
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Hum. Neurosci. , 19 February 2025
Sec. Brain Health and Clinical Neuroscience
Volume 19 - 2025 | https://doi.org/10.3389/fnhum.2025.1504575
This article is part of the Research Topic Bipolar Disorder: Where are we now? Treatment Response, Neural Correlates and Personality View all articles
Bipolar disorder (BD) is a chronic and debilitating mental illness affecting approximately 40 million people worldwide. Cognitive impairment is a core feature of BD, impacting daily functioning and persisting even during mood stability. Cognitive deficits are among the most reliable indicators of long-term functional outcomes in BD. Despite their significance, there are currently no widely available treatments targeting cognitive impairment in BD, largely due to our limited understanding of the underlying pathophysiology. A healthy blood–brain barrier (BBB) is essential for brain homeostasis, serving as a protective filter that restricts peripheral toxins, pathogens, and ions from entering the brain and disrupting neuronal function. Increased BBB permeability can allow harmful substances to infiltrate the brain, potentially leading to neuroinflammation, disrupted signaling, and damage to brain tissue, all of which may contribute to cognitive impairments in BD. Thus, BBB dysfunction could represent an upstream driver of cognitive impairment in BD, offering a potential target for disease-modifying interventions. This narrative review examined the evidence for the link between BBB permeability and cognitive deficits in BD. Our search yielded limited studies with mixed findings, highlighting the significant need for further research to explore this critical area and its potential for developing disease-modifying treatments.
Bipolar disorder (BD) is a heterogeneous and disabling mental illness that affects approximately 40 million people around the globe (Lai et al., 2024). People with BD experience high rates of functional impairment, both occupationally and socially (Burdick et al., 2022; Léda-Rêgo et al., 2020). Cognitive deficits are among the largest contributors to functional impairment in individuals with BD (Depp et al., 2012), and exist across various domains with varying degrees of impairment (Bora and Özerdem, 2017; Burdick et al., 2011). An estimated 40–60% of patients with BD have cognitive impairments, many with severe, global impairments (Burdick et al., 2014). Severe cognitive impairment is associated with worse treatment response (Manove and Levy, 2010), episode relapse (Miskowiak et al., 2022), and lower quality of life (Cotrena et al., 2016). Despite the centrality of cognitive impairment in BD, there exists a substantial gap in our understanding of the etiology of cognitive deficits in BD. It is unknown whether cognitive changes follow a neurodevelopmental course, or also include a neuroprogressive component (Millett and Burdick, 2021).
Existing evidence regarding the effect of premorbid intelligence quotient (IQ) on subsequent BD diagnosis is mixed. A few large cohort studies suggest that premorbid IQ does not predict the subsequent onset of BD, unlike schizophrenia (Zammit et al., 2004). A large Swedish cohort study found that the risk of developing BD may vary based on specific premorbid cognitive domains: both high performance in arithmetic and low performance in visuospatial tasks have been identified as potential risk factors (Tiihonen et al., 2005).
Consistently, broad cognitive impairment is observed around the time of the first episode (Bora and Pantelis, 2015). The trajectory of cognitive performance after the onset of illness is poorly understood. Longitudinal studies in BD—most of which span fewer than 10 years (Van Rheenen et al., 2020; Samamé et al., 2022)—suggest that cognitive impairments in BD do not meaningfully worsen after illness onset (Van Rheenen et al., 2020). However, more (hypo)manic episodes have been linked with increased cortical thinning in BD in longitudinal neuroimaging studies (Abé et al., 2023; Abé et al., 2015). Furthermore, a large, longitudinal cohort study of patients with psychotic disorders supported the notion that the etiology of cognitive impairments has both neurodevelopmental and neuroprogressive underpinnings and that the neuroprogressive effects in BD patients with psychosis may occur at a slower rate relative to those with schizophrenia spectrum disorders (SSDs). The authors reported that in those with “other” psychotic disorders (n = 216)—including BD (n = 106), major depression (n = 43), substance-induced (n = 30), and not otherwise specified (n = 33)—there was a loss of one IQ point every 7 years versus one IQ point lost every 3 years for SSDs in the “declining phase” (Jonas et al., 2022) This is in line with some evidence indicating a decline in cognitive function in BD patients with psychosis over longer timeframes (20-year follow-up) (Fett et al., 2020). Evidence also suggests that people with BD are at higher risk of developing dementia than the general population and people with unipolar depression (Velosa et al., 2020).
These knowledge gaps have hindered efforts to develop interventions to slow or prevent the onset of cognitive dysfunction and decline. To date, no treatments are widely available to successfully ameliorate (or improve) cognitive impairment in BD (Solé et al., 2017; Burdick et al., 2012). Considering cognition’s impact on “everyday” function and mood episode onset and recurrence, successfully targeting cognition will have significant impacts on patients’ quality of life.
