- 1School of Electrical and Computer Engineering, College of Engineering, University of Tehran, Tehran, Iran
- 2Department of Psychology, Faculty of Psychology and Education, University of Tehran, Tehran, Iran
- 3School of Cognitive Sciences, Institute for Research in Fundamental Sciences (IPM), Tehran, Iran
- 4Institute for Cognitive Science Studies (ICSS), Tehran, Iran
- 5Department of Neurosurgery, Sina Hospital, Tehran University of Medical Science, Tehran, Iran
Ablation surgeries are utilized to treat certain brain disorders. Recently, these surgeries have become more prevalent using techniques such as magnetic resonance guided focused ultrasound (MRgFUS) ablation and Gamma knife thalamotomy (GKT). However, as the thalamus plays a critical role in cognitive functions, the potential impact of these surgeries on functional connectivity and cognition is a matter of concern. Various approaches have been developed to locate the target for ablation and also investigate changes in functional connectivity before and after surgery. Functional magnetic resonance imaging (fMRI) and electroencephalogram (EEG) are widely used methods for assessing changes in functional connectivity and activity in clinical research. In this Review, we summarize the use of fMRI and EEG in thalamotomy surgeries. Our analysis shows that thalamotomy surgery can result in changes in functional connectivity in motor-related, visuomotor, and default-mode networks, as detected by fMRI. EEG data also indicate a reduction in over-activities observed in the preoperative state.
Introduction
The thalamus is a critical node in the brain network and acts as a hub in information transmission (Tomasi and Volkow, 2011). This area is extensively connected to the cerebral cortex via thalamocortical radiations (Coenen et al., 2014). This massive connectivity means that the thalamus plays a vital role in many cognitive, sensory, and executive functions (Hwang et al., 2017). Manipulating the thalamus, such as through thalamotomy, can have a significant impact on behavior and the brain networks (Halpern et al., 2019; Krishna et al., 2020; Lin et al., 2022), making it an effective treatment for essential tremor (ET), Parkinson’s disease (PD) (Zaaroor et al., 2017), Holmes tremor (HT) (Kim et al., 2002), epilepsy (Sitnikov et al., 2016), neurogenic pain (Sarnthein et al., 2006), multiple sclerosis (MS) (Mathieu et al., 2007), and dyskinesia (Lee, 1997). Monitoring the effects of thalamotomy surgery on brain function is essential to evaluate the success of procedure, predict patient outcomes, and adjust treatment plans accordingly.
Thalamotomy is an effective method to decrease dominant disease symptoms, such as tremors, unwanted motor activity, poor quality of life, and seizures (Lipsman et al., 2013). The first thalamotomy surgery have done using the stereotactic approach was a pallido- thalamotomy, which Spiegel and Wycis carried out in 1946 on a patient with Huntington’s Disease (HD) to reduce emotional reactivity by targeting the medial nucleus of the thalamus (Ea and Ht, 1950; Sarnthein et al., 2006). Today, using new thalamotomy methods such as, Magnetic resonance guided focused ultrasound (MRgFUS) (Stanziano et al., 2022), and gamma knife thalamotomy (GKT) (Tuleasca et al., 2021) these surgeries have become prevalent. However, the system-level description of thalamotomy effectiveness remains rudimentary. The improvement resulting from thalamotomy surgery is thought to arise from changing the activity and the functional connectivity in the associated regions (Jang et al., 2016). To evaluate the functional connectivity and activity in the brain network before and after thalamotomy, it is necessary to use methods such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) (Tuleasca et al., 2018d), beside of other methods such as positron emission topography (PET), magnetoencephalography (MEG) and functional near-infrared spectroscopy (fNIRS). fMRI and EEG is currently the dominant methods due the superior spatial resolution of fMRI and the temporal resolution of EEG, we also evaluate several studies that used other methods during thalamotomy surgery.
Combining EEG and fMRI in preoperative and postoperative conditions will enable a comprehensive evaluation of the functional connectivity and activity during thalamotomy. Although simultaneous EEG-fMRI studies exist (Abreu et al., 2019; Boerwinkle et al., 2022), there is a lack of studies that compare pre and post-thalamotomy conditions. Therefore, the objective of this study is to summarize the application of EEG and fMRI in thalamotomy surgeries. Analysis of the EEG in pre-and postoperative conditions shows a decrease in EEG over-activities after surgery, while, fMRI studies demonstrate changes in functional connectivity, particularly in motor (Hesselmann et al., 2006; Jang et al., 2016; Park et al., 2017; Tuleasca et al., 2021), visuomotor (Tuleasca et al., 2018a,c, 2020; Xiong et al., 2022a), and default-mode networks (Wen et al., 2016; Tuleasca et al., 2018b,e).
Methods
We conducted a PubMed search up to March 2023, entering ‘thalamotomy’ ablation surgery’ in combination (AND) with the following search terms and their corresponding abbreviations: functional, magnetic resonance (fMRI), electroencephalogram (MRI, and EEG), positron emission tomography (PET), single-photon emission computed tomography (SPECT), Magnetoencephalography (MEG), Functional near-infrared spectroscopy (fNIRS), Electrocorticography (ECoG) and was restricted to articles published in English. We followed Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines for meta-analysis (Moher et al., 2009). The PRISMA of the included search results is presented in Figure 1. All data included in this study were related to human diseases that used thalamotomy surgery as a clinical approach. Both unilateral and bilateral thalamotomy were considered. The following inquiries were our focus: We only included articles that met the following criteria: (1) they were written in English, (2) they measured functional connectivity or activity, (3) they involved human subjects, and (4) they provided quantitative or semiquantitative functional activity and connectivity data analyses before and after thalamotomy surgery. We excluded case reports, method articles, case series, and research papers that primarily focused on therapeutic interventions, such as thalamotomy, MRI-guided focused ultrasound, or deep brain stimulation (DBS) and pre-surgical planning.
