
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 13 March 2024
Sec. Interacting Minds and Brains
Volume 18 - 2024 | https://doi.org/10.3389/fnhum.2024.1363891
This article is part of the Research Topic Women In Interacting Minds and Brains View all 6 articles
 Yarden Menashri Sinai1†
Yarden Menashri Sinai1† Yaopeng X. J. Ma2†
Yaopeng X. J. Ma2† Michal Abba Daleski3
Michal Abba Daleski3 Sharon Gannot4
Sharon Gannot4 Ronny P. Bartsch2
Ronny P. Bartsch2 Ilanit Gordon1,3*
Ilanit Gordon1,3*Introduction: To date, studies focusing on the connection between psychological functioning and autonomic nervous system (ANS) activity usually adopted the one-dimensional model of autonomic balance, according to which activation of one branch of the ANS is accompanied by an inhibition of the other. However, the sympathetic and parasympathetic branches also activate independently; thus, co-activation and co-inhibition may occur, which is demonstrated by a two-dimensional model of ANS activity. Here, we apply such models to assess how markers of the autonomic space relate to several critical psychological constructs: emotional contagion (EC), general anxiety, and positive and negative affect (PA and NA). We also examined gender differences in those psychophysiological relations.
Methods: In the present study, we analyzed data from 408 healthy students, who underwent a 5-min group baseline period as part of their participation in several experiments and completed self-reported questionnaires. Electrocardiogram (ECG), electrodermal activity (EDA), and respiration were recorded. Respiratory sinus arrhythmia (RSA), pre-ejection period (PEP), as well as cardiac autonomic balance (CAB) and regulation (CAR) and cross-system autonomic balance (CSAB) and regulation (CSAR), were calculated.
Results: Notably, two-dimensional models were more suitable for predicting and describing most psychological constructs. Gender differences were found in psychological and physiological aspects as well as in psychophysiological relations. Women's EC scores were negatively correlated with sympathetic activity and positively linked to parasympathetic dominance. Men's PA and NA scores were positively associated with sympathetic activity. PA in men also had a positive link to an overall activation of the ANS, and a negative link to parasympathetic dominance.
Discussion: The current results expand our understanding of the psychological aspects of the autonomic space model and psychophysiological associations. Gender differences and strengths and weaknesses of alternative physiological models are discussed.
The ANS has an established role in psychophysiological research (Cacioppo et al., 2007). Most studies that have examined how ANS activity is related to psychological functions investigated discrete measures of the ANS quantifying either activity of the sympathetic (SNS) or the parasympathetic (PNS) branch (or their combined activity). PNS control is theorized to reflect our capacity to flexibly respond to changes in environmental cues and stressors (Thayer and Lane, 2000; Porges, 2007), including the ability to regulate emotions (Thayer and Lane, 2000; Beauchaine, 2015; Balzarotti et al., 2017; Pace-Schott et al., 2019). SNS governs a wide array of visceral functions, and, in situations of threat and stress, triggers the activation of both the cardiovascular and adrenal catecholamine systems, facilitating a fight-or-flight response (Jansen et al., 1995; Sapolsky et al., 2000; Obradović and Boyce, 2012). SNS is thought to reflect heightened arousal during behavioral inhibition and avoidant coping (Dawson et al., 2007; Obradović and Boyce, 2012), which are greatly linked to anxiety disorders (Hirschfeld, 2001).
There are several indices of ANS function, which have been extensively studied in psychological research in the past. Respiratory sinus arrhythmia (RSA) is one aspect of the coupling between the cardiac and the respiratory systems (Bartsch et al., 2014). It indicates periodic variations of the heart rate within a breathing cycle and is considered an indicator of cardiac PNS activity (Cacioppo et al., 1994; Malik et al., 1996; Berntson et al., 1997; Bartsch et al., 2012). Low resting RSA has been linked in several studies to anxiety disorders (Friedman and Thayer, 1998; Friedman, 2007; Chalmers et al., 2014), while high levels of resting RSA are considered to indicate more integration between peripheral and central nervous activity which allows for more flexible responses and greater emotional regulation (Thayer and Lane, 2000; Porges, 2007; Beauchaine, 2015; Balzarotti et al., 2017; Pace-Schott et al., 2019). Electrodermal activity (EDA), an indicator of the SNS that reflects activity of the eccrine sweat glands driven by cholinergic neurotransmission, has been widely studied in the context of anxiety disorders (Fowles, 1986; Shields et al., 1987; Dawson et al., 2007). Resting EDA levels, as well as EDA measured in a variety of settings, have been consistently related to anxiety (for reviews see: Hoehn-Saric and McLeod, 1988. Also: Balyan et al., 2016; Barlow, 2002; Boucsein, 2012). Another marker of SNS activity is pre-ejection period (PEP), defined as the length of time between an electrical signal reaching the left ventricle of the heart and indicating it to contract and the time when the aortic valve opens (Sherwood et al., 1990; Berntson et al., 1994a; Cacioppo et al., 1994; Benevides and Lane, 2013; Stone et al., 2020). PEP is highly predictive of cardiac sympathetic control (Cacioppo et al., 1994) and is thought to be linked to active coping (Brenner et al., 2005; Kelsey, 2012; Obradović and Boyce, 2012) and to stress sensitivity (Beauchaine, 2001). The association between PEP and anxiety is not consistent, with different studies reporting positive correlations (Pollatos et al., 2007), negative correlations (Kossowsky et al., 2012; Fu et al., 2018) or no associations at all (Burns et al., 1992; Sperry et al., 2018). RSA, EDA and PEP are all distinct indices of ANS, each indicating discrete aspects of the functioning of the ANS.
Due to evidence suggesting that the two branches of the ANS can activate independently (Berntson et al., 1991; Berntson and Cacioppo, 2007; Stone et al., 2020), the autonomic balance and regularity capacity model was offered to describe the full range of autonomic patterns that may occur (i.e., co-activation or co-habitation of the PNS and SNS, Berntson et al., 1991, 1993, 1994b; Berntson and Cacioppo, 2007). Cardiac autonomic balance (CAB) and cross-system autonomic balance (CSAB) quantify the differences between PNS (measured by RSA) and SNS (measured by PEP or EDA, respectively) activities and represent the relative influences of the ANS branches, with lower scores indicating SNS dominance and higher scores indicating PNS dominance. Cardiac autonomic regulation (CAR) and cross-system autonomic regulation (CSAR) represent the sum of PNS and SNS activities, and constitute markers of overall autonomic activation (i.e., co-activation or co-inhibition) of both PNS and SNS branches (Berntson et al., 2008; Stone et al., 2020). Since PEP and EDA capture distinct aspects of the SNS and their activities are not necessarily aligned (see, for example, Brenner et al., 2005; Kreibig et al., 2007; Stone et al., 2020), CAB, CAR, CSAB and CSAR may produce different representations of the ANS activity. While some studies have linked CAB, CAR, CSAB and CSAR to health problems (Berntson, 2019; Alen et al., 2020), anxiety symptoms (Stone et al., 2020) and psychopathologies (Bylsma et al., 2015; Brush et al., 2019; Cohen et al., 2020; Stone et al., 2020), only a few studies to date have examined the relationship between those indices at rest or baseline and several meaningful psychological functions in a healthy adult population.
In the current study we focused on four psychological indices which have a high influence on social interaction.
EC is the automatic tendency to catch another's emotions (Hatfield et al., 1992, 1993; Mayo et al., 2023) and can be manifested as a demonstration of similar postural, vocal, or facial expression or as similar neurophysiological or neurological patterns of activity (Schoenewolf, 1990; Hatfield et al., 1994, 2009, 2014; Barsade, 2002; Barsade et al., 2018). EC is related to empathy, attunement, and bonding (Hatfield et al., 1994, 2011; Spoor and Kelly, 2004; Decety and Ickles, 2009; Neves et al., 2018), as well as to stress (Feldman and Kaal, 2007), and has been suggested to facilitate social interactions (Hatfield et al., 1994; Butler, 2011). Individuals differ in their susceptibility to EC (Doherty, 1997; Hatfield et al., 2014; Barsade et al., 2018; Horesh et al., 2021), and women usually score higher than men in EC performance and questionnaires (Hall, 1978; Buck, 1984; Doherty et al., 1995; Surakka and Hietanen, 1998; Wild et al., 2001; Sonnby-Borgström et al., 2008; Magen and Konasewich, 2011; Manera et al., 2013; Fairbairn et al., 2015).
Associations between EC and physiological synchrony had been suggested and demonstrated many times (see Table 1 for details), yet the connection between EC as a trait and baseline physiological markers of the ANS remain largely unknown.
PA and NA are highly distinctive and almost orthogonal dimensions of affective structure (Russell, 1980, 1983; Stone, 1981; Zevon and Tellegen, 1982; Watson et al., 1984; Diener et al., 1985; Clark and Watson, 1988) and are commonly collected via self-reported mood questionnaires in psychological research (Clark and Watson, 1988; Clark et al., 1989; Futterman et al., 1992; Christie and Friedman, 2004; Fredrickson and Losada, 2005; Lai et al., 2005; Pressman and Cohen, 2005; Kubzansky and Thurston, 2007; Nabi et al., 2008; Oveis et al., 2009; Steptoe et al., 2009; Dockray and Steptoe, 2010; Drachen et al., 2010; Cribbet et al., 2011; Diamond et al., 2011; Shiota et al., 2011; Wang et al., 2013). PA represents feelings of enthusiasm, activeness, and alertness, with high PA being a state of high energy, concentration, and pleasurable engagement whereas low PA is a state of sadness and lethargy (Clark and Watson, 1988). NA reflects distress and unpleasurable engagement and includes a variety of aversive mood states, such as anger, contempt, disgust, guilt, fear, and nervousness, with low NA being a state of calmness and serenity (Clark and Watson, 1988). Both PA and NA are usually higher in women compared to men (for a review see Batz and Tay, 2018, and also: Fujita et al., 1991; Grossman and Wood, 1993; Thomsen et al., 2005; Zuckerman et al., 2017).
Many studies have examined the sympathetic and parasympathetic responses to PA (see Table 2) and to NA (see Table 3), with conflicting results. Thus, an examination of autonomic space model markers might be more suitable to describe the physiological manifestation of those important psychological constructs.
Anxiety is a trait depicting individuals' tendency to regard many situations as dangerous or alarming and to respond to them accordingly (Spielberger, 1966; Elwood et al., 2012; Sege et al., 2018). Anxiety is also a state of ANS arousal in which a person consciously experiences feelings of tension and worry (Spielberger, 1966). Women are usually reported to have higher levels of anxiety than men (Angst and Dobler-Mikola, 1985; Weissman and Merikangas, 1986; Anderson et al., 1987; Bourdon et al., 1988; Líndal and Stefánsson, 1993; Campbell and Rapee, 1994; Kessler et al., 1994, 1995; Lewinsohn et al., 1998; Poulton et al., 2001; Muris and Ollendick, 2002; Costello et al., 2003; Bruce et al., 2005; McLean et al., 2011).
State anxiety had been extensively studied in the context of ANS activity (see Table 4 for details), yet the relation between state anxiety and sympathetic and parasympathetic activities is complex.
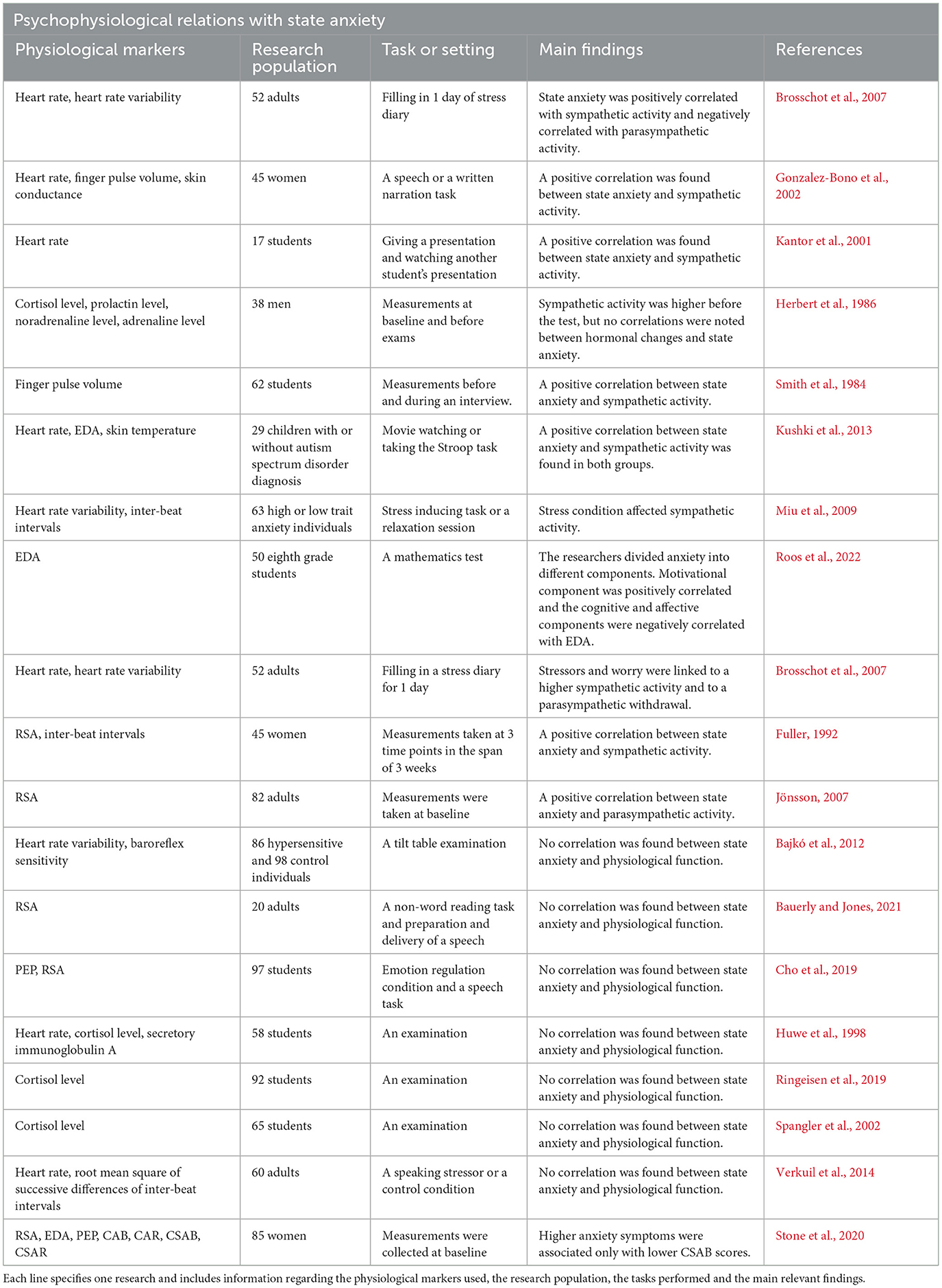
Table 4. This table details previously found associations between state anxiety and physiological activity.
Gender differences had been the focus of intense investigation and interest for more than a century, with research consecrating on various aspects of psychology, such as cognitive abilities, personality and social behaviors (for reviews see Hyde et al., 1990 and Hyde, 2014).
The psychological indices which interested us in the current study were also examined for gender differences in the literature. Specifically, gender differences had been widely studied in anxiety. Although women report more anxiety symptoms and have a greater risk than men to develop most kinds of anxiety disorders (Angst and Dobler-Mikola, 1985; Weissman and Merikangas, 1986; Anderson et al., 1987; Bourdon et al., 1988; Líndal and Stefánsson, 1993; Campbell and Rapee, 1994; Kessler et al., 1994, 1995; Lewinsohn et al., 1998; Poulton et al., 2001; Muris and Ollendick, 2002; Costello et al., 2003; Bruce et al., 2005; McLean et al., 2011), there appears to be no difference in baseline state anxiety between the genders (Carrillo et al., 2001; Brand and Schoonheim-Klein, 2009; Limbu et al., 2010; Strohmaier et al., 2020). In regard to EC, it had previously been suggested that women are better than men at reading the emotional displays of other individuals (Haviland and Malatesta, 1981; Hall, 1984), which may facilitate their ability to emphasize and mimic the emotional states of others (Hatfield et al., 1992). Indeed, EC in women is time and again being reported as higher than men's EC, under various conditions (Hall, 1978; Buck, 1984; Doherty et al., 1995; Surakka and Hietanen, 1998; Wild et al., 2001; Sonnby-Borgström et al., 2008; Magen and Konasewich, 2011; Manera et al., 2013; Fairbairn et al., 2015). Affective states may also be influenced by gender. Women are usually reported to experience higher levels of PA (for a review see Batz and Tay, 2018) and of NA (Fujita et al., 1991; Grossman and Wood, 1993; Thomsen et al., 2005; Zuckerman et al., 2017) than men, although some studies found no gender differences in levels of affect (Neumann and Waldstein, 2001; Cribbet et al., 2011; Volante et al., 2016).
