
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 30 June 2016
Sec. Neuroendocrine Science
Volume 10 - 2016 | https://doi.org/10.3389/fnins.2016.00318
This article is part of the Research Topic Chemicals in the environment and brain development: importance of neuroendocrinological approaches View all 16 articles
Endocrine-disrupting chemicals (EDCs) are diverse natural and synthetic chemicals that may alter various mechanisms of the endocrine system and produce adverse developmental, reproductive, metabolic, and neurological effects in both humans and wildlife. Research on EDCs has revealed that they use a variety of both nuclear receptor-mediated and non-receptor-mediated mechanisms to modulate different components of the endocrine system. The molecular mechanisms underlying the effects of EDCs are still under investigation. Interestingly, some of the effects of EDCs have been observed to pass on to subsequent unexposed generations, which can be explained by the gametic transmission of deregulated epigenetic marks. Epigenetics is the study of heritable changes in gene expression that occur without a change in the DNA sequence. Epigenetic mechanisms, including histone modifications, DNA methylation, and specific micro-RNAs (miRNAs) expression, have been proposed to mediate transgenerational transmission and can be triggered by environmental factors. MiRNAs are short non-coding RNA molecules that post-transcriptionally repress the expression of genes by binding to 3′-untranslated regions of the target mRNAs. Given that there is mounting evidence that miRNAs are regulated by hormones, then clearly it is important to investigate the potential for environmental EDCs to deregulate miRNA expression and action.
Endocrine-disrupting chemicals (EDCs) are diverse natural and synthetic chemicals that may alter various mechanisms of the endocrine system and produce adverse developmental, reproductive, metabolic, and neurological effects in both humans and wildlife (Henley and Korach, 2006). To date, close to 800 chemicals are known or suspected to be capable of interfering with hormone receptors and/or hormone synthesis and then play a larger role in the causation of many endocrine diseases and disorders (WHO | State of the science of endocrine disrupting chemicals, 2012). Excretion of EDCs is dependent on the nature of the chemical substances. If the substance is non-persistent it is usually predicted that they are metabolized by the liver then finally eliminated from the body through feces and urine. Persistent endocrine disruptors are accumulated especially in adipose tissue and they can be released slowly. One way of excretion of these persistent endocrine disruptors is thought to be from mother to child through breast feeding. It is observed in many studies that the daily intake of breast milk containing organic pollutants may exceed the tolerable limit. It has been established that some EDCs can act directly on hormone receptors as hormone mimics or antagonists. Others can act directly on proteins that control the delivery of a hormone to its target cell or tissue. In addition, EDCs may act synergistically and produce additive effects. Most studies on EDCs have focused on chemicals that affect the reproductive and thyroid axis. However, several studies have suggested that environmental chemicals could affect several physiological systems that lead to metabolic disorders or central nervous system dysfunctions (Casals-Casas and Desvergne, 2011). For instance, neurobehavioral disorders have been associated with hypothalamic-pituitary-adrenal (HPA) axis disruption induced by hydroxyl-polychlorinated biphenyl (PCB; Kimura-Kuroda et al., 2007).
It is particularly difficult to highlight only one mechanism of action shared by the set of EDCs. In fact, the main problem is that there are many and diverse EDCs including industrial chemicals, pesticides, pollutants, and plastic industry compounds. Nevertheless, research on EDCs has revealed that they use a variety of both nuclear receptor- and non-receptor- mediated mechanisms to modulate different components of the endocrine system. For instance Vinclozolin (VCZ), a widely used fungicide with antiandrogenic effects in mammals, is a competitive antagonist of androgen receptor (AR) ligand binding (Kelce et al., 1997). Several studies showed that exposure to VCZ induce masculinized females and feminized males in rodents (Buckley et al., 2006). Interestingly, some of the effects of VCZ have been observed to pass on to subsequent unexposed generations, which can be explained by the gametic transmission of deregulated epigenetic marks (Anway et al., 2005; Stouder and Paoloni-Giacobino, 2010; Guerrero-Bosagna et al., 2012; Skinner et al., 2013). Epigenetic mechanisms, including histone modifications, DNA methylation, and specific micro-RNAs (miRNAs) expression, have been proposed to mediate such transgenerational transmission (Reik et al., 2001; Del-Mazo et al., 2013).
This review provides an insight into the toxicological effects of EDCs and particularly new molecular mechanisms, i.e., miRNAs, involved in the EDCs induced endocrine disruption.
The term endocrine disruptors were first introduced by the group of Soto in 1993 that showed that EDCs induced developmental abnormalities (Colborn et al., 1993). The International Program on Chemical Safety (IPCS) in 2002 and World Health Organization in 2013 defined EDCs as “…an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations. A potential endocrine disruptor is an exogenous substance or mixture that possesses properties that might be expected to lead to endocrine disruption in an intact organism, or its progeny, or (sub) populations.” To date, EDCs include a large variety of chemical classes such as pesticides [methoxychlor, chlopyrifos, and dichlorodiphenyltrichloroethane (DDT)], pharmaceutical agents [diethylstrilbestrol (DES)], plastic packaging compounds [Bisphenol A (BPA), phthalates], and other industrial products that are used in daily life as fungicides VCZ or solvents/lubricants (dioxins). Some of them but not all are exposed in this paragraph.
A large number of chemicals are used as pesticides. The most important pesticides are organochlorines pesticides (OCPs), organophosphates, or triazines. The most emblematic of the banned OCPs is DDT and the exposure to it persists. The pesticides are involved in a large number of diseases including cancer, diabetes but also neurodegenerative disease as Parkinson or Alzheimer (Mostafalou et al., 2012; Mostafalou and Abdollahi, 2013).
The dioxins are a general name for a family of organochlorines including the polychlorinated dibenzodioxins (PCDDs), the polychlorinated dibenzofurans (PCDFs), and the polychlorinated biphenyls (PCBs). Dioxins are produced by various industrial processes and are commonly regarded as highly toxic compounds that are environmental pollutants and persistent organic pollutants. Among the PCDDs, the 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most potent and toxic compound and became known as a contaminant in Agent Orange, a herbicide used as a weapon in the Vietnam War (Schecter et al., 2006). TCDD was also released into the environment during the Seveso disaster (Sweeney and Mocarelli, 2000). The TCDD and the other toxins have been shown to be involved in different diseases including cancers, thyroid dysfunction, and nervous system degeneration but also type 2 diabetes (Pelclová et al., 2006; Schecter et al., 2006; Mostafalou et al., 2012; Mostafalou and Abdollahi, 2013).
An important number of EDCs are found in plastic products. World plastic production exceeded 300 million tons in 2010 (Halden, 2010). Most abundant of these plastics are phthalates and BPA. These are two most common EDCs and are associated with parental and social behavioral disturbances but also endocrine disease. Phthalates are mainly used as plasticizers in a wide range of common products, and are released into the environment. Phthalate exposure may be through direct use or by indirect means through leaching and general environmental contamination (Aurela et al., 1999). Food products are believed to be the main source of di-(2-ethylhexyl) phthalate (DEHP) and other phthalates in the general population. Fatty foods such as milk, butter, and meats are a major source. In several studies in human and rodents, high and even low doses of phthalates have been shown to change hormone levels as T3, T4, and thyroid-stimulating hormone and cause birth defects (Gayathri et al., 2004; Heudorf et al., 2007; Meeker et al., 2009). BPA is one of the other emblematic plastics used in polycarbonate plastic and polystyrene resins. Interestingly, it has been shown that BPA is detected in 95% of urine sample from a reference population of 394 adults in the United States (Calafat et al., 2005). This higher level of BPA in urine is associated with cardiovascular disease, sterility, and other reproductive diseases but also diabetes and liver abnormalities (Takeuchi et al., 2004; Sugiura-Ogasawara et al., 2005; Lang et al., 2008).
In the last few years, it has been noticed that the incidence of certain diseases of the reproductive axis has increased (WHO | State of the science of endocrine disrupting chemicals, 2012). It is well-established that estrogen and androgen are involved in sexual differentiation. In this context, EDCs may act as estrogen and or androgen antagonists and induce different sexual disorders in males and females (Diamanti-Kandarakis et al., 2009; Sweeney et al., 2015; Toppari et al., 2016). For instance, DES and phthalates exposure to rats are associated with cryptorchidism or micropenis (Fisher et al., 2003; Li et al., 2003). In human, it has been shown that breast milk dioxin concentration correlated positively with the risk of cryptorchidism in Denmark (Main et al., 2007). It has also been shown that perinatal exposure to low doses of dioxin can permanently reduce sperm quality (Mocarelli et al., 2011). In humans, exposure to PCBs caused a defect in the development of the reproductive system (Staessen et al., 2001). Recently, epidemiological, study suggested that prenatal exposure to PCBs may be also associated with increased risk for cryptorchidism (Koskenniemi et al., 2015).
EDCs are associated with some types of female reproductive axis disorder including polycystic ovarian syndrome (PCOS). PCOS is a problem in which a woman's hormones are out of balance. It can disrupt the menstrual cycle and makes it difficult to become pregnant. If it isn't treated, over time it can lead to serious health problems, such as diabetes and heart disease. Most women with PCOS grow many small cysts on their ovaries. Interestingly, women with PCOS have higher levels of BPA and increased testosterone in these women is consistent with decreased clearance of BPA (Takeuchi et al., 2004, 2006). The cause of PCOS is not fully understood, but the EDCs as well as BPA could play a role in the onset of PCOS. Female rats exhibited sexual precocity as a consequence of exposure to DTT (Rasier et al., 2007).
It has also been shown in the hypothalamic GT1-7 cell line that organochlorine pesticides such as methoxychlor and chlopyrifos altered gonadotropin-releasing hormone (GnRH) gene expression and biosynthesis (Gore, 2002) suggesting that EDCs could affect the different levels or reproductive axis. Interestingly, it has been revealed that the BPA-mediated inhibition of GnRH neuronal activity occurred independent of estrogen receptors via a non-canonical unknown pathway (Klenke et al., 2016).
Thyroid hormones (T3 and T4) are important for brain development, for the modulation of metabolism and are associated with many aspects of normal adult physiology. For these reasons, thyreotropic axis disruption induced a large scale of perturbation in adult physiology, development, and metabolism. It has been reported that numerous EDCs can directly affect the normal functioning of the thyroid gland. In numerous studies, it has been shown that different EDCs such as PCBs, BPA, or DTT have thyroid-disrupting effects in animals and humans (Patrick, 2009; Molehin et al., 2016).
