
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hum. Neurosci., 21 September 2022
Sec. Brain Health and Clinical Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.923695
This article is part of the Research TopicEndogenous Neuromodulation in the Infra-Low Frequency RegimeView all 18 articles
 Joannis N. Nestoros*
Joannis N. Nestoros* Nionia G. Vallianatou
Nionia G. VallianatouA 38-year-old army officer started therapy in 2020 with a four-year history of auditory hallucinations and delusions of reference, persecution and grandeur, symptoms that were resistant to traditional antipsychotic medications. He follows an integrative psychotherapy program that aims to reduce his anxiety, continues his antipsychotic medications, and has Infra-Low Frequency Neurofeedback. After his initial assessment he had a 40 min session of Infra-Low Frequency Neurofeedback before any other kind of intervention. Before and immediately after the session he completed the SCL-90 scale and the Visual Analog Scale covering 20 aspects of his psychological and physical state as well as his schizophrenic symptoms. This first Neurofeedback session had dramatic effects on his psychotic symptoms, levels of anxiety and psychosomatic condition, before his first psychotherapy session and/or any changes in his antipsychotic medication. The above results have great importance due to the severity and chronicity of schizophrenia. Informed consent was obtained from the participant for the publication of this case report (including all data and images).
Schizophrenia is without doubt one of the most fascinating topics of research because it involves biological, psychological, family and sociocultural factors. The evolution of the concept of schizophrenia since 1860 and its effects on current thinking has been discussed by Nestoros (1997a,b) as well as by others (Tandon et al., 2010; Keshavan et al., 2011). In summary, whereas we previously had in the scientific literature at least 26 different types of schizophrenia (Nestoros, 1997a,b, 2012a,b), we ended in DSM-5–first published in 2013–with only one type of schizophrenia characterized as a severe, chronic mental disorder with disturbances in thought, perception and behavior that resembles what was previously called schizophrenia, paranoid type.
In our opinion, certain two neurophysiological models for the etiology of schizophrenic symptoms that relate to one classical neurotransmitter, which is γ-aminobutyric acid (GABA) (Roberts, 1972, 1976), and one neuropeptide/neuromodulator which is cholecystokinin-CCK (Hockfelt et al., 1980a,b), although about 40 years old, when combined with very recent relevant research (Whissell et al., 2015, 2019; Ballaz and Bourin, 2021a,b; Ballaz et al., 2021; Ochoa-de la Paz et al., 2021), prove their contribution to understanding schizophrenia. The two models are not in conflict but complement each other Nestoros, 1980a. Firstly, the increase of extreme stress causes the hyperactivity of dopaminergic (Brisch et al., 2014) and the hypoactivity in GABAergic neurotransmission (Nuss, 2015). Diazepam is known to reduce dopamine release in nucleus accumbens (Gomez et al., 2017) and potentiates the inhibitory action of GABA (Haefely, 1978; Haefely et al., 1978a,b; Costa and Guidotti, 1979; Nestoros and Nistri, 1979; Nestoros, 1980b,c, 1982, 1997a,b); while cholecystokinin regulates dopaminergic (as well as GABAergic and glutaminergic) activity through a corticolimbic and a mesolimbic dimmer-switch process (Ballaz and Bourin, 2021a,b; Ballaz et al., 2021). The inhibitory action of γ-aminobutyric acid (GABA) is mediated predominantly by interneurons (Kelsom and Lu, 2013). The interneurons have a crucial role in neural processing, impinging on behaviors such as fear, social interaction, anxiety, locomotion, cognition, information processing and memory. So, dysfunctions in interneuron signaling affect the behavioral functions and have implications to the many psychiatric and neurological disorders (Ochoa-de la Paz et al., 2021). Interneurons have many subtypes and contribute differentially to behavioral patterns in health and disease. One classification of interneurons is perisomatic interneurons that can be divided into two different groups based on the expression of the molecular markers cholecystokinin (CCK-GABA neurons) and parvalbumin (PV-GABA neurons) (Whissell et al., 2015). CCK-GABA neurons are considered to be involved in the regulation of mood, anxiety and fear, since their function has been largely attributed to a modest population of neurons in amygdala (Vereczki et al., 2021). CCK-GABA neuron distribution is also localized in several regions of the hippocampus, thalamus and cortex (Whissell et al., 2019).
