- 1Research Center for Applied Mathematics and Machine Intelligence, Research Institute of Basic Theories, Zhejiang Lab, Hangzhou, China
- 2Department of Psychology, School of Humanities and Social Sciences, Beihang University, Beijing, China
- 3Department of Rehabilitation, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 4Department of Neurology, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Children with attention deficit hyperactivity disorder (ADHD) exhibit pervasive difficulties in speech perception. Given that speech processing involves both acoustic and linguistic stages, it remains unclear which stage of speech processing is impaired in children with ADHD. To investigate this issue, we measured neural tracking of speech at syllable and word levels using electroencephalography (EEG), and evaluated the relationship between neural responses and ADHD symptoms in 6–8 years old children. Twenty-three children participated in the current study, and their ADHD symptoms were assessed with SNAP-IV questionnaires. In the experiment, the children listened to hierarchical speech sequences in which syllables and words were, respectively, repeated at 2.5 and 1.25 Hz. Using frequency domain analyses, reliable neural tracking of syllables and words was observed in both the low-frequency band (<4 Hz) and the high-gamma band (70–160 Hz). However, the neural tracking of words in the high-gamma band showed an anti-correlation with the ADHD symptom scores of the children. These results indicate that ADHD prominently impairs cortical encoding of linguistic information (e.g., words) in speech perception.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is a common childhood mental disorder characterized by attention deficit, hyperactivity and/or impulsivity, and cognitive dysfunction (Mueller et al., 2017; Katya, 2018). Extensive evidence has demonstrated that children with ADHD exhibit difficulties in speech perception, e.g., recognizing speech in noisy environments or extracting target speech from competing speakers (Davidson and Prior, 1978; Keith et al., 1989; Cook et al., 1993; Pillsbury et al., 1995; Geffner et al., 1996; Gomez and Condon, 1999; Schafer et al., 2013; Lanzetta-Valdo et al., 2017; Blomberg et al., 2019). Given that speech perception is a complex process involving both low-level acoustic encoding and high-level linguistic processing, it remains unclear which stage of speech processing is prominently impaired in children with ADHD.
On the one hand, it has been hypothesized that children with ADHD have difficulties in neural encoding of acoustic features, especially for a complex auditory scene consisting of multiple acoustic distractors. Evidence shows that children with ADHD (vs. healthy control children) have reduced neural responses to target sounds under distractors (Loiselle et al., 1980; Jonkman et al., 1997; Gomes et al., 2012). It is also found that children with ADHD exhibit impairments in motor synchrony of acoustic rhythm (Rubia et al., 2001; Toplak and Tannock, 2005; Ben-Pazi et al., 2006; Puyjarinet et al., 2017), which is associated with the compromised neural encoding of the auditory envelope (Tierney and Kraus, 2013; Carr et al., 2014). Specifically, acoustic rhythm is carried by the auditory envelope (i.e., the temporal fluctuations of sound power), which captures acoustic information about duration, tempo, and stress (Kotz et al., 2018; Myers et al., 2019; Poeppel and Assaneo, 2020). When the envelope is corrupted in speech, neural encoding of the auditory envelope degrades (Doelling et al., 2014), and speech intelligibility drops (Ghitza, 2012). Since the auditory envelope provides important cues for syllabic boundaries in speech (Giraud and Poeppel, 2012; Ding et al., 2014; Poeppel and Assaneo, 2020), the neural encoding of the auditory envelope might reflect an intermediate neural process to link the auditory representation of acoustic speech features and phonological representation of syllables, and therefore play a critical role in speech perception (Giraud and Poeppel, 2012; Poeppel and Assaneo, 2020).
On the other hand, speech perception entails not only acoustic encoding, but also higher-level linguistic processing. Since children with ADHD usually suffer from co-occurring linguistic impairments (Seidman et al., 1998; Cohen et al., 2000; Litovsky, 2015; Mueller et al., 2017; Randell et al., 2019), it has also been hypothesized that degraded linguistic processing induces difficulties in speech perception for children with ADHD (Bellani et al., 2011; Laffere et al., 2021). This hypothesis is motivated by two sides of observations. First, ADHD children do not always show behavioral impairments in acoustic detection performance (Satterfield et al., 1990; Rothenberger et al., 2000; van Mourik et al., 2011; Michalek et al., 2014). Accordingly, electrophysiological evidence also finds comparable neural responses to target sounds (Rothenberger et al., 2000) and acoustic envelope (Laffere et al., 2021) between children with and without ADHD. Second, ADHD children exhibit significantly reduced word identification and speech discrimination ability compared with typically developing children (Norrelgen et al., 1999; Fuermaier et al., 2018). Moreover, abnormal neural activity and connectivity are also observed in ADHD children during word identification (Murias et al., 2007). All these findings suggest that ADHD might impair higher-level linguistic processing beyond low-level acoustic encoding in speech perception.
Despite converging evidence for neurocognitive deficits in speech perception for children with ADHD, few studies have analyzed the underlying neural mechanisms across multiple processing stages. One methodological challenge is the difficulty in dissociating neural representations of multiple stages in speech processing. Here, we adopted a hierarchical auditory linguistic sequence paradigm to quantify the neural responses tracking two levels of speech units, i.e., syllables and words (Ding et al., 2018; Jin et al., 2020). The rationale is that cortical activity tracks different levels of speech units during speech perception (Luo and Poeppel, 2007; Ding et al., 2016, 2018; Gao et al., 2020). When syllables and words are presented at a unique and constant rate in speech, the neural tracking responses to syllables and words are tagged at distinct frequencies (Ding et al., 2016). Neural tracking of syllables and words can reflect two stages of speech processing: Neural tracking of syllables is interconnected to acoustic encoding of speech features, i.e., speech envelope (Giraud and Poeppel, 2012; Poeppel and Assaneo, 2020), and neural tracking of words reflects higher-level linguistic processing (Martin and Doumas, 2017; Jin et al., 2020; Meyer et al., 2020; Lu et al., 2022). The locus of the ADHD effects is important because it can offer effective guidance for how the remediation should be targeted to ADHD children who have difficulty in speech perception (Laffere et al., 2021). The goal of the current study is twofold. First, we explored whether cortical activity of young children could track syllables and words in a continuous speech stream. Six-eight years old children with mild ADHD symptoms were recruited to take part in a speech detection task, and their neural responses were recorded using electroencephalogram (EEG). Second, we investigated whether ADHD symptoms are correlated with the attenuation in the neural tracking of syllables and words. The ADHD symptoms were collected from the children using the SNAP-IV Questionnaire, and the neural correlates of individual symptoms were analyzed in both the low-frequency and high-gamma bands.
2. Materials and methods
2.1. Participants
The study was approved by the Ethics Committee of Children’s Hospital of Zhejiang University (Approval number: 2020-IRBAL-023). For this study, 23 drug-naïve children (6–8 years old, mean 7.3 years old; 15 male) were recruited from Children’s Hospital of Zhejiang University. All children were referred for participation in the study by teachers or parents, and had a diagnosis of ADHD by a pediatrician. All children were right-handed, with no self-reported hearing loss or neurological disorders. Written informed consent was obtained before the experiment. Parents of these children completed the Chinese version of SNAP-IV ADHD Questionnaire (Gau et al., 2008). The SNAP-IV was designed to evaluate ADHD symptoms of children without serious comorbid conditions (Swanson et al., 2012; Zieff et al., 2022). The checklist contained three subsets of atypical behavior: inattention (INATT, nine items), hyperactivity/impulsivity (HYP/IMP, nine items), and oppositional defiant behaviors (ODD, eight items). Each item was graded from 0 to 3, with a higher score indicating an increased level of ADHD symptoms. The subscale scores were calculated by averaging scores of items within each subset.