In recent years, the blood–brain barrier (BBB) has garnered increased attention in the psychiatric field. This is for good reason, as the BBB is the interface between the peripheral circulation and the central nervous system and is crucial to maintaining homeostasis by protecting the brain from bloodborne toxins, as well as tightly regulating the influx and efflux of oxygen, ions, nutrients, and water (Sweeney et al., 2019). Increased BBB permeability (BBBP) has been observed in various neuropsychiatric and neurodegenerative disorders (Sweeney et al., 2018; Najjar et al., 2013) and has been linked with cognitive decline progression (Puig-Pijoan et al., 2024) and worse functional outcomes after neural injury (Ivanidze et al., 2018). A burgeoning body of evidence suggests that there is increased BBBP in psychiatric disorders as well (Futtrup et al., 2020; Cheng et al., 2022). However, how BBBP is related, if at all, to the onset or progression of cognitive impairment in BD remains to be known. The aim of this narrative review is to summarize the evidence linking BBBP to cognitive performance/impairment in BD, current gaps in our knowledge, and where to go from here.
This narrative review, conducted in December 2024, investigated the relationship between BBB integrity and cognitive function in BD. Searches were performed in PubMed and APA PsychNet (PsycInfo). The first search broadly explored the association between BD and BBBP using the terms: (bipolar disorder OR psychosis OR psychotic OR mood disorder) AND (blood brain barrier OR BBB OR S100B OR DCE-MRI). The second search narrowed the focus to include cognitive measures, using the terms: (bipolar disorder OR psychosis OR psychotic OR mood disorder) AND (blood brain barrier OR BBB OR S100B OR DCE-MRI) AND (cognition OR cognitive OR neurocognitive OR executive function OR processing speed OR attention). After removing duplicates, 614 articles were screened for relevance based on title and abstract within Covidence. This initial screening yielded 89 articles to extract for full-text review. Inclusion criteria required direct measurement of both cognitive function (using a cognitive test) and at least one measurement of BBBP or function in individuals with BD. Ultimately, two articles met these criteria, summarized in Table 1.
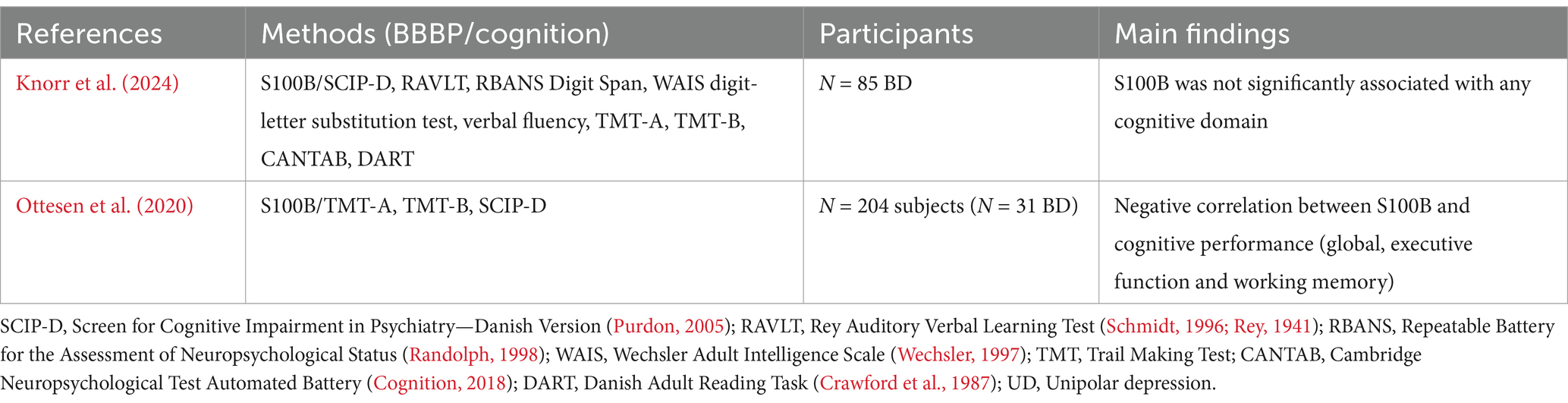
Table 1. Articles directly exploring the association between markers of BBB function and cognition in bipolar disorder.
Given the essential role of the BBB in neurovascular coupling, nutrient uptake, toxin and waste removal, and ionic homeostasis in the CNS, a connection between BBBP and cognitive impairment may seem clear. Numerous studies have supported an association between BBBP and cognitive impairment in a variety of disorders and diseases, reviewed here (Sweeney et al., 2018). In contrast, there is a paucity of direct evidence linking BBBP to cognitive performance in BD. To our knowledge, there are only two studies that have directly examined this relationship so far (Table 1).
A cross-sectional study by Ottesen et al. (2020) identified 204 twins from Danish registries. They analyzed S100B in these participants across three groups: the “affected” group consisted of individuals with an affective disorder (27% were diagnosed BD, and the remainder had unipolar depression, UD); the second group was the high-risk co-twins of the affected group; the third group was unaffected low-risk twins. The authors did not find a significant difference in S100B across groups, nor did they find a difference in S100B between BD and UD participants (Ottesen et al., 2020). However, they found a significant association between S100B and cognitive performance in all participants (Ottesen et al., 2020). Specifically, they found that higher S100B (i.e., higher BBBP) was associated with poorer global cognition (SCIP Total), executive function, and working memory (Ottesen et al., 2020). A major limitation of this analysis was the diagnostic heterogeneity of the “affected” group—making definitive statements about the relationship between cognition and S100B in BD versus others impossible.
