
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 23 June 2022
Sec. Brain Imaging and Stimulation
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.910846
This article is part of the Research TopicMultimodal Magnetic Resonance Imaging Methods to Explore the Visual Pathway and Brain Network Changes in Blindness DiseaseView all 17 articles
Purpose: To study the changes in functional connections between the left and right hemispheres of patients with high myopia (HM) and healthy controls (HCs) by resting functional magnetic resonance imaging (fMRI) based on voxel-mirrored homotopic connectivity (VMHC). To study the changes in resting-state functional connectivity (rsFC) between the left and right hemispheres of patients with HM and healthy controls (HCS) at rest by using resting functional magnetic resonance imaging (fMRI) based on voxel-mirror homotopy connectivity (VMHC).
Patients and Methods: A total of 89 patients with HM (41 men and 48 women) and 59 HCs (24 men and 35 women) were collected and matched according to gender, age, and education level. The VMHC method was used to evaluate the changes in rsFC between cerebral hemispheres, and a correlation analysis was carried out to understand the differences in brain functional activities between the patients with HM and the HCs.
Results: Compared with the HCs, the VMHC values of the putamen and fusiform in the HM group were significantly lower (voxel-level p < 0.01, Gaussian random field correction cluster level p < 0.05).
Conclusion: This study preliminarily confirmed the destruction of interhemispheric functional connection in some brain regions of the patients with HM and provided effective information for clarifying the neural mechanism of patients with HM.
High myopia (HM) is a worldwide health problem that has attracted widespread attention. It seriously affects the quality of life of patients. The rate of incidence is increasing year by year. It affects about 2.9% of the global population (Holden et al., 2015). HM refers to myopia with diopter > −6D, mostly axial myopia, which is characterized by pathological and degenerative changes in the retina, choroid, and sclera, resulting in visual impairment. Therefore, the harm is great (Hsu et al., 2014). Eyeground structures such as the retina and optic nerve are closely related to the brain, especially visual-related areas (Wang et al., 2020). One study has shown that the retinal nerve fiber layer of patients with HM becomes thinner and that the optic papilla is deformed (Qu et al., 2018). Zhai et al. (2016) found that the visual function of patients with HM was abnormal, the functional connection of the posterior cingulate cortex/precuneus decreased, and the function of other brain regions changed. Therefore, they believed that abnormal visual experiences would lead to abnormal brain structure and function. In addition, a correlation analysis based on functional connectivity density (FCD) mapping and seeding showed that HM can lead to changes in brain morphology and functional connectivity density (Li et al., 2012; Zhai et al., 2016). Consequently, HM may lead to changes in brain functional activities.
In order to evaluate the changes in brain activity, functional magnetic resonance imaging (fMRI) is increasingly conducted in studies on HM. MRI has the characteristics of multi-sequence, multi parameters, no ionizing radiation, routine imaging, and functional imaging. It can find changes in brain activity and function in patients with HM, and it has a great application value. A recent study used functional magnetic resonance imaging to evaluate the activity of occipital visual cortex in patients with lens induced high myopia (IHM) under different visual stimulation. The results showed that the activity of visual cortex in patients with IHM was significantly lower than that in the normal group (Mirzajani et al., 2017). Patients with HM have certain changes in visual pathway area and limbic system structure (Huang et al., 2018). Homotopic functional connectivity is the connection between mirror regions of the cerebral hemisphere, and it is also one of the most remarkable features of the internal functional structure of the brain (Salvador et al., 2005; Stark et al., 2008). It may reflect that inter-hemispheric communication plays a very important role in integrating brain function based on coherent cognition and behavior. At the same time, it has significant spatial variability related to function and is disturbed by a variety of pathological conditions (Mancuso et al., 2019). Previous studies related to MRI have found that HM affects brain neural activity and functional connection, but the specific mechanism has not been fully clarified.
