- 1Behavioral Neuroscience Lab, Institute of Psychology, SWPS University, Warsaw, Poland
- 2Center for Brain Science, RIKEN, Wako, Japan
Analysis and interpretation of studies on cognitive and affective dysregulation often draw upon the network paradigm, especially the Triple Network Model, which consists of the default mode network (DMN), the frontoparietal network (FPN), and the salience network (SN). DMN activity is primarily dominant during cognitive leisure and self-monitoring processes. The FPN peaks during task involvement and cognitive exertion. Meanwhile, the SN serves as a dynamic “switch” between the DMN and FPN, in line with salience and cognitive demand. In the cognitive and affective domains, dysfunctions involving SN activity are connected to a broad spectrum of deficits and maladaptive behavioral patterns in a variety of clinical disorders, such as depression, insomnia, narcissism, PTSD (in the case of SN hyperactivity), chronic pain, and anxiety, high degrees of neuroticism, schizophrenia, epilepsy, autism, and neurodegenerative illnesses, bipolar disorder (in the case of SN hypoactivity). We discuss behavioral and neurological data from various research domains and present an integrated perspective indicating that these conditions can be associated with a widespread disruption in predictive coding at multiple hierarchical levels. We delineate the fundamental ideas of the brain network paradigm and contrast them with the conventional modular method in the first section of this article. Following this, we outline the interaction model of the key functional brain networks and highlight recent studies coupling SN-related dysfunctions with cognitive and affective impairments.
1. Modern paradigms in neuroimaging studies—from modular to systemic perspectives
Understanding how the brain’s rich functionality emerges from its relatively fixed anatomical structure is one of the main challenges in neuroscience. The brain’s cognitive functions can be studied at many levels of complexity, ranging from the influence of particular genes and their interactions on behavior to the analysis of dynamic systems of interdependent structures creating intrinsic brain networks. For many years, the modular perspective of applying particular functions to specific structures has dominated cognitive science (Fodor, 1983; Barrett and Satpute, 2013), most often ascribing autonomic roles to the studied regions, treating them as independent specialized modules.
However, shortcomings of this paradigm have been noted (Fuster, 2000), and study results, like the discovery of cross-modal sensory processing modulations (Garner and Keller, 2022; McClure et al., 2022), have begun to undermine even the most basic assumptions, such as monomodality of first-order sensory poles (Cappe and Barone, 2005). The biggest questions being raised regard the apparent independence and specialization of structures. Studies making this assumption often give accurate but inconclusive results in the broader context, and the lack of an overarching model makes it difficult to draw unified conclusions. The function of the anterior insula (AI) is an apt example (Wager and Barrett, 2017). Its activity is regularly attached to a wide range of apparently unrelated processes from sensory and affective processing to higher-order cognition (Uddin et al., 2017), such as body and emotional awareness, pain (Liu et al., 2021), self-recognition and motivation (Craig, 2009), singing and music recognition (Zamorano et al., 2019), uncertainty, empathy, and risky decisions (Singer et al., 2009), visual consciousness (Salomon et al., 2018), time perception (Vicario et al., 2020), attention span (Nelson et al., 2010) and integration of internal interoceptive and external sensory signals (Chen et al., 2021), as well as homeostasis (Flynn, 1999).
The systemic perspective describes psychological functions as the result of interdependent processes driven by domain-general functional brain networks, which do not have strict spatial boundaries (Park and Friston, 2013; Uddin et al., 2019; Luo, 2021). Moreover, the decoupling of structural and functional networks is required to achieve the advanced context-sensitive integration that is typical for humans (Griffa et al., 2022).
2. Brain functional networks
The perspective that the human brain is organized into hierarchically modularized networks is now widely accepted (Wang et al., 2015). In contrast to the assumption of independent and functionally rigid modules similar to a set of specialized tools (Gigerenzer and Todd, 1999), functional neural networks are assessed as dynamic, elastic, and hierarchical (Gilmore et al., 2018). This is necessary in order to confront changing environmental factors and develop a wide range of context-dependent behaviors (Bressler and McIntosh, 2007; Bressler and Menon, 2010). Transitions between functional networks are a response to environmental changes (Sadaghiani and Kleinschmidt, 2013). Zerbi et al. (2019) showed that a rapid reconfiguration of the functional connectome occurred in response to a threat by the release of norepinephrine, which drastically increases global brain connectivity, primarily within the salience network.
Functional neural networks have emerged from the temporally organized coupling of activity across vastly dispersed brain regions. They are characterized by the functional interdependence of brain structures within their frameworks (Bressler and Menon, 2010). Functional networks are bounded by the anatomical structure of neural connections (Xie et al., 2021). The topology of functional networks is dependent on individual development (Shanmugan et al., 2022). Furthermore, Functional connectivity (FC) can be used to predict behavioral traits such as fluid intelligence or even personality factors (NEO-FFI; Li et al., 2022). FC is a powerful tool for exploring healthy brain organization as well as mental disorders and individual differences.
