
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurosci. , 20 June 2016
Sec. Auditory Cognitive Neuroscience
Volume 10 - 2016 | https://doi.org/10.3389/fnins.2016.00274
This article is part of the Research Topic The Evolution of Rhythm Cognition: Timing in Music and Speech View all 34 articles
 Andrea Ravignani1,2*
Andrea Ravignani1,2* W. Tecumseh Fitch3†
W. Tecumseh Fitch3† Frederike D. Hanke2†
Frederike D. Hanke2† Tamara Heinrich2†
Tamara Heinrich2† Bettina Hurgitsch4†
Bettina Hurgitsch4† Sonja A. Kotz5,6†
Sonja A. Kotz5,6† Constance Scharff7†
Constance Scharff7† Angela S. Stoeger3†
Angela S. Stoeger3† Bart de Boer1
Bart de Boer1Research on the evolution of human speech and music benefits from hypotheses and data generated in a number of disciplines. The purpose of this article is to illustrate the high relevance of pinniped research for the study of speech, musical rhythm, and their origins, bridging and complementing current research on primates and birds. We briefly discuss speech, vocal learning, and rhythm from an evolutionary and comparative perspective. We review the current state of the art on pinniped communication and behavior relevant to the evolution of human speech and music, showing interesting parallels to hypotheses on rhythmic behavior in early hominids. We suggest future research directions in terms of species to test and empirical data needed.
Humans are particularly vocal and musical animals. They flexibly learn new vocalizations and easily perceive and move to rhythm (Bolton, 1894; Fitch, 2009)1. Why do humans show these two traits that have only been described in relatively few other animals? Previous research led to conflicting hypotheses on how evolution has shaped human brains and physiology to produce complex vocalizations (Richman, 1993; Fitch, 2000; Galantucci et al., 2006; Fitch and Jarvis, 2013; Manson et al., 2013). Several contrasting hypotheses also exist on how and why human and other animals' brains can perceive complex rhythmic patterns (Merker et al., 2009; Honing et al., 2012; Merchant and Honing, 2013; Patel and Iversen, 2014; Ravignani et al., 2014a). Crucially, these hypotheses differ on assumptions about social structure, ecological conditions, and audio-motor abilities present in early hominids, also providing discordant predictions on rhythm and vocal learning skills in different living species (for reviews see Ravignani et al., 2013a, 2014a; Iversen, 2016; Wilson and Cook, 2016). An influential hypothesis in the field, the vocal learning—beat perception and synchronization hypothesis (Patel, 2006), states that vocal production learning (VPL) is a prerequisite for species to be able to extract a pulse from periodic acoustic events (like an internal metronome), and use this inferred pulse to synchronize movements to these external events in a predictive and flexible way (rhythmical entrainment). In fact, neural pathways between auditory and motor areas of the brain, which originally evolved for VPL, would also enable precisely timed movements to sounds (Kuypers, 1958, 1973; Jürgens et al., 1982). Only a few species are capable of VPL: that is, to modify existing vocalizations and to imitate novel sounds not belonging to their innate repertoire (Janik and Slater, 2000; Van Parijs et al., 2003). Humans, bats (Boughman, 1998; Knörnschild et al., 2010; Vernes, 2016), elephants (Poole et al., 2005; Stoeger et al., 2012), seals (Ralls et al., 1985), dolphins (Reiss and McCowan, 1993; Favaro et al., 2016), and whales (Foote et al., 2006), together with many bird species (Marler, 1970; Todt, 1975; Marler and Peters, 1977; Scharff and Nottebohm, 1991), have been shown capable of vocal learning (Schusterman, 2008; Petkov and Jarvis, 2012; Nowicki and Searcy, 2014).
Model species can be used to test hypotheses on how our ancestors evolved the neuropsychological prerequisites underpinning speech and music (see also Vernes, 2016). One can either pick model species, which are closely related to humans, and hence should share a specific trait by common ancestry (homology), or species that have a similar socio-ecology to humans, and hence independently evolved a similar trait by convergent evolution (analogy). If a living animal (i) shares much of its evolutionary history with humans, or (ii) was exposed to environmental conditions and evolutionary pressures similar to early hominids, then commonalities in selected behavioral traits may exist between the two (Fitch, 2010, 2014). This comparative approach is extremely powerful as a way of addressing questions such as (a) how humans acquired complex rhythmic and vocal imitation capacities, (b) why distantly related species but not our closest primate relatives evolved these capacities. Several biological factors may provide an answer to these questions, including brain anatomy, body morphology, social structure, habitat, and ecology. Hence suitable model species to investigate rhythm and VPL in our human lineage should, first and foremost, exhibit rhythm and VPL, and possibly be as close as possible to humans in anatomical, ecological, and evolutionary terms. To test the vocal learning—beat perception and synchronization hypothesis against alternative ones, we suggest below why pinnipeds—including vocal and less vocal species—provide an excellent group of model species.
Traditionally, VPL and rhythmic behavior have been investigated in primates, parrots or songbirds. Monkeys and non-human apes, like chimpanzees, are evolutionarily and cognitively close to humans, but exhibit limited vocal imitation and rhythmic patterning skills (Janik and Slater, 1997; Ravignani et al., 2013a; Repp and Su, 2013; see Gamba et al., 2016, for timing in lemur singing). In contrast, many bird species are excellent at learning to imitatively produce new vocalizations (Petkov and Jarvis, 2012). Moreover, when tested on non-vocal rhythmic tasks requiring precise temporal coordination, birds outperform primates, although direct primate-avian comparisons on identical tasks are lacking at present (Nagasaka et al., 2013; Hoeschele et al., 2015; Benichov et al., 2016; ten Cate et al., 2016). However, the last common ancestor of birds and humans lived about 300 million years ago (Kumar and Hedges, 1998), and birds have evolved a vocal production system (the syrinx) quite different from the human larynx (Fitch, 2010; Elemans et al., 2015). Hence, primates and birds each have only one of the desirable features to understand rhythm and VPL: non-human primates are evolutionary close to humans but exhibit scarce rhythm and VPL capacities, while birds have rhythmic capacities and VPL but are evolutionary distant from humans.
A third taxonomic group, previously overlooked in comparative research on human evolution (cf. Cook et al., 2013; Rouse et al., 2016), may be the solution to this conundrum. Pinnipeds exhibit VPL and rhythmic abilities (Table 1), and as mammals they are evolutionary closer to humans than birds: the last common ancestor of humans and pinnipeds lived about 65 MY ago (O'Leary et al., 2013). This clade includes more than 30 species of semiaquatic mammals divided in three families: Phocidae (e.g., harbor and gray seals), Otariidae (e.g., California sea lions and Cape fur seals), and Odobenidae (walruses). Pinniped phylogeny is controversial. However, recent molecular evidence suggests that the first split, separating Phocidae from other pinnipeds, occurred 33 MY ago (Arnason et al., 2006). This relatively old common origin—compare it with the 33 MY between humans and e.g., capuchin monkeys (Glazko and Nei, 2003), has provided ample time to adapt to many different ecological niches and environmental constraints. Accordingly, pinniped species exhibit variation in VPL capacities, social organization, mating systems, and habitats (Table 1). These dimensions conveniently have anthropological equivalents, each of them deemed crucial for at least one hypothesis on the evolution of speech and music (Fitch, 2000; Hagen and Bryant, 2003; Patel, 2006; Hagen and Hammerstein, 2009; Merker et al., 2009; Petkov and Jarvis, 2012; Merchant and Honing, 2013; Patel and Iversen, 2014; Ravignani, 2014; Ravignani et al., 2014a,b; for a comparative definition of speech).
