- Department of Psychology and Centre for Cognitive Neuroscience, University of Salzburg, Salzburg, Austria
Estradiol and progesterone levels vary along the menstrual cycle and have multiple neuroactive effects, including on the dopaminergic system. Dopamine relates to executive functions in an “inverted U-shaped” manner and its levels are increased by estradiol. Accordingly, dopamine dependent changes in executive functions along the menstrual cycle have been previously studied in the pre-ovulatory phase, when estradiol levels peak. Specifically it has been demonstrated that working memory is enhanced during the pre-ovulatory phase in women with low dopamine baseline levels, but impaired in women with high dopamine baseline levels. However, the role of progesterone, which peaks in the luteal cycle phase, has not been taken into account previously. Therefore, the main goals of the present study were to extend these findings (i) to the luteal cycle phase and (ii) to other executive functions. Furthermore, the usefulness of the eye blink rate (EBR) as an indicator of dopamine baseline levels in menstrual cycle research was explored. 36 naturally cycling women were tested during three cycle phases (menses–low sex hormones; pre-ovulatory–high estradiol; luteal–high progesterone and estradiol). During each session, women performed a verbal N-back task, as measure of working memory, and a single trial version of the Stroop task, as measure of response inhibition and cognitive flexibility. Hormone levels were assessed from saliva samples and spontaneous eye blink rate was recorded during menses. In the N-back task, women were faster during the luteal phase the higher their progesterone levels, irrespective of their dopamine baseline levels. In the Stroop task, we found a dopamine-cycle interaction, which was also driven by the luteal phase and progesterone levels. For women with higher EBR performance decreased during the luteal phase, whereas for women with lower EBR performance improved during the luteal phase. These findings suggest an important role of progesterone in modulating dopamine-cycle interactions. Additionally, we identified the eye blink rate as a non-invasive indicator of baseline dopamine function in menstrual cycle research.
Introduction
During the past decades, cognitive neuroscience has witnessed an increasing interest in the research of menstrual cycle and sex hormone effects on female brain and cognition. In naturally cycling women, estradiol and progesterone levels fluctuate along the menstrual cycle as a product of complex neuroendocrine interactions. In the early follicular phase (to which we will refer as menses phase), both estradiol and progesterone levels are low, whereas the late follicular phase (or pre-ovulatory phase), is characterized by a peak in estradiol levels, and the luteal phase by high levels of progesterone and medium of estradiol (Fehring et al., 2006; Sacher et al., 2013).
Both estradiol and progesterone are known to interact with several neurotransmitter systems, including norepinephrine, serotonin, acetylcholine and dopamine (DA) (Genazzani et al., 1997; McEwen and Alves, 1999; Mitsushima, 2010; Barth et al., 2015). In particular, estradiol seems to have a special impact on the dopaminergic system. Animal research has demonstrated numerous genomic and non-genomic estradiol effects on functional activity modulating DA. It increases the synthesis, release, reuptake and turnover of DA, affects downstream targets of its receptor and modifies basal firing rates of dopaminergic neurons (Tansey et al., 1983; Becker, 1990, 1999; Bazzett and Becker, 1994; Pasqualini et al., 1995; Bethea et al., 2002). The effect of progesterone on neurotransmitter systems is not as straightforward. While some studies assume that progesterone exerts opposite effects to estradiol (Fernández-Ruiz et al., 1990), others assume similar effects of both hormones (Sánchez et al., 2010) or a modulation of the estrogenic actions by progesterone (Alves et al., 2000; Barbosa-Vargas et al., 2009). Likewise, for the dopaminergic system, some studies reported an effect of progesterone only when primed with estrogen (Dluzen and Ramirez, 1987; Becker and Rudick, 1999), or effects in opposite direction depending on its concentration (Cabrera et al., 1993). Given the multitude of estrogenic effects on the dopaminergic system, these inconsistencies are not so surprising, as progesterone might facilitate some of these actions, while modulating or opposing others. However, along the menstrual cycle, it is very hard to dissociate the effects of progesterone from the effects of estradiol, since both hormones levels are high during the luteal phase.
Related to these neuroactive effects of estradiol and progesterone, cognitive performance and behavior change along the menstrual cycle as these hormones fluctuate. Specifically performance in tasks which reveal greater gender differences are mediated through sex hormone levels (Hampson, 1990; Kimura and Hampson, 1994; Phillips and Silverman, 1997; Andreano and Cahill, 2009). For instance, verbal abilities seem to be improved during pre-ovulatory (Hampson, 1990) or mid-luteal phase (Maki et al., 2002) whereas spatial abilities, like mental rotation, are improved during menses (Hampson, 1990; Hausmann et al., 2000), and multiple memory systems seem to be differently regulated by ovarian hormones (Phillips, 1992; Hussain et al., 2016). Changes have also been observed in higher cognitive functions, such as working memory (Jacobs and D'Esposito, 2011), cognitive control (Hatta and Nagaya, 2009), inhibitory control (Colzato et al., 2010), navigation task strategy (Hussain et al., 2016) or delayed reward selection (Smith et al., 2014) during the pre-ovulatory phase compared to the menses and/or luteal phase.
However, these findings are far from consistent (Maki et al., 2002; Rosenberg and Park, 2002; Schöning et al., 2007; Gasbarri et al., 2008; Mordecai et al., 2008; Solís-Ortiz and Corsi-Cabrera, 2008). Some of the discrepant results may be explained by the lack of methodological standardization regarding the timing of assessment, confirmation of cycle phase apart from self-reports, or use of biological samples, among others (Sacher et al., 2013; Sundström Poromaa and Gingnell, 2014). With regards to higher cognitive functions it has been suggested that these inconsistencies could also arise from the interactions between estradiol and the dopaminergic system (Colzato and Hommel, 2014). The dopaminergic system plays a key role in complex cognitive processes. Among these higher executive functions, “shifting,” “updating,” and “inhibition” (Miyake et al., 2000) can be distinguished. Since DA modulates higher cognitive functions in an inverted u-shaped manner, either insufficient or excessive levels of this neurotransmitter are related to a worse performance. The DA optimum to achieve the maximum performance is different for each function, and hence for each DA-dependent task (Cai and Arnsten, 1997; Feil et al., 2010; Cools and D'Esposito, 2011). Consequently, during the pre-ovulatory phase, the peak in estradiol leads to a rise in DA levels and therefore modifies the performance depending on the individual differences in DA baseline levels. In this way, the effects of menstrual cycle on higher cognitive functions would not be consistent unless differences in DA baseline levels between women are accounted for. This hypothesis has been previously reported for the working memory during high control interference trials (lure trials) (Jacobs and D'Esposito, 2011). Women with low baseline DA levels (indicated by the enzyme catechol-O-methyltransferase, COMT) presented enhanced performance during the pre-ovulatory phase related to an increase in estradiol whereas women with higher DA levels presented impaired performance. However the role of progesterone has not been fully characterized. Given the modulating effect of progesterone on the dopaminergic system (Frye and Sora, 2010) and its influence on estrogen impact (Dluzen and Ramirez, 1987; Yu and Liao, 2000), it is necessary to extend this research to the luteal phase, in which both estradiol and progesterone levels are higher compared to menses. Furthermore, the modulation of sex hormone effects by the DA baseline levels has not been generalized to other DA-dependent executive functions so far.
In the present study we used a verbal N-back task as measure of working memory (Braver et al., 1997), and a Stroop task as measure of cognitive flexibility (Stroop, 1935). The variant used for the N-back was similar to the task employed by Jacobs and D'Esposito (2011). The N-back task allows to not only study the ability to manipulate and retain information (as working memory is often described), i.e., “updating” via the accuracy in detecting targets, but also the attentional control of interference via lure trials (Engle and Kane, 2003; Gray et al., 2003). For the Stroop task, we used a single trial version (Dalrymple-Alford and Budayr, 1966), which demands more flexible control mechanism than the classical block-wise presentation, since congruent, neutral and incongruent trials are randomly presented avoiding anticipation. Therefore, this task does not only allow for the assessment of “inhibitory control” via the interference effect, but also for “shifting” via overall performance on the task. Furthermore, we used two different conditions, Stroop word and Stroop color, in order to explore the semantic and the color naming interference and facilitation separately. The parallel distributed processing (PDP) model (Cohen et al., 1990; MacLeod and MacDonald, 2000) suggests that interference arises from the processing of the word and color in parallel, therefore allowing us to explore the more automatic process of reading words (Stroop word), in contrast to naming colors (Stroop color) (MacLeod and Dunbar, 1988).
In humans, direct measurement of individual DA levels is only possible through techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) (Willeit et al., 2016). However, due to their invasiveness, other indirect markers are more commonly used. The spontaneous eye blink rate (EBR) has been suggested as a non-invasive indicator of striatal DA levels (Colzato and Hommel, 2014; see review Jongkees and Colzato, 2016). Its relation to dopamine levels is now well-established from animal, pharmacological and clinical studies. EBR correlates positively with dopamine levels in the caudate nucleus of non-human primates (Taylor et al., 1999). DA agonists and antagonists increase and decrease EBRs, respectively in animals (Korsgaard et al., 1985; Elsworth et al., 1991; Lawrence and Redmond, 1991; Kleven and Koek, 1996; Kaminer et al., 2011) and humans (Blin et al., 1990; Strakowski et al., 1996; Strakowski and Sax, 1998). Additionally, hypo-dopaminergic conditions like Parkinson's lead to a reduction of the spontaneous blink rate (Karson, 1983; Karson et al., 1984; Korosec et al., 2006), while hyper-dopaminergic conditions like schizophrenia lead to an increase of the spontaneous blink rate (Cheung et al., 1995; Chen et al., 1996). So far, several studies have used the EBR as a behavioral index of DA functioning, predicting performance in DA-dependent cognitive tasks (Dreisbach et al., 2005; Colzato et al., 2007, 2008, 2009; Chermahini and Hommel, 2010; Akbari Chermahini and Hommel, 2012; Dang et al., 2016). However, the usefulness of this indicator has not yet been established in menstrual cycle research. In the present study, spontaneous EBR was used as an indicator of baseline striatal DA levels as suggested by Jongkees and Colzato (2016) and Colzato and Hommel (2014).