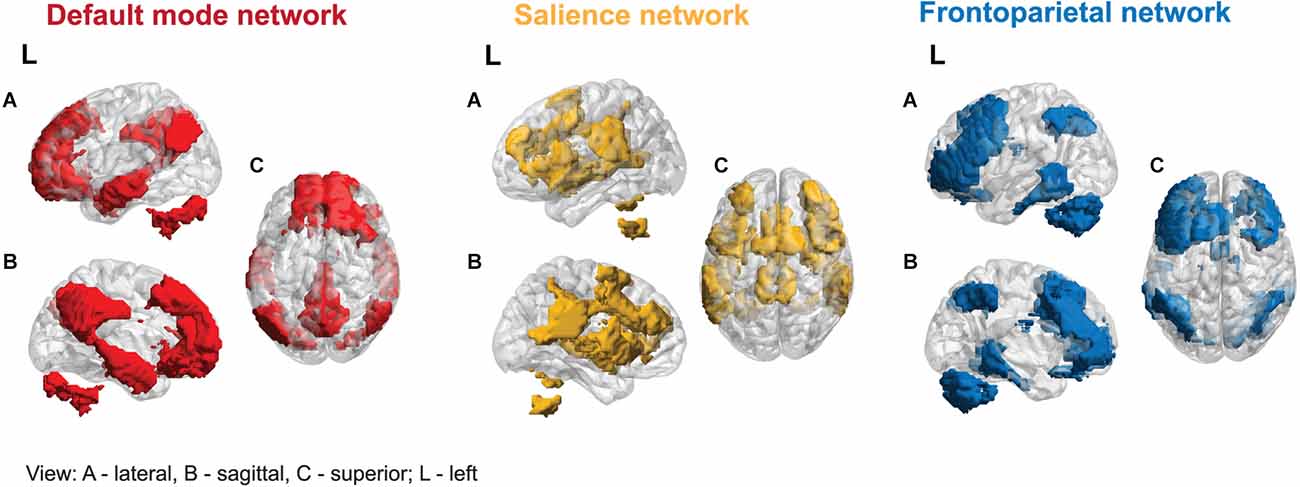
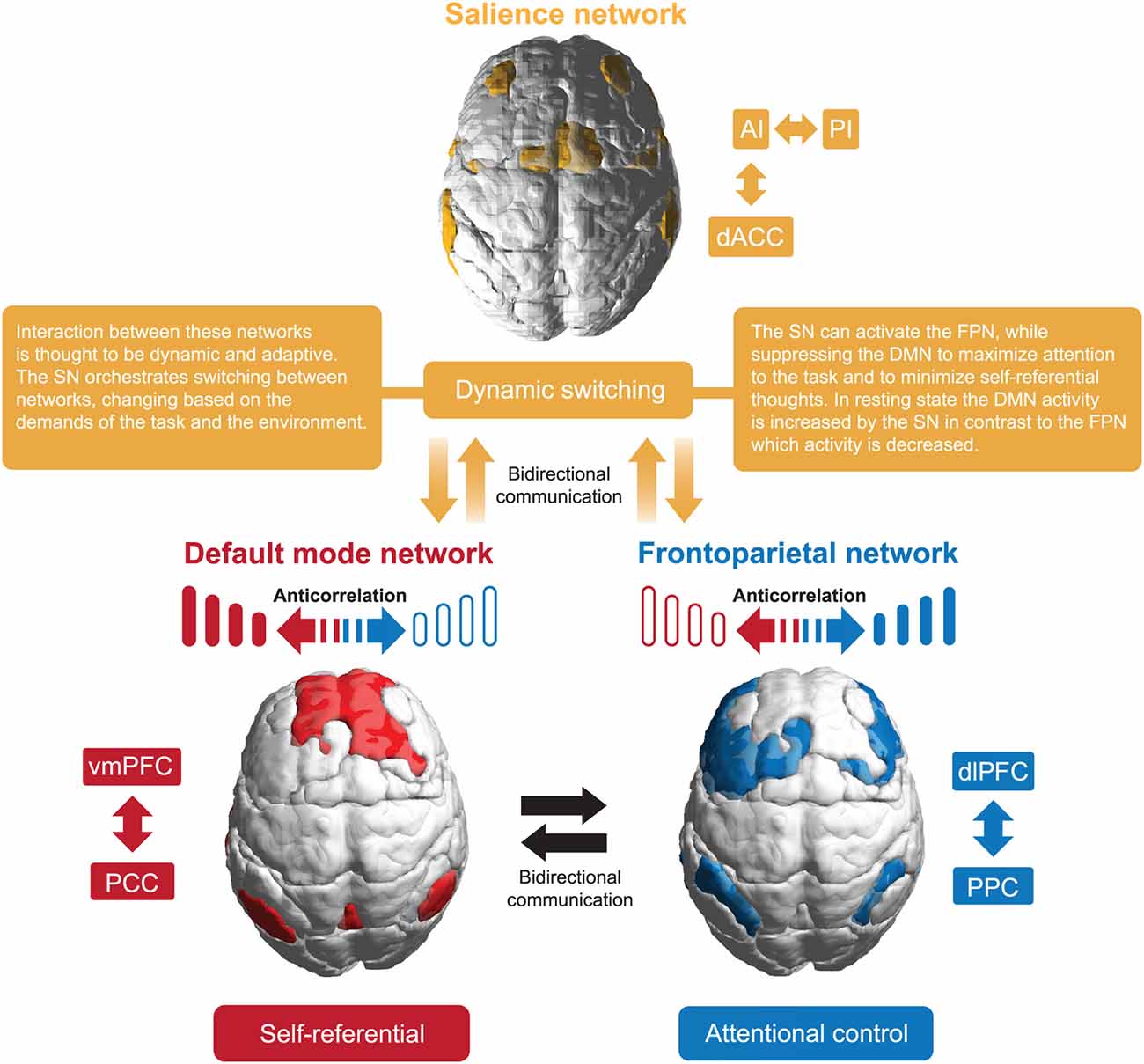
Uddin et al. (2019) identified six prevalent macro-scale brain networks. Based on convergent evidence from many studies, three networks: Default Mode Network (DMN), Frontoparietal Network (FPN), and the Salience Network (SN), are often called canonical (Ciric et al., 2017; Uddin et al., 2022), as their interactions play a role in almost all cognitive functions (see Figure 1). The abnormal functional organization of these networks and dynamic cross-network talk may underlie a wide range of psychiatric symptoms in the “triple-network model of psychopathology” (Menon, 2018; Menon et al., 2022).
The DMN was the first large-scale network identified in human subjects and, later, across all mammalian species studied to date (Garin et al., 2022). Its central nodes consist of the posterior cingulate cortex (PCC), precuneus, and ventromedial prefrontal cortex (VMPFC; Bressler and Menon, 2010). The DMN is often referred to as a task-negative network, characterized by a stable and replicable deactivation of its core nodes during tasks requiring cognitive effort in PET and fMRI studies (e.g., Raichle et al., 2001). Nonetheless, some nodes are active throughout cognitive processing, implying that DMN plays a more complex and dynamic role in cognition (Weber et al., 2022). It was shown that the DMN is active during tasks requiring autobiographical memory, prospective thinking, ego/allocentric spatial reference, and understanding of others’ intentions (Buckner et al., 2008; Spreng et al., 2009). Additionally, the DMN is crucial for high-level social cognitive processes, mediating individual variability in cognitive empathy response (Oliveira-Silva et al., 2023).
FPN activity is significantly negatively correlated with DMN (Uddin and Menon, 2009), and its activation is relatively strongest during cognitive effort. Its function is primarily related to task selection and executive function, using input from other brain networks to actively process information, and supporting higher-order cognitive functions, such as attentional control and working memory. The FPN is also essential for decision-making in the context of goal-directed behavior in rule-based problem-solving (Lindquist and Barrett, 2012). It connects the lateral posterior parietal cortex (PPC) and the dorsolateral prefrontal cortex (DLPFC; Seeley et al., 2007).
The SN includes the AI and dorsolateral cingulate cortex (dACC; Sridharan et al., 2008). It is distinguished by a unique cellular component, the von Economo neurons in the AI/dACC (Banovac et al., 2021), characterized by a large spindle-shaped body. The SN functions as a dynamic switch between concentration on self and the inner world, mediated by the DMN, and task-related and directed attention on outside stimuli maintained by the FPN. Additionally, the amygdala and other SN subcortical nodes co-activate in response to various experimental tasks, indicating a more domain-general role in identifying homeostatically most relevant competing internal and external stimuli (Chong et al., 2017; Seeley, 2019). Its function has been shown to be relevant for processing reward, motivation, emotion, and pain (Menon, 2015).
