- 1Department of Biomedical Sciences, University of Cagliari, Cagliari, Italy
- 2Advisory and Steering Group, URITo.N. - Unit for Research and Innovation on Forensic Toxicology, Neuroscience of Addiction and New Drugs. FT-DSS University of Florence, Florence, Italy
- 3Department of Diagnostic and Public Health, University of Verona, Verona, Italy
- 4Departmentof Pharmaceutical and Pharmacological Sciences, University of Padua, Padua, Italy
- 5Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy
New psychoactive substances (NPS) are a heterogeneous and rapidly evolving class of molecules available on the global illicit drug market (e.g smart shops, internet, “dark net”) as a substitute for controlled substances. The use of NPS, mainly consumed along with other drugs of abuse and/or alcohol, has resulted in a significantly growing number of mortality and emergency admissions for overdoses, as reported by several poison centers from all over the world. The fact that the number of NPS have more than doubled over the last 10 years, is a critical challenge to governments, the scientific community, and civil society [EMCDDA (European Drug Report), 2014; UNODC, 2014b; Trends and developments]. The chemical structure (phenethylamines, piperazines, cathinones, tryptamines, synthetic cannabinoids) of NPS and their pharmacological and clinical effects (hallucinogenic, anesthetic, dissociative, depressant) help classify them into different categories. In the recent past, 50% of newly identified NPS have been classified as synthetic cannabinoids followed by new phenethylamines (17%) (UNODC, 2014b). Besides peripheral toxicological effects, many NPS seem to have addictive properties. Behavioral, neurochemical, and electrophysiological evidence can help in detecting them. This manuscript will review existing literature about the addictive and rewarding properties of the most popular NPS classes: cannabimimetics (JWH, HU, CP series) and amphetamine-like stimulants (amphetamine, methamphetamine, methcathinone, and MDMA analogs). Moreover, the review will include recent data from our lab which links JWH-018, a CB1 and CB2 agonist more potent than Δ9-THC, to other cannabinoids with known abuse potential, and to other classes of abused drugs that increase dopamine signaling in the Nucleus Accumbens (NAc) shell. Thus the neurochemical mechanisms that produce the rewarding properties of JWH-018, which most likely contributes to the greater incidence of dependence associated with “Spice” use, will be described (De Luca et al., 2015a). Considering the growing evidence of a widespread use of NPS, this review will be useful to understand the new trends in the field of drug reward and drug addiction by revealing the rewarding properties of NPS, and will be helpful to gather reliable data regarding the abuse potential of these compounds.
Introduction
Over the last decade, New Psychoactive Substances (NPS) have become a global phenomenon. The emergence of these substances have been reported in almost 100 countries and territories, and more than 500 NPS have been identified worldwide based on reports by national governments, as well as the EU, and international institutions (UNODC, 2014a, 2015) (Figure 1). In 2014, in Europe alone, 101 NPS have been detected showing an increase of 25%, as compared to 2013 [EMCDDA (New psychoactive substances in Europe), 2015b]. NPS are able to mimic the effects of controlled substances and are mainly synthetic cannabinoids, stimulants, hallucinogens, and opioids.
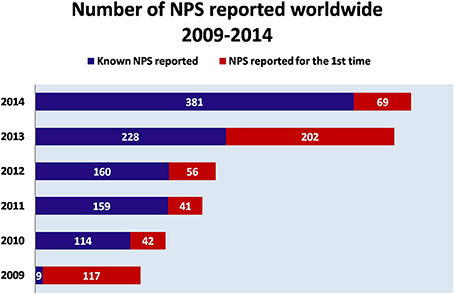
Figure 1. Number of NPS reported worldwide (2009–2014). Adapted from UNODC (2014a).  NPS reported for the first time
NPS reported for the first time Known NPS reported.
Known NPS reported.
Previous studies show that the use of NPS occurs among different subject groups: school students, partygoers, psychonauts, prisoners, and injecting drug users. Motivations for use include factors such as legal status, availability, and cost, as well as the desire to avoid detection and user preferences for particular pharmacological properties [González et al., 2013; Helander et al., 2013, 2014; EMCDDA (European Drug Report), 2015a; EMCDDA (New psychoactive substances in Europe), 2015b]. Although global research is not available yet about NPS, prevalence of use among the population, single national surveys (with respect to substances and subpopulations) show that the use of NPS amongst the general adult population is relatively low compared with the use of other illicit drugs. However, adolescents use more NPS than illicit drugs mostly because many of them are legal and easily available on the web [Drug Policy Department Italian Presidency of the Council of Ministers, 2014; Fraser, 2014; Kikura-Hanajiri et al., 2014; EMCDDA (New psychoactive substances in Europe), 2015b; Hondebrink et al., 2015; Palamar et al., 2015; Wood et al., 2015].
Similar to many known illicit drugs, NPS can cause severe physical and psychological symptoms that can even result in death [Drug Policy Department Italian Presidency of the Council of Ministers, 2013b; Fraser, 2014; EMCDDA (European Drug Report), 2015a; UNODC, 2015]. A recent analysis by the European Drug Emergencies Network, monitoring emergency admissions in the last 5 years in 10 EU countries, found that 9% of all drug-related emergencies involved NPS, primarily synthetic cannabinoids and cathinones (Wood et al., 2014). Consequently, there is increasing evidence that NPS play a great role in hospital emergencies and some drug-induced deaths [EMCDDA (European Drug Report), 2015a]. However, the analytical detection of NPS for emergency services is not technically available so far; their recognition by means of second level analysis requires standards solution, methodologies and analytical equipment not accessible to every laboratory yet [Drug Policy Department Italian Presidency of the Council of Ministers, 2013b; UNODC, 2014a; EMCDDA (European Drug Report), 2015a]. Therefore, their identification in biological samples, as well as in seized or collected samples, represents one major difficulty.
Notably, the internet is an important marketplace for the sale of NPS. Evidence is emerging of so-called “gray marketplaces”-online sites selling NPS which operate on both the surface as well as the deep web (Deluca et al., 2012; Drug Policy Department Italian Presidency of the Council of Ministers, 2013b; Burns et al., 2014; Corazza et al., 2014). Therefore, NPS can be sold via the internet to everyone, including young, underage teenagers, with complete anonymity and an easy avoidance of law enforcement and health controls [Drug Policy Department Italian Presidency of the Council of Ministers, 2013b; UNODC, 2014a; EMCDDA (European Drug Report), 2015a]. The growth of online and virtual drug markets strongly contributes to the uncontrolled widespread use of these substances, increasing health risks for consumers, and challenging drug control policies.
The largest increase in terms of newly reported NPS involves synthetic cannabinoids, synthetic cathinones, and phenethylamines [EMCDDA (European Drug Report), 2015a; EMCDDA (New psychoactive substances in Europe), 2015b]. The first synthetic cannabinoids were identified in 2008 in preparations called “herbal mixtures” or “herbal blends” (i.e., Spice) and sold as incense or air fresheners. Their effects are similar, if not superior, to those caused by cannabis consumption (Hohmann et al., 2014; Khullar et al., 2014; Mills et al., 2015). Recently, a survey of the use of synthetic cannabinoids among US students showed that Spice products were the second most used drug after marijuana, with a prevalence of 7.4–7.9% in those aged between 15 and 18 years (Johnston et al., 2013). Adolescence, a critical developmental period commonly associated with an increase in drug abuse in the human population, may be a stage of particular vulnerability to the effects of the new psychoactive drugs (Johnston et al., 2013). In fact, most of the brain receptor systems have been shown to mature slowly, reaching maximal levels around age 20. Indeed, the use of these drugs might influence neurodevelopment inducing psychiatric disorders or other mental deficits (Paus, 2005; Sussman et al., 2008). Several NPS such as mephedrone, pentedrone and MDPV which mimic the effects of amphetamine-like stimulants (ATS), can be classified as synthetic cathinones with stimulant and empathogenic properties, or as phenethylamines which can induce stimulant and hallucinogenic effects (UNODC, 2014a, 2015). Similar to other NPS, synthetic cannabinoids and ATS are largely available online and are often sold as research chemical components. They are mainly produced in Eastern Europe, Central Asia and China, and then shipped and sold to Europe and the USA (UNODC, 2015).
Currently, not all NPS are under international control. Many countries worldwide have established permanent control measures for some substances or issued temporary bans [EMCDDA (New psychoactive substances in Europe), 2015b; UNODC, 2015]. Only a few NPS have been reviewed by the mechanisms established under the international drug conventions. Existing laws covering issues unrelated to controlled drugs, such as consumer safety legislation, have been used in some countries such as Poland and UK; in others (Hungary, Finland, Italy, France, Denmark, etc.) existing drug laws or processes have been extended or adapted; additionally, in Ireland, Austria, Portugal, Romania, and Sweden new legislation has been designed [EMCDDA (New psychoactive substances in Europe), 2015b; UNODC, 2015].
The forensic identification of NPS is very difficult. These may concern the lack of knowledge on NPS available to the professionals performing analytical analysis. In addition, analytical methodologies are still not sufficient to detect the presence of all of the NPS in the analyzed samples and many laboratories lack appropriate analytical equipment for their recognition (Drug Policy Department Italian Presidency of the Council of Ministers, 2013a). These are all important aspects to take into account when considering the legal, health, and social consequences related to NPS.
To date, several behavioral, neurochemical, and electrophysiological studies have helped us to understand the pharmacological mechanisms of action of NPS. However, many of them have been focused on the acute toxicological consequences of NPS use. As they are relatively new and novel, there are no epidemiological studies to show the long-term effects of these psychoactive compounds. Also, there is not a lot of evidence on the addictive properties of NPS.
This work has been divided into two main parts based on pharmacological classification of the most popular and public health-concerning NPS classes: amphetamine-like stimulants and cannabimimetic drugs. Moreover, specific references to recent papers by the authors have been presented. A thorough analysis of the rewarding and reinforcing properties of NPS and their abuse liability will hopefully, provide to be useful for understanding the new disturbing trends in the field of drug addiction and provide strategies to tackle this growing problem.
NPS: From Chemistry to Pharmacological Effects
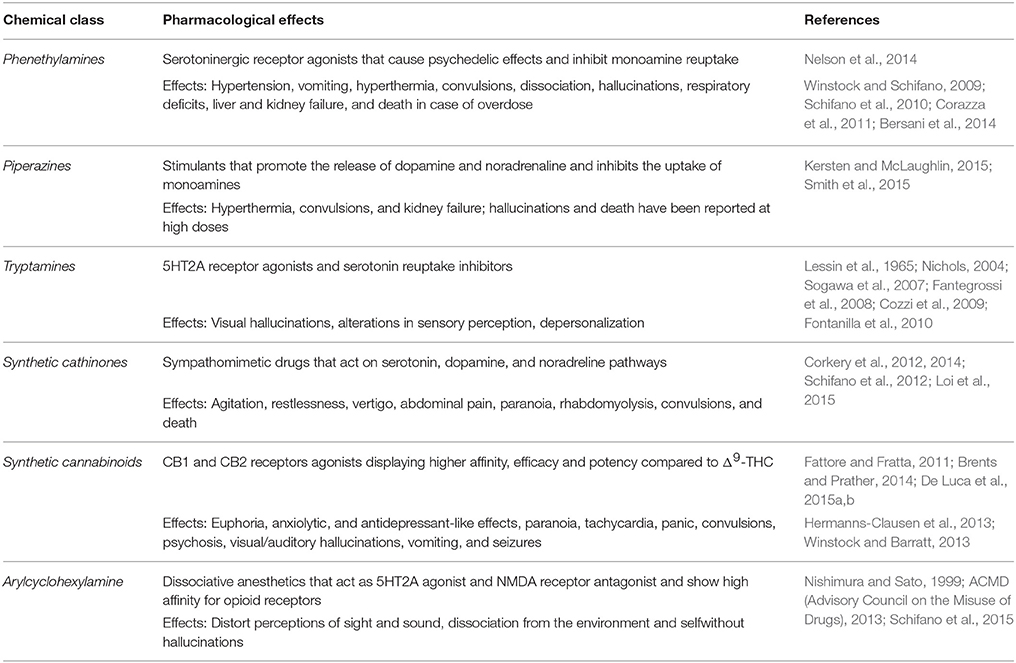
NPS can be divided into six chemical classes (Martinotti et al., 2015; Schifano et al., 2015): phenethylamines, piperazines, tryptamines, synthetic cathinones, alkylindoles (synthetic cannabinoids) and arylcyclohexylamines (see Table 1). Alternatively, a different classification is based on pharmacological and clinical effects: stimulants, entactogens, hallucinogens, and cannabis-like compounds.