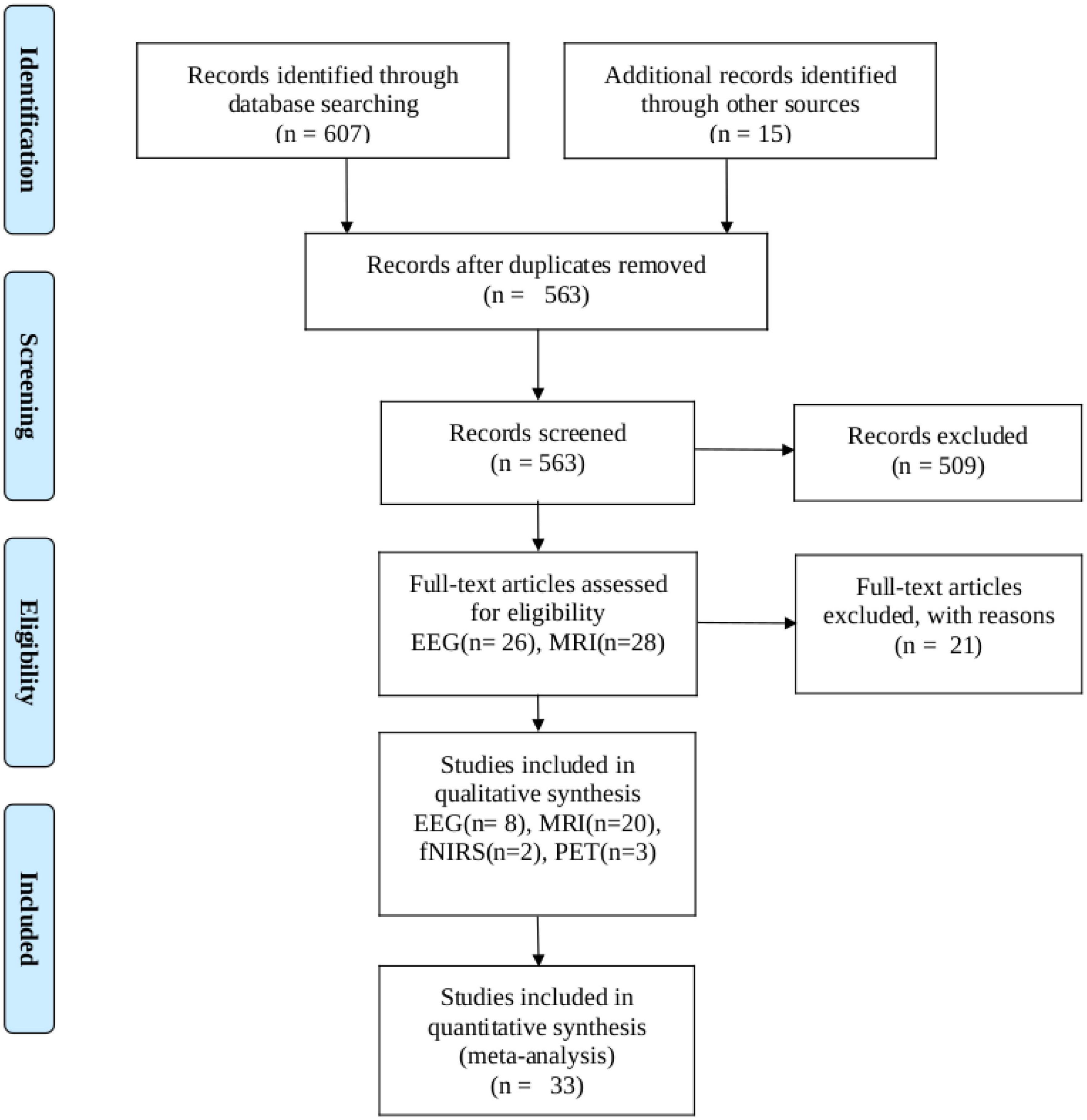
Figure 1. PRISMA flow diagram depicting the study selection process. The search was performed in PubMed up to 12 March 2023.
Results
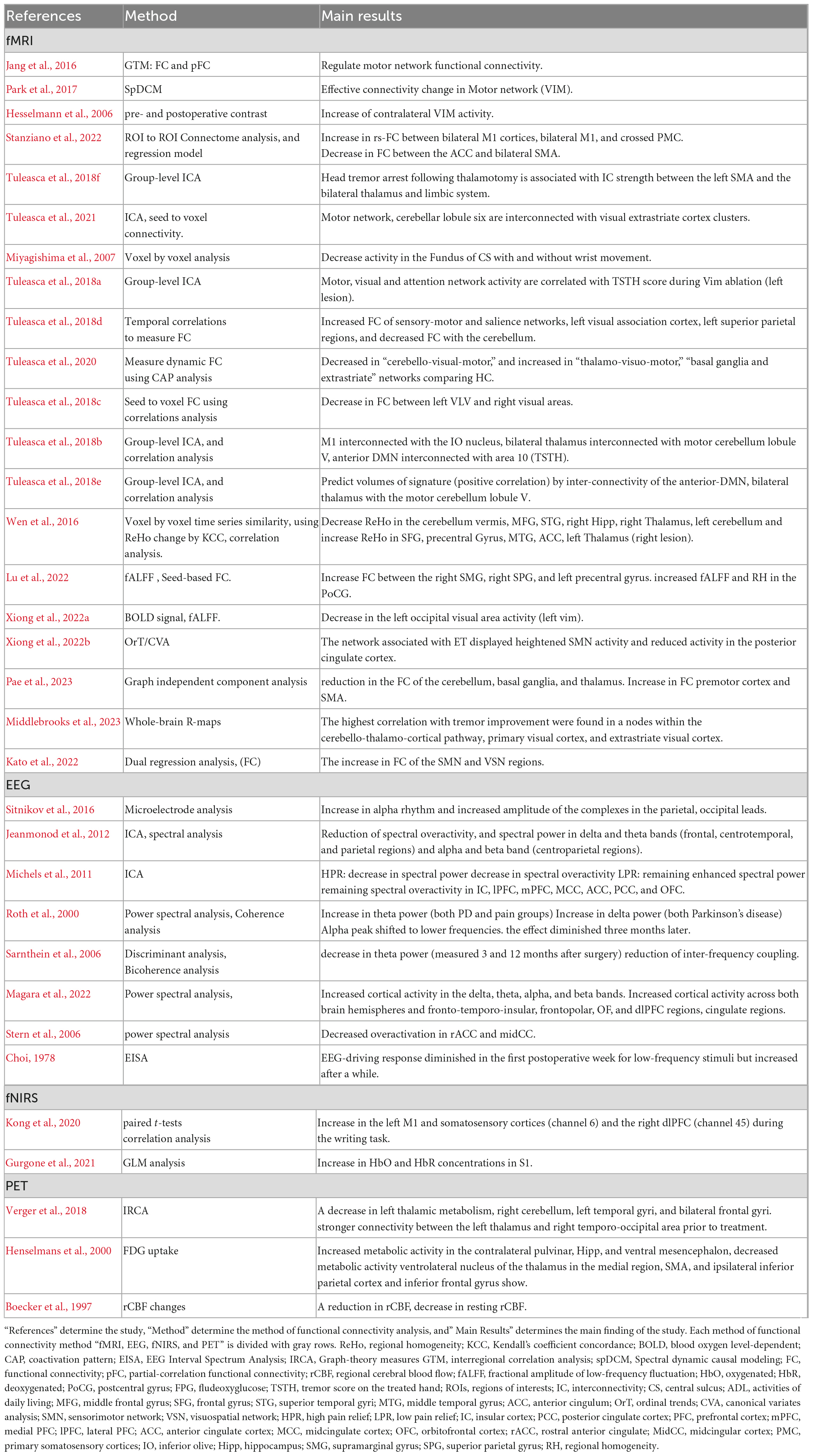
Our search turned up 607 results. Zotero (V6) reference manager imported the obtained references, and duplicates were removed. The validity of each title and abstract was verified separately by the authors. By searching through the references of the approved papers, one more paper was found that matched the criteria. In total, 33 publications met our inclusion criteria, with 20 of them being studies that used fMRI, 8 using EEG, 2 using fNIRS, and 3 using PET investigations.
