, we reconstructed a three-dimensional model of the brain via FreeSurfer (Martinos Center for Biomedical Imaging, Harvard University) and co-registered electrode locations. Based on this and a functional parcellation of the brain, we roughly estimated the electrode coverage on the primary motor cortex and the somatosensory cortex. At this stage, it turned out that the electrodes of S5 were actually only covering somatosensory areas. Figure 1 provides an overview of the electrode placement.
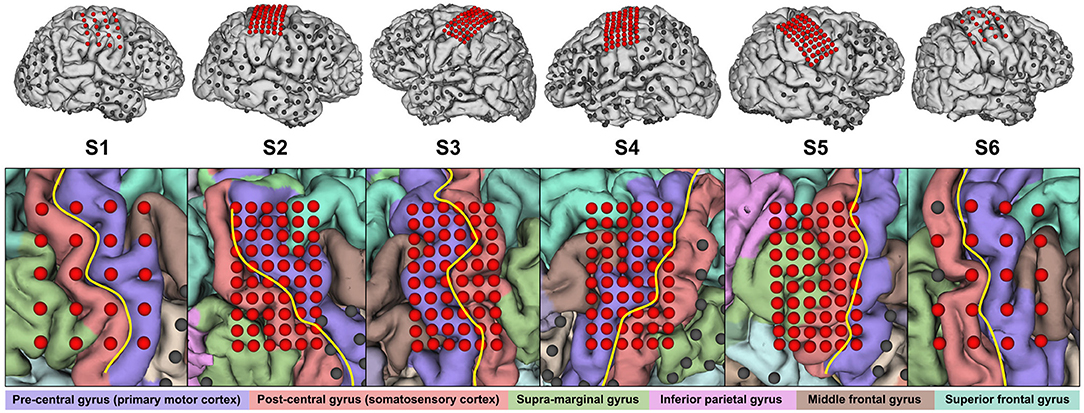
Figure 1. Electrode placement overview. Electrodes reported in Table 2 are highlighted in red. Not all of the remaining electrodes in the top row are visible due to occlusion. In the close-up view, the central sulcus is indicated in yellow and the identified gyri are shaded in respective colors.
In order to make our analyses reproducible by other researchers, we also evaluated the publicly available fingerflex dataset1 from Kai Miller.
All patients participated in a purely voluntary manner, after providing informed written consent, under experimental protocols approved by the Institutional Review Board of the University of Washington (no. 12193). All patient data was anonymized according to IRB protocol, in accordance with HIPAA mandate. These data originally appeared in the manuscript Human Motor Cortical Activity Is Selectively Phase- Entrained on Underlying Rhythms published in PLoS Computational Biology in 2012 (Miller et al., 2012).
This dataset contains nine subjects, which we integrate as S7 through S15 in this context. A brief summary is given in Table 3. All subjects used implanted platinum arrays (Ad-Tech Medical Instrument Corporation, Wisonsin, USA) with 2.3 mm exposed surface and 10 mm inter-electrode distance. The datasets comprised a variable number of channels, which all seemed to contain good ECoG data. In contrast to the data from our study in Asahikawa, it was difficult to assess the exact coverage of S7–S15; we thus used all channels for further processing. Please see the original publication for more details regarding the exact electrode locations.
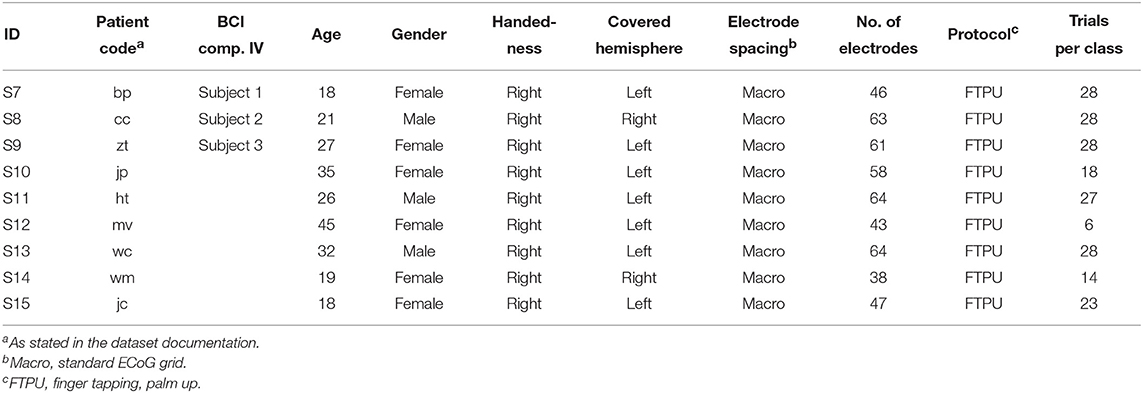
Table 3. Subjects S7–S15 and experiment overview of the public ECoG dataset (S7–S9 are identical with Subject 1–3 from the BCI Competition IV, respectively).
We recognized that the recordings from S7 to S9 are identical with Subject 1–3 from the BCI Competition IV, respectively, which is another highly popular public ECoG dataset (Tangermann et al., 2012).
Table 2 summarizes the conducted experiments, which all relate to hand motor functions at different abstraction levels. The rock-paper-scissors (RPS) experiment addresses high-level gestures, whereas the finger-tapping experiment aims at decoding individual finger movement. The latter is divided into the two variants palm down (FTPD) and palm up (FTPU). We will use the terms finger movement and finger tapping interchangeably throughout this publication.
All experiments were conducted at the bedside of the patient. Before each experiment, the patients received and confirmed all necessary instructions to successfully perform it. The respective tasks were triggered by a visual cue, shown on a computer monitor placed in front of the patient. A data glove (5DT Inc., Florida, USA) was used to capture the hand movements of all subjects. In all experiments, the contralateral hand relative to the implantation site carried out the movements. Figure 2 gives a visual impression of the setup. The experiments conducted with S1–S6 were repeated over the course of several days, depending on the condition and motivation of the subjects.
In the following, we describe the individual experiments more in detail.
The different hand poses involved in this experiment were inspired by the well-known hand game rock-paper-scissors (RPS), constituting a three-class experiment. The visual cues were shown on the screen for one second, interleaved by a scrambled picture distractor of randomized duration between 1.5 and 2.5 seconds. The subjects were instructed to form the requested gesture with their hand once the stimulus appeared, and to return into a relaxed hand position once the distractor showed up. One run included 20 trials per class. The rock-paper-scissors experiment was only conducted with S1–S6.
Here, the subjects were asked to perform two taps with the finger indicated on the screen for one second. Between the cues, a scrambled picture was shown for a randomized duration between 1.7 and 2.5 seconds, indicating that the subject should stay at rest. In the easier version, the palm-up version (FTPU), the subjects executed two repeated finger flexions, whereas in the palm-down version (FTPD), the subjects performed actual taps on a solid, planar surface. One run of this five-class experiment consisted of 10 trials per class. The palm-down version turned out to require a certain level of fine motor skills that was not present in all subjects, so only S4 completed it successfully.
Due to misunderstanding of the task instructions, S3 executed this experiment differently: instead of executing two discrete taps, he kept flexing the finger until a new instruction showed up on the screen. As described below, this required some additional processing steps to obtain usable data.