P. H. is a male, right-handed, 38-year-old unmarried army officer. He had a four-year history of auditory hallucinations and delusions of reference, persecution and grandeur, symptoms that were resistant to traditional antipsychotic medications (Clozapine 700 mg per day, Amisulpride 600 mg per day, Valproic acid 1,000 mg per day and Fluoxetine 40 mg per day). Even though the medications did not resolve the issues, this regime was maintained throughout, and continued unchanged through the neurofeedback training. The patient complied with DSM-5 and ICD-11 (2022) criteria for schizophrenia when assessed by the Army psychiatrists and by our center. He experienced the first auditory hallucinations during his adolescence. He started therapy in December 2020. In his initial evaluation he complained of the consistent presence of “loud voices” and many stereotypic behavior patterns that he was forced to execute because of these voices, such as whispering psalms, and/or quick glance movements. He was started on integrative psychotherapy, which aims to reduce his anxiety, continued his antipsychotic medications, and was started an Infra-Low Frequency Regime Neurofeedback following the Othmer and Othmer (2016, 2017, 2020) methodology.
After his initial assessment and before any other kind of intervention, he had a 1 h session of Infra-Low Frequency Neurofeedback. Before and immediately after the session he completed the SCL-90 scale (Derogatis, 1977) and the Visual Analog Scale, covering 20 aspects of his psychological and physical state as well as his schizophrenic symptoms (Nestoros, 1998). He was instructed to complete the scales answering to the time period “How I feel now”? This first Neurofeedback session had dramatic effects on his psychotic symptoms, levels of anxiety and psychosomatic condition, before his first psychotherapy session and/or any changes in his antipsychotic medication. This positive result convinced him to continue the psychotherapeutic and neurofeedback sessions until today. He presently reports (end of March 2022), after eighteen (18) neurofeedback sessions, minor levels of transient and manageable auditory hallucinations and delusions only under stress conditions, without stereotypic behavior patterns. He enjoys his administrative duties, assigned by the army, and 6 months ago started to date a young woman, moving away from his mother's home, where he had been living before. His antipsychotic medication remained the same throughout the above course of treatment, since the army psychiatrists felt that he was doing so well that there was no reason to change it.
Informed consent was obtained from the participant for the publication of this case report (including all data and images). We have placed the electrodes (recording, reference and ground electrodes) in particular areas of the head in accordance with the “Ten percent electrode system for recording EEG activity” (Chatrian et al., 1985) which is based on the “Ten-twenty electrode system” which was proposed by Jasper (1958). The placement of the electrodes and the time of Infra-Low Frequency Neurofeedback employed is shown on Figure 1. We trained P4-T4 (0–10 min), T3-T4 (10th−20th min), Fp2-T4 (20th−30th min and Fp1-T3 (30th−40th min for a total of 10 min each, in the above order. For the right hemisphere reward frequencies were set to 0.0001 Hz and for the left hemisphere at 0.0002 Hz, with percent success of multiple inhibits (with 10 inhibit bands covering the spectrum to 40 Hz) maintained at 95%. That is, the level of difficulty was kept at a level such that inhibits were engaged about 5% of the time. The Othmer and Othmer (2016, 2017, 2020) neurofeedback methodology was followed, using Cygnet Software and Bee Medic one-channel EEG Advance Media player. The patient watched a motion picture while his ILF/10, Delta, Theta, Alpha, Beta and HiBeta band activity was continuously monitored. The patient was attentive throughout the entire session. Before and immediately after the session he completed the SCL-90 (Derogatis, 1977) and the Visual Analog Scale (Nestoros, 1998) covering 20 aspects of his psychological and physical state as well as his schizophrenic symptoms. He was asked to complete both above Scales according to how he was feeling “now”.
As shown on Figure 1, Delta band activity predominated and was higher in all recorded areas throughout the session. ILF/10 amplitude ranked 2nd and was significantly lower than Delta. came Theta band activity, ranked 3rd, followed by Alpha. HiBeta amplitude was the lowest, with one exception between the 30th and the 33th min, at the beginning of the period of recording Fp1-T3, possibly because of anxiety-producing thoughts that were reflected in left frontal lobe activity.
Figure 2 shows the SCL-90 results before and after the first neurofeedback session. The term Somatization represents the somatic symptoms that were reported by the subject (headache, dizziness, pain in the heart or chest, nausea or upset stomach, etc.). The abbreviation OCD represents the obsessive-compulsive symptoms (“Unwanted thoughts, words or ideas that cannot leave your mind”, “Worrying about sloppiness or carelessness”, “Having to check and recheck what you do,” etc.); the abbreviation PI represents the paranoid ideation (“Feeling that others are to blame for most of your problems”, “Feeling that most people are not trustworthy”, “Feeling that other people watch you or talk about you”); and the abbreviation PSY represents the Psychoticism (that is the psychotic symptoms such as “The idea that somebody controls my thoughts”, “Hearing voices that other people do not hear”, “Other people can read my thoughts,” etc.). We evaluated the mean scores of each factor, and we can observe significant post-session differences in the factors “Anxiety”, “Paranoid Ideation” and “Psychoticism”.