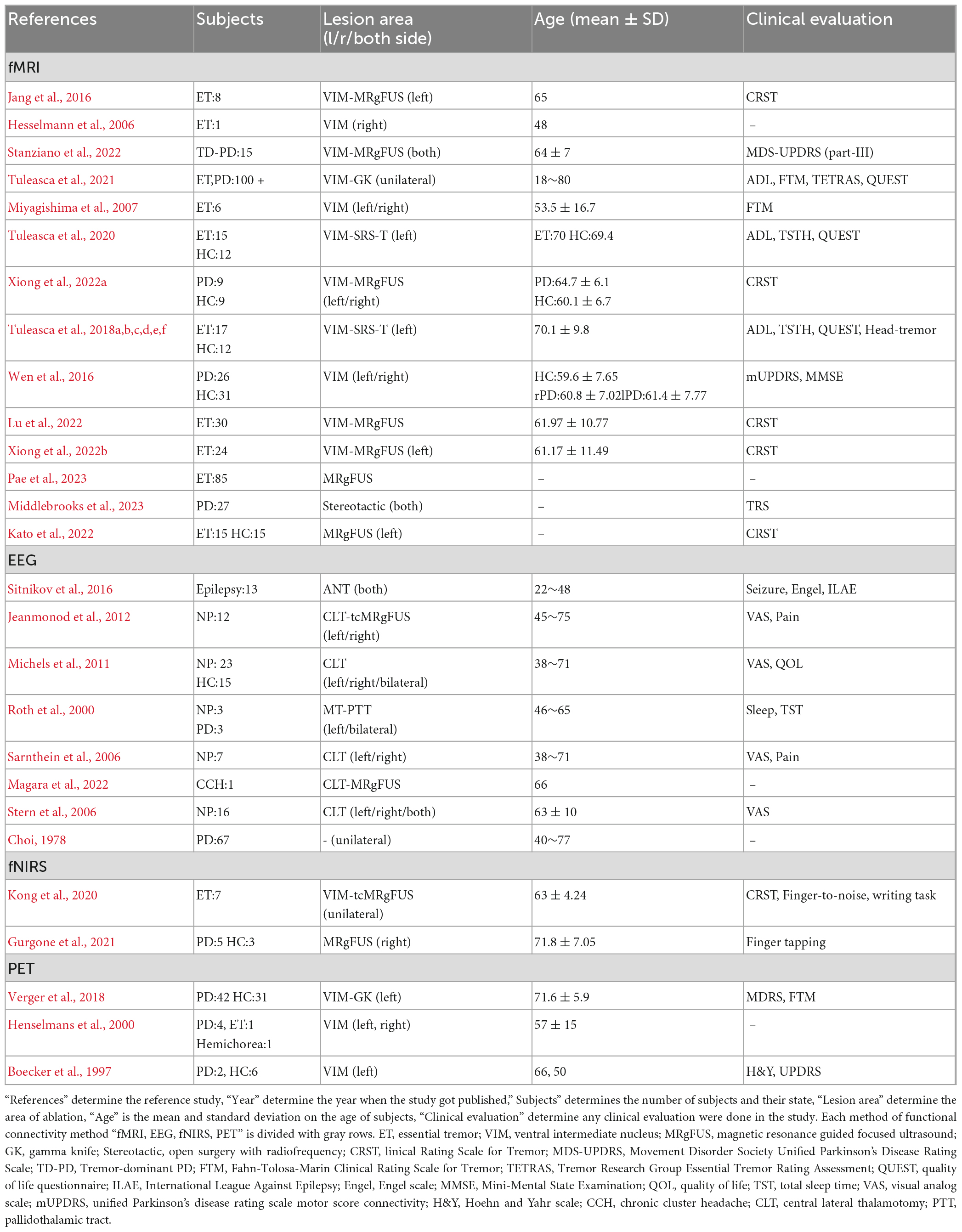
A flowchart of the selection process is presented in Figure 1. The main result and analysis of the included EEG, fMRI, fNIRS, and PET studies are all summarized in Table 1, and the demographic data are summarized in Table 2. There more evidences for fMRI, EEG as non-invasive recordings, which are methods that have been used more than the others in the clinic.
Functional magnetic resonance imaging
The fMRI is a technique used in ablation surgery research to determine the target location of ablation before surgery. Thalamotomy surgeries can affect both structural (Benito-Leon et al., 2018) and functional connectivity within brain network. While several studies have investigated structural alterations within the brain (sub) networks before and after thalamotomy surgery [for review see Bolton et al. (2022)], only a few studies investigated the functionality of the brain network before and after surgery. In this regard, this review article summarizes studies that focused on functional connectivity and activity of the brain intervened by thalamotomy (Table 1). The changes have been followed several times after the surgery to examine the reversibility. Mostly, three brain networks were mostly affected by thalamotomy surgery such as motor, visuomotor, default-mode networks.