As described in Miller et al. (2012), the subjects were cued with a word shown on a bedside monitor, indicating which finger to move. Each task lasted for two seconds, during which the subjects typically performed between two and five repeated finger flexions. A blank screen, shown for another two seconds, was interleaved between each task as a resting trial. Only the palm-up variant of the experiment was performed.
We captured the raw ECoG data with the g.HIamp biosignal amplifier (g.tec medical engineering GmbH, Austria) and used Simulink (The MathWorks, Inc., Massachusetts, USA) as the recording environment. Depending on the overall number of channels, we set the sampling rate to either 1.2 or 2.4 kHz. We used the g.HIsys Highspeed Online Processing toolbox (g.tec medical engineering GmbH) for the stimulus presentation and synchronous data acquisition and storage. The recorded data were saved on a hard drive and re-processed offline in MATLAB (The MathWorks, Inc.) for this study as described in this section.
As communicated by Miller et al. (2012), the ECoG data were recorded with the Synamps 2 biosignal amplifier (Compumedics Neuroscan, North Carolina, USA) at a sampling rate of 1 kHz and internal bandpass-filter from 0.3 to 200 Hz. The general-purpose software environment BCI2000 was used for stimulus presentation and synchronous data acquisition.
This subsection closely follows the concept of Gruenwald et al. (2017b), which outlines optimal bandpower estimation for real-time BCIs. If not otherwise mentioned, we processed data from all subjects regardless of their origin in the exactly same manner.
After excluding channels that were notably bad due to high impedance, we re-referenced the data by the common average. After that, a notch-filter cascade (recursive 6th-order Butterworth, bandwidth: 5 Hz) up to the 6th harmonic was used to remove interference peaks from the spectrum at integer multiples of the power line frequency.
Next, an optional spectral whitening filter (Oppenheim and Schafer, 2010) was applied to each channel. While the concept of whitening (or spectral equalization) is frequently used in time-frequency analysis (Miller et al., 2009; Yanagisawa et al., 2011; Pistohl et al., 2012), it is less known that it can also be performed in time domain by a simple finite-impulse response filter. This enables whitening for real-time applications, where time-frequency transformation is not an option.
The underlying principle of a whitening filter is that the input signal can be modeled as a Pth-order autoregressive (AR) process, e.g.,
with being a zero-mean white Gaussian noise with variance . In this publication, we use the tilde notation to link a random variable with its particular distribution and the term i.i.d. to indicate independent and identically distributed samples. The AR coefficients ap can be determined by the Yule-Walker equations that are applied to a sufficiently long signal fragment of y[n] (e.g., a few seconds). Equation 1 can now be seen as a linear time-invariant filter with impulse response ap:
It is intuitive that the filter output ȳ[n] resembles the white noise v[n] and therefore has a flat spectrum.
The positive effect of whitening on the signal-to-noise ratio of ECoG bandpower features was anticipated previously (Gruenwald et al., 2017b). Figure 3 provides an illustration, where the dynamic range of the signal within the cut-off frequencies can be roughly estimated to 25 dB. Whitening equalizes the pronounced 1/f-shape of the spectrum, which balances the frequency-specific contributions to the overall bandpower and thus increases signal fidelity.
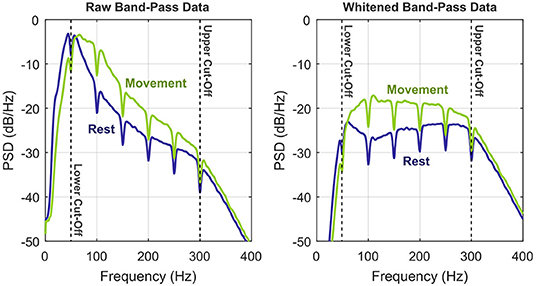
Figure 3. Illustration of the whitening procedure by means of power spectral densities of the preprocessed bandpass signals (S6, RPS, exemplary channel). To illustrate the benefits of whitening, the two conditions rest vs. movement (any class) are shown separately. Upper and lower corner frequencies of the bandpass filter are indicated by the vertical dashed lines.
Since an ECoG spectrum is rather smooth in absence of interference peaks, the filter order can be low. In practice, we found a 10th-order whitening filter sufficient.
After the optional whitening stage, we band-passed the signal (recursive 6th-order Butterworth) to our high-gamma frequency band of interest. We assessed several bands with respect to classification performance, and finally chose 50 to 300 Hz as our target. This may seem inappropriate in view of the fact that the data of S7–S15 was pre-filtered by a bandpass between 0.3 and 200 Hz. However, we observed that the whitening procedure was able to recover high-gamma components well above 200 Hz.
Given the bandpass signals, we then estimated the bandpower via a sliding variance window of 50 ms length, without overlap. A log-transform was appended, to improve signal stationarity and Gaussianity.
Then, the data were triggered, i.e., cut into signal fragments for each trial and class. Since S3 and S7–S15 exhibited a large movement onset jitter, we applied a trial-based correction. To this end, we used the signals captured by the data glove for aligning the individual trials of S3. Likewise, we corrected the onset jitter of S7 to S15 by a movement trigger already contained in the data. For the other subjects (S1, S2, and S4–S6), no explicit trial alignment was performed, since the onset jitter was already small enough for good classification results. However, we compensated for the systematic reaction and execution latency by shifting the grand average high-gamma onset to the center of the trial to guarantee symmetry.
We set our trial length to 0.75 seconds pre- and post-onset, respectively. Trials that were contaminated with pathological brain activity (such as inter-ictal spiking) were removed. No further trial exclusion was performed.
At this point, it is reasonable to establish a mathematical model that facilitates subsequent methodological derivations. To this end, we refer to the number of samples and channels as NS and NCh, respectively. The preprocessed and triggered data then constitute spatiotemporal feature matrices for trials i and classes c. Both trials and classes are expected to stem from a pool of NT trials and NC classes, respectively, e.g., T = {1, …, NT} and = {A, B, C, … } with || = NC. For the typical machine-learning scenario, we are further partitioning the set of trials into a training set TTrain (with known class labels) and a test set TTest (with unknown class labels), which are disjoint. Formally, these sets can be expressed as
Toward mathematical tractability, we decompose into row vectors with discrete-time index n:
The number of recorded channels may be high, particularly in ECoG experiments. This increases the computational demands and the risk of classifier overfitting. Consequently, a feature projection or selection stage usually precedes the classifier. Especially for ECoG, this can decrease dimensionality tremendously without losing information, since (1) only a limited amount of channels significantly contributes to class separation and (2) correlation across contributing channels may be high. Mathematically, this feature projection is implemented by a generic matrix with NF ≪ NCh, such that
Following a likewise decomposition as in (5), we denote the contracted spatiotemporal feature matrices by and write for the training and test sets
The subsections below describe strategies how to populate the projection matrix P.
Common spatial patterns (CSPs) are the de-facto standard for dimension reduction in EEG signal processing (Blankertz et al., 2008; Lotte et al., 2015) and are also popular in ECoG signal processing (Onaran et al., 2011; Kapeller et al., 2014; Xie et al., 2015; Jiang et al., 2017). This approach expects multivariate distributions of two classes A and B with covariances ΣA and ΣB, respectively. The CSP transformation matrix then simultaneously diagonalizes both ΣA and ΣB, where the element-wise ratio along the diagonals is strictly monotonic. Consequently, the first and the last CSP component maximize the variance for one class, while minimizing it for the other. Additional CSP components further contribute to this.
