
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 12 September 2022
Sec. Cognitive Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.955005
This article is part of the Research Topic Frontiers in Psychodynamic Neuroscience View all 18 articles
Genetic and early environmental factors are interwoven in the etiology of Borderline Personality Disorder (BPD). Epigenetic mechanisms offer the molecular machinery to adapt to environmental conditions. There are gaps in the knowledge about how epigenetic mechanisms are involved in the effects of early affective environment, development of BPD, and psychotherapy response. We reviewed the available evidence of the effects of psychotherapy on changes in DNA methylation and conducted a pilot study in a sample of 11 female adolescents diagnosed with BPD, exploring for changes in peripheral DNA methylation of FKBP5 gene, which encodes for a stress response protein, in relation to psychotherapy, on symptomatology and underlying psychological processes. For this purpose, measures of early trauma, borderline and depressive symptoms, psychotherapy outcome, mentalization, and emotional regulation were studied. A reduction in the average FKBP5 methylation levels was observed over time. Additionally, the decrease in FKBP5 methylation observed occurred only in those individuals who had early trauma and responded to psychotherapy. The results suggest an effect of psychotherapy on epigenetic mechanisms associated with the stress response. The finding that epigenetic changes were only observed in patients with early trauma suggests a specific molecular mechanism of recovery. The results should be taken with caution given the small sample size. Also, further research is needed to adjust for confounding factors and include endocrinological markers and therapeutic process variables.
Borderline Personality Disorder (BPD) is characterized by a general pattern of instability in affect regulation, impulse control, interpersonal relationships, and self-image. A revision of the data from The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) study in the United States estimates a lifetime prevalence of 2.7% (Trull et al., 2010). In mental health settings, the prevalence of BPD is expected to be 10% in outpatient and between 15% and 25% in inpatients (Leichsenring et al., 2011). The prevalence of BPD is relatively similar in adolescents and adults, and presents acute symptomatology, such as suicidal ideation, impulsive behaviors, and non-suicidal self-injury (NSSI; Stead et al., 2019). Adolescents with BPD have academic difficulties and social relationship problems (Kaess et al., 2017). Additionally, this disorder is a significant predictor of substance use and mood disorders (Chanen et al., 2008). Individuals with BPD symptoms at mean age of 14 years show lower life satisfaction, more general impairment, and need for services at mean age of 33 (Winograd et al., 2008).
Given the implications of BPD on the functioning and mental health of adolescents, a better understanding of the interplay between genetic and environmental factors that contribute to their symptomatic expression is highly needed.
The development of BPD pathology implies a complex etiopathogenic trajectory in which adverse early life events in the presence of genetic susceptibility that confer sensitivity to the environment, can lead to the development over time of the BPD phenotype or its underlying traits (Gunderson and Lyons-Ruth, 2008; Bulbena-Cabre et al., 2018). However, evidence of the underlying molecular mechanisms is largely unknown. A large-scale family study estimates the heritability of the disorder at 46% (95% CI = 39–53; Skoglund et al., 2021). Despite evidence from twin studies showing that BPD is highly heritable, genetic association studies are so far inconclusive (Calati et al., 2013; Amad et al., 2014).
Epigenetic processes are sensitive to environmental conditions and can operate as mechanisms that allow early environmental experiences to trigger phenotypic modifications without modifying the genotype (Weaver, 2007). A limited number of studies have shown relations between DNA methylation patterns and the presence of childhood stress in individuals with BPD symptoms, showing associations with genes involved in stress regulation and neuroplasticity. A positive correlation has been observed between the levels of DNA methylation of exon 1F the Glucocorticoid Receptor gene (NR3C1) promoting region, child maltreatment (physical abuse), and clinical severity in a sample of individuals with BPD (Martín-Blanco et al., 2014). Perroud et al. (2013) examined 115 individuals diagnosed with borderline personality disorder (BPD). All received intensive dialectical behavioral therapy. Those who responded to treatment showed a decrease in the percentage of exon I and IV BDNF DNA methylation. Changes in the methylation status were significantly related to changes in depressive symptoms, hopelessness, and impulsivity.
To date, there is no psychopharmacological treatment with robust evidence of effectiveness for the treatment of BPD. On the other hand, various types of specialized psychotherapy have shown efficacy in reducing symptoms and improving global functioning (Choi-Kain et al., 2017). These effects could occur by modification of the epigenetic profile. In line with the preceding, there are few recent studies relating BPD and the potential epigenetic effect of psychotherapeutic treatments, specifically on BDNF gene DNA methylation (Perroud et al., 2013; Thomas et al., 2018) and APBA3 and MCF2 genes DNA methylation (Knoblich et al., 2018). However, the exploration of the association between the change produced at the level of symptoms or personality functioning induced by psychotherapeutic interventions and changes at the molecular level is still very scarce (Jiménez et al., 2018).
Accordingly, the aim of the present study is to discuss the role of epigenetic changes as a mechanism of gene-environment interaction, its relevance for BPD, and current evidence on DNA methylation changes in individuals with BPD during psychotherapy. Second, the concept of mentalization as a capacity for the processing of the interpersonal context, its development from the early experiences of care, and its participation as a possible common factor in psychotherapy in BPD is exposed. Finally, the notion that subjective processing of the social environment can act on the genetic expression as a mechanism of adaptation of the BPD phenotype is discussed.
Epigenetic modifications refer to stable alterations of potential gene expression during development and cell proliferation, that are held through cell divisions and do not alter the DNA sequence (Jaenisch and Bird, 2003). These correspond to heritable patterns of DNA methylation and hydroxymethylation, posttranslational histone modifications, and gene expression regulation by non-coding RNAs (Zannas et al., 2015). The combination of these changes determines a specific pattern of gene expression, which is highly dynamic and permeable to environmental influences. It is also heterogeneous in different organisms, tissues, and cell types, and changes according to the stages of development. Therefore, they correspond to a complex set of mechanisms of “phenotypic plasticity” in response to environmental demands (Ecker et al., 2018).
Experimental models have studied the impact of early adversity as a function of maternal care. For instance, in rats, the effect of maternal care behavior like licking, grooming (LG), and back arch-nursing (ABN) on behavior of the offspring and DNA methylation in the glucocorticoid receptor gene (NR3C1) have been reported (Lutz and Turecki, 2014). Increased DNA methylation in promoter regions of GR gene (NR3C1; hence more inactive chromatin and therefore lower transcription) in the hippocampus of adult rats reared by mothers with low levels of LG-ABN compared to offspring reared by mothers with high LG-ABN was observed. This lower GR expression was associated with less negative feedback in the HPA axis and higher reactivity to stress (Lutz and Turecki, 2014).
In rats exposed to early stress (separation of mother and calf), increased secretion of corticosterone and a persistent increase in Arginine Vasopressin (AVP) expression in neurons of the paraventricular nucleus of the Hippocampus are observed. AVP acts by enhancing the action of Corticotropin Releasing Hormone (CRH) under sustained stress situations. This increase is associated with hypomethylation in the regulatory region CGI3 of AVP gene and with altered behaviors of stress coping (Murgatroyd et al., 2009).
In humans, a number of studies have explored the relationship between early adverse environment and changes in methylation patterns, using candidate genes and epigenome-wide strategies.
Regarding studies linking candidate genes studies related to the stress response and adverse events in childhood, a significant correlation between the number of adverse events reported and one exon 1F DNA methylation site (cg17860381, located in exon 1F) of NR3C1 gene was observed in lymphocytes of females who reported childhood adverse events, including physical, emotional and sexual abuse. Moreover, this pattern of adverse events and DNA methylation was correlated with borderline symptoms (Radtke et al., 2015).