The EDCs can affect the thyroid system at different levels such as the transport and/or biosynthesis of the thyroid hormones. It has been shown that PCBs have a high affinity with thyroxin specific binding protein which can affect the thyroid hormone transport (Rickenbacher et al., 1986; McKinney et al., 1987; Darnerud et al., 1996). More precisely, treatment of mice during gestation with PCB as 3,3′, 4,4′-tetrachlorobiphenyl (CB-77) leads to a decrease of free and total T4 in fetal plasma (Darnerud et al., 1996). More recently, the group of Seegal examined the effects of a mixture of PCBs and polybrominated diphenyl ethers (PBDEs) coexposure from gestational day 6 through postnatal day 21, alone and in combination, on T4 levels in rat offspring (Miller et al., 2012, 201). They observed that PCBs and PBDEs induces similar reductions in T4 levels and that coexposure to a mixture of PCBs and PBDEs has additive effects on T4 level in male and female offspring (Miller et al., 2012). In the study of Schmutzler et al., rats (female, ovariectomized) were treated for 12 weeks with different EDCs and an alteration in thyrotropin (TSH) and thyroid hormones (T4, T3) serum levels were observed (Schmutzler et al., 2004). In another set of studies, exposure to phthalates induced thyroid function alterations (Mitchell et al., 1985; Hinton et al., 1986; Price et al., 1988). Interestingly, the treatment of rats for periods of 3 months with di-(2-ethylhexyl) phthalate increased the number and size of lysosomes, hypertrophy of the Golgi apparatus, and dilation of the rough endoplasmic reticulum in thyroid cells and these changes are consistent with persistent hyperactivity in the gland (Price et al., 1988). It has also been shown that EDCs can alter deiodinase activity which is the peroxidase enzyme that is involved in the activation or deactivation of thyroid hormones (Meerts et al., 2002; Viluksela et al., 2004; Noyes et al., 2013).
In human, there is now growing evidence that PCBs but also BPA and phthalates have thyroid-disrupting effects (Boas et al., 2012; Campos and Freire, 2016). For instance, the group of Yoshinaga showed that exposure to hydroxylated-PCBs at environmental levels during the first trimester of pregnancy can affect neonatal thyroid hormone status (Hisada et al., 2014). It has also been shown that early exposure to certain environmental chemicals with endocrine-disruption activity as pesticides may interfere with neonatal thyroid hormone status (Freire et al., 2011).
There is strong evidence that there is a correlation between the increasing prevalence of neurodevelopmental disorders and the increase in exposure to pollutants over the past several decades (Weiss and Landrigan, 2000; Landrigan and Goldman, 2011a,b). For instance, since the 1970s, there have been dramatic increases in previously rare neurodevelopmental disorders such as autism which is characterized by some degree of impaired social behavior, communication and language, and a narrow range of interests and activities that are both unique to the individual and carried out repetitively. In the 1970s, autism's prevalence was estimated to be between 4 and 5 in 10,000 children (Wing et al., 1976) but today this value is estimated to be 1 in 110 children (Rice et al., 2007). In a review of the literature performed by de Cock et al., a positive association was found for autism in relation to exposure to different chemicals investigated, which included hazardous air pollutants, pesticides, and BPA (de Cock et al., 2012). In the same study, a relationship between attention deficit hyperactivity disorders and different EDCs including BCPs and pesticides such as chlorpyrifos has been done (de Cock et al., 2012).
The function of central nervous system (CNS) can be affected by EDCs and these effects can be induced by different mechanisms. The most important is the effects of EDCs on different endocrine axis important for CNS functions and development. Evidence that prenatal estrogen exposure is important in neuronal correct development emerged from reports of psychosis in patients prenatally exposed to the synthetic estrogen DES (Katz et al., 1987; Brown, 2009; Inadera, 2015; Negri-Cesi, 2015). Interestingly, several researches indicate that BPA is an estrogenic EDC that alters or interferes with normal endocrine development in various vertebrate and invertebrate species (vom Saal et al., 2007) suggesting a role of BPA in CNS disease. For instance, prenatal exposure to low dose of BPA disturbed neocortical histogenesis in mice (Nakamura et al., 2006, 2007).
As exposed above, BPA is a well-known xenoestrogen (Kuiper et al., 1998; Delfosse et al., 2014; Inadera, 2015). BPA has complex action in the CNS but primarily BPA was exhibited to bind both estrogen receptors α and β (ERα and ERβ) and has also been shown to act as an anti-androgen (Kuiper et al., 1998; Wolstenholme et al., 2011). Interestingly, it has been described endocrine and neuroendocrine abnormalities in schizophrenia (Marx and Lieberman, 1998; Stevens, 2002). In fact, estrogen has been associated with a neuroprotective effect but lower plasma levels of estrogens induced schizophrenia-like syndrome in males and females (Huber et al., 2001; Kaneda and Ohmori, 2005; Segal et al., 2007). Furthermore, neuronal disorders have also been associated with an impairment of HPA axis. For instance, the increase of glucocorticoid concentrations induced hippocampal nerve damage and schizophrenia (Cotter and Pariante, 2002). In rat, corticosterone exposures also lead to degeneration of the prefrontal cortex causing impairments in executive functions such as behavioral flexibility and working memory (Cerqueira et al., 2005). It has been established in baboons that HPA is potentially affected by estrogen (Pepe and Albrecht, 1998; Albrecht et al., 2005). In addition, it has been recently shown that perinatal exposure to low-dose of BPA caused HPA axis dysfunctions (Panagiotidou et al., 2014; Chen et al., 2015; Zhou et al., 2015). Particularly, the administration of low doses of BPA (2 μg/kg.day) to female breeders from gestation day 10 to lactation day 7 induced obvious anxiety/depression-like behaviors in the offspring (Chen et al., 2015). Notably, significant increase in serum corticosterone and adrenocorticotropin, and corticotropin-releasing hormone mRNA were detected in BPA-exposed rats before or after the mild stressor (Chen et al., 2015). Altogether these different observations strongly suggest that BPA and other EDCs could be associated to schizophrenia pathogenesis (Brown, 2009).
In addition to the reproductive and neuronal developmental effects, there is also evidence that metabolic disorders may be linked to EDCs (Casals-Casas et al., 2008; Newbold et al., 2008). Obesity, diabetes and metabolic syndrome are due to disruption of the energy storage balance endocrine system and thus are potentially sensitive to EDCs. This hypothesis is supported by different epidemiological and animal studies that have shown that a variety of EDCs can influence adipogenesis and obesity (Baillie-Hamilton, 2002; Casals-Casas et al., 2008; Elobeid and Allison, 2008; Newbold et al., 2008; Chen et al., 2009). For instance, the administration of DES to neonatal mice induced overweight associated with an increase of abdominal body fats and inflammatory biomarkers (Newbold et al., 2007). In rats, perinatal exposure to low doses of BPA increased adipogenesis and body weight in adult females (Somm et al., 2009). EDCs are also involved in glucose homeostasis defects. In accordance with this fact, epidemiological studies report that exposure to EDCs may affect the risk of type 2 diabetes (Remillard and Bunce, 2002; Huang et al., 2015; Song et al., 2016). Very low doses of BPA induced hyperinsulenemia and type 2 diabetes (Alonso-Magdalena et al., 2010). In the same way, low doses of BPA and dioxins altered α-cell function and glucagon release which lead to glucose homeostasis defect (Alonso-Magdalena et al., 2005). Interestingly, it has been established that EDCs such as BPA or dioxins are accumulated by adipose tissue and that they are released slowly and have induced glucose homeostasis impairment (Alonso-Magdalena et al., 2011). When administrated to mother mice, BPA induces metabolic disorders in adult male offspring such as an age-related change in food intake, an increase in body weight and liver weight, abdominal adipocyte mass, number and volume, and in serum leptin and insulin, but a decrease in serum adiponectin and in glucose tolerance (Angle et al., 2013). Furthermore, mother mice treated with BPA during gestation, at environmentally relevant doses, exhibit profound glucose intolerance and altered insulin sensitivity as well as increased body weight (Alonso-Magdalena et al., 2015).
EDCs often act via more than one mechanism. The target cells of the hormones bear receptors specific to a given hormone and will be activated by either a lipid-soluble (permeable to plasma membrane) or water-soluble hormone (binds cell-surface receptor; Casals-Casas and Desvergne, 2011; Wolstenholme et al., 2011; Maqbool et al., 2016). Lipid-soluble hormones (steroid hormones and hormones of the thyroid gland) diffuse through the plasma membrane to enter the target cell and bind to a nuclear receptor (NR) protein that will in turn activates expression of specific genes that influence specific physiological cell activities. Water-soluble hormones (such as insulin) bind to a receptor protein on the plasma membrane of the cell which leads to specific cellular transduction pathways (Casals-Casas and Desvergne, 2011; Maqbool et al., 2016; Wolstenholme et al., 2011). Because many EDCs are small lipophilic compounds, they can directly interact with a given NR, which presumably perturbs or modulates downstream gene expression.
In parallel with these classical pathways, it appears that EDCs not only involve genetics but also epigenetic mechanisms. Epigenetics is broadly defined as those heritable changes in the genome not dependent upon changes in genetic sequences (e.g., DNA methylation or histone modification). These epigenetic processes control tissue development by controlling gene expression. Thus, a major route by which hormones act during development is by changing the epigenome. These different epigenetic mechanisms also include miRNAs which are short non-coding RNA molecules that post-transcriptionally repress the expression of genes by binding to 3′-untranslated regions (3′UTR) of the target mRNAs. Recently, it appears that miRNAs can be involved in the action of EDCs (Cameron et al., 2016; Klinge, 2015). This part of the review focuses on the regulation of miRNAs by the EDCs which appear as a new molecular mechanism involved in endocrine disruption.
The miRNAs are short non-coding RNA with a size of 21–26 nucleotides that suppress target gene expression through the inhibition of gene translation and the increase of the degradation of target mRNAs (Bartel, 2004). These small regulatory molecules are involved in a large range of biological processes such as development, cell proliferation, apoptosis, synaptic plasticity, and energy metabolism (Bartel, 2004). The gene regulation and processing as well as the mode of action of miRNAs are conserved over the evolution of a species (Stricklin et al., 2005; Landgraf et al., 2007; Ruby et al., 2007b). In recent decades, research on miRNAs has deepened our understanding of their mechanisms of action and their biological functions. These regulatory RNAs are predicted to modulate the expression of ~30% of protein-coding genes (Lewis et al., 2005). The miRNA can affect translation and mRNA stability by means of RNA-RNA interactions. A number of algorithms allow the identification of the potentially targeted mRNA by miRNA and conversely miRNA modulator of mRNA. Although the regulation of genes by miRNAs is an active area of research, few targets of miRNAs have been experimentally validated in a physiological context.