The motor network, which is highly involved in tremor symptoms, is primarily affected by thalamotomy surgeries (Xiong et al., 2022a). There is a complex neural mechanisms underlying tremors which involve multiple brain regions and their interconnectivity. Thalamotomy has temporarily reconfigured the whole brain network and resulted in a reduction in the average connection among the motor network (Jeanmonod et al., 2012; Stanziano et al., 2022). Using cross-correlation and partial-correlation methods, Jang et al. (2016) observed an immediate increase inter-hemispheric similarity which reverted after 3 months, and no significant change in the direct connection between the thalamus and the motor cortex area. Moreover, thalamotomy caused selective and consistent changes in effective connectivity from the ventrolateral nuclei and the supplementary motor area (SMA) to the contralateral dentate nucleus (Park et al., 2017). Additionally, a reduction of the activity in the sensorimotor cortex and SMA is observed after thalamotomy (Miyagishima et al., 2007). The interconnectivity strength between the bilateral thalamus and limbic system with SMA predicted head tremors. So, the SMA proved to have a role in the motor network, indicating its effect on modulating head tremors through the abnormal connectivity in the thalamo limbic system (Tuleasca et al., 2018f). Moreover, some resting-state fMRI and task-based fMRI (T-fMRI) studies show no significant alteration in sensorimotor cortex (Park et al., 2017; Xiong et al., 2022a), and just the cingulate motor area is considerably activated (Hesselmann et al., 2006).
It seems that the visuomotor network can be affected by thalamotomy surgery too, according to studies by Kato et al. (2022), Tuleasca et al. (2018c), and Xiong et al., 2022a. They observed changes in functional connectivity within salience and bilateral motor networks as well as within areas involved in hand movement planning or language production (Tuleasca et al., 2018d). Moreover, these studies found interconnection between the ventral intermediate nucleus (VIM) and both the primary motor cortex and the contralateral cerebellum inconsistent with the adjustment role of VIM thalamotomy in the extra-pyramidal circuit (Hyam et al., 2012; Klein et al., 2012). Finally, both structural and functional neuroimaging suggested that the motor network and cerebellar lobule six are interconnected with visual extrastriate cortex clusters (Tuleasca et al., 2021). This suggests that visomotoor integration is complex and may involve multiple brain regions, including those that are targeted by thalamotomy surgery. Tuleasca et al. (2020) applied coactivation pattern (CAP) analysis to characterize three CAP groups: including cerebello-visuo-motor; thalamo-visuo-motor and, basal-ganglia and extrastriate cortex. They observed a decrease in cerebello- visuo-motor occurrence in pretherapy ET compared to healthy control, while thalamo-visuo-motor, basal ganglia, and extrastriate network increased. They suggested that there is a balance between the cerebellar circuitry and the thalamo-visuo-motor and basal ganglia networks, revealing the visual network’s role in tremor generation and suppression after treatment. In addition, Xiong et al. (2022a) have observed a longitudinal dynamic fractional amplitude of low-frequency fluctuations (fALFF) change in the left occipital cortex (Brodmann area 17) in PD patients after MRgFUS thalamotomy and concluded that the visuomotor networks were involved. Tuleasca et al. (2018c) also attempted to predict clinical responses by examining the relationship between pre-therapeutic left ventro-laterla-ventral (VLV) nucleus functional connectivity with right visual association area, left fusiform gyrus, left posterior cingulate. So, they concluded that visual areas played a significant role in this correlation. Overall, these studies highlight the important role of the visuomotor network in tremor generation and suppression and suggest that thalamotomy surgery can impact its associated brain regions.
It is possible that thalamotomy could cause change in default mode network (DMN) activity. Although further research is necessary to fully understand the potential effect. Wen et al. (2016) and Tuleasca et al. (2018e) have both reported on DMN activity in different ways, providing a starting point for understanding potential changes post-thalamotomy. Tuleasca et al. (2018e) focued on predicting volume size, while Wen et al. (2016) examined regional homogeneity (ReHo) changes. Wen et al.’s (2016) findings indicated that the DMN is convergence point for ReHo alteration in the middle temporal gyrus (MTG), anterior cingulum (ACC), and frontal regions.
These regions are involved in various cognitive and behavioral functions, such as memory retrieval, emotion regulation, and social cognition, which could be affected by thalamotomy (Jang et al., 2016; Xiong et al., 2022a).
To sum up, functional connectivity studies have utilized rs-fMRI or T-fMRI methods to examine brain networks associated with tremor circuitry, focusing on networks such as motor, sensorimotor visuo-motor, salience, and default-mode networks. These studies have found that overall activity and connectivity in the motor-related areas were decreased, suggesting changes inn network activity may be involved in tremor suppression after treatment. Further studies are needed to better understand the impact of thalamotomy on the various brain networks and associated cognitive and behavioral functions.
Electroencephalograms
The electroencephalogram (EEG) is a tool used to study functional connectivity and activity in the brain by measuring the power spectra and phase-locking activity at varying frequency bands in different brain regions. Prior to thalamotomy, EEG recordings showed abnormalities related to over-activity in high theta and low beta frequency bands (Stern et al., 2006). After thalamotomy, changes were observed in the EEG raw signal and power spectrum at different frequency bands, although the effect of on alpha, beta, and theta bands had contradictory results. Some studies reported a decrease in the over-activity in the rostral anterior cingulated cortex (rACC) and midbody of the corpus callosum (midCC) in alpha range (Stern et al., 2006), while others found an increase in alpha power after surgery (Michels et al., 2011). Results regarding beta power were also mixed, with some studies reporting an increase and others finding no change (Michels et al., 2011; Jeanmonod et al., 2012). There were reports of both increased and decreased theta power after thalamotomy surgery (Roth et al., 2000; Michels et al., 2011). It is important to note that these studies were conducted mainly before 2017, and therefore no conclusive evidence has been established to date. In general, activity in all frequency bands tended to decrease after surgery. However, the changes appeared to diminish with time during short post-surgery periods. While low-frequency stimuli initially decreased post-surgery and then increased in the second week (Choi, 1978), changes in power spectra appeared to persist in long post-surgery periods of 3 months to 1 year, lastly total sleep time and sleep efficiency increased after surgery (Roth et al., 2000).
With regard to EEG studies of thalamotomy, the findings are limited by small sample size, methodological differences, and the use of varying outcome measures. Overall, further research is needed to build on the existing knowledge regarding the effect of thalamotomy on EEG activity and functional connectivity, as well as the potential risks and benefits of the procedure in different patient populations.