Over the past few decades some evidence emerged suggesting that gender differences may exist in ANS activity (for reviews see Hinojosa-Laborde et al., 1999; Dart et al., 2002; Pothineni et al., 2016). Most studies found no differences in parasympathetic dominance in the form of high-frequency heart rate variability (HRV) or RSA between men and women (Frazier et al., 2004; Codispoti et al., 2008; Cribbet et al., 2011; Moodithaya and Avadhany, 2012; Botek et al., 2018).
Regarding SNS activity, however, the picture is more complicated (see Table 5 for details).
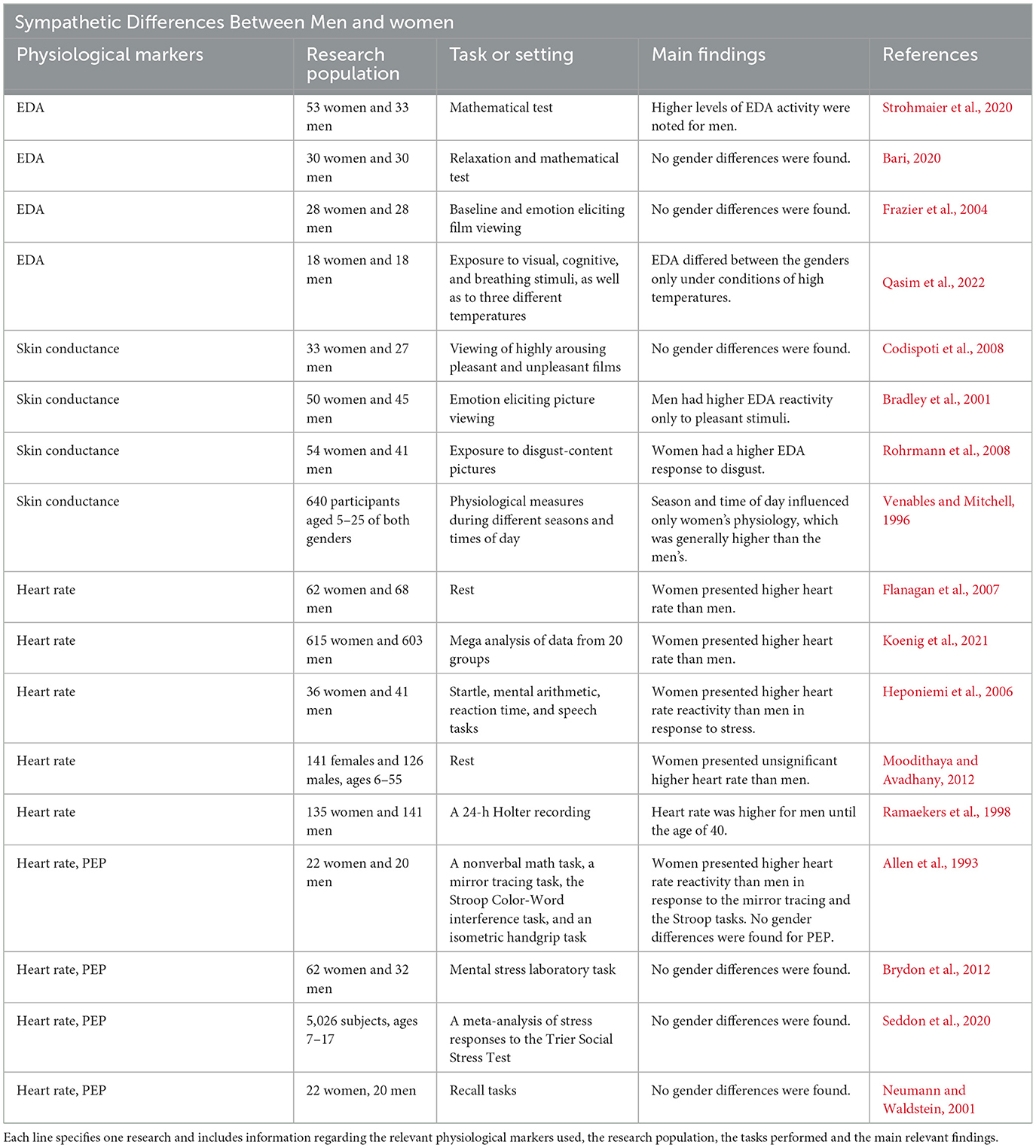
Table 5. This table presents previous findings regarding gender differences in sympathetic physiological activity, specifically in measures of EDA, skin conductance, heart rate and PEP.
Considering the above, the main goal of the current study was to determine whether a two-dimensional autonomic space approach is preferable to the usage of a single physiological measure in assessing the relation between markers of ANS activity and several important aspects of psychological function in healthy adults during a social baseline period. A secondary aim was to estimate gender differences in the manifestation of those psychophysiological correlations.
We focused on four psychological constructs, EC, PA, NA and state anxiety, all of which are highly influential on social interaction, and are widely studied in psychological literature in general and in the area of gender differences in particular. However, to our knowledge, only scant research (Stone et al., 2020) has examined CAB, CAR, CSAB or CSAR associations with anxiety, while PA, NA and EC have not yet been extensively tested in the context of the autonomic space models.
While gender differences in individual psychological functions had been extensively studied and physiological indices were also assessed for gender diversities in past research, variations between men and women in psychophysiological correlations are largely unknown. In the present study we aspired to bridge that knowledge gap as well as to further examine gender differences at the level of individual psychological and physiological indices.
EC is robustly reported as being higher in women compared to men, and we expected to find the same in our research. The link between EC as a trait and physiological activity, however, is largely unknown and was explored here with the autonomic space model for the first time.
Since both PA and NA are usually reported to be higher in women than in men, we hypothesized women to score higher than men in both dimensions of affect. Regarding physiological activities, most studies link PA to increased PNS activity in general and specifically to rise in RSA and some associate PA with sympathetic activity. We, therefore, hypothesized that PA would be positively correlated with RSA, EDA, PEP, CAR and CSAR, meaning that individuals who display higher PA scores will be characterized by higher levels of sympathetic, parasympathetic, and total ANS activation. Since more studies indicate higher PNS activity rather than SNS activity in association with higher PA, we speculated PA to positively correlate with CAB and CSAB, indicating parasympathetic dominance.
NA was usually associated in past research with sympathetic activity and parasympathetic withdrawal. Hence, we hypothesized NA to be positively correlated with EDA and PEP and negatively associated with RSA, meaning that participants with higher NA scores will demonstrate higher sympathetic and lower parasympathetic activities. We also assumed individuals with higher NA scores will have lower CAB and CSAB scores, indicating sympathetic dominance. The correlations between CAR and CSAR and NA had never been tested before, and we therefore had no prior hypothesis regarding them and examined those connections here for the first time.
The literature shows that women are more anxious and are more likely to develop an anxiety disorder than men. However, when measuring baseline levels of state anxiety, no gender differences were found in previous research. We therefore expected no gender differences in our measurements of baseline state anxiety.
The relations between state anxiety and physiological activities are complex. While the majority of studies indicate a positive correlation between state anxiety and sympathetic activity, the relations state anxiety has with PEP at rest are either negative or non-existent. RSA is usually reported to withdraw with a rise in state anxiety. We therefore hypothesized a positive correlation between state anxiety and EDA and a negative correlation with RSA and PEP.
CAB, CAR, CSAR, and CSAB had only been examined once in relation to anxiety (Stone et al., 2020). CSAB had a negative correlation with state anxiety, and this was the correlation we expected to find in our study, while for CAB, CAR, and CSAR there were no correlations detected. We could therefore produce no prior hypotheses concerning those three physiological indices.
Most studies to date report no gender differences in parasympathetic activity as measured by RSA or in PEP representation of sympathetic dominance. Although EDA reactivity is stronger in men, no gender differences were observed at rest. We therefore expected men and women to express roughly the same levels of all types of physiological indices. Table 6 summarizes the entirety of our hypotheses regarding psychophysiological relations (Table 6A) as well as gender differences in the distributions of psychological scores and physiological measures (Table 6B).
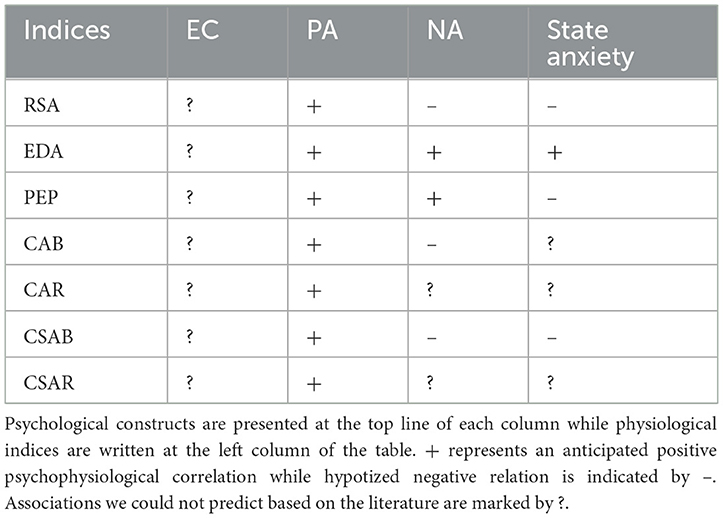
Table 6A. A summary of all hypotheses regarding psychophysiological correlations, based on past research.
Data was collected from four studies recently performed at the Social Neuroscience lab at Bar-Ilan University, Israel: (A) a study aimed to examine associations between physiological and behavioral synchrony to group cohesion and performance (Gordon et al., 2020a,b); (B) a study aimed to inspect the influence of political rivalry on group processes via the role of intermediate physiological mechanisms; (C) a study aimed to investigate physiological reactions to justice, and (D) a pilot study for study C (Gordon et al., 2021). After providing informed consent, all studies began with a 5-min social baseline procedure in which groups of three participants were asked to sit down quietly together with their eyes open, to not look at each other but to choose a location or object in the room and look toward it and not do anything else for a 5-min resting physiological recording period. We used the resting physiological data from the initial baseline period in the current study.
Undergraduate students (n = 498) studying at the Department of Psychology, Bar-Ilan University, participated in these studies. Study A included 145 participants (30 men, mean age = 22.51 years, SD = 2.1); 144 subjects participated in Study B (51 men, mean age = 23.43 years, SD = 3.39), study C involved 149 participants (50 men, mean age = 24.09 years, SD = 3.98), and 60 individuals took part in study D (16 men, mean age = 22.96 years, SD = 2.43). Ninety participants were removed from the physiological analysis (n = 41 from study A, n = 28 from study B, n = 17 from study C, and n = 4 from study D): n = 56 participants had incomplete physiological recordings, and n = 34 participants had unreliable recordings [e.g., heart rate was below 40 beats per minute (bpm) or above 110 bpm, or the breathing frequency was below 0.01 Hz or above 0.5 Hz, corresponding to breathing rates of 0.6 and 30 breaths per minute, respectively]. Overall, data from 408 participants were analyzed.
As can be seen, there is a large gap between the number of men and women participating in the study. We were limited to influence the composition of the study population due to the nature of this research, which is based on data previously gathered in past studies. However, any statistical analysis should be carefully considered with this imbalance in mind. Please see the discussion on this subject.
All participants provided written informed consent in accordance with the request of the University's Institutional Review Board (IRB). All procedures of the studies were done based on the ethical guidelines of the IRB approvals.
In all studies, participants were individually wired to a MindWare Mobile recording unit (MindWare Technologies LTD, Westerville, OH, USA), a well-validated and specialized hardware and software system for monitoring cardiac performance, autonomic balance, and respiratory activity. The recorder enabled, via seven electrodes, the measurement of electrocardiogram (ECG) (through which HRV can be derived), impedance cardiographic waveforms (enabling PEP calculation) and electrodermal activity (EDA).
ECG was recorded at a sampling rate of 500 Hz using a standard lead II configuration. It was analyzed with the MindWare Technology's HRV application software (version 3.1.4) as a single segment. The ECG signal was amplified by a gain of 1,000 and filtered with a hamming windowing function. The data was then visually inspected and manually edited by trained graduate and undergraduate students to ensure proper removal of artifacts and ectopic beats (Berntson et al., 1997).
RSA scores were calculated for each baseline period as a one 5-min segment using MindWare's HRV application. The procedure of determining RSA entails the detection of R-peaks (i.e., heartbeats) in the clean ECG recording and the calculation of an interbeat-interval (IBI) time series. This time series is resampled to 2 Hz (by 500 ms interpolation) in order to obtain equidistant IBI and detrended by removing the global linear trend. The resampled and detrended IBI time series is then analyzed by Fast Fourier Transform (FFT) (using a Hamming window function) to calculate the Heart Period Power Spectrum. MindWare automatically chooses a high frequency (HF) band—a range of 0.12–0.4 Hz (for 15.8% of the participants in our database) or 0.12–0.42 Hz (for 84.2% of participants). We used the respiration time series to verify that the average respiration frequency falls into the selected HF band. Finally, the spectral power of the HF band is calculated, and its natural logarithm equals the RSA score for that given baseline period. PEP scores were calculated as an average for the entire baseline period using the clean and filtered ECG as well as impedance waveforms, with Mindware Impedance Cardiographic software module. PEP is quantified as the time interval between the electrical invasion of the ventricular myocardium (Q-wave of the ECG) and the opening of the aortic valve (B point of the impedance waveform (dZ/dt), Sherwood et al., 1990). The Q-wave peak was automatically marked as the point in which the maximal change in slope occurred during the 35-milliseconds preceding the R-wave peak in the ECG record.
Skin conductance was obtained at a sampling rate of 500 Hz through two Ag/AgCl MindWare electrodes, placed on the non-dominant palm of each individual. It was analyzed using Mindware Technology's EDA application software (version 3.1.5) and visually inspected and manually edited by trained graduate students to ensure proper removal of artifacts. The skin conductance signal was smoothed with a rolling filter of 500 data points per block. For every 500 milliseconds, the level of skin conductance was outputted in MicroSiemens (threshold of 0.05). EDA was calculated as the mean of skin conductance scores in the entire recording period (Picard et al., 2016).
In order to calculate CAB, CAR, CSAB and CSAR we first calculated z-scores for all RSA, PEP and EDA values of all 408 participants, using MATLAB. CAB scores were calculated as the differences between subjects' RSA and PEP scores, while CAR equaled RSA and PEP's sums. CSAB was calculated as the individual's RSA z-score minus his or hers EDA z-score. CSAR was calculated as the sum of those z-scores.
Prior to baseline measurements, all participants were asked to complete several questionnaires. We focus here on three such measures detailed below. The Emotional Contagion Scale (Doherty, 1997) and a mood questionnaire (Watson et al., 1988) were included in all four studies. A short version of the General Anxiety questionnaire (Marteau and Bekker, 1992) was included only in studies C and D. In the entire dataset we analyzed for this report, 400 individuals reported on the Emotional Contagion scale, 404 individuals reported on their mood via the PANAS questionnaire, and 197 individuals rated General Anxiety. Missing data is due to the fact that not all individuals who participated in the study fully completed their self-report questionnaires. Furthermore, 404 of all participants reported on their gender.
The EC scale is a well-established 15-item measure of individual susceptibility to the emotions of others (Doherty, 1997). This trait-like measure estimates mimetic tendency to five basic emotions (love, happiness, sadness, fear, and anger), derived from afferent feedback caused by mimicry. There is a positive association between susceptibility and emotionality, self-esteem, empathy, affective orientation and sensitivity to others, and a negative correlation with emotional stability, alienation, and self-assertiveness. A higher score indicated a higher self-reported tendency to “catch” emotions. In the current sample, EC's Cronbach's alpha was 0.893.