A systematic review conducted by Turecki and Meaney (2016) regarding the effects of social environment on NR3C1 gene methylation in humans showed that there was a consistent relationship (16 out of 17 reviewed studies) between early life adversity and increased exon 1F DNA methylation across different tissues (blood, saliva, buccal cells, and brain tissue). However, the results are inconsistent when exploring the association between exon 1F methylation and psychopathology including post-traumatic stress disorder and Depression. These results are also inconsistent when exploring methylation in other sites of exon 1 of the same gene in relation to early adversity, suggesting the need for further research to determine the permeability and stability of DNA methylation of each specific site.
Different types of early adversity including physical, emotional, sexual abuse, or psychosocial deprivation are associated with altered DNA methylation in specific sites of the epigenome. The epigenome-wide studies that include a greater number of genetic loci, to date, are scarce, the sample sizes are small, assess early adverse events with different methods (Yang et al., 2013; Cecil et al., 2016; Kumsta et al., 2016; Perna et al., 2020; and Merrill et al., 2021) and their findings, are still inconsistent among them and with candidate genes studies.
A limited number of studies have explored the effect of psychotherapy on epigenetic changes in BPD.
One study was performed on a sample of 115 outpatients diagnosed with BPD (and 52 healthy controls) and extracted DNA from blood leukocytes before and after 4 weeks of Intensive Dialectical Behavioral psychotherapy (DBT) to measure CpG methylation of exons I and IV of the BDNF gene. Patients who responded to DBT, exhibited a decrease in DNA methylation of BDNF gene exons I and IV, whereas no association was found between BPD diagnosis and methylation levels (Perroud et al., 2013). In another study performed on 44 patients with BPD and 44 matched controls, DNA methylation of APBA3 and MCF2 genes was measured from blood samples. APBA 3 (neuronal adapter protein) is related to the production of β-amyloid, a component of amyloid plaques linked to Alzheimer’s disease and MCF2 is a guanine nucleotide exchange factor, involved in neurite outgrowth that has been associated with schizophrenia and autism-spectrum disorders. Individuals with BPD who respond to DBT therapy presented higher methylation in both genes after 12 weeks relative to non-responders (Knoblich et al., 2018).
A third study, involving a sample of 41 individuals with BPD and 41 healthy controls and assessing candidate gene DNA methylation, reported higher methylation levels in promoter IV of the BDNF gene in both saliva and blood samples of BPD patients. Twenty-six out of the 41 patients completed DBT psychotherapy and after 12 weeks, a decrease in methylation levels was observed only in saliva samples (Thomas et al., 2018).
Some results are discordant, for example, Perroud et al.’s (2013) study found significant DNA methylation change after therapy in blood samples while Thomas et al.’s (2018) work did not. One possible explanation may be that the first study reported an average of methylation from exon IV while the second reported individual CpG methylation. There were also differences in the methylation evaluation technique (high resolution melt analysis vs. pyrosequencing).
These initial findings suggest that psychotherapy may be associated with epigenetic changes in candidate genes related to neuroplasticity.
FKBP5 gene encodes for FK506, a glucocorticoid receptor co-chaperon whose levels are increased after stress exposure and decreases the ability of the glucocorticoid receptor to bind cortisol and to translocate to the nucleus, creating an ultrashort negative feedback for NR3C1 activation (Binder, 2009; Zannas and Binder, 2014). DNA methylation of FKBP5 gene promoting regions decrease gene transcription and might limit the effects over stress neuroendocrine response (Zannas and Binder, 2014).
An association has been described between a lower DNA methylation of intron 7 of the FKBP5 gene and the presence of child maltreatment in adults (Klengel et al., 2013) and preschool children (Tyrka et al., 2015). Moreover, high FKBP5 DNA methylation was found in infants who displayed resistant attachment behavior (Mulder et al., 2017).
Changes in FKBP5 DNA methylation have also been associated with other early stressors such as low socioeconomic status in childhood (Needham et al., 2015) and Holocaust survivors and their offspring (Yehuda et al., 2016).
These findings suggest a possible role for FKBP5 in the adaptation of molecular stress response systems in relation to the early environment through epigenetic modifications.
Interestingly, in a case-control study with individuals with BPD, FKBP5 intron 7 (bin 2), DNA methylation inversely correlate with empathic perspective taking and with anxiety symptoms. Furthermore, lower empathic perspective-taking abilities and anxiety correlated with childhood maltreatment. Although this study does not find differences between clinical and non-clinical samples, it reveals the relationship between empathy engagement and FKBP5 DNA methylation (Flasbeck and Brüne, 2021).
FKBP5 DNA methylation has been also associated with response to psychotherapy in PTSD (Yehuda et al., 2013), children with anxiety disorders (Roberts et al., 2015), and agoraphobia (Roberts et al., 2019). Bishop et al. (2018) also report significant findings in individuals with PTSD treated with Mindfulness-Based Stress Reduction (MBSR) therapy, but in the opposite direction, with responders having increased DNA methylation (intron 7, bin 2).
Mentalization can be understood as a mental activity that allows interpreting behavior in terms of intentional mental states of others (needs, desires, feelings, goals), constituting a form of social cognition (Fonagy and Luyten, 2009).
Through mentalization individuals realize that they have a mind that can mediate its experience with the world, have the capacity to distinguish the inner reality from external reality and include both, an intrapersonal mental context and emotional processes of interpersonal communication (Gergely et al., 2002).
The quality of early attachment relationships is critical for the development of mentalization, as they allow the internal states to be mirrored by an attentive and reliable caretaker. This process at the same time impacts the processes of emotional regulation and self-control (Fonagy and Luyten, 2009).
A mentalization deficiency occurs in subjects with BPD compared to a non-clinical sample and with subjects with other personality disorders, but only in the presence of child abuse (Fonagy et al., 1996). However, other studies show a superior capacity for mentalization in these patients. This may be because the expression of deficits could be in BPD, specifically activated in the context of attachment relationships under conditions of high emotional arousal (Antonsen et al., 2016), which increases the tendency to attribute mental states to others that exceed the information given by social cues (hypermentalization; Sharp and Fonagy, 2015).
Less certainty about mental states was reported measured by the Reflective Functioning Questionnaire (RFQ) in a sample of individuals with BPD compared to healthy controls (Morandotti et al., 2018). Moreover, individuals with BPD would not present difficulties in the processes of decoding mental states from observed behavior, but rather to make causal inferences and predictions that include context information and basic social knowledge (Németh et al., 2020). Lower values of mentalization ability measured with RFQ mediate the relationship between a diagnosis of BPD and insecure adult attachment, supporting the idea that the presence of negative internal work models reduces the ability to accurately distinguish the relationship between mental states and behavior (Badoud et al., 2018).
The treatment specifically developed to increase mentalization, Mentalization-based Treatment (MBT; Bateman and Fonagy, 2004) has shown to be effective compared to Structured Clinical Management in a sample of individuals with BPD in both reducing self-injurious behaviors and hospitalizations and in reducing symptoms and improving interpersonal functioning (Bateman and Fonagy, 2009). Also, at an 8-year follow-up, patients treated with MBT maintained a stable improvement over time compared with Treatment as Usual (TAU; Bateman and Fonagy, 2008).
Using the Psychotherapy Process Q-Set, an instrument that allows the rating of sessions according to how close they are to a prototypical session of their respective orientation, one study compared sessions of TFP, DBT, and therapy focused on mentalization. Interestingly, the prototype mentalization response correlated with all therapies, with a greater correlation on mentalizing the other (including the therapist) in TFP and more focused on the self in DBT (Goodman et al., 2015). These findings are in agreement with the statement that the development of mentalization corresponds to a common factor in BPD psychotherapies (Fonagy and Allison, 2014).
The capacity to navigate the interpersonal environment using the ability to mentalize can be improved by psychotherapies of different orientations in BPD. This change could be associated with biological changes at the epigenetic level specially in stress response systems.