Other methods
The more functional connectivity methods commonly used for evaluating thalamotomy surgery include fNIRs and PET. However, their application is limited as fNIRs are only capable of measuring cortical activities and cannot be used to assess deep structures, such as the thalamus and basal ganglia structures. Meanwhile, PET has poor spatial resolution and can only measure brain region activities. Two studies have utilized fNIRs to investigate changes following thalamotomy surgeries, and both studies found an increase in activity in the motor and somatosensory-related areas, as well as functional connectivity with prefrontal areas on the contralateral side (Kong et al., 2020; Gurgone et al., 2021). PET imaging studies have reported a decrease in frontal, parietal, and temporal regions during thalamotomy surgery, and the effect of the surgery on motor-related areas is controversial (Boecker et al., 1997; Henselmans et al., 2000). Furthermore, while one study found a decrease in functional connectivity between thalamus and temporal-occipital regions, however, this decrease diminished 1 week after surgery (Verger et al., 2018).
Discussion
Thalamotomy has proven to be effective in treating physical symptoms of various diseases such as parkinsonism, hyperkinetic movements, multiple sclerosis, and epilepsy (Spiegel et al., 1947; Ea and Ht, 1950; Elble, 2000; DeLong and Wichmann, 2007; Postuma et al., 2018). However, research examining the influence of thalamotomy on behavioral status is limited. Conducting an analysis on psychological and behavioral data before and after the procedure can help to classify and examine the neurological signs.
The types of surgery, brain recording methods, and brain network analysis influence the functional activity and connectivity results during thalamotomy surgery. Less invasive methods such as MRgFUS (Jang et al., 2016; Stanziano et al., 2022; Xiong et al., 2022a,b; Kato et al., 2022; Pae et al., 2023) and Gamma knife (Tuleasca et al., 2018a,b,c,d,e,f, 2020, 2021; Verger et al., 2018) techniques have emerged, which demonstrate differences in functional connectivity and activity outcomes in comparison with old surgery techniques due to the absence of unwanted tissue ablation in the electrode track.
While EEG was routinely employed to investigate the effects of thalamotomy surgery in the past, fMRI has become more widespread. Combining the both methods in preoperative and postoperative conditions can provide a more thorough examination of the effects of thalamotomy. Using more advanced equipment and greater numbers of electrodes in EEG studies can produce more consistent outcomes, allowing researchers to better understand the indirect impact of thalamotomy surgery on the resulting activity and overlapping functions of brain networks on the human cortex.
Table 1 demonstrates that despite the existence of various voxel-based functional connectivity techniques, such as fALFF (Xiong et al., 2022b), seed-based correlation analysis (SCA) (Jang et al., 2016), and ReHo (Wen et al., 2016), most present EEG and fMRI studies apply a data-driven ICA analysis. While new methods are continuously being developed, the brain is a dynamic and complex system that undergoes significant changes over time, making it a non-stationary network. To better model changes within the network, novel techniques such as Comprehensive Autonomic Predictor (CAP) (Tuleasca et al., 2020), Dynamic Causal Modeling (DCM) (Park et al., 2017), and windowed analysis may be more applicable. For future studies, more hypothesis-based approaches are recommended.
However, in terms of maintaining the brain’s equilibrium and functionality, how do the tremor circuit and other networks collaborate? The tremor circuit is not a subset of a specific network, so it’s possible that other essential contributors are present alongside the precentral gyrus, thalamus, and dentate nucleus. As reported by Miyagishima et al. (2007) the fundus of the central sulcus (Brodmann area 3a) is an additional critical relay in the tremor circuit, receiving proprioceptive inputs from the VIM nucleus. Therefore, a decrease in activation within the sensorimotor cortex and SMA can occur following thalamotomy. Tremors have been found to be associated with functional connection abnormalities in higher-level cortical and visual areas. The network-level functional connectivity changes in cortical, basal ganglia, and cerebellar systems have also been linked to tremors (DeSimone et al., 2019). Thus, among all the studies that have focused on the pathophysiology of essential tremor (ET), the role of the cerebellum’s dentate nuclei has been strongly supported (Jang et al., 2016; Park et al., 2017; Tuleasca et al., 2021; Stanziano et al., 2022). Tuleasca et al. (2020) have proposed a hypothesis that considers the broader impact of the visual system on tremor production. They suggest that if more than two brain networks are activated, it may be difficult to determine which one affects the other. This hypothesis indicates that the visual network could impact the motor network or vice versa. In an attempt to address such questions, Park et al. (2017) and Tuleasca et al. (2020) utilized DCM and CAP, respectively.
Passive movement in the brain is demonstrated by hemodynamic changes through fMRI and PET, while direct synchrony in the brain is depicted by EEG. However, functional association between basal ganglia and motor cortex (Brooks, 1999), as well as the asymmetric effect in postoperative patients by cortical metabolic (Baron et al., 1992), has been confirmed by both fMRI and PET studies. In PET scans comparing thalamotomy and pallidotomy of basal ganglia in pre- and post-surgical cases, the (pre)frontal cortex, lateral prefrontal, and parietal cortex were identified (Henselmans et al., 2000), and these are potential locations for future fMRI studies to track DMN changes. Basal ganglia connect with the SMA, dorsal prefrontal cortex, and frontal association areas. Consequently, insufficient activity in basal ganglia triggers compensatory overactivity of the lateral premotor and parietal cortex, which is primarily responsible for facilitating motor responses to visual and auditory cues. The association of subcortical activity areas that primarily receive input from basal ganglia demonstrates dysfunction of motor and visuomotor network activity in the tremor circuit (Jones, 1985).
Research studies that solely focused on structural changes have revealed alterations in the temporal pole and occipital cortex, indicating the significance of targeting specific visuomotor networks (Wen et al., 2016). Allowing researchers to understand the indirect effect of thalamotomy surgery on the human motor cortex and its impact on the overlapping activity of brain networks. While the majority of studies have focused on network connectivity alterations in imaging results, this hypothesis can be verified by comparing the functional connectivity and activity observed in EEG and fMRI studies in the future. According to Table 2, a common pattern noticed in both EEG and fMRI studies was the decrease-increase pattern reconfiguration in EEG studies (Brooks, 1999; Park et al., 2017) and the pattern evaluated with Clinical Rating Scale for Tremor (CRST) in fMRI studies (Jang et al., 2016; Park et al., 2017; Xiong et al., 2022b).
