- 1Biomedical Signals and Systems (BSS) Group, Faculty of Electrical Engineering, Mathematics and Computer Science (EEMCS), University of Twente, Enschede, Netherlands
- 2Department of Biophysics, Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands
- 3Centre for Computational Science and Mathematical Modelling, Coventry University, Coventry, United Kingdom
- 4School of Aerospace, Transport and Manufacturing, Cranfield University, Cranfield, United Kingdom
Parkinson’s disease (PD) is a neurodegenerative disorder that affects millions of people worldwide. Its slow and heterogeneous progression over time makes timely diagnosis challenging. Wrist-worn digital devices, particularly smartwatches, are currently the most popular tools in the PD research field due to their convenience for long-term daily life monitoring. While wrist-worn sensing devices have garnered significant interest, their value for daily practice is still unclear. In this narrative review, we survey demographic, clinical and technological information from 39 articles across four public databases. Wrist-worn technology mainly monitors motor symptoms and sleep disorders of patients in daily life. We find that accelerometers are the most commonly used sensors to measure the movement of people living with PD. There are few studies on monitoring the disease progression compared to symptom classification. We conclude that wrist-worn sensing technology might be useful to assist in the management of PD through an automatic assessment based on patient-provided daily living information.
1. Introduction
Parkinson’s disease (PD) is a rapidly growing neurological disorder that affects people worldwide, especially those over 65 years old. In the past three decades, the social burden of PD has doubled due to the ageing of the global population. If this trend continues, the predicted number of patients will exceed 12 million in the next 30 years, which will have a significant economic impact on our society (Rocca, 2018). There is an urgent need to identify new effective and affordable interventions to reduce the impact of PD on patients and society (G 2016 Parkinson’s Disease Collaborators, 2018).
Clinically, PD patients typically present motor symptoms for the first clinic visit, such as rest tremor, bradykinesia, and rigidity. Healthcare professionals can often diagnose PD when patients present these classical motor symptoms at a late stage, according to the UK Brain Bank Criteria (Calne et al., 1992). Freezing of gait (FoG), posture instability, or gait disturbances are also motor symptoms in daily life but aren’t belonging to the currently diagnosed criteria according to the UK brain bank. The Braak stages show that some prodromal non-motor symptoms (NMS), such as constipation and sleep disorder, can occur before Lewy bodies (LB) appear in the substantia nigra of the brain stem (Rietdijk et al., 2017). Early detection of prodromal parkinsonism is crucial to the effectiveness of disease-modifying interventions. Moreover, emergency measures are insufficient to stop the disease deterioration in the late phase. Therefore, it is essential to track small changes in disease severity during daily life and take effective action to slow down the progression of PD. A personalized disease management plan is considered to be an effective approach for addressing the highly heterogeneous preferences and practical requirements of individual patients.
Wearable devices are important tools for personalized PD management. In the past decade, wearable devices have developed rapidly, thanks to the advances in communication technology and the Internet of Things (IoT). Remote monitoring of the patient’s daily lives with wearable sensing technology can assist healthcare professionals to gain insights into patients’ health conditions and empower PD patients to improve treatment effectiveness and slow disease progression (Dorsey and Eric, 2020). Mobile computing devices (e.g., smartwatches) have expanded the use of “on-body” applications from clinical settings to daily life based on body sensor networks (BSN).
Wrist-worn digital devices, especially smartwatches, are currently the most popular smart consumer wearable tools for healthcare diagnosis and self-management due to their convenience for long-term monitoring (Chakrabarti et al., 2022). In 2021, a methodological review surveyed the electronic health (eHealth) technologies for PD detection in daily life from the past two decades, while the management of symptoms was not investigated (Zhang et al., 2021). In 2022, a five-decade review outlined the progress of digital technology and computational techniques applied to PD motor symptom monitoring (Chandrabhatla et al., 2022). Non-motor manifestations of PD, such as sleep disorders and depression, can significantly decrease patient quality of life (QoL), yet are overlooked in this review. A recent systematic review highlights the deployed sensorial and algorithmic aspects of PD diagnosis and management (Giannakopoulou et al., 2022). These reviews do not consider the effect of the controlled environment, such as a clinic or laboratory on the clinical use of wearable devices in PD patients. Some activities of daily living (ADLs) mainly occur at home or home-like setting, and sleep quality is difficult to measure longitudinally in a clinic or lab. It is therefore essential to explore the clinical application of commercial wrist-worn devices for in-home PD monitoring. Wrist-based sensors have advantages such as convenience, wear compliance, and relative availability in the commercial market. There is a lack of review on the use of smartwatches for health telemonitoring from public databases, while a survey shows that most PD-related research concerns self-management in a laboratory environment (King and Majid, 2018). A recent review investigates whether the smartwatch can replace the role of a Parkinson’s disease doctor and concludes that the immediate implications for patients and clinicians are limited (Bloem et al., 2023). Remote digital monitoring of PD can become an important development for disease management and care.
Although the telemonitoring of PD using wrist-worn sensing has attracted significant interest in the research community, its value in daily PD management is still unclear. In this narrative review, we investigate the potential role of wrist-worn devices in PD motor and non-motor symptom monitoring in a natural, free-living environment. We evaluate the current technological progress as well as discuss the potential challenges and important future research directions of wrist-based technology in managing PD better.
2. Materials and methods
2.1. Reference searching method
This narrative review aims to summarize the current state-of-art of using wrist-worn technologies for monitoring motor and non-motor signs of PD, the different sensor types used in studies, and the potential for these devices in daily practice for the remote management of PD. To gather sufficient information for this review, we search for peer-reviewed journal articles from PubMed, IEEE Xplore, Web of Science, and Google Scholar using AI-based software ASReview LAB in October 2022. The search includes keywords: (“Parkinson” OR “PD” OR “Parkinson’s disease”) AND (“daily” OR “daily life” OR “home” OR “daily living”) AND (“wrist” OR “wrist-worn” OR “smartwatch” OR “wristband”).
2.2. Article inclusion and exclusion criteria
We select the articles based on the following inclusion criteria: (1) studies that focus on the use of wrist-based sensing technology for monitoring the motor or non-motor signs of PD, and (2) studies that are conducted in daily life or at home. Articles are excluded if (1) patients under study are not diagnosed with PD by a neurologist, (2) the article is not written in English, (3) the experiment is set up in the hospital or laboratory, (4) the device is unwearable or difficult to daily use at home, such as the wrist exoskeleton, or (5) there is no sensor in the monitoring device.