2.2. Stimuli and procedures
The speech was created as an isochronous sequence of independent Chinese syllables, and every two syllables constituted a noun word (Figure 1A). Fifty common bisyllabic words were selected from a dataset of daily speech materials for 3–5 years old children (Liu et al., 2008), with a probe word “grandma.” Each syllable was independently synthesized using the Neospeech synthesizer (the male voice, Liang).1 All syllables were adjusted to the same intensity and duration (i.e., 400 ms) following the procedure in Ding et al. (2016). Each speech sequence contained 20 syllables (i.e., 10 disyllabic words), and the syllables were concatenated sequentially without any acoustic gap inserted. Therefore, the trial duration was 8 s with syllables and words presented at 2.5 and 1.25 Hz, respectively. In total, 35 trials were created. All disyllabic words were randomly distributed in the 35 trials, and each word was repeated seven times with no repeated words presented in the same trial.
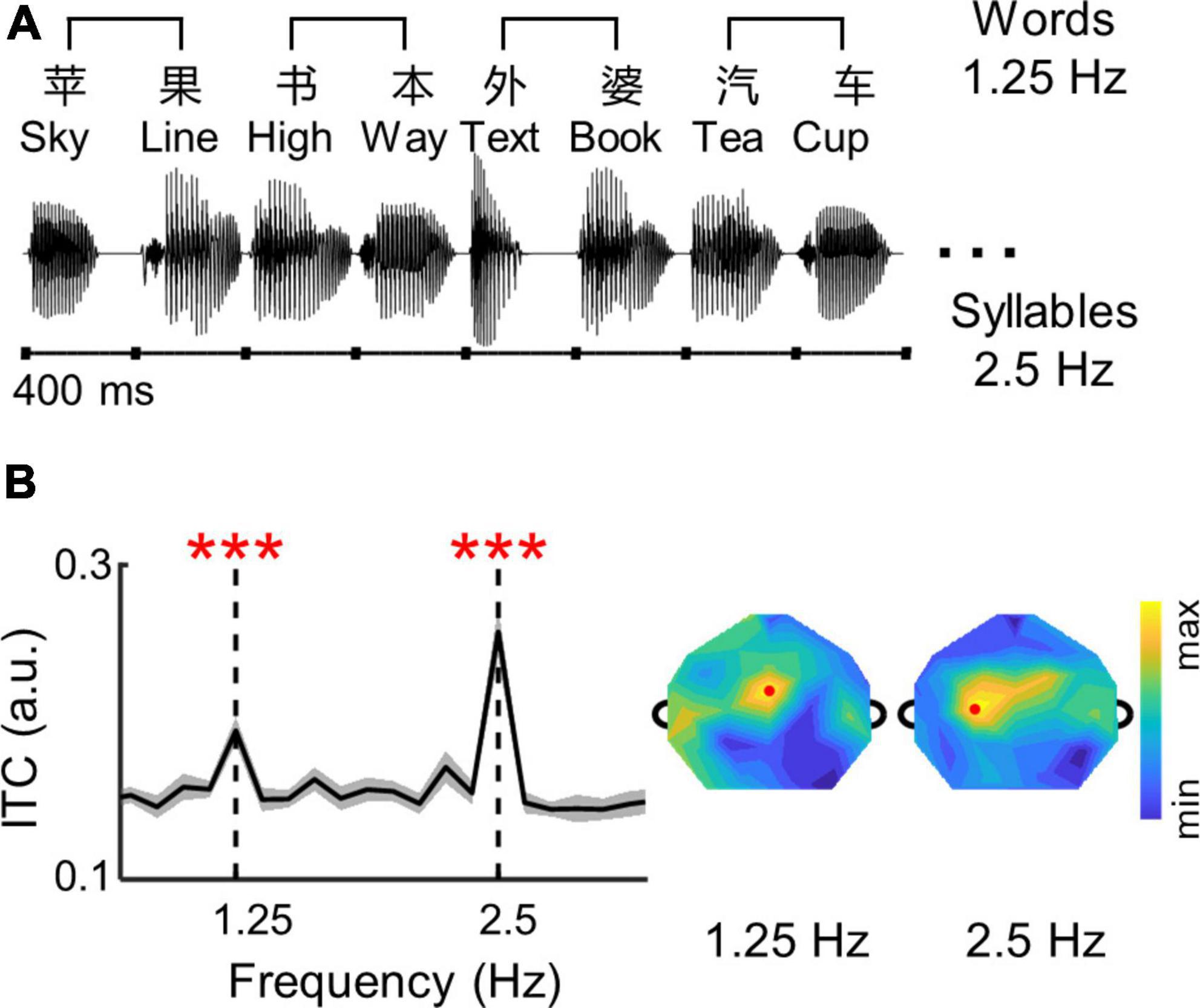
Figure 1. (A) Structure of the isochronous syllable sequences. Syllables are presented at a constant rate of 2.5 Hz. Therefore, bisyllabic words are presented at 1.25 Hz. Each trial consists of 10 bisyllabic words, lasting 8 s. The stimuli were in Chinese and English examples are shown for illustrative purposes. (B) The ITC values of neural responses are averaged over participants and channels. The shaded area indicates one standard error of the mean (SEM) across participants. The ITC spectrum of individual participants were shown in Supplementary Figure 3. Stars indicate significant peaks higher than their neighboring frequencies (***p < 0.001; bootstrap; FDR corrected). The topographies show distributions of ITC values. Red dots show channels with highest ITC values at 1.25 and 2.5 Hz.
For each participant, the 35 trials were presented in a random order. The participants were asked to detect whether the probe word “grandma” was presented in the speech by pressing a key (“1” for “yes” and “0” for “no”). The next trial was presented after an interval randomized between 1 and 2 s (uniform distribution) after the key press. After the experiment was finished, the SNAP-IV ADHD Questionnaire was collected from the parents of the children, and the ADHD symptom scores are shown in Supplementary Table 1.
2.3. EEG recording and analysis
All preprocessing and analysis in this study were performed using MATLAB (The MathWorks, Natick, MA, USA). EEG data was acquired on a 32-channel Hydrocele Geodesic Sensor Net (GSN) by Magstim EGI (Electrical Geodesic, Inc., Eugene, OR, USA) with 500 Hz sampling rate. The EEG recordings were referenced to the average of 32-channel recordings. Occasional large artifacts in EEG/EOG, that is, samples with magnitude >1 mV, were removed from the analysis (Luo and Ding, 2020). For the low-frequency band analyses, the EEG recordings were down-sampled to 120 Hz. Based on the frequency-tagging stimuli (see section “2.2. Stimuli and procedures”), the current study focused on word-rate and syllable-rate neural responses (1.25 and 2.5 Hz, respectively). To remove power-line noise (50 Hz) and slow drifts (<5 Hz), the EEG recordings were band-pass filtered between 0.6 and 20 Hz using a linear-phase finite impulse response (FIR) filter (4 s Hamming window, 6 dB attenuation at the cut-off frequencies). A linear-phase FIR filter causes a constant time delay to the input. The delay equals to N/2, where N was the window length of the filter (Oppenheim et al., 1997). The delay was compensated by removing the first N/2 samples in the filter output.
After data preprocessing, an 8-s epoch of EEG signal was obtained for each trial, and therefore the frequency resolution of the DFT analysis was 1/8 Hz. The EEG signal in the analysis window was transformed into the frequency domain using the discrete Fourier transform (DFT) without any additional smoothing window. Then, the inter-trial coherence (ITC) was calculated following (Bardouille and Ross, 2008):
where ∅i was the phase at the frequency bin i in trial n. The ITC is a scalar measure bounded between 0 and 1.
2.4. High-gamma amplitude
Most previous studies use invasive brain imaging (e.g., electrocorticography, ECoG) to investigate neural tracking of speech in high-gamma activity (Golumbic et al., 2013; Ding et al., 2016; Akbari et al., 2019). Nevertheless, recent studies provide evidence that reliable speech-tracking neural activity in high-gamma band can also be captured using non-invasive brain imaging technologies, e.g., EEG (Synigal et al., 2020), and MEG (Kulasingham et al., 2020). Therefore, the current study further analyzed the neural tracking responses to syllables and words in the high-gamma band.