Voxel-mirrored homotopic connectivity (VMHC) is a method proposed by Stark DE (Stark et al., 2008) to explore the differences in functional connections between voxels in bilateral hemispheric symmetrical systems and evaluate their coordination. It is a functional magnetic resonance imaging technology used to explore brain tissue patterns based on resting functional connections. This method needs to conduct anatomical structure correction to make the bilateral cerebral hemispheres basically symmetrical and calculate the time-series correlation between each voxel and its contralateral hemisphere allele. The lower the VMHC value, the worse the coordination of allelic voxel functional activities between the bilateral cerebral hemispheres. On the contrary, the higher VMHC value, the coordination will be better. Different MRI analysis methods have different emphases in expressing the functional changes of brain regions. VMHC not only directly compares functional connections between cerebral hemispheres in the resting state but also accurately and effectively evaluates changes in functional connections between cerebral hemispheres related to patients' behavior and cognition. It has certain efficiency, stability, and safety in studies on brain information integration. In recent years, more and more studies have focused on VMHC and found that it is related to a variety of diseases and functional states, and has a good correlation. Kelly et al. found that there is an association between long-term exposure to cocaine and large-scale brain circuit interruption supporting cognitive control through the VMHC method (Kelly et al., 2011). Luo et al. (2018) found that the VMHC value was lower in some brain regions of multi-domain amnestic mild cognitive impairment patients and showed more severe interhemispheric communication defects than that of single-domain amnestic mild cognitive impairment patients. In addition, there were VMHC abnormalities in multiple brain regions of patients with type 2 diabetes, indicating that the functional coordination between the same brain regions was generally impaired (Zhang Y. et al., 2021). In conclusion, rs-fMRI analysis based on VMHC can provide additional information beyond the classical FC index for understanding the neural mechanism of executive function changes between cerebral hemispheres, and VMHC is a reliable neural index for brain function reorganization. Through previous studies, it has also been found that VMHC has been used for the analysis of a variety of eye diseases, such as primary open-angle glaucoma (Liu et al., 2008), monocular blindness (Shao et al., 2018), and strabismus amblyopia (Zhang S. et al., 2021). We speculate that patients with HM, which affects brain visual imaging, may have abnormal brain functional activities. Therefore, this study discusses changes in cerebral hemisphere functional connection in patients with HM by VMHC analysis, to provide a new idea for the neural mechanism of HM.
Participants were selected from the hospital during the period from September 2018 to September 2020. The cohort included 89 patients with HM and 59 HCs without ametropia. The inclusion criteria for patients with myopia were as follows: (1) aged 18–60 years, (2) meeting the diagnostic criteria of high myopia, (3) right-hand dominance, and (4) no other ophthalmic diseases. The exclusion criteria were: (1) other ophthalmic diseases, (2) unilateral myopia, (3) abnormal brain structure such as tumor or subdural hematoma, (4) mental diseases and unable to cooperate, and (5) complications of HM including HM optic neuropathy and HM macular degeneration. The age, sex, hand advantage, and education level of the HCs were matched with those of the HM group and met the following criteria: (1) no ametropia and other ophthalmic diseases, (2) no mental diseases, and (3) normal routine brain magnetic resonance imaging. All the subjects underwent MRI scanning, and those with incomplete MRI data or excessive head movements were excluded. The study was approved by the medical ethics committee of the First Affiliated Hospital of Nanchang University, and all the participants were informed and agreed.
All MRI data were collected with a Siemens Trio 3.0T scanner by implementing an 8-channel head coil. MRI scanning was performed on each subject. Whole-brain T1-weighted images were obtained with magnetization-prepared gradient echo image (MPRAGE) with these parameters: repetition time = 1,900 ms, echo time (TE) = 2.26 ms, thickness = 1, no intersection gap, acquisition matrix =2 56 × 256, field of view (FOV) = 240 × 240 mm2, and flip angle = 12°. Functional images were obtained using a gradient-echo-planar imaging sequence.