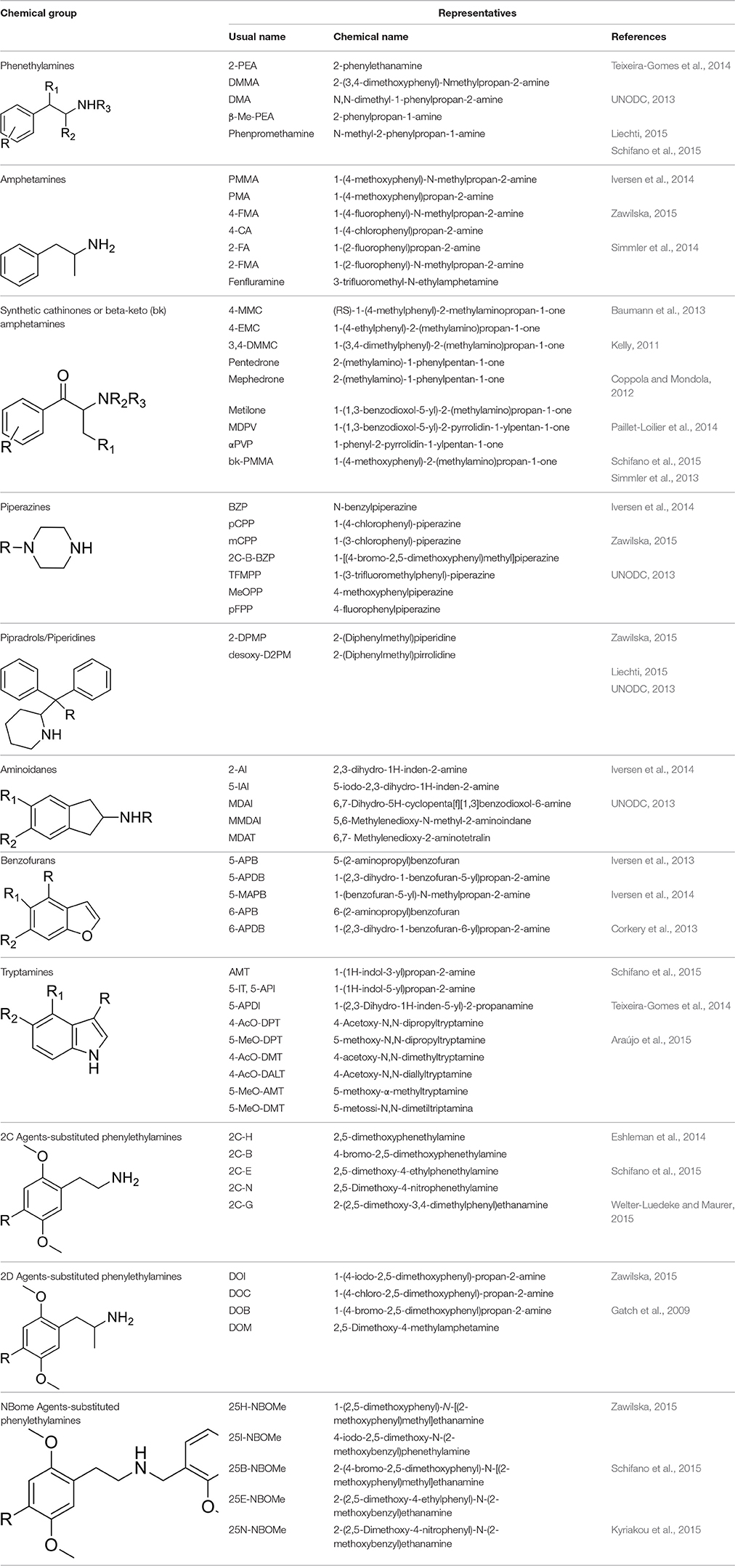
Phenethylamines, piperazines, tryptamines, and synthetic catinones exhibit stimulant and hallucinogenic effects, making up the distinct class of entactogens, which are described as psychoactive substances that enhance feelings of empathy, love, and emotional closeness to others (Schifano et al., 2007). Entactogens can be chemically divided into phenethylamines, amphetamines, synthetic cathinones, piperazines, pipradrols/piperidines, aminoindanes, benzofurans, and tryptamines (see Table 2). Stimulant drugs usually inhibit monoamine reuptake, increasing the quantity of noradrenaline, dopamine and serotonin in the synaptic cleft leading to sympathomimetic effects (Schifano, 2013). Phenethylamines are synthetic compounds commercially known as “party pills” (e.g., tablets of different colors/shapes, capsules, powder/crystal). They act on serotoninergic receptors leading to psychedelic effects and some of them inhibit the monoamine reuptake as well (Nelson et al., 2014); 3,4-methylenedioxy-methamphetamine (MDMA), widely known as “ecstasy,” is one of the most popular drugs among young people because of its stimulant effects. But, recently a growing use of new dangerous molecules on the recreational drug scene, such as 2C and its derivatives (e.g., “N-Bomb,” “B-Fly,” and “Dr. Death”), 2-D series drugs, 3C-bromo-Dragonfly, 4-MTA, 6-APB, 4,4′-DMAR and MPA, that are novel derivatives of classic psychedelic phenethylamines/MDMA-like drugs (Nelson et al., 2014) has been reported; several cases of intoxications have been reported with symptoms such as hypertension, vomiting, hyperthermia, convulsions, dissociation, hallucinations, respiratory deficits, liver, and kidney failure and death in case of overdose (Winstock and Schifano, 2009; Schifano et al., 2010; Corazza et al., 2011; Dean et al., 2013; Bersani et al., 2014; Le Roux et al., 2015; Maas et al., 2015). The lead compound in piperazines, N-Benzylpiperazin (BZP), has a typical central nervous system stimulant structure so it triggers the release of dopamine and norepinephrine and inhibits the uptake of dopamine, norepinephrine and serotonin (Smith et al., 2015). Although BZP is structurally similar to amphetamine, it is reported to have only one-tenth the potency (Wikström et al., 2004). However, at higher dosages, hallucinations can be reported as well (Kersten and McLaughlin, 2015). Before legal restrictions were placed on it, BZP was used as a safe alternative to amphetamines such as MDMA (Monteiro et al., 2013). Tryptamines (the most common is the lysergic acid diethylamide-LSD) are a group of monoamine alkaloids, very similar to the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) (Tittarelli et al., 2015), so they act both as 5HT2A receptor agonists and serotonin reuptake inhibitors (Lessin et al., 1965; Nichols, 2004; Fantegrossi et al., 2008; Cozzi et al., 2009; Fontanilla et al., 2010) provoking visual hallucinations, alterations in sensory perception, and depersonalization (Sogawa et al., 2007); novel tryptamines, as 5-MeO-AMT or 5-MeO-DMT, continue to appear on the online drug market and on the “dark net” (Araújo et al., 2015; Schifano et al., 2015; Teixeira-Gomes et al., 2014). Synthetic cathinones (mephedrone, methylone,butylone, MDPV, and α-PVP) are structural analogs of cathinones (a molecule present in the psychoactive plant Khat) and are available in tablets, capsules, powder/crystal and generally labeled as “bath salts” or “plant fertilizers” (Fass et al., 2012; German et al., 2014; Valente et al., 2014; Karila et al., 2015). Clinical effects most commonly reported with cathinones include anxiety, impaired concentration and memory, irritation of the nasal mucosa, headache, tachycardia, and hypertension. The typical clinical symptoms are indistinguishable from the acute effects of MDMA or cocaine (Prosser and Nelson, 2012; Baumann et al., 2013; Valente et al., 2014); among their psychoactive effects, agitation, restlessness, vertigo, abdominal pain, paranoia, rhabdomyolysis, convulsions, and death are included (Corkery et al., 2012, 2014; Schifano et al., 2012; Loi et al., 2015).
Synthetic cannabinoids belong to the alkylindoles and cyclohexylphenos classes which show high affinity for CB1 and CB2 cannabinoid receptors and act like Δ9-THC but with prolonged psychoactive effects and more side effects (Fattore and Fratta, 2011; Brents and Prather, 2014). As shown in Figure 2, they can be divided into naphtoylindoles (e.g., JWH- 018, JWH-073, JWH-210, WIN-55212), phenylacetylindoles (e.g., JWH-250 e JWH-251), benzoylindoles (e.g., WIN-48,098, AM-694, RSC-4), cyclohexylphenols (e.g., CP-47497, CP-55940, CP-55244) (Smith et al., 2015). They are generally consumed by inhalation through the consumption of cigarettes containing herbal substances as well as these synthetic molecules to obtain euphoria, anxiolytic, and antidepressant-like effects. However, reports presented by the EMCDDA (2009a) and by the Italian Early Warning System – N.E.W.S. (Anti-drug Policies Department) have shown effects like paranoia, tachycardia, panic, convulsions, psychosis, visual/auditory hallucinations, vomiting, and seizures (Hermanns-Clausen et al., 2013; Winstock and Barratt, 2013).
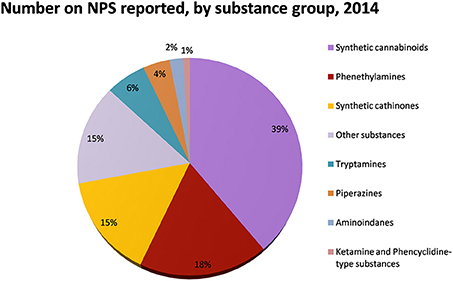
Figure 2. Number of NPS reported by substance group in 2014. Adapted from UNODC (2014a).
Finally, arylcyclohexylamine (ketamine, phencyclidine- PCP and methoxetamine) are dissociative anesthetics that distort perceptions of sight and sound and produce feelings of detachment (or dissociation) from the environment and self without hallucinations [Nishimura and Sato, 1999; ACMD (Advisory Council on the Misuse of Drugs), 2013]. Although present in the classification, the rewarding properties of the latter group will not be discussed in this review paper.
Human and Animal Studies on Amphetamine-like Stimulants Effects: Psychoactive Effects, Cognitive Deficits, Emotional Alterations, and Dependence
In the second part of the 90s, a global trend of escalating amphetamine-like stimulant use was observed and synthetic tryptamines appeared on illicit drug markets. Instead of replacing or displacing MDMA and cocaine, mephedrone, and other NPS from this group appear to have been added to the established repertoire of psychostimulant narcotics (Sanders et al., 2008; Zawilska, 2015).