High-gamma amplitude were extracted following the procedure in Foster et al. (2015). As shown in Figure 2A, continuous EEG recordings were filtered into a sequential of 10-Hz-width narrow band signal between 70 and 160 Hz (i.e., 70–80, 80–90,…, 150–160 Hz). To avoid artifacts of 100 and 150 Hz, surrounding filter bands were adjusted to 90–98, 102–110,…, 140–148, and 152–160 Hz. The amplitude (i.e., envelope) of each narrow band signal was extracted by Hilbert function in MATLAB. Then, each amplitude was normalized to its mean value in each band, and all amplitudes were averaged to obtain the high-gamma amplitude. In the frequency domain analysis, the high-gamma amplitudes were down-sampled to 60 Hz, and divided into 8-s trials. The ITC was calculated for the high-gamma amplitude following the same procedure as adopted in the analysis of the low-frequency signals.
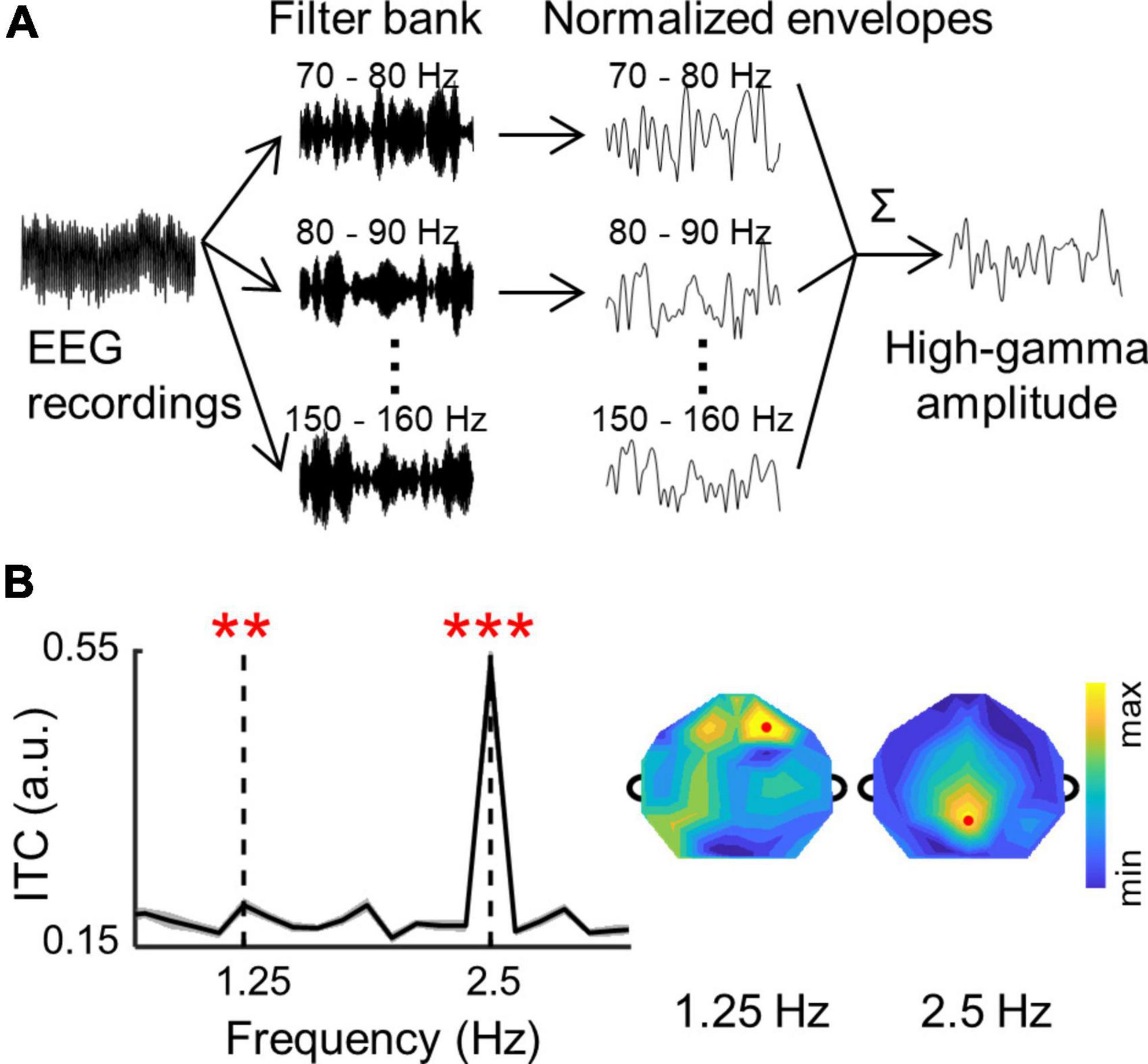
Figure 2. (A) A schematic illustration of the high-gamma amplitude analysis procedure (see section “2. Materials and methods”). (B) The ITC values in the high-gamma band are averaged over participants and channels. The shaded area indicates 1 SEM across participants. The ITC spectrum of individual participants were shown in Supplementary Figure 3. Stars indicate significant peaks higher than their neighboring frequencies (**p < 0.01; ***p < 0.001; bootstrap; FDR corrected). The topographies show distributions of ITC values in the high-gamma band. Red dots show channels with highest ITC values at 1.25 and 2.5 Hz.
2.5. Statistical test
The bootstrap significance test is a bias-corrected and accelerated procedure (Efron and Tibshirani, 1994). In the bootstrap procedure, data of all participants were resampled with replacement 10,000 times. To test the significance of the 1.25 and 2.5 Hz peaks in the ITC spectrum, the ITC at the peak frequency was compared with the mean ITC of the four neighboring frequency bins (two bins on each side, one-sided comparison). When multiple comparisons were performed, the p-value was further adjusted using the false discovery rate (FDR) correction (Benjamini and Hochberg, 1995). Moreover, Pearson’s correlation was calculated to quantify the relationship between neural responses and symptom scores. The significance of Pearson’s correlation coefficient for EEG channels was tested by cluster-based permutation analysis (Maris and Oostenveld, 2007). The approach was implemented using ft_statfun_correlationT function (based on 3,000 permutations) in the FieldTrip Matlab toolbox (Oostenveld et al., 2011). The cluster-based permutation analysis can combine spatially adjacent samples (i.e., neighboring channels) to correct for the multiple comparisons problem (Meyer et al., 2021).
2.6. Post hoc effect size calculation
A post hoc effect size analysis was performed to validate the appropriateness of the sample size to observe the 1.25 and 2.5 Hz responses. To simplify the analysis, we conducted a paired t-test to compare the ITC at the peak frequency with the mean ITC of the four neighboring frequency bins using the G*Power software (version 3.1) (Faul et al., 2007). The effect size d and power were reported in Supplementary Table 2, suggesting that the current study was powerful with the described sample size at the α level of 0.05.
3. Results
3.1. Behavioral performance
The experiment presented isochronous syllable sequences (Figure 1A), and the participants were asked to attend to the sequences and detect the probe word “grandma.” All participants finished the detection task, and the detection accuracy was 86.7% (SD = 6.3%). The Pearson’s correlation analysis revealed no significant correlation between the behavioral performance and ADHD symptom scores (INATT scores: p = 0.45, HYP/IMP scores: p = 0.14, ODD scores: p = 0.18, Pearson’s correlation, FDR corrected, Supplementary Figure 1).