2.3. Information extraction
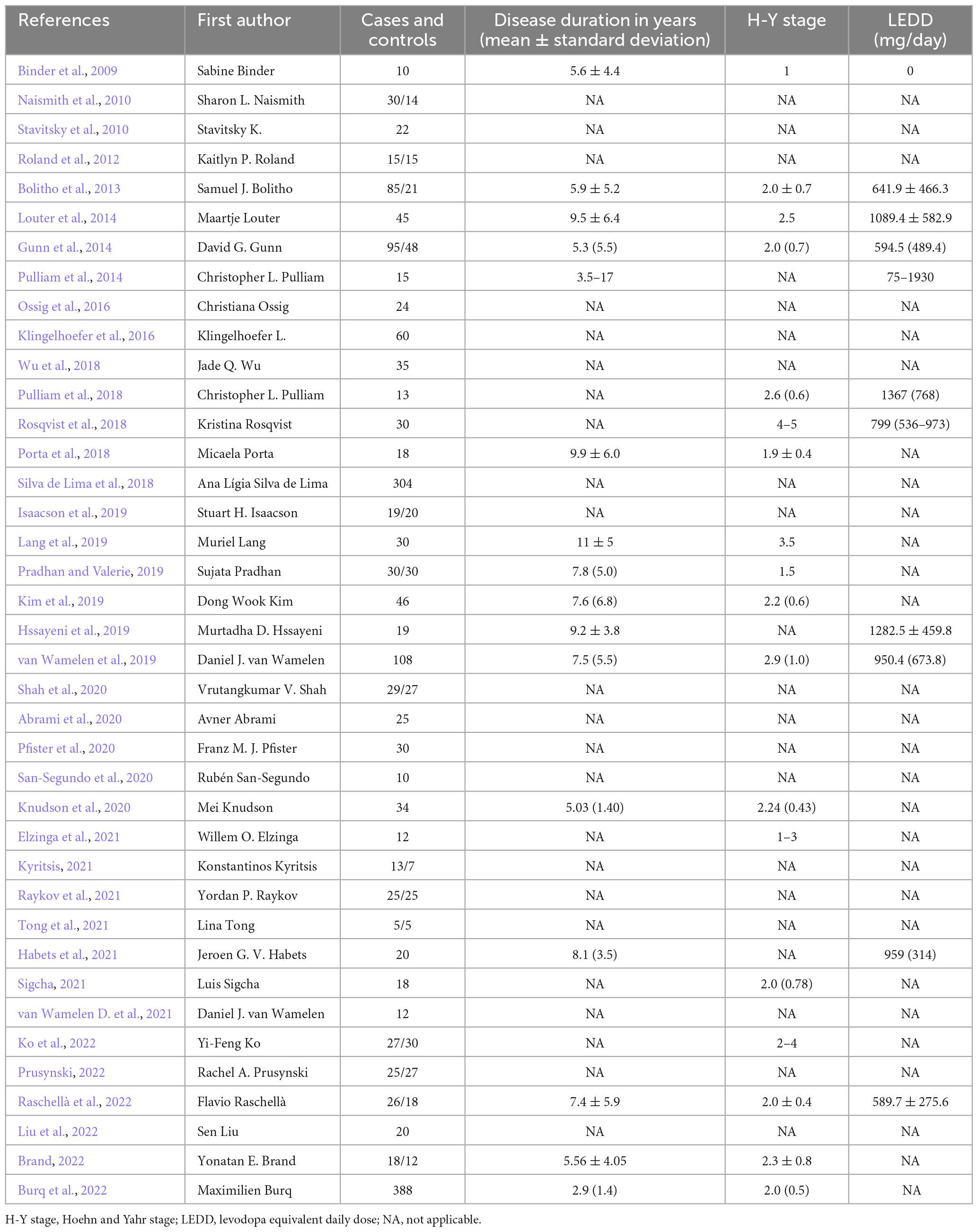
We extract four categories of data from selected articles: (1) Basic article information, including the authors and year of publication. We arrange the sources in chronological order to analyse the recent trend of published papers on wrist-based technology in PD monitoring. (2) The number of participants under study, including the number of PD patients and healthy controls, which are included to compare the credibility of different research. (3) Demographic information on the study population. To analyse the mild, moderate, and late-stage subgroups, we extract the disease duration of PD patients. To present the trend change in symptoms of PD, we also show the Hoehn and Yahr (H-Y) stage of PD patients (Goetz et al., 2004). To investigate the effect of total daily medication on patients’ daily symptoms, we extract the L-dopa equivalent daily dose (LEDD) (Julien et al., 2021). (4) Technical information: to differentiate the technical characteristic of commercial wrist-worn devices in references, we extract the outcome measures, location of the worn wrist, features, performances of wearable devices, sensor types, and clinical applications in the diagnosis and management of PD.
3. Results
3.1. Article selection and publication year
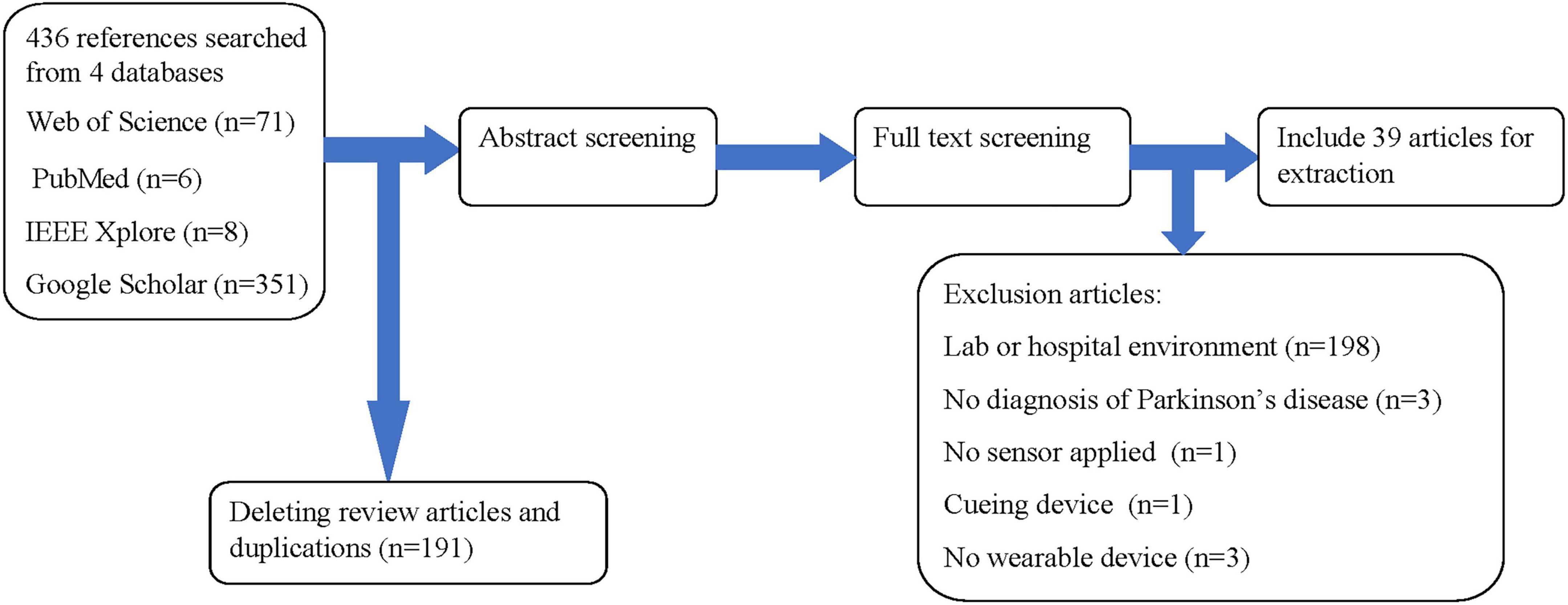
We find 436 articles by searching the keywords. The articles come from a variety of sources: 71 articles from the Web of Science, 6 from PubMed, 8 from IEEE Xplore, and 351 from Google Scholar. After deleting review and duplicated articles through the use of ENDNOTE 20 software, 39 are selected for review according to our inclusion and exclusion criteria as can be shown in Figure 1. In Figure 2, the publication year is presented. The number of publications about commercial wrist-worn wearable devices applied in PD evaluation has increased significantly in the past 4 years, i.e., from 2018 to 2022; there are 29 articles published, accounting for 74% of the total studies considered.