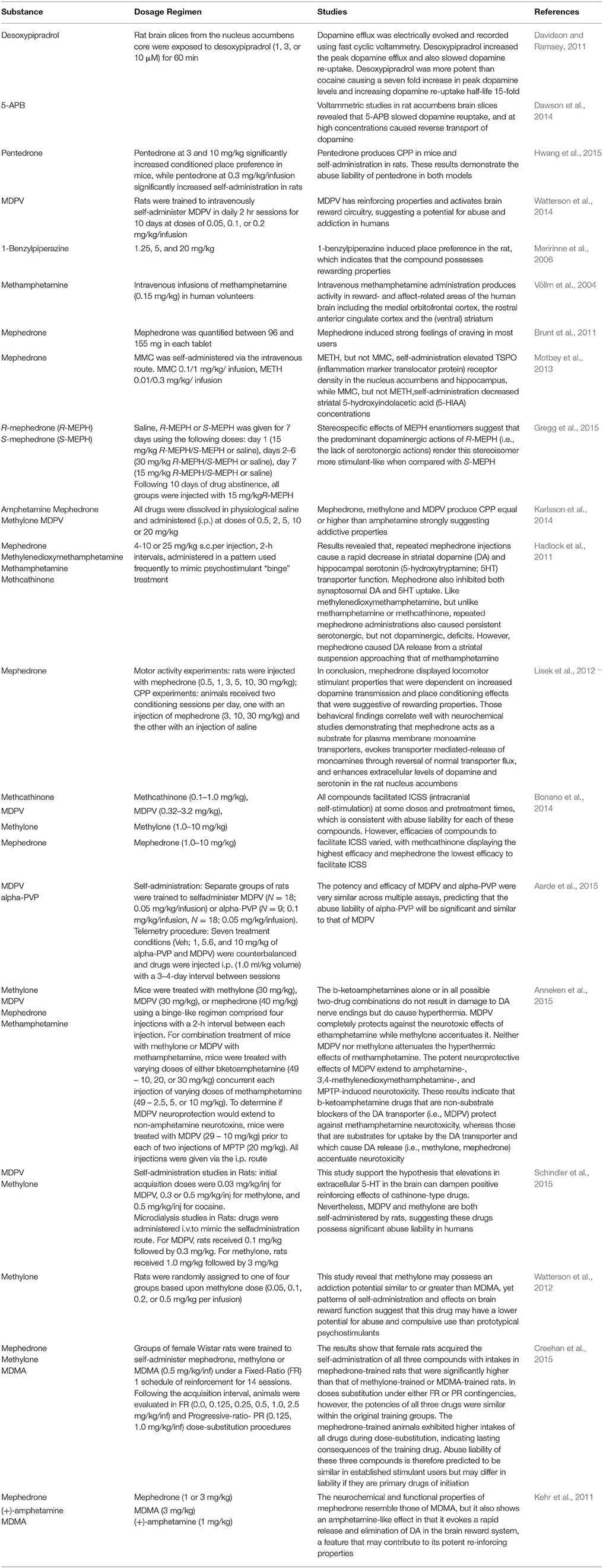
In animal models of addiction, cathinones have displayed potential rewarding and reinforcing effects. For example, mephedrone produces conditioned place preference (CPP), facilitates intracranial self-stimulation and is self-administered in rats (Hadlock et al., 2011; Lisek et al., 2012; Motbey et al., 2013; Bonano et al., 2014; Gregg et al., 2015). Prior studies demonstrated that MDPV and methylone, another synthetic cathinone, increase locomotor activity in rodents (López-Arnau et al., 2012; Marusich et al., 2012; Aarde et al., 2013; Gatch et al., 2013) and also enhance intracranial self-stimulation (Watterson et al., 2012, 2014; Bonano et al., 2014) and engender conditioned place preference (Karlsson et al., 2014), effects that are indicators of high abuse potential (Schindler et al., 2015). This evidence suggests that each compound could produce behavioral effects consistent with psychostimulant drugs displaying high abuse liability, possibly higher than amphetamine. In fact, in rats trained to self-administer MDPV or methamphetamine, dose-substitution studies demonstrated that behavior was dose-sensitive for both drugs, but MDPV showed greater potency and efficacy than methamphetamine (Paillet-Loilier et al., 2014). Moreover, in mice models, mephedrone, methylone, and MDPV produce CPP equal or higher than amphetamine, strongly suggesting their addictive properties (Karlsson et al., 2014). In addition, MDMA, methylone, and mephedrone are self-administered in female rats with a higher intake in mephedrone-trained rats compared to methylone-trained animals. This seems to suggest that mephedrone might have greater reinforcing effects compared to methylone or MDMA (Creehan et al., 2015), despite their shared mechanism of action. They are non-selective transporter substrates that increase the release of dopamine, norepinephrine and 5-HT in vitro (Baumann et al., 2012; Eshleman et al., 2013; Simmler et al., 2013). Importantly, the 5-HT-releasing ability of these drugs is more prevalent than their effects on dopamine in vivo (Baumann et al., 2008, 2012; Kehr et al., 2011; Wright et al., 2012) suggesting empathogen-like effects. Such findings indicate that self-administration of MDMA-like drugs is influenced by 5-HT release, but also drug pharmacokinetics, effects on noradrenergic systems, or non-transporter sites of action (Schindler et al., 2015).
In humans, synthetic cathinones produce psychotropic effects similar to MDMA and cocaine (Simmler et al., 2013). The typical dose range varies according to the different cathinone derivatives. However, according to information released from users in drug forums, where people discuss their experiences with recreational drugs (e.g., “Drugs-Forum,” “Urban 75,” “Erowid”), they usually start with a small dose and gradually increase it. This is in line with scientific reports which show that an excessive increase of noradrenergic signals could promote the onset of adverse effects and that the potency of a substance to activate the noradrenergic system is inversely correlated to the doses typically used recreationally (Simmler et al., 2013). All cathinones exhibit higher dopaminergic activity when compared with their non β-keto amphetamines analogs. Recent studies of the mechanisms by which b-ketoamphetamines interact with DAT, offer significant insight into why these drugs have such divergent effects on neurotoxicity. “Bath salts” have been classified as substrates and non-substrates based on whether or not they are transported by the DAT (Anneken et al., 2015). This increased dopaminergic property of the cathinones suggests higher stimulant-type effects and a greater risk for dependence (Aarde et al., 2013). Stimulant and entactogenic properties are typical of cathinones. In fact, desired or pleasant effects most often described by users include euphoria, intensification of sensory senses, increased sociability, increased energy, mental stimulation, empathy connection, openness, increased sensory perception, decreased inhibition, and sexual arousal; but side effects such as cognitive confusion, cognitive impairment, psychiatric irritability, aggression that sometimes progresses to violent or even criminal behavior, and self-destructive behavior have also been reported [IACP (International Association of Chiefs of Police), 2012]. MDPV and mephedrone have been directly implicated in a number of fatalities in medical literature. In one case involving MDPV, the cause of death was consistent with excited delirium syndrome, a condition associated with stimulant drug overdose and attributable to excessive dopaminergic transmission (Baumann et al., 2012).
However, amphetamine remains the prototype of psychostimulants causing agitation, insomnia, loss of appetite and, at higher doses, “amphetamines psychosis” characterized by paranoia, hallucinations and delusion (Iversen et al., 2014). In experimental animals, low doses of amphetamine cause hyperactivity and higher doses lead to stereotyped repetitive behaviors (Whelpton, 2007). The neurotoxic effects of amphetamines has been vastly studied and their ability to damage brain monoaminergic cells was shown by long-term deficits in dopaminergic and serotoninergic systems in several brain areas of animals (Teixeira-Gomes et al., 2014). One of the major neurotoxic actions of amphetamines observed in laboratory animals is the sustained depletion of monoamine brain levels. In addition to the damage to dopaminergic and serotoninergic neuronal systems, amphetamines can also induce neuronal death. For example, in several studies, MDMA administration in mice and rats produced neuronal death in several brain areas including the cortex, hippocampus, amygdala, ventromedial/ventrolateral thalamus, and teniatecta (Teixeira-Gomes et al., 2014). MDMA is still one of the most widely used recreational drugs and many NPS were designed to mimic its effects or as substitutes for MDMA in ecstasy pills.
The potency of abused psychostimulants to activate the brain reward circuitry increases the risk of potential for abuse and addiction in humans (Table 3). In contrast, a relative activation of the serotonin system would be linked to a reduction in abuse potential. Thus, the DAT/SERT inhibition ratio and dopamine/serotonin release potency has been proposed to predict the effects of psychostimulants in humans (Paillet-Loilier et al., 2014). Data currently available has shown that the frequent consumption of high doses of synthetic cathinones induce tolerance, dependence, craving, and withdrawal syndrome after sudden suspension [NDIC (National Drug Intelligence Center), 2011]. Indeed, Kehr et al. (2011) showed that mephedrone induces a stimulation of the dopamine transmission in the nucleus accumbens, that might be a starting point for developing drug-addiction (Volkow et al., 2003; Di Chiara et al., 2004). Although the typical dose range of MDPV appears to be between 5 and 30 mg in a single administration, some users reported tolerance with the consumption of a single dose, higher than 200 mg (Coppola and Mondola, 2012). Several users have reported a withdrawal syndrome after abrupt cessation of long-term use of methcathinone, mephedrone and MDPV (Winstock et al., 2011). Moreover, Gatch et al. (2013) showed that all of the cathinone derivatives fully substituted for methamphetamine or cocaine in drug discrimination tests. Results suggest that these drugs are comparable to cocaine and methamphetamine, and are likely to induce dependence (Iversen et al., 2014).
Synthetic Marijuana and the Cannabimimetics
Spice and CB1 “Super Agonists”
Synthetic Cannabimimetic agents (SC), also known as Cannabimimetics, are substances with pharmacological properties similar to delta-9-tetrahydrocannabinol (Δ9-THC) assessed by in vitro and in vivo animal studies such as binding studies and functional assays (Compton et al., 1992; EMCDDA, 2009b). SC have been detected in “Spice,” “K2,” and spice-like samples all over the world. Spice is a smokable herbal mixture marketed as a safe, legal alternative to Cannabis, composed by shredded plant material laced with a variety of SC compounds [NIDA (National Institute on Drug Abuse), 2012]. These compounds are “smokable” since they are small (typically 20–26 carbon atoms) and highly lipophilic molecules. A few hundred of SC of the JWH, HU, and CP series are currently available. They retain very high cannabinoid receptor binding affinity levels, with a dose-response efficacy significantly higher than Δ9-THC itself (Brents et al., 2011; Fattore and Fratta, 2011; Schifano et al., 2015). New legal regulations have been enacted to control the global diffusion of Spice. As a consequence of that, three subsequent generations of SC have been developed based on slight modifications of the first generation compounds such as JWH-018, CP 47,497, and HU-210 [ACMD (Advisory Council on the Misuse of Drugs), 2009] that are full CB1 agonists with affinities that are 4.5, 8.6, and 55 times that of Δ9-THC, respectively.
Different European countries, in 2009, and some states in the US, in 2010, banned the sale and use of first generation SC. These regulations induced an extreme reduction of these SC in the Spice/K2 preparations with a subsequent increase of newly synthetized SC, thus belonging to the “second” (e.g., AM-2201, MAM 2201, AM-694, RCS-4) and “third” (e.g., PB-22 “QUPIC,” 5F-PB-22, BB-22 “QUCHIC,” AB-PINACA) generation [ACMD (Advisory Council on the Misuse of Drugs), 2012, 2014] in order to avoid detection.
Several studies show that SC are remarkably different from and more dangerous than THC. Indeed, while THC is a partial CB1 agonist, in vitro studies have clearly shown that these compounds are full agonists with higher potency and efficacy as compared to Δ9-THC (Atwood et al., 2010, 2011; Marshell et al., 2014). More recent studies have been shown that selected third generation compounds, such as 5F-PB-22 and BB-22, retain greater CB1 receptor agonist potency (five- and seven- fold, respectively) and efficacy and a higher binding affinity (26- and 30-fold, respectively) at CB1 receptors compared to JWH-018 (De Luca et al., 2015b).
Moreover, studies performed in rats and mice showed that many SC displayed locomotor depressant effects and a characteristic tetrad profile at lower doses compared to Δ9-THC (Chaperon and Thiébot, 1999; Wiley et al., 2012, 2014; Gatch and Forster, 2014, 2015; Vigolo et al., 2015). In addition, JWH-018 and its congeners are readily metabolized to a series of cannabimimetics (Seely et al., 2012). That, together with the presence of several different SC in Spice/K2 products and their unpredictable dosing when consumed (Kronstrand et al., 2014), might explain their acute severe toxicity and even lethal medical complications in humans (Brents et al., 2011; Papanti et al., 2013; Brents and Prather, 2014; Brewer and Collins, 2014; Santacroce et al., 2015), leading to severe withdrawal syndrome and dependence as well in some cases (Zimmermann et al., 2009; Gunderson et al., 2012; Macfarlane and Christie, 2015). In addition, clinical evidence indicates that JWH-018 can generate/cause psychosis in vulnerable individuals (Every-Palmer, 2011). Notably, SC misuse has been associated with anxiety, agitation/panic attacks, paranoid ideation, suicidal ideation, and hallucinations (Fattore and Fratta, 2011; Wells and Ott, 2011; Thomas et al., 2012; Besli et al., 2015), and also been related to mood, cognitive (i.e., memory impairment, attention difficulties), neurological (i.e., dizziness, sensation changes, seizures, tremor) and psychotic (i.e., agitation, aggression, catatonia, paranoia, hallucinations, depersonalization, dissociation, prolonged psychosis, perceptual alterations) episodes, with a higher incidence in comparison to those seen with Δ9-THC use (Papanti et al., 2013; Spaderna et al., 2013; Van Amsterdam et al., 2015).