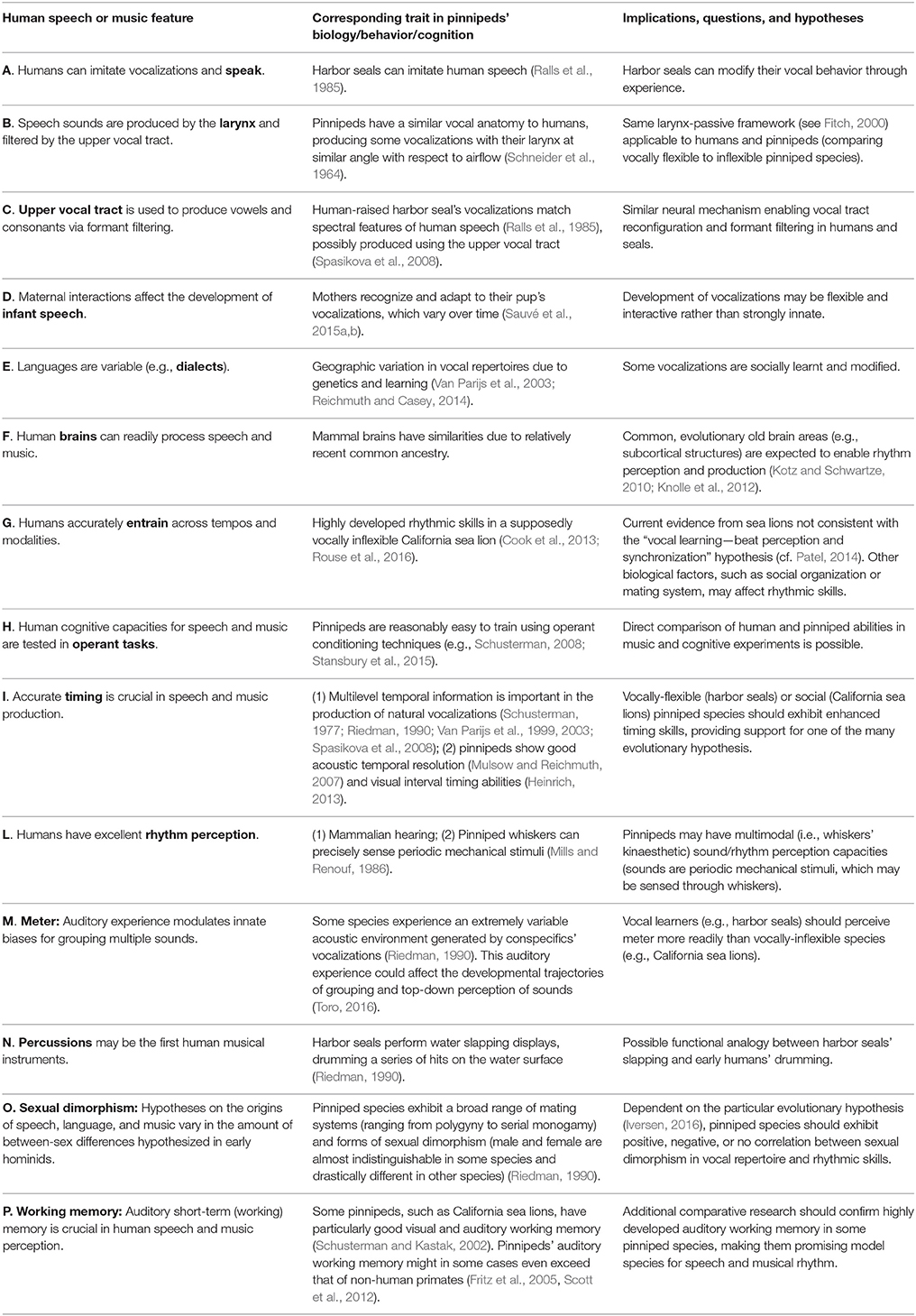
Table 1. Features of human speech and music (first column) are related to findings in pinniped biology (second column) to draw comparative conclusions and suggest further research (third column).
Notably, among the pinnipeds, harbor seals (Phoca vitulina) exhibit an excellent trade-off between VPL abilities and phylogenetic proximity to humans: among vocal learners, harbor seals have the closest vocal apparatus to humans (Schneider, 1962; Schneider et al., 1964; Ralls et al., 1985; Fitch, 2000; Table 1A,B). A human-raised harbor seal has even learned to imitate some human words and phrases (Ralls et al., 1985; Table 1C). So far, harbor seals have not been tested for rhythmic entrainment abilities; however, another pinniped species, the California sea lion (Zalophus californianus) was shown capable of non-vocal audio motor synchronization with precision previously exhibited only by avian species and humans (Cook et al., 2013; Rouse et al., 2016; Table 1G). With these few exceptions, pinniped communication, rhythm, and human speech have mostly remained unconnected areas of research until now. However, a lot of information is available on pinnipeds' natural vocal behavior, making the comparative study of pinniped communication and human speech a field ripe for research. We suggest that pinnipeds are ideal species to understand human speech, rhythm, and complex VPL at different levels (including physiology, behavior, neurobiology, and genetics). Pinnipeds' vocal anatomy, brain evolutionary history, socio-ecology, and broad range of environmental conditions conveniently map to human biology (Schneider, 1962; Ralls et al., 1985; Riedman, 1990; Van Parijs et al., 1999, 2003; Schusterman, 2008; Cook et al., 2013; Sauvé et al., 2015a,b; Table 1).
Then, why do humans and harbor seals produce flexible vocalizations? Taking ultimate and proximate causes into account and adopting a comparative approach (Table 2), we suggest several strands of empirical research in pinnipeds, which can shed light on the evolution of human rhythmicity.