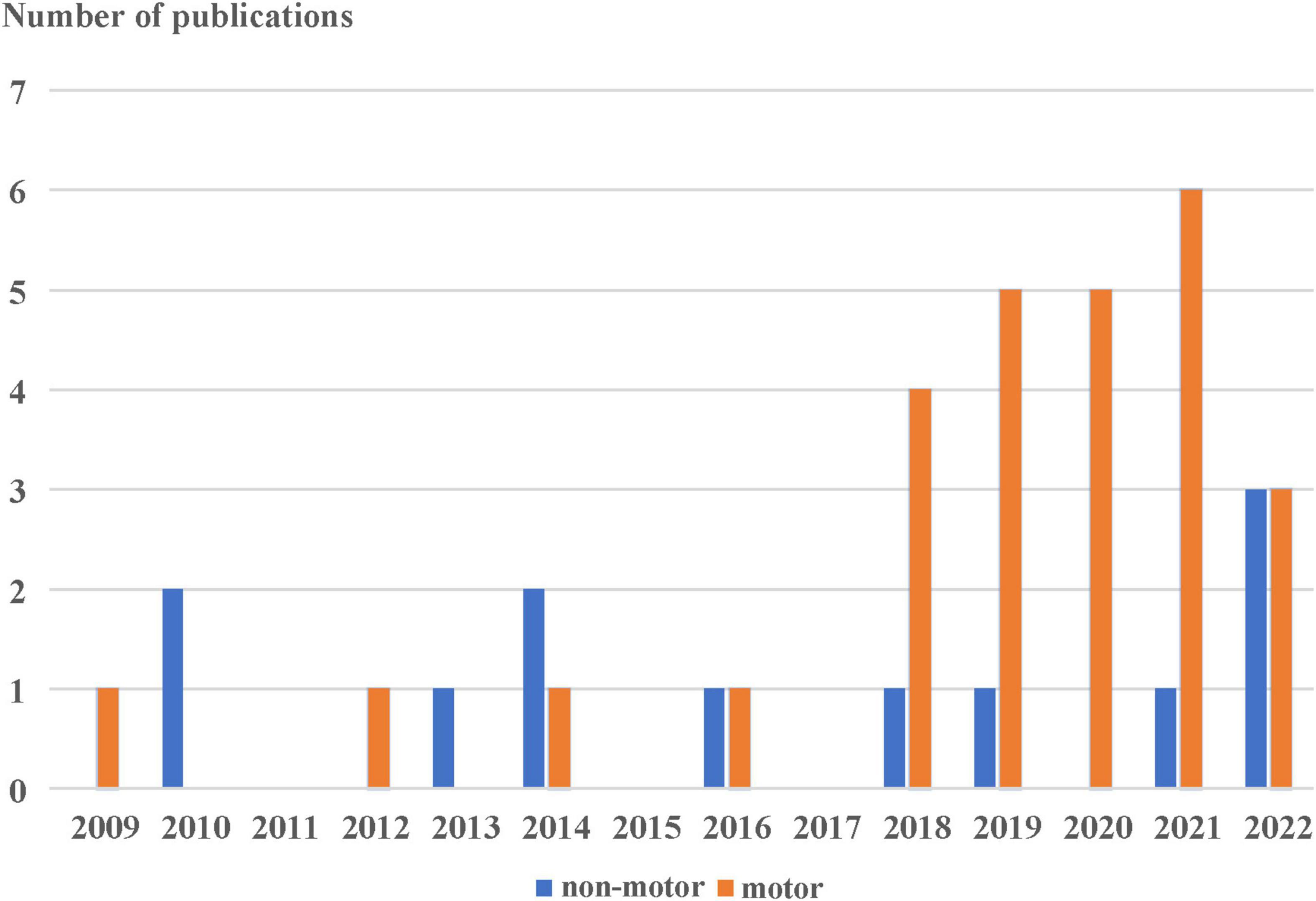
Figure 2. Published number of the study using the wrist-worn wearable in the monitoring of motor (orange) and non-motor (blue) symptoms of Parkinson’s disease.
3.2. Demographic and clinical characteristics
Table 1 provides information on the publication year, authors, number of participants, and demographics of the included studies. These studies use different research protocols with different numbers of patients and/or healthy controls. There are 14 case-control studies and 25 case studies. Most of the studies include a small number of patients (less than 100), with the exception of two papers with 304 and 388 participants, respectively. The participants in most of these studies are in the mild to moderate stages of PD, and only one study investigated the motor function of participants in the late stage of PD.
3.3. Motor symptoms monitoring
Between 2009 and 2017, we only find four publications focusing on the application of wrist technology for monitoring motor signs in PD. Figure 2 shows a strong increase in the use of wrist-based technology for monitoring PD motor signs in daily life, starting in 2018. The number of published papers on this topic increase by 4.5 times in the past 4 years, from 4 to 22. The majority of the studies (67%) focuses on motor symptoms of PD including rest tremor, bradykinesia, and gait.
Table 2 presents 26 studies that investigate the use of wrist-based wearable sensor devices for monitoring the motor features of PD patients over the past decade. These devices are typically worn for a mean of 6–7 days. To reduce the effect of motor symptoms on wearables’ sensitivity and specificity, the sensor is worn on the non-dominant wrist of patients. The motor commonly studied is tremor, which accounts for 34.6% (9/26) of all reviewed articles.
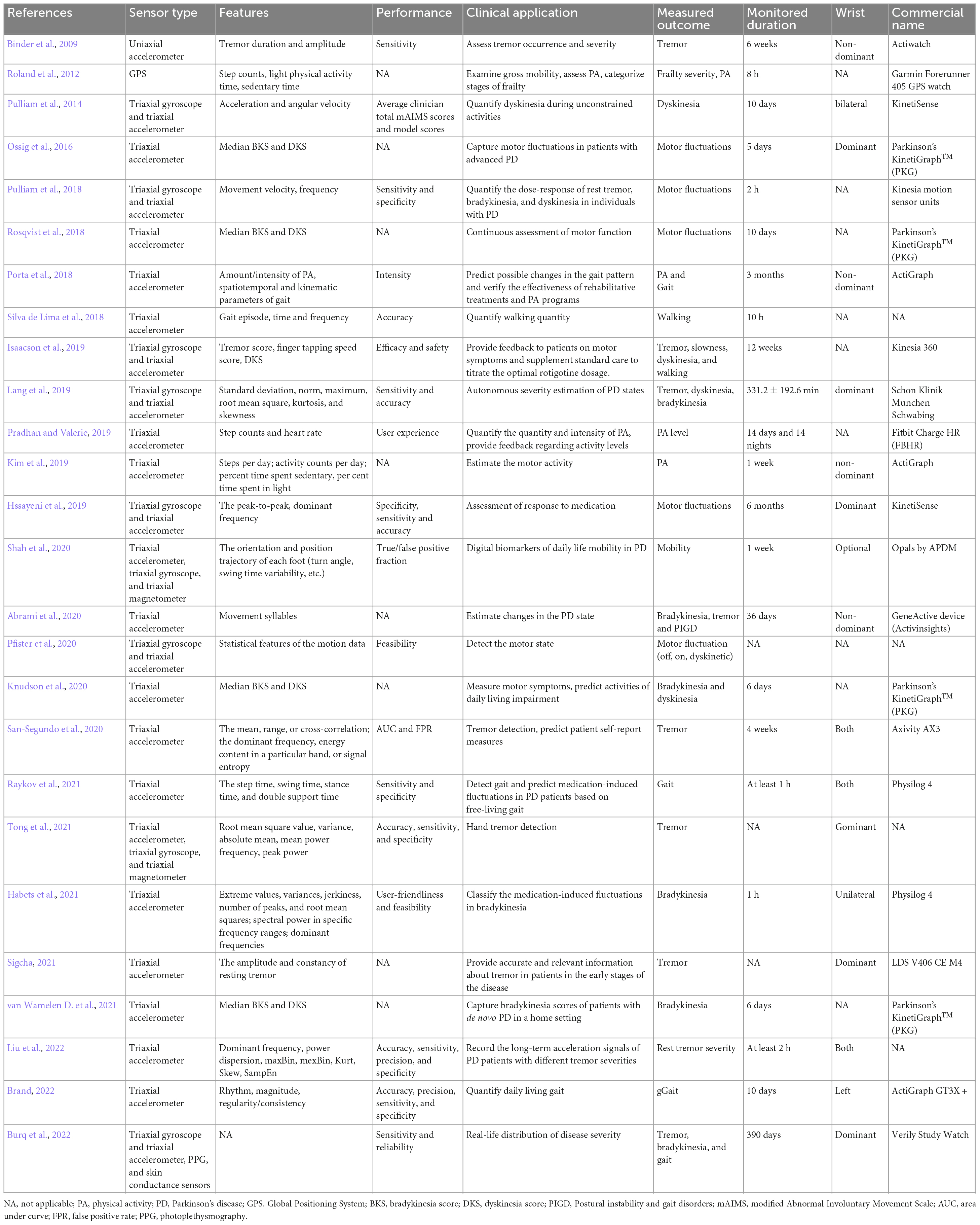
Table 2. Articles about wrist-worn commercial devices for telemonitoring motor signs in PD and related information.