In summary, the main goal of the current study is to extend the previous findings of DA dependent changes in cognitive performance across the menstrual cycle to the luteal cycle phase on the one hand and to a variety of executive functions on the other hand. Furthermore, we intend to demonstrate the usefulness of the EBR as DA indicator in menstrual cycle research. As demonstrated previously, we expect cognitive benefits in the pre-ovulatory phase compared to the menses phase, if EBR is low during menses; and impairment in those individuals with high EBR during menses for working memory functions. We hypothesize a similar interaction for inhibitory control and shifting of information as assessed with the Stroop task. Different DA optima will be explored by different EBR cut-offs. Regarding the luteal phase, increases or decreases in performance compared to the pre-ovulatory phase are expected and should shed light on the modulatory role of progesterone on the estrogenic actions in the dopaminergic system.
Materials and Methods
Participants
Forty-three healthy right-handed women were recruited for the study on the campus of the Faculty of Sciences of the University of Salzburg and through social media. All participants were German-speaking and most of them university students. A total of 7 participants were excluded prior to analyses because of inconsistencies between hormone values and cycle phase as calculated based on self-reports. Therefore, analyses were performed in 36 women with an age range between 18 and 33 years (Mage = 23.36, SD = 3.44). All of them had a regular menstrual cycle (Mcycle length = 28.67 days, SD = 2.48) and had not used hormonal contraceptives within the previous 6 months. Regular menstrual cycle was defined as ranging between 21 and 35 days and a variability of cycle length between individual cycles of less than 7 days (Fehring et al., 2006). Other exclusion criteria were neurological, psychiatric or endocrine disorders, and being under medication treatment. All participants received either course credits or 30 € for their participation.
Ethics Statement
Experiments were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), and all participants gave their informed written consent to participate in the study. The institutional guidelines of the University of Salzburg (Statutes of the University of Salzburg - see https://online.uni-salzburg.at/plus_online/wbMitteilungsblaetter.display?pNr=98160) state in §163 (1) that ethical approval is necessary for research on human subjects if it affects the physical or psychological integrity, the right for privacy or other important rights or interests of the subjects or their dependents. In §163 (2) it is stated that it is the responsibility of the PI to decide, whether (1) applies to a study or not. Therefore, we did not seek ethical approval for this study. Since it was non-invasive and performed on healthy adult volunteers, who gave their informed consent to participate, (1) did not apply. Data were processed in anonymized/deidentified form. Upon arrival at the lab, participants were assigned a subject ID (VP001, VP002, etc.), which was used throughout the study.
Procedure
In order to study every possible combination of hormonal levels, participants were tested in three sessions, time-locked to the subject's menstrual cycle as follows. First, during menses (low progesterone and estradiol); second in the pre-ovulatory phase (when estradiol levels peak and progesterone is still low), and last during the mid-luteal phase (high progesterone and estradiol), order counter-balanced. Menses phase spanned from the second day of menstruation to 7 days before ovulation (Mday = 3.92, SD = 2.34) depending on the individual cycle length. Pre-ovulatory appointments were scheduled 3 days before the expected ovulation (Mday = 11.93, SD = 3.07), which can be calculated subtracting 14 days to the length of the whole cycle, since luteal phase, the following period, is relatively stable among women (Fehring et al., 2006). The ovulation was confirmed by commercially available ovulation tests (Pregnafix® Ovulationstest), which test for the LH surge in urine. Mid-luteal phase ranged from day 3 post ovulation to 3 days before the onset of the next menstruation (Mday = 21.50, SD = 3.26), which was confirmed by follow up reports of the participants.
During each session participants filled in a brief questionnaire to assess food, sports and sleeping habits during the previous hours, and possible stressors within the last weeks. Afterwards EBR was measured and saliva samples taken as explained in the following sections. Then, both tasks were applied with a break in between them. EBR is supposed to be stable during daytime but increases in the evening (Barbato, 2000), and cortisol levels, which are known to interact with estradiol levels (Kirschbaum et al., 1999; Whirledge and Cidlowski, 2010), typically are highest in the morning, right after waking (Kirschbaum and Hellhammer, 1989; Pruesser et al., 1997; Schmidt-Reinwald et al., 1999). To avoid any confounds by these diurnal changes, data were always collected after 11 a.m. and before 5 p.m. and the three sessions of the same participants were scheduled approximately around the same hour.
Cognitive Paradigms
Stimulus presentation and data acquisition were done with Presentation® (Systems, 2011, Neurobehavioral Systems, http://www.neurobs.com/) in a CRT monitor (1,024 × 768 pixel resolution, 120 Hz refresh rate). As part of a larger study, participants completed two different tasks:
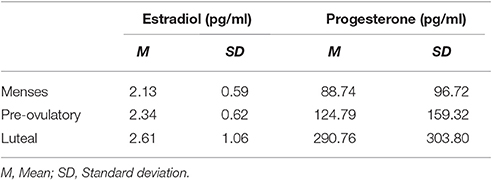
N-back Task
The N-back verbal task consisted of 4 levels of load: 0-, 1-, 2-, and 3-back; and 3 different trial types: targets, lures and non-lures. Upper case black letters were presented on a white background. Participants were instructed to respond to each letter by pressing the left mouse button when the answer was “yes” and the right one if it was “no.” In the 0-back level participants indicated whether or not the target letter X appeared. In this condition there were not lures, trial types consisted only of targets and non- targets. For the 1-back level the participants responded whether the letter matched the previous letter, and for 2- and 3-back whether it was identical to the one presented 2 or 3 trials before, respectively (Figure 1). Three different versions of N-back task were presented, one in each session, order counterbalanced. Each version consisted of 16 blocks of trials (4 blocks of each level) ordered in a Latin squares sequence. Every block included 20 stimuli which appeared every 2 sec and lasted 1 s each. Trials were pseudo-randomly ordered and their proportions were 20% targets, 65% non-lures, and 15% lures, except for the 0-back, in which targets were 20% and non-lures 80%. Blocks were preceded by 6 s of instructions each and separated from one another by 16 s (including instructions). The whole task was always preceded by a training version which included one shortened block of each level.
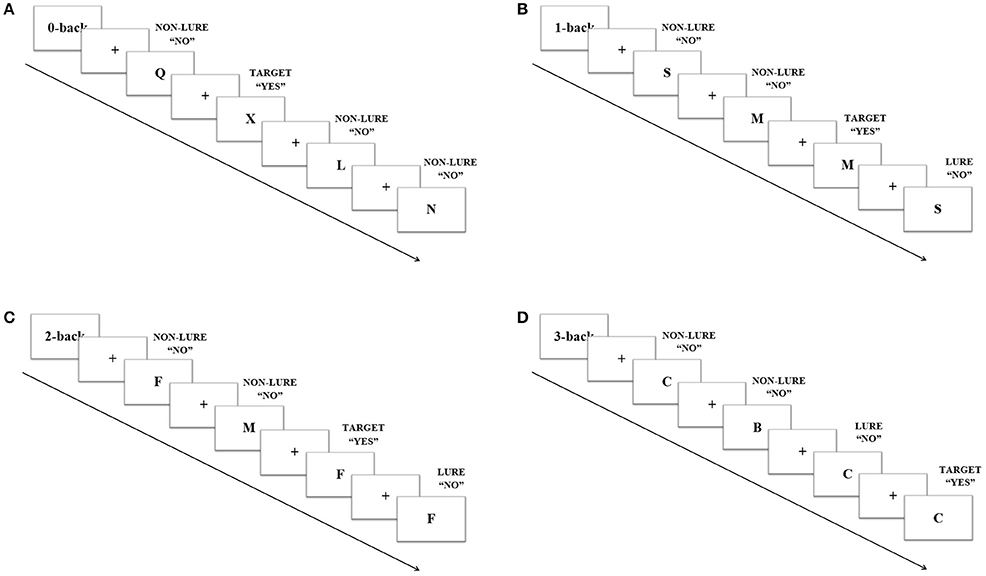
Figure 1. Example of four trials in the N-back task. Every trial appeared every 2 s and lasted 1 s each. Participants were instructed to press left mouse button when “yes” and the right one if “no.” For 0-back (A), targets were letter X; for 1-back (B), the target was when the letter matched the previous letter; and for 2-back (C) and 3-back (D) when it was identical to the one presented 2 or 3 trials before, respectively.