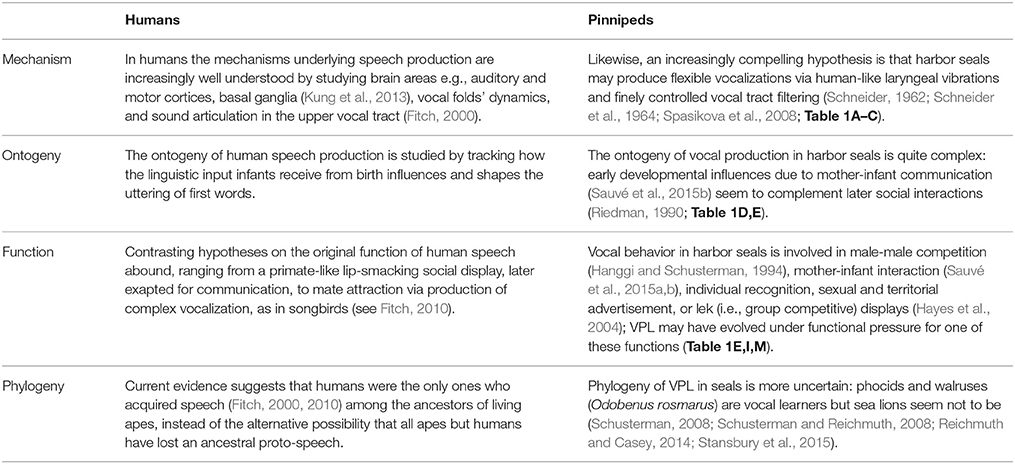
Table 2. The question of why a particular behavioral trait, such as vocal production learning, exists in a species can be answered taking ultimate and proximate causes into account (Tinbergen, 1963).
Pinnipeds produce many types of vocalizations, which can be recorded in air, enabling acoustic data collection with precise individual identification. Research in harbor seals, building on existing evidence on vocal imitation (Ralls et al., 1985), should investigate their ability to learn vocalizations (i) over developmental phases, and (ii) from each other in a social network (Janik and Slater, 2000; Tyack, 2008; Table 1A–F). This will reveal how seal vocalizations are imitated and transformed (Fitch, 2015b) similarly to human speech. In parallel, vocal flexibility in Otariids should be investigated across species, testing their ability to imitate new sounds. This will hopefully provide clear support for or against VPL capacities in this pinniped family considered, until now, the least vocally flexible. While performing this research, it will be important to keep an open-minded attitude toward vocal learning, as this seems to be a graded ability rather than an all-or-none trait (Petkov and Jarvis, 2012; Fitch, 2015a).
Comparative vocal and brain anatomy in pinnipeds can be fruitful strands of research (Table 1B,C,F). The angle of vocal folds with respect to the tracheal air stream is 76° (degrees) in harbor seals, while 17.5° in sea lions (Schneider et al., 1964). This suggests sea lions have a vocal folds' angle closer to elephants (45°); harbor seals' angle instead is closer to humans (90°) than to sea lions (Herbst et al., 2013). Does this difference in vocal anatomy map to a difference in types of sounds produced or just modalities of sound production?
Neuroanatomy may constitute a fruitful research avenue to understand the mechanisms behind successful entrainment in California sea lions. Although the shape of their brain is similar to that of other carnivores, analyses of brain folding show remarkable differences. In particular, California sea lions have more secondary folds and sulci, and a radically different pattern of folds and fissures than other carnivores such as canids, e.g., dogs, wolves, coyotes, and mustelids e.g., minks (Montie et al., 2009). This suggests evolutionary pressures and potentially similar mechanisms increased the size of the neocortex in sea lions showing an interesting parallel to human evolution. A further open question is how the evolution of different brain structures relates to VPL (Patel, 2014) and social organization across pinniped species. Comparative brain anatomy and imaging will elucidate whether evolutionary old brain circuits subserving VPL are still present in vocally inflexible pinnipeds, such as sea lions (Patel, 2014).
Timing experiments often investigate the attentional and cognitive processes involved in perceiving or estimating single time intervals, either independently or by comparison with a second interval (Grondin, 2010). These experiments have, for instance, shown similarities and differences between humans and other primates in estimating single interval durations in the visual and auditory modality (Merchant et al., 2003; Zarco et al., 2009; Mendez et al., 2011). In pinnipeds, recent data show that a harbor seal and a Cape fur seal (Arctocephalus pusillus) can accurately discriminate time intervals in the visual modality (Heinrich, 2013; Table 1I). In contrast, rhythm refers to the structure of multiple durational events, i.e., sequences of time intervals. Hence, single-interval timing research is essential (Merchant and Honing, 2013) though not enough to understand rhythm perception: in fact, perception of one interval influences perception of adjacent intervals (McAuley, 2010). Studying perception, reproduction, and entrainment to isochronous (metronome-like) sequences is the first step when moving from timing to rhythm research. In entrainment experiments, humans and other animals are tested on their ability to synchronize their movements to an external visual or auditory metronomic stimulus. Synchronization can arise spontaneously or be trained by the experimenter. Crucial experimental criteria for successful synchronization are: (i) flexibility, i.e., comparable performance at different tempos, (ii) multimodality i.e., ability to synchronize one's behavior in a sensory modality different from that of the external stimulus, and (iii) predictive rather than reactive behavior, i.e., zero or negative asynchrony, and unperturbed performance when one beat is missing (Patel et al., 2009a,b).
Extending previous entrainment studies in otariids (Cook et al., 2013; Rouse et al., 2016), harbor seals' and walruses' ability to entrain should be tested (Tables 1G,L). Successful synchronization in one of these vocal learners (Reichmuth and Casey, 2014) would provide an important data point in support of the VPL—rhythm link (Patel, 2006). Useful out-groups for synchronization experiments could be non-pinniped Canoidea, like dogs, exhibiting almost no VPL (Janik and Slater, 1997; Taylor et al., 2009). Harbor seals' and walruses' inability to synchronize would not refute Patel's hypothesis. However, failure to synchronize would refute alternative hypotheses, postulating individual territorial advertisement or lek displays as crucial factors for the evolution of rhythm (Hagen and Hammerstein, 2009; Ravignani, 2014).
As flexible synchronization requires the ability to represent an isochronous pulse (Iversen and Balasubramaniam, 2016), pinnipeds should be tested on their ability to discriminate between isochronous and non-isochronous temporal patterns. In birds, the ability to recognize isochronicity in acoustic sequences seems to positively correlate with VPL: pigeons perform much worse (Hagmann and Cook, 2010) than other birds capable of VPL, like zebra finches and starlings (Hulse et al., 1984; van der Aa et al., 2015). If this can be generalized, one would analogously expect harbor seals and walruses tested in comparable setups to outperform e.g., California sea lions and Cape fur seals. Finally, pinniped species naturally showing isochronous vocal behavior may be particularly promising to test in order to ascertain how VPL and natural isochronous behavior affect the ability to entrain. While vocalizations in the vocally inflexible Australian and California sea lions can be quite regular, the vocally flexible harbor seals vocalize with much less temporal regularity (Schusterman, 1977; Charrier et al., 2011).
Meter provides an additional dimension to rhythmic patterns, where individual events in time have different perceptual or acoustic “weights.” Meter is defined as hierarchical organization of temporal events (McAuley, 2010). Meter corresponds to hearing events in time as related, forming structured patterns, e.g., the alternation of weak/strong beats in music and stressed/unstressed syllables in speech (Fabb and Halle, 2012). Meter perception can occur in sequences of stimuli that are acoustically identical (Brochard et al., 2003), or instead based on stimuli that alternate in duration, frequency, or amplitude (McAuley, 2010; Toro and Nespor, 2015; Geambasu et al., 2016; Hoeschele and Fitch, 2016).