A more recent prospective, longitudinal study by Knorr et al. (2024) examined S100B solely in BD patients. This study aimed to assess the association between CSF and blood-based markers of neurodegeneration and cognitive performance in BD patients. The study recruited N = 85 patients with BD aged 18–69 while in remission, and they were followed for up to 1 year. In exploratory analyses, this study failed to find an association between S100B and cognitive performance across several domains (global cognition, verbal memory, executive function, psychomotor speed, and sustained attention) in patients (Knorr et al., 2024). However, they found a significant association between a biomarker of neurodegeneration (CSF Aβ42) and cognitive performance in patients (Knorr et al., 2024).
Here, we present a narrative review of existing literature that examines cognitive performance and BBBP in individuals with BD. The literature search revealed two studies that met our criteria for inclusion—highlighting the paucity of published research on this topic in BD. The studies found disparate results, one indicating a possible relationship between BBBP and cognitive impairment and the other indicating no relationship between these variables. This discrepancy may be influenced by several factors. The study by Ottesen et al. (2020) used a transdiagnostic approach in their exploratory analyses—making diagnosis-specific conclusions difficult. The “affected” group was primarily composed of UD patients (72% of this group), so the effects may be primarily driven by them. This is supported by the study by Knorr et al. (2024), which analyzed data in only BD patients and found no significant associations between cognition and S100B. These negative and conflicting findings may have been influenced by a variety of factors, including mood state (patients were in remission at baseline) and the non-specific marker used to measure BBBP—S100B.
Existing research on BBBP in mood disorders thus far has utilized the “low hanging fruit” markers such as S100B—a calcium-binding protein localized to astrocytic end feet and a peripheral marker of BBB disruption—reviewed in a recent meta-analysis (Futtrup et al., 2020). Futtrup et al. (2020) showed that S100B is elevated across several diagnostic groups, including BD, SSDs, and MDD. The preponderance of evidence exists for SSDs, with 24 studies (n = 1,107 patients) included in this population. By comparison, very few studies (n = 4 studies with n = 142 patients) have examined this marker in BD. Intriguingly, most of the studies that looked at S100B in BD were in the manic phase (Tsai and Huang, 2017; Machado-Vieira et al., 2004; Andreazza et al., 2007). In fact, one very small meta-analysis has reported increased BBBP in manic BD specifically, supporting this theory (da Rosa et al., 2016). More research needs to be done to validate these initial findings. A very recent systematic review explored evidence for BBBP in BD specifically. Overall, there were 55 studies examining BBBP in BD, 38 of which reported higher BBBP and BD (Wakonigg Alonso et al., 2024). A total of 29 studies examined serum or CSF markers, and of those, 16 supported higher BBBP in BD across a range of markers, including S100B, matrix metalloproteinases (MMPs), and tight junction molecules, among others (Wakonigg Alonso et al., 2024). Only one study was identified, which looked specifically at Qalb in BD and found it to be increased in BD (Zetterberg et al., 2014). Nearly all studies included in this systematic review used indirect markers, such as blood-based and CSF-based measures, and only one study used DCE-MRI (the reference standard) (Kamintsky et al., 2019). The diversity of measures used makes direct comparison difficult. Overall, these reviews support a link between increased BBBP and BD. However, there are limitations to using indirect measures of BBBP. Specifically, S100B is known to be highly expressed in astrocytic end feet. However, it has peripheral sources as well, including adipocytes (Michetti et al., 2019). This is a major confounder of many studies which do not statistically control for BMI, which tends to be elevated in BD patient populations. Similarly, the concentration of albumin in the CSF (which is normally low) could be higher not due to increased leakiness of brain barriers but because there is reduced volume of CSF (Asgari et al., 2017). It should be noted that increased albumin in the CSF is likely a marker of BCSFB leakiness, not BBB leakiness, although it is commonly reported as such in literature (Yakimov et al., 2024).
Only a handful of studies have directly measured BBBP using DCE-MRI in psychiatric patient populations. To our knowledge, only two studies have reported results using DCE-MRI in SSDs. One study by Cheng et al. (2022) observed increased Ktrans in the thalamus. This is intriguing because the thalamus is implicated in the pathophysiology of psychosis. They also observed a positive correlation between Ktrans and PANSS scores in patients. Another study by Moussiopoulou et al. (2023) observed elevated Ktrans in SSD patients compared to controls in several brain regions, including the thalamus. However, they did not observe an association between Ktrans and symptom severity on the PANSS or cognition. To our knowledge, there exists only one published study using DCE-MRI in patients with BD. Kamintsky et al. (2019) found increased BBBP in a subset of BD patients (10 of a total of 36 patients) which was associated with higher BMI, insulin resistance, and risk of cardiovascular disease. The authors also reported that higher Ktrans was associated with a more severe course of illness in a retrospective analysis.
Literature on other neuropsychiatric disorders has supported a relationship between BBBP and cognitive/functional outcomes. A retrospective study by Ivanidze et al. (2018) found that in patients with sub-arachnoid hemorrhage (SAH, n = 22), a ROC curve analysis of four BBBP DCE-MRI parameters (Ktrans, Ve, PS, and Kep) had an area under the curve of 0.89 for prediction of modified Rankin scores 3–6 (i.e., BBBP parameters can predict more severe functional disability outcomes post-stroke). The authors concluded that BBBP parameters might have prognostic utility for stroke outcomes. A more recent prospective study by Puig-Pijoan et al. (2024) reported that among patients with dementias (n = 273), cognitive decline was predicted by higher BBBP (Qalb). They concluded that increased BBBP might contribute to clinical worsening in patients with dementia. Overall, these findings suggest that BBBP may have prognostic utility across neuropsychiatric disorders and highlight the need for similar approaches in BD. To date, no study in BD patients has examined the relationship between BBBP on cognitive outcomes using DCE-MRI.