This study aims to explore changes in peripheral DNA methylation of FKBP5 gene, which encodes for a stress response protein, in relation to psychotherapy, symptomatology, and underlying psychological processes in a sample of 11 female adolescents diagnosed with BPD. For this purpose, measures of early trauma, borderline and depressive symptoms, psychotherapy outcome, mentalization, and emotion regulation were studied longitudinally at baseline, 3 and 6 months. Percentage DNA methylation levels of specific regions of FKBP5 gene intron 7 were measured at the same time interval. The design was a quasi-experimental, longitudinal, process-outcome study.
Participants were female adolescent patients, aged 15–20 years, with a BPD subthreshold cut-off of 3 or more criteria of the DSM IV-TR for BPD. Subthreshold-BPD was included based on impairment of quality of life, presence of self-injury, and suicidality similar to full-syndrome BPD female adolescents previously reported (Kaess et al., 2017). Participants were starting a psychotherapeutic process with a focus on difficulties in the development of their personality, eigth of them were of psychodynamic orientation and three were dialectical behavior therapy (DBT).
The exclusion criteria were the following: psychosis, pervasive developmental disorder, and unstable medical (non-psychiatric) disease.
A convenience sampling technique was used by contacting psychotherapists who work with adolescent populations and whose theoretical model and therapeutic approach include the development of the mentalizing capacity, including psychodynamic psychotherapies and DBT applied in private practice and in public and private outpatient treatment centers of Santiago de Chile. Therapists were clinical psychologists or psychiatrists with formal therapeutic training. Verbal and written information about the research and subject participation were provided. The study was approved by the ethics committee of Pontificia Universidad Católica de Chile.
Eligibility criteria was assessed through the Structured Clinical Interview for DSM-IV axis II Personality Disorders (SCID II; First et al., 1995) and Mini International Neuropsychiatric Interview (M.I.N.I-Kid; Sheehan et al., 2010). The presence of childhood trauma was evaluated using the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1997), while attachment patterns were evaluated usingthe Attachment Adolescent Questionnaire (AAQ; West et al., 1998). Once selected, the Brief Reflective Functioning Interview (BRFI; Rudden et al., 2005) and Difficulties in Emotion Regulation Scale (DERS; Gratz and Roemer, 2004) were applied at 0, 3, and 6 months. The Reflective Functioning Scale (RFS) coding system was applied to BRFI transcript by a certified coder (Fonagy et al., 1998). Also, as outcome measures the Youth Outcome Self-Report (Y-OQ-SR; Wells et al., 2003), Beck Depression Inventory (BDI-I; Beck et al., 1961), and Borderline Symptom List (BSL-23; Bohus et al., 2009) were applied at 0, 3 months and 6 months.
In addition, a blood sample was collected to perform the epigenetic analysis at 0, 3, and 6 months. For each participant, a nurse collected three samples of 5 ml of venous blood. The genomic DNA of the participants was extracted from 5 ml leukocytes of peripheral venous blood using tubes with EDTA as an anticoagulant.
The methylation status of three intron 7 CpG sites (ADS3828-FS2, ADS3828-FS1, and ADS6607-FS) was determined individually as an artificial C/T SNP using QCpG software (Pyrosequencing method, Qiagen). The methylation level at each CpG site was calculated as the percentage of the methylated alleles divided by the sum of all methylated and unmethylated alleles. The mean methylation level was calculated using methylation levels of all measured CpG sites within the targeted region of each gene1.
Data analysis was performed using R version 3.4.1 (R Core Team, 2016) and the R packages psych for descriptive data (Revelle, 2016). Baseline features were summarized using descriptive statistics. Given multiple points of data for each participant, a mixed-effect growth curve modeling strategy was followed to assess the change in time of DNA methylation and clinical parameters that account for shared variance within subjects while modeling between-subject differences using the nlme r package (Pinheiro et al., 2013).
Our model included average FKBP5 DNA methylation as the dependent variable, predicted by a linear function of time (i.e., Time = 0, 1, 2), dummy coded presence or absence of childhood trauma [based on at least one above-threshold score on the CTQ (Tyrka et al., 2015)], and genotype (where 1 means presence of T allele). Psychotherapy response was measured by the reliable change index (RCI) of the Y-OQ-SR. RCI was calculated using pre and post treatment score, standard deviation, and Cronbach alpha from the normative sample of the scale according to Jacobson and Truax formula (Jacobson and Truax, 1991; Bauer et al., 2004). The RCI allows to identify if the differences are greater than expected due to random error. An RCI that is greater than 1.96 correspond to the 97.5th percentile of a normal distribution and is equivalent to a statistically significant change (p < 0.05; Jacobson and Truax, 1991). The dependent variable was transformed to its natural logarithm to normalize the distribution of the residues. Visual inspections were performed to check assumptions of heteroscedasticity and normality of residues. Missing values (timepoints) were programmed to be omitted from each of the equations. Only random intercepts were accounted for, to avoid convergence problems due to the small sample size and number of parameters in the model.
The study was planned with 34 individuals based on a power calculation to detect a 10 percent difference in DNA methylation. Sampling was restricted by the onset of the COVID-19 pandemic. Two individuals dropped out of the study at baseline, and there were no significant differences in the number of symptoms. A total of 11 individuals completed the study. The mean age was 16.77 ± 1.64 years. Nine of the 11 patients fulfill the threshold of 5 BPD criteria and two patients fulfill the sub-threshold criteria with four symptoms. All patients were above the cut-off score for the presence of depression (BDI-I) of 13 for Chilean population (Valdés et al., 2017) and the mean was in the range of severe depressive symptomatology (Beck et al., 1988).
The presence of childhood trauma was determined if at least one of the subscales of the CTQ scored above the threshold for moderate trauma. Seven participants (63.6%) had scores in the “moderate to severe” trauma range on at least one of the subscales. No significant differences in DNA methylation were found at baseline between individuals with and without the presence of childhood trauma (0.79 vs. 0.78, p = 0.65).
Response to psychotherapy as measured by Y-OQ-SR and according to the Reliable Change Index was associated with a decrease in mean FKBP5 DNA methylation only in those participants who reported the presence of moderate to severe childhood trauma (β = −3.18, SE = 1.24, p = 0.04; Table 1). Fixed effects explain 0.42 of the variance (marginal R2: 0.42, conditional R2: 0.81).
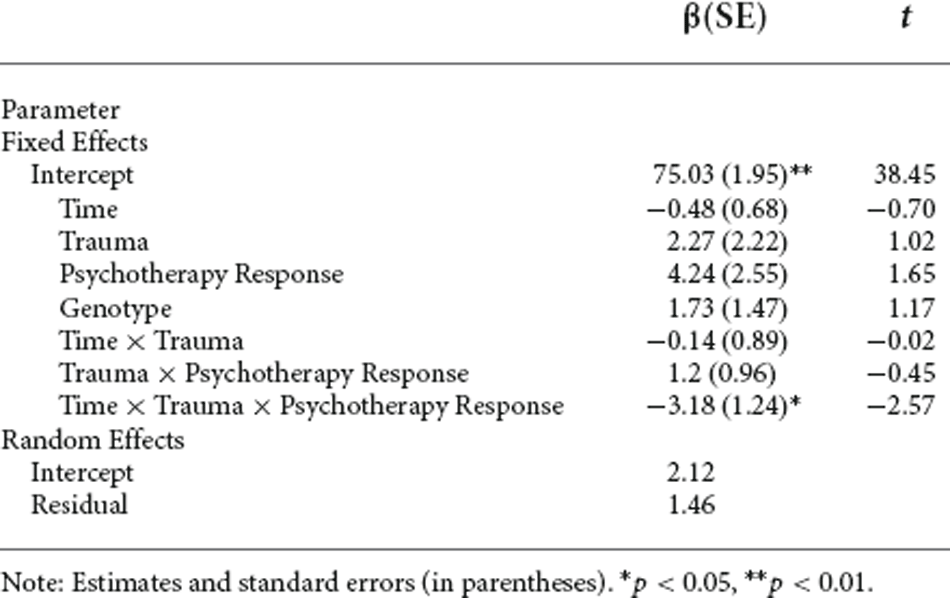
Table 1. Regression analyses of mean FKBP5 DNA methylation change in time according to trauma and psychotherapy response.