3.2. Neural tracking of syllables and words in low-frequency activity
The EEG responses to the isochronous speech are shown in Figure 1B. The ITC spectrum was averaged over participants and EEG electrodes. Significant ITC peaks were observed at the syllable rate (2.5 Hz; p = 0.0001, bootstrap, FDR corrected), and at the word rate (1.25 Hz; p = 0.0001, bootstrap, FDR corrected). The topography of ITC showed a central distribution. All these findings suggested that low-frequency cortical activity exhibited neural tracking of syllables and words during active speech comprehension in children with ADHD, similar to healthy adults (Ding et al., 2016, 2018).
3.3. Neural tracking of syllables and words in the high-gamma amplitude
The neural responses to words and syllables were also analyzed in the high-gamma band (see section “2. Materials and methods” for details, Figure 2A and Supplementary Figure 4). The ITC spectrum of high-gamma amplitude is shown in Figure 2B. Similar to the results in the low-frequency band, significant ITC peaks were also observed at the syllable rate (2.5 Hz; p = 0.0001, bootstrap, FDR corrected), and at the word rate (1.25 Hz; p = 0.004, bootstrap, FDR corrected) for high-gamma amplitude. Moreover, the topography of high-gamma ITC showed a central-frontal distribution at the word rate and a central-posterior distribution at the syllable rate, which is similar to recent studies on high-gamma activity (Synigal et al., 2020; Dor-Ziderman et al., 2021). These results showed that the neural tracking of words could also be observed in the high-gamma band, and different topographic distributions suggested distinct neural source for low-frequency and high-gamma activity.
3.4. Relationships between ADHD scores and neural tracking responses
To investigate whether cortical encoding of words and syllables was correlated with ADHD symptoms, we analyzed the relationship between low-frequency/high-gamma ITC and ADHD symptom scores. In the analysis, we selected the channel with highest averaged ITC values at the syllable or word rate, and calculated the Pearson’s correlation between the ITC values and ADHD symptom scores. At the syllable rate (Supplementary Figure 2), no signification correlation was observed between the ITC and ADHD symptom scores either in the low-frequency band (INATT scores: p = 0.44, HYP/IMP scores: p = 0.78, ODD scores: p = 0.44, Pearson’s correlation, FDR corrected) nor in the high-gamma band (INATT scores: p = 0.70, HYP/IMP scores: p = 0.70, ODD scores: p = 0.70, Pearson’s correlation, FDR corrected). At the word rate (Figure 3), no significant correlation was observed between ITC and ADHD symptom scores in the low-frequency band as well (INATT scores: p = 0.96, HYP/IMP scores: p = 0.96, ODD scores: p = 0.96, Pearson’s correlation, FDR corrected). In the high-gamma band, however, the word-rate ITC values was anti-correlated to the INATT scores (r = −0.538, p = 0.019, Pearson’s correlation, FDR corrected) and the HYP/IMP scores (r = −0.512, p = 0.019, Pearson’s correlation, FDR corrected) while the correlation between word-rate ITC and ODD scores was not significant (p = 0.26, Pearson’s correlation, FDR corrected). A data-driven analysis of individual electrodes further showed the topography of correlation in high-gamma band was located at central-frontal electrodes (Figure 3C; all p < 0.048, Pearson’s correlation, cluster-based permutation test), consistent with the distribution of word-rate response in the high-gamma band (topography in Figure 2). These findings revealed that word tracking activity in the high-gamma band specifically correlated to certain ADHD symptoms of inattention and hyperactivity.
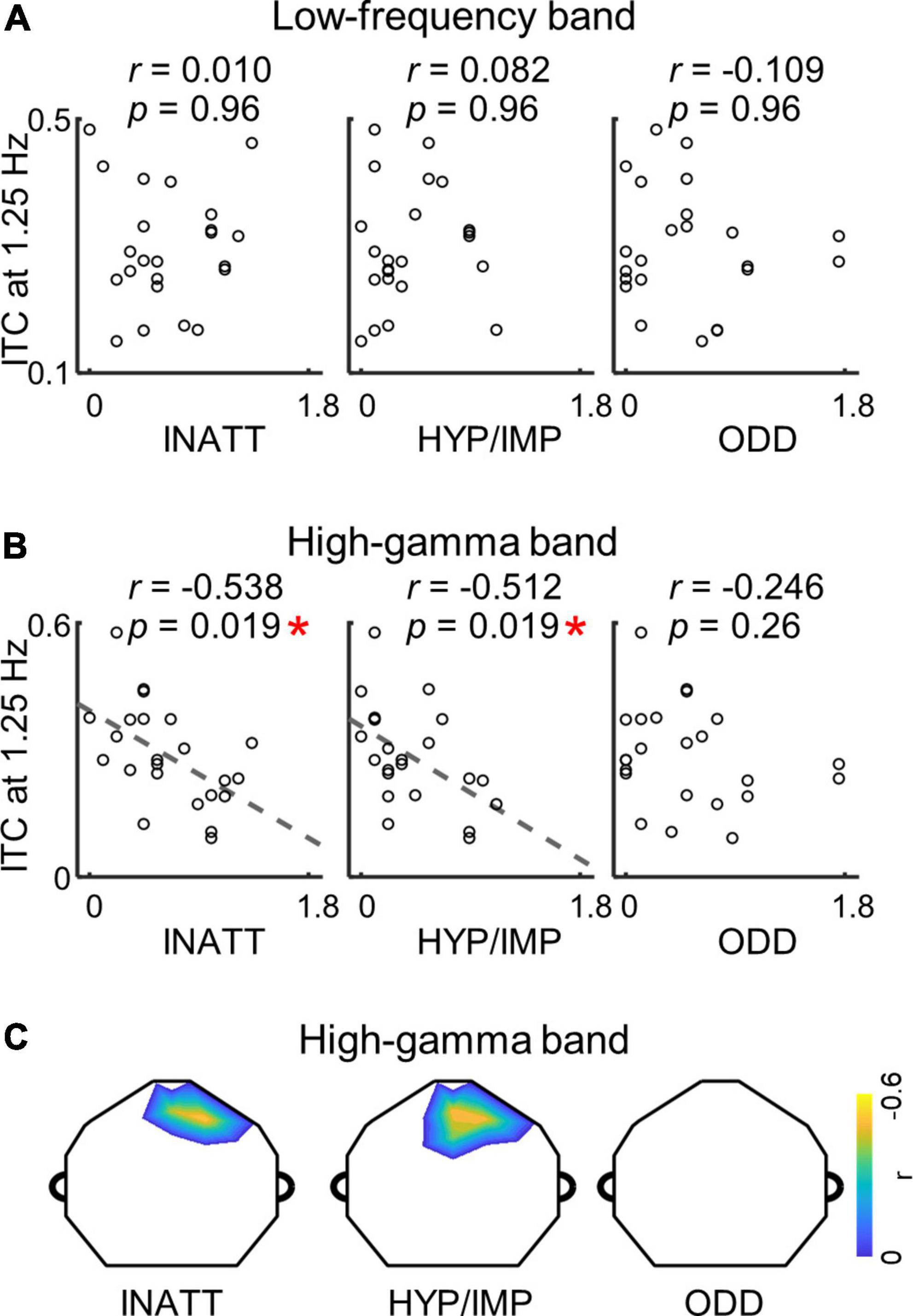
Figure 3. (A) No significant correlation is found between ADHD scores and word-rate ITC in the low-frequency band (all p > 0.05; Pearson’s correlation; FDR corrected). (B) Correlation between ADHD scores and word-rate ITC in the high-gamma band (*p < 0.05; Pearson’s correlation; FDR corrected). Each dot indicates a participant. The channel with the highest ITC values is selected for the correlation analysis. (C) The topographies show distributions of channels with significant correlation between ADHD scores and word-rate ITC in the high-gamma band (all p < 0.05; Pearson’s correlation; cluster-based permutation test).