PANAS is a reliable, brief, and valid mood scale that measures the state of two primary dimensions of mood—positive and negative affect (Watson et al., 1988). PA indicates how enthusiastic, alert, and active a person feels, with low positive affect being a state of sadness and apathy. PA is reflective of concentration, pleasurable engagement, and high energy, and is related to social activity and satisfaction. NA refers to subjective distress and unpleasurable engagement accompanied by unpleasant mood states, such as fear, disgust, anger, contempt, and nervousness. NA is associated with self-reported stress and health complaints, while low NA reflects a state of serenity and calmness. Both positive and negative dimensions are largely uncorrelated, exceedingly internally consistent, and quite stable over a 2-month period. A higher score in each dimension indicated higher reports of positive or negative mood. We used a 20-item version of PANAS in studies A and B (Cronbach's alpha of 0.853) and a 25-item version of the PANAS questionnaire in studies C and D (Cronbach's alpha of 0.721).
The Spielberger State-Trait Anxiety Inventory is a reliable, valid and one of the most widely used measure of state anxiety (Spielberger et al., 1970; Marteau and Bekker, 1992). It assesses the level of anxiety a person currently feels and is rather sensitive to fluctuations in transitory anxiety. A higher score indicated higher anxiety levels. Cronbach's alpha was 0.943 in our study.
To examine psychophysiological correlations for men and women separately, we created a four-dimensional comparison of data (see Figures 1, 2). To be consistent with previous literature regarding the two-space ANS model (Berntson et al., 2008; Brush et al., 2019), as well as for visualization purposes, we utilized z-scores of the physiological indices. Self-reported psychological measurements were split separately for each gender into high and low scores according to the median of each measurement. Normality of each physiological and psychological distribution was examined using a Shapiro-Wilk test. We next assessed whether each couple of distributions stem from separate populations. We probed for statistical significance by applying either one-sided t-test for data which follows normal distribution, or Mann-Whitney U-test for data which does not follow normal distribution.
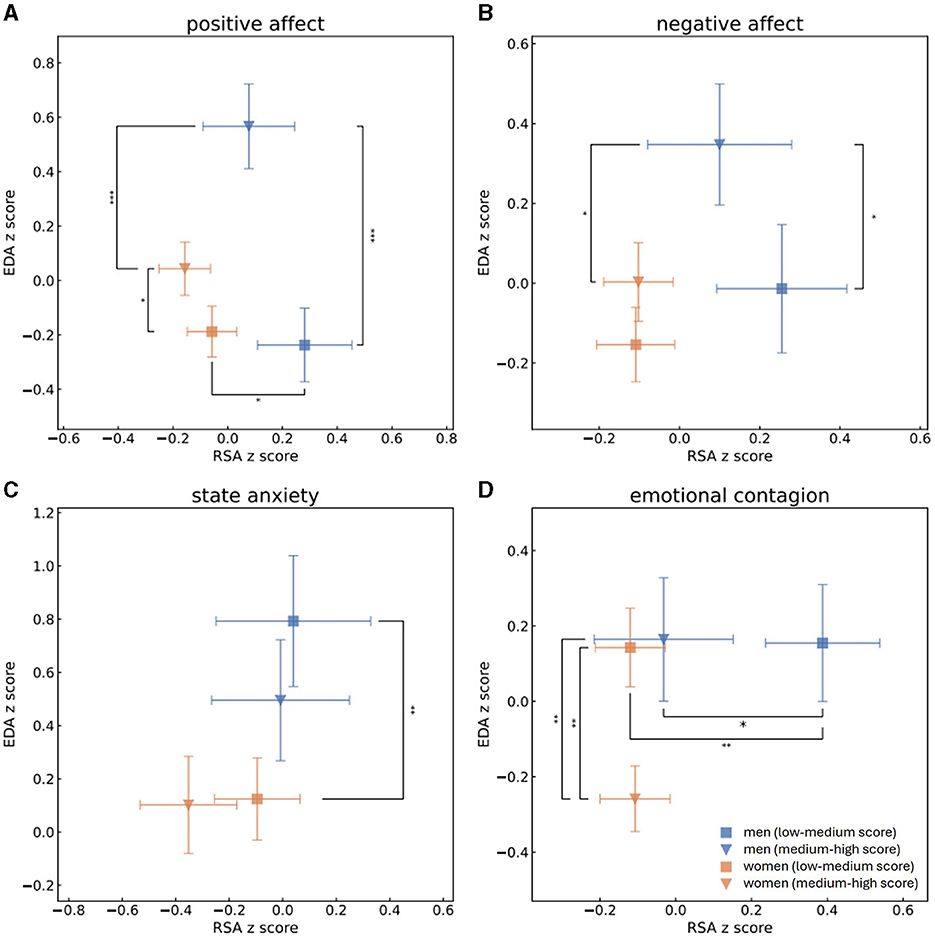
Figure 1. Mean and standard error values of the z-scores of RSA and EDA measurements of men with high (blue triangle) or low (blue square) scores and of women with high (orange triangle) or low (orange square) scores of PA (A), NA (B), state anxiety (C) and EC (D). This grouping to high vs. low scores was determined by splitting the population to scores (s) of PA, NA, state anxiety or EC according to the median. One-sided t-tests were performed to determine whether men and women who received high and low scores of PA, NA, state anxiety and EC were separated from each other in terms of parasympathetic activity (RSA z-scores). Mann-Whitney U-tests were performed to determine group separation in terms of sympathetic activity (EDA z-scores). Statistical significance is marked by * for p < 0.05, ** for p < 0.01 and *** for p < 0.001. Men and women who reported on high PA levels experienced higher levels of EDA. Higher scores of PA in men were associated with higher levels of sympathetic activities than higher scoring women, while men who received low PA scores had higher levels of parasympathetic activities than low scoring women. Men who received higher scores of NA were characterized by higher levels of sympathetic activities than either men who had low NA scores of women who had high NA scores. Regarding individuals who had low scores of state anxiety, men were identified by higher sympathetic recordings than women. Women characterized by higher levels of EC experienced lower levels of sympathetic activity than either women identified by lower levels of EC or melas who had high EC scores. Men who received lower EC scores had higher parasympathetic activity then either men who scored higher EC scores or women who had lower scores of EC.
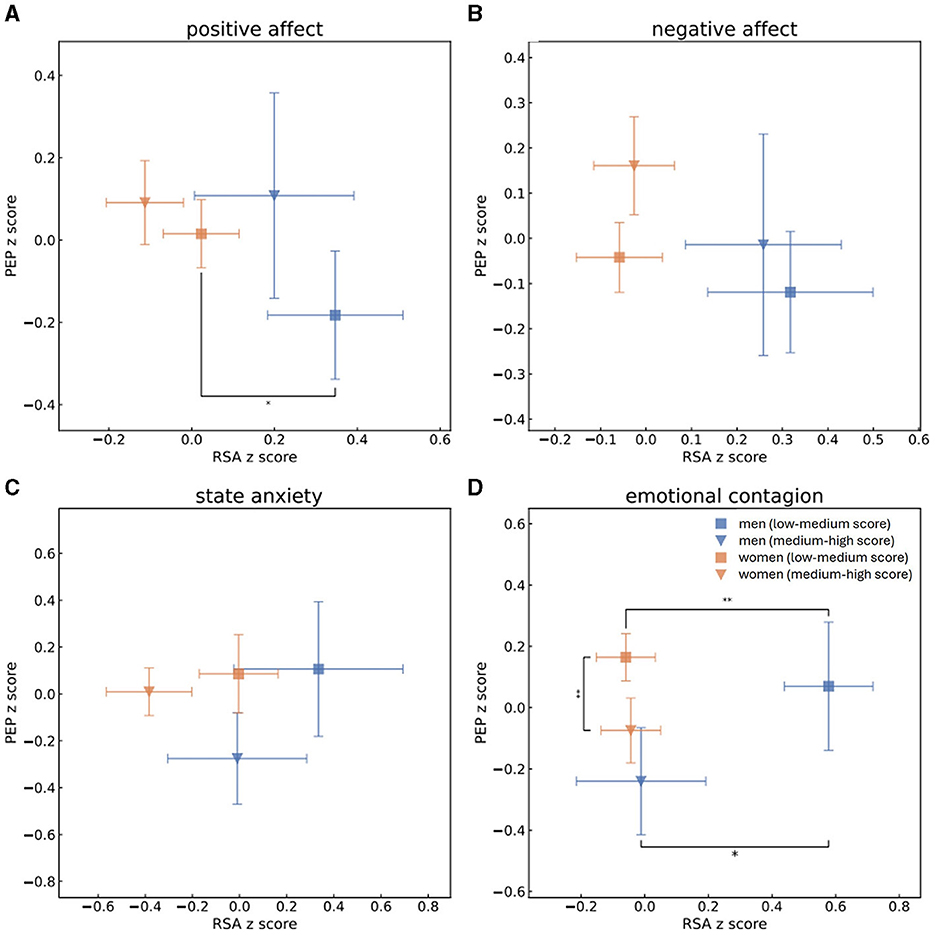
Figure 2. Mean and standard error values of the z-scores of RSA and PEP measurements of men with high (blue triangle) or low (blue square) scores and of women with high (orange triangle) or low (orange square) scores of PA (A), NA (B), state anxiety (C) and EC (D). This grouping to high vs. low scores was determined by splitting the population to scores (s) of PA, NA, state anxiety or EC according to the median. One-sided t-tests were performed to determine whether men and women who received high and low scores of PA, NA, state anxiety and EC were separated from each other in terms of parasympathetic activity (RSA z-scores). Mann-Whitney U-tests were performed to determine group separation in terms of sympathetic activity (PEP z-scores). Statistical significance is marked by * for p < 0.05 and ** for p < 0.01. In regard to low scores of PA, men were identified by higher levels of parasympathetic activity than women. Men who scored low EC scores were characterized by higher parasympathetic activity than either men who had high EC scores or women who scored low scores of EC, while women who had high EC scores were identified by lower sympathetic activity than women who received lower EC scores.
Table 7 provides a summary of gender differences that were found within physiological and self-reported psychological parameters. Physiological indices were calculated as mean scores of the entire database. Considering physiological aspects, men are distinctly higher than women in measures of normalized RSA scores (men: mean 0.1812, std 1.0893, women: mean −0.0561, std 0.9657), CAB (men: mean 0.365, std 1.6763, women: mean −0.0695, std 1.3586) and CSAR (men: mean 0.3407, std 1.4468, women: mean −0.1729, std 1.3276). There was no significant difference between the genders in measures of normalized EDA scores (men: mean 0.1707, std 0.9825, women: mean −0.0602, std 1.0013), normalized PEP scores (men: mean −0.0797, std 1.2060, women: mean 0.0237, std 0.9313), CAR (men: mean 0.2055, std 1.5857, women: mean −0.0171, std 1.3664) and CSAB (men: mean 0.0209, std 1.4272, women: mean −0.0650, std 1.4380). Regarding psychological constructs, women reported themselves as significantly higher than men in parameters of state anxiety (men: mean 2.8274, std 0.7574, women: mean 3.2255, std 1.0421) and of EC (men: mean 3.9788, std 0.9154, women: mean 4.5621, std 0.9881), whereas no differences were found between the genders in PA (men: mean 3.2690, std 1.0996, women: mean 3.2862, std 1.1366) and NA (men: mean 1.7579, std 0.6818, women: mean 1.7457, std 0.6846).
Spearman correlations were conducted between each pair of physiological and psychological indices (see Table 8). We found EC to be negatively correlated with the sympathetic EDA and PEP, as well as with CAR and CSAR. Both positive and negative affect dimensions were positively correlated with EDA and had negative links to CSAB. State anxiety had no significant correlations with any physiological measure. We then employed the Holm's method (Holm, 1979), designed to account for multiple hypothesis testing, to account for the relatively large number of correlations we conducted, and retested the significance of all Spearman's correlations. EC's relations to EDA and PEP as well as PA's correlation with EDA remained significant after this correction, while the other correlations did not.
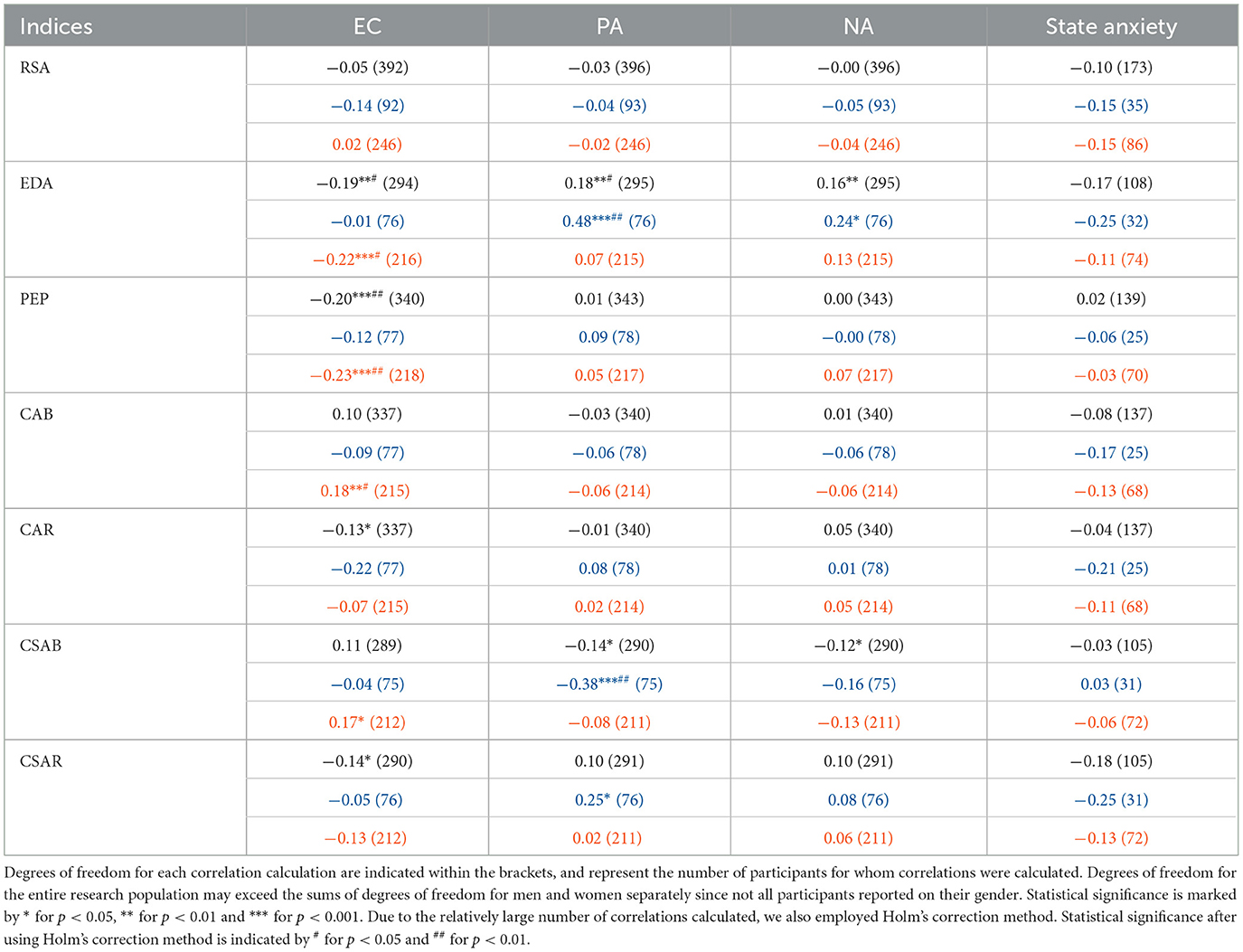
Table 8. This table details all the correlations between the physiological parameters and psychological indices, for men (middle lines, blue) and for women (bottom lines, orange) separately and for the entire research population together (upper lines, black), and is calculated by Spearman's rank correlation.