Rewarding and Reinforcing Properties of Cannabimimetics
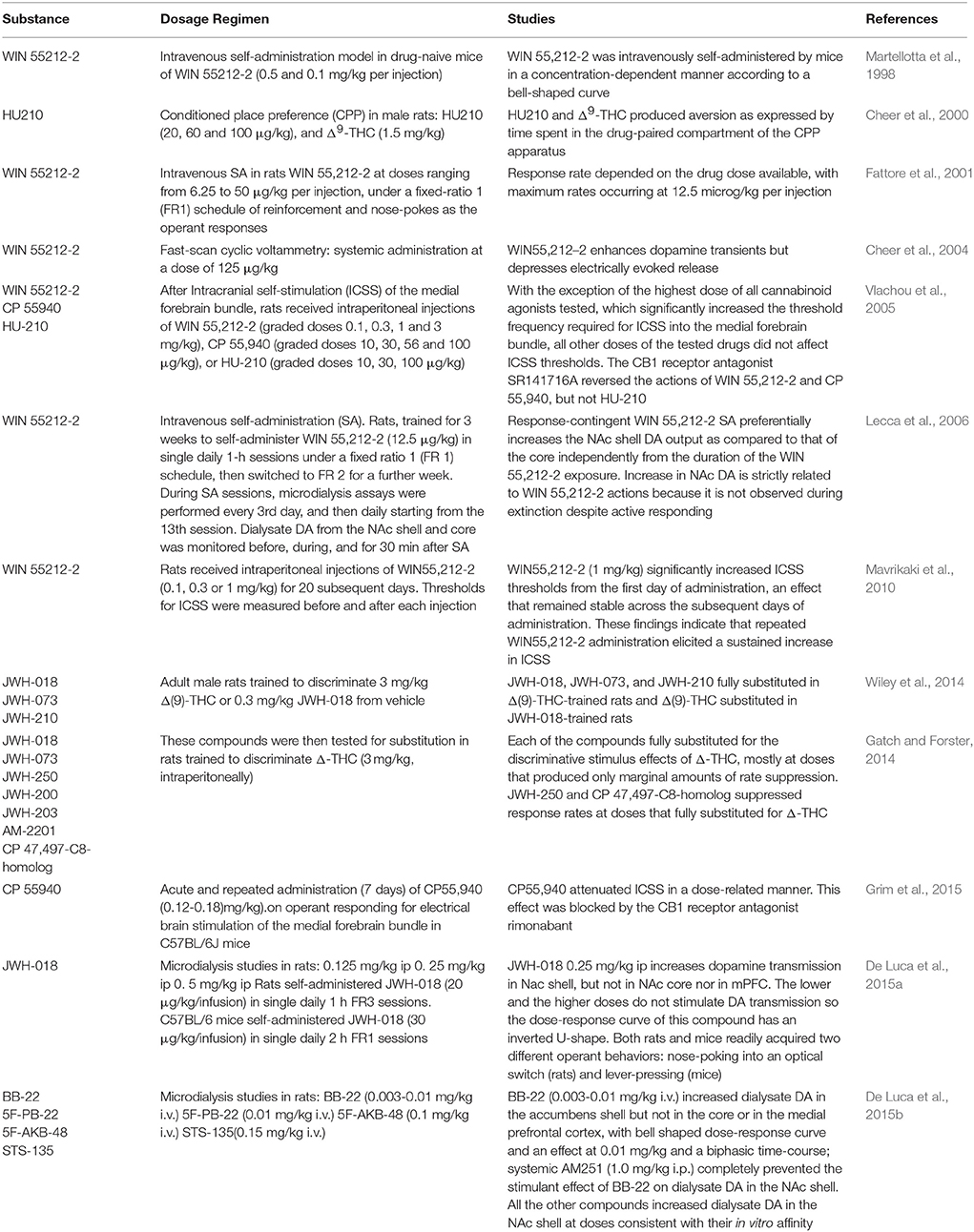
Recent literature shows that SC have emerged as new drugs of abuse. As previously reported, an incredibly huge number of SC have been detected in Marijuana substitutes (Denooz et al., 2013; Brents and Prather, 2014; Maxwell, 2014). Being CB1 receptor agonists with extremely high affinity, SC probably act in brain regions where CB1 receptors are heavily expressed, such as the amygdala, cingulate cortex, prefrontal cortex (PFC), ventral pallidum, caudate putamen, nucleus accumbens (NAc), ventral tegmental area (VTA), and lateral hypothalamus (Glass et al., 1997; Wang et al., 2003). All these brain regions have a recognized involvement in reward, addiction and cognitive functions (Koob and Volkow, 2010). Furthermore, CB1 receptors are located in limbic regions, such as VTA, NAc, ventral pallidum, CeA, BNST, and PFC (Herkenham et al., 1991; Glass et al., 1997; Wang et al., 2003); the integration of excitatory and inhibitory inputs, coming from these structures, influence, and modulate reward processing (Sidhpura and Parsons, 2011; Panagis et al., 2014). Several studies in mice and rats showed that these compounds affect the mesolimbic dopaminergic transmission and influence conditioned behaviors (Table 4). Similar to other drugs of abuse, THC activates dopamine (DA) transmission in the ventral striatum in humans (Volkow et al., 2003; Bossong et al., 2009). In addition, animal studies showed that both Δ9-THC and WIN 55.212-2, a CB1 and CB2 agonist, elicit dopamine release in the NAc (Chen et al., 1993; Cheer et al., 2004) with a specific activation of the NAc shell subregion (Tanda et al., 1997; Lecca et al., 2006; De Luca et al., 2012). The NAc plays a crucial role in brain reward circuits involved in motivational and cognitive functions (Heimer et al., 1991; Zahm and Brog, 1992). In particular, it has been shown that stimulation of DA transmission in the NAc shell is directly involved in the rewarding properties of both natural reward and addictive drugs (Di Chiara et al., 2004). Microdialysis studies on awake freely moving animals performed in our laboratories showed that JWH-018, at the dose of 0.25 mg/kg i.p., increases DA transmission in the NAc shell but not in the NAc core nor in mPFC (Figure 4). Surprisingly, DA transmission in the NAc shell was not stimulated after administration of lower (0.125 mg/kg ip) or higher (0.5 mg/kg ip) doses producing an inverted U-shape dose response curve for the effect of JWH-018 (De Luca et al., 2015a). Further studies in mice and rats showed a similar effect after the intraperitoneal administration of JWH-073 and JWH-250 as well (Ossato et al., 2016), and after the intravenous administration of BB-22 (De Luca et al., 2015b). Notably, as previously reported by De Luca et al. (2012), THC stimulated extracellular DA release in the NAc shell at a dose fourfold higher than JWH-018 when administered intraperitoneally. In addition, BB-22 stimulates NAc shell DA release at the dose of 0.01 mg/kg iv, while THC increases extracellular DA in the same area at dose of 0.15 mg/kg iv (Tanda et al., 1997). These results show that both JWH-018 and BB-22 are more potent than THC in inducing NAc shell DA release, suggesting a putative higher abuse liability of synthetic vs. natural cannabinoids. Electrophysiological studies show that the stimulation of DA extracellular levels in the NAc shell by JWH-018 is thought to be due to the activation of CB1 receptors located on presynaptic GABAergic afferents directed to VTA DA neurons, leading to a reduction of GABAA receptors mediated inhibition of DA neuronal activity in the VTA (Lupica and Riegel, 2005; Mátyás et al., 2008; Melis et al., 2014; De Luca et al., 2015a). Rewarding effects of cannabimimetics have also been assessed by different experimental paradigms such as intracranial self-stimulation (ICSS), place conditioning tests, drug-discrimination and intravenous self-administration (IVSA) studies. ICSS of the medial forebrain bundle is the operant conditioning method used in rodents to evaluate the role of the mesolimbic dopamine pathway in rewarding behavioral effects (Carlezon and Chartoff, 2007) and evaluating potential of abuse (Negus and Miller, 2014). Not surprisingly, to date no data on the effect of new SC on ICSS are available. Δ9-THC does not facilitate ICSS, but has a dose-dependent inhibitory influence on ICSS (Vlachou et al., 2007). Similarly, a depression of ICSS is observed after the administration of WIN55212-2, CP55940, HU210 (Antoniou et al., 2005; Vlachou et al., 2005; Mavrikaki et al., 2010). Differences in developing tolerance to depression of ICSS after repeated exposure to cannabinoids have been reported. Tolerance is completely developed after repeated exposure to Δ9-THC (Kwilasz and Negus, 2012) but partially developed after CP55940 (Grim et al., 2015), and not developed after WIN55212-2 administration (Mavrikaki et al., 2010), suggesting that the different affinity of Δ9-THC vs. SC for the CB1 receptors could play a role in developing this tolerance (Grim et al., 2015).
On the other hand, place conditioning tests in animals showed that WIN 55212-2 and HU210 established a robust place aversion (CPA), reversed by the CB1 receptor antagonist/inverse agonist SR 141716A, in a similar way as seen with Δ9-THC (Chaperon et al., 1998; Cheer et al., 2000; Valjent and Maldonado, 2000). In addition, the CB1 antagonist/inverse agonist AM281 did not induce conditioned place preference (CPP). However, a pre-treatment of 14 days with AM281 prior to the CPP test with the same drug, showed significant CPP (Botanas et al., 2015). Accordingly, it has been hypothesized that the endogenous cannabinoid system in the brain may act as a counter-reward system, and blocking or antagonizing this system would therefore produce the reward (Sañudo-Peña et al., 1997; Botanas et al., 2015). This could represent a limitation on the therapeutic use of CB1 antagonist/inverse agonists (Seely et al., 2011).
The psychopharmacological effects of SC have also been assessed by drug-discrimination studies. These experimental paradigms represent useful tools for evaluating the abuse liability of new drugs that might produce dependence (Solinas et al., 2006). Drug-discrimination studies in rats have showed that JWH-018, JWH-250 and CP 47,497-C8-homolog, UR-144, XLR-11, AKB-48 (APINACA), PB-22 (QUPIC), 5F-PB-22, and AB-FUBINACA fully substituted for the discriminative stimulus effects of Δ9-THC (Gatch and Forster, 2014, 2015; Wiley et al., 2014).
These studies typically serve as an integration of the results obtained by intravenous self-administration (SA) experiments. As for SA studies, while there is some disputable data concerning the reinforcing properties of Δ9-THC based on its ability to be persistently self-administered in squirrel monkeys (Tanda et al., 2000) but not in rodents, there is still favorable evidence available about SC SA. Thus it has been reported that monkeys, mice and rats acquire and maintain WIN 55.212-2 SA (Martellotta et al., 1998; Fattore et al., 2001; Justinova et al., 2004; Lecca et al., 2006). Recently, it has been reported that JWH-018 is self-administered in rodents (Figure 5). In the study by De Luca et al. (2015a) both rats and mice readily acquired two different operant behaviors: nose-poking into an optical switch (rats) and lever-pressing (mice). Rats self-administered JWH-018 at the dose of 20 μg/kg/infusion in daily 1 h FR3 sessions (Figure 5A). As expected, a reduction of SA after the injection of SR141617A (1 mg/kg ip, 30 min prior to the SA session) was observed, consistent with the lack of JWH-018 mediated reinforcement. Intriguingly, nose-poking for JWH-018 significantly increased from the first session (30th session, Figure 5A) performed after the administration of SR141617A for 2 consecutive days, confirming that these effects of JWH-018 are mediated through cannabinoid receptors. SA behavior did not decrease when JWH-018 was replaced by vehicle. A control group of rats trained for vehicle, failed to acquire SA behavior. It has been hypothesized that the absence of extinction-like response patterns was unrelated to response-contingent training for JWH-018 because the vehicle failed to induce responding. We think that this probably occurred as a result of a habit learning conditioned by JWH-018, in fact contextual cues were sufficient to maintain responding (De Luca et al., 2015a). This confirmed previous evidence showing that endocannabinoid signaling through CB1 receptors is significant for the habit formation (Hilário et al., 2007). Indeed, in mice, CB1 receptor knockdown can enhance or blunt habit formation, whereas Δ9-THC tolerance enhances habit formation; in humans, cannabis use enhances the stimulus-response/habit memory (for review, see Goodman and Packard, 2015).