4. Discussion
Speech perception is a complex process involving both acoustic encoding and linguistic processing, and the current study investigated which stage of speech was impaired in children with ADHD. When 6–8 years old children were engaged in a speech detection task, robust tracking responses to syllables and words were observed in both the low-frequency band and high-gamma band. We further analyzed the relationship between neural responses and ADHD symptoms. It was found that the ITC of neural response to words in the high-gamma band showed an anti-correlation with ADHD symptom scores of children, i.e., children with severer ADHD symptoms exhibited weaker word-tracking responses in the high-gamma band. These results indicated that ADHD prominently affected neural processing of linguistic information (e.g., words) during speech perception.
It is commonly observed that cortical activity tracks speech at different levels, corresponding to low-level acoustic features and high-level linguistic information (Luo and Poeppel, 2007; Ding and Simon, 2012; Brodbeck et al., 2018a,b; Keitel et al., 2018; Gao et al., 2020). Using the hierarchical auditory linguistic sequence paradigm, previous studies have found robust neural tracking responses to hierarchical speech units (e.g., syllables, words, and sentences) in adults and patients with a disorder of consciousness (Ding et al., 2016; Gui et al., 2020; Luo and Ding, 2020). Our results extended these findings and demonstrated that the hierarchical speech-tracking responses could also be observed in young children. Moreover, one line of studies has reported that cortical tracking of multiple speech features is differently modulated by selective attention (Golumbic et al., 2013; Kong et al., 2014; Ding et al., 2018; Luo and Ding, 2020; Yahav and Golumbic, 2021). Specifically, when a speech stream is presented, neural tracking of linguistic information (e.g., words, phrases, or sentences) is significantly decreased with attenuated attention, while neural tracking of acoustic features (e.g., speech envelope or rhythm) remains relatively stable. These results suggest that higher-level linguistic processing more relies on top-down attentional modulation in speech perception. Consistent with these findings, the current study found that children with severer ADHD symptoms exhibited weaker neural tracking of words (rather than syllables) in a single speech stream, indicating that the underlying attention deficits predominantly affected higher-level linguistic processing in children with ADHD.
Although cortical activity consistently tracks the temporal dynamics of speech in both low-frequency and high-gamma bands, they have non-redundant tracking properties and functional roles in speech perception (Nourski et al., 2009; Belitski et al., 2010; Golumbic et al., 2013; Mai et al., 2016; Synigal et al., 2020; Xu et al., 2023). For example, the high-gamma neural activity tracks speech with a short response latency, and remains robust for unintelligible speech (Nourski et al., 2009; Golumbic et al., 2013; Xu et al., 2023). In contrast, the low-frequency neural activity tracks speech with a long response latency, and decreases with degrading speech intelligibility (Luo and Poeppel, 2007; Xu et al., 2023). Based on these results, it has been proposed that high-gamma activity reflects automatic speech encoding in the early stage, while low-frequency activity reflects slow build-up processing in the late stage (Xu et al., 2023). Consistent with the proposal, evidence has shown that combing neural activity in the high-gamma and low-frequency bands can optimize accuracy for speech reconstruction (Golumbic et al., 2013; Akbari et al., 2019; Synigal et al., 2020). Here, our results showed that ADHD symptoms correlate to high-gamma responses but not to low-frequency responses, further supporting the previous suggestion that these two frequency bands represent systematically different mechanism and function for speech processing. Furthermore, evidence has shown that low-frequency and high-gamma responses exhibit distinct neural sources during speech perception (Crone et al., 2001; Edwards et al., 2009; Synigal et al., 2020). Specifically, low-frequency activity is distributed across temporal, frontal, and parietal lobes (Towle et al., 2008; Sinai et al., 2009), while high-gamma activity is mainly localized to the superior temporal gyrus (Crone et al., 2006; Golumbic et al., 2013). Consistent with these findings, the topographical plots in the current study also displayed different spatial distributions between low-frequency and high-gamma responses (Figure 1 vs. Figure 2).
Previous studies on healthy individuals have demonstrated that high-gamma neural activity is associated with processes of attention (Ray et al., 2008; Akimoto et al., 2014; Fiebelkorn and Kastner, 2019), working memory (Brovelli et al., 2005; Jensen et al., 2007; Wilsch and Obleser, 2016), and speech perception (Kubanek et al., 2013; Ding et al., 2016; Kulasingham et al., 2020). Therefore, it seems plausible to assume that the atypical cognitive function of ADHD children could stem from altered high-gamma neural activity (Herrmann and Demiralp, 2005). Previous EEG studies have shown evidence for significant differences between ADHD children and healthy controls in both resting-state and task-related neural activity in the gamma band (Lenz et al., 2008; Barry et al., 2010). A recent MEG study has also demonstrated that resting-state high-gamma power can predict cognitive performance and emotional state in ADHD children (Brovelli et al., 2005). Our results extended these findings, and demonstrated that the high-gamma neural response to words during speech perception is correlated with atypical symptoms in ADHD children. Since the cognitive function of attention and working memory (WM) is universally impaired in ADHD individuals (Hale et al., 2005, 2007; Halder and Kotnala, 2018; Ramos et al., 2020), the neural correlates of ADHD symptoms might reflect the influence of attention or WM impairments on linguistic processing in children with ADHD.
There are also limitations in the current study. It should be noted that the children who participated in the current study only had mild ADHD symptoms since we found children with severe ADHD were hard to remain stationary and complete all experimental procedures during EEG recordings. Although the current studies exhibited that ADHD symptoms were only correlated with neural impairment in linguistic processing, we cannot exclude the possibility that severer ADHD or different ADHD subtypes might further influence acoustic processing in speech perception. Future efforts are encouraged to adopt a more fine-grained paradigm and investigate how speech processing is impaired in children with severe ADHD or with different ADHD subtypes. Moreover, neither ADHD symptoms nor neural responses are correlated with behavioral performance in the current study (see Supplementary Figure 1). This is probably because word detection in a quiet listening condition is a relatively easy task (Luo and Ding, 2020; Liu et al., 2022), and most children achieved high performance (86.7 ± 6.3%) in the current study. Therefore, the measure of detection performance might not be sensitive enough to detect language impairment in children with ADHD. Future work could use the paradigm in more challenging or sophisticated settings (e.g., complex auditory scenes and difficult speech materials) to investigate the underlying neural correlates of individual behavioral performance in children with ADHD. Finally, the current study is also limited by its small sample size and lack of a clinical control group, which should be addressed by future studies. Nevertheless, it provides initial evidence for the relationship between task-related high-gamma activity and ADHD symptoms. Due to the evolving understanding in both the anatomical and functional level of high-gamma activity, focusing future studies on the high-gamma band bears the potential for better understanding of ADHD, and developing new tools for evaluation and therapy.
In sum, the current study demonstrates that cortical activity can track different levels of speech units (e.g., syllables and words) during speech perception in young children with ADHD, and ADHD predominantly impairs cortical encoding of linguistic information (e.g., words) in speech perception.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Children’s Hospital of Zhejiang University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
CL designed the research. YY and XZ performed the research. CL, JF, YL, XZ, and YG analyzed the data. CL and YG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32200860 and 32200862), Zhejiang Provincial Natural Science Foundation of China (LGF19H090020), Institute of Psychology, CAS (No. GJ202005), Exploratory Research Project of Zhejiang Lab (No. 2022RC0AN01), and Beihang University Sponsored Projects for Core Young Researchers in the Disciplines of Social Sciences and Humanities.
