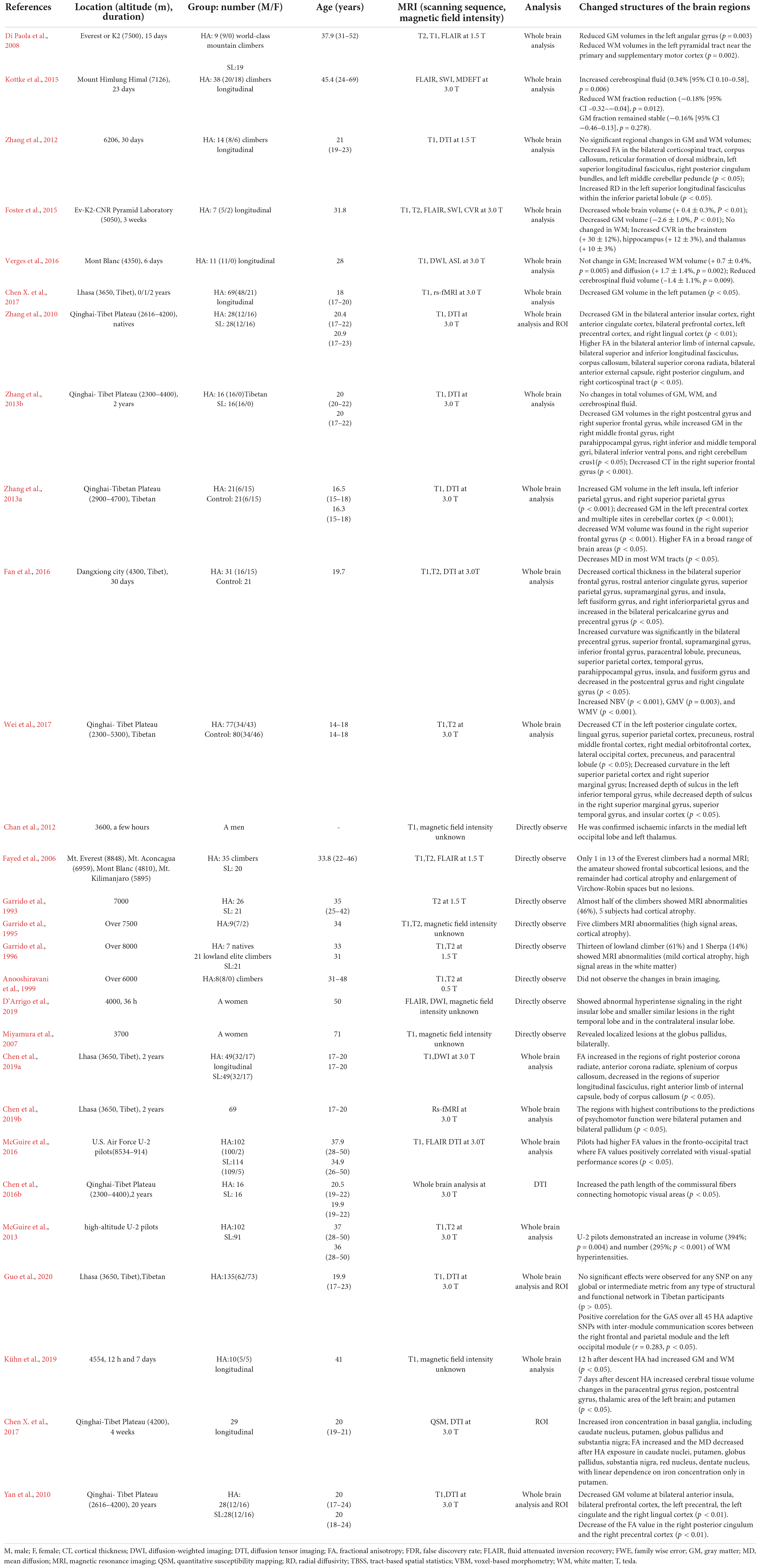
- 1Institute of Brain Diseases and Cognition, School of Medicine, Xiamen University, Xiamen, China
- 2Department of Physiology, School of Medicine, Xiamen University, Xiamen, China
With the advancement of in vivo magnetic resonance imaging (MRI) technique, more detailed information about the human brain at high altitude (HA) has been revealed. The present review aimed to draw a conclusion regarding changes in the human brain in both unacclimatized and acclimatized states in a natural HA environment. Using multiple advanced analysis methods that based on MRI as well as electroencephalography, the modulations of brain gray and white matter morphology and the electrophysiological mechanisms underlying processing of cognitive activity have been explored in certain extent. The visual, motor and insular cortices are brain regions seen to be consistently affected in both HA immigrants and natives. Current findings regarding cortical electrophysiological and blood dynamic signals may be related to cardiovascular and respiratory regulations, and may clarify the mechanisms underlying some behaviors at HA. In general, in the past 10 years, researches on the brain at HA have gone beyond cognitive tests. Due to the sample size is not large enough, the current findings in HA brain are not very reliable, and thus much more researches are needed. Moreover, the histological and genetic bases of brain structures at HA are also needed to be elucidated.
Introduction
High altitude (HA) is characterized as having lower atmospheric oxygen pressure, a cold climate, and strong ultraviolet rays, and is therefore inhospitable to human settlement. However, there are still a large number of people living in HA regions. More than 10 million highlanders permanently reside above 2,200 m in the Qinghai-Tibet Plateau (Kayser and Wu, 2006). Every year, hundreds of thousands of people move from lowlands to HA regions for work or study staying for several months to several years. Moreover, altitude training has been used as a routine in endurance sports around the world to improve performance (Lundby and Robach, 2016), and thus the effects of exercise under HA hypoxia on the brain may be most important for athletes performing HA training.
The brain accounts for roughly 2% of the total mass of human body, yet it consumes over 20% of the oxygen that the human body intakes (Raichle, 2010), and as a consequence, HA inevitably challenges the brain. Through afferent feedback, the adaptation that occurs in the cardiovascular and respiratory systems (Hoiland et al., 2018) may act on their corresponding control centers in the brain (Figure 1). Moreover, the changes in blood CO2 and pH may have direct effect on cerebral blood flow. Hypobaric and cold may be the other two important factors contributing to the alteration of the brain at HA. Aggravation of mental illness (Mizoguchi et al., 2011), impairment of visual sensitivity (Degache et al., 2012), development of acute mountain sickness (AMS) (DiPasquale et al., 2015), and damaged brain structure (Simmons et al., 2015) are more often seen with hypobaric vs. normobaric hypoxia. The cold ambient temperature at HA may result in hypothermia; however, hypothermia has been shown to have a neuroprotective effect against hypoxic encephalopathy (Gao et al., 2014; Kvistad et al., 2014). Most present-day HA natives are descendants of colonizers who arrived in HA regions tens of thousands of years ago. Positive natural selection in the Egl nine homolog 1 (EGLN1), endothelial PAS domain protein 1 (EPAS1), and peroxisome proliferator activated receptor alpha (PPARA) genes, all of which are associated with the hypoxia-inducible transcription factor (HIF) pathway (Simonson et al., 2010; Yi et al., 2010; Yang et al., 2017) has recently been found to be the mechanism underlying the adaptation of Tibetans, who have lived on the Qinghai-Tibet Plateau for millennia. The purpose of the present review was to assess how the combined action of a variety of HA environmental factors and genetic factors lead to changes in various regions of the brain.
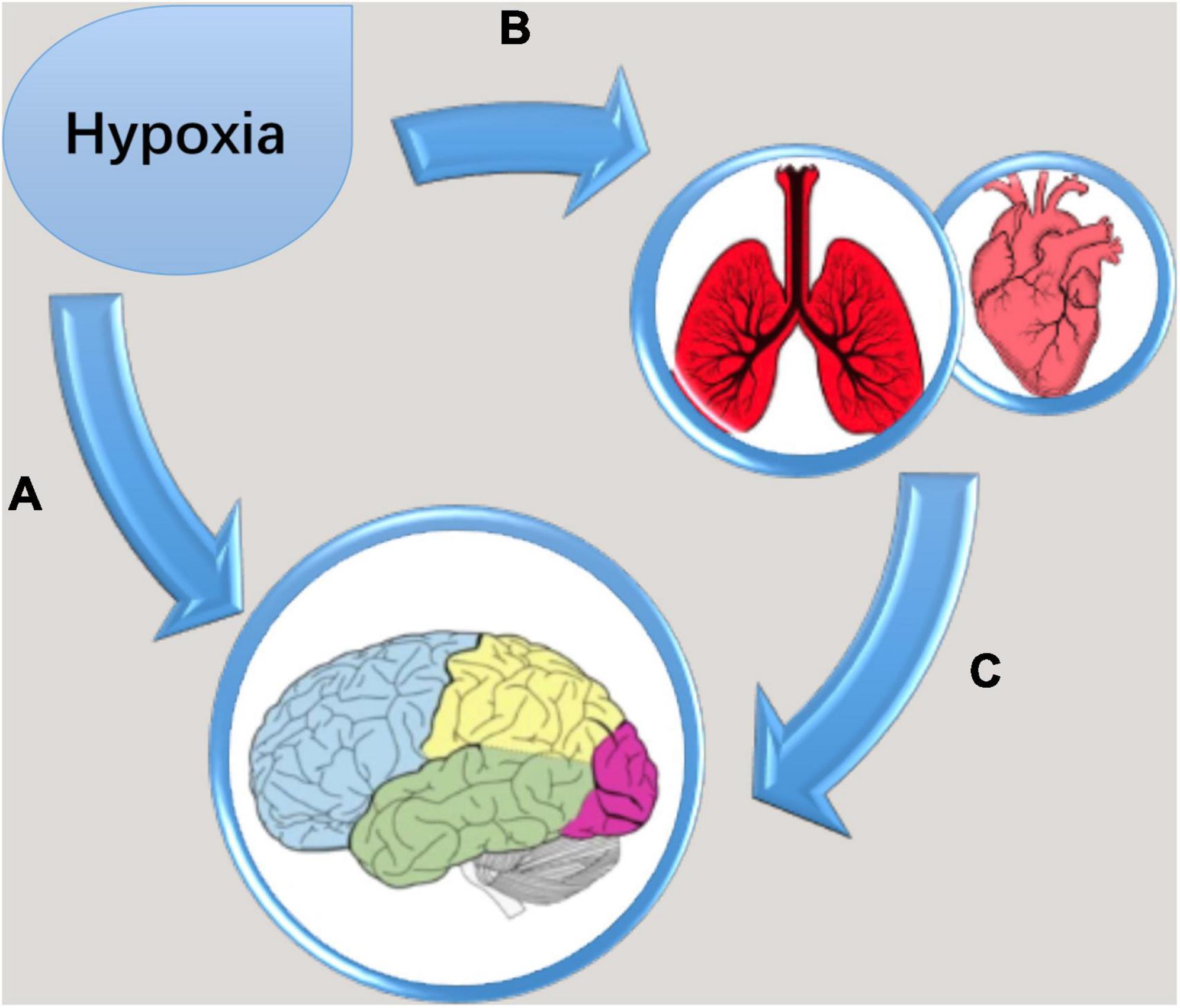
Figure 1. Diagrammatic drawing of cumulative effects of hypoxia on the brain. (A) Neurons in the brain directly suffer from the stress of low oxygen concentration; (B) cardiovascular and respiratory systems changed in adaptation to hypoxia; (C) through afferent feedback, the adaptation in the cardiovascular and respiratory systems act on their control centers in the brain.