In parallel with the discovery of new miRNA, the identification of the components of the miRNA maturation and processing machinery is an active area of research (Figure 1). The miRNA genes are located throughout the genome, within introns of protein-coding genes and rarely in exons (Rodriguez et al., 2004). Despite the small number of cases studied, it seems that the promoters of miRNAs have the same characteristics as those genes encoding proteins. The genes encode primary RNA (pri-miRNAs) conformation stem-loop, with one or two sequences which produce mature miRNAs (Hutvágner et al., 2001; Lagos-Quintana et al., 2001; Lau et al., 2001). The transcription machinery involves a RNA polymerase II (Lee et al., 2004; Bortolin-Cavaillé et al., 2009). The pri-miRNA is cleaved and polyadenylated at 3′ and 5′ capped in the same manner as the mRNAs (Figure 1; Cai et al., 2004). The steps of the pri-miRNA maturation require two endonucleases before they become functional miRNAs (Lee et al., 2003). The first step involves an RNA binding protein, the DiGeorge Syndrome Critical Region 8 (DGCR8) also called Partner of Drosha (PASHA) associated with Drosha (Denli et al., 2004; Gregory et al., 2004; Han et al., 2004). Drosha cleaves sequences on either side of the stem-loop of the pri-miRNA and gives the precursor miRNA (pre-miRNA). Pre-miRNA is exported from the nucleus to the cytoplasm by a karyopherin known as Exportin 5 (Yi et al., 2003; Bohnsack et al., 2004; Lund et al., 2004). In the second step, endonuclease DICER cleaves the pre-miRNA loop region in the cytoplasm, thereby releasing a double-stranded RNA of about 20 nucleotide pairs containing the mature miRNA (Bernstein et al., 2001; Grishok et al., 2001; Hutvágner et al., 2001; Ketting et al., 2001). Like Drosha, DICER is associated with an RNA binding-protein, the human immunodeficiency virus Transactivating Response RNA-Binding Protein (TRBP; Chendrimada et al., 2005; Gregory et al., 2005; Haase et al., 2005). One of the two strands is recognized by a protein of the family of the Argonautes (AGO), most commonly AGO2, which in turn recruits other elements of the RNA-induced silencing complex (RISC; Sontheimer, 2005). The other strand called “star strand” is degraded. An asterisk is associated with the name of the miRNA that is not incorporated into the RISC complex (e.g., miR-488*). However, for some miRNAs, both strands may be incorporated into the RISC complex. In this case, the end of the strand 5′ of the stem-loop is called “5p” and that of strand 3′ is called “3p” (e.g., miR-384-5p and miR-384-3p). In fact, new data indicates that a small fraction of the star strand is incorporated into the RISC complex for most miRNA families (Yang et al., 2011). For these reasons, the nomenclature scheme “–5p/–3p” is increasingly used instead of the terminology “mature/star.” The RISC complex/mature miRNA (miRISC) recognizes the target mRNA and induces degradation and/or inactivation of the latter (Figure 2).
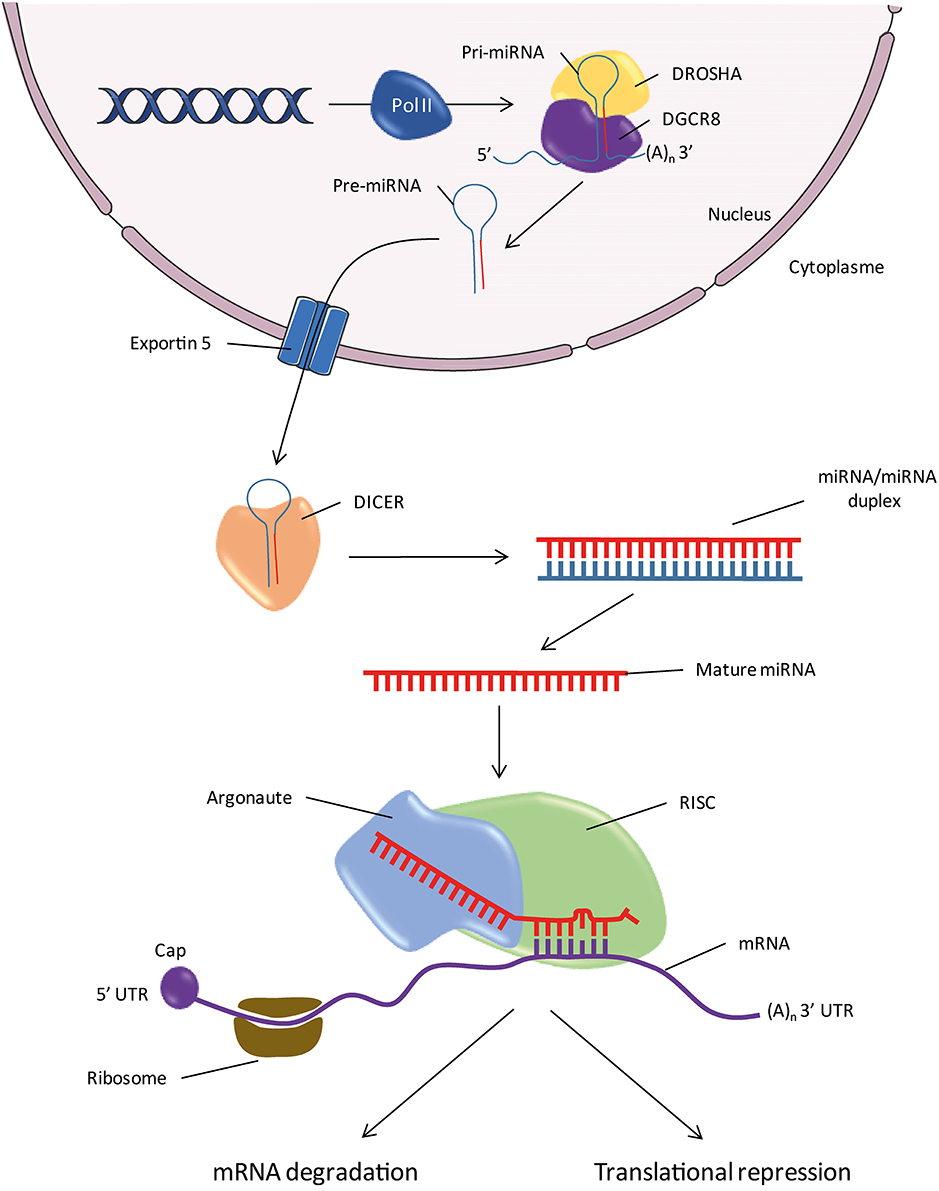
Figure 1. Biogenesis of miRNAs. Pol II, RNA Polymerase II; pri-miR, primary miRNA; pre-miR, precursor miRNA; RISC, RNA-Induced Silencing Complex; 5′ or 3′UTR, 5′ or 3′ untranslated region; DGCR8, DiGeorge Syndrome Critical Region 8; (A)n, Polyadenylation.
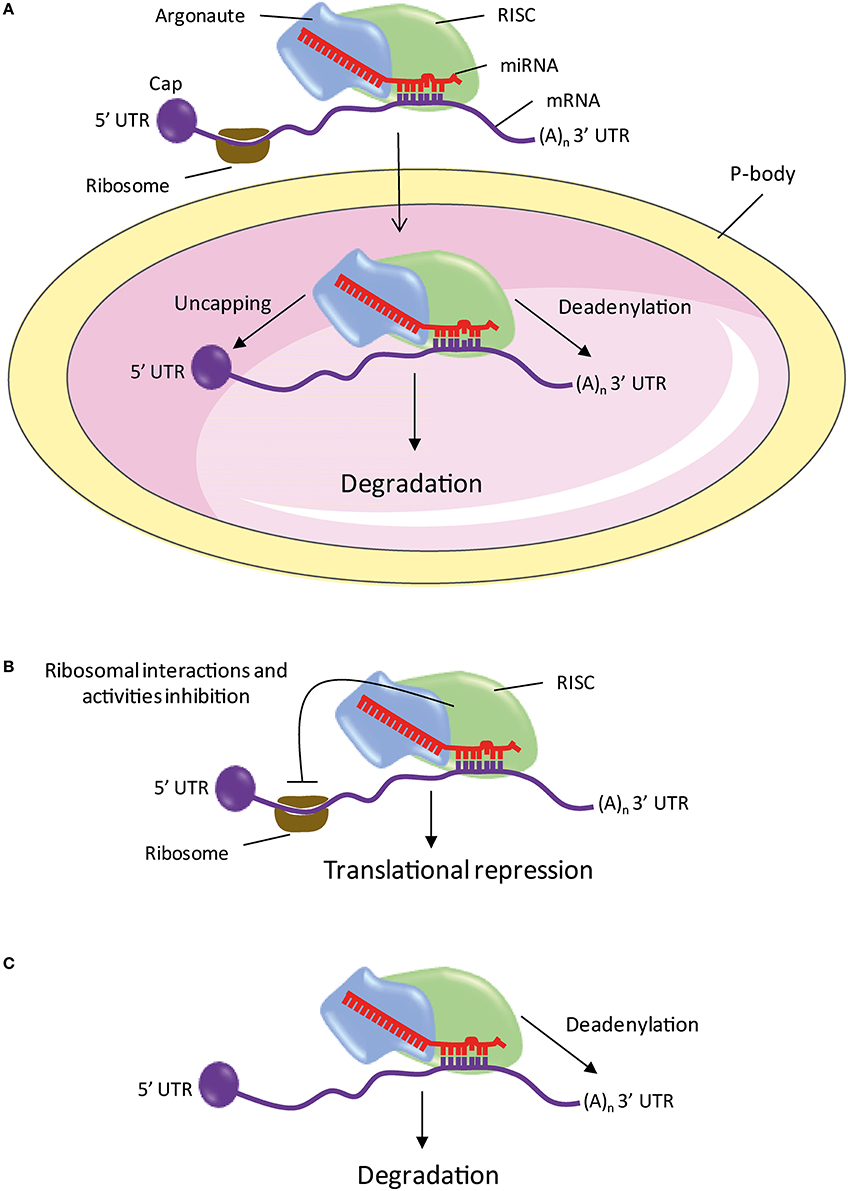
Figure 2. The different mechanisms of mRNA repression or degradation by miRNA. (A) The processing bodies. (B) Action on the initiation of translation and repression in post-initiation steps. (C) Deadenylation. miRNA, microRNA; P-bodies, processing bodies; RISC, RNA-Induced Silencing Complex; 5′ or 3′UTR, 5′ or 3′ untranslated region; (A)n, Polyadenylation.
However, various studies reveal that some families of miRNAs undergo non-canonical pathway maturation. Importantly, some studies described miRNAs called mirtrons which are located in the short sequence of introns. The mirtrons undergo a first processing step, independent of Drosha, by the splicing machinery to give miRNA with a lariat structure. The introns are then processed by the lariat-debranching enzyme to give the pre-miRNAs which carry-on its maturation by the canonical pathway (Okamura et al., 2007; Ruby et al., 2007a). It has also been reported in one case (miR-451) that the cleavage step by DICER is substituted with AGO2 (Cheloufi et al., 2010; Cifuentes et al., 2010).
The action of miRNAs depends on their specific interaction with their targets. In plants, miRNAs bind to their targets with perfect complementarity of bases, which induces a rapid cleavage of the transcript by the ribonuclease activity of AGO (Baumberger and Baulcombe, 2005). In metazoans, the majority of miRNAs partially bind to their targets primarily through a region of so-called seed sequence, located at positions 2–7 from the miRNA 5′-end (Doench and Sharp, 2004; Brennecke et al., 2005). This region binds perfectly on the 3′UTR via complementary base interactions. These interactions induce inhibition of the expression of the target mRNA through a blocking of the translation or a degradation of the transcript. The different mechanisms of mRNA repression or degradation by miRNA are briefly described below.