In the given context, CSPs operate on the triggered bandpass data within a pre-defined window, i.e., before power computation. For all datasets, we have located the peak of the grand high-gamma activation over trials and classes, and centered the CSP window about this peak. We set the window length to 0.3 seconds, since this yielded the best classification results. Denoting the CSP transformation by , the projection matrix PCSP is then column-wise populated with the first ⌈NF/2⌉ and the last ⌊NF/2⌋ columns of R. As will be discussed in section 2.8, we computed CSPs pairwise for each binary classification in a multi-class scenario.
Another common approach to reduce the dimensionality is a discrete feature (or channel) selection process. While the individual implementations differ considerably, feature selection is heavily used in the ECoG community (Kapeller et al., 2014; Xie et al., 2015; Bleichner et al., 2016; Hotson et al., 2016; Li et al., 2017; Pan et al., 2018).
Here, we use a straightforward approach for feature selection. First, we compute an activation score for each class and channel, which is the trial-averaged relative band-power increase from baseline (before high-gamma onset) to activation (after high-gamma onset). For each pair of classes, we then calculate the absolute difference of this activation score for each channel and sort the result in descending order. This way, the channels exhibiting the largest high-gamma activation difference for the two classes are ranked top. Consequently, the projection matrix PFS (which is more a selection matrix now) is established such that its NF columns logically index the first NF channels in the given ranking, respectively.
We now assume that, for each class, the feature matrices comprise a unique underlying activation pattern that is identical over trials. However, each repetition is subject to noise, most prominently from imperfect task execution and the uncertainty of feature estimation. We thus employ a multivariate Gaussian distribution to describe these components as follows:
In general, and are not known.
The independence constraint is expected to hold over samples n, trials i and classes c. While this requirement is intuitively hold over trials and classes, in fact it may be violated over samples. We have shown in Gruenwald et al. (2017b) that the signal processing pipeline yields high-gamma features with estimation noise that can be considered white; however, imperfect trial execution may impose temporally correlated noise on the data. We will also address this issue in section 4.
A standard tool to separate features of two classes is linear discriminant analysis (LDA). In a nutshell, LDA expects multivariate Gaussian distributions from two classes A and B and finds a projection vector that simultaneously maximizes the mean distance whilst minimizing the individual variances of the projected populations (Bishop, 2006). LDA-based classifiers are optimal in the maximum-likelihood sense if the two distributions are homoscedastic.
For convenience and if applicable, we hereafter use the generic class label c ∈ {A, B} to denote either of the two classes. In case we know the class associated with a particular variable, we denote this by subscript notation.
A common approach to classify spatiotemporal features with LDA is the training of several LDA instances over time and selecting the classifier which yields best performance. Thus, after introducing the well-known difference of means and pooled covariance matrix
the standard LDA projection vector equates to
Given an arbitrary input x(i)[n], the symmetric LDA score p(i)[n] is computed as
where the superscript (·)T denotes matrix transposition and the offset
centers the two projected class populations about zero. This can be verified by equating the means via Equations (13) and (14), where {·} denotes the expectation operator over trials. It is now evident that μpA[n] = −μpB[n], since
There are different approaches to apply an LDA classifier to spatiotemporal features. The most common strategy is to smooth the features over time, train the LDA classifier for each point in time, and then select the one which gives best performance. In the present context, best performance for LDA was achieved if the features within a trial were symmetrically smoothed by three samples in each direction. Toward the decision which classification time point to use, we investigated several options. Most robust results were obtained by adaptively selecting the time point of maximum high-gamma activation over all classes and trials. We subsequently denote this time point as nLDA.
Summarizing the LDA training procedure, the sample means [nLDA] and covariances [nLDA] are computed first, given labeled training data . Via Equations (10), (11), (12), and (14), the set then constitutes the LDA classifier.
Given a test trial and as the classifier, the LDA score is simply computed analogously to (13):
Since the two classes in question lead to LDA scores symmetric about zero, the natural threshold for classification is zero as well:
Computing the LDA weight vector requires the inversion of the pooled covariance matrix. This can become numerically unstable if the number of samples is not much larger than the feature dimensionality. To overcome this problem, a regularized LDA (rLDA) can be used where only the main diagonal of the sample covariance matrices is accounted for (also known as shrinking). Since this allows stable inversion even in high-dimensional feature space, rLDA is particularly appealing when applied to vectorized features , such that
to account for all spatiotemporal information at once.
Training the rLDA classifier is straightforward. After computing sample means and sample covariance matrices from the vectorized training data, the off-diagonal elements of the sample covariance matrices are set to zero. Equations (10), (11), (12), and (14) yield the rLDA classifier . Note that the temporal index n has now vanished.
Applying the rLDA classifier to test data follows analogously to section 2.6.1.2.
The major improvement of time-variant linear discriminant analysis (TVLDA) over standard LDA is that it utilizes information of all individually trained LDA classifiers over the whole trial, which makes it inherently time-variant. To derive the concept of TVLDA mathematically, we first interpret p(i)[n] (13) as an NS-dimensional vector:
In this notation, each class establishes the multivariate Gaussian distribution
with means and covariances equating to
The assumed temporal independence of the feature noise (cf. (9)) implicates the fact that Σpc must be diagonal. The elements of μpc are given by Equations (15) and (16), and the elements of Σpc are obtained after short calculus as
We now want to separate the two class populations {} and {} again in the LDA-sense. Consequently, the difference of means and pooled covariance are given as
To find an expression for the LDA projection vector , we trace back Equations (23) and (24), Equations (15), (16), and (25), and Equations (11) and (12), to finally arrive at the elegant result
In other words, the overall TVLDA score, denoted by z(i), is simply the sum of all intermediate LDA scores (13):
It is evident that , which can be shown by inserting (12) into Equations (15) and (16). Consequently, the difference of the expected TVLDA scores yields via (29)
which is the accumulated Kullback-Leibler divergence of the classes A and B under the homoscedasticity assumption of the (TV)LDA.
From the training data , the TVLDA parameters are computed based on the sample means and covariances via Equations (10), (11), (12), and (14).
For a test trial and a set of TVLDA parameters , the TVLDA score ẑ(i) follows according to Equations (29) and (13):
Evidently, also the TVLDA score is symmetric about zero, which leads to the classification scheme
We here resume section 2.5 by proposing a novel feature dimensionality reduction approach that is intrinsic to TVLDA, based on principal component analysis (PCA).
We can interpret the time-variant TVLDA weight vector as a spatiotemporal weight matrix , whose rows are given by w[n]:
Motivated by the nature of PCA, we now restrict the generic transformation matrix P to be orthogonal, i.e., with NF = NCh and P−1 = PT. Consequently, any transformation of by P transparently affects the TVLDA weight matrices Wx and Wy:
where the subscripts indicate which variable W is associated with. This relationship can be shown by substituting the projection scheme into the computation of the weight vector (12) via Equations (10) and (11).
The idea now is to find P, such that the weights in Wy are compressed into very few columns of Wx. Only these columns of Wx are then kept, leading to an effective reduction in dimensionality.