Accuracy and response time were recorded for each trial type and condition. Additionally, for the targets, as a measure of sensitivity, d prime (d') was calculated for all 4 n-back conditions, based on the signal detection theory (Swets et al., 1961). This discriminability index has been proposed to provide a more suitable index of working memory performance, and to be less susceptible to demographic variables (Haatveit et al., 2010). It describes the relationship between the signal and noise distributions and therefore offers information about the participant's discrimination between targets and non-targets (Wickens, 2001, p. 3–16). For its calculation we followed the formula d' = ZHit – ZFA (Macmillan and Creelman, 1990). The hit rate (Hit) is expressed by the proportion of correct answers to targets when they appear (hits target/number of targets), and the false alarm rate (FA), is expressed by the incorrect answers to non-targets, which include both lures and non-lures (incorrect non-target/number of non-targets). To adjust the perfect scores, values were replaced as previously reported by Haatveit et al. (2010), by 1−1/(2n) for perfect hits and 1/(2n) for zero false alarms, with n being the total number of targets (16) or non-targets (64). Following these formulas higher values of d' (ranging between ±4.28) indicate higher sensitivity.
Stroop Task
The Stroop task consisted of an adapted single trial version divided in a color and a word variant. Each condition of the task included three blocks in which 120 trials were presented in a random order every 1.5 s. In the Stroop word, the words “Blue” and “Red” appeared in red, blue or white color. In this condition, participants were told to press the left mouse button when the word “Blue” was presented and the right one when “Red” was, in this case regardless the color of the letters. There were also three trial types: congruent, when “Blue” was written in blue color or when “Red” was written in red color; incongruent, when “Blue” was written in red or when “Red” was written in blue; and neutral, when any of the words appeared written in white (Figure 2A). The proportions of the trials were: 33% congruent, 33% incongruent, and 33% neutral, all of the groups with half of the trials with the word “Blue” and half with the word “Red.” In the Stroop color, the words “Blue,” “Red,” or the string “XXX” appeared in uppercase letters on a black background either on blue or red color. Participants were asked to press the left mouse button when a string of letters written in blue appeared and the right one when it was red, always regardless the meaning of the word. There were three trial types: congruent, when “Blue” was written in blue color or when “Red” was written in red color; incongruent, when the former words were written in a mismatching color; and neutral, when the string “XXX” appeared (Figure 2B). The proportions of the trials were: 33% congruent, 33% incongruent, and 33% neutral, half red half blue in every group. For both conditions, participants completed a training version, which consisted of a shortened block with 12 trials in the same proportions as described.
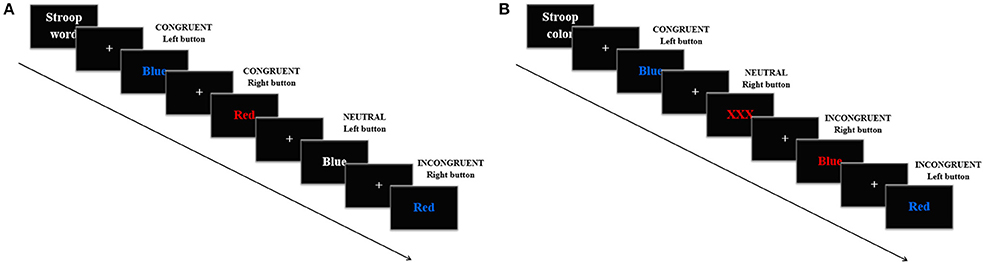
Figure 2. Example of four trials in the Strop task. Every trial appeared every 1.5 s. In the Stroop word (A), participants were told to press the left mouse button when the word “Blue” was presented and the right one when the word “Red” was presented. In the Stroop color (B), participants were asked to press the left mouse button when the letters were written in blue and the right one when the letters were written in red.
EBR Measurement
Spontaneous EBR was taken as an indirect measure of baseline DA levels. Fluctuations in the DA levels and hence in the EBR can be found related to estradiol levels. A decrease in EBR in older women has been related to an estradiol decline (Chen et al., 2003), and oral contraceptives are suggested to increase the EBR (Yolton et al., 1994). Therefore, we used the EBR recorded during menses as DA indicator, in order to ensure it was not affected by any hormonal influences.
For the recording, subjects were seated 1 m from a white wall with a black cross at their eyes height. They were left alone in the room and asked to fix their gaze at the cross silently and in resting conditions. Vertical and horizontal electro-oculograms (EOGs) were recorded with an EEG system (actiCAP, Brain Products GmbH, Germany) at a sampling rate of 500 Hz and impedances kept under 50 kΩ. Skin electrodes were placed above and below the right orbita, and at the outer canthi, referenced against the vertex electrode (Cz), and a grounding electrode was located on the forehead. An eye blink was defined as a sharp high amplitude wave with a voltage change of 100 uv in a time interval of 500 ms. The EBR was defined as the number of blinks per minute as averaged over six consecutive minutes. Signals were amplified using an ActiCHamp Amplifier (Brain Products GmbH, Germany). The analysis of the recorded blinks was performed online with Brain Vision Analyzer 2.1(Brain Products™ GmbH, Munich, Germany), in which two independent observers visually scored the number of blinks for the 6 min segment. The inter-rater agreement was 100%. The EBR during menses ranged from 2.00 to 42.33 blinks per min (MEBR = 16.14, SD = 10.73). This large spectrum, although indirectly, should provide the sufficient variability in dopaminergic activity between subjects.
Hormone Analysis
In order to assess estradiol and progesterone levels three saliva samples 2 ml each were collected throughout every session: one before the tasks, one after, and one in between. Until further analysis they were stored in a freezer at −20°C and solid particles were removed by centrifugation (3,000 rpm for 15 min, then 3,000 rpm for 10 min). For hormone analyses, the three samples were pooled in order to control for diurnal fluctuations in hormone levels and to ensure reliability of hormone assessment. Estradiol and progesterone levels were quantified using ELISA kits from DeMediTec Diagnostics which are based on the competition principle and microplate separation using polyclonal antisera coated to the wells. Sensitivity is 0.6 pg/ml for estradiol and 5.0 pg/ml for progesterone. Intra-assay variation (CV) is between 2.4 and 8.3% for estradiol and between 6.0 and 9.6% for progesterone. Inter-assay variation (CV) is between 2.8 and 12.0% for estradiol and between 8.6 and 10.1% for progesterone.
Statistical Analysis
Those sessions in which overall performance was below chance were excluded from the analysis. In particular this concerned two whole sessions from 2 different participants in the Stroop task, and every session from one participant in the N-back task. Statistical analyses were carried out in R 3.2.2., assessing the possible effect of the factors on each dependent variable through linear mixed models as follows. First, we used the lmer function of the lme4 package, modulating the participant number as random factor, so that we controlled for repeated measurements. In every analysis, the factors that were not relevant to explain the model were eliminated with the step function of the lmerTest package at its default settings. The specific models are described in the respective paragraph of the results section. In all models, both the dependent and continuous independent variables were z-standardized using the scale function. Therefore, the coefficients b of fixed effects in the models represent a standardized effect size based on standard deviations, similar to Cohen's d.
Results
Cycle Phase and Hormone Levels
In order to compare sex hormone levels between the different cycle phases, the fixed factor cycle phase was modeled for dependent variables estradiol and progesterone, respectively in the context of a linear mixed effects model.
Estradiol was significantly higher in the pre-ovulatory phase compared to menses phase [b = 0.17, SEb = 0.06, t(35) = 3.06, p < 0.01] and in luteal phase compared to menses phase [b = 0.27, SEb = 0.10, t(35) = 2.83, p < 0.01] and did not differ significantly between pre-ovulatory and luteal phases. Progesterone was significantly higher in the luteal phase compared to the pre-ovulatory phase [b = 0.33, SEb = 0.09, t(33) = 3.54, p < 0.05] and in luteal phase compared to the menses phase [b = 0.41, SEb = 0.11, t(34) = 3.89, p < 0.001] and did not differ significantly between pre-ovulatory and menses (all means are displayed in Table 1).
N-back Task
Since targets and lures assess functionally different processes, i.e., updating and inhibition, they were analyzed separately (Engle and Kane, 2003; Gray et al., 2003). For targets, linear mixed models were applied to RT, accuracy and sensitivity as dependent variables. For lures, linear mixed models were applied to RT and accuracy as dependent variables. For both, targets and lures, we included the participant number (PNr) as random factor and session on the one hand, as well as the interactive effects of load, cycle phase and EBR during menses on the other hand, as fixed variables (e.g., RT ~ 1|PNr + session + load*cycle*EBR). Load, session and EBR were included as continuous variables, while cycle phase was factorized, which allows the assessment of differences between the pre-ovulatory phase and menses on the one hand and the luteal phase and menses on the other hand. In order to assess differences between luteal phase and pre-ovulatory phase, all models were rerun after excluding menses. Thus, for each dependent variable, in the first model the high hormone phases were compared to menses, while in the second model, the luteal phase was compared to the pre-ovulatory phase.
Interactive Effects of Cycle Phase and EBR during Menses
Targets
Accuracy. For the accuracy with targets, the main effect of session was non-significant and thus removed from the models. We observed a significant main effect of load [b = −0.46, SEb = 0.04, t(380) = −11.88, p < 0.001], indicating that accuracy was reduced as the load increased. Furthermore, the main effects of cycle phase and EBR were not significant and did not interact with each other or with load in both models. Therefore, they were removed from the models.