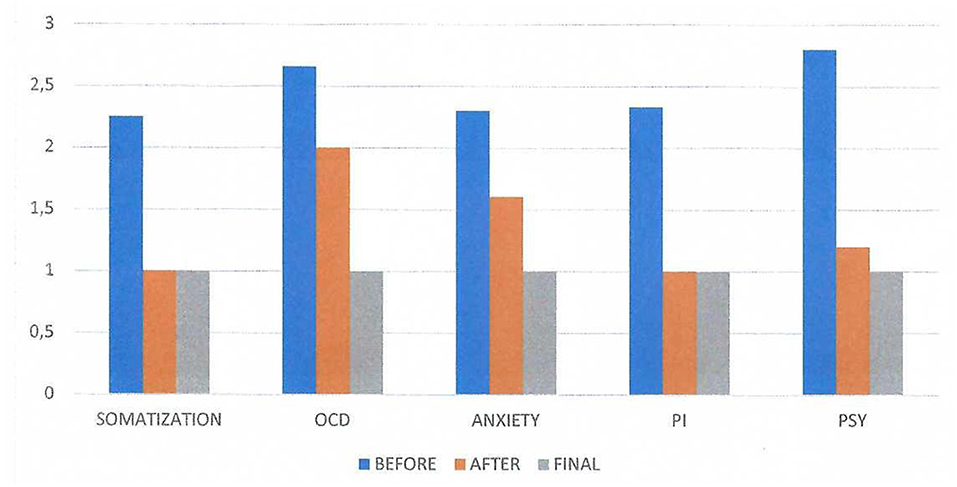
Figure 2. SCL-90 results on three conditions: before and after the first neurofeedback session and the results of the final session. The term Somatization represents the somatic symptoms that were reported by the subject before the session. There was no difference in the appearance of these symptoms after the session. The abbreviation OCD represents the obsessive-compulsive symptoms before and after the session, the abbreviation PI represents the paranoid ideation before and after the session and the abbreviation PSY represents the Psychoticism (that is the psychotic symptoms) before and after the session. We evaluated the mean scores of each factor and we can observe significant variation in the factors “Anxiety”, “Paranoid Ideation” and “Psychoticis”.
Figure 3 shows the results obtained by using the Visual Analog Scale (Nestoros, 1998) covering 20 aspects of his psychological and physical state as well as his psychotic symptoms (negative and positive) according to Andreasen (1984a,b). The abbreviation PST represents the general psychological state of the subject, PSY the psychosomatic symptoms, AH the auditory hallucinations, RD the delusions of reference, PD the persecutory delusions, CD the delusions of being controlled, DMR the delusions of mind reading, and TI the thought insertion. The self-assessment visual analog scale had for every item a vertical line 10 cm in length, where “0” represented “I have no such symptom” and “100” represented “The symptom is extremely annoying”. We evaluated the mean scores before and after the first neurofeedback session. We observe significant positive changes in the scales AH, PST, Anxiety, DMR and TI.
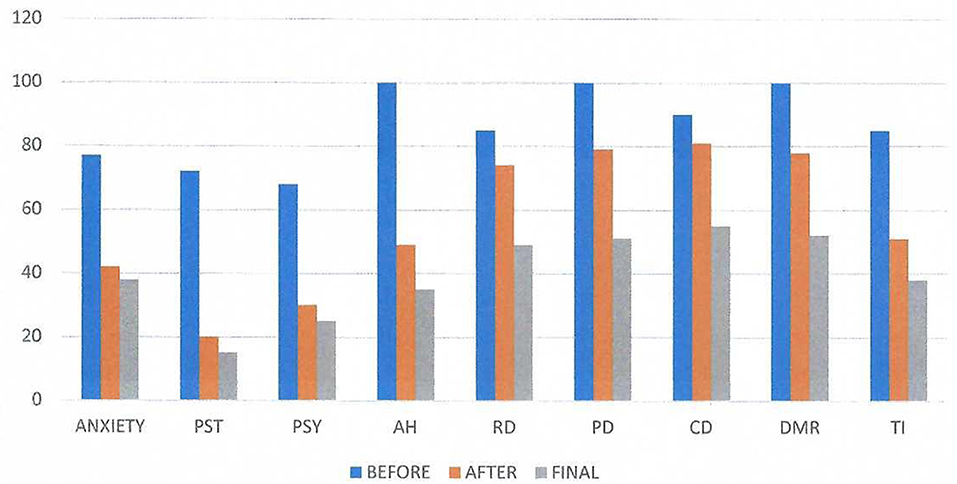
Figure 3. Visual Analog Results on three conditions: before and after the first neurofeedback session and the results of the final session. The abbreviation PST represents the general psychological state of the subject, PSY the psychosomatic symptoms, AH the auditory hallucinations, RD the delusions of reference, PD the persecutory delusions, CD the delusions of being controlled by outside forces, DMR the delusions of mind reading, and TI the thought insertion. The self-assessment scale had a length of 10 cm, where “O” represented “I have no such symptom” and “I00” represented “The symptom is extremely annoying”. We evaluated the mean scores. We observe great significance in the scales AH, PST, Anxiety, DMR, and TL.