Cerebral edema was first associated with HA due to the appearance of many mountain sickness symptoms during HA exposure (Ravenhill, 1913). In an earlier research program named “the American Medical Research Everest Expedition,” finger-tapping behavior was found to be impaired in climbers (West, 1984). After that, along with the advancement of many in vivo imaging techniques and analysis methods, more information about changes in brain structure and function in HA populations have been revealed. The present review focused on these new findings relating to the human brain in a natural HA environment. Acute HA exposure typically refers to being on an elevated plateau for several days to weeks, while chronic HA exposure refers to extended living or having permanent residence on a plateau (Javier et al., 2004). Therefore, this review presents a mix of acute exposure, chronic exposure, and life-long exposure literature.
Brain researches selected and search methodology for this review
Using “high altitude” or “plateau” with “brain” or “cerebral cortex” as keywords limited in Abstract, we retrieved 1,316 items in PubMed and 1,036 items in Web of Science. Finally, a total of 185 articles were distinguished to be related to brain research. These studies involve many aspects of the brain, including brain edema, blood-brain barrier injury, and protective treatments of drugs on brain injury. Brain edema presents extensive whole brain swelling. However, humans would like to know which brain regions were more vulnerable to injury and the cognitive impairments resulting from brain region damage. In fact, HA brain edema has been reviewed by researchers every few years (Hackett and Roach, 2004; Turner et al., 2021), so this review was no longer include the study of brain edema. In addition, there were several animal studies through exposing animals to a simulating HA hypoxia but not real HA environment, which did not include in this review. Magnetic resonance imaging (MRI) and electroencephalography (EEG) are the two advanced technologies to study the brains of living humans currently. MRI has high spatial resolution and can accurately locate millimeter level of brain regions with gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) can be accurately visualized. Nowadays, functional MRI (fMRI) and EEG have been intensively employed to explore neuronal activities within brains. Therefore, this review mainly summarizes the researches that used these two methods. With these considerations in mind, a total of 53 research articles from PubMed and Web of Science meet the criterion of this review (see Tables 1, 2).
Brain structures at high altitude
Gray matter and white matter changes
In a lot of earlier studies, high intensity signals on T2-weighted MR images were used to directly observe the severe damage of brain GM and WM. Cytotoxic edema, as in early hypoxic encephalopathy, leads to restriction of water motion, producing high signal intensity on T2-weighted MR images. Subcortical edema and cortical atrophy (enlarged cerebral sulci) were reported in groups of 26 and 21 mountain climbers ascending to altitudes of 7,000 and 8,000 m (Garrido et al., 1993, 1996) and in 35 climbers after ascents to peaks of different heights ranging from Mont Blanc (4,810 m) to Mt. Everest (8,848 m) (Fayed et al., 2006) compared with non-climbers. In a comparative longitudinal study, high-intensity signal and cortical atrophy were also detected in the occipital cortex in 2 of 9 climbers after reaching altitudes between 7,800 and 8,463 m (Garrido et al., 1995). However, no severe damage visible to the naked eye in brain imaging was observed in eight male climbers between 31 and 48 years of age a few days before and between 5 and 10 days after returning to sea level following ascent to altitudes of over 5,947 m (Anooshiravani et al., 1999), which may be due to small number of climbers and low intensity magnet which was a 0.5 T. Brain region identified by direct observation may be the brain region most affected by hypoxia. In general, serious brain structural damage can be found through direct observation, but the changes that are invisible to the naked eye need more detailed analysis.
In recent years, GM and WM structural changes in individuals at HA have been examined based on brain T1 MRI and diffusion tensor imaging (DTI), respectively (detailed information is shown in Table 1). GM volume, cortical thickness, and cortical surface area were quantitatively analyzed using voxel-based morphometry (VBM), while WM microstructure was measured using Tract-Based Spatial Statistics (TBSS). TBSS measures the fractional anisotropy (FA) and mean diffusion (MD) of WM fibers. FA represents the ratio between the length of the primary axis and the other two orthogonal axes, with a high anisotropy representing diffusion that is highly oriented in one direction. MD represents the overall free space available for water to self-diffuse, and thus is the average length of all three axes.
There were no consistent results regarding the alteration of the total brain volume. Total brain volume was first found unchanged after mountain climbers stayed at the Tanggula Mountains (6,260 m) for 30 days (Zhang et al., 2012) and at Mount Himlung Himal (7,126 m) for 23 days (Kottke et al., 2015). More recently, total brain volume was revealed to be reduced after HA exposure for 3 weeks at 5,050 m (Foster et al., 2015). Global GM volume, however, was found to increase after 30 days at 4,300 m (Fan et al., 2016). High altitude climbers suffers from cortical atrophy (Fayed et al., 2010). This inconsistency may be due to the variability of study designs. In studies conducted by Zhang et al. (2012) and Kottke et al. (2015), brain images were obtained before the start of the expedition and then several weeks after the return to sea level. Thus, the brain structure could have been influenced by reoxygenation-induced changes in cerebral blood flow (CBF) (Harris et al., 2013; Wang et al., 2018). In contrast, Fan et al. (2016) conducted the study at both the lowland and the plateau.
Regional GM changes were seen in the mountain climbers who repeated expeditions to Mount Everest (Di Paola et al., 2008), adult soldiers who had garrisoned the frontiers in the Qinghai-Tibet Plateau for 2 years (Zhang et al., 2013b), climbers who made a single ascent to for less than 36 h (D’Arrigo et al., 2019), and college freshmen who immigrated to the Qinghai-Tibet Plateau and were followed-up for 2 years (Chen X. et al., 2017, Chen et al., 2019a). To explore the influences of the environment on HA residents, Han immigrant adolescents (Zhang et al., 2010) and HA adolescents (Yan et al., 2010; Wei et al., 2017) living in the Qinghai-Tibet Plateau were studied, and decreased GM volume and changes in cortical thickness were detected. Two follow-up observations have also been conducted to investigate the persistent sequelae to brain structure after people return to lowlands. An earlier observation is from the study on college students who volunteered for a 30-day teaching on the Qinghai-Tibet Plateau (Fan et al., 2016), and a latter observation examined volunteers 12 h post-descent and again 3.5 months after a 7-day HA exposure at 4,554 m, respectively (Kühn et al., 2019). Among these HA-exposed populations, GM volume and cortical thickness showed discrepant changes, with some regions having increased but others having decreased.
For HA immigrants, the increase in GM may be associated with hypoxia-induced neurogenesis, gliogenesis, or vascular proliferation. Adult neocortices, such as the prefrontal cortex, inferior temporal cortex, and posterior parietal cortex, have the capability of neurogenesis (Gould et al., 1999), which may potentially be induced by stress (Magavi et al., 2000) or afferent feedback (function-activated effects). The brain is the source of behavior, but in turn, it is modified by the behaviors it produces. Glial cells comprise more than 85% of the total number of brain cells. They are sensitive to changes in oxygen partial pressure (Angelova et al., 2015), and can be activated by hypoxia (Pforte et al., 2005). Vasculature accounts for about 5% of GM (Zatorre et al., 2012). The capillary length per unit volume of tissue, dilation, and density in the cortex increased after 3 weeks of exposure to a hypoxic environment (LaManna et al., 1992; Boero et al., 1999). On the other hand, a decrease in cortical GM may be due to neuronal loss (Maiti et al., 2007). Therefore, unbalanced development between angiogenesis/gliogenesis and neuronal loss could determine regional cortical volume.
Few studies have observed changes in brain WM at HA. The change in total WM volume varies, as seen by increases after people stayed at 4,300 m for either 30 days (Fan et al., 2016) or 6 days (Verges et al., 2016), decreases after ascending Mount Everest (8,848 m) for several days (Di Paola et al., 2008) and Mount Himlung Himal (7,126 m) for 23 days (Kottke et al., 2015), no change after an expedition to 5,050 m for 3 weeks (Foster et al., 2015) and 6,206 m for 1 month (Zhang et al., 2012). The differing results may be due to different experimental conditions. In some studies, brain MRI scans were conducted before individuals ascended to and remained at a plateau after HA exposure (Fan et al., 2016; Verges et al., 2016). In this situation, the vasogenic edema that occurred in WM during HA exposure may have contributed to the increase in WM volume. In other studies, the images were obtained several days after the individuals returned to the lowlands, and thus any brain edema could have already been alleviated (Di Paola et al., 2008; Foster et al., 2015; Kottke et al., 2015). One study showed that total WM volume did not change after an ascent to 6,260 m for 30 days, but regional FA and radial diffusivity values both decreased (Zhang et al., 2012), indicating that only WM microstructure was impaired. Moreover, the number of commissural fibers connecting the bilateral visual cortices increased in adults who had immigrated to and remained on a plateau for 2 years (Chen et al., 2016b). Changes of FA of WM fibers were observed in a lot of studies on descendants of Han immigrants (Zhang et al., 2010), Tang-ku-la Mountains climbers (Zhang et al., 2012), soldiers who have garrisoned the frontiers in Qinghai-Tibet Plateau (Zhang et al., 2013b), subjects as volunteer teachers (Fan et al., 2016), and college freshmen who immigrated to Tibet and followed up for 2 years (Chen et al., 2019a), with some fibers have increased FA but others have decreased FA (Table 1).
The change in cerebrospinal fluid (CSF) volume also varies, with CSF volume showing decrease, increase, or no change. In the study conducted by Verges et al. (2016), the MRI scanning were performed in lowland before and within 6 h after returning to lowland, and this acute hypoxia markedly induced the increase in WM volume (0.7 ± 0.4%, p = 0.005), which may squeeze ventricle, and result in CSF volume reduction. In contrast, in the study of Kottke et al. (2015), a single sojourn to extreme altitudes was not associated with development of GM atrophy but lead to a decrease in brain WM fraction (–0.18%, p = 0.012), which may cause the ventricles to expand. In the observation of Zhang et al. (2012), because the subjects gradually reached their destination in the process of up to 10 days and returned to the plain within 10 days, the brain gradually adapted to hypoxia and various cerebrovascular barriers damaged by hypoxia (Michalicova et al., 2017) may recover to normal during slow reoxygenation, and so, only WM remained with local slight cytotoxic edema.
Brain structural network and genetic variants
In a recent study, the modulation of HA adaptive genetic variants (single nucleotide polymorphisms [SNPs]) of EGLN1, EPAS1, and PPARA on the organizational network of the brain was investigated in Tibetan natives in the Qinghai-Tibet Plateau. The study showed that the HA adaptive genetic variants regulated the topological organization of large-scale structural brain networks (Guo et al., 2020). These findings provide novel insights into the genetic substrate and dynamic evolution of HA adaptive phenotypes in native Tibetans.
Iron deposition in the brain
Iron is the most abundant transition metal found in the human brain, and is highly compartmentalized in subcortical nuclei. Iron plays an important role in the physiological functions and development of human brains (McAllum et al., 2020). MRI studies in patients with HACE revealed multiple hemosiderin depositions in the brain, predominantly in the corpus callosum (Kallenberg et al., 2008). In a recent study, brain iron levels were found to increase in the basal ganglions after 30 days of exposure to HA, and even remained slightly elevated 1 year after people return to sea level (Chen L. et al., 2017).