The standard solution to this problem is PCA, which we implement as a singular value decomposition (SVD) of Wy. In short, we factorize where and are orthogonal matrices, and is a matrix with zeros, except for the non-negative, decreasing singular values on the diagonal. The desired scores in the principal-component space of Wx are now given by the product US, such that we require
and obtain simply
Since V establishes an orthonormal projection, which can be seen as a rotation in high-dimensional space, all information is preserved. The principal components are ordered by their impact, so the projection matrix PPCA is simply populated by the first NF columns of V. Figure 4 provides an example of the PCA-based feature reduction method.
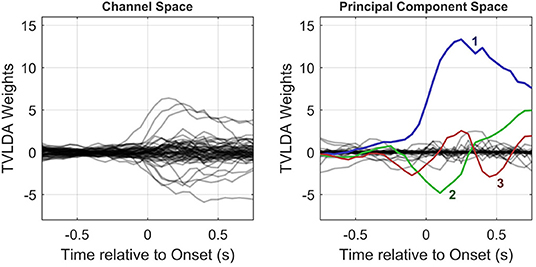
Figure 4. Column-wise visualization of the original and PCA-transformed spatiotemporal weight matrices Wy (left) and Wx (right), respectively. As illustrated in the right subplot, only few principal components with large amplitudes remain. This allows for substantial dimension reduction, as detailed in the text.
Importantly, the number of channels may be too high to yield invertible covariance matrices (i.e., NCh ≫ NT). Even if the covariance matrices are nonsingular, their inversion may be numerically unstable. To find a robust PCA decomposition and unless many more trials than channels are available, we therefore recommend smoothing the sample means and covariances over time before computing the weight matrix Wy that is subject to the SVD. In our case, we used bidirectional averaging of two samples in each direction to obtain the best results. For datasets comprising many trials, this bidirectional averaging did not impair results, and hence we recommend using it whenever applicable.
The number of trials may be extremely low, and thus even temporal averaging does not yield a usable PCA decomposition. In this case—and only in this case—we suggest adding a certain level of regularization to PCA: here, the off-diagonal elements of the TVLDA sample covariances are weighted with a factor between 0 and 1, where 0 is identical to complete diagonalization. We have observed that setting this factor to 0.75 (which equals a regularization of 25%) can substantially improve results, especially for datasets with particularly low trial count and a small number of channels capturing task-related activation. We will address this issue further in section 4.
Note that the proposed temporal averaging and regularization only apply for computing the initial Wy, but not for Wx after transformation.
So far, we have only addressed binary classification problems. Since many experiments entail more than two classes, the decision rules defined in Equations (18) and (32) must be extended. We do so by employing a one-vs.-one classification scheme. Consequently, each class is tested against each other class, yielding NC(NC − 1)/2 binary classification results. It would be straightforward to implement a voting approach that elects the winner based on the most votes; however, this approach would lead to frequent ties. Moreover, the quantitative information in the (TV)LDA scores would be lost. We thus propose to use a min-max approach for multi-class discrimination. First, we refer to the (TV)LDA score for class cp vs. cq as (cp, cq ∈ , cp ≠ cq). The smaller this value gets, the more certain (TV)LDA is that trial i belongs to class cp rather than to class cq. Taking the worst score over all classes (i.e., the maximum of over all q) then indicates how likely it is that trial i stems from class cp, relative to all other classes (the lower the more likely). Finally, the class that minimizes this score is elected:
Evidently, the feature-reduction techniques discussed in sections 2.5 and 2.7 follow this one-vs.-one scheme as well.
Here, we describe our framework for performance evaluation.
We performed 20 repetitions of a randomized 10 × 10 cross-validation to assess the expected performance of the system. All components (such as feature reduction and classification) were subject to this cross-validation to ensure that testing was done on completely unseen data.
In this publication, we mainly want to investigate the potentials of our proposed improvements, such as (1) spectral whitening, (2) PCA-based feature reduction instead of CSP and feature selection, and (3) TVLDA instead of LDA or rLDA. To this end, we identified seven method variants (or simply methods) that logically follow this path: for LDA with CSP and feature selection, we investigate the effect of whitening. Then, for whitened data, we incorporate rLDA and PCA as a feature reduction technique for LDA. Finally, for whitened data and PCA-based feature reduction, LDA is switched to TVLDA to arrive at the complete set of proposed improvements.
We quantify the performance of the respective methods by means of accuracy rates (or simply accuracies). This is the true positive rate, defined as the ratio between correctly classified trials and total number of trials, averaged over all classes. Since our evaluation framework is of statistical nature, a rigorous comparison between methods by means of accuracies is inappropriate. To resolve this, we here define a margin, within which we consider two methods to perform equal. Intuitively, we set this margin to 1/NT [%], since this represents the accuracy range that relates to one trial per class. This in turn is the actual quantization level of the respective dataset, and we hereafter refer to it as the quantization margin.
To facilitate interpretation and comparison further, we also introduce the term representative accuracy. The representative accuracy is an acceptable trade-off between classification accuracy and number of features needed. Since, at some point, increasing NF may only marginally contribute to better performance, we chose the smallest NF whose corresponding (i.e., representative) accuracy still lies within the quantization margin of the best result.
In this section, we present the results of the classifier performance evaluation. For the most comprehensive comparison, we included the number of features NF from 1 to 15 and evaluated the accuracies for each method variant and dataset.
Figure 5 gives a qualitative overview of the performance evaluation for S1–S6. At this stage, it is already evident that whitening dramatically improves decoding performance, regardless of the feature reduction technique. For CSP and feature selection, a gradual improvement can be observed in most datasets as NF increases. This is plausible as new information is added to the system. It is remarkable that this characteristic is different for PCA-based feature reduction: more features only slightly improve performance, if there is any improvement at all. For many datasets, the best performance is already achieved for very few PCA components and degrades as more are added to the system. The representative accuracy is indicated by the large dots. Note that the concept of representative accuracy does not apply to rLDA, since it directly operates on the vectorized feature space.