No change was observed over time in levels of mentalization, nor was there any significant association with changes in DNA methylation.
No significant relationship was observed between genotype, depressive symptoms, borderline symptoms, emotional regulation, and change in DNA methylation over time.
In this study, a reduction in FKBP5 DNA methylation was observed in responders to therapy and especially in the group with early trauma.
The finding of decreased FKBP5 DNA methylation associated with response to psychotherapy replicates the results of previous studies in individuals with PTSD who were treated with exposure therapy (Yehuda et al., 2013), children with anxiety disorders treated with cognitive behavioral therapy (Roberts et al., 2015) and individuals with Agoraphobia with or without panic disorder (Roberts et al., 2019). Bishop et al. (2018) also report significant findings in individuals with PTSD treated with Mindfulness-Based Stress Reduction (MBSR) therapy, but in the opposite direction, responders have increased DNA methylation (intron 7, bin 2). This study found a decrease in FKBP5 DNA methylation in BPD phenotype and with different types of psychotherapy (psychodynamic psychotherapy and dialectic behavior therapy) suggesting that psychotherapies, in general, can act as “environmental regulators” (Yehuda et al., 2013) through modification of expression of HPA-axis related genes across several mental disorders. DBT psychotherapy has previously been associated with DNA methylation change in other genes in individuals with BPD, but not with FKBP5 (Perroud et al., 2013; Knoblich et al., 2018; Thomas et al., 2018).
The identification of plasticity genes can contribute to the advance in the identification of molecular markers of stable improvement in BPD. Interesting candidates are genes associated with the HPA axis, NR3C1, and FKBP5. Both showed stability in methylation for 2 years in healthy adults suggesting that it may be markers of stable changes and individual differences in stress response regulation (Di Sante et al., 2018). More research is needed to determine the patterns of variability and stability in time of methylation patterns of different genes, in different developmental periods and in clinical samples.
A striking finding of this study is that only those who reported the presence of early trauma exhibited a decrease in DNA methylation. This suggests that in this group the biological mechanism for developing BPD could be different than the no early trauma group.
Although in this study no difference in DNA methylation of FKBP5 intron 7 was found at baseline and no effect of FKBP5 SNP1360780 risk T allele, several studies have reported a relationship between the presence of childhood trauma and decreased DNA methylation levels in this region across different populations, preschool children, low-income adult population, Holocaust offspring, postpartum women, individuals with MDD, and individuals with psychotic disorders (Klengel et al., 2013; Yehuda et al., 2013; Tyrka et al., 2015; Tozzi et al., 2018; Grasso et al., 2020; Misiak et al., 2020) in particular those individuals carrying the FKBP5 SNP1360780 risk T allele, suggesting the impact of early emotional environment on stress response systems and development of psychopathology throughout life.
No significant change in mentalization levels was observed, nor was an association found with response to psychotherapy or changes in DNA methylation. This is probably due to the difficulty of the instruments to detect changes in mentalizing capacity, which is highly context dependent. In individuals with BPD faced with interpersonal situations that generate emotional arousal, mentalizing capacity is deactivated and less sophisticated behavioral and emotional patterns are activated (Fonagy and Bateman, 2008). Instruments that can assess mentalization emerging from dyadic interaction such as psychotherapy sessions (Talia et al., 2019) could be more accurate and ecologically valid in finding episodes of mentalizing deactivations and mentalizing improvements across time.
In this study, only individuals who reported the presence of early trauma and who responded to psychotherapy exhibited a decrease in DNA methylation. Other studies have reported positive associations between the presence of early adverse events and response to psychotherapy, adult individuals treated for chronic depression responded better to psychotherapy than to psychopharmacological treatment if they had a history of childhood abuse (Nemeroff et al., 2003). Similarly, adolescents with non-suicidal self-injury (NSSI) had a better response to psychotherapy in reducing the frequency of NSSI if they had reported adverse childhood experiences (Edinger et al., 2020). This differential response to the presence of early trauma is suggestive of specific mechanisms not only of symptomatology development but also of distinct mechanisms of recovery. In this sample, the differential response to psychotherapy at the level of DNA methylation may imply that some individuals are more permeable at the molecular level to both negative (early trauma) and positive influences (psychotherapy) from their affective environment.
According to the differential sensitivity model, individuals carrying “plastic alleles” who, faced with an early sub-optimal affective environment, would be more susceptible to develop psychopathology but can be also more susceptible to respond to positive social environments (Hammen et al., 2015). Psychotherapeutic interventions, understood as a factor capable of modifying the relationship with the current social environment, may have a greater effect on individuals who carry plastic alleles (Leighton et al., 2017; Jiménez et al., 2018).
In accordance with the above, a GWAS study of twins reported a polygenic score based on differences in sensitivity to develop anxiety disorders according to positive or negative parenting. In a second sample, individuals with major differential sensitivity polygenic score responded better to individual cognitive behavioral therapy (Keers et al., 2016). These results suggest that those individuals who present a greater sensitivity to the environment present more emotional problems if they experienced negative parenting, but they will also be the ones who will benefit most from more intensive forms of psychotherapy (Choi-Kain et al., 2017).
Close human relationships regulate optimal stimulation and modulate arousal levels and attenuate stress in order to improve the adaptation to the social environment. This phenomenon has been called “psychobiological attunement”, and has been explored in mother-child dyads and peer relations and can be observed from its behavioral, physiological, and biochemical correlates (Field, 2012). For example, intrusive mothers can upregulate infants’ developing stress systems, increasing cortisol levels in saliva (Tarullo et al., 2017).
During the establishment of the therapeutic relationship, the formation of an alliance between patient and therapist can lead to the restoration of “epistemic trust”, that is, to restore an individual’s confidence in obtaining from another human being knowledge relevant to his or her adaptation to the social world (Fonagy and Allison, 2014). This would be particularly relevant with individuals with BPD, in whom insecure attachment patterns developed in sub-optimal interaction with their caregivers would involve chronic epistemic mistrust, i.e., a deficiency in the trustworthiness and relevance of interpersonal communication with the concomitant development of deficient behavioral and emotional patterns for establishing cooperation and the ability to repair ruptures in relationships with others, increase their mentalizing capacities and improve their adaptation to their social environment (Fonagy et al., 2015; Orme et al., 2019).
Psychotherapy as a special form of human relationship would then be constituted as a biologically embedded experience, capable of altering biological functions in a stable and long-term manner (Demetriou et al., 2015). Psychotherapy is constituted as a disrupter of the “external social recursion” that goes from the social environment to the neural systems, modifying the subjective perception of the interpersonal environment. At the same time, it is capable of changing the “internal physiologic recursion” that ranges from the Central Nervous System to gene expression, that includes hormonal systems, inflammatory molecules, and intracellular signal transduction (Slavich and Cole, 2013).
Psychotherapy focused on personality pathology may lead to changes in DNA methylation causing not only a symptomatic improvement but a reprogramming of the phenotypic adaptation to the interpersonal environment (Figure 1).