This area is a burgeoning topic in the literature, and more research is necessary to advance this field. It should be noted that much more work has been done so far examining the interrelated features of neurophysiology, including glucose metabolism (e.g., FDG-PET) and cerebral blood flow (CBF), which can complement the existing data on BBBP. For example, the preponderance of published literature examining CBF has suggested that there is hypoperfusion in patients with BD during mood episodes (Toma et al., 2018). Considering the potential mechanistic links between BBBP and CBF—specifically, increased BBBP may result in reduced CBF or vice versa—it may be hypothesized that areas of the brain with reduced CBF may be excellent candidates for examining BBBP (Xu et al., 2022). Even so, based on the existing evidence, there is little data to support a significant relationship between BBBP and cognition in BD patients.
Parsing cognitive heterogeneity in BD may be important for elucidating the effect of BBBP on cognition. Clustering analyses have shown an approximately 40% of BD patients have global/widespread impairments across cognitive domains, while others are “selectively” impaired or “intact” (Burdick et al., 2014; Burdick and Millett, 2021). Given the heterogeneity and likely multifaceted causes of cognitive impairment, there is a demand for more personalized treatment strategies. Despite the considerable research on pharmacological interventions (Miskowiak et al., 2016; Miskowiak et al., 2018), few have emerged as effective, widely available treatment options for cognitive impairments in BD. Cognitive remediation/training is the most supported intervention for addressing these impairments (Miskowiak et al., 2017; Xu et al., 2022). A multifaceted approach utilizing both psychological and pharmacological interventions may ultimately be most beneficial for effective amelioration of cognitive impairments in BD. If BBBP is shown to be a driver of cognitive impairments in some patients, vascular stabilizing medications may be useful adjuncts to more traditional treatments for mood and cognitive symptoms in BD (Hashimoto et al., 2021).
Based on the limited existing evidence, it can be hypothesized that during mood episodes, several processes—such as increased pro-inflammatory cytokines, decreased CBF, and excitotoxicity in the CNS—may disrupt the BBB (Figure 1). These disruptions in the BBB may contribute to cognitive impairment through several mechanisms. When the BBB is compromised, the neurovascular unit (NVU) becomes permeable to infiltration by toxins and peripheral proteins, leading to brain tissue damage, edema, and disruptions in cerebral blood flow (CBF) and neurovascular coupling (Stanimirovic and Friedman, 2012). These changes can further exacerbate neuronal damage and cause inflammation in the CNS.
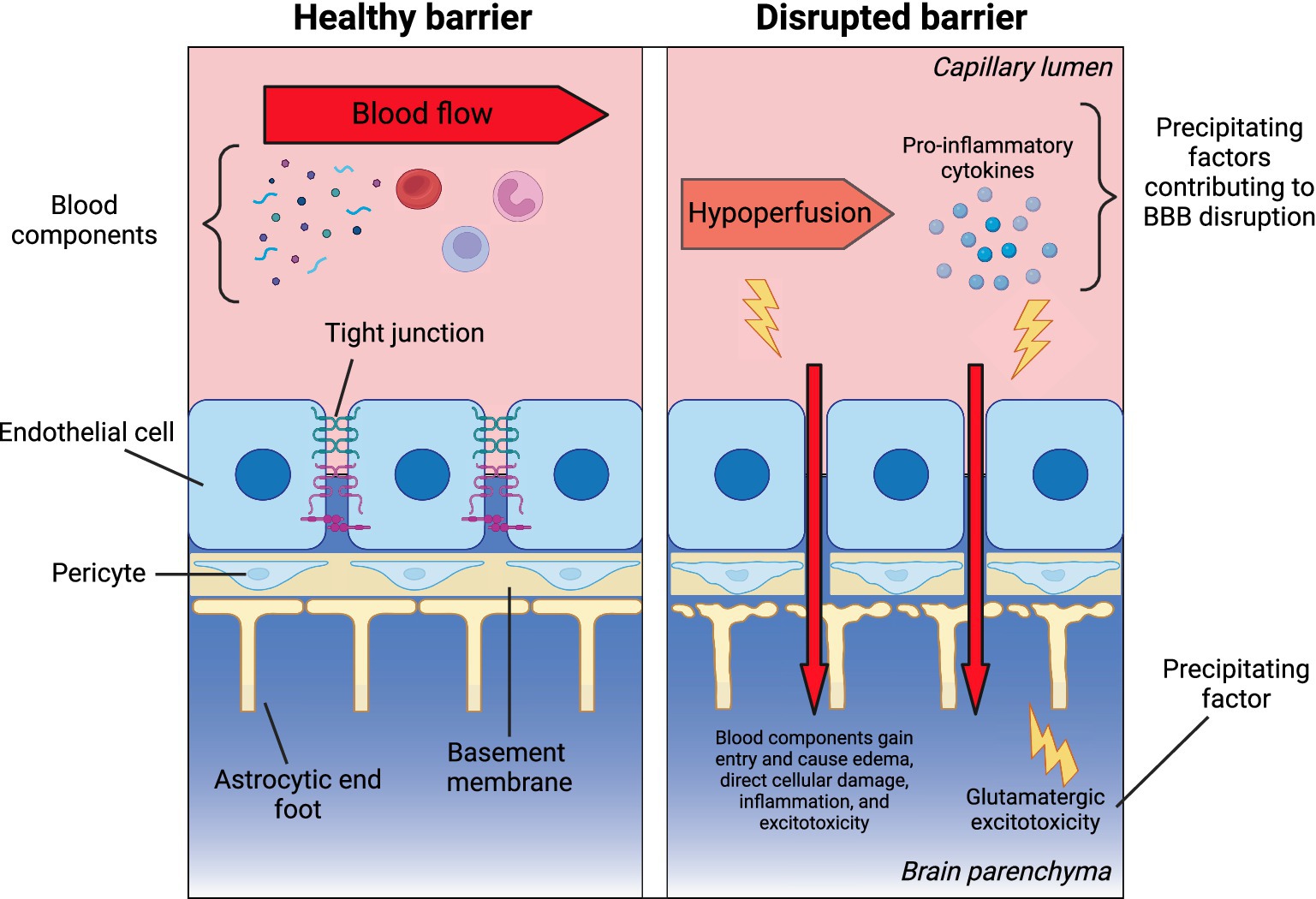
Figure 1. Theoretical framework: blood–brain barrier disruption in bipolar disorder. The left panel illustrates the key components of a healthy blood–brain barrier (BBB), separating the peripheral circulation and CNS. These include endothelial cells with tight junctions, the basement membrane, pericytes, and astrocytic end feet. The right panel presents a theoretical model of factors that may increase BBB permeability (BBBP), potentially leading to secondary damage from peripheral components entering the CNS through a leaky barrier. Created in BioRender. Millett, C. (2025), https://BioRender.com/j89y763.
In BD, hypoperfusion—particularly in the frontal cortices—has been linked to cognitive deficits, as these areas are critical for executive functions and emotion regulation (Toma et al., 2018). Additionally, elevated levels of circulating pro-inflammatory markers, which are commonly observed in BD, especially during acute episodes (Fernandes et al., 2016; Goldsmith et al., 2016), may drive BBB dysfunction and amplify neuroinflammatory responses, worsening cognitive outcomes. While the role of glutamate remains complex, evidence from magnetic resonance spectroscopy (MRS) studies suggests glutamatergic dysregulation exists in the anterior cingulate cortex (ACC), particularly during depressive episodes in BD (Ino et al., 2023). This complex interplay of vascular, inflammatory, and excitotoxic factors within disrupted BBB regions may collectively impair cognitive function in BD. These processes could theoretically impair neuronal firing and cognitive function, potentially persisting long after the initial insult. However, these ideas are currently theoretical and require testing in BD patients during acute illness.