Processing bodies (P-bodies) are cytoplasmic foci containing mRNA degradation enzymes and trinucleotide repeat-containing gene 6A protein (TNRC6A or GW182 for Drosophila). These are involved in the catabolism and/or storage of untranslated mRNA (Figure 2A; Eystathioy et al., 2002, 2003; Ingelfinger et al., 2002; van Dijk et al., 2002; Sheth and Parker, 2003). The GW182 proteins are also found in the miRISC complex where they play a key role in the repression induced by miRNAs (Jakymiw et al., 2005; Liu et al., 2005; Eulalio et al., 2008). In addition, the AGO and GW182 proteins, miRNAs and targeted mRNAs are found in the P-bodies (Ding et al., 2005; Liu et al., 2005; Pillai et al., 2005; Sen and Blau, 2005). These studies suggest that targeted mRNAs are repressed or degraded in the P-bodies.
The mechanism by which miRISC inhibits translation is controversial. Several studies indicate a blocking of the initiation of translation, while other studies suggest a repression in post-initiation steps (Figure 2B). Indeed, it has been shown that the miRNA targeted mRNAs are associated with fewer ribosomes during elongation than in mRNAs controls (Humphreys et al., 2005; Pillai et al., 2005; Bhattacharyya et al., 2006; Huang et al., 2007; Ding and Grosshans, 2009). The initiation is stopped by the blocking by miRISC of the interaction of the translation ribosomal subunit 60S with mRNA (Chendrimada et al., 2007; Wang et al., 2008). In addition, GW182 recognizes the 5′ cap of the mRNA and prevents the initiation of translation (Eulalio et al., 2008). In the other studies, two mechanisms inducing translation repression after initiation have been described. It has been shown that miRISC promotes the release of ribosomes during elongation, thus blocking translation (Petersen et al., 2006). Another study suggests that the elongation process is maintained without peptide production when mRNA is targeted by a miRNA (Nottrott et al., 2006). The authors suggest that the complex-related proteases miRISC could degrade the native peptides.
Studies showed that repression of many miRNA targets is associated with a deadenylation and degradation (Figure 2C; Lim et al., 2005; Giraldez et al., 2006; Wu et al., 2006; Wakiyama et al., 2007; Eulalio et al., 2009). Comparative analysis of large scale proteomic and transcriptomic changes, following overexpression or inhibition of a miRNA in mammalian cells show that the vast majority of targets repressed by a miRNA have decreased their level of mRNA reflecting a lower presence of protein (Baek et al., 2008; Selbach et al., 2008; Hendrickson et al., 2009; Guo et al., 2010). These studies show that repression induced by miRNAs predominantly results in mRNA degradation.
Numerous studies clearly indicated that different hormones modulate miRNA expression in different organs (Hu et al., 2013; Cameron et al., 2016; Derghal et al., 2015; Klinge, 2015). For instance, the treatment with thyroid hormones of hepatocytes cells AML 12 over-expressing miR-206 resulted in decreased miR-206 expression, and a significant increase in two predicted target genes (i.e., Mup1 and Gpd2; Dong et al., 2010).
It has also been shown that estradiol actively controls miRNA production in various tissues such as mammary and ovarian cells (Gupta et al., 2012). More precisely, estrogens modulate miRNA transcription by inactivating RNA polymerase II and precursor miRNA biogenesis by blocking Drosha-mediated processing (Gupta et al., 2012). It also been shown that estrogen regulates miRNA expression in brain and particularly in the hippocampus, the amygdala and paraventricular nucleus (Rao et al., 2013). Recently, it has been established that miR-27a/b and miR-494 regulate tissue factor pathway inhibitor α (TFPIα) expression suggesting a possible role of these miRNAs in the estrogen mediated downregulation of TFPIα involved in breast cancer (Ali et al., 2016).
Several studies indicate that gonadotropins as estrogen can affect miRNA expression (Cohen et al., 2016). In accordance with this, it has been observed variability in miRNA expression profiles in estrogen receptor-positive and -negative breast cancer phenotypes (Iorio et al., 2005; Mattie et al., 2006). As described recently miR-136-3p expression levels were increased after the administration of human chorionic gonadotropin to ovarian cells (Kitahara et al., 2013). Direct action of estrogen on miRNAs expression has been demonstrated in different studies. For instance, an aberrant miRNA expression has been characterized in estrogen-induced rat breast carcinogenesis (Kovalchuk et al., 2007). Using the microarray approach, it has been shown that estrogen can modulate the profile of miRNAs expression in zebrafish model and in human MCF-7 and ZR-75 breast cancer cells (Cohen et al., 2008; Bhat-Nakshatri et al., 2009; Maillot et al., 2009; Ferraro et al., 2012).
Altogether, these different observations suggest that the link between hormones, miRNAs and mRNA targets will lead to an improved understanding of how EDCs affect the different endocrine axis.
A few recent studies report the effect of several EDCs on the expression of miRNAs in fish, animals, or cell lines (Collotta et al., 2013; Vrijens et al., 2015). These disturbances of miRNAs expression profile by EDCs are associated with diseases of the CNS and reproductive axis as well as metabolic disorders (Vrijens et al., 2015).
In humans, it has been shown that several EDCs as DTT or BPA decreased the expression of miR-21 which has a key role in cancer especially in breast cancer development (Tilghman et al., 2012; Sicard et al., 2013). In addition, decreased expression of let-7f is also associated with breast cancer (Sakurai et al., 2012). In the work led by Tilghman et al., DTT (10 μM) or BPA (10 μM) activate ERα in MCF-7 breast cancer cells which down-regulated the expression of miR-21, let-7a-f, miR-15b, and miR-28b and increased the expression miR-638, miR-663, and miR-1915 (Tilghman et al., 2012). In addition, it has been exhibited an important role of miR-19 in BPA-mediated MCF-7 cell proliferation (Li et al., 2014). The xenoestrogens DES also showed a decrease of miR-34b expression in MCF-7 cells (Lee et al., 2011). In rats, the neonatal exposure to the estrogenic analog (i.e., estradiol benzoate) increased the expression of miR-29 in testicular tissue (Meunier et al., 2012). Increased miR-29 expression resulted in a decrease in DNA methyltransferases (DNMT1, 3a and 3b) and antiapoptotic myeloid cell leukemia sequence 1 (Mcl-1) protein levels. Together, the increased miR-29 combined with a subsequent reduction of DNMT and Mcl-1 protein levels may represent a basis of explanation for the adult expression of the germ cell apoptosis phenotype. Interestingly, BPA given to rats at moderate doses is associated with erectile dysfunction, cavernosal lipofibrosis and alterations of global gene transcription including a set of miRNAs expressed in the penile shaft (Kovanecz et al., 2014). In female, prenatal BPA treatment in sheep results in hypergonadotropism and ovarian cycle disruptions (Veiga-Lopez et al., 2013). Interestingly, in this study it has been shown that fetal ovarian miRNAs expression was altered by prenatal BPA with 45 down-regulated (>1.5-fold) at day 65 and 11 down-regulated at day 90 of gestation (Veiga-Lopez et al., 2013). In chicks, several miRNAs (miR-1623, miR-1552-3p, miR-1573, miR-124a, and miR-1764) were down-regulated in the DES-treated chick oviduct compared with control oviduct (Lim and Song, 2015). Interestingly, these miRNAs regulate the expression of vitelline membrane outer layer protein 1, a basic protein present in the outer layer of the vitelline membrane of eggs, plays essential roles in separating the yolk from the egg white (Lim and Song, 2015). There is a growing concern about the potential health effects of exposure to various EDCs during pregnancy and infancy. The placenta is expected to be an effective barrier protecting the developing embryo against some EDCs circulating in maternal blood. However, it has been shown recently that miR-146a was significant overexpressed and correlated significantly with BPA accumulation in the placenta from pregnant women living in a polluted area and undergoing therapeutic abortion due to fetal malformations (De Felice et al., 2015). This observation has been also established in HTR-8 and 3A human placental cells (Avissar-Whiting et al., 2010). These different studies highlight the fact that the EDCs induce miRNA-expression alterations in the reproductiveaxis.
In the context of CNS disease, Jiang et al. established by in silico approach that miR-146a is involved in Alzheimer's disease (Jiang et al., 2013). Interestingly, BPA exposure of human placental cell lines has been shown to alter miRNA expression levels, and specifically, miR-146a was strongly induced by BPA treatment (Avissar-Whiting et al., 2010). Then, miR-146a could be used as a biomarker for Alzheimer's disease after EDCs exposure.
Recently, it has been established that the expression of hepatic miRNA (miR-22b, miR-140, miR-210a, mir-301, miR-457b, and let-7d) is increased in fluoxetine (the active ingredient in Prozac®) exposed female zebrafish (Craig et al., 2014). Interestingly, the miRNAs that were up-regulated were predicted to be responsible for down-regulating pathways such as insulin signaling, cholesterol synthesis, and triglyceride synthesis (Craig et al., 2014). Recently, it was shown that miR-21, 221, 222, and 429 expression levels decreased in the liver of DDT-treated female Wistar rats, whereas increases were observed in cytochrome 1A1 and 2B1 mRNA (Chanyshev et al., 2014; Gulyaeva et al., 2016). By an original approach using DNA-Au bio bar code (DNA-Au) and G-quadruplex-based DNA enzyme, Meng et al. demonstrated that miR-21 expression is increased in BPA-treated human hepatocarcinoma BEL-7402 cells (Meng et al., 2013). In primary mouse hepatocyte, TCCD modulated the expression of miR-503-5p that targeting cyclin D2 which was involved in the discriminative process of p53 signaling and metabolism (Rieswijk et al., 2015). In addition, it also been shown that TCDD regulates the expression of miR-101a and miR-122 and that cyclooxygenase-2, a target gene of miR-101a, plays a significant role in liver damage in mice exposed to TCDD (Yoshioka et al., 2011). Altogether, these observations suggest that the EDCs can induce metabolic disorders through the disturbance of specific miRNAs in the liver.
Altogether, these different studies indicated that miRNAs profile changed in tissue exposed to different EDCs. Potentially, miRNAs can be considered as new biomarkers for EDCs exposure (Vrijens et al., 2015).
Despite the high number of studies generated in the past few years on the mechanism of how EDCs act on the different endocrine axis, much still needs to be learnt. To date, very few ecotoxicology studies have considered miRNA in the context of endocrine disruption. In this review, we have seen that exposure to EDCs may lead to modification of miRNAs expression associated with endocrine disruption. However, many questions remain open, for instance (i) what is the impact on the miRNAs expression in different tissues which have suffered chronic low level EDCs exposure, (ii) what are the effects of the exposure either to a single EDC or to a complex mixture of different chemicals. Further, studies are warranted to evaluate if miRNAs may act as a causal link between EDCs exposure and their effect on health or if they can be used as a diagnostic or prognostic tools.
LM and AD wrote the manuscript. MD and JT helped with manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by funding obtained from Aix-Marseille University, the “Région Provence-Alpes-Côte d'Azur,” the “Conseil Général des Bouches-du-Rhône” (PACA, CG13) and Benjamin Delessert foundation. AD is the recipient of a doctoral fellowship from the Ministry of Education. The authors are grateful to O. Knowles for critical reading of the manuscript.