RT. For the RT with targets, we observed significant main effects of session [b = −0.19, SEb = 0.03, t(379) = −5.48, p < 0.001] and load [b = 0.52, SEb = 0.03, t(379) = 15.20, p < 0.001]. RT became shorter with the number of sessions, and longer as the load increased. In the first model, luteal and pre-ovulatory phases did not differ from menses and cycle phase is removed from the model. In the second model, women were significantly faster in their answers to targets during the luteal phase compared to the pre-ovulatory phase [b = −0.16, SEb = 0.08, t(241) = −2.15, p < 0.05; Figure 3]. The main effect of EBR was not significant in both models and did not interact with the cycle effects or load. Therefore, it was removed from the models. The cycle effect did also not interact with load.
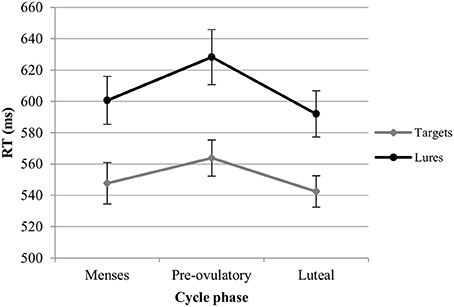
Figure 3. Reaction time (RT) for the N-back task along the menstrual cycle. Responses were significantly faster in the luteal phase compared to pre-ovulatory phase for both targets and lures.
Sensitivity. For the sensitivity with targets, we observed significant main effects of session [b = 0.11, SEb = 0.03, t(362) = 3.19, p < 0.01] and load [b = −0.65, SEb = 0.03, t(362) = −18.96, p < 0.001], indicating that sensitivity was increased along the sessions and reduced as the load increased. The main effects of cycle phase and EBR were not significant and did not interact with each other or with load in both models and were, therefore, removed from the models.
Lures
Accuracy. For the accuracy with lures, we observed significant main effects of session and load. Performance increased with the number of sessions [b = 0.09, SEb = 0.04, t(261) = 2.04, p < 0.05], and decreased with higher levels of load [b = −0.57, SEb = 0.04, t(261) = −13.73, p < 0.001]. The main effects of cycle phase and EBR were not significant and did not interact with each other or with load in both models. Therefore, they were removed from the models.
RT. For the RT with lures, we observed significant main effects of session [b = −0.25, SEb = 0.04, t(259) = −5.99, p < 0.001] and load [b = 0.40, SEb = 0.41, t(259) = 9.92, p < 0.001]. RT became shorter along the sessions and longer as the load increased. In the first model, luteal and pre-ovulatory phases did not differ from menses and cycle phase is removed from the model. In the second model, we found a significant difference between luteal and pre-ovulatory phase [b = −0.23, SEb = 0.10, t(161) = −2.24, p < 0.05]. Women were faster in responding to the lures during the luteal phase as compared to the pre-ovulatory phase (Figure 3). The main effect of EBR was non-significant and did not interact with load or the cycle effects in both models. Therefore, it was removed from the models. The cycle effect did also not interact with load.
Effect of Estradiol and Progesterone Levels
To explore, whether the cycle effects observed for the RT were attributable to estradiol or progesterone, the final models from above were rerun, replacing cycle phase by estradiol and progesterone values, respectively (RT ~ 1|PNr + session + load + hormone).
Targets
For the RT with targets, no effect of estradiol was found. However, we found a significant main effect of progesterone [b = −0.09, SEb = 0.04, t(363) = −2.17, p < 0.05]. The lower the progesterone levels of women, the slower were their reactions to targets (Figure 4).
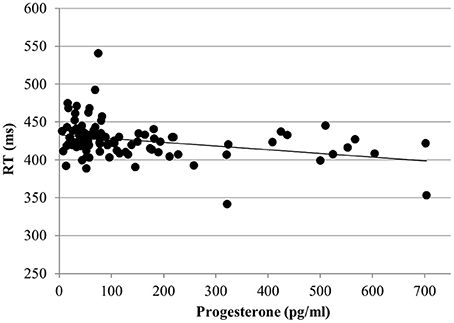
Figure 4. Relationship of progesterone levels to reaction time (RT) for the targets of the N-back task. Responses to targets were slower the lower the progesterone levels of women.
Lures
Both estradiol and progesterone did not affect the RT with lures, so they were removed from the model.
Stroop Task
For the Stroop task, word and color condition were analyzed separately, as they assess qualitatively different processes (MacLeod and Dunbar, 1988; Cohen et al., 1990). For both conditions, linear mixed models were applied to RT and accuracy as dependent variables, including the participant number (PNr) as random factor and session as well as the interactive effects of trial type, cycle phase and EBR as fixed factors (e.g., RT ~ 1|PNr + session + trial*cycle*EBR). Session and EBR were included as continuous variables, while trial type and cycle phase were factorized. Regarding trial type this allows for the assessment of interference effects (incongruent vs. neutral) on the one hand and facilitation effects (congruent vs. neutral) on the other hand. Regarding cycle phase, this allows the assessment of differences between the pre-ovulatory phase and menses on the one hand and the luteal phase and menses on the other hand. All models were rerun after excluding menses, allowing us to assess the differences between luteal phase and pre-ovulatory phase. Therefore, for each dependent variable, high hormone phases were compared to menses in the first model, whereas the luteal phase was compared to the pre-ovulatory phase in the second model.
Interactive Effect of Cycle Phase and EBR during Menses
Word condition
Accuracy. For the accuracy in the word condition, the main effect of session was non-significant and thus removed from the models. We found a significant main effect of trial type. The accuracy was significantly lower for the incongruent trials compared to the neutral ones [Interference effect; b = −0.32, SEb = 0.10, t(280) = −3.07, p < 0.01], but did not differ significantly between congruent and neutral trials [Facilitation effect; b = 0.08, SEb = 0.10, t(280) = 0.77, p = 0.44]. The main effects of cycle phase and EBR were non-significant and did not interact with trial type or each other and were thus removed from the model.
RT. For the RT in the word condition, we observed significant main effects of session [b = −0.15, SEb = 0.03, t(277) = −5.14, p < 0.001] and trial type. RT increased significantly along the number of sessions and were significantly slower for incongruent trials compared to neutral trials [Interference effect; b = 0.17, SEb = 0.07, t(277) = 2.50, p < 0.05]. There was no significant difference between RT for congruent and neutral trials [Facilitation effect; b = −0.06, SEb = 0.07, t(277) = −0.84, p = 0.40]. Furthermore, there was a significant main effect of phase on the RT, which did not interact with trial type. In the first model, we found a significant difference between luteal phase and menses [b = −0.18, SEb = 0.07, t(277) = −2.63, p < 0.01], while the pre-ovulatory phase and menses did not differ significantly [b = 0.12, SEb = 0.07, t(277) = 1.79, p = 0.07]. In the second model, we found a significant difference between luteal and pre-ovulatory phase [b = −0.31, SEb = 0.07, t(173) = −4.42, p < 0.001]. Women were significantly faster in responding during the luteal phase as compared to menses and the pre-ovulatory phase, irrespective of the type of trial (Figure 5). The main effect of EBR was non-significant and did not interact with trial type or phase and was thus removed from the model.
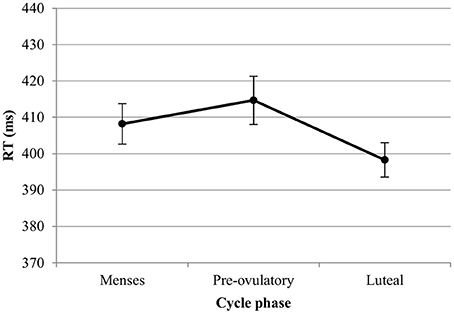
Figure 5. Reaction time (RT) for the Stroop word along the menstrual cycle. Responses were significantly faster in the luteal phase compare to menses and pre-ovulatory phase.
Color condition
Accuracy. For the accuracy in the color condition, significant main effects of session and trial type were found. Accuracy increased significantly with the number of sessions [b = 0.13, SEb = 0.05, t(275) = 2.73, p < 0.01]. Among the trial types, accuracy was significantly lower for incongruent trials compared to neutral trials [Interference effect: b = −0.25, SEb = 0.11, t(275) = −2.29, p < 0.05], whereas accuracy for congruent trials was significantly higher than for neutral trials [Facilitation effect: b = 0.22, SEb = 0.11, t(275) = 2.02, p < 0.05]. The main effects of cycle phase were non-significant in both models (all |b| < 0.22, all SEb < 0.12, all t < 1.92, all p > 0.05), as was the main effect of EBR [b = −0.03, SEb = 0.12, t(34) = −0.27, p = 0.79]. Also, the interactions between trial type and cycle phase as well as trial type and EBR were non-significant and thus removed from the model. There was a significant modulation of the accuracy by the interaction of the EBR during menses and the cycle phase of participants in both models. This interaction was driven by changes during the luteal phase compared to menses [b = −0.27, SEb = 0.11, t(275) = −2.47, p < 0.05] in the first model, while it was non-significant for the comparison of pre-ovulatory phase and menses [b < 0.004, SEb = 0.11, t(275) = −0.003, p > 0.99]. The interaction was also confirmed for the comparison of luteal phase to the pre-ovulatory phase in the second model [b = −0.25, SEb = 0.11, t(172) = −2.20, p < 0.05]. Irrespective of the number of sessions or the type of trial, in the luteal phase accuracy decreased more strongly the higher the EBR during menses (Figure 6).