In conclusion, the role of BBBP in the onset and progression of cognitive deficits in BD remains largely unknown. A critical next step is to employ neuroimaging techniques like DCE-MRI longitudinally to assess functional BBB changes in patients quantitatively. This should be done in conjunction with longitudinal cognitive and clinical assessments to develop causal models of cognitive dysfunction in BD. One particularly important area of inquiry is the relationship between BBBP, and the cognitive impairments frequently observed around a patient’s first episode. More granular investigation of this timeframe, including before illness onset, could significantly advance our understanding of the disorder’s trajectory. Furthermore, broadening the scope of investigation to encompass dysfunction in the BCSFB will provide a more complete picture. A deeper understanding of both the qualitative and quantitative features of barrier dysfunction, combined with advancements in neuromodulation techniques and their effects on the brain’s barriers, holds significant therapeutic potential. For instance, if increased BBBP is confirmed as a key driver of any aspect of BD pathophysiology, interventions aimed at strengthening the barrier could be prioritized. Conversely, if barrier dysfunction hinders drug delivery to the central nervous system, neuromodulation strategies that transiently open the barrier might enhance treatment efficacy.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
CM: Writing – original draft, Writing – review & editing. FM: Writing – review & editing. PS: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. CEM is partly supported by Breakthrough Discoveries for Thriving with Bipolar Disorders Foundation (BD2). PS has received funding from NINDS, NIAID, American Heart Association, Neiman Health Policy Institute, Blue Rock Therapeutics, and Siemens Lab Diagnostics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Gen AI was used in the creation of this manuscript. Generative AI (Chat GPT4 and Gemini 1.5) was used for spell check and editing.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abé, C., Ekman, C. J., Sellgren, C., Petrovic, P., Ingvar, M., and Landén, M. (2015). Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain 138, 3440–3448. doi: 10.1093/brain/awv266
Abé, C., Liberg, B., Klahn, A. L., Petrovic, P., and Landén, M. (2023). Mania-related effects on structural brain changes in bipolar disorder – a narrative review of the evidence. Mol. Psychiatry 28, 2674–2682. doi: 10.1038/s41380-023-02073-4
Andreazza, A. C., Cassini, C., Rosa, A. R., Leite, M. C., de Almeida, L. M. V., Nardin, P., et al. (2007). Serum S100B and antioxidant enzymes in bipolar patients. J. Psychiatr. Res. 41, 523–529. doi: 10.1016/j.jpsychires.2006.07.013
Asgari, M., de Zélicourt, D. A., and Kurtcuoglu, V. (2017). Barrier dysfunction or drainage reduction: differentiating causes of CSF protein increase. Fluids Barriers CNS 14, 1–11. doi: 10.1186/s12987-017-0063-4
Bora, E., and Özerdem, A. (2017). Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol. Med. 47, 2753–2766. doi: 10.1017/S0033291717001490
Bora, E., and Pantelis, C. (2015). Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr. Bull 41, 1095–1104. doi: 10.1093/schbul/sbu198
Burdick, K. E., Braga, R. J., Nnadi, C. U., Shaya, Y., Stearns, W. H., and Malhotra, A. K. (2012). Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J. Clin. Psychiatry 73, 103–112. doi: 10.4088/JCP.11m07299
Burdick, K. E., Goldberg, T. E., Cornblatt, B. A., Keefe, R. S., Gopin, C. B., Derosse, P., et al. (2011). The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology 36, 1587–1592. doi: 10.1038/npp.2011.36