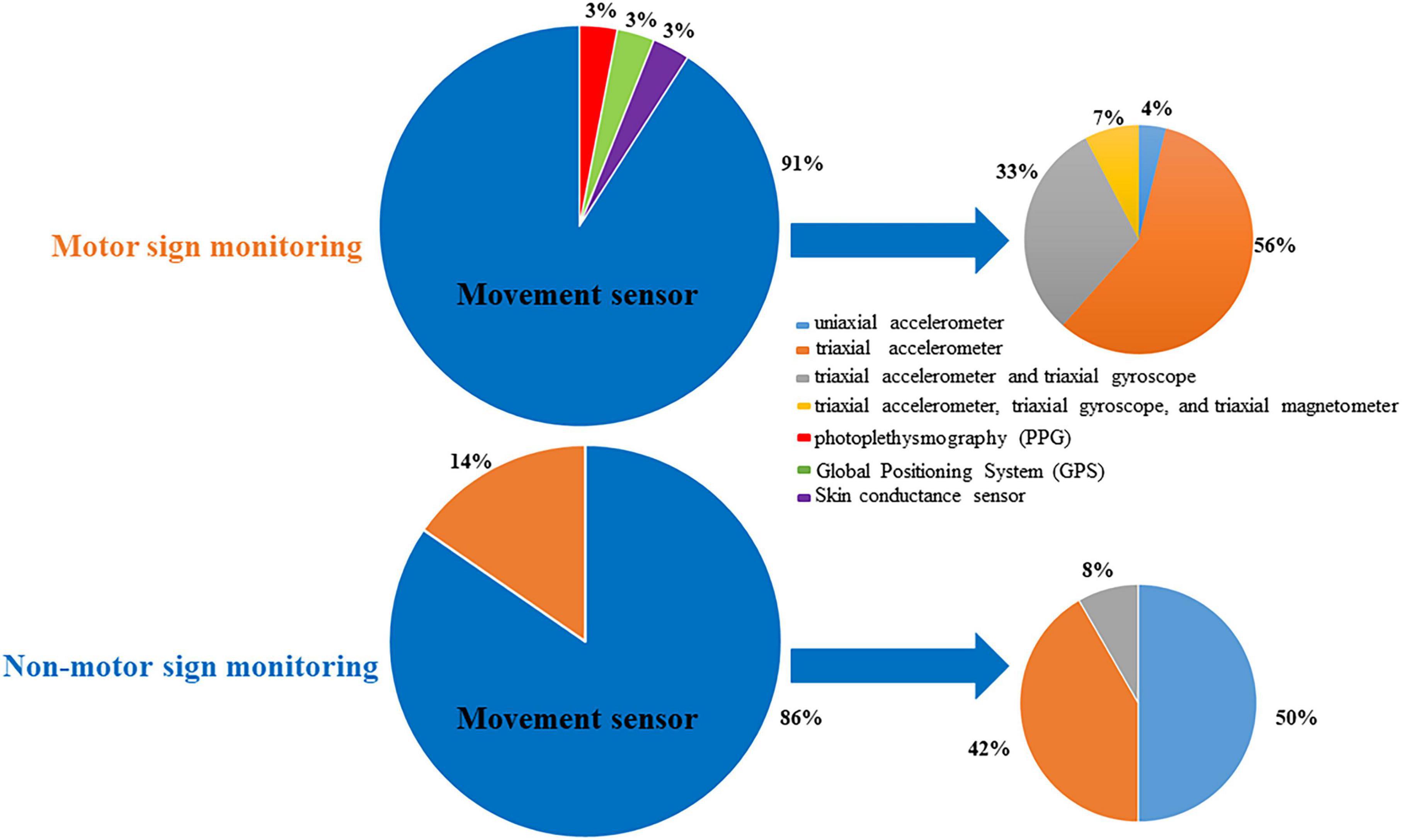
Various research groups have employed different types of sensors in their research protocols. The pie chart (Figure 3) above shows that the most commonly used sensor type for motor signs monitoring is the movement sensor (91%). Interestingly, the triaxial accelerometer is the most frequently used movement sensor (60%). The gyroscope is another type of motion sensor that is frequently used to measure orientation and angular velocity.
Figure 4 shows that the technical applications of wrist-worn sensors for monitoring rest tremor in PD include tracking tremor severity (n = 4) and detecting tremor signs (n = 3). In 2021, bradykinesia was first unobtrusively monitored by a wrist-worn accelerometer which indicated monitoring bradykinesia in non-invasive and non-intrusive ways to track the severity and progression of the symptom (Habets et al., 2021). Dyskinesia was first quantified in 2014 when the KinetiSense were worn on the bilateral wrists of PD patients during uncontrolled activities (Pulliam et al., 2014). Australian researchers developed the Personal KinetiGraph® (PKG®) (Global Kinetics Corporation Ltd.) to produce the median bradykinesia score (BKS) and dyskinesia score (DKS) for objectively estimating the bradykinesia and dyskinesia of PD. Table 2 shows that the United Kingdom and Netherlands research group uses PKG to early detect bradykinesia in newly diagnosed and previously untreated PD patients (van Wamelen D. et al., 2021). Swedish and German research groups use it to assess the motor fluctuation of advanced PD patients and aim to guide the self-management of PD patients (Ossig et al., 2016; Rosqvist et al., 2018).
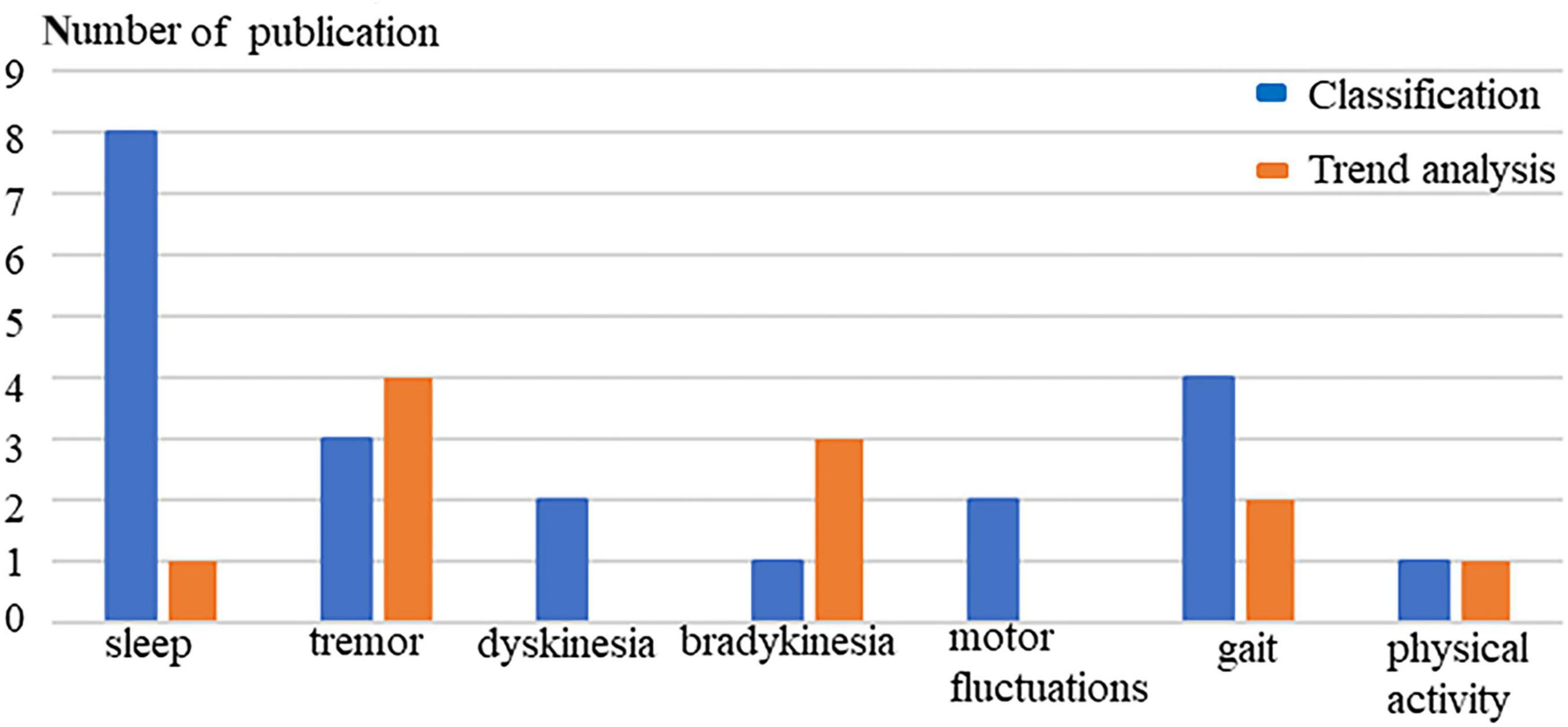
Figure 4. The technological application of wrist-worn sensors in monitoring PD symptoms. Classification (blue): Prediction and detection of symptoms; Trend analysis (orange): Progression or change of severity.
