
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 17 February 2015
Sec. Neuroendocrine Science
Volume 9 - 2015 | https://doi.org/10.3389/fnins.2015.00012
This article is part of the Research TopicFrom Sex Differences in Neuroscience to a Neuroscience of Sex Differences: New Directions and PerspectivesView all 17 articles
Testosterone influences the brain via organizational and activational effects. Numerous relevant studies on rodents and a few on humans focusing on specific behavioral and cognitive parameters have been published. The results are, unfortunately, controversial and puzzling. Dosing, timing, even the application route seem to considerably affect the outcomes. In addition, the methods used for the assessment of psychometric parameters are a bit less than ideal regarding their validity and reproducibility. Metabolism of testosterone contributes to the complexity of its actions. Reduction to dihydrotestosterone by 5-alpha reductase increases the androgen activity; conversion to estradiol by aromatase converts the androgen to estrogen activity. Recently, the non-genomic effects of testosterone on behavior bypassing the nuclear receptors have attracted the interest of researchers. This review tries to summarize the current understanding of the complexity of the effects of testosterone on brain with special focus on their role in the known sex differences.
Despite current efforts of the European commission to combat gender issues with respect to gender equality, men and women are different in several important aspects (Cahill, 2014). These aspects include cognitive functioning and behavioral traits. Some of these may be socially induced, but scientists have showed on intact animals that other factors such as genetics and gender itself are mostly responsible forthe sex differences in behavior and cognition. Therefore, the current research strategies are calling for including both males and females in the research in order to report the possible gender differences (Ruigrok et al., 2014). Indeed, the exact mechanisms and reasons of sex differences in brain structures that mediate some of these functional dissimilarities are unknown. Genetics and endocrine factors are the most prominent biological explanations and are interconnected. Testosterone is the major male sex hormone. It is present in women, although in much lower concentrations. Testosterone has also been intensively studied in relation to sex differences and behavioral functions. This review focuses on physiology of testosterone to give the reader understanding of the mechanisms and complexity of testosterone action and then tries to summarize the studies and experiments focusing on the functional changes in anxiety, depression, spatial abilities and memory. Readers interested in sex differences and brain structures might find the needed information in the recently published focused review (Filova et al., 2013).
Testosterone is produced mainly in Leydig cells of testes in males, and in ovaries in females. In both, testosterone can be synthetized in the adrenal gland cortex (Burger, 2002; Dohle et al., 2003). However, in addition to the classic steroidogenic organs such as gonads, adrenals and even placenta, the active biosynthesis of steroids also occurs in the brain (Mellon et al., 2001). This synthesis can be either de novo from the cholesterol, or testosterone is derived from classical steroids as is deoxycorticosterone or progesterone, which enter through blood stream into nervous system. The latter one depends on the enzymatic ability of the neural region or cell. The key regulatory enzyme is Steroidogenic acute regulatory protein (StAR) (Miller and Auchus, 2011). This phosphoprotein mediates the transfer of cholesterol from the outer to the inner mitochondrial membrane, from where cholesterol can be further processed by corresponding enzymes. The StAR gene is expressed solely in the steroidogenic tissues. However, StAR mRNA expression in a rat brain was first shown by Furukawa (Furukawa et al., 1998) and confirmed in humans and mouse brains in several regions by immunohistochemistry.
The complexity of testosterone mechanism of action is underlined by its metabolism and steroid nature. The classical view suggests genomic mechanism, i.e., after translocation into cytoplasm, testosterone binds the androgen receptor, and subsequently after transportation into the nucleus it binds on the hormone response element at DNA, where it activates or silences the expression of genes and subsequent protein synthesis (Tsai and O'malley, 1994). During recent years, a new pathway for non-genomic mechanism was shown. This can include activating the membrane receptors and thus activating the second messengers, or after translocation to the cell, testosterone can either directly activate second messenger intracellular cascade, or can bind to its respective receptor and as a complex of hormone-receptor it can activate the second messenger cascade (Michels and Hoppe, 2008). Additionally, testosterone can be changed into either estradiol by aromatase or into dihydrotestosterone by reductase. The pathway depends on the enzymatic equipment of the cells.
The synthesis of sex hormones is ultimately controlled by gonadotropin-releasing hormone (GnRH), which is produced by the hypothalamus and which stimulates the pituitary gland to release luteinizing hormone and follicle stimulating hormone, where LH increases the expression of StaR protein in target cells (Ubuka et al., 2014). GnRH secretion in adulthood is pulsatile and highest during sleep with subsequent highest peaks of testosterone to be during the early morning hours (Lord et al., 2014). Nevertheless, the testosterone levels decline gradually with aging, mainly due to the attrition of Leydig cells and hypothalamic GnRH pulse generation slow down. Rapid drop can be observed in the 6th decade of life in males.(Basaria, 2013). A higher incidence of mood disorders that occurs with aging is then related to decreased testosterone and/or other androgens. However, not all studies agree with this simple explanation. Sartorius et al. showed, that there was no decline in testosterone levels in males who self-reported to be in very good health. Indeed, a subgroup of patients who were smoking and/or obese was associated with age related decline in serum androgens (Sartorius et al., 2012). Similarly, Camacho et al. reported that lifestyle factors and body weight were more important in maintaining the plasma testosterone levels than aging itself (Camacho et al., 2013).
In any respect, the causal role of testosterone deficiency and behavioral disorders including effect on cognitive abilities is still debated. Therefore, in the next chapters we will try to summarize the main experimental studies on individual behavioral traits.
Our daily decision-making as well as response to stress is our everyday experience. Indeed, many factors contribute and even more factors modify the decision-making and stress reaction, with anxiety level to be one of them. Nevertheless, it has clearly been shown, and recently reviewed that women show higher anxiety in comparison to men (Mchenry et al., 2014). From all behavioral parameters, the anxiety seems to be most sensitive to testosterone. The most cited paper analyzing the effects of testosterone on anxiety in mice has shown in several experiments that testosterone—either endogenous or exogenous decreased anxiety in elevated plus maze (Aikey et al., 2002). In addition, the same study showed that this anxiolytic effect of testosterone is dose-dependent and very likely mediated by 5-alpha reductase that reduces testosterone to dihydrotestosterone. The study was conducted in male mice, but similar anxiolytic effects of single testosterone administration resulted in reduced fear of healthy women (Van Honk et al., 2005). In rats, a single testosterone injection did not reduce anxiety, however, a repeated administration had anxiolytic effects tested by the burying behavior test (Fernandez-Guasti and Martinez-Mota, 2005). A possible mechanism can include the androgen receptor, as its blockade has been shown to prevent the testosterone-induced anxiolysis. Similar results were obtained in our experiment. However, the anxiolytic effect was observed only in the light-dark box. We were not able to reproduce the anxiolytic effects of testosterone in the elevated plus maze and in the open field (Hodosy et al., 2012). On the other hand, flutamide alone had anxiolytic effects in the open field. This suggests that the association between testosterone and anxiety might not be linear. A number of experiments on gonadectomized rats from the lab of professor Frye further showed that dihydrotestosterone 3-alpha metabolites can be the mediators of testosterone anxiolytic effects (Edinger and Frye, 2004, 2005). In addition, blockade of the dihydrotestosterone transformation to 3-alpha androstanediol by a 3-alpha hydroxysteroid dehydrogenase inhibitor prevented the anxiolysis (Frye and Edinger, 2004). Age-related decline in cognitive and affective functions was associated with lower concentrations of testosterone metabolites in the hippocampus. Again, this effect blocked by administration of 3-alpha metabolites administration (Frye et al., 2010). Another mechanism of anxiolytic effect of testosterone was explained in recently published experiment, where exogenous or endogenous opioids could modulate anxiolysis (Khakpai, 2014). In this study, the gama aminobutyric acid systemthat has been proposed in the past, on the other hand, did not alleviate the anxiety level (Roohbakhsh et al., 2011). An important determinant of the postnatal association between anxiety and testosterone or its metabolites might be prenatal stress. Stress induced during gestation resulted in both, reduced testosterone and increased anxiety of the adult offspring (Walf and Frye, 2012). Taken together, the results are consistent and despite differences in the methodology it seems clear that testosterone reduces anxiety in both genders. Its higher concentrations in men might be the reason for the sex differences in anxiety. However, a very important study in rhesus monkeys showed that pharmacological castration reduced and testosterone supplementation normalized anxiety levels (Suarez-Jimenez et al., 2013). A result that is in contrast to the majority of literature of experiments in rodents. Of course, this discrepancy might be discussed with major differences in the methodology—other behavioral tests, and in the intervention—surgical vs. pharmacologic castration. But in general, experiments on monkeys are more relevant to human behavior and, thus, this study must not be overseen. Some of the animal experiments on testosterone and anxiety are summarized in Table 1.