Studies have demonstrated the impact of thalamotomy on visuomotor network changes (Tuleasca et al., 2018a,c, 2020; Xiong et al., 2022a). According to Table 2, the VAS is a standard clinical evaluation technique utilized in EEG studies (Sarnthein et al., 2006; Stern et al., 2006; Michels et al., 2011; Jeanmonod et al., 2012). This investigation suggests that the effect of thalamotomy on the visual network is based on an earlier pathological belief that thalamotomy alters the visual network. Studies on the thalamus from as early as 1,664 and 1,681 by Thomas Willis described the human thalamus as the “chambers of the Optic Nerves.” Luys (1865) reported that thalamic nuclei are correlated with sensory, motor, limbic, and intrinsic functions. Karl Friedrich Burdach, since 1,822, has followed the optic tract and the superior colliculum’s brachium to the lateral geniculate body (Jones, 1985).
Although studies have examined various networks, such as the visual network, default mode network (DMN), and motor networks, the role of the mediodorsal thalamic nucleus in olfaction remains an unanswered question, considering that all senses except olfaction pass through the thalamus.
Author contributions
M-HN, SE, ER, M-RD, A-HV, MSa, AE, MSh, and YB contributed to the study design. M-HN, SE, and ER performed the data collection and analysis. M-HN, SE, ER, M-RD, A-HV, and MSa contributed to manuscript drafting and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abreu, R., Leal, A., and Figueiredo, P. (2019). Identification of epileptic brain states by dynamic functional connectivity analysis of simultaneous eeg-fmri: A dictionary learning approach. Sci. Rep. 9:638. doi: 10.1038/s41598-018-36976-y
Baron, J., Levasseur, M., Mazoyer, B., Legault- Demare, F., Mauguiere, F., Pappata, S., et al. (1992). Thalamocortical diaschisis: Positron emission tomography in humans. J. Neurol. Neurosurg. Psychiatry 55, 935–942.
Benito-Leon, J., Mato-Abad, V., Louis, E. D., Antonio Hernandez-Tamames, J., Alvarez-Linera, J., Bermejo-Pareja, F., et al. (2018). White matter microstructural changes are related to cognitive dysfunction in essential tremor. Sci. Rep. 7:2978.
Boecker, H., Wills, A. J., Ceballos-Baumann, A., Samuel, M., Thomas, D. G. T., Marsden, C. D., et al. (1997). Stereotactic thalamotomy in tremor-dominant Parkinson’s disease: An H215O PET motor activation study. Ann. Neurol. 41, 108–111. doi: 10.1002/ana.410410118
Boerwinkle, V. L., Sussman, B. L., Wyckoff, S. N., Manjo’n, I., Fine, J. M., and Adelson, P. D. (2022). Discerning seizure-onset v. propagation zone: Pre-and-post-operative resting-state fmri directionality and boerwinkle neuroplasticity index. NeuroImage 35:103063. doi: 10.1016/j.nicl.2022.103063
Bolton, T. A., Van De Ville, D., Re’gis, J., Witjas, T., Girard, N., Levivier, M., et al. (2022). Graph theoretical analysis of structural covariance reveals the relevance of visuospatial and attentional areas in essential tremor recovery after stereotactic radiosurgical thalamotomy. Front. Aging Neurosci. 14:873605. doi: 10.3389/fnagi.2022.873605
Brooks, D. J. (1999). Functional imaging of Parkinson’s disease: Is it possible to detect brain areas for specific symptoms? J. Neural Transm. Suppl. 56, 139–153.
Choi, C. (1978). Photic EEG-driving responses in thalamotomized and medicated cases of Parkinson’s disease. Arch. Psychiatr. Nervenkr. 225, 97–105. doi: 10.1007/BF00343393
Coenen, V. A., Allert, N., Paus, S., Kronenbu¨rger, M., Urbach, H., and Ma¨dler, B. (2014). Modulation of the cerebello- thalamo-cortical network in thalamic deep brain stimulation for tremor: A diffusion tensor imaging study. Neurosurgery 75, 657–670. doi: 10.1227/NEU.0000000000000540
DeLong, M. R., and Wichmann, T. (2007). Circuits and circuit disorders of the basal Ganglia. Arch. Neurol. 64:20. doi: 10.1001/archneur.64.1.20
DeSimone, J. C., Archer, D. B., Vaillancourt, D. E., and Wagle Shukla, A. (2019). Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain 142, 1644–1659. doi: 10.1093/brain/awz085
Elble, R. J. (2000). Diagnostic criteria for essential tremor and differential diagnosis. Neurology 54, S2–S6.
Gurgone, S., Acri, G., Bonanno, L., Caridi, F., De Salvo, S., Marino, S., et al. (2021). Effect of MRgFUS treatment on cortical activity in Parkinson’s disease: A fNIRS study [JD]. Atti Della Accademia Perloritana Dei Pericolanti. Classe Sci. Fisiche Matematiche e Naturali 99, A5-1A5-A14. doi: 10.1478/AAPP.992A5
Halpern, C. H., Santini, V., Lipsman, N., Lozano, A. M., Schwartz, M. L., Shah, B. B., et al. (2019). Three-year follow- up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology 93, e2284–e2293. doi: 10.1212/WNL.0000000000008561
Henselmans, J. M. L., de Jong, B. M., Pruim, J., Staal, M. J., Rutgers, A. W. F., and Haaxma, R. (2000). Acute effects of thalamotomy and pallidotomy on regional cerebral metabolism, evaluated by PET. Clin. Neurol. Neurosurg. 102, 84–90. doi: 10.1016/s0303-8467(00)00070-6
Hesselmann, V., Maarouf, M., Hunsche, S., Lasek, K., Schaaf, M., Krug, B., et al. (2006). Functional MRI for immediate monitoring stereotactic thalamotomy in a patient with essential tremor. Eur. Radiol. 16, 2229–2233. doi: 10.1007/s00330-006-0211-8
Hwang, K., Bertolero, M. A., Liu, W. B., and D’Esposito, M. (2017). The human thalamus is an integrative hub for functional brain networks. J. Neurosci. 37, 5594–5607.