3.4. Non-motor symptoms monitoring
Table 3 shows 13 articles that have investigated non-motor symptoms (NMS) of PD in daily life with wrist-worn technology. The non-motor symptom monitors are mainly commercial actigraphy, including Actiwatch (n = 6), PKG (n = 2), ASUS VivoWatch BP (n = 1), Fitbit Charge HR (n = 1), and GENEActiv™ (n = 1). The actigraphy typically is worn on the non-dominant wrist. The reason is that the non-dominant wrist has fewer movements than the dominant one. Clearer data can be obtained when there are fewer movement artefacts. Therefore, PD patients can expect that the data can be acquired with fewer noises by wrist-worn on the non-dominant wrist. The duration of monitoring in these studies is typically 1 to 2 weeks. However, one study detects abnormal sleep phenomena in PD patients with a right wrist-worn device (ASUS VivoWatch BP) for 2 years (Ko et al., 2022).
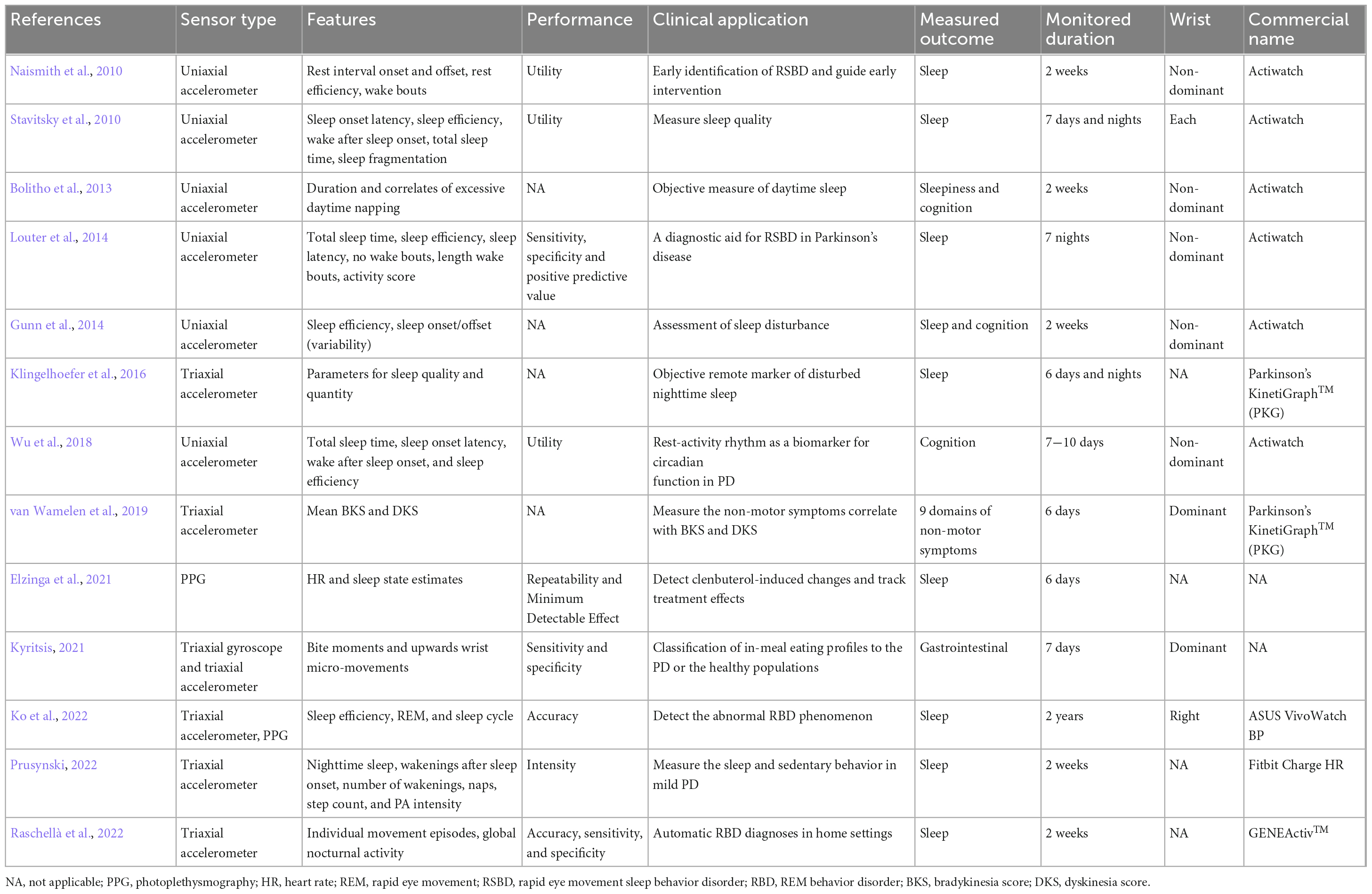
Table 3. Articles about wrist-worn commercial devices for telemonitoring non-motor signs in PD and related information.
Most NMS studies (8 out of 13) focus on sleep quality. Interestingly, one article classifies gastrointestinal symptoms in PD using Inertial Measurement Units (IMUs) (Kyritsis, 2021). Figure 3 shows that sensor types used to measure NMS include movement sensors (n = 12, 86%) and photoplethysmography (n = 2, 14%). The uniaxial accelerometer is the most frequently employed movement sensor (50%).
4. Discussion and conclusion
This review is the first to provide an overview of the use of wrist-worn devices for remote monitoring and managing PD patients in their daily living environment. Compared with the classification of PD symptoms, wrist-worn sensors have been less commonly used to daily track the progression of PD. In the last decade, researchers have primarily used movement sensors, i.e., accelerometers, to daily monitor motor and non-motor symptoms of PD.
4.1. Symptoms monitoring
In-home monitoring using wrist-worn technology has primarily focused on classifying and managing motor symptoms and sleep disorders of PD patients, leaving many non-motor symptoms unaddressed. With the development of digital mobile technology, technology-based objective measurements, particularly wrist-worn monitors have become popular among patients and clinicians in daily monitoring (King and Majid, 2018). Researchers have mainly used mobile devices to help PD patients detect, monitor, and manage motor symptoms while neglecting non-motor symptoms which are difficult to measure directly by wearable sensors every day. This is reflected in the fact that 67% of the studies reviewed focus on motor signs, compared to only 33% that focus on non-motor signs. Non-motor symptoms remain a neglected area of research in PD monitoring.
Previous works on PD motor monitoring mainly focused on three cardinal signs: tremor, dyskinesia, and bradykinesia. The wrist joint is most frequently affected by rigidity. Table 2 shows that there is no study on rigidity monitoring with the wrist-based sensor in a daily environment. A recent scoping review of wrist rigidity evaluation reported that some force and inertial sensors have been used to quantitatively assess the rigidity of wrists in PD (Alves et al., 2022). However, it is challenging to standardize the measurement of rigidity with accessible wearables. With the fast development of technology and computational techniques, it is likely that new analytical models for sensor application in rigidity emerge in the future.
Compared with 26 studies focusing on motor signs, only half of them have investigated the non-motor signs in daily life. In particular, sleep problems have been the focus of PD research. Rapid eye movement sleep behavior disorder is thought to be a prodromal symptom of PD and has been targeted for disease-modifying treatment (Naismith et al., 2010). Actiwatch is most frequently used to quantify sleep features, such as sleep efficiency, and sleep onset/offset (Naismith et al., 2010; Stavitsky et al., 2010; Bolitho et al., 2013; Gunn et al., 2014; Louter et al., 2014). Daniel et al. used PKG to measure the correlation between non-motor symptoms and bradykinesia and dyskinesia scores in PD patients. They suggested that the future of digital technologies may enable the reliable measurement of often under-reported and under-recognized non-motor signs (van Wamelen et al., 2019; van Wamelen D. J. et al., 2021). In addition to motor problems, non-motor symptoms impact 90% of PD patients. Some subjective non-motor symptoms, such as fatigue and depression, can significantly decrease the quality of life of PD patients and are outside the focus of Parkinson’s care and research. These non-motor problems are highly variable and present throughout the disease’s progress. The gap between these unmet needs of non-motor endpoints and technology platforms is currently large. New sensor technology and computational models, such as deep learning and semi-supervised learning may be applied to smart wrist-worn devices to improve the management of non-motor symptoms in PD.
4.2. Wearable healthcare sensor
Compared to traditional scales, body-worn sensors (BWS) record the data of patients’ symptoms in a long-term, real-time, and objective manner. Additionally, BWS may reduce the cost of time and money for patients seeking professional care. A large amount of data collected by BWS may be valuable for monitoring symptoms. BWS can be worn on the wrist, waist, or ankle, and provide PD patients and healthcare professionals with relevant information in daily-living conditions. Compared to locations of the body, the wrist-worn sensor is easy to view and simple to operate in daily life by older individuals.