The preprocessing reason brain imaging data processing and analysis toolbox (DPABI, http://rfmri.org/dpabi) are completed based on statistical parameter mapping (SPM12), which runs on MATLAB 8.4.0 (MathWorks, Natick, MA, United States). The main preprocessing steps were: discard the first 10 volumes, slice timing, spatial rearrangement, head motion correction, individual registration between high-resolution T1 and EPI images, spatial normalization, and spatial smoothing that can reduce the registration error and increase the normality of statistical data. The RS fMRI data set is registered in the space of Montreal Neurological Institute (MNI) and resampled to a 3 × 3 × 3 mm3 cube voxel; head movement data with a maximum translation of more than 2 mm or a maximum rotation of 2° were excluded from the final analysis. The preprocessed data are divided into typical frequency bands (0.01–0.1 Hz). Linear regression is performed on covariates such as head movement, whole-brain parenchyma, and cerebrospinal fluid signals to reduce the impact (including white matter, cerebrospinal fluid, and head movement parameters based on Friston 24 parameter model) (Friston et al., 1996).
The value of VMHC is calculated as the Pearson correlation coefficient between the voxel time series between each pair of mirror hemispheres (Zuo et al., 2010). The REST software was used to calculate the Pearson's correlation between each group of symmetrical alleles in both cerebral hemispheres one by one, and then the fisher-z transform was performed on these correlation values. The results of correlation values formed the VMHC diagram and were used for subsequent group-level analysis. To reduce the difference between different subjects, it is also necessary to standardize the mean of the whole brain VMHC map.
This study was tested with the SPSS software (version 13.0; SPSS Inc., Chicago, IL, United States). The changes in VMHC z-diagram between the healthy control group and the HM group were evaluated by two-sample t-tests. All important clusters were reported on MNI coordinates, and the T value of peak voxels was determined (voxel-level P < 0.01, Gaussian random field [GRF] correction, cluster-level P < 0.05).
The basic information of patients with HM and the healthy control group is shown in Table 1.
VMHC values changed between the HM group and the HC group (Figure 1); Compared with HCS, the VMHC in the lenticular nucleus and fusiform gyrus decreased in patients with HM (Figure 2, Table 2).
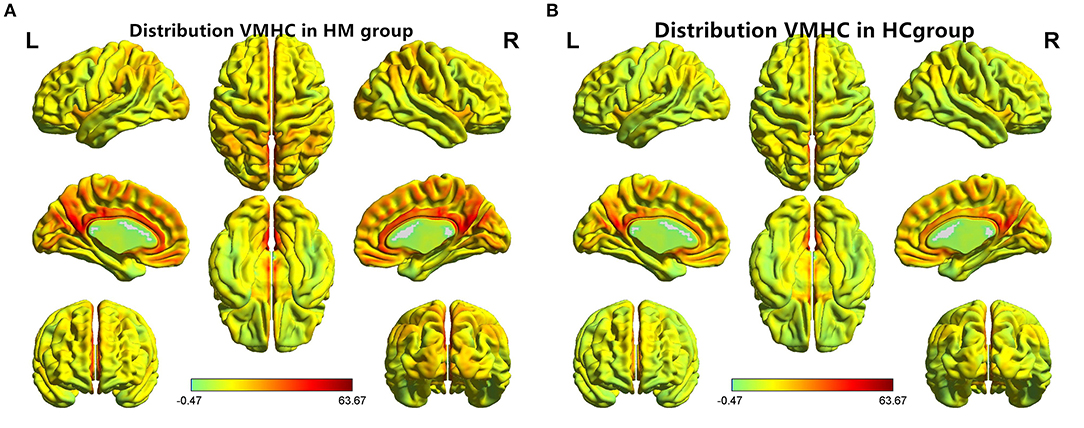
Figure 1. Distribution patterns of VMHC were observed at the group level in patients with (A) HM and (B) HCs. VMHC, voxel-mirrored homotopic connectivity; HM, high myopia; HCs, healthy controls.