Next, we split the data to men and women and recalculated all psychophysiological correlations. For men, PA is positively correlated with EDA and CSAR and negatively correlated with CSAB, while men's NA scores are positively correlated with their EDA measurements. After Holm's correction, the links PA have with EDA and CSAB remain significant. In women, however, only EC is significantly correlated with physiological activity, depicting positive links to CAB and CSAB and negative links to EDA and PEP. Most of those correlations remain significant after Holm's correction, and only the correlation between EC and CSAB does not.
In the next step, we split PA, NA, state anxiety and EC to high and low scores according to the median and compared the sympathetic and parasympathetic activities of groups of men and women who received high and low scores of each psychological aspect. A Shapiro-Wilk test was performed to check the normality of normalized RSA, EDA and PEP distributions. RSA z-scores of both men and women follow the normal distribution whereas EDA and PEP normalized scores of both genders do not. One-sided t-test was conducted for all RSA z-score distributions and revealed that men who received high EC marks (mean RSA: −0.056, std: 1.2) significantly differed from men who received low marks of EC (mean RSA:0.42, std: 0.95) in terms of their parasympathetic activities. This means that men who received low scores of EC also demonstrated significantly higher levels of parasympathetic activity during the baseline recording. Men who reported themselves as having low PA (mean RSA: 0.28, std: 1.1) and EC (mean RSA: 0.42, std: 0.95) had significantly higher RSA measurements than women who had low scores of PA (mean RSA: 0.002, std: 0.93) and EC (mean RSA: −0.07, std: 0.95). After applying the Holm's method for multiple hypothesis, however, only the separation between the genders in low EC scores remained significant. For all EDA and PEP distributions of both men and women Mann-Whitney U-tests were performed to determine whether there is a separation between individuals who received high or low scores in terms of sympathetic activity. Women's EDA recordings significantly differed between low (EDA median = −0.52, lower quartile = −0.91, upper quartile = 0.36) and high (EDA median = −0.17, lower quartile = −0.78, upper quartile = 0.664) PA and low (EDA median = −0.17, lower quartile = −0.77, upper quartile = 0.755) and high (EDA median = −0.54, lower quartile = −0.93, upper quartile = 0.219) EC scores, as did women's PEP recordings for scores of low (PEP median = 0.12, lower quartile = −0.29, upper quartile = 0.653) and high (PEP median = −0.018, lower quartile = −0.55, upper quartile = 0.385) EC and men's recordings of EDA for scores of low (EDA median = −0.38, lower quartile = −0.96, upper quartile = 0.3) and high (EDA median = 0.56, lower quartile = 0.093, upper quartile = 1.182) PA and low (EDA median = −0.13, lower quartile = −0.89, upper quartile = 0.466) and high (EDA median = 0.45, lower quartile = −0.44, upper quartile = 1.067) NA. All those separations remained significant after Holm's correction apart from women's EDA recordings for high and low PA marks. In other words, individuals who received low PA scores also demonstrated lower sympathetic activity as measured by EDA, men's sympathetic activity was higher in terms of EDA recordings for men who scored higher in NA, and women who had higher tendencies of EC demonstrated lower sympathetic activity, both in EDA and in PEP measures. In terms of separation between men and women, men's EDA measures were higher than women's who received high PA (men's EDA: median = 0.56, lower quartile = 0.093, upper quartile = 1.182; women's EDA: median = −0.17, lower quartile = −0.78, upper quartile = 0.664), NA (men's EDA: median = 0.45, lower quartile = −0.44, upper quartile = 1.067; women's EDA: median = −0.33, lower quartile = −0.78, upper quartile = 0.611) and EC (men's EDA: median = 0.19, lower quartile = −0.79, upper quartile = 0.941; women's EDA: median = −0.54, lower quartile = −0.93, upper quartile = 0.219) scores and in the groups who scored high anxiety marks (men's EDA: median = 1.0, lower quartile = 0.47, upper quartile = 1.186; women's EDA: median = 0.073, lower quartile = −0.65, upper quartile = 0.778). All of those separations remained significant after applying the Holm's method for multiple hypothesis. Figure 1 displays the mean normalized RSA and EDA scores of men and women within all experiments, while Figure 2 showcases mean normalizes RSA and PEP scores. Blue triangles and blue squares represent high and low scores (respectively) of men's PA (Figures 1A, 2A), NA (Figures 1B, 2B), state anxiety (Figures 1C, 2C), or EC (Figures 1D, 2D), while orange triangles and orange squares represent high and low scores (respectively) that women received in those psychological questionnaires. Error bars depict standard error.
The distributions of normalized RSA as well as normalized EDA scores or PEP scores across all subjects are plotted in Figures 3A, B, respectively. Women are shown by orange dots, men are plotted in blue while individuals who did not report their gender are depicted as gray dots. The distributions are plotted upon a bivariate representation of the autonomic space, primarily introduced by Berntson et al. (2008). The bivariate space depicts areas of coactivation of sympathetic and parasympathetic activity (CSAR in Figure 3A or CAR in Figure 3B), reciprocal parasympathetic activity (CSAB in Figure 3A or CAB in Figure 3B), reciprocal sympathetic activity and inhibition of both sympathetic and parasympathetic activity. The diagonal arrows of reciprocity (CSAB or CAB) represent a bipolar model, according to which an increase in sympathetic activity occurs on the expense of a decrease in parasympathetic activity and vice versa. The diagonal arrows of coactivation (CSAR or CAR) represent a mutual increase or decrease in the activity of both branches of autonomic nervous system. The general distribution of sympathetic and parasympathetic activities of participants in Figures 3A, B is not concentrated around either of those diagonal arrows, thus rejecting both the bipolar model and the notion of mutual activity or inhibition in favor of independency between the activities of the sympathetic and the parasympathetic branches of the ANS.
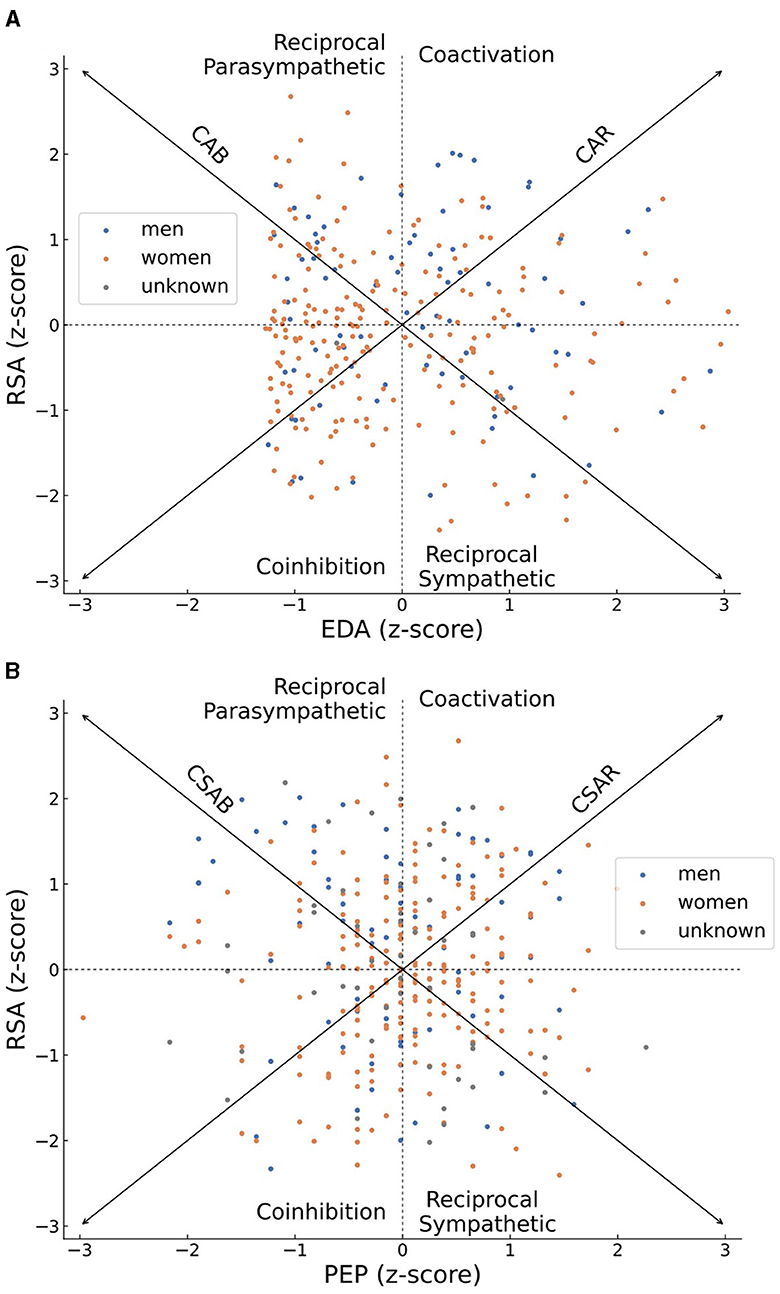
Figure 3. Scatter plots of the z-scores of individuals' parasympathetic (RSA) and sympathetic (EDA in A or PEP in B) activities, plotted on top of a bivariate representation of the autonomic space. Women's scores are colored in orange, men's scores are colored in blue, scores of individuals who did not report their gender are colored in gray. An individual who demonstrated an overall high parasympathetic activity during the recording period will be plotted on the high half of the graph, and an overall high sympathetic activity will place him or her at the right half of the graph. The overall distribution of sympathetic and parasympathetic activities of all participants is scattered and not concentrated across the CSAB or CSAR diagonal arrows, representing patterns of independent sympathetic and parasympathetic activities, and demonstrating a disagreement with either the bipolar model or the notion of total activation or disactivating of the autonomic nervous system.
In order to conclude which physiological measures best predict each psychological aspect, as well as to determine if one-dimensional or two-dimensional physiological framework is more suitable for psychophysiological research, we created a set of linear regression models for each psychological index. We compared the amounts of explained variability produced by each model, as well as each model's Akaike information criterion (AIC) and Bayesian information criterion (BIC), both criterions for model selection which indicate a better fitting model the lower their scores are. Each psychological construct was modeled separately for men and for women, as well as for both genders together.
Table 9 showcases linear regression models created to predict EC. When examining both genders together, the joint model of CSAB and CSAR explains the largest amount of variability (R-square = 0.0326) and produces the smallest values of AIC (828) and BIC (843), with only small differences from the model of EDA alone (R-square = 0.0300, AIC = 837, BIC = 848). This is also the case for the models created for women alone (joint model of CSAB + CSAR: R-square = 0.0398, AIC = 608, BIC = 621; EDA model: R-square = 0.0378, AIC = 615, BIC = 625). The comparison between models for men, however, yielded very different results, with CAB and CAR joint ability to predict EC far surpasses each of the other models (R-square = 0.0600). Linear regression fit of EC and CSAB for men and women are presented in Figure 4A.
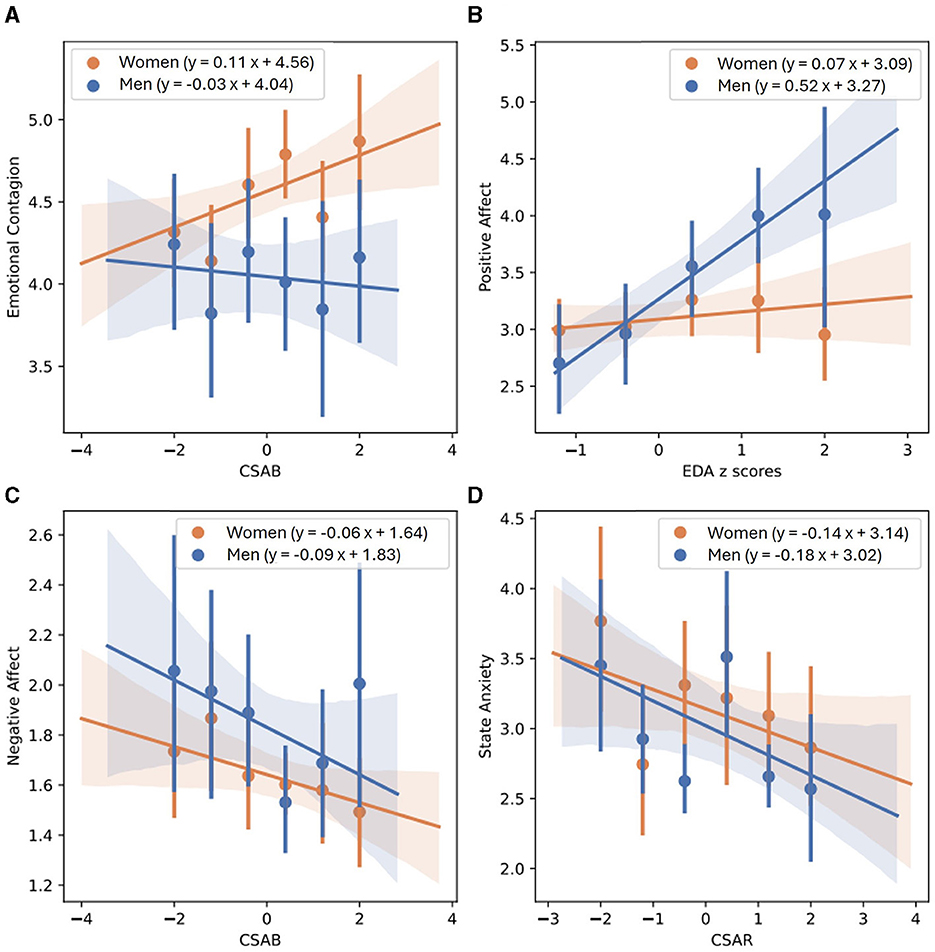
Figure 4. Linear regression between EC and CSAB (A), PA and normalized EDA (B), NA and CSAB (C) and state anxiety and CSAR (D), separated for men and women. Men's best fit of linear regression is depicted by a solid blue line, mean values of each bin in represented by blue dots, and blue shaded areas demonstrate confidence intervals obtained using the bootstrap method. Women's linear regression best fit, mean values of separate bins and confidence intervals are depicted in orange.
Table 10 demonstrates all linear regression models calculated to predict PA. EDA predicts PA more accurately than any other model, with R-square = 0.0304 and only slightly higher scores of AIC and BIC than the combined model of CSAB and CSAR (EDA model: AIC = 889, BIC = 900; CSAB + CSAR model: R-square = 0.0289, AIC = 877, BIC = 891). The models for men repeat the same conclusions, with EDA predicting 22.5% of unexplained variability compared to 22.1% predicted by CSAB and CSAR together, and almost exactly the same AIC and BIC scores (EDA model: AIC = 218, BIC = 225; CSAB + CSAR model: AIC = 218, BIC = 227). For women, however, no physiological model accounts for even 1% of unexplained variability. A linear regression fit of PA and EDA for men and women is shown in Figure 4B.
NA predicting models are presented in Table 11. When calculated for both genders together, CSAB and CSAR measures account for 1.94% of all unexplained variability whereas EDA alone accounts for 1.35%. The AIC and BIC scores of both models are also similar (EDA model: AIC = 600, BIC = 611; CSAB + CSAR model: AIC = 591, BIC = 605). For men the difference between EDA model and CSAB and CSAR joint model is a little bigger, although measurements of model fit remain largely the same (EDA model: R-square = 0.0263, AIC = 169, BIC = 176; CSAB + CSAR model: R-square = 0.0387, AIC = 169, BIC = 178). For women, however, the joint model of CAB and CAR is the best predictor for NA, accounting for 2% of unexplained variability, whereas PEP and CSAB and CSAR models account for 1.29–1.52%. The CAB and CAR model is also preferable for women in terms of model fit (CAB + CAR model: AIC = 403, BIC = 416; PEP model: AIC = 410, BIC = 421; CSAB + CSAR model: AIC = 421, BIC = 434). The linear regression fit of NA and CSAB is presented in Figure 4C, separated for men and women.
State anxiety predicting models are presented in Table 12. CSAB and CSAR joint model is the best one to predict state anxiety for both genders together, accounting for 6% of unexplained variability and producing AIC of 292 and BIC of 303. EDA only accounts for 2.95% of unexplained variability while all other models explain < 1%. CSAB and CSAR remain the preferred model to use when separating the data for men and for women as well, accounting for 12.4% of unexplained variability for men and 3.31% for women, although in both cases the AIC and BIC scores are lower for PEP and for CAB and CAR models. Men's and women's linear fits for state anxiety and CSAR are shown in Figure 4D.