Humans can perceive a range of metrical patterns but are biased toward specific metrical grouping patterns, partially depending on their native language (Iversen et al., 2008). In particular, a few perceptual laws, such the iambic-trochaic law (de la Mora et al., 2013), may explain most of rhythmic grouping in speech and music (Figure 1 in Supplementary Material). Rats, for instance, exhibit experience-modulated grouping biases: Like humans, they spontaneously group sequences when sounds alternate in pitch, but do not when sounds alternate in duration (de la Mora et al., 2013). However, rats can learn to group sounds of alternating durations: if exposed to short-long sequences, they will show the corresponding iambic bias when tested; if familiarized with long-short, rats will prefer trochaic grouping (Toro and Nespor, 2015).
Meter perception should be investigated across pinnipeds (Table 1M). As grouping is influenced by auditory experience, we would expect pinnipeds with a varied conspecific auditory input, like harbor seals, to require little training to discriminate metrical patterns. After probing pinnipeds' predictive timing by having them produce behavioral responses, temporal expectations could be explored by directly tapping into perception. Adapting non-invasive electrophysiology originally developed for humans and non-human primates, one could record event-related potentials corresponding to click sounds repeating at a constant rate, and compare these potentials to those evoked by click trains containing missing clicks or metrically-structured (accented) clicks (Rothermich et al., 2010; Schmidt-Kassow et al., 2011; Schwartze et al., 2011; Honing et al., 2012; Selezneva et al., 2013; Celma-Miralles et al., 2016; Cirelli et al., 2016).
Empirical evidence from human archeology, ethnomusicology and African apes' behavior suggest that percussion may have been the first form of musical expression in our hominid ancestors (Arcadi et al., 1998; Morley, 2003; Fitch, 2009). What was the function of rhythmic drumming in early hominids? A behavioral display in harbor seals may help answer this question: Accompanying vocalizations, harbor seals “drum” on the water, repeatedly slapping their flippers on the sea surface (Riedman, 1990; Wahlberg et al., 2002). Once again, hypotheses on the function of this slapping behavior mimic hypotheses proposed for human drumming (e.g., Kirschner and Tomasello, 2009). Slapping in harbor seals may function as signal in agonistic sexual displays (Riedman, 1990), or as a form of intrasexual competition to attract females (Nikolich, 2015). Another hypothesis regards water drumming as a form of territorial advertisement in agonistic contexts: in fact, during the breeding season, male seals produce slaps in response to other males either intruding a territory, or challenging an intruder (Hayes et al., 2004). Water slapping may hence indirectly play a role in establishing and maintaining dominance hierarchies, similar to chimpanzees' drumming (Arcadi et al., 1998; Ravignani et al., 2013b).
One hypothesis we suggest is that vocal displays and drumming displays may have the same territorial function but be used complementarily. Seals' slaps cover a different frequency band than, and have dramatically different durations from, roars. Slaps last about 0.002 s, contain most frequency between 5 and 20 kHz, and have (in-water) source intensity of 166–199 dB (Wahlberg et al., 2002). In contrast, roars last 2–3 s, are centered at frequencies of 200–300 Hz and have 150 dB intensity (Hayes et al., 2004). How far can each of these sounds travel so that they are still audible by seals? At 200 Hz, seals' hearing threshold is 32 dB (82 dB underwater); the sensitivity is much higher between 5 and 20 kHz, reaching 1–29 dB (60–62 dB in water; Reichmuth et al., 2013). Hence (1) slaps carry much farther than roars, (2) even if a slap and a roar reach a seal with the same sound intensity, a slap will be more conspicuous: slap might be perceived up to 30 times louder than a roar, and (3) slaps could be in principle perceived visually (Nikolich, 2015). Seals' water slaps hence seem to mimic many features of early human's territorial advertisement, which have been hypothesized to underlie the evolution of human musicality (Hagen and Hammerstein, 2009).
Future research should record individuals over time to: (i) analyse the fine-grained temporal structure of series of slaps (Babiszewska et al., 2015); (ii) test whether drumming and its temporal parameters are socially learnt, and if so (iii) compare the social dynamics of two transmitted rhythmic behaviors, across modalities (vocalizations vs. slapping), and (iv) relate water-slapping to similar percussive behaviors present in humans and chimpanzees (Fuhrmann et al., 2014; Whiten, 2015; Table 1N). Collection of slapping data will enable to test hypotheses postulating group and mating displays as necessary evolutionary steps toward human musicality (Fitch, 2009; Merker et al., 2009). In fact, if harbor seals' slaps show strong temporal interdependence between individuals, successful entrainment experiments in this species would support the hypothesis that rhythm may have evolved in humans as by-product of temporally-intertwined group displays (Merker et al., 2009).
Researchers of human evolution and pinniped communication have been suggesting, unbeknownst to each other, similar hypotheses for the evolution of human speech and music, on the one hand, and pinnipeds' vocal displays and non-vocal communication, on the other hand. Advocating the comparative method and the distinction between proximate and ultimate questions, we have shown how animal research can help formulate and test hypotheses about the evolution of human speech and music. We have briefly reviewed previous findings in pinniped biology, explicitly pointing out their relevance to the human sense of rhythm in music and speech. We have discussed crucial questions that pinniped research should address empirically, possibly using comparable stimuli, tasks, and analysis techniques across species, ultimately shedding light on the origins of rhythmic behaviors in humans.
Andrea Ravignani wrote the manuscript. All authors provided ideas and edited the manuscript.
Andrea Ravignani was supported by FWO grant V439315N (to Andrea Ravignani), and European Research Council grant 283435 ABACUS (to Bart de Boer).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AR is grateful to Peter Cook, Guido Dehnhardt, Maxime Garcia, Alina Gaugg, John Iversen, Vincent Janik, Lars Miersch, Benedikt Niesterok, Ana Rubio Garcia, Ruth Sonnweber, Amanda Stansbury, and Sonja Vernes for helpful discussions, comments, and insights.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnins.2016.00274
1. ^Rhythm is defined as a “serial pattern of durations marked by a series of events” (McAuley, 2010. pp. 166).