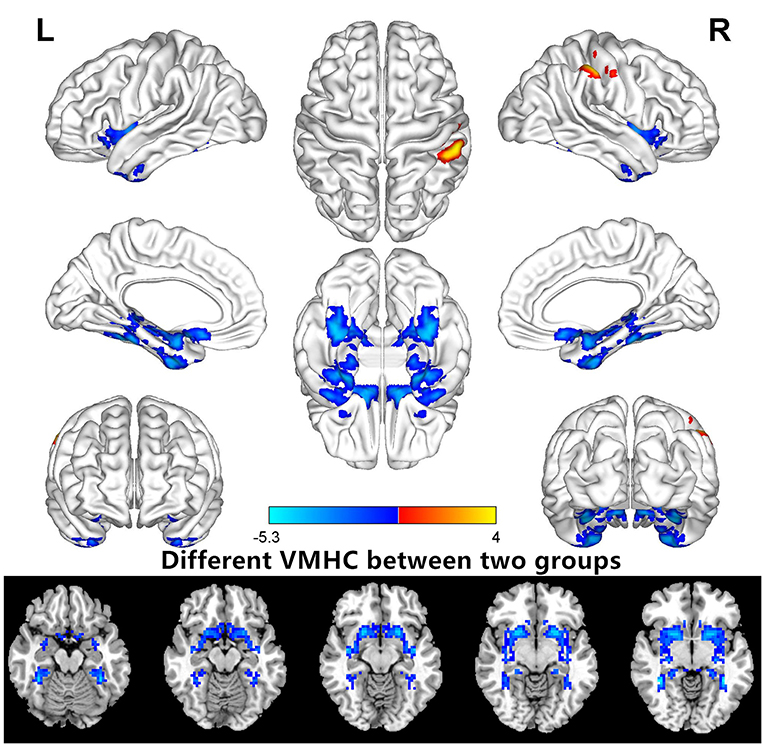
Figure 2. Different VMHCs between the HM and HC groups (voxel level P < 0.01, GRF correction, cluster level P < 0.05). VMHC, voxel-mirrored homotopic connectivity.
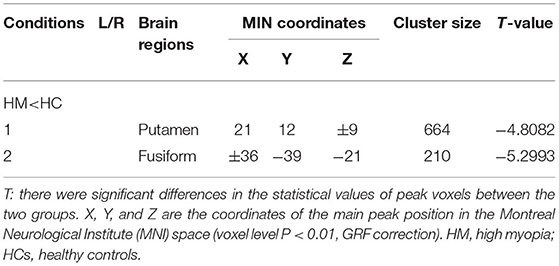
Table 2. Different voxel-mirrored homotopic connectivity (VMHC) values of brain areas between the HM and HC groups (voxel level P < 0.01, Gaussian random field, GRF, correction).
In this study, we evaluated the VMHC difference between the HM group and the HC group. The study found that compared with the HCs, the VMHC values of the putamen and fusiform gyrus of the patients with HM were decreased.
The putamen is a structure located under the cortex and a part of the dorsal striatum of the basal ganglia. It is considered to be related to reinforcement learning, motor control, and language function (Vinas-Guasch and Wu, 2017). Recently, it has been found that the putamen is closely related to processes that visually guided and internally guided force control in humans. A study on changes in visual movement and functional activities in brain regions found that 91% of caudate cells and 65% of putamen cells changed when monkeys performed visual tasks. Therefore, the activity of neurons in the caudate and putamen during a visuomotor task will be activated whether there is motor behavior or not (Romero et al., 2008). Li et al. found that the regional homology (ReHo) of bilateral putamen in patients with idiopathic rapid eye movement sleep behavior disorder (iRBD) was significantly reduced, the tracer uptake of bilateral putamen and left caudate nucleus was also significantly reduced, and abnormal spontaneous brain activity of bilateral putamen in patients with iRBD was detected when studying the correlation between iRBD and the putamen (Li et al., 2019). It can be seen from the above that the change in the putamen is related to the abnormal state of the body. When studying changes in brain structure between patients with HM and HCs, Huang et al. (2018) found that the global gray matter volume (GMV) of the bilateral putamen in patients with HM was increased. The authors speculated that HM may lead to structural changes in the bilateral putamen, to compensate for the motor function of HM. In our study, we found that compared with the HC group, the VMHC value of the putamen in patients with HM was decreased, which may be related to abnormal changes in this area due to the abnormal visual experience and behavior of HM. It is consistent with the above research results. Hence, we speculate that HM will lead to disorder in the putamen connection and affect visual function.