Albrecht, E. D., Aberdeen, G. W., and Pepe, G. J. (2005). Estrogen elicits cortical zone-specific effects on development of the primate fetal adrenal gland. Endocrinology 146, 1737–1744. doi: 10.1210/en.2004-1124
Ali, H. O., Arroyo, A. B., González-Conejero, R., Stavik, B., Iversen, N., Sandset, P. M., et al. (2016). The role of microRNA -27a/b and microRNA-494 in oestrogen mediated downregulation of tissue factor pathway inhibitor α. J. Thromb. Haemost. 14, 1226–1237. doi: 10.1111/jth.13321
Alonso-Magdalena, P., García-Arévalo, M., Quesada, I., and Nadal, Á. (2015). Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 156, 1659–1670. doi: 10.1210/en.2014-1952
Alonso-Magdalena, P., Laribi, O., Ropero, A. B., Fuentes, E., Ripoll, C., Soria, B., et al. (2005). Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ. Health Perspect. 113, 969–977. doi: 10.1289/ehp.8002
Alonso-Magdalena, P., Quesada, I., and Nadal, A. (2011). Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 7, 346–353. doi: 10.1038/nrendo.2011.56
Alonso-Magdalena, P., Vieira, E., Soriano, S., Menes, L., Burks, D., Quesada, I., et al. (2010). Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 118, 1243–1250. doi: 10.1289/ehp.1001993
Angle, B. M., Do, R. P., Ponzi, D., Stahlhut, R. W., Drury, B. E., Nagel, S. C., et al. (2013). Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 42, 256–268. doi: 10.1016/j.reprotox.2013.07.017
Anway, M. D., Cupp, A. S., Uzumcu, M., and Skinner, M. K. (2005). Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469. doi: 10.1126/science.1108190
Aurela, B., Kulmala, H., and Söderhjelm, L. (1999). Phthalates in paper and board packaging and their migration into Tenax and sugar. Food Addit. Contam. 16, 571–577. doi: 10.1080/026520399283713
Avissar-Whiting, M., Veiga, K. R., Uhl, K. M., Maccani, M. A., Gagne, L. A., Moen, E. L., et al. (2010). Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 29, 401–406. doi: 10.1016/j.reprotox.2010.04.004
Baek, D., Villén, J., Shin, C., Camargo, F. D., Gygi, S. P., and Bartel, D. P. (2008). The impact of microRNAs on protein output. Nature 455, 64–71. doi: 10.1038/nature07242
Baillie-Hamilton, P. F. (2002). Chemical toxins: a hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 8, 185–192. doi: 10.1089/107555302317371479
Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. doi: 10.1016/S0092-8674(04)00045-5
Baumberger, N., and Baulcombe, D. C. (2005). Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. U.S.A. 102, 11928–11933. doi: 10.1073/pnas.0505461102
Bernstein, E., Caudy, A. A., Hammond, S. M., and Hannon, G. J. (2001). Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366. doi: 10.1038/35053110
Bhat-Nakshatri, P., Wang, G., Collins, N. R., Thomson, M. J., Geistlinger, T. R., Carroll, J. S., et al. (2009). Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 37, 4850–4861. doi: 10.1093/nar/gkp500
Bhattacharyya, S. N., Habermacher, R., Martine, U., Closs, E. I., and Filipowicz, W. (2006). Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124. doi: 10.1016/j.cell.2006.04.031
Boas, M., Feldt-Rasmussen, U., and Main, K. M. (2012). Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 355, 240–248. doi: 10.1016/j.mce.2011.09.005
Bohnsack, M. T., Czaplinski, K., and Görlich, D. (2004). Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10, 185–191. doi: 10.1261/rna.5167604
Bortolin-Cavaillé, M.-L., Dance, M., Weber, M., and Cavaillé, J. (2009). C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 37, 3464–3473. doi: 10.1093/nar/gkp205
Brennecke, J., Stark, A., Russell, R. B., and Cohen, S. M. (2005). Principles of microRNA-target recognition. PLoS Biol. 3:e85. doi: 10.1371/journal.pbio.0030085
Brown, J. S. (2009). Effects of bisphenol-A and other endocrine disruptors compared with abnormalities of schizophrenia: an endocrine-disruption theory of schizophrenia. Schizophr. Bull. 35, 256–278. doi: 10.1093/schbul/sbm147
Buckley, J., Willingham, E., Agras, K., and Baskin, L. S. (2006). Embryonic exposure to the fungicide vinclozolin causes virilization of females and alteration of progesterone receptor expression in vivo: an experimental study in mice. Environ. Health Glob. Access Sci. Source 5:4. doi: 10.1186/1476-069X-5-4
Cai, X., Hagedorn, C. H., and Cullen, B. R. (2004). Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10, 1957–1966. doi: 10.1261/rna.7135204
Calafat, A. M., Kuklenyik, Z., Reidy, J. A., Caudill, S. P., Ekong, J., and Needham, L. L. (2005). Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113, 391–395. doi: 10.1289/ehp.7534
Cameron, B. E., Craig, P. M., and Trudeau, V. L. (2016). Implication of microRNA deregulation in the response of vertebrates to endocrine disrupting chemicals. Environ. Toxicol. Chem. SETAC, 35, 788–793. doi: 10.1002/etc.3063
Campos, É., and Freire, C. (2016). Exposure to non-persistent pesticides and thyroid function: a systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health. doi: 10.1016/j.ijheh.2016.05.006. [Epub ahead of print].
Casals-Casas, C., and Desvergne, B. (2011). Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 73, 135–162. doi: 10.1146/annurev-physiol-012110-142200
Casals-Casas, C., Feige, J. N., and Desvergne, B. (2008). Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int. J. Obes. 2005 (32 Suppl. 6), S53–S61. doi: 10.1038/ijo.2008.207
Cerqueira, J. J., Pêgo, J. M., Taipa, R., Bessa, J. M., Almeida, O. F. X., and Sousa, N. (2005). Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. Off. J. Soc. Neurosci. 25, 7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005
Chanyshev, M. D., Kosorotikov, N. I., Titov, S. E., Kolesnikov, N. N., and Gulyaeva, L. F. (2014). Expression of microRNAs, CYP1A1 and CYP2B1 in the livers and ovaries of female rats treated with DDT and PAHs. Life Sci. 103, 95–100. doi: 10.1016/j.lfs.2014.03.031
Cheloufi, S., Dos Santos, C. O., Chong, M. M. W., and Hannon, G. J. (2010). A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589. doi: 10.1038/nature09092
Chen, F., Zhou, L., Bai, Y., Zhou, R., and Chen, L. (2015). Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J. Biomed. Res. 29, 250–258. doi: 10.7555/JBR.29.20140058
Chen, J.-Q., Brown, T. R., and Russo, J. (2009). Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta 1793, 1128–1143. doi: 10.1016/j.bbamcr.2009.03.009
Chendrimada, T. P., Finn, K. J., Ji, X., Baillat, D., Gregory, R. I., Liebhaber, S. A., et al. (2007). MicroRNA silencing through RISC recruitment of eIF6. Nature 447, 823–828. doi: 10.1038/nature05841
Chendrimada, T. P., Gregory, R. I., Kumaraswamy, E., Norman, J., Cooch, N., Nishikura, K., et al. (2005). TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744. doi: 10.1038/nature03868
Cifuentes, D., Xue, H., Taylor, D. W., Patnode, H., Mishima, Y., Cheloufi, S., et al. (2010). A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328, 1694–1698. doi: 10.1126/science.1190809
Cohen, A., Burgos-Aceves, M. A., and Smith, Y. (2016). Estrogen repression of microRNA as a potential cause of cancer. Biomed. Pharmacother. Bioméd. Pharmacothérapie 78, 234–238. doi: 10.1016/j.biopha.2016.01.023
Cohen, A., Shmoish, M., Levi, L., Cheruti, U., Levavi-Sivan, B., and Lubzens, E. (2008). Alterations in micro-ribonucleic acid expression profiles reveal a novel pathway for estrogen regulation. Endocrinology 149, 1687–1696. doi: 10.1210/en.2007-0969
Colborn, T., vom Saal, F. S., and Soto, A. M. (1993). Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 101, 378–384. doi: 10.1289/ehp.93101378
Collotta, M., Bertazzi, P. A., and Bollati, V. (2013). Epigenetics and pesticides. Toxicology 307, 35–41. doi: 10.1016/j.tox.2013.01.017
Cotter, D., and Pariante, C. M. (2002). Stress and the progression of the developmental hypothesis of schizophrenia. Br. J. Psychiatry J. Ment. Sci. 181, 363–365. doi: 10.1192/bjp.181.5.363
Craig, P. M., Trudeau, V. L., and Moon, T. W. (2014). Profiling hepatic microRNAs in zebrafish: fluoxetine exposure mimics a fasting response that targets AMP-activated protein kinase (AMPK). PLoS ONE 9:e95351. doi: 10.1371/journal.pone.0095351
Darnerud, P. O., Morse, D., Klasson-Wehler, E., and Brouwer, A. (1996). Binding of a 3,3′, 4,4′-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology 106, 105–114. doi: 10.1016/0300-483X(95)03169-G
de Cock, M., Maas, Y. G. H., and van de Bor, M. (2012). Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Acta Paediatr. 101, 811–818. doi: 10.1111/j.1651-2227.2012.02693.x
De Felice, B., Manfellotto, F., Palumbo, A., Troisi, J., Zullo, F., Di Carlo, C., et al. (2015). Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med. Genomics 8:56. doi: 10.1186/s12920-015-0131-z
Delfosse, V., Grimaldi, M., le Maire, A., Bourguet, W., and Balaguer, P. (2014). Nuclear receptor profiling of bisphenol-A and its halogenated analogues. Vitam. Horm. 94, 229–251. doi: 10.1016/B978-0-12-800095-3.00009-2
Del-Mazo, J., Brieño-Enríquez, M. A., García-López, J., López-Fernández, L. A., and De-Felici, M. (2013). Endocrine disruptors, gene deregulation and male germ cell tumors. Int. J. Dev. Biol. 57, 225–239. doi: 10.1387/ijdb.130042jd
Denli, A. M., Tops, B. B. J., Plasterk, R. H. A., Ketting, R. F., and Hannon, G. J. (2004). Processing of primary microRNAs by the Microprocessor complex. Nature 432, 231–235. doi: 10.1038/nature03049
Derghal, A., Djelloul, M., Airault, C., Pierre, C., Dallaporta, M., Troadec, J.-D., et al. (2015). Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3′UTR. Front. Cell. Neurosci. 9:172. doi: 10.3389/fncel.2015.00172
Diamanti-Kandarakis, E., Bourguignon, J.-P., Giudice, L. C., Hauser, R., Prins, G. S., Soto, A. M., et al. (2009). Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342. doi: 10.1210/er.2009-0002
Ding, L., Spencer, A., Morita, K., and Han, M. (2005). The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19, 437–447. doi: 10.1016/j.molcel.2005.07.013
Ding, X. C., and Grosshans, H. (2009). Repression of C. elegans microRNA targets at the initiation level of translation requires GW182 proteins. EMBO J. 28, 213–222. doi: 10.1038/emboj.2008.275
Doench, J. G., and Sharp, P. A. (2004). Specificity of microRNA target selection in translational repression. Genes Dev. 18, 504–511. doi: 10.1101/gad.1184404
Dong, H., Paquette, M., Williams, A., Zoeller, R. T., Wade, M., and Yauk, C. (2010). Thyroid hormone may regulate mRNA abundance in liver by acting on microRNAs. PLoS ONE 5:e12136. doi: 10.1371/journal.pone.0012136
Elobeid, M. A., and Allison, D. B. (2008). Putative environmental-endocrine disruptors and obesity: a review. Curr. Opin. Endocrinol. Diabetes Obes. 15, 403–408. doi: 10.1097/MED.0b013e32830ce95c
Eulalio, A., Huntzinger, E., and Izaurralde, E. (2008). GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 15, 346–353. doi: 10.1038/nsmb.1405
Eulalio, A., Huntzinger, E., Nishihara, T., Rehwinkel, J., Fauser, M., and Izaurralde, E. (2009). Deadenylation is a widespread effect of miRNA regulation. RNA 15, 21–32. doi: 10.1261/rna.1399509
Eystathioy, T., Chan, E. K. L., Tenenbaum, S. A., Keene, J. D., Griffith, K., and Fritzler, M. J. (2002). A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13, 1338–1351. doi: 10.1091/mbc.01-11-0544
Eystathioy, T., Jakymiw, A., Chan, E. K. L., Séraphin, B., Cougot, N., and Fritzler, M. J. (2003). The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA 9, 1171–1173. doi: 10.1261/rna.5810203
Ferraro, L., Ravo, M., Nassa, G., Tarallo, R., De Filippo, M. R., Giurato, G., et al. (2012). Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm. Cancer 3, 65–78. doi: 10.1007/s12672-012-0102-1
Fisher, J. S., Macpherson, S., Marchetti, N., and Sharpe, R. M. (2003). Human “testicular dysgenesis syndrome”: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. Oxf. Engl. 18, 1383–1394. doi: 10.1093/humrep/deg273
Freire, C., Lopez-Espinosa, M.-J., Fernández, M., Molina-Molina, J.-M., Prada, R., and Olea, N. (2011). Prenatal exposure to organochlorine pesticides and TSH status in newborns from Southern Spain. Sci. Total Environ. 409, 3281–3287. doi: 10.1016/j.scitotenv.2011.05.037
Gayathri, N. S., Dhanya, C. R., Indu, A. R., and Kurup, P. A. (2004). Changes in some hormones by low doses of di (2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J. Med. Res. 119, 139–144.