According to a review of recent meta-analyses, the acute efficacy in schizophrenia was found to be modest (Haddad and Correll, 2018) with the antipsychotic drugs that were developed as dopamine D2 postsynaptic receptor antagonists (Seeman and Lapur, 2000). Furthermore, according to Heinz E. Lehmann, the McGill professor who pioneered the use of antipsychotic drugs in North America (Stip, 2015), schizophrenic delusions were affected between the fourth and sixth week of treatment. It was therefore an extremely pleasant surprise to observe schizophrenic patients improve to the point of being symptom-free within hours or a few days of high doses diazepam treatment (Nestoros et al., 1982). The results were so impressive that all patients were videotaped at baseline before treatment and within the next 24 h, in order to provide material for other colleagues to verify our findings. These results when presented at the Annual Meeting of the American Psychiatric Association, 2013, Toronto 1982, made headlines on Medical and other newspapers all over the world. Consequently, we found that high doses of diazepam improve neuroleptic-resistant chronic schizophrenic patients (Nestoros et al., 1983). In parallel with the benzodiazepines in schizophrenia studies, our research group studied the effects of CCK-33 in schizophrenic patients (Nair et al., 1982) and CCK-8 in neuroleptic-resistant schizophrenic subjects (Nair et al., 1983, 1985). Again, we documented on videotapes severe schizophrenic symptoms to improve 1 h after CCK intravenous administration.
The optimism resulting from the above psychopharmacological studies led the first author of the present paper to propose a novel Integrative Psychotherapy model for schizophrenia and other psychoses (Nestoros, 1997a,b, 2001, 2006, 2018) which was rigorously tested (Kalaitzaki and Nestoros, 2006; Zgantzouri et al., 2006; Kalaitzaki et al., 2009, 2010; Nestoros et al., 2016; Zgantzouri and Nestoros, 2017). Moreover, the first author (J.N.N.) of this paper has many times treated with success patients suffering from severe schizophrenic symptoms with an integrative approach utilizing psychotherapy, antipsychotic drugs, diazepam and CCK-8 and published these results in Greek in textbooks used by the University of Crete and other Greek Universities (Nestoros, 1993, 2012b). Since 2009, Neurofeedback was added to the integrative treatment of patients exhibiting schizophrenic symptoms. The results of the present case study of the first Neurofeedback session signifies its distinct contribution to their improvement.
Obviously, psychotherapy alone needs several months to several years to decrease schizophrenic symptoms and traditional antipsychotics at least several weeks. It is therefore suggested that the beneficial effects of Neurofeedback on schizophrenic symptoms are mediated by GABA and CCK.
Furthermore, a neurophysiological model of anxiety has been proposed (Nestoros, 1980b, 1981, 1984) conceptualizing anxiety as a state of diminished GABAergic neurotransmission resulting from too frequent recruitment of GABAergic neurons. Repetitive stimulation of the recurrent inhibitory pathway in rat hippocampus leads to a remarkable reduction of the effectiveness of GABA, with GABA receptors becoming insensitive to endogenous and iontophoretically applied GABA. This “disinhibition” is probably related to “fading” of the GABA response (Krnjevic, 1980a,b). The above phenomenon is counteracted by antianxiety drugs, including ethanol (Nestoros, 1980b,d). Since in the model of GABA receptor suggested by Costa and Guidotti (1979) an endogenous peptide inhibitor of GABA receptors was included, in view of recent evidence presented here, one can hypothesize that this peptide is CCK. One may speculate with Krešimir Krnjevic (see Ben-Ari et al., 2022) that GABA needs a mechanism to stop it from shutting down the entire nervous system.
The answer as to why neurofeedback benefits such a great variety of different brain dysfunctions may be found in mechanisms similar to the one suggesting that cholecystokinin regulates dopaminergic (as well as GABAergic and glutaminergic) activity through a corticolimbic and a mesolimbic dimmer-switch process (Ballaz and Bourin, 2021a,b; Ballaz et al., 2021).