Allocation of attentional resources to the most salient stimuli requires top-down sensitivity control and a bottom-up mechanism for filtering stimuli (Parr and Friston, 2017). A central role of the SN is filled by the insula, acting as a gatekeeper of executive control. Thanks to a widespread connectivity fingerprint, its posterior part integrates signals from within the body with external stimuli. Then, the interaction of the anterior and posterior parts of the insula moderates autonomic reactions and generates a signal sent to the anterior cingulate cortex (ACC), selectively intensifying salient stimuli that require further cortical analysis. The right dAIC is considered to be a unique brain region, functioning as a hub that influences both the FPN and DMN (Uddin, 2015). A strong negative correlation between the DMN and FPN relates to the higher efficiency of executive functions (Posner et al., 2016; see Figure 2).
3. Network dysfunction. The impact of network materiality on dysfunction
The correct SN response determines the appropriateness of behavior, and the AI plays a key role in the proper functioning of the entire network. Disorders within this structure are correlated with many cognitive-affective dysfunctions—including those associated with both psychiatric disorders and neurodegenerative diseases.
3.1. Deficits associated with an overactive salience network
Overactivity in the AI-dACC pathway is mainly associated with affective disorders (high anxiety) and neuroticism (Massullo et al., 2020). Findings indicate elevated AI activity in response to facial emotional expressions (Paulus and Stein, 2006), particularly in individuals with high levels of anxiety (Stein et al., 2007). Paulus et al. (2003) showed an association of AI with questionnaire measures—neuroticism and risk avoidance, as well as behavioral measures in making risky decisions in a gambling game. Higher activation within this structure was characteristic of riskier decisions and predicted the likelihood of choosing the safe option in the next choice. This suggests that people with elevated levels of neuroticism may interpret relatively safe situations as threatening (Feinstein et al., 2006). Hamilton et al. (2013), in a review article, presented findings showing the activity of key SN structures (AI and ACC) and the amygdala, in response to negative stimuli in depressed individuals. They also observed elevated AI activity in insomniacs when trying to fall asleep (Chen et al., 2014) and in the right AI among narcissistic individuals (Fan et al., 2011).
In addition, resting-state studies also contribute to our understanding of SN function and dysfunction. Seeley et al. (2007) noted a positive correlation between reported levels of pre-test anxiety and a measure of the strength of functional connections between AI and dACC. Markett et al. (2013), on the other hand, showed a correlation between Cloninger’s temperamental harm avoidance scale and the strength of connections between AI and ACC and AI and DLPFC. Stronger functional connectivity between dorsal ACC and new cortex regions has been reported in patients diagnosed with panic anxiety (Pannekoek et al., 2013).
It is noteworthy that SN hyperactivity is linked not only to psychological but also physical vulnerability. For example, the volume of gray matter within the insula and ACC, among others, is characteristically high in patients suffering from chronic pain (Borsook et al., 2013; Cauda et al., 2014), and the subjectively perceived level of pain is correlated with the strength of AI and ACC activations (Legrain et al., 2011).
All of the above results seem consistent with the SN model and suggest that AI overactivation leads to excessive sensitivity and anxiety arousal, and maybe a joint transdiagnostic characteristic in numerous conditions. This is likely related to the low excitability threshold of the structures that make up the SN (particularly the right AI) and the classification of excessive stimuli as important. This leads to the generalization and over-mobilization of stress reactions in non-threatening situations (Menon and Uddin, 2010; Hermans et al., 2014). In the case of narcissism, on the other hand, a proposed model of SN dysfunction relies on the inability of a dysregulated right AI to turn off the DMN, leading to an excessive concentration of thoughts on one’s self (Jankowiak-Siuda and Zajkowski, 2013).
Functional MRI data suggests that at least three subdivisions can be recognized within the insula on the basis of differential FC patterns: a dorsal anterior (dAI) involved in high-level cognitive control processes (activated by tasks requiring attention and redirecting information to the DLPFC-PPC loop), a ventral anterior (vAI) involved in affective processes (responsible for the flow of affective stimuli to specialized areas within the limbic cortex and medial prefrontal cortex), and a posterior insula (PI) involved in sensorimotor processing (Deen et al., 2011; Chang et al., 2013). Subjects with stronger connections in the ventral stream were characterized by stronger affective feelings, and subjects with stronger connections in the dorsal stream performed faster and more effectively on a cognitive task requiring the activity of attentional processes.
Alterations within all brain networks activity and connectivity in the Triple Network Model (TNM) may underlie Post Traumatic Stress Disorder (PTSD; Lebois et al., 2022). It is proposed that overactive and hyperconnected SN destabilizes intrinsically weakly connected and hypoactive DMN and FPN. In pursuance of this model, alternations in networks e.g., increased posterior SN connectivity to the PI may result in raised sensitivity to stimuli and potential threats, that contribute to avoidance and hypervigilance which characterize PTSD patients. Hyperactivation in the AI is linked with re-experiencing traumatic memories (Nicholson et al., 2020).
The SN with a low threshold for perceived saliency is not able to efficiently regulate the DMN and FPN switching (Weng et al., 2019). The impairment of cognitive control over salience processing in PTSD may be reflected in the reduced insular functional connectivity in the ACC and the supplementary motor region (Lee et al., 2022). The FPN and the DMN are weakly interconnected and hypoactive, which causes narrowed cognition and incapacity for top-down SN regulation in the FPN as well as dissociation and fear generalization in the DMN.
As shown by Fenster et al. (2018), low involvement in AI is linked to depersonalization and emotional detachment symptoms in PTSD. However, Akiki et al. (2017) suggest that alterations within the DMN may also underlie impairments in the processing of self-referential information. In addition, hyperconnectivity of the DMN with prefrontal FPN areas may limit the capacity of the FPN to engage in other cognitively demanding tasks, thus underpinning symptoms of reduced cognitive efficacy in the PTSD group. Charquero-Ballester et al. (2022) demonstrated positive correlations between activity of SN and severity of PTSD symptoms and showed that successful Cognitive Therapy for PTSD can normalize the dynamics of brain networks. The Triple Network Model offers a valuable way of comprehending the underlying neural mechanisms of PTSD, but it is unlikely to account for all PTSD abnormalities.
3.2. Deficits associated with underperformance of the salience network
Reduced strength of causal influence from the AI to the FPN and DMN has been linked to cognitive and affective deficits. Up until now, the best-documented links relate to schizophrenia, autism, and bipolar disorder.
Schizophrenia is characterized by impaired thinking and perception as well as shallow, maladaptive affect, which can be considered a defect of executive control. As shown by Limongi et al. (2020), the key SN nodes’ excitation-inhibition balance is impacted by the pathophysiology of glutamate neurotransmission. Reduced FC has been demonstrated between the SN and DMN (Buckner et al., 2009; Orliac et al., 2013) and between the SN and FPN (Moran et al., 2013), as well as within SN—between the AI and dACC (White et al., 2010). Structural MRI studies in people with schizophrenia have revealed a smaller volume of gray matter, encompassing all three networks (Palaniyappan et al., 2011; Krishnadas et al., 2014). The most recent research on individuals with schizophrenia revealed a general decrease in insula FC, as well as a reduction in the differentiation of connectivity profiles between insular subregions, which was associated with clinical symptom variability (Tian et al., 2019).