The fusiform gyrus is located in the middle and bottom of the visual joint cortex. It is the largest macro-anatomical structure in the advanced visual cortex and has a wide range of functions; however, it is highly controversial (Cohen et al., 2000; Huth et al., 2012; Cukur et al., 2013). At first, it was well-known for its facial-recognition function, but with the deepening of research, it was found that it was more involved in processing high-order visual information, especially related to face, body, and stimuli characterized by high spatial frequency, and was responsible for the recognition of secondary classification of objects (Weiner and Zilles, 2016; Rokem et al., 2017). Moreover, the fusiform gyrus has a special visual processing mechanism for text and objects (Chen et al., 2019). Tanja Kassuba et.al. found that the lateral occipital cortex (LO), fusiform gyrus, and intraparietal sulcus (IPS) play an important role in the integration of visual-tactile objects (Kassuba et al., 2014). In addition, a study on the frequency-dependent spontaneous neural activity of primary angle-closure glaucoma found that the ALFF values of multiple brain regions were changed. The ALFF values in the bilateral frontal lobe, right fusiform gyrus, and right posterior cerebellar lobe were higher in slow band 5 than in slow band 4. It was considered that the abnormal spontaneous neural activity of patients with PACG indicates that the cognitive, visual, and emotional functions of these patients are impaired (Jiang et al., 2019). In our study, we found that the VMHC value of the fusiform gyrus in the patients with HM was decreased. We speculated that abnormal visual experience may lead to structural changes in the fusiform gyrus and even affect its functions, such as visual and recognition functions.
However, there are some limitations to this experiment. For example, the sample size is not large enough, and the head movement and cerebrospinal fluid movement effects of the patients during examination have a certain impact on the experiment. However, to minimize such effects, we have strictly selected qualified examination results and eliminated the effects of movement on the brain through statistical methods. In addition, the results of this study were obtained in the resting state of participants. In future studies, we will also combine the resting-state and task-state fMRI to research the changes of abnormal brain regions in different states. At the same time, we will expand the sample size and improve the applicability and accuracy of the results.
In conclusion, compared with the HCs, VMHC values in different brain regions of the patients with HM have different changes, suggesting that HM may cause changes in functional connections between cerebral hemispheres in some regions, resulting in functional damage, which may become a breakthrough in studying the divine mechanism of changes in visual and motor functions in HM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
X-RW: guidance on the content of this article. X-LC: charts and diagrams graphic typesetting. S-YL, LS, HH, and P-PZ: clinical data collection. All authors contributed to the article and approved the submitted version.
This work was supported by Key research plan of Jiangxi Provincial Department of Science and Technology (No. 20192BBG70042), Jiangxi Province Education Department Key Foundation (No. GJJ160033), Health Development Planning Commission Science Foundation of Jiangxi Province (No. 20185118), Technology and Science Foundation of Jiangxi Province (No. 20141BBG70027), Jiangxi Province Education Department Scientific Research Foundation (No. GJJ13147), Health and Family Planning Commission Traditional Chinese Medicine Foundation of Jiangxi Province (No. 2017A001), and Basic Health Appropriate Technology Spark Promotion Program of Jiangxi Province (No. 20188007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank for the support of NSFC, etc.