Acknowledgments
We thank editor and two reviewers for their constructive comments. We also thank Mr. Yuhan Lu, Ms. Jiaying Zhang, Dr. Qian Wang, Dr. Yiguang Liu, and Dr. Jianfen Zhang for their constructive comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2023.1174720/full#supplementary-material
Footnotes
References
Akbari, H., Khalighinejad, B., Herrero, J. L., Mehta, A. D., and Mesgarani, N. (2019). Towards reconstructing intelligible speech from the human auditory cortex. Sci. Rep. 9:874. doi: 10.1038/s41598-018-37359-z
Akimoto, Y., Nozawa, T., Kanno, A., Ihara, M., Goto, T., Ogawa, T., et al. (2014). High-gamma activity in an attention network predicts individual differences in elderly adults’ behavioral performance. Neuroimage 100, 290–300. doi: 10.1016/j.neuroimage.2014.06.037
Bardouille, T., and Ross, B. (2008). MEG imaging of sensorimotor areas using inter-trial coherence in vibrotactile steady-state responses. Neuroimage 42, 323–331. doi: 10.1016/j.neuroimage.2008.04.176
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., and Dupuy, F. E. (2010). Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 121, 1871–1877. doi: 10.1016/j.clinph.2010.04.022
Belitski, A., Panzeri, S., Magri, C., Logothetis, N. K., and Kayser, C. (2010). Sensory information in local field potentials and spikes from visual and auditory cortices: Time scales and frequency bands. J. Comput. Neurosci. 29, 533–545. doi: 10.1007/s10827-010-0230-y
Bellani, M., Moretti, A., Perlini, C., and Brambilla, P. (2011). Language disturbances in ADHD. Epidemiol. Psychiatr. Sci. 20, 311–315. doi: 10.1017/S2045796011000527
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 57, 289–300.
Ben-Pazi, H., Shalev, R. S., Gross-Tsur, V., and Bergman, H. (2006). Age and medication effects on rhythmic responses in ADHD: Possible oscillatory mechanisms? Neoropsychologia 44, 412–416. doi: 10.1016/j.neuropsychologia.2005.05.022
Blomberg, R., Danielsson, H., Rudner, M., Soderlund, G. B. W., and Ronnberg, J. (2019). Speech processing difficulties in attention deficit hyperactivity disorder. Front. Psychol. 10:1536. doi: 10.3389/fpsyg.2019.01536
Brodbeck, C., Presacco, A., and Simon, J. (2018a). Neural source dynamics of brain responses to continuous stimuli: Speech processing from acoustics to comprehension. Neuroimage 172, 162–174. doi: 10.1016/j.neuroimage.2018.01.042
Brodbeck, C., Hong, L. E., and Simon, J. Z. (2018b). Rapid transformation from auditory to linguistic representations of continuous speech. Curr. Biol. 28, 3976–3983. doi: 10.1016/j.cub.2018.10.042
Brovelli, A., Lachaux, J. P., Kahane, P., and Boussaoud, D. (2005). High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage 28, 154–164. doi: 10.1016/j.neuroimage.2005.05.045
Carr, K. W., White-Schwoch, T., Tierney, A. T., Strait, D. L., and Kraus, N. (2014). Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proc. Natl. Acad. Sci. U.S.A. 111, 14559–14564. doi: 10.1073/pnas.1406219111
Cohen, N. J., Vallance, D. D., Barwick, M., Im, N., Menna, R., Horodezky, N. B., et al. (2000). The interface between ADHD and language impairment: An examination of language, achievement, and cognitive processing. J. Child Psychol. Psychiatry 41, 353–362. doi: 10.1017/S0021963099005442
Cook, J. R., Mausbach, T., Burd, L., Gascon, G. G., and Reynolds, B. W. (1993). A preliminary study of the relationship between central auditory processing disorder and attention deficit disorder. J. Psychiatry Neurosci. 18, 130–137. doi: 10.1111/j.1600-079X.1993.tb00502.x
Crone, N. E., Boatman, D., Gordon, B., and Hao, L. (2001). Induced electrocorticographic gamma activity during auditory perception. Clin. Neurophysiol. 112, 565–582. doi: 10.1016/S1388-2457(00)00545-9
Crone, N. E., Sinai, A., and Korzeniewska, A. (2006). “High-frequency gamma oscillations and human brain mapping with electrocorticography,” in Event-related dynamics of brain oscillations, eds C. Neuper and W. Klimesch (Amsterdam: Elsevier), 275–295.
Davidson, E. M., and Prior, M. R. (1978). Laterality and selective attention in hyperactive children. J. Abnorm. Child Psychol. 6, 475–481. doi: 10.1007/BF00926057
Ding, N., and Simon, J. Z. (2012). Emergence of neural encoding of auditory objects while listening to competing speakers. Proc. Natl. Acad. Sci. U.S.A. 109, 11854–11859. doi: 10.1073/pnas.1205381109
Ding, N., Chatterjee, M., and Simon, J. Z. (2014). Robust cortical entrainment to the speech envelope relies on the spectro-temporal fine structure. Neuroimage 88, 41–46. doi: 10.1016/j.neuroimage.2013.10.054
Ding, N., Melloni, L., Zhang, H., Tian, X., and Poeppel, D. (2016). Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164. doi: 10.1038/nn.4186
Ding, N., Pan, X., Luo, C., Su, N., Zhang, W., and Zhang, J. (2018). Attention is required for knowledge-based sequential grouping: Insights from the integration of syllables into words. J. Neurosci. 38, 1178–1188. doi: 10.1523/JNEUROSCI.2606-17.2017
Doelling, K. B., Arnal, L. H., Ghitza, O., and Poeppel, D. (2014). Acoustic landmarks drive delta-theta oscillations to enable speech comprehension by facilitating perceptual parsing. Neuroimage 85, 761–768. doi: 10.1016/j.neuroimage.2013.06.035
Dor-Ziderman, Y., Zeev-Wolf, M., Klein, E. H., Bar-Oz, D., Nitzan, U., Maoz, H., et al. (2021). High-gamma oscillations as neurocorrelates of ADHD: A MEG crossover placebo-controlled study. J. Psychiatr. Res. 137, 186–193. doi: 10.1016/j.jpsychires.2021.02.050
Edwards, E., Soltani, M., Kim, W., Dalal, S. S., Nagarajan, S. S., Berger, M. S., et al. (2009). Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J. Neurophysiol. 102, 377–386. doi: 10.1152/jn.90954.2008
Faul, F., Erdfelder, E., Lang, A.-G., and Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fiebelkorn, I. C., and Kastner, S. (2019). A rhythmic theory of attention. Trends. Cogn. Sci. 23, 87–101. doi: 10.1016/j.tics.2018.11.009
Foster, B. L., Rangarajan, V., Shirer, W. R., and Parvizi, J. (2015). Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron 86, 578–590. doi: 10.1016/j.neuron.2015.03.018
Fuermaier, A. B. M., Hupen, P., De Vries, S. M., Muller, M., Kok, F. M., Koerts, J., et al. (2018). Perception in attention deficit hyperactivity disorder. Atten. Deficit. Hyperact. Disord. 10, 21–47. doi: 10.1007/s12402-017-0230-0
Gao, Y., Zhang, J., and Wang, Q. (2020). Robust neural tracking of linguistic units relates to distractor suppression. Eur. J. Neurosci. 51, 641–650. doi: 10.1111/ejn.14552
Gau, S. S. F., Shang, C. Y., Liu, S. K., Lin, C. H., Swanson, J. M., Liu, Y. C., et al. (2008). Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale - parent form. Int. J. Methods Psychiatr. Res. 17, 35–44. doi: 10.1002/mpr.237
Geffner, D., Lucker, J. R., and Koch, W. (1996). Evaluation of auditory discrimination in children with ADD and without ADD. Child Psychiatry Hum. Dev. 26:169. doi: 10.1007/BF02353358
Ghitza, O. (2012). On the role of theta-driven syllabic parsing in decoding speech: Intelligibility of speech with a manipulated modulation spectrum. Front. Psychol. 3:238. doi: 10.3389/fpsyg.2012.00238
Giraud, A. L., and Poeppel, D. (2012). Cortical oscillations and speech processing: Emerging computational principles and operations. Nat. Neurosci. 15, 511–517. doi: 10.1038/nn.3063
Golumbic, E. M. Z., Ding, N., Bickel, S., Lakatos, P., Schevon, C. A., McKhann, G. M., et al. (2013). Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party”. Neuron 77, 980–991. doi: 10.1016/j.neuron.2012.12.037
Gomes, H., Duff, M., Ramos, M., Molholm, S., Foxe, J. J., and Halperin, J. (2012). Auditory selective attention and processing in children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 123, 293–302. doi: 10.1016/j.clinph.2011.07.030
Gomez, R., and Condon, M. (1999). Central auditory processing ability in children with ADHD with and without learning disabilities. J. Learn. Disabil. 32:150. doi: 10.1177/002221949903200205
Gui, P., Jiang, Y. W., Zang, D., Qi, Z. X., Tan, J. X., Tanigawa, H., et al. (2020). Assessing the depth of language processing in patients with disorders of consciousness. Nat. Neurosci. 23, 761–770. doi: 10.1038/s41593-020-0639-1
Halder, S., and Kotnala, S. (2018). Working memory, verbal comprehension, perceptual reasoning and processing speed in adhd and normal children: A comparative study. J. Indian Assoc. Child Adolesc. Mental Health 14, 60–79.