Giraldez, A. J., Mishima, Y., Rihel, J., Grocock, R. J., Van Dongen, S., Inoue, K., et al. (2006). Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79. doi: 10.1126/science.1122689
Gore, A. C. (2002). Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol. Cell. Endocrinol. 192, 157–170. doi: 10.1016/S0303-7207(02)00010-2
Gregory, R. I., Chendrimada, T. P., Cooch, N., and Shiekhattar, R. (2005). Human RISC Couples MicroRNA Biogenesis and Posttranscriptional Gene Silencing. Cell 123, 631–640. doi: 10.1016/j.cell.2005.10.022
Gregory, R. I., Yan, K.-P., Amuthan, G., Chendrimada, T., Doratotaj, B., Cooch, N., et al. (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240. doi: 10.1038/nature03120
Grishok, A., Pasquinelli, A. E., Conte, D., Li, N., Parrish, S., Ha, I., et al. (2001). Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34. doi: 10.1016/S0092-8674(01)00431-7
Guerrero-Bosagna, C., Covert, T. R., Haque, M. M., Settles, M., Nilsson, E. E., Anway, M. D., et al. (2012). Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod. Toxicol. 34, 694–707. doi: 10.1016/j.reprotox.2012.09.005
Gulyaeva, L. F., Chanyshev, M. D., Kolmykov, S. K., Ushakov, D. S., and Nechkin, S. S. (2016). Effect of xenobiotics on microrna expression in rat liver. Biomed. Khim. 62, 154–159. doi: 10.18097/pbmc20166202154
Guo, H., Ingolia, N. T., Weissman, J. S., and Bartel, D. P. (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840. doi: 10.1038/nature09267
Gupta, A., Caffrey, E., Callagy, G., and Gupta, S. (2012). Oestrogen-dependent regulation of miRNA biogenesis: many ways to skin the cat. Biochem. Soc. Trans. 40, 752–758. doi: 10.1042/BST20110763
Haase, A. D., Jaskiewicz, L., Zhang, H., Lainé, S., Sack, R., Gatignol, A., et al. (2005). TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6, 961–967. doi: 10.1038/sj.embor.7400509
Halden, R. U. (2010). Plastics and health risks. Annu. Rev. Public Health 31, 179–194. doi: 10.1146/annurev.publhealth.012809.103714
Han, J., Lee, Y., Yeom, K.-H., Kim, Y.-K., Jin, H., and Kim, V. N. (2004). The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027. doi: 10.1101/gad.1262504
Hendrickson, D. G., Hogan, D. J., McCullough, H. L., Myers, J. W., Herschlag, D., Ferrell, J. E., et al. (2009). Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 7:e1000238. doi: 10.1371/journal.pbio.1000238
Henley, D. V., and Korach, K. S. (2006). Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 147, S25–S32. doi: 10.1210/en.2005-1117
Heudorf, U., Mersch-Sundermann, V., and Angerer, J. (2007). Phthalates: toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634. doi: 10.1016/j.ijheh.2007.07.011
Hinton, R. H., Mitchell, F. E., Mann, A., Chescoe, D., Price, S. C., Nunn, A., et al. (1986). Effects of phthalic acid esters on the liver and thyroid. Environ. Health Perspect. 70, 195–210. doi: 10.1289/ehp.8670195
Hisada, A., Shimodaira, K., Okai, T., Watanabe, K., Takemori, H., Takasuga, T., et al. (2014). Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates. Int. J. Hyg. Environ. Health 217, 546–553. doi: 10.1016/j.ijheh.2013.10.004
Hu, Z., Shen, W.-J., Cortez, Y., Tang, X., Liu, L.-F., Kraemer, F. B., et al. (2013). Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland. PLoS ONE 8:e78040. doi: 10.1371/journal.pone.0078040
Huang, C.-Y., Wu, C.-L., Yang, Y.-C., Chang, J.-W., Kuo, Y.-C., Cheng, Y.-Y., et al. (2015). Association between Dioxin and diabetes mellitus in an endemic area of exposure in Taiwan: a population-based study. Medicine (Baltimore). 94:e1730. doi: 10.1097/md.0000000000001730
Huang, J., Liang, Z., Yang, B., Tian, H., Ma, J., and Zhang, H. (2007). Derepression of microRNA-mediated protein translation inhibition by apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) and its family members. J. Biol. Chem. 282, 33632–33640. doi: 10.1074/jbc.M705116200
Huber, T. J., Rollnik, J., Wilhelms, J., von zur Mühlen, A., Emrich, H. M., and Schneider, U. (2001). Estradiol levels in psychotic disorders. Psychoneuroendocrinology 26, 27–35. doi: 10.1016/S0306-4530(00)00034-2
Humphreys, D. T., Westman, B. J., Martin, D. I. K., and Preiss, T. (2005). MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. U.S.A. 102, 16961–16966. doi: 10.1073/pnas.0506482102
Hutvágner, G., McLachlan, J., Pasquinelli, A. E., Bálint, E., Tuschl, T., and Zamore, P. D. (2001). A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838. doi: 10.1126/science.1062961
Inadera, H. (2015). Neurological effects of bisphenol A and its analogues. Int. J. Med. Sci. 12, 926–936. doi: 10.7150/ijms.13267
Ingelfinger, D., Arndt-Jovin, D. J., Lührmann, R., and Achsel, T. (2002). The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8, 1489–1501. doi: 10.1017/S1355838202021726
Iorio, M. V., Ferracin, M., Liu, C.-G., Veronese, A., Spizzo, R., Sabbioni, S., et al. (2005). MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070. doi: 10.1158/0008-5472.CAN-05-1783
Jakymiw, A., Lian, S., Eystathioy, T., Li, S., Satoh, M., Hamel, J. C., et al. (2005). Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 7, 1267–1274. doi: 10.1038/ncb1334
Jiang, W., Zhang, Y., Meng, F., Lian, B., Chen, X., Yu, X., et al. (2013). Identification of active transcription factor and miRNA regulatory pathways in Alzheimer's disease. Bioinforma. Oxf. Engl. 29, 2596–2602. doi: 10.1093/bioinformatics/btt423
Kaneda, Y., and Ohmori, T. (2005). Relation between estradiol and negative symptoms in men with schizophrenia. J. Neuropsychiatry Clin. Neurosci. 17, 239–242. doi: 10.1176/jnp.17.2.239
Katz, D. L., Frankenburg, F. R., Benowitz, L. I., and Gilbert, J. M. (1987). Psychosis and prenatal exposure to diethylstilbestrol. J. Nerv. Ment. Dis. 175, 306–308. doi: 10.1097/00005053-198705000-00011
Kelce, W. R., Lambright, C. R., Gray, L. E., and Roberts, K. P. (1997). Vinclozolin and p,p'-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism. Toxicol. Appl. Pharmacol. 142, 192–200. doi: 10.1006/taap.1996.7966
Ketting, R. F., Fischer, S. E. J., Bernstein, E., Sijen, T., Hannon, G. J., and Plasterk, R. H. A. (2001). Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659. doi: 10.1101/gad.927801
Kimura-Kuroda, J., Nagata, I., and Kuroda, Y. (2007). Disrupting effects of hydroxy-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: a possible causal factor for developmental brain disorders? Chemosphere 67, S412–S420. doi: 10.1016/j.chemosphere.2006.05.137
Kitahara, Y., Nakamura, K., Kogure, K., and Minegishi, T. (2013). Role of microRNA-136-3p on the expression of luteinizing hormone-human chorionic gonadotropin receptor mRNA in rat ovaries. Biol. Reprod. 89, 114. doi: 10.1095/biolreprod.113.109207
Klenke, U., Constantin, S., and Wray, S. (2016). BPA directly decreases GnRH neuronal activity via noncanonical pathway. Endocrinology 157, 1980–1990. doi: 10.1210/en.2015-1924
Klinge, C. M. (2015). miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol. Cell. Endocrinol. 418(Pt 3), 273–297. doi: 10.1016/j.mce.2015.01.035
Koskenniemi, J. J., Virtanen, H. E., Kiviranta, H., Damgaard, I. N., Matomäki, J., Thorup, J. M., et al. (2015). Association between levels of persistent organic pollutants in adipose tissue and cryptorchidism in early childhood: a case-control study. Environ. Health Glob. Access Sci. Source 14, 78. doi: 10.1186/s12940-015-0065-0
Kovalchuk, O., Tryndyak, V. P., Montgomery, B., Boyko, A., Kutanzi, K., Zemp, F., et al. (2007). Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle Georget. Tex 6, 2010–2018. doi: 10.4161/cc.6.16.4549
Kovanecz, I., Gelfand, R., Masouminia, M., Gharib, S., Segura, D., Vernet, D., et al. (2014). Oral Bisphenol A (BPA) given to rats at moderate doses is associated with erectile dysfunction, cavernosal lipofibrosis and alterations of global gene transcription. Int. J. Impot. Res. 26, 67–75. doi: 10.1038/ijir.2013.37
Kuiper, G. G., Lemmen, J. G., Carlsson, B., Corton, J. C., Safe, S. H., van der Saag, P. T., et al. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139, 4252–4263.