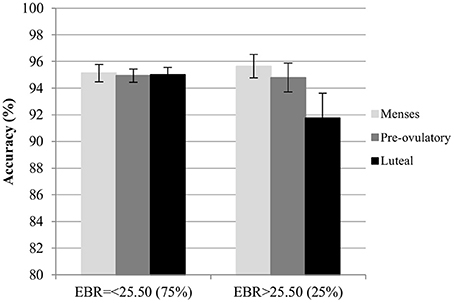
Figure 6. Mean accuracy for the Stroop color along the menstrual cycle. Performance was worse in global in the luteal phase compare to menses and pre-ovulatory for women with higher EBR during menses.
RT. For the RT in the color condition we also observed significant main effects of session and trial type. RT decreased significantly with the number of sessions [b = −0.13, SEb = 0.03, t(275) = −4.34, p < 0.001], and were significantly longer for incongruent trials compared to neutral trials [Interference effect; b = 0.27, SEb = 0.07, t(275) = 3.81, p < 0.001], while RT did not differ significantly between congruent and neutral trials [Facilitation effect; b < 0.004, SEb = 0.07, t(275) = −0.04, p = 0.97]. The main effect of cycle phase was non-significant in the first model [all |b| < 0.10, all SEb < 0.08, all t(275) < 1.40, all p > 0.05], as was the main effect of EBR [b = −0.06, SEb = 0.15, t(34) = −0.39, p = 0.70]. The interactions between trial type and cycle phase as well as trial type and EBR were also non-significant and thus removed from the model. There was a significant phase*EBR interaction in the first model, which was attributable to changes during the luteal phase compared to menses phase [b = 0.26, SEb = 0.07, t(275) = 3.68, p < 0.01], while no differences were observed between the pre-ovulatory phase and menses phase [b = 0.14, SEb = 0.07, t(275) = 1.94, p = 0.05]. During the luteal phase, the RT was shorter compared to the menses phase for women with lower EBR during menses, whereas it was significantly longer compared to the menses phase for those with higher EBR during menses (Figure 7). In the second model, the main effect of cycle phase was non-significant and did not interact with EBR or trial type, indicating that luteal and pre-ovulatory phases did not differ from each other. It was thus removed from the model.
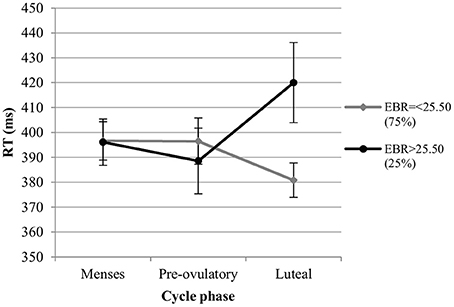
Figure 7. Interactive effects of cycle phase and EBR on reaction time (RT) for Stroop color. Responses were slower in the luteal phase compare to menses and pre-ovulatory phase for women with higher EBR during menses and faster in the luteal phase compare to the menses and pre-ovulatory phase for women with lower EBR during menses.
As different DA optima have been observed for different tasks, additional analyses were carried out to approximate the EBR cut off responsible for the interactions observed. The sample was split into groups by the quartiles of EBR during menses. The cut-off at which the interaction effects between EBR during menses and cycle phase disappeared was determined, by successively removing quartiles bottom up.
Accuracy. For the accuracy, the interaction remained in the upper 75% after removing the first quartile [b = −0.29, SEb = 0.13, t(206) = −2.30, p < 0.05] and in the upper 50% after removing the first two quartiles [b = −0.44, SEb = 0.15, t(134) = −3.01, p < 0.01]. However, the interaction disappeared from the model when only considering the upper quartile. Therefore, the cut off in the EBR for the accuracy in the Stroop color lay between the 50% and the 75% quartiles.
RT. For the RT, the interaction remained significant even in the highest quartile [b = 0.54, SEb = 0.13, t(62) = 4.01, p < 0.001]. Consequently, for the RT of the Stroop color, the cut off lay within the upper 25%.
Effect of Estradiol and Progesterone Levels
To determine whether the effects of cycle phase observed above were attributable to estradiol or progesterone, the final models from above were rerun, replacing cycle phase by estradiol and progesterone values respectively (word: RT ~ 1|PNr + session + trial type + hormone, color: e.g. RT ~ 1|PNr + session + trial type + hormone*EBR).
Word condition
RT. In the word condition, only progesterone, but not estradiol affected the RT [b = −0.12, SEb = 0.04, t(267) = −3.27, p < 0.01]. Irrespective of any other factor, participants were faster the higher their levels of progesterone (Figure 8).
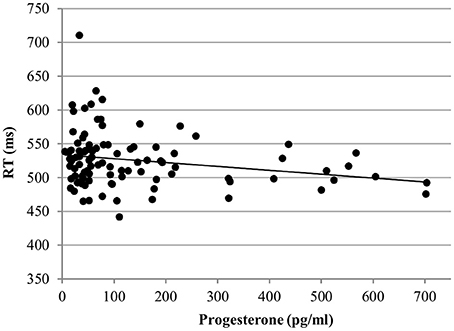
Figure 8. Relationship of progesterone levels to reaction time (RT) for Stroop word. Responses were faster the higher the progesterone levels of women.
Color condition
Accuracy. Both estradiol and progesterone did not affect accuracy and there were no interactions between EBR and estradiol or progesterone in the analysis of accuracy, so every factor was removed from the model.
RT. The RT was also not affected by estradiol levels and estradiol did not interact with EBR in the analysis of RT. The main effects of progesterone levels and EBR were non-significant [b = 0.02, SEb = 0.04, t(266) = 0.54, p = 0.59; b = 0.07, SEb = 0.15, t(33) = 0.45, p = 0.65, respectively]. However, RT was modulated by a significant interaction of progesterone with EBR during menses [b = 0.13, SEb = 0.05, t(266) = 2.70, p < 0.01]. This way, women with lower EBR during menses were slower the higher their progesterone levels, whereas women with higher EBR during menses were faster as their progesterone levels increased (Figure 9).
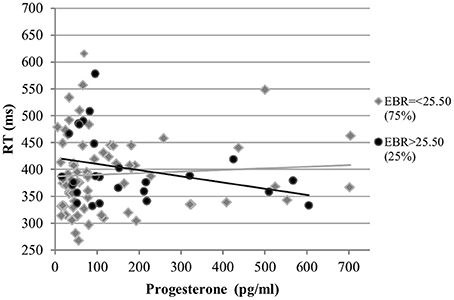
Figure 9. Interactive effects of progesterone and EBR on reaction time (RT) for Stroop color. Women with lower EBR during menses were slower the higher their progesterone levels, whereas women with higher EBR during menses were faster as their progesterone levels increased.
Discussion
The primary goal of this study was to investigate the modulating effect of DA baseline levels on menstrual cycle changes in DA dependant processes. On the one hand, we wanted to extend previous findings to (i) the luteal cycle phase and (ii) a variety of cognitive functions using different tasks. On the other hand, we wanted to explore the usefulness of the EBR as a non-invasive indicator of DA baseline levels in menstrual cycle research. To address these aims, we tested women during three cycle phases—menses (low sex hormones), pre-ovulatory (high estradiol and low progesterone), and luteal phase (high estradiol and progesterone)—on two different tasks, the N-back and the Stroop task, while assessing their EBR during menses.
In the N-back task for both targets (updating) and lures (inhibition), women were significantly faster during the luteal phase compared to the pre-ovulatory phase. For the targets (updating) this effect was related to the progesterone levels, i.e., women were faster, the higher their progesterone level. Similarly, for the Stroop word condition, women were significantly faster during the luteal phase as compared to menses and the pre-ovulatory phase. This effect was also related to progesterone levels, as participants were faster the higher their levels of progesterone. However, neither in the N-back task nor in the Stroop word task did we find an effect of cycle phase on accuracy. Thus, during the luteal phase women increased their response speed with no change in accuracy, which is indicative of better performance in both tasks. We interpret these findings as an evidence of improved verbal skills during the luteal phase compared to other phases. Both the verbal N-back task and Stroop word condition involve inner speech, and it is well documented that silent repetition of letters and single word reading tasks engage similar brain regions such as left inferior frontal gyrus (including BA 44, Broca's area) and other peri-sylvian regions (Paulesu et al., 1993; see Turkeltaub et al., 2002 for a meta-analysis). Our findings are consistent with previous reports, demonstrating enhanced verbal abilities during the luteal phase, including simple verbal output tasks (Hampson, 1990; Maki et al., 2002; Šimič and Santini, 2012). Our results indicate that this effect is attributable to progesterone, but not estradiol as suggested by previous studies (Maki et al., 2002). A study from Natale et al. (2001) found that in postmenopausal women, the addition of progestogens to estrogen therapy replacement, improved verbal memory compared to estrogen alone.
Contrary to our expectations we have not been able to replicate the DA-cycle phase interaction reported by previous studies for the lure trials of the N-back task (Jacobs and D'Esposito, 2011). One of the main reasons for this may be the methodological differences to distinguish high and low DA baseline levels (genotyping vs. EBR). In the study of Jacobs and D'Esposito (2011) the DA baseline level was indexed by the COMT genotype and its activity levels. This enzyme metabolizes DA, and its efficiency depends on a polymorphism (Val158Met) for the gene that encodes it. Specifically, the val/val allelic variant is more efficient compare to the met/met form and therefore leading to lower DA baseline levels. In this study, we used the EBR during menses as a continuous variable instead of splitting the sample in fixed groups. However, this variable doesn't allow us to directly infer the DA levels and we can just compare the individuals within the range sampled, without knowing where exactly they are situated in the normal Gaussian curve.
