
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Neuroinform. , 09 August 2024
Volume 18 - 2024 | https://doi.org/10.3389/fninf.2024.1461597
This article is a correction to:
Building a realistic, scalable memory model with independent engrams using a homeostatic mechanism
A corrigendum on
Building a realistic, scalable memory model with independent engrams using a homeostatic mechanism
by Kaster, M., Czappa, F., Butz-Ostendorf, M., and Wolf, F. (2024). Front. Neuroinform. 18:1323203. doi: 10.3389/fninf.2024.1323203
In the published article, there was an error in Figure 7 as published. The wrong image was included. Figures 7 and 8 were identical! The corrected Figure 7 and its caption appear below.
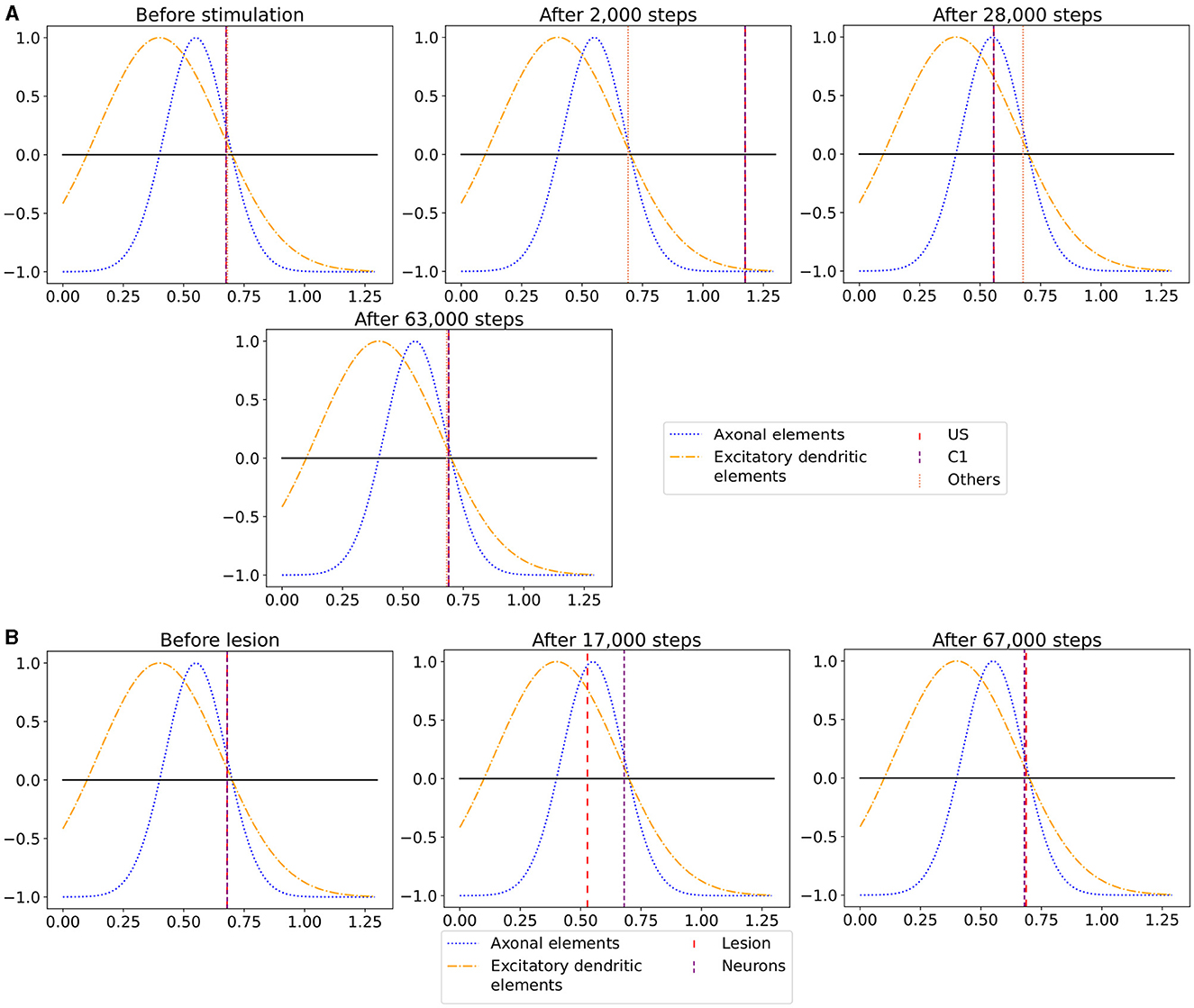
Figure 7. The average calcium level of groups of neurons of a single box (x-axis) with calcium-dependent growth curves (y-axis). (A) Calcium level before and after the ensembles US and C1 were stimulated at step 450,000. Subsequentially, their calcium levels increased and growth curves caused synaptic elements to decrease and synapses to prune. When stimulation stopped and synapses were pruned, activities and calcium levels, respectively, dropped below the homeostatic set-point which, in turn, triggered the growth of synaptic elements and potentially also the growth of new synapses. Note, that calcium levels below the set-point were in an optimal regime for axonal element formation while dendritic elements grew slower. A surplus of axonal elements may result in more long-range connections while a prolongued growth of dendritic elements extended the phase in which new engrams could form because it may take longer until activities return to a homeostatic set-point. (B) Average calcium levels during ablation studies in which we removed connectivity of 50% of the neurons in a box. Directly after the stimulation, calcium levels of lesioned neurons dropped due to the lack of input. As a result, the neurons start regrowing synaptic elements until enough synapses were formed to restore activity homeostasis. The homeostatic reorganization is comparable to engram formation after stimulation in A. Note, that even for higher deletion rates neurons will return average firing rates to the homeostatic set-point (data not shown) very much as in B, however without functional recovery of trained engrams.
In the published article, there was an error in Supplementary Figures S1 and S2. The order of the figures was reversed. The corrected order of the Figures S1 and S2 have been updated in the original article. References to the supplementary material in the main text remain unchanged.
In the published article, there was an error. There was a missing minus sign in Equation 5.
A correction has been made to 2 Materials and methods, 2.1 Model of structural plasticity, 2.1.2 Forming and pruning of synapses, Equation 5. This equation previously stated:
The corrected equation appears below:
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: learning, memory, homeostatic plasticity, structural plasticity, scalable
Citation: Kaster M, Czappa F, Butz-Ostendorf M and Wolf F (2024) Corrigendum: Building a realistic, scalable memory model with independent engrams using a homeostatic mechanism. Front. Neuroinform. 18:1461597. doi: 10.3389/fninf.2024.1461597
Received: 08 July 2024; Accepted: 11 July 2024;
Published: 09 August 2024.
Approved by:
Dong Song, University of Southern California, United StatesCopyright © 2024 Kaster, Czappa, Butz-Ostendorf and Wolf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marvin Kaster, bWFydmluLmthc3RlckB0dS1kYXJtc3RhZHQuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.