As for the Delta band activity that was found in the present study to be elicited by the Infra-Low Frequency Neurofeedback, the following thoughts may be of value. Firstly, it is known that Delta waves arise either in the thalamus or in the cortex (Kropotov, 2009). The particular waves can appear during sleep and in wakefulness (Assenza et al., 2015; Frohlich et al., 2021). As Watson (2018) points out in his review of Cognitive and Physiologic Impacts of the Infraslow Oscillation (ISO) (from 0.01 to 0.1 Hz), this rhythm coincides with the oscillation underlying resting state networks (RSNs) in awake human subjects, which are spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging (Fox and Raichle, 2007) and relates the phenomenon of ISOs to the proposal by Halász et al. (2004) that the brain needs to rest at times. Watson (2018) concludes his extensive review of the literature stating: “The ISO typically peaks in power at 0.02 Hz, is seen in multiple brain regions in mammalian species from mice to humans and occurs across wake and sleep. It organizes neurons, oscillatory patterns, sleep patterns, brainwide networks, is modulated by and modulates both pathological conditions and cognitive performance. Furthermore, the thalamus and in particular a glial effect on the thalamus is a candidate for the generation of these rhythms”. The possible involvement of glial cells is supported by the findings of MacVicar et al. (1989) that GABA- activated Cl− channels in astrocytes (a form of glial cells) in hippocampal slices, and by the findings of Deemyad et al. (2018) that astrocytes, although they do not fire action potentials themselves, integrate and drive action potential firing in inhibitory interneuron subnetworks. This indirectly induced firing of action potentials, mediated through GABA-activated Cl− channels in astrocytes, may explain why Infra-Low Frequency Neurofeedback can elicit Delta band activity. This merely implies that the frequency-based organization of resting states extends into the Delta band.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data contained within the article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (Vol. 5). Washington, DC: American Psychiatric Association.doi: 10.1176/appi.books.9780890425596
Andreasen, N. C. (1984a). Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: The University of Iowa.
Andreasen, N. C. (1984b). Scale for the Assessment of Negative Symptoms (SANS). Iowa City: The University of Iowa.
Assenza, G., Pellegrino, G., Tombini, M., Di Pino, G., and Di Lazzaro, V. (2015). Wakefulness delta waves increase after cortical plasticity induction. Clini. Neurophysiol. 126, 1221–1227. doi: 10.1016/j.clinph.2014.09.029
Ballaz, S., Espinosa, N., and Bourin, M. (2021). Does endogenous cholecystokinin modulate alcohol intake?. Neuropharmacology. 193, 108539. doi: 10.1016/j.neuropharm.2021.108539
Ballaz, S. J., and Bourin, M. (2021a). Cholecystokinin-mediated neuromodulation of anxiety and schizophrenia: a “dimmer-switch” hypothesis. Curr. Neuropharmacology. 19, 925–938. doi: 10.2174/1570159X18666201113145143
Ballaz, S. J., and Bourin, M. (2021b). Cholecystokinin-mediated neuromodulation of anxiety and schizophrenia: a “dimmer-switch” hypothesis. Curr. Neuropharmacol. 19, 925–938.
Ben-Ari, Y., Cherubini, E., and Avoli, M. (2022). Krešimir Krnjevic (1927–2021) and GABAergic inhibition: a lifetime dedication. Can. J. Physiol. Pharmacol. 100, 1–4. doi: 10.1139/cjpp-2021-0451
Brisch, R., Saniotis, A., Wolf, R., Bielau, H., Bernstein, H. G., Steiner, J., et al. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front. Psychiatry. 5, 47. doi: 10.3389/fpsyt.2014.00110
Chatrian, G. E., Lettich, E., and Nelson, P. L. (1985). Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am. J. EEG Technol. 25, 83–92. doi: 10.1080/00029238.1985.11080163
Costa, E., and Guidotti, A. (1979). Molecular mechanlsms in the receptor action of benzodiazepines. Ann. Review Pharmacol. Toxicol.19, 531–545. doi: 10.1146/annurev.pa.19.040179.002531
Deemyad, T., Lüthi, J., and Spruston, N. (2018). Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun. 9, 1–13. doi: 10.1038/s41467-018-06338-3
Derogatis, L. R. (1977). SCL-90-R: Administration, Scoring and Procedures Manual. Baltimore, MD: Clinical Psychometric Research.
Fox, M. D., and Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. doi: 10.1038/nrn2201
Frohlich, J., Toker, D., and Monti, M. M. (2021). Consciousness among delta waves: a paradox? Brain. 14, 2257–2277. doi: 10.1093/brain/awab095
Gomez, -A A, Fiorenza, A. M., Boschen, S. L., Sugi, A. H., Beckman, D., et al. (2017). Diazepam inhibits electrically evoked and tonic dopamine release in the nucleus accumbens and reverses the effect of amphetamine. ACS Chem. Neurosci. 8, 300–309. doi: 10.1021/acschemneuro.6b00358
Haddad, P. M., and Correll, C. U. (2018). The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 8, 303–318. doi: 10.1177/2045125318781475
Haefely, W., Kulcsar, A., Mohler, H., Pieri, L., Polc, P., and Schaffner, R. (1978a). “Possible involvement of CABA in the central actions of benzodiazepines”, in Mechanisms of Action of Benzodiazepines, Costa, E., and Greengard, P (eds). (New York: Raven Press). p. 131–151.