Although the depressive disorder is more prevalent in females (Bebbington, 1996) when compared to males, the prevalence of depression in males increases with age (Khera, 2013) as plasma testosterone drops. Consequently, many experiments and studies were performed to confirm the causative role of testosterone decline in depression pathogenesis. Indeed, these studies were not triggered by the lone fact of testosterone decline and sex difference in prevalence of depressive disorders. In depressive disorders with decreased libido and low testosterone, the androgen hormone replacement therapy was at least as effective as serotonin reuptake transporters (Kranz et al., 2014). Further it was investigated that testosterone can modulate serotonergic transmission, where serotonin plays a crucial role in depression development (Jovanovic et al., 2014). But it is not only the prevalence of depression that differ between sexes. In a study on opposite sex twins, it has been demonstrated that also the etiology of depression is different in men and women (Kendler and Gardner, 2014). Whether testosterone plays a major role in the sex differences in depression is unclear, but a number of studies indicate that it can affect the mood of depressive patients as well as healthy probands (Mchenry et al., 2014). Nevertheless, it is only one of many biological factors potentially responsible for the sex differences in depression. These were reviewed recently (Altemus et al., 2014). Observational studies on older men revealed that their depressive symptoms are associated with low plasma testosterone (Joshi et al., 2010). Low testosterone and depressive symptoms are both associated with the risk of falls, which are important for life expectancy in the elderly (Kurita et al., 2014). Similarly, in women testosterone concentrations are lower in depressive patients when compared to healthy controls (Kumsar et al., 2014). However, standard antidepressant treatment leads to normalization of testosterone. This suggests that the causality could be different than predicted—depression lowers testosterone. On the other hand, in both men and women, testosterone supplementation leads to improvement of depressive symptoms (Pope et al., 2003; Miller et al., 2009). However, not all interventional studies confirmed the anti-depressant effect of testosterone. At least in one published randomized controlled trial, the effects of testosterone were comparable to placebo effects (Seidman et al., 2001). Similarly, not all observational studies show a consistent picture. At least in one small study, depressive women had higher testosterone (Weber et al., 2000). When publication bias and the high intra- and inter-individual variability of testosterone are taken into account, these small negative or contradictory studies could be even more important. The meta-analyses of the published studies are also to be taken into account. In a meta-analysis of the effects of testosterone on depression, the anti-depressant effect was positive, at least in patients suffering from hypogonadism (Zarrour et al., 2009). The biology of the association between testosterone and depression has been reviewed recently (Mchenry et al., 2014). In an animal model of aging the associated depressive-like behavior correlated with lower testosterone (Egashira et al., 2010). Aged mice of both sexes benefited from testosterone supplementation. In the forced swim test the aged mice treated with testosterone or its metabolites spent less time immobile suggesting that the antidepressant effect of testosterone is mediated via several pathways including the androgen and the estrogen receptor (Frye and Walf, 2009). Another experiment on intact rats revealed that the effect of testosterone on depression is dose-dependent (Buddenberg et al., 2009). Interestingly, similar experiment on gonadectomized rats showed that the testosterone metabolite—3-alpha androstanediol, but not testosterone reverted the depression induced by gonadectomy (Frye et al., 2010). Selected animal experiments on the effects of testosterone on depression are compared in Table 2.
Spatial cognitive abilities as well as general cognition and memory decline with aging together with the testosterone levels. During the productive ages and even in early adulthood, men generally outperform women in spatial abilities (Linn and Petersen, 1985). Especially, mental rotation shows a clear sex difference in favor of men. Not surprisingly, observational studies have focused on the association between testosterone and spatial abilities. Some studies have found a positive relationship between testosterone and mental rotation in men (Silverman et al., 1999). Error rate as well as the reaction time negatively correlated with testosterone (Hooven et al., 2004). However, it is not only the actual concentration of testosterone that is studied in relation to spatial performance. Prenatal testosterone and its proxy—the finger length ratio (second to fourth digit) seem to have a stronger association with figure-disembedding and targeting, as additional spatial abilities (Falter et al., 2006). In this study, mental rotation was affected only by sex. In another study, actual testosterone was not associated with spatial abilities, but prenatal testosterone correlated positively with spatial abilities in women (Kempel et al., 2005). In line with these findings is the lack of an association between actual salivary testosterone levels and mental rotation in men and women (Puts et al., 2010). However, in a large observational study analyzing spatial abilities in adult men from various age categories, low testosterone was associated with better spatial visualization (Yonker et al., 2006). In a very interesting study, it was found that in men, the pubertal concentrations of testosterone are negatively associated with mental rotation in the adulthood (Vuoksimaa et al., 2012). In the same paper, the comparison of twins is reported. The twin with higher testosterone scored worse in the mental rotation tests. The results are contradictory, but may depend on the test used for the assessment of spatial abilities. When virtual Morris water maze was used, a positive correlation between testosterone and spatial navigation was found in women, but not in men (Burkitt et al., 2007). The size of the corpus callosum seems to add complexity in the relationship between spatial abilities and testosterone (Karadi et al., 2006). This might be one of the causes for negative findings in studies where some of the determinants are missing (Kubranska et al., 2014). Another cause is likely the selection of the tested population. In gifted children, a negative correlation between salivary testosterone and spatial abilities was found (Ostatnikova et al., 1996). In Chinese men, the accuracy in mental rotation tests was comparable to Americans, but the reaction times were longer indicating that cultural differences could add to the variability of published results (Yang et al., 2007). Last but not least, genetic factors likely modulate the effect of testosterone. We have previously shown that at least in gifted boys, genetic polymorphisms influencing testosterone metabolism affect also its relationship to mental rotation (Celec et al., 2009, 2013). Especially, the CAG short tandem repeat in the exon 1 of the androgen receptor gene seems to be important for the action of testosterone and its metabolites (Nowak et al., 2014). Despite all complexity, the current picture indicates that the association between testosterone and spatial abilities is curvilinear and sex-dependent. In women higher testosterone is associated with better mental rotation, in men lower testosterone is associated with better spatial abilities. This seems to be true both for actual testosterone (Moffat and Hampson, 1996) and for prenatal testosterone (Grimshaw et al., 1995). Supplementation of testosterone in older men results in improvement of spatial abilities, but it is accompanied with changes in estradiol metabolism and it is likely that this interferes with modifications of spatial abilities (Janowsky et al., 1994). Even in rats, testosterone administration affects the strategy of the animals in spatial tasks (Spritzer et al., 2013). However, the interaction between testosterone and mental rotation tests is bidirectional. It has been shown that mental rotation testing affects testosterone, at least in women (Durdiakova et al., 2012). In Table 3, published experimental data on the effects of testosterone on spatial abilities are summarized.
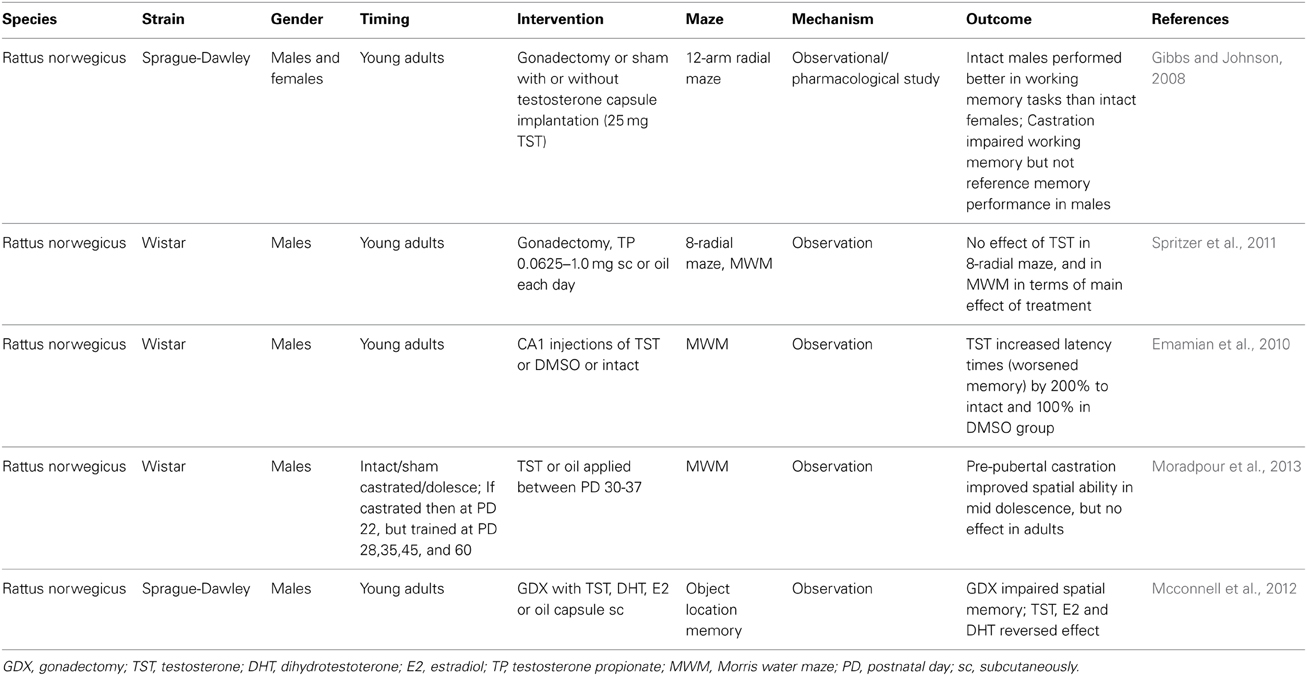
Table 3. Selected animal studies analyzing the relationship between testosterone and spatial abilities.