This paper aimed to examine the superiority of two-dimensional models in explaining individual differences in psychological and physiological factors as well as psychophysiological associations, compared with separate SNS and PNS activity indicators. A secondary goal was to evaluate gender differences in the manifestation of those indices and psychophysiological relations. To this end, participants have completed questionnaires measuring their EC, PA, NA, and state anxiety. Physiological data were recorded during a social baseline period, and EDA, PEP, and RSA were calculated. Each psychological index was inspected both in the bipolar one-dimensional model of autonomic balance, comparing psychological indices to sympathetic activity (represented by EDA and PEP scores) and to parasympathetic activity (marked as RSA score), and in the framework of autonomic space model, comparing EC, PA, NA, and state anxiety to CAB, CAR, CSAB, and CSAR. Cardiac autonomic balance (CAB) and cross-system autonomic balance (CSAB) indicate the influence of the parasympathetic relative to the sympathetic branches of the ANS. Cardiac autonomic regulation (CAR) and cross-system autonomic regulation (CSAR) mark the overall ANS activation. Most psychological indices were correlated to some aspect of physiological activity, with pronounce differences between men and women in terms of psychophysiological correlations.
We examined EC as a trait related to physiological activity for the first time, and found negative correlations to sympathetic activity, measured by EDA and PEP scores, and to overall activation of the ANS, measured by CAR and CSAR scores. The literature links EC with social interaction (Hatfield et al., 1994; Butler, 2011), empathy, bonding, and attunement (Hatfield et al., 1994, 2011; Spoor and Kelly, 2004; Decety and Ickles, 2009; Neves et al., 2018). Sympathetic withdrawal is also connected to social engagement (Porges, 2001); therefore, EDA and PEP can be expected to correlate negatively with EC. The negative association between EC and CAR and CSAR indicates a decrease in total ANS activity in individuals with higher tendencies to catch others' emotions. Both high levels of total ANS activation (Berntson et al., 2008) and low levels of SNS activation (Schwartz et al., 1992; Airaksinen, 1999; Hohnloser, 2005; Billman, 2006a,b) are predictive of good health, and it will therefore be interesting to examine EC connection to health in the future.
As expected, PA was positively associated with sympathetic activity, measured by EDA scores, in line with previous studies (Neumann and Waldstein, 2001; Frazier et al., 2004; Shiota et al., 2011). Unlike our hypotheses, however, higher levels of PA were linked to lower scores of CSAB, meaning that high PA was characterized by SNS dominance, and not correlated with PNS activity. Those divergences from our original expectations, and indeed the inconsistency of physiological response to PA among different papers, may be attributed to the differences in questionnaires used to quantify PA in the literature. While we used the PANAS questionnaire, Demaree et al. (2006) used the Self-Assessment Manikin (Bradley and Lang, 1994), Frazier et al. (2004) adopted a combination of the Semantic Differential Scale (Mehrabian and Russell, 1974) and the Self-Assessment Manikin whereas Neumann and Waldstein (2001) applied an Affect Grid (Russell et al., 1989) and a 28-item questionnaire (Russell, 1980). The comparison of findings between different articles is complicated under such circumstances.
Keeping with the existing literature on NA (Demaree and Everhart, 2004; Oveis et al., 2009; Drachen et al., 2010), higher NA scores were associated with higher SNS activity, measured by EDA. Also consistent with our hypothesis, NA was negatively correlated with CSAB, indicating a sympathetic dominance in individuals with higher NA score. We predicted NA to have a negative association with RSA. However, no connection to parasympathetic activity was noted. A possible explanation for that gap may lay in the differences in autonomic patterns which emerge in response to distinct negative emotions, such as fear, disgust, and anger (for a review, see Shiota et al., 2011). Both NA (Watson and Pennebaker, 1989; Knapp et al., 1992; Billings et al., 2000; Kubzansky and Kawachi, 2000; Meeks and Murrell, 2001; Kiecolt-Glaser et al., 2002; Suls and Bunde, 2005; Powell et al., 2008; Finch et al., 2012; Pressman et al., 2013; Ambrona and López-Pérez, 2014) and high sympathetic activity (Schwartz et al., 1992; Airaksinen, 1999; Hohnloser, 2005; Billman, 2006a,b) are repeatedly associated with negative health consequences. The eliciting of high SNS response may be the cause through which NA influences health, and indeed some studies have demonstrated how health deficiencies associated with negative emotions are mediated by differences in cultural background (Consedine et al., 2002; Miyamoto et al., 2013; Pressman et al., 2013; Curhan et al., 2014) or in psychological processes (Bastian et al., 2012). Further research should be conducted to determine whether indeed health impairments caused by NA can be attributed to sympathetic activity.
No physiological index could predict state anxiety. Although there are other studies which reported no relation between state anxiety and physiological activity (Huwe et al., 1998; Spangler et al., 2002; Bajkó et al., 2012; Verkuil et al., 2014; Cho et al., 2019; Ringeisen et al., 2019; Bauerly and Jones, 2021), state anxiety is characterized, among other properties, by arousal of the ANS (Spielberger, 1966), and this lack of correlation with any physiological index is therefor still surprising. It may be explained by differences in the construction of our research compared with the common anxiety literature. Most studies tested physiological responses to a stressful situation, meant to trigger anxiety, compared with a neutral stimulus. Our physiological measurements, however, were obtained during a social baseline period designed to be devoid of any affective triggers. Under these low-anxiety circumstances, it stands to reason that there are no significant differences in physiological arousal levels among participants, and therefore correlations will be difficult to find.
Men and women demonstrated many differences in the distribution of distinct psychological and psychological indices, in psychophysiological relations and even in in terms of the physiological model which best describes each of their psychological variabilities.
We expected men and women to present no differences in any type of physiological activity. Indeed, we found both genders to express roughly the same levels of the sympathetic EDA, as was suggested by Boucsein (2012) and reported by Strohmaier et al. (2020), although contradicting other findings (Frazier et al., 2004; Codispoti et al., 2008; Bari, 2020). The sympathetic PEP was also found to be similar for both genders, a finding that is in line with the common research on the subject (Allen et al., 1993; Neumann and Waldstein, 2001; Brydon et al., 2012; Seddon et al., 2020). We found, however, that men had significantly higher levels of parasympathetic activity, a surprising result based on past research (Frazier et al., 2004; Codispoti et al., 2008; Cribbet et al., 2011; Moodithaya and Avadhany, 2012; Botek et al., 2018). However, there are works linking low resting RSA levels to higher levels of anxiety disorders (Friedman and Thayer, 1998; Friedman, 2007; Chalmers et al., 2014), which are in turn reported as higher in women (Angst and Dobler-Mikola, 1985; Weissman and Merikangas, 1986; Anderson et al., 1987; Líndal and Stefánsson, 1993; Kessler et al., 1994, 1995; Lewinsohn et al., 1998; Costello et al., 2003; Bruce et al., 2005; McLean et al., 2011), and in this regard it stands to reason that women will express lower levels of baseline RSA. Women also demonstrated lower parasympathetic dominance (expressed by CAB), previously associated with greater health risk factors (Brush et al., 2019; Alen et al., 2020) as well as with higher levels of depressive symptoms (Stone et al., 2020). Time and again, women are reported to be about twice as likely as men to develop depression (for reviews see: Piccinelli and Wilkinson, 2000; Nolen-Hoeksema, 2001; Girgus and Yang, 2015). Further research is required to determine whether differences in physiological activity are associated to this elevated risk for women.
EC scores as well as EC's psychophysiological correlations differed for men and women. As expected, men exhibit lower EC scores than women, in line with previous research on the subject (Hall, 1978; Buck, 1984; Doherty et al., 1995; Surakka and Hietanen, 1998; Wild et al., 2001; Sonnby-Borgström et al., 2008; Magen and Konasewich, 2011; Manera et al., 2013; Fairbairn et al., 2015). Interestingly, there were also differences in psychophysiological correlations between the genders. While men EC scores exhibited no relations to any physiological activity, women reported higher levels of EC in accordance with lower sympathetic activity, measured by EDA and PEP, and with higher parasympathetic dominance, manifested by higher scores of CAB and CSAB. Those findings are partially in line with a recent study (Tracy and Giummarra, 2017) which examined gender differences in empathy to pain and its relation to parasympathetic activity, and found no such relations in men, and a negative correlation between baseline parasympathetic activity at rest and women's empathy trait. Our finding of women's association between EC and parasympathetic dominance, as well as our finding of higher parasympathetic activity in the group of men individuals who received higher scores of RSA, is in agreement with Stellar et al. (2015), who reported a positive relation between parasympathetic activity and compassionate tendencies. Notably, 72% of their participants were women. It is interesting to note that in our study, neither gender demonstrated any relation between EC and overall ANS activation, although such a connection was evident when taking both genders together into account.
In regard to affect, both genders reported similar levels of PA and NA. Although most past studies reported women to have higher PA and NA scores (Fujita et al., 1991; Grossman and Wood, 1993; Thomsen et al., 2005; Zuckerman et al., 2017; Batz and Tay, 2018), some reported no such differences (Neumann and Waldstein, 2001; Cribbet et al., 2011; Volante et al., 2016). We did find, however, differences in psychophysiological correlations between men and women. Women's PA and NA scores demonstrated no significant correlations with any physiological measure, similarly to several studies (Levenson et al., 1994; Demaree et al., 2006). Women who belonged to the group of higher PA scores, however, were characterized by higher EDA recordings, supporting other works which reported a positive correlation between PA and parasympathetic activity in groups consisting mostly of women (Oveis et al., 2009; Kop et al., 2011; Wang et al., 2013). Men reported higher levels of PA and of NA in accordance with higher sympathetic activity, measured by EDA. Previous studies have similarly showed a positive correlation between NA and sympathetic activity in men (Levenson et al., 1994) or for both men and women (Anderson et al., 2017), while Neumann and Waldstein (2001) demonstrated a link between PA and sympathetic activity for both genders. Men's levels of PA were also correlated with overall higher activation of the ANS, measured by CSAR, and with sympathetic dominance, indicated by lower levels of CSAB. While as one united group men and women's NA scores were related to sympathetic dominance, this relation cannot be found separately in any of the two groups. This separation to men and women also enabled our conclusion that all PA correlations with physiological activity measured in the general sample of both genders actually stemmed solely from the men's data.
As for state anxiety, women's levels of anxiety were significantly higher than men's levels, in line with many studies (Angst and Dobler-Mikola, 1985; Weissman and Merikangas, 1986; Anderson et al., 1987; Bourdon et al., 1988; Líndal and Stefánsson, 1993; Campbell and Rapee, 1994; Kessler et al., 1994, 1995; Lewinsohn et al., 1998; Poulton et al., 2001; Muris and Ollendick, 2002; Costello et al., 2003; Bruce et al., 2005; McLean et al., 2011), but contradicting others (Carrillo et al., 2001; Brand and Schoonheim-Klein, 2009; Limbu et al., 2010; Strohmaier et al., 2020). As for correlations between state anxiety and physiological activity, both genders demonstrated none. There are apparent gender differences in the physiological response to stress (for a review, see Donner and Lowry, 2013), and Kong et al. (2022) even showed that men's patterns of physiological activity can discriminate between high and low trait anxiety individuals much more reliably than women's patterns. Therefore, our results in this regard are unexpected.
The main goal of this study was to investigate the autonomic space models as frameworks for physiological correlations with psychological measures and to inspect their superiority over the one-dimensional ANS models. In our study, we chose to employ both the cardiac autonomic balance (CAB) and regulation (CAR) model and the refined framework of cross-system autonomic balance (CSAB) and regulation (CSAR). Since the CSAB-CSAR framework is based on EDA, a sympathetic index of the eccrine system (Fowles, 1986; Shields et al., 1987; Dawson et al., 2007), and RSA, a parasympathetic index of the cardiac system (Cacioppo et al., 1994; Malik et al., 1996; Berntson et al., 1997), whereas the CAB-CAR model represents purely cardiac activity (Sherwood et al., 1990; Berntson et al., 1994a; Cacioppo et al., 1994; Benevides and Lane, 2013; Stone et al., 2020), the usage of both frameworks allowed us to examine physiological correlates of psychological traits and processes in a comprehensive ANS context.
The distribution of sympathetic and parasympathetic scores in the two-dimensional autonomic space (Figure 3) suggests a clear preference for the autonomic space models. The classic one-dimensional model requires a decrease in parasympathetic activity to accompany an increase in sympathetic activity and vice versa (a slope of −1 in the two-dimensional space, concentrating around the CAB or CSAB diagonal arrows). This relationship does not manifest in our results, nor does a repeated co-activation or co-inhibition of the two ANS branches (a slope of +1 in the two-dimensional space, concentrating around the CAR or CSAR diagonal arrows). Our results reinforce that the PNS and the SNS can be activated independently from one another (Berntson et al., 1991; Berntson and Cacioppo, 2007; Stone et al., 2020). We will note that this clear support of the autonomic space model is only provided here for a baseline period, in which no stimulation was given to the participants. Further research is needed to determine whether this is the preferred physiological framework in cases of activity, interaction, or stimulation.
To further investigate which physiological model best predicts each psychological aspect, we conducted a series of linear regression calculations. When considering both genders together, we found that the CSAB-CSAR model best predicted the variability of EC, NA and state anxiety, whereas EDA fitted PA better. CSAB-CSAR model was the best fitting for most psychological constructs calculated for men and women separated as well, while only men's PA variability was better predicted by the one-dimensional EDA. We therefore conclude that at rest, two-dimensional models, and specifically the CSAB-CSAR model, are the most predictive of most psychological aspects.
Our study has several limitations. The first is the employment of self-reported questionnaires, subjecting the psychological indices to bias (Wang, 2015). The second limitation concerns the structural design of the research. The focus of our study was correlation measurements, rendering it impossible to conclude any reasoning or direction of effect between psychology and physiology. The third limitation regards the measurement context. Our physiological measurements were obtained during a baseline period, during which an individual is supposedly awake, at rest, and removed from any stimuli. However, the level of alertness a subject is experiencing during a baseline period is unclear, and there are possible stress-inducing stimuli that may affect them, such as electrode hookups and the company of other individuals. See Fishel et al. (2007) for a broader consideration of the baseline procedure. The fourth and final limitation concerns the research population. As in many psychophysiological studies, most of our subjects were women. A careful consideration of all results obtained for men is warranted, as well as the comparisons between the genders, due to this imbalance. A properly balanced research sample will be needed to strengthen and verify the associations we found here in future research.
Two strengths of this research are the relatively large number of subjects, thanks to which we can draw more robust conclusions (Machin et al., 1987; Whitley and Ball, 2002), and the usage of several physiological frameworks, allowing for a comprehensive understanding of the relationship between the psychological measures and the physiological activity. This paper was one of the first to examine the psychological aspects of the autonomic space model and expanded our understanding of the psychology-physiology link.
The data analyzed in this study is subject to the following licenses/restrictions: deidentified data will be made available upon request from the corresponding author. Requests to access these datasets should be directed to aWxhbml0LmdvcmRvbkBiaXUuYWMuaWw=.