JWH-018 self-administration studies performed in C57BL/6 mice show that animals acquired SA at the dose of 30 μg/kg/infusion in daily 2 h FR1 sessions (Figure 5B). Importantly, the specificity of mice responding behavior has been confirmed by the increase of SA under progressive-ratio (PR) schedule of reinforcement. During extinction phase, surprisingly, active lever-pressing did not decrease, while inactive lever-pressing increased becoming superimposable to the active ones. Unlike the SA experiments with rats, in these experiments, the drug associated cues were removed after the first three of a total of 12 sessions during the extinction phase. However, during the JWH-018 reinstatement, SA behavior was immediately reinstated and inactive lever-presses decreased since the SA behavior was specifically regulated by the drug infusion confirming the reinforcing properties of JWH-018. This may also prove that JWH-018 alters cortical processes important for the context updating and the automatic orientation of attention (D'Souza et al., 2012) with consequent disruption of cognitive functioning, emotional processing, and affective states as different SC make in humans (Zimmermann et al., 2009). Further research is needed to evaluate the impact of chronic exposure to SC.
Concluding Remarks
The review of the biomedical data here presented, clearly demonstrates the unsafe nature of these new drugs of abuse. This is particularly alarming since adolescents seem to be the most exposed subjects to these dangerous NPS. Indeed, users are often unaware of the consequences of ingesting synthetic compounds that are sold as “legal alternative” to classical drugs, and their unexpected, sometimes fatal adverse effects. Only awareness can reduce NPS use since stopping their synthesis and diffusion seems to be an improbable task and, morbidity and mortality reports keep increasing as NPS gain popularity worldwide. Awareness campaigns about these substances and their devastating effects should be organized to inform everyone, including clinicians, who should be able to recognize symptoms of intoxication induced by NPS (Simonato et al., 2013; Papanti et al., 2013; Schifano et al., 2015).
Ultimately, this paper intends to be helpful to drive governments and civil society to not underestimate the NPS issue, and to encourage the scientific community to deeply evaluate the pharmacology and toxicological effects of NPS and to develop effective treatments for NPS intoxication. Additionally, this paper intends to be useful for advising law enforcement agencies, which need updated information for the prevention and fight against trafficking and sale of NPS, and in the long run, hopefully contribute to better protect public health and safety.
Author Contributions
This is a review, different authors contributed as follows: CM: Section NPS: From Chemistry to Pharmacological Effects; Figures 1–3, Tables 1, 3, 4. GS and CR: Section–Introduction; MM and MM: Section–Human and Animal Studies on amphetamine-Like Stimulant Effects: Psychoactive Effects, Cognitive Deficits, Emotional Alterations, and Dependence -MM: Table 2. MDL: Section–Synthetic Marijuana and the Cannabimimetics, Section–Concluding Remarks and entire revision of the manuscript; Figures 4, 5 and Tables 3, 4.
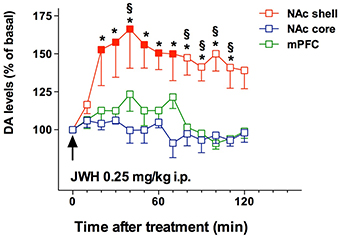
Figure 4. Effect of JWH-018 administration on DA transmission in the NAc shell, NAc core, and mPFC. Results are expressed as mean ± SEM of change in DA extracellular levels expressed as the percentage of basal values. The arrow indicates the start of JWH-018 i.p. injection at the dose of 0.25 mg/kg in the NAc shell (red squares), NAc core (blue squares), and mPFC (green squares). Solid symbol: p < 0.05 with respect to basal values; *p < 0.05 vsNAc core group; § p < 0.05 vs mPFC group; (NAc shell N = 10; NAc core N = 7; mPFC N = 11) (Two-way ANOVA, Tukey's HSD post hoc). Adapted from De Luca et al. (2015a).
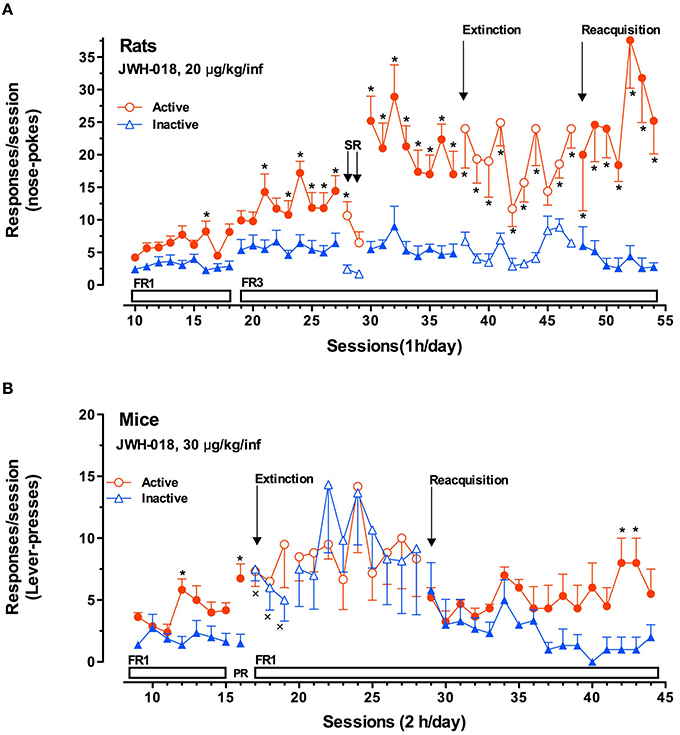
Figure 5. JWH-018 self-administration in rats and mice. (A) JWH-018 self-administration by Sprague-Dawley rats and involvement of CB1 cannabinoid receptors in this behavior. Number of active nose pokes (circles) that resulted in JWH-018 infusion (20 μg/kg/infusion) or inactive ones (triangles) during each 1-h daily session under FR1 and FR 3 during acquisition (1th to 37th sessions), extinction (38th To 47th sessions) and reacquisition (48th to 54thsessions) phases. On sessions 28th and 29th the effect of SR 141716A on the JWH-018 SA was tested. Results are expressed as mean ± SEM (N sessions 10–47 = 14, sessions 48–54 = 6) *p < 0.05 vs. inactive nose pokes; ANOVA followed by LSD post hoc test. (B) JWH-018 self-administration by C57BL/6 mice under fixed (FR1) and progressive (PR) reinforcement schedules. Number of active lever-presses (circles) that resulted in JWH-018 infusion (30 μg/kg/inf) or inactive lever-presses (triangles) during each 2 h daily session under FR1 (9th–15th sessions), and PR (16th session) reinforcement schedules. Results are expressed as mean ± SEM (N = 8), *p < 0.05 vs. inactive lever- presses; ANOVA followed by LSD post hoc test. Adapted from De Luca et al. (2015a).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research has been funded by the Dipartimento Politiche Antidroga, Presidenza del Consigliodei Ministri, Italy (projects INSIDE-018 to MA De Luca and NS-DRUGS to M Marti and MA De Luca), by Fondazione Banco di Sardegna (Prot. U404.2015/AI.338.MGB Prat.2015.0713), and by European Commission (Drug Prevention and Information Programme 2014-16, contract no. JUST/2013/DPIP/AG/4823, EU-MADNESS project). Some of the study cited in this review has been performed by MA De Luca, in collaboration with Prof. LH Parsons, at TSRI (La Jolla, CA) during the completion of her Fulbright Research Scholar (AY 2013-2014); Dr. De Luca would like to thank the U.S.-Italy Fulbright Commission (www.fulbright.it) for the assistance.
References
Aarde, S. M., Creehan, K. M., Vandewater, S. A., Dickerson, T. J., and Taffe, M. A. (2015). In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl.) 232, 3045–3055. doi: 10.1007/s00213-015-3944-8
Aarde, S. M., Huang, P. K., Creehan, K. M., Dickerson, T. J., and Taffe, M. A. (2013). The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71, 130–140. doi: 10.1016/j.neuropharm.2013.04.003
ACMD (Advisory Council on the Misuse of Drugs) (2009). Review of the ACMD, 2009 - Publications - GOV.UK. Available online at: https://www.gov.uk/government/publications/review-of-the-acmd-2009 (Accessed October 29, 2015).
ACMD (Advisory Council on the Misuse of Drugs) (2012). Methoxetamine report, 2012 - Publications - GOV.UK. Available at: https://www.gov.uk/government/publications/advisory-council-on-the-misuse-of-drugs-acmd-methoxetamine-report-2012 (Accessed October 29, 2015).
ACMD (Advisory Council on the Misuse of Drugs) (2014). IMSD 2014/ACMD 2014. Available online at: http://imsd-acmd2014.ksme.or.kr/main/ (Accessed October 29, 2015)
Anneken, J. H., Angoa-Pérez, M., and Kuhn, D. M. (2015). 3,4-Methylenedioxypyrovalerone prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: β-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J. Neurochem. 133, 211–222. doi: 10.1111/jnc.13048
Antoniou, K., Galanopoulos, A., Vlachou, S., Kourouli, T., Nahmias, V., Thermos, K., et al. (2005). Behavioral pharmacological properties of a novel cannabinoid 1′,1′-dithiolane delta8-THC analog, AMG-3. Behav. Pharmacol. 16, 499–510. doi: 10.1097/00008877-200509000-00024
Araújo, A. M., Carvalho, F., Bastos, M., de, L., Guedes de Pinho, P., and Carvalho, M. (2015). The hallucinogenic world of tryptamines: an updated review. Arch. Toxicol. 89, 1151–1173. doi: 10.1007/s00204-015-1513-x
Atwood, B. K., Huffman, J., Straiker, A., and MacKie, K. (2010). JWH018, a common constituent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB 1 receptor agonist. Br. J. Pharmacol. 160, 585–593. doi: 10.1111/j.1476-5381.2009.00582.x
Atwood, B. K., Lee, D., Straiker, A., Widlanski, T. S., and Mackie, K. (2011). CP47,497-C8 and JWH073, commonly found in “Spice” herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur. J. Pharmacol. 659, 139–145. doi: 10.1016/j.ejphar.2011.01.066
Baumann, M. H., Clark, R. D., and Rothman, R. B. (2008). Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol. Biochem. Behav. 90, 208–217. doi: 10.1016/j.pbb.2008.02.018
Baumann, M. H., Ayestas, M. A. Jr., Partilla, J. S., Sink, J. R., Shulgin, A. T., Daley, P. F., et al. (2012). The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37, 1192–1203. doi: 10.1038/npp.2011.304
Baumann, M. H., Partilla, J. S., and Lehner, K. R. (2013). Psychoactive “bath salts”: Not so soothing. Eur. J. Pharmacol. 698, 1–5. doi: 10.1016/j.ejphar.2012.11.020
Bersani, F. S., Corazza, O., Albano, G., Valeriani, G., Santacroce, R., Bolzan Mariotti Posocco, F., et al. (2014). 25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug. Biomed Res. Int. 2014:734749. doi: 10.1155/2014/734749
Besli, G. E., Ikiz, M. A., Yildirim, S., and Saltik, S. (2015). Synthetic cannabinoid abuse in adolescents: a case series. J. Emerg. Med. 49, 644–650. doi: 10.1016/j.jemermed.2015.06.053
Bonano, J. S., Glennon, R. A., De Felice, L. J., Banks, M. L., and Negus, S. S. (2014). Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl). 231, 199–207. doi: 10.1007/s00213-013-3223-5
Bossong, M. G., van Berckel, B. N. M., Boellaard, R., Zuurman, L., Schuit, R. C., Windhorst, A. D., et al. (2009). Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34, 759–766. doi: 10.1038/npp.2008.138
Botanas, C. J., de la Peña, J. B., Dela Pena, I. J., Tampus, R., Kim, H. J., Yoon, S. S., et al. (2015). Evaluation of the abuse potential of AM281, a new synthetic cannabinoid CB1 receptor antagonist. Eur. J. Pharmacol. 766, 135–141. doi: 10.1016/j.ejphar.2015.10.004
Brents, L. K., and Prather, P. L. (2014). The K2/Spice phenomenon: emergence, identification, legislation and metabolic characterization of synthetic cannabinoids in herbal incense products. Drug Metab. Rev. 46, 72–85. doi: 10.3109/03602532.2013.839700
Brents, L. K., Reichard, E. E., Zimmerman, S. M., Moran, J. H., Fantegrossi, W. E., and Prather, P. L. (2011). Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS ONE 6:e21917. doi: 10.1371/journal.pone.0021917
Brewer, T. L., and Collins, M. (2014). A review of clinical manifestations in adolescent and young adults after use of synthetic cannabinoids. J. Spec. Pediatr. Nurs. 19, 119–126. doi: 10.1111/jspn.12057
Brunt, T. M., Poortman, A., Niesink, R. J. M., and van den Brink, W. (2011). Instability of the ecstasy market and a new kid on the block: mephedrone. J. Psychopharmacol. 25, 1543–1547. doi: 10.1177/0269881110378370
Burns, L., Roxburgh, A, Bruno, R., and Van Buskirk, J. (2014). Monitoring drug markets in the Internet age and the evolution of drug monitoring systems in Australia. Drug Test. Anal. 6, 840–845. doi: 10.1002/dta.1613
Carlezon, W. A. Jr., and Chartoff, E. H. (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc. 2, 2987–2995. doi: 10.1038/nprot.2007.441
Chaperon, F., and Thiébot, M. H. (1999). Behavioral effects of cannabinoid agents in animals. Crit. Rev. Neurobiol. 13, 243–281.