Women have better verbal memory, while men have an advantage in visual-spatial memory (Lewin et al., 2001). Especially, the difference in spatial memory has been studied in detail (Shah et al., 2013). In a meta-analysis of animal experiments using radial and water mazes, it has been confirmed that males outperform females in spatial memory tasks (Jonasson, 2005). The positive effect of testosterone on memory was, however, well documented in both sexes. Numerous clinical studies in postmenopausal women and men in the andropause showed improvements of learning and memory after testosterone supplementation. Even a short 6-week testosterone treatment resulted in improved spatial and verbal memory of older men (Cherrier et al., 2001). Testosterone has even showed a positive effect on spatial and verbal memory in Alzheimer disease patients (Cherrier et al., 2005). In young women, a single dose of testosterone improved spatial memory (Postma et al., 2000). However, the mechanism of action is unclear, as testosterone is now rather considered as a precursor than as a final hormone. In contrast to some animal experiments, observational studies in elderly men showed that lower testosterone, especially its free fraction was associated with worse visual-spatial memory (Moffat et al., 2002). This might be related to the tasks used, as the testosterone levels in men are related to the learning strategies, especially for spatial memory (Choi and Silverman, 2002). The results are, however, inconsistent. In a study analyzing the effects of a single testosterone injection on elderly men the treatment caused a worsening of verbal memory (Wolf et al., 2000). Similarly, biweekly injections of testosterone during 90 days resulted in memory decline (Maki et al., 2007). In addition, patients with prostate cancer that need hormonal castration via androgen deprivation therapy had worse verbal memory than heathy controls. Interestingly, estradiol—the estrogen metabolite of testosterone reversed the negative effects of androgen deprivation (Beer et al., 2006). Similar findings were found in elderly men and women where estradiol slowed down the age-related memory decline (Carlson and Sherwin, 2000). It seems that the effect of testosterone is dose-dependent and could be curvilinear even within sexes. At least in men, it has been demonstrated that moderate dosing resulted in improved memory, but not low and very high increases of testosterone (Cherrier et al., 2007). Similar results were found in adult male rats where only moderate testosterone doses resulted in spatial memory improvements (Spritzer et al., 2011). A relatively high dose of testosterone had no effect on memory or other analyzed behavioral measures in postmenopausal women in a well-designed and large study (Kocoska-Maras et al., 2011). In another study, women with surgically induced menopause received testosterone or placebo in addition to estrogen supplementation. Testosterone in this case worsened the verbal memory (Moller et al., 2010). A smaller, but longer study on postmenopausal women showed the complete opposite—improvement of verbal memory after testosterone treatment (Davison et al., 2011). Similarly to other behavioral measures memory will be influenced also by prenatal concentrations of testosterone (Bull et al., 2010). This effect might be mediated by the organizational effect of testosterone on brain structures such as amygdala or hippocampus (Ackermann et al., 2012). It has been shown that prenatal and neonatal testosterone affects stress coping and the effects of stress on learning abilities, at least in rodents (Shors and Miesegaes, 2002). Of course, genetic factors might also play a role. At least in one study the APOE genotype interacts with testosterone regarding the influence on age-related cognitive decline (Panizzon et al., 2014). More such studies can be expected in the near future.
Animal experiments help us to uncover the molecular and physiological mechanisms behind the phenotype correlations seen in human studies. The organizational effect of testosterone on the hippocampus, the major memory structure in the brain has been described a long time ago in rats using various mazes (Roof and Havens, 1992; Roof, 1993). In birds, evidence exists for a low testosterone period needed during the development of brain functions such as vocal memory (Korsia and Bottjer, 1991). In aged rats, an important experiment showed that the positive effect was found only when testosterone was administered. The testosterone metabolite, dihydrotestosterone, which cannot be metabolized to estradiol did not showed this effect (Bimonte-Nelson et al., 2003). This indicates that the effect of testosterone on memory is mediated by estradiol and the effect of aromatase which converts testosterone to estradiol. However, in male deer mice it has been shown that aging but not testosterone affects memory (Perrot-Sinal et al., 1998). Testosterone might rather increase synaptic plasticity as shown in rats (Schulz and Korz, 2010). Increased plasticity, however, only enables improved memory. But whether the potential is used depends on other factors including environment and timing and form of learning. Another advantage of animal experiments is the possibility to surgically localize the administration of testosterone into specific brain structures, which is ethically not possible in humans. Such studies showed that in adult male rats administration of any dose of testosterone or the androgen receptor blocker flutamide resulted in worsening of spatial memory (Naghdi et al., 2001). Similar injections of flutamide into amygdala had no effect on spatial memory, but testosterone negatively affected spatial memory and learning (Naghdi et al., 2003). When histological analyses were conducted, it was found that the intrahippocampal injections of testosterone led to an increase in the number of astrocytes in the target area (Emamian et al., 2010). Co-administration of a protein kinase AII inhibitor resulted in a synergistic negative effect on spatial memory (Khorshidahmad et al., 2012). Interestingly, the injection of anastrozole—an aromatase inhibitor resulted in improvement of spatial learning and memory tested in the Morris water maze (Moradpour et al., 2006). This further confirms that the negative effect of testosterone on memory is localized to hippocampus and is mediated by estradiol. When dihydrotestosterone—the androgen metabolite of testosterone was injected into the CA1 region of the hippocampus, spatial memory was improved (Babanejad et al., 2012). Testosterone has very likely an important role in the physiology of brain functions, but it might also be useful in some pathologies. In castrated rats, testosterone was able to reverse the ethanol-induced memory deficit (Khalil et al., 2005). In diabetic rats, the memory impairment was partially reversed by testosterone administration as well (Nayebi et al., 2014). An experiment in mice contributed to the growing list of confounding variables with the length of the photoperiod. Castration and supplementation with testosterone had no effect when the photoperiod was long (16 h of light per day). On the contrary, in mice housed with a short photoperiod (8 h of light per day), the effects on spatial memory were clearly seen (Pyter et al., 2006). A selection of the numerous animal experiments focusing on testosterone and memory are presented in Table 4.
Functional magnetic resonance imaging (fMRI) is a neuroimaging procedure that uses MRI technology for measuring the brain activity. The principle lies in detection of associated changes in blood flow and is useful in mapping the brain functional areas (Hofer et al., 2013). Several studies were performed using human volunteers for spatial tasks, memory as well as mood disorders/traits.
As for spatial tasks and mental rotation, the fMRI data are valuably consistent. In line with the previous studies, the males outperformed females in spatial tasks. Additionally, the fMRI showed stronger activation of left inferior parietal lobe in males compared to females. Also, the testosterone levels correlated with activation levels during mental rotation task in males. In females, the early follicular and midluteal phases were associated with better outcome and higher estradiol concentrations (Schoning et al., 2007). Likewise, a study of van Hemmen et al. confirmed previously reported sex differences in neural activation during mental rotation. Moreover, participants with complete androgen insensitivity syndrome presented with female-like neural activation pattern in the parietal lobe, indicating that gonadal hormone exposure rather than genetic sex itself plays role in brain functions (Van Hemmen et al., 2014). The menstrual cycle and thus the involvement of sex hormones, including testosterone, in spatial abilities was further confirmed by Pletzer et al. In their study, error rates linked with deactivation of inferior parietal lobes and prefrontal lobes were higher during luteal phase for verbal tasks, while in the follicular phase, spatial abilities in females were confirmed (Pletzer et al., 2011).