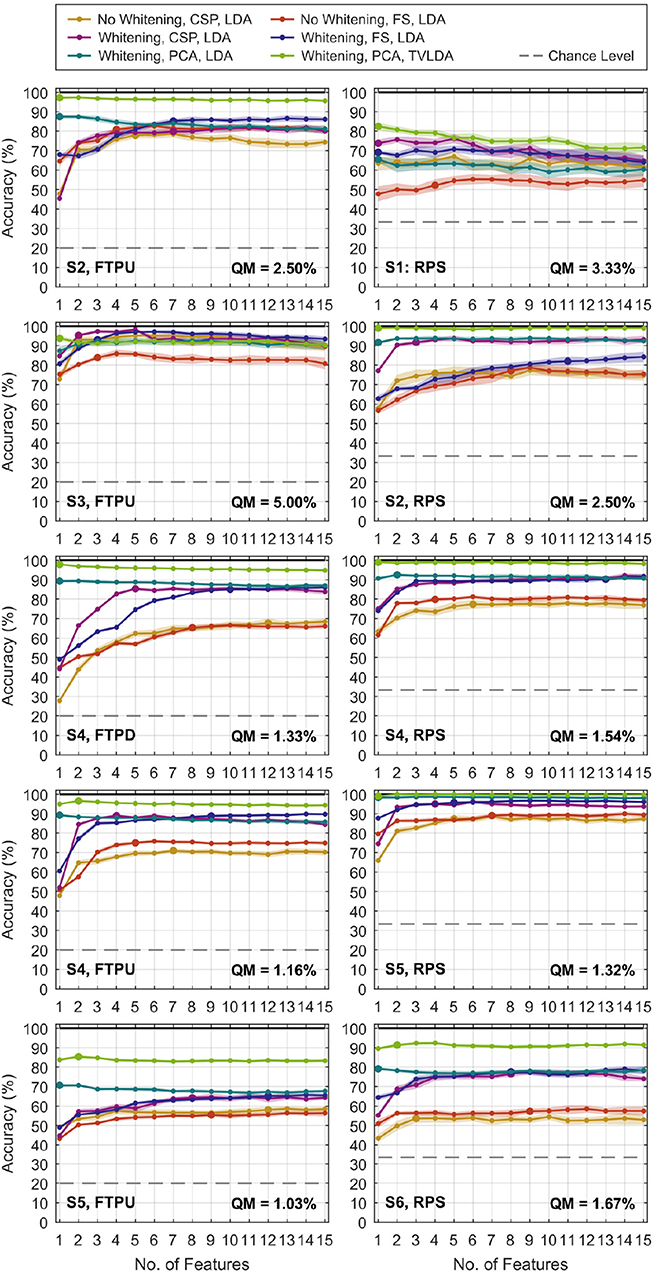
Figure 5. Classification accuracies versus number of features NF for selected finger-tapping (left) and rock-paper-scissors (right) datasets. Results for rLDA are not shown since NF does not apply. The dots represent the average of 20 repetitions of the randomized cross-validation, and the shaded area indicates the standard deviation. The pronounced dots relate to the representative accuracy, which is defined in the text. Feature selection is abbreviated by “FS” in the legend. The quantization margin is abbreviated by “QM”.
Table 4 lists the representative mean accuracies, standard deviations, and respective number of features versus methods and datasets. For better reading, we ordered the presentation by protocol and electrode grid density. Below, we summarize the most important findings. For brevity, we refer to TVLDA with PCA-based feature reduction and whitening just as TVLDA.
As summarized in Table 4, the accuracies increase systematically from the standard methods to TVLDA. Whitening already has a dramatic impact on the performance. For CSP and LDA, the improvement peaks at +22.9% (S6, RPS) with +12.3% on average. A similar trend can be observed for feature selection and LDA, where we improved by up to +20.3% (S6, RPS) and +10.4% on average.
For whitened data, rLDA performed worst on average with 71.5%. LDA with CSP and feature selection was slightly better with 74.0 and 76.1% on average. LDA with PCA was the best on average with 78.8%. At the same time, PCA turned out to be the most efficient feature reduction technique by far, needing only 1.1 components on average instead of 3.5 (CSP), 5.7 (feature selection), or the whole vectorized feature space (rLDA).
Overall, the best results were seen for whitened data and TVLDA, where TVLDA outperformed rLDA by +16.4%, LDA and CSP by +13.9%, and LDA and feature selection by +11.8% on average. Investigating the impact of using TVLDA instead of LDA for PCA-based feature reduction and whitened data, we obtained an improvement of +9.1% on average. To assess the robustness of each evaluated method, we computed the standard deviation of the accuracies over 20 repetitions of the randomized cross-validation. For the non-whitened data and LDA, we obtained an average standard deviation of ±2.2 and ±2.0% for CSP and feature selection, respectively. Whitening decreased these values to ±1.6% and ±1.5%, respectively, whereas rLDA showed an overall standard deviation of ±1.4%. TVLDA slightly diminished the overall standard deviation further to ±1.3%.
TVLDA performed best not only on average, but for every single dataset (within the quantization margin relative to the overall maximum). Only one or two PCA features (1.1 on average) are needed to achieve top performance. For the subsequent discussion, we thus refer to the results yielded by TVLDA.
Combining the results of the rock-paper-scissors experiment for the subjects with standard ECoG grids implanted, an overall accuracy of 86.9% was achieved. In contrast, the accuracy increases tremendously for subjects with high-density grids implanted, who scored 99.0% on average.
S7–S15, all with standard electrode grids implanted, scored 81.0% on average in the finger-tapping experiments. In general, all these data comprised fewer trials; for S12, even only 6 trials were available. The entries marked with an asterisk in Table 4 were thus obtained with a regularized PCA to avoid overfitting (cf. section 2.7).
For the patients with high-density grids implanted, the classification accuracy in the finger-tapping experiment was 94.2% over all subjects. Accounting only for the subjects with substantial sensorimotor coverage (thus excluding S5), the overall score increased to 96.4%.
Spectral whitening during the preprocessing stage has a tremendous impact on decoding performance. On average, the accuracy rises by +12.3% for CSP and LDA and by +10.4% for feature selection and LDA. Figure 3 illustrates the reason for this huge leap: whitening balances the information with respect to frequency and therefore substantially increases the signal-to-noise ratio. Employing multi-band features in the high-gamma band (Shenoy et al., 2007; Kubánek et al., 2009; Kapeller et al., 2014; Pan et al., 2018) may have a similar positive effect on the classification performance as whitening, but this comes at the cost of an expanded feature space.
When ECoG signals are offline analyzed in the time-frequency domain, spectral whitening is well established (Miller et al., 2009). Yanagisawa et al. (2011) and Pistohl et al. (2012) directly extracted bandpower features for classification from a time-frequency signal representation (such as short-time Fourier or wavelet transforms). However, this approach is computationally demanding and may not meet real-time constraints. We therefore strongly promote the proposed time-domain whitening filter to save valuable resources.
The evidence that TVLDA outperforms LDA on high-gamma features is overwhelming: for every single dataset, TVLDA delivers the best results. The grand average accuracy improvement relative to standard methods is +16.4% (vs. rLDA), +13.9% (vs. CSP and LDA) and +11.8% (vs. feature selection and LDA). These results were obtained with mostly one (sometimes two) internal PCA components for TVLDA, whereas CSP and feature selection require 3.5 and 5.7 components, respectively. Performance thus not only gets better, but is also achieved at lower system complexity. The fact that only few PCA components are necessary to achieve maximum performance leads to remarkable robustness against overfitting; TVLDA with PCA delivers 10 × 10 cross-validation results with a standard deviation of ±1.3% on average. If very few trials are available, a regularization term to PCA as discussed in section 2.7 can be applied to further enhance stability.
Before putting our results into the context of state-of-the art research, we want to emphasize that it was not our focus to maximize the absolute performance of our system, but rather to investigate the impact of structural and methodological advances proposed in this manuscript. In other words, we did not employ multiple frequency bands or add other features to improve overall performance, unlike other studies to which we compare our results. We did not reject badly or differently executed trials from the datasets. In view of good responsiveness of a real-time BCI, we kept our trial window short (±0.75 seconds, relative to movement onset), whereas longer trials would have increased classification accuracies for some datasets most certainly.
The three-class rock-paper-scissors experiment with standard electrodes yielded an average accuracy of 86.9%. In view of the experiment settings, this compares best to 68.3% (Yanagisawa et al., 2011), 83.8% and 95.8% (Kapeller et al., 2014), 92.7% (Xie et al., 2015), and 80.0% (Li et al., 2017). Whereas the cited reference results relate to the same protocol in general, they were obtained from multi-band features and substantially longer trial durations. Xie et al. (2015) also used alternative features besides bandpower.