Brain function after high altitude exposure
Detailed information is shown in Table 2.
Electrophysiological characteristics
Event-related potentials (ERPs), obtained by time-locked averaging EEG, have been used to evaluate the electrophysiological processing of cognitive activity in various HA residents. Most studies of ERP use task-activated electrode points in the relevant brain regions for analysis, called regions of interest analysis which can control class I errors in statistical testing and better discernment of the effects of high altitude exposure on specific brain functions. Given the high temporal resolution, ERPs have been used to record the precise temporal sequence of brain processes. The onset of stimulation is shown to evoke a characteristic negative potential (N1) of ERP with a peak latency around 120 ms and is used as index of the attentional allocation of the early visual processing (Wang et al., 2014). Negative potential (N2pc: N2-posterior-contralateral) is an electrophysiological mark that embodies the visuospatial attention focusing on a target stimulus that supports visual search, which occurs approximately 280 ms after the presentation of a stimulus (Luck and Hillyard, 1994). The negative potential (N2) occurs approximately 200 ms after the presentation of a stimulus reflected the processing of conflict detection (Rueda et al., 2004). The positive potential (P50) occurs approximately 50 ms after the presentation of a stimulus and reflects a predominantly pre-attentional inhibitory filter mechanism that was admitted to the early sensory gating (Lijffijt et al., 2009). The positive potential (P2) is related to top−down processing, matching sensory input information with memory representations, which occurs approximately 200 ms after the presentation of a stimulus (Luck and Hillyard, 1994). The positive potential (P300/P3) of ERP occurs approximately 300 ms after the presentation of a stimulus, requiring detection, counting, or cognitive processing by the participants. The P3 component has generally been considered a late stage of information processing. Moreover, the time-frequency signal processing approach has been widely employed for the study of the energy distribution of ERP data across time and frequency. In particular, the time-frequency amplitude has been used extensively to investigate alpha, beta, delta, and theta powers with regard to ERP data recorded during cognitive tasks.
Earlier EEG studies mainly tested the electrophysiological processing of visual stimuluses after short-term exposure to HA. Two previous studies have observed EEG patterns during exposure to HA-induced hypoxia over time. One study found that the occipital alpha component was 25.5% at sea level, which changed at 3,500 m to 45.7, 15.8, 28.0, 30.3, and 33.2% on days 2, 7, 14, 21, and 28, respectively. The average amplitude was 17.3 μV at sea level, which changed at 3,500 m to 23.3, 11.8, 16.2, 17.3, and 19.8 μV on days 2, 7, 14, 21, and 28, respectively. These results indicate a cortical depression in the initial phase of HA exposure, followed by a gradual build-up of EEG waves during acclimatization (Selvamurthy et al., 1978). Another study was conducted in the lowland soldiers 7 days before ascending to altitude, and on the 7th and 30th days at 3,800 m, which found that acute acclimatization only decreased theta power, while chronic acclimatization discriminately increased alpha and beta powers but decreased delta power (Zhao et al., 2016). This study also found decreased alpha power and increased beta power within 7 days of returning to lowlands, which indicated a sustained higher level of cortical excitation during reoxygenation. After 5 days of HA exposure EEG frequency was increased (Forster et al., 1975). This is the only study that attempted to clarify neuronal activity during the “HA deadaptation reaction” (Zhou et al., 2012).
Recently, serial task-induced ERPs were measured in immigrants who had lived at Lhasa (3560 m) for more than 2 years. The HA immigrants showed reduced attentional resources with smaller P3 amplitudes (Wang et al., 2014; Qiu et al., 2021), reduced attention reactions in visual search tasks with lower N2pc amplitudes (Zhang et al., 2018a), overactive performance monitoring with larger error-related negative and correct-related negative amplitudes (Ma et al., 2015a), impaired response inhibition in the conflict-monitoring stage (Ma et al., 2015b; Wang et al., 2021) and visual executive ability (Ma et al., 2015c) with smaller P3 amplitudes, slowed stimulus-driven behaviors and P3 magnitudes of resource allocation (Ma et al., 2018a), impaired spatial manipulation ability with larger rotation-related negativity amplitudes (Ma et al., 2018b), decreased P50 mean amplitude and delay activity amplitude of mental rotation (Li et al., 2021), impaired spatial working memory with lower P2 and impaired verbal and spatial working memory maintenance with late-positive potentials (Ma et al., 2019b), and decreased alpha event-related desynchronization at the parietal-occipital regions and beta event-related desynchronization at the central-parietal regions within the time window (400–700 ms) in the mental rotation task (Xiang et al., 2021). Taken together, these electrophysiological studies showed that prolonged exposure to HA mainly impairs the late processing stage of cognition due to insufficient attention resources.
Rare electrophysiological studies of brain function were conducted on HA natives. In Bolivian children living at 3,700 m, reductions in the amplitude of delta and beta frequencies were recorded (Richardson et al., 2011). In Tibetan natives, an overactive executive ability with larger P3 and an impaired orienting ability with larger N1 were recorded only at extreme altitudes above 4,200 m, while below this threshold no cognitive impairments were observed, suggesting the existence of a threshold for the influence of HA exposure on brain function (Zhang et al., 2018b). The N2 difference wave was smaller in the 4,500 m group than in the groups living below 4,000 m, which indicated that the altitude threshold for impairment of cognition may be 4,000 m (Ma et al., 2019a).
Brain neuronal activity
Given its high spatial resolution, functional magnetic resonance imaging (fMRI) is used to measure changes in blood dynamics caused by neuronal activity. In fMRI, the blood oxygenation level dependent (BOLD) effect is employed to identify and delineate neuronal activity. An initial fMRI study was conducted on individuals who stayed at Chacaltaya (5,260 m) for 5 weeks, and showed that the average magnitude of BOLD responses induced by visual stimulation was reduced in the occipital gyrus (Rostrup et al., 2005). Subsequently, serial fMRI studies were conducted on Han immigrant descendants who were born and raised in the Qinghai-Tibet Plateau for at least 17 years and later relocated to sea level for more than 1 year. These serial scans revealed the brain mechanisms underlying spatial working memory (Yan et al., 2011a), verbal working memory (Yan et al., 2011c), and food cravings (Yan et al., 2011d). Recently, a task fMRI study was conducted on military aviation personnel who have chronic intermittent exposure to HA, and found significant activation in the right middle frontal gyrus by a spatial working memory task (Tower of London paradigm) (Nisha et al., 2020). In soldiers who had garrisoned the frontiers in the Qinghai-Tibet Plateau for 2 years, the regional homogeneity (ReHo) of neuronal activity (Chen et al., 2016a), voxel-mirrored homo-topic connectivity in the bilateral visual cortex (Chen et al., 2016b), and amplitude of low-frequency fluctuations (ALFF) of resting-state neuronal activity (Zhang et al., 2017) were investigated. In the sea level college students who had immigrated to a plateau for 1 and 2 years, ReHo of neuronal activity was also studied (Chen X. et al., 2017; Wang et al., 2017), and further study on these students suggested that ReHo of neuronal activity in the bilateral putamen and bilateral pallidum can predict psychomotor impairment due to HA exposure (Chen et al., 2019b).
Brain functional network
One study showed decreases of neuronal co-activation within the left/right frontoparietal network, sensorimotor network, and auditory network at resting state in lowland students after they had their college study at Lhasa for 2 years (Chen et al., 2021). Another study revealed changes of topological property of functional network of some important regions, showing alterations of degree centrality and nodal efficiency within the network after HA exposure for 2 years (Xin et al., 2020). The brain functional network alteration in the resting state may be the functional basic of executive control impairment.
Conclusions of the findings in brain structures and functions
The regions of the brain found to be altered in the HA population included the bilateral insular cortex, occipital cortex, cingulate cortex, precentral gyrus, and hippocampus as well as cerebellum (summarized in Figure 2). Although multiple regions of the brain are affected during HA exposure, the most consistent findings involved the occipital visual cortex, covering lingual gyrus, fusiform gyrus, and precuneus (Figure 3), and insular cortex, including anterior insula and posterior insula (Figure 4). According to the review by Evans (2010), most of these regions are the key components of the cortico-limbic modulation of respiratory control and sensation. Supporting this review, GM structure and function in these regions showed correlations with pulmonary functions in several studies done on the HA population. For example, GM volume in the insular cortex correlated with vital capacity (Zhang et al., 2013a); BOLD signals in the insular cortex, thalamus, cerebellum, and precentral cortex correlated with inspiratory and expiratory reserve volumes (Yan et al., 2011b); the strength of functional connectivity in the right insular cortex to the right superior temporal gyrus showed significant positive correlation with the predicted forced expiratory volume in one second (FEV1) (Zhang et al., 2017); and GM volumes in the hippocampal and middle frontal gyri were significantly negatively correlated with vital capacity (Zhang et al., 2013a).
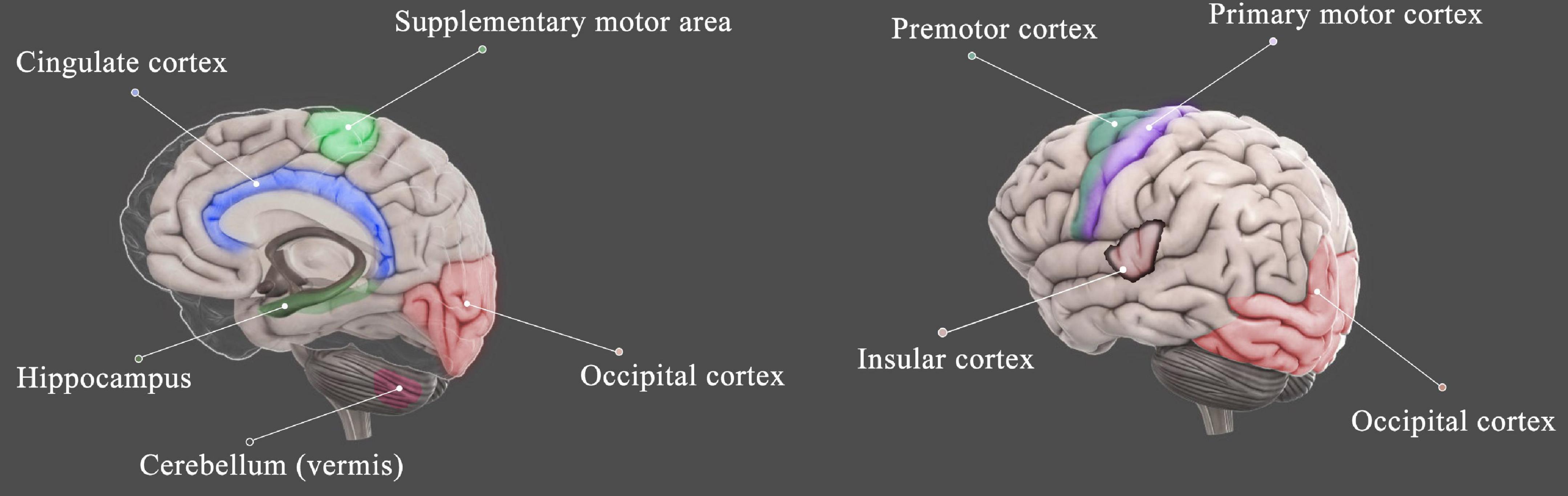
Figure 2. Schematic diagram shows the brain regions that were affected by HA exposure. The regions include the insular cortex (Zhang et al., 2010, 2013a, 2017; Yan et al., 2011b,d; Fan et al., 2016; Wei et al., 2017; Xin et al., 2020), occipital cortex (Zhang et al., 2010, 2013a, 2017; Yan et al., 2011a,b,c,d; Fan et al., 2016; Zhao et al., 2016; Wang et al., 2017, 2018; Wei et al., 2017; Xin et al., 2020; Xiang et al., 2021), cingulate cortex (Zhang et al., 2010, 2013b; Yan et al., 2011b; Fan et al., 2016; Verges et al., 2016; Chen X. et al., 2017; Wei et al., 2017; Wang et al., 2018), motor cortex (Zhang et al., 2010, 2013a; Chen et al., 2016a; Fan et al., 2016), cerebellum (Yan et al., 2011a; Zhang et al., 2012, 2013a,b; Xin et al., 2020), and hippocampus (Zhang et al., 2013b; Foster et al., 2015; Fan et al., 2016; Chen X. et al., 2017; Xin et al., 2020).