The analysis of behavior is not as straightforward as biochemical and molecular methods. Several alternatives exist for testing any brain function. However, the tests are variable and it is only a consensus, which can or should be used. The same applies to mazes used for the assessment of animal behavior. Even for the widely used Morris water maze several alternatives exist and numerous different parameters are used in the particular studies. An experiment showed that testosterone does not affect some of the measures analyzed in the water maze, but does affect other measures such as spatial working memory retention (Sandstrom et al., 2006).
One of the major factors that might explain the differences between the results of various studies is the variability of the examined populations. As mentioned above, the cultural differences, sex and age have all been shown to impact the physiological effects of testosterone. In animal experiments, chosen species and the particular strain is also of importance. Looking at the studies in non-human primates in contrast to the majority of rodent studies the results are mostly negative. For example, testosterone manipulations in rhesus monkeys did not alter their working and reference memory, although emotional processing was affected. Indeed, the treatment for testosterone might have not last long enough to affect the cognition (Kelly et al., 2014). Other possible explanations might be due to low number of animals included, but also to physiological differences including body size and the concluding testosterone kinetics (King et al., 2012). Specific behavioral tests might also be responsible for the differences observed (Lacreuse et al., 2012).
There are several possibilities as to what kind of biological samples should be used for the testosterone measurement. Plasma, saliva, urine are available and all have some strengths and weaknesses. The simple scheme of free—bioactive fraction of testosterone that should be assessed using salivary testosterone or plasma albumin and sex hormone binding globulin is not correct. Bound testosterone has its effects on target tissues and it is not clear which of the potential biological liquids is robust against technical and biological variability. One of the exotic possibilities is measurement of testosterone in the hair. The concentration in the hair might, however, be relevant as it integrates all the intra-individual variability of testosterone (Dettenborn et al., 2013).
Testosterone undergoes several biorhythms. In some studies, even the best-known circadian rhythm is not taken into account. Implants that slowly release testosterone totally ignore daily variations that occur physiologically. Other rhythms such as infradian cycles are completely forgotten when experiments are designed. But beyond cyclic variations, testosterone undergoes chaotic temporary changes that are usually described as noise. Although such research is lacking, it might be that it has some physiological role similarly to heart rhythm variability. In addition, the timing of behavioral analyses is of importance. While within 30 min after administration, non-genomic effects are important, later genomic effects are expected to be the major mediator. But this does not have to be true. Even later, the non-genomic effects are active in parallel with the gene expression changes. Only the study of the particular effects is more and more complicated, especially due to the complex kinetics of testosterone and the complex abilities being tested as proved in a focused experiment (Hawley et al., 2013). Additionally, physiological and also behavioral functions are exerted on a rhythmic basis. Timing the behavioral tests for light phase, while rodents usually are active during night can represent a major problem in animal behavior testing. Moreover, the central circadian clock is located in the suprachiasmatic nucleus of the hypothalamus and it receives signals directly from photoreceptors. GABA is thought to play a major role in coordinating the synchronized firing of suprachiasmatic neurons (Urbanski, 2011). However, steroid hormones may also exert their nongenomic function through GABA receptors. Disrupting the GABAergic system by untimed testosterone application, may be one other reason for controversy results in behavioral analysis. Alternatively, aging is strongly related to decline of circulating sex hormones, disrupting thus also circadian rhythms and leading to impaired sleep or cognitive functioning (Urbanski et al., 2014). Restoring natural circulating hormone pattern in older but also in younger animals could possibly lead also to more comprehensive results of sex hormones and behavior studies.
In most studies, testosterone is injected via i.p. or i.m. injections, but there are indices that to study the effects of testosterone on brain functions, the steroid has to be injected directly into the target brain structure. At least in one experiment directly comparing peripheral administration and intrahippocampal injections of testosterone it was shown that the peripheral route had no effect on learning and memory while central injections were effective (Harooni et al., 2008).
Testosterone in the experiments is sometimes used as butyrate, decanoate, undecanoate etc. These pharmacological forms have, however, variable kinetics and might therefore have also variable effects, especially in the brain, where the kinetics is of special importance (Filova et al., 2012). Dosing of testosterone seems to be of enormous importance. It varies between the experiments widely and should always be taken into account when evaluating the results. In experiments, moderate, but not very low or very high doses of testosterone had some effect on behavioral measures such as memory (Spritzer et al., 2011).
The effect of testosterone is influenced by several factors, but only some of them are known. These include genetic polymorphisms related to testosterone metabolism or other pathways related to cognitive functioning (Panizzon et al., 2014). Next generation sequencing and lower prices of genotyping will enable detailed studies focusing on the genetic factors and especially on the complex interactions between genetic, endocrine and other environmental factors.
Testosterone is currently seen more as a precursor of hormones. In most target tissues, testosterone is converted into metabolites such as dihydrotestosterone—a more potent androgen receptor ligand. The enzyme aromatase, on the other hand, can metabolize testosterone into estradiol—a ligand of the estrogen receptors. Further metabolites are being added to the list. But in general, it is of importance to recognize the role of the target tissue that can convert testosterone to inducers of very different signaling pathways. Without genetic or pharmacologic manipulation it is not possible to distinguish the effects when testosterone itself is administered.
The metabolism of testosterone makes studying the physiology of testosterone effects on the brain difficult. But the response of target tissues are similarly complex. Testosterone can be recognized by the androgen receptor inducing genomic effects—changes in gene expression. But the same testosterone can induce other signaling pathways that do not require changes in the use of the genomic information. These effects are called non-genomic and are studied for all steroid hormones. When testosterone is injected into the hippocampus together with a protein synthesis inhibitor that prevents genomic effects, spatial memory is improved in male rats (Naghdi et al., 2005). This points toward the possibility that non-genomic effects can be opposite to the genomic effects. But it also shows that doing such experiments and interpreting their results is difficult. Inhibition of protein synthesis is of course not specific. An alternative is to analyze the behavior rapidly after testosterone injection, as it take roughly 30 min to induce gene expression changes. But the kinetics of testosterone in vivo complicates the interpretation. Another option is the co-administration of androgen receptor and estrogen receptor blockers.
While fMRI results bring interesting data and knowledge on behavioral traits and spatial abilities in relation to testosterone levels and sex differences, the result obtained can show only association or correlation but not causal relationship of testosterone effect on behavior. Nevertheless, also according to the numerous published studies and animal experiments, testosterone seems to affect brain functions. The high number of relevant publications also indicates that it is a hot topic of interest. However, quantity is not quality and currently, despite numerous publications it is very difficult to conclude how testosterone affects cognitions and emotions. Most of the published literature agrees on the fact that testosterone is anxiolytic, anti-depressant and improves spatial abilities. But this picture is oversimplified. Many variables add to the complex interactions between testosterone and the brain. Memory, both, verbal and spatial, is a good example. Age, sex, current endocrine status, but also the timing of testosterone analysis or administration, status of the target tissues and several other factors influence the outcome of observational or interventional studies. It is, thus, clear that small studies can only describe a very small window of the whole complex physiology. Analyzing testosterone concentrations, choosing appropriate doses and pharmacological forms is difficult enough. The psychometrics behind behavioral tests in animal experiments and behind psychological tests in human studies is, nevertheless, lacking. Standardization in this area would surely improve our understanding of the neuroendocrinology of testosterone. More systematic research using the whole spectrum of available tools and looking at the various physiological aspects is needed. However, to be able to publish such research, journals should accept manuscripts based on the design and not on the results. Otherwise, the publication bias that is obvious in the so far published literature will continue to be a big issue. Many researchers in this field complain about negative results that are very difficult to publish in the relevant journals. The number of such unpublished observations and experiments is unknown. But based on our humble experience, the negative results will probably be more common than the published positive ones. And if the contradictory published findings are added, the picture gets even more confusing. Large systematic research projects with more cooperation between the most productive research teams is definitely needed.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This publication is the result of the implementation of the project University Science Park of Comenius University in Bratislava (ITMS 26240220086) supported by the Research and Development Operational Programme funded by the European Regional Development Fund.