We did however find an interaction between cycle phase and EBR during menses for the Stroop color condition. As the effect concerns the overall RT and accuracy, rather than the interference effect, it can be interpreted as an effect on the “shifting” function, or cognitive flexibility, rather than the “inhibitory control” function. In contrast, this interaction was not present in the Stroop word condition. These different findings can be understood in the context of the parallel distributed processing (PDP) model as explained in the introduction (Cohen et al., 1990; MacLeod and MacDonald, 2000). Reading the name of the colors (word condition) is more practiced and automatic than naming the color of words (color condition). Therefore, it is harder to inhibit the word reading in the color condition than inhibiting the color naming in the word condition. Consequently, the color condition elicits more response competition and requires more cognitive control than the word condition. This may explain why the DA-cycle interaction was found for the color, but not for the word condition.
Contrary to what we expected, the interaction effect in the color condition was driven by the luteal and not the pre-ovulatory phase. During the luteal phase, accuracy was lower and responses slower for women with high EBR during menses. This could have been explained by the higher values of estradiol during this phase, which, as it increases DA, would drive the DA level to exceed the optimal level for this task in women with already high DA baseline levels. However, we did not find our results to be related to estradiol levels, but to progesterone levels for RT. Women with higher EBR during menses were faster the higher their progesterone levels. This is counter-intuitive given that the same women show decreased performance during the luteal cycle phase, when progesterone levels peak. Thus, the increase in response times during the luteal cycle phase cannot be explained by the increased progesterone levels during that phase.
If progesterone exerted a positive effect on DA baseline levels like estradiol, we would expect an interaction in the opposite direction. As this is not the case, the most plausible explanation for our findings is that progesterone exerted a negative effect on DA baseline levels, thereby counteracting the positive effect of estradiol. Multiple findings support a differential effect of estradiol and progesterone on the central nervous system, although their combinatory effect is not clear. Specifically in the dopaminergic system, there is evidence for different modes of action between the two steroids (Morissette et al., 1990), combinatory but not synergistic effects (Morissette and Paolo, 1993), and opposite effects (Fernández-Ruiz et al., 1990). If progesterone counteracted the influence of estradiol on DA levels, this could have masked the relationship between estradiol and performance, and could explain why our findings don't relate to estradiol levels in a significant manner. Note however, that this could also be the result of a power failure or other factors driving the menstrual cycle effect that weren't taken into account in the present study. Furthermore, also note that the effect size of this interaction with progesterone was rather small and should therefore be interpreted with caution.
Currently, the literature on the effects of progesterone on the dopaminergic system in humans and its interaction with estradiol is inconclusive (Sun et al., 2016). This is also reflected by the discrepancies found in studies on executive functions. As an example, an improved performance for prefrontal executive control functions during the early luteal phase has previously been reported using the Wisconsin Card Sorting Test (Solis-Ortiz et al., 2004). In this EEG study they related their findings with the higher levels of progesterone and the role of this hormone in tasks demanding inner attention and cognitive control. In contrast, in the study by Hatta and Nagaya (2009) using the Stroop task, women showed and impaired performance during luteal phase compared to menses. These inconsistences may result from a lack of control for DA baseline levels.
The most important limitation of the present study, that may also explain why we were not able to find an effect of estradiol on performance, is that we did not adequately capture the pre-ovulatory peak of estradiol. Although estradiol was higher in the pre-ovulatory phase compared to menses, we found the highest estradiol values during the luteal phase, when progesterone is also high. Potential reasons for this are that estradiol levels fluctuate along the day due to the circadian rhythm (Shirtcliff et al., 2000), between different cycles (Becker et al., 2005) and sometimes exhibits different pattern from woman to woman (Gandara et al., 2007). In addition, insufficient analytical accuracy in individual assays have already been reported (Gao et al., 2015) and other studies have also failed to replicate the expected pattern of estradiol along the menstrual cycle in salivary samples, although the correlation with serum estradiol was high (Lu et al., 1999). One explanation for this could be inter-individual differences in the metabolism of estradiol within the salivary glands (Lu et al., 1999). However, since previous studies demonstrating interactive effects of estradiol and DA baseline levels on higher cognitive functions did not include the luteal cycle phase (Jacobs and D'Esposito, 2011), it is unclear whether the pre-ovulatory estradiol levels reported in their study were significantly higher than in the luteal phase.
In summary, we could not replicate previous results of DA-cycle interactions in the N-back task, but we found this interaction in the Stroop color condition. Thereby we were able to extend the idea of DA-cycle interactions to other cognitive functions, such as inhibitory control and shifting processes involved in this condition. Unlike previous studies, the inclusion of the luteal cycle phase also allowed us to relate the interactive effects of menstrual cycle and DA baseline levels to progesterone levels. The evidence presented here suggests that, not only estradiol, but the luteal phase and progesterone should also be considered in research on DA-cycle interactions. Last but not least, we have successfully identified the EBR as a useful indicator to differentiate baseline DA function in menstrual cycle research.
Author Contributions
BP designed and made the concept of the study. EH-L was responsible for data acquisition. Analysis and interpretation of the data were performed by both authors, BP and EH-L. EH-L drafted the manuscript, which was critically revised and finally approved by BP. Both authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was supported by the Austrian Science Fund (FWF), PhD Programme “Imaging the Mind: Connectivity and Higher Cognitive Function” [W 1233-G17].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the students of BP for their assistance during participant's recruitment and data acquisition. The authors thank Thomas Scherndl and Stefan Hawelka for statistical advice and the Pregnafix® company ATT Drogerievertriebs GmbH, for donating ovulation tests. Furthermore, we thank all participants for their time and willingness to contribute to this study.
References
Akbari Chermahini, S., and Hommel, B. (2012). More creative through positive mood? Not everyone! Front. Hum. Neurosci. 6:319. doi: 10.3389/fnhum.2012.00319
Alves, S. E., Mcewen, B. S., Hayashi, S., Korach, K. S., Pfaff, D. W., and Ogawa, S. (2000). Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ERα) gene-disrupted mice. J. Comp. Neurol. 427, 185–195. doi: 10.1002/1096-9861(20001113)427:2<185::AID-CNE2>3.0.CO;2-G
Andreano, J. M., and Cahill, L. (2009). Sex influences on the neurobiology of learning and memory. Learn. Mem. 16, 248–266. doi: 10.1101/lm.918309
Barbato, G. (2000). Diurnal variation in spontaneous eye- blink rate Diurnal variation in spontaneous eye-blink rate. Psychiatry Res. 93, 145–151. doi: 10.1016/S0165-1781(00)00108-6
Barbosa-Vargas, E., Pfaus, J. G., and Woodside, B. (2009). Sexual behavior in lactating rats: role of estrogen-induced progesterone receptors. Horm. Behav. 56, 246–253. doi: 10.1016/j.yhbeh.2009.05.004
Barth, C., Villringer, A., and Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 9:37. doi: 10.3389/fnins.2015.00037
Bazzett, T. J., and Becker, J. B. (1994). Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 637, 163–172. doi: 10.1016/0006-8993(94)91229-7
Becker, J. B. (1990). Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse 5, 157–164. doi: 10.1002/syn.890050211
Becker, J. B. (1999). Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 64, 803–812. doi: 10.1016/S0091-3057(99)00168-9
Becker, J. B., Arnold, A. P., Berkley, K. J., Blaustein, J. D., Eckel, L. A., Hampson, E., et al. (2005). Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. doi: 10.1210/en.2004-1142
Becker, J. B., and Rudick, C. N. (1999). Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol. Biochem. Behav. 64, 53–57. doi: 10.1016/S0091-3057(99)00091-X
Bethea, C. L., Lu, N. Z., Gundlah, C., and Streicher, J. M. (2002). Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocr. 23:225. doi: 10.1006/frne.2001.0225
Blin, O., Masson, G., Azulay, J., Fondarai, J., and Serratrice, G. (1990). Apomorphine-induced blinking and yawning in healthy volunteers. Br. J. Clin. Pharmacol. 30, 769–773. doi: 10.1111/j.1365-2125.1990.tb03848.x
Braver, T. S., Cohen, J. D., Nystrom, L., Jonides, J., Smith, E., and Noll, D. C. (1997). A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 62, 49–62.
Cabrera, R., Diaz, A., Pinter, A., and Belmar, J. (1993). In vitro progesterone effects on 3H-dopamine release from rat corpus striatum slices obtained under different endocrine conditions. Life Sci. 53, 1767–1777. doi: 10.1016/0024-3205(93)90164-X
Cai, J. X., and Arnsten, A. F. (1997). Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J. Pharmacol. Exp. Ther. 283, 183–189.
Chen, E. Y. H., Lam, L. C. W., Chen, R. Y. L., and Nguyen, D. G. H. (1996). Blink rate, neurocognitive impairments, and symptoms in schizophrenia. Biol. Psychiatry 40, 597–603. doi: 10.1016/0006-3223(95)00482-3
Chen, W. H., Chiang, T. J., Hsu, M. C., and Liu, J. S. (2003). The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson's disease. Clin. Neurol. Neurosurg. 105, 90–92. doi: 10.1016/S0303-8467(02)00107-5
Chermahini, S. A., and Hommel, B. (2010). The (b)link between creativity and dopamine: spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition 115, 458–465. doi: 10.1016/j.cognition.2010.03.007
Cheung, V., Chen, E. Y. H., Chen, R. Y. L., Woo, M. F., and Yee, B. K. (1995). A comparison between schizophrenia patients and healthy controls on the expression of attentional blink in a rapid serial visual presentation (RSVP) paradigm. Schizophr. Bull. 28, 443–458.