Arcadi, A. C., Robert, D., and Boesch, C. (1998). Buttress drumming by wild chimpanzees: temporal patterning, phrase integration into loud calls, and preliminary evidence for individual distinctiveness. Primates 39, 505–518. doi: 10.1007/BF02557572
Arnason, U., Gullberg, A., Janke, A., Kullberg, M., Lehman, N., Petrov, E. A., et al. (2006). Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol. Phylogenet. Evol. 41, 345–354. doi: 10.1016/j.ympev.2006.05.022
Babiszewska, M., Schel, A. M., Wilke, C., and Slocombe, K. E. (2015). Social, contextual, and individual factors affecting the occurrence and acoustic structure of drumming bouts in wild chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 156, 125–134. doi: 10.1002/ajpa.22634
Benichov, J. I., Globerson, E., and Tchernichovski, O. (2016). Finding the beat: from socially coordinated vocalizations in songbirds to rhythmic entrainment in humans. Front. Hum. Neurosci. 10:255. doi: 10.3389/fnhum.2016.00255
Boughman, J. W. (1998). Vocal learning by greater spear-nosed bats. Proc. Biol. Sci. 265, 227–233. doi: 10.1098/rspb.1998.0286
Brochard, R., Abecasis, D., Potter, D., Ragot, R., and Drake, C. (2003). The “Ticktock” of our internal clock: direct brain evidence of subjective accents in isochronous sequences. Psychol. Sci. 14, 362–366. doi: 10.1111/1467-9280.24441
Celma-Miralles, A., de Menezes, R. F., and Toro, J. M. (2016). Look at the beat, feel the meter: top-down effects of meter induction on auditory and visual modalities. Front. Hum. Neurosci. 10:108. doi: 10.3389/fnhum.2016.00108
Charrier, I., Ahonen, H., and Harcourt, R. G. (2011). What makes an Australian sea lion (Neophoca cinerea) male's bark threatening? J. Comp. Psychol. 125, 385. doi: 10.1037/a0024513
Cirelli, L. K., Spinelli, C., Nozaradan, S., and Trainor, L. J. (2016). Measuring neural entrainment to beat and meter in infants: effects of music background. Front. Neurosci. 10:229. doi: 10.3389/fnins.2016.00229
Cook, P., Rouse, A., Wilson, M., and Reichmuth, C. J. (2013). A California sea lion (Zalophus californianus) can keep the beat: motor entrainment to rhythmic auditory stimuli in a non vocal mimic. J. Comp. Psychol. 127, 1–16. doi: 10.1037/a0032345
de la Mora, D. M., Nespor, M., and Toro, J. M. (2013). Do humans and nonhuman animals share the grouping principles of the iambic-trochaic law? Atten. Percept. Psychophys. 75, 92–100. doi: 10.3758/s13414-012-0371-3
Elemans, C., Rasmussen, J. H., Herbst, C. T., Döring, D. N., Zollinger, S. A., Brumm, H., et al. (2015). Universal mechanisms of sound production and control in birds and mammals. Nat. commun. 6:8978. doi: 10.1038/ncomms9978
Fabb, N., and Halle, M. (2012). “Grouping in the stressing of words, in metrical verse, and in music,” in Language and Music as Cognitive Systems, eds P. Rebuschat, M. Rohmeier, J. A. Hawkins, and I. Cross (Oxford, UK: Oxford University Press), 4–21.
Favaro, L., Neves, S., Furlati, S., Pessani, D., Martin, V., and Janik, V. M. (2016). Evidence suggests vocal production learning in a cross-fostered Risso's dolphin (Grampus griseus). Animal Cogn. 19, 847–853. doi: 10.1007/s10071-016-0961-x
Fitch, W. T. (2000). The evolution of speech: a comparative review. Trends Cogn. Sci. 4, 258–267. doi: 10.1016/S1364-6613(00)01494-7
Fitch, W. T. (2009). “The biology and evolution of rhythm: unraveling a paradox,” in Language and Music as Cognitive Systems, eds P. Rebuschat, M. Rohrmeier, J. A. Hawkins, and I. Cross (Oxford, UK: Oxford University Press), 73–95.
Fitch, W. T. (2014). Toward a computational framework for cognitive biology: unifying approaches from cognitive neuroscience and comparative cognition. Phys. Life Rev. 11, 329–364. doi: 10.1016/j.plrev.2014.04.005
Fitch, W. T. (2015a). “The biology and evolution of musical rhythm: an update,” in Structures in the Mind: Essays on Language, Music, and Cognition in Honor of Ray Jackendoff, eds I. Toivonen, P. Csúri, and E. van der Zee (Cambridge, MA: MIT Press), 293–324.
Fitch, W. T. (2015b). Four principles of bio-musicology. Philos. Trans R. Soc. B: Biol. Sci. 370:20140091. doi: 10.1098/rstb.2014.0091
Fitch, W. T., and Jarvis, E. D. (2013). “Birdsong and other animal models for human speech, song, and vocal learning,” in Language, Music, and the Brain: a Mysterious Relationship, ed M. A. Arbib (Cambridge, MA: MIT Press), 499–539.
Foote, A. D., Griffin, R. M., Howitt, D., Larsson, L., Miller, P. J., and Hoelzel, A. R. (2006). Killer whales are capable of vocal learning. Biol. Lett. 2, 509–512. doi: 10.1098/rsbl.2006.0525
Fritz, J., Mishkin, M., and Saunders, R. C. (2005). In search of an auditory engram. Proc. Natl. Acad. Sci. U.S.A. 102, 9359–9364. doi: 10.1073/pnas.0503998102
Fuhrmann, D., Ravignani, A., Marshall-Pescini, S., and Whiten, A. (2014). Synchrony and motor mimicking in chimpanzee observational learning. Sci. Rep. 4, 1–7. doi: 10.1038/srep05283
Galantucci, B., Fowler, C. A., and Turvey, M. T. (2006). The motor theory of speech perception reviewed. Psychon. Bull. Rev. 13, 361–377. doi: 10.3758/BF03193857
Gamba, M., Torti, V., Estienne, V., Randrianarison, R., Valente, D., Rovara, P., et al. (2016). The indris have got rhythm! Timing and pitch variation of a primate song examined between sexes and age classes. Front. Neurosci. 10:249. doi: 10.3389/fnins.2016.00249
Geambasu, A., Ravignani, A., and Levelt, C. C. (2016). Preliminary experiments on human sensitivity to rhythmic structure in a grammar with recursive self-similarity. Front. Neurosci. 10:281. doi: 10.3389/fnins.2016.00281
Glazko, G. V., and Nei, M. (2003). Estimation of divergence times for major lineages of primate species. Mol. Biol. Evol. 20, 424–434. doi: 10.1093/molbev/msg050
Grondin, S. (2010). Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Atten. Percept. Psychophys. 72, 561–582. doi: 10.3758/APP.72.3.561
Hagen, E. H., and Bryant, G. A. (2003). Music and dance as a coalition signaling system. Human Nat. 14, 21–51. doi: 10.1007/s12110-003-1015-z
Hagen, E. H., and Hammerstein, P. (2009). Did Neanderthals and other early humans sing? seeking the biological roots of music in the territorial advertisements of primates, lions, hyenas, and wolves. Musicae Sci. 13, 291–320. doi: 10.1177/1029864909013002131