Ackermann, S., Spalek, K., Rasch, B., Gschwind, L., Coynel, D., Fastenrath, M., et al. (2012). Testosterone levels in healthy men are related to amygdala reactivity and memory performance. Psychoneuroendocrinology 37, 1417–1424. doi: 10.1016/j.psyneuen.2012.01.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aikey, J. L., Nyby, J. G., Anmuth, D. M., and James, P. J. (2002). Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 42, 448–460. doi: 10.1006/hbeh.2002.1838
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Altemus, M., Sarvaiya, N., and Epperson, C. N. (2014). Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35:4. doi: 10.1016/j.yfrne.2014.05.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Babanejad, S., Naghdi, N., and Haeri Rohani, S. A. (2012). Microinjection of dihydrotestosterone as a 5alpha-reduced metabolite of testosterone into CA1 region of hippocampus could improve spatial learning in the adult male rats. Iran J. Pharm. Res. 11, 661–669.
Basaria, S. (2013). Reproductive aging in men. Endocrinol. Metab. Clin. North Am. 42, 255–270. doi: 10.1016/j.ecl.2013.02.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bebbington, P. (1996). The origins of sex differences in depressive disorder: bridging the gap. Int. Rev. Psychiatry 8, 295–332. doi: 10.3109/09540269609051547
Beer, T. M., Bland, L. B., Bussiere, J. R., Neiss, M. B., Wersinger, E. M., Garzotto, M., et al. (2006). Testosterone loss and estradiol administration modify memory in men. J. Urol. 175, 130–135. doi: 10.1016/S0022-5347(05)00049-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Benice, T. S., and Raber, J. (2009). Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn. Mem. 16, 479–485. doi: 10.1101/lm.1428209
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bimonte-Nelson, H. A., Singleton, R. S., Nelson, M. E., Eckman, C. B., Barber, J., Scott, T. Y., et al. (2003). Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol. 181, 301–312. doi: 10.1016/S0014-4886(03)00061-X
Buddenberg, T. E., Komorowski, M., Ruocco, L. A., Silva, M. A., and Topic, B. (2009). Attenuating effects of testosterone on depressive-like behavior in the forced swim test in healthy male rats. Brain Res. Bull. 79, 182–186. doi: 10.1016/j.brainresbull.2009.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bull, R., Davidson, W. A., and Nordmann, E. (2010). Prenatal testosterone, visual-spatial memory, and numerical skills in young children. Learn. Individ. Differ. 20, 246–250. doi: 10.1016/j.lindif.2009.12.002
Burger, H. G. (2002). Androgen production in women. Fertil. Steril. 77(Suppl. 4), S3–S5. doi: 10.1016/S0015-0282(02)02985-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Burkitt, J., Widman, D., and Saucier, D. M. (2007). Evidence for the influence of testosterone in the performance of spatial navigation in a virtual water maze in women but not in men. Horm. Behav. 51, 649–654. doi: 10.1016/j.yhbeh.2007.03.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Camacho, E. M., Huhtaniemi, I. T., O'neill, T. W., Finn, J. D., Pye, S. R., Lee, D. M., et al. (2013). Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European male ageing study. Eur. J. Endocrinol. 168, 445–455. doi: 10.1530/EJE-12-0890
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carlson, L. E., and Sherwin, B. B. (2000). Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause 7, 168–177. doi: 10.1097/00042192-200007030-00007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carrier, N., and Kabbaj, M. (2012). Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm. Behav. 61, 678–685. doi: 10.1016/j.yhbeh.2012.03.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Celec, P., Ostatnikova, D., Holesova, Z., Minarik, G., Ficek, A., Kelemenova, S., et al. (2009). Spatial abilities in prepubertal intellectually gifted boys and genetic polymorphisms related to testosterone metabolism. J. Psychophysiol. 23, 1–6. doi: 10.1027/0269-8803.23.1.1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Celec, P., Tretinárová, D., Minárik, G., Ficek, A., Szemes, T., Lakatošová, S., et al. (2013). Genetic polymorphisms related to testosterone metabolism in intellectually gifted boys. PLoS ONE 8:e54751. doi: 10.1371/journal.pone.0054751
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cherrier, M. M., Asthana, S., Plymate, S., Baker, L., Matsumoto, A. M., Peskind, E., et al. (2001). Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57, 80–88. doi: 10.1212/WNL.57.1.80
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cherrier, M. M., Matsumoto, A. M., Amory, J. K., Asthana, S., Bremner, W., Peskind, E. R., et al. (2005). Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 64, 2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cherrier, M. M., Matsumoto, A. M., Amory, J. K., Johnson, M., Craft, S., Peskind, E. R., et al. (2007). Characterization of verbal and spatial memory changes from moderate to supraphysio logical increases in serum testosterone in healthy older men. Psychoneuroendocrinology 32, 72–79. doi: 10.1016/j.psyneuen.2006.10.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Choi, J., and Silverman, I. (2002). The relationship between testosterone and route-learning strategies in humans. Brain Cogn. 50, 116–120. doi: 10.1016/S0278-2626(02)00015-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Clark, A. S., Mitre, M. C., and Brinck-Johnsen, T. (1995). Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 679, 64–71. doi: 10.1016/0006-8993(95)00202-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dalla, C., Antoniou, K., Papadopoulou-Daifoti, Z., Balthazart, J., and Bakker, J. (2005). Male aromatase-knockout mice exhibit normal levels of activity, anxiety and “depressive-like” symptomatology. Behav. Brain Res. 163, 186–193. doi: 10.1016/j.bbr.2005.04.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dalla, C., Edgecomb, C., Whetstone, A. S., and Shors, T. J. (2008). Females do not express learned helplessness like males do. Neuropsychopharmacology 33, 1559–1569. doi: 10.1038/sj.npp.1301533
Davison, S. L., Bell, R. J., Gavrilescu, M., Searle, K., Maruff, P., Gogos, A., et al. (2011). Testosterone improves verbal learning and memory in postmenopausal women: results from a pilot study. Maturitas 70, 307–311. doi: 10.1016/j.maturitas.2011.08.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dettenborn, L., Hinkelmann, K., Muhtz, C., Gao, W., Wingenfeld, K., Spitzer, C., et al. (2013). Hair testosterone and visuospatial memory in middle-aged men and women with and without depressive symptoms. Psychoneuroendocrinology 38, 2373–2377. doi: 10.1016/j.psyneuen.2013.03.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dohle, G. R., Smit, M., and Weber, R. F. (2003). Androgens and male fertility. World J. Urol. 21, 341–345. doi: 10.1007/s00345-003-0365-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Durdiakova, J., Hodosy, J., Kubranska, A., Ostatnikova, D., and Celec, P. (2012). The effect of mental rotation on changes in plasma testosterone and cortisol levels. Cent. Eur. J. Biol. 7, 1005–1012. doi: 10.2478/s11535-012-0084-6
Edinger, K. L., and Frye, C. A. (2004). Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav. Neurosci. 118, 1352–1364. doi: 10.1037/0735-7044.118.6.1352
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Edinger, K. L., and Frye, C. A. (2005). Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5 alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology 30, 418–430. doi: 10.1016/j.psyneuen.2004.11.001
Egashira, N., Koushi, E., Okuno, R., Shirakawa, A., Mishima, K., Iwasaki, K., et al. (2010). Depression-like behavior and reduced plasma testosterone levels in the senescence-accelerated mouse. Behav. Brain Res. 209, 142–147. doi: 10.1016/j.bbr.2010.01.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Emamian, S., Naghdi, N., Sepehri, H., Jahanshahi, M., Sadeghi, Y., and Choopani, S. (2010). Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav. Brain Res. 208, 30–37. doi: 10.1016/j.bbr.2009.11.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Falter, C. M., Arroyo, M., and Davis, G. J. (2006). Testosterone: activation or organization of spatial cognition? Biol. Psychol. 73, 132–140. doi: 10.1016/j.biopsycho.2006.01.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fernandez-Guasti, A., and Martinez-Mota, L. (2005). Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology 30, 762–770. doi: 10.1016/j.psyneuen.2005.03.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Filova, B., Majzunova, M., Malinova, M., Ostatnikova, D., Celec, P., and Hodosy, J. (2012). Factors determining the kinetics of a single dose of testosterone in rats. Arch. Biol. Sci. 64, 859–863. doi: 10.2298/ABS1203859F
Filova, B., Ostatnikova, D., Celec, P., and Hodosy, J. (2013). The effect of testosterone on the formation of brain structures. Cells Tissues Organs 197, 169–177. doi: 10.1159/000345567
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Forgie, M. L., and Kolb, B. (1998). Sex differences in the effects of frontal cortex injury: role of differential hormonal experience in early development. Behav. Neurosci. 112, 141–153. doi: 10.1037/0735-7044.112.1.141
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frick, K. M., Fernandez, S. M., Bennett, J. C., Prange-Kiel, J., Maclusky, N. J., and Leranth, C. (2004). Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur. J. Neurosci. 19, 3026–3032. doi: 10.1111/j.0953-816X.2004.03427.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frye, C. A., and Edinger, K. L. (2004). Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol. Biochem. Behav. 78, 473–481. doi: 10.1016/j.pbb.2004.04.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frye, C. A., Edinger, K. L., Lephart, E. D., and Walf, A. A. (2010). 3 alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front. Aging Neurosci. 2:15. doi: 10.3389/fnagi.2010.00015
Frye, C. A., and Seliga, A. M. (2001). Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 1, 371–381. doi: 10.3758/CABN.1.4.371
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frye, C. A., and Walf, A. A. (2009). Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol. Behav. 97, 266–269. doi: 10.1016/j.physbeh.2009.02.022
Furukawa, A., Miyatake, A., Ohnishi, T., and Ichikawa, Y. (1998). Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J. Neurochem. 71, 2231–2238. doi: 10.1046/j.1471-4159.1998.71062231.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Galea, L. A., Kavaliers, M., and Ossenkopp, K. P. (1996). Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J. Exp. Biol. 199, 195–200.