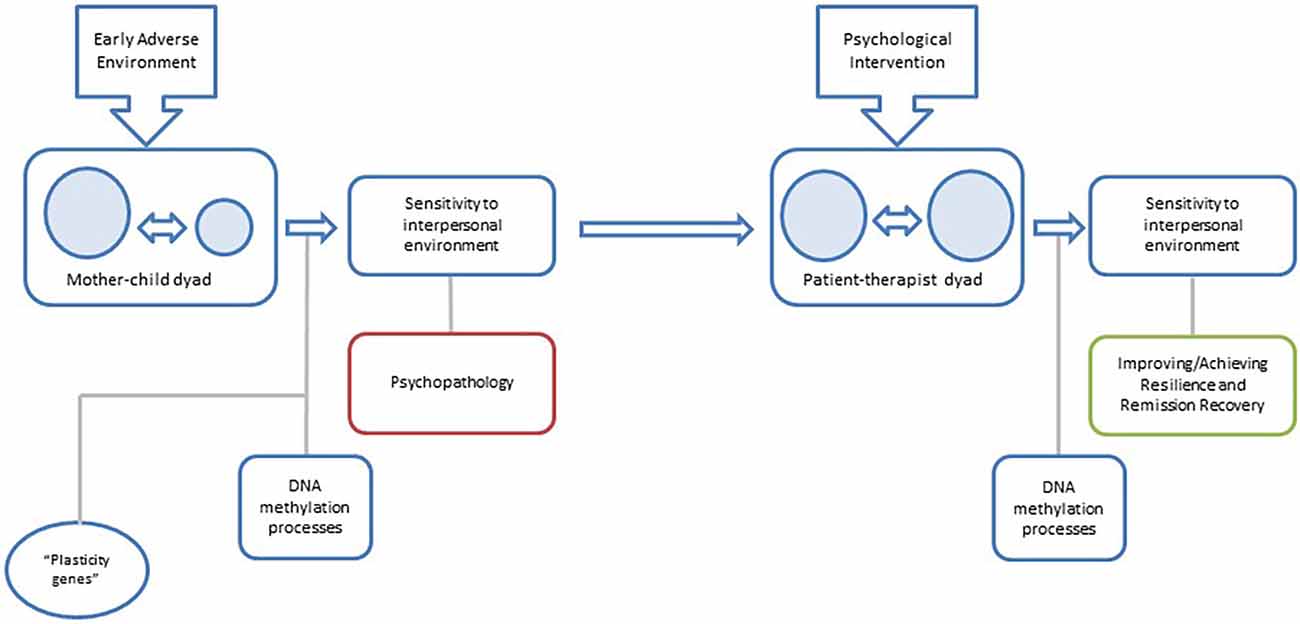
Figure 1. Proposal for a model of the relationship between changes in DNA methylation and psychotherapy in BPD: early mother-child interaction configures interpersonal sensitivity patterns on the child through DNA methylation processes, and the subsequent development of the borderline phenotype. In later stages of development, an appropriate patient-therapist interaction will be able to reconfigure interpersonal sensitivity patterns and reduce borderline symptomatology through stable changes in DNA methylation.
This study has several limitations; as a pilot study, our sample size was small relative to the number of predictors used in the final model, a known factor associated with type I and II errors. As such, our findings should be taken with caution and not be interpreted as conclusive but as a valuable indication of the direction for further inquiry in the study of the molecular changes associated with psychotherapy for personality problems, as they aim to inspire further studies with an adequate sample size. For the same reason, other concomitant environmental factors were not incorporated as covariates that may interact with DNA methylation such as physical factors, i.e., nutrition, alcohol, drugs, contraceptives, and sleep deprivation (Nilsson et al., 2016; Sarabi et al., 2017; Gabbianelli and Damiani, 2018) and other social factors such as socioeconomic status (Maddock et al., 2018) should be taken into account.
The absence of healthy controls is another important limitation because in the childhood and adolescent population there may be methylation changes associated with development.
In this regard, studies exploring longitudinal changes using a genome-wide DNA methylation strategy in adolescents show that in a range of 3–6 months, there is one group of genes that is highly variable over time and another that varies between individuals, but remains stable over time (Lévesque et al., 2014). Moreover, a study in 51 adult individuals showed stability of FKBP5 DNA methylation for 2 years, suggesting that it could be a trait marker of stable changes and individual differences in stress response regulation (Di Sante et al., 2018). The present study was able to compare and found differences between responders and non-responders to psychotherapy i.e., those individuals who had no significant clinical change over time operate as controls, in a manner similar to other studies that longitudinally explored FKBP5DNA methylation changes in relation to psychotherapy (Yehuda et al., 2013; Bishop et al., 2018).
In this study, BPD symptomatology was assessed through an instrument based on symptom intensity according to the DSM-IV categorization (Bohus et al., 2009). The study of personality can be broadened by resorting to a dimensional approach in line with the Alternative Model of Personality Disorders of DSM-5 (American Psychiatric Association, 2013) and the International Classification of Diseases, 11th Revision [ICD-11, (ICD-11—Mortality and Morbidity Statistics, 2021)], for example characterizing the Functioning Levels of Personality, which include identity, self-direction, empathy, and intimacy (Zimmermann et al., 2012). These dimensions of personality functioning are more directly connected with the focus of the therapeutic work and, therefore, allow a greater understanding of the mechanisms of change in psychotherapy of personality.
Along with outcome indicators, psychotherapy process measures such as emotional regulation and mentalization were included which has not been reported in previous studies. Other process measures can be incorporated, such as therapeutic alliance (Horvath et al., 2011), characteristics of the therapist (e.g., warmth), and the patient (e.g., expectations; Wampold, 2015). Prospective, longitudinal studies designs with the use of repeated measures could allow exploration of the interaction between DNA methylation changes and different factors of the therapy process.
Differences in the direction of DNA methylation change may be due to different regions of FKBP5 having been explored, for example, the region near promoter exon 1 (Yang et al., 2021) or intron 7 different CpG sites (Roberts et al., 2019; Bishop et al., 2018). Potentially, a reduction in methylation could generate a downstream lower expression of the FK506-binding protein 5 and reduce glucocorticoid receptor resistance and the impairment of negative feedback loop (Yang et al., 2021), however, functional inferences are not possible given that FKBP5 expression and endocrine markers of hypothalamic-pituitary-adrenal axis function were not measured. Yehuda et al. (2013) found that variation in FKBP5 DNA methylation was associated with treatment response and correlated with measures of plasma cortisol and glucocorticoid sensitivity, implying a functional impact at the HPA axis level of changes in DNA methylation.
Despite the limitations, the present work proposes a design that allows us to explore the explanatory relationships between the therapeutic process and changes at the epigenetic level, in other words, to advance in the understanding of the molecular mechanisms of psychic change.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by ethics committee of Pontificia Universidad Católica de Chile. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
YQ, LB, LH, and JJ contributed to the conception and design of the study. CH contributed to data analysis. YQ wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by The National Fund for Science and Technology (Grant N° 1150166), The National Agency of Research and Development (Grant N° 21150647), and by the Fund for Innovation and Competitiveness (FIC) of the Chilean Ministry of Economy, Development and Tourism, through the Millennium Scientific Initiative, Grant N° IS130005.