For the rock-paper-scissors experiment with high-density electrodes, TVLDA delivered almost perfect accuracies of 99.0% on average over three subjects. A similar experiment was recently conducted by Pan et al. (2018), who reported an accuracy of up to 90%. Bleichner et al. (2016) achieved 85.5% and Branco et al. (2017) attained 85.0% accuracy with high-density grids, but for an experiment involving four gestures.
For standard electrode grids and the finger-tapping experiment, TVLDA scored 81.0% on average over all subjects. This is well in line with state-of-the art results, such as such as 86.3% (Onaran et al., 2011), 80.3% (Kubánek et al., 2009), and 77.0% (Shenoy et al., 2007). For solid differentiation of five individual fingers, however, the spatial sampling of standard-sized grids may be too coarse. In particular, we observed considerable confusion between the ring finger and little finger, which are in fact difficult to move independently. Combining these two classes improved decoding performance to 88.1% in a four-class scenario, which seems a more usable setup in this context. Interestingly, this result with 88.1% is higher than the 86.9% we obtained for only three classes. We thus suspect that the electrode coverage of S1 was not particularly fortunate for the rock-paper-scissors experiment, or the movements were not executed consistently or pronounced enough. Based on our experimental evidence, we believe that the rock-paper-scissors experiment with proper sensorimotor coverage of standard-sized electrodes and good subject participation should yield around 90% accuracy and above with TVLDA (as in S6).
The finger-tapping experiment with high-density sensorimotor coverage resulted in 96.4% on average. This is comparable to Hotson et al. (2016), who scored a maximum of 96.5% in a single subject, but with posterior selection of the best LDA evaluation time point. With both standard and high-density electrodes implanted, Chestek et al. (2013) reported 79.3% in a similar experiment.
TVLDA with PCA has further advantages beyond high classification accuracy. The architecture needs only minor extensions compared to standard LDA. Additionally required components encompass a time-domain whitening filter for preprocessing, the summation over several LDA scores, temporal smoothing of sample means and covariances for PCA, and an SVD of the spatiotemporal TVLDA weight matrix. All of these elements are strictly deterministic and can be implemented easily. Training a system that implements TVLDA is fully automatic and done in less than a second. Moreover, all shortcomings and difficulties of CSPs and feature-selection approaches disappear, since no external feature reduction is required. TVLDA is more robust than any other assessed approach, even when only few trials are available. With only one or two PCA components, TVLDA already attains maximum performance.
Choosing the optimal number of principal components for TVLDA may be straightforward in the given context, where performance vs. number of features was evaluated via cross-validation. In fact, one could have chosen just the first principal component for all datasets with still very good results. TVLDA may however be applied to more complicated datasets, where more than one principal component is required. In this case, cross-validation is still an option to determine the optimal number of principal components. A more theoretical approach that efficiently estimates the true number of underlying principal components via Bayesian model selection was proposed by Minka (2001).
We already mentioned that the temporal independence of the noise as stated in (9) may be violated by inconsistent trial repetitions of the subject. In this case, the assumptions of a diagonal covariance matrix for TVLDA as in (24) is not justified any more. In fact, a good estimate of the true covariance matrix can be obtained from the training statistics of the LDA scores (22) with considerable effort. We tested this option, but it did not yield any improvements. On the contrary, TVLDA turned out to become less stable. We therefore resorted to the variant proposed in this manuscript, which can also be seen as a form of regularization.
As evidenced by Figure 3, our high-gamma band of choice covered several harmonics of the power-line frequency. Since power-line interference can be huge, especially for ECoG data, it must be addressed. Applying notch filters is a robust solution, although they remove the complete signal within the specified frequency band. As a consequence, we expect to have lost up to 10% of the signal power (harmonic spacing: 50/60 Hz, notch filter bandwidth: 5 Hz). In reality, it may be much less than 10% though, since the filter cut-offs are not infinitely steep. A more sophisticated interference cancelation approach that removes only unwanted signal components could have maintained a higher signal-to-noise ratio. This may have led to slightly better performance, especially for whitened data.
Our evaluation is based on retrospective analysis of offline data. However, since the signal processing pipeline is strictly causal, we are confident that the whole system can be put to the online context in a straightforward manner, yielding comparable results. Based on the experimental evidence and our experience with TVLDA, 20 trials per class for training should already be enough for reasonable online classification performance, provided that the coverage is good and high-density grids are used. Of course, more training data can often improve results.
It should be noted that TVLDA is trial-based per se, so it needs a trigger to perform classification. An interesting undertaking would be the adaptation of TVLDA for asynchronous BCIs. For training, triggered and labeled data will still be necessary (as for most supervised classifiers). During a free run, the previously trained, asynchronous TVLDA may then continuously process the incoming data stream in sliding windows. This yields one classification result at a time, including idle time periods. To reduce this large number of produced false positives, we suggest two strategies. First, the TVLDA scores themselves may be taken into account, such that only scores that exceed a minimum of certainty actually trigger a classifier output. This threshold may be determined during training. As an alternative, a baseline class could be added to the framework to explicitly account for the idle state.
In any case, TVLDA is a window-based classifier and thus requires a consistent spatiotemporal activation pattern for successful classification. Truly continuous BCI control may be difficult to implement with TVLDA.
In its design as proposed here, TVLDA requires each trial to be completed until it is classified. For real-time applications, the trial window should therefore be as short as possible. We can imagine however an adaptive TVLDA that does not necessarily accumulate the LDA scores over the whole trial. Rather, it would raise a classification output whenever the accumulated LDA scores up to the current time point exceed a certain threshold that allows a reliable decision.
TVLDA may also find usage in different application fields apart from classification. For example, as shown in (30), the TVLDA score relates to the accumulated Kullback-Leibler divergence, which can be used for statistical evaluation such as in trial-based functional brain mapping. Here, a particular task is usually compared to a resting condition. Applying TVLDA at each channel separately would then yield a robust measure how much the respective channel is involved in carrying out the task.
Another potential use case of TVLDA would be the reconstruction of task-related activation patterns. The PCA of the TVLDA weight matrix readily provides a decoupled spatial and temporal representation of the underlying cortical processes that are specifically discriminating between the two classes. A similar tool was published by Kobak et al. (2016), who proposed a demixing PCA (dPCA). Here, PCA was extended with task-related information to reduce data dimensionality and to reveal unique activation patterns specific to each task. Unlike TVLDA, dPCA was designed to simplify the analysis and visualization of multi-dimensional neural recordings in animals, but it may also be used for classification.
One fundamental assumption of TVLDA is that each trial is the exact repetition of each other trial. While this leads to a convenient signal model, it may not reflect reality. Most likely, the overall amplitude of the underlying activation curve may vary over trials due to adaptation, high-gamma attenuation, learning, or fatigue. It may be worthwile to study a potential extension of TVLDA that allows for these fluctuations or trends. This may be inspired by Williams et al. (2018), who have recently shown that tensor component analysis (TCA)—a multilinear extension of PCA—provides a powerful framework for decomposing triggered neural data into electrode factors, time factors, and trial factors.