Haefely, W., Polc, P., Schaffner, R., Keller, H. H., Pieri, L., and Mohler, H. (1978b). “Facilitation of GABAergic transmission by drugs,” in GABA-Neuro-Transmitters, Krogsgaard-Larsen, P., Scheel-Kruger, J., and Kofod, H. (Munksgaard, Copenhagen: 12th Alfred Benzon Symposium). p. 357–375.
Haefely, W. E. (1978). “Behavioral and Neuropharmacological Aspects of Drugs Used in Anxiety and Related States”, in Psychopharmacology: A Generation of Progress, Lipton, M. A., Dimascio, A., and Killam, K. F. (eds). (New York: Raven Press). p. 1359–1374.
Halász, P., Terzano, M., Parrino, L., and Bódizs, R. (2004). The nature of arousal in sleep. J. Sleep Res. 13, 1–23. doi: 10.1111/j.1365-2869.2004.00388.x
Hockfelt, T., Johnson, O., Ljungdahl, A., Lundberg, J. M., and Schultzberg, M. (1980b). Peptidergic neurons. Nature. 284, 515–521. doi: 10.1038/284515a0
Hockfelt, T., Rehfeld, J. F., Skirboll, L., Ivenmark, B., Goldstein, M., and Markey, K. (1980a). Evidence, for coexistence of dopamine and CCK in mesolimbic neurons. Nature. 285, 476–478. doi: 10.1038/285476a0
ICD-11. (2022). International Classification id Diseases for Mortality and Morbidity Statistics, 11th Edn. Geneva: World Health Organization.
Jasper, H. H. (1958). The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 10, 371–375.
Kalaitzaki, A., Birtchnell, J., and Nestoros, J. (2010). Does family interrelating change over the course of individual treatment?. Clini. Psychol. Psychotherapy. 17, 463–481. doi: 10.1002/cpp.687
Kalaitzaki, A. E., Birtchnell, J., and Nestoros, J. N (2009). Interrelating within the families of young psychotherapy outpatients. Clini. Psychol. Psychotherapy. 16, 199–215. doi: 10.1002/cpp.613
Kalaitzaki, A. E., and Nestoros, J. N. (2006). “Integrating individual and family therapy in improving negative interrelating within families of persons with schizophrenic symptoms”, in New Approaches to Integrative Psychotherapy, O'Leary, E., and Murphy, M. (eds) (London: Brunner-Roudledge). p. 141–154.
Kelsom, C., and Lu, W. (2013). Development and specification of GABAergic cortical interneurons. Cell Biosci. 3, 1–19. doi: 10.1186/2045-3701-3-19
Keshavan, M. S., Nasrallah, H. A., and Tandon, R. (2011). Schizophrenia, “Just the Facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophrenia Res. 127, 3–13. doi: 10.1016/j.schres.2011.01.011
Krnjevic, K. (1980a). “Action of GABA on hippocampal, neurons”, in Amino Acid Transmitters, Mandel, P., and DeFeudis, F. V. (New York, Raven Press).
Krnjevic, K. (1980b). “Desensitizatlon of GABA”. in GABA and Glutamate as Transmitters, Costa, E. (eds) (New York, Raven Press.).
Kropotov, J. D. (2009). Quantitative EEG, Event-Related Potentials and Neurotherapy. London: Academic Press.
MacVicar, B. A., Tse, F. W., Crichton, S. A., and Kettenmann, H. (1989). GABA-activated Cl-channels in astrocytes of hippocampal slices. J. Neurosci. 9, 3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989
Nair, N. P., Lal, S., and Bloom, D. M. (1985). Cholecystokinin peptides, dopamine and schizophrenia–a review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 9, 515–524. doi: 10.1016/0278-5846(85)90011-9
Nair, N. P. V., Bloom, D. M., Nestoros, J. N., and Schwartz, G. (1983). Therapeutic efficacy of cholecystokinin in neuroleptic-resistant schizophrenic subjects. Psychopharmacology Bull. 19, 134–136.
Nair, N. V., Bloom, D. M., and Nestoros, J. N. (1982). Cholecystokinin appears to have antipsychotic properties. Prog. Neuropsychopharmacol. Biol. Psychiatry. 6, 509–512. doi: 10.1016/S0278-5846(82)80140-1
Nestoros, J. N. (1980a). Benzodiazepines in the treatment of schizophrenia: a need for reassessment. Int. Pharmacopsychiat. 15, 171–179. doi: 10.1159/000468434
Nestoros, J. N. (1980b). Anti-anxiety Agents and Synaptic Transmission in the Brain: Electrophysiological Studies. [Ph.D. Thesis]. Montreal: McGill University.