We thank The National Agency of Research and Development, the Millenium Institute for Depression and Personality Research (MIDAP), Sussanne Schlüter-Müller MD, Klaus Schmeck, MD, and Ronan Zimmermann, PhD from the Personality Research and Research Department | Child and Adolescent Psychiatric Hospital, Psychiatric Hospitals of the University of Basel (UPK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amad, A., Ramoz, N., Thomas, P., Jardri, R., and Gorwood, P. (2014). Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci. Biobehav. Rev. 40, 6–19. doi: 10.1016/j.neubiorev.2014.01.003
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890425596
Antonsen, B. T., Johansen, M. S., Ro, F. G., Kvarstein, E. H., and Wilberg, T. (2016). Is reflective functioning associated with clinical symptoms and long-term course in patients with personality disorders? Compr. Psychiatry 64, 46–58. doi: 10.1016/j.comppsych.2015.05.016
Badoud, D., Prada, P., Nicastro, R., Germond, C., Luyten, P., Perroud, N., et al. (2018). Attachment and reflective functioning in women with borderline personality disorder. J. Pers. Disord. 32, 17–30. doi: 10.1521/pedi_2017_31_283
Bateman, A. W., and Fonagy, P. (2004). Mentalization-based treatment of BPD. J. Pers. Disord. 18, 36–51. doi: 10.1521/pedi.18.1.36.32772
Bateman, A., and Fonagy, P. (2008). 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. Am. J. Psychiatry 165, 631–638. doi: 10.1176/appi.ajp.2007.07040636
Bateman, A., and Fonagy, P. (2009). Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am. J. Psychiatry 166, 1355–1364. doi: 10.1176/appi.ajp.2009.09040539
Bauer, S., Lambert, M. J., and Nielsen, S. L. (2004). Clinical significance methods: a comparison of statistical techniques. J. Pers. Assess. 82, 60–70. doi: 10.1207/s15327752jpa8201_11
Beck, A. T., Steer, R. A., and Garbin, M. G. (1988). Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100. doi: 10.1016/0272-7358(88)90050-5
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. doi: 10.1001/archpsyc.1961.01710120031004
Bernstein, D. P., Ahluvalia, T., Pogge, D., and Handelsman, L. (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348. doi: 10.1097/00004583-199703000-00012
Binder, E. B. (2009). The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34, S186–S195. doi: 10.1016/j.psyneuen.2009.05.021
Bishop, J. R., Lee, A. M., Mills, L. J., Thuras, P. D., Eum, S., Clancy, D., et al. (2018). Methylation of FKBP5 and SLC6A4 in relation to treatment response to mindfulness based stress reduction for posttraumatic stress disorder. Front. Psychiatry 9:418. doi: 10.3389/fpsyt.2018.00418
Bohus, M., Kleindienst, N., Limberger, M. F., Stieglitz, R. D., Domsalla, M., Chapman, A. L., et al. (2009). The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology 42, 32–39. doi: 10.1159/000173701
Bulbena-Cabre, A., Bassir Nia, A., and Perez-Rodriguez, M. M. (2018). Current knowledge on gene-environment interactions in personality disorders: an update. Curr. Psychiatry Rep. 20:74. doi: 10.1007/s11920-018-0934-7
Calati, R., Gressier, F., Balestri, M., and Serretti, A. (2013). Genetic modulation of borderline personality disorder: systematic review and meta-analysis. J. Psychiatr. Res. 47, 1275–1287. doi: 10.1016/j.jpsychires.2013.06.002
Cecil, C. A. M., Smith, R. G., Walton, E., Mill, J., McCrory, E. J., and Viding, E. (2016). Epigenetic signatures of childhood abuse and neglect: implications for psychiatric vulnerability. J. Psychiatr. Res. 83, 184–194. doi: 10.1016/j.jpsychires.2016.09.010
Chanen, A. M., Jovev, M., Djaja, D., McDougall, E., Yuen, H. P., Rawlings, D., et al. (2008). Screening for borderline personality disorder in outpatient youth. J. Pers. Disord. 22, 353–364. doi: 10.1521/pedi.2008.22.4.353
Choi-Kain, L. W., Finch, E. F., Masland, S. R., Jenkins, J. A., and Unruh, B. T. (2017). What works in the treatment of borderline personality disorder. Curr. Behav. Neurosci. Rep. 4, 21–30. doi: 10.1007/s40473-017-0103-z
Demetriou, C. A., van Veldhoven, K., Relton, C., Stringhini, S., Kyriacou, K., and Vineis, P. (2015). Biological embedding of early-life exposures and disease risk in humans: a role for DNA methylation. Eur. J. Clin. Invest. 45, 303–332. doi: 10.1111/eci.12406
Di Sante, J., Ismaylova, E., Nemoda, Z., Gouin, J. P., Yu, W. J., Caldwell, W., et al. (2018). Peripheral DNA methylation of HPA axis-related genes in humans: cross-tissue convergence, two-year stability and behavioural and neural correlates. Psychoneuroendocrinology 97, 196–205. doi: 10.1016/j.psyneuen.2018.07.019
Ecker, S., Pancaldi, V., Valencia, A., Beck, S., and Paul, D. S. (2018). Epigenetic and transcriptional variability shape phenotypic plasticity. Bioessays 40:1700148. doi: 10.1002/bies.201700148
Edinger, A., Fischer-Waldschmidt, G., Parzer, P., Brunner, R., Resch, F., and Kaess, M. (2020). The impact of adverse childhood experiences on therapy outcome in adolescents engaging in nonsuicidal self-injury. Front. Psychiatry 11:505661. doi: 10.3389/fpsyt.2020.505661
First, B., Spitzer, R., Gibbon, M., Williams, J., Davies, M., Borus, J., et al. (1995). The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part II: multi-site test-retest reliability study. J. Pers. Disord. 9, 92–104. doi: 10.1521/pedi.1995.9.2.92
Flasbeck, V., and Brüne, M. (2021). Association between childhood maltreatment, psychopathology and DNA methylation of genes involved in stress regulation: evidence from a study in Borderline Personality Disorder. PLoS One 16:e0248514. doi: 10.1371/journal.pone.0248514
Fonagy, P., and Allison, E. (2014). The role of mentalizing and epistemic trust in the therapeutic relationship. Psychotherapy (Chic) 51, 372–380. doi: 10.1037/a0036505
Fonagy, P., and Bateman, A. (2008). The development of borderline personality disorder–a mentalizing model. J. Pers. Disord. 22, 4–21. doi: 10.1521/pedi.2008.22.1.4
Fonagy, P., and Luyten, P. (2009). A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Dev. Psychopathol. 21, 1355–1381. doi: 10.1017/S0954579409990198
Fonagy, P., Leigh, T., Steele, M., Steele, H., Kennedy, T., Mattoon, G., et al. (1996). The relation of attachment status, psychiatric classification and the response to psychotherapy. J. Consult. Clin. Psychol. 64, 22–31. doi: 10.1037/0022-006x.64.1.22
Fonagy, P., Luyten, P., and Allison, E. (2015). Epistemic Petrification and the restoration of epistemic trust: a new conceptualization of borderline personality disorder and its psychosocial treatment. J. Pers. Disord. 29, 575–609. doi: 10.1521/pedi.2015.29.5.575
Fonagy, P., Steele, M., Steele, H., and Target, M. (1998). Reflective-Functioning Manual for Application to Adult Attachment Interviews (Ver 5). London: University College London.
Gabbianelli, R., and Damiani, E. (2018). Epigenetics and neurodegeneration: role of early-life nutrition. J. Nutr. Biochem. 57, 1–13. doi: 10.1016/j.jnutbio.2018.01.014
Gergely, G., Jurist, E. L., Fonagy, P. (2002). Affect Regulation, Mentalization and the Development of the Self (1st ed.). London: Routledge.