Lagos-Quintana, M., Rauhut, R., Lendeckel, W., and Tuschl, T. (2001). Identification of novel genes coding for small expressed RNAs. Science 294, 853–858. doi: 10.1126/science.1064921
Landgraf, P., Rusu, M., Sheridan, R., Sewer, A., Iovino, N., Aravin, A., et al. (2007). A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129, 1401–1414. doi: 10.1016/j.cell.2007.04.040
Landrigan, P. J., and Goldman, L. R. (2011a). Children's vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. (Millwood) 30, 842–850. doi: 10.1377/hlthaff.2011.0151
Landrigan, P. J., and Goldman, L. R. (2011b). Protecting children from pesticides and other toxic chemicals. J. Expo. Sci. Environ. Epidemiol. 21, 119–120. doi: 10.1038/jes.2011.1
Lang, I. A., Galloway, T. S., Scarlett, A., Henley, W. E., Depledge, M., Wallace, R. B., et al. (2008). Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300, 1303–1310. doi: 10.1001/jama.300.11.1303
Lau, N. C., Lim, L. P., Weinstein, E. G., and Bartel, D. P. (2001). An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858–862. doi: 10.1126/science.1065062
Lee, Y., Ahn, C., Han, J., Choi, H., Kim, J., Yim, J., et al. (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419. doi: 10.1038/nature01957
Lee, Y., Kim, M., Han, J., Yeom, K.-H., Lee, S., Baek, S. H., et al. (2004). MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060. doi: 10.1038/sj.emboj.7600385
Lee, Y.-M., Lee, J.-Y., Ho, C.-C., Hong, Q.-S., Yu, S.-L., Tzeng, C.-R., et al. (2011). miRNA-34b as a tumor suppressor in estrogen-dependent growth of breast cancer cells. Breast Cancer Res. 13, R116. doi: 10.1186/bcr3059
Lewis, B. P., Burge, C. B., and Bartel, D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20. doi: 10.1016/j.cell.2004.12.035
Li, S., Hursting, S. D., Davis, B. J., McLachlan, J. A., and Barrett, J. C. (2003). Environmental exposure, DNA methylation, and gene regulation: lessons from diethylstilbesterol-induced cancers. Ann. N.Y. Acad. Sci. 983, 161–169. doi: 10.1111/j.1749-6632.2003.tb05971.x
Li, X., Xie, W., Xie, C., Huang, C., Zhu, J., Liang, Z., et al. (2014). Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 28, 1553–1560. doi: 10.1002/ptr.5167
Lim, L. P., Lau, N. C., Garrett-Engele, P., Grimson, A., Schelter, J. M., Castle, J., et al. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773. doi: 10.1038/nature03315
Lim, W., and Song, G. (2015). Differential expression of vitelline membrane outer layer protein 1: hormonal regulation of expression in the oviduct and in ovarian carcinomas from laying hens. Mol. Cell. Endocrinol. 399, 250–258. doi: 10.1016/j.mce.2014.10.015
Liu, J., Rivas, F. V., Wohlschlegel, J., Yates, J. R., Parker, R., and Hannon, G. J. (2005). A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266. doi: 10.1038/ncb1333
Lund, E., Güttinger, S., Calado, A., Dahlberg, J. E., and Kutay, U. (2004). Nuclear export of microRNA precursors. Science 303, 95–98. doi: 10.1126/science.1090599
Maillot, G., Lacroix-Triki, M., Pierredon, S., Gratadou, L., Schmidt, S., Bénès, V., et al. (2009). Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 69, 8332–8340. doi: 10.1158/0008-5472.CAN-09-2206
Main, K. M., Kiviranta, H., Virtanen, H. E., Sundqvist, E., Tuomisto, J. T., Tuomisto, J., et al. (2007). Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ. Health Perspect. 115, 1519–1526. doi: 10.1289/ehp.9924
Maqbool, F., Mostafalou, S., Bahadar, H., and Abdollahi, M. (2016). Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 145, 265–273. doi: 10.1016/j.lfs.2015.10.022
Marx, C. E., and Lieberman, J. A. (1998). Psychoneuroendocrinology of schizophrenia. Psychiatr. Clin. North Am. 21, 413–434. doi: 10.1016/S0193-953X(05)70013-7
Mattie, M. D., Benz, C. C., Bowers, J., Sensinger, K., Wong, L., Scott, G. K., et al. (2006). Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol. Cancer 5:24. doi: 10.1186/1476-4598-5-24
McKinney, J., Fannin, R., Jordan, S., Chae, K., Rickenbacher, U., and Pedersen, L. (1987). Polychlorinated biphenyls and related compound interactions with specific binding sites for thyroxine in rat liver nuclear extracts. J. Med. Chem. 30, 79–86. doi: 10.1021/jm00384a014
Meeker, J. D., Sathyanarayana, S., and Swan, S. H. (2009). Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2097–2113. doi: 10.1098/rstb.2008.0268
Meerts, I. A. T. M., Assink, Y., Cenijn, P. H., Van Den Berg, J. H. J., Weijers, B. M., Bergman, A., et al. (2002). Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol. Sci. Off. J. Soc. Toxicol. 68, 361–371. doi: 10.1093/toxsci/68.2.361
Meng, X., Zhou, Y., Liang, Q., Qu, X., Yang, Q., Yin, H., et al. (2013). Electrochemical determination of microRNA-21 based on bio bar code and hemin/G-quadruplet DNAenzyme. Analyst 138, 3409–3415. doi: 10.1039/c3an36788f
Meunier, L., Siddeek, B., Vega, A., Lakhdari, N., Inoubli, L., Bellon, R. P., et al. (2012). Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: role of microRNA miR-29 family in the down-regulation of DNA methyltransferases and Mcl-1. Endocrinology 153, 1936–1947. doi: 10.1210/en.2011-1109
Miller, V. M., Sanchez-Morrissey, S., Brosch, K. O., and Seegal, R. F. (2012). Developmental coexposure to polychlorinated biphenyls and polybrominated diphenyl ethers has additive effects on circulating thyroxine levels in rats. Toxicol. Sci. Off. J. Soc. Toxicol. 127, 76–83. doi: 10.1093/toxsci/kfs089
Mitchell, F. E., Price, S. C., Hinton, R. H., Grasso, P., and Bridges, J. W. (1985). Time and dose-response study of the effects on rats of the plasticizer di(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 81, 371–392. doi: 10.1016/0041-008X(85)90409-0
Mocarelli, P., Gerthoux, P. M., Needham, L. L., Patterson, D. G., Limonta, G., Falbo, R., et al. (2011). Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ. Health Perspect. 119, 713–718. doi: 10.1289/ehp.1002134
Molehin, D., Dekker Nitert, M., and Richard, K. (2016). Prenatal exposures to multiple thyroid hormone disruptors: effects on glucose and lipid metabolism. J. Thyroid Res. 2016:8765049. doi: 10.1155/2016/8765049
Mostafalou, S., and Abdollahi, M. (2013). Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 268, 157–177. doi: 10.1016/j.taap.2013.01.025
Mostafalou, S., Eghbal, M. A., Nili-Ahmadabadi, A., Baeeri, M., and Abdollahi, M. (2012). Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicol. Ind. Health 28, 840–851. doi: 10.1177/0748233711425073
Nakamura, K., Itoh, K., Sugimoto, T., and Fushiki, S. (2007). Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci. Lett. 420, 100–105. doi: 10.1016/j.neulet.2007.02.093
Nakamura, K., Itoh, K., Yaoi, T., Fujiwara, Y., Sugimoto, T., and Fushiki, S. (2006). Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J. Neurosci. Res. 84, 1197–1205. doi: 10.1002/jnr.21020
Negri-Cesi, P. (2015). Bisphenol A interaction with brain development and functions. Dose Response 13:1559325815590394. doi: 10.1177/1559325815590394
Newbold, R. R., Padilla-Banks, E., Jefferson, W. N., and Heindel, J. J. (2008). Effects of endocrine disruptors on obesity. Int. J. Androl. 31, 201–208. doi: 10.1111/j.1365-2605.2007.00858.x
Newbold, R. R., Padilla-Banks, E., Snyder, R. J., and Jefferson, W. N. (2007). Perinatal exposure to environmental estrogens and the development of obesity. Mol. Nutr. Food Res. 51, 912–917. doi: 10.1002/mnfr.200600259
Nottrott, S., Simard, M. J., and Richter, J. D. (2006). Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 13, 1108–1114. doi: 10.1038/nsmb1173
Noyes, P. D., Lema, S. C., Macaulay, L. J., Douglas, N. K., and Stapleton, H. M. (2013). Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ. Sci. Technol. 47, 10012–10021. doi: 10.1021/es402650x
Okamura, K., Hagen, J. W., Duan, H., Tyler, D. M., and Lai, E. C. (2007). The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 130, 89–100. doi: 10.1016/j.cell.2007.06.028
Panagiotidou, E., Zerva, S., Mitsiou, D. J., Alexis, M. N., and Kitraki, E. (2014). Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J. Endocrinol. 220, 207–218. doi: 10.1530/JOE-13-0416
Patrick, L. (2009). Thyroid disruption: mechanism and clinical implications in human health. Altern. Med. Rev. 14, 326–346.