Hale, T. S., Bookheimer, S., Mcgough, J. J., Phillips, J. M., and Mccracken, J. T. (2007). Atypical brain activation during simple & complex levels of processing in adult ADHD: An fMRI study. J. Atten. Disord. 11, 125–140.
Hale, T. S., McCracken, J. T., McGough, J. J., Smalley, S. L., Phillips, J. M., and Zaidel, E. (2005). Impaired linguistic processing and atypical brain laterality in adults with ADHD. Clin. Neurosci. Res. 5, 255–263. doi: 10.1016/j.cnr.2005.09.006
Herrmann, C. S., and Demiralp, T. (2005). Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 116, 2719–2733. doi: 10.1016/j.clinph.2005.07.007
Jensen, O., Kaiser, J., and Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324. doi: 10.1016/j.tins.2007.05.001
Jin, P. Q., Lu, Y. H., and Ding, N. (2020). Low-frequency neural activity reflects rule-based chunking during speech listening. Elife 9:e55613. doi: 10.7554/eLife.55613
Jonkman, L. M., Kemner, C., Verbaten, M. N., Koelega, H. S., and Camfferman, G. (1997). Event-related potentials and performance of attention-deficit hyperactivity disorder: Children and normal controls in auditory and visual selective attention tasks. Biol. Psychiatry 41, 595–611. doi: 10.1016/S0006-3223(96)00073-X
Katya, R. (2018). Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front. Hum. Neurosci 12:100. doi: 10.3389/fnhum.2018.00100
Keitel, A., Gross, J., and Kayser, C. (2018). Perceptually relevant speech tracking in auditory and motor cortex reflects distinct linguistic features. PLoS Biol. 16:e2004473. doi: 10.1371/journal.pbio.2004473
Keith, R. W., Rudy, J., Donahue, P. A., and Katbamna, B. (1989). Comparison of SCAN results with other auditory and language measures in a clinical population. Ear Hear. 10, 382–386. doi: 10.1097/00003446-198912000-00011
Kong, Y. Y., Mullangi, A., and Ding, N. (2014). Differential modulation of auditory responses to attended and unattended speech in different listening conditions. Hear. Res. 316, 73–81. doi: 10.1016/j.heares.2014.07.009
Kotz, S. A., Ravignani, A., and Fitch, W. T. (2018). The evolution of rhythm processing. Trends. Cogn. Sci. 22, 896–910. doi: 10.1016/j.tics.2018.08.002
Kubanek, J., Brunner, P., Gunduz, A., Poeppel, D., and Schalk, G. (2013). The tracking of speech envelope in the human cortex. PLoS One 8:e53398. doi: 10.1371/journal.pone.0053398
Kulasingham, J. P., Brodbeck, C., Presacco, A., Kuchinsky, S. E., and Simon, J. Z. (2020). High gamma cortical processing of continuous speech in younger and older listeners. Neuroimage 222:117291. doi: 10.1016/j.neuroimage.2020.117291
Laffere, A., Dick, F., Holt, L. L., and Tierney, A. (2021). Attentional modulation of neural entrainment to sound streams in children with and without ADHD. Neuroimage 224:117396. doi: 10.1016/j.neuroimage.2020.117396
Lanzetta-Valdo, B. P., Oliveira, G. A. D., Ferreira, J. T. C., and Palacios, E. M. N. (2017). Auditory processing assessment in children with attention deficit hyperactivity disorder: An open study examining methylphenidate effects. Int. Arch. Otorhinolaryngol. 21, 72–78. doi: 10.1055/s-0036-1572526
Lenz, D., Krauel, K., Schadow, J., Baving, L., Duzel, E., and Herrmann, C. S. (2008). Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Res. 1235, 117–132. doi: 10.1016/j.brainres.2008.06.023
Litovsky, R. (2015). Development of the auditory system. Handb. Clin. Neurol. 129:55. doi: 10.1016/B978-0-444-62630-1.00003-2
Liu, S., Han, D., Wu, X., Mo, L., and Kong, Y. (2008). The phonetic development of normal-hearing pre-school children. J. Clin. Otorhinolaryngol. Head Neck Surg. 22, 301–303.
Liu, Y. G., Luo, C., Zheng, J., Liang, J. Y., and Ding, N. (2022). Working memory asymmetrically modulates auditory and linguistic processing of speech. Neuroimage 264:119698. doi: 10.1016/j.neuroimage.2022.119698
Loiselle, D. L., Stamm, J. S., Maitinsky, S., and Whipple, S. C. (1980). Evoked potential and behavioral signs of attentive dysfunctions in hyperactive boys. Psychophysiology 17, 193–201. doi: 10.1111/j.1469-8986.1980.tb00134.x
Lu, Y. H., Jin, P. Q., Pan, X. Y., and Ding, N. (2022). Delta-band neural activity primarily tracks sentences instead of semantic properties of words. Neuroimage 251:118979. doi: 10.1016/j.neuroimage.2022.118979
Luo, C., and Ding, N. (2020). Cortical encoding of acoustic and linguistic rhythms in spoken narratives. Elife 9:e60433. doi: 10.7554/eLife.60433
Luo, H., and Poeppel, D. (2007). Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron 54, 1001–1010. doi: 10.1016/j.neuron.2007.06.004
Mai, G., Minett, J. W., and Wang, S. Y. (2016). Delta, theta, beta, and gamma brain oscillations index levels of auditory sentence processing. Neuroimage 133, 516–528. doi: 10.1016/j.neuroimage.2016.02.064
Maris, E., and Oostenveld, R. (2007). Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. doi: 10.1016/j.jneumeth.2007.03.024
Martin, A. E., and Doumas, L. A. A. (2017). A mechanism for the cortical computation of hierarchical linguistic structure. PLoS Biol. 15:e2000663. doi: 10.1371/journal.pbio.2000663
Meyer, L., Sun, Y., and Martin, A. E. (2020). “Entraining” to speech, generating language? Lang. Cogn. Neurosci. 35, 1138–1148. doi: 10.1080/23273798.2020.1827155
Meyer, M., Lamers, D., Kayhan, E., Hunnius, S., and Oostenveld, R. (2021). Enhancing reproducibility in developmental EEG research: BIDS, cluster-based permutation tests, and effect sizes. Dev. Cogn. Neurosci 52:101036. doi: 10.1016/j.dcn.2021.101036
Michalek, A. M. P., Watson, S. M., Ash, I., Ringleb, S., and Raymer, A. (2014). Effects of noise and audiovisual cues on speech processing in adults with and without ADHD. Int. J. Audiol. 53, 145–152. doi: 10.3109/14992027.2013.866282
Mueller, A., Hong, D. S., Shepard, S., and Moore, T. (2017). Linking ADHD to the neural circuitry of attention. Trends. Cogn. Sci. 21, 474–488. doi: 10.1016/j.tics.2017.03.009
Murias, M., Swanson, J. M., and Srinivasan, R. (2007). Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb. Cortex 17, 1788–1799. doi: 10.1093/cercor/bhl089
Myers, B. R., Lense, M. D., and Gordon, R. L. (2019). Pushing the envelope: Developments in neural entrainment to speech and the biological underpinnings of prosody perception. Brain Sci. 9:70. doi: 10.3390/brainsci9030070
Norrelgen, F., Lacerda, F., and Forssberg, H. (1999). Speech discrimination and phonological working memory in children with ADHD. Dev. Med. Child Neurol. 41, 335–339. doi: 10.1017/S0012162299000730
Nourski, K. V., Reale, R. A., Oya, H., Kawasaki, H., Kovach, C. K., Chen, H. M., et al. (2009). Temporal envelope of time-compressed speech represented in the human auditory cortex. J. Neurosci. 29, 15564–15574. doi: 10.1523/JNEUROSCI.3065-09.2009
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci 2011:156869. doi: 10.1155/2011/156869
Pillsbury, H. C., Grose, J. H., Coleman, W. L., Conners, C. K., and Hall, J. W. (1995). Binaural function in children with attention-deficit hyperactivity disorder. Arch. Otolaryngol. Head Neck Surg. 121, 1345–1350.