The studies involving humans were approved by Department of Psychology IRB Bar-Ilan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YMS: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. YXJM: Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. MAD: Writing – original draft. SG: Methodology, Supervision, Writing – review & editing. RPB: Methodology, Supervision, Writing – review & editing. IG: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. IG reports grant support from the Israel Science Foundation grants 20 #434/2021 and a VATAT data science award together with RPB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Airaksinen, K. E. (1999). Autonomic mechanisms and sudden death after abrupt coronary occlusion. Ann. Med. 31, 240–245. doi: 10.3109/07853899908995886
Alen, N. V., Deer, L. K., and Hostinar, C. E. (2020). Autonomic nervous system activity predicts increasing serum cytokines in children. Psychoneuroendocrinology 119:104745.? doi: 10.1016/j.psyneuen.2020.104745
Allen, M. T., Stoney, C. M., Owens, J. F., and Matthews, K. A. (1993). Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosom. Med. 55, 505–517. doi: 10.1097/00006842-199311000-00006
Ambrona, T., and López-Pérez, B. (2014). A longitudinal analysis of the relationship between positive and negative affect and health. Psychology 5, 859–863. doi: 10.4236/psych.2014.58097
Anderson, A. P., Mayer, M. D., Fellows, A. M., Cowan, D. R., Hegel, M. T., and Buckey, J. C. (2017). Relaxation with immersive natural scenes presented using virtual reality. Aerosp. Med. Hum. Perform. 88, 520–526. doi: 10.3357/AMHP.4747.2017
Anderson, J. C., Williams, S., McGee, R., and Silva, P. A. (1987). DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch. Gen. Psychiatry 44, 69–76. doi: 10.1001/archpsyc.1987.01800130081010
Angst, J., and Dobler-Mikola, A. (1985). The zurich study. Eur. Arch. Psychiatry Neurol. Sci. 235, 179–186. doi: 10.1007/BF00380990
Bajkó, Z., Szekeres, C. C., Kovács, K. R., Csapó, K., Molnár, S., Soltész, P., et al. (2012). Anxiety, depression and autonomic nervous system dysfunction in hypertension. J. Neurol. Sci. 317, 112–116. doi: 10.1016/j.jns.2012.02.014
Balyan, K. Y., Tok, S., Tatar, A., Binboga, E., and Balyan, M. (2016). The Relationship among personality, cognitive anxiety, somatic anxiety, physiological arousal, and performance in male athletes. J. Clin. Sport Psychol. 10, 48–58. doi: 10.1123/jcsp.2015-0013
Balzarotti, S., Biassoni, F., Colombo, B., and Ciceri, M. R. (2017). Cardiac vagal control as a marker of emotion regulation in healthy adults: a review. Biol. Psychol. 130, 54–66.? doi: 10.1016/j.biopsycho.2017.10.008
Bari, D. S. (2020). Gender differences in tonic and phasic electrodermal activity components. Sci. J. Univ. Zakho 8, 29–33. doi: 10.25271/sjuoz.2020.8.1.670
Barlow, D. H. (2002). Anxiety and Its Disorders—The Nature and Treatment of Anxiety and Panic, 2nd Edn. New York, NY: Guilford Press.
Barsade, S. G. (2002). The ripple effect: emotional contagion and its influence on group behavior. Adm. Sci. Q., 47, 644–675. doi: 10.2307/3094912
Barsade, S. G., Coutifaris, C. G., and Pillemer, J. (2018). Emotional contagion in organizational life. Res. Org. Behav. 38, 137–151. doi: 10.1016/j.riob.2018.11.005
Bartsch, R. P., Liu, K. K. L., Ma, Q. D. Y, and Ivanov, P. C. (2014). Three independent forms of cardio-respiratory coupling: transitions across sleep stages. Comput. Cardiol. 41, 781–784.
Bartsch, R. P., Schumann, A. Y., Kantelhardt, J. W., Penzel, T., and Ivanov, P. C. (2012). Phase transitions in physiologic coupling. Proc. Natl. Acad. Sci. U. S. A. 109, 10181–10186. doi: 10.1073/pnas.1204568109
Bastian, B., Kuppens, P., Hornsey, M. J., Park, J., Koval, P., and Uchida, Y. (2012). Feeling bad about being sad: the role of social expectancies in amplifying negative mood. Emotion 12, 69–80. doi: 10.1037/a0024755
Batz, C., and Tay, L. (2018). “Gender differences in subjective well-being,” in Handbook of Well-being, eds E. Diener, S. Oishi, and L. Tay (Salt Lake City, UT: DEF Publishers).
Bauerly, K. R., and Jones, R. M. (2021). The impact of self-reported levels of anxiety on respiratory sinus arrhythmia levels in adults who stutter. J. Commun. Disord. 90:106084. doi: 10.1016/j.jcomdis.2021.106084
Beauchaine, T. (2001). Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol. 13, 183–214.? doi: 10.1017/S0954579401002012
Beauchaine, T. P. (2015). Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Curr. Opin. Psychol. 3, 43–47.? doi: 10.1016/j.copsyc.2015.01.017
Benevides, T. W., and Lane, S. J. (2013). A review of cardiac autonomic measures: considerations for examination of physiological response in children with autism spectrum disorder. J. Autism Dev. Disord. 45, 560–575. doi: 10.1007/s10803-013-1971-z
Berntson, G. G. (2019). Presidential address 2011: autonomic modes of control and health. Psychophysiology 56:e13306.? doi: 10.1111/psyp.13306
Berntson, G. G., and Cacioppo, J. T. (2007). “Integrative physiology: homeostasis, allostasis, and the orchestration of systemic physiology,” in Handbook of Psychophysiology, 3rd Edn, eds J. T. Cacioppo, L. G. Tassinary, and G. G. Berntson (Cambridge: Cambridge University Press), 433–452.
Berntson, G. G., Cacioppo, J. T., Binkley, P. F., Uchino, B. N., Quigley, K. S., and Fieldstone, A. (1994a). Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology 31:599–608.? doi: 10.1111/j.1469-8986.1994.tb02352.x
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol. Rev. 98:459.? doi: 10.1037/0033-295X.98.4.459
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychol. Bull. 114:296.? doi: 10.1037/0033-2909.114.2.296
Berntson, G. G., Cacioppo, J. T., Quigley, K. S., and Fabro, V. T. (1994b). Autonomic space and psychophysiological response. Psychophysiology 31, 44–61.? doi: 10.1111/j.1469-8986.1994.tb01024.x
Berntson, G. G., Norman, G. J., Hawkley, L. C., and Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology 45, 643–652.? doi: 10.1111/j.1469-8986.2008.00652.x
Berntson, G. G., Thomas bigger, J., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., et al. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x
Billings, D. W., Folkman, S., Acree, M., and Moskowitz, J. T. (2000). Coping and physical health during caregiving: the roles of positive and negative affect. J. Pers. Soc. Psychol. 79, 131–142. doi: 10.1037/0022-3514.79.1.131
Billman, G. E. (2006a). A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol. Ther. 111, 808–835. doi: 10.1016/j.pharmthera.2006.01.002
Billman, G. E. (2006b). Heart rate response to onset of exercise: evidence for enhanced cardiac sympathetic activity in animals susceptible to ventricular fibrillation. Am. J. Physiol. 291, 429–435. doi: 10.1152/ajpheart.00020.2006
Botek, M., Krejčí, J., and McKune, A. (2018). Sex differences in autonomic cardiac control and oxygen saturation response to short-term normobaric hypoxia and following recovery: effect of aerobic fitness. Front. Endocrinol. 9:697. doi: 10.3389/fendo.2018.00697
Boucsein, W. (2012). “Applications of electrodermal recording,” in Electrodermal Activity, 2nd Edn, ed W. Boucsein (New York, NY: Springer), 259–523.
Bourdon, K. H., Boyd, J. H., Rae, D. S., Burns, B. J., Thompson, J. W., and Locke, B. Z. (1988). Gender differences in phobias: results of the ECA community survey. J. Anxiety Disord. 2, 227–241. doi: 10.1016/0887-6185(88)90004-7
Bradley, M. M., Codispoti, M., Sabatinelli, D., and Lang, P. J. (2001). Emotion and motivation II: sex differences in picture processing. Emotion 1, 300–319. doi: 10.1037/1528-3542.1.3.300
Bradley, M. M., and Lang, P. J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Therapy Exp. Psychiatry 25, 49–59. doi: 10.1016/0005-7916(94)90063-9
Brand, H. S., and Schoonheim-Klein, M. (2009). Is the OSCE more stressful? Examination anxiety and its consequences in different assessment methods in dental education. Eur. J. Dental Educ. 13, 147–153. doi: 10.1111/j.1600-0579.2008.00554.x
Brenner, S. L., Beauchaine, T. P., and Sylvers, P. D. (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology 42, 108–115.? doi: 10.1111/j.1469-8986.2005.00261.x
Brosschot, J. F., VanDijk, E. V., and Thayer, J. F. (2007). Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int. J. Psychophysiol. 63, 39–47. doi: 10.1016/j.ijpsycho.2006.07.016
Bruce, S. E., Yonkers, K. A., Otto, M. W., Eisen, J. L., Weisberg, R. B., Pagano, M., et al. (2005). Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am. J. Psychiatry 162, 1179–1187. doi: 10.1176/appi.ajp.162.6.1179
Brush, C. J., Olson, R. L., Ehmann, P. J., Bocchine, A. J., Bates, M. E., Buckman, J. F., et al. (2019). Lower resting cardiac autonomic balance in young adults with current major depression. Psychophysiology 56:e13385.? doi: 10.1111/psyp.13385
Brydon, L., O'Donnell, K., Wright, C. E., Wawrzyniak, A. J., Wardle, J., and Steptoe, A. (2012). Circulating leptin and stress-induced cardiovascular activity in humans. Obesity 16, 2642–2647. doi: 10.1038/oby.2008.415
Burns, J. W., Friedman, R., and Katkin, E. S. (1992). Anger expression, hostility, anxiety, and patterns of cardiac reactivity to stress. Behav. Med. 18, 71–78. doi: 10.1080/08964289.1992.9935174
Butler, E. A. (2011). Temporal interpersonal emotion systems: the “TIES” that form relationships. Person. Soc. Psychol. Rev. 15, 367–393. doi: 10.1177/1088868311411164
Bylsma, L. M., Yaroslavsky, I., Rottenberg, J., Jennings, J. R., George, C. J., Kiss, E., et al. (2015). Juvenile onset depression alters cardiac autonomic balance in response to psychological and physical challenges. Biol. Psychol. 110, 167–174. doi: 10.1016/j.biopsycho.2015.07.003
Cacioppo, J. T., Berntson, G. G., Binkley, P. F., Quigley, K. S., Uchino, B. N., and Fieldstone, A. (1994). Autonomic cardiac control. II. noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology 31, 586–598.? doi: 10.1111/j.1469-8986.1994.tb02351.x
Cacioppo, J. T., Tassinary, L. G., and Berntson, G., (eds.). (2007). Handbook of Psychophysiology. Cambridge: Cambridge University Press.?
Campbell, M. A., and Rapee, R. M. (1994). The nature of feared outcome representations in children. J. Abnorm. Child Psychol. 22, 99–111. doi: 10.1007/BF02169258
Carrillo, E., Moya-Albiol, L., González-Bono, E., Salvador, A., Ricarte, J., and Gómez-Amor, J. (2001). Gender differences in cardiovascular and electrodermal responses to public speaking task: the role of anxiety and mood states. Int. J. Psychophysiol. 42, 253–264. doi: 10.1016/S0167-8760(01)00147-7
Chalmers, J. A., Quintana, D. S., Norment, T., Abbott, M. J.-A., Kemp, A. H., and Paulo, B. (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry 5:80. doi: 10.3389/fpsyt.2014.00080
Cho, S., White, K. H., Yang, Y., and Soto, J. A. (2019). The role of trait anxiety in the selection of emotion regulation strategies and subsequent effectiveness. Pers. Individ. Dif., 147, 326–331. doi: 10.1016/j.paid.2019.04.035
Christie, I. C., and Friedman, B. H. (2004). Autonomic specificity of discrete emotion and dimensions of affective space: a multivariate approach. Int. J. Psychophysiol. 51, 143–153. doi: 10.1016/j.ijpsycho.2003.08.002
Clark, L. A., and Watson, D. (1988). Mood and the mundane: relations between daily life events and self-reported mood. J. Pers. Soc. Psychol. 54, 296–308. doi: 10.1037/0022-3514.54.2.296
Clark, L. A., Watson, D., and Leeka, J. (1989). Diurnal variaiton in the positive affects. Motiv. Emot. 13, 205–234. doi: 10.1007/BF00995536
Codispoti, M., Surcinelli, P., and Baldaro, B. (2008). Watching emotional movies: affective reactions and gender differences. Int. J. Psychophysiol. 69, 90–95. doi: 10.1016/j.ijpsycho.2008.03.004
Cohen, J. R., Thomsen, K. N., Tu, K. M., Thakur, H., McNeil, S., and Menon, S. V. (2020). Cardiac autonomic functioning and post-traumatic stress: a preliminary study in youth at-risk for PTSD. Psychiatry Res. 284:112684.? doi: 10.1016/j.psychres.2019.112684
Consedine, N. S., Magai, C., Cohen, C. I., and Gillespie, M. (2002). Ethnic variation in the impact of negative emotion and emotion inhibition on the health of older adults. J. Gerontol. 57, 396–408. doi: 10.1093/geronb/57.5.P396
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., and Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry 60, 837–844. doi: 10.1001/archpsyc.60.8.837
Cribbet, M. R., Williams, P. G., Gunn, H. E., and Rau, H. K. (2011). Effects of tonic and phasic respiratory sinus arrhythmia on affective stress responses. Emotion 11, 188–193. doi: 10.1037/a0021789
Curhan, K. B., Sims, T., Markus, H. R., Kitayama, S., Karasawa, M., Kawakami, N., et al. (2014). Just how bad negative affect is for your health depends on culture. Psychol. Sci. 25, 2277–2280. doi: 10.1177/0956797614543802
Dart, A. M., Du, X. J., and Kingwell, B. A. (2002). Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res. 53, 678–687. doi: 10.1016/S0008-6363(01)00508-9
Dawson, M. E., Schell, A. M., and Filion, D. L. (2007). “The electrodermal system,” in Handbook of Psychophysiology, 3rd Edn, eds J. T. Cacioppo, L. G. Tassinary, and G. G. Berntson (New York, NY: Cambridge University Press), 159–181.
Demaree, H. A., and Everhart, D. E. (2004). Healthy high-hostiles: reduced parasympathetic activity and decreased sympathovagal flexibility during emotional processing. Pers. Individ. Dif. 36, 457–469. doi: 10.1016/S0191-8869(03)00109-0
Demaree, H. A., Pu, J., Robinson, J. L., Schmeichel, B. J., and Everhart, D. E. (2006). Predicting facial valence to stimuli from resting RSA: not a function of active emotion regulation. Cogn. Emot. 20, 161–176. doi: 10.1080/02699930500260427
Diamond, L. M., Hicks, A. M., and Otter-Henderson, K. D. (2011). Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Pers. Soc. Psychol. Bull. 37, 731–744. doi: 10.1177/0146167211400620
Diener, E., Larsen, R. J., Levine, S., and Emmons, R. A. (1985). Intensity and frequency: dimensions underlying positive and negative affect. J. Pers. Soc. Psychol. 48, 1253–1265. doi: 10.1037/0022-3514.48.5.1253
Dimitroff, S. J., Kardan, O., Necka, E. A., Decety, J., Berman, M. G., and Norman, G. J. (2017). Physiological dynamics of stress contagion. Sci. Rep. 7:6168. doi: 10.1038/s41598-017-05811-1
Dockray, S., and Steptoe, A. (2010). Positive affect and psychobiological processes. Neurosci. Biobehav. Rev., 35, 69–75. doi: 10.1016/j.neubiorev.2010.01.006
Doherty, R. W. (1997). The emotional contagion scale: a measure of individual differences. J. Nonverb. Behav. 21, 131–154. doi: 10.1023/A:1024956003661
Doherty, R. W., Orimoto, L., Singelis, T. M., Hatfield, E., and Hebb, J. (1995). Emotional contagion: gender and occupational differences. Psychol. Women Q. 19, 355–371. doi: 10.1111/j.1471-6402.1995.tb00080.x
Donner, N. C., and Lowry, C. A. (2013). Sex differences in anxiety and emotional behavior. Pflugers Archiv 465, 601–626. doi: 10.1007/s00424-013-1271-7
Drachen, A., Nacke, L. E., Yannakakis, G., and Pedersen, A. L. (2010). “Correlation between heart rate, electrodermal activity and player experience in first-person shooter games,” in Proceedings of the 5th ACM SIGGRAPH Symposium on Video Games, 49–54.