Hyam, J. A., Owen, S. L., Kringelbach, M. L., Jenkinson, N., Stein, J. F., Green, A. L., et al. (2012). Contrasting connectivity of the ventralis intermedius and ventralis oralis posterior nuclei of the motor thalamus demonstrated by probabilistic tractography. Neurosurgery 70, 162–169. doi: 10.1227/NEU.0b013e3182262c9a
Jang, C., Park, H.-J., Chang, W. S., Pae, C., and Chang, J. W. (2016). Immediate and longitudinal alterations of functional networks after thalamotomy in essential tremor. Front. Neurol. 7:184. doi: 10.3389/fneur.2016.00184
Jeanmonod, D., Werner, B., Morel, A., Michels, L., Zadicario, E., Schiff, G., et al. (2012). Transcranial magnetic resonance imaging–guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 32:E1. doi: 10.3171/2011.10.FOCUS11248
Kato, S., Maesawa, S., Bagarinao, E., Nakatsubo, D., Tsugawa, T., Mizuno, S., et al. (2022). Magnetic resonance–guided focused ultrasound thalamotomy restored distinctive resting-state networks in patients with essential tremor. J. Neurosurg. 138, 306–317. doi: 10.3171/2022.5.jns22411
Kim, M., Son, B., Miyagi, Y., and Kang, J. (2002). Vim thalamotomy for holmes’ tremor secondary to midbrain tumour. J. Neurol. Neurosurg. Psychiatry 73, 453–455. doi: 10.1136/jnnp.73.4.453
Klein, J., Barbe, M., Seifried, C., Baudrexel, S., Runge, M., Maarouf, M., et al. (2012). The tremor network targeted by successful vim deep brain stimulation in humans. Neurology 78, 787–795.
Kong, D., Zong, R., Zhang, T., Pan, L., and Yu, X. (2020). Clinical effect of unilateral transcranial magnetic resonance-guided focused ultrasound thalamotomy in patients with essential tremor as evaluated by functional near-infrared spectroscopy. Durham, NC: Research Square Platform LLC, doi: 10.21203/rs.3.rs-20984/v1
Krishna, V., Sammartino, F., Cosgrove, R., Ghanouni, P., Schwartz, M., Gwinn, R., et al. (2020). Predictors of outcomes after focused ultrasound thalamotomy. Neurosurgery 87, 229–237.
Lee, K.-H. (1997). Mri-guided stereotactic thalamotomy for cerebral palsy patients with mixed dyskinesia. Stereotact. Funct. Neurosurg. 69, 300–310. doi: 10.1159/000099891
Lin, S.-J., Rodriguez-Rojas, R., Baumeister, T. R., Lenglos, C., Pineda-Pardo, J. A., Ma´n˜ez- Miro´, J., et al. (2022). Neuroimaging signatures predicting motor improvement to focused ultrasound subthalamotomy in Parkinson’s disease. NPJ Parkinsons Dis. 8, 1–9. doi: 10.1038/s41531-022-00332-9
Lipsman, N., Schwartz, M. L., Huang, Y., Lee, L., Sankar, T., Chapman, M., et al. (2013). Mr-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. The Lancet Neurology 12, 462–468. doi: 10.1016/S1474-4422(13)70048-6
Lu, H., Lin, J., Xiong, Y., Deng, L., Wang, X., Zhang, D., et al. (2022). Assessing the impact of MR-guided focused ultrasound thalamotomy on brain activity and connectivity in patients with essential tremor. Neurosurg. Focus 53:E5. doi: 10.3171/2022.9.focus22228
Magara, A. E., Gallay, M. N., Moser, D., and Jeanmonod, D. (2022). Complete resolution of chronic cluster headache following central lateral thalamotomy using incisionless MRI-guided focused ultrasound with 6 years of follow-up: Illustrative case. J. Neurosurg. 4:CASE22259. doi: 10.3171/case22259
Mathieu, D., Kondziolka, D., Niranjan, A., Flickinger, J., and Lunsford, L. D. (2007). Gamma knife thalamotomy for multiple sclerosis tremor. Surg. Neurol. 68, 394–399.
Michels, L., Moazami-Goudarzi, M., and Jeanmonod, D. (2011). Correlations between EEG and clinical outcome in chronic neuropathic pain: Surgical effects and treatment resistance. Brain Imaging Behav. 5, 329–348. doi: 10.1007/s11682-011-9135-2
Middlebrooks, E. H., Popple, R. A., Greco, E., Okromelidze, L., Walker, H. C., Lakhani, D. A., et al. (2023). Connectomic basis for tremor control in stereotactic radiosurgical thalamotomy. Am. J. Neuroradiol. 44, 157–164. doi: 10.3174/ajnr.a7778
Miyagishima, T., Takahashi, A., Kikuchi, S., Watanabe, K., Hirato, M., Saito, N., et al. (2007). Effect of ventralis intermedius thalamotomy on the area in the sensorimotor cortex activated by passive hand movements: fMR imaging study. Stereotact. Funct. Neurosurg. 85, 225–234. doi: 10.1159/000103261
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, T. P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed1000097
Pae, C., Kim, M. J., Chang, W. S., Jung, H. H., Chang, K. W., Eo, J., et al. (2023). Differences in intrinsic functional networks in patients with essential tremor who had good and poor long-term responses after thalamotomy performed using MR-guided ultrasound. J. Neurosurg. 138, 318–328. doi: 10.3171/2022.5.jns22324
Park, H.-J., Pae, C., Friston, K., Jang, C., Razi, A., Zeidman, P., et al. (2017). Hierarchical dynamic causal modeling of resting-state fMRI reveals longitudinal changes in effective connectivity in the motor system after thalamotomy for essential tremor. Front. Neurol. 8:346. doi: 10.3389/fneur.201700346
Postuma, R. B., Poewe, W., Litvan, I., Lewis, S., Lang, A. E., Halliday, G., et al. (2018). Validation of the mds clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 33, 1601–1608.
Roth, C., Jeanmonod, D., Magnin, M., Morel, A., and Achermann, P. (2000). Effects of medial thalamotomy and pallido-thalamic tractotomy on sleep and waking EEG in pain and Parkinsonian patients. Clin. Neurophysiol 111, 1266–1275. doi: 10.1016/s1388-2457(00)00295-9
Sarnthein, J., Stern, J., Aufenberg, C., Rousson, V., and Jeanmonod, D. (2006). Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 129, 55–64. doi: 10.1093/brain/awh631
Sitnikov, A. R., Grigoryan, Y. A., and Mishnyakova, L. P. (2016). Bilateral radiofrequency anterior thalamotomy in intractable epilepsy patients. Zh. Vopr. Neirokhir. Im. N. N. Burdenko 80, 25–34. doi: 10.17116/neiro201680325-34
Spiegel, E. A., Wycis, H. T., Marks, M., and Lee, A. J. (1947). Stereotaxic apparatus for operations on the human brain. Science 106, 349–350. doi: 10.1126/science.106.2754.349
Stanziano, M., Golfre‘ Andreasi, N., Messina, G., Rinaldo, S., Palermo, S., Verri, M., et al. (2022). Resting state functional connectivity signatures of MRgFUS Vim thalamotomy in Parkinson’s disease: A preliminary study. Front. Neurol. 12:786734. doi: 10.3389/fneur.2021.786734
Stern, J., Jeanmonod, D., and Sarnthein, J. (2006). Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. NeuroImage 31, 721–731. doi: 10.1016/j.neuroimage.2005.12.042
Tomasi, D., and Volkow, N. D. (2011). Association between functional connectivity hubs and brain networks. Cerebral cortex 21, 2003–2013.