Chaperon, F., Soubrié, P., Puech, A. J., and Thiébot, M. H. (1998). Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 135, 324–332. doi: 10.1007/s002130050518
Cheer, J. F., Kendall, D. A., and Marsden, C. A. (2000). Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl). 151, 25–30. doi: 10.1007/s002130000481
Cheer, J. F., Wassum, K. M., Heien, M. L. A. V., Phillips, P. E. M., and Wightman, R. M. (2004). Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J. Neurosci. 24, 4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004
Chen, J., Marmur, R., Pulles, A., Paredes, W., and Gardner, E. L. (1993). Ventral tegmental microinjection of delta 9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain Res. 621, 65–70. doi: 10.1016/0006-8993(93)90298-2
Compton, D. R., Johnson, M. R., Melvin, L. S., and Martin, B. R. (1992). Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J. Pharmacol. Exp. Ther. 260, 201–209.
Coppola, M., and Mondola, R. (2012). Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food.” Toxicol. Lett. 211, 144–149. doi: 10.1016/j.toxlet.2012.03.009
Corazza, O., Schifano, F., Farre, M., Deluca, P., Davey, Z., Torrens, M., et al. (2011). Designer drugs on the internet: a phenomenon out-of-control? the emergence ofhallucinogenic drug Bromo-Dragonfly. Curr. Clin. Pharmacol. 6, 125–129. doi: 10.2174/157488411796151129
Corazza, O., Valeriani, G., Bersani, F. S., Corkery, J., Martinotti, G., Bersani, G., et al. (2014). “Spice,” “kryptonite,” “black mamba”: an overview of brand names and marketing strategies of novel psychoactive substances on the web. J. Psychoactive Drugs 46, 287–294. doi: 10.1080/02791072.2014.944291
Corkery, J. M., Schifano, F., and Ghodse, A. H. (2012). “Mephedrone-related fatalities in the United Kingdom: contextual, clinical and practicalissues,” in Pharmacology, ed L. Gallelli (Rijeka: InTech), 355–380.
Corkery, J. M., Elliott, S., Schifano, F., Corazza, O., and Ghodse, A. H. (2013). MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; “sparkle”; “mindy”) toxicity: a brief overview and update. Hum. Psychopharmacol. Clin. Exp. 28, 345–355. doi: 10.1002/hup.2298
Corkery, J. M., Claridge, H., Loi, B., Goodair, C., and Schifano, F. (2014). Drug Related Deaths in the, UK. NPSAD Annual Report 2013. London: International Centre for Drug Policy; St. George's University of London.
Cozzi, N. V., Gopalakrishnan, A., Anderson, L. L., Feih, J. T., Shulgin, A. T., Daley, P. F., et al. (2009). Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J. Neural Transm. 116, 1591–1599. doi: 10.1007/s00702-009-0308-8
Creehan, K. M., Vandewater, S. A., and Taffe, M. A. (2015). Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology 92, 90–97. doi: 10.1016/j.neuropharm.2015.01.003
Davidson, C., and Ramsey, J. (2011). Desoxypipradrol is more potent than cocaine on evoked dopamine efflux in the nucleus accumbens. J. Psychopharmacol. 26, 1036–1041. doi: 10.1177/0269881111430733
Dawson, P., Opacka-Juffry, J., Moffatt, J. D., Daniju, Y., Dutta, N., Ramsey, J., et al. (2014). The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog. Neuro Psychopharmacol. Biol. Psychiatry 48, 57–63. doi: 10.1016/j.pnpbp.2013.08.013
De Luca, M. A., Bimpisidis, Z., Melis, M., Marti, M., Caboni, P., Valentini, V., et al. (2015a). Stimulation of in vivo dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology 99, 705–714. doi: 10.1016/j.neuropharm.2015.08.041
De Luca, M. A., Solinas, M., Bimpisidis, Z., Goldberg, S. R., and Di Chiara, G. (2012). Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 63, 161–168. doi: 10.1016/j.neuropharm.2011.10.018
De Luca, M. A., Castelli, M. P., Loi, B., Porcu, A., Martorelli, M., Miliano, C., et al. (2015b). Native CB1 receptor affinity, intrisic activity and accumbens shell dopamine stimulant properties of third generation spice/K2 cannabinoids: BB-22, 5F-PB-22, 5F-AKB-48 and STS-135. Neuropharmacology. 105, 630–638. doi: 10.1016/j.neuropharm.2015.11.017
Dean, B. V., Stellpflug, S. J., Burnett, A. M., and Engebretsen, K. M. (2013). 2C or not 2C: phenethylamine designer drug review. J. Med. Toxicol. 9, 172–178. doi: 10.1007/s13181-013-0295-x
Deluca, P., Davey, Z., Corazza, O., Di Furia, L., Farre, M., Flesland, L. H., et al. (2012). Identifying emerging trends in recreational drug use; outcomes from the Psychonaut Web Mapping Project. Prog. Neuro Psychopharmacol. Biol. Psychiatry 39, 221–226. doi: 10.1016/j.pnpbp.2012.07.011
Denooz, R., Vanheugen, J.-C., Frederich, M., de Tullio, P., and Charlier, C. (2013). Identification and structural elucidation of four cannabimimetic compounds (RCS-4, AM-2201, JWH-203 and JWH-210) in seized products. J. Anal. Toxicol. 37, 56–63. doi: 10.1093/jat/bks095
Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M. A., Spina, L., Cadoni, C., et al. (2004). Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 47, 227–241. doi: 10.1016/j.neuropharm.2004.06.032
Drug Policy Department Italian Presidency of the Council of Ministers (2013a). National Action Plan on New Psychoactive Substances, Rome.
Drug Policy Department Italian Presidency of the Council of Ministers (2013b). New Psychoactive Substances. Rome.
Drug Policy Department Italian Presidency of the Council of Ministers (2014). Annual Report on Drug. Rome.
D'Souza, D. C., Fridberg, D. J., Skosnik, P. D., Williams, A., Roach, B., Singh, N., et al. (2012). Dose-related modulation of event-related potentials to novel and target stimuli by intravenous delta9-THC in humans. Neuropsychopharmacology 37, 1632–1646. doi: 10.1038/npp.2012.8
EMCDDA (European Drug Report) (2014). Trends and Developments. Available online at: http://www.emcdda.europa.eu/publications/edr/trends-developments/2014 (Accessed November 14, 2015).
EMCDDA (2009a). Annual Report on the State of the Drugs Problem in Europe. Available online at: http://www.emcdda.europa.eu/publications/annual-report/2009 (Accessed October 29, 2015).
EMCDDA (European Drug Report) (2015a). Trends and Developments. Available online at: http://www.emcdda.europa.eu/publications/edr/trends-developments/2015 (Accessed November 14, 2015).
EMCDDA (New psychoactive substances in Europe) (2015b). An Update from the EU Early Warning System. Available online at: http://www.emcdda.europa.eu/publications/2015/new-psychoactive-substances (Accessed December 14, 2015).
Eshleman, A. J., Forster, M. J., Wolfrum, K. M., Johnson, R. A., Janowsky, A., and Gatch, M. B. (2014). Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl.) 231, 875–888. doi: 10.1007/s00213-013-3303-6
Eshleman, A. J., Wolfrum, K. M., Hatfield, M. G., Johnson, R. A., Murphy, K. V., and Janowsky, A. (2013). Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 85, 1803–1815. doi: 10.1016/j.bcp.2013.04.004
Every-Palmer, S. (2011). Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 117, 152–157. doi: 10.1016/j.drugalcdep.2011.01.012
Fantegrossi, W. E., Murnane, K. S., and Reissig, C. J. (2008). The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 75, 17–33. doi: 10.1016/j.bcp.2007.07.018
Fass, J. A., Fass, A. D., and Garcia, A. S. (2012). Synthetic Cathinones (Bath Salts): legal status and patterns of abuse. Ann. Pharmacother. 46, 436–441. doi: 10.1345/aph.1Q628
Fattore, L., and Fratta, W. (2011). Beyond THC: The new generation of cannabinoid designer drugs. Front. Behav. Neurosci. 5:60. doi: 10.3389/fnbeh.2011.00060
Fattore, L., Cossu, G., Martellotta, C. M., and Fratta, W. (2001). Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl). 156, 410–416. doi: 10.1007/s002130100734
Fontanilla, D., Johannessen, M., Hajipour, A. R., Cozzi, N. V., Jackson, B., and Ruoho, A. E. (2010). NIH Public Access. Science, Vol. 323, 934–937. doi: 10.1126/science.1166127
Fraser, F. (2014). New Psychoactive Substances – Evidence Review, Safer Communities Analytical Unit. Edinburgh: Scottish Government Social Research.
Gatch, M. B., and Forster, M. J. (2014). Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav. Pharmacol. 25, 750–757. doi: 10.1097/FBP.0000000000000093
Gatch, M. B., and Forster, M. J. (2015). Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behav. Pharmacol. 26, 460–468. doi: 10.1097/FBP.0000000000000150
Gatch, M. B., Rutledge, M. A., Carbonaro, T., and Forster, M. J. (2009). Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology (Berl). 204, 715–724. doi: 10.1007/s00213-009-1501-z
Gatch, M. B., Taylor, C. M., and Forster, M. J. (2013). Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav. Pharmacol. 24, 437–447. doi: 10.1097/FBP.0b013e328364166d
German, C. L., Fleckenstein, A. E., and Hanson, G. R. (2014). Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 97, 2–8. doi: 10.1016/j.lfs.2013.07.023
Glass, M., Dragunow, M., and Faull, R. L. (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318. doi: 10.1016/S0306-4522(96)00428-9
González, D., Ventura, M., Caudevilla, F., Torrens, M., and Farre, M. (2013). Consumption of new psychoactive substances in a Spanish sample of research chemical users. Hum. Psychopharmacol. 28, 332–340. doi: 10.1002/hup.2323
Goodman, J., and Packard, M. G. (2015). The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiol. Learn. Mem. 125, 1–14. doi: 10.1016/j.nlm.2015.06.008
Gregg, R. A., Baumann, M. H., Partilla, J. S., Bonano, J. S., Vouga, A., Tallarida, C. S., et al. (2015). Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol. 172, 883–894. doi: 10.1111/bph.12951
Grim, T. W., Wiebelhaus, J. M., Morales, A. J., Negus, S. S., and Lichtman, A. H. (2015). Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice. Drug Alcohol Depend. 150, 31–37. doi: 10.1016/j.drugalcdep.2015.01.022
Gunderson, E. W., Haughey, H. M., Ait-Daoud, N., Joshi, A. S., and Hart, C. L. (2012). “Spice” and “K2” Herbal Highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am. J. Addict. 21, 320–326. doi: 10.1111/j.1521-0391.2012.00240.x
Hadlock, G. C., Webb, K. M., McFadden, L. M., Chu, P. W., Ellis, J. D., Allen, S. C., et al. (2011). 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 339, 530–536. doi: 10.1124/jpet.111.184119
Heimer, L., Zahm, D. S., Churchill, L., Kalivas, P. W., and Wohltmann, C. (1991). Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41, 89–125. doi: 10.1016/0306-4522(91)90202-Y
Helander, A., Beck, O., Hägerkvist, R., and Hultén, P. (2013). Identification of novel psychoactive drug use in Sweden based on laboratory analysis–initial experiences from the STRIDA project. Scand. J. Clin. Lab Invest. 73, 400–406. doi: 10.3109/00365513.2013.793817
Helander, A., Bäckberg, M., Hultén, P., Al-Saffar, Y., and Beck, O. (2014). Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci. Int. 243, 23–29. doi: 10.1016/j.forsciint.2014.02.022
Herkenham, M., Lynn, A. B., Johnson, M. R., Melvin, L. S., de Costa, B. R., and Rice, K. C. (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583.