Elwood, L. S., Wolitzky-Taylor, K., and Olatunji, B. O. (2012). Measurement of anxious traits: a contemporary review and synthesis. Anxiety Stress Coping 25, 647–666. doi: 10.1080/10615806.2011.582949
Fairbairn, C. E., Sayette, M. A., Aalen, O. O., and Frigessi, A. (2015). Alcohol and emotional contagion: an examination of the spreading of smiles in male and female drinking groups. Clin. Psychol. Sci. 3, 686–701. doi: 10.1177/2167702614548892
Feldman, D. B., and Kaal, K. J. (2007). Vicarious trauma and assumptive worldview: beliefs about the world in acquaintances of trauma victims. Traumatology 13, 21–31. doi: 10.1177/1534765607305437
Finch, J. F., Baranik, L. E., Steven, Y. L., and West, G. (2012). Physical health, positive and negative affect, and personality: a longitudinal analysis. J. Res. Pers. 46, 537–545. doi: 10.1016/j.jrp.2012.05.013
Fishel, S. R., Muth, E. R., and Hoover, A. W. (2007). Establishing appropriate physiological baseline procedures for real-time physiological measurement. J. Cogn. Eng. Decis. Making 1, 286–308. doi: 10.1518/155534307X255636
Flanagan, D. E., Vaile, J. C., Petley, G. W., Phillips, D. I., Godsland, I. F., Owens, P., et al. (2007). Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regul. Pept. 140, 37–42. doi: 10.1016/j.regpep.2006.11.009
Fowles, D. C. (1986). “The eccrine system and electrodermal activity,” in psychophysiology: Systems, Processes, and Applications, eds M. G. H. Coles, E. Donchin, and S. W. Porges (New York, NY: Guilford), 51–96.
Frazier, T. W., Strauss, M. E., and Steinhauer, S. R. (2004). Respiratory sinus arrhythmia as an index of emotional response in young adults. Psychophysiology 41, 75–83. doi: 10.1046/j.1469-8986.2003.00131.x
Fredrickson, B. L., and Losada, M. F. (2005). Positive affect and the complex dynamics of human flourishing. Am. Psychol. 60, 678–686. doi: 10.1037/0003-066X.60.7.678
Friedman, B. H. (2007). An autonomic flexibility—neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 74, 85–199. doi: 10.1016/j.biopsycho.2005.08.009
Friedman, B. H., and Thayer, J. F. (1998). Anxiety and autonomic flexibility: a cardiovascular approach. Biol. Psychol. 47, 243–263. doi: 10.1016/S0301-0511(97)00027-6
Fu, P., Gibson, C. J., Mendes, W. B., Schembri, M., and Huang, A. J. (2018). Anxiety, depressive symptoms, and cardiac autonomic function in perimenopausal and postmenopausal women with hot flashes: a brief report. Menopause 25, 1470–1475. doi: 10.1097/GME.0000000000001153
Fujita, F., Diener, E., and Sandvik, E. (1991). Gender differences in negative affect and well-being: the case for emotional intensity. J. Pers. Soc. Psychol. 61, 427–434. doi: 10.1037/0022-3514.61.3.427
Fuller, B. F. (1992). The effects of stress-anxiety and coping styles on heart rate variability. Int. J. Psychophysiol. 12, 81–86. doi: 10.1016/0167-8760(92)90045-D
Futterman, A. D., Kemeny, M. E., Shapiro, D., Polonsky, W., and Fahey, J. L. (1992). Immunological variability associated with experimentally-induced positive and negative affective states. Psychol. Med. 22, 231–238. doi: 10.1017/S003329170003289X
Girgus, J. S., and Yang, K. (2015). Gender and depression. Curr. Opin. Psychol. 4, 53–60. doi: 10.1016/j.copsyc.2015.01.019
Gonzalez-Bono, E., Moya-Albiol, L., Salvador, A., Carrillo, E., Ricarte, J., and GomezAmor, J. (2002). Anticipatory autonomic response to a public speaking task in women – The role of trait anxiety. Biol. Psychol. 60, 37–49, doi: 10.1016/S0301-0511(02)00008-X
Gordon, I., Gilboa, A., Cohen, S., and Kleinfeld, T. (2020a). The relationship between physiological synchrony and motion energy synchrony during a joint group drumming task. Physiol. Behav. 224:113074. doi: 10.1016/j.physbeh.2020.113074
Gordon, I., Gilboa, A., Cohen, S., Milstein, N., Haimovich, N., Pinhasi, S., et al. (2020b). Physiological and behavioral synchrony predict group cohesion and performance. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-65670-1
Gordon, I., Wallot, S., and Berson, Y. (2021). Group-level physiological synchrony and individual-level anxiety predict positive affective behaviors during a group decision-making task. Psychophysiology 58:e13857. doi: 10.1111/psyp.13857
Grossman, M., and Wood, W. (1993). Sex differences in intensity of emotional experience: a social role interpretation. J. Pers. Soc. Psychol. 65, 1010–1022. doi: 10.1037/0022-3514.65.5.1010
Hall, J. A. (1978). Gender effects in decoding nonverbal cues. Psychol. Bull. 85, 845–857. doi: 10.1037/0033-2909.85.4.845
Hall, J. A. (1984). Nonverbal Sex Differences: Communication Accuracy and Expressive Style. Baltimore, MD: Johns Hopkins University Press.
Hatfield, E., Bensman, L., Thornton, P. D., and Rapson, R. L. (2014). New perspectives on emotional contagion: a review of classic and recent research on facial mimicry and contagion. Interpersona 8, 159–179. doi: 10.5964/ijpr.v8i2.162
Hatfield, E., Cacioppo, J. T., and Rapson, R. L. (1992). Primitive emotion contagion. Rev. Person. Soc. Psychol. 14, 151–177. doi: 10.1017/CBO9781139174138
Hatfield, E., Cacioppo, J. T., and Rapson, R. L. (1993). Emotional contagion. Curr. Direct. Psychol. Sci. 2, 96–100. doi: 10.1111/1467-8721.ep10770953
Hatfield, E., Cacioppo, J. T., and Rapson, R. L. (1994). “Emotional contagion,” in Studies in Emotion and Social Interaction (Cambridge: Cambridge University Press).
Hatfield, E., Rapson, R. L., and Le, Y.-C. L. (2009). “Emotional contagion and empathy,” in The Social Neuroscience of Empathy, eds J. Decety, and W. Ickes (Cambridge, MA: MIT Press), 19–30.
Hatfield, E., Rapson, R. L., and Le, Y. C. L. (2011). “Emotional contagion and empathy,” in The Social Neuroscience of Empathy, ed J. Decety, and W. Ickes (MIT Press), 19–30.
Haviland, J. J., and Malatesta, C. Z. (1981). “The development of sex differences in nonverbal signals: fallacies, facts, and fantasies,” in Gender and Nonverbal Behavior, Springer Series in Social Psychology, eds C. Mayo and N. M. Henley (New York, NY: Springer).
Heponiemi, T., Ravaja, N., Elovainio, M., Näätänen, P., and Keltikangas-Järvinen, L. (2006). Experiencing positive affect and negative affect during stress: relationships to cardiac reactivity and to facial expressions. Scand. J. Psychol. 47, 327–337. doi: 10.1111/j.1467-9450.2006.00527.x
Herbert, J., Moore, G., De La Riva, C., and Watts, F. (1986). Endocrine responses and examination anxiety. Biol. Psychol. 22, 215–226. doi: 10.1016/0301-0511(86)90027-X
Hinojosa-Laborde, C., Chapa, I., Lange, D., and Haywood, J. R. (1999). Gender differences in sympathetic nervous system regulation. Clin. Exp. Pharmacol. Physiol. 26, 122–126. doi: 10.1046/j.1440-1681.1999.02995.x
Hirschfeld, R. M. A. (2001). The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim. Care Comp. J. Clin. Psychiatry 3, 244–254. doi: 10.4088/PCC.v03n0609
Hoehn-Saric, R., and McLeod, D. R. (1988). The peripheral sympathetic nervous system. Its role in normal and pathologic anxiety. Psychiatr. Clin. N. Am. 11, 375–386. doi: 10.1016/S0193-953X(18)30504-5
Hohnloser, S. H. (2005). Ventricular arrhythmias: antiadrenergic therapy for the patient with coronary artery disease. J. Cardiovasc. Pharmacol. Ther. 10, S23–31. doi: 10.1177/10742484050100i404
Horesh, D., Hasson-Ohayon, I., and Harwood-Gross, A. (2021). The contagion of psychopathology across different psychiatric disorders: a comparative theoretical analysis. Brain Sci. 12:67. doi: 10.3390/brainsci12010067
Huwe, S., Hennig, J., and Netter, P. (1998). Biological, emotional, behavioral, and coping reactions to examination stress in high and low state anxious subjects. Anxiety Stress Coping 11, 47–65. doi: 10.1080/10615809808249313
Hyde, J. S. (2014). Gender similarities and differences. Annu. Rev. Psychol. 65, 373–398. doi: 10.1146/annurev-psych-010213-115057
Hyde, J. S., Fennema, E., and Lamon, S. J. (1990). Gender differences in mathematics performance: a meta-analysis. Psychol. Bull. 107, 139–155. doi: 10.1037/0033-2909.107.2.139
Jansen, A. S., Nguyen, X. V., Karpitskiy, V., Mettenleiter, T. C., and Loewy, A. D. (1995). Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270, 644–646. doi: 10.1126/science.270.5236.644
Jönsson, P. (2007). Respiratory sinus arrythmia as a function of state anxiety in healthy individuals. Int. J. Psychophysiol. 63, 48–54. doi: 10.1016/j.ijpsycho.2006.08.002
Kantor, L., Endler, N. S., Heslegrave, R. J., and Kocovski, N. L. (2001). Validating self-report measures of state and trait anxiety against a physiological measure. Curr. Psychol., 20, 207–215. doi: 10.1007/s12144-001-1007-2
Kelsey, R. M. (2012). “Beta-adrenergic cardiovascular reactivity and adaptation to stress: the cardiac pre-ejection period as an index of effort,” in How Motivation Affects Cardiovascular Response: Mechanisms and Applications, eds R. A. Wright, and G. H. E. Gendolla (Washington, DC: American Psychological Association), 43–60.
Kessler, R. C., McGonagle, K. A., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., et al. (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the united states: results from the national comorbidity survey. Arch. Gen. Psychiatry 51, 8–19. doi: 10.1001/archpsyc.1994.03950010008002
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., and Nelson, C. B. (1995). Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry 52, 1048–1060. doi: 10.1001/archpsyc.1995.03950240066012
Kettunen, J., Ravaja, N., Naatanen, P., and Keltikangas-Jarvinen, L. (2000). The relationship of respiratory sinus arrhythmia to the co-activation of autonomic and facial responses during the Rorschach test. Psychophysiology 37, 242–250. doi: 10.1111/1469-8986.3720242
Kiecolt-Glaser, J. K., McGuire, L., Robles, T. F., and Glaser, R. (2002). Psychoneuroimmunology: psychological influences on immune function and health. J. Consult. Clin. Psychol. 70, 537–547. doi: 10.1037/0022-006X.70.3.537
Knapp, P. H., Levy, E. M., Giorgi, R. G., Black, P. H., Fox, B. H., and Heeren, T. C. (1992). Short-term immunological effects of induced emotion. Psychosom. Med. 54, 133–148. doi: 10.1097/00006842-199203000-00002
Koenig, J., Abler, B., Agartz, I., Åkerstedt, T., Andreassen, O. A., Anthony, M., et al. (2021). Cortical thickness and resting-state cardiac function across the lifespan: a cross-sectional pooled mega-analysis. Psychophysiology 58:e13688. doi: 10.1111/psyp.13688
Kong, F., Wen, W., Liu, G., Xiong, R., and Yang, X. (2022). Autonomic nervous pattern analysis of trait anxiety. Biomed. Signal Process. Control 71, 103129. doi: 10.1016/j.bspc.2021.103129
Kop, W. J., Synowski, S. J., Newell, M. E., Schmidt, L. A., Waldstein, S. R., and Fox, N. A. (2011). Autonomic nervous system reactivity to positive and negative mood induction: the role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 86, 230–238. doi: 10.1016/j.biopsycho.2010.12.003
Kossowsky, J., Wilhelm, F. H., Roth, W. T., and Schneider, S. (2012). Separation anxiety disorder in children: disorder-specific responses to experimental separation from the mother. J. Child Psychol. Psychiatry 53, 178–187. doi: 10.1111/j.1469-7610.2011.02465.x
Kreibig, S. D., Wilhelm, F. H., Roth, W. T., and Gross, J. J. (2007). Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology 44, 787–806. doi: 10.1111/j.1469-8986.2007.00550.x
Kubzansky, L. D., and Kawachi, I. (2000). Going to the heart of the matter: do negative emotions cause coronary heart disease? J. Psychosom. Res. 48, 323–337. doi: 10.1016/S0022-3999(99)00091-4
Kubzansky, L. D., and Thurston, R. C. (2007). Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Arch. Gen. Psychiatry 64, 1393–1401. doi: 10.1001/archpsyc.64.12.1393
Kushki, A., Drumm, E., Pla Mobarak, M., Tanel, N., Dupuis, A., Chau, T., et al. (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE 8:e59730. doi: 10.1371/journal.pone.0059730
Lai, J. C., Evans, P. D., Ng, S. H., Chong, A. M., Siu, O. T., Chan, C. L., et al. (2005). Optimism, positive affectivity, and salivary cortisol. Br. J. Health Psychol. 10(Pt 4), 467–484. doi: 10.1348/135910705X26083
Levenson, R. W., Carstensen, L. L., and Gottman, J. M. (1994). Influence of age and gender on affect, physiology, and their interrelations: A Study of long-term marriages. J. Pers. Soc. Psychol. 67, 56–68. doi: 10.1037/0022-3514.67.1.56
Lewinsohn, P. M., Gotlib, I. H., Lewinsohn, M., Seeley, J. R., and Allen, N. B. (1998). Gender differences in anxiety disorders and anxiety symptoms in adolescents. J. Abnorm. Psychol. 107, 109–117. doi: 10.1037/0021-843X.107.1.109
Limbu, N., Sinha, R., Sinha, M., and Paudel, B. H. (2010). Gender difference in electroencephalographic and anxiety status in response to single bout of physical exercise. Health Renaiss. 8, 152–157. doi: 10.3126/hren.v8i3.4207
Líndal, E., and Stefánsson, J. G. (1993). The lifetime prevalence of anxiety disorders in Iceland as estimated by the US National Institute of Mental Health Diagnostic Interview Schedule. Acta Psychiatr. Scand. 88, 29–34. doi: 10.1111/j.1600-0447.1993.tb03410.x
Machin, D., Campbell, M. J., Fayers, P., and Pinol, A. (1987). Sample Size Tables for Clinical Studies. Oxford: Blackwell Science Ltd.
Magen, E., and Konasewich, P. A. (2011). Women support providers are more susceptible than men to emotional contagion following brief supportive interactions. Psychol. Women Q. 35, 611–616. doi: 10.1177/0361684311423912
Malik, M., Bigger, J. T., Camm, A. J., Kleiger, R. E., Malliani, A., Moss, A. J., et al. (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381.? doi: 10.1093/oxfordjournals.eurheartj.a014868
Manera, V., Grandi, E., and Colle, L. (2013). Susceptibility to emotional contagion for negative emotions improves detection of smile authenticity. Front. Hum. Neurosci. 7:6. doi: 10.3389/fnhum.2013.00006
Marteau, T. M., and Bekker, H. (1992). The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 31, 301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x
Mayo, O., Horesh, D., Korisky, A., Milstein, N., Zadok, E., Tomashin, A., et al. (2023). I feel you: prepandemic physiological synchrony and emotional contagion during COVID-19. Emotion. 23, 753–763. doi: 10.1037/emo0001122
McLean, C. P., Asnaani, A., Litz, B. T., and Hofmann, S. G. (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45, 1027–1035. doi: 10.1016/j.jpsychires.2011.03.006
Meeks, S., and Murrell, S. A. (2001). Contribution of education to health and life satisfaction in older adults mediated by negative affect. J. Aging Health 13, 92–119. doi: 10.1177/089826430101300105
Mehrabian, A., and Russell, J. A. (1974). An Approach to Environmental Psychology. Cambridge, MA: MIT.