Cohen, J. D., Dunbar, K., and McClelland, J. L. (1990). On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol. Rev. 97, 332–361. doi: 10.1037/0033-295X.97.3.332
Colzato, L. S., Hertsig, G., van den Wildenberg, W. P. M., and Hommel, B. (2010). Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience 167, 709–715. doi: 10.1016/j.neuroscience.2010.02.029
Colzato, L. S., and Hommel, B. (2014). Effects of estrogen on higher-order cognitive functions in unstressed human females may depend on individual variation in dopamine baseline levels. Front. Neurosci. 8:66. doi: 10.3389/fnins.2014.00065
Colzato, L. S., Slagter, H. A., Spapé, M. M. A., and Hommel, B. (2008). Blinks of the eye predict blinks of the mind. Neuropsychologia 46, 3179–3183. doi: 10.1016/j.neuropsychologia.2008.07.006
Colzato, L. S., Van Den Wildenberg, W. P. M., Van Wouwe, N. C., Pannebakker, M. M., and Hommel, B. (2009). Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp. Brain Res. 196, 467–474. doi: 10.1007/s00221-009-1862-x
Colzato, L. S., van Wouwe, N. C., and Hommel, B. (2007). Spontaneous eyeblink rate predicts the strength of visuomotor binding. Neuropsychologia 45, 2387–2392. doi: 10.1016/j.neuropsychologia.2007.03.004
Cools, R., and D'Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125. doi: 10.1016/j.biopsych.2011.03.028
Dalrymple-Alford, E. C., and Budayr, B. (1966). Examination of some aspects of the stroop color-word test. Percept. Mot. Skills 23, 1211–1214. doi: 10.2466/pms.1966.23.3f.1211
Dang, J., Xiao, S., Liu, Y., Jiang, Y., and Mao, L. (2016). Individual differences in dopamine level modulate the ego depletion effect. Int. J. Psychophysiol. 99, 121–124. doi: 10.1016/j.ijpsycho.2015.11.013
Dluzen, D. E., and Ramirez, V. D. (1987). Intermittent infusion of progesterone potentiates whereas continuous infusion reduces amphetamine-stimulated dopamine release from ovariectomized estrogen-primed rat striatal fragments superfused in vitro. Brain Res. 406, 1–9. doi: 10.1016/0006-8993(87)90762-1
Dreisbach, G., Müller, J., Goschke, T., Strobel, A., Schulze, K., Lesch, K.-P., et al. (2005). Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav. Neurosci. 119, 483–490. doi: 10.1037/0735-7044.119.2.483
Elsworth, J. D., Lawrence, M. S., Roth, R. H., Taylor, J. R., Mailman, R. B., Nichols, D. E., et al. (1991). D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J. Pharmacol. Exp. Ther. 259, 595–600. doi: 10.1016/S0065-2113(08)60255-2
Engle, R. W., and Kane, M. J. (2003). Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol. Learn. Motiv. 44, 145–199. doi: 10.1016/S0079-7421(03)44005-X
Fehring, R. J., Schneider, M., and Raviele, K. (2006). Variability in the phases of the menstrual cycle. JOGNN - J. Obstet. Gynecol. Neonatal Nurs. 35, 376–384. doi: 10.1111/j.1552-6909.2006.00051.x
Feil, J., Sheppard, D., Fitzgerald, P. B., Yücel, M., Lubman, D. I., and Bradshaw, J. L. (2010). Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 35, 248–275. doi: 10.1016/j.neubiorev.2010.03.001
Fernández-Ruiz, J. J., de Miguel, R., Hernández, M. L., and Ramos, J. A. (1990). Time-course of the effects of ovarian steroids on the activity of limbic and striatal dopaminergic neurons in female rat brain. Pharmacol. Biochem. Behav. 36, 603–606. doi: 10.1016/0091-3057(90)90262-G
Frye, C. A., and Sora, I. (2010). Progesterone reduces hyperactivity of female and male dopamine transporter knockout mice. Behav. Brain Res. 209, 59–65. doi: 10.1016/j.bbr.2010.01.015
Gandara, B. K., Leresche, L., and Mancl, L. (2007). Patterns of salivary estradiol and progesterone across the menstrual cycle. Ann. N.Y. Acad. Sci. 1098, 446–450. doi: 10.1196/annals.1384.022
Gao, W., Stalder, T., and Kirschbaum, C. (2015). Quantitative analysis of estradiol and six other steroid hormones in human saliva using a high throughput liquid chromatography–tandem mass spectrometry assay. Talanta 143, 353–358. doi: 10.1016/j.talanta.2015.05.004
Gasbarri, A., Pompili, A., D'Onofrio, A., Cifariello, A., Tavares, M. C., and Tomaz, C. (2008). Working memory for emotional facial expressions: role of the estrogen in young women. Psychoneuroendocrinology 33, 964–972. doi: 10.1016/j.psyneuen.2008.04.007
Genazzani, A. R., Lucchesi, A., Stomati, M., Catarsi, S., Genazzani, A. D., Criscuolo, M., et al. (1997). Effects of sex steroid hormones on the neuroendocrine system. Eur. J. Contracept. Reprod. Heal. Care 2, 63–69.
Gray, J. R., Chabris, C. F., and Braver, T. S. (2003). Neural mechanisms of general fluid intelligence. Nat. Neurosci. 6, 316–322. doi: 10.1038/nn1014
Haatveit, B. C., Sundet, K., Hugdahl, K., Ueland, T., Melle, I., and Andreassen, O. A. (2010). The validity of d prime as a working memory index: results from the “Bergen n -back task.” J. Clin. Exp. Neuropsychol. 32, 871–880. doi: 10.1080/13803391003596421
Hampson, E. (1990). Variations in sex-related cognitive-abilities across the menstrual- cycle. Brain Cogn. 14, 26–43.
Hatta, T., and Nagaya, K. (2009). Menstrual cycle phase effects on memory and Stroop task performance. Arch. Sex Behav. 38, 821–827. doi: 10.1007/s10508-008-9445-7
Hausmann, M., Slabbekoorn, D., Van Goozen, S. H. M., Cohen-Kettenis, P. T., and Güntürkün, O. (2000). Sex hormones affect spatial abilities during the menstrual cycle. Behav. Neurosci. 114, 1245–1250. doi: 10.1037/0735-7044.114.6.1245
Hussain, D., Hanafi, S., Konishi, K., Brake, W. G., and Bohbot, V. D. (2016). Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology 70, 108–117. doi: 10.1016/j.psyneuen.2016.05.008
Jacobs, E., and D'Esposito, M. (2011). Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J. Neurosci. 31, 5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011
Jongkees, B. J., and Colzato, L. S. (2016). Spontaneous eye blink rate as predictor of dopamine-related cognitive function-A review. Neurosci. Biobehav. Rev. 71, 58–82. doi: 10.1016/j.neubiorev.2016.08.020
Kaminer, J., Powers, A. S., Horn, K. G., Hui, C., and Evinger, C. (2011). Behavioral/Systems/Cognitive characterizing the spontaneous blink generator: an animal model. J. Neurosci. 31, 11256–11267. doi: 10.1523/JNEUROSCI.6218-10.2011
Karson, C. N. (1983). Spontaneous eye-blink rates and dopaminergic systems. Brain 106, 643–653. doi: 10.1093/brain/106.3.643
Karson, C. N., Burns, R. S., LeWitt, P. A., Foster, N. L., and Newman, R. P. (1984). Blink rates and disorders of movement. Neurology 34, 677–678. doi: 10.1212/WNL.34.5.677
Kimura, D., and Hampson, E. (1994). Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Curr. Dir. Psychol. Sci. 3, 57–61.