Goodman, G., Edwards, K., and Chung, H. (2015). The relation between prototypical processes and psychological distress in psychodynamic therapy of five inpatients with borderline personality disorder. Clin. Psychol. Psychother. 22, 83–95. doi: 10.1002/cpp.1875
Grasso, D. J., Drury, S., Briggs-Gowan, M., Johnson, A., Ford, J., Lapidus, G., et al. (2020). Adverse childhood experiences, posttraumatic stress and FKBP5 methylation patterns in postpartum women and their newborn infants. Psychoneuroendocrinology 114:104604. doi: 10.1016/j.psyneuen.2020.104604
Gratz, K. L., and Roemer, L. (2004). Multidimensional Assessment of emotion regulation and dysregulation: development, factor structure and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess. 26, 41–54. doi: 10.1023/B:JOBA.0000007455.08539.94
Gunderson, J. G., and Lyons-Ruth, K. (2008). BPD’s interpersonal hypersensitivity phenotype: a gene-environment-developmental model. J. Pers. Disord. 22, 22–41. doi: 10.1521/pedi.2008.22.1.22
Hammen, C., Bower, J. E., and Cole, S. W. (2015). Oxytocin receptor gene variation and differential susceptibility to family environment in predicting youth borderline symptoms. J. Pers. Disord. 29, 177–192. doi: 10.1521/pedi_2014_28_152
Horvath, A. O., Del Re, A. C., Flückiger, C., and Symonds, D. (2011). Alliance in individual psychotherapy. Psychotherapy (Chic) 48, 9–16. doi: 10.1037/a0022186
ICD-11—Mortality and Morbidity Statistics (2021). Available online at: https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f941859884
Jacobson, N. S., and Truax, P. (1991). Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 59, 12–19. doi: 10.1037//0022-006x.59.1.12
Jaenisch, R., and Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254. doi: 10.1038/ng1089
Jiménez, J. P., Botto, A., Herrera, L., Leighton, C., Rossi, J. L., Quevedo, Y., et al. (2018). Psychotherapy and genetic neuroscience: an emerging dialog. Front. Genet. 9:257. doi: 10.3389/fgene.2018.00257
Kaess, M., Fischer-Waldschmidt, G., Resch, F., and Koenig, J. (2017). Health related quality of life and psychopathological distress in risk taking and self-harming adolescents with full-syndrome, subthreshold and without borderline personality disorder: rethinking the clinical cut-off? Borderline Personal. Disord. Emot. Dysregul. 4:7. doi: 10.1186/s40479-017-0058-4
Keers, R., Coleman, J. R. I., Lester, K. J., Roberts, S., Breen, G., Thastum, M., et al. (2016). A genome-wide test of the differential susceptibility hypothesis reveals a genetic predictor of differential response to psychological treatments for child anxiety disorders. Psychother. Psychosom. 85, 146–158. doi: 10.1159/000444023
Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J. C., Pariante, C. M., et al. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16, 33–41. doi: 10.1038/nn.3275
Knoblich, N., Gundel, F., Brückmann, C., Becker-Sadzio, J., Frischholz, C., and Nieratschker, V. (2018). DNA methylation of APBA3 and MCF2 in borderline personality disorder: potential biomarkers for response to psychotherapy. Eur. Neuropsychopharmacol. 28, 252–263. doi: 10.1016/j.euroneuro.2017.12.010
Kumsta, R., Marzi, S. J., Viana, J., Dempster, E. L., Crawford, B., Rutter, M., et al. (2016). Severe psychosocial deprivation in early childhood is associated with increased DNA methylation across a region spanning the transcription start site of CYP2E1. Transl. Psychiatry 6:e830. doi: 10.1038/tp.2016.95
Leichsenring, F., Leibing, E., Kruse, J., New, A. S., and Leweke, F. (2011). Borderline personality disorder. Lancet 377, 74–84. doi: 10.1016/S0140-6736(10)61422-5
Leighton, C., Botto, A., Silva, J. R., Jiménez, J. P., and Luyten, P. (2017). Vulnerability or sensitivity to the environment? Methodological issues, trends and recommendations in gene-environment interactions research in human behavior. Front. Psychiatry 8:106. doi: 10.3389/fpsyt.2017.00106
Lévesque, M. L., Casey, K. F., Szyf, M., Ismaylova, E., Ly, V., Verner, M. P., et al. (2014). Genome-wide DNA methylation variability in adolescent monozygotic twins followed since birth. Epigenetics 9, 1410–1421. doi: 10.4161/15592294.2014.970060
Lutz, P. E., and Turecki, G. (2014). DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience 264, 142–156. doi: 10.1016/j.neuroscience.2013.07.069
Maddock, J., Wulaningsih, W., Fernandez, J. C., Ploubidis, G. B., Goodman, A., Bell, J., et al. (2018). Associations between body size, nutrition and socioeconomic position in early life and the epigenome: a systematic review. PLoS One 13:e0201672. doi: 10.1371/journal.pone.0201672
Martín-Blanco, A., Ferrer, M., Soler, J., Salazar, J., Vega, D., Andión, O., et al. (2014). Association between methylation of the glucocorticoid receptor gene, childhood maltreatment and clinical severity in borderline personality disorder. J. Psychiatr. Res. 57, 34–40. doi: 10.1016/j.jpsychires.2014.06.011
Merrill, S. M., Gladish, N., Fu, M. P., Moore, S. R., Konwar, C., Giesbrecht, G. F., et al. (2021). Associations of peripheral blood DNA methylation and estimated monocyte proportion differences during infancy with toddler attachment style. Attach. Hum. Dev. 1, 1–30. . [Online ahead of print]. doi: 10.1080/14616734.2021.1938872
Misiak, B., Karpiński, P., Szmida, E., Grąźlewski, T., Jabłoński, M., Cyranka, K., et al. (2020). Adverse childhood experiences and methylation of the FKBP5 gene in patients with psychotic disorders. J. Clin. Med. 9:E3792. doi: 10.3390/jcm9123792
Morandotti, N., Brondino, N., Merelli, A., Boldrini, A., De Vidovich, G. Z., Ricciardo, S., et al. (2018). The Italian version of the Reflective Functioning Questionnaire: validity data for adults and its association with severity of borderline personality disorder. PLoS One 13:e0206433. doi: 10.1371/journal.pone.0206433
Mulder, R. H., Rijlaarsdam, J., Luijk, M. P. C. M., Verhulst, F. C., Felix, J. F., Tiemeier, H., et al. (2017). Methylation matters: FK506 binding protein 51 (FKBP5) methylation moderates the associations of FKBP5 genotype and resistant attachment with stress regulation. Dev. Psychopathol. 29, 491–503. doi: 10.1017/S095457941700013X
Murgatroyd, C., Patchev, A. V., Wu, Y., Micale, V., Bockmühl, Y., Fischer, D., et al. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 12, 1559–1566. doi: 10.1038/nn.2436
Needham, B. L., Smith, J. A., Zhao, W., Wang, X., Mukherjee, B., Kardia, S. L., et al. (2015). Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics 10, 958–969. doi: 10.1080/15592294.2015.1085139
Nemeroff, C. B., Heim, C. M., Thase, M. E., Klein, D. N., Rush, A. J., Schatzberg, A. F., et al. (2003). Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl. Acad. Sci. U S A 100, 14293–14296. doi: 10.1073/pnas.2336126100
Németh, N., Péterfalvi, Á., Czéh, B., Tényi, T., and Simon, M. (2020). Examining the relationship between executive functions and mentalizing abilities of patients with borderline personality disorder. Front. Psychol. 11:1583. doi: 10.3389/fpsyg.2020.01583
Nilsson, E. K., Bostrom, A. E., Mwinyi, J., and Schioth, H. B. (2016). Epigenomics of total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS 20, 334–342. doi: 10.1089/omi.2016.0041
Orme, W., Bowersox, L., Vanwoerden, S., Fonagy, P., and Sharp, C. (2019). The relation between epistemic trust and borderline pathology in an adolescent inpatient sample. Borderline Personal. Disord. Emot. Dysregul. 6:13. doi: 10.1186/s40479-019-0110-7
Perna, L., Zhang, Y., Wild, B., Kliegel, M., Ihle, A., Schöttker, B., et al. (2020). Childhood exposure to hunger: associations with health outcomes in later life and epigenetic markers. Epigenomics 12, 1861–1870. doi: 10.2217/epi-2019-0333
Perroud, N., Salzmann, A., Prada, P., Nicastro, R., Hoeppli, M. E., Furrer, S., et al. (2013). Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl. Psychiatry 3:e207. doi: 10.1038/tp.2012.140
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. R., and Development Core Team (2013). nlme: Linear and nonlinear mixed effects models. R Package Version 3, 1–108. Available online at: https://CRAN.R-project.org/package=nlme.
Pyrosequencing method. Available online at: https://www.epigendx.com/d/service/pyrosequencing/dna-methylation.
R Core Team (2016). R: a language and environment for statistical computing. Vienna: Austria. Available online at: https://www.R-project.org/.