Chen, Y., Xiang, C., Liu, W., Zhu, P., Ye, L., Li, B., et al. (2019). Application of amplitude of lowfrequency fluctuation to altered spontaneous neuronal activity in classical trigeminal neuralgia patients: A restingstate functional MRI study. Mol. Med. Rep 20, 1707–1715. doi: 10.3892/mmr.2019.10404
Cohen, L., Dehaene, S., Naccache, L., Lehericy, S., Dehaene-Lambertz, G., Henaff, M., et al. (2000). The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 123, 291–307. doi: 10.1093/brain/123.2.291
Cukur, T., Huth, A., Nishimoto, S., and Gallant, J. (2013). Functional subdomains within human FFA. J. Neurosci. 33, 16748–16766. doi: 10.1523/JNEUROSCI.1259-13.2013
Friston, K. J., Williams, S., Howard, R., Frackowiak, R., and Turner, R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. doi: 10.1002/mrm.1910350312
Holden, B., Wilson, D., Jong, M., Sankaridurg, P., Fricke, T., Smith, E. L. I. I., et al. (2015). Myopia: a growing global problem with sight-threatening complications. Commun. Eye Health. 28, 35. Available online at: https://pubmed.ncbi.nlm.nih.gov/26692649/
Hsu, C., Chen, S., Li, A., and Lee, F. (2014). Systolic blood pressure, choroidal thickness, and axial length in patients with myopic maculopathy. J. Chin. Med. Assoc. 77, 487–491. doi: 10.1016/j.jcma.2014.06.009
Huang, X, Hu, Y., Zhou, F., Wu, Y., Xu, X., Wu, Y., et al. (2018). Altered whole-brain gray matter volume in high myopia patients: a voxel-based morphometry study. Neuroreport. 29, 760–767. doi: 10.1097/WNR.0000000000001028
Huth, A., Nishimoto, S., Vu, A., and Gallant, J. (2012). A continuous semantic space describes the representation of thousands of object and action categories across the human brain. Neuron. 76, 1210–1224. doi: 10.1016/j.neuron.2012.10.014
Jiang, F., Yu, C., Zuo, M., Zhang, C., Wang, Y., Zhou, F., et al. (2019). Frequency-dependent neural activity in primary angle-closure glaucoma. Neuropsychiatr. Dis. Treat. 15, 271–282. doi: 10.2147/NDT.S187367
Kassuba, T., Klinge, C., Holig, C., Roder, B., and Siebner, H. (2014). Short-term plasticity of visuo-haptic object recognition. Front. Psychol. 5, 274. doi: 10.3389/fpsyg.2014.00274
Kelly, C., Zuo, X., Gotimer, K., Cox, C., Lynch, L., Brock, D., et al. (2011). Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry. 69, 684–692. doi: 10.1016/j.biopsych.2010.11.022
Li, G., Chen, Z., Zhou, L., Yao, M., Luo, N., Kang, W., et al. (2019). Abnormal intrinsic brain activity of the putamen is correlated with dopamine deficiency in idiopathic rapid eye movement sleep behavior disorder. Sleep Med. 75, 73–80. doi: 10.1016/j.sleep.2019.09.015
Li, Q., Guo, M., Dong, H., Zhang, Y., Fu, Y., and Yin, X. (2012). Voxel-based analysis of regional gray and white matter concentration in high myopia. Vision. Res. 58, 45–50. doi: 10.1016/j.visres.2012.02.005
Liu, T., Zeng, D., Zeng, C., and He, X. (2008). Association between MYOC.mt1 promoter polymorphism and risk of primary open-angle glaucoma: a systematic review and meta-analysis. Med. Sci. Monit. 14, A87–A93. Available online at: https://pubmed.ncbi.nlm.nih.gov/18591929/
Luo, X., Li, K., Zeng, Q., Huang, P., Jiaerken, Y., Qiu, T., et al. (2018). Decreased bilateral FDG-PET uptake and inter-hemispheric connectivity in multi-domain amnestic mild cognitive impairment patients: a preliminary study. Front. Aging. Neurosci. 10, 161. doi: 10.3389/fnagi.2018.00161
Mancuso, L., Costa, T., Nani, A., Manuello, J., Liloia, D., Gelmini, G., et al. (2019). The homotopic connectivity of the functional brain: a meta-analytic approach. Sci. Rep. 9, 3346. doi: 10.1038/s41598-019-40188-3