Galea, L. A., Kavaliers, M., Ossenkopp, K. P., and Hampson, E. (1995). Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm. Behav. 29, 106–125. doi: 10.1006/hbeh.1995.1008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gibbs, R. B., and Johnson, D. A. (2008). Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149, 3176–3183. doi: 10.1210/en.2007-1645
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goel, N., and Bale, T. L. (2008). Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology 149, 6399–6405. doi: 10.1210/en.2008-0433
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goudsmit, E., Van De Poll, N. E., and Swaab, D. F. (1990). Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in young and middle-aged animals. Behav. Neural Biol. 53, 6–20. doi: 10.1016/0163-1047(90)90729-P
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grimshaw, G. M., Sitarenios, G., and Finegan, J. A. (1995). Mental rotation at 7 years: relations with prenatal testosterone levels and spatial play experiences. Brain Cogn. 29, 85–100. doi: 10.1006/brcg.1995.1269
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gutierrez-Garcia, A. G., Contreras, C. M., Vasquez-Hernandez, D. I., Molina-Jimenez, T., and Jacome-Jacome, E. (2009). Testosterone reduces cumulative burying in female Wistar rats with minimal participation of estradiol. Pharmacol. Biochem. Behav. 93, 406–412. doi: 10.1016/j.pbb.2009.06.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harooni, H. E., Naghdi, N., Sepehri, H., and Rohani, A. H. (2008). Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitory avoidance learning and memory in adult male rats. Behav. Brain Res. 188, 71–77. doi: 10.1016/j.bbr.2007.10.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hawley, W. R., Grissom, E. M., Martin, R. C., Halmos, M. B., Bart, C. L., and Dohanich, G. P. (2013). Testosterone modulates spatial recognition memory in male rats. Horm. Behav. 63, 559–565. doi: 10.1016/j.yhbeh.2013.02.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Healy, S. D., Braham, S. R., and Braithwaite, V. A. (1999). Spatial working memory in rats: no differences between the sexes. Proc. Biol. Sci. 266, 2303–2308. doi: 10.1098/rspb.1999.0923
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herrera-Perez, J. J., Martinez-Mota, L., Chavira, R., and Fernandez-Guasti, A. (2012). Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Horm. Behav. 61, 623–630. doi: 10.1016/j.yhbeh.2012.02.015
Hodosy, J., Zelmanova, D., Majzunova, M., Filova, B., Malinova, M., Ostatnikova, D., et al. (2012). The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol. Biochem. Behav. 102, 191–195. doi: 10.1016/j.pbb.2012.04.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hofer, P., Lanzenberger, R., and Kasper, S. (2013). Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur. Neuropsychopharmacol. 23, 79–88. doi: 10.1016/j.euroneuro.2012.04.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hooven, C. K., Chabris, C. F., Ellison, P. T., and Kosslyn, S. M. (2004). The relationship of male testosterone to components of mental rotation. Neuropsychologia 42, 782–790. doi: 10.1016/j.neuropsychologia.2003.11.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isgor, C., and Sengelaub, D. R. (1998). Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm. Behav. 34, 183–198. doi: 10.1006/hbeh.1998.1477
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isgor, C., and Sengelaub, D. R. (2003). Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J. Neurobiol. 55, 179–190. doi: 10.1002/neu.10200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Janowsky, J. S., Oviatt, S. K., and Orwoll, E. S. (1994). Testosterone influences spatial cognition in older men. Behav. Neurosci. 108, 325–332. doi: 10.1037/0735-7044.108.2.325
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jonasson, Z. (2005). Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci. Biobehav. Rev. 28, 811–825. doi: 10.1016/j.neubiorev.2004.10.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jones, B. A., and Watson, N. V. (2005). Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 85, 135–141. doi: 10.1016/j.physbeh.2005.03.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Joshi, D., Van Schoor, N. M., De Ronde, W., Schaap, L. A., Comijs, H. C., Beekman, A. T. F., et al. (2010). Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin. Endocrinol. 72, 232–240. doi: 10.1111/j.1365-2265.2009.03641.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jovanovic, H., Kocoska-Maras, L., Radestad, A. F., Halldin, C., Borg, J., Hirschberg, A. L., et al. (2014). Effects of estrogen and testosterone treatment on serotonin transporter binding in the brain of surgically postmenopausal women—a PET study. Neuroimage 106C, 47–54.
Karadi, K., Kallai, J., Kover, F., Nemes, J., Makany, T., and Nagy, F. (2006). Endogenous testosterone concentration, mental rotation, and size of the corpus callosum in a sample of young Hungarian women. Percept. Mot. Skills 102, 445–453. doi: 10.2466/pms.102.2.445-453
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kelly, B., Maguire-Herring, V., Rose, C. M., Gore, H. E., Ferrigno, S., Novak, M. A., et al. (2014). Short-term testosterone manipulations do not affect cognition or motor function but differentially modulate emotions in young and older male rhesus monkeys. Horm. Behav. 66, 731–742. doi: 10.1016/j.yhbeh.2014.08.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kempel, P., Gohlke, B., Klempau, J., Zinsberger, P., Reuter, M., and Hennig, J. (2005). Second-to-fourth digit length, testosterone and spatial ability. Intelligence 33, 215–230. doi: 10.1016/j.intell.2004.11.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kendler, K. S., and Gardner, C. O. (2014). Sex differences in the pathways to major depression: a study of opposite-sex twin Pairs. Am. J. Psychiatry 171, 426–435. doi: 10.1176/appi.ajp.2013.13101375
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Khakpai, F. (2014). The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav. Brain Res. 263, 9–15. doi: 10.1016/j.bbr.2014.01.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Khalil, R., King, M. A., and Soliman, M. R. (2005). Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology 75, 87–92. doi: 10.1159/000087188
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Khera, M. (2013). Patients with testosterone deficit syndrome and depression. Arch. Esp. Urol. 66, 729–736.
Khorshidahmad, T., Tabrizian, K., Vakilzadeh, G., Nikbin, P., Moradi, S., Hosseini-Sharifabad, A., et al. (2012). Interactive effects of a protein kinase AII inhibitor and testosterone on spatial learning in the Morris water maze. Behav. Brain Res. 228, 432–439. doi: 10.1016/j.bbr.2011.12.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
King, H. M., Kurdziel, L. B., Meyer, J. S., and Lacreuse, A. (2012). Effects of testosterone on attention and memory for emotional stimuli in male rhesus monkeys. Psychoneuroendocrinology 37, 396–409. doi: 10.1016/j.psyneuen.2011.07.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
King, J. A., Barkley, R. A., Delville, Y., and Ferris, C. F. (2000). Early androgen treatment decreases cognitive function and catecholamine innervation in an animal model of ADHD. Behav. Brain Res. 107, 35–43. doi: 10.1016/S0166-4328(99)00113-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kocoska-Maras, L., Zethraeus, N., Radestad, A. F., Ellingsen, T., Von Schoultz, B., Johannesson, M., et al. (2011). A randomized trial of the effect of testosterone and estrogen on verbal fluency, verbal memory, and spatial ability in healthy postmenopausal women. Fertil. Steril. 95, 152–157. doi: 10.1016/j.fertnstert.2010.05.062
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kolb, B., and Stewart, J. (1995). Changes in the neonatal gonadal hormonal environment prevent behavioral sparing and alter cortical morphogenesis after early frontal cortex lesions in male and female rats. Behav. Neurosci. 109, 285–294. doi: 10.1037/0735-7044.109.2.285
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Korsia, S., and Bottjer, S. W. (1991). Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J. Neurosci. 11, 2362–2371.