Pelclová, D., Urban, P., Preiss, J., Lukás, E., Fenclová, Z., Navrátil, T., et al. (2006). Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Rev. Environ. Health 21, 119–138. doi: 10.1515/REVEH.2006.21.2.119
Pepe, G. J., and Albrecht, E. D. (1998). Central integrative role of oestrogen in the regulation of placental steroidogenic maturation and the development of the fetal pituitary-adrenocortical axis in the baboon. Hum. Reprod. Update 4, 406–419. doi: 10.1093/humupd/4.4.406
Petersen, C. P., Bordeleau, M.-E., Pelletier, J., and Sharp, P. A. (2006). Short RNAs repress translation after initiation in mammalian cells. Mol. Cell 21, 533–542. doi: 10.1016/j.molcel.2006.01.031
Pillai, R. S., Bhattacharyya, S. N., Artus, C. G., Zoller, T., Cougot, N., Basyuk, E., et al. (2005). Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 309, 1573–1576. doi: 10.1126/science.1115079
Price, S. C., Chescoe, D., Grasso, P., Wright, M., and Hinton, R. H. (1988). Alterations in the thyroids of rats treated for long periods with di-(2-ethylhexyl) phthalate or with hypolipidaemic agents. Toxicol. Lett. 40, 37–46. doi: 10.1016/0378-4274(88)90181-6
Rao, Y. S., Mott, N. N., Wang, Y., Chung, W. C. J., and Pak, T. R. (2013). MicroRNAs in the aging female brain: a putative mechanism for age-specific estrogen effects. Endocrinology 154, 2795–2806. doi: 10.1210/en.2013-1230
Rasier, G., Parent, A.-S., Gérard, A., Lebrethon, M.-C., and Bourguignon, J.-P. (2007). Early maturation of gonadotropin-releasing hormone secretion and sexual precocity after exposure of infant female rats to estradiol or dichlorodiphenyltrichloroethane. Biol. Reprod. 77, 734–742. doi: 10.1095/biolreprod.106.059303
Reik, W., Dean, W., and Walter, J. (2001). Epigenetic reprogramming in mammalian development. Science 293, 1089–1093. doi: 10.1126/science.1063443
Remillard, R. B. J., and Bunce, N. J. (2002). Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ. Health Perspect. 110, 853–858. doi: 10.1289/ehp.02110853
Rice, C. E., Baio, J., Van Naarden Braun, K., Doernberg, N., Meaney, F. J., Kirby, R. S., et al. (2007). A public health collaboration for the surveillance of autism spectrum disorders. Paediatr. Perinat. Epidemiol. 21, 179–190. doi: 10.1111/j.1365-3016.2007.00801.x
Rickenbacher, U., McKinney, J. D., Oatley, S. J., and Blake, C. C. (1986). Structurally specific binding of halogenated biphenyls to thyroxine transport protein. J. Med. Chem. 29, 641–648. doi: 10.1021/jm00155a010
Rieswijk, L., Brauers, K. J. J., Coonen, M. L. J., van Breda, S. G. J., Jennen, D. G. J., and Kleinjans, J. C. S. (2015). Evaluating microRNA profiles reveals discriminative responses following genotoxic or non-genotoxic carcinogen exposure in primary mouse hepatocytes. Mutagenesis 30, 771–784. doi: 10.1093/mutage/gev036
Rodriguez, A., Griffiths-Jones, S., Ashurst, J. L., and Bradley, A. (2004). Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902–1910. doi: 10.1101/gr.2722704
Ruby, J. G., Jan, C. H., and Bartel, D. P. (2007a). Intronic microRNA precursors that bypass Drosha processing. Nature 448, 83–86. doi: 10.1038/nature05983
Ruby, J. G., Stark, A., Johnston, W. K., Kellis, M., Bartel, D. P., and Lai, E. C. (2007b). Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 17, 1850–1864. doi: 10.1101/gr.6597907
vom Saal, F. S., Akingbemi, B. T., Belcher, S. M., Birnbaum, L. S., Crain, D. A., Eriksen, M., et al. (2007). Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24, 131–138. doi: 10.1016/j.reprotox.2007.07.005
Sakurai, M., Miki, Y., Masuda, M., Hata, S., Shibahara, Y., Hirakawa, H., et al. (2012). LIN28: a regulator of tumor-suppressing activity of let-7 microRNA in human breast cancer. J. Steroid Biochem. Mol. Biol. 131, 101–106. doi: 10.1016/j.jsbmb.2011.10.007
Schecter, A., Birnbaum, L., Ryan, J. J., and Constable, J. D. (2006). Dioxins: an overview. Environ. Res. 101, 419–428. doi: 10.1016/j.envres.2005.12.003
Schmutzler, C., Hamann, I., Hofmann, P. J., Kovacs, G., Stemmler, L., Mentrup, B., et al. (2004). Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology 205, 95–102. doi: 10.1016/j.tox.2004.06.041
Segal, M., Avital, A., Berstein, S., Derevenski, A., Sandbank, S., and Weizman, A. (2007). Prolactin and estradiol serum levels in unmedicated male paranoid schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 378–382. doi: 10.1016/j.pnpbp.2006.09.016
Selbach, M., Schwanhäusser, B., Thierfelder, N., Fang, Z., Khanin, R., and Rajewsky, N. (2008). Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63. doi: 10.1038/nature07228
Sen, G. L., and Blau, H. M. (2005). Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–636. doi: 10.1038/ncb1265
Sheth, U., and Parker, R. (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. doi: 10.1126/science.1082320
Sicard, F., Gayral, M., Lulka, H., Buscail, L., and Cordelier, P. (2013). Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. J. Am. Soc. Gene Ther. 21, 986–994. doi: 10.1038/mt.2013.35
Skinner, M. K., Guerrero-Bosagna, C., Haque, M., Nilsson, E., Bhandari, R., and McCarrey, J. R. (2013). Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 8:e66318. doi: 10.1371/journal.pone.0066318
Somm, E., Schwitzgebel, V. M., Toulotte, A., Cederroth, C. R., Combescure, C., Nef, S., et al. (2009). Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 117, 1549–1555. doi: 10.1289/ehp.11342
Song, Y., Chou, E. L., Baecker, A., You, N.-C. Y., Song, Y., Sun, Q., et al. (2016). Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: a systematic review and meta-analysis. J. Diabetes. 8, 516–532. doi: 10.1111/1753-0407.12325
Sontheimer, E. J. (2005). Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell Biol. 6, 127–138. doi: 10.1038/nrm1568
Staessen, J. A., Nawrot, T., Hond, E. D., Thijs, L., Fagard, R., Hoppenbrouwers, K., et al. (2001). Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet Lond. Engl. 357, 1660–1669. doi: 10.1016/S0140-6736(00)04822-4
Stevens, J. R. (2002). Schizophrenia: reproductive hormones and the brain. Am. J. Psychiatry 159, 713–719. doi: 10.1176/appi.ajp.159.5.713
Stouder, C., and Paoloni-Giacobino, A. (2010). Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reprod. Camb. Engl. 139, 373–379. doi: 10.1530/rep-09-0340
Stricklin, S. L., Griffiths-Jones, S., and Eddy, S. R. (2005). C. elegans noncoding RNA genes. WormBook Online Rev. C Elegans Biol, 1–7. doi: 10.1895/wormbook.1.1.1
Sugiura-Ogasawara, M., Ozaki, Y., Sonta, S., Makino, T., and Suzumori, K. (2005). Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. Oxf. Engl. 20, 2325–2329. doi: 10.1093/humrep/deh888
Sweeney, M. F., Hasan, N., Soto, A. M., and Sonnenschein, C. (2015). Environmental endocrine disruptors: effects on the human male reproductive system. Rev. Endocr. Metab. Disord. 16, 341–357. doi: 10.1007/s11154-016-9337-4
Sweeney, M. H., and Mocarelli, P. (2000). Human health effects after exposure to 2,3,7,8-TCDD. Food Addit. Contam. 17, 303–316. doi: 10.1080/026520300283379
Takeuchi, T., Tsutsumi, O., Ikezuki, Y., Kamei, Y., Osuga, Y., Fujiwara, T., et al. (2006). Elevated serum bisphenol A levels under hyperandrogenic conditions may be caused by decreased UDP-glucuronosyltransferase activity. Endocr. J. 53, 485–491. doi: 10.1507/endocrj.K06-032
Takeuchi, T., Tsutsumi, O., Ikezuki, Y., Takai, Y., and Taketani, Y. (2004). Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 51, 165–169. doi: 10.1507/endocrj.51.165
Tilghman, S. L., Bratton, M. R., Segar, H. C., Martin, E. C., Rhodes, L. V., Li, M., et al. (2012). Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS ONE 7:e32754. doi: 10.1371/journal.pone.0032754
Toppari, J., Rodprasert, W., and Koskenniemi, J. J. (2016). Exposure variation and endocrine disruption of the male reproductive system. Horm. Res. Paediatr. 85. doi: 10.1159/000446436. [Epub ahead of print].
van Dijk, E., Cougot, N., Meyer, S., Babajko, S., Wahle, E., and Séraphin, B. (2002). Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 21, 6915–6924. doi: 10.1093/emboj/cdf678
Veiga-Lopez, A., Luense, L. J., Christenson, L. K., and Padmanabhan, V. (2013). Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884. doi: 10.1210/en.2012-2129
Viluksela, M., Raasmaja, A., Lebofsky, M., Stahl, B. U., and Rozman, K. K. (2004). Tissue-specific effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the activity of 5′-deiodinases I and II in rats. Toxicol. Lett. 147, 133–142. doi: 10.1016/j.toxlet.2003.10.025
Vrijens, K., Bollati, V., and Nawrot, T. S. (2015). MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 123, 399–411. doi: 10.1289/ehp.1408459
Wakiyama, M., Takimoto, K., Ohara, O., and Yokoyama, S. (2007). Let-7 microRNA-mediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev. 21, 1857–1862. doi: 10.1101/gad.1566707
Wang, B., Yanez, A., and Novina, C. D. (2008). MicroRNA-repressed mRNAs contain 40S but not 60S components. Proc. Natl. Acad. Sci. U.S.A. 105, 5343–5348. doi: 10.1073/pnas.0801102105
Weiss, B., and Landrigan, P. J. (2000). The developing brain and the environment: an introduction. Environ. Health Perspect. 108(Suppl. 3), 373–374. doi: 10.1289/ehp.00108s3373
WHO | State of the science of endocrine disrupting chemicals (2012). WHO. Available online at: http://www.who.int/ceh/publications/endocrine/en/ (Accessed February 28, 2016).
Wing, L., Yeates, S. R., Brierley, L. M., and Gould, J. (1976). The prevalence of early childhood autism: comparison of administrative and epidemiological studies. Psychol. Med. 6, 89–100. doi: 10.1017/S0033291700007522
Wolstenholme, J. T., Rissman, E. F., and Connelly, J. J. (2011). The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 59, 296–305. doi: 10.1016/j.yhbeh.2010.10.001
Wu, L., Fan, J., and Belasco, J. G. (2006). MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. U.S.A. 103, 4034–4039. doi: 10.1073/pnas.0510928103
Yang, J.-S., Phillips, M. D., Betel, D., Mu, P., Ventura, A., Siepel, A. C., et al. (2011). Widespread regulatory activity of vertebrate microRNA* species. RNA 17, 312–326. doi: 10.1261/rna.2537911
Yi, R., Qin, Y., Macara, I. G., and Cullen, B. R. (2003). Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 17, 3011–3016. doi: 10.1101/gad.1158803
Yoshioka, W., Higashiyama, W., and Tohyama, C. (2011). Involvement of microRNAs in dioxin-induced liver damage in the mouse. Toxicol. Sci. Off. J. Soc. Toxicol. 122, 457–465. doi: 10.1093/toxsci/kfr130
Zhou, R., Chen, F., Feng, X., Zhou, L., Li, Y., and Chen, L. (2015). Perinatal exposure to low-dose of bisphenol A causes anxiety-like alteration in adrenal axis regulation and behaviors of rat offspring: a potential role for metabotropic glutamate 2/3 receptors. J. Psychiatr. Res. 64, 121–129. doi: 10.1016/j.jpsychires.2015.02.018
Keywords: micro-RNA, endocrine disruptors, environment
Citation: Derghal A, Djelloul M, Trouslard J and Mounien L (2016) An Emerging Role of micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 10:318. doi: 10.3389/fnins.2016.00318
Received: 28 February 2016; Accepted: 23 June 2016;
Published: 30 June 2016.
Edited by:
Fumihiko Maekawa, National Institute for Environmental Studies, JapanReviewed by:
Guillaume Gourcerol, Rouen University Hospital, FranceCopyright © 2016 Derghal, Djelloul, Trouslard and Mounien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lourdes Mounien, bG91cmRlcy5tb3VuaWVuQHVuaXYtYW11LmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.