Mirzajani, A., Ghorbani, M., Rasuli, B., and Mahmoud-Pashazadeh, A. (2017). Effect of induced high myopia on functional MRI signal changes. Phys. Med. 37, 32–36. doi: 10.1016/j.ejmp.2017.04.004
Qu, D., Lin, Y., Jiang, H., Shao, Y., Shi, Y., Airen, S., et al. (2018). Retinal nerve fiber layer (RNFL) integrity and its relations to retinal microvasculature and microcirculation in myopic eyes. Eye Vis. 5, 25. doi: 10.1186/s40662-018-0120-3
Rokem, A., Takemura, H., Bock, A., Scherf, K., Behrmann, M., Wandell, B., et al. (2017). The visual white matter: The application of diffusion MRI and fiber tractography to vision science. J. Vis. 17, 4. doi: 10.1167/17.2.4
Romero, M., Bermudez, M., Vicente, A., Perez, R., and Gonzalez, F. (2008). Activity of neurons in the caudate and putamen during a visuomotor task. Neuroreport. 19, 1141–1145. doi: 10.1097/WNR.0b013e328307c3fc
Salvador, R., Suckling, J., Schwarzbauer, C., and Bullmore, E. (2005). Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos. Trans. R. Soc. Lond B Biol. Sci. 360, 937–946. doi: 10.1098/rstb.2005.1645
Shao, Y., Bao, J., Huang, X., Zhou, F., Ye, L., Min, Y., et al. (2018). Comparative study of interhemispheric functional connectivity in left eye monocular blindness versus right eye monocular blindness: a resting-state functional MRI study. Oncotarget. 9, 14285–14295. doi: 10.18632/oncotarget.24487
Stark, D., Margulies, D., Shehzad, Z., Reiss, P., Kelly, A., Uddin, L., et al. (2008). Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 28, 13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008
Vinas-Guasch, N., and Wu, Y. (2017). The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct. Funct. 222, 3991–4004. doi: 10.1007/s00429-017-1450-y
Wang, H., Li, S., Chen, X., Wang, Y., Li, J., and Wang, Z. (2020). Cerebral blood flow alterations in high myopia: an arterial spin labeling study. Neural. Plast. 2020, 6090262. doi: 10.1155/2020/6090262
Weiner, K., and Zilles, K. (2016). The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 83, 48–62. doi: 10.1016/j.neuropsychologia.2015.06.033
Zhai, L., Li, Q., Wang, T., Dong, H., Peng, Y., Guo, M., et al. (2016). Altered functional connectivity density in high myopia. Behav. Brain Res. 303, 85–92. doi: 10.1016/j.bbr.2016.01.046
Zhang, S., Gao, G., Shi, W., Li, B., Lin, Q., Shu, H., et al. (2021). Abnormal interhemispheric functional connectivity in patients with strabismic amblyopia: a resting-state fMRI study using voxel-mirrored homotopic connectivity. BMC Ophthalmol. 21, 255. doi: 10.1186/s12886-021-02015-0
Zhang, Y., Wang, J., Wei, P., Zhang, J., Zhang, G., Pan, C., et al. (2021). Interhemispheric resting-state functional connectivity abnormalities in type 2 diabetes patients. Ann. Palliat. Med. 10, 8123–8133. doi: 10.21037/apm-21-1655
Keywords: high myopia, voxel-mirrored homotopic connectivity, functional magnetic resonance imaging, putamen, fusiform gyrus
Citation: Cheng Y, Chen X-L, Shi L, Li S-Y, Huang H, Zhong P-P and Wu X-R (2022) Abnormal Functional Connectivity Between Cerebral Hemispheres in Patients With High Myopia: A Resting FMRI Study Based on Voxel-Mirrored Homotopic Connectivity. Front. Hum. Neurosci. 16:910846. doi: 10.3389/fnhum.2022.910846
Received: 01 April 2022; Accepted: 19 April 2022;
Published: 23 June 2022.
Edited by:
Xin Huang, Jiangxi Provincial People's Hospital, ChinaReviewed by:
Yu Lin Zhong, Jiangxi Provincial People's Hospital, ChinaCopyright © 2022 Cheng, Chen, Shi, Li, Huang, Zhong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Rong Wu, d3hyOTgwMjFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.