Nestoros, J. N. (1980c). “Electrophysiological evidence that GABA potentiation in vivo correlates with benzodiazepine receptor affinity”, in GABA Neuro-transmission: Current development in Physiology and Neurochemistry, Lal, H. (Ankho International, Inc., Fayetteville) p. 849–851. doi: 10.1016/0361-9230(80)90142-2
Nestoros, J. N. (1980d). Ethanol selectively potentiates GABA-mediated neurotransmission in feline celebral cortex. Science. 209, 708–710. doi: 10.1126/science.7394531
Nestoros, J. N. (1981). Anxiety as a state of diminished GABAergic neurotransmission resulting from too frequent recruitment of GABAergic neurons: a neurophysiological model. Prog. Neuro-Psychopharmac. 5, 591–594. doi: 10.1016/0364-7722(81)90053-9
Nestoros, J. N. (1982). Benzodiazepines and GABA receptors are functionally related: Further electrophysiological evidence in vivo. Prog. Neuro-Psychopharmac. and Biol. Psychiat. 6, 417–420. doi: 10.1016/S0278-5846(82)80119-X
Nestoros, J. N. (1984). GABAergic mechanisms and anxiety: an overview and a new neurophysiological model. Can. J. Psychiatry. 29, 520–529. doi: 10.1177/070674378402900614
Nestoros, J. N. (1993). In the world of psychosis. Eric's Odyssey and other cases (Ston kosmo tis psychosis: H Odysseia tou Eric kai alles periptoses) (in Greek). Athens: Ellinika Grammata.
Nestoros, J. N. (1997a). “Integrative Psychotherapy of Individuals with Schizophrenic Symptoms”, in Psychotherapy: New perspectives on Theory, Practice and Research, Hawkins, P. J., and Nestoros, J. N. (eds) (Athens: Ellinika Grammata Publishers) p. 321–363.
Nestoros, J. N. (1997b). “A model of training in the methodology of individual psychotherapy research: The case of schizophrenia as a paradigm”, in Psychotherapy: New perspectives on Theory, Practice and Research, Hawkins, P. J., and Nestoros, J. N. (Athens: Ellinika Grammata Publishers) p. 633–681.
Nestoros, J. N. (1998). Visual Analogue for psychological State and Psychotic Symptoms, Laboratory of Clinical Psychology, Department of Psychology, University of Crete. Based on Visual Analoague Scale – Pain (VASP), Department of Psychiatry, Vanderbilt University. Rethymno: University of Crete.
Nestoros, J. N. (2001). Synthetiki psychotherapy: an integrative psychotherapy for individuals with schizophrenic symptoms. J. Contemp. Psychother. 31, 51–59. doi: 10.1023/A:1010230915700
Nestoros, J. N. (2006). “Recent developments in an integrative approach of the psychotherapy of individuals suffering from schizophrenic symptoms”, in New Approaches to Integrative Psychotherapy, O' Leary, E., and Murphy, M. (London: Brunner-Roudledge) p. 74–88.
Nestoros, J. N. (2012a). Integrative psychotherapy (Synthetiki Psychotherapia) (in Greek). 2nd expanded Edition. Athens: Pedio publications.
Nestoros, J. N. (2012b). In the world of psychosis (Ston kosmo tis psychosis) (in Greek). 2nd expanded Edition. Athens: Pedio publications.