Orliac et al. (2013) noted negative moderate correlations between left striatum connectivity (included in the SN) and levels of hallucinations and depression. The researchers interpret this as a potential confirmation of the “relevance dysfunction” (aberrant salience) hypothesis in schizophrenia, proposed by Kapur (2003). It assumes that dysfunctional connections of the corticothalamic-parietal loop lead to chaotic discharges of dopaminergic neurons, disrupting the stimulus relevance selection taking place in the SN (Menon et al., 2022; Pugliese et al., 2022). On the other hand, Palaniyappan et al. (2013) highlighted the disruption between the SN and FPN. Granger causality analysis indicated a significantly reduced effect of SN on FPN activity, manifested by the inability to strongly engage executive structures and “mute” the DMN during cognitive effort.
The theory of predictive coding (PC) and Bayesian inference offers a comprehensive principle of brain function with the potential to link various levels of observation into a more unified model of schizophrenia (e.g., Adams et al., 2022 or Limongi et al., 2018). PC defines a biological scheme, where the brain can be seen as a computational organ generating predictions to infer the probable causes of the sensory signals, which can be compared with actual sensory samples (Friston, 2010). Bottom-up sensory evidence (information from the sensory milieu) ascends brain hierarchical architecture, where the lower levels of the brain receive predictive signals from higher levels of the brain, which encode prior beliefs. The accuracy of prediction is cyclically tested—when the incoming sensory input violates predictions, a prediction error (PE) is created and sent forward to update higher-level expectations (Bayesian belief updating; Friston, 2019). Agents weigh new evidence and prior knowledge according to the level of confidence placed in a prediction or PE, which determines the impact on belief updates. The insular cortex in this framework is seen as an integrator of low-level sensory PEs with interoceptive expectations, regulating emotion and affective salience (Barrett and Simmons, 2015).
In addition, the SN plays an essential role in the bidirectional circulation of prior beliefs, in order to execute functional integration and activation of task stimuli (Limongi et al., 2020). Royer et al. (2020) showed an insula microstructural gradient transition with changes in local affiliation: from the granular posterior, through ventral, up to agranular dorsal anterior subregions. The shift in gradient corresponded with an FC transition from primarily sensorimotor (unimodal) to modulatory and association (transmodal) networks, analogous to the hierarchical organization of other subcortical systems responsible for perceptual, control, and higher-level cognitive functions. Therefore, the multidimensional cytoarchitecture of the insular cortex (and the whole SN) is well suited for computing and transmitting the accuracy of ascending sensory PEs. The FC hierarchical gradient is considered to be a large-scale neural architecture for the PC and allostasis—predictive regulation of the body’s energy resources, which is vital for every aspect of a living organism (Katsumi et al., 2022).
The view that brain inference systems are changed in schizophrenia is supported by well-documented deficits in cognitive decision-making in numerous studies (e.g., Schmack et al., 2015; Kirihara et al., 2020). Failures of inference can explain a wide range of psychotic symptoms and traits (Friston et al., 2016). Neurotransmitter alterations underlie imprecision in the PC hierarchical mechanism, particularly in the post-synaptic gain of cortical NMDA receptors and GABAergic neurons with elevated dopaminergic neuromodulation. Disturbed neural PE signals induce misattribution of the salience of stimuli. The participation of the insula in monitoring the disruption of predictions is compatible with its function in processing salient stimuli and neuropathology in the assignment of behavioral salience to non-target stimuli in schizophrenia (Sridharan et al., 2008).
Furthermore, Luo et al. (2020) showed that control signals from rAI are improperly elevated and directed towards both the FPN and DMN, disrupting the contextually congruent assignment of brain resources in patients with schizophrenia. Liddle et al. (2016) used magnetoencephalography (MEG) to measure beta oscillations in the insula during a saliency modulation task to compare activity during task-relevant and task-irrelevant stimulus processing. Beta oscillations were chosen as they mediate endogenous long-range integrative signals or prior expectations to recurring environmental stimuli. When compared to healthy controls, schizophrenia patients had more beta synchronization in the insula when processing irrelevant stimuli over relevant ones (stronger reaction to disruption of prediction; Fries, 2015).
Empirical studies also link schizophrenia symptoms to abnormal signaling of PEs (particularly in the brain areas of reward, value-based decision-making), lack of long-term stability of internal models and priors (Sterzer et al., 2019). A DCM study of the PC provided further evidence of abnormal connectivity in the neuropathology and pathophysiology of schizophrenia (Fogelson et al., 2014). The long-standing unpredictability about upcoming sensory inputs finally leads to stimulus avoidance and psychomotor poverty, which is observed in clinical conditions (Corlett et al., 2016). In conclusion, the symptoms of schizophrenia are consistent with a decrease in high-level precision or a failure of sensory attenuation (an overestimate of the trustworthiness of the PEs), leading to false inferences and failure in cognitive control as well as the possibility of hallucinations and delusions (Sterzer et al., 2018).
Autism spectrum disorder (ASD) belongs to a group of developmental disorders characterized by qualitative abnormalities in social interactions and behavioral patterns, as well as a limited and repetitive repertoire of interests and activities (ICD-10). A meta-analysis of fMRI studies found that AI and ACC are regularly less active in people with autism, compared to a control group, during social tasks (Di Martino et al., 2009). Uddin and Menon’s (2009) model of dysfunction in autism posits that the disorder is caused by deficits in communication between sensory and limbic structures and the insula. This leads to the SN’s “underestimation” of the importance of social stimuli, which explains the phenotype of characteristic dysfunctions in responding to social stimuli. Moreover, changes in the FC pattern among the dAIC, DMN, and FPN correlate with the severity of ASD symptoms (Uddin et al., 2015). Gonzalez-Gadea et al. (2015), using the PC framework, implied that persons with ASD may have reduced precision adjustment when confronted with uncertainty because of rigid expectations (Van de Cruys et al., 2014). The predisposition to suppress bottom-up inputs and the attentional bias toward anticipated stimuli may hinder the ability to adjust precision in dynamic real-world contexts. This result is consistent with previous research on predictive coding in ASD (Lawson et al., 2014), which indicates that autistic persons struggle to contextualize sensory input in light of their preexisting beliefs and that these deficits primarily manifest in situations of uncertainty (Gomot and Wicker, 2012).
Meta-analysis of bipolar disorder (BD) patients focused on rs-fMRI and analysis of effective connectivity have shown that functional integration within and among three core brain networks (SN, DMN, and FPN) is abnormal (Sha et al., 2019; Yoon et al., 2021; Zhang et al., 2022). Altered connectivity patterns were dependent on mood, as well as the type of BD (Zhang et al., 2022). BD patients expressed altered connectivity both within networks (FPN, SN) and between (DMN-SN, DMN-FPN). There were also differences between stages of the disorder: compared to the depression stage, patients with euthymic stage expressed a hyperconnectivity among the FPN and reduced connectivity between SN and FPN and SN and DMN (Zhang et al., 2022). Martino and Magioncalda (2022) and Magioncalda and Martino (2022) suggested that the lack of integration between SN, DMN, and FPN may be due to changes in neurotransmitter signaling which can be observed during the manic and depressive phases of BD.