Burdick, K. E., and Millett, C. E. (2021). Cognitive heterogeneity is a key predictor of differential functional outcome in patients with bipolar disorder. Eur. Neuropsychopharmacol. 53, 4–6. doi: 10.1016/j.euroneuro.2021.06.008
Burdick, K. E., Millett, C. E., Yocum, A. K., Altimus, C. M., Andreassen, O. A., Aubin, V., et al. (2022). Predictors of functional impairment in bipolar disorder: results from 13 cohorts from seven countries by the global bipolar cohort collaborative. Bipolar Disord. 24, 709–719. doi: 10.1111/bdi.13208
Burdick, K. E., Russo, M., Frangou, S., Mahon, K., Braga, R. J., Shanahan, M., et al. (2014). Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol. Med. 44, 3083–3096. doi: 10.1017/s0033291714000439
Cheng, Y., Wang, T., Zhang, T., Yi, S., Zhao, S., Li, N., et al. (2022). Increased blood-brain barrier permeability of the thalamus correlated with symptom severity and brain volume alterations in patients with schizophrenia. Biol. Psychiatr. Cogn. Neurosci. Neuroimaging. 7, 1025–1034. doi: 10.1016/j.bpsc.2022.06.006
Cognition, C. (2018). CANTAB, cognitive assessment software. All right reserved. Cambridge Cognition.
Cotrena, C., Branco, L. D., Shansis, F. M., and Fonseca, R. P. (2016). Executive function impairments in depression and bipolar disorder: association with functional impairment and quality of life. J. Affect. Disord. 190, 744–753. doi: 10.1016/j.jad.2015.11.007
Crawford, J. R., Besson, J. A., Parker, D. M., Sutherland, K. M., and Keen, P. L. (1987). Estimation of premorbid intellectual status in depression. Br. J. Clin. Psychol. 26, 313–314. doi: 10.1111/j.2044-8260.1987.tb01366.x
da Rosa, M. I., Simon, C., Grande, A. J., Barichello, T., Oses, J. P., and Quevedo, J. (2016). Serum S100B in manic bipolar disorder patients: systematic review and meta-analysis. J. Affect. Disord. 206, 210–215. doi: 10.1016/j.jad.2016.07.030
Depp, C. A., Mausbach, B. T., Harmell, A. L., Savla, G. N., Bowie, C. R., Harvey, P. D., et al. (2012). Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 14, 217–226. doi: 10.1111/j.1399-5618.2012.01011.x
Fernandes, B. S., Steiner, J., Molendijk, M. L., Dodd, S., Nardin, P., Gonçalves, C. A., et al. (2016). C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 3, 1147–1156. doi: 10.1016/S2215-0366(16)30370-4
Fett, A. K. J., Velthorst, E., Reichenberg, A., Ruggero, C. J., Callahan, J. L., Fochtmann, L. J., et al. (2020). Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the Suffolk County mental health project. JAMA Psychiatr. 77, 387–396. doi: 10.1001/jamapsychiatry.2019.3993
Futtrup, J., Margolinsky, R., Eriksen Benros, M., Moos, T., Routhe, L. J., Rungby, J., et al. (2020). Blood-brain barrier pathology in patients with severe mental disorders: A systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav. Immun. Health 6:100102. doi: 10.1016/j.bbih.2020.100102
Goldsmith, D. R., Rapaport, M. H., and Miller, B. J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709. doi: 10.1038/mp.2016.3
Hashimoto, Y., Campbell, M., Tachibana, K., Okada, Y., and Kondoh, M. (2021). Claudin-5: a pharmacological target to modify the permeability of the blood-brain barrier. Biol. Pharm. Bull. 44, 1380–1390. doi: 10.1248/bpb.b21-00408
Ino, H., Honda, S., Yamada, K., Horita, N., Tsugawa, S., Yoshida, K., et al. (2023). Glutamatergic Neurometabolite levels in bipolar disorder: a systematic review and Meta-analysis of proton magnetic resonance spectroscopy studies. Biol. Psychiatr. Cogn. Neurosci. Neuroimaging 8, 140–150. doi: 10.1016/j.bpsc.2022.09.017
Ivanidze, J., Ferraro, R. A., Giambrone, A. E., Segal, A. Z., Gupta, A., and Sanelli, P. C. (2018). Blood-brain barrier permeability in aneurysmal subarachnoid hemorrhage: correlation with clinical outcomes. AJR 211, 891–895. doi: 10.2214/AJR.17.18237
Jonas, K., Lian, W., Callahan, J., Ruggero, C. J., Clouston, S., Reichenberg, A., et al. (2022). The course of general cognitive ability in individuals with psychotic disorders. JAMA Psychiatry 79, 659–666. doi: 10.1001/jamapsychiatry.2022.1142
Kamintsky, L., Cairns, K. A., Veksler, R., Bowen, C., Beyea, S. D., Friedman, A., et al. (2019). Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. NeuroImage: Clinical 26:102049. doi: 10.1016/j.nicl.2019.102049