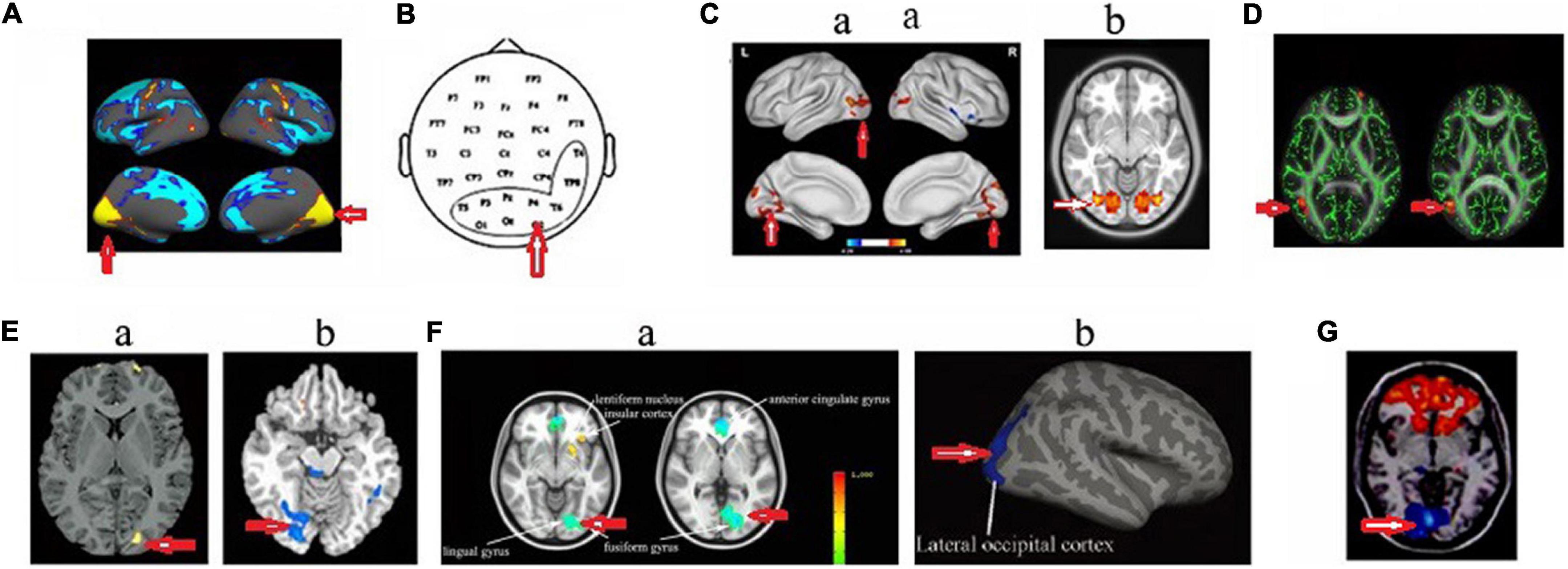
Figure 3. Changed structure and function in visual cortex in HA population. (A) Increased cortical thickness in sea-level college students who had a 30-day teaching at HA (Fan et al., 2016); (B) increased beta power in soldiers who had garrisoned at HA for 1 month (Zhao et al., 2016); (C) increased amplitude of low-frequency fluctuations (Zhang et al., 2017) (a) and voxel-mirrored homotopic connectivity (Chen et al., 2016b) (b) in soldiers who had garrisoned the frontiers at HA for 2 years; (D) The increased fractional anisotropy in Tibetan adolescents descending to sea level for 4 years (Zhang et al., 2013a); (E) Decreased gray matter volume (Zhang et al., 2010) (a) and decreased cerebrovascular reactivity (Yan et al., 2011b) (b) in the descendants of Han population who have immigrated to HA for several generations; (F) decreased cerebral blood flow (Wang et al., 2018) (a) and cortical thickness (Wei et al., 2017) (b) in HA Tibetan natives; (G) decreased the amplitude of low-frequency fluctuations in college students who studied at HA for 1 year (Wang et al., 2017). The arrow indicates the visual cortex.
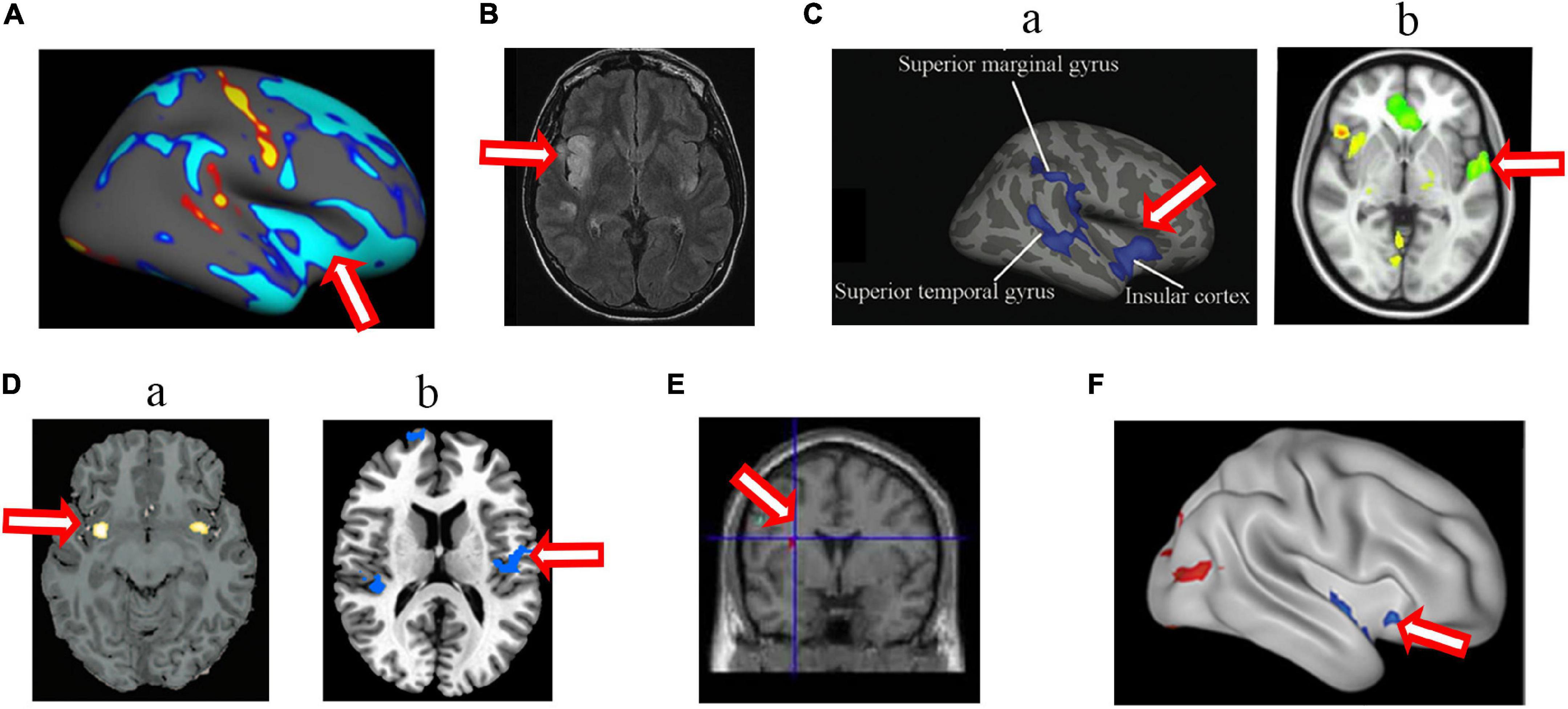
Figure 4. Changed structure and function in insular cortex in HA population. (A) The decreased cortical thickness in sea-level college students who had a 30-day teaching at HA (Fan et al., 2016); (B) hyperintense signaling in a woman after a rapid ascent to mountain (D’Arrigo et al., 2019); (C) the decreased sulcus depth (Wei et al., 2017) (a) and decreased cerebral blood flow (Wang et al., 2018) (b) in HA Tibetan natives; (D) the decreased gray matter volume (Zhang et al., 2010) (a) and longer dwelay of hemodynamic response (Yan et al., 2011b) (b) in the descendants of Han population who have immigrated to HA for several generations; (E) the increased gray matter volume in Tibetan adolescents descending to sea level for 4 years (Zhang et al., 2013a); (F) the decreased ALFF in soldiers who had garrisoned the frontiers at HA for 2 years (Zhang et al., 2017). The arrow indicates the insular cortex.
In addition, structural and functional changes in the motor cortex, including Brodmann area 4, Brodmann area 6, and supplementary motor area (Figure 5), and cerebellum (mainly in vermis) were found in HA natives as well as HA immigrants. The cerebellum has diverse connections to the cerebrum, brain stem, and spinal cord, takes part in motor control, and is associated mainly with motor skills, visual-motor coordination, and balance (Swinny et al., 2005). Therefore, the abnormal structures of the motor cortex and cerebellum suggest deficits in motor coordination in HA population; however, there are no behavior tests so far to support these brain findings.