Hagmann, C. E., and Cook, R. G. (2010). Testing meter, rhythm, and tempo discriminations in pigeons. Behav. Processes 85, 99–110. doi: 10.1016/j.beproc.2010.06.015
Hanggi, E. B., and Schusterman, R. J. (1994). Underwater acoustic displays and individual variation in male harbour seals, Phoca vitulina. Anim. Behav. 48, 1275–1283. doi: 10.1006/anbe.1994.1363
Hayes, S. A., Kumar, A., Costa, D. P., Mellinger, D. K., Harvey, J. T., Southall, B. L., et al. (2004). Evaluating the function of the male harbour seal, Phoca vitulina, roar through playback experiments. Anim. Behav. 67, 1133–1139. doi: 10.1016/j.anbehav.2003.06.019
Herbst, C. T., Svec, J. G., Lohscheller, J., Frey, R., Gumpenberger, M., Stoeger, A. S., et al. (2013). Complex vibratory patterns in an elephant larynx. J. Exp. Biol. 216, 4054–4064. doi: 10.1242/jeb.091009
Hoeschele, M., and Fitch, W. T. (2016). Phonological perception by birds: budgerigars can perceive lexical stress. Anim. Cogn. 19, 643–654. doi: 10.1007/s10071-016-0968-3
Hoeschele, M., Merchant, H., Kikuchi, Y., Hattori, Y., and ten Cate, C. (2015). Searching for the origins of musicality across species. Philos. Trans. R. Soc. B: Biol. Sci. 370:20140094. doi: 10.1098/rstb.2014.0094
Honing, H., Merchant, H., Háden, G. P., Prado, L., and Bartolo, R. (2012). Rhesus monkeys (Macaca mulatta) detect rhythmic groups in music, but not the beat. PLoS ONE 7:e51369. doi: 10.1371/journal.pone.0051369
Hulse, S. H., Humpal, J., and Cynx, J. (1984). Discrimination and generalization of rhythmic and arrhythmic sound patterns by european starlings (Sturnus vulgaris). Music Percept. 1, 442–446. doi: 10.2307/40285272
Iversen, J. R. (2016). “In the beginning was the beat: evolutionary origins of musical rhythm in humans,” in The Cambridge Companion to Percussion, ed R. Hartenberger (Cambridge, UK: Cambridge University Press), 281–295. doi: 10.1017/CBO9781316145074.022
Iversen, J. R., and Balasubramaniam, R. (2016). Synchronization and temporal processing. Curr. Opin. Behav. Sci. 8, 175–180. doi: 10.1016/j.cobeha.2016.02.027
Iversen, J. R., Patel, A. D., and Ohgushi, K. (2008). Perception of rhythmic grouping depends on auditory experience. J. Acoust. Soc. Am. 124, 2263–2271. doi: 10.1121/1.2973189
Janik, V. M., and Slater, P. B. (1997). Vocal learning in mammals. Adv. Study Behav. 26, 59–99. doi: 10.1016/S0065-3454(08)60377-0
Janik, V. M., and Slater, P. J. B. (2000). The different roles of social learning in vocal communication. Anim. Behav. 60, 1–11. doi: 10.1006/anbe.2000.1410
Jürgens, U., Kirzinger, A., and Von Cramon, D. (1982). The effects of deep-reaching lesions in the cortical face area on phonation a combined case report and experimental monkey study. Cortex 18, 125–139. doi: 10.1016/S0010-9452(82)80024-5
Kirschner, S., and Tomasello, M. (2009). Joint drumming: social context facilitates synchronization in preschool children. J. Exp. Child Psychol. 102, 299–314. doi: 10.1016/j.jecp.2008.07.005
Knolle, F., Schröger, E., Baess, P., and Kotz, S. A. (2012). The cerebellum generates motor-to-auditory predictions: ERP lesion evidence. J. Cogn. Neurosci. 24, 698–706. doi: 10.1162/jocn_a_00167
Knörnschild, M., Nagy, M., Metz, M., Mayer, F., and Von Helversen, O. (2010). Complex vocal imitation during ontogeny in a bat. Biol. Lett. 6, 156–159. doi: 10.1098/rsbl.2009.0685
Kotz, S. A., and Schwartze, M. (2010). Cortical speech processing unplugged: a timely subcortico-cortical framework. Trends Cogn. Sci. 14, 392–399. doi: 10.1016/j.tics.2010.06.005
Kumar, S., and Hedges, S. B. (1998). A molecular timescale for vertebrate evolution. Nature 392, 917–920. doi: 10.1038/31927
Kung, S.-J., Chen, J. L., Zatorre, R. J., and Penhune, V. B. (2013). Interacting cortical and basal ganglia networks underlying finding and tapping to the musical beat. J. Cogn. Neurosci. 25, 401–420. doi: 10.1162/jocn_a_00325
Kuypers, H. (1958). Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. J. Comp. Neurol. 110, 221–255. doi: 10.1002/cne.901100205
Kuypers, H. (1973). The anatomical organization of the descending pathways and their contributions to motor control especially in primates. Hum. Reflexes Pathophysiol. Mot. Syst. Methodol. Hum. Reflexes 3, 38–68. doi: 10.1159/000394127
Manson, J. H., Bryant, G. A., Gervais, M. M., and Kline, M. A. (2013). Convergence of speech rate in conversation predicts cooperation. Evol. Hum. Behav. 34, 419–426. doi: 10.1016/j.evolhumbehav.2013.08.001
Marler, P. (1970). A comparative approach to vocal learning: song development in white-crowned sparrows. J. Comp. Physiol. Psychol. 71, 1. doi: 10.1037/h0029144
Marler, P., and Peters, S. (1977). Selective vocal learning in a sparrow. Science 198, 519–521. doi: 10.1126/science.198.4316.519
McAuley, J. D. (2010). “Tempo and rhythm,” in Springer Handbook of Auditory Research, Vol. 36: Music Perception, eds M. R. Jones, R. R. Fay, and A. N. Popper (New York, NY: Springer), 165–199.
Mendez, J. C., Prado, L., Mendoza, G., and Merchant, H. (2011). Temporal and spatial categorization in human and non-human primates. Front. Integr. Neurosci. 5:50. doi: 10.3389/fnint.2011.00050
Merchant, H., Battaglia-Mayer, A., and Georgopoulos, A. P. (2003). Interception of real and apparent motion targets: psychophysics in humans and monkeys. Exp. Brain Res. 152, 106–112. doi: 10.1007/s00221-003-1514-5
Merchant, H., and Honing, H. (2013). Are non-human primates capable of rhythmic entrainment? Evidence for the gradual audiomotor evolution hypothesis. Front. Neurosci. 7:27. doi: 10.3389/fnins.2013.0027
Merker, B., Madison, G. S., and Eckerdal, P. (2009). On the role and origin of isochrony in human rhythmic entrainment. Cortex 45, 4–17. doi: 10.1016/j.cortex.2008.06.011
Mills, F. H., and Renouf, D. (1986). Determination of the vibration sensitivity of harbour seal Phoca vitulina (L.) vibrissae. J. Exp. Mar. Biol. Ecol. 100, 3–9. doi: 10.1016/0022-0981(86)90151-6
Montie, E. W., Pussini, N., Schneider, G. E., Battey, T. W., Dennison, S., Barakos, J., et al. (2009). Neuroanatomy and volumes of brain structures of a live California sea lion (Zalophus californianus) from magnetic resonance images. Anat. Rec. 292, 1523–1547. doi: 10.1002/ar.20937
Morley, I. (2003). The Evolutionary Origins and Archaeology of Music. Ph.D. thesis, Darwin College, Cambridge University.