4. Discussion, limitations, and directions for further research
This mini-review segregates SN dysfunctions into hyperactivity and hypoactivity, which can lead to a simplistic perception of the mechanisms of the described deficits. However, it should be noted that the actual role of the SN in the presented disorders is more elusive. First, SN dysfunctions are a unifying feature of a whole range of deficits, but this does not mean that they are the only or even the main cause. Second, the relationships between and within SN structures themselves are complex and varied, which is one reason why the dysfunctions themselves are different. Third, atypical connections or activations within SNs are not sufficient conditions for cognitive-affective dysfunction to occur.
The second point, which entails exploring the more intricate interactions and conditional dependencies that distinguish the mechanisms underlying various disorders, seems most intriguing from the standpoint of future research. Attempts have been made to specify these mechanisms, such as the briefly described neural models of dysfunction in autism (Uddin and Menon, 2009), narcissism (Jankowiak-Siuda and Zajkowski, 2013) or schizophrenia (Kapur, 2003; Palaniyappan and Liddle, 2012). However, most of them are not yet supported by enough empirical evidence to fully validate all the hypotheses they pose; for now, they mainly serve to steer further research.
It must also be taken into account that the functional connectivity data derived from imaging studies suffer from limited spatial and temporal resolution, limiting the inference to sufficiently large brain areas and sufficiently slow dynamical processes. Coupling these findings with methods capturing millisecond dynamics (such as single or multi-electrode arrays; Spira and Hai, 2013) could lead to new insights and fuller understanding of the processes governing the network dynamics.
Author contributions
JS: visualization, writing—original draft, writing—review and editing. JT: writing—original draft. WZ: conceptualization, writing—original draft, writing—review and editing. KJ-S: conceptualization, funding acquisition, project administration, writing—original draft, writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was partially supported by a Grant from SWPS University of Social Sciences and Humanities SUB/IPsy/04/2021/04.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, R. A., Vincent, P., Benrimoh, D., Friston, K. J., and Parr, T. (2022). Everything is connected: inference and attractors in delusions. Schizophr. Res. 245, 5–22. doi: 10.1016/j.schres.2021.07.032
Akiki, T. J., Averill, C. L., and Abdallah, C. G. (2017). A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep. 19:81. doi: 10.1007/s11920-017-0840-4
Banovac, I., Sedmak, D., Judaš, M., and Petanjek, Z. (2021). Von economo neurons - primate-specific or commonplace in the mammalian brain? Front. Neural Circuits 15:714611. doi: 10.3389/fncir.2021.714611
Barrett, L. F., and Satpute, A. B. (2013). Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 23, 361–372. doi: 10.1016/j.conb.2012.12.012
Barrett, L. F., and Simmons, W. K. (2015). Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429. doi: 10.1038/nrn3950
Borsook, D., Edwards, R., Elman, I., Becerra, L., and Levine, J. (2013). Pain and analgesia: the value of salience circuits. Prog. Neurobiol. 104, 93–105. doi: 10.1016/j.pneurobio.2013.02.003
Bressler, S. L., and McIntosh, A. R. (2007). “The role of neural context in large-scale neurocognitive network operations,” in Handbook of Brain Connectivity, Eds. V. K. Jirsa and A. McIntosh (Berlin, Heidelberg: Springer Berlin Heidelberg), 403–419.
Bressler, S. L., and Menon, V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14, 277–290. doi: 10.1016/j.tics.2010.04.004
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network: anatomy, function and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability and relation to Alzheimer’s disease. J. Neurosci. 29, 1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009
Cappe, C., and Barone, P. (2005). Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur. J. Neurosci. 22, 2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x
Cauda, F., Palermo, S., Costa, T., Torta, R., Duca, S., Vercelli, U., et al. (2014). Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin. 4, 676–686. doi: 10.1016/j.nicl.2014.04.007
Chang, L. J., Yarkoni, T., Khaw, M. W., and Sanfey, A. G. (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex 23, 739–749. doi: 10.1093/cercor/bhs065
Charquero-Ballester, M., Kleim, B., Vidaurre, D., Ruff, C., Stark, E., Tuulari, J. J., et al. (2022). Effective psychological therapy for PTSD changes the dynamics of specific large-scale brain networks. Hum. Brain Mapp. 43, 3207–3220. doi: 10.1002/hbm.25846
Chen, M. C., Chang, C., Glover, G. H., and Gotlib, I. H. (2014). Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 97, 1–8. doi: 10.1016/j.biopsycho.2013.12.016
Chen, W. G., Schloesser, D., Arensdorf, A. M., Simmons, J. M., Cui, C., Valentino, R., et al. (2021). The emerging science of interoception: sensing, integrating, interpreting and regulating signals within the self. Trends Neurosci. 44, 3–16. doi: 10.1016/j.tins.2020.10.007
Chong, J. S. X., Ng, G. J. P., Lee, S. C., and Zhou, J. (2017). Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Struct. Funct. 222, 1635–1644. doi: 10.1007/s00429-016-1297-7
Ciric, R., Nomi, J. S., Uddin, L. Q., and Satpute, A. B. (2017). Contextual connectivity: A framework for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci. Rep. 7:6537. doi: 10.1038/s41598-017-06866-w
Corlett, P. R., Honey, G. D., and Fletcher, P. C. (2016). Prediction error, ketamine and psychosis: an updated model. J. Psychopharmacol. 30, 1145–1155. doi: 10.1177/0269881116650087
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
Deen, B., Pitskel, N. B., and Pelphrey, K. A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex 21, 1498–1506. doi: 10.1093/cercor/bhq186
Di Martino, A., Ross, K., Uddin, L. Q., Sklar, A. B., Castellanos, F. X., and Milham, M. P. (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry 65, 63–74. doi: 10.1016/j.biopsych.2008.09.022
Fan, Y., Wonneberger, C., Enzi, B., de Greck, M., Ulrich, C., Tempelmann, C., et al. (2011). The narcissistic self and its psychological and neural correlates: an exploratory fMRI study. Psychol. Med. 41, 1641–1650. doi: 10.1017/S003329171000228X
Feinstein, J. S., Stein, M. B., and Paulus, M. P. (2006). Anterior insula reactivity during certain decisions is associated with neuroticism. Soc. Cogn. Affect. Neurosci. 1, 136–142. doi: 10.1093/scan/nsl016
Fenster, R. J., Lebois, L. A. M., Ressler, K. J., and Suh, J. (2018). Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci. 19, 535–551. doi: 10.1038/s41583-018-0039-7
Flynn, F. G. (1999). Anatomy of the insula functional and clinical correlates. Aphasiology 13, 55–78. doi: 10.1080/026870399402325
Fodor, J. A. (1983). The Modularity of Mind. Cambridge, MA, and London, England: MIT Press. Available online at: https://books.google.pl/books?id=0vg0AwAAQBAJ.