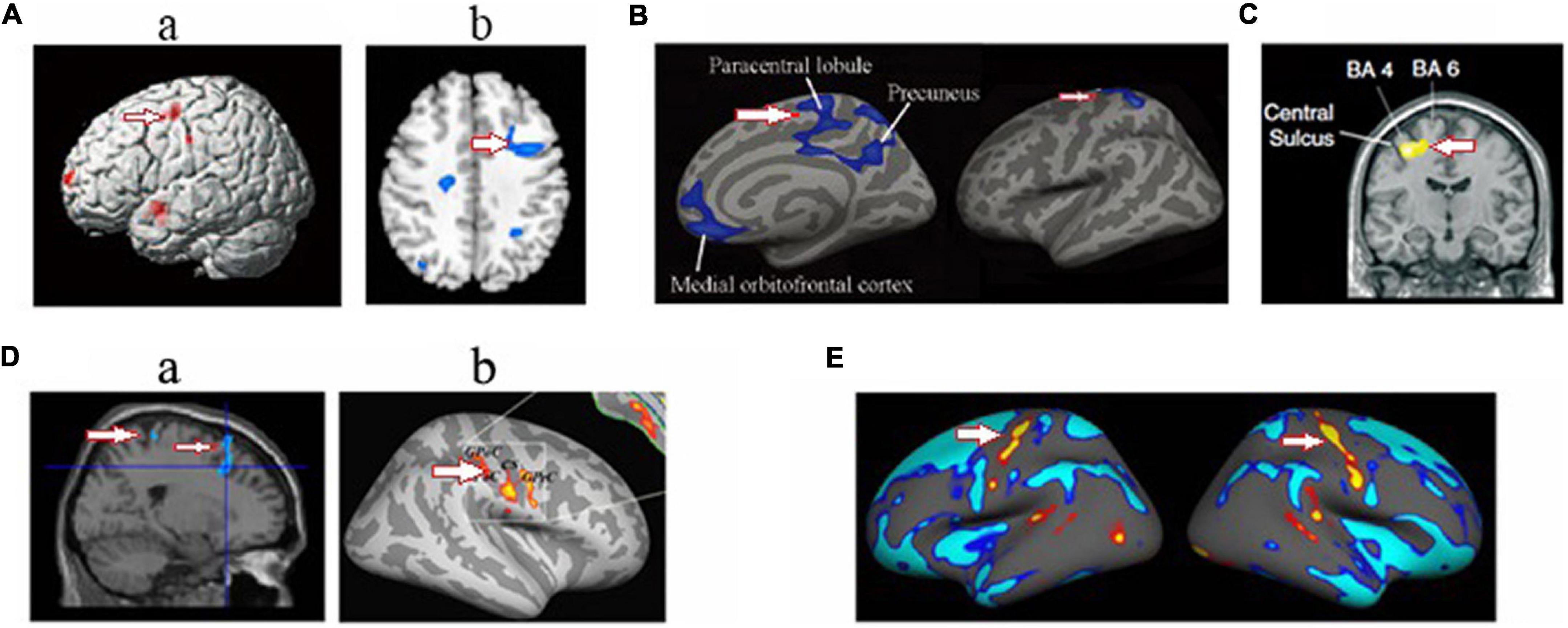
Figure 5. Changed structure and function in motor cortex in HA population. (A) The decreased gray matter volume (Zhang et al., 2010) (a) and cerebrovascular reactivity (Yan et al., 2011b) (b) in the descendants of Han population who have immigrated to HA for several generations; (B) the decreased cortical thickness in HA Tibetan natives (Wei et al., 2017); (C) the decreased white matter fiber volume projecting from motor cortex in Everest climbers (Di Paola et al., 2008). (D) the decreased the gray matter volume (Zhang et al., 2013b) (a) and increased regional homogeneity in soldiers who had garrisoned the frontiers at HA for 2 years (Chen et al., 2016a) (b); (E) the increased cortical thickness in sea-level college students who had a 30-day teaching at HA (Fan et al., 2016). The arrow indicates the motor cortex.
The hippocampus is thought to be a core brain region for spatial memory (Bellmund et al., 2018). Evidence from animal studies have showed that apoptotic death in the hippocampus may be involved in HA-induced memory impairments (Maiti et al., 2008), while the increased p-CREB, long-term potentiation, and synapses of the hippocampus were related to enhanced spatial learning and memory in mice exposed to intermittent hypobaric hypoxia (Zhang et al., 2005). Unexpectedly, however, only two MRI studies revealed impairments in the hippocampi of individuals who were exposed to HA for 2 years (Zhang et al., 2013b; Chen X. et al., 2017), which does not account for the changes in spatial cognition detected in various HA individuals. We suggest that the impaired visual cortex may be responsible for deficits in spatial cognition.
Many factors may contribute to the structural and functional changes of the visual cortex. The brain and retina have the highest oxygen uptake per unit mass of any body system (Parker and West, 1973; Country, 2017). Existing data show that vessel engorgement and tortuosity, optic disc hyperemia, and hemorrhages are often seen in Tibetans (Morris et al., 2006). Epidemiological data show that the prevalence of blindness in HA Tibetans was higher in Lhasa Tibetans than in lowland populations in the 50–59 years age range (Wang et al., 2013). The affected retinal vasculature decreases the blood supply to visual photoreceptor cells, reduces the activity in these cells, and thus decreases the excitement to visual cortical cells. The optic nerve axons are also thought to be injured by hypoxia (Chan et al., 2015). More importantly, the visual cortex is always directly affected by hypoxia, as during hypoxic events, neuronal activity and metabolism in the visual cortex are reduced (Rostrup et al., 2005; Vestergaard et al., 2016). Changes in the visual cortex may also be an outcome of increased exposure to ultraviolet (UV) radiation at HA (Wei et al., 2017). UV light has been shown to alter neuronal activity in the cortical structures involved in visual processing (Amir and Robinson, 1996), and environmental UV rays can induce apoptosis in the retinal and laminar ganglions within the visual system and brain (de Oliveira Miguel et al., 2002; Hollmann et al., 2016).
Altered neuronal activity in the insular cortex likely contributed to the regulation of ventilatory and cardiovascular functions while in the HA environment. As mentioned above, the relationship between the insular cortex and respiratory function has been demonstrated in HA populations. The insular cortex is connected with the hypothalamus, midbrain, pontine, and medulla oblongata (Gaytan and Pasaro, 1998; Bowman et al., 2013), as well as the diaphragm (Lois et al., 2009), all of which are involved in respiratory and cardiovascular sensation and control. Electrophysiological recording showed neural activity in the right anterior insular cortex mediated resting spontaneous breathing (Evans et al., 2009), while stimulation of the anterior insular cortex produced a set of specific alterations in the respiratory pattern (Aleksandrov et al., 2007). The injection of l-glutamate into the insular cortex induced apnea in rats (Cui et al., 2012). Numerous fMRI studies have shown that the anterior insular cortex plays an important role in air hunger (Evans, 2010; Parshall et al., 2012; Binks et al., 2014). The left insular cortex principally regulates parasympathetic activity and the right regulates sympathetic nerve activity. A structural impairment in the anterior insular cortex (Zhang et al., 2010; Wei et al., 2017) may be one reason why HA natives had a blunted hypoxic ventilatory response (West, 2002). The findings regarding the insular cortex support the hypothesis that the anterior insular and cingulate cortices are needed to process perturbation of the homoeostatic balance in extreme environments (Paulus et al., 2009; Zhang et al., 2010).
Nowadays, a large number of sea−level people migrate to high altitudes to travel or to work (Tianyi and Kayser, 2006). A review of the effects of altitude on the brain helps these people overcome the fear of high altitude. In addition, hypoxia is a very common disease in clinic. High altitude hypoxia is a natural model, and its damaged brain regions provide a theoretical basis for the therapeutic targets of clinical hypoxia diseases.
Conclusion
The microstructural and functional alterations to the human brain in response to acute HA-induced stress or chronic acclimatization to HA environments have been studied in vivo mainly in the past 10 years. Among various HA populations, the most consistent findings involve changes in the visual, motor and insular cortices, and it has been found that the altered insular cortex may be related to cardiovascular or respiratory regulation.
At present, there are relatively few studies on HA brain, and thus the reliability of the results among these studies needs to be further confirmed. Some subjective or objective factors as follows may affect the results. (1) In MRI experiments, data acquisition and analysis are required to be carried out by different researchers; however, due to the limited conditions in the actual research (these data collection are difficult), some studies may not be double-blind. However, seeing that these results are relatively consistent, we suggest that the factor of human expectation is small. Insular cortex is the visceral sensory center, which can be expected to be affected by hypoxia, but the changes in the visual and motor cortices cannot be expected. Generally speaking, we think the results are relatively reliable. (2) VBM involves a voxel-wise comparison of the local concentration of GM between groups. Corrections for multiple comparisons are needed to avoid statistical class I errors. The results showed in some researches, however, cannot stand correction. With or without correction, similar HA exposure showed different results in different studies. For example, to study the effect of 2-year HA exposure on human brain, Zhang et al. (2013b) showed GM volume changes in multiple brain regions without statistical correction, while Chen X. et al. (2017) showed only one differential brain region under statistical correction. Of course, the subjects in Zhang et al. (2013b) are soldiers, and thus the physical training may partially aggravate the effect of hypoxia. (3) The procedure of VBM involves spatially normalizing high-resolution images from all the subjects in the study into the same stereotactic space, segmenting GM from the spatially normalized images and smoothing GM segments. Voxel-wise parametric statistical tests are performed by comparing the smoothed GM images from the two groups. All these steps involved in VBM could produce non-uniformity artifact. (4) Some studies reported no change in total brain volume, but only local changes. One way to think about it is that local brain changes account for only a small part of brain volume, and are not enough to affect the total brain. (5) Some longitudinal studies were conducted before and after HA exposure, in which subjects were scanned and rescanned at an interval, and thus MRI instrument-related factors may affect the morphological measurements. (6) At present, there are few high-intensity MRI machines on the HA areas. Previously, we have repeatedly performed MRI scans in Lhasa and found this phenomenon. (7) There were no lowland control group in some studies. In fact, in addition to the influence of plateau environment, subjects will be challenged by the changes of diet and cultural customs. In the future, when there will be more high-intensity MRI machines in the plateau area, and the machine performance cannot be disturbed by factors such as low pressure, it will be convenient to recruit a large number of HA population to carry out large sample research. At that time, the impact of HA environment on the brain will be more truly revealed.
There are still many questions that need to be answered, such as which mechanisms, neuronal, glial, and/or vascular, contribute to brain structure alterations? Exploring the genetic basis of brain structures in HA natives helps to understand the mechanisms of HA adaptation in the native population. The difference that exists between individual brains in response to HA is also an interesting issue. How about brain readaptation to lowlands after HA immigrants return to lowlands?
According to literature, there are several strategies that may be employed to protect brain injury in HA environment. (1) Cerebral edema contributes much to the severity of the ultimate brain damage. Gradual ascents are proven to be the best strategy for preventing HA cerebral edema (Houston and Dickinson, 1975; Basnyat and Murdoch, 2003; Joyce et al., 2018). Moreover, cerebral edema can be eliminated when HA immigrants leave a lower oxygen environment, such as descending to lowlands, being supplied with oxygen (Pines, 1978; Hackett and Roach, 2001; West et al., 2004; Davis and Hackett, 2017; Williamson et al., 2018), or inhaling a higher concentration of oxygen (West, 2003). (2) In the recent studies, salidroside has been illuminated as a promising neuroprotectant for preventing and treating ischemic stroke (see the review by Fan et al., 2020). Therefore, with higher efficacy and better safety profiles, salidroside can be used for preventing acute cerebral edema. (3) Reviews showed that the beneficial effects of living at HA on the cardiovascular and cerebrovascular systems, morbidity, and mortality are influenced not only by the level of altitude but also by lifestyle behaviors like physical activity, nutrition, smoking, and alcohol consumption (Burtscher, 2014; Mallet et al., 2021). Keep this in mind, we believe that forming a good lifestyle, such as no smoking and no drinking and proper exercise, will help to reduce brain damage. (4) Dietary restriction has been proved to increase the length of life in several species. Moderate dietary restriction, but not extreme dietary restriction, can reduce neurovascular damage after hypoxic-ischemia and confer long-term protection in neonatal brain (Tu et al., 2012) and thus dietary restriction may be an alternative protective strategy for brain hypoxic injury.