Knorr, U., Simonsen, A. H., Zetterberg, H., Blennow, K., Willkan, M., Forman, J., et al. (2024). Biomarkers for neurodegeneration impact cognitive function: a longitudinal 1-year case–control study of patients with bipolar disorder and healthy control individuals. Int. J. Bipolar Disord. 12:324. doi: 10.1186/s40345-023-00324-5
Lai, J., Li, S., Wei, C., Chen, J., Fang, Y., Song, P., et al. (2024). Mapping the global, regional and national burden of bipolar disorder from 1990 to 2019: trend analysis on the global burden of disease study 2019. Br. J. Psychiatr. 224, 36–46. doi: 10.1192/bjp.2023.127
Léda-Rêgo, G., Bezerra-Filho, S., and Miranda-Scippa, Â. (2020). Functioning in euthymic patients with bipolar disorder: a systematic review and meta-analysis using the functioning assessment short test. Bipolar Disord. 22, 569–581. doi: 10.1111/bdi.12904
Machado-Vieira, R., Schmidt, A. P., Ávila, T. T., Kapczinski, F., Soares, J. C., Souza, D. O., et al. (2004). Increased cerebrospinal fluid levels of S100B protein in rat model of mania induced by ouabain. Life Sci. 76, 805–811. doi: 10.1016/j.lfs.2004.07.021
Manove, E., and Levy, B. (2010). Cognitive impairment in bipolar disorder: an overview. Postgrad. Med. 122, 7–16. doi: 10.3810/pgm.2010.07.2170
Michetti, F., D’Ambrosi, N., Toesca, A., Puglisi, M. A., Serrano, A., Marchese, E., et al. (2019). The S100B story: from biomarker to active factor in neural injury. J. Neurochem. 148, 168–187. doi: 10.1111/jnc.14574
Millett, C. E., and Burdick, K. E. (2021). Defining heterogeneous cognitive trajectories in bipolar disorder: a perspective. Harv. Rev. Psychiatry 29, 298–302. doi: 10.1097/HRP.0000000000000297
Miskowiak, K. W., Burdick, K. E., Martinez-Aran, A., Bonnin, C. M., Bowie, C. R., Carvalho, A. F., et al. (2018). Assessing and addressing cognitive impairment in bipolar disorder: the international society for bipolar disorders targeting cognition task force recommendations for clinicians. Bipolar Disord. 20, 184–194. doi: 10.1111/bdi.12595
Miskowiak, K. W., Burdick, K. E., Martinez-Aran, A., Bonnin, C. M., Bowie, C. R., Carvalho, A. F., et al. (2017). Methodological recommendations for cognition trials in bipolar disorder by the international society for bipolar disorders targeting cognition task force. Bipolar Disord. 19, 614–626. doi: 10.1111/bdi.12534
Miskowiak, K. W., Mariegaard, J., Jahn, F. S., and Kjærstad, H. L. (2022). Associations between cognition and subsequent mood episodes in patients with bipolar disorder and their unaffected relatives: a systematic review. J. Affect. Disord. 297, 176–188. doi: 10.1016/j.jad.2021.10.044
Miskowiak, K., Rush, A., Gerds, T., Vinberg, M., and Kessing, L. (2016). Targeting treatments to improve cognitive function in mood disorder: suggestions from trials using erythropoietin. J. Clin. Psychiatry 77, e1639–e1646. doi: 10.4088/JCP.15m10480
Moussiopoulou, J., Yakimov, V., Rauchmann, B. S., Toth, H., Melcher, J., Jäger, I., et al. (2023). Increased blood–brain barrier leakage in schizophrenia spectrum disorders compared to healthy controls in dynamic contrast-enhanced magnetic resonance imaging. medRxiv 12:23299782. doi: 10.1101/2023.12.12.23299782v1
Najjar, S., Pearlman, D. M., Devinsky, O., Najjar, A., and Zagzag, D. (2013). Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J. Neuroinflamm. 10:142. doi: 10.1186/1742-2094-10-142
Ottesen, N. M., Meluken, I., Frikke-Schmidt, R., Plomgaard, P., Scheike, T., Kessing, L. V., et al. (2020). S100B and brain derived neurotrophic factor in monozygotic twins with, at risk of and without affective disorders. J. Affect. Disord. 274, 726–732. doi: 10.1016/j.jad.2020.05.015
Puig-Pijoan, A., Jimenez-Balado, J., Fernández-Lebrero, A., García-Escobar, G., Navalpotro-Gómez, I., Contador, J., et al. (2024). Risk of cognitive decline progression is associated to increased blood-brain-barrier permeability: a longitudinal study in a memory unit clinical cohort. Alzheimer’s Dementia 20, 538–548. doi: 10.1002/alz.13433
Purdon, S. E. (2005). Screen for cognitive impairment in psychiatry: Administration and psychometric properties. Alberta: PNL, Inc.