Fogelson, N., Litvak, V., Peled, A., Fernandez-del-Olmo, M., and Friston, K. (2014). The functional anatomy of schizophrenia: a dynamic causal modeling study of predictive coding. Schizophr. Res. 158, 204–212. doi: 10.1016/j.schres.2014.06.011
Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220–235. doi: 10.1016/j.neuron.2015.09.034
Friston, K. (2010). The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138. doi: 10.1038/nrn2787
Friston, K. J. (2019). Waves of prediction. PLoS Biol. 17:e3000426. doi: 10.1371/journal.pbio.3000426
Friston, K., Brown, H. R., Siemerkus, J., and Stephan, K. E. (2016). The disconnection hypothesis (2016). Schizophr. Res. 176, 83–94. doi: 10.1016/j.schres.2016.07.014
Garin, C. M., Hori, Y., Everling, S., Whitlow, C. T., Calabro, F. J., Luna, B., et al. (2022). An evolutionary gap in primate default mode network organization. Cell Rep. 39:110669. doi: 10.1016/j.celrep.2022.110669
Garner, A. R., and Keller, G. B. (2022). A cortical circuit for audio-visual predictions. Nat. Neurosci. 25, 98–105. doi: 10.1038/s41593-021-00974-7
Gigerenzer, G., and Todd, P. M. (1999). “Fast and frugal heuristics: the adaptive toolbox,” in Simple Heuristics That Make Us Smart, Eds. G. Gigerenzer and P. M. Todd (New York, NY; Oxford: Oxford University Press), 3–34.
Gilmore, J. H., Knickmeyer, R. C., and Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 19, 123–137. doi: 10.1038/nrn.2018.1
Gomot, M., and Wicker, B. (2012). A challenging, unpredictable world for people with autism spectrum disorder. Int. J. Psychophysiol. 83, 240–247. doi: 10.1016/j.ijpsycho.2011.09.017
Gonzalez-Gadea, M. L., Chennu, S., Bekinschtein, T. A., Rattazzi, A., Beraudi, A., Tripicchio, P., et al. (2015). Predictive coding in autism spectrum disorder and attention deficit hyperactivity disorder. J. Neurophysiol. 114, 2625–2636. doi: 10.1152/jn.00543.2015
Griffa, A., Amico, E., Liégeois, R., Van De Ville, D., and Preti, M. G. (2022). Brain structure-function coupling provides signatures for task decoding and individual fingerprinting. Neuroimage 250:118970. doi: 10.1016/j.neuroimage.2022.118970
Hamilton, J. P., Chen, M. C., and Gotlib, I. H. (2013). Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol. Dis. 52, 4–11. doi: 10.1016/j.nbd.2012.01.015
Hermans, E. J., Henckens, M. J. A. G., Joëls, M., and Fernández, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 37, 304–314. doi: 10.1016/j.tins.2014.03.006
Jankowiak-Siuda, K., and Zajkowski, W. (2013). A neural model of mechanisms of empathy deficits in narcissism. Med. Sci. Monit. 19, 934–941. doi: 10.12659/MSM.889593
Kapur, S. (2003). Psychosis as a state of aberrant salience: a framework linking biology, phenomenology and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23. doi: 10.1176/appi.ajp.160.1.13
Katsumi, Y., Theriault, J. E., Quigley, K. S., and Barrett, L. F. (2022). Allostasis as a core feature of hierarchical gradients in the human brain. Netw. Neurosci. 6, 1010–1031. doi: 10.1162/netn_a_00240
Kirihara, K., Tada, M., Koshiyama, D., Fujioka, M., Usui, K., Araki, T., et al. (2020). A predictive coding perspective on mismatch negativity impairment in schizophrenia. Front. Psychiatry 11:660. doi: 10.3389/fpsyt.2020.00660
Krishnadas, R., Palaniyappan, L., Lang, J., McLean, J., and Cavanagh, J. (2014). Psychoticism and salience network morphology. Pers. Individ. Differ. 57, 37–42. doi: 10.1016/j.paid.2013.09.016
Lawson, R. P., Rees, G., and Friston, K. J. (2014). An aberrant precision account of autism. Front. Hum. Neurosci. 8:302. doi: 10.3389/fnhum.2014.00302
Lebois, L. A. M., Kumar, P., Palermo, C. A., Lambros, A. M., O’Connor, L., Wolff, J. D., et al. (2022). Deconstructing dissociation: a triple network model of trauma-related dissociation and its subtypes. Neuropsychopharmacology 47, 2261–2270. doi: 10.1038/s41386-022-01468-1
Lee, D., Lee, J. E., Lee, J., Kim, C., and Jung, Y.-C. (2022). Insular activation and functional connectivity in firefighters with post-traumatic stress disorder. BJPsych Open 8:e69. doi: 10.1192/bjo.2022.32
Legrain, V., Iannetti, G. D., Plaghki, L., and Mouraux, A. (2011). The pain matrix reloaded. Prog. Neurobiol. 93, 111–124. doi: 10.1016/j.pneurobio.2010.10.005
Li, Y., Cai, H., Li, X., Qian, Y., Zhang, C., Zhu, J., et al. (2022). Functional connectivity of the central autonomic and default mode networks represent neural correlates and predictors of individual personality. J. Neurosci. Res. 100, 2187–2200. doi: 10.1002/jnr.25121
Liddle, E. B., Price, D., Palaniyappan, L., Brookes, M. J., Robson, S. E., Hall, E. L., et al. (2016). Abnormal salience signaling in schizophrenia: the role of integrative beta oscillations. Hum. Brain Mapp. 37, 1361–1374. doi: 10.1002/hbm.23107
Limongi, R., Bohaterewicz, B., Nowicka, M., Plewka, A., and Friston, K. J. (2018). Knowing when to stop: Aberrant precision and evidence accumulation in schizophrenia. Schizophr. Res. 197, 386–391. doi: 10.1016/j.schres.2017.12.018
Limongi, R., Jeon, P., Mackinley, M., Das, T., Dempster, K., Théberge, J., et al. (2020). Glutamate and dysconnection in the salience network: neurochemical, effective connectivity and computational evidence in schizophrenia. Biol. Psychiatry 88, 273–281. doi: 10.1016/j.biopsych.2020.01.021
Lindquist, K. A., and Barrett, L. F. (2012). A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn. Sci. 16, 533–540. doi: 10.1016/j.tics.2012.09.005
Liu, C.-C., Moosa, S., Quigg, M., and Elias, W. J. (2021). Anterior insula stimulation increases pain threshold in humans: a pilot study. J. Neurosurg. 135, 1487–1492. . [Online ahead of print]. doi: 10.3171/2020.10.JNS203323
Luo, L. (2021). Architectures of neuronal circuits. Science 373:eabg7285. doi: 10.1126/science.abg7285
Luo, Q., Pan, B., Gu, H., Simmonite, M., Francis, S., Liddle, P. F., et al. (2020). Effective connectivity of the right anterior insula in schizophrenia: The salience network and task-negative to task-positive transition. Neuroimage Clin. 28:102377. doi: 10.1016/j.nicl.2020.102377
Magioncalda, P., and Martino, M. (2022). A unified model of the pathophysiology of bipolar disorder. Mol. Psychiatry 27, 202–211. doi: 10.1038/s41380-021-01091-4
Markett, S., Weber, B., Voigt, G., Montag, C., Felten, A., Elger, C., et al. (2013). Intrinsic connectivity networks and personality: the temperament dimension harm avoidance moderates functional connectivity in the resting brain. Neuroscience 240, 98–105. doi: 10.1016/j.neuroscience.2013.02.056
Martino, M., and Magioncalda, P. (2022). Tracing the psychopathology of bipolar disorder to the functional architecture of intrinsic brain activity and its neurotransmitter modulation: a three-dimensional model. Mol. Psychiatry 27, 793–802. doi: 10.1038/s41380-020-00982-2
Massullo, C., Carbone, G. A., Farina, B., Panno, A., Capriotti, C., Giacchini, M., et al. (2020). Dysregulated brain salience within a triple network model in high trait anxiety individuals: a pilot EEG functional connectivity study. Int. J. Psychophysiol. 157, 61–69. doi: 10.1016/j.ijpsycho.2020.09.002
McClure, J. P., Erkat, O. B., Corbo, J., and Polack, P.-O. (2022). Estimating how sounds modulate orientation representation in the primary visual cortex using shallow neural networks. Front. Syst. Neurosci. 16:869705. doi: 10.3389/fnsys.2022.869705
Menon, V. (2015). “Large-scale functional brain organization,” in Brain Mapping: An Encyclopedic Reference, Ed. A. W. Toga (New York, NY: Elsevier), 449–459.