Author contributions
XZ: investigation and writing—original draft. JZ: conceptualization, writing—review and editing, and funding acquisition. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81871519 and 82171864).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aleksandrov, V., Ivanova, T., and Aleksandrova, N. (2007). Prefrontal control of respiration. J. Physiol. Pharmacol. 58, 17–23.
Amir, S., and Robinson, B. (1996). Fos expression in rat visual cortex induced by ocular input of ultraviolet light. Brain Res. 716, 213–218. doi: 10.1016/0006-8993(96)00025-X
Angelova, P. R., Kasymov, V., Christie, I., Sheikhbahaei, S., Turovsky, E., Marina, N., et al. (2015). Functional Oxygen Sensitivity of Astrocytes. J. Neurosci. 35, 10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015
Anooshiravani, M., Dumont, L., Mardirosoff, C., Soto-Debeuf, G., and Delavelle, J. (1999). Brain magnetic resonance imaging (MRI) and neurological changes after a single high altitude climb. Med. Sci. Sports Exerc. 31, 969–972. doi: 10.1097/00005768-199907000-00008
Basnyat, B., and Murdoch, D. R. (2003). High-altitude illness. Lancet 361, 1967–1974. doi: 10.1016/S0140-6736(03)13591-X
Bellmund, J. L. S., Gardenfors, P., Moser, E. I., and Doeller, C. F. (2018). Navigating cognition: Spatial codes for human thinking. Science 362, 654–654. doi: 10.1126/science.aat6766
Binks, A. P., Evans, K. C., Reed, J. D., Moosavi, S. H., and Banzett, R. B. (2014). The time-course of cortico-limbic neural responses to air hunger. Respir. Physiol. Neurobiol. 204, 78–85. doi: 10.1016/j.resp.2014.09.005
Boero, J. A., Ascher, J., Arregui, A., Rovainen, C., and Woolsey, T. A. (1999). Increased brain capillaries in chronic hypoxia. J. Appl. Physiol. 86, 1211–1219. doi: 10.1152/jappl.1999.86.4.1211
Bowman, B. R., Kumar, N. N., Hassan, S. F., McMullan, S., and Goodchild, A. K. (2013). Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J. Comp. Neurol. 521, 213–232. doi: 10.1002/cne.23175
Burtscher, M. (2014). Effects of living at higher altitudes on mortality: A narrative review. Aging Dis. 5, 274–280. doi: 10.14336/AD.2014.0500274
Chan, T., Wong, W. W., Chan, J. K., Ma, J. K., and Mak, H. K. (2012). Acute ischaemic stroke during short-term travel to high altitude. Hong Kong Med. J. 18, 63–65.
Chan, K. C., Kancherla, S., Fan, S.-J., and Wu, E. X. (2015). Long-term effects of neonatal hypoxia-ischemia on structural and physiological integrity of the eye and visual pathway by multimodal MRI. Investig. Ophthalmol. Vis. Sci. 56, 1–9. doi: 10.1167/iovs.14-14287
Chen, J., Li, J., Han, Q., Lin, J., Yang, T., Chen, Z., et al. (2016b). Long-term acclimatization to high-altitude hypoxia modifies interhemispheric functional and structural connectivity in the adult brain. Brain Behav. 6:e00512. doi: 10.1002/brb3.512
Chen, J., Fan, C., Li, J., Han, Q., Lin, J., Yang, T., et al. (2016a). Increased Intraregional Synchronized Neural Activity in Adult Brain After Prolonged Adaptation to High-Altitude Hypoxia: A Resting-State fMRI Study. High Alt. Med. Biol. 17, 16–24. doi: 10.1089/ham.2015.0104
Chen, L., Cai, C., Yang, T., Lin, J., Cai, S., Zhang, J., et al. (2017). Changes in brain iron concentration after exposure to high-altitude hypoxia measured by quantitative susceptibility mapping. Neuroimage 147, 488–499. doi: 10.1016/j.neuroimage.2016.12.033
Chen, X., Li, H., Zhang, Q., Wang, J., Zhang, W., Liu, J., et al. (2019a). Combined fractional anisotropy and subcortical volumetric abnormalities in healthy immigrants to high altitude: A longitudinal study. Hum. Brain Mapp. 40, 4202–4212. doi: 10.1002/hbm.24696
Chen, X., Zhang, Q., Wang, J., Xin, Z., Chen, J., and Luo, W. (2019b). Combined machine learning and functional magnetic resonance imaging allows individualized prediction of high-altitude induced psychomotor impairment: The role of neural functionality in putamen and pallidum. Biosci. Trends 13, 98–104. doi: 10.5582/bst.2019.01002
Chen, X., Liu, J., Wang, J., Xin, Z., Zhang, Q., Zhang, W., et al. (2021). Altered resting-state networks may explain the executive impairment in young health immigrants into high-altitude area. Brain Imaging Behav. 15, 147–156. doi: 10.1007/s11682-019-00241-1
Chen, X., Zhang, Q., Wang, J., Liu, J., Zhang, W., Qi, S., et al. (2017). Cognitive and neuroimaging changes in healthy immigrants upon relocation to a high altitude: A panel study. Hum. Brain Mapp. 38, 3865–3877. doi: 10.1002/hbm.23635
Country, M. W. (2017). Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 1672, 50–57. doi: 10.1016/j.brainres.2017.07.025
Cui, L., Wang, J.-H., Wang, M., Huang, M., Wang, C.-Y., Xia, H., et al. (2012). Injection of L-glutamate into the insular cortex produces sleep apnea and serotonin reduction in rats. Sleep Breath 16, 845–853. doi: 10.1007/s11325-011-0586-x
D’Arrigo, A. M., Altavilla, R., Bartesaghi, F., Floro, S., Campiglio, L., Secchi, M., et al. (2019). Bilateral ischemia of the insular cortex after high altitude climbing: A case report. J. Clin. Neurosci. 67, 276–277. doi: 10.1016/j.jocn.2019.05.049
Davis, C., and Hackett, P. (2017). Advances in the Prevention and Treatment of High Altitude Illness. Emerg. Med. Clin. N. Am. 35, 241–260. doi: 10.1016/j.emc.2017.01.002
de Oliveira Miguel, N. C., Meyer-Rochow, V. B., and Allodi, S. (2002). Ultrastructural study of first and second order neurons in the visual system of the crab Ucides cordatus following exposure to ultraviolet radiation. Micron 33, 627–637. doi: 10.1016/s0968-4328(02)00030-6
Degache, F., Larghi, G., Faiss, R., Deriaz, O., and Millet, G. (2012). Hypobaric versus normobaric hypoxia: Same effects on postural stability? High Alt. Med. Biol. 13, 40–45. doi: 10.1089/ham.2011.1042
Di Paola, M., Bozzali, M., Fadda, L., Musicco, M., Sabatini, U., and Caltagirone, C. (2008). Reduced oxygen due to high-altitude exposure relates to atrophy in motor-function brain areas. Eur. J. Neurol. 15, 1050–1057. doi: 10.1111/j.1468-1331.2008.02243.x
DiPasquale, D. M., Strangman, G. E., Harris, N. S., and Muza, S. R. (2015). Hypoxia, Hypobaria, and Exercise Duration Affect Acute Mountain Sickness. Aerosp. Med. Hum. Perform. 86, 614–619.
Evans, K. C. (2010). Cortico-limbic circuitry and the airways: Insights from functional neuroimaging of respiratory afferents and efferents. Biol. Psychol. 84, 13–25. doi: 10.1016/j.biopsycho.2010.02.005
Evans, K. C., Dougherty, D. D., Schmid, A. M., Scannell, E., McCallister, A., Benson, H., et al. (2009). Modulation of spontaneous breathing via limbic/paralimbic-bulbar circuitry: An event-related fMRI study. Neuroimage 47, 961–971. doi: 10.1016/j.neuroimage.2009.05.025
Fan, C., Zhao, Y., Yu, Q., Yin, W., Liu, H., Lin, J., et al. (2016). Reversible Brain Abnormalities in People Without Signs of Mountain Sickness During High-Altitude Exposure. Sci. Rep. 6:33596. doi: 10.1038/srep33596
Fan, F., Yang, L., Li, R., Zou, X., Li, N., Meng, X., et al. (2020). Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 129:110458. doi: 10.1016/j.biopha.2020.110458
Fayed, N., Diaz, L., Dávila, J., and Medrano, J. (2010). Hematological indices, mountain sickness and MRI brain abnormalities in professional and amateur mountain climbers after altitude exposure. Neurol. Res. 32, 144–147. doi: 10.1179/174313209X414551
Fayed, N., Modrego, P. J., and Morales, H. (2006). Evidence of brain damage after high-altitude climbing by means of magnetic resonance imaging. Am. J. Med. 119, e161–e166. doi: 10.1016/j.amjmed.2005.07.062
Forster, H. V., Soto, R. J., Dempsey, J. A., and Hosko, M. J. (1975). Effect of sojourn at 4,300 m altitude on electroencephalogram and visual evoked response. J. Appl. Physiol. 39, 109–113. doi: 10.1152/jappl.1975.39.1.109
Foster, G. E., Davies-Thompson, J., Dominelli, P. B., Heran, M. K., Donnelly, J., duManoir, G. R., et al. (2015). Changes in cerebral vascular reactivity and structure following prolonged exposure to high altitude in humans. Physiol. Rep. 3, e12647. doi: 10.14814/phy2.12647
Gao, X. Y., Huang, J.-O., Hu, Y.-F., Gu, Y., Zhu, S.-Z., Huang, K.-B., et al. (2014). Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury. Sci. Rep. 4:7091. doi: 10.1038/srep07091
Garrido, E., Castello, A., Ventura, J. L., Capdevila, A., and Rodriguez, F. A. (1993). Cortical atrophy and other brain magnetic resonance imaging (MRI) changes after extremely high-altitude climbs without oxygen. Int. J. Sports Med. 14, 232–234. doi: 10.1055/s-2007-1021169
Garrido, E., Segura, R., Capdevila, A., Aldoma, J., Rodriguez, F. A., Javierra, C., et al. (1995). New evidence from magnetic resonance imaging of brain changes after climbs at extreme altitude. Eur. J. Appl. Physiol. Occup. Physiol. 70, 477–481. doi: 10.1007/BF00634375
Garrido, E., Segura, R., Capdevila, A., Pujol, J., Javierre, C., and Ventura, J. L. (1996). Are Himalayan Sherpas better protected against brain damage associated with extreme altitude climbs? Clin. Sci. 90, 81–85. doi: 10.1042/cs0900081
Gaytan, S. P., and Pasaro, R. (1998). Connections of the rostral ventral respiratory neuronal cell group: An anterograde and retrograde tracing study in the rat. Brain Res. Bull. 47, 625–642. doi: 10.1016/s0361-9230(98)00125-7
Gould, E., Reeves, A. J., Graziano, M. S., and Gross, C. G. (1999). Neurogenesis in the neocortex of adult primates. Science 286, 548–552. doi: 10.1126/science.286.5439.548