Different kinds of sensor types have different functions in clinical applications of PD monitoring. The accelerometer is the most frequently used wrist-worn sensor in monitoring PD motor and non-motor signs in the natural living environment. Among motion sensors, the triaxial accelerometer most frequently detects motor signs of PD, while the uniaxial accelerometer is most frequently used for monitoring sleep signs. The possible reason is that a single triaxial accelerometer can classify the signals of basic daily movements in a PD patient’s activity, while the uniaxial accelerometer only classifies postural orientations during rest. In addition to movement sensors, photoplethysmography (PPG) sensors are also frequently used in monitoring non-motor and motor signs (Elzinga et al., 2021; Burq et al., 2022; Ko et al., 2022). PPG is a simple and low-cost wearable device for monitoring blood flow and blood oxygenation. Wrist PPG signal is widely used in heart rate monitors. The role of PPG in PD clinical practice still has a lot of potential. However, a single sensor is probably insufficient to detect all relevant signs of the body in PD patients. Accelerometers and other sensors do not offer sufficient and practical data for the real-time assessment of motor signals. Global Positioning System (GPS) sensors do not penetrate solid walls and are affected by large structures, which means that patients cannot use GPS indoors or in the underground environment. In the future, researchers should design multimodal measurements to monitor different clinical manifestations of PD. IMU and other multimodal sensors can help capture more clinically meaningful signs in daily monitoring and are expected to better monitor PD manifestation than a single-modal sensor. For example, Maximilien et al. studied motion sensors connected with skin conductance sensors and PPG to capture multimodal data of PD for standard virtual motor exams (Burq et al., 2022). Meanwhile, it is important to balance the amount of information being collected with the number of sensors when developing a standard technology measurement platform. Also, it is important that these data systems have high standards for patient data security and privacy.
4.3. Value in the daily management
Wrist-worn sensors might have the potential to help clinicians and patients to detect PD symptoms at an early stage through increasing the awareness of patients and offering clinicians deep insight into patients’ daily life situations, predict the motor and non-motor signs and subsequently track PD symptom severity in daily naturalistic environments. From Figure 4, we find that classification of wrist-based technology is mainly in sleep, gait, and tremor of PD, because wrist-worn accelerometers can easily classify activity patterns in daily living. Moreover, wrist-worn devices have been widely used by the public with the popularity of smartwatches. The common usage of the devices can timely offer healthcare professionals opportunities to detect PD symptoms in the high-risk population at an early stage. Wrist wearables mainly analyse the trend of rest tremor and bradykinesia, but have not been used very often to track NMS. The lack of proper sensing technology may be the cause of the gap which will need to be addressed in future studies. In addition, wrist-worn devices might be helpful in the daily management of PD patients, but further research must be done as the correlations so far with clinical scales are not very high.
In line with our findings, a recent in-depth analysis based on over 50 articles supported that little focus has been placed on the management of PD NMS via wearable sensors, compared to much work that has been attributed to PD motor symptom management (Mughal et al., 2022). Wrist-worn devices can be used to detect prodromal non-motor signs in daily life, such as sleep disorders, which can serve as digital biomarkers in the prodromal phase of PD. Actigraphy can be used to measure abnormal sleep-related features in daily life and guide portable interventions for precision medicine in patients with specific phenotypes. In 2022, a review reported that lifestyle intervention is the first test to prevent PD (Janssen Daalen et al., 2022). Digital remote devices can provide exercise interventions to prodromal patients in daily life, monitored by experts, which may also help early detection of specific prodromal NMS, objectively measure the outcome of disease progression, and guide long-term self-management. However, the NMS is a field which is still to be explored. There is no device certified under the United States Food and Drug Administration (FDA) or European Medicines Agency (EMA), so articles found so far only explore the possibility to use them, and NMS device development and validation are still needed to be done in future.
Furthermore, if the phenotype of patients is better defined, diagnosing and managing the complex disease can be more accurate. Currently, late-stage patients are often ignored, and more advanced sensor technology is needed to address their specific needs. Early diagnosis of PD before clinic visits and the use of sensor technology for self-management at home are two areas where there is potential for clinical applications of sensor technology for PD.
4.4. Current pitfalls in using wrist-worn devices in the clinical PD management
Considering the convenience of smartwatches in daily practice, we propose to use wrist-worn monitoring in health self-management. However, there are also some limitations that may hinder the wrist-worn monitoring technology to be translated into PD clinical management in daily life. (1) One of the most important pitfalls is the lack of standardization of different wrist-based objective measurements. For example, commercial wrist-worn devices have different measurement protocols and different feature extraction algorithms behind PD symptoms. The heterogeneity can hinder the standardization of clinical practice guidelines about using wrist-worn devices for PD management. In future, the obligation of certification by a recognized agency of medication, such as the FDA or EMA, can set standard technology assessment criteria for different competitors’ devices. (2) Another pitfall is about missing medical device certificates in most wrist-worn devices. The certification of the devices indicates the safe and reliable usage for customers to use it track the disease and manage their health. Before the clinical trials of wearable devices are completed, the technology cannot be approved by the professional authority, which becomes the main barrier to pushing forward at-home monitoring. Effective collaborations between private companies and academic initiatives can further advance this field. (3) The limitation of using wrist-worn movement sensors for the complete human body movement analysis is another pitfall for clinical PD management. Wrist technology has the limitation of motion data being detected only from the arm, rather than the whole body in the field of human movement science. Meanwhile, false negative and positives events should be considered in detecting some PD symptoms from the wrist location. For example, the wrist worn devices misses several movements in the rest of the body a part of missing the axial symptoms. In addition, the high degrees of freedom in the arm adds to the randomness of movements in the arms, provokes overestimation of several movements and false positives events (Gjoreski et al., 2016; Shcherbina et al., 2017). The current possible solution for accurate PD motor symptom monitoring is to use multiple sensors placed at different body parts, for example, combining a smartwatch with a smartphone, or using non-wearable sensor technologies such as wall-mounted devices that can non-invasively capture the body movement patterns. In many cases, the accuracy of the calculated variables is still poor, and the measurement accuracy of many wearables is even not validated for people with PD. To increase the accuracy of wrist-worn techniques in PD motor symptom monitoring, we should further develop the ambulatory human movement analysis field that aims to capture the whole body movement using the minimal number of movement sensors on the body. According to the current ambulatory human movement analysis techniques, we would recommend that PD researchers and healthcare professionals use waist- or chest-worn sensor to better characterize axial movements, such as, bradykinesia and dyskinesia and better model the gait patterns of human subjects than only using the wrist-worn sensor. (4) Researchers should also consider that PPG and electrodermal activity sensors are strongly influenced by the wearing of wrist-worn devices during non-motor symptom monitoring. The loose wearing of the devices can decrease the quality of signals given the technical mechanism of the sensors. However, wearing too tight can affect the usability of the device and then increase users’ dropout rate in using the devices for their PD management. It is essential for healthcare professionals to find a balance between the correct and comfortable usage of the wrist-worn device and provide their patients with some practical guidelines. Moreover, the relevant algorithms to extract physiological variables from PPG and electrodermal activity are still necessary to be validated according to the actual daily life usage of the wrist-worn devices for people with PD.
4.5. Limitation
There are a couple of limitations to this narrative review that should be noted. Firstly, this narrative review does not use systematic statistical methods to assess the quality of studies, and may make it less reproducible compared to a systematic review. Additionally, the review might contain selection and evaluation biases. Secondly, the review does not provide a systematic discussion about the data analysis techniques employed in the studies that are surveyed. The clear description of algorithms behind the extracted features enables healthcare professionals and patients to make decisions based on transparent information for PD disease management. However, we noticed that most clinical articles we reviewed did not describe the algorithms clearly. In addition, this review focuses on the potential value of investigating wrist technology in the management in daily life, and the feature extraction algorithm and monitoring performance are out of the scope of this review. Therefore, we did not include information about feature extraction algorithms and relevant monitoring performances in this review. We recommend that it is valuable to have a technique-oriented review discussing data analysis methods behind the usage of wrist-worn devices in PD management to contribute to the accuracy of PD symptom monitoring in daily life.