Mulsow, J., and Reichmuth, C. (2007). Electrophysiological assessment of temporal resolution in pinnipeds. Aquat. Mammals 33:122. doi: 10.1578/AM.33.1.2007.122
Nagasaka, Y., Chao, Z. C., Hasegawa, N., Notoya, T., and Fujii, N. (2013). Spontaneous synchronization of arm motion between Japanese macaques. Sci. Rep. 3:1151. doi: 10.1038/srep01151
Nikolich, K. A. (2015). The Vocal Breeding Behaviour of Harbour Seals (Phoca vitulina) in Georgia Strait, Canada: Temporal Patterns and Vocal Repertoire. Western Washington University Masters Thesis Collection.
Nowicki, S., and Searcy, W. A. (2014). The evolution of vocal learning. Curr. Opin. Neurobiol. 28, 48–53. doi: 10.1016/j.conb.2014.06.007
O'Leary, M. A., Bloch, J. I., Flynn, J. J., Gaudin, T. J., Giallombardo, A., Giannini, N. P., et al. (2013). The placental mammal ancestor and the post–K-Pg radiation of placentals. Science 339, 662–667. doi: 10.1126/science.1229237
Patel, A. D. (2006). Musical rhythm, linguistic rhythm, and human evolution. Music Percept. 24, 99–104. doi: 10.1525/mp.2006.24.1.99
Patel, A. D. (2014). The evolutionary biology of musical rhythm: was darwin wrong? PLoS Biol. 12:e1001821. doi: 10.1371/journal.pbio.1001821
Patel, A. D., and Iversen, J. R. (2014). The evolutionary neuroscience of musical beat perception: the Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 8:57. doi: 10.3389/fnsys.2014.00057
Patel, A. D., Iversen, J. R., Bregman, M. R., and Schulz, I. (2009a). Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr. Biol. 19, 827–830. doi: 10.1016/j.cub.2009.03.038
Patel, A. D., Iversen, J. R., Bregman, M. R., and Schulz, I. (2009b). Studying synchronization to a musical beat in nonhuman animals. Ann. N.Y. Acad. Sci. 1169, 459–469. doi: 10.1111/j.1749-6632.2009.04581.x
Petkov, C. I., and Jarvis, E. D. (2012). Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 4:12. doi: 10.3389/fnevo.2012.00012
Poole, J. H., Tyack, P. L., Stoeger-Horwath, A. S., and Watwood, S. (2005). Animal behaviour: elephants are capable of vocal learning. Nature 434, 455–456. doi: 10.1038/434455a
Ralls, K., Fiorelli, P., and Gish, S. (1985). Vocalizations and vocal mimicry in captive harbor seals, Phoca vitulina. Can. J. Zool. 63, 1050–1056. doi: 10.1139/z85-157
Ravignani, A. (2014). Chronometry for the chorusing herd: Hamilton's legacy on context-dependent acoustic signalling-a comment on Herbers (2013). Biol. Lett. 10:20131018. doi: 10.1098/rsbl.2013.1018
Ravignani, A., Bowling, D. L., and Fitch, W. T. (2014a). Chorusing, synchrony and the evolutionary functions of rhythm. Front. Psychol. 5:1118. doi: 10.3389/fpsyg.2014.01118
Ravignani, A., Gingras, B., Asano, R., Sonnweber, R., Matellán, V., and Fitch, W. T. (2013a). “The evolution of rhythmic cognition: new perspectives and technologies in comparative research,” in Proceedings of the 35th Annual Conference of the Cognitive Science Society, eds M. P. M. Knauff, N. Sebanz, and I. Wachsmuth (Berlin: Cognitive Science Society).
Ravignani, A., Martins, M., and Fitch, W. (2014b). Vocal learning, prosody, and basal ganglia: don't underestimate their complexity. Behav. Brain Sci. 37, 570–571. doi: 10.1017/S0140525X13004184
Ravignani, A., Olivera, V. M., Gingras, B., Hofer, R., Hernández, C. R., Sonnweber, R.-S., et al. (2013b). Primate drum kit: a system for studying acoustic pattern production by non-human primates using acceleration and strain sensors. Sensors 13, 9790–9820. doi: 10.3390/s130809790
Reichmuth, C., and Casey, C. (2014). Vocal learning in seals, sea lions, and walruses. Curr. Opin. Neurobiol. 28, 66–71. doi: 10.1016/j.conb.2014.06.011
Reichmuth, C., Holt, M. M., Mulsow, J., Sills, J. M., and Southall, B. L. (2013). Comparative assessment of amphibious hearing in pinnipeds. J. Comp. Physiol. A 199, 491–507. doi: 10.1007/s00359-013-0813-y
Reiss, D., and McCowan, B. (1993). Spontaneous vocal mimicry and production by bottlenose dolphins (Tursiops truncatus): evidence for vocal learning. J. Comp. Psychol. 107:301. doi: 10.1037/0735-7036.107.3.301
Repp, B. H., and Su, Y.-H. (2013). Sensorimotor synchronization: a review of recent research (2006-2012). Psychon. Bull. Rev. 20, 403–452. doi: 10.3758/s13423-012-0371-2
Richman, B. (1993). On the evolution of speech: singing as the middle term. Curr. Anthropol. 34, 721–722. doi: 10.1086/204217
Riedman, M. (1990). The Pinnipeds: Seals, Sea Lions, and Walruses. Berkeley, CA: University of California Press.
Rothermich, K., Schmidt-Kassow, M., Schwartze, M., and Kotz, S. A. (2010). Event-related potential responses to metric violations: rules versus meaning. Neuroreport 21, 580–584. doi: 10.1097/WNR.0b013e32833a7da7
Rouse, A. A., Cook, P. F., Large, E. W., and Reichmuth, C. (2016). Beat keeping in a sea lion as coupled oscillation: implications for comparative understanding of human rhythm. Front. Neurosci. 10:257. doi: 10.3389/fnins.2016.00257
Sauvé, C. C., Beauplet, G., Hammill, M. O., and Charrier, I. (2015a). Acoustic analysis of airborne, underwater, and amphibious mother attraction calls by wild harbor seal pups (Phoca vitulina). J. Mammal 96, 591–602. doi: 10.1093/jmammal/gyv064
Sauvé, C. C., Beauplet, G., Hammill, M. O., and Charrier, I. (2015b). Mother-pup vocal recognition in harbour seals: influence of maternal behaviour, pup voice and habitat sound properties. Anim. Behav. 105, 109–120. doi: 10.1016/j.anbehav.2015.04.011
Scharff, C., and Nottebohm, F. (1991). A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 11, 2896–2913.