Miu, A. C., Heilman, R. M., and Miclea, M. (2009). Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton. Neurosci. 145, 99–103, doi: 10.1016/j.autneu.2008.11.010
Miyamoto, Y., Morozink Boylon, J., Coe, C. L., Curhan, K. B., Levine, C. S., Markus, H. R., et al. (2013). Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain Behav. Immun. 34, 79–85. doi: 10.1016/j.bbi.2013.07.173
Moodithaya, S., and Avadhany, S. T. (2012). Gender differences in age-related changes in cardiac autonomic nervous function. J. Aging Res. 2012:679345. doi: 10.1155/2012/679345
Muris, P., and Ollendick, T. H. (2002). The assessment of contemporary fears in adolescents using a modified version of the Fear Survey Schedule for Children-Revised. J. Anxiety Disord. 16, 567–584. doi: 10.1016/S0887-6185(02)00106-8
Nabi, H., Kivimaki, M., De Vogli, R., Marmot, M. G., and Singh-Manoux, A. (2008). Positive and negative affect and risk of coronary heart disease: Whitehall II prospective cohort study. BMJ 30:337a118. doi: 10.1136/bmj.a118
Neumann, S. A., and Waldstein, S. R. (2001). Similar patterns of cardiovascular response during emotional activation as a function of affective valence and arousal and gender. J. Psychosom. Res. 50, 245–253. doi: 10.1016/S0022-3999(01)00198-2
Neves, L., Cordeiro, C., Scott, S. K., Castro, S. L., and Lima, C. F. (2018). High emotional contagion and empathy are associated with enhanced detection of emotional authenticity in laughter. Q. J. Exp. Psychol. 71, 2355–2363. doi: 10.1177/1747021817741800
Noah, J. A., Ono, Y., Shimada, S., Tachibana, A., and Bronner, S. (2015). Changes in sympathetic tone during cooperative game play. Soc. Behavi. Person. 43, 1123–1134. doi: 10.2224/sbp.2015.43.7.1123
Nolen-Hoeksema, S. (2001). Gender differences in depression. Curr. Dir. Psychol. Sci. 10, 173–176. doi: 10.1111/1467-8721.00142
Obradović, J., and Boyce, W. T. (2012). Developmental psychophysiology of emotion processes. Monogr. Soc. Res. Child Dev. 77, 120–128.? doi: 10.1111/j.1540-5834.2011.00670.x
Oveis, C., Gruber, J., Haidt, J., Cohen, A. B., Shiota, M. N., and Keltner, D. (2009). Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion 9, 265–270. doi: 10.1037/a0015383
Pace-Schott, E. F., Amole, M. C., Aue, T., Balconi, M., Bylsma, L. M., Critchley, H., et al. (2019). Physiological feelings. Neurosci. Biobehav. Rev. 103, 267–304.? doi: 10.1016/j.neubiorev.2019.05.002
Park, S., Choi, S. J., Mun, S., and Whang, M. C. (2019). Measurement of emotional contagion using synchronization of heart rhythm pattern between two persons: application to sales managers and sales force synchronization. Physiol. Behav. 200, 148–158. doi: 10.1016/j.physbeh.2018.04.022
Picard, R. W., Fedor, S., and Ayzenberg, Y. (2016). Multiple arousal theory and daily-life electrodermal activity asymmetry. Emot. Rev. 8, 62–75. doi: 10.1177/1754073914565517
Piccinelli, M., and Wilkinson, G. (2000). Gender differences in depression: critical review. Br. J. Psychiatry 177, 486–492. doi: 10.1192/bjp.177.6.486
Pollatos, O., Herbert, B. M., Kaufmann, C., Auer, D. P., and Schandry, R. (2007). Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. Int. J. Psychophysiol. 65, 167–173. doi: 10.1016/j.ijpsycho.2007.03.005
Porges, S. W. (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 42, 123–146. doi: 10.1016/S0167-8760(01)00162-3
Porges, S. W. (2007). The polyvagal perspective. Biol. Psychol. 74, 116–143.? doi: 10.1016/j.biopsycho.2006.06.009
Pothineni, N. V., Shirazi, L. F., and Mehta, J. L. (2016). Gender differences in autonomic control of the cardiovascular system. Curr. Pharm. Des. 22, 3829–3834. doi: 10.2174/1381612822666160518125810
Poulton, R., Milne, B. J., Craske, M. G., and Menzies, R. G. (2001). A longitudinal study of the etiology of separation anxiety. Behav. Res. Ther. 39, 1395–1410. doi: 10.1016/S0005-7967(00)00105-4
Powell, R., Johnston, M., and Johnston, D. W. (2008). The effects of negative affectivity on self-reported activity limitations in stroke patients: testing the symptom perception, disability and psychosomatic hypotheses. Psychol. Health 23, 195–206. doi: 10.1080/14768320701204153
Pressman, S. D., and Cohen, S. (2005). Does positive affect influence health? Psychol. Bull. 131, 925–971. doi: 10.1037/0033-2909.131.6.925
Pressman, S. D., Gallagher, M. W., and Lopez, S. J. (2013). Is the emotion-health connection a “first-world problem”? Psychol. Sci. 24, 544–549. doi: 10.1177/0956797612457382
Qasim, M. S., Bari, D. S., and Martinsen, Ø. G. (2022). Influence of ambient temperature on tonic and phasic electrodermal activity components. Physiol. Meas. 43:ac72f4. doi: 10.1088/1361-6579/ac72f4
Ramaekers, D., Ector, H., Aubert, A. E., Rubens, A., and Van de Werf, F. (1998). Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur. Heart J. 19, 1334–1341. doi: 10.1053/euhj.1998.1084
Ringeisen, T., Lichtenfeld, S., Becker, S., and Minkley, N. (2019). Stress experience and performance during an oral exam: the role of self-efficacy, threat appraisals, anxiety, and cortisol. Anxiety Stress Coping 32, 50–66. doi: 10.1080/10615806.2018.1528528
Rohrmann, S., Hopp, H., and Quirin, M. (2008). Gender differences in psychophysiological responses to disgust. J. Psychophysiol. 22, 65–75. doi: 10.1027/0269-8803.22.2.65
Roos, A. L., Goetz, T., Krannich, M., Donker, M., Bieleke, M., Caltabiano, A., et al. (2022). Control, anxiety and test performance: self-reported and physiological indicators of anxiety as mediators. Br. J. Educ. Psychol. 93, 72–89. doi: 10.1111/bjep.12536
Russell, J. A. (1980). A circumplex model of affect. J. Pers. Soc. Psychol. 39, 1161–1178. doi: 10.1037/h0077714
Russell, J. A. (1983). Pancultural aspects of the human conceptual organization of emotions. J. Pers. Soc. Psychol. 45, 1281–1288. doi: 10.1037/0022-3514.45.6.1281
Russell, J. A., Weiss, A., and Mendelsohn, G. A. (1989). Affect Grid: a single-item scale of pleasure and arousal. J. Pers. Soc. Psychol. 37, 493–502. doi: 10.1037/0022-3514.57.3.493
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. doi: 10.1210/edrv.21.1.0389
Saxbe, D., and Repetti, R. L. (2010). For better or worse? Coregulation of couples' cortisol levels and mood states. J. Person. Soc. Psychol. 98, 92–103. doi: 10.1037/a0016959
Schoenewolf, G. (1990). Emotional contagion: behavioral induction in individuals and groups. Mod. Psychoanal. 15, 49–61.
Schwartz, P., La Rovere, M., and Vanoli, E. (1992). Autonomic nervous system and sudden cardiac death: experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation 85, 177–191.
Seddon, J. A., Rodriguez, V. J., Provencher, Y., Raftery-Helmer, J., Hersh, J., Labelle, P. R., et al. (2020). Meta-analysis of the effectiveness of the Trier Social Stress Test in eliciting physiological stress responses in children and adolescents. Psychoneuroendocrinology 116:104582. doi: 10.1016/j.psyneuen.2020.104582
Sege, C. T., Bradley, M. M., and Lang, P. J. (2018). Avoidance and escape: defensive reactivity and trait anxiety. Behav. Res. Ther. 104, 62–68. doi: 10.1016/j.brat.2018.03.002
Sherwood, A., Allen, M. T., Fahrenberg, J., Kelsey, R. M., Lovallo, W. R., and Van Doornen, L. J. (1990). Methodological guidelines for impedance cardiography. Psychophysiology 27, 1–23.? doi: 10.1111/j.1469-8986.1990.tb02171.x
Shields, S., MacDowell, K. A., Fairchild, S. B., and Campbell, M. L. (1987). Is meditation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology 24, 312–319. doi: 10.1111/j.1469-8986.1987.tb00301.x
Shiota, M. N., Neufeld, S. L., Yeung, W. H., Moser, S. E., and Perea, E. F. (2011). Feeling good: autonomic nervous system responding in five positive emotions. Emotion 11, 1368–1378. doi: 10.1037/a0024278
Smith, T. W., Houston, B. K., and Zurawski, R. M. (1984). Finger pulse volume as a measure of anxiety in response to evaluative threat. Psychophysiology 21, 260–264. doi: 10.1111/j.1469-8986.1984.tb02932.x
Sonnby-Borgström, M., Jönsson, P., and Svensson, O. (2008). Gender differences in facial imitation and verbally reported emotional contagion from spontaneous to emotionally regulated processing levels. Scand. J. Psychol. 49, 111–122. doi: 10.1111/j.1467-9450.2008.00626.x
Spangler, G., Pekrun, R., Kramer, K., and Hofmann, H. (2002). Students' emotions, physiological reactions, and coping in academic exams. Anxiety Stress Coping 15, 413–432. doi: 10.1080/1061580021000056555
Sperry, S. H., Kwapil, T. R., Eddington, K. M., and Silvia, P. J. (2018). Psychopathology, everyday behaviors, and autonomic activity in daily life: an ambulatory impedance cardiography study of depression, anxiety, and hypomanic traits. Int. J. Psychophysiol. 129, 67–75. doi: 10.1016/j.ijpsycho.2018.04.008
Spielberger, C. D. (1966). “Theory and research on anxiety,” in Anxiety and Behavior, ed C. D. Spielberger (San Diego, CA: Academic Press), 3–20.
Spielberger, C. D., Gorsuch, R. L., and Lushene, R. E. (1970). Manualfor the State-Trait Anxieg Inventov (Self-Evaluation Questionnaire). Palo Alto, CA: Consulting Psychologists Press.
Spoor, J. R., and Kelly, J. R. (2004). The evolutionary significance of affect in groups: communication and group bonding. Group Process. Intergr. Relat. 7, 398–412. doi: 10.1177/1368430204046145
Stellar, J. E., Cohen, A., Oveis, C., and Keltner, D. (2015). Affective and physiological responses to the suffering of others: compassion and vagal activity. J. Pers. Soc. Psychol. 108, 572–585. doi: 10.1037/pspi0000010
Steptoe, A., Dockray, S., and Wardle, J. (2009). Positive affect and psychobiological processes relevant to health. J. Pers. 77, 1747–1776. doi: 10.1111/j.1467-6494.2009.00599.x
Stone, A. A. (1981). The association between perceptions of daily experiences and self- and spouse-rated mood. J. Res. Person. 5, 510–522. doi: 10.1016/0092-6566(81)90047-7
Stone, L. B., McCormack, C. C., and Bylsma, L. M. (2020). Cross system autonomic balance and regulation: associations with depression and anxiety symptoms. Psychophysiology 57:e13636.? doi: 10.1111/psyp.13636
Strohmaier, A. R., Schiepe-Tiska, A., and Reiss, K. M. (2020). A comparison of self-reports and electrodermal activity as indicators of mathematics state anxiety.: An application of the control-value theory. Front. Learn. Res. 8, 16–32. doi: 10.14786/flr.v8i1.427
Suls, J., and Bunde, J. (2005). Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective disorders. Psychol. Bull. 131, 260–300. doi: 10.1037/0033-2909.131.2.260
Surakka, V., and Hietanen, J. K. (1998). Facial and emotional reactions to Duchenne and non-Duchenne smiles. Int. J. Psychophysiol. 29, 23–33. doi: 10.1016/S0167-8760(97)00088-3
Thayer, J. F., and Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 61, 201–216.? doi: 10.1016/S0165-0327(00)00338-4
Thomsen, D. K., Mehlsen, M. Y., Viidik, A., Sommerlund, B., and Zachariae, R. (2005). Age and gender differences in negative affect–Is there a role for emotion regulation? Pers. Individ. Dif. 38, 1935–1946. doi: 10.1016/j.paid.2004.12.001
Tracy, L. M., and Giummarra, M. J. (2017). Sex differences in empathy for pain: what is the role of autonomic regulation? Psychophysiology 54, 1549–1558. doi: 10.1111/psyp.12895
Venables, P. H., and Mitchell, D. A. (1996). The effects of age, sex and time of testing on skin conductance activity. Biol. Psychol. 43, 87–101. doi: 10.1016/0301-0511(96)05183-6
Verkuil, B., Brosschot, J. F., and Thayer, J. F. (2014). Cardiac reactivity to and recovery from acute stress: temporal associations with implicit anxiety. Int. J. Psychophysiol. 92, 85–91. doi: 10.1016/j.ijpsycho.2014.03.002
Volante, M., Babu, S. V., Chaturvedi, H., Newsome, N., Ebrahimi, E., Roy, T., et al. (2016). Effects of virtual human appearance fidelity on emotion contagion in affective inter-personal simulations. IEEE Trans. Vis. Comput. Graph. 22, 1326–1335. doi: 10.1109/TVCG.2016.2518158
Wang, Z., Lü, W., and Qin, R. (2013). Respiratory sinus arrhythmia is associated with trait positive affect and positive emotional expressivity. Biol. Psychol. 93, 190–196. doi: 10.1016/j.biopsycho.2012.12.006
Waters, S. F., West, T. V., Karnilowicz, H. R., and Mendes, W. B. (2017). Affect contagion between mothers and infants: examining valence and touch. J. Exp. Psychol. 146, 1043–1051. doi: 10.1037/xge0000322
Waters, S. F., West, T. V., and Mendes, W. B. (2014). Stress contagion: physiological covariation between mothers and infants. Psychol. Sci. 25 934–942. doi: 10.1177/0956797613518352
Watson, D., Clark, L. A., and Tellegen, A. (1984). Cross-cultural convergence in the structure of mood: AJapanese replication and a comparison with U. S. findings. J. Person. Soc. Psychol. 47, 127–144. doi: 10.1037/0022-3514.47.1.127
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
Watson, D., and Pennebaker, J. W. (1989). Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol. Rev. 96, 234–254. doi: 10.1037/0033-295X.96.2.234
Weissman, M. M., and Merikangas, K. R. (1986). The epidemiology of anxiety and panic disorders: an update. J. Clin. Psychiatry 47(Suppl.), 11–17.
Whitley, E., and Ball, J. (2002). Statistics review 4: sample size calculations. Crit. Care 6:335. doi: 10.1186/cc1521
Wild, B., Erb, M., and Bartels, M. (2001). Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: quality, quantity, time course and gender differences. Psychiatry Res. 102, 109–124. doi: 10.1016/S0165-1781(01)00225-6
Zevon, M. A., and Tellegen, A. (1982). The structure of mood change: an idiographic/nomothetic analysis. J. Pers. Soc. Psychol. 43, 111–122. doi: 10.1037/0022-3514.43.1.111
Zhou, A. M., Morales, S., Youatt, E. A., and Buss, K. A. (2022). Autonomic nervous system activity moderates associations between temperament and externalizing behaviors in early childhood. Dev. Psychobiol. 64:e22323. doi: 10.1002/dev.22323
Keywords: cardiac autonomic balance (CAB), cardiac autonomic regulation (CAR), cross-system autonomic balance (CSAB), cross-system autonomic regulation (CSAR), respiratory sinus arrythmia (RSA), electrodermal activity (EDA), pre-ejection period (PEP), gender
Citation: Menashri Sinai Y, Ma YXJ, Abba Daleski M, Gannot S, Bartsch RP and Gordon I (2024) Unveiling gender differences in psychophysiological dynamics: support for a two-dimensional autonomic space approach. Front. Hum. Neurosci. 18:1363891. doi: 10.3389/fnhum.2024.1363891
Received: 31 December 2023; Accepted: 09 February 2024;
Published: 13 March 2024.
Edited by:
Yaara Yeshurun, Tel Aviv University, IsraelReviewed by:
Enrica Laura Santarcangelo, University of Pisa, ItalyCopyright © 2024 Menashri Sinai, Ma, Abba Daleski, Gannot, Bartsch and Gordon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilanit Gordon, aWxhbml0LmdvcmRvbkBiaXUuYWMuaWw=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.