Hermanns-Clausen, M., Kneisel, S., Szabo, B., and Auwärter, V. (2013). Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 108, 534–544. doi: 10.1111/j.1360-0443.2012.04078.x
Hilário, M. R., Clouse, E., Yin, H. H., and Costa, R. M. (2007). Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci. 1:6. doi: 10.3389/neuro.07.006.2007
Hohmann, N., Mikus, G., and Czock, D. (2014). Effects and risks associated with novel psychoactive substances: mislabeling and sale as bath salts, spice, and research chemicals. Dtsch. Arztebl. Int. 111, 139–147. doi: 10.3238/arztebl.2014.0139
Hondebrink, L., Nugteren-van Lonkhuyzen, J. J., Van Der Gouwe, D., and Brunt, T. M. (2015). Monitoring new psychoactive substances (NPS) in The Netherlands: data from the drug market and the Poisons Information Centre. Drug Alcohol Depend. 147, 109–115. doi: 10.1016/j.drugalcdep.2014.11.033
Hwang, J.-Y., Kim, J.-S., Oh, J.-H., Hong, S.-I., Ma, S.-X., Jung, Y.-H., et al. (2015). The new stimulant designer compound pentedrone exhibits rewarding properties and affects dopaminergic activity. Addict. Biol. doi: 10.1111/adb.12299 [Epub ahead of print].
IACP (International Association of Chiefs of Police) (2012). The 2012 Annual Report of the Drug Recognition Expert Section, Alexandria, VA.
Iversen, L., Gibbons, S., Treble, R., Setola, V., Huang, X.-P., and Roth, B. L. (2013). Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 700, 147–151. doi: 10.1016/j.ejphar.2012.12.006
Iversen, L., White, M., and Treble, R. (2014). Designer psychostimulants: pharmacology and differences. Neuropharmacology 87, 59–65. doi: 10.1016/j.neuropharm.2014.01.015
Johnston, L. D., O'Malley, P. M., Bachman, J. G., and Schulenberg, J. E. (2013). Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings. Bethesda, MD: National Institute on Drug Abuse, 2013. Available online at www.monitoringthefuture.org
Justinova, Z., Tanda, G., Munzar, P., and Goldberg, S. R. (2004). The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl). 173, 186–194. doi: 10.1007/s00213-003-1693-6
Karila, L., Megarbane, B., Cottencin, O., and Lejoyeux, M. (2015). Synthetic cathinones: a new public health problem. Curr. Neuropharmacol. 13, 12–20. doi: 10.2174/1570159X13666141210224137
Karlsson, L., Andersson, M., Kronstrand, R., and Kugelberg, F. C. (2014). Mephedrone, Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin. Pharmacol. Toxicol. 115, 411–416. doi: 10.1111/bcpt.12253
Kehr, J., Ichinose, F., Yoshitake, S., Goiny, M., Sievertsson, T., Nyberg, F., et al. (2011). Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 164, 1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x
Kelly, J. P. (2011). Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 3, 439–453. doi: 10.1002/dta.313
Kersten, B. P., and McLaughlin, M. E. (2015). Toxicology and management of novel psychoactive drugs. J. Pharm. Pract. 28, 50–65. doi: 10.1177/0897190014544814
Khullar, V., Jain, A., and Sattari, M. (2014). Emergence of New Classes of Recreational Drugs—Synthetic Cannabinoids and Cathinones. J. Gen. Intern. Med. 29, 1200–1204. doi: 10.1007/s11606-014-2802-4
Kikura-Hanajiri, R., Kawamura, N. U. M., and Goda, Y. (2014). Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test. Anal. 6, 832–839. doi: 10.1002/dta.1584
Koob, G. F., and Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. doi: 10.1038/npp.2009.110
Kronstrand, R., Brinkhagen, L., Birath-Karlsson, C., Roman, M., and Josefsson, M. (2014). LC-QTOF-MS as a superior strategy to immunoassay for the comprehensive analysis of synthetic cannabinoids in urine. Anal. Bioanal. Chem. 406, 3599–3609. doi: 10.1007/s00216-013-7574-x
Kwilasz, A. J., and Negus, S. S. (2012). Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J. Pharmacol. Exp. Ther. 343, 389–400. doi: 10.1124/jpet.112.197780
Kyriakou, C., Marinelli, E., Frati, P., Santurro, A., Afxentiou, M., Zaami, S., et al. (2015). NBOMe : new potent hallucinogens – pharmacology, analytical methods, toxicities, fatalities : a review. Eur. Rev. Med. Pharmacol. Sci. 19, 3270–3281.
Le Roux, G., Bruneau, C., Lelièvre, B., Deguigne, M. B., Turcant, A., Harry, P., et al. (2015). Recreational phenethylamine poisonings reported to a French poison control center. Drug Alcohol Depend. 154, 46–53. doi: 10.1016/j.drugalcdep.2015.05.048
Lecca, D., Cacciapaglia, F., Valentini, V., and Di Chiara, G. (2006). Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology (Berl). 188, 63–74. doi: 10.1007/s00213-006-0475-3
Liechti, M. (2015). Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med. Wkly. 145:w14043. doi: 10.4414/smw.2015.14043
Lisek, R., Xu, W., Yuvasheva, E., Chiu, Y.-T., Reitz, A. B., Liu-Chen, L.-Y., et al. (2012). Mephedrone (“bath salt”) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 126, 257–262. doi: 10.1016/j.drugalcdep.2012.04.021
Loi, B., Corkery, J. M., Claridge, H., Goodair, C., Chiappini, S., Gimeno Clemente, C., et al. (2015). Deaths of individuals aged 16-24 years in the UK after usingmephedrone. Hum. Psychopharmacol. 30, 225–232. doi: 10.1002/hup.2423
Lessin, A. W., Long, R. F., and Parkes, M. W. (1965). Central Stimulant Actions of Alpha-Alkyl Substituted Tryptamines in Mice. Br. J. Pharmacol. Chemother. 24, 49–67. doi: 10.1111/j.1476-5381.1965.tb02079.x
López-Arnau, R., Martínez-Clemente, J., Pubill, D., Escubedo, E., and Camarasa, J. (2012). Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br. J. Pharmacol. 167, 407–420. doi: 10.1111/j.1476-5381.2012.01998.x
Lupica, C. R., and Riegel, A. C. (2005). Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48, 1105–1116. doi: 10.1016/j.neuropharm.2005.03.016
Maas, A., Wippich, C., Madea, B., and Hess, C. (2015). Driving under the influence of synthetic phenethylamines: a case series. Int. J. Legal Med. 129, 997–1003. doi: 10.1007/s00414-015-1150-1
Macfarlane, V., and Christie, G. (2015). Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 34, 147–153. doi: 10.1111/dar.12225
Marshell, R., Kearney-Ramos, T., Brents, L. K., Hyatt, W. S., Tai, S., Prather, P. L., et al. (2014). In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ(9)-THC in mice: inhalation versus intraperitoneal injection. Pharmacol. Biochem. Behav. 124, 40–47. doi: 10.1016/j.pbb.2014.05.010
Martellotta, M. C., Cossu, G., Fattore, L., Gessa, G. L., and Fratta, W. (1998). Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85, 327–330. doi: 10.1016/S0306-4522(98)00052-9
Martinotti, G., Lupi, M., Carlucci, L., Cinosi, E., Santacroce, R., Acciavatti, T., et al. (2015). Novel psychoactive substances: use and knowledge among adolescents and young adults in urban and rural areas. Hum Psychopharmacol. 30, 295–301. doi: 10.1002/hup.2486
Marusich, J. A., Grant, K. R., Blough, B. E., and Wiley, J. L. (2012). Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33, 1305–1313. doi: 10.1016/j.neuro.2012.08.003
Mátyás, F., Urbán, G. M., Watanabe, M., Mackie, K., Zimmer, A., Freund, T. F., et al. (2008). Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology 54, 95–107. doi: 10.1016/j.neuropharm.2007.05.028
Mavrikaki, M., Markaki, E., Nomikos, G. G., and Panagis, G. (2010). Chronic WIN55,212-2 elicits sustained and conditioned increases in intracranial self-stimulation thresholds in the rat. Behav. Brain Res. 209, 114–118. doi: 10.1016/j.bbr.2010.01.024
Maxwell, J. C. (2014). Psychoactive substances–some new, some old: a scan of the situation in the U.S. Drug Alcohol Depend. 134, 71–77. doi: 10.1016/j.drugalcdep.2013.09.011
Melis, M., Sagheddu, C., De Felice, M., Casti, A., Madeddu, C., Spiga, S., et al. (2014). Enhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in sardinian alcohol-preferring rats. J. Neurosci. 34, 12716–12724. doi: 10.1523/JNEUROSCI.1844-14.2014
Meririnne, E., Kajos, M., Kankaanpää, A., and Seppälä, T. (2006). Rewarding properties of 1-benzylpiperazine, a new drug of abuse, in rats. Basic Clin. Pharmacol. Toxicol. 98, 346–350. doi: 10.1111/j.1742-7843.2006.pto_243.x
Mills, B., Yepes, A., and Nugent, K. (2015). Synthetic Cannabinoids. Am. J. Med. Sci. 350, 59–62. doi: 10.1097/MAJ.0000000000000466
Monteiro, M. S., Bastos, M. D. L., Guedes de Pinho, P., and Carvalho, M. (2013). Update on 1-benzylpiperazine (BZP) party pills. Arch. Toxicol. 87, 929–947. doi: 10.1007/s00204-013-1057-x
Motbey, C. P., Clemens, K. J., Apetz, N., Winstock, A. R., Ramsey, J., Li, K. M., et al. (2013). High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J. Psychopharmacol. 27, 823–836. doi: 10.1177/0269881113490325
Negus, S. S., and Miller, L. L. (2014). Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol. Rev. 66, 869–917. doi: 10.1124/pr.112.007419
Nelson, M. E., Bryant, S. M., and Aks, S. E. (2014). Emerging drugs of abuse. Dis. Mon. 60, 110–132. doi: 10.1016/j.disamonth.2014.01.001
Nichols, D. E. (2004). Hallucinogens. Pharmacol. Ther. 101, 131–181. doi: 10.1016/j.pharmthera.2003.11.002
NIDA (National Institute on Drug Abuse) (2012). Monitoring the Future 2012 Survey Results. Available online at: https://www.drugabuse.gov/related-topics/trends-statistics/infographics/monitoring-future-2012-survey-results (Accessed October 29, 2015).
Nishimura, M., and Sato, K. (1999). Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci. Lett. 274, 131–134. doi: 10.1016/S0304-3940(99)00688-6
Ossato, A., Canazza, I., Trapella, C., Vincenzi, F., De Luca, M. A., Rimondo, C., et al. (2016). Effect of JWH-250, JWH-073 and their interaction on “tetrad,” sensorimotor, neurological and neurochemical responses in mice. Prog Neuropsychopharmacol. Biol Psychiatry. 15, 31–50. doi: 10.1016/j.pnpbp.2016.01.007
Paillet-Loilier, M., Cesbron, A., Le Boisselier, R., Bourgine, J., and Debruyne, D. (2014). Emerging drugs of abuse: current perspectives on substituted cathinones. Subst. Abuse Rehabil. 5, 37–52. doi: 10.2147/SAR.S37257
Palamar, J. J., Martins, S. S., Su, M. K., and Ompad, D. C. (2015). Self-reported use of novel psychoactive substances in a US nationally representative survey: Prevalence, correlates, and a call for new survey methods to prevent underreporting. Drug Alcohol Depend. 156, 112–119. doi: 10.1016/j.drugalcdep.2015.08.028
Panagis, G., Mackey, B., and Vlachou, S. (2014). Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step Back into the Future. Front. Psychiatry 5:92. doi: 10.3389/fpsyt.2014.00092
Papanti, D., Schifano, F., Botteon, G., Bertossi, F., Mannix, J., Vidoni, D., et al. (2013). “Spiceophrenia”: a systematic overview of “spice”-related psychopathological issues and a case report. Hum. Psychopharmacol. 28, 379–389. doi: 10.1002/hup.2312
Paus, T. (2005). Mapping brain maturation and cognitive development during adolescence. TrendsCogn Sci. 9, 60–68. doi: 10.1016/j.tics.2004.12.008
Prosser, J. M., and Nelson, L. S. (2012). The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol. 8, 33–42. doi: 10.1007/s13181-011-0193-z
Sanders, B., Lankenau, S. E., Bloom, J. J., and Hathazi, D. (2008). “Research chemicals”: tryptamine and phenethylamine use among high-risk youth. Subst. Use Misuse. 43, 389–402. doi: 10.1080/00952990701202970
Santacroce, R., Corazza, O., Martinotti, G., Bersani, F. S., Valeriani, G., and Di Giannantonio, M. (2015). Psyclones: a roller coaster of life? Hidden synthetic cannabinoids and stimulants in apparently harmless products. Hum. Psychopharmacol. 30, 265–271. doi: 10.1002/hup.2410
Sañudo-Peña, M. C., Tsou, K., Delay, E. R., Hohman, A. G., Force, M., and Walker, J. M. (1997). Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci. Lett. 223, 125–128.