4.6. Conclusion
Wrist-worn monitoring technology with medical device certification has the potential to improve early intervention and personalized care management of PD patients based on daily health information exchange. Wrist-worn PPG sensor combined with motion sensors could assist to estimate motor symptoms, analyze daily activity, and even address some unmet needs for non-motor symptoms of PD patients in daily life. However, only a few studies have addressed the need of non-motor symptom monitoring among PD patients. More emphasis should be placed on non-motor symptom monitoring using wrist-worn devices in the future. In addition, clinical healthcare professionals and patients should pay attention to the shortcomings of commercialized wrist-worn devices, such as, the lack of reliability, sufficient quality, and clinical validation. More wrist-worn technology assessment and clinical validation studies are recommended in future to increase the technique trustworthiness and effectiveness in the clinical management of PD in daily life.
Author contributions
PL made the draft of manuscript. PL and YW made the main design flow of the review. RW and YW gave the main comments to the manuscript. YZ and FH gave the grammatical and other revised suggestions to the draft. All authors approved the final revision to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrami, A., Heisig, S., Ramos, V., Thomas, K., Ho, B., Caggiano, V., et al. (2020). Using an unbiased symbolic movement representation to characterize Parkinson’s disease states. Sci. Rep. 10:7377. doi: 10.1038/s41598-020-64181-3
Alves, C. M., Rezende, A., Marques, I., Mendes, L., de Sá, A., Vieira, M., et al. (2022). Wrist rigidity evaluation in Parkinson’s disease: a scoping review. Healthcare 10:2178. doi: 10.3390/healthcare10112178
Binder, S., Deuschl, G., and Volkmann, J. (2009). Effect of cabergoline on parkinsonian tremor assessed by long-term actigraphy. Eur. Neurol. 61, 149–153. doi: 10.1159/000186505
Bloem, B. R., Post, E., and Hall, D. A. (2023). An apple a day to keep the Parkinson’s disease doctor away? Ann. Neurol. doi: 10.1002/ana.26612 [Epub ahead of print].
Bolitho, S., Naismith, S., Salahuddin, P., Terpening, Z., Grunstein, R., Lewis, S., et al. (2013). Objective measurement of daytime napping, cognitive dysfunction and subjective sleepiness in Parkinson’s disease. PLoS One 8:e81233. doi: 10.1371/journal.pone.0081233
Brand, Y. (2022). Gait detection from a wrist-worn sensor using machine learning methods: a daily living study in older adults and people with Parkinson’s disease. Sensors 22:7094. doi: 10.3390/s22187094
Burq, M., Rainaldi, E., Ho, K., Chen, C., Bloem, B., Evers, L., et al. (2022). Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. NPJ Digital Med. 5:65. doi: 10.1038/s41746-022-00607-8
Calne, D., Snow, B., and Lee, C. (1992). Criteria for diagnosing Parkinson’s disease. Ann. Neurol. 32, S125–S127. doi: 10.1002/ana.410320721
Chakrabarti, S., Biswas, N., Jones, L. D., Kesari, S., and Ashili, S. (2022). Smart consumer wearables as digital diagnostic tools: a review. Diagnostics 12:2110. doi: 10.3390/diagnostics12092110
Chandrabhatla, A. S., Pomeraniec, I., and Ksendzovsky, A. (2022). Co-evolution of machine learning and digital technologies to improve monitoring of Parkinson’s disease motor symptoms. NPJ Digital Med. 5:32. doi: 10.1038/s41746-022-00568-y
Dorsey, E. R., and Eric, J. T. (2020). Telemedicine 2020 and the next decade. Lance 395:859. doi: 10.1016/S0140-6736(20)30424-4
Elzinga, W., Prins, S., Borghans, L., Gal, P., Vargas, G., Groeneveld, G., et al. (2021). Detection of clenbuterol-induced changes in heart rate using at-home recorded smartwatch data: randomized controlled trial. JMIR Format. Res. 5:e31890. doi: 10.2196/31890
G 2016 Parkinson’s Disease Collaborators (2018). Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 11, 939–953. doi: 10.1016/S1474-4422(18)30295-3
Giannakopoulou, K., Roussaki, I., and Demestichas, K. (2022). Internet of things technologies and machine learning methods for Parkinson’s disease diagnosis, monitoring and management: a systematic review. Sensors 22:1799. doi: 10.3390/s22051799
Gjoreski, M., Gjoreski, H., Luštrek, M., and Gams, M. (2016). How accurately can your wrist device recognize daily activities and detect falls? Sensors 16:800. doi: 10.3390/s16060800
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G., Counsell, C., et al. (2004). Movement disorder society task force report on the hoehn and yahr staging scale: status and recommendations. Mov. Dis. Offi. J. Mov. Dis. Soc. 19, 1020–1028. doi: 10.1002/mds.20213
Gunn, D. G., Naismith, S., Bolitho, S., and Lewis, S. (2014). Actigraphically-defined sleep disturbance in Parkinson’s disease is associated with differential aspects of cognitive functioning. J. Clin. Neurosci. Offi.. Neurosurg. Soc. Austr. 21, 1112–1115. doi: 10.1016/j.jocn.2013.09.017
Habets, J. G., Herff, C., Kubben, P., Kuijf, M., Temel, Y., Evers, L., et al. (2021). Rapid dynamic naturalistic monitoring of bradykinesia in Parkinson’s disease using a wrist-worn accelerometer. Sensors 21:7876. doi: 10.3390/s21237876
Hssayeni, M., Burack, M., Jimenez-Shahed, J., and Ghoraani, B. (2019). Assessment of response to medication in individuals with Parkinson’s disease. Med. Eng. Phys. 67, 33–43. doi: 10.1016/j.medengphy.2019.03.002
Isaacson, S., Boroojerdi, B., Waln, O., McGraw, M., Kreitzman, D., Klos, K., et al. (2019). Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson’s disease starting transdermal rotigotine patch: a pilot study. Parkins. Related Dis. 64, 132–137. doi: 10.1016/j.parkreldis.2019.01.025
Janssen Daalen, J. M., Schootemeijer, S., Richard, E., Darweesh, S., and Bloem, B. (2022). Lifestyle interventions for the prevention of parkinson disease: a recipe for action. Neurology 99, 42–51. doi: 10.1212/WNL.0000000000200787
Julien, C., Hache, G., Dulac, M., Dubrou, C., Castelnovo, G., Giordana, C., et al. (2021). The clinical meaning of levodopa equivalent daily dose in Parkinson’s disease. Fund. Clin. Pharmacol. 35, 620–630. doi: 10.1111/fcp.12646
Kim, D. W., Hassett, L., Nguy, V., and Allen, N. (2019). A comparison of activity monitor data from devices worn on the wrist and the waist in people with Parkinson’s disease. Mov. Dis. Clin. Pract. 6, 693–699. doi: 10.1002/mdc3.12850
King, C. E., and Majid, S. (2018). A survey of smartwatches in remote health monitoring. J. Health. Inform. Res. 2, 1–24. doi: 10.1007/s41666-017-0012-7
Klingelhoefer, L., Rizos, A., Sauerbier, A., McGregor, S., Martinez-Martin, P., Reichmann, H., et al. (2016). Night-time sleep in Parkinson’s disease - the potential use of Parkinson’s KinetiGraph: a prospective comparative study. Eur. J. Neurol. 23, 1275–1288. doi: 10.1111/ene.13015
Knudson, M., Thomsen, T., and Kjaer, T. (2020). Comparing objective and subjective measures of parkinson’s disease using the Parkinson’s KinetiGraph. Front. Neurol. 11:570833. doi: 10.3389/fneur.2020.570833
Ko, Y. F., Kuo, P., Wang, C., Chen, Y., Chuang, P., Li, S., et al. (2022). Quantification analysis of sleep based on smartwatch sensors for Parkinson’s disease. Biosensors 12:74. doi: 10.3390/bios12020074