Nestoros, J. N. (2018). Hellenic Integrative Psychotherapy: A Total Holistic Approach. Psychology. 9, 1731–1760. doi: 10.4236/psych.2018.97103
Nestoros, J. N., Nair, N. P. V., Pulman, J. R., and Schwartz, G. (1983). High doses of diazepam improve neuroleptic-resistant chronic schizophrenic patients. Psychopharmacology. 81, 42–47. doi: 10.1007/BF00439272
Nestoros, J. N., and Nistri, A. (1979). Effects of microiontophoretically applied flurazepam on responses of celebral cortical neurones to putative neurotransmitters. Can. J. Physiol. Pharmacol. 57, 1324–1329. doi: 10.1139/y79-198
Nestoros, J. N., Seliniotaki, T., Vergoti, A., and Benioudakis, M. (2016). “Interrelating Within the Families of Schizophrenics Before Their First Psychotic Episode”, in Relating Theory: Clinical, Forensic and other Applications, Birtchnell, J., Newberry, M., and Kalaitzaki, A. (London: Palgrave MacMillan) p. 210–226. doi: 10.1057/978-1-137-50459-3_15
Nestoros, J. N., Suranyi-Cadotte, R. E., Spees, R. C., Schwartz, G., and Nair, N. P. V. (1982). Diazepam in high doses is effective in schizophrenia. Prog. Neuro-Psychopharmac. and Biol. Psychiat. 6, 513–516. doi: 10.1016/S0278-5846(82)80141-3
Nuss, P. (2015). Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 11, 165. doi: 10.2147/NDT.S58841
Ochoa-de la Paz, L. D., Gulias-Cañizo, R., Ruíz-Leyja, E. D., Sánchez-Castillo, H., and Parod,í, J. (2021). The role of GABA neurotransmitter in the human central nervous system, physiology, and pathophysiology. Revista mexicana de neurociencia. 22, 67–76. doi: 10.24875/RMN.20000050
Othmer, S., and Othmer, S. (2016). Infra-low-frequency neurofeedback for optimum performance. Biofeedback. 44, 81–89. doi: 10.5298/1081-5937-44.2.07
Othmer, S., and Othmer, S. (2017). “Toward a Frequency-based Theory of Neurofeedback”, in Rhythmic Stimulation Procedures in Neuromodulation, Evans J. R., and Turner, R. A. (eds). (London, Academic Press) p. 254–307. doi: 10.1016/B978-0-12-803726-3.00008-0
Othmer, S., and Othmer, S. (2020). “Toward a Theory of Infra-Low Frequency Neurofeedback”, in Restoring the Brain, Kirk, H. W. (ed). (Routledge) p. 56–79. doi: 10.4324/9780429275760-3
Roberts, E. (1972). Prospects of research on schizophrenia: An hypothesis suggesting that there is a defect in the GABA system in schizophrenia. Neurosci. Res. Program Bull. 10, 468–482.
Roberts, E. (1976). “Disinhibition as an organizing principle in the nervous system: The role of the GABA system,” in Application to Neurologic and Psychiatric Disorders, in GABA in Nervous System Function, eds E. Roberts, T. N. Chase, and D. B. Tower (New York, NY: Raven Press).
Seeman, P., and Lapur, S. (2000). Schizophrenia: More dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 97, 7673–7675. doi: 10.1073/pnas.97.14.7673
Stip, E. (2015). Who pioneered the use of antipsychotics in North America? Can J. Psychiatry. 60, S5–S13.
Tandon, R., Nasrallah, H. A., and Keshavan, M. S. (2010). Schizophrenia, “Just the Facts” 5. Treatment and prevention past, present, and future. Schizophrenia Res. 122, 1–23. doi: 10.1016/j.schres.2010.05.025
Vereczki, V. K., Müller, K., Krizsán, É., Máté, Z., Fekete, Z., Rovira-Esteban, L., et al. (2021). Total number and ratio of GABAergic neuron types in the mouse lateral and basal amygdala. J. Neurosci. 41, 4575–4595. doi: 10.1523/JNEUROSCI.2700-20.2021
Watson, B. O. (2018). Cognitive and physiologic impacts of the infraslow oscillation. Front. Syst. Neurosci. 44. doi: 10.3389/fnsys.2018.00044
Whissell, P. D., Bang, J. Y., Khan, I., Xie, Y. F., Parfitt, G. M., Grenon, M., et al. (2019). Selective activation of cholecystokinin-expressing GABA (CCK-GABA) neurons enhances memory and cognition. Eneuro. 6. doi: 10.1523/ENEURO.0360-18.2019
Whissell, P. D., Cajanding, J. D., Fogel, N., and Kim, J. C. (2015). Comparative density of CCK-and PV-GABA cells within the cortex and hippocampus. Front. Neuroanat. 9, 124. doi: 10.3389/fnana.2015.00124
Zgantzouri, K. A., and Nestoros, J. N. (2017). Psychotherapy process research in schizophrenia paranoid type: the investigation of delusion formation through the evaluation of in-session events (research article). Int. J. Psychiatry. 2, 1–14. doi: 10.33140/IJP/02/02/00002
Keywords: schizophrenia, Neurofeedback, antipsychotic treatment, Infra-Low Frequency Regime, rapid improvement
Citation: Nestoros JN and Vallianatou NG (2022) Infra-Low Frequency Neurofeedback rapidly ameliorates schizophrenia symptoms: A case report of the first session. Front. Hum. Neurosci. 16:923695. doi: 10.3389/fnhum.2022.923695
Received: 19 April 2022; Accepted: 27 July 2022;
Published: 21 September 2022.
Edited by:
Siegfried Othmer, EEG Info, United StatesReviewed by:
Fabian Bazzana, University of Turin, ItalyCopyright © 2022 Nestoros and Vallianatou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joannis N. Nestoros, aW5mb0BhbXBoaWFyYWlhLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.