Kirschbaum, C., and Hellhammer, D. H. (1989). Salivary cortisol in psychobiological reasearch: an overview. Neuropsychobiology 22, 150–169. doi: 10.1159/000118611
Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., and Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 61, 154–162. doi: 10.1097/00006842-199903000-00006
Kleven, M. S., and Koek, W. (1996). Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 279, 1211–1219. doi: 10.1016/S0065-2113(08)60255-2
Korosec, M., Zidar, I., Reits, D., Vanderwerf, F., and Evinger, C. (2006). FC10.1 Reflex blink effects on smooth pursuit maintenance in patients with Parkinson's disease. Clin. Neurophysiol. 117, 1–2. doi: 10.1016/j.clinph.2006.06.030
Korsgaard, S., Gerlach, J., and Christensson, E. (1985). Behavioral aspects of serotonin-dopamine interaction in the monkey. Eur. J. Pharmacol. 118, 245–252. doi: 10.1016/0014-2999(85)90135-9
Lawrence, M. S., and Redmond, D. E. (1991). MPTP lesions and dopaminergic drugs alter eye blink rate in African green monkeys. Pharmacol. Biochem. Behav. 38, 869–874. doi: 10.1016/0091-3057(91)90255-Z
Lu, Y. C., Bentley, G. R., Gann, P. H., Hodges, K. R., and Chatterton, R. T. (1999). Salivary estradiol and progesterone levels in conception and nonconception cycles in women: evaluation of a new assay for salivary estradiol. Fertil. Steril. 71, 863–868. doi: 10.1016/S0015-0282(99)00093-X
MacLeod, C. M., and Dunbar, K. (1988). Training and Stroop-like interference: evidence for a continuum of automaticity. J. Exp. Psychol. Learn. Mem. Cogn. 14, 126–135. doi: 10.1037/0278-7393.14.1.126
MacLeod, C. M., and MacDonald, P. A. (2000). Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn. Sci. 4, 383–391. doi: 10.1016/S1364-6613(00)01530-8
Macmillan, N. A., and Creelman, C. D. (1990). Response bias: characteristics of detection theory, threshold theory, and “nonparametric” indexes. Psychol. Bull. 107, 401–413. doi: 10.1037/0033-2909.107.3.401
Maki, P. M., Rich, J. B., and Shayna Rosenbaum, R. (2002). Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia 40, 518–529. doi: 10.1016/S0028-3932(01)00126-9
McEwen, B. S., and Alves, S. E. (1999). Estrogen actions in the central nervous system. Endocr. Rev. 20, 279–307. doi: 10.1210/edrv.20.3.0365
Mitsushima, D. (2010). Sex steroids and acetylcholine release in the hippocampus. Vitam. Horm. 82, 263–277. doi: 10.1016/S0083-6729(10)82014-X
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100. doi: 10.1006/cogp.1999.0734
Mordecai, K. L., Rubin, L. H., and Maki, P. M. (2008). Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm. Behav. 54, 286–293. doi: 10.1016/j.yhbeh.2008.03.006
Morissette, M., Lévesque, D., Bélanger, A., and Di Paolo, T. (1990). A physiological dose of estradiol with progesterone affects striatum biogenic amines. Can. J. Physiol. Pharmacol. 68, 1520–1526. doi: 10.1139/y90-231
Morissette, M., and Di Paolo, T. (1993). Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J. Neurochem. 60, 1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x
Natale, V., Albertazzi, P., Zini, M., and Di Micco, R. (2001). Exploration of cyclical changes in memory and mood in postmenopausal women taking sequential combined oestrogen and progestogen preparations. Br. J. Obstet. Gynaecol. 108, 286–290. doi: 10.1016/S0306-5456(00)00070-X
Šimič, N., and Santini, M. (2012). Verbal and spatial functions during different phases of the menstrual cycle. Psychiatr. Danub. 24, 73–79.
Pasqualini, C., Olivier, V., Guibert, B., Frain, O., and Leviel, V. (1995). Acute stimulatory effect of estradiol on striatal dopamine synthesis. J. Neurochem. 65, 1651–1657. doi: 10.1038/2091178c0
Paulesu, E., Frith, C. D., and Frackowiak, R. S. (1993). The neural correlates of the verbal component of working memory. Nature 362, 342–345. doi: 10.1038/362342a0
Phillips, K., and Silverman, I. (1997). Differences in the relationship of menstrual cycle phase to spatial performance on two- and three-dimensional tasks. Horm. Behav. 32, 167–175. doi: 10.1006/hbeh.1997.1418
Phillips, S. (1992). Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology 17, 497–506. doi: 10.1016/0306-4530(92)90008-U
Pruesser, J. C., Wolf, O. T., Hellhammer, D. H., Buske-Kirschbaum, A., von Auer, K., JObst, S., et al. (1997). Free cortisol levels after awaking: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 61, 2539–2549.
Rosenberg, L., and Park, S. (2002). Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology 27, 835–841. doi: 10.1016/S0306-4530(01)00083-X
Sacher, J., Okon-Singer, H., and Villringer, A. (2013). Evidence from neuroimaging for the role of the menstrual cycle in the interplay of emotion and cognition. Front. Hum. Neurosci. 7:374. doi: 10.3389/fnhum.2013.00374
Sánchez, M. G., Bourque, M., Morissette, M., and Di Paolo, T. (2010). Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, e43–e71. doi: 10.1111/j.1755-5949.2010.00163.x
Schmidt-Reinwald, A., Prussner, J. C., Hellhammer, D. H., Federenko, I., Rohleder, N., Schumeyer, T., et al. (1999). The cortisol response to awakening in relation to pharmacological and psychological challenge tests, and a 12-hour circadian cortisol rhythm. Life Sci. 64, 1653–1660.
Schöning, S., Engelien, A., Kugel, H., Schäfer, S., Schiffbauer, H., Zwitserlood, P., et al. (2007). Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia 45, 3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011
Shirtcliff, E. A., Granger, D. A., Schwartz, E. B., Curran, M. J., Booth, A., and Overman, W. H. (2000). Assessing estradiol in biobehavioral studies using Saliva and blood spots: simple radioimmunoassay protocols, reliability, and comparative validity. Horm. Behav. 38, 137–147. doi: 10.1006/hbeh.2000.1614
Smith, C. T., Sierra, Y., Oppler, S. H., and Boettiger, C. A. (2014). Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J. Neurosci. 34, 5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014
Solís-Ortiz, S., and Corsi-Cabrera, M. (2008). Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology 33, 989–998. doi: 10.1016/j.psyneuen.2008.04.003
Solis-Ortiz, S., Guevara, M. A., and Corsi-Cabrera, M. (2004). Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: an electroencephalographic study. Psychoneuroendocrinology 29, 1047–1057. doi: 10.1016/j.psyneuen.2003.10.007
Strakowski, S. M., and Sax, K. W. (1998). Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol. Psychiatry. 44, 1171–1177. doi: 10.1016/S0006-3223(97)00454-X
Strakowski, S. M., Sax, K. W., Setters, M. J., and Keck, P. E. (1996). Enhanced response to repeated d-amphetamine challenge: evidence for behavioral sensitization in humans. Biol. Psychiatry. 40, 872–880. doi: 10.1016/0006-3223(95)00497-1
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662. doi: 10.1037/h0054651
Sun, J., Walker, A. J., Dean, B., van den Buuse, M., and Gogos, A. (2016). Progesterone: the neglected hormone in schizophrenia? A focus on progesterone-dopamine interactions. Psychoneuroendocrinology 74, 126–140. doi: 10.1016/j.psyneuen.2016.08.019
Sundström Poromaa, I., and Gingnell, M. (2014). Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 8:380. doi: 10.3389/fnins.2014.00380
Swets, J. A., Tanner, W. P. Jr., and Birdsall, T. G. (1961). Decision processes in perception. Psychol. Rev. 68, 301–340. doi: 10.1037/h0040547
Systems, N. (2011). Neurobehavioral Systems. 6427. Available online at: http://www.neurobs.com/
Tansey, E. M., Arbuthnott, G. W., Fink, G., and Whale, D. (1983). Oestradiol-17β increases the firing rate of antidromically identified neurones of the rat neostriatum. Neuroendocrinology 37, 106–110.
Taylor, J. R., Elsworth, J. D., Lawrence, M. S., Sladek, J. R., Roth, R. H., and Redmond, D. E. (1999). Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp. Neurol. 158, 214–220. doi: 10.1006/exnr.1999.7093
Turkeltaub, P. E., Eden, G. F., Jones, K. M., and Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. doi: 10.1006/nimg.2002.1131
Whirledge, S., and Cidlowski, J. A. (2010). Glucocorticoids, stress, and fertility. Minerva Endocrinol. 35, 109–125. doi: 10.1586/eem.10.1
Wickens, T. D. (2001). Elementary Signal Detection Theory, Vol. 1. New York, NY: Oxford University Press.
Willeit, M., Popovic, A., Bartova, L., Sauerzopf, U., Bauer, M., and Praschak-Rieder, N. (2016). “In vivo imaging of dopamine metabolismand dopamine transporter function in the human brain,” in Neurotransmitter Transporters, eds H. Bönisch and H. H. Sitte (New York, NY: Springer Science+Business Media), 203–220. doi: 10.1007/978-1-4939-3765-3_12
Yolton, D. P., Yolton, R. L., López, R., Bogner, B., Stevens, R., and Rao, D. (1994). The effects of gender and birth control pill use on spontaneous blink rates. J. Am. Optom. Assoc. 65, 763–770.
Keywords: menstrual cycle, estradiol, progesterone, dopamine, executive functions, eye blink rate, working memory, cognitive flexibility
Citation: Hidalgo-Lopez E and Pletzer B (2017) Interactive Effects of Dopamine Baseline Levels and Cycle Phase on Executive Functions: The Role of Progesterone. Front. Neurosci. 11:403. doi: 10.3389/fnins.2017.00403
Received: 05 May 2017; Accepted: 28 June 2017;
Published: 13 July 2017.
Edited by:
Pierrette Gaudreau, Université de Montréal, CanadaReviewed by:
Agnes Lacreuse, University of Massachusetts Amherst, United StatesLorenza S. Colzato, Leiden University, Netherlands
Copyright © 2017 Hidalgo-Lopez and Pletzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esmeralda Hidalgo-Lopez, ZXNtZXJhbGRhLmhpZGFsZ29sb3BlekBzYmcuYWMuYXQ=
Belinda Pletzer, YmVsaW5kYS5wbGV0emVyQGdtYWlsLmNvbQ==
 Esmeralda Hidalgo-Lopez
Esmeralda Hidalgo-Lopez Belinda Pletzer
Belinda Pletzer