Radtke, K. M., Schauer, M., Gunter, H. M., Ruf-Leuschner, M., Sill, J., Meyer, A., et al. (2015). Epigenetic modifications of the glucocorticoid receptor gene are associated with the vulnerability to psychopathology in childhood maltreatment. Transl. Psychiatry 5:e571. doi: 10.1038/tp.2015.63
Revelle, W. (2016). psych: Procedures for Personality and Psychological Research. Evanston, IL: Northwestern University. Available online at: https://cran.r-project.org/web/packages/psych/psych.tif.
Roberts, S., Keers, R., Breen, G., Coleman, J., Jöhren, P., Kepa, A., et al. (2019). DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 180, 150–158. doi: 10.1002/ajmg.b.32650
Roberts, S., Keers, R., Lester, K. J., Coleman, J. R., Breen, G., Arendt, K., et al. (2015). HPA axis related genes and response to psychological therapies: genetics and epigenetics. Depress. Anxiety 32, 861–870. doi: 10.1002/da.22430
Rudden, M. G., Milrod, B., and Target, M. (2005). The Brief Reflective Functioning Interview. New York: Weill Cornell Medical College.
Sarabi, M. M., Ghareghani, P., Khademi, F., and Zal, F. (2017). Oral contraceptive use may modulate global genomic DNA methylation and promoter methylation of APC1 and ESR1. Asian Pac. J. Cancer Prev. 18, 2361–2366. doi: 10.22034/APJCP.2017.18.9.2361
Sharp, C., and Fonagy, P. (2015). Practitioner review: borderline personality disorder in adolescence - recent conceptualization, intervention and implications for clinical practice. J. Child Psychol. Psychiatry 56, 1266–1288. doi: 10.1111/jcpp.12449
Sheehan, D. V., Sheehan, K. H., Shytle, R. D., Janavs, J., Bannon, Y., Rogers, J. E., et al. (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J. Clin. Psychiatry 71, 313–326. doi: 10.4088/JCP.09m05305whi
Skoglund, C., Tiger, A., Rück, C., Petrovic, P., Asherson, P., Hellner, C., et al. (2021). Familial risk and heritability of diagnosed borderline personality disorder: a register study of the Swedish population. Mol. Psychiatry 26, 999–1008. doi: 10.1038/s41380-019-0442-0
Slavich, G. M., and Cole, S. W. (2013). The emerging field of human social genomics. Clin. Psychol. Sci. 1, 331–348. doi: 10.1177/2167702613478594
Stead, V. E., Boylan, K., and Schmidt, L. A. (2019). Longitudinal associations between non-suicidal self-injury and borderline personality disorder in adolescents: a literature review. Borderline Personal. Disord. Emot. Dysregul. 6:3. doi: 10.1186/s40479-019-0100-9
Talia, A., Miller-Bottome, M., Katznelson, H., Pedersen, S. H., Steele, H., Schröder, P., et al. (2019). Mentalizing in the presence of another: measuring reflective functioning and attachment in the therapy process. Psychother. Res. 29, 652–665. doi: 10.1080/10503307.2017.1417651
Tarullo, A. R., St. John, A. M., and Meyer, J. S. (2017). Chronic stress in the mother-infant dyad: maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant. Behav. Dev. 47, 92–102. doi: 10.1016/j.infbeh.2017.03.007
Thomas, M., Knoblich, N., Wallisch, A., Glowacz, K., Becker-Sadzio, J., Gundel, F., et al. (2018). Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin. Epigenetics 10:109. doi: 10.1186/s13148-018-0544-6
Tozzi, L., Farrell, C., Booij, L., Doolin, K., Nemoda, Z., Szyf, M., et al. (2018). Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology 43, 1138–1145. doi: 10.1038/npp.2017.290
Trull, T. J., Jahng, S., Tomko, R. L., Wood, P. K., and Sher, K. J. (2010). Revised NESARC personality disorder diagnoses: gender, prevalence and comorbidity with substance dependence disorders. J. Personal. Disord. 24, 412–426. doi: 10.1521/pedi.2010.24.4.412
Turecki, G., and Meaney, M. J. (2016). Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol. Psychiatry 79, 87–96. doi: 10.1016/j.biopsych.2014.11.022
Tyrka, A. R., Ridout, K. K., Parade, S. H., Paquette, A., Marsit, C. J., Seifer, R., et al. (2015). Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Dev. Psychopathol. 27, 1637–1645. doi: 10.1017/S0954579415000991
Valdés, C., Morales-Reyes, I., Pérez, J. C., Medellín, A., Rojas, G., Krause, M., et al. (2017). Propiedades psicométricas del inventario de depresión de Beck IA para la población chilena (Psychometric properties of a spanish version of the Beck depression inventory IA). Revista Médica de Chile 145, 1005–1012. doi: 10.4067/s0034-98872017000801005
Wampold, B. E. (2015). How important are the common factors in psychotherapy? an update. World Psychiatry 14, 270–277. doi: 10.1002/wps.20238
Weaver, I. C. (2007). Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let’s call the whole thing off. Epigenetics 2, 22–28. doi: 10.4161/epi.2.1.3881
Wells, M. G., Burlingame, G. M., and Rose, P. M. (2003). Youth outcome questionnaire self report. Wilmington, DE: American Professional Credentialing Services.
West, M., Rose, M. S., Spreng, S., Sheldon-Keller, A., and Adam, K. (1998). Adolescent attachment questionnaire: a brief assessment of attachment in adolescence. J. Youth Adolesc. 27, 661–673. doi: 10.1023/A:1022891225542
Winograd, G., Cohen, P., and Chen, H. (2008). Adolescent borderline symptoms in the community: prognosis for functioning over 20 years. J. Child Psychol. Psychiatry 49, 933–941. doi: 10.1111/j.1469-7610.2008.01930.x
Yang, B. Z., Zhang, H., Ge, W., Weder, N., Douglas-Palumberi, H., Perepletchikova, F., et al. (2013). Child abuse and epigenetic mechanisms of disease risk. Am. J. Prev. Med. 44, 101–107. doi: 10.1016/j.amepre.2012.10.012
Yang, R., Xu, C., Bierer, L. M., Flory, J. D., Gautam, A., Bader, H. N., et al. (2021). Longitudinal genome-wide methylation study of PTSD treatment using prolonged exposure and hydrocortisone. Transl. Psychiatry 11:398. doi: 10.1038/s41398-021-01513-5
Yehuda, R., Daskalakis, N. P., Bierer, L. M., Bader, H. N., Klengel, T., Holsboer, F., et al. (2016). Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol. Psychiatry 80, 372–380. doi: 10.1016/j.biopsych.2015.08.005
Yehuda, R., Daskalakis, N. P., Desarnaud, F., Makotkine, I., Lehrner, A. L., Koch, E., et al. (2013). Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front. Psychiatry 4:118. doi: 10.3389/fpsyt.2013.00118
Zannas, A. S., and Binder, E. B. (2014). Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 13, 25–37. doi: 10.1111/gbb.12104
Zannas, A. S., Wiechmann, T., Gassen, N. C., and Binder, E. B. (2015). Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology 41, 261–274. doi: 10.1038/npp.2015.235
Keywords: borderline personality disorder, DNA methylation, mentalization, psychotherapy, epigenetics
Citation: Quevedo Y, Booij L, Herrera L, Hernández C and Jiménez JP (2022) Potential epigenetic mechanisms in psychotherapy: a pilot study on DNA methylation and mentalization change in borderline personality disorder. Front. Hum. Neurosci. 16:955005. doi: 10.3389/fnhum.2022.955005
Received: 27 May 2022; Accepted: 18 August 2022;
Published: 12 September 2022.
Edited by:
Filippo Cieri, Cleveland Clinic, United StatesCopyright © 2022 Quevedo, Booij, Herrera, Hernández and Jiménez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Pablo Jiménez, amppbWVuZXpAbWVkLnVjaGlsZS5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.