Guo, Z., Fan, C., Li, T., Gesang, L., Yin, W., Wang, N., et al. (2020). Neural network correlates of high-altitude adaptive genetic variants in Tibetans: A pilot, exploratory study. Hum. Brain Mapp. 41, 2406–2430. doi: 10.1002/hbm.24954
Hackett, P. H., and Roach, R. C. (2001). High-altitude illness. N. Engl. J. Med. 345, 107–114. doi: 10.1056/NEJM200107123450206
Hackett, P. H., and Roach, R. C. (2004). High altitude cerebral edema. High Alt. Med. Biol. 5, 136–146. doi: 10.1089/1527029041352054
Harris, A. D., Murphy, K., Diaz, C. M., Saxena, N., Hall, J. E., Liu, T. T., et al. (2013). Cerebral blood flow response to acute hypoxic hypoxia. NMR Biomed. 26, 1844–1852. doi: 10.1002/nbm.3026
Hoiland, R. L., Howe, C. A., Coombs, G. B., and Ainslie, P. N. (2018). Ventilatory and cerebrovascular regulation and integration at high-altitude. Clin. Auton. Res. 28, 423–435. doi: 10.1007/s10286-018-0522-2
Hollmann, G., Linden, R., Giangrande, A., and Allodi, S. (2016). Increased p53 and decreased p21 accompany apoptosis induced by ultraviolet radiation in the nervous system of a crustacean. Aquat. Toxicol. 173, 1–8. doi: 10.1016/j.aquatox.2015.12.025
Houston, C. S., and Dickinson, J. (1975). Cerebral form of high-altitude illness. Lancet 2, 758–761. doi: 10.1016/s0140-6736(75)90735-7
Javier, V.-O., Gualberto, B.-C., Eduardo, G., and Bernardino, A. (2004). Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 14, 197–224. doi: 10.1007/s11065-004-8159-4
Joyce, K. E., Lucas, S. J. E., Imray, C. H. E., Balanos, G. M., and Wright, A. D. (2018). Advances in the available non-biological pharmacotherapy prevention and treatment of acute mountain sickness and high altitude cerebral and pulmonary oedema. Expert Opin. Pharmacother. 19, 1891–1902. doi: 10.1080/14656566.2018.1528228
Kallenberg, K., Dehnert, C., Dorfler, A., Schellinger, P. D., Bailey, D. M., Knauth, M., et al. (2008). Microhemorrhages in nonfatal high-altitude cerebral edema. J. Cereb. Blood Flow Metab. 28, 1635–1642. doi: 10.1038/jcbfm.2008.55
Kayser, B., and Wu, T. (2006). High Altitude Adaptation in Tibetans. High Alt. Med. Biol. 7:193. doi: 10.1089/ham.2006.7.193
Kottke, R., Pichler Hefti, J., Rummel, C., Hauf, M., Hefti, U., and Merz, T. M. (2015). Morphological Brain Changes after Climbing to Extreme Altitudes–A Prospective Cohort Study. PLoS One 10:e0141097. doi: 10.1371/journal.pone.0141097
Kühn, S., Gerlach, D., Noblé, H.-J., Weber, F., Rittweger, J., Jordan, J., et al. (2019). An Observational Cerebral Magnetic Resonance Imaging Study Following 7 Days at 4554 m. High Alt. Med. Biol. 20, 407–416. doi: 10.1089/ham.2019.0056
Kvistad, C. E., Thomassen, L., Waje-Andreassen, U., Logallo, N., and Naess, H. (2014). Body temperature and major neurological improvement in tPA-treated stroke patients. Acta Neurol. Scand. 129, 325–329. doi: 10.1111/ane.12184
LaManna, J. C., Vendel, L. M., and Farrell, R. M. (1992). Brain adaptation to chronic hypobaric hypoxia in rats. J. Appl. Physiol. 72, 2238–2243. doi: 10.1152/jappl.1992.72.6.2238
Li, Z., Xue, X., Li, X., Bao, X., Yu, S., Wang, Z., et al. (2021). Neuropsychological effect of working memory capacity on mental rotation under hypoxia environment. Int. J. Psychophysiol. 165, 18–28. doi: 10.1016/j.ijpsycho.2021.03.012
Lijffijt, M., Lane, S. D., Meier, S. L., Boutros, N. N., Burroughs, S., Steinberg, J. L., et al. (2009). P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology 46, 1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x
Lois, J. H., Rice, C. D., and Yates, B. J. (2009). Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J. Appl. Physiol. 106, 138–152. doi: 10.1152/japplphysiol.91125.2008
Luck, S. J., and Hillyard, S. A. (1994). Spatial filtering during visual search: Evidence from human electrophysiology. J. Exp. Psychol. 20, 1000–1014. doi: 10.1037//0096-1523.20.5.1000
Lundby, C., and Robach, P. (2016). Does ‘altitude training’ increase exercise performance in elite athletes? Exp. Physiol. 101, 783–788. doi: 10.1113/EP085579
Ma, H., Zhang, D., Li, X., Ma, H., Wang, N., and Wang, Y. (2019b). Long-term exposure to high altitude attenuates verbal and spatial working memory: Evidence from an event-related potential study. Brain Behav. 9:e01256. doi: 10.1002/brb3.1256
Ma, H., Han, B., and Wang, Y. (2019a). Different neurocognitive patterns of conflict control in Tibetans living above and below 4,000 m. PeerJ 7:e7269. doi: 10.7717/peerj.7269
Ma, H., Huang, X., Liu, M., Ma, H., and Zhang, D. (2018a). Aging of stimulus-driven and goal-directed attentional processes in young immigrants with long-term high altitude exposure in Tibet: An ERP study. Sci. Rep. 8:17417. doi: 10.1038/s41598-018-34706-y
Ma, H., Li, X., Liu, M., Ma, H., and Zhang, D. (2018b). Mental Rotation Effect on Adult Immigrants with Long-term Exposure to High Altitude in Tibet: An ERP Study. Neuroscience 386, 339–350. doi: 10.1016/j.neuroscience.2018.06.038
Ma, H., Wang, Y., Wu, J., Liu, H., Luo, P., and Han, B. (2015a). Overactive Performance Monitoring Resulting from Chronic Exposure to High Altitude. Aerosp. Med. Hum. Perform. 86, 860–864. doi: 10.3357/AMHP.4261.2015
Ma, H., Wang, Y., Wu, J., Luo, P., and Han, B. (2015b). Long-Term Exposure to High Altitude Affects Response Inhibition in the Conflict-monitoring Stage. Sci. Rep. 5:13701. doi: 10.1038/srep13701
Ma, H., Wang, Y., Wu, J., Wang, B., Guo, S., Luo, P., et al. (2015c). Long-Term Exposure to High Altitude Affects Conflict Control in the Conflict-Resolving Stage. PLoS One 10:e0145246. doi: 10.1371/journal.pone.0145246
Magavi, S. S., Leavitt, B. R., and Macklis, J. D. (2000). Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955. doi: 10.1038/35016083
Maiti, P., Singh, S. B., Mallick, B., Muthuraju, S., and Ilavazhagan, G. (2008). High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J. Chem. Neuroanat. 36, 227–238. doi: 10.1016/j.jchemneu.2008.07.003
Maiti, P., Singh, S. B., Muthuraju, S., Veleri, S., and Ilavazhagan, G. (2007). Hypobaric hypoxia damages the hippocampal pyramidal neurons in the rat brain. Brain Res. 1175, 1–9. doi: 10.1016/j.brainres.2007.06.106
Mallet, R. T., Burtscher, J., Richalet, J. P., Millet, G. P., and Burtscher, M. (2021). Impact of High Altitude on Cardiovascular Health: Current Perspectives. Vasc. Health Risk Manag. 17, 317–335. doi: 10.2147/VHRM.S294121
McAllum, E. J., Hare, D. J., Volitakis, I., McLean, C. A., Bush, A. I., Finkelstein, D. I., et al. (2020). Regional iron distribution and soluble ferroprotein profiles in the healthy human brain. Prog. Neurobiol. 186:101744. doi: 10.1016/j.pneurobio.2019.101744
McGuire, S. A., Boone, G. R., Sherman, P. M., Tate, D. F., Wood, J. D., Patel, B., et al. (2016). White matter integrity in high-altitude pilots exposed to hypobaria. Aerosp. Med. Hum. Perform. 87, 983–988. doi: 10.3357/AMHP.4585.2016
McGuire, S., Sherman, P., Profenna, L., Grogan, P., Sladky, J., Brown, A., et al. (2013). White matter hyperintensities on MRI in high-altitude U-2 pilots. Neurology 81, 729–735. doi: 10.1212/WNL.0b013e3182a1ab12
Michalicova, A., Banks, W. A., Legath, J., and Kovac, A. (2017). Tauopathies - Focus on Changes at the Neurovascular Unit. Curr. Alzheimer Res. 14, 790–801. doi: 10.2174/1567205014666170203143336
Miyamura, M., Matsumoto, K., Takahashi, Y., and Matsumoto, N. (2007). A case of acute mountain sickness followed by globus pallidus syndrome. Brain Nerve 59, 1283–1286.
Mizoguchi, H., Fukaya, K., Mori, R., Itoh, M., Funakubo, M., and Sato, J. (2011). Lowering barometric pressure aggravates depression-like behavior in rats. Behav. Brain Res. 218, 190–193. doi: 10.1016/j.bbr.2010.11.057
Morris, D. S., Somner, J., Donald, M. J., McCormick, I. J., Bourne, R. R., Huang, S. S., et al. (2006). “The eye at altitude,” in Hypoxia And Exercise, eds R. Roach, P. Wagner, and P. Hackett (Berlin: Springer), 249–270.
Nisha, S., Fathinul, F. A., Aida, A., Salasiah, M., Hamed, S., Rohit, T., et al. (2020). The objective assessment of the effects on cognition functioning among military personnel exposed to hypobaric-hypoxia: A pilot fMRI study. Med. J. Malays. 75:62.
Parker, J. F., and West, V. R. (1973). Bioastronautics Data Book, Second Edition. Washington, D.C.: NASA Special Publication, 3006.
Parshall, M. B., Schwartzstein, R. M., Adams, L., Banzett, R. B., Manning, H. L., Bourbeau, J., et al. (2012). An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 185, 435–452. doi: 10.1164/rccm.201111-2042ST
Paulus, M. P., Potterat, E. G., Taylor, M. K., Van Orden, K. F., Bauman, J., Momen, N., et al. (2009). A neuroscience approach to optimizing brain resources for human performance in extreme environments. Neurosci. Biobehav. Rev. 33, 1080–1088. doi: 10.1016/j.neubiorev.2009.05.003
Pforte, C., Henrich-Noack, P., Baldauf, K., and Reymann, K. G. (2005). Increase in proliferation and gliogenesis but decrease of early neurogenesis in the rat forebrain shortly after transient global ischemia. Neuroscience 136, 1133–1146. doi: 10.1016/j.neuroscience.2005.08.043
Qiu, Q., Lv, P., Zhongshen, Y., Yuan, F., Zhang, X., Zhou, X., et al. (2021). Electrophysiological mechanisms underlying hypoxia-induced deficits in visual spatial and non-spatial discrimination. Physiol. Rep. 9:e15036. doi: 10.14814/phy2.15036
Raichle, M. E. (2010). Two views of brain function. Trends Cogn. Sci. 14, 180–190. doi: 10.1016/j.tics.2010.01.008
Ravenhill, T. H. (1913). Some Experiences of Mountain Sickness in the Andes. J. Trop. Med. Hyg. 16, 313–320.