In this work, we have outlined a novel classification method for invasive motor-control BCIs that extends LDA to account for time-variant features. We named it TVLDA, for time-variant linear discriminant analysis. At the same time, we proposed an optimized feature extraction path for high-gamma bandpower that utilizes time-domain whitening for improved performance. We assessed the performance of TVLDA by evaluating data from 15 epilepsy patients with implanted subdural grids. Based on 19 experiments involving three high-level gestures and individual finger movement, we systematically demonstrated the superiority of TVLDA over several reference methods based on LDA.
TVLDA establishes a new benchmark for invasive motor-control BCIs, especially for those with high-density electrodes implanted on sensorimotor areas. To our knowledge, 99.0% for the recognition of three high-level gestures and 96.4% for individual finger identification are the highest consistent accuracies ever reported for these kinds of experiments.
Among the strengths of TVLDA is its ability to dramatically reduce feature dimensionality through a novel projection scheme based on PCA. This leads to robust performance, even for experiments with very few trials. As a valuable consequence, TVLDA makes any preceding feature reduction stage obsolete. The implementation of TVLDA is straight forward and requires only few adaptations compared to standard LDA.
It is evident that TVLDA is not limited to motor-based classification tasks. Rather, it can be used for any experimental setup that produces spatio-temporal activation patterns for classification—potentially even in EEG or other non-brain imaging approaches, such as electrooculography (EOG), electrocardiography (ECG), electromyography (EMG), and the like. TVLDA may also find use in different ECoG applications, such trial-based functional brain mapping.
Overall, we believe that we have developed a valuable tool that will open the door for invasive brain-computer interfaces with almost perfect multi-class control in the near future. However, additional work is necessary to further validate TVLDA with different ECoG environments, as well as with EEG and other imaging methods for clinical and scientific applications.
The recordings of S7–S15 analyzed for this study can be found in the fingerflex dataset provided by Kai Miller (https://stacks.stanford.edu/file/druid:zk881ps0522/fingerflex.zip).
This study was carried out in accordance with the recommendations of the institutional review board of Asahikawa Medical University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review board of Asahikawa Medical University.
JG developed the methods, was involved in data acquisition, performed data processing, and prepared the manuscript. AZ assisted in data processing, results evaluation, and interpretation. CK was involved in data acquisition and contributed to methods development. KK supervised the clinical study. JS provided scientific input. CG founded g.tec medical engineering GmbH and supervised the project.
This work was funded by the Eurostars RapidMaps 2020 project.
Several authors of this publication are employed at g.tec medical engineering GmbH, Austria. As part of a Ph.D. program, our motivation for conducting the study and developing the methods was entirely scientific. However, the described algorithms may be commercialized by g.tec medical engineering GmbH in the future.
The authors want to thank Michael Jordan for preparing the renderings in Figure 1, Brendan Z. Allison for proofreading, and the reviewers for their valuable comments.
Bishop, C. M. (2006). “Fisher's linear discriminant analysis,” in Pattern Recognition and Machine Learning, Information Science and Statistics, eds M. Jordan, J. Kleinberg, and B. Schoelkopf (New York, NY: Springer), 186–189.
Blankertz, B., Lemm, S., Treder, M., Haufe, S., and Mueller, K.-R. (2011). Single-trial analysis and classification of ERP components–A tutorial. NeuroImage 56, 814–825. doi: 10.1016/j.neuroimage.2010.06.048
Blankertz, B., Tomioka, R., Lemm, S., Kawanabe, M., and Mueller, K.-R. (2008). Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Process. Mag. 25, 41–56. doi: 10.1109/MSP.2008.4408441
Bleichner, M. G., Freudenburg, Z. V., Jansma, J. M., Aarnoutse, E. J., Vansteensel, M. J., and Ramsey, N. F. (2016). Give me a sign: decoding four complex hand gestures based on high-density ECoG. Brain Struct. Funct. 221, 203–216. doi: 10.1007/s00429-014-0902-x
Bostanov, V. (2004). BCI competition 2003–data sets Ib and IIb: feature extraction from event-related brain potentials with the continuous wavelet transform and the t-value scalogram. IEEE Trans. Biomed. Eng. 51, 1057–1061. doi: 10.1109/TBME.2004.826702
Branco, M. P., Freudenburg, Z. V., Aarnoutse, E. J., Bleichner, M. G., Vansteensel, M. J., and Ramsey, N. F. (2017). Decoding hand gestures from primary somatosensory cortex using high-density ECoG. NeuroImage 147, 130–142. doi: 10.1016/j.neuroimage.2016.12.004
Chestek, C. A., Gilja, V., Blabe, C. H., Foster, B. L., Shenoy, K. V., Parvizi, J., et al. (2013). Hand posture classification using electrocorticography signals in the gamma band over human sensorimotor brain areas. J. Neural Eng. 10:026002. doi: 10.1088/1741-2560/10/2/026002
Garrett, D., Peterson, D. A., Anderson, C. W., and Thaut, M. H. (2003). Comparison of linear, nonlinear, and feature selection methods for EEG signal classification. IEEE Trans. Neural Syst. Rehabil. Eng. 11, 141–144. doi: 10.1109/TNSRE.2003.814441
Gruenwald, J., Kapeller, C., Guger, C., Ogawa, H., Kamada, K., and Scharinger, J. (2017a). “Comparison of alpha/beta and high-gamma band for motor-imagery based BCI control: a qualitative study,” in 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC) (IEEE) (Banff, AB), 2308–2311.
Gruenwald, J., Kapeller, C., Kamada, K., Scharinger, J., and Guger, C. (2017b). “Optimal bandpower estimation and tracking via Kalman filtering for real-time brain-computer interfaces,” in 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER) (Shanghai), 605–608.
Hoffmann, U., Vesin, J.-M., Ebrahimi, T., and Diserens, K. (2008). An efficient P300-based brain–computer interface for disabled subjects. J. Neurosci. Methods 167, 115–125. doi: 10.1016/j.jneumeth.2007.03.005
Hotson, G., McMullen, D. P., Fifer, M. S., Johannes, M. S., Katyal, K. D., Para, M. P., et al. (2016). Individual finger control of a modular prosthetic limb using high-density electrocorticography in a human subject. J. Neural Eng. 13:026017. doi: 10.1088/1741-2560/13/2/026017
Jiang, T., Jiang, T., Wang, T., Mei, S., Liu, Q., Li, Y., et al. (2017). Characterization and decoding the spatial patterns of hand extension/flexion using high-density ECoG. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 370–379. doi: 10.1109/TNSRE.2016.2647255
Kapeller, C., Ogawa, H., Schalk, G., Kunii, N., Coon, W. G., Scharinger, J., et al. (2018). Real-time detection and discrimination of visual perception using electrocorticographic signals. J. Neural Eng. 15:036001. doi: 10.1088/1741-2552/aaa9f6
Kapeller, C., Schneider, C., Kamada, K., Ogawa, H., Kunii, N., Ortner, R., et al. (2014). “Single trial detection of hand poses in human ECoG using CSP based feature extraction,” in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (Chicago, IL), 4599–4602.