Schifano, F. (2013). “Novel psychoactive substances also known as ‘legal highs',” in Annual Report of the Chief MedicalOfficer. Public Mental Health Priorities: Investing in the Evidence, ed S. C Davies (London: Department of Health), 259.
Schifano, F., Corkery, J. M., and Cuffolo, G. (2007). Smokable (“ice,” “crystal meth”) and non smokable amphetamine-type stimulants: Clinical pharmacological and epidemiological issues, with special reference to the UK. Ann. Ist Super. Sanita 43, 110–115.
Schifano, F., Corkery, J., Naidoo, V., Oyefeso, A., and Ghodse, H. (2010). Overview ofamphetamine-type stimulant mortality data–UK, 1997-2007. Neuropsychobiology 61, 122–130. doi: 10.1159/000279302
Schifano, F., Corkery, J., and Ghodse, A. H. (2012). Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone;‘meow meow') in the UK. J. Clin. Psychopharmacol. 32, 7104. doi: 10.1097/JCP.0b013e318266c70c
Schifano, F., Orsolini, L., Duccio Papanti, G., and Corkery, J. M. (2015). Novel psychoactive substances of interest for psychiatry. World Psychiatry 14, 15–26. doi: 10.1002/wps.20174
Schindler, C. W., Thorndike, E. B., Goldberg, S. R., Lehner, K. R., Cozzi, N. V., Brandt, S. D., et al. (2015). Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl). doi: 10.1007/s00213-015-4057-0. [Epub ahead of print].
Seely, K. A., Prather, P. L., James, L. P., and Moran, J. H. (2011). Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol. Interv. 11, 36–51. doi: 10.1124/mi.11.1.6
Seely, K. A., Brents, L. K., Radominska-Pandya, A., Endres, G. W., Keyes, G. S., Moran, J. H., et al. (2012). A major glucuronidated metabolite of JWH-018 is a neutral antagonist at CB1 receptors. Chem. Res. Toxicol. 39, 234–243. doi: 10.1021/tx3000472
Sidhpura, N., and Parsons, L. H. (2011). Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology 61, 1070–1087. doi: 10.1016/j.neuropharm.2011.05.034
Simmler, L. D., Buser, T. A., Donzelli, M., Schramm, Y., Dieu, L.-H., Huwyler, J., et al. (2013). Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 168, 458–470. doi: 10.1111/j.1476-5381.2012.02145.x
Simmler, L. D., Rickli, A., Schramm, Y., Hoener, M. C., and Liechti, M. E. (2014). Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 88, 237–244. doi: 10.1016/j.bcp.2014.01.024
Simonato, P., Corazza, O., Santonastaso, P., Corkery, J., Deluca, P., Davey, Z., et al. (2013). Novel psychoactive substances as a novel challenge for health professionals: results from an Italian survey. Hum. Psychopharmacol. 28, 324–331. doi: 10.1002/hup.2300
Smith, J. P., Sutcliffe, O. B., and Banks, C. E. (2015). An overview of recent developments in the analytical detection of new psychoactive substances (NPSs). Analyst 140, 4932–4948. doi: 10.1039/C5AN00797F
Sogawa, C., Sogawa, N., Tagawa, J., Fujino, A., Ohyama, K., Asanuma, M., et al. (2007). 5-Methoxy-N,N-diisopropyltryptamine (Foxy), a selective and high affinity inhibitor of serotonin transporter. Toxicol. Lett. 170, 75–82. doi: 10.1016/j.toxlet.2007.02.007
Solinas, M., Panlilio, L. V., Justinova, Z., Yasar, S., and Goldberg, S. R. (2006). Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 1, 1194–1206. doi: 10.1038/nprot.2006.167
Spaderna, M., Addy, P. H., and D'souza, D. C. (2013). Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl) 228, 525–540. doi: 10.1007/s00213-013-3188-4
Sussman, S., Skara, S., and Ames, S. L. (2008). Substance abuse among adolescents. Substance Use Misuse 43, 1802–1828. doi: 10.1080/10826080802297302
Tanda, G., Pontieri, F. E., and Di Chiara, G. (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050. doi: 10.1126/science.276.5321.2048
Tanda, G., Munzar, P., and Goldberg, S. R. (2000). Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat. Neurosci. 3, 1073–1074. doi: 10.1038/80577
Teixeira-Gomes, A., Costa, V. M., Feio-Azevedo, R., de Lourdes Bastos, M., Carvalho, F., and Capela, J. P. S. (2014). The neurotoxicity of amphetamines during the adolescent period. Int. J. Dev. Neurosci. 41, 1–18. doi: 10.1016/j.ijdevneu.2014.12.001
Thomas, S., Bliss, S., and Malik, M. (2012). Suicidal ideation and self-harm following K2 use. J. Okla. State Med. Assoc. 105, 430–433.
Tittarelli, R., Mannocchi, G., Pantano, F., and Romolo, F. S. (2015). Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 13, 26–46. doi: 10.2174/1570159X13666141210222409
Vigolo, A., Ossato, A., Trapella, C., Vincenzi, F., Rimondo, C., Seri, C., et al. (2015). Novel halogenated derivates of JWH-018: behavioral and binding studies in mice. Neuropharmacology 95, 68–82. doi: 10.1016/j.neuropharm.2015.02.008
UNODC (2015). The Challenge of Synthetic Drugs in East and South-East Asia and Oceania. Trends and Patterns of Amphetamine-type Stimulants and New Psychoactive Substances. World Drugs Report. Wien: Global SMART Programme.
Valente, M. J., Guedes de Pinho, P., de Lourdes Bastos, M., Carvalho, F., and Carvalho, M. (2014). Khat and synthetic cathinones: a review. Arch. Toxicol. 88, 15–45. doi: 10.1007/s00204-013-1163-9
Valjent, E., and Maldonado, R. (2000). A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology 147, 436–438. doi: 10.1007/s002130050013
Van Amsterdam, J., Brunt, T., and van den Brink, W. (2015). The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J. Psychopharmacol. 29, 254–263. doi: 10.1177/0269881114565142
Vlachou, S., Nomikos, G. G., and Panagis, G. (2005). CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology (Berl) 179, 498–508. doi: 10.1007/s00213-004-2050-0
Vlachou, S., Nomikos, G. G., Stephens, D. N., and Panagis, G. (2007). Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav. Pharmacol. 18, 311–319. doi: 10.1097/FBP.0b013e3282186cf2
Volkow, N. D., Fowler, J. S., and Wang, G.-J. (2003). The addicted human brain: insights from imaging studies. J. Clin. Invest. 111, 1444–1451. doi: 10.1172/JCI18533
Völlm, B. A., de Araujo, I. E., Cowen, P. J., Rolls, E. T., Kringelbach, M. L., Smith, K. A., et al. (2004). Methamphetamine activates reward circuitry in drug naïve human subjects. Neuropsychopharmacology 29, 1715–1722. doi: 10.1038/sj.npp.1300481
Wang, X., Dow-Edwards, D., Keller, E., and Hurd, Y. L. (2003). Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694.
Watterson, L. R., Hood, L., Sewalia, K., Tomek, S. E., Yahn, S., Johnson, C. T., et al. (2012). The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “Bath Salts.” J. Addict. Res. Ther. pii (Suppl. 9):002. doi: 10.4172/2155-6105.S9-002
Watterson, L. R., Kufahl, P. R., Nemirovsky, N. E., Sewalia, K., Grabenauer, M., Thomas, B. F., et al. (2014). Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict. Biol. 19, 165–174. doi: 10.1111/j.1369-1600.2012.00474.x
Wells, D. L., and Ott, C. A. (2011). The “new” marijuana. Ann. Pharmacother. 45, 414–417. doi: 10.1345/aph.1P580
Welter-Luedeke, J., and Maurer, H. H. (2015). New psychoactive substances. Ther. Drug Monit. 38, 4–11. doi: 10.1097/FTD.0000000000000240
Whelpton, R. (2007). Speed, Ecstasy, Ritalin: the science of amphetamines. Br. J. Clin. Pharmacol. 63, 763–763. doi: 10.1111/j.1365-2125.2006.02818.x
Wikström, M., Holmgren, P., and Ahlner, J. (2004). A2 (N-benzylpiperazine) a new drug of abuse in Sweden. J. Anal. Toxicol. 28, 67–70. doi: 10.1093/jat/28.1.67
Wiley, J. L., Marusich, J. A., Martin, B. R., and Huffman, J. W. (2012). 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 123, 148–153. doi: 10.1016/j.drugalcdep.2011.11.001
Wiley, J. L., Marusich, J. A., and Huffman, J. W. (2014). Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 97, 55–63. doi: 10.1016/j.lfs.2013.09.011
Winstock, A. R., and Barratt, M. J. (2013). The 12-month prevalence and nature of adverse experiences resulting in emergency medical presentations associated with the use of synthetic cannabinoid products. Hum. Psychopharmacol. Clin. Exp. 28, 390–393. doi: 10.1002/hup.2292
Winstock, A., and Schifano, F. (2009). “Disorders relating to the use of ecstasy, other ‘party drugs' and khat,” in New Oxford Textbook of Psychiatry eds M. Gelder, N. Andreasen, J. J. Lopez-Ibor, and J. Geddes (Oxford: Oxford University Press), 494–502.
Winstock, A. R., Mitcheson, L. R., Deluca, P., Davey, Z., Corazza, O., and Schifano, F. (2011). Mephedrone, new kid for the chop? Addiction 106, 154–161. doi: 10.1111/j.1360-0443.2010.0330.x
Wood, D. M., Heyerdahl, F., Yates, C. B., Dines, A. M., Giraudon, I., and Hovda, K. E. (2014). The european drug emergencies network (Euro-DEN). Clin. Toxicol. (Phila). 52, 239–241. doi: 10.3109/15563650.2014.898771
Wood, D. M., Sedefov, R., Cunningham, A., and Dargan, P. I. (2015). Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin. Toxicol. 53, 85–92. doi: 10.3109/15563650.2015.1004179
Wright, M. J. Jr., Angrish, D., Aarde, S. M., Barlow, D. J., Buczynski, M. W., Creehan, K. M., et al. (2012). Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in wistar and sprague-dawley rats. PLoS ONE 7:e44652. doi: 10.1371/journal.pone.0044652
Zahm, D. S., and Brog, J. S. (1992). On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50, 751–767. doi: 10.1016/0306-4522(92)90202-D
Zawilska, J. B. (2015). “Legal Highs”–An Emerging Epidemic of Novel Psychoactive Substances. Int. Rev. Neurobiol. 120, 273–300. doi: 10.1016/bs.irn.2015.02.009
Keywords: novel psychoactive substances, NPS, cannabinoids, psychostimulants, JWH-018, Spice
Citation: Miliano C, Serpelloni G, Rimondo C, Mereu M, Marti M and De Luca MA (2016) Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 10:153. doi: 10.3389/fnins.2016.00153
Received: 02 November 2015; Accepted: 21 March 2016;
Published: 19 April 2016.
Edited by:
Styliani Vlachou, Dublin City University, IrelandReviewed by:
Joseph F. Cheer, University of Maryland School of Medicine, USAViviana Trezza, University “Roma Tre,” Italy
Copyright © 2016 Miliano, Serpelloni, Rimondo, Mereu, Marti and De Luca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Antonietta De Luca, ZGVsdWNhQHVuaWNhLml0
 Cristina Miliano
Cristina Miliano Giovanni Serpelloni
Giovanni Serpelloni Claudia Rimondo
Claudia Rimondo Maddalena Mereu
Maddalena Mereu Matteo Marti
Matteo Marti Maria Antonietta De Luca
Maria Antonietta De Luca