Menon, V. (2018). The triple network model, insight and large-scale brain organization in autism. Biol. Psychiatry 84, 236–238. doi: 10.1016/j.biopsych.2018.06.012
Menon, V., Palaniyappan, L., and Supekar, K. (2022). Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol. Psychiatry S0006322322016377. [Online ahead of print]. doi: 10.1016/j.biopsych.2022.09.029
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Moran, L. V., Tagamets, M. A., Sampath, H., O’Donnell, A., Stein, E. A., Kochunov, P., et al. (2013). Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol. Psychiatry 74, 467–474. doi: 10.1016/j.biopsych.2013.02.029
Nelson, S. M., Dosenbach, N. U. F., Cohen, A. L., Wheeler, M. E., Schlaggar, B. L., and Petersen, S. E. (2010). Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 214, 669–680. doi: 10.1007/s00429-010-0260-2
Nicholson, A. A., Harricharan, S., Densmore, M., Neufeld, R. W. J., Ros, T., McKinnon, M. C., et al. (2020). Classifying heterogeneous presentations of PTSD via the default mode, central executive and salience networks with machine learning. Neuroimage Clin. 27:102262. doi: 10.1016/j.nicl.2020.102262
Oliveira-Silva, P., Maia, L., Coutinho, J., Moreno, A. F., Penalba, L., Frank, B., et al. (2023). Nodes of the default mode network implicated in the quality of empathic responses: a clinical perspective of the empathic response. Int. J. Clin. Health Psychol. 23:100319. doi: 10.1016/j.ijchp.2022.100319
Orliac, F., Naveau, M., Joliot, M., Delcroix, N., Razafimandimby, A., Brazo, P., et al. (2013). Links among resting-state default-mode network, salience network and symptomatology in schizophrenia. Schizophr. Res. 148, 74–80. doi: 10.1016/j.schres.2013.05.007
Palaniyappan, L., and Liddle, P. F. (2012). Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 37, 17–27. doi: 10.1503/jpn.100176
Palaniyappan, L., Mallikarjun, P., Joseph, V., White, T. P., and Liddle, P. F. (2011). Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr. Res. 129, 163–168. doi: 10.1016/j.schres.2011.03.020
Palaniyappan, L., Simmonite, M., White, T. P., Liddle, E. B., and Liddle, P. F. (2013). Neural primacy of the salience processing system in schizophrenia. Neuron 79, 814–828. doi: 10.1016/j.neuron.2013.06.027
Pannekoek, J. N., Veer, I. M., van Tol, M.-J., van der Werff, S. J. A., Demenescu, L. R., Aleman, A., et al. (2013). Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J. Affect. Disord. 145, 29–35. doi: 10.1016/j.jad.2012.07.006
Park, H.-J., and Friston, K. (2013). Structural and functional brain networks: from connections to cognition. Science 342:1238411. doi: 10.1126/science.1238411
Parr, T., and Friston, K. J. (2017). Working memory, attention and salience in active inference. Sci. Rep. 7:14678. doi: 10.1038/s41598-017-15249-0
Paulus, M. P., Rogalsky, C., Simmons, A., Feinstein, J. S., and Stein, M. B. (2003). Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage 19, 1439–1448. doi: 10.1016/s1053-8119(03)00251-9
Paulus, M. P., and Stein, M. B. (2006). An insular view of anxiety. Biol. Psychiatry 60, 383–387. doi: 10.1016/j.biopsych.2006.03.042
Posner, J., Cha, J., Wang, Z., Talati, A., Warner, V., Gerber, A., et al. (2016). Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology 41, 1759–1767. doi: 10.1038/npp.2015.342
Pugliese, V., de Filippis, R., Aloi, M., Rotella, P., Carbone, E. A., Gaetano, R., et al. (2022). Aberrant salience correlates with psychotic dimensions in outpatients with schizophrenia spectrum disorders. Ann. Gen. Psychiatry 21:25. doi: 10.1186/s12991-022-00402-5
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 676–682. doi: 10.1073/pnas.98.2.676
Royer, J., Paquola, C., Larivière, S., Vos de Wael, R., Tavakol, S., Lowe, A. J., et al. (2020). Myeloarchitecture gradients in the human insula: histological underpinnings and association to intrinsic functional connectivity. Neuroimage 216:116859. doi: 10.1016/j.neuroimage.2020.116859
Sadaghiani, S., and Kleinschmidt, A. (2013). Functional interactions between intrinsic brain activity and behavior. Neuroimage 80, 379–386. doi: 10.1016/j.neuroimage.2013.04.100
Salomon, R., Ronchi, R., Dönz, J., Bello-Ruiz, J., Herbelin, B., Faivre, N., et al. (2018). Insula mediates heartbeat related effects on visual consciousness. Cortex 101, 87–95. doi: 10.1016/j.cortex.2018.01.005
Schmack, K., Schnack, A., Priller, J., and Sterzer, P. (2015). Perceptual instability in schizophrenia: probing predictive coding accounts of delusions with ambiguous stimuli. Schizophr. Res. Cogn. 2, 72–77.