Tuleasca, C., Bolton, T., Re’gis, J., Najdenovska, E., Witjas, T., Girard, N., et al. (2020). Normalization of aberrant pretherapeutic dynamic functional connectivity of extrastriate visual system in patients who underwent thalamotomy with stereotactic radiosurgery for essential tremor: A resting-state functional MRI study. J. Neurosurg. 132, 1792–1801. doi: 10.3171/2019.2.JNS183454
Tuleasca, C., Najdenovska, E., Re’gis, J., Witjas, T., Girard, N., Champoudry, J., et al. (2018a). Clinical response to Vim’s thalamic stereotactic radiosurgery for essential tremor is associated with distinctive functional connectivity patterns. Acta Neurochir. 160, 611–624. doi: 10.1007/s00701-017-3456-x
Tuleasca, C., Najdenovska, E., Re’gis, J., Witjas, T., Girard, N., Champoudry, J., et al. (2018b). Pretherapeutic functional neuroimaging predicts tremor arrest after thalamotomy. Acta Neurol. Scand. 137, 500–508. doi: 10.1111/ane.12891
Tuleasca, C., Najdenovska, E., Re’gis, J., Witjas, T., Girard, N., Champoudry, J., et al. (2018c). Pretherapeutic motor thalamus resting-state functional connectivity with visual areas predicts tremor arrest after thalamotomy for essential tremor: Tracing the cerebello-thalamo-visuo-motor network. World Neurosurg. 117, e438–e449. doi: 10.1016/j.wneu.2018.06.049
Tuleasca, C., Najdenovska, E., Re’gis, J., Witjas, T., Girard, N., Champoudry, J., et al. (2018d). Ventrolateral motor thalamus abnormal connectivity in essential tremor before and after thalamotomy: A resting- state functional magnetic resonance imaging study. World Neurosurg. 113, e453–e464. doi: 10.1016/j.wneu.2018.02.055
Tuleasca, C., Re’gis, J., Najdenovska, E., Witjas, T., Girard, N., Bolton, T., et al. (2018e). Pretherapeutic resting-state fMRI profiles are associated with MR signature volumes after stereotactic radiosurgical thalamotomy for essential tremor. J. Neurosurgery 129, 63–71. doi: 10.3171/2018.7.GKS18752
Tuleasca, C., Re’gis, J., Najdenovska, E., Witjas, T., Girard, N., Champoudry, J., et al. (2018f). Pretherapeutic functional imaging allows prediction of head tremor arrest after thalamotomy for essential tremor: The role of altered interconnectivity between thalamolimbic and supplementary motor circuits. World Neurosurgery 112, e479–e488. doi: 10.1016/j.wneu.2018.01.063
Tuleasca, C., Witjas, T., Levivier, M., Girard, N., Cretol, A., Levy, N., et al. (2021). The brain connectome after gamma knife radiosurgery of the ventro-intermediate nucleus for tremor: Marseille-Lausanne radiobiology study protocol. Stereotact. Funct. Neurosurgery 99, 387–392. doi: 10.1159/000514066
Verger, A., Witjas, T., Carron, R., Eusebio, A., Boutin, E., Azulay, J.-P., et al. (2018). Metabolic positron emission tomography response to gamma knife of the ventral intermediate nucleus in essential tremor. Neurosurgery 84, E294–E303. doi: 10.1093/neuros/nyy340
Wen, Z., Zhang, J., Li, J., Dai, J., Lin, F., and Wu, G. (2016). Altered activation in cerebellum contralateral to unilateral thalamotomy may mediate tremor suppression in Parkinson’s disease: A short- term regional homogeneity fMRI study. PLoS ONE 11:e0157562. doi: 10.1371/journal.pone.0157562
Xiong, Y., Han, D., He, J., Zong, R., Bian, X., Duan, C., et al. (2022a). Correlation of visual area with tremor improvement after MRgFUS thalamotomy in Parkinson’s disease. J. Neurosurgery 136, 681–688.
Xiong, Y., Lin, J., Bian, X., Lu, H., Zhou, J., Zhang, D., et al. (2022b). Treatment-specific network modulation of MRI-guided focused ultrasound thalamotomy in essential tremor. Neurotherapeutics 19, 1920–1931. doi: 10.1007/s13311-022-01294-9
Keywords: ablation surgery, thalamotomy, functional connectivity, activity, fMRI, EEG
Citation: Nili M-HHK, Esfahan SM, Bagheri Y, Vahabie A-H, Sanayei M, Ertiaei A, Shirani M, Dehaqani M-RA and Rezayat E (2023) The variation of functional connectivity and activity before and after thalamotomy surgery (review). Front. Hum. Neurosci. 17:1108888. doi: 10.3389/fnhum.2023.1108888
Received: 26 November 2022; Accepted: 06 April 2023;
Published: 28 April 2023.
Edited by:
Ali Motie Nasrabadi, Shahed University, IranReviewed by:
Jean Regis, Aix Marseille Université, FranceBenito de Celis Alonso, Meritorious Autonomous University of Puebla, Mexico
Copyright © 2023 Nili, Esfahan, Bagheri, Vahabie, Sanayei, Ertiaei, Shirani, Dehaqani and Rezayat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ehsan Rezayat, cmV6YXlhdEB1dC5hYy5pcg==
†These authors have contributed equally to this work
 Mohammad-Hossein H. K. Nili
Mohammad-Hossein H. K. Nili Shahrzad M. Esfahan
Shahrzad M. Esfahan Yamin Bagheri2,3
Yamin Bagheri2,3 Abdol-Hossein Vahabie
Abdol-Hossein Vahabie Abolhassan Ertiaei
Abolhassan Ertiaei Ehsan Rezayat
Ehsan Rezayat