Kobak, D., Brendel, W., Constantinidis, C., Feierstein, C. E., Kepecs, A., Mainen, Z. F., et al. (2016). Demixed principal component analysis of neural population data. eLife 5:e10989. doi: 10.7554/eLife.10989
Kubánek, J., Miller, K. J., Ojemann, J. G., Wolpaw, J. R., and Schalk, G. (2009). Decoding flexion of individual fingers using electrocorticographic signals in humans. J. Neural Eng. 6:066001. doi: 10.1088/1741-2560/6/6/066001
Lee, FY-T., Scherer, R., Leeb, R., Neuper, C., Bischof, H., and Pfurtscheller, G. (2005). “A comparative analysis of multi-class EEG classification for brain computer interface,” in Computer Vision Winter Workshop (Zell an der Pram), 195–204. Available online at: https://graz.pure.elsevier.com/en/publications/a-comparative-analysis-of-multi-class-eeg-classification-for-brai
Li, Y., Zhang, S., Jin, Y., Cai, B., Controzzi, M., Zhu, J., et al. (2017). Gesture decoding using ECoG signals from human sensorimotor cortex: a pilot study. Behav. Neurol. 2017:3435686. doi: 10.1155/2017/3435686
Lotte, F., Bougrain, L., and Clerc, M. (2015). “Electroencephalography (EEG)-based brain–computer interfaces,” in Wiley Encyclopedia of Electrical and Electronics Engineering, ed J. G. Webster (Hoboken, NJ: John Wiley & Sons, Inc), 44.
Lotte, F., Congedo, M., Lécuyer, A., Lamarche, F., and Arnaldi, B. (2007). A review of classification algorithms for EEG-based brain-computer interfaces. J. Neural Eng. 4, R1–R13. doi: 10.1088/1741-2552/aab2f2
Miller, K. J., Hermes, D., Honey, C. J., Hebb, A. O., Ramsey, N. F., Knight, R. T., et al. (2012). Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput. Biol. 8:e1002655. doi: 10.1371/journal.pcbi.1002655
Miller, K. J., Zanos, S., Fetz, E. E., den Nijs, M., and Ojemann, J. G. (2009). Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J. Neurosci. 29, 3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009
Minka, T. P. (2001). “Automatic choice of dimensionality for PCA,” in Advances in Neural Information Processing Systems, eds T. G. Dietterich, S, Becker, and Z. Ghahramani (Vancouver, BC: MIT Press), 598–604. Available online at: http://www.cs.cmu.edu/Groups/NIPS/NIPS2001/
Ogawa, H., Kamada, K., Kapeller, C., Hiroshima, S., Prueckl, R., and Guger, C. (2014). Rapid and minimum invasive functional brain mapping by real-time visualization of high gamma activity during awake craniotomy. World Neurosurg. 82, 912.e1–912.e10. doi: 10.1016/j.wneu.2014.08.009
Onaran, I., Ince, N. F., and Cetin, A. E. (2011). “Classification of multichannel ECoG related to individual finger movements with redundant spatial projections,” in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE) (Boston, MA), 5424–5427.
Oppenheim, A. V., and Schafer, R. W. (2010). “Chapter 11.2.2: Modeling of random signals,” in Discrete-Time Signal Processing: International Edition, eds M. J. Horton and A. Gilfillan (Upper Saddle River, NJ: Pearson Education, Inc.), 921.
Pan, G., Li, J.-J., Qi, Y., Yu, H., Zhu, J.-M., Zheng, X.-X., et al. (2018). Rapid decoding of hand gestures in electrocorticography using recurrent neural networks. Front. Neurosci. 12:555. doi: 10.3389/fnins.2018.00555
Pfurtscheller, G., and Lopes da Silva, F. (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857. doi: 10.1016/S1388-2457(99)00141-8
Pistohl, T., Schulze-Bonhage, A., Aertsen, A., Mehring, C., and Ball, T. (2012). Decoding natural grasp types from human ECoG. NeuroImage 59, 248–260. doi: 10.1016/j.neuroimage.2011.06.084
Prueckl, R., and Guger, C. (2009). “A brain-computer interface based on steady state visual evoked potentials for controlling a robot,” in Bio-Inspired Systems: Computational and Ambient Intelligence, eds J. Cabestany, F. Sandoval, A. Prieto, and J. M. Corchado (Berlin; Heidelberg: Springer), 690–697.
Scherer, R., Mueller, G., Neuper, C., Graimann, B., and Pfurtscheller, G. (2004). An asynchronously controlled EEG-based virtual keyboard: improvement of the spelling rate. IEEE Trans. Biomed. Eng. 51, 979–984. doi: 10.1109/TBME.2004.827062
Shenoy, P., Miller, K. J., Ojemann, J. G., and Rao, R. P. (2007). “Finger movement classification for an electrocorticographic BCI,” in 2007 3rd International IEEE/EMBS Conference on Neural Engineering (IEEE) (Kohala Coast), 192–195.
Tangermann, M., Müller, K.-R., Aertsen, A., Birbaumer, N., Braun, C., Brunner, C., et al. (2012). Review of the BCI competition IV. Front. Neurosci. 6:55. doi: 10.3389/fnins.2012.00055
Williams, A. H., Kim, T. H., Wang, F., Vyas, S., Ryu, S. I., Shenoy, K. V., et al. (2018). Unsupervised discovery of demixed, low-dimensional neural dynamics across multiple timescales through tensor component analysis. Neuron 98, 1099–1115.e8. doi: 10.1016/j.neuron.2018.05.015
Wolpaw, J. R., Birbaumer, N., McFarland, D. J., Pfurtscheller, G., and Vaughan, T. M. (2002). Brain–computer interfaces for communication and control. Clin. Neurophysiol. 113, 767–791. doi: 10.1016/S1388-2457(02)00057-3
Wolpaw, J. R., and Wolpaw, E. W. (2012). Brain-Computer Interfaces: Principles and Practice, 1st Edition. Oxford; New York, NY: Oxford University Press.
Wu, S.-L., Wu, C.-W., Pal, N. R., Chen, C.-Y., Chen, S.-A., and Lin, C.-T. (2013). “Common spatial pattern and linear discriminant analysis for motor imagery classification,” in 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB) (IEEE) (Singapore), 146–151.
Xie, T., Zhang, D., Wu, Z., Chen, L., and Zhu, X. (2015). Classifying multiple types of hand motions using electrocorticography during intraoperative awake craniotomy and seizure monitoring processes–case studies. Front. Neurosci. 9:353. doi: 10.3389/fnins.2015.00353
Xu, R., Jiang, N., Lin, C., Mrachacz-Kersting, N., Dremstrup, K., and Farina, D. (2014). Enhanced low-latency detection of motor intention from EEG for closed-loop brain-computer interface applications. IEEE Trans. Biomed. Eng. 61, 288–296. doi: 10.1109/TBME.2013.2294203
Keywords: brain-computer interface, electrocorticography, movement decoding, linear discriminant analysis, spectral whitening
Citation: Gruenwald J, Znobishchev A, Kapeller C, Kamada K, Scharinger J and Guger C (2019) Time-Variant Linear Discriminant Analysis Improves Hand Gesture and Finger Movement Decoding for Invasive Brain-Computer Interfaces. Front. Neurosci. 13:901. doi: 10.3389/fnins.2019.00901
Received: 28 February 2019; Accepted: 12 August 2019;
Published: 26 September 2019.
Edited by:
Cuntai Guan, Nanyang Technological University, SingaporeReviewed by:
Kazutaka Takahashi, University of Chicago, United StatesCopyright © 2019 Gruenwald, Znobishchev, Kapeller, Kamada, Scharinger and Guger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Gruenwald, Z3J1ZW53YWxkQGd0ZWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.