Seeley, W. W. (2019). The salience network: a neural system for perceiving and responding to homeostatic demands. J. Neurosci. 39, 9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007
Sha, Z., Wager, T. D., Mechelli, A., and He, Y. (2019). Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry 85, 379–388. doi: 10.1016/j.biopsych.2018.11.011
Shanmugan, S., Seidlitz, J., Cui, Z., Adebimpe, A., Bassett, D. S., Bertolero, M. A., et al. (2022). Sex differences in the functional topography of association networks in youth. Proc. Natl. Acad. Sci. U S A 119:e2110416119. doi: 10.1073/pnas.2110416119
Singer, T., Critchley, H. D., and Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. doi: 10.1016/j.tics.2009.05.001
Spira, M. E., and Hai, A. (2013). Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94. doi: 10.1038/nnano.2012.265
Spreng, R. N., Mar, R. A., and Kim, A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510. doi: 10.1162/jocn.2008.21029
Sridharan, D., Levitin, D. J., and Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U S A 105, 12569–12574. doi: 10.1073/pnas.0800005105
Stein, M. B., Simmons, A. N., Feinstein, J. S., and Paulus, M. P. (2007). Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry 164, 318–327. doi: 10.1176/ajp.2007.164.2.318
Sterzer, P., Adams, R. A., Fletcher, P., Frith, C., Lawrie, S. M., Muckli, L., et al. (2018). The predictive coding account of psychosis. Biol. Psychiatry 84, 634–643. doi: 10.1016/j.biopsych.2018.05.015
Sterzer, P., Voss, M., Schlagenhauf, F., and Heinz, A. (2019). Decision-making in schizophrenia: a predictive-coding perspective. Neuroimage 190, 133–143. doi: 10.1016/j.neuroimage.2018.05.074
Tian, Y., Zalesky, A., Bousman, C., Everall, I., and Pantelis, C. (2019). Insula functional connectivity in schizophrenia: Subregions, gradients and symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 399–408. doi: 10.1016/j.bpsc.2018.12.003
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. doi: 10.1038/nrn3857
Uddin, L. Q., Betzel, R. F., Cohen, J. R., Damoiseaux, J. S., De Brigard, F., Eickhoff, S., et al. (2022). Controversies and progress on standardization of large-scale brain network nomenclature. Open Science Framework [Preprint]. doi: 10.31219/osf.io/25za6
Uddin, L. Q., and Menon, V. (2009). The anterior insula in autism: under-connected and under-examined. Neurosci. Biobehav. Rev. 33, 1198–1203. doi: 10.1016/j.neubiorev.2009.06.002
Uddin, L. Q., Nomi, J. S., Hébert-Seropian, B., Ghaziri, J., and Boucher, O. (2017). Structure and function of the human insula. J. Clin. Neurophysiol. 34, 300–306. doi: 10.1097/WNP.0000000000000377
Uddin, L. Q., Supekar, K., Lynch, C. J., Cheng, K. M., Odriozola, P., Barth, M. E., et al. (2015). Brain state differentiation and behavioral inflexibility in autism. Cereb. Cortex 25, 4740–4747. doi: 10.1093/cercor/bhu161
Uddin, L. Q., Yeo, B. T. T., and Spreng, R. N. (2019). Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 32, 926–942. doi: 10.1007/s10548-019-00744-6
Van de Cruys, S., Evers, K., Van der Hallen, R., Van Eylen, L., Boets, B., De-Wit, L., et al. (2014). Precise minds in uncertain worlds: predictive coding in autism. Psychol. Rev. 121, 649–675. doi: 10.1037/a0037665
Vicario, C. M., Nitsche, M. A., Salehinejad, M. A., Avanzino, L., and Martino, G. (2020). Time processing, interoception and insula activation: a mini-review on clinical disorders. Front. Psychol. 11:1893. doi: 10.3389/fpsyg.2020.01893
Wager, T. D., and Barrett, L. F. (2017). From affect to control: functional specialization of the insula in motivation and regulation. bioRxiv [Preprint]. doi: 10.1101/102368
Wang, Z., Dai, Z., Gong, G., Zhou, C., and He, Y. (2015). Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist 21, 290–305. doi: 10.1177/1073858414537560
Weber, S., Aleman, A., and Hugdahl, K. (2022). Involvement of the default mode network under varying levels of cognitive effort. Sci. Rep. 12:6303. doi: 10.1038/s41598-022-10289-7
Weng, Y., Qi, R., Zhang, L., Luo, Y., Ke, J., Xu, Q., et al. (2019). Disturbed effective connectivity patterns in an intrinsic triple network model are associated with posttraumatic stress disorder. Neurol. Sci. 40, 339–349. doi: 10.1007/s10072-018-3638-1
White, T. P., Joseph, V., Francis, S. T., and Liddle, P. F. (2010). Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res. 123, 105–115. doi: 10.1016/j.schres.2010.07.020
Xie, X., Cai, C., Damasceno, P. F., Nagarajan, S. S., and Raj, A. (2021). Emergence of canonical functional networks from the structural connectome. Neuroimage 237:118190. doi: 10.1016/j.neuroimage.2021.118190
Yoon, S., Kim, T. D., Kim, J., and Lyoo, I. K. (2021). Altered functional activity in bipolar disorder: a comprehensive review from a large-scale network perspective. Brain Behav. 11:e01953. doi: 10.1002/brb3.1953
Zamorano, A. M., Zatorre, R. J., Vuust, P., Friberg, A., Birbaumer, N., and Kleber, B. (2019). Singing training predicts increased insula connectivity with speech and respiratory sensorimotor areas at rest. bioRxiv [Preprint]. doi: 10.1101/793083
Zerbi, V., Floriou-Servou, A., Markicevic, M., Vermeiren, Y., Sturman, O., Privitera, M., et al. (2019). Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5. doi: 10.1016/j.neuron.2019.05.034
Keywords: triple network model, default mode network (DMN), the frontoparietal network (FPN), salience network (SN), cognitive dysfunctions, affective dysfunctions
Citation: Schimmelpfennig J, Topczewski J, Zajkowski W and Jankowiak-Siuda K (2023) The role of the salience network in cognitive and affective deficits. Front. Hum. Neurosci. 17:1133367. doi: 10.3389/fnhum.2023.1133367
Received: 28 December 2022; Accepted: 22 February 2023;
Published: 20 March 2023.
Edited by:
Yaara Yeshurun, Tel Aviv University, IsraelReviewed by:
Jean Theberge, St Joseph’s Health Care, CanadaCopyright © 2023 Schimmelpfennig, Topczewski, Zajkowski and Jankowiak-Siuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamila Jankowiak-Siuda, a2phbmtvd2lhay1zaXVkYUBzd3BzLmVkdS5wbA==
 Jakub Schimmelpfennig
Jakub Schimmelpfennig Jan Topczewski1
Jan Topczewski1 Wojciech Zajkowski
Wojciech Zajkowski Kamila Jankowiak-Siuda
Kamila Jankowiak-Siuda