Poeppel, D., and Assaneo, M. F. (2020). Speech rhythms and their neural foundations. Nat. Rev. Neurosci. 21, 322–334. doi: 10.1038/s41583-020-0304-4
Puyjarinet, F., Begel, V., Lopez, R., Dellacherie, D., and Dalla Bella, S. (2017). Children and adults with Attention-Deficit/Hyperactivity Disorder cannot move to the beat. Sci. Rep. 7:11550. doi: 10.1038/s41598-017-11295-w
Ramos, A. A., Hamdan, A. C., and Machado, L. (2020). A meta-analysis on verbal working memory in children and adolescents with ADHD. Clin. Neuropsychol. 34, 873–898. doi: 10.1080/13854046.2019.1604998
Randell, R., Somerville-Brown, L., and Chen, W. (2019). How relevant is higher-order language deficit (HOLD) to children with complex presentations of attention-deficit hyperactivity disorder? Atten. Deficit Hyperact. Disord. 11, 325–332. doi: 10.1007/s12402-018-0279-4
Ray, S., Niebur, E., Hsiao, S. S., Sinai, A., and Crone, N. E. (2008). High-frequency gamma activity (80-150 Hz) is increased in human cortex during selective attention. Clin. Neurophysiol. 119, 116–133. doi: 10.1016/j.clinph.2007.09.136
Rothenberger, A., Banaschewski, T., Heinrich, H., Moll, G. H., Schmidt, M. H., and van’t Klooster, B. (2000). Comorbidity in ADHD-children: Effects of coexisting conduct disorder or tic disorder on event-related brain potentials in an auditory selective-attention task. Eur. Arch. Psychiatry Clin. Neurosci. 250, 101–110. doi: 10.1007/s004060070042
Rubia, K., Taylor, E., Smith, A. B., Oksannen, H., Overmeyer, S., and Newman, S. (2001). Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br. J. Psychiatry 179, 138–143. doi: 10.1192/bjp.179.2.138
Satterfield, J. H., Schell, A. M., Nicholas, T. W., Satterfield, B. T., and Freese, T. E. (1990). Ontogeny of selective attention effects on event-related potentials in attention-deficit hyperactivity disorder and normal boys. Biol. Psychiatry 28, 879–903. doi: 10.1016/0006-3223(90)90569-N
Schafer, E. C., Mathews, L., Mehta, S., Hill, M., and Moloney, M. (2013). Personal FM systems for children with autism spectrum disorders (ASD) and/or attention-deficit hyperactivity disorder (ADHD): An initial investigation. J. Commun. Disord. 46, 30–52. doi: 10.1016/j.jcomdis.2012.09.002
Seidman, L. J., Biederman, J., Weber, W., Hatch, M., and Faraone, S. V. (1998). Neuropsychological function in adults with attention-deficit hyperactivity disorder. Biol. Psychiatry 44, 260–268. doi: 10.1016/S0006-3223(97)00392-2
Sinai, A., Crone, N. E., Wied, H. M., Franaszczuk, P. J., Miglioretti, D., and Boatman-Reich, D. (2009). Intracranial mapping of auditory perception: Event-related responses and electrocortical stimulation. Clin. Neurophysiol. 120, 140–149. doi: 10.1016/j.clinph.2008.10.152
Swanson, J. M., Schuck, S., Porter, M. M., Carlson, C., Hartman, C. A., Sergeant, J. A., et al. (2012). Categorical and dimensional definitions and evaluations of symptoms of ADHD: History of the SNAP and the SWAN rating scales. Int. J. Educ. Psychol. Assess. 10, 51–70.
Synigal, S. R., Teoh, E. S., and Lalor, E. C. (2020). Including measures of high gamma power can improve the decoding of natural speech from EEG. Front. Hum. Neurosci. 14:130. doi: 10.3389/fnhum.2020.00130
Tierney, A. T., and Kraus, N. (2013). The ability to tap to a beat relates to cognitive, linguistic, and perceptual skills. Brain Lang. 124, 225–231. doi: 10.1016/j.bandl.2012.12.014
Toplak, M. E., and Tannock, R. (2005). Tapping and anticipation performance in attention deficit hyperactivity disorder. Percept. Mot. Skills 100, 659–675. doi: 10.2466/PMS.100.3.659-675
Towle, V. L., Yoon, H. A., Castelle, M., Edgar, J. C., Biassou, N. M., Frim, D. M., et al. (2008). ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain 131, 2013–2027. doi: 10.1093/brain/awn147
van Mourik, R., Sergeant, J. A., Heslenfeld, D., Konig, C., and Oosterlaan, J. (2011). Auditory conflict processing in ADHD. J. Child Psychol. Psychiatry 52, 265–274. doi: 10.1111/j.1469-7610.2010.02339.x
Wilsch, A., and Obleser, J. (2016). What works in auditory working memory? A neural oscillations perspective. Brain Res. 1640, 193–207. doi: 10.1016/j.brainres.2015.10.054
Xu, N., Zhao, B. T., Luo, L., Zhang, K., Shao, X. Q., Luan, G. M., et al. (2023). Two stages of speech envelope tracking in human auditory cortex modulated by speech intelligibility. Cereb. Cortex 33, 2215–2228. doi: 10.1093/cercor/bhac203
Yahav, P. H. S., and Golumbic, E. Z. (2021). Linguistic processing of task-irrelevant speech at a cocktail party. Elife 10:e65096. doi: 10.7554/eLife.65096
Keywords: ADHD, speech perception, neural tracking, acoustic encoding, linguistic processing
Citation: Luo C, Gao Y, Fan J, Liu Y, Yu Y and Zhang X (2023) Compromised word-level neural tracking in the high-gamma band for children with attention deficit hyperactivity disorder. Front. Hum. Neurosci. 17:1174720. doi: 10.3389/fnhum.2023.1174720
Received: 27 February 2023; Accepted: 18 April 2023;
Published: 05 May 2023.
Edited by:
Xing Tian, New York University Shanghai, ChinaReviewed by:
Changxin Zhang, East China Normal University, ChinaDan Zhang, Tsinghua University, China
Copyright © 2023 Luo, Gao, Fan, Liu, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayue Gao, Z2FvX3lheXVlQGJ1YWEuZWR1LmNu; Xin Zhang, NjUxMDAzOUB6anUuZWR1LmNu; Yonglin Yu, eXV5b25nbGluMTk5OEB6anUuZWR1LmNu; Cheng Luo, bHVvX2NoZW5nQHpoZWppYW5nbGFiLmNvbQ==
†These authors share first authorship
 Cheng Luo
Cheng Luo Yayue Gao
Yayue Gao Jianing Fan
Jianing Fan Yang Liu
Yang Liu Yonglin Yu
Yonglin Yu Xin Zhang
Xin Zhang