Richardson, C., Hogan, A. M., Bucks, R. S., Baya, A., Virues-Ortega, J., Holloway, J. W., et al. (2011). Neurophysiological evidence for cognitive and brain functional adaptation in adolescents living at high altitude. Clin. Neurophysiol. 122, 1726–1734. doi: 10.1016/j.clinph.2011.02.001
Rostrup, E., Larsson, H. B., Born, A. P., Knudsen, G. M., and Paulson, O. B. (2005). Changes in BOLD and ADC weighted imaging in acute hypoxia during sea-level and altitude adapted states. Neuroimage 28, 947–955. doi: 10.1016/j.neuroimage.2005.06.032
Rueda, M. R., Posner, M. I., Rothbart, M. K., and Davis-Stober, C. P. (2004). Development of the time course for processing conflict: An event-related potentials study with 4 year olds and adults. BMC Neurosci. 5:39. doi: 10.1186/1471-2202-5-39
Selvamurthy, W., Saxena, R. K., Krishnamurthy, N., Suri, M. L., and Malhotra, M. S. (1978). Changes in EEG pattern during acclimatization to high altitude (3500 m) in man. Aviat. Space Environ. Med. 49, 968–971.
Simmons, D., Bursaw, A., Sherman, P., Kochunov, P., and Mcguire, S. (2015). Repeated exposure to hypobaria in U2 pilots is associated with reduced aggregate cortical thickness. Neurology 84:161.
Simonson, T. S., Yang, Y., Huff, C. D., Yun, H., Qin, G., Witherspoon, D. J., et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75. doi: 10.1126/science.1189406
Swinny, J. D., van der Want, J. J., and Gramsbergen, A. (2005). Cerebellar development and plasticity: Perspectives for motor coordination strategies, for motor skills, and for therapy. Neural Plast. 12, 153–160. doi: 10.1155/NP.2005.153
Tianyi, W. U., and Kayser, B. (2006). High altitude adaptation in tibetans. High Alt. Med. Biol. 7:193. doi: 10.1089/ham.2006.7.193
Tu, Y. F., Lu, P. J., Huang, C. C., Ho, C. J., and Chou, Y. P. (2012). Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke 43, 491–498. doi: 10.1161/STROKEAHA.111.629931
Turner, R. E. F., Gatterer, H., Falla, M., and Lawley, J. S. (2021). High-altitude cerebral edema: Its own entity or end-stage acute mountain sickness? J. Appl. Physiol. 131, 313–325. doi: 10.1152/japplphysiol.00861.2019
Verges, S., Rupp, T., Villien, M., Lamalle, L., Tropres, I., Poquet, C., et al. (2016). Multiparametric Magnetic Resonance Investigation of Brain Adaptations to 6 Days at 4350 m. Front. Physiol. 7:393. doi: 10.3389/fphys.2016.00393
Vestergaard, M. B., Lindberg, U., Aachmann-Andersen, N. J., Lisbjerg, K., Christensen, S. J., Law, I., et al. (2016). Acute hypoxia increases the cerebral metabolic rate–a magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 36, 1046–1058. doi: 10.1177/0271678x15606460
Wang, F., Bao, H., Kong, D., and Li, C. (2017). Adaptive modulation of brain in normal adult living in plain to high altitude areas: Studies on resting-state fMRI. J. Pract. Radiol. 33, 1–4.
Wang, G. Q., Bai, Z. X., Shi, J., Luo, S., Chang, H. F., and Sai, X. Y. (2013). Prevalence and risk factors for eye diseases, blindness, and low vision in Lhasa, Tibet. Int. J. Ophthalmol. 6, 237–241. doi: 10.3980/j.issn.2222-3959.2013.02.24
Wang, J., Zheng, L., Wang, Z., Wu, X., Ma, N., Zhang, T., et al. (2021). Alteration of Behavioral Inhibitory Control in High-Altitude Immigrants. Front. Behav. Neurosci. 15:712278. doi: 10.3389/fnbeh.2021.712278
Wang, X., Wei, W., Yuan, F., Li, S., Lin, J., and Zhang, J. (2018). Regional cerebral blood flow in natives at high altitude: An arterial spin labeled mri study. J. Magn. Reson. Imaging 48, 708–717. doi: 10.1002/jmri.25996
Wang, Y., Ma, H., Fu, S., Guo, S., Yang, X., Luo, P., et al. (2014). Long-Term Exposure to High Altitude Affects Voluntary Spatial Attention at Early and Late Processing Stages. Sci. Rep. 4:4443. doi: 10.1038/srep04443
Wei, W., Wang, X., Gong, Q., Fan, M., and Zhang, J. (2017). Cortical Thickness of Native Tibetans in the Qinghai-Tibetan Plateau. AJNR Am. J. Neuroradiol. 38, 553–560. doi: 10.3174/ajnr.A5050
West, J. B. (1984). Human physiology at extreme altitudes on Mount Everest. Science 223, 784–788. doi: 10.1126/science.6364351
West, J. B. (2002). Commuting to high altitude: Value of oxygen enrichment of room air. High Alt. Med. Biol. 3, 223–235. doi: 10.1089/15270290260131948
West, J. B. (2003). Improving oxygenation at high altitude: Acclimatization and O2 enrichment. High Alt. Med. Biol. 4, 389–398. doi: 10.1089/152702903769192340
West, J. B., American College of, P., and American Physiological, S. (2004). The physiologic basis of high-altitude diseases. Ann. Intern. Med. 141, 789–800. doi: 10.7326/0003-4819-141-10-200411160-00010
Williamson, J., Oakeshott, P., and Dallimore, J. (2018). Altitude sickness and acetazolamide. BMJ 361:k2153. doi: 10.1136/bmj.k2153
Xiang, Z. Q., Huang, Y. L., Luo, G. L., Ma, H. L., and Zhang, D. L. (2021). Decreased Event-Related Desynchronization of Mental Rotation Tasks in Young Tibetan Immigrants. Front. Hum. Neurosci. 15:664039. doi: 10.3389/fnhum.2021.664039
Xin, Z., Chen, X., Zhang, Q., Wang, J., Xi, Y., Liu, J., et al. (2020). Alteration in topological properties of brain functional network after 2-year high altitude exposure: A panel study. Brain Behav. 10:e01656. doi: 10.1002/brb3.1656
Yan, X., Zhang, J., Gong, Q., and Weng, X. (2011a). Adaptive influence of long term high altitude residence on spatial working memory: An fMRI study. Brain Cogn. 77, 53–59. doi: 10.1016/j.bandc.2011.06.002
Yan, X., Zhang, J., Gong, Q., and Weng, X. (2011c). Appetite at high altitude: An fMRI study on the impact of prolonged high-altitude residence on gustatory neural processing. Exp. Brain Res. 209, 495–499. doi: 10.1007/s00221-010-2516-8)
Yan, X., Zhang, J., Gong, Q., and Weng, X. (2011d). Prolonged high-altitude residence impacts verbal working memory: An fMRI study. Exp. Brain Res. 208, 437–45. doi: 10.1007/s00221-010-2494-x
Yan, X., Zhang, J., Gong, Q., and Weng, X. (2011b). Cerebrovascular reactivity among native-raised high altitude residents: An fMRI study. BMC Neurosci. 12:94. doi: 10.1186/1471-2202-12-94
Yan, X., Zhang, J., Shi, J., Gong, Q., and Weng, X. (2010). Cerebral and functional adaptation with chronic hypoxia exposure: A multi-modal MRI study. Brain Res. 1348, 21–29. doi: 10.1016/j.brainres.2010.06.024
Yang, J., Jin, Z. B., Chen, J., Huang, X. F., Li, X. M., Liang, Y. B., et al. (2017). Genetic signatures of high-altitude adaptation in Tibetans. Proc. Natl. Acad. Sci. U.S.A. 114, 4189–4194. doi: 10.1073/pnas.1617042114
Yi, X., Liang, Y., Huerta-Sanchez, E., Jin, X., Cuo, Z. X., Pool, J. E., et al. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78. doi: 10.1126/science.1190371
Zatorre, R. J., Fields, R. D., and Johansen-Berg, H. (2012). Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536.
Zhang, D., Ma, H., Huang, J., Zhang, X., Ma, H., and Liu, M. (2018a). Exploring the impact of chronic high-altitude exposure on visual spatial attention using the ERP approach. Brain Behav. 8:e00944. doi: 10.1002/brb3.944
Zhang, D., Zhang, X., Ma, H., Wang, Y., Ma, H., and Liu, M. (2018b). Competition among the attentional networks due to resource reduction in Tibetan indigenous residents: Evidence from event-related potentials. Sci. Rep. 8:610. doi: 10.1038/s41598-017-18886-7
Zhang, H., Lin, J., Sun, Y., Huang, Y., Ye, H., Wang, X., et al. (2012). Compromised white matter microstructural integrity after mountain climbing: Evidence from diffusion tensor imaging. High Alt. Med. Biol. 13, 118–125. doi: 10.1089/ham.2011.1073
Zhang, J. X., Chen, X. Q., Du, J. Z., Chen, Q. M., and Zhu, C. Y. (2005). Neonatal exposure to intermittent hypoxia enhances mice performance in water maze and 8-arm radial maze tasks. J. Neurobiol. 65, 72–84. doi: 10.1002/neu.20174
Zhang, J., Chen, J., Fan, C., Li, J., Lin, J., Yang, T., et al. (2017). Alteration of Spontaneous Brain Activity After Hypoxia-Reoxygenation: A Resting-State fMRI Study. High Alt. Med. Biol. 18, 20–26. doi: 10.1089/ham.2016.0083
Zhang, J., Yan, X., Shi, J., Gong, Q., Weng, X., and Liu, Y. (2010). Structural modifications of the brain in acclimatization to high-altitude. PLoS One 5:e11449. doi: 10.1371/journal.pone.0011449
Zhang, J., Zhang, H., Li, J., Chen, J., Han, Q., Lin, J., et al. (2013b). Adaptive modulation of adult brain gray and white matter to high altitude: Structural MRI studies. PLoS One 8:e68621. doi: 10.1371/journal.pone.0068621
Zhang, J., Zhang, H., Chen, J., Fan, M., and Gong, Q. (2013a). Structural modulation of brain development by oxygen: Evidence on adolescents migrating from high altitude to sea level environment. PLoS One 8:e67803. doi: 10.1371/journal.pone.0067803
Zhao, J. P., Zhang, R., Yu, Q., and Zhang, J. X. (2016). Characteristics of EEG activity during high altitude hypoxia and lowland reoxygenation. Brain Res. 1648, 243–249. doi: 10.1016/j.brainres.2016.07.013
Keywords: brain, high altitude, hypoxia, visual cortex, motor cortex, insular cortex, MRI
Citation: Zhang X and Zhang J (2022) The human brain in a high altitude natural environment: A review. Front. Hum. Neurosci. 16:915995. doi: 10.3389/fnhum.2022.915995
Received: 08 April 2022; Accepted: 25 July 2022;
Published: 15 September 2022.
Edited by:
Stephane Perrey, Université de Montpellier, FranceReviewed by:
Fali Li, University of Electronic Science and Technology of China, ChinaShinsuke Hidese, Teikyo University, Japan
Copyright © 2022 Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaxing Zhang, emhhbmdqaWF4aW5nQHhtdS5lZHUuY24=
 Xinjuan Zhang
Xinjuan Zhang Jiaxing Zhang
Jiaxing Zhang