Kranz, G. S., Wadsak, W., Kaufmann, U., Savli, M., Baldinger, P., Gryglewski, G., et al. (2014). High-dose testosterone treatment increases serotonin transporter binding in transgender people. Biol. Psychiatry. doi: 10.1016/j.biopsych.2014.09.010. [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kubranska, A., Lakatosova, S., Schmidtova, E., Durdiakova, J., Celec, P., and Ostatnikova, D. (2014). Spatial abilities are not related to testosterone levels and variation in the androgen receptor in healthy young men. Gen. Physiol. Biophys. 33, 311–319. doi: 10.4149/gpb_2014005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kumsar, S., Kumsar, N. A., Saglam, H. S., Kose, O., Budak, S., and Adsan, O. (2014). Testosterone levels and sexual function disorders in depressive female patients: effects of antidepressant treatment. J. Sex. Med. 11, 529–535. doi: 10.1111/jsm.12394
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kurita, N., Horie, S., Yamazaki, S., Otoshi, K., Otani, K., Sekiguchi, M., et al. (2014). Low Testosterone levels, depressive symptoms, and falls in older men: a cross-sectional study. J. Am. Med. Dir. Assoc. 15, 30–35. doi: 10.1016/j.jamda.2013.11.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lacreuse, A., Gore, H. E., Chang, J., and Kaplan, E. R. (2012). Short-term testosterone manipulations modulate visual recognition memory and some aspects of emotional reactivity in male rhesus monkeys. Physiol. Behav. 106, 229–237. doi: 10.1016/j.physbeh.2012.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lewin, C., Wolgers, G., and Herlitz, A. (2001). Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology 15, 165–173. doi: 10.1037/0894-4105.15.2.165
Linn, M. C., and Petersen, A. C. (1985). Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 56, 1479–1498. doi: 10.2307/1130467
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lord, C., Sekerovic, Z., and Carrier, J. (2014). Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol. Biol. (Paris) 62, 302–310. doi: 10.1016/j.patbio.2014.07.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maki, P. M., Ernst, M., London, E. D., Mordecai, K. L., Perschler, P., Durso, S. C., et al. (2007). Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J. Clin. Endocrinol. Metab. 92, 4107–4114. doi: 10.1210/jc.2006-1805
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martinez-Mota, L., Ulloa, R. E., Herrera-Perez, J., Chavira, R., and Fernandez-Guasti, A. (2011). Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol. Behav. 104, 900–905. doi: 10.1016/j.physbeh.2011.05.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mcconnell, S. E., Alla, J., Wheat, E., Romeo, R. D., Mcewen, B., and Thornton, J. E. (2012). The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm. Behav. 61, 479–486. doi: 10.1016/j.yhbeh.2012.01.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mchenry, J., Carrier, N., Hull, E., and Kabbaj, M. (2014). Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 35:1. doi: 10.1016/j.yfrne.2013.09.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mellon, S. H., Griffin, L. D., and Compagnone, N. A. (2001). Biosynthesis and action of neurosteroids. Brain Res. Brain Res. Rev. 37, 3–12. doi: 10.1016/S0165-0173(01)00109-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Michels, G., and Hoppe, U. C. (2008). Rapid actions of androgens. Front. Neuroendocrinol. 29:4. doi: 10.1016/j.yfrne.2007.08.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miller, K. K., Perlis, R. H., Papakostas, G. I., Mischoulon, D., Iosifescu, D. V., Brick, D. J., et al. (2009). Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr. 14, 688–694.
Miller, W. L., and Auchus, R. J. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151. doi: 10.1210/er.2010-0013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moffat, S. D., and Hampson, E. (1996). A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology 21, 323–337. doi: 10.1016/0306-4530(95)00051-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moffat, S. D., Zonderman, A. B., Metter, E. J., Blackman, M. R., Harman, S. M., and Resnick, S. M. (2002). Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J. Clin. Endocrinol. Metab. 87, 5001–5007. doi: 10.1210/jc.2002-020419
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mohaddes, G., Naghdi, N., Khamnei, S., Khatami, S., and Haeri, A. (2009). Effect of spatial learning on hippocampal testosterone in intact and castrated male rats. Iran. Biomed. J. 13, 49–58.
Moller, M. C., Bartfai, A. B., and Radestad, A. F. (2010). Effects of testosterone and estrogen replacement on memory function. Menopause 17, 983–989. doi: 10.1097/gme.0b013e3181dc2e40
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moradpour, F., Naghdi, N., and Fathollahi, Y. (2006). Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav. Brain Res. 175, 223–232. doi: 10.1016/j.bbr.2006.08.037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moradpour, F., Naghdi, N., Fathollahi, Y., Javan, M., Choopani, S., and Gharaylou, Z. (2013). Pre-pubertal castration improves spatial learning during mid-adolescence in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 105–112. doi: 10.1016/j.pnpbp.2013.07.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Naghdi, N., Majlessi, N., and Bozorgmehr, T. (2005). The effect of intrahippocampal injection of testosterone enanthate (an androgen receptor agonist) and anisomycin (protein synthesis inhibitor) on spatial learning and memory in adult, male rats. Behav. Brain Res. 156, 263–268. doi: 10.1016/j.bbr.2004.05.032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Naghdi, N., Nafisy, N., and Majlessi, N. (2001). The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 897, 44–51. doi: 10.1016/S0006-8993(00)03261-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Naghdi, N., Oryan, S., and Etemadi, R. (2003). The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucleus of the amygdala in Morris water maze. Brain Res. 972, 1–8. doi: 10.1016/S0006-8993(03)02227-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nayebi, A. M., Pourrabi, S., and Hossini, S. (2014). Testosterone ameliorates streptozotocin-induced memory impairment in male rats. Acta Pharmacol. Sin. 35, 752–757. doi: 10.1038/aps.2014.6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nowak, N. T., Diamond, M. P., Land, S. J., and Moffat, S. D. (2014). Contributions of sex, testosterone, and androgen receptor CAG repeat number to virtual Morris water maze performance. Psychoneuroendocrinology 41, 13–22. doi: 10.1016/j.psyneuen.2013.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Osborne, D. M., Edinger, K., and Frye, C. A. (2009). Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr). 31, 191–198. doi: 10.1007/s11357-009-9114-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ostatnikova, D., Laznibatova, J., and Dohnanyiova, M. (1996). Testosterone influence on spatial ability in prepubertal children. Stud. Psychol. (Bratisl). 38, 237–245.
Panizzon, M. S., Hauger, R., Xian, H., Vuoksimaa, E., Spoon, K. M., Mendoza, S. P., et al. (2014). Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiol. Aging 35, e1771–e1778. doi: 10.1016/j.neurobiolaging.2013.12.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Parrilla-Carrero, J., Figueroa, O., Lugo, A., Garcia-Sosa, R., Brito-Vargas, P., Cruz, B., et al. (2009). The anabolic steroids testosterone propionate and nandrolone, but not 17alpha-methyltestosterone, induce conditioned place preference in adult mice. Drug Alcohol Depend. 100, 122–127. doi: 10.1016/j.drugalcdep.2008.09.014
Perrot-Sinal, T. S., Kavaliers, M., and Ossenkopp, K. P. (1998). Spatial learning and hippocampal volume in male deer mice: relations to age, testosterone and adrenal gland weight. Neuroscience 86, 1089–1099. doi: 10.1016/S0306-4522(98)00131-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pletzer, B., Kronbichler, M., Ladurner, G., Nuerk, H. C., and Kerschbaum, H. (2011). Menstrual cycle variations in the BOLD-response to a number bisection task: implications for research on sex differences. Brain Res. 1420, 37–47. doi: 10.1016/j.brainres.2011.08.058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pope, H. G., Cohane, G. H., Kanayama, G., Siegel, A. J., and Hudson, J. I. (2003). Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am. J. Psychiatry 160, 105–111. doi: 10.1176/appi.ajp.160.1.105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Postma, A., Meyer, G., Tuiten, A., Van Honk, J., Kessels, R. P. C., and Thijssen, J. (2000). Effects of testosterone administration on selective aspects of object-location memory in healthy young women. Psychoneuroendocrinology 25, 563–575. doi: 10.1016/S0306-4530(00)00010-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Puts, D. A., Cardenas, R. A., Bailey, D. H., Burriss, R. P., Jordan, C. L., and Breedlove, S. M. (2010). Salivary testosterone does not predict mental rotation performance in men or women. Horm. Behav. 58, 282–289. doi: 10.1016/j.yhbeh.2010.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pyter, L. M., Trainor, B. C., and Nelson, R. J. (2006). Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus). Eur. J. Neurosci. 23, 3056–3062. doi: 10.1111/j.1460-9568.2006.04821.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raber, J., Bongers, G., Lefevour, A., Buttini, M., and Mucke, L. (2002). Androgens protect against apolipoprotein E4-induced cognitive deficits. J. Neurosci. 22, 5204–5209.