Kyritsis, K. (2021). Assessment of real life eating difficulties in Parkinson’s disease patients by measuring plate to mouth movement elongation with inertial sensors. Sci. Rep. 11:1632. doi: 10.1038/s41598-020-80394-y
Lang, M., Pfister, F., Frohner, J., Abedinpour, K., Pichler, D., Fietzek, U., et al. (2019). A multi-layer gaussian process for motor symptom estimation in people with Parkinson’s disease. IEEE Trans. Bio Med. Eng. 66, 3038–3049. doi: 10.1109/TBME.2019.2900002
Liu, S., Yuan, H., Liu, J., Lin, H., Yang, C., and Cai, X. (2022). Comprehensive analysis of resting tremor based on acceleration signals of patients with Parkinson’s disease. Technol. Health Care Offi. J. Eur. Soc. Eng. Med. 30, 895–907. doi: 10.3233/THC-213205
Louter, M., Arends, J., Bloem, B., and Overeem, S. (2014). Actigraphy as a diagnostic aid for REM sleep behavior disorder in Parkinson’s disease. BMC Neurol. 14:76. doi: 10.1186/1471-2377-14-76
Mughal, H., Javed, A. R., Rizwan, M., Almadhor, A. S., and Kryvinska, N. (2022). Parkinson’s disease management via wearable sensors: a systematic review. IEEE Access 10, 35219–35237. doi: 10.1109/ACCESS.2022.3162844
Naismith, S. L., Rogers, N. L., Mackenzie, J., Hickie, I. B., and Lewis, S. J. G. (2010). The relationship between actigraphically defined sleep disturbance and REM sleep behaviour. Disorder in Parkinson’s disease. Clin. Neurol. Neurosurg. 112, 420–423. doi: 10.1016/j.clineuro.2010.02.011
Ossig, C., Gandor, F., Fauser, M., Bosredon, C., Churilov, L., Reichmann, H., et al. (2016). Correlation of quantitative motor state assessment using a kinetograph and patient diaries in advanced PD: data from an observational study. PLoS One 11:e0161559. doi: 10.1371/journal.pone.0161559
Pfister, F. M., Um, T., Pichler, D., Goschenhofer, J., Abedinpour, K., Lang, M., et al. (2020). High-resolution motor state detection in Parkinson’s disease using convolutional neural networks. Sci. Rep. 10:5860. doi: 10.1038/s41598-020-61789-3
Porta, M., Pilloni, G., Pili, R., Casula, C., Murgia, M., Cossu, G., et al. (2018). Association between objectively measured physical activity and gait patterns in people with parkinson’s disease: results from a 3-month monitoring. Parkinson’s Dis. 2018:7806574. doi: 10.1155/2018/7806574
Pradhan, S., and Valerie, E. K. (2019). Quantifying physical activity in early parkinson disease using a commercial activity monitor. Parkins. Related Dis. 66, 171–175. doi: 10.1016/j.parkreldis.2019.08.001
Prusynski, R. A. (2022). The association between sleep deficits and sedentary behavior in people with mild parkinson disease. Disability Rehabilit. 44, 5585–5591. doi: 10.1080/09638288.2021.1940320
Pulliam, C. L., Burack, M. A., Heldman, D. A., Giuffrida, J. P., and Mera, T. O. (2014). Motion sensor dyskinesia assessment during activities of daily living. J. Parkinson’s Dis. 4, 609–615. doi: 10.3233/JPD-140348
Pulliam, C., Heldman, D., Brokaw, E., Mera, T., Mari, Z., and Burack, M. (2018). Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans. Bio Med. Eng. 65, 159–164. doi: 10.1109/TBME.2017.2697764
Raschellà, F., Scafa, S., Puiatti, A., Martin Moraud, E., and Ratti, P. (2022). Actigraphy enables home screening of rapid eye movement behavior disorder in Parkinson’s disease. Ann. Neurol. 2022:26517. doi: 10.1002/ana.26517
Raykov, Y. P., Evers, L., Badawy, R., Bloem, B., Heskes, T., Meinders, M., et al. (2021). Probabilistic modelling of gait for robust passive monitoring in daily life. IEEE J. Biomed. Health Inform. 25, 2293–2304. doi: 10.1109/JBHI.2020.3037857
Rietdijk, C. D, Perez-Pardo, P., Garssen, J., Wezel, R. J., and Kraneveld, A. D (2017). Exploring Braak’s hypothesis of Parkinson’s disease. Front. Neurol. 8:37. doi: 10.3389/fneur.2017.00037
Rocca, W. (2018). The burden of Parkinson’s disease: a worldwide perspective. Lancet Neurol. 17, 928–929. doi: 10.1016/S1474-4422(18)30355-7
Roland, K. P., Cornett, K., Theou, O., Jakobi, J., and Jones, G. (2012). Physical activity across frailty phenotypes in females with Parkinson’s disease. J. Aging Res. 2012:468156. doi: 10.1155/2012/468156
Rosqvist, K., Horne, M., Hagell, P., Iwarsson, S., Nilsson, M., Odin, P., et al. (2018). Levodopa effect and motor function in late stage Parkinson’s disease. J. Parkinson’s Dis. 8, 59–70. doi: 10.3233/JPD-171181
San-Segundo, R., Zhang, A., Cebulla, A., Panev, S., Tabor, G., Stebbins, K., et al. (2020). Parkinson’s disease tremor detection in the wild using wearable accelerometers. Sensors 20:5817. doi: 10.3390/s20205817
Shah, V. V., McNames, J., Mancini, M., Carlson-Kuhta, P., Nutt, J., El-Gohary, M., et al. (2020). Digital biomarkers of mobility in Parkinson’s disease during daily living. J. Parkinson’s Dis. 10, 1099–1111. doi: 10.3233/JPD-201914
Shcherbina, A., Mattsson, C., Waggott, D., Salisbury, H., Christle, J., Hastie, T., et al. (2017). Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J. Pers Med. 7:3. doi: 10.3390/jpm7020003
Sigcha, L. (2021). Automatic resting tremor assessment in parkinson’s disease using smartwatches and multitask convolutional neural networks. Sensors 21:291. doi: 10.3390/s21010291
Silva de Lima, A. L., Evers, L., Hahn, T., de Vries, N., Daeschler, M., Boroojerdi, B., et al. (2018). Impact of motor fluctuations on real-life gait in Parkinson’s patients. Gait Post. 62, 388–394. doi: 10.1016/j.gaitpost.2018.03.045
Stavitsky, K., Saurman, J., McNamara, P., and Cronin-Golomb, A. (2010). Sleep in Parkinson’s disease: a comparison of actigraphy and subjective measures. Parkins. Related Dis. 16, 280–283. doi: 10.1016/j.parkreldis.2010.02.001
Tong, L., He, J., and Peng, L. (2021). CNN-based PD hand tremor detection using inertial sensors. IEEE Sensors Lett. 5, 1–4. doi: 10.1109/lsens.2021.3074958
van Wamelen, D. J., Hota, S., Podlewska, A., Leta, V., Trivedi, D., Rizos, A., et al. (2019). Non-motor correlates of wrist-worn wearable sensor use in Parkinson’s disease: an exploratory analysis. NPJ Parkinson’s Dis. 5:94. doi: 10.1038/s41531-019-0094-4
van Wamelen, D. J., Sringean, J., Trivedi, D., Carroll, C., Schrag, A., Odin, P., et al. (2021). Digital health technology for non-motor symptoms in people with Parkinson’s disease: futile or future? Parkins. Related Dis. 89, 186–194. doi: 10.1016/j.parkreldis.2021.07.032
van Wamelen, D., Urso, D., and Ray Chaudhuri, K. (2021). How time rules: diurnal motor patterns in de novo Parkinson’s disease. J. Parkinson’s Dis. 11, 695–702. doi: 10.3233/JPD-202352
Wu, J. Q., Li, P., Stavitsky Gilbert, K., Hu, K., and Cronin-Golomb, A. (2018). Circadian rest-activity rhythms predict cognitive function in early Parkinson’s disease independently of sleep. Mov. Dis. Clin. Pract. 5, 614–619. doi: 10.1002/mdc3.12692
Keywords: Parkinson’s disease, wrist-worn, sensor, daily life, monitoring, management
Citation: Li P, van Wezel R, He F, Zhao Y and Wang Y (2023) The role of wrist-worn technology in the management of Parkinson’s disease in daily life: A narrative review. Front. Neuroinform. 17:1135300. doi: 10.3389/fninf.2023.1135300
Received: 31 December 2022; Accepted: 28 March 2023;
Published: 12 April 2023.
Edited by:
Antonio Fernández-Caballero, University of Castilla-La Mancha, SpainCopyright © 2023 Li, van Wezel, He, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Li, cGVuZ2xpMDYwOEBnbWFpbC5jb20=
 Peng Li
Peng Li Richard van Wezel
Richard van Wezel Fei He
Fei He Yifan Zhao
Yifan Zhao Ying Wang
Ying Wang