Schmidt-Kassow, M., Rothermich, K., Schwartze, M., and Kotz, S. A. (2011). Did you get the beat? late proficient French-German learners extract strong-weak patterns in tonal but not in linguistic sequences. Neuroimage 54, 568–576. doi: 10.1016/j.neuroimage.2010.07.062
Schneider, R. (1962). Vergleichende Untersuchungen am Kehlkopf der Robben (Mammalia, Carnivora, Pinnipedia). Morph. Jb. 103, 177–262.
Schneider, R., Krumbach, T., and Kükenthal, W. G. (1964). Der Larynx der Säugetiere. Berlin: de Gruyter.
Schusterman, R. J. (1977). Temporal patterning in sea lion barking (Zalophus californianus). Behav. Biol. 20, 404–408. doi: 10.1016/S0091-6773(77)90964-6
Schusterman, R. J. (2008). “Vocal learning in mammals with special emphasis on pinnipeds,” in The Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication, eds D. K. Oller and U. Griebel (Cambridge, MA: MIT Press), 41–70.
Schusterman, R. J., and Kastak, D. (2002). “Problem solving and memory,” in Marine Mammal Biology: An Evolutionary Approach, ed A. R. Hoelzel (Oxford: Blackwell Publishing), 388–415.
Schusterman, R. J., and Reichmuth, C. (2008). Novel sound production through contingency learning in the Pacific walrus (Odobenus rosmarus divergens). Anim. Cogn. 11, 319–327. doi: 10.1007/s10071-007-0120-5
Schwartze, M., Rothermich, K., Schmidt-Kassow, M., and Kotz, S. A. (2011). Temporal regularity effects on pre-attentive and attentive processing of deviance. Biol. Psychol. 87, 146–151. doi: 10.1016/j.biopsycho.2011.02.021
Scott, B. H., Mishkin, M., and Yin, P. (2012). Monkeys have a limited form of short-term memory in audition. Proc. Natl. Acad. Sci. U.S.A. 109, 12237–12241. doi: 10.1073/pnas.1209685109
Selezneva, E., Deike, S., Knyazeva, S., Scheich, H., Brechmann, A., and Brosch, M. (2013). Rhythm sensitivity in macaque monkeys. Front. Syst. Neurosci. 7:49. doi: 10.3389/fnsys.2013.00049
Spasikova, M., Fitch, W. T., Reichmuth, C., and Schusterman, R. J. (2008). Acoustic production mechanisms in pinnipeds (conference abstract). J. Acoust. Soc. Am. 123, 3771. doi: 10.1121/1.2935383
Stansbury, A. L., De Freitas, M., Wu, G.-M., and Janik, V. M. (2015). Can a gray seal (Halichoerus grypus) generalize call classes? J. Comp. Psychol. 129, 412. doi: 10.1037/a0039756
Stoeger, A. S., Mietchen, D., Oh, S., De Silva, S., Herbst, C. T., Kwon, S., et al. (2012). An Asian elephant imitates human speech. Curr. Biol. 22, 2144–2148. doi: 10.1016/j.cub.2012.09.022
Taylor, A. M., Reby, D., and McComb, K. (2009). Context-related variation in the vocal growling behaviour of the domestic dog (Canis familiaris). Ethology 115, 905–915. doi: 10.1111/j.1439-0310.2009.01681.x
ten Cate, C., Spierings, M., Hubert, J., and Honing, H. (2016). Can birds perceive rhythmic patterns? A review and experiments on a songbird and a parrot species. Front. Psychol. 7:730. doi: 10.3389/fpsyg.2016.00730
Tinbergen, N. (1963). On aims and methods of ethology. Zeitschrift Tierpsychol. 20, 410–433. doi: 10.1111/j.1439-0310.1963.tb01161.x
Todt, D. (1975). Social learning of vocal patterns and modes of their application in grey parrots (Psittacus erithacus). Zeitschrift Tierpsychol. 39, 178–188. doi: 10.1111/j.1439-0310.1975.tb00907.x
Toro, J. M. (2016). Something Old, something new combining mechanisms during language acquisition. Curr. Dir. Psychol. Sci. 25, 130–134. doi: 10.1177/0963721416629645
Toro, J. M., and Nespor, M. (2015). Experience-dependent emergence of a grouping bias. Biol. Lett. 11, 20150374. doi: 10.1098/rsbl.2015.0374
Tyack, P. L. (2008). Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J. Comp. Psychol. 122, 319. doi: 10.1037/a0013087
van der Aa, J., Honing, H., and ten Cate, C. (2015). The perception of regularity in an isochronous stimulus in zebra finches (Taeniopygia guttata) and humans. Behav. Processes 115, 37–45. doi: 10.1016/j.beproc.2015.02.018
Van Parijs, S. M., Corkeron, P. J., Harvey, J., Hayes, S. A., Mellinger, D. K., Rouget, P. A., et al. (2003). Patterns in the vocalizations of male harbor seals. J. Acoust. Soc. Am. 113, 3403–3410. doi: 10.1121/1.1568943
Van Parijs, S. M., Hastie, G. D., and Thompson, P. M. (1999). Geographical variation in temporal and spatial vocalization patterns of male harbour seals in the mating season. Anim. Behav. 58, 1231–1239. doi: 10.1006/anbe.1999.1258
Wahlberg, M., Lunneryd, S.-G., and Westerberg, H. (2002). The source level of harbour seal flipper slaps. Aquat. Mammals 28, 90–92.
Whiten, A. (2015). Experimental studies illuminate the cultural transmission of percussive technologies in Homo and Pan. Philos. Trans. R. Soc. B 370:20140359. doi: 10.1098/rstb.2014.0359
Wilson, M., and Cook, P. F. (2016). Rhythmic entrainment: Why humans want to, fireflies can't help it, pet birds try, and sea lions have to be bribed. Psychon. Bull. Rev. doi: 10.3758/s13423-016-1013-x. [Epub ahead of print].
Keywords: evolution of speech, evolution of music, evolution of language, vocal learning, entrainment, timing, synchronization, seal
Citation: Ravignani A, Fitch WT, Hanke FD, Heinrich T, Hurgitsch B, Kotz SA, Scharff C, Stoeger AS and de Boer B (2016) What Pinnipeds Have to Say about Human Speech, Music, and the Evolution of Rhythm. Front. Neurosci. 10:274. doi: 10.3389/fnins.2016.00274
Received: 04 March 2016; Accepted: 31 May 2016;
Published: 20 June 2016.
Edited by:
Virginia Penhune, Concordia University, CanadaReviewed by:
Hugo Merchant, Universidad Nacional Autónoma de México, MexicoCopyright © 2016 Ravignani, Fitch, Hanke, Heinrich, Hurgitsch, Kotz, Scharff, Stoeger and de Boer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Ravignani, YW5kcmVhLnJhdmlnbmFuaUBnbWFpbC5jb20=
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.