Randolph, C. (1998). RBANS manual: Repeatable battery for the assessment of neuropsychological status. San Antonio, TX: The Psychological Corporation.
Rey, A. (1941). Psychological examination of traumatic encephalopathy. Archieves Psychol. 7, 286–340.
Samamé, C., Cattaneo, B. L., Richaud, M. C., Strejilevich, S., and Aprahamian, I. (2022). The long-term course of cognition in bipolar disorder: a systematic review and meta-analysis of patient-control differences in test-score changes. Psychol. Med. 52, 217–228. doi: 10.1017/S0033291721004517
Schmidt, M. (1996). Rey auditory verbal learning test: RAVLT: A handbook. Torrance, CA: Western Psychological Services.
Solé, B., Jiménez, E., Torrent, C., Reinares, M., Del Mar, B. C., Torres, I., et al. (2017). Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int. J. Neuropsychopharmacol. 20, 670–680. doi: 10.1093/ijnp/pyx032
Stanimirovic, D. B., and Friedman, A. (2012). Pathophysiology of the neurovascular unit: disease cause or consequence? J. Cerebral Blood Flow Metab. 32, 1207–1221. doi: 10.1038/jcbfm.2012.25
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018). Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150. doi: 10.1038/nrneurol.2017.188
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: from physiology to disease and Back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Tiihonen, J., Haukka, J., Henriksson, M., Cannon, M., Kieseppä, T., Laaksonen, I., et al. (2005). Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. Am. J. Psychiatry 162, 1904–1910. doi: 10.1176/appi.ajp.162.10.1904
Toma, S., Macintosh, B. J., Swardfager, W., and Goldstein, B. I. (2018). Cerebral blood flow in bipolar disorder: A systematic review. J. Aff. Disorders 241, 505–513. doi: 10.1016/j.jad.2018.08.040
Tsai, M. C., and Huang, T. L. (2017). Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr. Psychiatry 74, 27–34. doi: 10.1016/j.comppsych.2016.12.008
Van Rheenen, T. E., Lewandowski, K. E., Bauer, I. E., Kapczinski, F., Miskowiak, K., Burdick, K. E., et al. (2020). Current understandings of the trajectory and emerging correlates of cognitive impairment in bipolar disorder: an overview of evidence. Bipolar Disord 22, 13–27. doi: 10.1111/bdi.12821
Velosa, J., Delgado, A., Finger, E., Berk, M., Kapczinski, F., and de Azevedo Cardoso, T. (2020). Risk of dementia in bipolar disorder and the interplay of lithium: a systematic review and meta-analyses. Acta Psychiatr. Scand. 2020:acps.13153. doi: 10.1111/acps.13153
Wakonigg Alonso, C., McElhatton, F., O’Mahony, B., Campbell, M., Pollak, T. A., and Stokes, P. R. A. (2024). The blood-brain barrier in bipolar disorders: a systematic review. J. Affect. Disord. 361, 434–444. doi: 10.1016/j.jad.2024.06.032
Wechsler, D. (1997). Wechsler Adult Intelligence Scale III. San Antonio, TX: The Psychological Corporation.
Xu, W. Q., Bai, Q., Dong, Q., Guo, M., and Cui, M. (2022). Blood–brain barrier dysfunction and the potential mechanisms in chronic cerebral Hypoperfusion induced cognitive impairment. Front. Cell Neurosci. 16:870674. doi: 10.3389/fncel.2022.870674
Yakimov, V., Moussiopoulou, J., Hasan, A., and Wagner, E. (2024). The common misconception of blood-brain barrier terminology in psychiatry and neurology. Eur. Arch. Psychiatr. Clin. Neurosci. 274, 1779–1781. doi: 10.1007/s00406-023-01726-3
Zammit, S., Allebeck, P., David, A. S., Dalman, C., Hemmingsson, T., Lundberg, I., et al. (2004). A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry 61, 354–360. doi: 10.1001/archpsyc.61.4.354
Keywords: cognitive deficits, biomarkers, neurovascular permeability, mood disorders, mania
Citation: Millett CE, Monir F and Sanelli P (2025) The role of blood–brain barrier dysfunction in cognitive impairments in bipolar disorder—a narrative review. Front. Hum. Neurosci. 19:1504575. doi: 10.3389/fnhum.2025.1504575
Received: 02 October 2024; Accepted: 27 January 2025;
Published: 19 February 2025.
Edited by:
Eva Z Reininghaus, Medical University of Graz, AustriaReviewed by:
Mohammad Mofatteh, Queen’s University Belfast, United KingdomCopyright © 2025 Millett, Monir and Sanelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caitlin E. Millett, Y21pbGxldHRAbm9ydGh3ZWxsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.