Roof, R. L. (1993). Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav. Brain Res. 53, 1–10. doi: 10.1016/S0166-4328(05)80261-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roof, R. L., and Havens, M. D. (1992). Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 572, 310–313. doi: 10.1016/0006-8993(92)90491-Q
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roohbakhsh, A., Moghaddam, A. H., and Delfan, K. M. (2011). Anxiolytic-like effect of testosterone in male rats: GARA(C) receptors are not involved. Iran. J. Basic Med. Sci. 14, 376–382.
Ruigrok, A. N., Salimi-Khorshidi, G., Lai, M. C., Baron-Cohen, S., Lombardo, M. V., Tait, R. J., et al. (2014). A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50. doi: 10.1016/j.neubiorev.2013.12.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sandstrom, N. J., Kim, J. H., and Wasserman, M. A. (2006). Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 50, 18–26. doi: 10.1016/j.yhbeh.2005.09.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sartorius, G., Spasevska, S., Idan, A., Turner, L., Forbes, E., Zamojska, A., et al. (2012). Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin. Endocrinol. (Oxf). 77, 755–763. doi: 10.1111/j.1365-2265.2012.04432.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schoning, S., Engelien, A., Kugel, H., Schafer, S., Schiffbauer, H., Zwitserlood, P., et al. (2007). Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia 45, 3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schulz, K., and Korz, V. (2010). Hippocampal testosterone relates to reference memory performance and synaptic plasticity in male rats. Front. Behav. Neurosci. 4:187. doi: 10.3389/fnbeh.2010.00187
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seidman, S. N., Spatz, E., Rizzo, C., and Roose, S. P. (2001). Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trial. J. Clin. Psychiatry 62, 406–412. doi: 10.4088/JCP.v62n0602
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shah, D. S., Prados, J., Gamble, J., De Lillo, C., and Gibson, C. L. (2013). Sex differences in spatial memory using serial and search tasks. Behav. Brain Res. 257, 90–99. doi: 10.1016/j.bbr.2013.09.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shors, T. J., and Miesegaes, G. (2002). Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc. Natl. Acad. Sci. U.S.A. 99, 13955–13960. doi: 10.1073/pnas.202199999
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Silverman, I., Kastuk, D., Choi, J., and Phillips, K. (1999). Testosterone levels and spatial ability in men. Psychoneuroendocrinology 24, 813–822. doi: 10.1016/S0306-4530(99)00031-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spritzer, M. D., Daviau, E. D., Coneeny, M. K., Engelman, S. M., Prince, W. T., and Rodriguez-Wisdom, K. N. (2011). Effects of testosterone on spatial learning and memory in adult male rats. Horm. Behav. 59, 484–496. doi: 10.1016/j.yhbeh.2011.01.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spritzer, M. D., Fox, E. C., Larsen, G. D., Batson, C. G., Wagner, B. A., and Maher, J. (2013). Testosterone influences spatial strategy preferences among adult male rats. Horm. Behav. 63, 800–812. doi: 10.1016/j.yhbeh.2013.03.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spritzer, M. D., Gill, M., Weinberg, A., and Galea, L. A. (2008). Castration differentially affects spatial working and reference memory in male rats. Arch. Sex. Behav. 37, 19–29. doi: 10.1007/s10508-007-9264-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Suarez-Jimenez, B., Gore, H. E., Hachey, J., King, H. M., and Lacreuse, A. (2013). Testosterone modulation of anxiety in gonadally-suppressed male rhesus monkeys: a role for gonadotropins? Pharmacol. Biochem. Behav. 104, 97–104. doi: 10.1016/j.pbb.2013.01.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsai, M. J., and O'malley, B. W. (1994). Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63, 451–486. doi: 10.1146/annurev.bi.63.070194.002315
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ubuka, T., Son, Y. L., Tobari, Y., Narihiro, M., Bentley, G. E., Kriegsfeld, L. J., et al. (2014). Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor. Front. Endocrinol. (Lausanne). 5:8. doi: 10.3389/fendo.2014.00008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Urbanski, H. F. (2011). Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology 93, 211–222. doi: 10.1159/000327399
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Urbanski, H. F., Sorwell, K. G., Garyfallou, V. T., Garten, J., Weiss, A., Renner, L., et al. (2014). Androgen supplementation during aging: development of a physiologically appropriate protocol. Rejuvenation Res. 17, 150–153. doi: 10.1089/rej.2013.1518
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Hemmen, J., Veltman, D. J., Hoekzema, E., Cohen-Kettenis, P. T., Dessens, A. B., and Bakker, J. (2014). Neural activation during mental rotation in complete androgen insensitivity syndrome: the influence of sex hormones and sex chromosomes. Cereb. Cortex. doi: 10.1093/cercor/bhu280. [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Honk, J., Peper, J. S., and Schutter, D. (2005). Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biol. Psychiatry 58, 218–225. doi: 10.1016/j.biopsych.2005.04.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vuoksimaa, E., Kaprio, J., Eriksson, C. J. P., and Rose, R. J. (2012). Pubertal testosterone predicts mental rotation performance of young adult males. Psychoneuroendocrinology 37, 1791–1800. doi: 10.1016/j.psyneuen.2012.03.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Walf, A. A., and Frye, C. A. (2012). Gestational or acute restraint in adulthood reduces levels of 5alpha-reduced testosterone metabolites in the hippocampus and produces behavioral inhibition of adult male rats. Front. Cell. Neurosci. 6:40. doi: 10.3389/fncel.2012.00040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weber, B., Lewicka, S., Deuschle, M., Colla, M., and Heuser, I. (2000). Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology 25, 765–771. doi: 10.1016/S0306-4530(00)00023-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wolf, O. T., Preut, R., Hellhammer, D. H., Kudielka, B. M., Schurmeyer, T. H., and Kirschbaum, C. (2000). Testosterone and cognition in elderly men: a single testosterone injection blocks the practice effect in verbal fluency, but has no effect on spatial or verbal memory. Biol. Psychiatry 47, 650–654. doi: 10.1016/S0006-3223(99)00145-6
Yang, C. F. J., Hooven, C. K., Boynes, M., Gray, P. B., and Pope, H. G. (2007). Testosterone levels and mental rotation performance in Chinese men. Horm. Behav. 51, 373–378. doi: 10.1016/j.yhbeh.2006.12.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yonker, J. E., Eriksson, E., Nilsson, L. G., and Herlitz, A. (2006). Negative association of testosterone on spatial visualization in 35 to 80 year old men. Cortex 42, 376–386. doi: 10.1016/S0010-9452(08)70364-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zarrour, F. A., Artz, S., Griffith, J., Sirbu, C., and Kommor, M. (2009). Testosterone and depression: systematic review and meta-analysis. J. Psychiatr. Pract. 15, 289–305. doi: 10.1097/01.pra.0000358315.88931.fc
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zuloaga, D. G., Jordan, C. L., and Breedlove, S. M. (2011). The organizational role of testicular hormones and the androgen receptor in anxiety-related behaviors and sensorimotor gating in rats. Endocrinology 152, 1572–1581. doi: 10.1210/en.2010-1016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: androgen, cognition, emotions, hippocampus, behavioral neuroendocrinology
Citation: Celec P, Ostatníková D and Hodosy J (2015) On the effects of testosterone on brain behavioral functions. Front. Neurosci. 9:12. doi: 10.3389/fnins.2015.00012
Received: 25 August 2014; Accepted: 12 January 2015;
Published online: 17 February 2015.
Edited by:
Belinda Pletzer, University of Salzburg, AustriaReviewed by:
Henryk Urbanski, Oregon National Primate Research Center, USACopyright © 2015 Celec, Ostatníková and Hodosy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Celec, Institute of Molecular Biomedicine, Comenius University, Sasinkova 4, 811 08 Bratislava, Slovakia e-mail:cGV0ZXJjZWxlY0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.